Abstract
The rickettsial pathogen Anaplasma marginale expresses a variable immunodominant outer membrane protein, major surface protein 2 (MSP2), involved in antigenic variation and long-term persistence of the organism in carrier animals. MSP2 contains a central hypervariable region of about 100 amino acids that encodes immunogenic B-cell epitopes that induce variant-specific antibodies during infection. Previously, we have shown that MSP2 is encoded on a polycistronic mRNA transcript in erythrocyte stages of A. marginale and defined the structure of the genomic expression site for this transcript. In this study, we show that the same expression site is utilized in stages of A. marginale infecting tick salivary glands. We also analyzed the variability of this genomic expression site in Oklahoma strain A. marginale transmitted from in vitro cultures to cattle and between cattle and ticks. The structure of the expression site and flanking regions was conserved except for sequence that encoded the MSP2 hypervariable region. At least three different MSP2 variants were encoded in each A. marginale population. The major sequence variants did not change on passage of A. marginale between culture, acute erythrocyte stage infections, and tick salivary glands but did change during persistent infections of cattle. The variant types found in tick salivary glands most closely resembled those present in bovine blood at the time of acquisition of infection, whether infection was acquired from an acute or from a persistent rickettsemia. These variations in structure of an expression site for a major, immunoprotective outer membrane protein have important implications for vaccine development and for obtaining an improved understanding of the mechanisms of persistence of ehrlichial infections in humans, domestic animals, and reservoir hosts.
Anaplasma marginale is an animal pathogen of major economic importance to livestock production throughout many areas of North and South America, Africa, Australia, and Asia (22). Anaplasmosis causes economic losses in the United States of approximately $300 million/year (cost in 1986 U.S. dollars) (20). A. marginale is classified as a genogroup II ehrlichial agent, closely related taxonomically to other animal and human ehrlichial pathogens (9). Genogroup I and II ehrlichial agents include Cowdria ruminantium, causative agent of heartwater disease in ruminants; Ehrlichia canis, a causative agent of canine ehrlichiosis; Ehrlichia chaffeensis, which causes human monocytic ehrlichiosis; and agents related or identical to Ehrlichia phagocytophila and Ehrlichia ewingii that cause animal and human granulocytic ehrlichioses. The human ehrlichioses are classified as emerging diseases, with >500 cases confirmed since 1985 and an estimated 5% fatality rate.
There are a number of common features to these ehrlichial infections. After an initial acute phase infections may persist for long periods, even with antibiotic treatment (2, 10, 11, 16, 17, 29). In persistent infections caused by A. marginale, use of DNA probes and quantitative PCR revealed recurrent cyclic peaks of rickettsemia that probably continue for the lifetime of an infected animal (13, 19). A. marginale organisms express an outer membrane protein, major surface protein 2 (MSP2), approximately 36 kDa in size, which is strongly recognized by B and T cells from infected animals and partially protects immunized animals against challenge (8, 12, 23, 24). MSP2 is significantly similar to the major outer membrane protein of other ehrlichial organisms in amino acid sequence and is also encoded by a multigene family (25). Like outer membrane proteins of an agent of human granulocytic ehrlichiosis, MSP2 contains a single hypervariable region in the central part of the molecule (14). In the recurrent peaks of a single infection caused by A. marginale there are at least three different genetic and antigenic variants of MSP2 expressed in each peak (15). We have demonstrated previously that the predominant msp2 mRNA transcript in erythrocyte stages of A. marginale is a polycistronic mRNA containing msp2 and three other genes. Also, we have demonstrated that msp2 variation proceeds through the formation of different sequence mosaics in the expression site for this polycistronic mRNA (4). The availability of the sequence of this msp2 expression site, together with the recent development of an in vitro culture system for A. marginale (21), permits analysis of the extent and limitations of msp2 variation as organisms cycle between their different stages in infected tick and mammalian cells.
MATERIALS AND METHODS
Derivation of A. marginale populations.
An Oklahoma strain of A. marginale was propagated by in vitro culture, as described (6). Briefly, infected blood was collected in 1998 from a calf with clinical anaplasmosis from Wetumka, Okla., and was subinoculated into a susceptible, splenectomized calf. Blood collected at peak rickettsemia was frozen, thawed, and used as inoculum on confluent tick cell monolayers derived from lxodes scapularis embryos. Colonies of A. marginale were apparent in low numbers at 9 days postexposure, and infection in monolayers reached 100% (terminal cultures) by 4 to 5 weeks postexposure. Cultures were passaged by placing terminal cultures onto fresh tick cell monolayers at a dilution of 1:5 or 1:10. By the third passage development of the cultured Oklahoma strain was similar to that of the Virginia strain described previously (21), and a 1:5 dilution resulted in 100% infection in 10 to 12 days.
After two serial passages were achieved, a 25-cm2 flask with an infection of ∼90% was used to infect a splenectomized calf (PA408). Cells were pipetted from the flask and disrupted with a ground-glass homogenizer. Cells were suspended in 1 ml of medium and inoculated intravenously. Calf PA408 was monitored daily for clinical signs and appearance of rickettsiae in Diff-Quik-stained blood films. Calf PA408 developed clinical anaplasmosis with a prepatent period of 20 days, a peak rickettsemia of 34%, and a minimum packed cell volume of 12%. When the A. marginale rickettsemia was approximately 30%, 200 male Dermacentor variabilis ticks were acquisition fed on calf PA408 for 7 days during the ascending rickettsemia. After the infected ticks were removed and held for 7 days in a humidity chamber, they were transmission fed on a second splenectomized calf (calf PA407), which was monitored as described previously. Male D. variabilis ticks which fed on calf PA408 transmitted A. marginale to calf PA407 with a prepatent period of 22 days, a peak rickettsemia of 51% and a minimum PCV of 13.5%. Calf PA407 recovered from acute anaplasmosis and remained a carrier of A. marginale, with recurrent cycles of erythrocytic rickettsemia approximately every 4 to 6 weeks, of generally decreasing amplitude with time of infection. Another group of D. variabilis ticks were acquisition fed on calf PA407 during the peak of persistent rickettsemia that occurred on 28 September 1998 and which represented the fifth microscopically detected peak of A. marginale in calf PA407. These ticks were transmission fed on a sheep for 7 days, to allow salivary gland stages of A. marginale to fully develop. Samples were taken from all A. marginale populations for DNA isolation and analysis of the polycistronic msp2 expression site (see Fig. 1).
FIG. 1.
Derivation of cyclically transmitted A. marginale populations for analysis of sequence diversity in the polycistronic msp2 expression site.
A second cyclic transmission was performed to investigate more closely the relationship between msp2 expression site variants acquired by ticks from a persistently infected calf (calf PA417) and transmitted to a second calf (calf PA420). Calf PA417 was infected with the Oklahoma strain of A. marginale by transmission feeding with D. variabilis ticks. Calf PA417 experienced a rickettsemia peak of 64%, with a prepatent period of 23 days and subsequent rickettsemia peaks of decreasing amplitude. A group of D. variabilis ticks were acquisition fed on PA417 on days 111 to 118 (16 to 23 September 1999) after the peak of acute rickettsemia and subsequently transmission fed on calf PA420, which experienced an acute rickettsemia of 45%. Blood samples were taken from calf PA417 just prior to the beginning of tick feeding (13 September 1999; 0.9% rickettsemia), during tick feeding (20 September 1999; 1.3% rickettsemia), and just after ticks were removed from the calf (27 September 1999; 0.5% rickettsemia). Samples were also taken for DNA isolation from salivary glands of the infected ticks after transmission feeding and from the acute transmitted rickettsemia in calf PA420. The sequence of the hypervariable region was determined from 10 independent clones of the msp2 expression site in each A. marginale population.
To determine if there were differences in msp2 expression site variants transmitted by different tick species, Dermacentor andersoni and D. variabilis ticks were acquisition fed at the same time on A. marginale-infected calf PA411 during the acute rickettsemia. These ticks were subsequently transmission fed on different naive calves before dissection and removal of salivary glands for isolation of DNA for msp2 expression site analysis.
Genome and sequence analysis.
Erythrocyte stages of A. marginale were concentrated by passage of infected blood through a cellulose column (C-6288; Sigma, St. Louis, Mo.). Genomic DNA was isolated from erythrocytic stages of A. marginale by lysis with sodium dodecyl sulfate and lysozyme and treatment with proteinase K and ribonuclease, followed by phenol-chloroform extraction and ethanol precipitation (5), or by using a kit for genomic DNA isolation (Qiagen, Valencia, Calif.). DNA from A. marginale-infected tick salivary glands and cultured tick cells was extracted with NucleoSpin nucleic acid purification kits (Clontech, Palo Alto, Calif.). Genomic DNA was analyzed by restriction enzyme digestion followed by agarose gel electrophoresis and Southern blotting, as described (4), using probes specific for msp2 or orf2, orf3, or orf4 of the polycistronic msp2 expression site. The 3.9-kbp genomic expression site for msp2 was also amplified as described previously (4) using oligonucleotide primers AB767 (ACGCGCTTGAATAAATCGTT) and AB752 (CACCGGTTGATGAAGTTTGC). In some cases, primers AB750 (GGATTTTGTGGTCGGGTTTGTAT) and AB752 were used to similarly amplify a 2.9-kbp segment of the expression site lacking orf4 and its 5′ flanking region. PCR amplified products were analyzed by gel electrophoresis and sequenced directly or cloned into the pCR-XL-TOPO vector (Invitrogen, Carlsbad, Calif.) and plasmid DNA was isolated and sequenced. Sequencing was performed at the University of Florida DNA Sequencing Core Laboratory (Gainesville, Fla.) using ABI Prism Big Dye Terminator cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, Calif.). The fluorescently labeled extension products were analyzed on an Applied Biosystems model 373 Stretch DNA Sequencer (Perkin-Elmer Corp.). Oligonucleotide primers were designed using OLIGO 5.0 (Molecular Biology Insights, Cascade, Co.) software and synthesized by Genosys Biotechnologies (The Woodlands, Tex.). Nucleotide sequences were analyzed using the University of Wisconsin Genetics Computer Group programs available through the Biological Computing Facility of the Interdisciplinary Center for Biotechnology Research at the University of Florida. Nucleotide and amino acid alignments were made using the program PILEUP and displayed using PRETTY and PLOTSIMILARITY. msp2 expression site variability was calculated from aligned amino acid sequences using the following formula: number of different amino acids at a given position/frequency of the most common amino acid at that position (18).
Southern blotting of A. marginale genomic DNA.
Probes specific to msp2, orf2, orf3, or orf4 were prepared and used in Southern blotting of digested A. marginale genomic DNA as described previously (4). DNA probes were labeled with fluorescein-dUTP, hybridized, washed under high-stringency conditions (60°C; 0.1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% SDS), and detected by chemiluminescence (Illuminator Chemiluminescent Detection System; Stratagene, La Jolla, Calif.). Molecular size standards were Illuminator nonradioactive markers (Stratagene).
RNA isolation and RT-PCR.
Total RNA was isolated from infected tick salivary glands preserved in RNAlater (Ambion, Austin, Tex.) using the RNAqueous kit (Ambion). Isolated RNA was digested with DNase I (DNA-free; Ambion) before use in reverse transcription (RT)-PCRs. RNA transcripts of the msp2 gene were reverse transcribed into DNA using the RETRO script kit (Ambion) and primer AB198 (5′AAGGCAAACCTAACACCCAACTCACCACCA3′), which anneals to the conserved 3′ end of the coding region of the msp2 gene. Primary RT-PCRs used oligonucleotide primers AB765 (5′GGAACAACCCCAATACCATC3′) and AB766 (5′GTATGTCGATTCGCGGAAGAGCCTGTTGT3′), which amplify a 3.2-kb segment of the msp2 polycistronic transcript; a 200 μM concentration of each deoxynucleoside triphosphate, and 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer). Secondary (nested) RT-PCRs used primers AB192 (5′CTATCCTTGAAGCTAATCTTG3′) plus AB783 (5′AGTATCACATTGGGGAGGTTT3′) to amplify a segment containing the 3′ end of orf4, all of orf2 and -3, and the 5′ end of msp2, of size 2.1 kbp. Control reactions were conducted similarly, but without reverse transcriptase in the initial RT reaction. Products of RT-PCR were analyzed by agarose gel electrophoresis and Southern blotting using an orf2-specific DNA probe. RT-PCR products were also cloned into the pCR-XL-TOPO vector (Invitrogen), and plasmid DNA was isolated and sequenced to verify the structure of amplified DNA.
Nucleotide sequence accession numbers.
The sequences reported here have been assigned GenBank accession numbers AF317720 to AF317726.
RESULTS
Structural conservation of the polycistronic msp2 expression site in A. marginale from culture, cattle, and ticks.
Figure 1 shows the derivation of A. marginale organisms used for analysis of the msp2 expression site. Genomic DNA was extracted from each of the seven different populations of A. marginale, and the ∼3.9-kbp msp2 expression site was amplified by PCR and sequenced. The structure and sequence were similar to that described previously for the msp2 polycistronic expression site from Florida and Idaho strains of A. marginale (4). There were three open reading frames upstream of the msp2 gene, encoding polypeptides predicted by the PSORT algorithm (http://psort.nibb.ac.jp) to be outer membrane proteins. As in Florida and Idaho strains (4), orf3 and orf4 encoded polypeptides significantly similar to the outer membrane protein OMP1b of E. chaffeensis. When the different Oklahoma strain DNA sequences were aligned there were very few changes observed in orf2, orf3, or orf4 between the different life cycle stages and populations of the Oklahoma strain A. marginale (a total of 5 amino acid changes between all seven populations in polypeptides encoded by orf2, orf3, and orf4). In contrast, many differences were present in the central msp2 hypervariable region including substitutions, insertions and deletions (Fig. 2a). There was more variability, particularly in orf4 when the expression site sequences of acute erythrocyte stages from Oklahoma, Florida, and Idaho strains were aligned (Fig. 2b). No changes were present between Oklahoma (all stages), Florida, and Idaho strains in the 5′-flanking region of orf4, which was previously shown to contain the +1 site for transcription and a predicted prokaryotic promoter (4).
FIG. 2.
PLOTSIMILARITY profiles of nucleotide sequence variability in msp2 expression sites show greatest variability in the central region of the msp2 gene. (a) The 7 different populations from the Oklahoma strain of A. marginale described in Fig. 1 are compared; (b) Florida, Idaho, and Oklahoma strain acute bloodstream populations are compared. A similarity score of 1.0 indicates identical sequence in a sliding window of 10 nucleotides; a decreasing score from 1.0 to 0.0 indicates increasing variation.
MSP2 is encoded on a polycistronic transcript in A. marginale from tick salivary glands.
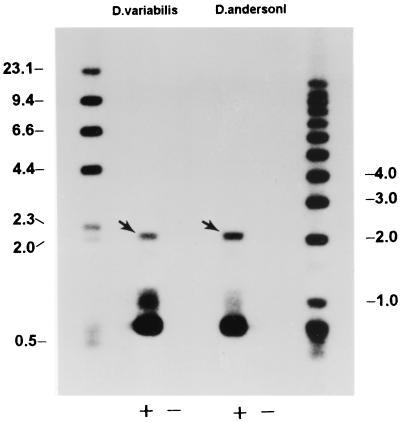
To demonstrate whether or not this genomic site encoded an msp2-containing mRNA transcript in tick salivary gland stages of A. marginale, RT-PCR was used to amplify a fragment that contained linked msp2, orf2, orf3, and orf4 genes. The expected fragment of 2.1 kbp was amplified from total RNA prepared from salivary glands of both D. variabilis and D. andersoni infected with A. marginale (Fig. 3). No PCR products were detected in control reactions without reverse transcriptase. Sequencing of the cloned 2.1-kbp RT-PCR product (Fig. 3) revealed that it contained the expected linked regions containing msp2, orf2, orf3, and orf4. Hence, this genomic site appears to be transcribed into msp2 mRNA in A. marginale isolated from infected tick salivary glands, as has been shown previously for bloodstream stages (4). As in Florida and Idaho strains of A. marginale, there were multiple copies of the msp2 gene in Oklahoma strain genomic DNA. Only a single band was detected, however, when using DNA probes containing orf2, orf3, and orf4 (Fig. 4). The multiple msp2 copies were polymorphic between the different strains, and only the msp2 copy derived from the expression site also contained contiguous coding sequence for orf2, orf3, and orf4 (Fig. 4). Therefore, the other msp2 copies could not be expressed as a polycistronic mRNA containing all 4 open reading frames without recombination.
FIG. 3.
A polycistronic RNA transcript containing the msp2 gene is present in A. marginale-infected salivary glands from D. variabilis and D. andersoni ticks. RT-PCR analysis of isolated RNA from infected salivary glands with AB198 as the RT primer, AB765 and AB766 as primary PCR primers, and AB192 and AB783 as secondary (nested) PCR primers. A 2.1-kbp product was specifically amplified in reactions containing reverse transcriptase enzyme (+) but was not present in control reactions without reverse transcriptase (−). This 2.1-kbp band hybridized to an orf2 probe (arrow). Cloning and sequencing of the 2.1-kbp product demonstrated that it contained sequence from msp2, orf2, orf3, and orf4. Low-molecular-weight hybridizing bands are also present in RNA (lanes labeled +), which may represent amplified products from partially degraded A. marginale RNA.
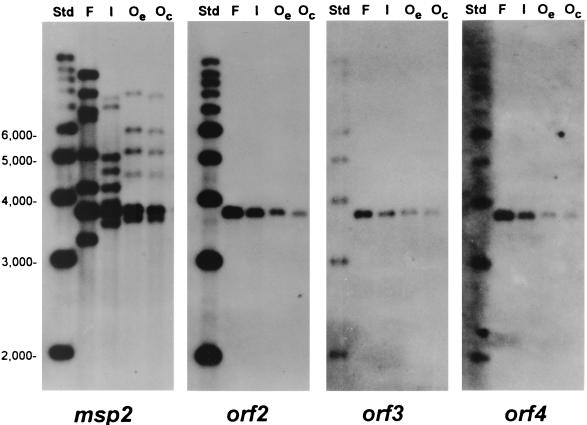
FIG. 4.
Structure of msp2 and orf2 to orf4 in genomic DNA of Florida, South Idaho, and Oklahoma strains of A. marginale. Southern blots of Florida (F), South Idaho (I), Oklahoma acute erythrocyte stage (Oe), or culture stage (Oc) genomic DNA digested with the restriction enzyme FspI and hybridized with probes specific for either msp2, orf2, orf3, or orf4 (probe is shown at bottom of figure). FspI cleaves 41 nucleotides 5′ to orf4 and 268 nucleotides 3′ to msp2 to release a fragment of 3.76 kbp that contains the complete polycistronic msp2 expression site sequence (see Fig. 2) from all genomic DNAs. Molecular size standards (Std) are shown in the left lane of each blot. Multiple msp2-related sequences are present in genomic DNA of all strains; only msp2 sequences located in the expression site are contiguous with orf2, orf3, and orf4.
Polymorphism in the msp2 hypervariable region in cyclically transmitted rickettsiae.
Three to five different variants were found in each A. marginale population. Some of these variants were shared between different populations; therefore, a total of 24 different variants of this msp2 expression site were identified in the 49 clones examined. The amino acid sequences of the different MSP2 hypervariable regions found in each life cycle stage of A. marginale are shown aligned in Fig. 5. They differ from one another by multiple insertions, deletions, and substitutions, with some sequences appearing to be “mosaics” of others, e.g., Ok407per2VarC is identical to Ok407acVarB in the first part of the MSP2 hypervariable region but identical to Ok407per2VarB in the last part. These differences among the variants suggest that templated intragenic recombination may be occurring between the multiple genomic msp2 copies and the polycistronic expression site.
FIG. 5.
Multiple different msp2 variants are present in the polycistronic expression site in each population of A. marginale. The major variant type is conserved during passage of A. marginale between culture, acute erythrocyte stage infection, and tick salivary glands but is not conserved in persistent cattle infections. The expression site was amplified by PCR using primers which annealed 288 bp 3′ to the termination codon of msp2 (AB752) and to the intercistronic sequence between orf3 and orf4 (AB750) to generate a product of 2.9 kbp from A. marginale genomic DNA that contained msp2, orf2, and orf3. The PCR product was cloned in pCR-XL-TOPO vector (Invitrogen), and independent colonies containing a 2.9-kbp insert were selected for sequencing of cloned plasmid DNA. The hypervariable region of the msp2 gene was sequenced on both strands in seven independent clones derived by PCR amplification from genomic DNA of each of the A. marginale populations described in Fig. 1. DNA sequences were translated to amino acids, and the different variant sequences were aligned with PILEUP. The proportion of each sequence variant in that population is indicated in brackets; e.g., the major sequence variant detected in cultured A. marginale was variant A, which was found in three of seven independent clones of the expression site. Identical amino acids shared between all variants are indicated by a dash and are shown on the bottom row of the alignment. Variant types present in different A. marginale populations are indicated by identical symbols to the left of the sequence alignments.
We examined where identical variant sequences were found in the polycistronic expression site in different A. marginale populations (Fig. 5). A predominant variant found in in vitro-cultured A. marginale was OkculVarA. The identical MSP2 variant was also present in the first (acute) bloodstream rickettsemia of the animal infected with these cultures (Ok408acVarA), the ticks that acquisition fed during this acute rickettsemia (OksgacVarA), and also the first bloodstream rickettsemia of the animal infected by these ticks (Ok407acVarA). This was the predominant, but not the only variant type in each of these A. marginale populations. Minor variants were also conserved in the transmission cycle: acute bloodstream rickettsemia to ticks to acute bloodstream rickettsemia (variants Ok408acVarB, OksgacVarB, and Ok407acVarB are identical). Despite this conservation of variant types found in the acute bovine rickettsemias and in the ticks that fed on them, there were also minor variants present in an acute rickettsemia not found elsewhere. For example Ok408acVarD was quite dissimilar to the other three variants in the acute-stage rickettsemia of animal PA408 and was not observed again in subsequent populations.
As the infection progressed in animal PA407 from acute to persistent relapsing rickettsemias, more diversity was observed in this polycistronic msp2 expression site (Fig. 6). The variants found were different, both from those observed in the acute rickettsemias and from those in cultured A. marginale or in the ticks that initiated the bovine infections (Fig. 5). This increase in diversity of the expression site parallels the increase in diversity of msp2 mRNA observed previously in persistent infections (15). Interestingly, when ticks acquired infection from a persistent rather than from an acute stage of a bovine infection, the variants found in the tick salivary glands (Oksgper variants [Fig. 5]) also appeared similar to the erythrocyte stage variants circulating at the time of tick feeding. One of the tick stage variants (OksgperVarB) was identical to an erythrocyte stage variant circulating during tick acquisition feeding (Ok407per1VarB).
FIG. 6.
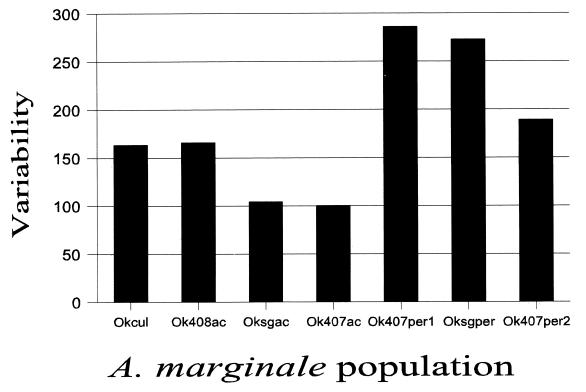
Msp2 expression site variability increases in persistent cattle infection. The variability of each population was calculated over the hypervariable region of the msp2 expression site using the seven independent clones sampled from each of the seven A. marginale populations in Fig. 1, the sequence alignment in Fig. 5, and the following formula: number of different amino acids at a given position/frequency of the most common amino acid at that position. For example, at position 7 in population okcul there is H (histidine) in six clones and Y (tyrosine) in one clone of the msp2 expression site, for a variability of 2/0.857 (=2.3). Values were obtained similarly for all variable positions in the alignment and added, to obtain a total population variability for okcul of 163.8.
To more closely examine this relationship between the circulating variants in persistent infection and those acquired and transmitted by the tick vector, we performed a second series of cyclic transmissions. Since acquisition feeding occurs over 7 days, we sampled the circulating expression site variants in persistently infected calf PA417 before, during, and just after tick feeding, as well as in tick salivary glands and the acute transmitted rickettsemia in calf PA420. The relationship between the MSP2 hypervariable region sequences obtained is shown in the dendrogram in Fig. 7, with brackets to the right of the figure indicating identical variants found in the different rickettsial populations. The same circulating variant was observed prior to, during, and after tick feeding (ok417-9-13VarA is identical to ok417-9-20VarG and ok417-9-27VarD) as well as in the salivary glands of infected ticks (oksg-VarA) and in the acute rickettsemia transmitted to calf PA420 (ok420-VarA). Five other variant types were also shared between some, but not all, A. marginale populations. These included variant types shared in the three samplings of the persistent infection in PA417 and also present in tick salivary glands (ok417-9-13VarC, ok417-9-20VarC, ok417-9-27VarA, and oksg-VarG), other variants shared between circulating bloodstream variants and ticks (ok417-9-20VarB and oksg-VarD; ok417-9-27VarG and oksg-VarH), and an identical circulating variant in calf PA417 and in the acute rickettsemia of calf PA420 (ok417-9-20VarA and ok420-VarD).
FIG. 7.
Circulating msp2 expression site variants in persistently infected cattle are those transmitted by ticks to naive cattle. Ten independent clones of the msp2 expression site were sampled from each of five different populations of A. marginale. These populations were derived from persistently infected calf PA417 just prior to tick feeding (ok417-9-13 variants), during tick feeding (ok417-9-20 variants), and just after tick feeding (ok417-9-27 variants) and also from salivary glands of D. variabilis that acquired infection from calf PA417 (oksg variants) and from the acute bloodstream rickettsemia that was transmitted by those ticks to calf PA420 (ok420 variants). The 50 clones were sequenced over the hypervariable region, translated to amino acids, and compared using PILEUP as explained in Fig. 5. The figure is the dendrogram output from PILEUP that shows the clustering of similar and identical sequences in the different A. marginale populations. Brackets to the right identify identical variants in different A. marginale populations; e.g., variant type ok417-9-13VarA was found in 50% (5 of 10) of the expression site clones sampled from calf PA417 prior to tick feeding. Identical variant types were found in other samplings of calf PA417 during and after tick feeding, in salivary glands of ticks that acquired infection from calf PA417, and in the acute rickettsemia that was transmitted to calf PA420. The asterisk indicates that three of ten expression site clones from tick salivary gland stages of A. marginale had changes that would lead to synthesis of truncated MSP2. In two of ten (oksg-VarB) clones the change was the same base substitution that introduced a termination codon into the hypervariable region; in oksg-VarC it was a single base deletion that changed the reading frame leading to termination shortly after the deletion. We cannot exclude the possibility that these changes are due to PCR and/or cloning errors; it is also possible that one result of recombination mechanisms can be variant types with truncated MSP2.
It is necessary to evaluate the artifactual contribution to sequence diversity in the above data that could result from PCR and sequencing errors. Previously, it was demonstrated that major and minor msp2 hypervariable region sequences observed in genomic clones of the polycistronic expression site corresponded to those found in msp2 mRNA in the same sample (4). However, some sequence changes could result from PCR-derived mutations. To assess this possibility we analyzed msp2 sequence in the conserved region of the polycistronic expression site upstream of the hypervariable region. In 15 independent clones of the expression site from different A. marginale populations, comparing 239 bp per clone of upstream sequence, there were base changes at four positions. At two of these positions there was an identical base substitution in 7 of 15 clones; therefore, this change probably represents a true sequence polymorphism. At the other two positions there was a base substitution unique to 1 of 15 clones; therefore, these may represent artifactual changes. This gives a potential error rate of 2 of 3,585 bp or potentially a 1-bp change for every four or five hypervariable region sequences. This error rate cannot explain the extensive base substitutions, insertions, and deletions observed in the hypervariable region of the msp2 expression site in the different rickettsial populations (Fig. 5).
Similar variants are present in salivary glands of different tick species acquisition fed on the same bloodstream rickettsemia.
D. andersoni and D. variabilis ticks were allowed to acquire A. marginale by feeding at the same time on an acute rickettsemia in calf PA411. The sequence of the msp2 hypervariable region was determined in seven independent clones of the polycistronic msp2 expression site from salivary gland DNA isolated from both D. andersoni and D. variabilis. Of the seven expression site clones from D. andersoni, five encoded the same MSP2 hypervariable region sequence and this was identical to a sequence found in two expression site clones from D. variabilis DNA. A second variant sequence was present in three expression site clones from D. variabilis DNA and also in one clone from D. andersoni DNA. Two other variant types were unique to D. variabilis, and one was unique to D. andersoni. These data do not support any substantial differences in elaboration of msp2 expression site variants in these different tick species.
DISCUSSION
The sequence data reveal conservation in overall structure of a polycistronic expression site for the msp2 gene in different strains and life cycle stages of A. marginale. In infections of both tick cells and mammalian erythrocytes the expression site contains three genes 5′ to msp2. DNA containing these three genes and msp2 is transcribed into a polycistronic RNA in tick salivary gland and erythrocyte stages and in all strains of A. marginale examined. msp2 and the three upstream genes are predicted to encode outer membrane proteins. Unlike msp2, multiple hybridizing copies of the three upstream genes are not found in A. marginale genomic DNA. The sequence of the three upstream genes and 5′ and 3′ flanking regions are conserved between different strains and stages, with the exception of some amino acid substitutions between strains, particularly in the polypeptide encoded by orf4. Greater polymorphism is found within the msp2 coding region itself, both between strains and between different stages in the life cycle of a single strain. This polymorphism is largely confined to a central hypervariable region of the msp2 gene in the polycistronic site encoding about 100 amino acids. Many variant forms of this hypervariable region exist in single populations of A. marginale, whether derived from culture, infected tick salivary glands, or infected bovine erythrocytes. The variant forms differ from one another by multiple insertions, deletions, and substitutions. Analysis of variant forms present in populations of A. marginale derived by cyclical transmission between culture, cattle, and ticks reveals most diversity in this expression site during persistent infections in the bovine host.
The above data and other published data on variation of MSP2 epitopes during persistent infection (14, 15) are consistent with the following hypothetical model for antigenic variation of A. marginale. The complete and incomplete genes encoding MSP2 (4, 23) may be silent until recombined into the polycistronic expression site containing the three upstream genes and promoter region. The recombination events introduce gene segments encoding the MSP2 hypervariable region into the expression site, probably via gene conversion employing flanking conserved sequences. This generates complex mosaics of sequence in the expression site which encode epitopes that are exposed on the surface of A. marginale. These epitopes are targeted by T and B cells (8) which results in the elimination of some variant types, the selection of other variants, and the sequential peaks of rickettsemia that are observed in persistent cattle infections (19). Immune selection based on MSP2 does not operate in ticks or in naive cattle prior to the first peak of acute rickettsemia. If there is a constant rate of msp2 recombination affecting a minority of the A. marginale population at any time, one may not detect substantial changes in MSP2 variants until there is immune selection.
Features of this model have similarities to antigenic variation in other organisms. In African trypanosomes, gene conversion of a polycistronic expression site by pseudogenes encoding a surface glycoprotein generates new antigenic variants in chronic infections (3, 26). In the family Picomaviridae, a virus population may consist of a swarm of slightly different individual genomes, and distinct repertoires of antigenic variants are observed in the presence and absence of immune selection (7). Similar molecular evasion mechanisms may have evolved in different organisms to allow persistent infections and onward transmission.
It has been proposed that, no matter which bloodstream variants of MSP2 are ingested by the tick, there is reversion to expression of a small number of specific “tick stage” sequence variants of MSP2 on transmission (27). These tick stage variants were the major variants present in tick salivary glands and in the first rickettsemia peak of cattle infected with a South Idaho strain of A. marginale (27). We did not obtain evidence for reversion in the present study. In contrast, our data are most consistent with the presence of a large number of different variants in persistent infections and transmission of the circulating bloodstream variants through ticks to naive cattle. That more shared variants were not observed in both PA417 and PA420 erythrocyte stage infections as well as in infected tick salivary glands is probably due to the sample size, i.e., the sequencing of only 10 independent clones of the msp2 expression site from each population. Possible explanations for differences from the results of Rurangirwa et al. (27) are the use of splenectomized calves in the present study, expression of tick stage MSP2 variants from a different expression site, or strain differences in msp2 expression. The infection of splenectomized calves with the Oklahoma strain results in microscopically visible relapsing peaks of rickettsemia which are easily monitored as a source of organisms and DNA. Although the type and extent of MSP2 expression site diversity between different bloodstream populations was similar in this study to that observed previously with spleen-intact calves (4), it is possible that the diversity of bloodstream variants observed can be influenced by splenectomy. Arguing against a different locus for MSP2 expression in the tick are, firstly, RT-PCR data showing that a polycistronic RNA encoding MSP2 is transcribed from the same genomic locus in A. marginale from tick salivary glands as erythrocyte stages. Secondly, the tick stage variants SGV1 and SGV2, identified in South Idaho strain A. marginale (27), utilized the same polycistronic expression site as described in this study (4). This suggests a similar mechanism of expression of tick stage SGV1 and SGV2 variants to other bloodstream variants. In contrast to their first results, substantial diversity in tick stage antigenic types was found by Rurangirwa et al. using two other strains of A. marginale (28). They suggested that there may be strain-specific selection for certain MSP2 variants in ticks. It is possible that we did not find restriction of MSP2 variants because the progenitor population of A. marginale (okcul) was already selected by growth in tick cell culture.
It is unlikely that a vaccine could be developed based on MSP2 variants present in tick salivary glands and conserved in the first rickettsemia peak as was initially proposed (27). Our analysis of salivary gland stages and acute bloodstream rickettsemias within a single strain of A. marginale identified numerous sequence variants in this polycistronic expression site. There is some basis for development of “region-specific” vaccines, as variants of one strain tended to group together in multiple alignment profiles (data not shown). This perhaps relates to observations made with different anaplasmosis vaccines that have been tested against field challenge, with less protection generally afforded to animals by immunization with geographically heterologous strains of A. marginale (22). Any optimism in this regard must be counterbalanced by consideration of the >20 msp2 expression site variants found (Fig. 5) in a few transmissions with one strain. A greater possibility for vaccine development may be to identify exposed T- and B-cell epitopes on other outer membrane proteins. Those epitopes encoded by the more conserved orf234 represent potential vaccine targets.
In conclusion, analysis of a polycistronic msp2 expression site in A. marginale from culture, tick salivary glands, and acutely or persistently infected cattle reveals sequence conservation between these stages of the Oklahoma strain throughout most of this expression site, including 5′ and 3′ flanking regions. The exception is in the expression site region encoding the central hypervariable region of MSP2. This region of the expression site is very polymorphic within individual populations of A. marginale. Although polymorphic, the major sequence variants present did not change on passage of A. marginale between culture, acute-stage erythrocyte infections, and tick salivary glands but did change during persistent infections of the bovine host. The sequence variants found in tick salivary glands most closely resembled those present in the blood at the time of acquisition of infection, whether infection was acquired from an animal with an acute or a persistent rickettsemia. These variations in structure of an expression site for a major, immunoprotective outer membrane protein have important implications for vaccine development against A. marginale and related ehrlichial pathogens. The data on msp2 variation suggest an unusual flexibility in the small 1.2-Mb genome (1) that may be employed in adaptation to and persistence in different host environments.
ACKNOWLEDGMENTS
This investigation was supported by USDA grant 9802528, National Institutes of Health grant AI45580, project 1669 of the Oklahoma Agriculture Experiment Station, and the endowed chair in Food Animal Research, College of Veterinary Medicine (K. M. Kocan).
We thank J. D. De La Fuente for helpful discussions.
REFERENCES
- 1.Alleman A R, Kamper S M, Viseshakul N, Barbet A F. Analysis of the Anaplasma marginale genome by pulsed-field electrophoresis. J Gen Microbiol. 1993;139:2439–2444. doi: 10.1099/00221287-139-10-2439. [DOI] [PubMed] [Google Scholar]
- 2.Andrew H R, Norval R A. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet Parasitol. 1989;34:261–266. doi: 10.1016/0304-4017(89)90056-3. [DOI] [PubMed] [Google Scholar]
- 3.Barbet A F, Kamper S M. The importance of mosaic genes to trypanosome survival. Parasitol Today. 1993;9:63–66. doi: 10.1016/0169-4758(93)90039-i. [DOI] [PubMed] [Google Scholar]
- 4.Barbet A F, Lundgren A, Yi J, Rurangirwa F R, Palmer G H. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect Immun. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet A F, Palmer G H, Myler P J, McGuire T C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987;55:2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blouin E F, Barbet A F, Yi J, Kocan K M, Saliki J T. Establishment and characterization of an Oklahoma isolate of Anaplasma marginale in cultured Ixodes scapularis cells. Vet Parasitol. 2000;87:301–313. doi: 10.1016/s0304-4017(99)00183-1. [DOI] [PubMed] [Google Scholar]
- 7.Borrego B, Novella I S, Giralt E, Andreu D, Domingo E. Distinct repertoire of antigenic variants of foot-and-mouth disease virus in the presence or absence of immune selection. J Virol. 1993;67:6071–6079. doi: 10.1128/jvi.67.10.6071-6079.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown W C, Zhu D, Shkap V, McGuire T C, Blouin E F, Kocan K M, Palmer G H. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect Immun. 1998;66:5414–5422. doi: 10.1128/iai.66.11.5414-5422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 10.Dumler J S, Sutker W L, Walker D H. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 11.Egenvall A, Lilliehook I, Bjoersdorff A, Engvall E O, Karlstam E, Artursson K, Heldtander M, Gunnarsson A. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet Rec. 2000;146:186–190. doi: 10.1136/vr.146.7.186. [DOI] [PubMed] [Google Scholar]
- 12.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. . (Erratum 66:2400.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrus S, Waner T, Aizenberg I, Foley J E, Poland A M, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. 1998;36:73–76. doi: 10.1128/jcm.36.1.73-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal Z, Rikihisa Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J Clin Microbiol. 1994;32:1644–1649. doi: 10.1128/jcm.32.7.1644-1649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabat E A, Wu T T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann N Y Acad Sci. 1971;190:382–393. doi: 10.1111/j.1749-6632.1971.tb13550.x. [DOI] [PubMed] [Google Scholar]
- 19.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCallon B R. Prevalence and economic aspects of anaplasmosis. In: Jones E W, editor. Proceedings of the 6th National Anaplasmosis Conference. Stillwater, Okla: Heritage Press; 1973. pp. 1–3. [Google Scholar]
- 21.Munderloh U G, Blouin E F, Kocan K M, Ge N L, Edwards W L, Kurtti T J. Establishment of the tick (Acari:Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales:Anaplasmataceae) in tick cell culture. J Med Entomol. 1996;33:656–664. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- 22.Palmer G H. Anaplasma vaccines. In: Wright I G, editor. Veterinary protozoan and hemoparasite vaccines. Boca Raton, Fla: CRC Press; 1989. pp. 1–29. [Google Scholar]
- 23.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer G H, Oberle S M, Barbet A F, Goff W L, Davis W C, McGuire T C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 26.Roth C, Bringaud F, Layden R, Baltz T, Eisen H. Active late-appearing variable surface antigen genes in Trypanosoma equiperdum are constructed entirely from pseudogenes. Proc Natl Acad Sci. 1989;86:9375–9379. doi: 10.1073/pnas.86.23.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rurangirwa F R, Stiller D, Palmer G H. Strain diversity in major surface protein 2 expression during tick transmission of Anaplasma marginale. Infect Immun. 2000;68:3023–3027. doi: 10.1128/iai.68.5.3023-3027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telford S R, Dawson J E. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet Microbiol. 1996;52:103–112. doi: 10.1016/0378-1135(96)00064-8. [DOI] [PubMed] [Google Scholar]