Abstract
Isocitrate dehydrogenase (IDH) mutant gliomas are the most common adult, malignant primary brain tumors diagnosed in patients younger than 50, constituting an important cause of morbidity and mortality. In recent years, there has been significant progress in understanding the molecular pathogenesis and biology of these tumors, sparking multiple efforts to improve their diagnosis and treatment. In this consensus review from the Society for Neuro-Oncology (SNO), the current diagnosis and management of IDH-mutant gliomas will be discussed. In addition, novel therapies, such as targeted molecular therapies and immunotherapies, will be reviewed. Current challenges and future directions for research will be discussed.
Keywords: D-2HG, glioma, Isocitrate dehydrogenase (IDH)
There has been longstanding appreciation of the fact that most diffusely infiltrating gliomas affecting patients in the third and fourth decades of life have a different natural history from that of patients diagnosed with glioblastoma.1 The discovery of mutations in isocitrate dehydrogenase (IDH) in 2008,2 in a subset of diffusely infiltrating gliomas started a new era in the molecular classification of gliomas. The 2016 WHO classification of central nervous system tumors incorporated IDH-mutation status in the classification of diffusely infiltrating gliomas, and the recent 2021 WHO classification uses this molecular alteration to define a distinct family of tumors that range from low-grade to high-grade and that are molecularly different from glioblastoma, which are IDH-wildtype.3–5 In this consensus review from SNO, recent advances in the diagnosis and management of IDH-mutant gliomas are discussed, as well as current challenges and future directions for research.
Epidemiology of IDH-mut Gliomas
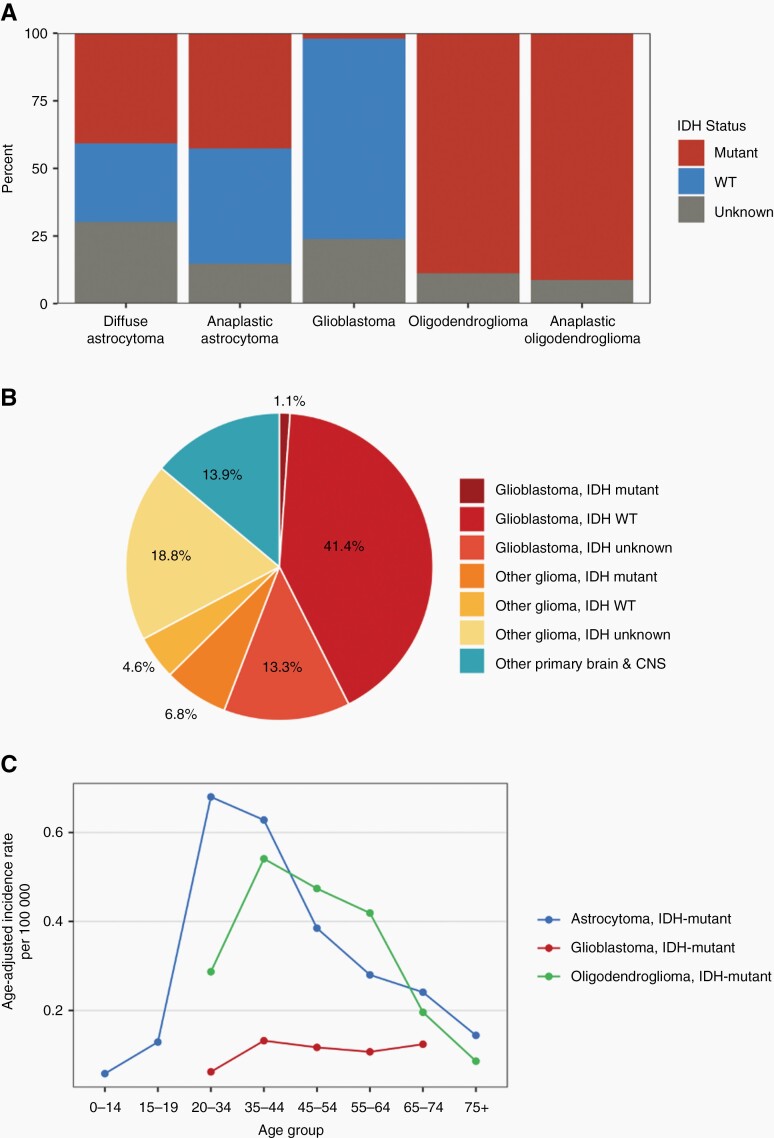
Beginning January 1, 2018, central cancer registries began collecting information on certain molecular alterations, such as IDH-mutation and 1p/19q codeletion status. This information is reported to the Centers for Disease Control and Prevention (CDC)’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology, and End Results Program and are included in the Central Brain Tumor Registry of the United States (CBTRUS) dataset. For cases diagnosed in 2018, the overall age-adjusted incidence (AAI) of IDH-mutant gliomas in the US was 0.70/100,000 persons, which includes astrocytoma, IDH-mutant, grade 2–4 (AAI: 0.43) and oligodendroglioma, IDH-mutant, 1p/19q codeleted, grade 2–3 (AAI: 0.27). Over 40% of astrocytoma grade 2–3, and around 2% of astrocytoma grade 4, diagnosed in 2018 were IDH-mutant (Figure 1A).6 IDH-mutant tumors accounted for approximately 12% of all glioma diagnoses in 2018 (Figure 1B). Incidence of both astrocytoma, IDH-mutant, grade 2–4 and oligodendroglioma, IDH-mutant, 1p/19q codeleted, grade 2–3 was highest among patients ages 35-44 (AAI of 0.76 and 0.54 respectively) (Figure 1C). In the United States, the incidence increases with age and peaks between 30 and 34 years for diffuse astrocytomas and between 40 and 44 years for oligodendrogliomas, with incidence subsequently decreasing with advancing age, Figure 1B.6 Similar to the case of glioblastoma, IDH-wildtype, the incidence of IDH-mutant gliomas is higher in males than in females (approximate ratio of 1.3 M:F).6 In addition, the incidence IDH-mutant gliomas in the CBTRUS dataset are more common in Whites compared to other racial groups.6
Figure 1.
(A) Distribution of IDH status among pathologically-confirmed diffuse astrocytoma, grade 2 (ICD-O-3: 9400/3), astrocytoma, grade 3 (ICD-O-3: 9401/3), astrocytoma, grade 4/glioblastoma (ICD-O-3: 9440/3, 9441/3, 9442/3, 9445/3), oligodendroglioma, grade 2 (ICD-O-3: 9450/3), and oligodendroglioma, grade 3 (ICD-O-3: 9451/3) using the 2016 WHO classification system. IDH status determined by NAACCR Item #3816: Brain Molecular Markers, where applicable. (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2018). *Oligodendroglioma, IDH-mutant, grade 2–3 is additionally 1p/19q codeleted. (B) Relative frequency of IDH-mutant gliomas among all pathologically-confirmed primary malignant gliomas. (CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2018). (C) Age-adjusted incidence per 100,000 for pathologically-confirmed astrocytoma, IDH-mutant, grade 2–4 (ICD-O-3: 9400/3, 9401/3, 9445/3), and oligodendroglioma, IDH-mutant, 1p/19q codeleted, grade 2–3 (ICD-O-3: 9450/3, 9451/3) by age group. IDH status determined by NAACCR Item #3816: Brain Molecular Markers, where applicable. Rates are not presented when fewer than 16 cases were reported for a specific age group. (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2018). Note: These data are based on tumors classified using CNS WHO 2016 diagnostic criteria, therefore the legacy terminiology is used within this figure.
The only well-established environmental risk factor for the development of gliomas is ionizing radiation.7 However, it is not clear that exposure to ionizing radiation specifically leads to the development of oncogenic IDH mutations. Patients with Ollier disease, a non-hereditary skeletal disorder associated with somatic mosaic IDH1 and IDH2 mutations,8 are at increased risk of developing IDH-mutant gliomas.9 In addition, a low frequency variant in the 8q24/CCDC26 region (rs55705857) has been linked to an increased risk of developing an IDH-mutant glioma.10 Given the low incidence of gliomas in general and more specifically IDH-mutant gliomas, testing for this germline variant is not standard practice.
Mechanisms of Oncogenesis in IDH-mutant Glioma
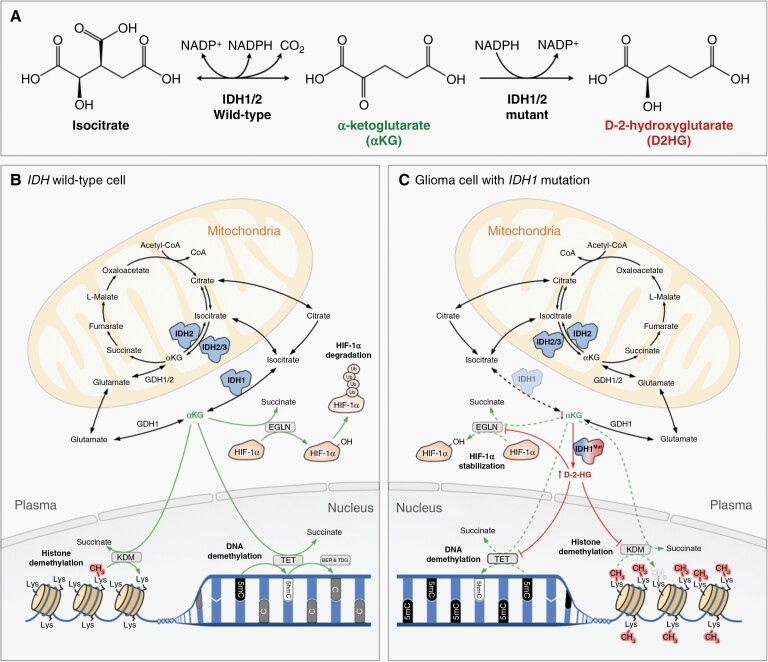
While mutation of both IDH1 and IDH2 isoforms are observed in a range of cancers,11 the majority of IDH-mutant gliomas have a heterozygous point mutation in IDH1 that causes an arginine-to-histidine substitution at amino acid 132 (IDH1 R132H).12,13 This gain-of-function mutation disrupts the conversion of isocitrate to alpha-ketoglutarate (α-KG) and instead favors the production of large amounts of the “oncometabolite” D-2-hydroxyglutarate (D-2HG).14 Mutant IDH1 promotes transformation in human astrocytes,15,16 while treatment with a mutant IDH-specific inhibitor that blocks D-2HG production impairs glioma growth in preclinical studies,17,18D-2HG is structurally similar to α-KG and promotes oncogenesis in part through competitive inhibition of tumor suppressors in the α-KG-dependent dioxygenase family, which includes ten-eleven translocation (TET) DNA modifying enzymes and jumonji C domain-containing (JmjC) histone demethylases (KDMs).19,20 In addition to this inhibitory role of D-2HG on its direct targets, D-2HG also contributes to gliomagenesis by directly stimulating EglN prolyl hydroxylase activity and thereby decreasing HIF1α activity.15 HIF1α suppresses gliomagenesis in mouse models21 and HIF1α levels in IDH1 mutant glioma are characteristically low22 (Figure 2).
Figure 2.
Functions of normal and mutated IDH enzymes. (A) Normal IDH1 and IDH2 proteins use NADP + as an electron acceptor to catalyze the oxidative decarboxylation of isocitrate, producing α-ketoglutarate (αKG) and CO2. However, mutant IDH1 and IDH2 produce D-2-hydroxyglutarate (D-2-HG) from α-KG using NADPH as an electron donor. (B) The normal isoforms IDH1 and IDH2 catalyze αKG production in the cytoplasm and mitochondria, respectively. As a critical intermediate in the Krebs cycle, αKG is involved in many biological metabolic processes. A superfamily of enzymes called αKG-dependent dioxygenases (αKGDs), including TET, KDM, and EglN, can decarboxylate αKG to succinate while hydroxylating different substrates for various further changes, such as DNA demethylation, histone demethylation, and ubiquitination of transcription factor HIF-1α. (C) Mutant IDH1 produces high level D-2-HG. As a structural analog of αKG, excessive D-2-HG competitively inhibits the catalytic efficiency of the TETs and KDMs while paradoxically stimulating EglN activity. Decreased TET and KDM activity causes DNA and histone hypermethylation, respectively, while increased EglN activity lowers HIF. Collectively, these changes affect gene expression, cell division and differentiation.
Interference with the normal activity of the TET and JmjC KDM dioxygenases can disrupt DNA and histone methylation patterns, respectively, and thus epigenetic states. Indeed, IDH-mutant gliomas exhibit a signature of global DNA hypermethylation, known as the Glioma CpG Island Methylator Phenotype (G-CIMP).23,24 DNA hypermethylation contributes to gliomagenesis through multiple mechanisms. Mutant IDH-driven epigenetic reprogramming is thought to cause a differentiation block,25 supported by recent work demonstrating genetically similar stem-like populations in both IDH-mutant astrocytomas and IDH-mutant oligodendrogliomas.26,27 It is hypothesized that epigenetic reprogramming causes inappropriate activation of growth-promoting signaling. In support of this, it has been reported that DNA hypermethylation at cohesion and CCCTC-binding factor (CTCF) binding sites can result in aberrant activation of the platelet-derived growth factor receptor alpha (PDGFRA), a well-established glioma oncogene.28
Mutant IDH enzyme activity causes metabolic reprogramming, from direct depletion of α-KG from the Krebs cycle to support D-2HG production.29,30 Mutant IDH enzymes also consume NADPH to produce D-2HG, thereby reducing the availability of this redox cofactor for de novo lipogenesis and enhancing dependence on exogenous lipids to promote biomass accumulation.31D-2HG also, likely as bystander effects, inhibits various metabolic enzymes, leading to increased dependence on glutaminolysis for glutamate production,32,33 and nicotinamide phosphoribotransferase (NAMPT) for nicotinamide adenine dinucleotide (NAD) biosynthesis,34 among other processes. While altered metabolic programming is evident in IDH-mutant glioma cells, it remains unclear whether this contributes to or detracts from cellular fitness. These bystander effects may create vulnerabilities to specific agents, some of which are undergoing clinical trial testing (Table 2).
Table 2.
Targeted Therapies in Clinical Trial in Patients with IDH-mutant Glioma
| Mechanism of action | Therapy | Phase | Design | Tumor type | Trial |
|---|---|---|---|---|---|
| Treatment naive | |||||
| IDH inhibitor | Vorasidenib | III | • Randomized, placebo-controlled • Primary outcome: PFS |
• Non-enhancing tumors IDH-mutant glioma • Within 5 years of initial surgery • No prior RT or chemotherapy |
NCT04164901 |
| IDH inhibitor | DS-1001 | II | • Primary outcomes: ORR, incidence of TEAEs | • Treatment-naïve grade 2 IDH-mutant glioma | NCT04458272 |
| Glutaminase inhibitor | Telaglenastat + RT and TMZ | I | • Determine MTD and RP2D | • Grade 2 or 3 IDH-mutant glioma • No prior RT or TMZ |
NCT03528642 |
| CDK4/6 inhibitor | Palbociclib | II | • Non-randomized • Primary outcomes: PFS-6 |
• Grade 3 oligodendroglioma (newly diagnosed and recurrent) | NCT02530320 |
| Recurrent after treatment | |||||
| Demethylating agent | ASTX7272 (decitabine + cedazuridine) | 0/I | • Phase I dose escalation to determine MTD • Phase 0 surgical expansion for PD effects |
• Recurrent IDH-mutant glioma • Non-enhancing tumors |
NCT03922555 |
| Demethylating agent | 5-azacytidine | II | • Single arm • Primary outcome: PFS-6 |
• Recurrent grade 2 or 3 IDH-mutant glioma after standard treatment | NCT03666559 |
| PARP inhibitor | Niraparib | 0 | • Phase 0 to examine PK, PD and presence of chromosomal fusion | • Recurrent grade 2-4 IDH-mutant astrocytoma (Arm B only) | NCT05076513 |
| PARP inhibitor | Pamiparib + metronomic TMZ | I/II | • Alkylator resistant and not-alkylator resistant arms in Phase II • Surgical arm includes grade 4 tumors • Primary outcomes: MTD and radiologic response |
• Recurrent grade 2-4 IDH-mutant glioma | NCT03914742 |
| PPARP inhibitor | Olaparib + Durvalumab | II | • Non-randomized • Primary outcomes: ORR |
• Recurrent IDH-mutant glioma at first or second relapse (Cohort A) | NCT03991832 |
| CDK4/6 inhibitor | Palbociclib | II | • Non-randomized • Primary outcomes: PFS-6 |
• Grade 3 oligodendroglioma (newly diagnosed and recurrent) | NCT02530320 |
| CDK4/6 inhibitor | Abemaciclib | II | • Non-randomized, single arm • Primary outcomes: PFS-6 |
• Recurrent oligodendroglioma | NCT03220646 |
| DHODH inhibitor | BAY2402234 | 0 | • Phase 0 for PD effects | • Grade 4 recurrent IDH-mutant glioma | Pending |
| Immune checkpoint inhibitor + IDH inhibitor | Nivolumab + Ivosidenib | II | • Non-randomized, single arm • Primary outcomes: PFS-6, ORR |
• Recurrent IDH-mutant glioma • Enhancing disease |
NCT04056910 |
The central role of IDH mutations in driving glioma formation is highlighted by ubiquitous expression throughout the tumor and retention of the mutation during the disease course in most cases.35–37 Controversy exists, however, regarding whether sustained D-2HG levels are required throughout the course of IDH-mutant gliomagenesis. The mutant copy of the IDH1 gene is spontaneously lost in some primary glioma cultures, xenografts, and a subset of recurrent tumors in patients.38–40 Mutant IDH-specific inhibitors have not exhibited anti-tumor efficacy in many IDH-mutant glioma xenografts models34,41 and only prevented cellular transformation during a brief window of time after mutant IDH1 expression in human astrocytes ex vivo.16 Experiments that used an inducible mutant IDH1 system in astrocytes also revealed only partial reversal of mutant-IDH induced epigenetic changes upon loss of the mutant enzyme.42 These data raise the possibility that D-2HG is largely dispensable once an oncogenic program has been established, with implications regarding the appropriate window of opportunity for the therapeutic application of mutant IDH inhibitors.
Pathology and Classification of IDH-mutant Gliomas
IDH-mutant gliomas comprising astrocytoma and oligodendroglioma are diffusely infiltrating tumors. Both histologic and molecular data are essential for classification and grading. In WHO grade 2–4 IDH-mutant astrocytomas, the histologic appearance depends on tumor subtype and grade. Grade 2 astrocytomas are characterized by moderate cellularity, well differentiated astrocytic cells, a fibrillary matrix forming microcysts and low proliferation. Grade 3 astrocytomas are distinguished from grade 2 by increased evidence of proliferation and pleomorphia; however, thresholds between grade 2 and grade 3 are not well-established.4 Histologic features found in grade 4 IDH-mutant astrocytomas include microvascular proliferation or necrosis. IDH1 or IDH2 hotspot mutations are very frequently accompanied by alpha thalassemia/mental retardation (ATRX) and TP53 mutations, resulting in nuclear loss of ATRX expression and frequently strong nuclear p53 expression. Homozygous deletion of CDKN2A/B leads to classification as grade 4 astrocytoma, irrespective of microvascular proliferation or necrosis.4,43
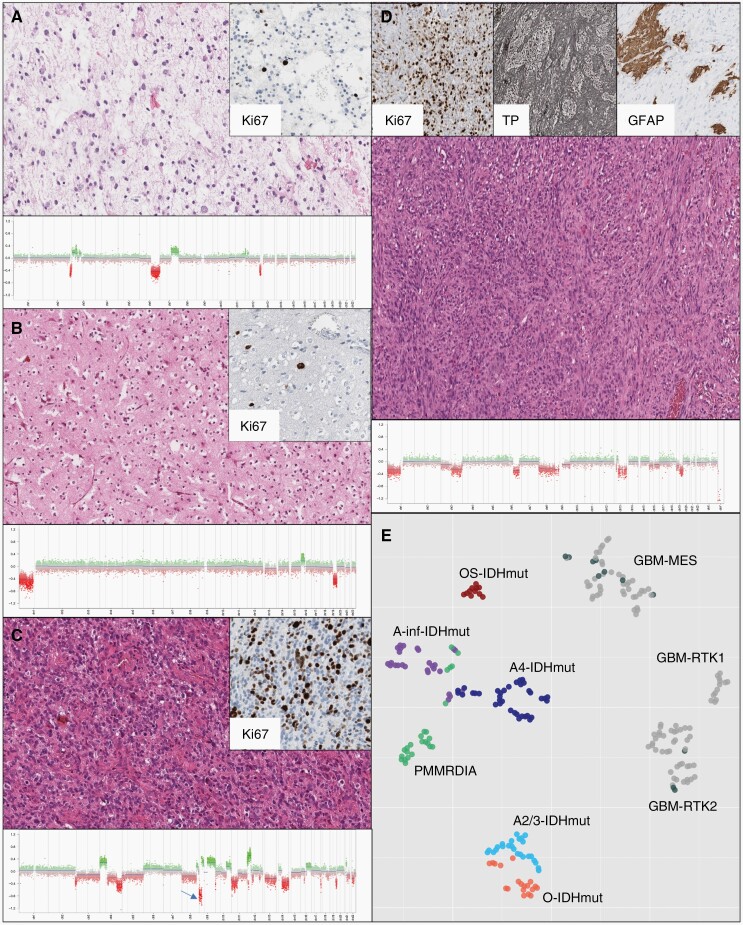
IDH-mutant, 1p/19q codeleted oligodendrogliomas WHO grades 2 and 3 exhibit morphologies dominated by a honeycomb or fried egg pattern which, however, is not exclusive to this tumor type. Other frequent features include calcifications and a capillary pattern resembling chicken wire. Grade 3 tumors demonstrate higher rates of proliferation than grade 2, as well as vascular proliferation or necrosis. By definition, the diagnosis of oligodendroglioma requires an IDH1 or IDH2 mutation accompanied by combined 1p/19q loss, making these tests obligatory for WHO compliant diagnosis. Nearly always, IDH-mutant 1p/19q codeleted oligodendrogliomas carry telomerase reverse transcriptase (TERT) promoter hotspot mutations and frequently CIC or FUBP1 mutations, however these molecular features are not required for classification. In cases of conflicting molecular and histological findings, i.e., 1p/19q loss in an IDH-mutant glioma with astrocytoma appearance, the molecular findings are decisive for the integrated diagnosis of oligodendroglioma. Likewise, an IDH-mutant tumor with oligodendroglial morphology lacking 1p/19q loss will receive the integrated diagnosis of astrocytoma. Methylation analysis can distinguish different types of IDH-mutant glioma (Figure 3). Molecular hallmarks are summarized in Table 1.
Figure 3.
Morphologic and molecular aspects in IDH-mutant glioma: (A) Astrocytoma IDH-mut grade 2 with few Ki67 positive nuclei and little copy number variations lacking homozygous CDKN2A/B deletion. (B) Oligodendroglioma IDH-mutant 1p/19q codeleted grade 2 with fried egg pattern. Copy number variation plot (CNVP) shows the defining 1p/19q codeletion (C) Astrocytoma IDH-mut grade 4 with high Ki67 count and CNVP demonstrating presence of homozygous CDKN2A/B deletion (arrow). (D) Oligosarcoma-IDH-mut, high Ki67 count, abundance of agyrophilic fibers (TP stain) and focal GFAP binding. CNVP shows multiple alterations. (E) tSNE analysis of methylation data separating IDH-mutant glioma. Glioblastomas-IDH wild-type of the methylation classes RTK1, RTK2 and MES are coanalyzed. Gliosarcoma (black) colocalize with glioblastomas and are separate from oligosarcoma-IDH-mut.
Table 1.
Molecular Hallmarks of IDH-mutant Glioma
| IDH-mutant glioma | Molecular hallmarks |
|---|---|
| Astrocytoma, IDH-mutant | All IDH1 or IDH2 (~ 90% of IDH1R132H-type), ATRX (> 90%), TP53, CDKN2A/B |
| Infratentorial astrocytoma, IDH.mutant | All IDH1 or IDH2 (< 25% of IDH1R132H-type), ATRX (< 50%), TP53, CDKN2A/B |
| Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) | All IDH1 or IDH2 (~ 90% of IDH1R132H-type), ATRX (> 90%), TP53, CDKN2A/B, all MLH1 or MSH6 or MSH2 |
| Oligodendroglioma, IDH-mutant and 1p/19q codeleted | All IDH1 or IDH2, all 1p/19q codel, pTERT, CIC, FUBP1, NOTCH1 |
| Oligosarcoma, IDH-mutant | All IDH1 or IDH2, all 1p/19q codel (in few cases obscured by duplication), pTERT (~ 50%), CDKN2A/B |
Novel subsets of IDH-mutant glioma deserving special mention are the rare infratentorial IDH-mutant astrocytomas,44 the primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA)45 and the oligosarcoma.46 Infratentorial IDH-mutant astrocytomas carry a high frequency of non-canonical IDH1 or IDH2 mutations, resulting in failed detection by IDH1-R132H immunohistochemistry. Further, these tumors have a much lower incidence of ATRX mutations. PMMRDIA exhibit very aggressive growth and may be considered for alternative therapy approaches.
Molecular Pathogenesis and Genomics
As detailed above, IDH-mutant gliomas are classified as astrocytomas or oligodendrogliomas based on lineage-defining genomic alterations. While these distinct molecular alterations are associated with differences in clinical behavior and response to therapy, the functional consequences of the changes are still under investigation.
Loss of one copy of the entire short arm of chromosome 1p along with one copy of the long arm of chromosome 19q is characteristic of oligodendroglial tumors. This is the result of an unbalanced translocation between the two chromosomes, which results in elimination of the 1p/19q fusion chromosome, leaving only one copy of 1p and 19q apiece.47 True whole-arm 1p/19q codeletion does not occur in the absence of IDH mutations.48,49 The remaining CIC allele on 19q, encoding the “Protein capicua homolog”, is inactivated in approximately half of all oligodendroglial tumors.50 CIC inactivation has been associated with worse oligodendroglioma outcomes,51 although others have not found such a connection.49 CIC interacts with chromatin regulators to transcriptionally repress targets in the receptor tyrosine kinase/MAPK pathway,52 and there is evidence that CIC deletion promotes proliferation of neural stem cells,53 suggesting a direct role for CIC in glioma formation.
The majority of oligodendroglial tumors also display abnormal telomerase activity through the acquisition of mutations in the promoter of the TERT gene,54 permitting tumor cells to overcome cellular senescence to allow unlimited replication. The acquisition of TERT promoter mutations (nearly always C228T or C250T, the same as in glioblastoma) occurs early in gliomagenesis. These mutations may promote TERT activation by allowing the specific binding of a complex involving the GABPA transcription factor, thereby facilitating the binding of RNA polymerase 2, which increases TERT expression.55
In contrast, IDH-mutant astrocytic tumors usually have inactivating mutations in the ATRX gene, which encodes for a chromatin remodeler. Through mechanisms not yet completely understood, ATRX inactivation promotes telomere maintenance through alternative lengthening of telomeres (ALT).56–58 Over 70% of IDH-mutant astrocytomas have mutations in ATRX and TP53.59
Despite these well-described clinical and molecular differences, single-cell RNA sequencing analyses suggest that IDH-mutant astrocytomas and IDH-mutant oligodendrogliomas arise from a common glial progenitor cell and share a largely similar developmental hierarchy, composed of proliferating neural stem-like cells and non-proliferating cells with astrocytic or oligodendroglial differentiation.26,27 Oligodendrogliomas have more oligodendroglial-like and astrocyte-like cells, with more neurons in the nonneoplastic background, whereas astrocytomas have more stem-like cells, and contain more activated microglia and macrophages.27
Both IDH-mutant astrocytomas and oligodendrogliomas exhibit the genome-wide DNA hypermethylation phenotype known as G-CIMP, as mentioned above.23,24 The extent of hypermethylation can be described as “G-CIMP-high” and “G-CIMP-low”, with the G-CIMP-low signature conferring worse prognosis.60 This epigenetic state is not static, as IDH-mutant gliomas can lose much of their DNA methylation during tumor progression.60–62 Such changes to the epigenetic landscape are thought to drive glioma formation and progression by activating programs for self-renewal and proliferation through dysregulated gene expression, as discussed previously.
During tumor progression, IDH-mutant gliomas acquire additional genomic insults that converge on activation of RTK, MYC and CDK-Retinoblastoma (RB) pathways.37,63–65 Evidence is mounting that therapy drives the acquisition of some genomic alterations. Treatment with the alkylating chemotherapy temozolomide (TMZ) leads to a hypermutation phenotype associated with acquired defects in DNA mismatch repair genes.37,65,66 Such hypermutation is eventually found in approximately 60% and 30% of post-TMZ oligodendrogliomas and astrocytomas, respectively.66 TMZ-induced hypermutation is more common when gliomas have methylation at the O6-methylguanine-DNA methyltransferase (MGMT) promoter; 67MGMT promoter methylation occurs in about 85% and 98% of IDH-mutant astrocytomas and oligodendrogliomas, respectively.68 Unlike what has been observed for IDH-wildtype glioblastoma, MGMT promoter methylation is not clearly predictive for response to TMZ in IDH-mutant tumors,69 though it is associated with improved survival.70 Not only are hypermutant tumors resistant to further treatment with alkylating chemotherapies owing primarily to mismatch repair (MMR) pathway mutations (recently reviewed in71), they seem inherently more aggressive,66 potentially through activation of pathways known to drive glioma progression, including CDK-Rb and PI3K pathways.37,65 Despite the concern regarding the development of a hypermutated phenotype, the benefit of adding temozolomide to radiation therapy outweighs the risks and results in improved survival compared to radiation therapy alone.72 Radiation therapy has also recently been implicated in the development of genomic alterations that are associated with worse outcomes. Kocacavuk and colleagues noted both small and large DNA deletions following RT in a variety of cancer types.73 In IDH-mutant gliomas, RT frequently results in homozygous deletion of the tumor suppressor CDKN2A which is linked to shorter survival time.73
Diagnostic Imaging and Response Assessment
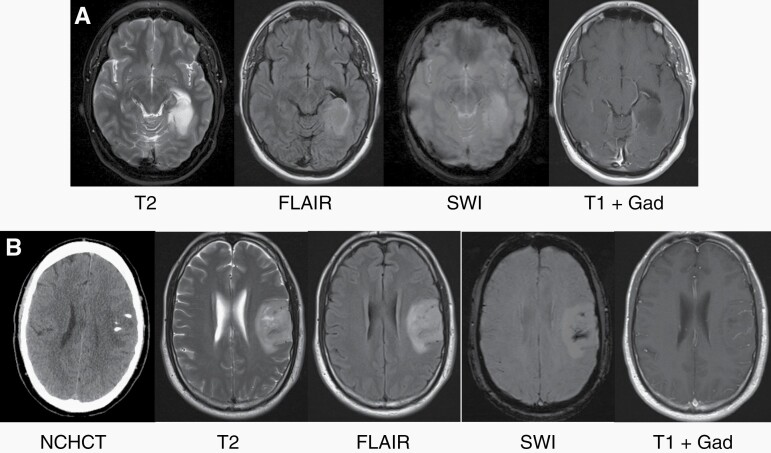
As discussed below, most patients with IDH-mutant gliomas present to medical attention after a seizure,74–76 or the tumors are discovered incidentally when brain magnetic resonance imaging (MRI) is obtained for evaluation of complaints unrelated to the tumor.77,78 On MRI, IDH-mutant gliomas are commonly located in the frontal lobes, characterized by expansive lesions with hyperintense signal in T2-weighted and T2-fluid-attenuated inversion recovery (FLAIR) images, (Figure 4). After intravenous gadolinium (Gad) chelate contrast administration, lesion enhancement is often not seen in grade 2 IDH-mutant tumors but is a common finding in astrocytoma, IDH-mutant, grade 3 and 4, and oligodendroglioma, IDH-mutant, 1p/19q codeleted, grade 3. Astrocytoma, IDH-mutant, can exhibit suppression of T2 signal on the FLAIR sequence in the core vs rim of the tumor compared to a more homogeneous signal in standard T2-weighted sequence (T2/FLAIR mismatch; see Figure 4A).79 This radiological sign is highly specific for astrocytoma, IDH-mutant (specificity, 100% in a recent meta-analysis), although it has low sensitivity (42%).80 Oligodendrogliomas more frequently involve the cortex, and identification of calcifications on computed tomography (CT) or susceptibility-weighted imaging (SWI) is highly specific for these tumors, Figure 4B.
Figure 4.
Imaging characteristics of IDH-mutant gliomas. (A) MRI features of astrocytoma, IDH-mutant, grade 2. This left posterior temporal lobe tumor demonstrates hyperintensity on T2-weighted and fluid-attenuated inverted recovery (FLAIR) sequences. Note the decreased signal within the core of the tumor on FLAIR compared to T2 (T2/FLAIR mismatch). The tumor does not demonstrate decreased signal on susceptibility-weighted imaging (SWI) or contrast enhancement after administration of Gadolinium (Gad). (B) MRI features of oligodendroglioma, IDH-mutant, 1p/19q codeleted, grade 2. This left frontal tumor demonstrates hyperdensities on non-contrast head CT (NHCHT), consistent with calcifications (also seen on SWI). The tumor is hyperintense on T2 and FLAIR and does not demonstrate contrast enhancement after administration of Gad.
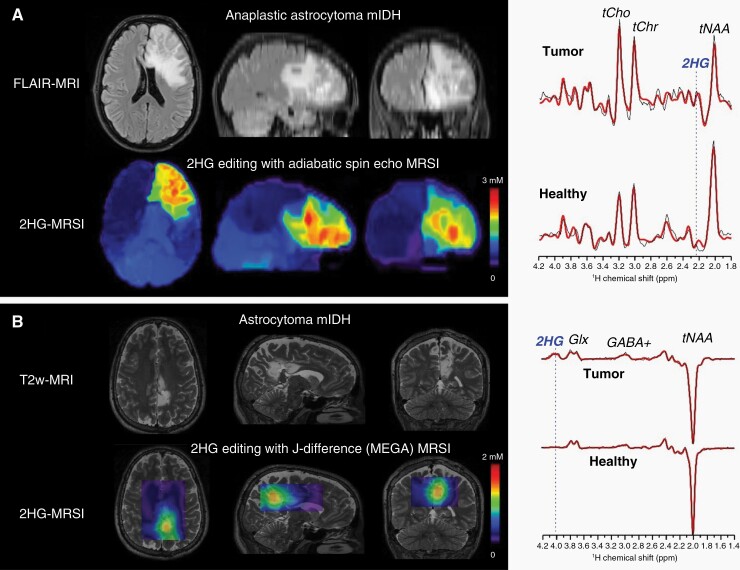
Advanced MRI techniques, such as MR spectroscopy imaging (MRSI), leverage the unique metabolic characteristics of these tumors and are increasingly being used for diagnosis and response assessment. In IDH-mutant gliomas, the oncometabolite D-2HG accumulates within the tumor to very high (1–50 mM) concentrations that are orders of magnitude above the wild-type levels.81,82 The presence of this metabolite can then be assessed using D-2HG edited MRSI, which has been shown to high sensitivity and specificity for D-2HG detection.83 Changes in the D-2HG signal are being explored for monitoring treatment response in certain clinical settings.81,84–86 Interestingly, these MRSI D-2HG maps reveal spatial heterogeneity of tumor metabolism and show areas of involvement that go beyond the areas of increased T2 signal, demonstrating the infiltrative nature of these tumors and the fact that tumor size is likely underestimated by the assessments made on standard T2-weighted sequences, Figure 5. Other advanced imaging techniques that remain in the experimental realm likewise take advantage of the unique metabolic properties of IDH-mutant gliomas, such as the use of imaging contrast that is pH and protein-content-sensitive in chemical exchange saturation transfer (CEST)87 or dynamic polarization of 13C-labeled probes to monitor specific metabolites by hyperpolarized MRI.88
Figure 5.
D -2-hydroxyglutarate (2-HG) imaging at 3T in IDH-mutant glioma (IDHm) patients: (A) 2-HG edited whole-brain magnetic resonance spectroscopic imaging (MRSI) using adiabatic spin echo with long echo time modulation, TE = 97 ms (TE1/TE2 = 32/65 ms). (B) 2-HG edited 3D MRSI using J-difference (MEGA-LASER editing, TE = 68 ms) technique. Metabolic heterogeneity can be seen in 2-HG images of large tumors (A). Spectra shown on the right indicate the positions of 2-HG signals edited by the two imaging methods.
Currently, the uniform assessment of treatment response and disease progression in neuro-oncology relies on standard anatomical MRI due to its wider availability and ease of use. However, confounding effects often limit the reliability of anatomical MRI in IDH-mutant gliomas, and the future adoption of standardized advanced metabolic and physiological MRI is expected to improve clinical practice. The Response Assessment in Neuro-Oncology (RANO) criteria for low-grade gliomas is the most widely used set of criteria for response assessment in clinical trials of low-grade IDH-mutant gliomas,89 although the RANO criteria for high-grade gliomas are also used in tumors that demonstrate contrast enhancement.90,91 The RANO criteria for gliomas is in the process of being updated and in RANO 2.0 a single response criteria for all gliomas is planned, regardless of their grade.92,93 Volumetric assessments are also being evaluated to monitor treatment response in clinical trials of IDH-mutant gliomas.94,95
Medical Management and Supportive Care
IDH-mutant gliomas have a less aggressive clinical behavior than glioblastoma. The majority of patients present with seizures (focal or generalized), and seizure at presentation is more common in IDH-mutant gliomas than in glioblastoma.74–76,96 The higher incidence of seizures at presentation in IDH-mutant gliomas is thought to result from the infiltrative nature of these tumors (often affecting the cerebral cortex such as in oligodendroglioma) and the fact that the oncometabolite D-2HG released by tumor cells has a structure analogous to that of glutamate, and has been experimentally shown to activate neuronal NMDA receptors, thereby creating excitatory postsynaptic potentials that can lower the seizure threshold.75 The management of tumor-related epilepsy in patients with IDH-mutant gliomas is similar to that of patients with other brain tumors, and is usually approached with the administration of nonhepatic microsomal enzyme inducing antiepileptic agents such as levetiracetam or lacosamide,76,97 although there are data suggesting that the seizures in patients with IDH-mutant gliomas are more treatment-refractory than their wild-type counterparts.98 IDH-mutant gliomas are sometimes discovered incidentally when brain magnetic resonance imaging (MRI) is obtained for evaluation of complaints that are often unrelated to the tumor (e.g., evaluation of a new headache that is eventually determined to correspond to a primary headache, such as migraine).77,78 As in the case of other brain tumors, prophylactic anti-epileptic drugs are not recommended for patients with incidentally found IDH-mutant gliomas who have not experienced seizures.97,99 Corticosteroids are frequently used in the peri-operative setting, as well as for the management of acute symptomatic worsening during progression and pseudo-progression as they are effective in the treatment of peritumoral edema.96 Although D-2HG may have an antithrombotic effect, the risk of venous thromboembolism (VTE) in patients with IDH-mutant gliomas is higher than in the general population.100,101 No specific guidelines are available for the treatment of VTE in patients with IDH-mutant gliomas. Patients are managed with low-molecular-weight heparin or direct oral anticoagulants (DOACs) in alignment with the treatment of VTE in patients with cancer.102–104
Surgical Management
IDH-mutant gliomas most often present as a single focus on imaging, and there is substantial evidence of benefit for a maximal safe resection, notwithstanding their diffuse infiltrative growth pattern. Surgical resection accomplishes the important goals of establishing a tissue diagnosis, providing the substrate for in-depth molecular analysis, reducing seizure frequency, as well as providing the critical first step in their effective treatment. Not only does initial resection lead to a more accurate diagnosis than stereotactic biopsy,105,106 due to less sampling error, but importantly, several studies have demonstrated surgical resection (versus biopsy-only) contributes to significantly improved clinical outcomes.107–111 More extensive surgical resection is associated with improved overall survival. With regards to surgical technique, particularly notable in patients with astrocytoma, IDH-mutant, those undergoing maximal resection of the areas of hyperintense T2/FLAIR signal achieve better overall survival.112–114 This resection benefit is observed in patients with both symptomatic and asymptomatic (incidentally-discovered) tumors.110 In addition, the extent of surgical resection is also associated with seizure control, with maximal resections associated with a higher percentage of seizure-free patients post-operatively.115,116 There are also emerging data in support of supramaximal resection of IDH-mutant gliomas at diagnosis improving progression-free and overall survival.117,118 From the standpoint of surgical technique, more extensive resections can be challenging, with the potential to increase the risk of post-operative deficits; however, a range of pre-operative and intraoperative mapping techniques have been developed and can decrease this risk, while permitting the aggressive surgical management of gliomas.119,120 In all cases, a post-operative brain MRI with contrast should be obtained within 24–48 h of the surgery document the extent of resection and to provide a post-operative baseline. In the immediate post-operative setting, it is difficult to distinguish between residual non-enhancing tumor and cerebral edema from surgery. Therefore, a follow-up MRI performed 1–2 months after resection, when surgery-induced changes have resolved, allows for the most accurate assessment of extent of resection in this situation. Although data are thus far limited in support of re-resection of IDH-mutant gliomas at the time of progression,121 re-resection is often pursued and it is recommended in society guidelines122,123 in cases where a prolonged response to radiation or chemotherapy is not anticipated.
Radiation Therapy
In the mid-1970s, data began to emerge that demonstrated patients with low-grade gliomas who underwent incomplete tumor resection followed by radiation therapy had better overall survival than patients who only had an incomplete resection.124 Despite initial reports suggesting that high-dose radiation (59.4–64.8 Gy) was more effective than lower-dose radiation (45–50.4 Gy) for low-grade gliomas, large randomized clinical trials demonstrated no differences in progression-free or overall survival between doses. Further, a higher incidence of radiation necrosis was observed with doses greater than 60 Gy.125,126 In addition, a large, randomized trial showed that early post-operative radiation improved progression-free survival but not overall survival when compared to delayed radiation provided at the time of radiographic progression.127 The main rationale for delaying radiation in patients with grade 2, IDH-mutant gliomas is to preserve cognitive function in younger patients with an expected median overall survival in excess of 10 years, as there is increasing evidence that radiation can lead to worse neurocognitive function in multiple domains, predominantly in attention and processing speed.128,129 Cognitive disability in the memory domain was mainly reported for the use of fraction doses exceeding 2 Gy.129 However, most long-term cognitive sequelae of RT are linked to higher doses, larger treatment fields and older RT techniques. With modern radiation techniques (intensity-modulated RT, image-guided RT, hippocampal sparing) and lower-dose levels, limited neurocognitive damages are expected. In fact, the EORTC 22033 trial showed no deleterious effect on memory function when treatment with radiation was compared to treatment with temozolomide in a subgroup of patients using a prospective repeated neurocognitive test battery during a 12-month follow-up period,130 but a much longer follow-up is required for meaningful conclusions. Post-operative radiation treatment is standardly offered to patients with astrocytoma, IDH-mutant, grade 3 or 4 or oligodendroglioma, IDH-mutant, grade 3 due to concerns for a faster growth rate compared to grade 2 IDH-mutant tumors. However, it remains unclear whether a true difference in behavior exists between grade 2 and grade 3 IDH-mutant tumors.
For the treatment of grade 2 IDH-mutant gliomas, the recommended radiation dosing is between 45 and 54 Gy in 25–30 fractions (1.8–2.0 Gy fractions).123,131 Clinical target volumes (CTV) are best defined using areas of increased T2 or FLAIR signal on MRI [including the gross tumor volume, (GTV) and/or resection cavity] and adding a 1–2 cm anatomically constrained margin. For grade 3 and 4 IDH-mutant gliomas, recommended total radiation doses include 59.4 Gy or 60 Gy, administered in 1.8 or 2.0 Gy fractions, respectively.123 GTVs are best defined using post-contrast T1 and T2 or FLAIR images and expanded to add a 1–2 cm anatomically constrained margin to determine the CTV. Acute radiation toxicities—including scalp erythema, alopecia and fatigue—are transient and typically self-resolve, though alopecia can be permanent. Pseudo-progression, in which treatment causes a self-limited increase in contrast enhancement that mimics tumor progression, can be observed in patients with IDH-mutant glioma, peaking between 3 and 78 months of radiation completion.132 Delayed toxicities, such as radiation necrosis, can develop within months of treatment completion, while others, like the stroke-like migraine attacks after radiation therapy (SMART) syndrome, can develop years later.133,134 Patients with oligodendrogliomas receiving radiation doses greater than 54 Gy appear to have a higher risk for developing radiation necrosis than those with astrocytoma.135 When feasible, proton radiation can be considered for the treatment of young patients with IDH-mutant glioma to protect non-involved brain regions at risk of radiation effects (optic nerves, optic chiasm, pituitary gland and hippocampus),136 although no definitive evidence is available to demonstrate decreased toxicity with proton-based radiation compared to the much more commonly available photon-based radiation. The effect of these two modalities on cognition is currently being evaluated in the NRG-BN005 phase II study, which randomizes grade 2 and 3 IDH-mutant glioma patients to proton vs photon radiation therapy.
Initial Diagnosis: Standard Therapy and Clinical Trials
Grade 2 IDH-mutant Astrocytoma/Grade 2 Oligodendroglioma
Young patients without neurologic symptoms related to the tumor who have undergone gross total resection and are found to have IDH-mutant CNS WHO grade 2 tumors may be observed without immediate cytotoxic treatment. Traditionally, patients less than age of 40 have been considered “young”, though this designation is derived from the pre-molecular era observation that age older than 40 is associated with poor prognosis; there is ongoing debate as to whether the cut-off of 40 is still relevant for IDH-mutant gliomas. Otherwise, post-surgical radiation and alkylating chemotherapy is generally accepted as the standard of care for patients with newly diagnosed IDH-mutant glioma. The use of chemotherapy following radiation therapy, is guided by data from randomized trials in patients with grade 2 and 3 glioma that were initiated before the current molecularly driven diagnostic criteria were put in place. As such, some portion of the trial populations do not fit within the current diagnostic criteria for oligodendroglioma or astrocytoma. However, retrospective molecular analysis of available tumor samples has aided our ability to apply results from legacy diagnostic categories to the current WHO classification in the best possible manner.137
NRG Oncology/RTOG 9802138 randomized 251 “high risk” (age > 40 with any amount of resection or age ≤ 40 with a subtotal resection or biopsy) patients with WHO grade 2 glioma (using pre-WHO 2021 criteria) to radiation therapy (RT) alone or RT followed by six cycle of procarbazine, lomustine and vincristine (PCV) combination chemotherapy. In this population, which included patients with both IDH-mutant and IDH wild-type astrocytomas, oligodendrogliomas and (morphological) oligoastrocytomas, the addition of PCV to RT significantly improved median overall survival (OS) [hazard ratio (HR) = 0.59; P = .003].138 In a post hoc genomic analysis of 42% (106/251) of tumors from eligible patients enrolled in RTOG 9802, all IDH-mutant subgroups, regardless of codeletion status, appeared to benefit from RT + PCV. For patients with oligodendrogliomas (n = 37 with 1p/19q codeletion), median OS was not reached in the RT + PCV arm compared to 13.9 years for RT alone (HR 0.21; P = .029). For patients with IDH-mutant astrocytoma (n = 43 with 1p/19q non-codeleted), median OS was 11.4 years with RT + PCV compared to 4.3 years with RT alone (HR 0.38; P = .013).139 It is worth noting that these significant differences were detected despite the relatively small number of available molecularly characterized patients in each group.
As discussed in the RT section, there has been interest in delaying RT for patients with grade 2 gliomas. To this end, temozolomide monotherapy has been investigated in several phase II trials,140,141 as well as in a randomized phase III study,142 in high risk, low-grade gliomas. Median OS following chemotherapy alone was inferior to RT + PCV in these studies, even when analyzed by molecular subgroups, however, TMZ monotherapy resulted in sufficient disease stability to allow for a meaningful delay in receipt of RT. The initial design of the CODEL study (Alliance for Clinical Trials in Oncology N0577/EORTC 26081-22086/NRG 1071/CEC.6) in patients with newly diagnosed grade 3 oligodendrogliomas involved three arms: RT alone, RT plus concomitant and adjuvant TMZ and TMZ alone. CODEL was redesigned after publication of results from RTOG 9402 and EORTC 26951 to remove the TMZ only arm. Although the number of patients included in the analysis was small, TMZ alone was associated with significantly shorter PFS compared to RT and RT + TMZ.143 RT followed by chemotherapy remains the standard approach in patients with IDH-mutant glioma but these data suggest a strategy of TMZ monotherapy that could be used in select patients deemed to be too high risk for immediate RT.
Grade 3 Oliogodendroglioma
EORTC 26951144 and RTOG 9402145 investigated the benefit of adding PCV to RT in patients with histologically defined grade 3 oligodendrogliomas. In EORTC 26951, PCV × 6 cycles was administered after RT; in RTOG 9402, an intensive regimen of PCV × 4 cycles was given prior to RT. Like RTOG 9802, these two trials were designed prior to the discovery of the IDH-mutation, leading to the inclusion of heterogeneous patient populations. Combination therapy improved OS compared to radiation alone in EORTC 26951 but not in RTOG 9402 when the entire population was considered; however, in both studies median OS was significantly prolonged in patients with IDH-mutant and 1p/19q codeleted tumors and who were treated on the combination therapy arm compared to those treated with radiation alone: (RTOG 9402: median OS 13.2 years with PCV + RT versus 7.3 years with RT; HR0.61, 95% CI .04–.94, P = .02; EORTC 26951: OS 14.2 years with RT + PCV versus 9.3 years with RT; HR0.60, 95% CI .35–1.03, P = .063). Since similar improvements in median OS was seen in both studies when PCV was added to treatment with RT, but significant toxicity occurred with the intensive PCV regimen, including two patient deaths due to neutropenia, the National Comprehensive Cancer Network Guidelines Panel for CNS Tumors recommends treating patients as per the EORTC 26951 study with RT followed by six cycles of PCV.119
Grade 3 IDH-mutant Astrocytoma
In a post hoc analysis of RTOG 9402, the investigators also observed a survival benefit from PCV + RT compared to RT alone in participants whose tumors were IDH-mutated but not 1p19q codeleted (i.e., grade 3 astrocytoma, per the 2021 WHO classification of gliomas), with a median overall survival of 9.4 years compared to 5.7 years with RT alone (HR 0.59; 95% CI .40–0.86).146 More recently, the benefit of temozolomide administered either concurrently with RT, following RT or both was investigated in the CATNON trial (EORTC 26053-22054), which enrolled 751 patients with 1p/19q non-codeleted grade 3 gliomas into a two-by-two factorial design.72 Of the 660 tumors evaluable for mutation status, 67% exhibited mutations in IDH1 or IDH2. In this IDH-mutant grade 3 astrocytoma subset, the addition of adjuvant temozolomide to RT improved overall survival compared to no adjuvant temozolomide [median OS 117 months compared to 78 months; HR 0.48 (95% CI .35–0.67); P < .0001], while no statistically significant benefit was seen with concurrent temozolomide [117 months compared to 92 months; HR 0.80 (95% CI .58–1.10); P = .17].
Although PCV can be harder for patients to tolerate in terms of typically more fatigue, nausea, and bone marrow suppression, as well as being more time-consuming for neuro-oncologists to prescribe and monitor, to date there are no phase 3 data showing an improvement in survival with radiation and temozolomide for the treatment of grade 2 IDH-mutant gliomas or grade 3 oligodendrogliomas. The CODEL trial was initially designed to compare the efficacy of RT alone, RT + TMZ or TMZ monotherapy in patients with newly diagnosed 1p/19q codeleted grade 3 oligodendroglioma. Although the trial population is small, a significantly shorter PFS was observed in the patients receiving TMZ monotherapy compared to RT, suggesting that TMZ alone is inferior.143 Moreover, with the final results of EORTC 26951 and RTOG 9402 showing an improvement in survival with upfront RT and PCV, the CODEL trial was redesigned to compare head on RT + TMZ to RT + PCV in patients with newly diagnosed grade 2 or 3 oligodendroglioma. Unfortunately results from this trial will likely not be available for many years.
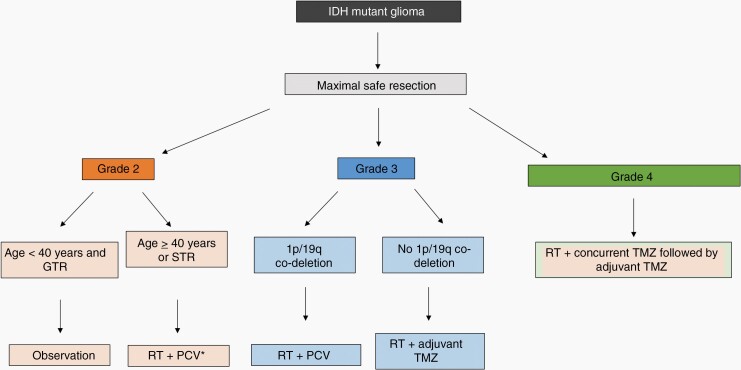
Taken together, data from RTOG 9802, RTOG 9402, EORTC 26951, and CATNON have consistently shown a clear benefit from the combination of RT and chemotherapy compared to RT alone. The use of TMZ or PCV as the chemotherapy regimen of choice varies by institution and geographic location because prospective, randomized comparisons have been limited by small numbers of patients. For many, this decision is driven by 1p/19q codeletion status, with PCV favored for IDH-mutant oligodendrogliomas and TMZ favored for IDH-mutant astrocytomas.147 Because vincristine has been shown to have minimal ability to penetrate the blood brain barrier in an experimental system,148 some providers use procarbazine and lomustine (PC) instead of PCV. A suggested treatment algorithm is found in Figure 6.
Figure 6.
Suggested management algorithm for patients with newly diagnosed IDH-mutant glioma, based on histopathological grade and 1p/19q codeletion status.
Several questions remain regarding optimal treatment of IDH-mutant gliomas: do all people with newly diagnosed IDH-mutant, 1p/19q codeleted gliomas who meet “high risk” criteria need to start treatment with radiation and chemotherapy immediately after surgery? Can PCV as the current standard chemoradiotherapy for patients with oligodendrogliomas be safely and effectively replaced by temozolomide? How should one weight more efficacy versus more toxicity, specifically considering the overall favorable prognosis of more than a decade median overall survival time? The ongoing POLCA (NCT02444000) and NOA-18 (NCT05331521) trials are focusing on this question by omitting radiation from the front-line treatments and considering health-related quality of life, neurological function and cognition relevant (patient-centered) endpoints. And, finally, how should patients with grade 4 IDH-mutant astrocytomas be treated, with RT following by adjuvant temozolomide along the lines of the CATNON trial or with concurrent chemoradiation followed by adjuvant temozolomide similar to the treatment strategy of IDH-wildtype glioblastoma?
A number of clinical trials are underway for treatment-naïve patients with IDH-mutant gliomas (Tables 2 and 3). IDH inhibitors, which selectively inhibit IDH-mutant enzymes to decrease production of D-2HG, are the most advanced in the clinical trial pipeline. In preclinical studies, IDH inhibitors demonstrated the ability to slow tumor growth and promote differentiation in some patient-derived glioma models.17,18,149 Ivosidenib, an IDH1-mutant specific inhibitor, and vorasidenib, a brain-penetrant pan-IDH-mutant inhibitor, both demonstrated promising signs of efficacy in phase I trials. In the ivosidenib trial, 30 of 35 patients (85%) without enhancing tumor achieved a best response of stable disease150 and in the vorasidenib trial, 90% of 22 such patients achieved stable disease or better, including 1 partial response and 3 minor responses.151 Based on these data, a phase III randomized, placebo-controlled trial of vorasidenib is currently underway in patients with non-enhancing IDH-mutant gliomas, grade 2 who are within 5 years of initial diagnosis and have never received RT or chemotherapy (INDIGO study, NCT04164901), with a primary endpoint of PFS. A phase II trial of a different IDH1 inhibitor, DS-100b, is similarly enrolling patients with grade 2 IDH-mutant glioma who are RT and chemotherapy-naïve (NCT04458272). Another small molecule inhibitor of mutant IDH, LY3410738, is in earlier stages of clinical testing (NCT04521686). Furthermore, based on preclinical data showing an IDH-mutation-specific dependence on glutaminase (GLS) for glutamate production,33 a phase Ib trial of the GLS inhibitor telaglenastat in combination with RT and TMZ in patients with newly diagnosed IDH-mutant gliomas, grade 2 or grade 3 is underway (NCT03528642), with plans to expand to a phase II/III study in the near future. Additionally, a phase 1 trial (NOA16; NCT02454634) has explored the safety and immunogenicity of a long peptide vaccine (IDH1-vac) targeting mutant IDH in patients with newly diagnosed astrocytomas grade 3 and 4 with IDH1 R132H mutations. When integrated into standard of care, IDH1-vac resulted in a high number of pseudoprogressions and encouraging survival rates at 3 years (OS: 84%; PFS: 63%).152 A phase 2 trial is planned.
Table 3.
Immunotherapy Clinical Trials for Progressive IDH-mutant Glioma
| Strategy | Registry no. | Institution | Immunotherapy | Phase | Treatment regimen | Status |
|---|---|---|---|---|---|---|
| Vaccine | NCT02193347 | Duke University Medical Center |
PEPIDH1M | I | PEPIDH1M ± RT + TMZ | Active, not recruiting |
| Vaccine + ICI | NCT03893903 | German Cancer Research Center |
IDH1-vac + anti-PD-L1 |
I | IDH1 vaccine ± avelumab | Recruiting |
| ICI | NCT03991832 | University Health Network, Toronto |
Anti-PD-L1 | II | Durvalumab + olaparib | Recruiting |
| ICI | NCT03925246 | Hôpitaux de Paris | Anti-PD-1 | II | Nivolumab | Active, not recruiting |
| ICI + IDHi | NCT04056910 | University of Pittsburgh Medical Center |
Anti-PD-1 | II | Nivolumab + ivosidenib | Recruiting |
| ICI + RT | NCT02968940 | NYU Langone Health | Anti-PD-L1 | II | Avelumab + HFRT | Completed |
| ICI | NCT03718767 | National Institutes of Health Clinical Center |
Anti-PD-1 | II | Nivolumab | Recruiting |
| ICI | NCT03557359 | Columbia University Medical Center |
Anti-PD-1 | II | Nivolumab | Recruiting |
Progression: Standard Therapy
Lower-grade IDH-mutant gliomas, despite maximal safe resection and subsequent therapies invariably develop progressive increase in T2/FLAIR signal with or without contrast enhancement, indicative of progressive tumor. The evolving imaging changes related to prior therapies usually have a time-limited effect. For instance, the time frame for contrast enhancement related to pseudo-progression from radiation and chemotherapy can take place over a period of time longer than a year.153 True progression is uncommon for most IDH-mutant gliomas during the first 18 months, unless there are poor prognostic factors such as CDKN2A/B homozygous deletion.4 Additionally, after radiation and chemotherapy it is not uncommon to have evolving non-enhancing post-surgical or post-radiation changes on imaging. These imaging changes can wax for many years after radiation treatment (range 3–78 months) and can be mistaken for non-enhancing tumor growth.132,154 At progression, all therapeutic modalities should be considered. Resection is recommended for tumors amenable to re-resection, and in some circumstances a biopsy should be considered for unresectable tumors in order to confirm progression and to obtain tissue samples for molecular profiling. Data in support of re-resection are limited,121 but when feasible, resection at the time of progression is a common practice and is recommended in the United States National Comprehensive Cancer Network (NCCN) guidelines.123 In patients who underwent resection and received adjuvant therapies at diagnosis, additional treatment considerations for progressive disease include clinical trials, chemotherapy and RT. Clinical trials, if available to the patient, should be strongly considered because none of the standard therapies are curative and because several therapeutic strategies based on recently discovered vulnerabilities of IDH-mutant gliomas are currently being evaluated in clinical trials (see sections on Targeted Therapy and Immunotherapy below). For patients with prior response to alkylating therapy, a re-challenge with temozolomide or another alkylating agent (lomustine) can be considered. Re-irradiation, particularly if there is progression outside the initial radiation field and sufficient time has elapsed since treatment completion, is an option to achieve temporary disease control. Even after gross total resection at progression, observation should only be considered for low-risk patients, especially if the interval between diagnosis or first resection and secondary resection is several years. In patients who were under observation after initial resection, when they develop tumor progression adjuvant therapy with a clinical trial or radiation and chemotherapy (with procarbazine/lomustine/vincristine or temozolomide as discussed above) should be considered. For patients progressing after surgery alone, treatment regimens based on radiotherapy alone or chemotherapy alone are not supported by the available evidence, as single modality therapy compromises both PFS and OS. The addition of bevacizumab to temozolomide in patients with progressive astrocytoma, IDH-mutant, grades 2 or 3 did not demonstrate an improvement in progression-free or overall survival.155
Progression: Targeted Therapy
At progression, targeted treatment options are currently limited to clinical trial settings (Table 2). There has been much interest in exploiting selective IDH-mutant glioma vulnerabilities with poly(ADP-ribose) polymerase (PARP) inhibitors. D-2HG impairs the fidelity of homologous recombination-mediated double strand DNA break repair, rendering IDH-mutant cells dependent on alternative DNA repair mechanisms, including those mediated by PARP. In preclinical models, IDH-mutant cancers are sensitive to PARP inhibitors, particularly when administered in conjunction with temozolomide, in a wide range of tumors with these mutations.156–158 Case studies have suggested that PARP inhibitors may be active against IDH-mutant cancers as a monotherapy in patients.159–161 The brain-penetrant PARP inhibitor niraparib is under investigation as monotherapy in one arm of a surgical window-of-opportunity study in patients with recurrent grade 2–4 IDH-mutant astrocytoma (NCT05076513). The safety and preliminary efficacy of the PARP inhibitor pamiparib in combination with temozolomide is being examined in a phase I/II trial enrolling patients with recurrent grade 2-4 IDH-mutant glioma (NCT03914742). Both trials are ongoing, and data are not yet mature. Another Phase 2 trial is examining the safety and effectiveness of a combination of pembrolizumab, olaparib, and temozolomide in recurrent IDH-mutant glioma (NCT05188508). Limited clinical activity was observed in the OLAGLI trial testing olaparib monotherapy in patients with recurrent IDH-mutant gliomas,162 although a subset of outlier responders were identified. Nonetheless, these data suggest that clinical benefit from PARP inhibitors may require co-administration with another agent, such as a DNA damaging agent or another DDR inhibitor.
Targeting the DNA hypermethylator phenotype associated with IDH-mutant status with demethylating agents is another targeting strategy actively under investigation in patients with recurrent tumors. ASTX727 is an oral drug containing the DNA methyltransferase (DMNT) inhibitor decitabine in combination with cedazuridine, which decreases systemic decitabine metabolism. ASTX727 is currently under study in a phase 0/1 trial in recurrent or progressive non-enhancing IDH-mutant gliomas (NCT03922555) to determine drug safety and pharmacodynamic parameters. The DNMT inhibitor 5-azacytidine is being tested in a phase II trial in patients with recurrent IDH-mutant glioma of any grade.
Homozygous deletion of the tumor suppressor gene CDKN2A/B is frequently observed in IDH-mutant gliomas at recurrence37,64,163 and has been associated with prior RT.73 Inhibitors of cyclin-dependent kinase (CDK) 4/6, which are widely used for advanced hormone-positive breast cancer, are being employed to target CDK-Rb pathway dysregulation caused by the absence of CDKN2A. The approach is currently under study in patients with recurrent oligodendroglioma (NCT02530320, NCT03220646). With the incorporation of CDKN2A status into the 2021 WHO CNS diagnostic criteria, interest in targeting dependencies associated with CDKN2A loss is likely to increase.
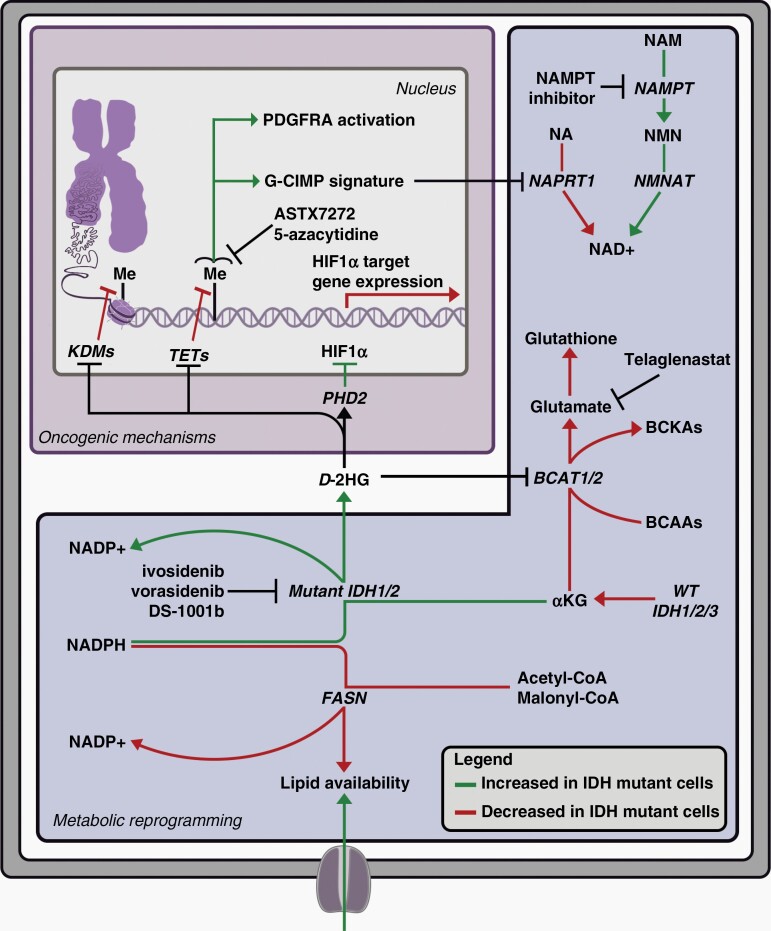
Other approaches that target mutant IDH-driven metabolic vulnerabilities based on published preclinical data are in the early stages of trial development, including the use of inhibitors of nicotinamide phosphoribosyltransferase (NAMPT),34 poly(ADP-ribose) glycohydrolase (PARG)164 and dihydroorotate dehydrogenase (DHODH).165 Active clinical trials are summarized in Table 2. A schematic of select molecular and metabolic pathways druggable with available targeted therapies is presented in Figure 7.
Figure 7.
Targeted therapies for IDH-mutant gliomas. Oncogenic mechanisms and metabolic reprogramming caused by mutant IDH and excess D-2-hydroxyglutarate (D-2-HG). Targeting strategies under investigation are noted at relevant points.
Progression: Immunotherapy
Recent studies have demonstrated important interactions between tumor cells in IDH-mutant gliomas and their tumor microenvironment. Important for immunotherapy considerations, IDH-mutant gliomas display reduced T-cell infiltration that is thought to result from D-2HG suppression of T-cell activity, an effect that has been experimentally reversed by inhibition of the neomorphic enzymatic function of the mutant IDH1.149,166,167 In order to revert D-2HG-mediated T-cell inhibition, an ongoing clinical trial (NCT04056910) is evaluating the strategy of combining an IDH1 inhibitor with an anti-PD1 immune checkpoint inhibitor (ICI) in patients with recurrent IDH-mutant glioma. The canonical IDH1 mutation, R132H, represents an attractive clonal neoantigen for a vaccine therapeutic strategy. Two vaccines targeting the IDH1 R132H mutation are currently being evaluated in patients with progressive IDH-mutant gliomas (Table 3). Although retrospective analysis of the response to ICI immunotherapy in IDH-mutant glioma patients with hypermutation induced by radiation and alkylating chemotherapy has not demonstrated an improvement in overall survival,66 a number of clinical trials are evaluating treatment with ICIs in patients with progressive disease (Table 3).
Challenges, Areas of Uncertainty, Future Directions
Significant progress towards understanding IDH-mutant-driven gliomagenesis has been made since the seminal discovery in 2008 of recurrent, frequent IDH mutations in lower-grade gliomas and “secondary” glioblastomas (Table 1). Genomic profiling resulted in an improved classification system that more accurately predicts clinical behavior, response to treatment and prognosis (Table 4). Despite these advances, standard treatment still consists of conventional therapies involving irradiation and chemotherapy. Treatment decisions continue to rely heavily on data generated from large randomized clinical trials that were designed before the advent of molecular classification of glioma and, as such, were not powered to robustly identify impact of therapy on patients in the IDH-mutant glioma subgroup. This issue will likely persist as the rapidly evolving knowledge base outpaces the necessarily long follow-up periods required to accurately document progression-free and overall survival times observed in this patient population. The development of evidence-based surrogate endpoints (clinical or radiographic) that strongly correlate with survival will be an important factor for testing promising novel treatments in an efficient manner. Further prognostic separation of IDH-mutant glioma by molecular subclassification will be critical for developing treatment strategies to prolong survival in situations where radiation plus chemotherapy is not adequate.
Table 4.
Summary Box: Key Points
| Key points | |
|---|---|
| Classification | • Astrocytoma, IDH-mutant: associated with ATRX and TP53 mutation, WHO grade 2-4 depending on histologic features and CDKN2A homozygous deletion status • Oligodendroglioma, IDH-mutant: characterized by 1p/19q codeletion and TERT promoter mutation, WHO grade 2-3 based on histologic features and CDKN2A homozygous deletion status |
| Mechanisms of gliomagenesis | • Mutant IDH enzyme activity promotes D-2-hydroxyglutarate (D-2HG) formation, leading to altered epigenetic state and metabolic reprogramming • D-2HG suppresses T-cell function, damping down tumor-directed immune response |
| Imaging features | • Frontal, hyperintense lesions on T2/FLAIR imaging; contrast enhancement more frequent in grade 3 and grade 4 tumors • T2—FLAIR mismatch sign has high specificity for astrocytic IDH-mutant gliomas |
| Treatment guidelines | • Maximal safe resection is associated with improved outcomes • Observation after surgery can be considered for low-risk patients with grade 2 IDH-mutant gliomas following a gross total resection • Radiation followed by chemotherapy is the standard of care for patients with high risk, grade 2 IDH-mutant gliomas and all patients with grade 3 and grade 4 IDH-mutant gliomas |
| Therapies in development | • Direct IDH inhibitors are under investigation for treatment of grade 2, non-enhancing, IDH-mutant glioma • Canonical IDH1 R132H mutation is a clonal neoantigen being targeted by vaccine-based therapy in clinical trials |
The survival benefit observed in the second interim analysis of the CATNON trial strongly supports the use of temozolomide for patients with IDH-mutant gliomas,72 however, IDH-mutant gliomas are particularly susceptible to the development of temozolomide-induced hypermutation after such treatment.37,65,66,168 A thorough understanding of factors that predispose some patients to hypermutation will be instrumental in securing the benefits of temozolomide adjuvant treatment while avoiding a therapy-induced transformation to a more aggressive tumor. Additionally, the benefits of temozolomide in gliomas may also be due to metabolic sensitivities imparted by the IDH-mutation.34,169
In the future, we expect that a further detailed understanding of direct and indirect effects of mutant IDH in the context of glioma will assist in utilizing the appropriate targeted treatments at the optimal time during the course of IDH-mutant glioma disease. More detail is needed to fully understand how D-2HG-driven effects are modified by concurrent lineage-specific alterations (TP53 and ATRX mutation, 1p/19q codeletion), if and when D-2HG becomes dispensable for glioma growth and how tumor microenvironmental factors cooperate to promote gliomagenesis, to optimally time the use of direct IDH inhibitors. In view of the wealth of data arising from genomic analysis of paired tumor samples from initial diagnosis and at recurrence, a systematic approach to tumor resampling at recurrence will be critical to investigate many of these lingering questions at a molecular level.
Additionally, as targeted agents on the horizon directed at IDH-mutant tumor cells or the immune system move into the standard of care armamentarium in the near future, we support a strong emphasis on the incorporation of surgical window-of-opportunity trials. Examination of intra-tumoral drug penetration in both enhancing and non-enhancing tumor compartments and confirmation of target engagement based on pharmacodynamic assays are necessary for the optimization of novel treatments. As evidenced by the variation in outcomes, correlative studies to analyze genetic, epigenetic, or metabolic biomarkers associated with clinical and radiographic responders will be valuable for optimization of patient selection.
At the patient level, much remains to be learned about how sex, germline single nucleotide polymorphisms (SNPs), diet and environment influences tumor behavior and response to treatment. Equally important to advances in treatment, more needs to be done to improve quality of life for patients who are expected to survive for decades, particularly those with grade 2 IDH-mutant gliomas and oligodendrogliomas. This may eventually involve replacing upfront radiation treatment with IDH inhibitors, immunotherapy or other novel therapies, or integrating newer approaches into the standard of care to maximize neurologic function and preserve independence.
Contributor Information
Julie J Miller, Stephen E. and Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
L Nicolas Gonzalez Castro, Harvard Medical School, Boston, MA, USA; Center for Neuro-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA; Department of Neurology, Brigham and Women’s Hospital, Boston, MA, USA.
Samuel McBrayer, Children’s Medical Center Research Institute, University of Texas Southwestern Medical Center, 6000 Harry Hines Blvd, Dallas, Texas, 75235, USA.
Michael Weller, Department of Neurology, University Hospital Zurich, Frauenklinikstrasse 26, 8091 Zurich, Switzerland.
Timothy Cloughesy, UCLA Neuro-Oncology Program, Los Angeles, CA, USA.
Jana Portnow, Oncology, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Ovidiu Andronesi, Harvard Medical School, Boston, MA, USA; Department of Radiology, Massachusetts General Hospital, Boston, MA, USA.
Jill S Barnholtz-Sloan, Informatics and Data Science (IDS), Center for Biomedical Informatics and Information Technology (CBIIT), Trans-Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute (NCI), Bethesda, MD, USA.
Brigitta G Baumert, Cantonal Hospital Graubunden, Institute of Radiation-Oncology, Chur, Switzerland.
Mitchell S Berger, Department of Neurosurgery, University of California-San Francisco, San Francisco, California, USA.
Wenya Linda Bi, Harvard Medical School, Boston, MA, USA; Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA, USA.
Ranjit Bindra, Department of Therapeutic Radiology, Brain Tumor Center, Yale School of Medicine, New Haven, CT, USA.
Daniel P Cahill, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Susan M Chang, Department of Neurosurgery, University of California-San Francisco, San Francisco, California, USA.
Joseph F Costello, Department of Neurosurgery, University of California-San Francisco, San Francisco, California, USA.
Craig Horbinski, Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA; Department of Neurological Surgery, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA; Northwestern Medicine Malnati Brain Tumor Institute of the Robert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Raymond Y Huang, Harvard Medical School, Boston, MA, USA; Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Robert B Jenkins, Individualized Medicine Research, Mayo Clinic, Department of Laboratory Medicine and Pathology, Rochester, Minnesota 55901, USA.
Keith L Ligon, Harvard Medical School, Boston, MA, USA; Department of Pathology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Ingo K Mellinghoff, Department of Neurology, Evnin Family Chair in Neuro-Oncology, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
L Burt Nabors, Department of Neurology, Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, Alabama, USA.
Michael Platten, CCU Neuroimmunology and Brain Tumor Immunology, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, 69120 Heidelberg, Germany.
David A Reardon, Harvard Medical School, Boston, MA, USA; Center for Neuro-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA; Department of Neurology, Brigham and Women’s Hospital, Boston, MA, USA.
Diana D Shi, Harvard Medical School, Boston, MA, USA; Department of Radiation Oncology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
David Schiff, Division of Neuro-Oncology, Department of Neurology, University of Virginia Health System, Charlottesville, Virginia, USA.
Wolfgang Wick, Neuro-Oncology at the German Cancer Research Center (DKFZ), Program Chair of Neuro-Oncology at the National Center for Tumor Diseases (NCT), and Neurology and Chairman at the Neurology Clinic in Heidelberg, Heidelberg, Germany.
Hai Yan, Genetron Health Inc, Gaithersburg, Maryland 20879, USA.
Andreas von Deimling, Department of Neuropathology, University Hospital Heidelberg, and, Clinical Cooperation Unit Neuropathology, German Cancer Research Center (DKFZ), and, DKTK, INF 224, 69120 Heidelberg, Germany.
Martin van den Bent, Brain Tumour Centre, Erasmus MC Cancer Institute, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands.
William G Kaelin, Harvard Medical School, Boston, MA, USA; Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Patrick Y Wen, Harvard Medical School, Boston, MA, USA; Center for Neuro-Oncology, Dana-Farber Cancer Institute, Boston, MA, USA; Department of Neurology, Brigham and Women’s Hospital, Boston, MA, USA.
Conflict of interest statement. J.J.M.: has received research funding from Karyopharm (to Massachusetts General Hospital). L.N.G.C.: nothing to disclose; S.M. has served as a paid consultant to Agios Pharmaceuticals. M.W. has received research grants from Apogenix, Merck, Sharp & Dohme, Merck (EMD), Philogen and Quercis, and honoraria for lectures or advisory board participation or consulting from Adastra, Bayer, Bristol Meyer Squibb, Medac, Merck, Sharp & Dohme, Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen and y-Mabs. T.C. is cofounder, major stock holder, consultant and board member of Katmai Pharmaceuticals, member of the board and paid consultant for the 501c3 Global Coalition for Adaptive Research, holds stock in Chimerix and receives milestone payments and possible future royalties, member of the scientific advisory board for Break Through Cancer, member of the scientific advisory board for Cure Brain Cancer Foundation, has provided paid consulting services to Sagimet, Clinical Care Options, Ideology Health, Servier, Jubilant, Immvira, Gan & Lee, BrainStorm, Katmai, Sapience, Inovio, Vigeo Therapeutics, DNATrix, Tyme, SDP, Novartis, Roche, Kintara, Bayer, Merck, Boehinger Ingelheim, VBL, Amgen, Kiyatec, Odonate Therapeutics QED, Medefield, Pascal Biosciences, Bayer, Tocagen, Karyopharm, GW Pharma, Abbvie, VBI, Deciphera, VBL, Agios, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, Medqia Trizel, Medscape and has contracts with UCLA for the Brain Tumor Program with Oncovir, Merck, Oncoceutics, Novartis, Amgen, Abbvie, DNAtrix, Beigene, BMS, AstraZeneca, Kazia, Agios, Boston Biomedical, Deciphera, Tocagen, Orbus, AstraZenica, Karyopharm. The Regents of the University of California (T.F.C. employer) has licensed intellectual property co-invented by TFC to Katmai Pharmaceuticals. J.P.: nothing to disclose. O.A.: nothing to disclose. J.S.B.-S.: Paid employee of NCI/NIH. B.G.B.: nothing to disclose. M.S.B.: nothing to disclose. W.L.B.: nothing to disclose. R.B.: nothing to disclose. D.P.C. has consulted for the Massachusetts Institute of Technology, Advise Connect Inspire, Lilly, GlaxoSmithKline, Boston Pharmaceuticals, and Iconovir and serves on the advisory board of Pyramid Biosciences, which includes an equity interest. He has received honoraria and travel reimbursement from Merck for invited lectures, and from the US NIH and DOD for clinical trial and grant review. S.M.C.: nothing to disclose. J.F.C. has ownership interest in Telo Therapeutics. C.H.: nothing to disclose. R.Y.H. has consulted for Nuvation Bio, has reserved research funding from Bristol-Myers-Squib (to the Brigham and Women’s Hospital) and is a member of the advisory board for Vysioneer. R.B.J.: nothing to disclose. K.L.L.: nothing to disclose. I.K.M. received research funding from Amgen, General Electric, Lilly, Kazia Therapeutics, and Servier Pharmaceuticals; other funding from Agios, Black Diamond Therapeutics, Debiopharm Group, Puma Biotechnology, Servier Pharmaceuticals, Voyager Therapeutics, DC Europa Ltd, Kazia Therapeutics, Novartis, Cardinal Health, Roche, Vigeo Therapeutics, Samus Therapeutics, Prelude Therapeutics, and AstraZeneca. L.B.N. serves on scientific advisory board for Chimerix and chairs the data safety monitoring committee for CNS Pharma. M.P. has consulted for Bayer, is a member of the HI-TRON Scientific Management Board, and holds patents for peptides for use in treating or diagnosing IDH1R132H positive cancers (EP2800580B1). D.A.R.: nothing to disclose. D.D.S.: nothing to disclose. D.S. has served on an advisory board with AstraZeneca on the use of PARP inhibitors for IDH-mutant gliomas, and co-chairs a study using PARP inhibitors for the treatment of IDH-mutant gliomas. W.W. reports to be inventor and patent-holder on ‘Peptides for use in treating or diagnosing IDH1R132H positive cancers’ (EP2800580B1) and ‘Cancer therapy with an oncolytic virus combined with a checkpoint inhibitor’ (US11027013B2). He consulted for Apogenix, Astra Zeneca, Bayer, Enterome, Medac, MSD, and Roche/Genentech with honoraria paid to the Medical Faculty at the University of Heidelberg. H.Y. is the chief scientific officer and has ownership interest in Genetron Holdings and receives royalties from Agios, Genetron and Personal Genome Diagnostics (PGDX). H.Y. holds a patent related to genetic alterations in IDH and other genes in malignant glioma (US Patent 8,685,660B2) issued, licensed and with royalties paid by Agios; a patent for genetic alterations in IDH and other genes in malignant glioma issued, licensed and with royalties paid by PGDX; a patent on methods for the rapid and sensitive detection of hotspot mutations (US 10,633,711B2) issued, licensed and with royalties paid by Genetron Holdings; a patent on homozygous and heterozygous IDH1 gene- defective human astrocytoma cell lines (US 9,695,400B2); and a patent on homozygous and heterozygous IDH1 gene- defective cell lines derived from human colorectal cells (US 9,074,221B2). A.v.D. is holding patents for the IDHR132H antibody and for methylation based tumor diagnostics. All terms are being managed by the German Cancer Research Center in accordance with its conflict of interest policies. M.v.d.B. has received honoraria from Agios, Carthera, Nerviano, Genenta, Boheringer-Ingelheim, Chimerix. W.G.K. receives compensation for roles as an Eli Lilly and LifeMine Therapeutics Board Director, as a founder of Tango Therapeutics and Cedilla Therapeutics, and as a scientific advisor for Fibrogen, IconOVir Bio, Circle Pharma, Nextext Invest, and Casdin Capital. PYW has consulted for Astra Zeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines. P.Y.B. has served in advisory boards for Agios, Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines.
References
- 1. Bailey P, Cushing H.. A Classification of the Tumors of the Glioma Group on a Histogenetic Basis with a Correlated Study of Prognosis. Philadelphia, London and Montreal: Lippincott; 1926. [Google Scholar]
- 2. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008; 321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020; 139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(12 Suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ostrom QT, Adel Fahmideh M, Cote DJ, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019; 21(11):1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011; 43(12):1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnet C, Thomas L, Psimaras D, et al. Characteristics of gliomas in patients with somatic IDH mosaicism. Acta Neuropathol Commun. 2016; 4(3):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkins RB, Xiao Y, Sicotte H, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012; 44(10):1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021; 18(10):645–661. [DOI] [PubMed] [Google Scholar]
- 12. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009; 118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 13. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009; 462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012; 483(7390):484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johannessen TA, Mukherjee J, Viswanath P, et al. Rapid conversion of mutant IDH1 from driver to passenger in a model of human gliomagenesis. Mol Cancer Res. 2016; 14(10):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013; 340(6132):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Machida Y, Nakagawa M, Matsunaga H, et al. A potent blood-brain barrier-permeable mutant IDH1 Inhibitor suppresses the growth of glioblastoma with IDH1 mutation in a patient-derived orthotopic xenograft model. Mol Cancer Ther. 2020; 19(2):375–383. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011; 12(5):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011; 19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blouw B, Song H, Tihan T, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003; 4(2):133–146. [DOI] [PubMed] [Google Scholar]
- 22. Zagzag D, Zhong H, Scalzitti JM, et al. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000; 88(11):2606–2618. [PubMed] [Google Scholar]
- 23. Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010; 17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012; 483(7390):479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012; 483(7390):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tirosh I, Venteicher AS, Hebert C, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016; 539(7628):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017; 355(6332):eaai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flavahan WA, Drier Y, Liau BB, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016; 529(7584):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reitman ZJ, Jin G, Karoly ED, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA. 2011; 108(8):3270–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grassian AR, Parker SJ, Davidson SM, et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014; 74(12):3317–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Badur MG, Muthusamy T, Parker SJ, et al. Oncogenic R132 IDH1 mutations limit NADPH for de novo lipogenesis through (D)2-hydroxyglutarate production in fibrosarcoma sells. Cell Rep. 2018; 25(4):1018–1026.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010; 70(22):8981–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McBrayer SK, Mayers JR, DiNatale GJ, et al. Transaminase inhibition by 2-hydroxyglutarate impairs glutamate biosynthesis and redox homeostasis in glioma. Cell. 2018; 175(1):101–116.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015; 28(6):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009; 174(4):1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011; 29(34):4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014; 343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin G, Reitman ZJ, Duncan CG, et al. Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Res. 2013; 73(2):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luchman HA, Chesnelong C, Cairncross JG, Weiss S. Spontaneous loss of heterozygosity leading to homozygous R132H in a patient-derived IDH1 mutant cell line. Neuro Oncol. 2013; 15(8):979–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mazor T, Chesnelong C, Pankov A, et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proc Natl Acad Sci USA. 2017; 114(40):10743–10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma S, Jiang B, Deng W, et al. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015; 6(11):8606–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turcan S, Makarov V, Taranda J, et al. Mutant-IDH1-dependent chromatin state reprogramming, reversibility, and persistence. Nat Genet. 2018; 50(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018; 136(1):153–166. [DOI] [PubMed] [Google Scholar]
- 44. Banan R, Stichel D, Bleck A, et al. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol. 2020; 140(4):569–581. [DOI] [PubMed] [Google Scholar]
- 45. Suwala AK, Stichel D, Schrimpf D, et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2021; 141(1):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Suwala AK, Felix M, Friedel D, et al. Oligosarcomas, IDH-mutant are distinct and aggressive. Acta Neuropathol. 2022; 143(2):263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006; 66(20):9852–9861. [DOI] [PubMed] [Google Scholar]
- 48. Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010; 74(23):1886–1890. [DOI] [PubMed] [Google Scholar]
- 49. Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012; 226(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011; 333(6048):1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gleize V, Alentorn A, Connen de Kerillis L, et al. CIC inactivating mutations identify aggressive subset of 1p19q codeleted gliomas. Ann Neurol. 2015; 78(3):355–374. [DOI] [PubMed] [Google Scholar]
- 52. Wong D, Yip S. Making heads or tails - the emergence of capicua (CIC) as an important multifunctional tumour suppressor. J Pathol. 2020; 250(5):532–540. [DOI] [PubMed] [Google Scholar]
- 53. Yang R, Chen LH, Hansen LJ, et al. Cic loss promotes gliomagenesis via aberrant neural stem cell proliferation and differentiation. Cancer Res. 2017; 77(22):6097–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013; 110(15):6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bell RJ, Rube HT, Kreig A, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015; 348(6238):1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012; 3(7):709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011; 333(6041):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015; 372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016; 164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mazor T, Pankov A, Johnson BE, et al. DNA methylation and somatic mutations converge on the cell cycle and define similar evolutionary histories in brain tumors. Cancer Cell. 2015; 28(3):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Souza CF, Sabedot TS, Malta TM, et al. A distinct DNA methylation shift in a subset of glioma CpG island methylator phenotypes during tumor recurrence. Cell Rep. 2018; 23(2):637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wakimoto H, Tanaka S, Curry WT, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014; 20(11):2898–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bai H, Harmancı AS, Erson-Omay EZ, et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet. 2016; 48(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019; 25(18):5537–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020; 580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mathur R, Zhang Y, Grimmer MR, et al. MGMT promoter methylation level in newly diagnosed low-grade glioma is a predictor of hypermutation at recurrence. Neuro Oncol. 2020; 22(11):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Horbinski C, McCortney K, Stupp R. MGMT promoter methylation is associated with patient age and 1p/19q status in IDH-mutant gliomas. Neuro Oncol. 2021; 23(5):858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013; 81(17):1515–1522. [DOI] [PubMed] [Google Scholar]
- 70. Lam K, Eldred BSC, Kevan B, et al. Prognostic value of O (6)-methylguanine-DNA methyltransferase methylation in isocitrate dehydrogenase mutant gliomas. Neurooncol Adv. 2022; 4(1):vdac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leelatian N, Hong CS, Bindra RS. The role of mismatch repair in glioblastoma multiforme treatment response and resistance. Neurosurg Clin N Am. 2021; 32(2):171–180. [DOI] [PubMed] [Google Scholar]
- 72. van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021; 22(6):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kocakavuk E, Anderson KJ, Varn FS, et al. Radiotherapy is associated with a deletion signature that contributes to poor outcomes in patients with cancer. Nat Genet. 2021; 53(7):1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schiff D. Low-grade gliomas. Continuum. 2015; 21(2 Neuro-oncology):345–354. [DOI] [PubMed] [Google Scholar]
- 75. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017; 88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gonzalez Castro LN, Milligan TA. Seizures in patients with cancer. Cancer. 2020; 126(7):1379–1389. [DOI] [PubMed] [Google Scholar]
- 77. Morris Z, Whiteley WN, LongstrethWT, Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009; 339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Opoku-Darko M, Eagles ME, Cadieux M, Isaacs AM, Kelly JJP. Natural history and growth patterns of incidentally discovered diffusely infiltrating low-grade gliomas: a volumetric study. World Neurosurg. 2019; 132:e133–e139. [DOI] [PubMed] [Google Scholar]
- 79. Patel SH, Poisson LM, Brat DJ, et al. T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res. 2017; 23(20):6078–6085. [DOI] [PubMed] [Google Scholar]
- 80. Park SI, Suh CH, Guenette JP, Huang RY, Kim HS. The T2-FLAIR mismatch sign as a predictor of IDH-mutant, 1p/19q-noncodeleted lower-grade gliomas: a systematic review and diagnostic meta-analysis. Eur Radiol. 2021; 31(7):5289–5299. [DOI] [PubMed] [Google Scholar]
- 81. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012; 4(116):116ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou M, Zhou Y, Liao H, et al. Diagnostic accuracy of 2-hydroxyglutarate magnetic resonance spectroscopy in newly diagnosed brain mass and suspected recurrent gliomas. Neuro Oncol. 2018; 20(9):1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Branzoli F, Di Stefano AL, Capelle L, et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro Oncol. 2018; 20(7):907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Andronesi OC, Loebel F, Bogner W, et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clin Cancer Res. 2016; 22(7):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de la Fuente MI, Young RJ, Rubel J, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 2016; 18(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choi C, Raisanen JM, Ganji SK, et al. Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol. 2016; 34(33):4030–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Demetriou E, Kujawa A, Golay X. Pulse sequences for measuring exchange rates between proton species: from unlocalised NMR spectroscopy to chemical exchange saturation transfer imaging. Prog Nucl Magn Reson Spectrosc. 2020; 120–121:25–71. [DOI] [PubMed] [Google Scholar]
- 88. Chaumeil MM, Lupo JM, Ronen SM. Magnetic resonance (MR) metabolic imaging in glioma. Brain Pathol. 2015; 25(6):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011; 12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 90. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 91. Wen PY, Chang SM, Van den Bent MJ, et al. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017; 35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wen PY. RANO 2.0. Paper presented at: Society for Neuro-Oncology RANO Meeting; 01/28/2022, 2022.
- 93. Ellingson BM, Gerstner ER, Lassman AB, et al. Hypothetical generalized framework for a new imaging endpoint of therapeutic activity in early phase clinical trials in brain tumors. Neuro Oncol. 2022; 24(8):1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huang RY, Young RJ, Ellingson BM, et al. Volumetric analysis of IDH-mutant lower-grade glioma: a natural history study of tumor growth rates before and after treatment. Neuro Oncol. 2020; 22(12):1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ellingson BM, Kim GHJ, Brown M, et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: evidence from a phase I trial of Ivosidenib. Neuro Oncol. 2021; 24(5):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017; 18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 97. Ruda R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012; 14(Suppl 4):iv55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jo J, Nevel K, Sutyla R, et al. Predictors of early, recurrent, and intractable seizures in low-grade glioma. Neurooncol Pract. 2021; 8(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Walbert T, Harrison RA, Schiff D, et al. SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 2021; 23(11):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016; 132(6):917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Diaz M, Jo J, Smolkin M, Ratcliffe SJ, Schiff D. Risk of venous thromboembolism in grade II–IV gliomas as a function of molecular subtype. Neurology. 2021; 96(7):e1063–e1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019; 20(10):e566–e581. [DOI] [PubMed] [Google Scholar]
- 103. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020; 38(5):496–520. [DOI] [PubMed] [Google Scholar]
- 104. Roth P, Pace A, Le Rhun E, et al. Neurological and vascular complications of primary and secondary brain tumours: EANO-ESMO clinical practice guidelines for prophylaxis, diagnosis, treatment and follow-up. Ann Oncol. 2021; 32(2):171–182. [DOI] [PubMed] [Google Scholar]
- 105. Glantz MJ, Burger PC, HerndonJE, 2nd, et al. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991; 41(11):1741–1744. [DOI] [PubMed] [Google Scholar]
- 106. Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001; 3(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012; 308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 108. Jakola AS, Unsgard G, Myrmel KS, et al. Surgical strategy in grade II astrocytoma: a population-based analysis of survival and morbidity with a strategy of early resection as compared to watchful waiting. Acta Neurochir. 2013; 155(12):2227–2235. [DOI] [PubMed] [Google Scholar]
- 109. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018; 20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ius T, Ng S, Young JS, et al. The benefit of early surgery on overall survival in incidental low grade glioma patients: a multicenter study. Neuro Oncol. 2021; 24(4):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jakola AS, Pedersen LK, Skjulsvik AJ, et al. The impact of resection in IDH-mutant WHO grade 2 gliomas: a retrospective population-based parallel cohort study. J Neurosurg. 2022:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008; 26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 113. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014; 16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer. 2021; 149(5):23–33. [DOI] [PubMed] [Google Scholar]
- 115. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012; 70(4):921–928; discussion 928. [DOI] [PubMed] [Google Scholar]
- 116. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012; 53(1):51–57. [DOI] [PubMed] [Google Scholar]
- 117. Rossi M, Gay L, Ambrogi F, et al. Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol. 2021; 23(5):812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Motomura K, Chalise L, Ohka F, et al. Impact of the extent of resection on the survival of patients with grade II and III gliomas using awake brain mapping. J Neurooncol. 2021; 153(2):361–372. [DOI] [PubMed] [Google Scholar]
- 119. Gogos AJ, Young JS, Morshed RA, et al. Triple motor mapping: transcranial, bipolar, and monopolar mapping for supratentorial glioma resection adjacent to motor pathways. J Neurosurg. 2020; 134(6):1728–1737. [DOI] [PubMed] [Google Scholar]
- 120. Gogos AJ, Young JS, Morshed RA, Hervey-Jumper SL, Berger MS. Awake glioma surgery: technical evolution and nuances. J Neurooncol. 2020; 147(3):515–524. [DOI] [PubMed] [Google Scholar]
- 121. Shofty B, Haim O, Costa M, et al. Impact of repeated operations for progressive low-grade gliomas. Eur J Surg Oncol. 2020; 46(12):2331–2337. [DOI] [PubMed] [Google Scholar]
- 122. Schiff D, Van den Bent M, Vogelbaum MA, et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro Oncol. 2019; 21(7):837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18(11):1537–1570. [DOI] [PubMed] [Google Scholar]
- 124. Leibel SA, Sheline GE, Wara WM, Boldrey EB, Nielsen SL. The role of radiation therapy in the treatment of astrocytomas. Cancer. 1975; 35(6):1551–1557. [DOI] [PubMed] [Google Scholar]
- 125. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996; 36(3):549–556. [DOI] [PubMed] [Google Scholar]
- 126. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002; 20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 127. van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005; 366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 128. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009; 8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 129. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002; 360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 130. Klein M, Drijver AJ, van den Bent MJ, et al. Memory in low-grade glioma patients treated with radiotherapy or temozolomide: a correlative analysis of EORTC study 22033-26033. Neuro Oncol. 2021; 23(5):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Breen WG, Anderson SK, Carrero XW, et al. Final report from Intergroup NCCTG 86–72–51 (Alliance): a phase III randomized clinical trial of high-dose versus low-dose radiation for adult low-grade glioma. Neuro Oncol. 2020; 22(6):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. van West SE, de Bruin HG, van de Langerijt B, et al. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro Oncol. 2017; 19(5):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dietrich J. Neurotoxicity of cancer therapies. Continuum. 2020; 26(6):1646–1672. [DOI] [PubMed] [Google Scholar]
- 134. Winter SF, Klein JP, Vaios EJ, et al. Clinical presentation and management of SMART syndrome. Neurology. 2021; 97(3):118–120. [DOI] [PubMed] [Google Scholar]
- 135. Ahmad H, Martin D, Patel SH, et al. Oligodendroglioma confers higher risk of radiation necrosis. J Neurooncol. 2019; 145(2):309–319. [DOI] [PubMed] [Google Scholar]
- 136. Tabrizi S, Yeap BY, Sherman JC, et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiother Oncol. 2019; 137(8):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kim MM, Hattangadi-Gluth JA, Redmond KJ, et al. Back to the future: charting the direction of lower grade glioma trials with lessons from the present and past. Int J Radiat Oncol Biol Phys. 2022; 112(1):30–34. [DOI] [PubMed] [Google Scholar]
- 138. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016; 374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG Oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020. doi: 10.1200/JCO.19.02983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wahl M, Phillips JJ, Molinaro AM, et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 2017; 19(2):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ruda R, Pellerino A, Pace A, et al. Efficacy of initial temozolomide for high-risk low grade gliomas in a phase II AINO (Italian Association for Neuro-Oncology) study: a post-hoc analysis within molecular subgroups of WHO 2016. J Neurooncol. 2019; 145(1):115–123. [DOI] [PubMed] [Google Scholar]
- 142. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016; 17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jaeckle KA, Ballman KV, van den Bent M, et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 2021; 23(3):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 145. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014; 32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Boyle FM, Eller SL, Grossman SA. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro Oncol. 2004; 6(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018; 24(8):1192–1203. [DOI] [PubMed] [Google Scholar]
- 150. Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 2020; 38(29):3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mellinghoff IK, Penas-Prado M, Peters KB, et al. Vorasidenib, a dual inhibitor of mutant IDH1/2, in recurrent or progressive glioma; results of a first-in-human phase I trial. Clin Cancer Res. 2021; 27(16):4491–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Platten M, Bunse L, Wick A, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021; 592(7854):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tran AN, Lai A, Li S, et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol. 2014; 16(3):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017; 134(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. van den Bent MJ, Klein M, Smits M, et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): a randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018; 19(9):1170–1179. [DOI] [PubMed] [Google Scholar]
- 156. Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017; 9(375):eaal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017; 77(7):1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gbyli R, Song Y, Liu W, et al. In vivo anti-tumor effect of PARP inhibition in IDH1/2 mutant MDS/AML resistant to targeted inhibitors of mutant IDH1/2. Leukemia. 2022; 36(5):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Molenaar RJ, Radivoyevitch T, Nagata Y, et al. IDH1/2 mutations sensitize acute myeloid leukemia to PARP inhibition and this is reversed by IDH1/2-mutant inhibitors. Clin Cancer Res. 2018; 24(7):1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wang Y, Wild AT, Turcan S, et al. Targeting therapeutic vulnerabilities with PARP inhibition and radiation in IDH-mutant gliomas and cholangiocarcinomas. Sci Adv. 2020; 6(17):eaaz3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Eder JP, Doroshow DB, Do KT, et al. Clinical efficacy of olaparib in IDH1/IDH2-mutant mesenchymal sarcomas. JCO Precis Oncol. 2021; 5(11):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ducray F, Sanson M, Chinot OL, et al. Olaparib in recurrent IDH-mutant high-grade glioma (OLAGLI). J Clin Oncol. 2021; 39(15_suppl):2007–2007. [Google Scholar]
- 163. Barthel FP, Johnson KC, Varn FS, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019; 576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nagashima H, Lee CK, Tateishi K, et al. Poly(ADP-ribose) glycohydrolase inhibition sequesters NAD+ to potentiate the metabolic lethality of alkylating chemotherapy in IDH mutant tumor cells. Cancer Discov. 2020; 10(11):1672–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shi DD, Savani MR, Levitt MM, et al. De novo pyrimidine synthesis is a targetable vulnerability in IDH mutant glioma. bioRxiv. 2021. doi: 10.1101/2021.11.30.470443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017; 127(4):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lu M, Cloughesy TF, Wen PY, et al. Impact of mutant IDH (mIDH) inhibition on DNA hydroxymethylation, tumor cell function, and tumor immune microenvironment (TIME) in resected mIDH1 lower-grade glioma (LGG). J Clin Oncol. 2021; 39(15_suppl):2008–2008. [Google Scholar]
- 168. Yu Y, Villanueva-Meyer J, Grimmer MR, et al. Temozolomide-induced hypermutation is associated with distant recurrence and reduced survival after high-grade transformation of low-grade IDH-mutant gliomas. Neuro Oncol. 2021; 23(11):1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tateishi K, Higuchi F, Miller JJ, et al. The Alkylating chemotherapeutic temozolomide induces metabolic stress in IDH1-mutant cancers and potentiates NAD(+) depletion-mediated cytotoxicity. Cancer Res. 2017; 77(15):4102–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]