Abstract
Probiotics could improve cognitive functions in patients with neurological disorders such as Alzheimer’s disease, but the effects on cognitive function in healthy older adults without cognitive impairment need further study. The purpose of this study was to investigate the effect of Bifidobacterium longum BB68S (BB68S) on cognitive functions among healthy older adults without cognitive impairment. A randomized, double-blind, placebo-controlled trial was conducted with 60 healthy older adults without cognitive impairment who were divided into probiotic or placebo groups and required to consume either a sachet of probiotic (BB68S, 5 × 1010 CFU/sachet) or placebo once daily for 8 weeks. The Montreal Cognitive Assessment (MoCA) was used as an inclusion screening tool to screen elderly participants with healthy cognitive function in our study, and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess cognitive function in subjects before and after intervention as an assessment tool. BB68S significantly improved subjects’ cognitive functions (total RBANS score increased by 18.89 points after intervention, p < 0.0001), especially immediate memory, visuospatial/constructional, attention, and delayed memory domains. BB68S intervention increased the relative abundances of beneficial bacteria Lachnospira, Bifidobacterium, Dorea, and Cellulosilyticum, while decreasing those of bacteria related to cognition impairment, such as Collinsella, Parabacteroides, Tyzzerella, Bilophila, unclassified_c_Negativicutes, Epulopiscium, Porphyromonas, and Granulicatella. In conclusion, BB68S could improve cognitive functions in healthy elderly adults without cognitive impairment, along with having beneficial regulatory effects on their gut microbiota. This study supports probiotics as a strategy to promote healthy aging and advances cognitive aging research.
Keywords: probiotic, cognitive function, healthy older adults, gut microbiota
1. Introduction
Research in the field of aging and neuroscience reveals that the human brain shrinks with the process of normal aging, while the ventricles expand and both gray and white matter are reduced [1], resulting in alterations in neurological function that cause age-related cognitive decline [2,3]. As the population ages, a great deal of the elderly are at risk of age-related cognitive decline, which not only threatens their personal lives but also poses a challenge to society [4]. Therefore, it is necessary to develop efficacious interventions and preventative strategies for age-related cognitive decline and promote healthy aging of the global population.
The gut microbiota was first defined by Lederberg and McCray, accentuating the importance of microorganisms located in the human body in health and diseases [5]. Compelling evidence shows that the gut microbiota plays a critical role in powerfully modulating brain activity through the gut-brain axis [6,7,8]. Furthermore, owing to its reciprocal relationship with age, the gut microbiota changes during host aging, is altered in age-related diseases, and plays a role in modifying age-related health impairment of the host [9,10,11]. Accumulating evidence links the gut microbiota to the cognitive function of older adults. Regulating the gut microbiota as a prevention therapy for age-related cognitive decline has become an active research strategy.
Probiotics, live microorganisms that provide health benefits when administered regularly in the host, substantially affect the composition as well as metabolic output of the gut microbiome [12,13,14]. Probiotics can impact brain health and host behaviors through the microbiota-gut-brain axis [15,16,17]. Their use in the treatment of neurological diseases is being actively investigated [18,19,20]. Numerous animal studies have confirmed that probiotics can improve brain health, along with many human studies that have also explored the impact of probiotic intervention on cognitive function, which have achieved some positive results [21,22,23]. However, existing research has mainly focused on patients with cognitive dysfunction, such as mild cognitive impairment, Alzheimer’s disease, and severe depressive disorder [24,25]. Studies aimed at healthy elderly people without cognitive impairment are scarce. Therefore, more population studies targeting the healthy elderly are urgently needed to supplement evidence on the role of probiotics in improving the cognitive function of this specific population.
Bifidobacterium longum BB68S (BB68S, CGMCC No. 14168) is a potential probiotic. The 16S ribonucleic acid (rRNA) sequence of B. longum BB68S is available in the NCBI database under sequence number OP984807. Our previous in vivo and in vitro study evaluated and verified its safety [26]. In an intervention trial, we observed that BB68S can relieve constipation and regulate the gut microbiota [27]. Considering its other potential functions, we aimed to investigate the effects of BB68S in improving cognitive function in healthy older adults. We also focused on providing clinical evidence for validating the properties and effects of probiotics on cognitive aging and healthy aging, thereby supporting their better implementation in the expanding fields of healthcare and alternative medicine.
2. Methods
2.1. Study Design
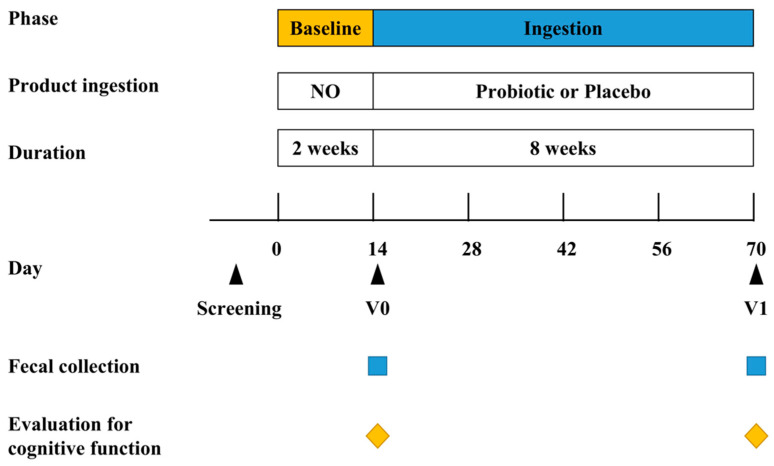
To investigate the effects of BB68S on the cognitive function of healthy elderly people aged 60–75 years, we designed a randomized, double-blind, placebo-controlled trial. The sample size was estimated according to Assessment of Neurological Status (ANS) scores demonstrated by Xiao et al. [28]. We calculated that 30 subjects per group were needed with an α-error of 0.05 and β-error of 0.20. This 10-week study included a 2-week baseline period and an 8-week ingestion period (Figure 1). To remove the impact of any previous intake, a 2-week baseline period was required. The participants were solicited to not change their normal dietary patterns throughout the baseline period and were not allowed to consume probiotics or any dietary supplements with probiotics. On day 14 ± 1, their fecal samples were collected and the cognitive functions of the participants were evaluated. The participants were randomly divided into either the placebo or probiotic groups. During the ingestion period, the subjects were required to consume a sachet of probiotic (BB68S, 5 × 1010 CFU/sachet) or placebo (maltodextrin powder without probiotics) after lunch or dinner daily for 8 weeks. Throughout the intervention period, the subjects were asked to abstain from consuming any other probiotics or any dietary supplements with probiotics while they were required to be consistent with their daily diet and exercise, thus excluding their corresponding influence on the test results. To monitor the stability of their lifestyle, subjects were requested to record their daily dietary intake. The subjects received adequate training before recording in their daily diaries. In addition, any adverse events or symptoms of discomfort were also required to be recorded. On day 70 ± 1, upon the completion of the ingestion period, fecal samples were collected in a sterile tube and stored at −80 °C until further analyses. The cognitive functions of the subjects were again evaluated using the RBANS scores. The study was authorized by the China Agricultural University Ethics Committee (CAUHR-2021021) and filed under registration number ChiCTR2200062331 (http://www.chictr.org.cn, accessed on 21 November 2022). Written informed permission was acquired from each subject.
Figure 1.
Study design. Probiotic: lyophilized powder containing B. longum BB68S. Placebo: maltodextrin. V0–V1: visit 0–visit 1.
2.2. Participants
The subjects’ personal information was collected, including age, sex, disease status, and drug use in the first round of screening. Experienced researchers assessed their physical conditions based on medication history and dietary habits, and patients were screened according to the inclusion and exclusion criteria.
2.3. Inclusion and Exclusion Criteria
We selected subjects based on the following inclusion criteria: (i) aged 60–75 years at the time of screening and (ii) with healthy cognitive function judged by the Montreal Cognitive Assessment (MoCA) screening tool. The specific criteria were as follows: total MoCA scores were 19 or higher for those with 6 or fewer years of education, 22 or higher for those with 7–12 years of education, and 24 or higher for those with 12 or more years of education. In our study, we used the Chinese Beijing Version of MoCA (http://www.mocatest.org, accessed on 21 November 2022) to define the cognitive health of the elderly. The exclusion criteria were as follows: (i) obvious cognitive decline, or diagnosis with Alzheimer’s disease, mild cognitive impairment, etc.; (ii) hearing, visual, or communication disabilities or difficulties, incapable of living independently, or surgical history of digestive system resection; (iii) history or clinical trace of nervous system disease or psychosis; (iv) history of serious diseases, such as those of the heart, liver, kidney, or hematopoietic system; (v) use of the test-related product (other probiotic products or antibiotics, anti-inflammatory medications, gastrointestinal medicine) within a short period; (vi) unable to eat the test product according to the regulations, and (vii) participation in other clinical studies.
2.4. Randomization and Blinding
During the screening process, demographic data were collected, and the examination for cognitive ability assessment was conducted. The research assistant who was otherwise not involved in the study completed randomization using SAS program version 9.4 with Pocock and Simon minimum randomization methods and was required to conceal the randomization results from all researchers and subjects. The randomization method performed dynamic randomization to maximally balance the baseline characteristics between the two groups. The randomized data were stratified by factors including sex (male, female), age (60~64 years old, 65~69 years old, ≥75 years old), and MoCA scores (19~21, 22~24, ≥25). The subjects were evenly and randomly allocated to the probiotic or placebo groups. The research team members remained blind to each participant’s assigned group until all data were collected.
2.5. Fecal Sample Collection
Fecal samples were collected before and after intervention. We provided a cryogenic storage box (4 °C) and a sterile tube with a scoop inside the lid, instructing subjects to gather fecal samples into the tubes within 24 h before visiting and transporting them in the cryogenic storage box to the research site. The tubes were kept tightly sealed and stored in the cryogenic storage box until they were sent to the laboratory, following which, the samples were immediately stored at −80 °C until further analyses.
2.6. Evaluation of Cognitive Functions
Before and after intervention, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) as an assessment tool to assess the subjects’ cognitive function. During the assessment, the subjects were placed in a quiet and undisturbed environment and simultaneously evaluated by two researchers experienced in using the evaluation form. One of the two researchers was required to speak uniformly and clearly to inform subjects of the description of each item, to ensure that they heard all instructions, and finally compute the average of the original scores of the 12 items recorded simultaneously by the two researchers. The twelve averages were then converted to five global domain scores as the final score for the corresponding subjects.
2.7. Gut Microbiota Analysis
2.7.1. Genomic DNA Extraction, Amplification, and Sequencing
The bacterial genomic DNA was extracted from fecal samples according to the method described by Tang et al. [29]. Purity was determined by 1% agarose gel electrophoresis and high-quality DNA (OD260/280 ≥ 1.5, ≥ 150 ng) was amplified using the 338F-806R primer [30] in the hypervariable V3–V4 region of the bacterial 16S rRNA gene. High-quality PCR products were sequenced on the Illumina MiSeq PE300 platform.
2.7.2. Processing of Sequencing Data
The original reads were demultiplexed and quality-filtered using the fastp 0.20.0 tool [31] and integrated using FLASH 1.2.7 [32]. UPARSE 7.1 (available at http://drive5.com/uparse/, accessed on 21 November 2022) was used to cluster the operational taxonomic units (OTUs) using a 97% similarity criterion [33], and chimeric sequences were discovered and eliminated. RDP Classifier version 2.2 [34] was used with a confidence threshold of 0.7 to evaluate the taxonomy of each OTU representative sequence according to the 16S rRNA database (Silva v138).
2.8. Statistical Analysis
When describing the baseline characteristics, we used the intent-to-treat dataset, comprising all subjects who underwent random assignment to a treatment group, and when analyzing the main results, the per-protocol set was adopted. Normality tests were performed on all continuous variables using the Kolmogorov-Smirnov test. We calculated means (SDs) for continuous variables and counts (percentages) for categorical variables. The independent samples t-test was used for the comparison of continuous variables and the chi-square test was used for the comparison of categorical variables. For the primary outcome, RBANS scores at week 8, an analysis of covariance model adjusted for baseline scores was used to examine the difference between the probiotic and placebo groups. With a significant threshold setting at p < 0.05, data analysis was performed using IBM SPSS statistics 23.0.
3. Results
3.1. Baseline Characteristics
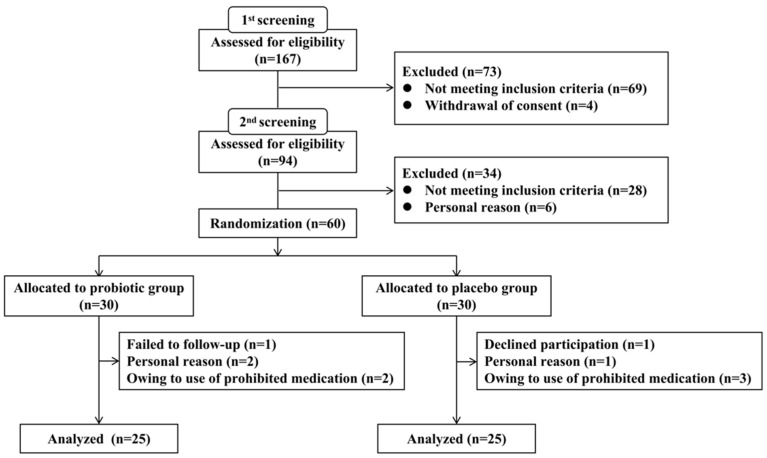
A total of 167 subjects were recruited for screening, and 60 subjects were chosen based on the inclusion criteria. The subjects were randomized to give 30 subjects in each of the placebo and probiotic groups. Ten subjects withdrew their consent and dropped out, including five from each of the placebo and probiotic groups, with 50 subjects finally finishing the trial (Figure 2). The intervention was carried out with 83% compliance and no clinically significant adverse events occurred. Table 1 summarizes the demographic and clinical variables at baseline, including age, weight, BMI, sex, education level, MoCA score, and RBANS score. These characteristics were comparable between the groups (all p-values were greater than 0.05).
Figure 2.
Trial profile.
Table 1.
Demographic data of the study subjects.
| BB68S | Placebo | p-Value | |
|---|---|---|---|
| Age (years) | 64.10 ± 3.40 | 64.50 ± 3.79 | 0.67 1 |
| Sex | |||
| Male (%) | 12 (40) | 13 (43) | 0.77 2 |
| Female Male (%) | 18 (60) | 17 (57) | |
| Height (cm) | 164.79 ± 8.18 | 165.09 ± 9.05 | 0.89 1 |
| Weight (kg) | 67.13 ± 13.50 | 67.21 ± 12.50 | 0.98 1 |
| BMI (kg/m2) | 24.55 ± 3.47 | 24.49 ± 3.03 | 0.95 1 |
| Education | |||
| Elementary or less | 10 | 10 | |
| Junior-high school | 13 | 12 | |
| High school or more | 7 | 8 | |
| MoCA total score | 23.03 ± 2.50 | 22.97 ± 2.43 | 0.92 1 |
| RBANS total score | 186.90 ± 17.22 | 186.93 ± 15.62 | 0.99 1 |
1 Independent sample’s t-test. 2 Chi-square test.
3.2. Primary Outcomes for Cognitive Function
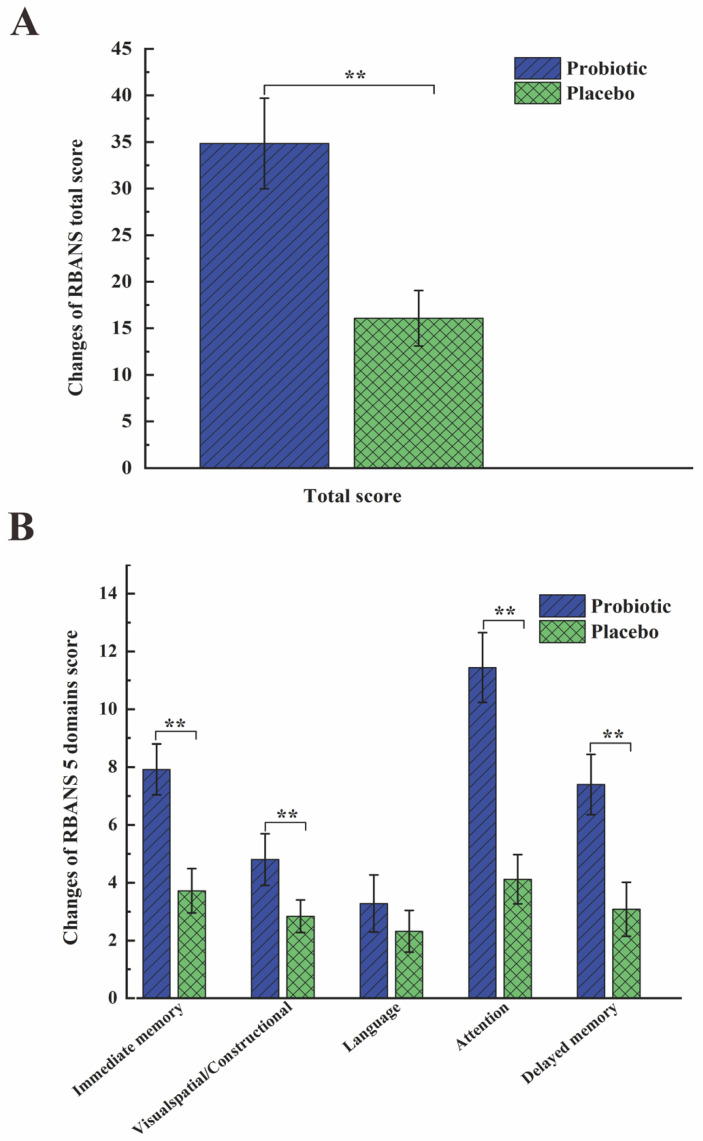
Each subject underwent RBANS assessment at baseline and at week 8, and the effects of BB68S on cognitive functioning were evaluated (Table 2). BB68S intervention significantly enhanced the cognitive function total score by 18.89 points (95% CI 14.98 to 22.80, p < 0.0001). As shown in Table, the BB68S group had significantly higher scores in 4 domains than the control group after 8 weeks of intervention. The score differences (95% confidence intervals) between groups were 4.36 (2.95 to 5.76, p < 0.0001) for immediate memory, 2.01 (1.18 to 2.83, p < 0.0001) for visuospatial/constructional, 7.29 (4.77 to 9.80, p < 0.0001) for attention, and 4.28 (95% CI 2.26 to 6.30, p < 0.0001) for delayed memory. BB68S’s effect on language was nonsignificant (p = 0.141). Changes in RBANS scores after the intervention were calculated and are exhibited in Figure 3 for the two groups. Subjects in the BB68S group had significantly greater changes in total score and in almost all domains, except for the language domain.
Table 2.
The effects of BB68S on RBANS scores.
| Baseline | Week 8 | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 25) |
BB68S (n = 25) |
Placebo (n = 25) |
BB68S (n = 25) |
Difference (95% CI) | p-Value | |
| Total score | 185.88 ± 16.77 | 186.88 ± 18.32 | 201.96 ± 17.14 | 221.72 ± 16.20 | 18.89 (14.98 to 22.80) | <0.0001 |
| Immediate memory | 31.56 ± 5.03 | 32.20 ± 5.69 | 35.28 ± 5.09 | 40.12 ± 4.35 | 4.36 (2.95 to 5.76) | <0.0001 |
| List learning | 20.28 ± 3.03 | 20.84 ± 3.82 | 22.56 ± 3.19 | 25.52 ± 4.00 | 2.49 (1.24 to 3.74) | <0.0001 |
| Story memory | 11.28 ± 3.54 | 11.36 ± 2.93 | 12.72 ± 3.49 | 14.60 ± 2.06 | 1.82 (0.81 to 2.84) | <0.0001 |
| Visuospatial/Constructional | 30.56 ± 4.08 | 30.68 ± 5.13 | 33.40 ± 3.33 | 35.48 ± 2.35 | 2.01 (1.18 to 2.83) | <0.0001 |
| Figure copy | 16.32 ± 2.69 | 16.48 ± 2.54 | 17.64 ± 2.18 | 19.04 ± 1.02 | 1.33 (0.62 to 2.04) | <0.0001 |
| Line orientation | 14.24 ± 2.85 | 14.20 ± 2.60 | 15.76 ± 2.33 | 16.44 ± 2.22 | 0.71 (0.03 to 1.39) | 0.042 |
| Language | 29.76 ± 3.56 | 30.36 ± 4.39 | 32.08 ± 3.66 | 33.64 ± 3.87 | 1.09 (−0.38 to 2.56) | 0.141 |
| Picture naming | 9.68 ± 0.75 | 9.60 ± 0.91 | 9.92 ± 0.28 | 9.76 ± 0.60 | −0.14 (−0.36 to 0.09) | 0.233 |
| Semantic fluency | 20.08 ± 3.37 | 20.76 ± 3.48 | 22.16 ± 3.65 | 23.88 ± 3.57 | 1.23 (−0.29 to 2.74) | 0.11 |
| Attention | 55.44 ± 9.99 | 55.20 ± 9.80 | 59.56 ± 10.34 | 66.64 ± 8.68 | 7.29 (4.77 to 9.80) | <0.0001 |
| Digit span | 14.16 ± 1.95 | 14.12 ± 1.96 | 14.36 ± 1.85 | 14.44 ± 1.73 | 0.10 (−0.66 to 0.87) | 0.785 |
| Coding | 41.28 ± 9.63 | 41.08 ± 10.21 | 45.20 ± 10.11 | 52.20 ± 8.40 | 7.17 (4.62 to 9.71) | <0.0001 |
| Delayed memory | 38.56 ± 4.91 | 38.44 ± 4.54 | 41.64 ± 5.10 | 45.84 ± 4.17 | 4.28 (2.26 to 6.30) | <0.0001 |
| List recall | 4.96 ± 2.23 | 4.00 ± 2.25 | 6.00 ± 2.18 | 6.60 ± 2.08 | 1.06 (−0.03 to 2.14) | 0.056 |
| List recognition | 18.04 ± 2.17 | 18.28 ± 1.81 | 18.84 ± 1.80 | 18.72 ± 1.67 | −0.25 (−1.03 to 0.54) | 0.526 |
| Story recall | 6.84 ± 1.97 | 7.20 ± 2.10 | 7.44 ± 2.38 | 9.56 ± 2.18 | 1.96 (0.75 to 3.17) | 0.002 |
| Figure recall | 8.72 ± 2.69 | 8.96 ± 2.49 | 9.36 ± 1.91 | 10.96 ± 2.61 | 1.53 (0.29 to 2.77) | 0.017 |
Mean ± SD is used to indicate values. Effects of BB68S are indicated by the difference (95% CI) between the two groups, calculated from the generalized linear model (GLM).
Figure 3.
Changes in RBANS scores from baseline at 8 weeks. (A) Changes of RBANS total score from baseline at week 8. (B) Changes of RBANS total score from baseline at week 8. Values are indicated as mean and SE with error bars. ** p < 0.01.
3.3. Results of Gut Microbiota Composition
To determine the relationship between the improvement in cognitive function and the gut microbiota, we performed gut microbiome analysis for all participants. After the amplification and cloning of bacterial sequences, purified amplicons were sequenced for a total of 50 fecal samples. Finally, 1133 OTUs from all samples were annotated, which were divided into 13 phyla, 23 classes, 56 orders, 109 families, 283 genera, and 600 species.
3.3.1. Results of Alpha-Diversity
After intervention, the BB68S group had increased Sob, Chao, Ace, and Shannon indexes and a reduced Simpson index, but not all changes were statistically significant (p > 0.05) (Table 3). This indicated that BB68S showed a tendency towards improving the alpha diversity of the gut microbiota but did not alter it significantly.
Table 3.
The effects of BB68S on the α-diversity.
| Baseline | Week 8 | a p-Value | b p-Value | |||
|---|---|---|---|---|---|---|
| Placebo | BB68S | Placebo | BB68S | |||
| Sobs | 229.44 ± 83.66 | 233.96 ± 90.34 | 236.32 ± 73.97 | 241.32 ± 91.81 | 0.76 | 0.67 |
| Ace | 282.86 ± 98.31 | 276.57 ± 106.21 | 278.72 ± 87.53 | 286.95 ± 106.95 | 0.67 | 0.63 |
| Chao | 284.75 ± 97.13 | 273.67 ± 112.03 | 280.71 ± 84.41 | 293.49 ± 114.6 | 0.56 | 0.56 |
| Shannon | 3.28 ± 0.65 | 3.22 ± 0.97 | 3.29 ± 0.59 | 3.22 ± 0.77 | 0.95 | 0.91 |
| Simpson | 0.1 ± 0.07 | 0.14 ± 0.22 | 0.11 ± 0.08 | 0.11 ± 0.09 | 0.77 | 0.95 |
Mean ± SD is used to indicate values; a p-values indicate the change from baseline to Week 8 for the BB68S group; b p-values indicate the difference between the 2 groups at Week 8; a and b were both calculated using Wilcoxon rank-sum tests.
3.3.2. Results of Beta-Diversity
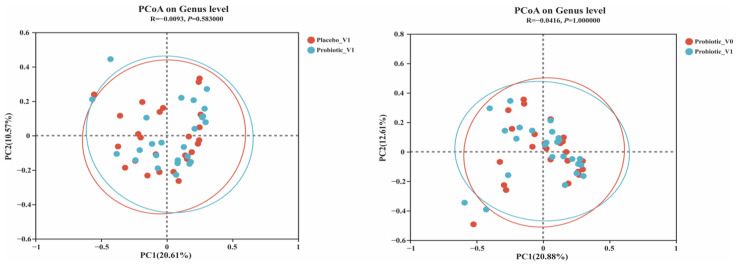
In Figure 4, the results of the principal coordinate analysis showed that the V0 and V1 regions of the probiotic group and the V1 regions between the BB68S and placebo groups did not differ significantly (p > 0.05). These results indicated that the BB68S intervention did not have a substantial impact on the beta diversity of the gut microbiota.
Figure 4.
Principal coordinate analysis (PCoA) of the gut microbiota at the operational taxonomic unit (OTU) level. (A) Differences in V4 between the Placebo and Probiotic groups. (B) Differences between V0 and V1 in the Probiotic group. Placebo_V1: samples at V1 in the placebo group, Probiotic_V1: samples at V1 in the probiotic group, Probiotic_V0: samples at V0 in the probiotic group.
3.3.3. Results of the Species Composition Analysis
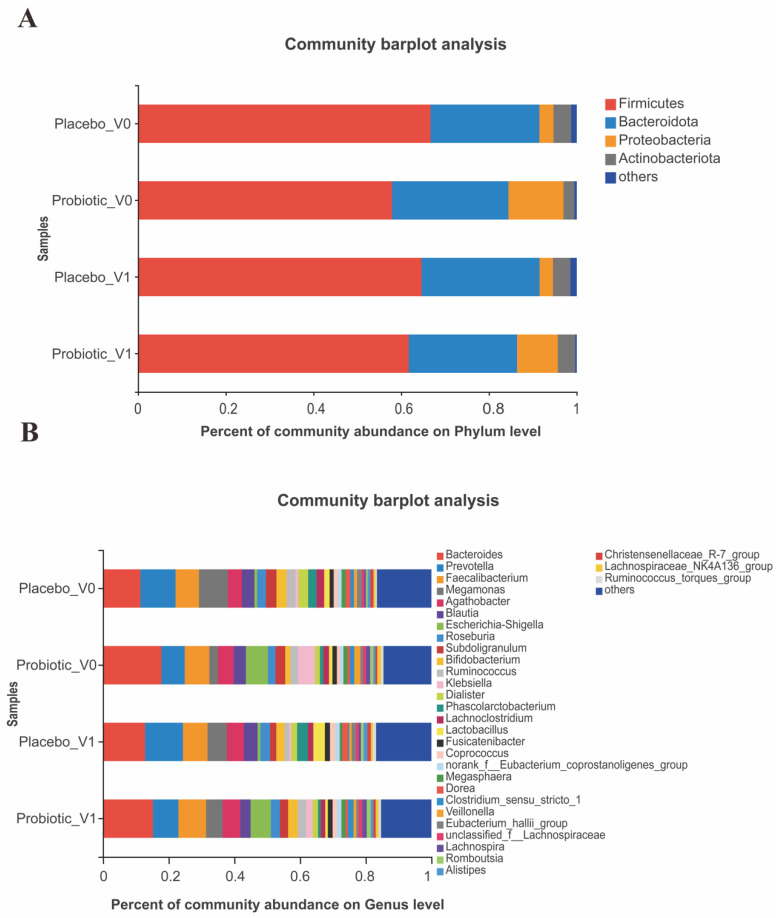
Figure 5 displays the composition of the gut microbiota between the BB68S and placebo groups at the phylum and genus levels.
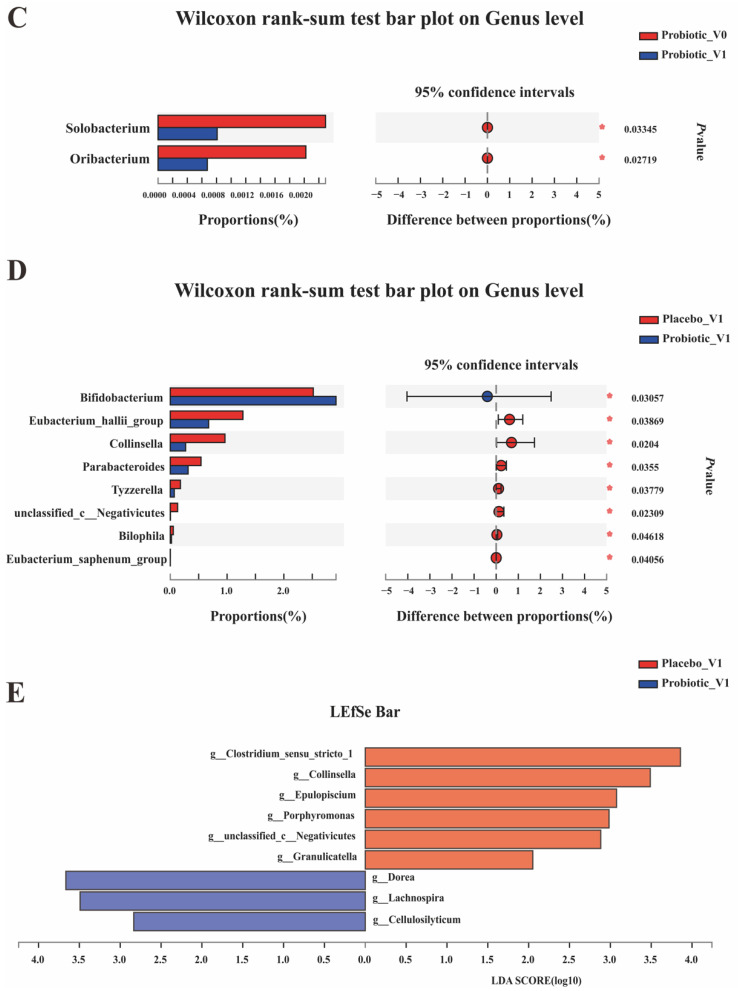
Figure 5.
Effects of BB68S intervention on the composition of the gut microbiota. (A) Relative abundances of main phyla between groups. (B) Relative abundances of main genera ≥1% between groups. (C) Significantly different genera levels after intervention in the BB68S group. (D) Significantly different genera levels between 2 groups after intervention. (E) LEfSe analysis indicated significant differences between the 2 groups after intervention. * p ≤ 0.05. Placebo_V0: samples of the placebo group at baseline, Probiotic_V0: samples of the probiotic group at baseline, Placebo_V1: samples of the placebo group after intervention, Probiotic_V1: samples of the BB68S group after intervention.
At the phylum level (Figure 5A), the gut microbiota mainly included Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. After intervention, the BB68S group had increased relative abundances of Firmicutes and Actinobacteria with a decreased relative abundance of Proteobacteria.
Figure 5B shows the bacteria with relative abundances of more than 1% at the genus level, including Bacteroides, Prevotella, Faecalibacterium, Megamonas, Agathobacter, Blautia, Escherichia-Shigella, Roseburia, Subdoligranulum, Bifidobacterium, Ruminococcus, Klebsiella, Dialister, Phascolarctobacterium, Lachnoclostridium, Lactobacillus, Fusicatenibacter, and Coprococcus.
Figure 5C,D exhibits the differences in the fecal bacterial population at the genus level. After BB68S intervention, the relative abundances of Solobacterium and Oribacterium decreased significantly (Figure 5C). Compared to the control, the BB68S group had a significantly higher relative abundance of Bifidobacterium and lower relative abundances of Eubacterium_hallif_group, Collinsella, Parabacteroides, Tyzzerella, Bilophila, Eubacterium_saphenum_group, and unclassified_c_Negativicutes after 8 weeks of intervention (Figure 5D).
In Figure 5E, we employed LEfSe analysis to further determine if different groups of bacterial taxa were specifically enriched after intervention. The results showed that the bacteria taxa significantly enriched in the control group were Clostridium_sensu_stricto_1, Collinsella, Epulopiscium, Porphyromonas, unclassified_c_Negativicutes, and Granulicatella, while in the BB68S group, these taxa were Dorea, Lachnospira, and Cellulosilyticum.
4. Discussion
This study was a randomized, double-blind, placebo-controlled trial conducted among healthy elderly people, showing the effects of probiotics on cognitive function in the healthy elderly. The subjects had good tolerance to the probiotic intervention in this study and no side effects were reported.
To our knowledge, among articles assessing the effect of probiotics on cognitive function, only two studies published to date have been conducted on the healthy elderly [35,36] and they were limited by the lack of consideration given to the subjects’ education level and lack of strict assessment criteria for cognitive health. In addition, the existing research tended to include some active and educated volunteers who cared about age-related memory problems and did not represent the general population. In a study of memory among middle-aged and elderly people, a relatively small but significant difference in the subjects’ education level between the two treatment groups impacted the results [37]. In our study, we recruited subjects with different education levels, which better represents the general population.
MoCA, developed by Nasreddine in 2004, is an assessment tool with 30 items used for rapid evaluation of cognitive function. Nine cognitive areas are assessed. The total score of the scale is 30 points. The higher the score, the better the global cognitive function. Its validity has been studied in various clinical settings [38,39,40,41,42]. In addition to being a concise cognitive assessment tool, MoCA is specific and useful to distinguish cognitive health from cognitive impairment. Based on MoCA, the criteria used to judge cognitive health are different for individuals with different education levels, which improves the screening sensitivity and effectiveness [43]. A total score of 19 points or more is considered representative of normal cognitive function among those who have been educated for less than or equal to 6 years, and scores of 22 and 24 points apply to those who have been educated for between 7 and 12 years and more than 12 years, respectively. In a study including Chinese healthy elderly with different levels of education, MoCA was sensitive, reliable, and highly accepted for the screening of cognitive function [43]. In our study, MoCA was used as a tool to cognitively screen healthy elderly people. Since we recruited the elderly without cognitive functional impairments in this study, rather than focusing on those who responded to the treatment effect, such as patients with neurological diseases, and strict screening tools (MoCA) with high sensitivity and effectiveness were used, our research is more applicable to a universal medical strategy for the elderly and has broader social significance.
In addition, this study used RBANS to evaluate the cognitive level of subjects. RBANS, a short assessment scale, was developed by Randolph in 1998 [44]. It contains 12 items, which are divided into 5 global areas that include immediate memory, visual space/structure, language, attention, and delayed memory. It is widely used in cognitive research at home and abroad to assess the cognitive level of normal people and patients, showing high reliability and validity [45,46,47,48]. In previous clinical studies, RBANS has been used to assess cognitive improvement after intervention and has good sensitivity [48]. The scale analysis for various aspects of brain functions shows the effect of the intervention on different cognitive domains.
In our study, BB68S intervention significantly improved cognitive function (total RBANS score increased by 18.89 points, p < 0.0001) compared to that of controls. Among the 12 items, list learning, story memory, figure copy, line orientation, coding, story recall, and figure recall scores of the probiotic group improved significantly. Among the 5 domains, only language did not significantly improve. All of the other four domains improved significantly in the BB68S group. The scores increased as follows: 4.36 points for immediate memory, 2.01 points for visuospatial/constructional, 7.19 points for attention, and 4.28 points for delayed memory (all p-values were less than 0.0001). In contrast, another study evaluating the effect of drinking lactic acid bacteria-fermented milk on memory only observed significant improvement in memory (the score increased by 4 points), with no change in total RBANS score and other domain scores [49]. Bifidobacterium longum BB68S significantly improved the cognitive function of healthy elderly people, providing strong evidence for future research and investigation.
Changes in the gut microbiota were essential to our study. Notably, the microbial composition of the probiotics group changed after the intervention, and the relative abundances of Solobacterium and Oribacterium, which cause inflammation, decreased significantly. Studies have shown that the relative abundances of Solobacterium and Oribacterium in the pro-inflammatory microbiota associated with colorectal cancer and reflux esophagitis were significantly reduced after the consumption of probiotics [50,51]. Based on Wilcoxon rank-sum and LEfSe analyses, many taxa showed differential abundance between the BB68S and control groups. The relative abundance of Bifidobacterium increased markedly after BB68S intervention. Some studies have shown that Bifidobacterium plays beneficial roles in the body, including regulating the gut microbiota and improving immune function [52]. The relative abundances of Collinsella, Parabacteroides, Tyzzerella, Bilophila, Eubacterium_saphenum_group, and unclassified_c_Negativicutes decreased significantly after BB68S intervention. Collinsella is an inflammation-related bacterium. Many studies have reported the association between the increased abundance of Collinsella and inflammatory diseases [53]. An increase in the abundance of Parabacteroides induced depression-like behavior in SAMP6 mice [54]. Tyzzerella has been negatively associated with 17-HAMD scores, playing a key role in metabolic disorders in patients with postpartum depression [55]. Hippocampal IL-1β levels have shown a positive correlation with the relative abundance of Tyzzerella, indicating that its increase might lead to neuroinflammation to a certain extent [56]. Bilophila is an obligate anaerobic pathobiont that is harmful to the hippocampus and cognitive behaviors [57]. In addition, unclassified_c_Negativicutes was found to be more abundant in patients suffering from Parkinson’s disease than in controls [58]. LEfSe analysis indicated that Dorea was enriched in the probiotic-treated group. These bacteria produce acetate and lactate that serve as substrates for butyrate production, and they are positively related to the immune response and negatively related to depression [59]. Many studies have shown that Lachnospira is negatively related to anxiety, depression, Parkinson’s disease, and psychiatric disorders [60,61,62]. The relative abundance of Lachnospira increased significantly following probiotic consumption according to the LEfSe analysis. Porphyromonas gingivalis is increasingly implicated in Alzheimer’s disease, cancer, and arthritis. The LEfSe results showed that probiotic intervention significantly reduced the relative abundance of the Porphyromonas genus [63].
The present study had some limitations that need to be addressed. First, our research lacked an analysis of the metabolites in the peripheral system and intestinal flora, and thus cannot explain the mechanism of action of the probiotics. In the coming research, we will attempt to carry out exploratory biomarker analysis to fully understand how probiotics improve the cognitive function of healthy elderly individuals. Second, although cognitive functions improved significantly, changes in some cognitive domains and intestinal flora were not significant. Eight weeks of study may not be sufficient to observe these results. Therefore, further research is needed with a longer duration. Third, although we instructed the subjects not to adjust their habitual diet during the intervention period, we did not strictly control their diet; therefore, we could not rule out the impact of dietary changes on gut microbiota. Studies with more rigorous designs (e.g., strict diet control) are needed in the future. Despite these limitations, our study also had some advantages. This study included subjects with diverse education levels and used highly sensitive screening tools specifically for different levels of education. We strictly and effectively evaluated cognitive function and measured the significant effects of probiotics on cognitive function with a short-term intervention among the healthy elderly.
In conclusion, our research showed that Bifidobacterium longum BB68S could improve cognitive function and has a beneficial regulatory effect on the gut microbiota in healthy elderly individuals. This study provides some evidence supporting probiotics as an alternative strategy to advance cognitive aging and promote healthy aging.
Author Contributions
Conceptualization, S.S., R.W. and J.H.; methodology, S.S.; software, S.S., Q.Z. and Y.S.; validation, Q.Z. and Y.S.; formal analysis, S.G., Q.W., R.W. and J.H.; investigation, R.W. and J.H.; resources, S.G., Q.W., R.W. and J.H.; data curation, S.S.; writing—original draft preparation, S.S.; visualization, Q.Z. and Y.S.; supervision, Q.Z., Y.S., R.W. and J.H.; project administration, R.W. and J.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of China Agricultural University (CAUHR-2021021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Key R&D Program of China (grant number 2021YFD1600204).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Liu Q.Y., Pan Y.C., Shu H.Y., Zhang L.J., Li Q.Y., Ge Q.M., Shao Y., Zhou Q. Brain Activity in Age-Related Macular Degeneration Patients from the Perspective of Regional Homogeneity: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 2022;14:865430. doi: 10.3389/fnagi.2022.865430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander G.E., Ryan L., Bowers D., Foster T.C., Bizon J.L., Geldmacher D.S., Glisky E.L. Characterizing Cognitive Aging in Humans with Links to Animal Models. Front. Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafto M.A., Tyler L.K. Language in the Aging Brain: The Network Dynamics of Cognitive Decline and Preservation. Science. 2014;346:583–587. doi: 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- 4.Harper S. Economic and Social Implications of Aging Societies. Science. 2014;346:587–591. doi: 10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- 5.Lederberg J., McCray A.T. ‘Ome Sweet’ Omics—A Genealogical Treasury of Words. Scientist. 2001;15:8. [Google Scholar]
- 6.Buffington S.A., Dooling S.W., Sgritta M., Noecker C., Murillo O.D., Felice D.F., Turnbaugh P.J., Costa-Mattioli M. Dissecting the Contribution of Host Genetics and the Microbiome in Complex Behaviors. Cell. 2021;184:1740–1756. doi: 10.1016/j.cell.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jameson K.G., Hsiao E.Y. Linking the Gut Microbiota to a Brain Neurotransmitter. Trends Neurosci. 2018;41:413–414. doi: 10.1016/j.tins.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z.J., Mu X.H., Cao Q.N., Shi Y., Hu X.S., Zheng H. Honeybee Gut Lactobacillus Modulates Host Learning and Memory Behaviors Via Regulating Tryptophan Metabolism. Nat. Commun. 2022;13:2037. doi: 10.1038/s41467-022-29760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costabile A., Bergillos-Meca T., Rasinkangas P., Korpela K., de Vos W.M., Gibson G.R. Effects of Soluble Corn Fiber Alone or in Synbiotic Combination with Lactobacillus Rhamnosus GG and the Pilus-Deficient Derivative Gg-Pb12 on Fecal Microbiota, Metabolism, and Markers of Immune Function: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Elderly (Saimes Study) Front. Immunol. 2017;8:01443. doi: 10.3389/fimmu.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh T.S., Shanahan F., O’Toole P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nature Reviews Gastroenterol. Hepatol. 2022;19:565–584. doi: 10.1038/s41575-022-00605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Haiminen N., Carrieri A.P., Hu R., Jiang L.J., Parida L., Russell B., Allaband C., Zarrinpar A., Vazquez-Baeza Y., et al. Human Skin, Oral, and Gut Microbiomes Predict Chronological Age. Msystems. 2020;5:e00630-19. doi: 10.1128/mSystems.00630-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 13.Wieers G., Belkhir L., Enaud R., Leclercq S., de Foy J.M.P., Dequenne I., de Timary P., Cani P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020;9:00454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan F., Polk D.B. Probiotics and Probiotic-Derived Functional Factors-Mechanistic Insights into Applications for Intestinal Homeostasis. Front. Immunol. 2020;11:01428. doi: 10.3389/fimmu.2020.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialecka-Debek A., Granda D., Szmidt M.K., Zielinska D. Gut Microbiota, Probiotic Interventions, and Cognitive Function in the Elderly: A Review of Current Knowledge. Nutrients. 2021;13:2514. doi: 10.3390/nu13082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X.Q., Yu D.K., Xue L., Li H., Du J.R. Probiotics Modulate the Microbiota-Gut-Brain Axis and Improve Memory Deficits in Aged Samp8 Mice. Acta Pharm. Sin. B. 2020;10:475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naomi R., Embong H., Othman F., Ghazi H.F., Maruthey N., Bahari H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients. 2022;14:20. doi: 10.3390/nu14010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romijn A.R., Rucklidge J.J., Kuijer R.G., Frampton C. A Double-Blind, Randomized, Placebo-Controlled Trial of Lactobacillus Helveticus and Bifidobacterium Longum for the Symptoms of Depression. Aust. N. Z. J. Psychiatry. 2017;51:810–821. doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelhamid M., Zhou C.Y., Jung C.G., Michikawa M. Probiotic Bifidobacterium Breve Mcc1274 Mitigates Alzheimer’s Disease-Related Pathologies in Wild-Type Mice. Nutrients. 2022;14:2543. doi: 10.3390/nu14122543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv T., Ye M., Luo F., Hu B., Wang A., Chen J., Yan J., He Z., Chen F., Qian C., et al. Probiotics Treatment Improves Cognitive Impairment in Patients and Animals: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021;120:159–172. doi: 10.1016/j.neubiorev.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai K., Toshimitsu T., Okada E., Anzai S., Shiraishi I., Inamura N., Kobayashi S., Sashihara T., Hisatsune T. Effects of Lactiplantibacillus Plantarum Oll2712 on Memory Function in Older Adults with Declining Memory: A Randomized Placebo-Controlled Trial. Nutrients. 2022;14:4300. doi: 10.3390/nu14204300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thangaleela S., Sivamaruthi B.S., Kesika P., Chaiyasut C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms. 2022;10:2268. doi: 10.3390/microorganisms10112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesika P., Suganthy N., Sivamaruthi B.S., Chaiyasut C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021;264:118627. doi: 10.1016/j.lfs.2020.118627. [DOI] [PubMed] [Google Scholar]
- 25.Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, Probiotics and Neurodegenerative Diseases: Deciphering the Gut Brain Axis. Cell. Mol. Life Sci. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X.M., Ge S.Y., Sang Y., Liu Z.L. Milk and Milk Product. Springer; New York, NY, USA: 2019. Safety Evaluation of Bifidobacteirum Longum Bb68S in vivo and vitro; pp. 43–46. [Google Scholar]
- 27.Li G.H., Ge S.Y., Sang Y., Liu Z.L., Wang R. Milk and Milk Product. Springer; New York, NY, USA: 2019. Effect of Bifidobacterium Longum Bb68S on Defaecation and Intestinal Flora in Constipation People; pp. 22–25. [Google Scholar]
- 28.Xiao J.Z., Katsumata N., Bernier F., Ohno K., Yamauchi Y., Odamaki T., Yoshikawa K., Ito K., Kaneko T. Probiotic Bifidobacterium Breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2020;77:139–147. doi: 10.3233/JAD-200488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J., Zhou X., Wu X., Lin S., Ming B., Zhong J., Wang B., Dong L. Gut Microbiota Aberration in Patients of Systemic Sclerosis and Bleomycin-Induced Mice Model. Front. Cell. Infect. Microbiol. 2021;11:647201. doi: 10.3389/fcimb.2021.647201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao R., Wan C., Wang Z. The Relationship of Gastric Microbiota and Helicobacter Pylori Infection in Pediatrics Population. Helicobacter. 2020;25:e12676. doi: 10.1111/hel.12676. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Zhou Y., Chen Y., Gu J. Fastp: An Ultra-Fast All-in-One Fastq Preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magoc T., Salzberg S.L. Flash: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar R.C. Uparse: Highly Accurate Otu Sequences from Microbial Amplicon Reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian Classifier for Rapid Assignment of Rrna Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung Y.C., Jin H.M., Cui Y., Kim D.S., Jung J.M., Park J.I., Jung E.S., Choi E.K., Chae S.W. Fermented Milk of Lactobacillus Helveticus Idcc3801 Improves Cognitive Functioning during Cognitive Fatigue Tests in Healthy Older Adults. J. Funct. Foods. 2014;10:465–474. doi: 10.1016/j.jff.2014.07.007. [DOI] [Google Scholar]
- 36.Kim C.S., Cha L.N., Sim M., Jung S., Chun W.Y., Baik H.W., Shin D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. Biol. Sci. Med. Sci. 2021;76:32–40. doi: 10.1093/gerona/glaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddarth P., Li Z., Miller K.J., Ercoli L.M., Merril D.A., Henning S.M., Heber D., Small G.W. Randomized Placebo-Controlled Study of the Memory Effects of Pomegranate Juice in Middle-Aged and Older Adults. Am. J. Clin. Nutr. 2020;111:170–177. doi: 10.1093/ajcn/nqz241. [DOI] [PubMed] [Google Scholar]
- 38.Gaviria M., Pliskin N., Kney A. Cognitive Impairment in Patients with Advanced Heart Failure and Its Implications on Decision-Making Capacity. Congest. Heart Fail. 2011;17:175–179. doi: 10.1111/j.1751-7133.2011.00242.x. [DOI] [PubMed] [Google Scholar]
- 39.Olson R.A., Chhanabhai T., McKenzie M. Feasibility Study of the Montreal Cognitive Assessment (Moca) in Patients with Brain Metastases. Support. Care Cancer. 2008;16:1273–1278. doi: 10.1007/s00520-008-0431-3. [DOI] [PubMed] [Google Scholar]
- 40.Gill D.J., Freshman A., Blender J.A., Ravina B. The Montreal Cognitive Assessment as a Screening Tool for Cognitive Impairment in Parkinson’s Disease. Mov. Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 41.Nazem S., Siderowf A.D., Duda J.E., Have T.T., Colcher A., Horn S.S., Moberg P.J., Wilkinson J.R., Hurtig H.I., Stern M.B., et al. “Normal” Global Cognition According to Mini-Mental State Examination Score. J. Am. Geriatr. Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cumming T.B., Bernhardt J., Linden T. The Montreal Cognitive Assessment: Short Cognitive Evaluation in a Large Stroke Trial. Stroke. 2011;42:2642–2644. doi: 10.1161/STROKEAHA.111.619486. [DOI] [PubMed] [Google Scholar]
- 43.Chen K.L., Xu Y., Chu A.Q., Ding D., Liang X.N., Nasreddine Z.S., Dong Q., Hong Z., Zhao Q.H., Guo Q.H. Validation of the Chinese Version of Montreal Cognitive Assessment Basic for Screening Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2016;64:e285–e290. doi: 10.1111/jgs.14530. [DOI] [PubMed] [Google Scholar]
- 44.Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (Rbans): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 45.Gontkovsky S.T., Mold J.W., Beatty W.W. Age and Educational Influences on Rbans Index Scores in a Nondemented Geriatric Sample. Clin. Neuropsychol. 2002;16:258–263. doi: 10.1076/clin.16.3.258.13844. [DOI] [PubMed] [Google Scholar]
- 46.Duff K., Patton D., Schoenberg M.R., Mold J., Scott J.G., Adams R.L. Age- and Education-Corrected Independent Normative Data for the Rbans in a Community Dwelling Elderly Sample. Clin. Neuropsychol. 2003;17:351–366. doi: 10.1076/clin.17.3.351.18082. [DOI] [PubMed] [Google Scholar]
- 47.Beatty W.W., Mold J.W., Gontkovsky S.T. Rbans Performance: Influences of Sex and Education. J. Clin. Exp. Neuropsychol. 2003;25:1065–1069. doi: 10.1076/jcen.25.8.1065.16732. [DOI] [PubMed] [Google Scholar]
- 48.Nakatsu D., Fukuhara T., Chaytor N.S., Phatak V.S., Avellino A.M. Repeatable Battery for the Assessment of Neuropsychological Status (Rbans) as a Cognitive Evaluation Tool for Patients with Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2016;56:51–61. doi: 10.2176/nmc.oa.2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohsawa K., Nakamura F., Uchida N., Mizuno S., Yokogoshi H. Lactobacillus Helveticus-Fermented Milk Containing Lactononadecapeptide (Nippltqtpvvvppflqpe) Improves Cognitive Function in Healthy Middle-Aged Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 2018;69:369–376. doi: 10.1080/09637486.2017.1365824. [DOI] [PubMed] [Google Scholar]
- 50.Marchesan J.T., Morelli T., Moss K., Barros S.P., Ward M., Jenkins W., Aspiras M.B., Offenbacher S. Association of Synergistetes and Cyclodipeptides with Periodontitis. J. Dent. Res. 2015;94:1425–1431. doi: 10.1177/0022034515594779. [DOI] [PubMed] [Google Scholar]
- 51.Liang T., Liu F., Liu L.J., Zhang Z.Y., Dong W.X., Bai S., Ma L.F., Kang L.L. Effects of Helicobacter Pylori Infection on the Oral Microbiota of Reflux Esophagitis Patients. Front. Cell. Infect. Microbiol. 2021;11:10112268. doi: 10.3389/fcimb.2021.732613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz L., Delgado S., Ruas-Madiedo P., Sanchez B., Margolles A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017;8:02345. doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Soest A.P.M., Hermes G.D.A., Berendsen A.A.M., van de Rest O., Zoetendal E.G., Fuentes S., Santoro A., Franceschi C., de Groot L.C.P.G.M., de Vos W.M. Associations between Pro- and Anti-Inflammatory Gastro-Intestinal Microbiota, Diet, and Cognitive Functioning in Dutch Healthy Older Adults: The Nu-Age Study. Nutrients. 2020;12:3471. doi: 10.3390/nu12113471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Nguyen A., Basson A.R., Dark-Fleury L., Hsu K., Osme A., Menghini P., Pizarro T.T., Cominelli F. Parabacteroides Distasonis Induces Depressive-Like Behavior in a Mouse Model of Crohn’s Disease. Brain Behav. Immun. 2021;98:245–250. doi: 10.1016/j.bbi.2021.08.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y.M., Chen C., Yu H.B., Yang Z.X. Fecal Microbiota Changes in Patients with Postpartum Depressive Disorder. Front. Cell. Infect. Microbiol. 2020;10:567268. doi: 10.3389/fcimb.2020.567268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant C.V., Loman B.R., Bailey M.T., Pyter L.M. Manipulations of the Gut Microbiome Alter Chemotherapy-Induced Inflammation and Behavioral Side Effects in Female Mice. Brain Behav. Immun. 2021;95:401–412. doi: 10.1016/j.bbi.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson C.A., Iniguez A.J., Yang G.E., Fang P., Pronovost G.N., Jameson K.G., Rendon T.K., Paramo J., Barlow J.T., Ismagilov R.F., et al. Alterations in the Gut Microbiota Contribute to Cognitive Impairment Induced by the Ketogenic Diet and Hypoxia. Cell Host Microbe. 2021;29:1378–1392. doi: 10.1016/j.chom.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleury V., Zekeridou A., Lazarevic V., Gaia N., Giannopoulou C., Genton L., Cancela J., Girard M., Goldstein R., Bally J.F., et al. Oral Dysbiosis and Inflammation in Parkinson’s Disease. J. Park. Dis. 2021;11:619–631. doi: 10.3233/JPD-202459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi Y., Shen L., Shi W., Xia F., Zhang H., Wang Y., Zhang J., Wang Y., Sun X., Zhang Z., et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2021;27:1329–1340. doi: 10.1158/1078-0432.CCR-20-3445. [DOI] [PubMed] [Google Scholar]
- 60.Yuan X.M., Chen B.Q., Duan Z.L., Xia Z.Q., Ding Y., Chen T., Liu H.Z., Wang B.S., Yang B.L., Wang X.Y., et al. Depression and Anxiety in Patients with Active Ulcerative Colitis: Crosstalk of Gut Microbiota, Metabolomics and Proteomics. Gut Microbes. 2021;13:1987775. doi: 10.1080/19490976.2021.1987779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun H.R., Zhao F.Y., Liu Y.Y., Ma T., Jin H., Quan K.Y., Leng B., Zhao J.W., Yuan X.L., Li Z.G., et al. Probiotics Synergized with Conventional Regimen in Managing Parkinson’s Disease. NPJ Park. Dis. 2022;8:62. doi: 10.1038/s41531-022-00327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J.J., Ma Y.L., Bao Z.W., Gui X.H., Li A.N., Yang Z.L., Li M.D. Clostridiales Are Predominant Microbes That Mediate Psychiatric Disorders. J. Psychiatr. Res. 2020;130:48–56. doi: 10.1016/j.jpsychires.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Miller D.P., Scott D.A. Inherently and Conditionally Essential Protein Catabolism Genes of Porphyromonas Gingivalis. Trends Microbiol. 2021;29:54–64. doi: 10.1016/j.tim.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.