Abstract
This review examined 3655 articles on benzalkonium chloride (BKC), benzethonium chloride (BZT) and chloroxylenol (CHO) aiming to understand their impact on antimicrobial resistance. Following the application of inclusion/exclusion criteria, only 230 articles were retained for analysis; 212 concerned BKC, with only 18 for CHO and BZT. Seventy‐eight percent of studies used MIC to measure BKC efficacy. Very few studies defined the term ‘resistance’ and 85% of studies defined ‘resistance’ as <10‐fold increase (40% as low as 2‐fold) in MIC. Only a few in vitro studies reported on formulated products and when they did, products performed better. In vitro studies looking at the impact of BKC exposure on bacterial resistance used either a stepwise training protocol or exposure to constant BKC concentrations. In these, BKC exposure resulted in elevated MIC or/and MBC, often associated with efflux, and at time, a change in antibiotic susceptibility profile. The clinical relevance of these findings was, however, neither reported nor addressed. Of note, several studies reported that bacterial strains with an elevated MIC or MBC remained susceptible to the in‐use BKC concentration. BKC exposure was shown to reduce bacterial diversity in complex microbial microcosms, although the clinical significance of such a change has not been established. The impact of BKC exposure on the dissemination of resistant genes (notably efflux) remains speculative, although it manifests that clinical, veterinary and food isolates with elevated BKC MIC carried multiple efflux pump genes. The correlation between BKC usage and gene carriage, maintenance and dissemination has also not been established. The lack of clinical interpretation and significance in these studies does not allow to establish with certainty the role of BKC on AMR in practice. The limited literature and BZT and CHO do not allow to conclude that these will impact negatively on emerging bacterial resistance in practice.
Keywords: benzalkonium chloride, benzethonium chloride, chloroxylenol, cross‐resistance, resistance
INTRODUCTION
There are many terms used in the literature to describe biocide ‘resistance’, including tolerance, decreased susceptibility, reduced susceptibility, insusceptibility, multidrug resistance, intrinsic or innate resistance, acquired resistance, acquired reduced susceptibility, co‐resistance and cross‐resistance (Gerba & Müller, 2015; Maillard et al., 2013; Meade et al., 2021). Some of these terms clearly reflect the lack of consensus within the scientific community. It also adds to the difficulty in comparing studies where the term ‘resistance’ is loosely used. In this review, the use of hyphenated ‘resistance’ reflects the terminology used in the source article, whilst resistance refers to bacterial survival in a biocidal product or bacterial survival at an in‐use concentration of a biocide in a product. Decreased susceptibility refers to an upward change in MIC or MBC of a biocide. Tolerance refers only to bacterial growth in the presence of a low (MIC range) concentration of a biocide (Gerba & Müller, 2015).
Bacterial decreased susceptibility and resistance to biocides has been reported since the 1950s, notably with quaternary ammonium compounds (Maillard, 2018; Maillard et al., 2013). To date, bacterial decreased susceptibility and resistance has been described with all biocides, including highly reactive ones such as alkylating and oxidizing agents (Maillard, 2018; Maillard et al., 2013). Biocides have general multiple and non‐specific target sites against bacteria, which suggests that bacterial resistance might be difficult to emerge when a biocide is used at a high concentration, generally above the minimum bactericidal concentration; this holds mostly true (Gerba & Müller, 2015; Maillard, 2007; Maillard, 2018; Meade et al., 2021).
The clinical or industrial implications of bacteria with a reduced biocide susceptibility are unclear at present. Bacteria surviving in a biocidal product have led to documented bacterial infections in patients, although misuse of the product or the presence of intrinsically resistant bacterial spores was the reason for the presence of bacterial pathogens (Weber et al., 2007). Bacterial decreased susceptibility to cationic agents such as biguanides and quaternary ammonium compounds, and phenolics such as triclosan has been widely reported in vitro and is often perceived to present a higher risk for the development of bacterial resistance to antimicrobials compared to highly reactive biocides such as oxidizing‐ and alkylating‐based biocides. This perceived increased risk is related to product mechanism of action, product usage or persistence in the environment (http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf, accessed 16/11/21). Evidence of decreased susceptibility to biocides mainly comes from in vitro investigations and is often defined as an increase in the minimum inhibitory concentrations (MIC), regardless of the in‐use concentration of the biocide, the composition of the formulation or its application (Maillard, 2018; Maillard et al., 2013).
Since 2016, the US Food and Drug Administration (US‐FDA) issued several rules to determine the safety and effectiveness of consumer antibacterial‐based products, particularly those containing benzalkonium chloride (BKC), benzethonium chloride (BZT) and chloroxylenol (CHO) (Safety and Effectiveness of Consumer Antiseptics: Topical Antimicrobial Drug Products for Over‐the‐Counter Human Use, Safety and Effectiveness of Health Care Antiseptics: Topical Antimicrobial Drug Products for Over‐the‐Counter Human Use and Safety and Effectiveness of Consumer Antiseptic Rubs: Topical Antimicrobial Drug Products for Over‐the‐Counter Human Use). These rules are based partly on the concern that long‐term exposure to certain active ingredients, such as triclosan, may be associated with bacterial resistance and thus pose a health risk.
This review aims to provide an in‐depth analysis of the peer‐reviewed literature regarding the potential for the topical antimicrobial active ingredients benzalkonium chloride, benzethonium chloride and chloroxylenol to confer antimicrobial ‘resistance’ in bacteria. This review assessed, compiled and critically appraised the relevant literature available from scientific databases regarding the impact of bacterial exposure to these three biocides on antimicrobial ‘resistance’.
SELECTION OF RELEVANT LITERATURE
The databases searched were Scopus, PubMed, Web of Science and Google Scholar. In addition, a private database, comprising co‐prising of scientific articles, reviews and industrial reports (provided by the American Cleaning Institute) was used. A total of 3655 scientific articles that mentioned BKC, BZT and CHO were analysed. After duplicate outputs were removed and the exclusion/inclusion criteria applied, only 230 were retained for an in‐depth analysis. The literature was analysed up to 25 January 2022.
Exclusion and inclusion criteria
This review concerns vegetative bacteria of interest, particularly those mentioned in the US‐FDA Safety and Effectiveness of Consumer Antiseptic Rubs; Topical Antimicrobial Drug Products for Over‐the‐Counter Human Use (https://www.federalregister.gov/documents/2019/04/12/2019‐06791/safety‐and‐effectiveness‐of‐consumer‐antiseptic‐rubs‐topical‐antimicrobial‐drug‐products‐for; accessed 19/04/2022). Biocide efficacy against bacterial biofilm was not considered, but the impact of biocide exposure on biofilm formation and composition was. Bacterial (endo)spores are intrinsically resistant to BKC, BZT and CHO and were excluded. Only peer‐reviewed scientific articles written in English were considered and not peer‐reviewed review articles.
To focus the review on relevant literature effectively, initially, several keywords were used including commercial and chemical names for benzalkonium chloride, benzethonium chloride and chloroxylenol, but also ‘quaternary ammonium compounds’ or QAC, and phenolics. Then, exclusion criteria included lack of mentioning BKC, BZT and CHO, lack of detailed MIC/MBC (minimum bactericidal concentration) determination protocols or other protocols to determine bactericidal efficacy, lack of a detailed antibiotic susceptibility determination protocol, lack of mentioning ‘resistance’ and non‐relevant micro‐organisms such as fungi, protozoa and spores.
Typically keywords and exclusion criteria to retain eligible documents followed a screen from (i) abstract level and (ii) full‐text level. Each retained article was then assessed for its relevance to this review in terms of bacterial susceptibility or resistance to the three actives. Finally, a detailed analysis of the test protocol used (MIC/MBC determination, antibiotic susceptibility assay), expression of resistance genes, evidence of stress response, mechanisms of resistance, maintenance of resistance genes and virulence assay, was performed for all the selected articles.
‘Resistance’ and definitions
Overall, only a minority of studies defined the term ‘resistance’. The majority of studies used the term ‘resistance’ to describe an increase in MIC between 2‐ and 10‐folds (87%) and as low as 2‐fold (40 %). Some studies defined ‘resistance’ as an MIC for an isolate above the MIC for 90% or 99% of bacteria of the same species. In this review, the hyphenated term ‘resistance’ was used to reflect the terminology reported in these studies. Where appropriate the use of decreased susceptibility or tolerance were used as defined in the introduction. Very few studies investigated formulations or in‐use or during use concentrations reflecting product usage in practice (see below). The during use concentration reflects potential dilution of the product upon usage, whilst the in‐use concentration is the concentration to use recommended by the manufacturer (Wesgate et al., 2016). Where bacteria survived, the in use or during use concentration of a product the term resistance was used.
When chemotherapeutic antibiotics were concerned, the term ‘resistance’ means clinical resistance and refers to breakpoints given by antimicrobial test standards. Yet, 27% of studies did not use recognized antibiotic standard testing protocols, whilst the majority do not refer to associated clinical breakpoints (Figure 1), but instead defined ‘resistance’ as a decrease in susceptibility, recorded as zone of inhibition or MIC, with no clinical significance. Where clinical significance (or lack of) is mentioned, this referred to clinical breakpoint given for a specific antibiotic from an established organization, such as for example, the British Society for Antimicrobial Chemotherapy (Andrews, 2009), the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2014) or the International Standard Organisation (ISO, 2006).
FIGURE 1.
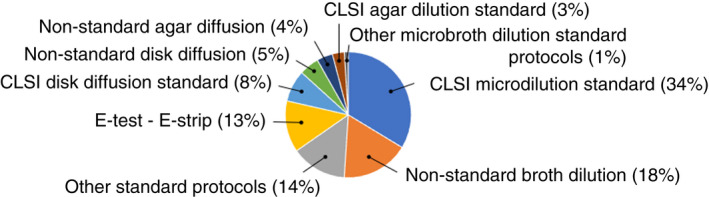
Protocols used for the determination of antibiotic susceptibility of bacterial isolates. (based on 98 scientific articles).
BENZALKONIUM CHLORIDE AND BACTERIAL ‘RESISTANCE’
Benzalkonium chloride (CAS: 8001‐54‐5) is a cationic biocide with a broad‐spectrum activity except for bacterial endospores (Leggett et al., 2016). Its bactericidal efficacy has been associated with an alteration of cytoplasmic membrane permeability following dissociation of cellular membrane lipid bilayers, resulting in leakage of cellular contents (Knauf et al., 2018).
Two hundred and twelve peer‐reviewed scientific papers investigating bacterial susceptibility, decreased susceptibility or ‘resistance’ to benzalkonium chloride (BKC) were retained and analysed (Table S1). The majority of the papers were classified as food‐related (31%), clinically related (29%) and laboratory‐related (25%) studies (Figure 2). The laboratory‐related studies concern the use of bacterial strains from culture collection, laboratory‐derived mutants or genetic constructs to investigate specific mechanisms of resistance, for the majority (80%), efflux pumps (Figure 3). The majority of articles investigated specific pathogens, such as staphylococci (mostly Staphylococcus aureus), Listeria monocytogenes, pseudomonads (mostly Pseudomonas aeruginosa), Escherichia coli, Salmonella enterica, Klebsiella pneumonia and Acinetobacter baumannii. A few studies concerned enterococci, Burkholderia spp., Stenotrophomonas spp., Serratia marcescens, Enterobacter spp. and Campylobacter spp. (Table S1).
FIGURE 2.
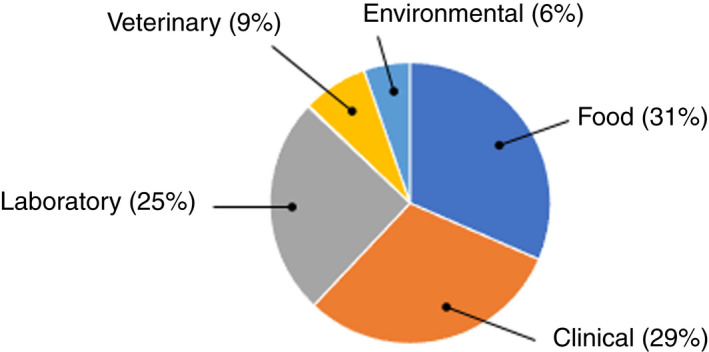
Main areas covered from peer‐reviewed scientific articles on BKC. Food includes all food pathogens; Clinical includes all human pathogens; Laboratory refers to the use of constructs regardless of the bacterium studied; Veterinary includes animal pathogens; Environmental including environmental and plant bacteria. (based on 212 scientific articles).
FIGURE 3.
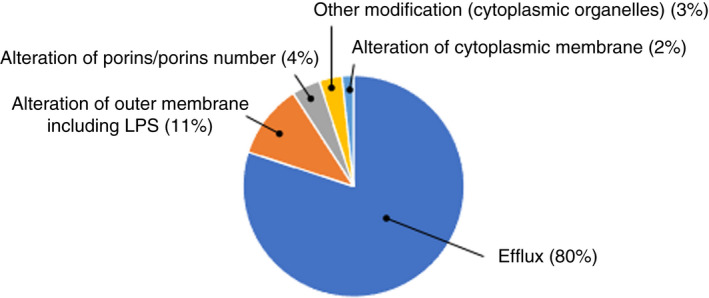
Mechanisms associated with a decrease in BKC susceptibility or/and a change in antibiotic susceptibility profile. Efflux includes direct or indirect measurements, using efflux inhibitors or/and fluorescent substrates, or gene expression. (based on 120 scientific articles).
Susceptibility determination protocols
Most studies measured a change in MIC or MBC based on standard efficacy protocols, particularly the Clinical and Laboratory Standards Institute (CLSI) microdilution broth protocol (Figure 4). For most studies (Table S1), ‘resistance’ is based on a change in MIC as low as 2‐fold increase (40%) and 10‐fold increase (85%) (Figure 5). A < 10‐fold increase in MIC observed could be considered as marginal (Maillard et al., 2013; Russell & McDonnell, 2000). A more substantial increase (>10‐fold) in MIC was only reported in six studies (Braoudaki & Hilton, 2005; Chaplin, 1951; Correa et al., 2008; Guo et al., 2015; Joynson et al., 2002; Loughlin et al., 2002). The CLSI microbroth dilution protocol was the most frequently used (34% of studies) to determine changes in antibiotic susceptibility profiles (Figure 1).
FIGURE 4.
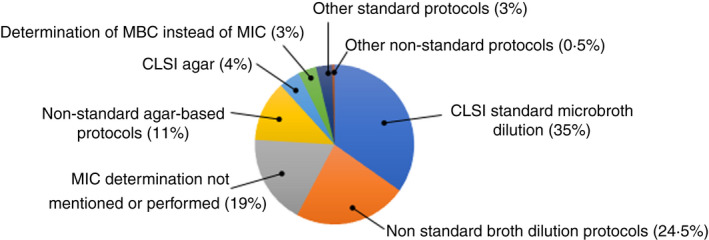
Protocols used for the determination of antimicrobial activity of BKC. (based on 212 scientific articles).
FIGURE 5.
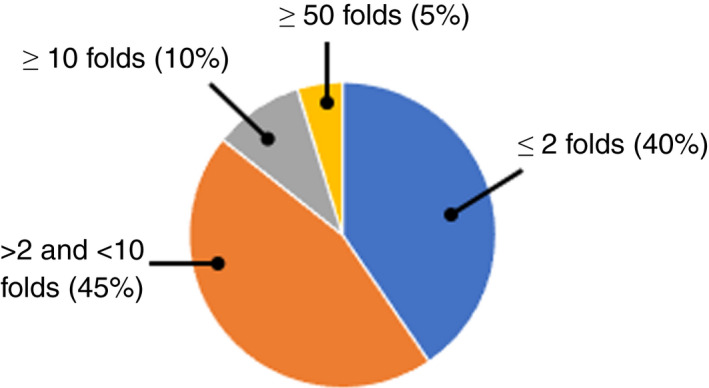
Percentage of studies assessing BKC ‘resistance’ based on increased folds in MIC. (based on 45 scientific articles).
Impact of bacterial exposure to BKC
Bacterial exposure to low (sub‐MIC) concentrations of BKC was investigated in several studies, the majority of which related to food and laboratory environments. These in vitro studies generally aimed to understand the mechanisms driving a reduction in antimicrobial susceptibility in adapted strains.
Thirty‐five peer‐reviewed articles (17% of retained BKC studies) investigated the impact of BKC exposure on increase in BKC MIC (Adair et al., 1975; Braoudaki & Hilton, 2004, 2005; Capita et al., 2019; Chaplin, 1951; Chuanchuen et al., 2008; Condell et al., 2012; Curiao et al., 2015; Dejoies et al., 2021; Fernández‐Cuenca et al., 2015; Guérin et al., 2021; Joynson et al., 2002; Kawamura‐Sato et al., 2008; Kim et al., 2018; Knapp et al., 2013, 2015; Knauf et al., 2017; Langsrud et al., 2004; Ligowska‐Marzeta et al., 2019; Loughlin et al., 2002; Marzoli et al., 2021; Mavri & Smole Možina, 2013; Nhung et al., 2015; Noll et al., 2020; Pagedar et al., 2012; Puangseree et al., 2021; Rakic‐Martinez et al., 2011; Romeu et al., 2020; Sánchez et al., 2015; Soumet et al., 2012; Tabata et al., 2003; Tandukar et al., 2013; To et al., 2002; Voumard et al., 2020; Zhang et al., 2012). Nineteen of these used an artificial stepwise training protocol that relies on increasing BKC concentrations in a stepwise fashion (Table 1).
TABLE 1.
Impact of BKC exposure/treatment on antimicrobial susceptibility in bacteria
| Bacteria | Exposure/treatment | MIC before exposure | MIC post exposure | Reference |
|---|---|---|---|---|
| Stepwise training | ||||
| P. aeruginosa ATCC 9027 | Subculture in increasing concentrations of BKC | ND | MIC: >200 mg l−1 | Adair et al. (1975) |
| E. coli O157; S enterica serovar enteritidis, typhimurium, virchow | Growth in increasing concentration ‐ starting sub‐MIC level | MIC: 400 mg l−1 S. enterica, virchow and enterica, <100 mg l−1 virchow | MIC: >1000 mg l−1 for E. coli | Braoudaki and Hilton (2004) |
| S enterica serovar enteritidis, typhimurium, virchow | Growth in increasing concentration ‐ starting sub‐MIC level | MIC for S. enterica serovar enteritidis 32 mg l−1, virchow 256 mg l−1 and typhimurium 64 mg l−1 | MIC for S. enterica serovar enteritidis 256 mg l−1; virchow 256 mg l−1 and typhimurium from 64 mg l−1 | Braoudaki and Hilton (2005) |
| Cronobacter sakazakii and enterocolitica | Subculture in increasing concentrations of BKC; starting sub‐MIC level |
MIC: 15 mg l−1 for C. sakazakii MIC: 20 mg l−1 for Y. enterocolitica |
MIC: 56.95 mg l−1 for C. sakazakii MIC: 50.63 mg l−1 for Y. enterocolitica |
Capita et al. (2019) |
| S. marcescens (Breed's no. 1377) | Subculture in increasing concentrations of BKC from highest concentration showing growth | MIC: 50–100 mg l−1 | MIC: 100,000 mg l−1 in three passages over a period of 15 days, and 50,000 mg l−1 in eight passages during 45 days | Chaplin (1951) |
| S. enterica isolates from poultry (n = 125) and swine (n = 132) | Exposure to sub‐inhibitory concentrations | MIC: 32–256 mg l−1 ‐ most at 64 mg l−1 | MIC: 128–256 mg l−1 | Chuanchuen et al. (2008) |
| 189 Salmonella strains, including 48 serotypes from various origins (such as clinical sources, food, the environment and water) | Growth in (0.25 or 0.5 MIC). Biocide formulation concentration increased in a stepwise manner until the biocide concentration reached 4X MIC | MIC:15 mg l−1 | MIC: 50 mg l−1 | Condell et al. (2014) |
| E. coli and K. pneumoniae mutants with different TRI susceptibility | Growth in sub‐inhibitory concentrations (0.5 MIC), followed by agar with concentration of 2.5 to 33 × MIC | MIC: 16 mg l−1 | MIC: 32 mg l−1 | Curiao et al. (2015) |
| P. aeruginosa NCIMB 10421 clinical isolates of P. aeruginosa | Subculture in increasing concentrations of BKC (2‐fold steps) | MIC: 50 mg l−1 | MIC: 580 mg l−1 after 25 subcultures | Joynson et al. (2002) |
| P. aeruginosa | Subculture in increasing concentrations of BKC until cessation of growth | MIC: 200 mg l−1 | Growth in BKC up to 1600 mg l−1 | Kim et al. (2018) |
| 16 P. aeruginosa from hospital environment | Subculture in increasing concentrations of BKC | MIC: 15–30 mg l−1 | MIC: >500 mg l−1 for clinical isolate; Reference standard culture strain PAO1: 500 mg l−1 | Loughlin et al. (2002) |
| Three C. jejuni (NCTC11168, ATCC33560 and the K49/4 poultry isolate) and two C. coli (ATCC33559, and the 137 poultry isolate) | Subculture in increasing concentrations of BKC | MIC: 0.5–2 mg l−1 | 1–4‐fold increase in MIC; ATCC33559 strain: 4‐fold MIC increase after 15 days of exposure | Mavri and Smole Možina (2000) |
| 6 antimicrobial‐susceptible E. coli and 6 antimicrobial‐susceptible non‐typhoidal Salmonella (NTS) isolates | Subculture in increasing concentrations of BKC: 0.5 MIC exposure over 12 days | MIC: 21–30 mg l−1 for Salmonella and 12–27 mg l−1 for E. coli | Post exposure MIC increase: <2‐fold to 2‐fold in one Salmonella and one E. coli | Nhung et al. (2015) |
| L. monocytogenes SLCC2540 | Subculture in increasing concentrations of BKC; starting concentration of 0.002 mg l−1; growth in increasing concentration of 0.001 mg l−1 every 48 h |
MICL: 0.004 mg l−1 MBC: 0.011 mg l−1 |
MIC: 0.008 mg l−1 MBC: unchanged |
Noll et al. (2020) |
| 81 E. coli from biofilm collected from raw milk line, pasteurizer inlet, outlet and, milk tank of a small scale and commercial scale dairy | Subculture in increasing concentrations of BKC; starting with sub‐MIC (well immediately below MIC step of 10 mg l−1) | MIC: 50–250 mg l−1 | MIC: 70–350 mg l−1; largest increased from 50 to 130 mg l−1 | Pagedar et al. (2012) |
| E. coli veterinary isolates | Subculture in increasing concentrations of BKC starting 1/4 MIC for 24 h | MIC range from 4 to 64 mg l−1 | Only 2/24 isolates showed increased MIC >4‐fold (6‐ and 9‐folds increased). All the others <2‐fold | Puangseree et al. (2021) |
| 9 avian and porcine E. coli strains and E. coli ATCC25922 | Subculture in increasing concentrations of BKC daily for 7 days | MIC: 16–32 mg l−1 | 2‐fold increase in MIC | Soumet et al. (2012) |
| P. aeruginosa ATCC 10145 and deletion mutants | Subculture in increasing concentrations of BKC | MIC: 18.2 mg l−1 | MIC: 51.2 mg l−1 | Tabata et al. (2003) |
| Two resistant (4 mg l−1) and four sensitive (1 mg l−1) L. monocytogenes strain | Subculture in increasing concentrations of BKC (1–10 mg l−1) | MIC: 1–4 mg l−1 | MIC increased to 5–6 mg l−1 for sensitive strains and to 8 mg l−1 for resistant ones | To et al. (2002) |
| GROWTH IN BKC (sub‐MIC level) | ||||
| E. faecium Aus0004 and S. aureus HG003 | Growth in sub‐inhibitory concentration; 1/2 MIC | E. faecium Aus0004 MIC: 4 mg l−1; S. aureus HG003 MIC: 2 mg l−1 | ND | Dejoies et al. (2021) |
| 49 clonally unrelated isolates of A. baumannii | Growth in BKC 0.25 × MIC (10 mg l−1) for 30 days ‐ one clinical isolate only | MIC: 1–15.6 mg l−1 ‐ majority at 3.9 and 7.8 mg l−1 | 2–16‐fold increase | Fernández‐Cuenca et al. (2015) |
| 205 L. monocytogenes food isolates | Exposure to 0.6 mg l−1 BKC in broth for 24 h at 37°C. | MIC range from 0.63 to 5 mg l−1 | MIC increased to 4 mg l−1 | Guérin et al. (2021) |
| 283 Acinetobacter spp. strains from 97 hospitals | Repeated exposure to 1/2 MIC for 72 h | MIC: mostly 5–10 mg l−1 | MIC: 10–50 mg l−1 | Kawamura‐Sato et al. (2018) |
| S. enterica serovar Typhimurium strains (SL1344 and 14028S) | Exposure to during use concentration ‐ 0.015 mg l−1 or 0.004 mg l−1 | Salmonella SL1344 MIC: 0.03 mg l−1 14028S MIC: 0.004 mg l−1; MBC 0.003 and 0.008 mg l−1 | Salmonella SL1344 MIC: 3 mg l−1 14028S MIC: 0.2 mg l−1; MBC 8 and 20 mg l−1 | Knapp et al. (2015) |
| Burkholderia lata strain 283 | Exposure to 50 mg l−1 BKC; 5 min or to mid‐log phase at 20°C | MIC: 0.5 mg l−1 | No change in MIC | Knapp et al. (2013) |
| A. baumannii standard culture collection strain and mutants | Exposure to 32 mg l−1 in agar for 24 h then growth in 16 mg l−1 | MIC: 16 mg l−1 | MIC: 32 mg l−1 | Knauf et al. (2017) |
| S. aureus laboratory constructs | Pre‐exposure test in 5 mg l−1 BKC for 60 min | MIC: 4 mg l−1 in parent strain; 1 mg l−1 in ΔnorA; 8 mg l−1 in norA construct; 16 mg l−1 in pCN38‐qacA qacR construct | ND (increased expression of norA and qacA) | LaBreck et al. (2020) |
| E. coli ATCC 11775 and E. coli DSM 682 | Sub‐MIC growth 5 mg l−1 overnight or 20 mg l−1 during exponential phase | MIC: 25 mg l−1 for 1175 and DSM682 | Exposure to 5 mg l−1: 1175; MIC 25 mg l−1; DSM682: MIC: 35 mg l−1; exposure to 20 mg l−1 1175; MIC 35 mg l−1; DSM682 MIC: 45 mg l−1 | Langsrud et al. (2004) |
| E. coli CFT073 | Exposure to 2 mg l−1 until sufficient increase in OD | MIC: 8 mg l−1 | ND | Ligowska‐Marzeta et al. (2019) |
| 24 coagulase‐negative Staphylococci isolates (12 isolates with decreased susceptibility to CHX and 12 isolates with decreased susceptibility to BKC) | Repeated exposures (3 passages) to 1/2 MIC (range 1–4 mg l‐1) BKC for 24 h at 37C | MIC range from 2 to 8 mg l−1 and MBC from 2 to 16 mg l−1 | 2‐fold increase in BKC MIC in 58% of CHX isolates and 83% of BKC isolates |
Marzoli et al. (2021) |
| L monocytogenes; food and veterinary isolates | Repeated exposure to 10 mg l−1 BKC | MIC: 10 mg l−1 | MIC: 30 mg l−1 | Rakic‐Martinez et al. (2011) |
| Salmonella Enteritidis NCTC13349 and one food isolate | Exposure to 1/2 MBEC (400 mg l−1 BKC) for 6 days every other day | MBEC: NCTC13349 800 mg l−1 MBEC isolate: 1600 mg l−1 | ND | Romeu et al. (2020) |
| S. maltophilia D457, and isogenic mutant D457R, overexpressing SmeDEF | Growth on agar containing 128 mg l−1 BKC | MIC: 128 mg l−1 | MIC: 256 mg l−1 | Sánchez et al. (2015) |
| Three aerobic communities from water effluent. BKCs unexposed (DP, fed a mixture of dextrin/peptone), BKCs exposed (DPB, fed a mixture of dextrin/peptone and BKCs) and BKCs enriched (B, fed only BKCs). BKC concentration 50 mg l−1 | Exposure of community to 50 mg l−1 for 4 years | ND | DPB MIC: 250 mg l−1, B MIC: 460 mg l−1 | Tandukar et al. (2013) |
| P. aeruginosa ATCC27853 | Repeated (10 cycles) exposure to BKC | MIC: 80 mg l−1 | MIC: Max 150 mg l−1 | Voumard et al. (2020) |
Note: term resistant/resistance as reported in the paper but does not correspond to the definition of resistance used in the main document.
Abbreviations: BKC, Benzalkonium chloride, ND, not determined; TRI, triclosan.
A high BKC MIC post stepwise training (100,000 mg l−1) was reported in S. marcescens in only one early study (Chaplin, 1951) and such results have not been reported since albeit with other bacteria. Other studies used single or continuous exposure to the same BKC concentration over different time periods and reported a more modest increase in BKC MIC (2–4‐fold increase) (Table 1). Only one study reported 100‐fold increase in MIC S. enterica serovar Typhimurium following exposure to during use concentration ‐ 0.015 or 0.004 mg l−1 (Knapp et al., 2015). Overall, the highest MIC (460 mg l−1) was reported in a complex microcosm (Tandukar et al., 2013).
Based on the retained literature on BKC, 54% (114/212) of the studies investigated the antibiotic susceptibility profile of isolates. Of those, 73% (83/114) used accepted standardized antibiotic protocols such as those recommended by CLSI, BSAC or the use of e‐test (Figure 1). Most of these studies investigated the antibiotic susceptibility profile of clinical and environmental isolates (Table S1). Bacteria adapted to increasing concentrations of BKC or following BKC single exposure were often found to have a reduced susceptibility to antibiotics in mostly Gram‐negative bacteria such as P. aeruginosa (Adair et al., 1975; Joynson et al., 2002; Kim et al., 2018; Loughlin et al., 2002; Morita et al., 2003; Pagedar et al., 2011; Voumard et al., 2020), E. coli (Curiao et al., 2015; Forbes et al., 2016; Langsrud et al., 2004; Nhung et al., 2015; Pagedar et al., 2012; Pereira et al., 2021; Puangseree et al., 2021), Burkholderia spp.(Geftic et al., 1979; Knapp et al., 2013, 2015), Salmonella spp. (Braoudaki & Hilton, 2004; Chuanchuen et al., 2008; Condell et al., 2012; Nhung et al., 2015), Klebsiella pneumoniae (Curiao et al., 2015), A. baumannii (Morrissey et al., 2004), L. monocytogenes (Guérin et al., 2021; Pasquali et al., 2018; Rakic‐Martinez et al., 2011), Campylobacter spp. (Mavri & Smole Možina, 2013), but also against staphylococci (Forbes et al., 2016; Houari & Di Martino, 2007; Marzoli et al., 2021), or against diverse isolates from food sources (Gadea et al., 2017) or water effluents (Tandukar et al., 2013). Only three studies reported a clinical change in antibiotic susceptibility (Knapp et al., 2013; Pereira et al., 2021; Puangseree et al., 2021). Out of 16 studies investigating the effect of BKC pre‐exposure on changes in antibiotic profile, only four investigated the persistence of this change (Guérin et al., 2021; Knapp et al., 2013; Mavri & Smole Možina, 2013; Voumard et al., 2020). Increase in antibiotic MIC (Mavri & Smole Možina, 2013), change in antibiotic susceptibility (Guérin et al., 2021) or clinical resistance (Knapp et al., 2013) was found to be unstable in the absence of BKC. The remaining studies did not investigate the stability of susceptibility profile change and did not consider the clinical significance of the observed changes (Table S1).
Capita et al. (2019) reported a change in ciprofloxacin clinical susceptibility from sensitive to intermediate in Cronobacter sakazakii. Other studies observed no change in antibiotic susceptibility profile (Braoudaki & Hilton, 2004; Furi et al., 2013; Knapp et al., 2015) or fitness cost (Curiao et al., 2015; Furi et al., 2013; Sánchez et al., 2015; Soumet et al., 2012) after BKC exposure. One recent study observed that E. coli mutants (with altered porins and efflux pumps) which evolved for 500 generations in BKC 4 mg l−1, showed higher fitness in competition assay with the wild strain (Pereira et al., 2021). Maertens et al. (2020) observed that E. coli isolates pre‐exposure to 6.75 mg l−1 BKC decreased the bactericidal activity of ciprofloxacin, presumably following a decrease in membrane potential inducing dormancy in some bacteria within the cell population. Such observation has not been confirmed in situ. Formation of viable but non‐culturable cells (VBNC) following BKC exposure (0.008 mg l−1 at 37°C for 24 h) has also recently been reported in L. monocytogenes. Following stepwise training, adapted bacteria showed a 2‐fold increase in BKC MIC and decreased antibiotic susceptibility to ceftriaxone, gentamicin, linezolid, tetracycline and trimethoprim/sulphamethoxazole. These observations likely resulted from an alteration in L. monocytogenes membrane permeability (Noll et al., 2020). Knapp et al. (2013) reported clinical susceptibility changes for ceftazidime, imipenem and ciprofloxacin in Burkholderia lata following exposure to BKC. These changes were however random and were only present in 50% of the experimental repeats. Adaptation of bacteria to BKC in terms of increased MIC has been found to be stable in S. enterica (Braoudaki & Hilton, 2004, 2005; Chuanchuen et al., 2008) and in P. aeruginosa (Voumard et al., 2020), but transient in S. aureus (Buzón‐Durán et al., 2017) and B. lata. (Knapp et al., 2013). A recent study based on P. aeruginosa pre‐exposure to BKC (40–70 mg l−1; corresponding to 50% or 88% MIC) showed no change in clinical susceptibility to antibiotics of the isolates despite a stable increase to BKC MIC to 150 mg l−1 (Voumard et al., 2020). Roedel et al. (2021) investigated ESBL/AmpC producing and no‐producing E. coli and reported that no veterinary isolates with reduced susceptibility to BKC were found on farms, and that reduced phenotypic susceptibility to BKC and antibiotic resistance were not linked. Nordholt et al. (2021) investigated the presence of phenotypically tolerant E. coli subpopulations to BKC exposure and reported on persisters with reduced cell surface charge and mutations in the lpxM locus, which is involved in lipid A biosynthesis. In addition, they observed that although these persisters had a fitness cost in the presence of BKC, these bacteria had a better growth rate in the presence of antibiotics. Of practical significance, the study by Knapp et al. (2015) that tested the impact of bacterial short exposure (1 min) to BKC‐containing products, reflecting the during use exposure of the product, demonstrated no change in antibiotic susceptibility profile.
Whilst to date, most studies are based on a pre‐exposure model, a few studies stand out by exploring the impact of co‐exposure of BKC with a chemotherapeutic antibiotic. Pietsch et al. (2021) investigated the impact of BKC co‐exposure with some antibiotics against P. aeruginosa and observed a decreased activity in meropenem (although not significant), but no changes in activity when BKC was used jointly with ciprofloxacin or gentamicin. Short et al. (2021) reported on co‐exposure between BKC (1–4 mg l−1) and aminoglycosides against A. baumannii and observed antagonism between BKC and aminoglycosides whereby gentamicin bactericidal activity was severely reduced. In addition, this study showed that BKC exposure increased the frequency of emerging A. baumannii mutants with reduced susceptibility to aminoglycosides following decreased intracellular accumulation of the antibiotics. Although of significance in this in vitro investigation, the authors stated that the likelihood of such co‐exposure in practice was likely to be rare (Short et al., 2021). However, an earlier study by Morita et al. (2003) did not show any difference in norfloxacin efficacy in P. aeruginosa when BKC was added at 20 mg l−1 (0.33 × MIC) to the growth media.
Bacterial stress response following BKC exposure
The expression of stress response as a result of BKC exposure has been mentioned in a few studies. Ceragioli et al. (2010) looked at gene expression in Bacillus cereus exposed to BKC (0.5 to 7.0 mg l−1) during mid‐exponential growth. A common response to BKC exposure included expression of genes involved in the general and oxidative stress responses. A specific response to BKC exposure included expression of genes involved in fatty acid metabolism. In Listeria monocytogenes, exposure to BKC 50 mg l−1 for 10 min resulted in elevated c‐di‐GMP levels and the synthesis of an exopolysaccharide that promoted cell aggregation, inhibited motility in semi‐solid media and desiccation (Chen et al., 2014). Food isolates of L. monocytogenes exposed to 10 mg l−1 BKC (although exposure was not explicit in the paper) resulted in enrichment of bacterial genomes with genes involved in alkaline and oxidative stress (Pasquali et al., 2018). Peyrat et al. (2008) demonstrated that deletion of sigB, a gene involved in bacterial stress response, affected the ability of L. monocytogenes to survive lethal concentration (40 mg l−1) of BKC, although the deletion of sigB did not affect BKC MIC. Similarly, van der Veen and Abee (2010) showed sigB deletion decreased the viability planktonic and sessile L. monocytogenes exposed to BKC (20 mg l−1 for 15 min). Conversely, overexpression of sigB in L. monocytogenes was associated with a decreased susceptibility to BKC (10 mg l−1 for 5 min) (Tamburro et al., 2015). Investigating bacterial cell injuries caused by food processing method, Siderakou et al. (2021) showed that 6 h incubation in BKC at 100 mg l−1 at 4°C or 20°C did not result in the formation of injured L. monocytogenes subpopulations, but significantly affected their survival. The exposure of A. baumannii to 6 mg l−1 BKC resulted in the expression of 227 genes involved in bacterial fitness and 335 genes involved with cell envelope maintenance, drug efflux, proteostasis and oxidative stress defence (Knauf et al., 2017). In Klebsiella pneumoniae, the Cpx envelope stress response system was shown to play a role in response to BKC exposure (Srinivasan et al., 2012). Exposure of growing (mid‐log growth phase) Desulfovibrio vulgaris Hildenborough to BKC (1.25 mg l−1) significantly changed gene expression (103 upregulated and 95 downregulated), mostly genes involved with cell envelope biogenesis, outer membrane, metabolism, energy production and conversion, translation, ribosomal structure and biogenesis, although the clinical significance of such finding was unclear (Lee et al., 2010). Merchel Piovesan Pereira et al. (2020) reported the overexpression of several chaperones and cochaperonins, such as dnaK, dnaJ, groL, groS, htpG, hscA, cpxP and clpB in E. coli following exposure to BKC 3.63 mg l−1 for 30 min.
Role of efflux in decreased susceptibility to BKC
The presence of efflux pump genes or expression of efflux has been implicated as a mechanism responsible for a decrease in MIC, regardless of bacterial genera, in 80% of the retained studies (Figure 3). Of those, 42% (50/120) mentioned Qac‐based efflux pumps. Carriage of efflux pump genes was observed to be widespread in Gram‐positive bacteria including staphylococci (Kaatz and Seo, 2004 ; Kaatz et al., 2005; Bjorland et al., 2005; Correa et al., 2008; Couto et al., 2008, 2013, 2015; DeMarco et al., 2007; Falcão‐Silva et al., 2009; Costa et al., 2010, 2013; Furi et al., 2013; Heir et al., 1999a, 1999b; Ho & Branley, 2012; Wong et al. 2013; Ignak et al., 2017; Lee et al., 2020; Marzoli et al., 2021; Morrissey et al., 2004; Shamsudin et al., 2012; Sidhu et al., 2002; Slifierz et al., 2015; Worthing et al., 2018; Zhang et al., 2012; Zmantar et al., 2011; Kroning et al., 2020 ), Lactococcus spp. (Fernández Márquez et al., 2014), Enterococcus spp. (Fernández Márquez et al., 2014; Alotaibi et al. 2017; Ignak et al., 2017; Morrissey et al., 2004), Lactobacillus spp. (Fernández Márquez et al., 2014) and Bacillus spp. (Fernández Márquez et al., 2014), and Gram‐negative bacteria such as K. pneumoniae (Morrissey et al., 2004; Abuzaid etal., 2012; Wand et al., 2015; Wang et al., 2008), E. coli (Paulsen et al., 1993; Hansen et al., 2007; Kumar and Doerrler, 2014; Deus et al., 2017; Fernández Márquez et al., 2014; Guo et al., 2015; Bay et al., 2017; Jiang et al., 2017; Puangseree et al., 2021; Wang et al., 2008), P. aeruginosa (Stoitsova et al., 2008; Amsalu et al., 2020; Gholamrezazadeh et al., 2018; Goodarzi et al., 2021; Namaki et al., 2022; Romão et al., 2011), Enterobacter spp. (Boutarfi et al., 2019; Fernández Márquez et al., 2014; Morrissey et al., 2004; Wang et al., 2008), Helicobacter spp. (Fernández Márquez et al., 2014), P. mirabilis (Jiang et al., 2017), L. monocytogenes (Soumet et al., 2005; Romanova et al., 2006; Müller et al., 2013, 2014; Bland et al., 2021; Cherifi et al., 2020; Chmielowska et al., 2021; Cooper et al., 2021; Dutta et al., 2013; Gray et al., 2021; Kremer et al., 2017; Meier et al., 2017; Mereghetti et al., 2000; Møretrø et al., 2017; Roedel et al., 2019; Tamburro et al., 2015), Citrobacter freundii (Kücken et al., 2000), Stenotrophomonas maltophilia (Kücken et al., 2000; Wang et al., 2008), A. baumannii (Srinivasan et al., 2011; Khosravi et al., 2021; Liu et al., 2017; Rajamohan et al., 2010a, 2010b; Wang et al., 2008), Salmonella spp. (Heir et al., 1998; Morrissey et al., 2004; Aksoy et al., 2019), Yesinia enterocolitica (Bengoechea and Skurnik, 2000), Legionella pneumophila (Ferhat et al., 2009) and Flavobacterium spp. (Wang et al., 2008). However, the role of BKC in the dissemination of these genes was not reported, or when mentioned, speculative. Morrissey et al. (2004) investigated the association of an elevated BKC MIC with the presence of qac genes qacI, qacE and qacK in 3319 clinical isolates of various bacterial genera; no association was found, although the definition of an elevated MIC (ECOFF's value) was arbitrary. Other studies have looked at bacterial construct to study the impact of a particular efflux pump, resulting at time in a decreased bacterial susceptibility to BKC (Braga et al., 2011; Chittrakanwong et al., 2021; Guérin et al., 2016; Huang et al., 2004; Kaatz & Seo, 1995; LaBreck et al., 2020; Li et al., 2008; Maseda et al., 2009; Matsuo et al., 2014; Nishino et al., 2006; Nordholt et al., 2021; Paulsen et al., 1995; Pereira et al., 2021; Rajamohan et al., 2010a; Srinivasan et al., 2009, 2014).
Decrease in antibiotic susceptibility has been associated with efflux in Gram‐positive bacteria, for example, staphylococci (Bjorland et al., 2005; Correa et al., 2008; Marzoli et al., 2021; Zhang et al., 2011; Zmantar et al., 2011), Lactococcus spp. (Fernández Márquez et al., 2014) and Lactobacillus spp. (Fernández Márquez et al., 2014), and Gram‐negative bacteria such as E. coli (Fernández Márquez et al., 2014; Pereira et al., 2021; Puangseree et al., 2021), Enterobacter spp. (Boutarfi et al., 2019), P. aeruginosa (Amsalu et al., 2020; Namaki et al., 2022; Romão et al., 2011), Helicobacter spp. (Fernández Márquez et al., 2014), Campylobacter spp. (Mavri & Smole Možina, 2013), A. baumannii (Khosravi et al., 2021; Srinivasan et al., 2009) and L. monocytogenes (Gray et al., 2021; Kremer et al., 2017). In six studies, both a change in efflux and membrane properties were associated with decreased susceptibility to BKC (Bischofberger et al., 2020; Fernández‐Cuenca et al., 2015; Langsrud et al., 2004; Mavri & Smole Možina, 2013; Nagai et al., 2003; Pereira et al., 2021). A few investigations looked at ad litteram ‘inducible resistance’ (as defined in this review inducible reduced susceptibility) when bacteria were exposed to BKC. Beier et al. (2014) showed that BKC was associated with low induced resistance (compared to other biocides) in P. aeruginosa veterinary isolates at rates of 10–13%; here, inducible resistance was defined as growth of the organism above the MIC. Morita et al. (2003) showed indirectly the induction of efflux in P. aeruginosa in the presence of BKC (6 mg l−1); P. aeruginosa was able to grow in the presence of both BKC (6 mg l−1) and norfloxacin (1 mg l−1), whilst growth in the presence of norfloxacin (1 mg l−1) alone was inhibited.
With S. aureus harbouring a luciferase QacR (efflux pump gene repressor) construct, Galluzzi et al. (2003) showed that BKC (1 mg l−1) exposure repressed the expression of QaCR. A follow‐up study showed that BKC (12 ng ml−1) induces the light emission of the luciferase QacR construct by two‐fold, demonstrating the low threshold impact of BKC‐induced response (Galluzzi et al., 2004). Likewise, Grkovic et al. (2003) showed that BKC (1 mg l−1) was a moderate inducer of QacA.
Braga et al. (2011) observed that efflux gene expression (qacZ) in Enterococcus faecalis was not inducible by BKC, although qacZ expression was associated with a decreased susceptibility in E. faecalis. Merchel Piovesan Merchel Piovesan Pereira et al. (2020) showed that long exposure (8–12 h) to BKC 3.63 mg l−1 led to the expression of a biofilm phenotype in E. coli. In Stenotrophomonas maltophilia, overexpression of smeDEF (efflux pump) was associated with a 2‐fold decrease in BKC MIC (from 128 to 256 mg l−1) and a reduced susceptibility to quinolones and chloramphenicol (Sánchez et al., 2015). Bioinformatics predicted BKC should induce smeDEF expression leading to transient S. maltophilia resistance to antibiotics since BKC binds to SmeT, the repressor of smeDEF. However, phenotypic assays showed that this was not the case (Sánchez et al., 2015). Chittrakanwong et al. (2021) observed that the addition of BKC (2–8 mg l−1) during the exponential growth phase of S. maltophilia resulted in derepressing MfsR activity, a QAC‐sensing regulator controlling the expression of the major facilitator superfamily efflux transporter mfsQ, enabling bacterial growth in 75 mg l−1 BKC.
Other mechanisms involved in decreased BKC susceptibility
Other studies have associated a decrease in BKC susceptibility with a change in bacterial cytoplasmic membrane composition (Bisbiroulas et al., 2011; Bischofberger et al., 2020; Ceragioli et al., 2010; Guérin‐Méchin et al., 2004; Jennings et al., 2017; Maertens et al., 2020; Noll et al., 2020; Voumard et al., 2020), outer membrane protein (Ishikawa et al., 2002; Pereira et al., 2021; Srinivasan et al., 2012; Tabata et al., 2003) or outer membrane (Chen et al., 2014; Ishikawa et al., 2002; Joynson et al., 2002; Loughlin et al., 2002; Manniello et al., 1978; Nordholt et al., 2021). One study attributed a decrease in BKC susceptibility associated with an increase in MIC to some antibiotics to a combination of mutations and efflux (Kim et al., 2018). Another study on Acinetobacter baumannii suggested that decreased susceptibility to BKC is associated with ribosomal protein mutations that protect A. baumannii against BKC‐induced protein aggregation (Knauf et al., 2017).
BKC exposure and impact on biofilm
Only a few studies investigated the effect of BKC exposure at sub‐MIC concentration on biofilm formation and evidence from different studies is at time contradictory. BKC was shown to induce biofilm formation in Staphylococcus epidermidis (Houari & Di Martino, 2007), K. pneumoniae (Elekhnawy et al., 2021) and L. monocytogenes (Bonneville et al., 2020; Piercey et al., 2017), or to increase biofilm biomass in E. coli (Machado et al., 2012; Yu et al., 2021), P. aeruginosa (Machado et al., 2012) and A. baumannii (Rajamohan et al., 2009). Others have however shown that BKC exposure decreases bacterial ability to form biofilms in S. aureus (Buzón‐Durán et al., 2017) and in L. monocytogenes (Ortiz et al., 2014a). BKC pre‐exposure also decreased susceptibility of Salmonella enterica (Mangalappalli‐Illathu & Korber, 2006) and L. monocytogenes (Ortiz et al., 2014a) biofilms to BKC. Different concentrations of BKC may impact differently on biofilm formation. Chaieb et al. (2011) observed that BKC (1 mg l−1) treatment induced biofilm formation in Staphylococcus epidermidis, whereas higher concentrations (2, 3, 4 and 5 mg l−1) decreased biofilm formation altogether. Pang et al. (2020) reported no change in Salmonella Enteritidis biofilm susceptibility to BKC 100 or 200 mg l−1 following biofilm pre‐exposure to 20 mg l−1 BKC for 5 days.
A few studies have looked at the impact of BKC treatment on complex biofilm communities. Gray et al. (2021) showed that bacterial isolates from water treatment plants that were able to grow on 250–500 mg l−1 BKC accounted for 0.2% of the overall culturable communities and were mainly Pseudomonas spp. A high proportion of these isolates with colistin resistance were observed, but no direct link between BKC insusceptibility and colistin resistance was demonstrated. Tandukar et al. (2013) observed a decrease in diversity of a water effluent microcosm following exposure to BKC 50 mg l−1 for 4 years. In addition, an increased BKC MIC (<2‐fold) of surviving bacteria was observed. Bastian et al. (2009) analysed a bacterial microcosm that was exposed to a product containing10–25% BKC and concluded a change in microflora occurred as a result of selection over a 3‐year application of the antifungal product. Forbes et al. (2017) exposed domestic drain biofilms daily with BKC‐containing products (100 mg l−1 BKC) for 6 months and observed an enrichment of bacteria, particularly pseudomonads, able to grow at BKC concentrations up to 100 mg l−1. Although a change in antibiotic susceptibility profile was observed, no bacteria became clinically resistant to the antibiotics tested. In addition, bacteria with reduced BKC and antibiotic susceptibility were susceptible to higher BKC concentrations, and BKC formulation significantly enhanced this effect. Chacón et al. (2021) observed a decrease in alpha diversity values and an expansion of the relative abundance of Alphaproteobacteria in a complex microcosm from a domestic water treatment plant following in vitro exposure to 10 mg l−1 BKC for 96 h. In addition to the change in bacterial community diversity, a significant increase in the qacE/qacEΔ1 genes was observed.
BKC exposure and gene maintenance transfer
Only a very few studies reflected on gene transfer conferring a reduced susceptibility to BKC. None of them investigated the impact of BKC on gene transfer or maintenance. Bjorland et al. (2001) described the occurrence of a small QAC resistance plasmid in 3 out of 31 veterinary S. aureus isolates belonging to the same clone suggesting a lateral transfer of plasmid‐borne QAC resistance. The authors speculated that maintenance of the plasmid results from the continuous use of BKC‐based preparations, but they did not offer tangible data supporting their assertion. In addition, isolates carrying the plasmid showed only a 2–3‐fold increased BKC MIC (2.5–3 mg l−1).
Harrison et al. (2020) observed an increase in the abundance of sul1 and Bla TEM in a complex microcosm exposed to BKC (0.0001–0.5 mg l−1) for 14 days. BKC was reported to positively select bacterial resistance to ciprofloxacin and sulphamethoxazole, but negatively select for resistance to ampicillin, streptomycin, rifampicin, erythromycin, trimethoprim and tetracycline (to note the antibiotic clinical resistance determination was not standard). The authors concluded that widespread use of BKC might impact bacterial resistance profiles in the environment but called for a better understanding of the mechanisms of resistance and the active expression of resistance genes in the environment. Although this in vitro study enabled to investigate the use of a specific antimicrobial against a complex microcosm, in the environment, the impact of a specific compound on a microcosm is difficult to ascertain because of the documented number of compounds, including antibiotics (Felis et al., 2020; Rodriguez‐Mozaz et al., 2020) particularly found in water effluents.
Yamamoto et al. (1988) showed the occurrence of S. aureus transconjugants with an elevated BKC MIC (6.25 mg l−1) compared to the recipient strain (MIC of 3.13 mg l−1) but not to the donor (MIC of 12.5 mg l−1). However, the MIC determination did not use a standard protocol and gene(s) transferred were not identified. Correa et al. (2008) proposed that qac genes are horizontally spread amongst different clones based on cluster analysis of 21 isolates of Staphylococcus haemolyticus. BKC MIC ranged between 1 and 8 mg l−1, but a number of isolates were resistant to gentamicin (21), erythromycin (15), ciprofloxacin (18), chloramphenicol (7) and tetracycline (1). The impact of BKC exposure to the qac genes dissemination was not studied.
Jiang et al. (2017) reported that QAC resistance genes were present amongst 52 Proteus mirabilis isolates from cooked meats (BKC MIC ranged from 4 to >32 mg l−1, but the majority of isolates had an MIC of 24 mg l−1). qacH was associated with non‐classic class 1 integrons located on conjugative plasmids. The impact of BKC exposure to the qacH dissemination was not studied. In another study with 179 E. coli food isolates (BKC MIC ranged from 4 to 64 mg l−1, with 44% isolates showing an MIC of 32 mg l−1), Jiang et al. (2017) showed that qacH‐associated integrons located on 100 kb plasmids in two isolates could be transferred to an E. coli recipient. The impact of BKC exposure to the qacH dissemination was not studied. Katharios‐Lanwermeyer et al. (2012) showed that heavy metal (cadmium) and BKC could be co‐selected during conjugation with Listeria spp. harbouring the BKC resistance cassette bcrABC. The paper mentioned that BKC‐‘resistant’ strains have a higher ability for conjugation, but the MIC protocol is not described, and ‘resistant’ strains are not defined. Kücken et al. (2000) showed that BKC MIC in a naive E. coli (MIC of 20 mg l−1) increased to 80 mg l−1 following transformation with a plasmid containing an integron qacE. However, there was no correlation between increased BKC MIC and the presence of qacE or qacE∆1.
BKC and in situ investigations
Only one study to date has looked at the impact on household microflora of BKC‐containing products; a liquid kitchen spray containing 0.08% alkyl dimethyl benzyl ammonium chlorides and 0.02% alkyl benzyl ammonium chlorides, and an ‘all‐purpose’ surface cleaner containing 2.7% alkyl benzyl ammonium chlorides; together with an antimicrobial handwashing soap containing 0.2% triclosan (Carson et al., 2008). In this in situ study, the authors used an ad hoc definition of resistance. After one year of assigned product usage, bacterial isolates with high BKC MICs were more likely to have high MICs for triclosan and be resistant to one or more antibiotics. The change in antibiotic susceptibility profile was random and there was no follow‐up to investigate the stability of these profiles or the clinical significance of these findings. In addition, it is unclear whether the change in antibiotic susceptibility profile was driven by the BKC products only.
BKC products or formulations
Overall, only 15% (31/212) of the retained studies tested a product formulation containing BKC. None of the studies referred to a Chemical Abstracts Service (CAS) number. Not all studies that used formulations provided details of the content of the formulation. Specific product information might be difficult to find since some products are no longer available. Nevertheless, when a BKC formulation or product was used, it was clear that the formulation achieved a better efficacy than BKC used on its own. Cowley et al. (2015) repeatedly exposed isolates (different genera) to formulations with and without BKC. MIC and MBC were 11‐fold lower for formulations in general. Exposure to BKC formulations did not increase MIC or MBC to the same extent as formulations without BKC. In addition, adapted strains were not stable. Lee et al. (2020) observed an increased in BKC MIC in S. epidermidis (MIC: 0.00098 mg l−1 for methicillin‐susceptible S. epidermidis and 0.00231 mg l−1 for methicillin‐resistant S. epidermidis) isolated from patient treated with Xalatan (0.005%; using BKC as a preservative; the duration of treatment was not disclosed). Isolates with high BKC MIC harboured efflux encoding genes qacC/smr. Kawamura‐Sato et al. (2008) showed that Acinetobacter spp. isolates adapted to BKC and showing increased MIC were not able to grow in the presence of an in‐use concentration of BKC (2000 mg l−1). Likewise, Condell et al. (2012) showed that adapted S. enterica isolates with increased BKC MIC could not grow in a 50% dilution of a BKC‐containing product. Bland et al. (2021) showed that 45/48 L. monocytogenes environmental isolates collected from produce handling and processing facilities in the USA had their growth characteristic severally affected by the presence of 1.56 mg l−1 of BKC product. Two of three isolates that were not affected by BKC harboured the bcrAB cassette suggesting efflux was responsible for the unaltered growth of these isolates in BKC. Forbes et al. (2016) reported that exposure to BKC formulation was more likely to increase antibiotic susceptibility than adaptation to the simple BKC aqueous solution. Worthing et al. (2018) reported elevated BKC‐product MIC and MBC in staphylococcal veterinary isolates, but all MBC values were well below the BKC‐product recommended usage concentration. Avrain et al. (2003) reported that antibiotic‐resistant Campylobacter spp. veterinary isolates did not have a reduced susceptibility to the BKC‐product tested. Following routine use of a BKC‐based product, Peyrat et al. (2008) isolated Campylobacter strains with decreased BKC‐MIC but with no change in antibiotic susceptibility profile. Nhung et al. (2015) reported, however, a weak correlation between increased BKC‐product MIC and decreased antibiotic susceptibility in E. coli and S. enterica following stepwise exposure to a BKC product, although none of the changes were clinically significant. Stickler and Thomas (1976) reported a correlation between increased BKC‐product MIC and CHX product in Providencia stuartii. Knapp et al. (2015) tested the efficacy and impact of exposure to the ‘during use’ concentration of BKC product (shampoo) against industrial isolates of P. aeruginosa, B. cepacia and K. pneumoniae, and two S. enterica serovar Typhimurium, and reported no changes in susceptibility profile to BKC or antibiotics. Beier, Andrews et al. (2021), Beier, Byrd et al. (2021) investigated the susceptibility of S. aureus (Beier, Andrews, et al., 2021; Beier, Byrd et al., 2021) and C. jejuni (Beier, Andrews, et al., 2021; Beier, Byrd et al., 2021) isolates to BKC and other biocides as well as antibiotic susceptibility profile. Whilst some isolates showed elevated MIC to several biocides and resistance to some antibiotics, they remained susceptible to a BKC product (Beier, Andrews, et al., 2021; Beier, Byrd et al., 2021). Bassani et al. (2021) observed no differences in bacterial susceptibility to BKC or antibiotics in S. Heidelberg isolates over a 10‐year period.
When a complex microcosm was investigated, Forbes et al. (2017) exposed domestic (kitchen) drain biofilms daily with BKC‐containing products (100 mg l−1 BKC) for 6 months and observed an enrichment of bacteria, particularly pseudomonads, able to grow at concentrations up to 100 mg l−1 BKC. Although a change in antibiotic susceptibility profile was observed, no bacteria became clinically resistant to the antibiotics tested. In addition, bacteria with reduced BKC and antibiotic susceptibility were susceptible to higher BKC concentrations. Likewise, Bastian et al. (2009) reported a decrease in bacterial diversity in a microcosm that was exposed to Devor Mousse® that contains10–25% BKC and concluded the change in microflora resulted from selection following the 3‐year application of the antifungal product. Tandukar et al. (2013) observed a decrease in the diversity of a water effluent microcosm following exposure to BKC 50 mg l−1 for 4 years. In addition, an increase in BKC MIC (<2‐fold) of surviving bacteria was observed.
BENZETHONIUM CHLORIDE AND BACTERIAL ‘RESISTANCE’
Benzethonium chloride (CAS Number 121‐54‐0) is a synthetic quaternary ammonium compound with a broad spectrum of activity used for preservation, antisepsis and disinfection. BZT has been less studied than BKC and there are only six relevant studies on bacterial ‘resistance’ to BZT (Table S2). Most of these publications (four of six) deal with food bacteria, notably Listeria monocytogenes (Casey et al., 2014; Collins et al., 2012), Salmonella enterica serovar Typhimurium (Chen et al., 2007) and E. coli (Deus et al., 2017). One study investigated Streptococcus mutants (Oyanagi et al., 2012) and another industrial isolates of Burkholderia spp (Rushton et al., 2013).
In these studies, measurement of activity was based mainly on the use of a microdilution broth (Collins et al., 2012; Deus et al., 2017; Rushton et al., 2013) but not necessarily following the standard CLSI protocol.
Only three publications investigated the effect of pre‐exposure to BZT. Collins et al. (2012) grew L. monocytogenes in different concentrations of BZT and investigated BZT effects on different L. monocytogenes mutants. Their study was not focussed on BZT but used BZT as one of the biocides investigated. They concluded that mutants with a deletion in the liaS gene that encodes for intramembrane‐sensing histidine kinases, which are involved in stress response, were more tolerant to BZT than the wild‐type bacteria. This publication did not look at cross‐resistance to chemotherapeutic antibiotics.
Rushton et al. (2013) exposed Burkholderia cenocepacia complex (Bcc) subcultures to increasing concentrations of BZT in an agar‐based protocol. BZT at 1 g l−1 failed to inhibit the growth of 93% (77/83) of Bcc strains. They observed that 14 Bcc strains (predominantly B. cenocepacia) showed a high tolerance for BZT, with MBCs up to 10 times >1 g l−1. There was no cross‐resistance to other preservatives such as isothiazolinones or chemotherapeutic antibiotics. The adapted Bcc strains displayed altered growth pattern with longer lag phase and shorter culture doubling time.
Casey et al. (2004) studied gene regulation (transcriptome and RNA‐seq analysis) following incubation of L. monocytogenes 6179 with BZT 4 mg l−1 at 14°C to early stationary phase. They identified approximately 600 genes that showed a 4‐fold or greater change in relative expression in the BZT‐treated sample compared to the control. The functions of genes that were upregulated concern chemotaxis, fatty acid metabolism, flagellar assembly, cobalamin (Vitamin B12) biosynthesis, peptidoglycan biosynthesis and phosphotransferase system. Of interest is the upregulation of a multidrug transporter pump: 4‐fold increase in qacH and 3‐fold increase in ykkC and lmrB, which encode efflux transporters. They did not study changes in MIC/MBC and antibiotic susceptibility profile.
Oyanagi et al. (2012) performed a suspension test with 0.1 g l−1 BZT against S. mutans MT8148 and Streptococcus sobrinus 6715 and observed only a 50% reduction in bacterial number within a 10‐s contact time. They did not study antibiotic susceptibility profile.
Deus et al. (2017) investigated the susceptibility of 174 human and veterinary isolates of extended‐spectrum beta‐lactamases E. coli to several biocides including BZT. The broth microdilution performed to determine susceptibility profile was not standard, but custom made. The MIC distribution was unimodal and presented a narrow range. The isolates showing elevated MIC to BZT (n = 6; 32 μg/ml) harboured a number of efflux pump genes involved in QAC tolerance; these included emrE, mdfA, sugE, sugE, oqxA, oqxB, qacE, qacEΔ1, qacF, qacG and qacH.
Out of the six peer‐reviewed papers on BZT, only one study investigated the effect of BZT exposure on antibiotic susceptibility changes. The study by Rushton et al. (2013) managed to produce stable mutants with increased BZT MBC through the use of stepwise training. No change in antibiotic susceptibility was observed. Casey et al. (2014) investigated L. monocytogenes response to BZT (4 mg l−1) exposure and notably the upregulation of efflux pump genes. The significance of gene upregulation is unclear since no change in susceptibility data was provided. Correlation between BZT usage, and efflux gene carriage and dissemination, has not been made in the only study available (Deus et al., 2017). No information on the impact of BZT on transfer or maintenance of resistant genes could be found.
CHLOROXYLENOL AND BACTERIAL ‘RESISTANCE’
Chloroxylenol (CAS number 88–04‐0 check) also known as para‐chloro‐meta‐xylenol (PCMX) is a halophenol used for disinfection and antisepsis. It is a membrane‐active agent with a broad spectrum of activity. Studies published in the peer‐reviewed literature on chloroxylenol (CHO) and antimicrobial ‘resistance’ (Table S3) concern a wide range of environments including healthcare and veterinary (Gilbert et al., 2002; Johnson et al., 2002; Tambe et al., 2011; Young et al., 2011), home (Cole et al., 2003; Moken et al., 1997), food (Collins et al., 2012) and industry (Lear et al., 2002, 2006) (Table S3). Several studies investigated the impact of bacterial biofilms on biocide efficacy (Das et al., 1998; Gilbert et al., 2002; Johnson et al., 2002). These studies did not necessarily focus on CHO but use CHO as an additional biocide amongst other actives.
Several studies measure biocide susceptibility as MIC or/and MBC using a microdilution broth based on standard protocols (Collins et al., 2012; Gilbert et al., 2002; Johnson et al., 2002; Tambe et al., 2011). Others used ad hoc protocols which makes the comparison of susceptibility data difficult (Cole et al., 2003; Conroy et al., 2010; De Majumdar et al., 2015; Young et al., 2011).
The study by Cole et al. (2003) is an in situ randomized trial focussing on the home environment. A total of 1268 isolates were collected following the use of household products in selected homes and tested for their susceptibility to several biocides and antibiotics. MIC for CHO ranged from 62 to 250 mg l−1. Antibiotic testing was conducted using a standard CLSI test. Overall, there was no meaningful correlation between antibiotic resistance in Gram‐positive and Gram‐negative human pathogens. Collins et al. (2012) grew L. monocytogenes in different concentrations of CHO and investigated CHO effects on different L. monocytogenes mutants. They concluded that mutants with a deletion in the liaS gene, which encodes for intramembrane‐sensing histidine kinases involved in stress response, were more tolerant to CHO than the wild type. This publication did not look at cross‐resistance. De Majumdar et al. (2015) observed that overexpressing ramA in K. pneumoniae resulted in increased tolerance to CHO and other compounds, including chemotherapeutic antibiotics, probably resulting from a change in membrane lipopolysaccharide composition. Conroy et al. (2010) investigated potential substrates of two RND‐type transport systems. The authors use the Biolog system to measure susceptibility and concluded these pumps confer CHO ‘moderate’ resistance; this level of resistance was not defined. Overall, this study showed that CHO was a substrate for these pumps that may contribute to an elevated MIC to CHO. Likewise, Moken et al. (1997) related the presence of acrAB to decrease susceptibility to CHO in E. coli.
Das et al. (1998) and Gilbert et al. (2002) investigated the impact of biofilms on decreased CHO susceptibility in Staphylococcus epidermidis NCTC 11047 and a mucoid isolate of E. coli. Measurement of biocide susceptibility was based on optical density (OD) measurement. There is no standard test for that type of biofilm testing. Das et al. (1998) observed that CHO was equally efficacious against planktonic and sessile bacteria, whilst Gilbert et al. (2002) concluded that pre‐exposure of biofilms to sub‐lethal concentrations of CHO did not impact biofilm changes in susceptibility to the biocide. A study from Young et al. (2011) merely looked at susceptibility of veterinary isolates to CHO using ad hoc methodology and did not measure susceptibility to chemotherapeutic antibiotics.
Perhaps, the most interesting articles are those of Lear et al. (2002, 2006) and Tambe et al. (2011). Lear et al. (2002) investigated CHO resistance in industrial isolates where CHO is manufactured. In addition, a range of protocols were used to explore the development of bacterial resistance to CHO, including the heavy inoculum approach, exposing 109 CFU spread on a tryptone soya agar plate containing 100–500 mg l−1 CHO, and repeated exposure to sub‐MIC (50 and 100 mg l−1) of CHO. They observed that industrial isolates tolerant (MIC > 500 mg l−1) to CHO were mostly Pseudomonas aeruginosa (24 isolates), Alcaligenes xylosoxidans (1 isolate) and Pseudomonas fluorescens (1 isolate). Five isolates of Pseudomonas stutzeri were identified with a CHO MIC range of 300–500 mg l−1, whilst a culture collection strain had an MIC of 200–220 mg l−1. The observed CHO tolerance was stable without the presence of CHO. The heavy inoculum or the repeated exposure approaches did not yield any resistant Ps. stutzeri to CHO. In addition, using a standard suspension test, Lear et al. (2002) showed that the CHO tolerant Ps. stutzeri was as susceptible to CHO at 250 mg l−1 as the culture collection strain. Follow‐up investigation on bacterial tolerance to CHO could not link high bacterial tolerance (measured based on MIC) to bactericidal efficacy (at 10 mg l−1 of CHO) or significant change in antibiotic susceptibility profile (Lear et al., 2006).
Tambe et al. (2011) investigated the effect of CHO sub‐MIC exposure on increased insusceptibility in S. epidermidis. They observed no changes in CHO MIC or MBC (remaining at 250 mg l−1) after 20 passages.
Only two studies investigated the effect of CHO exposure on bacterial cross‐resistance to antibiotics (Cole et al., 2003; Lear et al., 2006). No significant changes were observed and relevance to clinical significance was not explored. The three in vitro studies (Johnson et al., 2002; Lear et al., 2002; Tambe et al., 2011) that investigated pre‐exposure to a sub‐lethal concentration of CHO did not find evidence of a change in MIC or MBC. The study investigating CHO resistance in industrial isolates which were likely to be continuously exposed to CHO did not find any evidence of decrease in bacterial susceptibility to CHO (Lear et al., 2002).
CONCLUSION
The use of products containing BKC, BZT and CHO has been viewed as a concern for potential development of decreased bacterial susceptibility to these biocides and/or chemotherapeutic antibiotics. A total of 3655 scientific papers mentioning these biocides were analysed. The vast majority did not make the inclusion criteria and overall, very few relevant papers specifically dealing with antimicrobial resistance were retained. Most of the studies related to BKC, with 212 papers retained, and very few were relevant to CHO (12) and BZT (6).
One evident issue with the scientific literature was the lack of standardization of the method used to measure susceptibility to biocides and antibiotics. For example, with the literature on BKC the majority of studies 161/212 (76%) reports MIC data which does not reflect product usage in practice. Only 27% of these (43/161) used a standardized protocol, principally the CLSI broth microdilution method or agar dilution‐based method. In addition, only 10% (21/212) of the retained studies provided information on MBC and only 10% (22/212) performed bactericidal tests such as a suspension test. The use of an increase in MIC as a sole indicator of resistance has been criticized as not reflecting product usage in practice, but to just provide an indication that a biocide can alter a bacterial phenotype (Maillard et al., 2013; Russell & McDonnell, 2000). It has long been argued that a change in MIC does not necessarily indicate a bacterium will be resistant to a biocide, particularly when one considers that the concentration used in a product is often considerably higher (>1000 fold) than the MIC (Maillard et al., 2013; Russell & McDonnell, 2000). To add some perspective, the handbook of Pharmaceutical Excipients (Rowe et al., 2009) reports BKC in‐use concentrations for the preservation of pharmaceutical preparations of 100–200 mg l−1 and as low as 20 mg l−1 for otic formulations. In practice, BKC‐based soap products contain between 1 and 10 g l−1 BKC, BKC‐based sanitizing products contain BKC concentrations ranging from 200 to 400 mg l−1, whilst hospital disinfectants typically contain 1.2–2.4 g l−1 BKC. A BKC concentration of 1 g l−1 represents the low ends of the active eligibility monograph ranges for use in antiseptic products covered by the OTC antiseptic. Increases in MIC following repeated exposure to the same BKC concentration (Table 1) rather than stepwise training (repeated exposure in increasing BKC concentration), resulted in BKC MIC < 50 mg l−1 in single bacterial species (Kawamura‐Sato et al., 2008). Two recent manuscripts investigated the effect of co‐exposure of BKC with an antibiotic and demonstrated a reduced antibiotic activity (Pietsch et al., 2021; Short et al., 2021). Whilst the science is interesting, the likelihood of co‐exposure occurring in practice is remote.
Clinical, veterinary and environmental isolates showing an elevated BKC MIC have been shown to carry many efflux pump genes. Correlation between BKC usage, and efflux gene carriage and dissemination, has not been made in any in vitro studies. The clinical significance of a decreased antibiotic susceptibility in isolate with a decreased susceptibility to BKC has not been well studied. Where clinical resistance to antibiotics was observed, a direct correlation with BKC usage was not established. Furthermore, there is no information on the impact of BKC on transfer or maintenance of resistance genes. Bacterial isolates with a decreased BKC susceptibility have not been shown to be more virulent (in animal assays) and increased fitness (measured by growth rate) of isolates with a decreased BKC susceptibility, when studied, was shown, perhaps not surprisingly, to be elevated in the presence of BKC. Amongst the 230 retained papers, only one in situ paper raised a concern that repeated BKC exposure selects for bacteria leading to a clinical change in antibiotic susceptibility. It is however unclear whether the change in antibiotic susceptibility profile was driven by the BKC products only as the handwashing product used in this study contained triclosan, which is known to affect antimicrobial susceptibility.
The limited number of relevant studies on CHO and BZT does not allow to conclude that the use of these actives could lead to increases in susceptibility to CHO or BZC, or/and chemotherapeutic antibiotics. There is no information on the impact of CHO or BZC on transfer or maintenance of resistant genes.
Despite the abundant literature on antimicrobial ‘resistance’, the practicality of the findings remains limited. The use of recognized standard for testing the activity of biocides and antibiotics would enable a better comparison between data and provide some clinical significance. The debate about how to use MIC data is not over since the determination of MIC is less time consuming and can be automated. The use of relevant test concentration such as the ‘during use’ concentration would enable to interpret better the practical significance of an increased MIC. The rise of isolate with multiple efflux genes carriage may be a concern, but the clinical significance of such carriage needs to be established. Likewise, the impact of biocides (generally) on maintenance of efflux genes (or others), forced mutations and gene transfer still required further investigations. At the end of the day, the use of biocides to control/eliminate pathogens remains essential in many environments, and this needs to be appropriately balanced realistic risks of emerging antimicrobial resistance.
AUTHOR CONTRIBUTION
Jean‐Yves Maillard conducted the search for, read and analysed, all 3655 articles, wrote the entire review, design the tables and figures, and edited the manuscript.
CONFLICT OF INTEREST
The American Cleaning Institute commissioned Biocide Consult Ltd to write a comprehensive report on BKC, BZT and CHO resistance to help with a submission of a document on the impact of BKC, BZT and CHO on AMR to the US FDA. This review is based partly on that report. [Correction added on 21 October 2022, after first online publication: The American Clinical Institute has been corrected to The American Cleaning Institute in this version]
Jean‐Yves Maillard is the Director of Biocide Consult ltd.
Supporting information
Table S1
Table S2
Table S3
Maillard, J‐Y (2022) Impact of benzalkonium chloride, benzethonium chloride and chloroxylenol on bacterial antimicrobial resistance. Journal of Applied Microbiology, 133, 3322–3346. Available from: 10.1111/jam.15739
DATA AVAILABILITY STATEMENT
Supplementary tables on which this manuscript is based are available as supplementary materials and have been uploaded alongside the manuscript
REFERENCES
- Abuzaid, A. , Hamouda, A. & Amyes, S.G. (2012) Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacΔE and qacE efflux pump genes and antibiotic resistance. The Journal of Hospital Infection, 81, 87–91. [DOI] [PubMed] [Google Scholar]
- Adair, F.W. , Liauw, H.L. , Geftic, S.G. & Gelzer, J. (1975) Reduced virulence of Pseudomonas aeruginosa grown in the presence of benzalkonium chloride. Journal of Clinical Microbiology, 1, 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarestrup, F.M. & Hasman, H. (2004) Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Veterinary Microbiology, 100, 83–89. [DOI] [PubMed] [Google Scholar]
- Aksoy, A. , El Kahlout, K.E.M. & Yardimci, H. (2019) Comparative evaluation of the effects of benzalkonium chloride, iodine, glutaraldehyde and hydrogen peroxide disinfectants against avian salmonellae focusing on genotypic resistance pattern of the salmonellae serotypes toward benzalkonium chloride. Brazilian Journal of Poultry Science, 22, 1–12. [Google Scholar]
- Alotaibi, S.M.I. , Ayibiekea, A. , Pedersen, A.F. , Ayibiekea A., Pedersen A.F., Jakobsen L., Pinholt M., Gumpert H., Hammerum A.M., Westh H., Ingmer H. (2017) Susceptibility of vancomycin‐resistant and ‐sensitive enterococcus faecium obtained from Danish hospitals to benzalkonium chloride, chlorhexidine and hydrogen peroxide biocides. Journal of Medical Microbiology 66, 1744–1751. [DOI] [PubMed] [Google Scholar]
- Alqurashi, A.M. , Day, M.J. & Russell, A.D. (1996) Susceptibility of some strains of enterococci and streptococci to antibiotics and biocides. The Journal of Antimicrobial Chemotherapy, 38, 745. [DOI] [PubMed] [Google Scholar]
- Amsalu, A. , Sapula, S.A. , De Barros Lopes, M. , Hart, B.J. , Nguyen, A.H. , Drigo, B. et al. (2020) Efflux pump‐driven antibiotic and biocide cross‐resistance in Pseudomonas aeruginosa isolated from different ecological niches: a case study in the development of multidrug resistance in environmental hotspots. Microorganisms, 8, 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, J.M. (2009) BSAC working party on susceptibility testing. BSAC standardized disc susceptibility testing method (version 8). The Journal of Antimicrobial Chemotherapy, 64, 454–489. [DOI] [PubMed] [Google Scholar]
- A'shimi, M.H.N. , Alattraqchi, A.G. , Rani, F.M. , Rahman, N.I.A. , Ismail, S. , Abdullah, F.H. et al. (2019) Biocide susceptibilities and biofilm‐forming capacities of Acinetobacter baumannii clinical isolates from Malaysia. Journal of Infection in Developing Countries, 13, 626–633. [DOI] [PubMed] [Google Scholar]
- Avrain, L. , Allain, L. , Vernozy‐Rozand, C. & Kempf, I. (2003) Disinfectant susceptibility testing of avian and swine campylobacter isolates by a filtration method. Veterinary Microbiology, 96, 35–40. [DOI] [PubMed] [Google Scholar]
- Bassani, J. , Paravisi, M. , Wilsmann, D.E. , Borges, K.A. , Furian, T.Q. , Salle, C.T.P. et al. (2021) Antimicrobial and disinfectant resistance of salmonella Heidelberg from Brazilian flocks did not increase for ten years (2006‐2016). Pesquisa Veterinaria Brasileira, 41, e06818. [Google Scholar]
- Bastian, F. , Alabouvette, C. , Jurado, V. & Saiz‐Jimenez, C. (2009) Impact of biocide treatments on the bacterial communities of the Lascaux cave. Naturwissenschaften, 96, 863–868. [DOI] [PubMed] [Google Scholar]
- Bay, D.C. , Stremick, C.A. , Slipski, C.J. & Turner, R.J. (2017) Secondary multidrug efflux pump mutants alter Escherichia coli biofilm growth in the presence of cationic antimicrobial compounds. Research in Microbiology, 168, 208–221. [DOI] [PubMed] [Google Scholar]
- Beier, R.C. , Foley, S.L. , Davidson, M.K. , White, D.G. , McDermott, P.F. , Bodeis‐Jones, S. et al. (2014) Characterization of antibiotic and disinfectant susceptibility profiles among Pseudomonas aeruginosa veterinary isolates recovered during 1994‐2003. Journal of Applied Microbiology, 118, 326–342. [DOI] [PubMed] [Google Scholar]
- Beier, R.C. , Andrews, K. , Hume, M.E. , Sohail, M.U. , Harvey, R.B. , Poole, T.L. et al. (2021) Disinfectant and antimicrobial susceptibility studies of Staphylococcus aureus strains and ST398‐MRSA and ST5‐MRSA strains from swine mandibular lymph node tissue, commercial pork sausage meat and swine feces. Microorganisms, 9, 2401. 10.3390/microorganisms9112401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier, R.C. , Byrd, J.A. , Andrews, K. , Caldwell, D. , Crippen, T.L. , Anderson, R.C. et al. (2021) Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen campylobacter jejuni isolated from the litter of broiler chicken houses. Poultry Science, 100, 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoechea, J.A. & Skurnik, M. (2000) Temperature‐regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia . Molecular Microbiology, 37, 67–80. [DOI] [PubMed] [Google Scholar]
- Bisbiroulas, P. , Psylou, M. , Iliopoulou, I. , Diakogiannis, I. , Berberi, A. & Mastronicolis, S.K. (2011) Adaptational changes in cellular phospholipids and fatty acid composition of the food pathogen listeria monocytogenes as a stress response to disinfectant sanitizer benzalkonium chloride. Letters in Applied Microbiology, 52, 275–280. [DOI] [PubMed] [Google Scholar]
- Bischofberger, A.M. , Baumgartner, M. , Pfrunder‐Cardozo, K.R. , Allen, R.C. & Hall, A.R. (2020) Associations between sensitivity to antibiotics, disinfectants and heavy metals in natural, clinical and laboratory isolates of Escherichia coli . Environmental Microbiology, 22, 2664–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorland, J. , Sunde, M. & Waage, S. (2001) Plasmid‐borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus . Journal of Clinical Microbiology, 39, 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorland, J. , Steinum, T. , Sunde, M. , Waage, S. & Heir, E. (2003) Novel plasmid‐borne gene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus intermedius . Applied and Environmental Microbiology, 47, 3046–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorland, J. , Steinum, T. , Kvitle, B. , Waage, S. , Sunde, M. & Heir, E. (2005) Widespread distribution of disinfectant resistance genes among staphylococci of bovine and caprine origin in Norway. Journal of Clinical Microbiology, 43, 4363–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland, R.N. , Johnson, J.D. , Waite‐Cusic, J.G. , Weisberg, A.J. , Riutta, E.R. , Chang, J.H. et al. (2021) Application of whole genome sequencing to understand diversity and presence of genes associated with sanitizer tolerance in listeria monocytogenes from produce handling sources. Food, 10, 2454. 10.3390/foods10102454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville, L. , Ortiz, S. , Maia, V. , Brito, L. & Martínez‐Suárez, J.V. (2020) Strain and growth conditions may regulate resistance of listeria monocytogenes biofilms to benzalkonium chloride. Applied Sciences, 10, 988. [Google Scholar]
- Boutarfi, Z. , Rebiahi, S.‐A. , Morghada, T. , Pulido, R.P. , Burgos, M.J.G. , Mahdi, F. et al. (2019) Biocide tolerance and antibiotic resistance of Enterobacter spp. isolated from an Algerian hospital environment. Journal of Global Antimicrobial Resistance, 18, 291–297. [DOI] [PubMed] [Google Scholar]
- Braga, T.M. , Marujo, P.E. , Pomba, C. & Lopes, M.F. (2011) Involvement, and dissemination, of the enterococcal small multidrug resistance transporter QacZ in resistance to quaternary ammonium compounds. The Journal of Antimicrobial Chemotherapy, 66, 283–286. [DOI] [PubMed] [Google Scholar]
- Braoudaki, M. & Hilton, A.C. (2004) Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross‐resistance to antimicrobial agents. Journal of Clinical Microbiology, 42, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braoudaki, M. & Hilton, A.C. (2005) Mechanisms of resistance in salmonella enterica adapted to erythromycin, benzalkonium chloride and triclosan. International Journal of Antimicrobial Agents, 25, 31–37. [DOI] [PubMed] [Google Scholar]
- Bridier, A. , Briandet, R. , Thomas, V. & Dubois‐Brissonnet, F. (2011) Comparative biocidal activity of peracetic acid, benzalkonium chloride and ortho‐phthalaldehyde on 77 bacterial strains. The Journal of Hospital Infection, 78, 208–213. [DOI] [PubMed] [Google Scholar]
- Burgos, M.J. , Aguayo, M.C. , Pulido, R.P. , Gálvez, A. & López, R.L. (2014) Multilocus sequence typing and antimicrobial resistance in enterococcus faecium isolates from fresh produce. Antonie Van Leeuwenhoek, 105, 413–421. [DOI] [PubMed] [Google Scholar]
- Buzón‐Durán, L. , Alonso‐Calleja, C. , Riesco‐Peláez, F. & Capita, R. (2017) Effect of sub‐inhibitory concentrations of biocides on the architecture and viability of MRSA biofilms. Food Microbiology, 65, 294–301. [DOI] [PubMed] [Google Scholar]
- Capita, R. , Vicente‐Velasco, M. , Rodríguez‐Melcón, C. , García‐Fernández, C. , Carballo, J. & Alonso‐Calleja, C. (2019) Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica . Scientific Reports, 9, 15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, R.T. , Larson, E. , Levy, S.B. , Marshall, B.M. & Aiello, A.E. (2008) Use of antibacterial consumer products containing quaternary ammonium compounds and drug resistance in the community. The Journal of Antimicrobial Chemotherapy, 62, 1160–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, A. , Fox, E.M. , Schmitz‐Esser, S. , Coffey, A. , McAuliffe, O. & Jordan, K. (2014) Transcriptome analysis of listeria monocytogenes exposed to biocide stress reveals a multi‐system response involving cell wall synthesis, sugar uptake, and motility. Frontiers in Microbiology, 5, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceragioli, M. , Mols, M. , Moezelaar, R. , Ghelardi, E. , Senesi, S. & Abee, T. (2010) Comparative transcriptomic and phenotypic analysis of the responses of Bacillus cereus to various disinfectant treatments. Applied and Environmental Microbiology, 76, 3352–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón, L. , Arias‐Andres, M. , Mena, F. , Rivera, L. , Hernandez, L. , Achi, R. et al. (2021) Short‐term exposure to benzalkonium chloride in bacteria from activated sludge alters the community diversity and the antibiotic resistance profile. Journal of Water and Health, 19, 895–906. [DOI] [PubMed] [Google Scholar]
- Chaieb, K. , Zmantar, T. , Souiden, Y. , Mahdouani, K. & Bakhrouf, A. (2011) XTT assay for evaluating the effect of alcohols, hydrogen peroxide and benzalkonium chloride on biofilm formation of Staphylococcus epidermidis . Microbial Pathogenesis, 50, 1–5. [DOI] [PubMed] [Google Scholar]
- Chaplin, C.E. (1951) Bacterial resistance to quaternary ammonium disinfectants. Journal of Bacteriology, 63, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.H. , Köseoğlu, V.K. , Güvener, Z.T. , Myers‐Morales, T. , Reed, J.M. , D'Orazio, S.E.F. et al. (2014) Cyclic di‐GMP‐dependent signaling pathways in the pathogenic Firmicute listeria monocytogenes . PLoS Pathogens, 10(8), e1004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Cui, S. , McDermott, P.F. , Zhao, S. , White, D.G. , Paulsen, I. et al. (2007) Contribution of target gene mutations and efflux to decreased susceptibility of salmonella enterica serovar typhimurium to fluoroquinolones and other antimicrobials. Antimicrobial Agents and Chemotherapy, 51, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherifi, T. , Arsenault, J. , Pagotto, F. , Quessy, S. , Côté, J.C. , Neira, K. et al. (2020) Distribution, diversity and persistence of listeria monocytogenes in swine slaughterhouses and their association with food and human listeriosis strains. PLoS One, 15(8), e0236807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielowska, C. , Korsak, D. , Szuplewska, M. , Grzelecka, M. , Maćkiw, E. , Stasiak, M. et al. (2021) Benzalkonium chloride and heavy metal resistance profiles of listeria monocytogenes strains isolated from fish, fish products and food‐producing factories in Poland. Food Microbiology, 98, 103756. 10.1016/j.fm.2021.103756 [DOI] [PubMed] [Google Scholar]
- Chittrakanwong, J. , Charoenlap, N. , Mongkolsuk, S. & Vattanaviboon, P. (2021) mfsQ encoding an MFS efflux pump mediates adaptive protection of Stenotrophomonas maltophilia against benzalkonium chloride. Canadian Journal of Microbiology, 67(6), 491–495. [DOI] [PubMed] [Google Scholar]
- Chuanchuen, R. , Pathanasophon, P. , Khemtong, S. , Wannaprasat, W. & Padungtod, P. (2008) Susceptibilities to antimicrobials and disinfectants in salmonella isolates obtained from poultry and swine in Thailand. The Journal of Veterinary Medical Science, 70, 595–601. [DOI] [PubMed] [Google Scholar]
- Coelho, J.R. , Carriço, J.A. , Knight, D. , Martínez, J.L. , Morrissey, I. , Oggioni, M.R. et al. (2013) The use of machine learning methodologies to analyse antibiotic and biocide susceptibility in Staphylococcus aureus . PLoS One, 8(2), e55582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, E.C. , Addison, R.M. , Rubino, J.R. , Leese, K.E. , Dulaney, P.D. , Newell, M.S. et al. (2003) Investigation of antibiotic and antibacterial agent cross‐resistance in target bacteria from homes of antibacterial product users and nonusers. Journal of Applied Microbiology, 95, 664–676. [DOI] [PubMed] [Google Scholar]
- Collins, B. , Guinane, C.M. , Cotter, P.D. , Hill, C. & Ross, R.P. (2012) Assessing the contributions of the LiaS histidine kinase to the innate resistance of listeria monocytogenes to nisin, cephalosporins, and disinfectants. Applied and Environmental Microbiology, 78, 2923–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condell, O. , Iversen, C. , Cooney, S. , Power, K.A. , Walsh, C. , Burgess, C. et al. (2012) Efficacy of biocides used in the modern food industry to control salmonella enterica, and links between biocide tolerance and resistance to clinically relevant antimicrobial compounds. Applied and Environmental Microbiology, 78, 3087–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy, O. , Kim, E.H. , McEvoy, M.M. & Rensing, C. (2010) Differing ability to transport nonmetal substrates by two RND‐type metal exporters. FEMS Microbiology Letters, 308, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A.L. , Carrillo, C.D. , Deschenes, M. & Blais, B.W. (2021) Genomic markers for quaternary ammonium compound resistance as a persistence indicator for listeria monocytogenes contamination in food manufacturing environments. Journal of Food Protection, 84, 389–398. [DOI] [PubMed] [Google Scholar]
- Correa, J.E. , De Paulis, A. , Predari, S. , Sordelli, D.O. & Jeric, P.E. (2008) First report of qacG, qacH and qacJ genes in staphylococcus haemolyticus human clinical isolates. The Journal of Antimicrobial Chemotherapy, 62, 956–960. [DOI] [PubMed] [Google Scholar]
- Couto, I. , Costa, S.S. , Viveiros, M. , Martins, M. & Amaral, L. (2008) Efflux‐mediated response of Staphylococcus aureus exposed to ethidium bromide. The Journal of Antimicrobial Chemotherapy, 62, 504–513. [DOI] [PubMed] [Google Scholar]
- Couto, N. , Belas, A. , Tilley, P. , Couto, I. , Gama, L.T. , Kadlec, K. et al. (2013) Biocide and antimicrobial susceptibility of methicillin‐resistant staphylococcal isolates from horses. Veterinary Microbiology, 166, 299–303. [DOI] [PubMed] [Google Scholar]
- Couto, N. , Belas, A. , Kadlec, K. , Schwarz, S. & Pomba, C. (2015) Clonal diversity, virulence patterns and antimicrobial and biocide susceptibility among human, animal and environmental MRSA in Portugal. The Journal of Antimicrobial Chemotherapy, 70, 2483–2487. [DOI] [PubMed] [Google Scholar]
- Costa, S.S. , Ntokou, E. , Martins, A. , Viveiros, M. , Pournaras, S. , Couto, I. et al. (2010) Identification of the plasmid‐encoded qacA efflux pump gene in meticillin‐resistant Staphylococcus aureus (MRSA) strain HPV107, a representative of the MRSA Iberian clone. International Journal of Antimicrobial Agents, 36, 557–561. [DOI] [PubMed] [Google Scholar]
- Costa, S.S. , Junqueira, E. , Palma, C. et al. (2013) Resistance to antimicrobials mediated by efflux pumps in Staphylococcus aureus . Antibiotics, 2, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley, N.L. , Forbes, S. , Amézquita, A. , McClure, P. , Humphreys, G.J. & McBain, A.J. (2015) Effects of formulation on microbicide potency and mitigation of the development of bacterial insusceptibility. Applied and Environmental Microbiology, 81, 7330–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiao, T. , Marchi, E. , Viti, C. , Oggioni, M.R. , Baquero, F. , Martinez, J.L. et al. (2015) Polymorphic variation in susceptibility and metabolism of triclosan‐resistant mutants of Escherichia coli and Klebsiella pneumoniae clinical strains obtained after exposure to biocides and antibiotics. Antimicrobial Agents and Chemotherapy, 59, 3413–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, J.R. , Bhakoo, M. , Jones, M.V. & Gilbert, P. (1998) Changes in the biocide susceptibility of Staphylococcus epidermidis and Escherichia coli cells associated with rapid attachment to plastic surfaces. Journal of Applied Microbiology, 84, 852–858. [DOI] [PubMed] [Google Scholar]
- Dejoies, L. , Le Neindre, K. , Reissier, S. , Felden, B. & Cattoir, V. (2021) Distinct expression profiles of regulatory RNAs in the response to biocides in Staphylococcus aureus and enterococcus faecium . Scientific Reports, 11, 6892. 10.1038/s41598-021-86376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Majumdar, S. , Yu, J. , Fookes, M. et al. (2015) Elucidation of the RamA regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLOS Path, 11(1), e1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco, C.E. , Cushing, L.A. , Frempong‐Manso, E. , Seo, S.M. , Jaravaza, T.A. & Kaatz, G.W. (2007) Efflux‐related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 51, 3235–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus, D. , Krischek, C. , Pfeifer, Y. , Sharifi, A.R. , Fiegen, U. , Reich, F. et al. (2017) Comparative analysis of the susceptibility to biocides and heavy metals of extended‐spectrum β‐lactamase‐producing Escherichia coli isolates of human and avian origin, Germany. Diagnostic Microbiology and Infectious Disease, 88, 88–92. [DOI] [PubMed] [Google Scholar]
- Dutta, V. , Elhanafi, D. & Kathariou, S. (2013) Conservation and distribution of the benzalkonium chloride resistance cassette bcrABC in listeria monocytogenes . Applied and Environmental Microbiology, 79, 6067–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekhnawy, E.A. , Sonbol, F.I. , Elbanna, T.E. & Abdelaziz, A.A. (2021) Evaluation of the impact of adaptation of Klebsiella pneumoniae clinical isolates to benzalkonium chloride on biofilm formation. Egyptian Journal of Medical Human Genetics, 22, 51. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) . (2014) Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0.0.
- Falcão‐Silva, V.S. , Silva, D.A. , Souza Mde, F. & Siqueira‐Junior, J.P. (2009) Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae). Phytotherapy Research, 23, 1367–1370. [DOI] [PubMed] [Google Scholar]
- Felis, E. , Kalka, J. , Sochacki, A. , Kowalska, K. , Bajkacz, S. , Harnisz, M. et al. (2020) Antimicrobial pharmaceuticals in the aquatic environment ‐ occurrence and environmental implications. European Journal of Pharmacology, 866, 172813. [DOI] [PubMed] [Google Scholar]
- Ferhat, M. , Atlan, D. , Vianney, A. , Lazzaroni, J.C. , Doublet, P. & Gilbert, C. (2009) The TolC protein of legionella pneumophila plays a major role in multi‐drug resistance and the early steps of host invasion. PLoS One, 4(11), e7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Cuenca, F. , Tomás, M. , Caballero‐Moyano, F.J. , Bou, G. , Martínez‐Martínez, L. , Vila, J. et al. (2015) Reduced susceptibility to biocides in Acinetobacter baumannii: association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps. The Journal of Antimicrobial Chemotherapy, 70, 3222–3229. [DOI] [PubMed] [Google Scholar]
- Fernández Márquez, M.L. , Grande Burgos, M.J. , López Aguayo, M.C. , Pérez Pulido, R. , Gálvez, A. & Lucas, R. (2014) Characterization of biocide‐tolerant bacteria isolated from cheese and dairy small‐medium enterprises. Food Microbiology, 62, 77–81. [DOI] [PubMed] [Google Scholar]
- Forbes, S. , Knight, C.G. , Cowley, N.L. , Amézquita, A. , McClure, P. , Humphreys, G. et al. (2016) Variable effects of exposure to formulated microbicides on antibiotic susceptibility in firmicutes and proteobacteria. Applied and Environmental Microbiology, 82, 3591–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, S. , Cowley, N. , Humphreys, G. , Mistry, H. , Amézquita, A. & McBain, A.J. (2017) Formulation of biocides increases antimicrobial potency and mitigates the enrichment of nonsusceptible bacteria in multispecies. Applied and Environmental Microbiology, 83, e3054‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furi, L. , Ciusa, M.L. , Knight, D. , di Lorenzo, V. , Tocci, N. , Cirasola, D. et al. (2013) Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory‐generated mutants of Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 57, 3488–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea, R. , Fernández Fuentes, M.Á. , Pérez Pulido, R. , Gálvez, A. & Ortega, E. (2017) Effects of exposure to quaternary‐ammonium‐based biocides on antimicrobial susceptibility and tolerance to physical stresses in bacteria from organic foods. Food Microbiology, 63, 58–71. [DOI] [PubMed] [Google Scholar]
- Galluzzi, L. , Virtanen, P. & Karp, M. (2003) A real‐time analysis of QacR‐regulated multidrug resistance in Staphylococcus aureus . Biochemical and Biophysical Research Communications, 301, 24–30. [DOI] [PubMed] [Google Scholar]
- Galluzzi, L. , Virtanen, P. & Karp, M. (2004) Discarding multidrug resistance inducers, the possible role of a biosensing reporter in antimicrobial discovery. Luminescence, 19, 225–227. [DOI] [PubMed] [Google Scholar]
- Geftic, S.G. , Heymann, H. & Adair, F.W. (1979) Fourteen‐year survival of pseudomonas cepacia in a salts solution preserved with benzalkonium chloride. Applied and Environmental Microbiology, 37, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba, C.P. & Müller, V. (2015) Quaternary ammonium biocides: efficacy in application. Applied and Environmental Microbiology, 81, 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamrezazadeh, M. , Shakibaie, M.R. , Monirzadeh, F. , Masoumi, S. & Hashemizadeh, Z. (2018) Effect of nano‐silver, nano‐copper, deconex and benzalkonium chloride on biofilm formation and expression of transcription regulatory quorum sensing gene (rh1R) in drug‐resistance Pseudomonas aeruginosa burn isolates. Burns, 44, 700–708. [DOI] [PubMed] [Google Scholar]
- Gilbert, P. , Das, J.R. , Jones, M.V. & Allison, D.G. (2002) Assessment of resistance towards biocides following the attachment of micro‐organisms to, and growth on, surfaces. Journal of Applied Microbiology, 91, 248–254. [DOI] [PubMed] [Google Scholar]
- Goodarzi, R. , Yousefimashouf, R. , Taheri, M. , Nouri, F. & Asghari, B. (2021) Susceptibility to biocides and the prevalence of biocides resistance genes in clinical multidrug‐resistant Pseudomonas aeruginosa isolates from Hamadan, Iran. Molecular Biology Reports, 48, 5275–5281. [DOI] [PubMed] [Google Scholar]
- Gray, J.A. , Chandry, P.S. , Kaur, M. , Kocharunchitt, C. , Bowman, J.P. & Fox, E.M. (2021) Characterisation of listeria monocytogenes food‐associated isolates to assess environmental fitness and virulence potential. International Journal of Food Microbiology, 350, 109247. 10.1016/j.ijfoodmicro.2021.109247 [DOI] [PubMed] [Google Scholar]
- Gray, H.K. , Arora‐Williams, K.K. , Young, C. , Bouwer, E. , Davis, M.F. & Preheim, S.P. (2021) Contribution of time, taxonomy, and selective antimicrobials to antibiotic and multidrug resistance in wastewater bacteria. Environmental Science & Technology, 54, 15946–15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovic, S. , Hardie, K.M. , Brown, M.H. & Skurray, R.A. (2003) Interactions of the QacR multidrug‐binding protein with structurally diverse ligands: implications for the evolution of the binding pocket. Biochemistry, 42, 15226–15236. [DOI] [PubMed] [Google Scholar]
- Guérin, F. , Lallement, C. , Isnard, C. , Dhalluin, A. , Cattoir, V. & Giard, J.C. (2016) Landscape of resistance‐nodulation‐cell division (RND)‐type efflux pumps in Enterobacter cloacae complex. Antimicrobial Agents and Chemotherapy, 60, 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin, A. , Bridier, A. , Le Grandois, P. , Sévelle, Y. , Palm, F. , Félix, B. et al. (2021) Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in listeria monocytogenes. Pathogens, 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin‐Méchin, L. , Leveau, J.Y. & Dubois‐Brissonnet, F. (2004) Resistance of spheroplasts and whole cells of Pseudomonas aeruginosa to bactericidal activity of various biocides: evidence of the membrane implication. Microbiological Research, 159, 51–57. [DOI] [PubMed] [Google Scholar]
- Guo, L. , Long, M. , Huang, Y. , Wu, G. , Deng, W. , Yang, X. et al. (2015) Antimicrobial and disinfectant resistance of Escherichia coli isolated from giant pandas. Journal of Applied Microbiology, 119, 55–64. [DOI] [PubMed] [Google Scholar]
- Hansen, L.H. , Jensen, L.B. , Sørensen, H.I. & Sørensen, S.J. (2007) Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. The Journal of Antimicrobial Chemotherapy, 60, 145–147. [DOI] [PubMed] [Google Scholar]
- Harrison, K.R. , Kappell, A.D. & McNamara, P.J. (2020) Benzalkonium chloride alters phenotypic and genotypic antibiotic resistance profiles in a source water used for drinking water treatment. Environmental Pollution, 257, 113472. [DOI] [PubMed] [Google Scholar]
- Heir, E. , Sundheim, G. & Holck, A.L. (1998) The staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiology Letters, 163, 49–56. [DOI] [PubMed] [Google Scholar]
- Heir, E. , Sundheim, G. & Holck, A.L. (1999a) Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. International Journal of Food Microbiology, 48, 211–219. [DOI] [PubMed] [Google Scholar]
- Heir, E. , Sundheim, G. & Holck, A.L. (1999b) The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. Journal of Applied Microbiology, 86, 378–388. [DOI] [PubMed] [Google Scholar]
- Heir, E. , Lindstedt, B.A. , Røtterud, O.J. , Vardund, T. , Kapperud, G. & Nesbakken, T. (2004) Molecular epidemiology and disinfectant susceptibility of listeria monocytogenes from meat processing plants and human infections. International Journal of Food Microbiology, 96, 85–96. [DOI] [PubMed] [Google Scholar]
- Ho, J. & Branley, J. (2012) Prevalence of antiseptic resistance genes qacA/B and specific sequence types of methicillin‐resistant Staphylococcus aureus in the era of hand hygiene. The Journal of Antimicrobial Chemotherapy, 67, 1549–1550. [DOI] [PubMed] [Google Scholar]
- Houari, A. & Di Martino, P. (2007) Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Letters in Applied Microbiology, 45, 652–656. [DOI] [PubMed] [Google Scholar]
- Huang, J. , O'Toole, P.W. , Shen, W. , Amrine‐Madsen, H. , Jiang, X. , Lobo, N. et al. (2004) Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 48, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.W. , Liou, R.S. , Lin, Y.T. , Huang, H.H. & Yang, T.C. (2014) A linkage between SmeIJK efflux pump, cell envelope integrity, and σE‐mediated envelope stress response in Stenotrophomonas maltophilia . PLoS One, 9(11), e111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignak, S. , Nakipoglu, Y. & Gurler, B. (2017) Frequency of antiseptic resistance genes in clinical staphylococci and enterococci isolates in Turkey. Antimicrobial Resistance & Infection Control, 6, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, S. , Matsumura, Y. , Yoshizako, F. & Tsuchido, T. (2002) Characterization of a cationic surfactant‐resistant mutant isolated spontaneously from Escherichia coli . Journal of Applied Microbiology, 92, 261–268. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization . (2006) ISO: 20776–1. Clinical laboratory testing and in vitro diagnostic test systems: susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1. Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. London, UK: British Standard Institute. [Google Scholar]
- Jennings, M.C. , Forman, M.E. , Duggan, S.M. , Minbiole, K.P.C. & Wuest, W.M. (2017) Efflux pumps might not be the major drivers of QAC resistance in methicillin‐resistant Staphylococcus aureus . ChemBioChem, 18, 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. , Xu, Y. , Li, Y. , Zhang, K. , Liu, L. , Wang, H. et al. (2017) Characterization and horizontal transfer of qacH‐associated class 1 integrons in Escherichia coli isolated from retail meats. International Journal of Food Microbiology, 258, 12–17. [DOI] [PubMed] [Google Scholar]
- Jiang, X. , Yu, T. , Liu, L. , Li, Y. , Zhang, K. , Wang, H. et al. (2017) Examination of quaternary ammonium compound resistance in Proteus mirabilis isolated from cooked meat products in China. Frontiers in Microbiology, 8, 2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. , Ren, S. , Geng, Y. , Yu, T. , Li, Y. , Liu, L. et al. (2020) The sug operon involves in resistance to quaternary ammonium compounds in listeria monocytogenes EGD‐e. Applied Microbiology and Biotechnology, 104, 7093–7104. [DOI] [PubMed] [Google Scholar]
- Johnson, S.A. , Goddard, P.A. , Iliffe, C. , Timmins, B. , Rickard, A.H. , Robson, G. et al. (2002) Comparative susceptibility of resident and transient hand bacteria to Para‐chloro‐meta‐xylenol and triclosan. Journal of Applied Microbiology, 93, 336–344. [DOI] [PubMed] [Google Scholar]
- Joynson, J.A. , Forbes, B. & Lambert, R.J. (2002) Adaptive resistance to benzalkonium chloride, amikacin and tobramycin: the effect on susceptibility to other antimicrobials. Journal of Applied Microbiology, 93, 96–107. [DOI] [PubMed] [Google Scholar]
- Kaatz, G.W. & Seo, S.M. (1995) Inducible NorA‐mediated multidrug resistance in Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 39, 2650–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz, G.W. , Seo, S.M. , O'Brien, L. , Wahiduzzaman, M. & Foster, T.J. (2000) Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 44, 1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz, G.W. & Seo, S.M. (2004) Effect of substrate exposure and other growth condition manipulations on norA expression. The Journal of Antimicrobial Chemotherapy, 54, 364–369. [DOI] [PubMed] [Google Scholar]
- Kaatz, G.W. , McAleese, F. & Seo, S.M. (2005) Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrobial Agents and Chemotherapy, 49, 1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastbjerg, V.G. , Larsen, M.H. , Gram, L. & Ingmer, H. (2010) Influence of sublethal concentrations of common disinfectants on expression of virulence genes in listeria monocytogenes . Applied and Environmental Microbiology, 76, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura‐Sato, K. , Wachino, J. , Kondo, T. , Ito, H. & Arakawa, Y. (2008) Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. The Journal of Antimicrobial Chemotherapy, 61, 568–576. [DOI] [PubMed] [Google Scholar]
- Kawamura‐Sato, K. , Wachino, J. , Kondo, T. , Ito, H. & Arakawa, Y. (2010) Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. The Journal of Antimicrobial Chemotherapy, 65, 1975–1983. [DOI] [PubMed] [Google Scholar]
- Katharios‐Lanwermeyer, S. , Rakic‐Martinez, M. , Elhanafi, D. , Ratani, S. , Tiedje, J.M. & Kathariou, S. (2012) Coselection of cadmium and benzalkonium chloride resistance in conjugative transfers from nonpathogenic listeria spp. to other listeriae. Applied and Environmental Microbiology, 78, 7549–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi, A.D. , Montazeri, E.A. & Maki, S.R. (2021) Antibacterial effects of Octenicept, and benzalkonium chloride on Acinetobacter baumannii strains isolated from clinical samples and determination of genetic diversity of isolates by RAPD‐PCR method. Molecular Biology Reports, 48, 7423–7431. [DOI] [PubMed] [Google Scholar]
- Kim, M. , Weigand, M.R. , Oh, S. , Hatt, J.K. , Krishnan, R. , Tezel, U. et al. (2018) Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Applied and Environmental Microbiology, 84, 1201–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp, L. , Rushton, L. , Stapleton, H. , Sass, A. , Stewart, S. , Amezquita, A. et al. (2013) The effect of cationic microbicide exposure against Burkholderia cepacia complex (bcc); the use of Burkholderia lata strain 383 as a model bacterium. Journal of Applied Microbiology, 115, 1117–1126. [DOI] [PubMed] [Google Scholar]
- Knapp, L. , Amézquita, A. , McClure, P. , Stewart, S. & Maillard, J.‐Y. (2015) Development of a protocol for predicting bacterial resistance to microbicides. Applied and Environmental Microbiology, 81, 2652–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf, G.A. , Cunningham, A.L. , Kazi, M.I. , Riddington, I.M. , Crofts, A.A. , Cattoir, V. et al. (2017) Exploring the antimicrobial action of quaternary amines against Acinetobacter baumannii . mBio, 9, e02394‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf, G.A. , Cunningham, A.L. , Kazi, M.I. , Riddington, I.M. , Crofts, A.A. , Cattoir, V. et al. (2018) Exploring the antimicrobial action of quaternary amines against Acinetobacter baumannii . MBio, 9(1), e02394‐17. 10.1128/mBio.02394-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, P.H. , Lees, J.A. , Koopmans, M.M. , Ferwerda, B. , Arends, A.W.M. , Feller, M.M. et al. (2017) Benzalkonium tolerance genes and outcome in listeria monocytogenes meningitis. Clinical Microbiology and Infection, 23, 265–265.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroning, I.S. , Hubert, L. , Kleiubing, N.R. , Jaskulski, I.B. , Scheik, L.K. , Ramires, T. et al. (2020) New spa types, resistance to sanitisers and presence of efflux pump genes in Staphylococcus aureus from milk. International Dairy Journal, 109, 104712. [Google Scholar]
- Kücken, D. , Feucht, H. & Kaulfers, P. (2000) Association of qacE and qacE1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram‐negative bacteria. FEMS Microbiology Letters, 183, 95–98. [DOI] [PubMed] [Google Scholar]
- Kumar, S. & Doerrler, W.T. (2014) Members of the conserved DedA family are likely membrane transporters and are required for drug resistance in Escherichia coli . Antimicrobial Agents and Chemotherapy, 58, 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBreck, P.T. , Bochi‐Layec, A.C. , Stanbro, K. , Dabbah‐Krancher, G. , Simons, M.P. & Merrell, D.C. (2020) Systematic analysis of efflux pump‐mediated antiseptic resistance in Staphylococcus aureus suggests a need for greater antiseptic stewardship. mSphere, 5, e00959–e00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, R.J. , Joynson, J. & Forbes, B. (2001) The relationships and susceptibilities of some industrial, laboratory and clinical isolates of Pseudomonas aeruginosa to some antibiotics and biocides. Journal of Applied Microbiology, 91, 972–984. [DOI] [PubMed] [Google Scholar]
- Lambert, R.J. (2004) Comparative analysis of antibiotic and antimicrobial biocide susceptibility data in clinical isolates of methicillin‐sensitive Staphylococcus aureus, methicillin‐resistant Staphylococcus aureus and Pseudomonas aeruginosa between 1989 and 2000. Journal of Applied Microbiology, 97, 699–711. [DOI] [PubMed] [Google Scholar]
- Langsrud, S. , Sundheim, G. & Holck, A.L. (2004) Cross‐resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress‐inducers. Journal of Applied Microbiology, 96, 201–208. [DOI] [PubMed] [Google Scholar]
- Lavilla Lerma, L. , Benomar, N. , del Carmen Casado Muñoz, M. , Gálvez, A. & Abriouel, H. (2015) Correlation between antibiotic and biocide resistance in mesophilic and psychrotrophic pseudomonas spp. isolated from slaughterhouse surfaces throughout meat chain production. Food Microbiology, 51, 33e44. [DOI] [PubMed] [Google Scholar]
- Lear, J.C. , Maillard, J.‐Y. , Dettmar, P.W. , Goddard, P.A. & Russell, A.D. (2002) Chloroxylenol‐ and triclosan‐tolerant bacteria from industrial sources. Journal of Industrial Microbiology & Biotechnology, 29, 238–242. [DOI] [PubMed] [Google Scholar]
- Lear, J.C. , Maillard, J.‐Y. , Dettmar, P.W. , Goddard, P.A. & Russell, A.D. (2006) Chloroxylenol‐ and triclosan‐tolerant bacteria from industrial sources ‐ susceptibility to antibiotics and other biocides. International Biodeterioration & Biodegradation, 57, 51–56. [Google Scholar]
- Lee, L.F. , Huang, Y.J. & Chen, C.W. (2003) Repressed multidrug resistance genes in Streptomyces lividans . Archives of Microbiology, 180, 176–184. [DOI] [PubMed] [Google Scholar]
- Lee, M.H. , Caffrey, S.M. , Voordouw, J.K. & Voordouw, G. (2010) Effects of biocides on gene expression in the sulfate‐reducing bacterium Desulfovibrio vulgaris Hildenborough. Applied Microbiology and Biotechnology, 87, 1109–1118. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Iwasaki, T. , Ohtani, S. et al. (2020) Benzalkonium chloride resistance in Staphylococcus epidermidis on the ocular surface of glaucoma patients under long‐term administration of eye drops. Translational Vision Science & Technology, 9(8), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett, M. , Setlow, P. , Sattar, S.A. & Maillard, J.‐Y. (2016) Assessing the activity of microbicides against bacterial spores: knowledge and pitfalls. Journal of Applied Microbiology, 120, 1174–1180. [DOI] [PubMed] [Google Scholar]
- Li, D.W. , Onishi, M. , Kishino, T. , Matsuo, T. , Ogawa, W. , Kuroda, T. et al. (2008) Properties and expression of a multidrug efflux pump AcrAB‐KocC from Klebsiella pneumoniae . Biological & Pharmaceutical Bulletin, 31, 577–582. [DOI] [PubMed] [Google Scholar]
- Ligowska‐Marzeta, M. , Hancock, V. , Ingmer, H. & Aarestrup, F.M. (2019) Comparison of gene expression profiles of uropathogenic Escherichia coli CFT073 after prolonged exposure to subinhibitory concentrations of different biocides. Antibiotics, 8, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.J. , Fu, L. , Huang, M. , Zhang, J.P. , Wu, Y. , Zhou, Y.S. et al. (2017) Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem‐resistant Acinetobacter baumannii . Journal of Medical Microbiology, 66, 13–17. [DOI] [PubMed] [Google Scholar]
- Loughlin, M.F. , Jones, M.V. & Lambert, P.A. (2002) Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane‐active agents but not to clinically relevant antibiotics. The Journal of Antimicrobial Chemotherapy, 49, 631–639. [DOI] [PubMed] [Google Scholar]
- Luna, V.A. , Hall, T.J. , King, D.S. & Cannons, A.C. (2010) Susceptibility of 169 USA300 methicillin‐resistant Staphylococcus aureus isolates to two copper‐based biocides, CuAL42 and CuWB50. The Journal of Antimicrobial Chemotherapy, 65, 939–941. [DOI] [PubMed] [Google Scholar]
- Machado, I. , Lopes, S.P. , Sousa, A.M. & Pereira, M.O. (2012) Adaptive response of single and binary Pseudomonas aeruginosa and Escherichia coli biofilms to benzalkonium chloride. Journal of Basic Microbiology, 52, 43–52. [DOI] [PubMed] [Google Scholar]
- Machado, I. , Coquet, L. , Jouenne, T. & Pereira, M.O. (2013) Proteomic approach to Pseudomonas aeruginosa adaptive resistance to benzalkonium chloride. Journal of Proteomics, 89, 273–279. [DOI] [PubMed] [Google Scholar]
- Maertens, H. , Demeyere, K. , De Reu, K. , Dewulf, J. , Vanhauteghem, D. , Van Coillie, E. et al. (2020) Effect of subinhibitory exposure to quaternary ammonium compounds on the ciprofloxacin susceptibility of Escherichia coli strains in animal husbandry. BMC Microbiology, 20, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard, J.‐Y. (2007) Bacterial resistance to biocides in the healthcare environment: shall we be concerned? The Journal of Hospital Infection, 65(suppl 2), 60–72. [DOI] [PubMed] [Google Scholar]
- Maillard, J.‐Y. (2018) Resistance of bacteria to biocides. Microbiol . Spectrum, 6(2), ARBA_0006‐2017. 10.1128/microbiolspec.ARBA-0006-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard, J.‐Y. , Bloomfield, S. , Rosado Coelho, J. , Collier, P. , Cookson, B. , Fanning, S. et al. (2013) Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microbial Drug Resistance, 19, 344–354. [DOI] [PubMed] [Google Scholar]
- Mangalappalli‐Illathu, A.K. & Korber, D.R. (2006) Adaptive resistance and differential protein expression of salmonella enterica serovar Enteritidis biofilms exposed to benzalkonium chloride. Antimicrobial Agents and Chemotherapy, 50, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manniello, J.M. , Heymann, H. & Adair, F.W. (1978) Resistance of spheroplasts and whole cells of pseudomonas cepacia to polymyxin B. Antimicrobial Agents and Chemotherapy, 14, 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, M. , McCusker, M.P. , McCabe, E.M. , O'Leary, D. , Duffy, G. & Fanning, S. (2013) Evidence of metabolic switching and implications for food safety from the phenome(s) of salmonella enterica serovar Typhimurium DT104 cultured at selected points across the pork production food chain. Applied and Environmental Microbiology, 79, 5437–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzoli, F. , Turchi, B. , Pedonese, F. , Torracca, B. , Bertelloni, F. , Cilia, G. et al. (2021) Coagulase negative staphylococci from ovine bulk‐tank milk: effects of the exposure to sub‐inhibitory concentrations of disinfectants for teat‐dipping. Comp Immuno Microbiol . Infectious Diseases, 76, 101656. 10.1016/j.cimid.2021.101656 [DOI] [PubMed] [Google Scholar]
- Maseda, H. , Hashida, Y. , Konaka, R. , Shirai, A. & Kourai, H. (2009) Mutational upregulation of a resistance‐nodulation‐cell division‐type multidrug efflux pump, SdeAB, upon exposure to a biocide, cetylpyridinium chloride, and antibiotic resistance in Serratia marcescens . Antimicrobial Agents and Chemotherapy, 53, 5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, T. , Ogawa, W. , Tsuchiya, T. & Kuroda, T. (2014) Overexpression of vmeTUV encoding a multidrug efflux transporter of Vibrio parahaemolyticus causes bile acid resistance. Gene, 541, 19–25. [DOI] [PubMed] [Google Scholar]
- Mavri, A. & Smole Možina, S. (2013) Development of antimicrobial resistance in campylobacter jejuni and campylobacter coli adapted to biocides. International Journal of Food Microbiology, 160, 304–312. [DOI] [PubMed] [Google Scholar]
- Meade, E. , Slattery, M.A. & Garvey, M. (2021) Biocidal resistance in clinically relevant microbial species: a major public health risk. Pathogens, 10, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, A.B. , Guldimann, C. , Markkula, A. , Pöntinen, A. , Korkeala, H. & Tasara, T. (2017) Comparative phenotypic and genotypic analysis of Swiss and Finnish listeria monocytogenes isolates with respect to benzalkonium chloride resistance. Frontiers in Microbiology, 8, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchel Piovesan Pereira, B. , Wang, X. & Tagkopoulos, I. (2020) Short‐ and long‐term transcriptomic responses of Escherichia coli to biocides: a systems analysis. Applied and Environmental Microbiology, 86, e00708‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereghetti, L. , Quentin, R. , Marquet‐Van Der Mee, N. & Audurier, A. (2000) Low sensitivity of listeria monocytogenes to quaternary ammonium compounds. Applied and Environmental Microbiology, 66, 5083–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen, B. , Rudi, K. , Bore, E. & Langsrud, S. (2012) Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of Escherichia coli with inheritable characteristics. International Journal of Molecular Sciences, 13, 4101–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moken, M.C. , McMurry, L.M. & Levy, S.B. (1997) Selection of multiple‐antibiotic‐resistant (mar) mutants of Escherichia coli by using the disinfectant pine oil: roles of the mar and acrAB loci. Antimicrobial Agents and Chemotherapy, 41, 2770–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møretrø, T. , Schirmer, B.C.T. , Heir, E. , Fagerlund, A. , Hjemli, P. & Langsrud, S. (2017) Tolerance to quaternary ammonium compound disinfectants may enhance growth of listeria monocytogenes in the food industry. International Journal of Food Microbiology, 241, 215–224. [DOI] [PubMed] [Google Scholar]
- Morita, Y. , Murata, T. , Mima, T. , Shiota, S. , Kuroda, T. , Mizushima, T. et al. (2003) Induction of mexCD‐oprJ operon for a multidrug efflux pump by disinfectants in wild‐type Pseudomonas aeruginosa PAO1. The Journal of Antimicrobial Chemotherapy, 51, 991–994. [DOI] [PubMed] [Google Scholar]
- Morrissey, I. , Oggioni, M.R. , Knight, D. et al. (2004) Evaluation of epidemiological cut‐off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically‐relevant microorganisms. PLoS One, 9(1), e86669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullapudi, S. , Siletzky, R.M. & Kathariou, S. (2008) Heavy‐metal and benzalkonium chloride resistance of listeria monocytogenes isolates from the environment of Turkey‐processing plants. Applied and Environmental Microbiology, 74, 1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, A. , Rychli, K. , Muhterem‐Uyar, M. , Zaiser, A. , Stessl, B. , Guinane, C.M. et al. (2013) Tn6188 ‐ a novel transposon in listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS One, 8(10), e76835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, A. , Rychli, K. , Zaiser, A. , Wieser, C. , Wagner, M. & Schmitz‐Esser, S. (2014) The listeria monocytogenes transposon Tn6188 provides increased tolerance to various quaternary ammonium compounds and ethidium bromide. FEMS Microbiology Letters, 361, 166–173. [DOI] [PubMed] [Google Scholar]
- Murray, J.L. , Kwon, T. , Marcotte, E.M. & Whiteley, M. (2015) Intrinsic antimicrobial resistance determinants in the superbug Pseudomonas aeruginosa . MBio, 6(6), e01603‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, K. , Murata, T. , Ohta, S. , Zenda, H. , Ohnishi, M. & Hayashi, T. (2003) Two different mechanisms are involved in the extremely high‐level benzalkonium chloride resistance of a Pseudomonas fluorescens strain. Microbiology and Immunology, 47, 709–715. [DOI] [PubMed] [Google Scholar]
- Namaki, M. , Habibzadeh, S. , Vaez, H. , Arzanlou, M. , Safarirad, S. , Bazghandi, S.A. et al. (2022) Prevalence of resistance genes to biocides in antibiotic‐resistant Pseudomonas aeruginosa clinical isolates. Molecular Biology Reports, 49, 2149–2155. 10.1007/s11033-021-07032-2 [DOI] [PubMed] [Google Scholar]
- Nhung, N.T. , Thuy, C.T. , Trung, N.V. et al. (2015) Induction of antimicrobial resistance in Escherichia coli and non‐Typhoidal salmonella strains after adaptation to disinfectant commonly used on farms in Vietnam. Antibiotics, 4, 480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino, K. , Latifi, T. & Groisman, E.A. (2006) Virulence and drug resistance roles of multidrug efflux systems of salmonella enterica serovar Typhimurium. Molecular Microbiology, 59, 126–141. [DOI] [PubMed] [Google Scholar]
- Noll, M. , Trunzer, K. , Vondran, A. , Vincze, S. , Dieckmann, R. , al Dahouk, S. et al. (2020) Benzalkonium chloride induces a VBNC state in Listeria monocytogenes . Microorganisms, 8, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordholt, N. , Kanaris, O. , Schmidt, S.B.I. & Schreiber, F. (2021) Persistence against benzalkonium chloride promotes rapid evolution of tolerance during periodic disinfection. Nature Communications, 12, 6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggioni, M.R. , Coelho, J.R. , Furi, L. , Knight, D.R. , Viti, C. , Orefici, G. et al. (2015) Significant differences characterise the correlation coefficients between biocide and antibiotic susceptibility profiles in Staphylococcus aureus . Current Pharmaceutical Design, 21, 2054–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz, S. , López, V. & Martínez‐Suárez, J.V. (2014a) The influence of subminimal inhibitory concentrations of benzalkonium chloride on biofilm formation by listeria monocytogenes . International Journal of Food Microbiology, 189, 106–112. [DOI] [PubMed] [Google Scholar]
- Ortiz, S. , López, V. & Martínez‐Suárez, J.V. (2014b) Control of listeria monocytogenes contamination in an Iberian pork processing plant and selection of benzalkonium chloride‐resistant strains. Food Microbiology, 39, 1–8. [DOI] [PubMed] [Google Scholar]
- Orús, P. , Gomez‐Perez, L. , Leranoz, S. & Berlanga, M. (2015) Increasing antibiotic resistance in preservative‐tolerant bacterial strains isolated from cosmetic products. International Microbiology, 18, 51–59. [DOI] [PubMed] [Google Scholar]
- Oyanagi, T. , Tagami, J. & Matin, K. (2012) Potentials of mouthwashes in disinfecting cariogenic bacteria and biofilms leading to inhibition of caries. The Open Dentistry Journal, 6, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagedar, A. , Singh, J. & Batish, V.K. (2011) Efflux mediated adaptive and cross resistance to ciprofloxacin and benzalkonium chloride in Pseudomonas aeruginosa of dairy origin. Journal of Basic Microbiology, 51, 289–295. [DOI] [PubMed] [Google Scholar]
- Pagedar, A. , Singh, J. & Batish, V.K. (2012) Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. The Journal of Dairy Research, 79, 383–389. [DOI] [PubMed] [Google Scholar]
- Pang, X. , Chen, L. & Yuk, H.‐G. (2020) Stress response and survival of salmonella Enteritidis in single and dual species biofilms with Pseudomonas fluorescens following repeated exposure to quaternary ammonium compounds. International Journal of Food Microbiology, 325, 108643. [DOI] [PubMed] [Google Scholar]
- Paulsen, I.T. , Littlejohn, T.G. , Rådström, P. , Sundström, L. , Sköld, O. , Swedberg, G. et al. (1993) The 3' conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrobial Agents and Chemotherapy, 37, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, I.T. , Brown, M.H. , Dunstan, S.J. & Skurray, R.A. (1995) Molecular characterization of the staphylococcal multidrug resistance export protein QacC. Journal of Bacteriology, 177, 2827–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali, F. , Palma, F. , Guillier, L. , Lucchi, A. , De Cesare, A. & Manfreda, G. (2018) Listeria monocytogenes sequence types 121 and 14 repeatedly isolated within one year of sampling in a rabbit meat processing plant: persistence and ecophysiology. Frontiers in Microbiology, 9, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, B.M.P. , Wang, X.K. & Tagkopoulos, I. (2021) Biocide‐induced emergence of antibiotic resistance in Escherichia coli . Frontiers in Microbiology, 12, 640923. 10.3389/fmicb.2021.640923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrat, M.B. , Soumet, C. , Maris, P. & Sanders, P. (2008) Phenotypes and genotypes of campylobacter strains isolated after cleaning and disinfection in poultry slaughterhouses. Veterinary Microbiology, 128, 313–326. [DOI] [PubMed] [Google Scholar]
- Piercey, M.J. , Ells, T.C. , Macintosh, A.J. & Truelstrup Hansen, L. (2017) Variations in biofilm formation, desiccation resistance and benzalkonium chloride susceptibility among listeria monocytogenes strains isolated in Canada. International Journal of Food Microbiology, 257, 254–261. [DOI] [PubMed] [Google Scholar]
- Pietsch, F. , Heidrich, G. , Nordholt, N. & Schreiber, F. (2021) Prevalent synergy and antagonism among antibiotics and biocides in Pseudomonas aeruginosa . Frontiers in Microbiology, 11, 615618. 10.3389/fmicb.2020.615618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puangseree, J. , Jeamsripong, S. , Prathan, R. , Pungpian, C. & Chuanchuen, R. (2021) Resistance to widely‐used disinfectants and heavy metals and cross resistance to antibiotics in Escherichia coli isolated from pigs, pork and pig carcass. Food Control, 24, 107892. 10.1016/j.foodcont.2021.107892 [DOI] [Google Scholar]
- Rajamohan, G. , Srinivasan, V.B. & Gebreyes, W.A. (2009) Biocide‐tolerant multidrug‐resistant Acinetobacter baumannii clinical strains are associated with higher biofilm formation. The Journal of Hospital Infection, 73, 287–289. [DOI] [PubMed] [Google Scholar]
- Rajamohan, G. , Srinivasan, V.B. & Gebreyes, W.A. (2010a) Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii . The Journal of Antimicrobial Chemotherapy, 65, 1919–1925. [DOI] [PubMed] [Google Scholar]
- Rajamohan, G. , Srinivasan, V.B. & Gebreyes, W.A. (2010b) Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. The Journal of Antimicrobial Chemotherapy, 65, 228–232. [DOI] [PubMed] [Google Scholar]
- Rakic‐Martinez, M. , Drevets, D.A. , Dutta, V. , Katic, V. & Kathariou, S. (2011) Listeria monocytogenes strains selected on ciprofloxacin or the disinfectant benzalkonium chloride exhibit reduced susceptibility to ciprofloxacin, gentamicin, benzalkonium chloride, and other toxic compounds. Applied and Environmental Microbiology, 77, 8714–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos‐Castillo, A.G. , Fontecha Umaña, F. & Rodríguez‐Jerez, J.J. (2018) Long‐term antibacterial efficacy of disinfectants based on benzalkonium chloride and sodium hypochlorite tested on surfaces against resistant gram‐positive bacteria. Food Control, 93, 219–225. [Google Scholar]
- Rodriguez‐Mozaz, S. , Vaz‐Moreira, I. , Giustina, S.V.D. , Llorca, M. , Barceló, D. , Schubert, S. et al. (2020) Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environment International, 140, 105733. [DOI] [PubMed] [Google Scholar]
- Roedel, A. , Dieckmann, R. , Brendebach, H. , Hammerl, J.A. , Kleta, S. , Noll, M. et al. (2019) Biocide‐tolerant listeria monocytogenes isolates from German food production plants do not show cross‐resistance to clinically relevant antibiotics. Applied and Environmental Microbiology, 85, e01253‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedel, A. , Vincze, S. , Projahn, M. , Roesler, U. , Robe, C. , Hammerl, J.A. et al. (2021) Genetic but no phenotypic associations between biocide tolerance and antibiotic resistance in Escherichia coli from German broiler fattening farms. Microorganisms, 9, 651. 10.3390/microorganisms9030651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova, N. , Favrin, S. & Griffiths, M.W. (2002) Sensitivity of listeria monocytogenes to sanitizers used in the meat processing industry. Applied and Environmental Microbiology, 68, 6405–6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova, N.A. , Wolffs, P.F. , Brovko, L.Y. & Griffiths, M.W. (2006) Role of efflux pumps in adaptation and resistance of listeria monocytogenes to benzalkonium chloride. Applied and Environmental Microbiology, 72, 3498–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão, C. , Miranda, C.A. , Silva, J. , Mandetta Clementino, M. , de Filippis, I. & Asensi, M. (2011) Presence of qacEΔ1 gene and susceptibility to a hospital biocide in clinical isolates of Pseudomonas aeruginosa resistant to antibiotics. Current Microbiology, 63, 16–21. [DOI] [PubMed] [Google Scholar]
- Romeu, M.J. , Rodrigues, D. & Azeredo, J. (2020) Effect of sublethal chemical disinfection on the biofilm forming ability, resistance to antibiotics and expression of virulence genes of salmonella Enteritidis biofilm‐surviving cells. Biofouling, 36, 101–112. [DOI] [PubMed] [Google Scholar]
- Rose, H. , Baldwin, A. , Dowson, C.G. & Mahenthiralingam, E. (2009) Biocide susceptibility of the Burkholderia cepacia complex. The Journal of Antimicrobial Chemotherapy, 63, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, R.C. , Sheskey, P.J. & Quinn, M.E. (Eds.). (2009) Handbook of pharmaceutical excipients, 6th edition. London: Pharmaceutical Press. [Google Scholar]
- Russell, A.D. & McDonnell, G. (2000) Concentration: a major factor in studying biocidal action. The Journal of Hospital Infection, 44, 1–3. [DOI] [PubMed] [Google Scholar]
- Rushton, L. , Sass, A. , Baldwin, A. , Dowson, C.G. , Donoghue, D. & Mahenthiralingam, E. (2013) Key role for efflux in the preservative susceptibility and adaptive resistance of Burkholderia cepacia complex bacteria. Antimicrobial Agents and Chemotherapy, 57, 2972–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, M.B. , Decorosi, F. , Viti, C. , Oggioni, M.R. , Martínez, J.L. & Hernández, A. (2015) Predictive studies suggest that the risk for the selection of antibiotic resistance by biocides is likely low in Stenotrophomonas maltophilia . PLoS One, 10(7), e0132816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsudin, M.N. , Alreshidi, M.A. , Hamat, R.A. , Alshrari, A.S. , Atshan, S.S. & Neela, V. (2012) High prevalence of qacA/B carriage among clinical isolates of meticillin‐resistant Staphylococcus aureus in Malaysia. The Journal of Hospital Infection, 81, 206–208. [DOI] [PubMed] [Google Scholar]
- Short, F.L. , Lee, V. , Mamun, R. , Malmberg, R. , Li, L. , Espinosa, M.I. et al. (2021) Benzalkonium chloride antagonises aminoglycoside antibiotics and promotes evolution of resistance. eBioMedicine, 73, 103653. 10.1016/j.ebiom.2021.103653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderakou, D. , Zilelidou, E. , Poimenidou, S. , Tsipra, I. , Ouranou, E. , Papadimitriou, K. et al. (2021) Assessing the survival and sublethal injury kinetics of listeria monocytogenes under different food processing‐related stresses. International Journal of Food Microbiology, 346, 109159. 10.1016/j.ijfoodmicro.2021.109159 [DOI] [PubMed] [Google Scholar]
- Sidhu, M.S. , Heir, E. , Leegaard, T. , Wiger, K. & Holck, A. (2002) Frequency of disinfectant resistance genes and genetic linkage with beta‐lactamase transposon Tn552 among clinical staphylococci. Antimicrobial Agents and Chemotherapy, 46, 2797–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifierz, M.J. , Friendship, R.M. & Weese, J.S. (2015) Methicillin‐resistant Staphylococcus aureus in commercial swine herds is associated with disinfectant and zinc usage. Applied and Environmental Microbiology, 81, 2690–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumet, C. , Fourreau, E. , Legrandois, P. & Maris, P. (2012) Resistance to phenicol compounds following adaptation to quaternary ammonium compounds in Escherichia coli . Veterinary Microbiology, 158, 147–152. [DOI] [PubMed] [Google Scholar]
- Soumet, C. , Ragimbeau, C. & Maris, P. (2005) Screening of benzalkonium chloride resistance in listeria monocytogenes strains isolated during cold smoked fish production. Letters in Applied Microbiology, 41, 291–296. [DOI] [PubMed] [Google Scholar]
- Srinivasan, V.B. & Rajamohan, G. (2013) KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR‐type efflux pump involved in broad‐spectrum antimicrobial resistance. Antimicrobial Agents and Chemotherapy, 57, 4449–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, V.B. , Rajamohan, G. & Gebreyes, W.A. (2009) Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy, 53, 5312–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, V.B. , Rajamohan, G. , Pancholi, P. , Marcon, M. & Gebreyes, W.A. (2011) Molecular cloning and functional characterization of two novel membrane fusion proteins in conferring antimicrobial resistance in Acinetobacter baumannii . The Journal of Antimicrobial Chemotherapy, 66, 499–504. [DOI] [PubMed] [Google Scholar]
- Srinivasan, V.B. , Vaidyanathan, V. , Mondal, A. & Rajamohan, G. (2012) Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH‐K2044. PLoS One, 7(4), e33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, V.B. , Venkataramaiah, M. , Mondal, A. , Vaidyanathan, V. , Govil, T. & Rajamohan, G. (2012) Functional characterization of a novel outer membrane porin KpnO, regulated by PhoBR two‐component system in Klebsiella pneumoniae NTUH‐K2044. PLoS One, 7(7), e41505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, V.B. , Singh, B.B. , Priyadarshi, N. , Chauhan, N.K. & Rajamohan, G. (2014) Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae . PLoS One, 9(5), e96288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickler, D.J. & Thomas, B. (1976) Sensitivity of providence to antiseptics and disinfectants. Journal of Clinical Pathology, 29, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitsova, S.O. , Braun, Y. , Ullrich, M.S. & Weingart, H. (2008) Characterization of the RND‐type multidrug efflux pump MexAB‐OprM of the plant pathogen pseudomonas syringae . Applied and Environmental Microbiology, 74, 3387–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufya, N. , Allison, D.G. & Gilbert, P. (2003) Clonal variation in maximum specific growth rate and susceptibility towards antimicrobials. Journal of Applied Microbiology, 95, 1261–1267. [DOI] [PubMed] [Google Scholar]
- Tabata, A. , Nagamune, H. , Maeda, T. , Murakami, K. , Miyake, Y. & Kourai, H. (2003) Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR. Antimicrobial Agents and Chemotherapy, 47, 2093–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambe, S.M. , Sampath, L. & Modak, S.M. (2011) In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. The Journal of Antimicrobial Chemotherapy, 47, 589–598. [DOI] [PubMed] [Google Scholar]
- Tamburro, M. , Ripabelli, G. , Vitullo, M. , Dallman, T.J. , Pontello, M. , Amar, C.F.L. et al. (2015) Gene expression in listeria monocytogenes exposed to sublethal concentration of benzalkonium chloride. Comparative Immunology, Microbiology and Infectious Diseases, 40, 31–39. [DOI] [PubMed] [Google Scholar]
- Tandukar, M. , Oh, S. , Tezel, U. , Konstantinidis, K.T. & Pavlostathis, S.G. (2013) Long‐term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environmental Science & Technology, 47, 9730–9738. [DOI] [PubMed] [Google Scholar]
- To, M.S. , Favrin, S. , Romanova, N. & Griffiths, M.W. (2002) Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of listeria monocytogenes . Applied and Environmental Microbiology, 68, 5258–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen, S. & Abee, T. (2010) Importance of SigB for listeria monocytogenes static and continuous‐flow biofilm formation and disinfectant resistance. Applied and Environmental Microbiology, 76, 7854–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voumard, M. , Venturelli, L. , Borgatta, M. , Croxatto, A. , Kasas, S. , Dietler, G. et al. (2020) Adaptation of Pseudomonas aeruginosa to constant sub‐inhibitory concentrations of quaternary ammonium compounds. Environmental Science: Water Research & Technology, 6, 1139–1152. [Google Scholar]
- Wand, M.E. , Baker, K.S. , Benthall, G. , McGregor, H. , McCowen, J.W.I. , Deheer‐Graham, A. et al. (2015) Characterization of pre‐antibiotic era Klebsiella pneumoniae isolates with respect to antibiotic/disinfectant susceptibility and virulence in galleria mellonella . Antimicrobial Agents and Chemotherapy, 59, 3966–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Zhan, Q. , Mi, Z. , Huang, Z. & Chen, G. (2008) Distribution of the antiseptic‐resistance gene qacE1 in 283 clinical isolates of Gram‐negative bacteria in China. The Journal of Hospital Infection, 69, 394–396. [DOI] [PubMed] [Google Scholar]
- Weber, D.J. , Rutala, W.A. & Sickbert‐Bennett, E.E. (2007) Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrobial Agents and Chemotherapy, 12, 4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesgate, R. , Grascha, P. & Maillard, J.‐Y. (2016) The use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. American Journal of Infection Control, 44, 458–464. [DOI] [PubMed] [Google Scholar]
- Wong, H.S. , Townsend, K.M. , Fenwick, S.G. , Trengove, R.D. & O'Handley, R.M. (2010) Comparative susceptibility of planktonic and 3‐day‐old salmonella Typhimurium biofilms to disinfectants. Journal of Applied Microbiology, 108, 2222–2228. [DOI] [PubMed] [Google Scholar]
- Wong, T.Z. , Zhang, M. , O'Donoghue, M. & Boost, M. (2013) Presence of antiseptic resistance genes in porcine methicillin‐resistant Staphylococcus aureus . Veterinary Microbiology, 162, 977–979. [DOI] [PubMed] [Google Scholar]
- Worthing, K.A. , Marcus, A. , Abraham, S. , Trott, D.J. & Norris, J.M. (2018) Qac genes and biocide tolerance in clinical veterinary methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus and staphylococcus pseudintermedius . Veterinary Microbiology, 216, 153–158. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T. , Tamura, Y. & Yokota, T. (1988) Antiseptic and antibiotic resistance plasmid in Staphylococcus aureus that possesses ability to confer chlorhexidine and acrinol resistance. Antimicrobial Agents and Chemotherapy, 32, 932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R. , Buckley, L. , McEwan, N. & Nuttall, T. (2011) Comparative in vitro efficacy of antimicrobial shampoos: a pilot study. Veterinary Dermatology, 23, 36–40. [DOI] [PubMed] [Google Scholar]
- Yu, T. , Ma, M.Y. , Sun, Y.X. , Xu, X.B. , Qiu, S.X. , Yin, J.L. et al. (2021) The effect of sublethal concentrations of benzalkonium chloride on the LuxS/AI‐2 quorum sensing system, biofilm formation and motility of Escherichia coli . International Journal of Food Microbiology, 353, 109313. 10.1016/j.ijfoodmicro.2021.109313 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , O'Donoghue, M.M. , Ito, T. , Hiramatsu, K. & Boost, M.V. (2011) Prevalence of antiseptic‐resistance genes in Staphylococcus aureus and coagulase‐negative staphylococci colonising nurses and the general population in Hong Kong. The Journal of Hospital Infection, 78, 113–117. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , O'Dononghue, M. & Boost, M.V. (2012) Characterization of staphylococci contaminating automated teller machines in Hong Kong. Epidemiology and Infection, 140, 1366–1371. [DOI] [PubMed] [Google Scholar]
- Zmantar, T. , Kouidhi, B. , Miladi, H. & Bakhrouf, A. (2011) Detection of macrolide and disinfectant resistance genes in clinical Staphylococcus aureus and coagulase‐negative staphylococci. BMC Research Notes, 4, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
Supplementary tables on which this manuscript is based are available as supplementary materials and have been uploaded alongside the manuscript


