Abstract
The endocannabinoid system (ECS) is comprised of a set of lipid-derived messengers (the endocannabinoids, ECBs), proteins that control their production and degradation, and cell-surface cannabinoid (CB) receptors that transduce their actions. ECB molecules such as 2-arachidonoyl-sn-glycerol (2-AG) and anandamide (arachidonoyl ethanolamide) are produced on demand and deactivated through enzymatic actions tightly regulated both temporally and spatially, serving homeostatic roles in order to respond to various challenges to the body. Key components of the ECS are present in the hypothalamus-pituitary-gonadal (HPG) axis, which plays critical roles in the development and regulation of the reproductive system in both males and females. ECB signaling controls the action at each stage of the HPG axis through CB receptors expressed in the hypothalamus, pituitary, and reproductive organs such as the testis and ovary. It regulates the secretion of hypothalamic gonadotropin-releasing hormone (GnRH), pituitary follicle-stimulating hormone (FSH) and luteinizing hormone (LH), estrogen, testosterone, and affects spermatogenesis in males. Δ9-tetrahydrocannabinol (THC) and other phytocannabinoids from Cannabis sativa affect a variety of physiological processes by altering, or under certain conditions hijacking, the ECB system. Therefore, phytocannabinoids, in particular THC, may modify the homeostasis of the HPG axis by altering CB receptor signaling and cause deficits in reproductive function. While the ability of phytocannabinoids, THC and/or cannabidiol (CBD), to reduce pain and inflammation provides promising opportunities for therapeutic intervention for genitourinary and degenerative disorders, important questions remain regarding their unwanted long-term effects. It is nevertheless clear that the therapeutic potential of modulating the ECS calls for further scientific and clinical investigation.
Keywords: Cannabinoids, Endocannabinoids, Male, Reproduction, Spermatogenesis, Therapeutics
INTRODUCTION
The prevalence of cannabis use in the United States increases every year [1,2], and it is likely to keep growing as the availability of cannabis-based products continues to increase and risk perception by the general public of their adverse effects is lowered [3,4,5]. In particular, recent approval of the cannabidiol (CBD) medicine, Epidiolex® (GW Pharmaceuticals, Cambridge, UK), by the U.S. Food and Drug Administration (FDA) for the treatment of seizures associated with severe forms of epilepsy further accelerated the use of this non-psychotropic cannabinoid [5,6,7,8,9]. Furthermore, off-label uses of CBD from both physician’s recommendation and self-treatment are increasing [6,7,8,9].
Initial research on the plant Cannabis sativa (marijuana) focused on understanding its toxicity to humans because it was considered an addictive, illegal, and only harmful psychotropic drug without medical benefits; however, thanks to recent research we now have a much better understanding of the plant [10,11,12,13]. Δ9-tetrahydrocannabinol (THC), the main psychoactive and intoxicating substance of cannabis, was first described in the 1940s [14,15], and fully characterized in 1964 by precisely defining the structure to be (–)-trans-Δ9-tetrahydrocannabinol [16]. Two isoforms of cannabinoid (CB) receptors were discovered, cloned, and determined to be the direct bodily target proteins for THC in the 1990s [17,18,19]. THC activates and under certain conditions hijacks/over-stimulates CB receptors to exert its various pharmacological effects [10]. Interestingly, research later revealed the existence of unique lipid neurotransmitters that are produced by the body that serve as agonists for CB receptors: the endocannabinoids (ECBs) [12]. In addition, the biosynthetic and metabolizing pathways regulating local levels of ECBs have been characterized [10,13]. Research suggests that the endocannabinoid system (ECS) is a complex but essential signaling system found in most body organs where it serves regulatory roles for many biological functions including control of emotions, learning and memory, regulation of food intake and energy metabolism, regulation of body temperature, pain reception, immune response and inflammation, and maintaining body homeostasis.
Research during the last two decades also proposed that the ECS is a key modulator for reproductive functions in males and females. In this mini-review, we provide a brief overview of current knowledge about the ECS in the male reproductive system. Most, if not all, molecular components of the ECS are present in the male reproductive system, where ECB signals are thought to control the homeostasis of the hypothalamus-pituitary-gonadal (HPG) axis and critical testicular physiology, including spermatogenesis and the functions of Leydig and Sertoli cells [20,21,22,23]. Then, we discuss the potential therapeutic utility of CBs in treating male genitourinary disorders, but also their possible side effects on male reproduction.
THE ENDOCANNABINOID SYSTEM
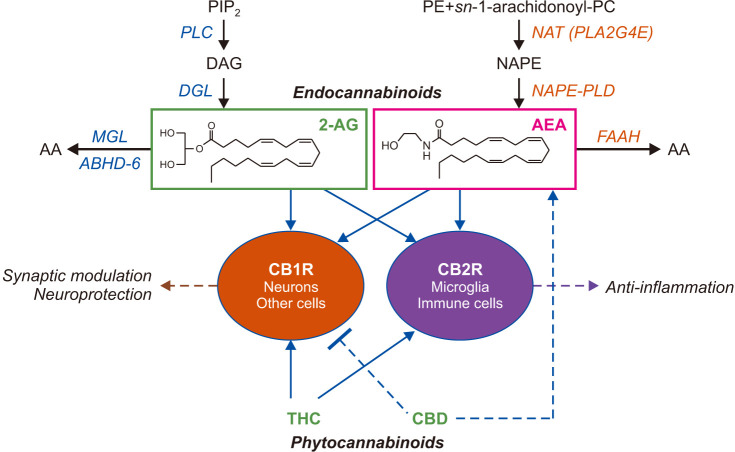
The ECS is comprised of two G protein-coupled cell-surface receptors, CB1 and CB2, two lipid-derived ECB molecules — arachidonoyl ethanolamide (anandamide, AEA) and 2-arachidonoyl-sn-glycerol (2-AG) — and proteins involved in the formation, transport, and deactivation of ECB molecules [10] (Fig. 1). The activation of the ECS is regulated through the expression of CB1 and CB2 receptors and their coupling with intracellular signaling pathways, as well as temporal and local changes in the concentration of ECB molecules, which are produced on demand through cleavage of distinct phospholipid precursors.
Fig. 1. Simplified overview of the endocannabinoid (ECB) system. The ECB system is comprised of the CB1 and CB2 cannabinoid receptors (CB1R and CB2R), the endogenous ligands for CB receptors, anandamide (arachidonoyl ethanolamide, AEA) and 2-arachidonoyl-sn-glycerol (2-AG), and proteins involved in the biosynthesis and inactivation of ECBs. Receptor-operated phospholipase C (PLC) converts phosphatidylinositol-4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG). DAG is hydrolyzed by diacylglycerol lipase (DGL) forming 2-AG. 2-AG is subjected to hydrolytic cleavage catalyzed by monoacylglycerol lipase (MGL) or, to a lesser extent, α,β-hydrolase domain-containing protein 6 (ABHD-6). The biosynthesis of AEA starts from the production of N-arachidonoyl-phosphatidylethanolamine (NAPE), through the transfer of an arachidonate group from the sn-1 position of 1,2-diarachidonoyl-phosphatidylcholine (PC) to the free amino group of phosphatidylethanolamine (PE). NAPE is converted to AEA, catalyzed by a unique phospholipase D (PLD). AEA is degraded by the intracellular serine amidase, fatty acid amide hydrolase (FAAH). These ECB molecules bind and activate both CB1R and CB2R, which are also targeted by exogenously administered phytocannabinoids.
1. Cannabinoid receptors
CB receptors are found in the central nervous system (CNS) and various peripheral organs where they serve important regulatory functions in synaptic plasticity, signal transduction, and inflammation [24]. In humans, the CB1 receptor is encoded by the CNR1 gene whereas the CNR2 gene encodes CB2 receptors. CB1 and CB2 receptors are highly homologous, sharing 48% identity in amino acid sequence. Both receptors signal through the transducing G proteins, Gi and Go [25,26]. The CB1 receptor is one of the most abundantly expressed receptors in the brain and mainly localized to the presynaptic axon terminals of both excitatory glutamatergic and inhibitory γ-amino-butyric acid (GABA)-ergic neurons [26]. As expected from their subcellular localization, CB1 receptors regulate neural activities by controlling synaptic transmission mediated by well-known classical neurotransmitters, such as glutamate and GABA. In the neuronal presynaptic axons, activation of CB1 receptors inhibits Ca2+ channel activity to reduce neurotransmitter release, and elevates K+ channel activity to suppress membrane excitability [10,26,27]. In addition, neuronal activity, such as elevation of intracellular calcium concentration in the postsynaptic spines, may trigger temporal biosynthesis of ECB molecules, which acts retrogradely on presynaptic CB1 receptors across the synaptic cleft to modulate neurotransmitter release. Therefore, it was proposed that ECB signaling serves as a prompt negative feedback mechanism for synaptic activities, i.e., a synaptic circuit breaker [27]. Other brain cells, such as astrocytes and microglia, also express CB1 receptors [28,29,30], but their precise functions need to be elucidated further. Outside the CNS, CB1 receptors are expressed in the peripheral nervous system, including liver, pancreas, small intestine, and skeletal muscle [24,31] and have been linked to diverse influences exerted by ECB messengers to maintain bodily homeostasis [32], such as control of lipogenesis in the liver [33].
The CB2 receptor is mainly found in cellular constituents of the immune systems–including monocyte-derived cells and lymphocytes [34]. CB2 receptor signaling mainly plays a role in regulating inflammation, cytokine release, cell migration, and apoptosis. It is of note that the psychoactive properties of cannabis, an unwanted side effect when used for medical purposes, are mediated by the CB1 receptor in the CNS, but not by the CB2 receptor, making it a particularly appealing target for drug development.
Although only limited information is available, the presence of CB receptors in the reproductive system has been reported and their functional importance has been proposed. In humans, protein expression of both isoforms of CB receptors has been detected, albeit at a low level, in post-meiotic germ, Leydig, and peritubular cells [35]. Transcripts encoding both isoforms of the CB receptors were also found in germ cells, and their differential distribution has been noted [35].
2. Endocannabinoid molecules
While THC and other synthetic CB receptor agonists hijack the endogenous CB receptor-mediated signaling to exert their pharmacological effects, ECB molecules serve homeostatic roles through activating CB receptors to respond to various challenges to the CNS and the periphery. To achieve this, the production and deactivation/hydrolysis of ECB molecules is tightly controlled through precise enzymatic actions [10,13].
AEA and 2-AG are the two best-characterized ECB molecules found in mammalian tissues [36,37,38]. During the 1990s, the first ECB molecule was discovered and named ‘anandamide’ after the Sanskrit word ‘nanda’, meaning ‘happiness’ [36]. Later, it was further discovered that another lipid molecule, 2-AG, which is present in large amounts in the brain, also binds and activates CB receptors to play a major role in neuronal synapses [37,38].
As shown in Fig. 1, AEA formation starts with the transfer of an arachidonate group from the sn-1 position of 1,2-diarachidonoyl-phosphatidylcholine to the free amino group of phosphatidylethanolamine (PE), which produces the AEA precursor N-arachidonoyl-PE (NAPE) [39,40]. This reaction is catalyzed by the calcium-dependent N-acyl transferase (NAT) activity of an isoform of phospholipase A2, PLA2G4E [41]. Hydrolytic cleavage of NAPE by an isoform of phospholipase D (PLD), the NAPE-PLD, produces AEA [42,43]. After biosynthesis, AEA diffuses out of the cell into the external milieu and activates CB receptors. AEA is deactivated through internalization into cells followed by intracellular hydrolysis catalyzed by the serine amidase fatty acid amide hydrolase (FAAH) [44] (Fig. 1).
Like AEA, 2-AG is also produced upon demand (Fig. 1). The membrane phospholipid that serves as its precursor, phosphatidylinositol-4,5-bisphosphate (PIP2), is first hydrolyzed by phospholipase C (PLC), probably PLC-β and/or PLC-ε [45,46], to produce 1,2-diacylglycerol (DAG). DAG is then cleaved by the α or β isoform of diacylglycerol lipase (DGL or DAGL) to generate 2-AG [47,48,49]. In excitatory glutamatergic neurons of the brain, PLC and DGL-α are physically and functionally linked to type-5 metabotropic glutamate receptors in a multimolecular complex (the ‘endocannabinoid signalosome’) that enables efficient retrograde signaling from the postsynaptic dendritic spine to the axon terminal [49]. 2-AG is inactivated by enzymatic activities of the lipid hydrolases, monoacylglycerol lipase (MGL or MAGL) and, to a lesser extent, α/β-hydrolase domain-containing protein 6 (ABHD-6) [50,51,52] (Fig. 1).
The reproductive organs of mammals contain the entire repertoire of proteins needed to produce and degrade ECB molecules [35,53]. The main biosynthesizing enzymes, DGL and NAPE-PLD, were detected in germ cells and somatic cells, respectively [35,54,55,56]. In addition, abundant expression of the 2-AG-hydrolyzing enzyme, MGL, was observed in Sertoli cells, whereas the AEA-hydrolyzing enzyme, FAAH, was found in late spermatocytes and post-meiotic germ cells [35]. The presence of ECB molecules was also confirmed in the human testis [35].
PHYSIOLOGICAL ROLES OF ENDOCANNABINOID IN MALE REPRODUCTION
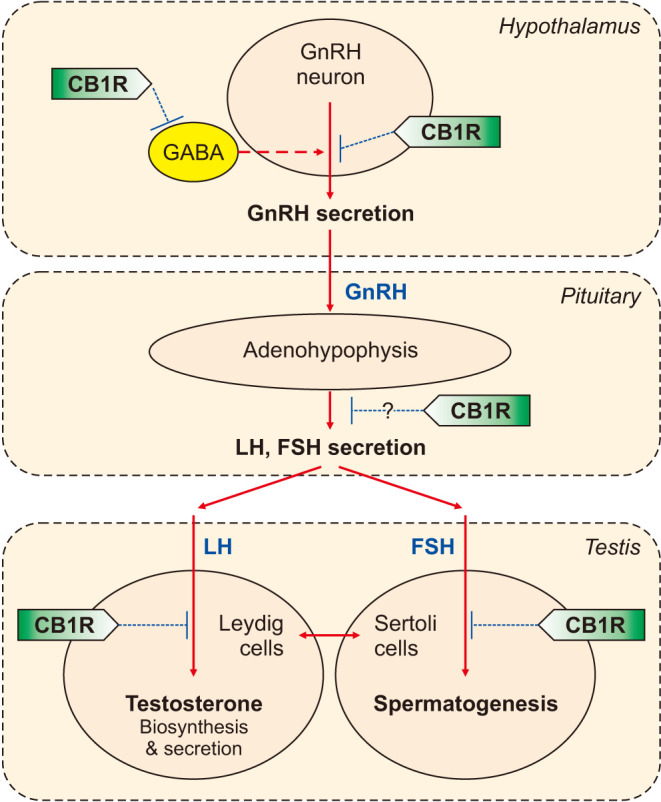
The ECS has been described as a critical modulator in the control of male and female reproduction at multiple stages of the HPG axis through CB receptors distributed in the hypothalamus, pituitary, and reproductive organs such as the testis. Centrally, the ECS affects neuronal activities of hypothalamic gonadotropin-releasing hormone (GnRH)-secreting neurons and secretion of pituitary hormones, and locally, produces direct effects on the gonads, affecting the synthesis and secretion of sex hormones and spermatogenesis [22] (Fig. 2).
Fig. 2. Endocannabinoid (ECB) signaling in the hypothalamus-pituitary-gonadal (HPG) axis. The HPG axis is a tightly regulated endocrine system, and the gonadotropin-releasing hormone (GnRH), released in a pulsatile manner from the hypothalamus, is the prime modulator of the system. GnRH stimulates the release of pituitary follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary, which are actively involved in gametogenesis regulation while also driving the synthesis and release of gonadal steroid hormones. The ECB system, through the activation of the cannabinoid CB1 receptor signaling, is involved in the regulation of the HPG axis at multiple stages: (1) ECBs suppress the release of GnRH in the hypothalamus; (2) reduction of GnRH, in turn, suppresses the release of LH and FSH in the adenohypophysis where CB1 receptor may play a role; and (3) direct action of ECBs on the Leydig and Sertoli cells reduces testosterone release and modulates spermatogenesis.
1. The endocannabinoid system in the hypothalamic control of the male hypothalamus-pituitary-gonadal axis
In the brain, ECBs are produced in a neuronal activity-dependent manner in the post-synapses, and their primary role is to control the release of excitatory and inhibitory neurotransmitters by activating CB1 receptors located at presynaptic axon terminals, serving as retrograde messengers [10,11,12,13]. The ECS may operate using a similar synaptic negative-feedback mechanism in the HPG axis. The HPG axis is a tightly regulated endocrine system, and the decapeptide hormone, GnRH, is released in a pulsatile manner from the hypothalamus as the prime modulator of reproduction. GnRH stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary, which are actively involved in gametogenesis regulation while also driving the synthesis and release of gonadal steroid hormones [22]. LH up-regulates testosterone secretion in the testis, and high levels of testosterone down-regulates GnRH in the brain to eventually lower the release of LH. The negative feedback loop mechanism of testis on the hypothalamo-pituitary unit has an imperative role in the maintenance of testosterone levels [57].
Research demonstrated a role of the ECS at both the hypothalamus and pituitary level along the gonadal axis [53] (Fig. 2). First, evidence indicates that hypothalamic GnRH neurons produce and secrete at least two different ECBs, 2-AG and AEA [58]. These lipids messengers, in turn, activate hypothalamic CB1 receptors and inhibit the release of GnRH to regulate diverse functions of GnRH, including the onset of puberty, ovulation, lactational infertility, and menopause [58]. In mice, systemic or intracerebroventricular administration of AEA produced significant reductions in circulating levels of LH and testosterone [59,60]. The effect of AEA on LH secretion was mediated by activation of CB1 receptors expressed in GnRH neurons, whose activation leads to the inhibition of pulsatile GnRH release [53,59]. Interestingly, an alternative neuronal circuital mechanism by which the ECS may affect GnRH neuron activity by modifying GABAergic synaptic activity has been proposed [61] (Fig. 2). Neuronal GABA is typically an inhibitory neurotransmitter; however, research found that it exerts a paradoxical excitatory effect on mature GnRH neurons [62], and that GABAergic afferent into kisspeptin neurons and/or GnRH neurons is an important positive regulator for the HPG axis [62,63,64,65,66]. Accordingly, ECS-mediated activation of CB1 receptors, expressed on GABAergic inputs into GnRH neurons, resulted in decreased GnRH neuron firing rate and consequent reduction of GnRH release [58,64]. Therefore, activation of CB1 receptors in the hypothalamus may inhibit GnRH release either through direct inhibition of GnRH neuronal activity or indirect effects on the GABAergic activation of GnRH neurons (Fig. 2).
Next, the existence of CB1 receptors, both the mRNA and proteins, and ECB molecules in the anterior pituitary of rodents has been observed [67,68,69]. The inhibitory effect of the ECS on hormonal secretion in the anterior pituitary has been also proposed [21]. These results suggest the role of the ECS as a neuromodulator at the pituitary level, but more research is needed to have a clear understanding on the underlying mechanism.
2. Endocannabinoid signaling in spermatogenesis
The presence of complete enzymatic machinery to synthesize and metabolize ECBs has been demonstrated in male reproductive organs [21,35]. Earlier studies have reported the presence of NAPE-PLD and FAAH, the biosynthetic and degradative enzymes for AEA, respectively, in human testis [55]. A recent study also found expression of NAPE-PLD in Leydig cells, Sertoli cells, and round spermatid nuclei, which indicates that these cells synthesize AEA [35]. Therefore, these data suggest a role of the ECS, mainly mediated by AEA, in reproductive regulation of late spermatocytes and spermatids.
Both CB1 and CB2 receptors have been found in post-meiotic germ, Leydig, and peritubular cells [35], and their functional relevance has been proposed [53]. In Leydig cells, activation of CB1 receptors negatively affects testosterone biosynthesis by decreasing Leydig cell responsiveness to LH. Within Sertoli cells, the ECS, specifically the production and degradation of AEA, plays an important role in controlling spermatogenic output by maintaining a balance between the cell’s survival and death [53]. Apoptosis via transient receptor potential vanilloid 1 (TRPV1) channels is induced through the orchestrated biosynthesis of AEA by NAPE-PLD [70]. This effect is antagonized by FSH, which increases the expression of FAAH through the activation of adenylyl cyclase (AC) and cAMP/protein kinase A (PKA) signaling [70,71,72,73]. FSH also triggers the phosphatidylinositol-3-kinase (PI3K) pathway, which in turn induces the expression of aromatase and leads to increased production of estradiol from testosterone [70,72]. Increased levels of estradiol act to an estrogen-responsive element in the Faah promoter, resulting in elevation of FAAH protein expression [73].
THERAPEUTIC POTENTIAL OF CANNABINOIDS FOR MALE GENITOURINARY SYSTEM DISORDERS
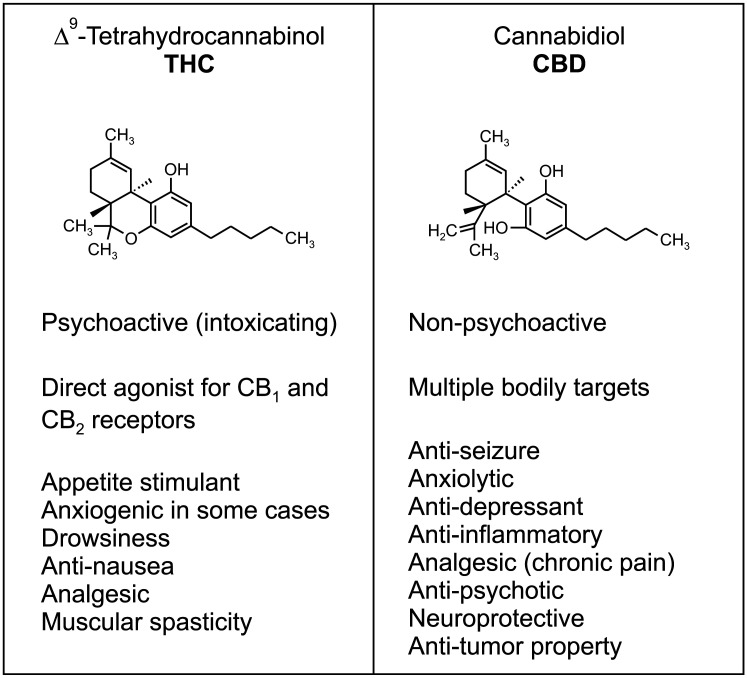
The Cannabis sativa plant contains more than 100 compounds that share the CB chemical scaffold [10,14]. Among them, THC is a main terpenophenolic constituent of cannabis and is responsible for the majority of the plant’s reinforcing (intoxicating) effects, by binding to and activating CB1 receptors [10,11,16,18,19] (Fig. 3). THC also activates CB2 receptors that contribute to other less-well understood effects such as those exerted on the immune system [10,11]. Recent research has documented that a variety of human disorders are accompanied by dysfunction in the ECS; therefore, pharmacological interventions that normalize dysfunctional ECB signaling, i.e. temporally activating CB receptors by THC could be potential therapeutics for diseases associated with hypo-cannabinergic pathology [10] (Fig. 3).
Fig. 3. Two main phytocannabinoids, THC and CBD. THC and CBD are two of the most well-known cannabinoids in the Cannabis plant with potential therapeutic utilities. They have distinct pharmacological properties and targets in the body. The intoxicating effects of THC, such as euphoria, relaxation, and sometimes paranoia, is associated with short-term memory deficits and increased risk for psychiatric disorders including psychosis, depression, anxiety, and substance use disorders. These problems are not caused by CBD, which displays anxiolytic, anti-psychotic, and anti-inflammatory and analgesic effects. Unanswered questions regarding potential side effects of phytocannabinoids along with the therapeutic potential of endocannabinoid modulation requires further investigation.
CBD is a major non-psychoactive compound found in cannabis plant and proposed to have anti-inflammatory, analgesic, anxiolytic, neuroprotective, and anti-seizure effects [74,75] (Fig. 3). In 2018, the CBD-based drug (Epidiolex®) was approved by the FDA for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older. Then in 2020, the FDA approved Epidiolex® oral solution for the treatment of seizures associated with tuberous sclerosis complex in patients one year of age and older [76,77,78]. Beneficial effects of CBD in brain disorders include neuroprotective activity via anti-inflammatory and anti-oxidative properties [79,80,81,82,83], sedative effects that decrease anxiety [87,88], and as described, an anti-epileptic effect that reduces seizure frequency [86,87,88,89]. The therapeutic potential of CBD has also been proposed further for treating ischemic stroke [90], schizophrenia [91,92], and Alzheimer’s disease [93]. Although the exact cellular and/or molecular targets of CBD in the body remain unclear, current research postulates that CBD may serve as a negative allosteric modulator of CB1 receptors or increase ECB tone by elevating AEA levels, among other broad-spectrum mechanisms [82,83,94].
According to Clinicaltrials.gov run by the National Institute of Health (NIH) in the United States, as of August 8, 2022, about 407 clinical studies have been initiated and/or completed to test the therapeutic applications of CBD for neuropsychiatric diseases, cancer, and chronic disorders accompanied with inflammation and pain, such as osteoarthritis pain [95]. With regard to human genitourinary diseases, two main areas of therapeutic interest are treating pain associated with various conditions and lower urinary tract symptoms (LUTS) [96] (Table 1).
Table 1. Therapeutic utility of cannabinoids for genitourinary disorders.
| Condition | Disease | Test drug | Dose and duration | Status | Remark | Clinical Trials Identifier |
|---|---|---|---|---|---|---|
| Pain | CIPN | CBD | Various dosages, 3 times per day, 12 weeks | Clinical-ongoing | Hemp-based CBD | NCT04398446 |
| Pain after ureteroscopy | CBD oil (Epidiolex) | 20 mg per day for 3 days | Clinical-completed | Ureteroscopy for kidney stones | NCT04387617 | |
| CP/CPPS | ASP3652 | Various dosages, twice daily for 12 weeks | Clinical-ongoing | Peripheral FAAH inhibitor | NCT01391338 | |
| Chronic cystitis | Nabilone and Dronabinol | 1 mg Nabilone and 2.5 mg Dronabinol, once daily for 6 months | Case report | Synthetic THC analogues | Ref [119] | |
| Interstitial cystitis/painful bladder syndrome | AEA and PEA | 25 mg/kg AEA and 10–30 mg/kg PEA, single dose | Preclinical | Rat model of visceral and somatic inflammatory pain | Ref [120] | |
| LUTS | Detrusor overactivity | Sativex | THC and CBD; max daily doses set at 130 mg THC and 120 mg CBD, for 10 weeks | Clinical-completed | Multiple sclerosis patients | NCT00678795 |
| Overactive bladder | Medical Cannabis | Inhaled dried buds or sublingual oil extract, for 8 weeks | Clinical-ongoing | Parkinson’s disease patients | NCT05106504 | |
| Renal disease | Pruritus | Nabilone | 0.5 mg, 1–2 per day for 3 weeks | Clinical-ongoing | End-stage renal disease patients | NCT05180968 |
| Diabetic nephropathy | GFB-024 | Ascending doses ranging from 5–300 mg for 10 weeks | Clinical-completed | Peripheral CB1 receptor agonist | NCT04880291 | |
| Cancer | Prostate cancer | CBD oil (Epidiolex) | 600–800 mg, once daily for up to 12 weeks | Clinical-completed | Biochemically recurrent prostate cancer | NCT04428203 |
AEA: anandamide, CBD: cannabidiol, CIPN: chemotherapy-induced neuropathy, CP/CPPS: chronic abacterial prostatitis/chronic pelvic pain syndrome, FAAH: fatty acid amide hydrolase, LUTS: lower urinary tract symptoms, PEA: palmitoylethanolamide, THC: Δ9-tetrahydrocannabinol.
A phase 2 study is currently underway to test hemp-based CBD for chemotherapy-induced neuropathy (CIPN) among non-metastatic breast, colorectal, uterine, and ovarian cancer patients who received neoadjuvant or adjuvant therapy that included neurotoxic chemotherapeutic agents. Also, another ongoing study investigates the efficacy of ASP3652, a peripherally restricted FAAH inhibitor that elevates tissue levels of AEA, in the treatment of patients with chronic abacterial prostatitis/chronic pelvic pain syndrome (CP/CPPS). A recently completed Phase 2 study assessed the effect of CBD oil on pain after ureteroscopy for kidney stones. In addition, AEA and palmitoylethanolamide (PEA), an ECB-related lipid molecule, were tested in animal model for interstitial cystitis/painful bladder syndrome.
In multiple sclerosis (MS) patients, use of the oral mucosal spray Sativex, a mixture of THC and CBD, displayed a significant effect on overactive bladder [97]. Cannabis-derivatives also demonstrated mixed degrees of improvement in incontinence, frequency, nocturia in multiple clinical trials with MS patients [96].
Additional conditions that CB medications have been implicated includes treating pruritus associated with end-stage renal dysfunction and various cancers. The anticancer effects of CBs against prostate cancer have been limited to preclinical in vitro studies so far, and translation to human conditions has been sluggish (Table 1).
PHYTOCANNABINOIDS: A DOUBLE-EDGED SWORD FOR MALE GENITOURINARY SYSTEM?
Since the ECS regulates major bodily functions, unwanted side effects may occur when manipulating its activity, which should be anticipated in advance and carefully considered during drug development. Indeed, strong evidence obtained from preclinical studies indicated that administration of cannabis extracts acts on the gonadal axis and reduces its function. Human studies also demonstrated that exposure to cannabis or its derivatives is associated with reduced sperm count and motility along with abnormal morphology, and may negatively impact male fertility [58,98,99,100]. In addition, cannabis consumption decreased the levels of plasma testosterone in human users compared to non-users [98], which was associated with reduced plasma LH [58].
The reproductive toxicity of cannabis was mainly reproduced by administration with THC in animal models [58,101,102,103]. In these studies, exposure to THC altered homeostasis of the HPG axis, and long-term administration of THC significantly decreased spermatogenesis. This phenomenon can be presumed to be the result of THC over-activating CB receptors distributed in the CNS and testis. In contrast, CBD has been suggested to be generally well tolerated by humans because the reported adverse events are mild [74,75,76]. However, CBD is not risk-free [104]. Clinical studies found that CBD causes adverse effects, including drug-drug interactions, hepatic abnormalities, fatigue, vomiting, diarrhea, somnolence, insomnia, and suicidal thoughts [104]. In addition, animal studies found that chronic high doses of CBD produce developmental toxicity and affect CNS function, among other peripheral effects including changes in organ weight, hepatocellular injuries, and hypotension [104]. Importantly, concerns have been raised about the adverse effects of CBD on male reproductive system [105]. Evidence indicates that exposure to CBD is associated with a reduction in mammalian testis size, the number of germ and Sertoli cells, fertilization rates, spermatogenesis, and plasma concentrations of hypothalamic, pituitary and gonadal hormones including a decrease in testosterone [100,105,106,107,108]. In sexually mature rhesus monkeys, oral administration of 30–300 mg/kg body weight/day CBD for 90 days caused reductions of testicular size and spermatogenesis [109]. A number of studies have reported that preincubation of sperm with CBD inhibited fertilization in sea urchins, a relevant model to study fertilization because of their similarity to human embryos in the early developmental stages [110,111]. The underlying mechanisms by which CBD negatively influences male reproductive system have not been elucidated, but may involve damages to Sertoli cells [112]. Finally, it is notable that CBs may interfere the ECB system required for normal development [113,114,115,116], and early life exposure to THC or CBD affected maturation of the HPG axis in rodents. In 21-day-old male Swiss mice, 15 or 30 mg/kg/day CBD administered orally for 34 consecutive days followed by a 35-day recovery period caused a decrease in the number of Sertoli cells, abnormalities in sperm morphology, and decreases in plasma testosterone levels [107,108,117,118]. Therefore, long-term adverse effects of chronic THC or CBD administration during early life could be a significant human health issue that needs scientific attention.
CONCLUSIONS
Preclinical and clinical evidence demonstrates the central role that the ECS plays in regulating many of the body’s key processes, including homeostasis of the HPG axis and male reproductive functions. The ability of phytocannabinoids to reduce pain and inflammation provides promising opportunities for therapeutic intervention for genitourinary and degenerative disorders. However, more scientific evidence should be obtained to fully address the general public’s interest in utilizing cannabis products for human disorders and as health supplements. Important knowledge gaps remain, including the role of ECS during early life development of the reproductive system and the underlying mechanisms by which CBD negatively influences male reproductive functions.
Despite these unanswered questions, it is clear that the therapeutic potential of ECB modulation calls for further basic and clinical investigation. Continued study to better understand the complexity of the ECS will provide new insights into the pathogenesis of reproductive and genitourinary disorders, allowing researchers to identify new ways to leverage this signaling system for therapeutic benefit. Drugs can be designed that selectively act on aspects of the ECS required for therapeutic purposes while avoiding unwanted side effects. Alternately, research exploring the uncontrolled use of cannabis (i.e., recreational and substance use disorder) might provide further evidence of its deleterious impacts on human reproductive function.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the USA Department of the Army (Grant No. GW210033 [to KMJ]).
- Conceptualization: KMJ.
- Investigation: JL, ES, KMJ.
- Writing: JL, ES, KMJ.
References
- 1.Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, et al. Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry. 2020;77:165–171. doi: 10.1001/jamapsychiatry.2019.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future national survey results on drug use 1975-2020: overview, key findings on adolescent drug use [Internet] Ann Arbor (MI): Institute for Social Research, University of Michigan; c2021. [cited 2022 Aug 8]. Available from: http://www.monitoringthefuture.org//pubs/monographs/mtf-overview2020.pdf . [Google Scholar]
- 3.Keyes KM, Schulenberg JE, O'Malley PM, Johnston LD, Bachman JG, Li G, et al. The social norms of birth cohorts and adolescent marijuana use in the United States, 1976-2007. Addiction. 2011;106:1790–1800. doi: 10.1111/j.1360-0443.2011.03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacek LR, Mauro PM, Martins SS. Perceived risk of regular cannabis use in the United States from 2002 to 2012: differences by sex, age, and race/ethnicity. Drug Alcohol Depend. 2015;149:232–244. doi: 10.1016/j.drugalcdep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadland SE, Knight JR, Harris SK. Medical marijuana: review of the science and implications for developmental-behavioral pediatric practice. J Dev Behav Pediatr. 2015;36:115–123. doi: 10.1097/DBP.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leas EC, Hendrickson EM, Nobles AL, Todd R, Smith DM, Dredze M, et al. Self-reported cannabidiol (CBD) use for conditions with proven therapies. JAMA Netw Open. 2020;3:e2020977. doi: 10.1001/jamanetworkopen.2020.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Failing CJ, Boehnke KF, Riebschleger M. Cannabidiol (CBD) use among children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2021;19:171. doi: 10.1186/s12969-021-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3:152–161. doi: 10.1089/can.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soleymanpour M, Saderholm S, Kavuluru R. Therapeutic claims in cannabidiol (CBD) marketing messages on Twitter. Proceedings (IEEE Int Conf Bioinformatics Biomed) 2021;2021:3083–3088. doi: 10.1109/bibm52615.2021.9669404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung KM, Piomelli D. In: Neuroscience in the 21st century. Pfaff D, Volkow N, editors. New York (NY): Springer; 2016. Cannabinoids and endocannabinoids; pp. 1811–1841. [Google Scholar]
- 11.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 12.Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams R. Marihuana: Harvey lecture, February 19, 1942. Bull N Y Acad Med. 1942;18:705–730. [PMC free article] [PubMed] [Google Scholar]
- 15.Wollner HJ, Matchett JR, Levine J, Loewe S. Isolation of a physiologically active tetrahydrocannabinol from cannabis sativa resin. J Am Chem Soc. 1942;64:26–29. [Google Scholar]
- 16.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 17.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 18.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 19.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 20.Hillard CJ. Endocannabinoids and the endocrine system in health and disease. Handb Exp Pharmacol. 2015;231:317–339. doi: 10.1007/978-3-319-20825-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayed TS, Balasinor NH, Nishi K. Diverse role of endocannabinoid system in mammalian male reproduction. Life Sci. 2021;286:120035. doi: 10.1016/j.lfs.2021.120035. [DOI] [PubMed] [Google Scholar]
- 22.Bovolin P, Cottone E, Pomatto V, Fasano S, Pierantoni R, Cobellis G, et al. Endocannabinoids are involved in male vertebrate reproduction: regulatory mechanisms at central and gonadal level. Front Endocrinol (Lausanne) 2014;5:54. doi: 10.3389/fendo.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meccariello R, Battista N, Bradshaw HB, Wang H. Updates in reproduction coming from the endocannabinoid system. Int J Endocrinol. 2014;2014:412354. doi: 10.1155/2014/412354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- 25.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 27.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 28.Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- 29.Gómez Del Pulgar T, De Ceballos ML, Guzmán M, Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2002;277:36527–36533. doi: 10.1074/jbc.M205797200. [DOI] [PubMed] [Google Scholar]
- 30.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunos G, Tam J. The case for peripheral CB1 receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163:1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunos G, Osei-Hyiaman D. Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1101–G1104. doi: 10.1152/ajpgi.00057.2008. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen JE, Rolland AD, Rajpert-De Meyts E, Janfelt C, Jørgensen A, Winge SB, et al. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci Rep. 2019;9:12866. doi: 10.1038/s41598-019-49177-y. Erratum in: Sci Rep 2020;10:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 37.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 38.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 39.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 40.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogura Y, Parsons WH, Kamat SS, Cravatt BF. A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat Chem Biol. 2016;12:669–671. doi: 10.1038/nchembio.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 43.Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol Disord Drug Targets. 2013;12:7–16. doi: 10.2174/1871527311312010005. [DOI] [PubMed] [Google Scholar]
- 44.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 45.Bennett CF, Balcarek JM, Varrichio A, Crooke ST. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature. 1988;334:268–270. doi: 10.1038/334268a0. [DOI] [PubMed] [Google Scholar]
- 46.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 47.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 49.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. Erratum in: Proc Natl Acad Sci U S A 2002;99:13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung KM, Clapper JR, Fu J, D'Agostino G, Guijarro A, Thongkham D, et al. 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maccarrone M, Rapino C, Francavilla F, Barbonetti A. Cannabinoid signalling and effects of cannabis on the male reproductive system. Nat Rev Urol. 2021;18:19–32. doi: 10.1038/s41585-020-00391-8. [DOI] [PubMed] [Google Scholar]
- 54.Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, Antonangelo C, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150:4692–4700. doi: 10.1210/en.2009-0057. [DOI] [PubMed] [Google Scholar]
- 55.Lewis SE, Rapino C, Di Tommaso M, Pucci M, Battista N, Paro R, et al. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS One. 2012;7:e47704. doi: 10.1371/journal.pone.0047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimaldi P, Rossi G, Catanzaro G, Maccarrone M. Modulation of the endocannabinoid-degrading enzyme fatty acid amide hydrolase by follicle-stimulating hormone. Vitam Horm. 2009;81:231–261. doi: 10.1016/S0083-6729(09)81010-8. [DOI] [PubMed] [Google Scholar]
- 57.Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64:735–742. doi: 10.1095/biolreprod64.3.735. [DOI] [PubMed] [Google Scholar]
- 58.Gammon CM, Freeman GM, Jr, Xie W, Petersen SL, Wetsel WC. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology. 2005;146:4491–4499. doi: 10.1210/en.2004-1672. Erratum in: Endocrinology 2006;147:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun. 2001;284:363–368. doi: 10.1006/bbrc.2001.4977. [DOI] [PubMed] [Google Scholar]
- 60.Scorticati C, Fernández-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, et al. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci U S A. 2004;101:11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farkas I, Kalló I, Deli L, Vida B, Hrabovszky E, Fekete C, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Front Neurosci. 2014;8:387. doi: 10.3389/fnins.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berg T, Silveira MA, Moenter SM. Prepubertal development of GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J Neurosci. 2018;38:2283–2293. doi: 10.1523/JNEUROSCI.2304-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farkas I, Vastagh C, Farkas E, Bálint F, Skrapits K, Hrabovszky E, et al. Glucagon-like peptide-1 excites firing and increases GABAergic miniature postsynaptic currents (mPSCs) in gonadotropin-releasing hormone (GnRH) neurons of the male mice via activation of nitric oxide (NO) and suppression of endocannabinoid signaling pathways. Front Cell Neurosci. 2016;10:214. doi: 10.3389/fncel.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Giorgio NP, Bizzozzero-Hiriart M, Libertun C, Lux-Lantos V. Unraveling the connection between GABA and kisspeptin in the control of reproduction. Reproduction. 2019;157:R225–R233. doi: 10.1530/REP-18-0527. [DOI] [PubMed] [Google Scholar]
- 66.Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol. 2005;17:591–599. doi: 10.1111/j.1365-2826.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 67.Murphy LL, Muñoz RM, Adrian BA, Villanúa MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5(6 Pt B):432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- 68.Wenger T, Fernández-Ruiz JJ, Ramos JA. Immunocytochemical demonstration of CB1 cannabinoid receptors in the anterior lobe of the pituitary gland. J Neuroendocrinol. 1999;11:873–878. doi: 10.1046/j.1365-2826.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- 69.González S, Manzanares J, Berrendero F, Wenger T, Corchero J, Bisogno T, et al. Identification of endocannabinoids and cannabinoid CB(1) receptor mRNA in the pituitary gland. Neuroendocrinology. 1999;70:137–145. doi: 10.1159/000054468. [DOI] [PubMed] [Google Scholar]
- 70.Maccarrone M, Cecconi S, Rossi G, Battista N, Pauselli R, Finazzi-Agrò A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology. 2003;144:20–28. doi: 10.1210/en.2002-220544. [DOI] [PubMed] [Google Scholar]
- 71.Rossi G, Gasperi V, Paro R, Barsacchi D, Cecconi S, Maccarrone M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology. 2007;148:1431–1439. doi: 10.1210/en.2006-0969. [DOI] [PubMed] [Google Scholar]
- 72.McDonald CA, Millena AC, Reddy S, Finlay S, Vizcarra J, Khan SA, et al. Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol Endocrinol. 2006;20:608–618. doi: 10.1210/me.2005-0245. [DOI] [PubMed] [Google Scholar]
- 73.Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, et al. The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci. 2012;69:4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.World Health Organization (WHO) Cannabidiol (CBD): critical review report [Internet] Geneva: WHO; c2018. [cited 2022 Aug 8]. Available from: https://cdn.who.int/media/docs/default-source/controlled-substances/whocbdreportmay2018-2.pdf?sfvrsn=f78db177_2&download=true . [Google Scholar]
- 76.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–1067. doi: 10.1007/s40263-018-0578-5. Erratum in: CNS Drugs 2019;33:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 78.Tang R, Fang F. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;377:699. doi: 10.1056/NEJMc1708349. [DOI] [PubMed] [Google Scholar]
- 79.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 80.Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:294–299. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- 81.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silvestro S, Schepici G, Bramanti P, Mazzon E. Molecular targets of cannabidiol in experimental models of neurological disease. Molecules. 2020;25:5186. doi: 10.3390/molecules25215186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–645. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 85.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7(1 Suppl):82–88. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 86.Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. Cannabis-based products for pediatric epilepsy: an updated systematic review. Seizure. 2020;75:18–22. doi: 10.1016/j.seizure.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. Cannabis-based products for pediatric epilepsy: a systematic review. Epilepsia. 2019;60:6–19. doi: 10.1111/epi.14608. [DOI] [PubMed] [Google Scholar]
- 88.Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78:1791–1804. doi: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- 89.Lattanzi S, Brigo F, Cagnetti C, Trinka E, Silvestrini M. Efficacy and safety of adjunctive cannabidiol in patients with lennox-gastaut syndrome: a systematic review and meta-analysis. CNS Drugs. 2018;32:905–916. doi: 10.1007/s40263-018-0558-9. [DOI] [PubMed] [Google Scholar]
- 90.Hayakawa K, Mishima K, Fujiwara M. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals (Basel) 2010;3:2197–2212. doi: 10.3390/ph3072197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Batalla A, Janssen H, Gangadin SS, Bossong MG. The potential of cannabidiol as a treatment for psychosis and addiction: who benefits most? A systematic review. J Clin Med. 2019;8:1058. doi: 10.3390/jcm8071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 93.Watt G, Karl T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer's disease. Front Pharmacol. 2017;8:20. doi: 10.3389/fphar.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verrico CD, Wesson S, Konduri V, Hofferek CJ, Vazquez-Perez J, Blair E, et al. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain. 2020;161:2191–2202. doi: 10.1097/j.pain.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pham MN, Hudnall MT, Nadler RB. Marijuana, lower urinary tract symptoms, and pain in the urologic patient. Urology. 2020;139:8–13. doi: 10.1016/j.urology.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 97.Maniscalco GT, Aponte R, Bruzzese D, Guarcello G, Manzo V, Napolitano M, et al. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: a pilot prospective study. Neurol Sci. 2018;39:97–102. doi: 10.1007/s10072-017-3148-6. [DOI] [PubMed] [Google Scholar]
- 98.Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med. 1974;290:872–874. doi: 10.1056/NEJM197404182901602. [DOI] [PubMed] [Google Scholar]
- 99.Dixit VP, Sharma VN, Lohiya NK. The effect of chronically administered cannabis extract on the testicular function of mice. Eur J Pharmacol. 1974;26:111–114. doi: 10.1016/0014-2999(74)90081-8. [DOI] [PubMed] [Google Scholar]
- 100.List A, Nazar B, Nyquist S, Harclerode J. The effects of delta9-tetrahydrocannabinol and cannabidiol on the metabolism of gonadal steroids in the rat. Drug Metab Dispos. 1977;5:268–272. [PubMed] [Google Scholar]
- 101.Wenger T, Rettori V, Snyder GD, Dalterio S, McCann SM. Effects of delta-9-tetrahydrocannabinol on the hypothalamic-pituitary control of luteinizing hormone and follicle-stimulating hormone secretion in adult male rats. Neuroendocrinology. 1987;46:488–493. doi: 10.1159/000124870. [DOI] [PubMed] [Google Scholar]
- 102.Kumar MS, Chen CL. Effect of an acute dose of delta 9-THC on hypothalamic luteinizing hormone releasing hormone and met-enkephalin content and serum levels of testosterone and corticosterone in rats. Subst Alcohol Actions Misuse. 1983;4:37–43. [PubMed] [Google Scholar]
- 103.Rettori V, Aguila MC, Gimeno MF, Franchi AM, McCann SM. In vitro effect of delta 9-tetrahydrocannabinol to stimulate somatostatin release and block that of luteinizing hormone-releasing hormone by suppression of the release of prostaglandin E2. Proc Natl Acad Sci U S A. 1990;87:10063–10066. doi: 10.1073/pnas.87.24.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17:974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carvalho RK, Andersen ML, Mazaro-Costa R. The effects of cannabidiol on male reproductive system: a literature review. J Appl Toxicol. 2020;40:132–150. doi: 10.1002/jat.3831. [DOI] [PubMed] [Google Scholar]
- 106.Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O'Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85:1888–1900. doi: 10.1111/bcp.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvalho RK, Rocha TL, Fernandes FH, Gonçalves BB, Souza MR, Araújo AA, et al. Decreasing sperm quality in mice subjected to chronic cannabidiol exposure: new insights of cannabidiol-mediated male reproductive toxicity. Chem Biol Interact. 2022;351:109743. doi: 10.1016/j.cbi.2021.109743. [DOI] [PubMed] [Google Scholar]
- 108.Carvalho RK, Santos ML, Souza MR, Rocha TL, Guimarães FS, Anselmo-Franci JA, et al. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. J Appl Toxicol. 2018;38:1215–1223. doi: 10.1002/jat.3631. Erratum in: J Appl Toxicol 2018;38:1545. [DOI] [PubMed] [Google Scholar]
- 109.Rosenkrantz H, Fleischman RW, Grant RJ. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol Appl Pharmacol. 1981;58:118–131. doi: 10.1016/0041-008x(81)90122-8. [DOI] [PubMed] [Google Scholar]
- 110.Schuel H, Schuel R, Zimmerman AM, Zimmerman S. Cannabinoids reduce fertility of sea urchin sperm. Biochem Cell Biol. 1987;65:130–136. doi: 10.1139/o87-018. [DOI] [PubMed] [Google Scholar]
- 111.Schuel H, Berkery D, Schuel R, Chang MC, Zimmerman AM, Zimmerman S. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. I. Inhibition of the acrosome reaction induced by egg jelly. Mol Reprod Dev. 1991;29:51–59. doi: 10.1002/mrd.1080290109. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Wu Q, Li X, Von Tungeln LS, Beland FA, Petibone D, et al. In vitro effects of cannabidiol and its main metabolites in mouse and human Sertoli cells. Food Chem Toxicol. 2022;159:112722. doi: 10.1016/j.fct.2021.112722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fride E. Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J Neuroendocrinol. 2008;20 Suppl 1:75–81. doi: 10.1111/j.1365-2826.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 114.Gaffuri AL, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology. 2012;90:19–39. doi: 10.1159/000339075. [DOI] [PubMed] [Google Scholar]
- 115.Galve-Roperh I, Aguado T, Rueda D, Velasco G, Guzmán M. Endocannabinoids: a new family of lipid mediators involved in the regulation of neural cell development. Curr Pharm Des. 2006;12:2319–2325. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- 116.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 117.Kaplan JS, Wagner JK, Reid K, McGuinness F, Arvila S, Brooks M, et al. Cannabidiol exposure during the mouse adolescent period is without harmful behavioral effects on locomotor activity, anxiety, and spatial memory. Front Behav Neurosci. 2021;15:711639. doi: 10.3389/fnbeh.2021.711639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalterio S, Steger R, Mayfield D, Bartke A. Early cannabinoid exposure influences neuroendocrine and reproductive functions in mice: II. Postnatal effects. Pharmacol Biochem Behav. 1984;20:115–123. doi: 10.1016/0091-3057(84)90111-4. [DOI] [PubMed] [Google Scholar]
- 119.Krenn H, Daha LK, Oczenski W, Fitzgerald RD. A case of cannabinoid rotation in a young woman with chronic cystitis. J Pain Symptom Manage. 2003;25:3–4. doi: 10.1016/s0885-3924(02)00601-2. [DOI] [PubMed] [Google Scholar]
- 120.Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]