Abstract
An updated LncTarD 2.0 database provides a comprehensive resource on key lncRNA–target regulations, their influenced functions and lncRNA-mediated regulatory mechanisms in human diseases. LncTarD 2.0 is freely available at (http://bio-bigdata.hrbmu.edu.cn/LncTarD or https://lnctard.bio-database.com/). LncTarD 2.0 was updated with several new features, including (i) an increased number of disease-associated lncRNA entries, where the current release provides 8360 key lncRNA–target regulations, with 419 disease subtypes and 1355 lncRNAs; (ii) predicted 3312 out of 8360 lncRNA–target regulations as potential diagnostic or therapeutic biomarkers in circulating tumor cells (CTCs); (iii) addition of 536 new, experimentally supported lncRNA–target regulations that modulate properties of cancer stem cells; (iv) addition of an experimentally supported clinical application section of 2894 lncRNA–target regulations for potential clinical application. Importantly, LncTarD 2.0 provides RNA-seq/microarray and single-cell web tools for customizable analysis and visualization of lncRNA–target regulations in diseases. RNA-seq/microarray web tool was used to mining lncRNA–target regulations in both disease tissue samples and CTCs blood samples. The single-cell web tools provide single-cell lncRNA–target annotation from the perspectives of pan-cancer analysis and cancer-specific analysis at the single-cell level. LncTarD 2.0 will be a useful resource and mining tool for the investigation of the functions and mechanisms of lncRNA deregulation in human disease.
INTRODUCTION
Long noncoding RNA (lncRNA) has been substantially linked to many human diseases, especially cancer (1–4). Some studies have found that lncRNA dysregulation in breast cancer (5,6), lung cancer (7), Alzheimer disease (8) and other diseases (9–12). Currently, many lncRNA-related databases have been published concerning lncRNA sequence, expression, function, regulation, and disease associations. For example, experimentally supported or predicted lncRNA–disease associations have been generated and stored in some databases, such as LncRNADisease 2.0 (13), Lnc2Cancer 3.0 (14), and MNDR v3.0 (15). Some databases have been established to collect experimentally validated or predicted lncRNA-associated regulatory relationships (such as ceRNA interactions based on scRNA-sequencing or bulk RNA-sequencing datasets), such as LnCeCell (16), LncRNA2Target 2.0 (17), LncACTdb 3.0 (18) and DIANA-LncBase v3 (19). Some databases have been established to document and annotate genetic variants of lncRNAs in diseases, such as Lnc2Meth (20) and LincSNP 3.0 (21). To facilitate the study of mechanisms of lncRNA deregulation in the pathogenesis of disease, we previously reported the first version of the LncTarD (22) database, which integrated disease-associated lncRNA–target regulations, key downstream targets, lncRNA-dependent mechanisms and biological functions in human diseases into a comprehensive resource.
With the increasing interest in human lncRNAs, a large number of disease-associated lncRNA–target regulations have been reported in recent years. The correlations between lncRNAs (23,24) and their target regulation mechanisms such as sponge (25,26), methylation (27–29) and transcriptional regulation (30,31), have also been widely reported. The reports of disease-related lncRNA–target regulations supported by a large number of experiments provide a basis for studying their clinical application. Previous studies have found that circulating tumor cells (CTCs) and circulating lncRNAs and their targets have broad application prospects for cancer diagnosis (32,33). For example, circulating RNA UCA1 regulates MYO6 expression through miR-143 in colorectal cancer, affecting cell proliferation and migration (34). LncRNAs have also been found to regulate tumor stem cells (CSCs) through their targets, and then affect tumor maintenance and spreading (35–37). MALAT1 has been reported to bind directly to the mRNA of SOX2, and promote the stemness of gastric cancer cells, and may be a potential target for gastric cancer (38). Considering these newly discovered lncRNA–target regulations, as well as publicly available data resources, it was necessary to update the LncTarD database with more resources and improved tools.
Therefore, we updated LncTarD to version 2.0 (LncTarD 2.0) (Figure 1 and Table 1). The current release includes 8,360 experimentally supported key lncRNA–target regulations in 419 disease subtypes and their clinical application. LncTarD 2.0 also provides two interactive web-based tools to characterize the dynamic lncRNA–target regulations in human diseases based on scRNA-seq and bulk RNA-seq datasets of tissue samples and CTCs blood samples. Collectively, LncTarD 2.0 aims to integrate the experimentally supported key lncRNA–target regulations, their influenced functions, lncRNA-mediated regulatory mechanisms and potential clinical application in human diseases.
Figure 1.
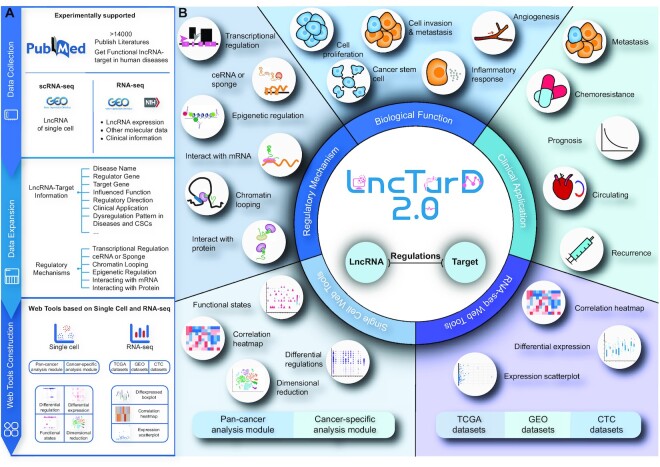
Data expansion and features of LncTarD 2.0. (A) Summary of the contents of the database, including the collection of articles reporting lncRNA–target regulations, the addition of lncRNA–target regulations information and mechanisms, and the construction of single-cell and RNA-seq/microarray web tools. (B) Data on the regulatory mechanisms, biological functions, and clinical application of lncRNA–target regulations in diseases are included. A panel of tools has been developed to mine, visualize, and analyze lncRNAs at single cell and RNA-seq/microarray levels.
Table 1.
Comparison of the data included in LncTarD 1.0 and LncTarD 2.0
| Features | LncTarD 1.0 | LncTarD 2.0 | Fold increase |
|---|---|---|---|
| lncRNA–target regulations | 2822 | 8360 | 2.96 |
| Diseases | 177 | 419 | 2.36 |
| lncRNAs | 475 | 1355 | 2.85 |
| PCG | 774 | 1743 | 2.25 |
| miRNA | 391 | 506 | 1.29 |
| Biological functions | 140 | 286 | 2.04 |
| Cancer stem cell | - | 536 | New |
| Clinical applications | - | 2894 | New |
| Circulating tumor cell | - | 3312 | New |
| Single cell Web Tools | - | 73 datasets | New |
| RNA seq Web Tools-GEO | 90 datasets | 351 datasets | 3.9 |
| RNA seq Web Tools-CTC | - | 17 datasets | New |
IMPROVED EXPANSION AND NEW FEATURES
Data expansion and preprocessing
LncTarD 2.0 was updated to contain experimentally supported key lncRNA–target regulations, their influenced functions, and lncRNA-mediated regulatory mechanisms associated with various human diseases (Table 1). In the first instance, we screened >6000 studies in the PubMed database. Among them, 5523 reports from 2019 to 2021 contained relevant information, which was incorporated into the database. All searches followed similar keyword combinations as the ones used in LncTarD 1.0.
Next, we extracted the experimentally supported associations between lncRNA–target gene regulation and disease, and these relationships were experimentally confirmed. Compared with LncTarD 1.0, we added the effect of lncRNA–target regulations on CSCs, expression pattern in CTCs, and clinical application. If lncRNA–target regulations were confirmed to be biomarkers related to metastasis, recurrence, circulation, drug resistance or prognosis in cancer, we extracted such information to characterize the clinical application of the lncRNA–target regulations. Similarly, if lncRNA–target regulations were confirmed to affect the functions of CSCs such as stemness, proliferation, invasion and expansion in cancer, we extracted this information to characterize the effect of lncRNA–target regulations on CSCs. In addition, we collected 17 RNA-seq datasets of CTCs from the PubMed database. In this step, we also recorded detailed information including lncRNAs, target genes and cancer names, direction of regulation, experimental method for each interaction, expression pattern of lncRNA, experimental method and lncRNA microarray, positively or negatively influenced biological functions, lncRNA-mediated regulatory mechanisms, information from the PubMed database and a brief description of functional lncRNA–target regulation in human diseases from the original studies. Furthermore, we added more detailed data to demonstrate the lncRNAs and their targets cancer associations more comprehensively. We collected other names of lncRNAs and their targets including aliases, synonyms, Entrez IDs and Ensembl ID (39). We also updated the location information of lncRNAs on chromosomes. Finally, we used a standardized classification scheme, the Disease Ontology, to annotate human diseases (40).
Experimentally supported regulatory mechanisms and clinical applications of lncRNAs and their targets in cancer
To provide a comprehensive resource to study the role of lncRNAs in cancer, we manually curated lncRNA–target regulations that can serve as cancer biomarkers. First, we collected 2894 experimentally supported clinical applications of lncRNA–target regulations. Second, we used 17 CTCs RNA-seq datasets including 877 CTC samples of seven cancer types to characterize the potential of lncRNA–target regulations as diagnosis or therapeutic response markers. The differential expression of lncRNA–target regulations between CTCs and normal blood samples was analyzed using R package limma (41). Genes with absolute fold-change > 2 and false discovery rate (FDR) < 0.05 in the same CTC dataset were considered dysregulated lncRNAs or target genes in CTCs. We predicted 3312 out of 8360 lncRNA–target regulations that were differentially expressed in CTC samples as potential diagnostic or therapeutic biomarkers in blood. Finally, we added new 536 experimentally supported key lncRNA–target regulations, which play important roles in plasticity and self-renewal properties of CSCs in 71 human disease subtypes. Furthermore, the current release includes 8360 key lncRNA–target regulations from PubMed literature associations with 419 disease subtypes, 1355 lncRNAs, 1743 protein-coding genes, 506 miRNAs and 286 biological functions. The current version of LncTarD records 5531 experimentally supported functional regulations including ceRNA or sponge (3719 entries), transcriptional regulation (595 entries), epigenetic regulation (436 entries), interaction with protein (706 entries), interaction with mRNA (62 entries), and chromatin looping (13 entries), and 2829 expression associations in human diseases.
Newly developed single-cell web tool for discovery and analysis of lncRNA–target regulations
Single-cell RNA sequencing (scRNA-seq) is a high-throughput method to measure and compare the levels of gene expression at single-cell resolution; thus, developing an efficient method for analyzing large datasets is essential. A rapid and comprehensive approach could enable the analysis of cancer pathology and the discovery of lncRNA–target regulations as cancer biomarkers. In LncTarD 2.0, we designed a single-cell web tool, which can be employed to characterize and understand lncRNA–target regulations according to the provided single-cell datasets. A total of 73 scRNA-seq datasets concerning lncRNA expression were collected from Lnc2Cancer 3.0 and Gene Expression Omnibus (GEO) (42). The R package Seurat and SingleR were used for standardization, quality control, and cell type identification of scRNA-seq data (Supplementary methods). A total of 168 197 high-quality cells were obtained in 32 cancer types. Moreover, we characterized the association of an lncRNA–target pair with a specific cell by testing the statistical independence of their expression values (43). The lncRNA–target pairs with significant expression correlation (with a significance level of less than 0.01) in a cell were identified (Supplementary methods). LncTarD 2.0 provides interactive and customizable functions including two powerful modules: (i) Pan-cancer analysis module of key lncRNA–target regulations, which mainly provides three tools to graphically display the differences/similarities of lncRNA–target regulations among cancer types at the single-cell level and (ii) Cancer-specific analysis module of key lncRNA–target regulations, which mainly provides three tools to reveal the cancer cell subpopulation specificity of lncRNA–target regulations and intratumor heterogeneity at the single-cell level.
Tools in pan-cancer and cancer-specific analysis modules provide the following: (i) differential regulations analysis, which provides the landscape of differential lncRNA–target regulations across multiple single-cell datasets and cancer cell subpopulations; (ii) differential expression analysis, which provides the landscape of differential expression of lncRNAs and target genes across multiple single-cell datasets and cancer cell subpopulations; (iii) functional states analysis, which provides the landscape of differential lncRNA–target regulations associated with a given cell state (such as stemness, angiogenesis and inflammation) across multiple single-cell datasets and cancer cell subpopulations. AUCell algorithm was used to determine the activity of lncRNA–target regulations within a cell (44). The Wilcoxon rank-sum test was used to estimate the significance of differences in activity of lncRNA–target regulations among different scRNA-seq datasets or subpopulations (45) (Supplementary methods). Furthermore, all tools in pan-cancer and cancer-specific analysis modules contained more complex functions for mining a given lncRNA–target pair, including general information (including associated disease subtype, biological functions, regulatory mechanisms, dysregulation in CTCs), UMAP/tSNE-based cell clustering, cell clustering maps of cell-specific lncRNA–target regulations, heat map and box plots of differential expression of lncRNA and targets, and lncRNA–target networks. All of the above functions were performed using the R package Seurat (version 4.0.4).
Updated RNA-seq/microarray web tool for mining and deeper understanding of lncRNA–target regulations
The rapid growth of RNA-seq/microarray data offers more chances for data mining and deeper understanding of the important role of lncRNA dysregulation in disease pathogenesis and tumor heterogeneity. In this work, we first obtained RNA-seq datasets containing lncRNA–target expression information from The Cancer Genome Atlas (TCGA), including transcriptome data of 33 cancer types and 11 373 TCGA pan-cancer patients (46). Second, we extracted 351 sets of microarray and RNA-seq datasets from the literature and downloaded them in the GEO. Similarly, we obtained 17 RNA-seq datasets of CTCs from the literature, including 877 CTC samples and seven cancer types. Three functions of the RNA-seq/microarray tools were established: TCGA analysis module of key lncRNA–target regulations, GEO analysis module of key lncRNA–target regulations and CTC analysis module of key lncRNA–target regulations. These tools allow valuable analysis for mining cancer-related lncRNA–target regulation biomarkers, exploring the biological function of lncRNAs, and promoting clinical application of blood therapy and diagnosis of diseases.
Tools in TCGA, GEO and CTC analysis modules mainly provided the following: (i) to demonstrate the differential expression patterns of lncRNA–target regulation, R package DESeq2 (47) and limma (41) were used for differential analysis of RNA-seq/microarray data; (ii) box plot diagrams were used to compare the expression of specific lncRNAs and targets between cancer and normal samples; (iii) to show the dynamic expression correlation of functional lncRNA–target regulations in human disease, Pearson correlation coefficients between lncRNAs and key targets in each of the datasets were calculated, and a t test of the Pearson correlation coefficient was used to estimate the correlation significance; (iv) scatter plots were used to display the expression of lncRNA–target in certain TCGA/GEO/CTC datasets.
DATABASE CONSTRUCTION AND IMPROVED USER INTERFACE
All data in LncTarD 2.0 were stored and managed using MySQL (version 5.6.50). The web interfaces were built in PHP (version 7.3) on Linux and Apache platform. The LncTarD 2.0 is freely available at https://lnctard.bio-database.com. The old version LncTarD 1.0 is still in service. Users can enter it from the LncTarD 2.0 homepage or go directly to http://bio-bigdata.hrbmu.edu.cn/LncTarD1.0/. LncTarD 2.0 exhibits a user-friendly interface and provides flexible routes for data access, enabling users to query the database in just a few steps: (i) From ‘Home’, ‘Browse’ and ‘Search’ pages, users can quickly obtain detailed information on lncRNA–target regulations in human diseases (Figure 2A–E); (ii) From the ‘RNA-seq/microarray Web Tools’ page (Figure 2F), users can obtain detailed data on lncRNAs and their target genes, including differential expression analysis and correlation analysis based on TCGA, GEO, and CTC datasets; (iii) In ‘Single Cell Web Tools’ page (Figure 3A and B), from the perspective of pan-cancer and cancer-specific analysis, single-cell web tools can perform complex functions and characterize key lncRNA–target regulations from scRNA-seq datasets, including differential regulations analysis, differential expression analysis, and functional states analysis at the pan-cancer and cancer-specific levels. In addition, we also provide specific tools for mining lncRNA–target regulations, including UMAP/tSNE-based cell clustering and gene expression, differential expression of lncRNAs and target genes among cell subsets, and characterization of lncRNA–target association in a single cell; (iv) LncTarD 2.0 is a fully open resource, and users can obtain all data from the ‘Download’ page; (v) The ‘Submit’ page enables researchers to submit novel experimentally supported lncRNA–target associations. (vi) In ‘Help’ page, users can receive a detailed tutorial about how to use LncTarD 2.0.
Figure 2.
Workflow and application of LncTarD 2.0. (A) The interface of the home module. (B) Browsing for all lncRNA–target regulations. (C) Search for all lncRNA–target regulations. (D) Network module for disease-associated lncRNA–target regulations. (E) The module contains detailed information about lncRNAs and target genes. (F) RNA-seq/microarray web tools characterize lncRNA–target regulations.
Figure 3.
Workflow and case study using web tools in LncTarD 2.0. (A) Single-cell web tools were used to characterize dynamic lncRNA–target regulations in pan-cancer and cancer-specific analysis modules. The Wilcoxon rank-sum test was used to identify differential lncRNA–target regulations by comparing the activity of lncRNA–target between a given scRNA-seq dataset and other scRNA-seq datasets. The Wilcoxon rank-sum test was used to compare the expression levels of differential lncRNAs and genes between a given single-cell scRNA-seq dataset and other scRNA-seq datasets. (B) UMAP/tSNE-based cell clustering and cell clustering maps of cell-specific lncRNA–target regulations were performed using the R package Seurat. Differential expression of lncRNAs and targets was identified using the Wilcoxon rank-sum test.
A case study to identify risk lncRNA–target regulations in NSCLC using web-based tools
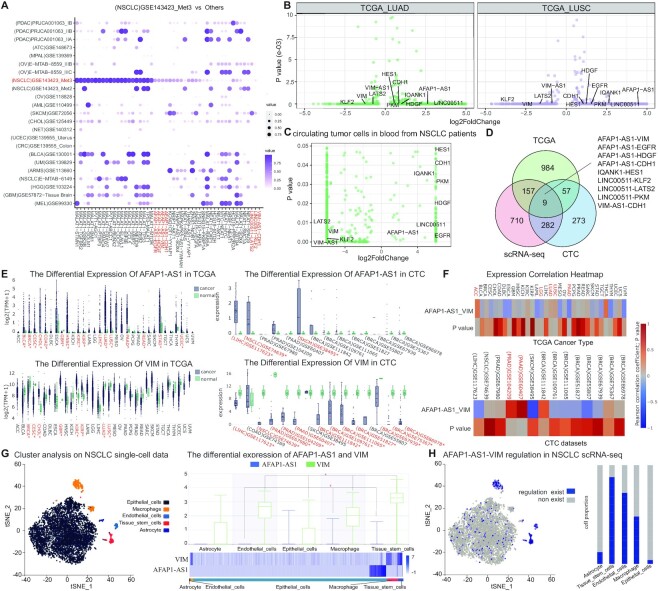
To demonstrate the usage and potential application of LncTarD database, we performed a case study to identify risk lncRNA–target regulations in non-small cell lung cancer (NSCLC) by integrating single-cell sequencing data and sequencing data from NSCLC tissue and CTCs using web-based tools. First, we identified differential lncRNA–target regulations in at least one of NSCLC scRNA-seq datasets by comparing their regulation activity with other single-cell datasets (Figure 4A). Next, genes in the differential lncRNA–target regulations were required to be differentially expressed in bulk sequence data of solid lung cancers from TCGA (Figure 4B). Finally, genes in the differential lncRNA–target regulations were required to be consistently differentially expressed in CTCs in blood from NSCLC patients (Figure 4C). Consequently, we identified nine risk lncRNA–target regulations as potential clinical biomarkers in NSCLC, including AFAP1-AS1-VIM, AFAP1-AS1-EGFR, AFAP1-AS1-HDGF, AFAP1-AS1-CDH1, IQANK1-HES1, LINC00511-KLF2, LINC00511-LATS2, LINC00511-PKM and VIM-AS1-CDH1 (Figure 4D). For example, AFAP1-AS1 was significantly upregulated in both the TCGA cohort and CTCs in blood samples of patients with NSCLC (Figure 4E). VIM was significantly downregulated in both the TCGA cohort and CTC data for NSCLC (Figure 4E). Moreover, AFAP1-AS1 expression showed significant positive correlations with VIM expression in the TCGA cohort (R = 0.1, P < 0.05; Figure 4F). It has been shown that AFAP1-AS1 is a potential biomarker for predicting the prognostic risk of non-small cell lung cancer (48). VIM has been reported to be a biomarker of epithelial-to-mesenchymal transition, which plays a key role in promoting cell migration (49). AFAP1-AS1 has been reported to promote cancer cell proliferation, cell invasion, and epithelial-to-mesenchymal transition via modulation of VIM in gallbladder cancer (50). We therefore speculated that AFAP1-AS1-VIM regulation may be a potential risk factor for NSCLC. Furthermore, using the single-cell web tool, we found that AFAP1-AS1 and VIM were both upregulated in non-small cell lung cancer stem cell subpopulation compared with endothelial cell subpopulation (Figure 4G). The cell proportion of AFAP1-AS1-VIM regulation was higher in breast cancer B cell subpopulation (fold change of 1.7; Figure 4H), suggesting that they possibly exert a regulatory effect on non-small cell lung cancer stem cell subpopulation and may be critical factors in lung tumor microenvironment.
Figure 4.
A case study to identify risk lncRNA–target regulations in NSCLC. (A) the top ranked differential lncRNA–target regulations in NSCLC scRNA-seq datasets by comparing regulation activity with other single-cell datasets. Differential expression of lncRNAs and genes in (B), TCGA cohort and in (C) CTC datasets. (D) an integrative pipeline to identity risk lncRNA–target regulations in NSCLC. (E) Differential expression of AFAP1-AS1 and VIM in TCGA cohort and in CTC datasets. (F) Pearson correlation coefficients and significance P-values between AFAP1-AS1 and VIM in TCGA cohort. (G) cell clustering maps and differential expression analysis of AFAP1-AS1 and VIM in NSCLC cell subpopulations. (H) Distribution of the AFAP1-AS1-VIM regulation in the tumour microenvironment of NSCLC (left panel) and the cell proportion of AFAP1-AS1-VIM regulation in cell subpopulations (right panel).
CONCLUSION
With the increase of reports on experimentally supported key lncRNA–target regulations, in this study, we expanded the LncTarD database according to the reported lncRNA–target regulations and increased the expression of regulations in tissue samples, single-cell samples and blood samples. With the continuous research on the pathogenesis of different diseases, we believe that more lncRNAs and lncRNA–target regulations will be reported. In the future, we will integrate multi-omics data of diseases, use more datasets and tools, and continue to maintain and update the LncTarD database, further understanding the important role of lncRNA dysregulation in disease pathogenesis and tumor heterogeneity.
DATA AVAILABILITY
All the data could be downloaded from http://bio-bigdata.hrbmu.edu.cn/LncTarD.
Supplementary Material
Contributor Information
Hongying Zhao, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Xiangzhe Yin, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Haotian Xu, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Kailai Liu, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Wangyang Liu, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Lixia Wang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Caiyu Zhang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Lin Bo, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Xicheng Lan, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Shihua Lin, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Ke Feng, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Shangwei Ning, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yunpeng Zhang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Li Wang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province [UNPYSCT-2020174]; Excellent Youth Project of Provincial scientific research Institute [CZKYF2022-1-C006]; Hei Long Jiang Postdoctoral Special Foundation [LBH-TZ1018]. Funding for open access charge: University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province [UNPYSCT-2020174]; Excellent Youth Project of Provincial scientific Research Institute [CZKYF2022-1-C006]; Hei Long Jiang Postdoctoral Special Foundation [LBH-TZ1018].
Conflict of interest statement. None declared.
REFERENCES
- 1. Yang Z., Jiang S., Shang J., Jiang Y., Dai Y., Xu B., Yu Y., Liang Z., Yang Y.. LncRNA: shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res. Rev. 2019; 52:17–31. [DOI] [PubMed] [Google Scholar]
- 2. Doherty G.J., Petruzzelli M., Beddowes E., Ahmad S.S., Caldas C., Gilbertson R.J.. Cancer treatment in the genomic era. Annu. Rev. Biochem. 2019; 88:247–280. [DOI] [PubMed] [Google Scholar]
- 3. Tan Y.-T., Lin J.-F., Li T., Li J.-J., Xu R.-H., Ju H.-Q.. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun. (Lond.). 2021; 41:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao H., Liu X., Yu L., Lin S., Zhang C., Xu H., Leng Z., Huang W., Lei J., Li T.et al.. Comprehensive landscape of epigenetic-dysregulated lncRNAs reveals a profound role of enhancers in carcinogenesis in BC subtypes. Mol. Ther. Nucleic Acids. 2021; 23:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bi M., Zheng L., Chen L., He J., Yuan C., Ma P., Zhao Y., Hu F., Tang W., Sheng M.. ln RNA LINC01234 promotes triple-negative breast cancer progression through regulating the miR-429/SYNJ1 axis. Am. J. Transl. Res. 2021; 13:11399–11412. [PMC free article] [PubMed] [Google Scholar]
- 6. Liang H., Yu T., Han Y., Jiang H., Wang C., You T., Zhao X., Shan H., Yang R., Yang L.et al.. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer. 2018; 17:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He Y., Jiang X., Duan L., Xiong Q., Yuan Y., Liu P., Jiang L., Shen Q., Zhao S., Yang C.et al.. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating wnt signaling pathway. Mol. Cancer. 2021; 20:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Q., Ling Z., Zhang J., Yu H., Wang Y., Xue Y., Wang C., Zhao J., Cao J., Duan S.et al.. lncRNA MIR600HG knockdown alleviates cognitive impairment in alzheimer's disease through NEDD4L mediated PINK1 degradation. J. Alzheimers Dis. 2022; 85:1783–1794. [DOI] [PubMed] [Google Scholar]
- 9. Huang L., Dai G.. Long non-coding RNA DCST1-AS1/hsa-miR-582-5p/HMGB1 axis regulates colorectal cancer progression. Bioengineered. 2022; 13:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Guo K., Qian K., Shi Y., Sun T., Wang Z.. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021; 12:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao S., Chu Q., Liu X., Zhao X., Qin L., Li G., Liu Q.. Long noncoding RNA HEIH promotes proliferation, migration and invasion of retinoblastoma cells through miR-194-5p/WEE1 axis. Onco Targets Ther. 2020; 13:12033–12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi J., Wang L., Yin X., Wang L., Bo L., Liu K., Feng K., Lin S., Xu Y., Ning S.et al.. Comprehensive characterization of clonality of driver genes revealing their clinical relevance in colorectal cancer. J. Transl. Med. 2022; 20:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao Z., Yang Z., Huang Z., Zhou Y., Cui Q., Dong D. LncRNADisease 2.0: an updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019; 47:D1034–D1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Y., Shang S., Guo S., Li X., Zhou H., Liu H., Sun Y., Wang J., Wang P., Zhi H.et al.. Lnc2Cancer 3.0: an updated resource for experimentally supported lncRNA/circRNA cancer associations and web tools based on RNA-seq and scRNA-seq data. Nucleic Acids Res. 2021; 49:D1251–D1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ning L., Cui T., Zheng B., Wang N., Luo J., Yang B., Du M., Cheng J., Dou Y., Wang D. MNDR v3.0: mammal ncRNA-disease repository with increased coverage and annotation. Nucleic Acids Res. 2021; 49:D160–D164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang P., Guo Q., Hao Y., Liu Q., Gao Y., Zhi H., Li X., Shang S., Guo S., Zhang Y.et al.. LnCeCell: a comprehensive database of predicted lncRNA-associated ceRNA networks at single-cell resolution. Nucleic Acids Res. 2021; 49:D125–D133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng L., Wang P., Tian R., Wang S., Guo Q., Luo M., Zhou W., Liu G., Jiang H., Jiang Q.. LncRNA2Target v2.0: a comprehensive database for target genes of lncRNAs in human and mouse. Nucleic Acids Res. 2019; 47:D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang P., Guo Q., Qi Y., Hao Y., Gao Y., Zhi H., Zhang Y., Sun Y., Zhang Y., Xin M.et al.. LncACTdb 3.0: an updated database of experimentally supported ceRNA interactions and personalized networks contributing to precision medicine. Nucleic Acids Res. 2022; 50:D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karagkouni D., Paraskevopoulou M.D., Tastsoglou S., Skoufos G., Karavangeli A., Pierros V., Zacharopoulou E., Hatzigeorgiou A.G.. DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 2020; 48:D101–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhi H., Li X., Wang P., Gao Y., Gao B., Zhou D., Zhang Y., Guo M., Yue M., Shen W.et al.. Lnc2Meth: a manually curated database of regulatory relationships between long non-coding RNAs and DNA methylation associated with human disease. Nucleic Acids Res. 2018; 46:D133–D138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao Y., Li X., Shang S., Guo S., Wang P., Sun D., Gan J., Sun J., Zhang Y., Wang J.et al.. LincSNP 3.0: an updated database for linking functional variants to human long non-coding RNAs, circular RNAs and their regulatory elements. Nucleic Acids Res. 2021; 49:D1244–D1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao H., Shi J., Zhang Y., Xie A., Yu L., Zhang C., Lei J., Xu H., Leng Z., Li T.et al.. LncTarD: a manually-curated database of experimentally-supported functional lncRNA–target regulations in human diseases. Nucleic Acids Res. 2020; 48:D118–D126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu S., Bu X., Kan A., Luo L., Xu Y., Chen H., Lin X., Lai Z., Wen D., Huang L.et al.. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022; 528:16–30. [DOI] [PubMed] [Google Scholar]
- 24. Wang H., Li J., Xu W., Li C., Wu K., Chen G., Cui J.. The mechanism underlying arsenic-induced PD-L1 upregulation in transformed BEAS-2B cells. Toxicol. Appl. Pharmacol. 2022; 435:115845. [DOI] [PubMed] [Google Scholar]
- 25. Wu W.J., Yin H., Hu J.J., Wei X.Z.. Long noncoding RNA LINC00313 modulates papillary thyroid cancer tumorigenesis via sponging miR-4429. Neoplasma. 2018; 65:933–942. [DOI] [PubMed] [Google Scholar]
- 26. Yang X., Cai J.-B., Peng R., Wei C.-Y., Lu J.-C., Gao C., Shen Z.-Z., Zhang P.-F., Huang X.-Y., Ke A.-W.et al.. The long noncoding RNA NORAD enhances the TGF-β pathway to promote hepatocellular carcinoma progression by targeting miR-202-5p. J. Cell. Physiol. 2019; 234:12051–12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang X., Wang L., Xie S., Chen Y., Song S., Lu Y., Lu D. Long noncoding RNA MEG3 blocks telomerase activity in human liver cancer stem cells epigenetically. Stem Cell Res. Ther. 2020; 11:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan L., Tang Y., Li H., Li P., Ye Y., Cen J., Gui C., Luo J., Cao J., Wei J.. N6-Methyladenosine modification of LncRNA DUXAP9 promotes renal cancer cells proliferation and motility by activating the PI3K/AKT signaling pathway. Front. Oncol. 2021; 11:641833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L., Yu L., Shi J., Li F., Zhang C., Xu H., Yin X., Wang L., Lin S., Litvinova A.et al.. Functional regulations between genetic alteration-driven genes and drug target genes acting as prognostic biomarkers in breast cancer. Sci. Rep. 2022; 12:10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Y., Ren W., Li Q., Duan C., Lin X., Bi Z., You K., Hu Q., Xie N., Yu Y.et al.. LncRNA Uc003xsl.1-Mediated activation of the NFκB/IL8 axis promotes progression of triple-negative breast cancer. Cancer Res. 2022; 82:556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh A.P., Luo H., Matur M., Eshelman M.A., Hamamoto K., Sharma A., Lesperance J., Huang S.. A coordinated function of lncRNA HOTTIP and miRNA-196b underpinning leukemogenesis by targeting FAS signaling. Oncogene. 2022; 41:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang N., Meng X., Mi H., Chi Y., Li S., Jin Z., Tian H., He J., Shen W., Tian H.et al.. Circulating lncRNA XLOC_009167 serves as a diagnostic biomarker to predict lung cancer. Clin. Chim. Acta. 2018; 486:26–33. [DOI] [PubMed] [Google Scholar]
- 33. Castro-Giner F., Aceto N.. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020; 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luan Y., Li X., Luan Y., Zhao R., Li Y., Liu L., Hao Y., Oleg Vladimir B., Jia L.. Circulating lncRNA UCA1 promotes malignancy of colorectal cancer via the miR-143/MYO6 axis. Mol. Ther. Nucleic Acids. 2020; 19:790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu S., Sun Y., Hou Y., Yang L., Wan X., Qin Y., Liu Y., Wang R., Zhu P., Teng Y.et al.. A novel lncRNA ROPM-mediated lipid metabolism governs breast cancer stem cell properties. J. Hematol. Oncol. 2021; 14:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCabe E.M., Rasmussen T.P.. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin. Cancer Biol. 2021; 75:38–48. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y., Zhu P., Luo J., Wang J., Liu Z., Wu W., Du Y., Ye B., Wang D., He L.et al.. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019; 38:e101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiao Y., Pan J., Geng Q., Wang G.. LncRNA MALAT1 increases the stemness of gastric cancer cells via enhancing SOX2 mRNA stability. FEBS Open Bio. 2019; 9:1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Howe K.L., Achuthan P., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Azov A.G., Bennett R., Bhai J.et al.. Ensembl 2021. Nucleic Acids Res. 2021; 49:D884–D891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schriml L.M., Mitraka E., Munro J., Tauber B., Schor M., Nickle L., Felix V., Jeng L., Bearer C., Lichenstein R.et al.. Human disease ontology 2018 update: classification, content and workflow expansion. Nucleic Acids Res. 2019; 47:D955–D962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K.. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M.et al.. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013; 41:D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dai H., Li L., Zeng T., Chen L.. Cell-specific network constructed by single-cell RNA sequencing data. Nucleic Acids Res. 2019; 47:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holland C.H., Tanevski J., Perales-Patón J., Gleixner J., Kumar M.P., Mereu E., Joughin B.A., Stegle O., Lauffenburger D.A., Heyn H.et al.. Robustness and applicability of transcription factor and pathway analysis tools on single-cell RNA-seq data. Genome Biol. 2020; 21:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han X., Zhou Z., Fei L., Sun H., Wang R., Chen Y., Chen H., Wang J., Tang H., Ge W.et al.. Construction of a human cell landscape at single-cell level. Nature. 2020; 581:303–309. [DOI] [PubMed] [Google Scholar]
- 46. Tomczak K., Czerwińska P., Wiznerowicz M.. The cancer genome atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015; 19:A68–A77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Love M.I., Huber W., Anders S.. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014; 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng J., Liang Y., Liu C., He S., Wang S.. The up-regulation of long non-coding RNA AFAP1-AS1 is associated with the poor prognosis of NSCLC patients. Biomed. Pharmacother. 2015; 75:420–432. [DOI] [PubMed] [Google Scholar]
- 49. Richardson A.M., Havel L.S., Koyen A.E., Konen J.M., Shupe J., Wiles W.G., Martin W.D., Grossniklaus H.E., Sica G., Gilbert-Ross M.et al.. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin. Cancer Res. 2018; 24:420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma F., Wang S.-H., Cai Q., Zhang M.-D., Yang Y., Ding J.. Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis and promotes cells proliferation and invasion in gallbladder cancer. Biomed. Pharmacother. 2016; 84:1249–1255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data could be downloaded from http://bio-bigdata.hrbmu.edu.cn/LncTarD.