Abstract
Brain development relies on both experience and genetically defined programs. Time windows where certain brain circuits are particularly receptive to external stimuli, resulting in heightened plasticity, are referred to as “critical periods”. Sleep is thought to be essential for normal brain development. Importantly, studies have shown that sleep enhances critical period plasticity and promotes experience-dependent synaptic pruning in the developing mammalian brain. Therefore, normal plasticity during critical periods depends on sleep. Problems falling and staying asleep occur at a higher rate in Autism Spectrum Disorder (ASD) relative to typical development. In this review, we explore the potential link between sleep, critical period plasticity, and ASD. First, we review the importance of critical period plasticity in typical development and the role of sleep in this process. Next, we summarize the evidence linking ASD with deficits in synaptic plasticity in rodent models of high-confidence ASD gene candidates. We then show that the high-confidence rodent models of ASD that show sleep deficits also display plasticity deficits. Given how important sleep is for critical period plasticity, it is essential to understand the connections between synaptic plasticity, sleep, and brain development in ASD. However, studies investigating sleep or plasticity during critical periods in ASD mouse models are lacking. Therefore, we highlight an urgent need to consider developmental trajectory in studies of sleep and plasticity in neurodevelopmental disorders.
Keywords: Autism, Sleep, Synaptic plasticity, Critical periods of development
Graphical abstract
Highlights
-
•
Synaptic plasticity depends on adequate amounts of sleep, especially during critical periods of brain development.
-
•
We provide a thorough review of ASD mouse models linking genetic mutations and brain plasticity deficits.
-
•
ASD mutations that lead to plasticity deficits often co-present with sleep deficits, when sleep have been performed.
-
•
Future studies using ASD mouse models should study sleep and plasticity simultaneously starting in early life.
Abbreviations
- ASD
Autism Spectrum Disorder
- NREMS
Non rapid eye movement sleep
- REMS
Rapid eye movement sleep
- ODP
Ocular dominance plasticity
- CNV
Copy Number Variants
1. Introduction
Brain development relies on both experience and genetically defined programs. Brain circuits are established through neuronal proliferation and migration. Experience remodels circuits through both synaptogenesis and pruning, a process referred to as synaptic plasticity (Hübener and Bonhoeffer, 2014). Time windows where certain brain circuits are particularly receptive to external stimuli, resulting in heightened plasticity, are referred to as “critical periods” (Dehorter and Del Pino, 2020; Hensch, 2004; Rice and Barone, 2000). While heightened plasticity allows us to shape neuronal circuits, this also introduces vulnerability. Therefore, disruptions early in life can result in neuronal circuits that respond differently to experiences later on (Hensch, 2003, 2004; Knudsen, 2004; Rice and Barone, 2000). In the last four decades, developmental windows for our brain's ability to attune to experience have been widely studied, emphasizing the importance of critical periods in the developing brain (Rice and Barone, 2000). During early postnatal development, and while critical periods occur, young mammals spend most of their time sleeping. Sleep is thought to be essential for normal brain development (Frank and Heller, 1997; Krueger et al., 2016; Ribeiro, 2012; Roffwarg et al., 1966; Shaffery and Roffwarg, 2009). Studies have shown that sleep enhances critical period plasticity (Frank et al., 2001) and promotes experience-dependent synaptic pruning in the developing cortex (Li et al., 2017; Zhou et al., 2020). Therefore, normal plasticity during critical periods depends on adequate amounts of sleep.
Problems falling and staying asleep occur at a higher rate in individuals with Autism Spectrum Disorder (ASD) than in individuals with typical development, affecting up to 93% of individuals (Petruzzelli et al., 2021). Sleep problems in individuals with ASD are predictive of the severity of ASD core symptoms (Cohen et al., 2014) and lead to a reduced quality of life of individuals and caregivers (Hodge et al., 2014). Although sleep problems are often thought of as a co-occurring condition, the fundamental role of sleep in brain development points to sleep problems as a potential core factor in ASD. Recent studies have shown that problems falling asleep in the first year of life precede an ASD diagnosis and are associated with hippocampal overgrowth in infants at high-risk for ASD (MacDuffie et al., 2020b). Recent studies have also shown that problems falling asleep predict impairments in behavior regulation later in children with ASD (Tesfaye et al., 2021). However, studies investigating the mechanisms that link sleep early in life and ASD are lacking. ASD is diagnosed behaviorally on average between 4 and 5 years of age based on symptoms in two core domains, social deficits and repetitive behavior (van ’t Hof et al., 2021). Altered trajectories of development in individuals that go on to be diagnosed with ASD can be detected earlier in life (Zwaigenbaum et al., 2015). For example, over-expansion of the cortical surface area between 6 and 12 months of age and subsequent brain volume overgrowth is predictive of later ASD diagnosis in high-risk infants (Hazlett et al., 2017). The high rate of cortical surface and brain volume expansion that occurs after birth in mammals is thought to facilitate the contributions of postnatal experience (Hill et al., 2010). Therefore, alterations in cortical and brain growth early in life can affect how experience molds the brain. The rapid brain growth in the postnatal years is accompanied by high rates of synaptogenesis and pruning, fundamental substrates of synaptic plasticity. Deficits in plasticity early in life are thought to be important contributors to the ASD phenotype (Cohen et al., 2014; LeBlanc and Fagiolini, 2011). Given the relationship between sleep and plasticity, it is reasonable to propose that sleep and plasticity deficits during critical periods of development are inextricably linked in ASD.
In this review, we explore the potential link between plasticity, especially during critical periods of development, sleep, and ASD. First, we review the importance of critical period plasticity in typical development and the role of sleep in this process. Next, we summarize the evidence linking ASD with deficits in synaptic plasticity in mouse models of high-confidence ASD gene candidates, and we show that almost all the high-confidence mouse models of ASD (SFARI gene score of 1 or Syndromic) that show sleep deficits also display plasticity deficits. Given how important sleep is in early life, and for critical period plasticity in particular, this review highlights the importance of understanding connections between synaptic plasticity, sleep, and brain development in ASD.
The importance of critical periods in typical brain development.
The brain's typical development relies on multiple processes, such as cell proliferation, migration, circuit formation and maturation, all driven by genetic and environmental stimuli (Dehorter and Del Pino, 2020). Experience promotes rewiring and remodeling through a process known as synaptic plasticity (Hübener and Bonhoeffer, 2014). Synaptic plasticity refers to the ability of synapses to change in numbers, through genesis or pruning, or strength in response to use or disuse. Changes in strength can in turn happen through different mechanisms. Hebbian plasticity is defined as changes in the connection strength (either strengthening or weakening) between two neurons resulting from correlated firing (Hebb, 2005). Long-term potentiation (LTP) and long-term depression (LTD) are two forms of Hebbian plasticity. Homeostatic plasticity, such as synaptic scaling, is a form of plasticity that attempts to stabilize neuron or neuronal circuit activity in the event of a perturbation, such as a change in synapse number or strength that may alter excitability.
Critical periods of development, in which brain circuits are particularly plastic, have been recognized for nearly a century (Levelt and Hübener, 2012). It is important to note that the critical period of plasticity is simply an extreme form of plasticity; while this is elevated in early development, different types of plasticity can occur throughout our lifespan. The timeline for brain development differs across species, but critical periods have been established in both humans and animals. Critical periods also differ in timing based on input modality. During postnatal development, critical periods occur at ages in which other important processes for brain development also occur at a high rate, in particular synaptogenesis and pruning.
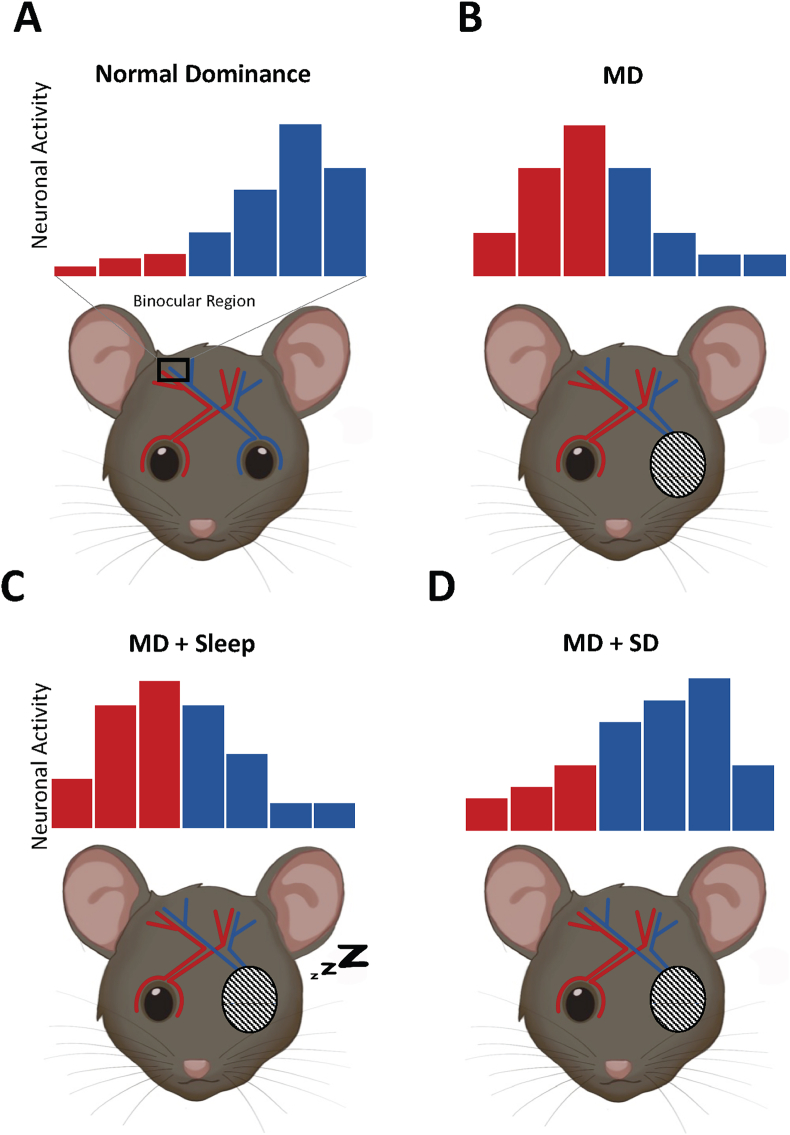
The visual system has long been favored as a model for critical period plasticity because experience strongly regulates the maturation of the visual cortex (Espinosa and Stryker, 2012; Levelt and Hübener, 2012). The best-studied example of critical period plasticity is the ocular dominance plasticity model (ODP) in the visual cortex. This model allows for measures of neuronal activity in vivo in unanesthetized brains in a system whose cellular processes are well established. In 1959, Hubel and Wiesel discovered that specific neurons in a cat's primary visual cortex were activated through stimuli presented to one eye or the other, termed ocular dominance (Hubel and Wiesel, 1959, 1962). They used monocular deprivation (MD) to deprive one eye of visual experience, which caused a decrease in the cortical neurons' response associated with the deprived eye and increased response to neurons associated with the spared eye in the binocular cortex (example depicted in Fig. 1). MD in a young kitten resulted in the irreversible loss of responsiveness in those cells even though there was no damage to the retina (Hubel and Wiesel, 1970; Wiesel and Hubel, 1963a, 1963b). In contrast, the same MD in an adult cat did not result in a loss of visual acuity. Importantly, this critical period is not a simple age-dependent mechanism, as animals that are only exposed to complete darkness in early life have a delayed onset of this critical period (Cynader, 1983; Mower, 1991). Therefore, the critical period for ODP is both age and experience dependent. The consolidation of ODP is divided into three mechanistically distinct stages. In the first stage, there is a decrease in response to the deprived eye, which is hypothesized to result from the loss of deprived-eye connections or depression in their synaptic efficacy. The dramatic loss of response to inputs from the deprived eye is thought to occur through Hebbian plasticity. In the second stage of ODP, there is an increase of response to inputs from both eyes, but most notably the open eye. This stage is thought to operate through both homeostatic and Hebbian plasticity. Restoring binocular vision by reopening the deprived eye during the critical period induces a third stage of plasticity, the rapid restoration of both eyes' responses to baseline levels, which is dependent on neurotrophic growth factor signaling. The ODP model has been used to demonstrate the role of sleep on critical period plasticity as described below.
Fig. 1.
The Ocular Dominance Plasticity model. A) In the binocular region of the visual cortex most neurons respond to input from the contralateral eye while very few respond to input from the ipsilateral eye input only. Input from eyes is color coded, red represent left, blue represent right. An example of neuronal activity sampled from the left side will receive most input from the contralateral side (blue). B) Monocular deprivation (MD) results in a shift of response from the non-deprived eye C) Sleep is necessary to consolidate the effects of MD. D) When sleep deprivation (SD) follows MD, the shift in response is not seen. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
1.1. The role of sleep in the consolidation of plasticity
Sleep is a naturally reoccurring and reversible brain state (Sun et al., 2020). Sleep or sleep-like states occur in all vertebrates and are widely present in invertebrates (Sun et al., 2020). Sleep states in mammals are traditionally defined using electroencephalographic recordings (EEG) and are categorized into two major stages: rapid-eye-movement (REM) sleep and non-rapid-eye movement (NREM) sleep. NREMS is characterized by high amplitude, slow frequency oscillations in the EEG (slow waves, less than 1 Hz), spindles (0.5–2s bursts of 10–16 Hz), a relaxed EMG, and a decrease in body temperature. Slow-wave activity (SWA) or the synchronization of low frequency (<4 Hz) membrane potentials of many neurons in the cortical membrane, is a major component of NREMS (Siegel, 2009). SWA is homeostatically regulated, as it is enhanced across the brain after prolonged periods of wake. REMS is characterized by low amplitude, fast frequency EEG, muscle atonia and increased body temperature (Siegel, 2009).
Scientists have yet to reach a universal consensus on the function of sleep and it is likely that it serves multiple functions. Nonetheless, we know sleep is important for cognitive function. Acute sleep loss can lead to impairments in learning, attention, and emotional regulation (Krause et al., 2017). Chronic sleep disturbances are common in neurodevelopmental disorders such as ASD (Cohen et al., 2014). The complexity of the molecular mechanism of sleep provides evidence for multiple functions leading to multiple hypotheses. One such theory supported by many sleep researchers is that sleep is essential for the connectivity or plasticity of the brain (Krueger et al., 2016). While the function of sleep is not fully understood, several functional and morphological studies in vivo provide direct evidence that sleep is important for synaptic plasticity during development and into adulthood (Frank, 2015; Renouard et al., 2022). The mechanisms underlying this relationship are less clear.
During early postnatal development, and while critical periods occur, mammals spend most of their time sleeping (Roffwarg et al., 1966). Sleep states in rodents cannot be identified using EEG until approximately postnatal (P) day 12 (Frank and Heller, 2003; Rensing et al., 2018), which corresponds to approximately a 40 day old human infant (Workman et al., 2013, https://www.translatingtime.org). Early in development REMS is the predominant sleep state. Gradually REMS decreases, while wake and NREMS amount increase (Frank et al., 2017; Medina et al., 2022; Rensing et al., 2018). In rodents, the decrease of REMS stabilizes around P20 while NREMS continues to increase until P30 (Frank et al., 2017), which correspond to 7–16 months in humans (Workman et al., 2013, https://www.translatingtime.org). REMS deprivation in P28 rodents prolonged the critical period for synaptic plasticity in the visual cortex (Shaffery et al., 2002). Most of the work regarding to role of sleep in ODP, however, has been done in kittens during the peak of their critical period (∼P30, corresponding to a 4-month-old human infant). Those studies have shown that sleep enhances plasticity during the critical period for ODP, while REMS deprivation inhibits experience-dependent plasticity in the cortex while (Dumoulin Bridi et al., 2015; Frank et al., 2001). Sleep can both strengthen and weaken different synapses in response to experience during wake (Aton et al., 2009; Frank et al., 2001; Frank, 2015; Puentes-Mestril and Aton, 2017; Zhou et al., 2020). In addition, REM sleep has been shown to be required for phosphorylation of kinases involved in plasticity following MD, such as ERK (Aton et al., 2009; Dumoulin Bridi et al., 2015; Renouard et al., 2018). Overall, these studies provide evidence that sleep, and in particular REM sleep, are essential for the consolidation of plasticity, especially during critical periods of development. Therefore, sleep alterations during early life may have long lasting effects on experience-dependent synapse remodeling.
1.2. Autism spectrum disorders and underlying mechanisms
Autism spectrum disorder (ASD) is the most prevalent neurodevelopmental disorder in the United States (Scandurra et al., 2019). ASD is called a spectrum disorder, as individuals on the spectrum display a wide range of symptoms; however, many impairments occur in the first two years of life which includes the critical period for normal brain development (Meredith, 2015). ASD diagnosis is challenging as it is behaviorally diagnosed and therefore often relies on the identification of symptomatic behaviors and professional clinical judgement. These behaviors may not be displayed until the disorder is well established. The current diagnostic criteria for ASD require symptoms in two core domains: deficits in social interactions and restrictive/repetitive behaviors. The average age of diagnosis in the United States is four years old (Zuckerman et al., 2016). Evidence suggests that early diagnosis and intervention can significantly improve treatment outcomes and, therefore, individuals' quality of life (Cohen et al., 2014; Verhoeff et al., 2018). While there is a genetic basis for ASD, most ASD cases occur for unknown reasons. Therefore, identifying biomarkers may aid in early detection and diagnosis is crucial.
Critical period dysregulation may pinpoint the key developmental stages at which synaptic abnormalities are present in neurodevelopmental disorders such as ASD. Some research supports that neurodevelopmental disorders are caused by the deregulation of critical periods for synaptic brain circuitry, leading to disruptions in proper development, and altered behavior. At the synaptic level, embryonic and early postnatal spine dysmorphology present in some ASD mouse models (Cruz-Martín et al., 2010; Galvez and Greenough, 2005) supports the hypothesis that abnormal circuitry may not be a consequence of impaired cognitive and behavioral impairment. This suggests that abnormalities in mechanisms that regulate synaptic circuitry are present before significant cognitive and behavioral abnormalities are detectable. The ability to measure molecular abnormalities in early postnatal development would give rise to the possibility of earlier intervention, thereby increasing the therapeutic potential for individuals with ASD.
ASD encompasses a set of highly heterogenous neurodevelopmental disorders that share common behavioral abnormalities. One common underlying theme in ASD is the functional alteration of both excitatory and inhibitory synapses. Brain function relies on informational transfer between excitatory and inhibitory networks. A dysfunctional balance between excitatory and inhibitory networks can lead to alterations in structural processes leading to electrophysiological changes that then lead to behavioral abnormalities. A mutation in a single gene can play a major role in cellular processes leading to complex effects in network phenotypes. Multiple ASD-associated genes are thought to influence plasticity in different cell types and at different levels, as they encode proteins involved in synaptic plasticity, neuronal excitability, and connectivity; as well as chromatin remodelers that allow changes brought upon by experience to be encoded long-term through epigenetic changes.
1.3. Prevalence of sleep problems in ASD
Sleep problems occur at a much higher rate in children with ASD. It is estimated that sleep problems affect 40–93% of children with ASD, compared to 11%–37% of typically developing children (Petruzzelli et al., 2021). Three key features characterize ASD sleep problems: problems falling asleep, problems staying asleep, and overall less sleep (Hodge et al., 2014). Sleep problems predict the severity of ASD core diagnostic symptoms and associated challenging behaviors (Cohen et al., 2014), and often increase with age (Hodge et al., 2014), which imposes a severe burden on caregivers. Recent studies using parent reports of sleep on a cohort of baby siblings of individuals with ASD (who are at a higher risk for a diagnosis) found that problems falling asleep in the first year of life precede an autism diagnosis and are associated with altered patterns of brain development, specifically hippocampal overgrowth (MacDuffie et al., 2020b). Baby-sibling studies have also shown that sleep problems in autistic children as young as four years old are associated with increased ‘higher-order’ restricted and repetitive behaviors later in childhood (MacDuffie et al., 2020a). This suggests that sleep problems early in life are predictive of symptom severity across the lifespan. This view is supported by recent studies showing that problems falling asleep predict impairments in behavior regulation later in children with ASD (Tesfaye et al., 2021). However, whether there is a causal mechanism linking sleep problems and brain dysfunction during development remains unknown.
There are several monogenic syndromes associated with ASD. The recapitulation of these syndromes in animal models has provided insights into mechanisms associated with the disorder. Genes linked to ASD and available mouse models can be found in the Simons Foundation Autism Research Initiative (SFARI) gene database. In that database genes are categorized according to the strength of evidence linking them to ASD based on human genetic studies as follows: S (Syndromic), 1 (High Confidence), 2 (Strong Candidate), 3 (Suggestive Evidence), 4 (Minimal Evidence), 5 (Hypothesized). Few of the mouse ASD models have been studied in regard to sleep and whether they recapitulate all features of the clinical sleep phenotype, which are: reduced sleep time, sleep fragmentation, and increased latency to fall asleep (Wintler et al., 2020). Some ASD mouse models display reduced sleep, even fewer also display sleep fragmentation. The Shank3ΔC mouse model is the only high-confidence ASD mouse model, so far, to also display increased sleep latency.
Sleep patterns across development including the response to sleep loss have been investigated in only one mouse model, Shank3ΔC mice. Studies show that Shank3ΔC mice sleep less throughout their lifespan and show reduced NREM sleep compared to wildtype littermates but paradoxically display dramatically increased levels of REM sleep at postnatal day 23 (Medina et al., 2022). At this age mice have achieved 70% of their maximal brain growth and are roughly equivalent to 9-10 month-old human infants based on brain volume (Workman et al., 2013) Shank3ΔC mice at P23 also show EEG spectral signatures during wake that may be indicative of cortical overgrowth (Medina et al., 2022). This time in rodents coincides with critical periods when the brain is exquisitely sensitive to changes in the environment. Therefore, studies in young mice, although limited, support the hypothesis from infant studies that sleep may be altered early in life and be a core feature of the ASD phenotype. In turn this predicts that sleep problems early in life may be highly correlated and perhaps predictive of alterations in brain plasticity such as an imbalance of excitation and inhibitory networks which are well documented in ASD (Bagni and Zukin, 2019).
1.4. Deficits in synaptic plasticity in ASD
Syndromic mouse models of ASD that recapitulate the human phenotype provide valuable insights into general mechanisms underlying ASD. To establish the number of ASD mouse models that have also been associated with deficits in synaptic plasticity we reviewed studies within the SFARI gene database (January 2021 release). The original dataset included copy number variants (CNV) and genes that were either syndromic (S) or had an evidence score of 1 (high confidence) and encompassed 105 genes and 438 related articles. Based on primary research articles we determined which genes or genomic regions influenced plasticity. We defined an effect on plasticity based on whether a mutation in the gene or a CNV produced one or more of the following phenotypes in mice: 1) change in dendritic spine morphology or number, 2) change in plasticity measured by electrophysiology, 3) change in learning/memory behavioral task and 4) whether a sleep phenotype has been investigated. The results are displayed in Table 1 and include 5 CNVs and 66 genes.
Table 1.
High-Confidence ASD Genes and CNVs associated with neuroplasticity. Summary of genes and CNVs extracted from the SFARI gene database (January 13th, 2021, release) for which mutations are associated with deficits in plasticity based on mouse model studies. Only copy number variants (CNV) or ASD genes that were either syndromic (S) or had an evidence score of 1 (high confidence) were considered. Column 1: gene symbol; column 2: brief description of the gene; column 3: Articles supporting a role for the gene/CNV in neuroplasticity; columns 4–6: “Yes” indicates a change in neuroplasticity reported in the literature in either: 1) dendritic spine morphology, 2) electrophysiological measures of plasticity (LTD, LTP, EPSC, IPSC, EPSP, IPSP), or 3) learning/memory behavioral task. “No” indicates article looked at and/or measured neuroplasticity but found no change between control and gene (i.e., enhancement or deficit was not indicated in the article). “N/a” indicates no article addressed measured plasticity in this manner; column 7: list articles that investigate sleep in a mouse model of the gene/CNV, “No” indicates it has not yet been investigated.
| Gene/CNV | Description | Citations | Dendritic spine morphology | Electro-physiology | Behavior | Sleep |
|---|---|---|---|---|---|---|
| m7qF3 | 16p11.2 duplication | Arbogast et al. (2016) | N/A | No | Yes | No |
| m7qF3 | 16p11.2 deletion | Arbogast et al. (2016); Bertero et al. (2018); Grissom et al. (2018); Horev et al. (2011); Portmann et al. (2014); Stoppel et al. (2018); Tian et al. (2015); Yang et al. (2015) | Yes | Yes | Yes | Angelakos et al. (2017); Lu et al. (2019) |
| m7qC | 15q13.3 deletion | Fejgin et al. (2014); Uddin et al. (2018) | Yes | Yes | Yes | No |
| m7qB5-qC | 15q11-q13 duplication | Isshiki et al. (2014); Kloth et al. (2015); Nakai et al. (2017); Piochon et al. (2014) | Yes | Yes | Yes | No |
| M11qB1.3-qB2 | 17p11.2 duplication | Lacaria et al. (2012) | N/A | N/A | Yes | No |
| Actl6b | Actin like 6B | Wenderski et al. (2020) | N/A | N/A | Yes | No |
| Adnp | Activity-dependent neuroprotector homeobox | Hacohen-Kleiman et al. (2018) | Yes | N/A | Yes | No |
| Ahi1 | Abelson helper integration site 1 | Sacai et al. (2020) | N/a | Yes | No | No |
| Ank2 | Ankyrin 2, neuronal | Yang et al. (2019) | No | Yes | Yes | No |
| Arid1b | AT-rich interaction domain 1B | Jung et al. (2017); Shibutani et al. (2017); Smith et al. (2020) | Yes | Yes | Yes | No |
| Arx | Aristaless related homeobox | Dubos et al. (2018) | N/A | Yes | Yes | No |
| Auts2 | Activator of transcription and developmental regulator | Hori et al. (2020) | Yes | Yes | Yes | No |
| Cacna1c | Calcium voltage-gated channel subunit alpha1 C | Langwieser et al. (2010); Moosmang et al. (2005) | No | Yes | Yes | Kumar et al. (2015) |
| Camk2a | Calcium/calmodulin dependent protein kinase II alpha | Stephenson et al. (2017) | Yes | Yes | No | No |
| Caprin1 | RNA granule protein 105 (Caprin 1) | Ohashi et al. (2016) | N/A | N/A | Yes | No |
| Cdkl5 | Cyclin-dependent kinase-like 5 | Tang et al. (2019) | Yes | Yes | Yes | No |
| Chd2 | Chromodomain helicase DNA binding protein 2 | Kim et al. (2018) | N/a | Yes | Yes | No |
| Chd8 | Chromodomain helicase DNA binding protein 8 | Durak et al. (2016); Ellingford et al. (2021); Gompers et al. (2017); Jung et al. (2018); Katayama et al. (2016); Kawamura et al. (2021); Platt et al. (2017) | Yes | Yes | Yes | No |
| Cntnap2 | Contactin associated protein-like 2 | Antoine et al. (2019); Brunner et al. (2015); Kloth et al. (2015); Peñagarikano et al. (2011); Sacai et al. (2020) | Yes | Yes | Yes | Thomas et al. (2017) |
| Ctnnb1 | Catenin (cadherin associated protein), beta 1 | Dong et al. (2016) | N/A | N/A | Yes | No |
| Cul3 | Cullin 3 | Z. Dong et al. (2020); Rapanelli et al. (2021) | Yes | Yes | No | No |
| Deaf1 | Transcription factor | Vulto-van Silfhout et al. (2014) | N/A | N/A | Yes | No |
| Dip2a | Disco interacting protein 2 homolog A | Ma et al. (2019) | Yes | Yes | No | No |
| Dlg4 | Discs, large homolog 4 (Drosophila) | Feyder et al. (2010) | Yes | N/A | Yes | No |
| Dmd | Dystrophin | Daoud et al. (2008) | Yes | Yes | Yes | No |
| Dscam | DS cell adhesion molecule | Maynard and Stein (2012) | Yes | N/A | N/A | No |
| Dyrk1a | Dual specificity tyrosine-Y-phosphorylation regulated kinase 1A | Altafaj et al. (2001); Arranz et al. (2019); Fotaki et al. (2002) | Yes | N/A | Yes | No |
| Fmr1 | Fragile X mental retardation protein 1 | Antoine et al. (2019); Bhattacharya et al. (2012); Comery et al. (1997); Costa et al. (2012); Koekkoek et al. (2005); Lim et al. (2014); Padmashri et al. (2013); Qin et al. (2011); Shang et al. (2009); Testa-Silva et al. (2012) | Yes | Yes | Yes | Boone et al. (2018) |
| Foxp1 | Forkhead box P1 | Araujo et al. (2017); Bacon et al. (2015) | Yes | Yes | Yes | No |
| Foxp2 | Forkhead box P2 | Enard et al. (2009) | Yes | Yes | N/a | No |
| Gabrb3 | Gamma-aminobutyric acid type A receptor beta 3 subunit | DeLorey et al. (1998) | N/A | N/A | Yes | Wisor et al. (2002) |
| Gria2 | Glutamate receptor, ionotropic, AMPA 2 | Chung et al. (2003) | N/A | Yes | N/A | No |
| Grin2b | Glutamate receptor, ionotropic, N-methyl D-aspartate 2B | Brigman et al. (2010) | Yes | Yes | Yes | No |
| Iqsec2 | IQ motif and Sec7 domain 2 | Rogers et al. (2019) | N/A | Yes | Yes | No |
| Kmt2a | Lysine methyltransferase 2A | Shen et al. (2016) | Yes | Yes | Yes | No |
| Mecp2 | Methyl-CpG binding protein 2 | Jentarra et al. (2010); Na et al. (2012); Wood et al. (2009); Wood and Shepherd (2010) | Yes | Yes | Yes | Dong et al. (2020); Johnston et al. (2014) |
| Mef2c | Myocyte Enhancer Factor 2C | Barbosa et al. (2008); Harrington et al. (2016) | Yes | Yes | Yes | Bjorness et al. (2020) |
| mTor | Mechanistic target of rapamycin kinase | Rial et al. (2020) | Yes | Yes | N/A | No |
| Nbea | Neurobeachin | Medrihan et al. (2009); Muellerleile et al. (2020); Nuytens et al. (2013) | Yes | Yes | Yes | No |
| Nf1 | Neurofibromin 1 | Costa et al. (2001); Molosh et al. (2014) | N/A | Yes | Yes | Anastasaki et al. (2019) |
| Nlgn3 | Neuroligin 3 | Baudouin et al. (2012); Etherton et al. (2011); Isshiki et al. (2014); Martella et al. (2018); Pizzarelli and Cherubini (2013); Tabuchi et al. (2007); Zhang et al. (2015) | Yes | Yes | Yes | Liu et al. (2017) |
| Nr2f1 | Nuclear receptor subfamily 2 group F member 1 | K. Zhang et al. (2020) | N/A | Yes | Yes | No |
| Nr3c2 | Nuclear receptor subfamily 3, group C, member 2 | Berger et al. (2006) | No | No | Yes | No |
| Nrxn1 | Neurexin 1 | Etherton et al. (2009) | N/a | Yes | Yes | No |
| Nrxn2 | Neurexin2 | Born et al. (2015) | No | Yes | No | No |
| Pogz | Pogo transposable element derived with ZNF domain | Matsumura et al. (2020) | Yes | Yes | Yes | No |
| Ptchd1 | Patched domain containing 1 | Ung et al. (2018); Wells et al. (2016) | Yes | Yes | Yes | No |
| Pten | Phosphatase and tensin homolog | Sánchez-Puelles et al. (2020); Takeuchi et al. (2013); Williams et al. (2015) | Yes | Yes | Yes | No |
| Rai1 | Retinoic acid induced 1 | W.-H. L. Huang et al. (2018) | Yes | N/A | N/A | Diessler et al. (2017) |
| Reln | Reelin | Rice et al. (2001) | Yes | N/A | N/A | No |
| Rims1 | Regulating synaptic membrane exocytosis 1 | Powell et al. (2004); Schoch et al. (2002); Wang et al. (2018) | No | Yes | Yes | Lonart et al. (2008) |
| Rps6ka3 | Ribosomal protein s6 kinase, 90 kDa, polypeptide 3 | Morice et al. (2013) | Yes | Yes | Yes | No |
| Scn1a | Sodium channel, voltage-gated, type 1, alpha subunit | Bahceci et al. (2020); Han et al. (2012); Kalume et al. (2015); Rubinstein et al. (2015); Tai et al. (2020) | N/A | Yes | Yes | Kalume et al. (2015); Papale et al. (2013) |
| Scn2a | Sodium voltage-gated channel alpha subunit 2 | Middleton et al. (2018); Shin et al. (2019) | N/A | Yes | Yes | Ma et al. (2022) |
| Scn8a | Sodium voltage-gated channel alpha subunit 8 | Papale et al. (2013); Woodruff-Pak et al. (2006) | N/A | Yes | Yes | Papale et al. (2010) |
| Setd5 | SET domain containing 5 | Deliu et al. (2018, p. 5); Moore et al. (2019) | Yes | Yes | Yes | No |
| Shank2 | SH3 and multiple ankyrin repeat domains 2 | Ha et al. (2016); Lim et al. (2017); Won et al. (2012) | Yes | Yes | Yes | No |
| Shank3 | SH3 and multiple ankyrin repeat domains 3 | Bozdagi et al., 2010, Bozdagi et al., 2013; Jaramillo et al., 2016, Jaramillo et al., 2017; Kabitzke et al. (2018); Kouser et al. (2013); Mei et al. (2016); Speed et al. (2015, p. 21); Tatavarty et al. (2020); Wang et al., 2011, Wang et al., 2016, Wang et al., 2020 | Yes | Yes | Yes | Bian et al. (2022); Ingiosi et al. (2019); Medina et al. (2022) |
| Slc1a2 | Solute carrier family 1 (glial high affinity glutamate transporter), member 2 | Aida et al. (2015) | N/A | Yes | N/A | No |
| Slc6a1 | Solute carrier family 6, member 1 | Gong et al. (2009) | No | Yes | Yes | Xu et al. (2013) |
| Slc9a6 | Solute carrier family 9, subfamily A | Ouyang et al. (2013) | Yes | N/A | N/A | No |
| Syngap1 | Synaptic Ras GTPase activating protein 1a | Clement et al. (2012); Kim et al. (2003); Muhia et al. (2012) | Yes | Yes | Yes | Sullivan et al. (2020) |
| Tbr1 | T-box, brain 1 | Fazel Darbandi et al., 2018, Fazel Darbandi et al., 2020; Huang et al., 2014, Huang et al., 2019; Lee et al. (2015); Yook et al. (2019) | Yes | Yes | Yes | No |
| Tbx1 | T-box 1 | Hiramoto et al. (2011) | N/A | N/A | Yes | No |
| Tcf4 | Transcription factor 4 | Kennedy et al. (2016, p. 4); Li et al. (2019) | Yes | Yes | Yes | No |
| Trio | Trio Rho guanine nucleotide exchange factor | Katrancha et al. (2019) | Yes | Yes | N/A | No |
| Tsc1 | Tuberous sclerosis 1 | Haji et al. (2020) | Yes | N/A | Yes | Rensing et al. (2020); K. Zhang et al. (2020) |
| Tsc2 | Tuberous sclerosis 2 | Antoine et al. (2019); Tang et al. (2014) | Yes | Yes | N/A | No |
| Tshz3 | Teashirt zinc finger homeobox 3 | Caubit et al. (2016) | N/A | Yes | N/A | No |
| Ube3a | Ubiquitin protein ligase E3A | Greer et al. (2010); Jiang et al. (1998); Kaphzan et al. (2012); Smith et al. (2011); Sun et al. (2015); Yashiro et al. (2009) | Yes | Yes | Yes | Colas et al. (2005); Copping and Silverman (2021); Ehlen et al. (2015) |
| Upf3b | Regulator of nonsense mediated mRNA decay | L. W.-H. Huang et al. (2018) | Yes | N/A | Yes | No |
While ASD encompasses a wide range of neurodevelopmental disorders, identifying common pathways may aid the understanding and treatment of ASD core features. ASD risk genes identified so far belong to convergent biological pathways relating to transcription, chromatin structure, and synaptic function. Although the earliest ASD risk genes to be identified were known to function at the synapse, it has become clear that a distinct subset of ASD genes are chromatin-modifiers and RNA-binding proteins. Of the 66 ASD risk genes we identified to have a role in synaptic plasticity based on animal model studies, 14 are associated with the cell membrane and known to function at the synapse (e.g.: Ctnnb1, Dscam, Shank2, Shank3, Ank2, Camk2a, Dlg4, Dmd, Fmr1, Gria2, Grin2b, Nrxn1, Nlgn3, Rims1). In addition, a large fraction of genes in Table 1 act in the nucleus as transcription/chromatin regulators e.g., Pogz, Ube3a, Nr3c2, Foxp2, Adnp, Mecp2, Chd2, Foxp1, Tcf4, Fmr1, Tsc1. Some genes have both a role at the synapse and in the nucleus (Ctnnb1, Shank3). This likely reflects the fact that synaptic plasticity requires changes at the synapse to be stabilized through changes in transcription, translation, and epigenetic modifications (Hegde and Smith, 2019).
1.5. Abnormal sleep in ASD and its potential impact on plasticity
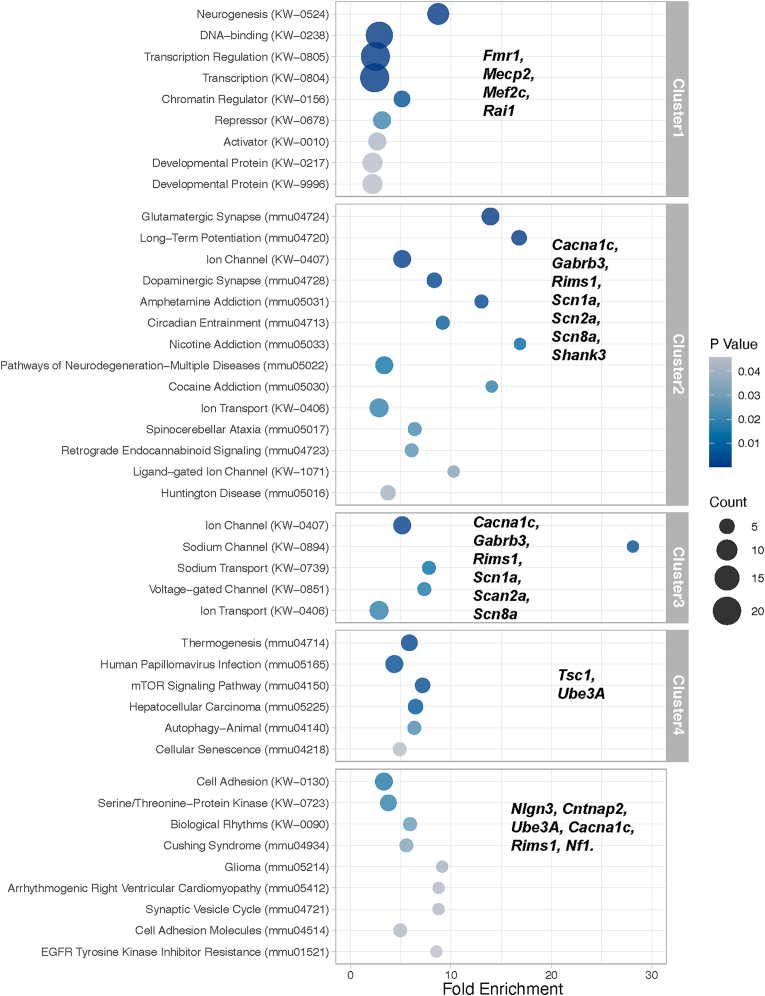
Abnormal sleep in ASD and deficits in synaptic plasticity are not mutually exclusive phenotypes. Table 2 provides additional details for genes in Table 1 for which mouse mode studies also display a sleep deficit. A reduction in sleep amount is defined as reduction in either NREMS, REMS or total sleep. Deficits in sleep quality are defined as fragmented sleep or altered spectral power in the EEG during sleep states. Deficits in the response to sleep loss are defined as either increases in latency to fall asleep or altered buildup of sleep pressure after sleep deprivation. Mice over 8 weeks were considered as adults. Table 2 includes 1 CNV and 18 high-confidence genes (SFARI gene score of 1 or Syndromic) for which mutations have been implicated in both plasticity and sleep deficits using mouse models (16p.11.2, Cacna1c, Cntnap2, Fmr1, Gabrb3, Mecp2, Mef2c, Nf1, Nlgn3, Rims1, Rai1, Shank3, Scn1a, Scn2a, Scn8a, Slc6a1, Syngap1, Tsc1, Ube3a).
Table 2.
High-Confidence ASD Genes and CNVs associated with neuroplasticity and sleep deficits. Additional details for genes in Table 1 for which mouse mode studies also display a sleep deficit. We restricted ourselves only to published studies that measure sleep in vivo with methods that allow differentiation between sleep state to quantify sleep amounts and quality of sleep. A reduction in sleep amount was defined as reduction in either NREMS or REMS or total sleep. Deficits in sleep quality were defined as fragmented sleep or altered spectral power in the EEG during sleep states. Deficits in the response to sleep loss were defined as either increases in latency to fall asleep or altered buildup of sleep pressure after sleep deprivation. Mice over 8 weeks were considered as adults. Table 2 includes 19 high-confidence ASD mouse models for which mutations have been implicated in both plasticity and sleep deficits (16p.11.2, Cacna1c, Cntnap2, Fmr1, Gabrb3, Mecp2, Mef2c, Nf1, Nlgn3, Rims1, Rai1, Scn1a, Scn2a, Scn8a, Shank3, Slc6a1, Syngap1, Tsc1, Ube3a).
| Gene/CNV | Citation | Reduced Sleep Amounts | Reduced Sleep Quality | Deficits in Response to Sleep Loss | Sex | Age |
|---|---|---|---|---|---|---|
| 16p11.2 (m7qF3) | Angelakos et al. (2017) | Yes | Abnormal EEG spectral power | Not assessed | Male and Female | Adult |
| Lu et al. (2019) | Yes | Fragmented, abnormal EEG spectral power | Not assessed | Male | Young (6–7 wks) | |
| Cacna1c | Kumar et al. (2015) | Yes | Fragmented, abnormal EEG spectral power | Alterations in REMS rebound | Male | Not specified |
| Cntnap2 | Thomas et al. (2017) | No | Fragmented | No difference in sleep pressure accumulation or sleep latency | Male | Adult |
| Fmr1 | Boone et al. (2018) | Yes | Fragmented | Not assessed | Male | Adult |
| Gabrb3 | Wisor et al. (2002) | Yes | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Adult |
| Mecp2 | Johnston et al. (2014) | Yes | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Young (6–7 wks) |
| H.-W. Dong et al. (2020) | Yes | Fragmented | Not assessed | Female | Young (5–8 wks) Old (20–24 wks) | |
| Mef2c | Bjorness et al. (2020) | No | Abnormal EEG spectral power (modest) | Deficits in sleep pressure accumulation | Male | Adult |
| Nf1 | Anastasaki et al. (2019) | No | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Adult |
| Nlgn3 | Liu et al. (2017) | No | Abnormal EEG spectral power | Not assessed | Male | Adult |
| Rai1 | Diessler et al. (2017) | No | Abnormal EEG spectral power | No | Male | Adult |
| Rims1 | Lonart et al. (2008) | Yes | Fragmented | Alterations in REMS rebound | Male | Adult |
| Scn1a | Kalume et al. (2015) | No | Fragmented | Deficits in sleep pressure accumulation | Not specified | Young (P30-33) |
| Papale et al. (2013) | Yes | No | No | Male | Adult | |
| Scn2a | Ma et al. (2022) | Yes | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Not specified |
| Scn8a | Papale et al. (2010) | Yes | Fragmented | No difference in sleep pressure accumulation | Male | Adult |
| Shank3 | Bian et al. (2022) | Yes | Abnormal EEG spectral power | Not assessed | Male and Female | Young (P35-42) |
| Ingiosi et al. (2019) | Yes | Fragmented, abnormal EEG spectral power | Increased sleep latency, no difference in sleep pressure accumulation | Male | Adult | |
| Medina et al. (2022) | Yes | Fragmented, abnormal EEG spectral power | Increased sleep latency, no difference in sleep pressure accumulation | Male | Longitudinal (P23–P60) | |
| Slc6a1 | Xu et al. (2013) | Yes | Abnormal EEG power | Deficits in sleep pressure accumulation | Male | Not specified |
| Syngap1 | Sullivan et al. (2020) | Yes | Abnormal EEG power | Not assessed | Male and Female | Adult |
| Tsc1 | Rensing et al. (2020) | Yes, age dependent | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Young (P9-21) |
| B. Zhang et al. (2020) | Yes | No | Not assessed | Male and Female | Young (4 wks) | |
| Ube3a | Ehlen et al. (2015) | Yes | Fragmented, abnormal EEG spectral power | Deficits in sleep pressure accumulation | Not specified | Adult (6–8 wks) |
| Colas et al. (2005) | Yes | Fragmented | Deficits in sleep pressure accumulation | Female | Adult | |
| Copping and Silverman (2021) | Yes | Fragmented, abnormal EEG spectral power | Not assessed | Male and Female | Adult |
To summarize the relationship between the ASD mouse models displaying plasticity deficits outlined in Table 1 and those also displaying sleep deficits outlined in Table 2, we performed a functional enrichment analysis using Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov) and data from the following databases: Uniprot and KEGG. We first determined which biological processes, molecular functions or pathways were statistically overrepresented among genes in Table 1 compared to what is expected by chance from the whole mouse genome. Significant statistical enrichment was defined as p < 0.05 and was found in several categories As excepted several genes mapped to multiple enriched biological processes, molecular functions and pathways. Subsequently we clustered the enriched terms to find common broad biological functions. Despite the small list of genes, this analysis resulted in four main clusters: transcription/chromatin regulation, synapse function, voltages gated ion channels and mTOR signaling; as well as a handful of pathways or processes that did not cluster. We then mapped the identity of ASD mouse models displaying sleep deficits outlined in Table 2 to these functional clusters. Fig. 2 shows the results of the enrichment and clustering and provide a summary of the functions of the genes that lie at the intersection between plasticity, sleep and ASD. These biological processes are well-known be affected in ASD as well and plasticity. However, the role of sleep in these processes, especially in the larger cluster involving transcription and chromatin regulation is less understood.
Fig. 2.
Summary of biological processes and pathways affected by ASD genes at the intersection of sleep and plasticity. Functional Annotation Enrichment analysis and subsequent clustering of genes in Table 1. Genes highlighted in bold are those for which mutations also affect sleep as outlined in Table 2. KW: KEGG pathway identifier, mmu: Uniprot biological process or molecular function identifier.
Synapse formation and chromatin regulation are processes well-known to influence brain development. Therefore, the convergence of ASD genetic models that show plasticity and sleep deficits on these biological processes may explain why sleep has such a profound effect on critical period plasticity. It is also likely that in ASD a sleep deficit may cause or contribute to deficits in synaptic plasticity from very early on. Nonetheless, very few studies have evaluated the role of ASD-associated genes on either sleep or plasticity early in life. The Shank3 mouse model is the only one in which sleep, and the impact of sleep loss has been examined early in life and in adults. Shank3ΔC−/- have been shown to develop an abnormal response to sleep loss during the early developmental period (Medina et al., 2022), while early-life sleep disruption in Shank3ΔC−/+ has been shown to affect behavior in adulthood (Lord et al., 2022). Mutations in Shank3 have also been shown to lead to deficits in homeostatic plasticity during the critical period, although those studies were done in a different mutant line (Shank3B) from the sleep studies (Tatavarty et al., 2020). There are currently no studies that have concurrently examined sleep and synaptic plasticity deficits early in life using an ASD mouse model. Recent studies demonstrate that sleep loss during development leads to long lasting impairments on social behavior and that increasing the quality of sleep during adolescence in a mouse model of ASD rescues social deficits in adulthood (Bian et al., 2022). The effect of early-life sleep disruption on social behavior and cognition has also been demonstrated in prairie voles in the absence of any genetic mutations (Jones et al., 2019, 2021). Therefore, it is likely the effects of sleep loss are more pronounced at earlier developmental stages in which sleep is the predominant brain state. Overall, further examination of the interaction between sleep and plasticity during early life in ASD is necessary.
2. Concluding remarks
Animal models have been proven useful to understand mechanisms underlying deficits in ASD. In this review, we support the known link between the genetic basis of ASD and deficits in synaptic plasticity and provide a comprehensive table of ASD mouse models that show plasticity deficits (Table 1). We also highlight that there is a considerable overlap between the ASD mouse models that show deficits in sleep and those that show deficits in plasticity (Table 2). This suggest that the genetic basis of both sleep and plasticity deficits in ASD is overlapping. This overlap may be particularly prominent during critical periods of brain development in which both plasticity and sleep are maximal. Recent studies suggest that sleep problems in ASD arise early in the postnatal period and are predictive of the severity of ASD core symptoms. Therefore, sleep problems: 1) may serve as an early biomarker for ASD and 2) may have functional consequences on plasticity during brain development. Further research is needed to understand the interaction between sleep and plasticity in ASD. Specifically, it is important to look early in life for the functional consequences of both sleep and plasticity deficits. Investigating sleep and plasticity during critical periods of development may be key to understanding long-term consequences of abnormal sleep on synaptic plasticity during both typical and atypical brain development.
Funding
This work was supported by the K01NS104172 and R56NS124805 from NIH/NINDS to Peixoto L.
Non-financial disclosure
The authors have no conflicts of interests to declare.
CRediT authorship contribution statement
Elizabeth Medina: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing, Visualization. Sarah Peterson: Conceptualization, Data curation, Writing – review & editing. Kaitlyn Ford: Data curation. Visualization. Kristan Singletary: Writing – review & editing, Visualization. Lucia Peixoto: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing, Visualization.
Declaration of competing interest
The authors have no financial arrangements or connections to declare.
Acknowledgements
We thank Dr. Marcos Frank for valuable discussions.
Mark R. Opp
References
- Aida T., Yoshida J., Nomura M., Tanimura A., Iino Y., Soma M., Bai N., Ito Y., Cui W., Aizawa H., Yanagisawa M., Nagai T., Takata N., Tanaka K.F., Takayanagi R., Kano M., Götz M., Hirase H., Tanaka K. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015;40:1569–1579. doi: 10.1038/npp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altafaj X., Dierssen M., Baamonde C., Martí E., Visa J., Guimerà J., Oset M., González J.R., Flórez J., Fillat C., Estivill X. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum. Mol. Genet. 2001;10:1915–1923. doi: 10.1093/hmg/10.18.1915. [DOI] [PubMed] [Google Scholar]
- Anastasaki C., Rensing N., Johnson K.J., Wong M., Gutmann D.H. Neurofibromatosis type 1 (Nf1)-mutant mice exhibit increased sleep fragmentation. J. Sleep Res. 2019;28 doi: 10.1111/jsr.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakos C.C., Watson A.J., O'Brien W.T., Krainock K.S., Nickl-Jockschat T., Abel T. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism Res. Off. J. Int. Soc. Autism Res. 2017;10:572–584. doi: 10.1002/aur.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine M.W., Langberg T., Schnepel P., Feldman D.E. Increased excitation-inhibition ratio Stabilizes Synapse and circuit excitability in four autism mouse models. Neuron. 2019;101:648–661. doi: 10.1016/j.neuron.2018.12.026. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo D.J., Toriumi K., Escamilla C.O., Kulkarni A., Anderson A.G., Harper M., Usui N., Ellegood J., Lerch J.P., Birnbaum S.G., Tucker H.O., Powell C.M., Konopka G. Foxp1 in forebrain pyramidal neurons controls gene expression required for Spatial learning and Synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:10917–10931. doi: 10.1523/JNEUROSCI.1005-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast T., Ouagazzal A.-M., Chevalier C., Kopanitsa M., Afinowi N., Migliavacca E., Cowling B.S., Birling M.-C., Champy M.-F., Reymond A., Herault Y. Reciprocal effects on neurocognitive and metabolic phenotypes in mouse models of 16p11.2 deletion and duplication Syndromes. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz J., Balducci E., Arató K., Sánchez-Elexpuru G., Najas S., Parras A., Rebollo E., Pijuan I., Erb I., Verde G., Sahun I., Barallobre M.J., Lucas J.J., Sánchez M.P., de la Luna S., Arbonés M.L. Impaired development of neocortical circuits contributes to the neurological alterations in DYRK1A haploinsufficiency syndrome. Neurobiol. Dis. 2019;127:210–222. doi: 10.1016/j.nbd.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton S.J., Seibt J., Dumoulin M., Jha S.K., Steinmetz N., Coleman T., Naidoo N., Frank M.G. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C., Schneider M., Le Magueresse C., Froehlich H., Sticht C., Gluch C., Monyer H., Rappold G.A. Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol. Psychiatr. 2015;20:632–639. doi: 10.1038/mp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C., Zukin R.S. A Synaptic perspective of fragile X Syndrome and autism Spectrum disorders. Neuron. 2019;101:1070–1088. doi: 10.1016/j.neuron.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahceci D., Anderson L.L., Occelli Hanbury Brown C.V., Zhou C., Arnold J.C. Adolescent behavioral abnormalities in a Scn1a+/- mouse model of Dravet syndrome. Epilepsy Behav. EB. 2020;103 doi: 10.1016/j.yebeh.2019.106842. [DOI] [PubMed] [Google Scholar]
- Barbosa A.C., Kim M.-S., Ertunc M., Adachi M., Nelson E.D., McAnally J., Richardson J.A., Kavalali E.T., Monteggia L.M., Bassel-Duby R., Olson E.N. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin S.J., Gaudias J., Gerharz S., Hatstatt L., Zhou K., Punnakkal P., Tanaka K.F., Spooren W., Hen R., De Zeeuw C.I., Vogt K., Scheiffele P. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- Berger S., Wolfer D.P., Selbach O., Alter H., Erdmann G., Reichardt H.M., Chepkova A.N., Welzl H., Haas H.L., Lipp H.-P., Schütz G. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc. Natl. Acad. Sci. U.S.A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero A., Liska A., Pagani M., Parolisi R., Masferrer M.E., Gritti M., Pedrazzoli M., Galbusera A., Sarica A., Cerasa A., Buffelli M., Tonini R., Buffo A., Gross C., Pasqualetti M., Gozzi A. Autism-associated 16p11.2 microdeletion impairs prefrontal functional connectivity in mouse and human. Brain J. Neurol. 2018;141:2055–2065. doi: 10.1093/brain/awy111. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Kaphzan H., Alvarez-Dieppa A.C., Murphy J.P., Pierre P., Klann E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76:325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W.-J., Brewer C.L., Kauer J.A., de Lecea L. Adolescent sleep shapes social novelty preference in mice. Nat. Neurosci. 2022;25:912–923. doi: 10.1038/s41593-022-01076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness T.E., Kulkarni A., Rybalchenko V., Suzuki A., Bridges C., Harrington A.J., Cowan C.W., Takahashi J.S., Konopka G., Greene R.W. An essential role for MEF2C in the cortical response to loss of sleep in mice. Elife. 2020;9 doi: 10.7554/eLife.58331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C.E., Davoudi H., Harrold J.B., Foster D.J. Abnormal Sleep architecture and hippocampal circuit dysfunction in a mouse model of fragile X Syndrome. Neuroscience. 2018;384:275–289. doi: 10.1016/j.neuroscience.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Born G., Grayton H.M., Langhorst H., Dudanova I., Rohlmann A., Woodward B.W., Collier D.A., Fernandes C., Missler M. Genetic targeting of NRXN2 in mice unveils role in excitatory cortical synapse function and social behaviors. Front. Synaptic Neurosci. 2015;7:3. doi: 10.3389/fnsyn.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O., Sakurai T., Papapetrou D., Wang X., Dickstein D.L., Takahashi N., Kajiwara Y., Yang M., Katz A.M., Scattoni M.L., Harris M.J., Saxena R., Silverman J.L., Crawley J.N., Zhou Q., Hof P.R., Buxbaum J.D. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O., Tavassoli T., Buxbaum J.D. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol. Autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman J.L., Wright T., Talani G., Prasad-Mulcare S., Jinde S., Seabold G.K., Mathur P., Davis M.I., Bock R., Gustin R.M., Colbran R.J., Alvarez V.A., Nakazawa K., Delpire E., Lovinger D.M., Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D., Kabitzke P., He D., Cox K., Thiede L., Hanania T., Sabath E., Alexandrov V., Saxe M., Peles E., Mills A., Spooren W., Ghosh A., Feliciano P., Benedetti M., Luo Clayton A., Biemans B. Comprehensive analysis of the 16p11.2 deletion and null Cntnap2 mouse models of autism Spectrum disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubit X., Gubellini P., Andrieux J., Roubertoux P.L., Metwaly M., Jacq B., Fatmi A., Had-Aissouni L., Kwan K.Y., Salin P., Carlier M., Liedén A., Rudd E., Shinawi M., Vincent-Delorme C., Cuisset J.-M., Lemaitre M.-P., Abderrehamane F., Duban B., Lemaitre J.-F., Woolf A.S., Bockenhauer D., Severac D., Dubois E., Zhu Y., Sestan N., Garratt A.N., Lydia Kerkerian-Le G., Fasano L. TSHZ3 deletion causes an autism syndrome and defects in cortical projection neurons. Nat. Genet. 2016;48:1359–1369. doi: 10.1038/ng.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.J., Steinberg J.P., Huganir R.L., Linden D.J. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Clement J.P., Aceti M., Creson T.K., Ozkan E.D., Shi Y., Reish N.J., Almonte A.G., Miller B.H., Wiltgen B.J., Miller C.A., Xu X., Rumbaugh G. Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses. Cell. 2012;151:709–723. doi: 10.1016/j.cell.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Conduit R., Lockley S.W., Rajaratnam S.M., Cornish K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J. Neurodev. Disord. 2014;6 doi: 10.1186/1866-1955-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas D., Wagstaff J., Fort P., Salvert D., Sarda N. Sleep disturbances in Ube3a maternal-deficient mice modeling Angelman syndrome. Neurobiol. Dis. 2005;20:471–478. doi: 10.1016/j.nbd.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Comery T.A., Harris J.B., Willems P.J., Oostra B.A., Irwin S.A., Weiler I.J., Greenough W.T. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copping N.A., Silverman J.L. Abnormal electrophysiological phenotypes and sleep deficits in a mouse model of Angelman Syndrome. Mol. Autism. 2021;12:9. doi: 10.1186/s13229-021-00416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L., Spatuzza M., D'Antoni S., Bonaccorso C.M., Trovato C., Musumeci S.A., Leopoldo M., Lacivita E., Catania M.V., Ciranna L. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol. Psychiatr. 2012;72:924–933. doi: 10.1016/j.biopsych.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Costa R.M., Yang T., Huynh D.P., Pulst S.M., Viskochil D.H., Silva A.J., Brannan C.I. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat. Genet. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- Cruz-Martín A., Crespo M., Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Brain Res. 1983;284:155–164. doi: 10.1016/0165-3806(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Daoud F., Candelario-Martínez A., Billard J.-M., Avital A., Khelfaoui M., Rozenvald Y., Guegan M., Mornet D., Jaillard D., Nudel U., Chelly J., Martínez-Rojas D., Laroche S., Yaffe D., Vaillend C. Role of mental retardation-associated dystrophin-gene product Dp71 in excitatory synapse organization, synaptic plasticity and behavioral functions. PLoS One. 2008;4 doi: 10.1371/journal.pone.0006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehorter N., Del Pino I. Shifting developmental trajectories during critical periods of brain formation. Front. Cell. Neurosci. 2020;14:283. doi: 10.3389/fncel.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E., Arecco N., Morandell J., Dotter C.P., Contreras X., Girardot C., Käsper E.-L., Kozlova A., Kishi K., Chiaradia I., Noh K.-M., Novarino G. Haploinsufficiency of the intellectual disability gene SETD5 disturbs developmental gene expression and cognition. Nat. Neurosci. 2018;21:1717–1727. doi: 10.1038/s41593-018-0266-2. [DOI] [PubMed] [Google Scholar]
- DeLorey T.M., Handforth A., Anagnostaras S.G., Homanics G.E., Minassian B.A., Asatourian A., Fanselow M.S., Delgado-Escueta A., Ellison G.D., Olsen R.W. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. Off. J. Soc. Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diessler S., Kostic C., Arsenijevic Y., Kawasaki A., Franken P. Rai1 frees mice from the repression of active wake behaviors by light. Elife. 2017;6 doi: 10.7554/eLife.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F., Jiang J., McSweeney C., Zou D., Liu L., Mao Y. Deletion of CTNNB1 in inhibitory circuitry contributes to autism-associated behavioral defects. Hum. Mol. Genet. 2016;25:2738–2751. doi: 10.1093/hmg/ddw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H.-W., Erickson K., Lee J.R., Merritt J., Fu C., Neul J.L. Detection of neurophysiological features in female R255X MeCP2 mutation mice. Neurobiol. Dis. 2020;145 doi: 10.1016/j.nbd.2020.105083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Chen W., Chen C., Wang H., Cui W., Tan Z., Robinson H., Gao N., Luo B., Zhang L., Zhao K., Xiong W.-C., Mei L. CUL3 deficiency causes Social deficits and anxiety-like behaviors by impairing excitation-inhibition balance through the promotion of cap-dependent translation. Neuron. 2020;105:475–490. doi: 10.1016/j.neuron.2019.10.035. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos A., Meziane H., Iacono G., Curie A., Riet F., Martin C., Loaëc N., Birling M.-C., Selloum M., Normand E., Pavlovic G., Sorg T., Stunnenberg H.G., Chelly J., Humeau Y., Friocourt G., Hérault Y. A new mouse model of ARX dup24 recapitulates the patients' behavioral and fine motor alterations. Hum. Mol. Genet. 2018;27:2138–2153. doi: 10.1093/hmg/ddy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin Bridi M.C., Aton S.J., Seibt J., Renouard L., Coleman T., Frank M.G. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durak O., Gao F., Kaeser-Woo Y.J., Rueda R., Martorell A.J., Nott A., Liu C.Y., Watson L.A., Tsai L.-H. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat. Neurosci. 2016;19:1477–1488. doi: 10.1038/nn.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen J.C., Jones K.A., Pinckney L., Gray C.L., Burette S., Weinberg R.J., Evans J.A., Brager A.J., Zylka M.J., Paul K.N., Philpot B.D., DeBruyne J.P. Maternal Ube3a loss disrupts Sleep homeostasis but leaves circadian rhythmicity largely intact. J. Neurosci. Off. J. Soc. Neurosci. 2015;35:13587–13598. doi: 10.1523/JNEUROSCI.2194-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingford R.A., Panasiuk M.J., de Meritens E.R., Shaunak R., Naybour L., Browne L., Basson M.A., Andreae L.C. Cell-type-specific synaptic imbalance and disrupted homeostatic plasticity in cortical circuits of ASD-associated Chd8 haploinsufficient mice. Mol. Psychiatr. 2021;26:3614–3624. doi: 10.1038/s41380-021-01070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W., Gehre S., Hammerschmidt K., Hölter S.M., Blass T., Somel M., Brückner M.K., Schreiweis C., Winter C., Sohr R., Becker L., Wiebe V., Nickel B., Giger T., Müller U., Groszer M., Adler T., Aguilar A., Bolle I., Calzada-Wack J., Dalke C., Ehrhardt N., Favor J., Fuchs H., Gailus-Durner V., Hans W., Hölzlwimmer G., Javaheri A., Kalaydjiev S., Kallnik M., Kling E., Kunder S., Mossbrugger I., Naton B., Racz I., Rathkolb B., Rozman J., Schrewe A., Busch D.H., Graw J., Ivandic B., Klingenspor M., Klopstock T., Ollert M., Quintanilla-Martinez L., Schulz H., Wolf E., Wurst W., Zimmer A., Fisher S.E., Morgenstern R., Arendt T., de Angelis M.H., Fischer J., Schwarz J., Pääbo S. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Espinosa J.S., Stryker M.P. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M., Földy C., Sharma M., Tabuchi K., Liu X., Shamloo M., Malenka R.C., Südhof T.C. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M.R., Blaiss C.A., Powell C.M., Südhof T.C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel Darbandi S., Robinson Schwartz S.E., Pai E.L.-L., Everitt A., Turner M.L., Cheyette B.N.R., Willsey A.J., State M.W., Sohal V.S., Rubenstein J.L.R. Enhancing WNT Signaling restores cortical neuronal Spine maturation and Synaptogenesis in Tbr1 mutants. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel Darbandi S., Robinson Schwartz S.E., Qi Q., Catta-Preta R., Pai E.L.-L., Mandell J.D., Everitt A., Rubin A., Krasnoff R.A., Katzman S., Tastad D., Nord A.S., Willsey A.J., Chen B., State M.W., Sohal V.S., Rubenstein J.L.R. Neonatal Tbr1 dosage controls cortical layer 6 connectivity. Neuron. 2018;100:831–845. doi: 10.1016/j.neuron.2018.09.027. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejgin K., Nielsen J., Birknow M.R., Bastlund J.F., Nielsen V., Lauridsen J.B., Stefansson H., Steinberg S., Sorensen H.B.D., Mortensen T.E., Larsen P.H., Klewe I.V., Rasmussen S.V., Stefansson K., Werge T.M., Kallunki P., Christensen K.V., Didriksen M. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol. Psychiatr. 2014;76:128–137. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Feyder M., Karlsson R.-M., Mathur P., Lyman M., Bock R., Momenan R., Munasinghe J., Scattoni M.L., Ihne J., Camp M., Graybeal C., Strathdee D., Begg A., Alvarez V.A., Kirsch P., Rietschel M., Cichon S., Walter H., Meyer-Lindenberg A., Grant S.G.N., Holmes A. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams' syndrome. Am. J. Psychiatr. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotaki V., Dierssen M., Alcántara S., Martínez S., Martí E., Casas C., Visa J., Soriano E., Estivill X., Arbonés M.L. Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol. Cell Biol. 2002;22:6636–6647. doi: 10.1128/MCB.22.18.6636-6647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G. Sleep and synaptic plasticity in the developing and adult brain. Curr. Top. Behav. Neurosci. 2015;25:123–149. doi: 10.1007/7854_2014_305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G., Heller H.C. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J. Sleep Res. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Heller H.C. Development of REM and slow wave sleep in the rat. Am. J. Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Issa N.P., Stryker M.P. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Ruby N.F., Heller H.C., Franken P. Development of circadian Sleep regulation in the rat: a longitudinal Study under constant conditions. Sleep. 2017;40:zsw077. doi: 10.1093/sleep/zsw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R., Greenough W.T. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am. J. Med. Genet. A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Gompers A.L., Su-Feher L., Ellegood J., Copping N.A., Riyadh M.A., Stradleigh T.W., Pride M.C., Schaffler M.D., Wade A.A., Catta-Preta R., Zdilar I., Louis S., Kaushik G., Mannion B.J., Plajzer-Frick I., Afzal V., Visel A., Pennacchio L.A., Dickel D.E., Lerch J.P., Crawley J.N., Zarbalis K.S., Silverman J.L., Nord A.S. Germline Chd8 haploinsufficiency alters brain development in mouse. Nat. Neurosci. 2017;20:1062–1073. doi: 10.1038/nn.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N., Li Y., Cai G.-Q., Niu R.-F., Fang Q., Wu K., Chen Z., Lin L.-N., Xu L., Fei J., Xu T.-L. GABA transporter-1 activity modulates hippocampal theta oscillation and theta burst stimulation-induced long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:15836–15845. doi: 10.1523/JNEUROSCI.4643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer P.L., Hanayama R., Bloodgood B.L., Mardinly A.R., Lipton D.M., Flavell S.W., Kim T.-K., Griffith E.C., Waldon Z., Maehr R., Ploegh H.L., Chowdhury S., Worley P.F., Steen J., Greenberg M.E. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N.M., McKee S.E., Schoch H., Bowman N., Havekes R., O'Brien W.T., Mahrt E., Siegel S., Commons K., Portfors C., Nickl-Jockschat T., Reyes T.M., Abel T. Male-specific deficits in natural reward learning in a mouse model of neurodevelopmental disorders. Mol. Psychiatr. 2018;23:544–555. doi: 10.1038/mp.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S., Lee D., Cho Y.S., Chung C., Yoo Y.-E., Kim J., Lee J., Kim W., Kim H., Bae Y.C., Tanaka-Yamamoto K., Kim E. Cerebellar Shank2 regulates excitatory Synapse density, motor coordination, and Specific repetitive and anxiety-like behaviors. J. Neurosci. Off. J. Soc. Neurosci. 2016;36:12129–12143. doi: 10.1523/JNEUROSCI.1849-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen-Kleiman G., Sragovich S., Karmon G., Gao A.Y.L., Grigg I., Pasmanik-Chor M., Le A., Korenková V., McKinney R.A., Gozes I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Invest. 2018;128:4956–4969. doi: 10.1172/JCI98199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji N., Riebe I., Aguilar-Valles A., Artinian J., Laplante I., Lacaille J.-C. Tsc1 haploinsufficiency in Nkx2.1 cells upregulates hippocampal interneuron mTORC1 activity, impairs pyramidal cell synaptic inhibition, and alters contextual fear discrimination and spatial working memory in mice. Mol. Autism. 2020;11:29. doi: 10.1186/s13229-020-00340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Tai C., Westenbroek R.E., Yu F.H., Cheah C.S., Potter G.B., Rubenstein J.L., Scheuer T., de la Iglesia H.O., Catterall W.A. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A.J., Raissi A., Rajkovich K., Berto S., Kumar J., Molinaro G., Raduazzo J., Guo Y., Loerwald K., Konopka G., Huber K.M., Cowan C.W. MEF2C regulates cortical inhibitory and excitatory synapses and behaviors relevant to neurodevelopmental disorders. Elife. 2016;5 doi: 10.7554/eLife.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., Elison J.T., Swanson M.R., Zhu H., Botteron K.N., Collins D.L., Constantino J.N., Dager S.R., Estes A.M., Evans A.C., Fonov V.S., Gerig G., Kostopoulos P., McKinstry R.C., Pandey J., Paterson S., Pruett J.R., Schultz R.T., Shaw D.W., Zwaigenbaum L., Piven J., IBIS Network, Clinical Sites, Data Coordinating Center, Image Processing Core, Statistical Analysis Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. Psychology Press; 2005. The Organization of Behavior: A Neuropsychological Theory. [Google Scholar]
- Hegde A.N., Smith S.G. Recent developments in transcriptional and translational regulation underlying long-term synaptic plasticity and memory. Learn. Mem. Cold Spring Harb. N. 2019;26:307–317. doi: 10.1101/lm.048769.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K. Critical period regulation. Annu. Rev. Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch T.K. Controlling the critical period. Neurosci. Res. 2003;47:17–22. doi: 10.1016/S0168-0102(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Hill J., Inder T., Neil J., Dierker D., Harwell J., Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto T., Kang G., Suzuki G., Satoh Y., Kucherlapati R., Watanabe Y., Hiroi N. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum. Mol. Genet. 2011;20:4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge D., Carollo T.M., Lewin M., Hoffman C.D., Sweeney D.P. Sleep patterns in children with and without autism spectrum disorders: developmental comparisons. Res. Dev. Disabil. 2014;35:1631–1638. doi: 10.1016/j.ridd.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Horev G., Ellegood J., Lerch J.P., Son Y.-E.E., Muthuswamy L., Vogel H., Krieger A.M., Buja A., Henkelman R.M., Wigler M., Mills A.A. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Yamashiro K., Nagai T., Shan W., Egusa S.F., Shimaoka K., Kuniishi H., Sekiguchi M., Go Y., Tatsumoto S., Yamada Mitsuyo, Shiraishi R., Kanno K., Miyashita S., Sakamoto A., Abe M., Sakimura K., Sone M., Sohya K., Kunugi H., Wada K., Yamada Mitsuhiko, Yamada K., Hoshino M. AUTS2 regulation of Synapses for proper Synaptic inputs and Social communication. iScience. 2020;23 doi: 10.1016/j.isci.2020.101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Shum E.Y., Jones S.H., Lou C.-H., Dumdie J., Kim H., Roberts A.J., Jolly L.A., Espinoza J.L., Skarbrevik D.M., Phan M.H., Cook-Andersen H., Swerdlow N.R., Gecz J., Wilkinson M.F. A Upf3b-mutant mouse model with behavioral and neurogenesis defects. Mol. Psychiatr. 2018;23:1773–1786. doi: 10.1038/mp.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.-N., Chuang H.-C., Chou W.-H., Chen C.-Y., Wang H.-F., Chou S.-J., Hsueh Y.-P. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat. Neurosci. 2014;17:240–247. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- Huang T.-N., Yen T.-L., Qiu L.R., Chuang H.-C., Lerch J.P., Hsueh Y.-P. Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice. Mol. Autism. 2019;10:5. doi: 10.1186/s13229-019-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.-H., Wang D.C., Allen W.E., Klope M., Hu H., Shamloo M., Luo L. Early adolescent Rai1 reactivation reverses transcriptional and social interaction deficits in a mouse model of Smith-Magenis syndrome. Proc. Natl. Acad. Sci. U.S.A. 2018;115:10744–10749. doi: 10.1073/pnas.1806796115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 1962;160:106–154.2. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Receptive fields of single neurones in the cat's striate cortex. J. Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübener M., Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159:727–737. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Ingiosi A., Schoch H., Wintler T.P., Singletary K.G., Righelli D., Roser L., Medina E., Risso D., Frank M.G., Peixoto L. Shank3 modulates Sleep and expression of circadian transcription factors. Elife. 2019;8 doi: 10.7554/eLife.42819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki M., Tanaka S., Kuriu T., Tabuchi K., Takumi T., Okabe S. Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nat. Commun. 2014;5:4742. doi: 10.1038/ncomms5742. [DOI] [PubMed] [Google Scholar]
- Jaramillo T.C., Speed H.E., Xuan Z., Reimers J.M., Escamilla C.O., Weaver T.P., Liu S., Filonova I., Powell C.M. Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res. Off. J. Int. Soc. Autism Res. 2017;10:42–65. doi: 10.1002/aur.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo T.C., Speed H.E., Xuan Z., Reimers J.M., Liu S., Powell C.M. Altered striatal Synaptic function and abnormal behaviour in Shank3 exon4–9 deletion mouse model of autism. Autism Res. Off. J. Int. Soc. Autism Res. 2016;9:350–375. doi: 10.1002/aur.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentarra G.M., Olfers S.L., Rice S.G., Srivastava N., Homanics G.E., Blue M., Naidu S., Narayanan V. Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC Neurosci. 2010;11:19. doi: 10.1186/1471-2202-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.H., Armstrong D., Albrecht U., Atkins C.M., Noebels J.L., Eichele G., Sweatt J.D., Beaudet A.L. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Johnston M.V., Ammanuel S., O'Driscoll C., Wozniak A., Naidu S., Kadam S.D. Twenty-four hour quantitative-EEG and in-vivo glutamate biosensor detects activity and circadian rhythm dependent biomarkers of pathogenesis in Mecp2 null mice. Front. Syst. Neurosci. 2014;8:118. doi: 10.3389/fnsys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., Chau A.Q., Olson R.J., Moore C., Wickham P.T., Puranik N., Guizzetti M., Cao H., Meshul C.K., Lim M.M. Early life sleep disruption alters glutamate and dendritic spines in prefrontal cortex and impairs cognitive flexibility in prairie voles. Curr. Res. Neurobiol. 2021;2 doi: 10.1016/j.crneur.2021.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., Opel R.A., Kaiser M.E., Chau A.Q., Quintana J.R., Nipper M.A., Finn D.A., Hammock E.A.D., Lim M.M. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E.-M., Moffat J.J., Liu J., Dravid S.M., Gurumurthy C.B., Kim W.-Y. Arid1b haploinsufficiency disrupts cortical interneuron development and mouse behavior. Nat. Neurosci. 2017;20:1694–1707. doi: 10.1038/s41593-017-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Park Haram, Choi Y., Kang H., Lee E., Kweon H., Roh J.D., Ellegood J., Choi W., Kang J., Rhim I., Choi S.-Y., Bae M., Kim S.-G., Lee J., Chung C., Yoo T., Park Hanwool, Kim Y., Ha S., Um S.M., Mo S., Kwon Y., Mah W., Bae Y.C., Kim H., Lerch J.P., Paik S.-B., Kim E. Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat. Neurosci. 2018;21:1218–1228. doi: 10.1038/s41593-018-0208-z. [DOI] [PubMed] [Google Scholar]
- Kabitzke P.A., Brunner D., He D., Fazio P.A., Cox K., Sutphen J., Thiede L., Sabath E., Hanania T., Alexandrov V., Rasmusson R., Spooren W., Ghosh A., Feliciano P., Biemans B., Benedetti M., Clayton A.L. Comprehensive analysis of two Shank3 and the Cacna1c mouse models of autism spectrum disorder. Gene Brain Behav. 2018;17:4–22. doi: 10.1111/gbb.12405. [DOI] [PubMed] [Google Scholar]
- Kalume F., Oakley J.C., Westenbroek R.E., Gile J., de la Iglesia H.O., Scheuer T., Catterall W.A. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol. Dis. 2015;77:141–154. doi: 10.1016/j.nbd.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphzan H., Hernandez P., Jung J.I., Cowansage K.K., Deinhardt K., Chao M.V., Abel T., Klann E. Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors. Biol. Psychiatr. 2012;72:182–190. doi: 10.1016/j.biopsych.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Nishiyama M., Shoji H., Ohkawa Y., Kawamura A., Sato T., Suyama M., Takumi T., Miyakawa T., Nakayama K.I. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature. 2016;537:675–679. doi: 10.1038/nature19357. [DOI] [PubMed] [Google Scholar]
- Katrancha S.M., Shaw J.E., Zhao A.Y., Myers S.A., Cocco A.R., Jeng A.T., Zhu M., Pittenger C., Greer C.A., Carr S.A., Xiao X., Koleske A.J. Trio haploinsufficiency causes neurodevelopmental disease-associated deficits. Cell Rep. 2019;26:2805–2817. doi: 10.1016/j.celrep.2019.02.022. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura A., Katayama Y., Kakegawa W., Ino D., Nishiyama M., Yuzaki M., Nakayama K.I. The autism-associated protein CHD8 is required for cerebellar development and motor function. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.108932. [DOI] [PubMed] [Google Scholar]
- Kennedy A.J., Rahn E.J., Paulukaitis B.S., Savell K.E., Kordasiewicz H.B., Wang J., Lewis J.W., Posey J., Strange S.K., Guzman-Karlsson M.C., Phillips S.E., Decker K., Motley S.T., Swayze E.E., Ecker D.J., Michael T.P., Day J.J., Sweatt J.D. Tcf4 regulates Synaptic plasticity, DNA methylation, and memory function. Cell Rep. 2016;16:2666–2685. doi: 10.1016/j.celrep.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee H.-K., Takamiya K., Huganir R.L. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:1119–1124. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Khoshkhoo S., Frankowski J.C., Zhu B., Abbasi S., Lee S., Wu Y.E., Hunt R.F. Chd2 is necessary for neural circuit development and long-term memory. Neuron. 2018;100:1180–1193. doi: 10.1016/j.neuron.2018.09.049. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth A.D., Badura A., Li A., Cherskov A., Connolly S.G., Giovannucci A., Bangash M.A., Grasselli G., Peñagarikano O., Piochon C., Tsai P.T., Geschwind D.H., Hansel C., Sahin M., Takumi T., Worley P.F., Wang S.S.-H. Cerebellar associative sensory learning defects in five mouse autism models. Elife. 2015;4 doi: 10.7554/eLife.06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. J. Cognit. Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Koekkoek S.K.E., Yamaguchi K., Milojkovic B.A., Dortland B.R., Ruigrok T.J.H., Maex R., De Graaf W., Smit A.E., VanderWerf F., Bakker C.E., Willemsen R., Ikeda T., Kakizawa S., Onodera K., Nelson D.L., Mientjes E., Joosten M., De Schutter E., Oostra B.A., Ito M., De Zeeuw C.I. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kouser M., Speed H.E., Dewey C.M., Reimers J.M., Widman A.J., Gupta N., Liu S., Jaramillo T.C., Bangash M., Xiao B., Worley P.F., Powell C.M. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:18448–18468. doi: 10.1523/JNEUROSCI.3017-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A.J., Ben Simon E., Mander B.A., Greer S.M., Saletin J.M., Goldstein-Piekarski A.N., Walker M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J.M., Frank M.G., Wisor J.P., Roy S. Sleep function: toward elucidating an enigma. Sleep Med. Rev. 2016;28:46–54. doi: 10.1016/j.smrv.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Dedic N., Flachskamm C., Voulé S., Deussing J.M., Kimura M. Cacna1c (Cav1.2) modulates electroencephalographic rhythm and rapid eye movement Sleep recovery. Sleep. 2015;38:1371–1380. doi: 10.5665/sleep.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaria M., Spencer C., Gu W., Paylor R., Lupski J.R. Enriched rearing improves behavioral responses of an animal model for CNV-based autistic-like traits. Hum. Mol. Genet. 2012;21:3083–3096. doi: 10.1093/hmg/dds124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langwieser N., Christel C.J., Kleppisch T., Hofmann F., Wotjak C.T., Moosmang S. Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:8367–8375. doi: 10.1523/JNEUROSCI.4164-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J.J., Fagiolini M. Autism: a “critical period” disorder? Neural Plast. 2011 doi: 10.1155/2011/921680. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.-J., Lee H., Huang T.-N., Chung C., Shin W., Kim K., Koh J.-Y., Hsueh Y.-P., Kim E. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat. Commun. 2015;6:7168. doi: 10.1038/ncomms8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt C.N., Hübener M. Critical-Period plasticity in the visual cortex. Annu. Rev. Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Li H., Zhu Y., Morozov Y.M., Chen X., Page S.C., Rannals M.D., Maher B.J., Rakic P. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol. Psychiatr. 2019;24:1235–1246. doi: 10.1038/s41380-019-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ma L., Yang G., Gan W.-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.-S., Hoang E.T., Viar K.E., Stornetta R.L., Scott M.M., Zhu J.J. Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev. 2014;28:273–289. doi: 10.1101/gad.232470.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.-S., Kim H., Yu N.-K., Kang S.J., Kim T., Ko H.-G., Lee J., Yang J.-E., Ryu H.-H., Park T., Gim J., Nam H.J., Baek S.H., Wegener S., Schmitz D., Boeckers T.M., Lee M.G., Kim E., Lee J.-H., Lee Y.-S., Kaang B.-K. Enhancing inhibitory synaptic function reverses spatial memory deficits in Shank2 mutant mice. Neuropharmacology. 2017;112:104–112. doi: 10.1016/j.neuropharm.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Liu J.J., Grace K.P., Horner R.L., Cortez M.A., Shao Y., Jia Z. Neuroligin 3 R451C mutation alters electroencephalography spectral activity in an animal model of autism spectrum disorders. Mol. Brain. 2017;10:10. doi: 10.1186/s13041-017-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonart G., Tang X., Simsek-Duran F., Machida M., Sanford L.D. The role of active zone protein Rab3 interacting molecule 1 alpha in the regulation of norepinephrine release, response to novelty, and sleep. Neuroscience. 2008;154:821–831. doi: 10.1016/j.neuroscience.2008.03.047. [DOI] [PubMed] [Google Scholar]
- Lord J.S., Gay S.M., Harper K.M., Nikolova V.D., Smith K.M., Moy S.S., Diering G.H. Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice. Mol. Autism. 2022;13:35. doi: 10.1186/s13229-022-00514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.-C., Pollack H., Lefante J.J., Mills A.A., Tian D. Altered sleep architecture, rapid eye movement sleep, and neural oscillation in a mouse model of human chromosome 16p11.2 microdeletion. Sleep. 2019;42 doi: 10.1093/sleep/zsy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Zhang L.-Q., He Z.-X., He X.-X., Wang Y.-J., Jian Y.-L., Wang X., Zhang B.-B., Su C., Lu J., Huang B.-Q., Zhang Y., Wang G.-Y., Guo W.-X., Qiu D.-L., Mei L., Xiong W.-C., Zheng Y.-W., Zhu X.-J. Autism candidate gene DIP2A regulates spine morphogenesis via acetylation of cortactin. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Eaton M., Liu Y., Zhang J., Chen X., Tu X., Shi Y., Que Z., Wettschurack K., Zhang Z., Shi R., Chen Y., Kimbrough A., Lanman N.A., Schust L., Huang Z., Yang Y. Deficiency of autism-related Scn2a gene in mice disrupts sleep patterns and circadian rhythms. Neurobiol. Dis. 2022;168 doi: 10.1016/j.nbd.2022.105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., Munson J., Greenson J., Ward T.M., Rogers S.J., Dawson G., Estes A. Sleep problems and trajectories of restricted and repetitive behaviors in children with neurodevelopmental disabilities. J. Autism Dev. Disord. 2020 doi: 10.1007/s10803-020-04438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., Shen M.D., Dager S.R., Styner M.A., Kim S.H., Paterson S., Pandey J., St John T., Elison J.T., Wolff J.J., Swanson M.R., Botteron K.N., Zwaigenbaum L., Piven J., Estes A.M. Sleep onset problems and subcortical development in infants later diagnosed with autism Spectrum disorder. Am. J. Psychiatr. 2020;177:518–525. doi: 10.1176/appi.ajp.2019.19060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G., Meringolo M., Trobiani L., De Jaco A., Pisani A., Bonsi P. The neurobiological bases of autism spectrum disorders: the R451C-neuroligin 3 mutation hampers the expression of long-term synaptic depression in the dorsal striatum. Eur. J. Neurosci. 2018;47:701–708. doi: 10.1111/ejn.13705. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Seiriki K., Okada S., Nagase M., Ayabe S., Yamada I., Furuse T., Shibuya H., Yasuda Y., Yamamori H., Fujimoto M., Nagayasu K., Yamamoto K., Kitagawa K., Miura H., Gotoda-Nishimura N., Igarashi H., Hayashida M., Baba M., Kondo M., Hasebe S., Ueshima K., Kasai A., Ago Y., Hayata-Takano A., Shintani N., Iguchi T., Sato M., Yamaguchi S., Tamura M., Wakana S., Yoshiki A., Watabe A.M., Okano H., Takuma K., Hashimoto R., Hashimoto H., Nakazawa T. Pathogenic POGZ mutation causes impaired cortical development and reversible autism-like phenotypes. Nat. Commun. 2020;11:859. doi: 10.1038/s41467-020-14697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard K.R., Stein E. DSCAM contributes to dendrite arborization and spine formation in the developing cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:16637–16650. doi: 10.1523/JNEUROSCI.2811-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E., Schoch H., Ford K., Wintler T., Singletary K.G., Peixoto L. Shank3 influences mammalian sleep development. J. Neurosci. Res. 2022 doi: 10.1002/jnr.25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L., Rohlmann A., Fairless R., Andrae J., Döring M., Missler M., Zhang W., Kilimann M.W. Neurobeachin, a protein implicated in membrane protein traffic and autism, is required for the formation and functioning of central synapses. J. Physiol. 2009;587:5095–5106. doi: 10.1113/jphysiol.2009.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y., Monteiro P., Zhou Y., Kim J.-A., Gao X., Fu Z., Feng G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith R.M. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015;50:180–188. doi: 10.1016/j.neubiorev.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Middleton S.J., Kneller E.M., Chen S., Ogiwara I., Montal M., Yamakawa K., McHugh T.J. Altered hippocampal replay is associated with memory impairment in mice heterozygous for the Scn2a gene. Nat. Neurosci. 2018;21:996–1003. doi: 10.1038/s41593-018-0163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molosh A.I., Johnson P.L., Spence J.P., Arendt D., Federici L.M., Bernabe C., Janasik S.P., Segu Z.M., Khanna R., Goswami C., Zhu W., Park S.-J., Li L., Mechref Y.S., Clapp D.W., Shekhar A. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nat. Neurosci. 2014;17:1583–1590. doi: 10.1038/nn.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.M., Seidman J.S., Ellegood J., Gao R., Savchenko A., Troutman T.D., Abe Y., Stender J., Lee D., Wang S., Voytek B., Lerch J.P., Suh H., Glass C.K., Muotri A.R. Setd5 haploinsufficiency alters neuronal network connectivity and leads to autistic-like behaviors in mice. Transl. Psychiatry. 2019;9:24. doi: 10.1038/s41398-018-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.-A., Storm D.R., Hofmann F., Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice E., Farley S., Poirier R., Dallerac G., Chagneau C., Pannetier S., Hanauer A., Davis S., Vaillend C., Laroche S. Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome. Neurobiol. Dis. 2013;58:156–168. doi: 10.1016/j.nbd.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Mower G.D. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res. Dev. Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Muellerleile J., Blistein A., Rohlmann A., Scheiwe F., Missler M., Schwarzacher S.W., Jedlicka P. Enhanced LTP of population spikes in the dentate gyrus of mice haploinsufficient for neurobeachin. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhia M., Willadt S., Yee B.K., Feldon J., Paterna J.-C., Schwendener S., Vogt K., Kennedy M.B., Knuesel I. Molecular and behavioral changes associated with adult hippocampus-specific SynGAP1 knockout. Learn. Mem. Cold Spring Harb. N. 2012;19:268–281. doi: 10.1101/lm.026351.112. [DOI] [PubMed] [Google Scholar]
- Na E.S., Nelson E.D., Adachi M., Autry A.E., Mahgoub M.A., Kavalali E.T., Monteggia L.M. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai N., Nagano M., Saitow F., Watanabe Yasuhito, Kawamura Y., Kawamoto A., Tamada K., Mizuma H., Onoe H., Watanabe Yasuyoshi, Monai H., Hirase H., Nakatani J., Inagaki H., Kawada T., Miyazaki T., Watanabe M., Sato Y., Okabe S., Kitamura K., Kano M., Hashimoto K., Suzuki H., Takumi T. Serotonin rebalances cortical tuning and behavior linked to autism symptoms in 15q11-13 CNV mice. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1603001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuytens K., Gantois I., Stijnen P., Iscru E., Laeremans A., Serneels L., Van Eylen L., Liebhaber S.A., Devriendt K., Balschun D., Arckens L., Creemers J.W.M., D'Hooge R. Haploinsufficiency of the autism candidate gene Neurobeachin induces autism-like behaviors and affects cellular and molecular processes of synaptic plasticity in mice. Neurobiol. Dis. 2013;51:144–151. doi: 10.1016/j.nbd.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Ohashi R., Takao K., Miyakawa T., Shiina N. Comprehensive behavioral analysis of RNG105 (Caprin1) heterozygous mice: reduced social interaction and attenuated response to novelty. Sci. Rep. 2016;6 doi: 10.1038/srep20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q., Lizarraga S.B., Schmidt M., Yang U., Gong J., Ellisor D., Kauer J.A., Morrow E.M. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R., Reiner B.C., Suresh A., Spartz E., Dunaevsky A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:19715–19723. doi: 10.1523/JNEUROSCI.2514-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale L.A., Makinson C.D., Christopher Ehlen J., Tufik S., Decker M.J., Paul K.N., Escayg A. Altered sleep regulation in a mouse model of SCN1A-derived genetic epilepsy with febrile seizures plus (GEFS+) Epilepsia. 2013;54:625–634. doi: 10.1111/epi.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale L.A., Paul K.N., Sawyer N.T., Manns J.R., Tufik S., Escayg A. Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory. J. Biol. Chem. 2010;285:16553–16561. doi: 10.1074/jbc.M109.090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O., Abrahams B.S., Herman E.I., Winden K.D., Gdalyahu A., Dong H., Sonnenblick L.I., Gruver R., Almajano J., Bragin A., Golshani P., Trachtenberg J.T., Peles E., Geschwind D.H. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M.G., Matera E., Giambersio D., Marzulli L., Gabellone A., Legrottaglie A.R., Margari A., Margari L. Subjective and electroencephalographic Sleep parameters in children and adolescents with autism Spectrum disorder: a Systematic review. J. Clin. Med. 2021;10:3893. doi: 10.3390/jcm10173893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C., Kloth A.D., Grasselli G., Titley H.K., Nakayama H., Hashimoto K., Wan V., Simmons D.H., Eissa T., Nakatani J., Cherskov A., Miyazaki T., Watanabe M., Takumi T., Kano M., Wang S.S.-H., Hansel C. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 2014;5:5586. doi: 10.1038/ncomms6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzarelli R., Cherubini E. Developmental regulation of GABAergic signalling in the hippocampus of neuroligin 3 R451C knock-in mice: an animal model of Autism. Front. Cell. Neurosci. 2013;7:85. doi: 10.3389/fncel.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt R.J., Zhou Y., Slaymaker I.M., Shetty A.S., Weisbach N.R., Kim J.-A., Sharma J., Desai M., Sood S., Kempton H.R., Crabtree G.R., Feng G., Zhang F. Chd8 mutation leads to autistic-like behaviors and impaired striatal circuits. Cell Rep. 2017;19:335–350. doi: 10.1016/j.celrep.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann T., Yang M., Mao R., Panagiotakos G., Ellegood J., Dolen G., Bader P.L., Grueter B.A., Goold C., Fisher E., Clifford K., Rengarajan P., Kalikhman D., Loureiro D., Saw N.L., Zhengqui Z., Miller M.A., Lerch J.P., Henkelman M., Shamloo M., Malenka R.C., Crawley J.N., Dolmetsch R.E. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep. 2014;7:1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C.M., Schoch S., Monteggia L., Barrot M., Matos M.F., Feldmann N., Südhof T.C., Nestler E.J. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes-Mestril C., Aton S.J. Linking network activity to Synaptic plasticity during Sleep: hypotheses and recent data. Front. Neural Circ. 2017;11 doi: 10.3389/fncir.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Entezam A., Usdin K., Huang T., Liu Z.-H., Hoffman G.E., Smith C.B. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol. Dis. 2011;42:85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M., Tan T., Wang W., Wang X., Wang Z.-J., Zhong P., Frick L., Qin L., Ma K., Qu J., Yan Z. Behavioral, circuitry, and molecular aberrations by region-specific deficiency of the high-risk autism gene Cul3. Mol. Psychiatr. 2021;26:1491–1504. doi: 10.1038/s41380-019-0498-x. [DOI] [PubMed] [Google Scholar]
- Renouard L., Bridi M.C.D., Coleman T., Arckens L., Frank M.G. Anatomical correlates of rapid eye movement sleep-dependent plasticity in the developing cortex. Sleep. 2018;41 doi: 10.1093/sleep/zsy124. [DOI] [PubMed] [Google Scholar]
- Renouard L., Hayworth C., Rempe M., Clegern W., Wisor J., Frank M.G. REM sleep promotes bidirectional plasticity in developing visual cortex in vivo. Neurobiol. Sleep Circadian Rhythms. 2022;12 doi: 10.1016/j.nbscr.2022.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing N., Johnson K.J., Foutz T.J., Friedman J.L., Galindo R., Wong M. Early developmental electroencephalography abnormalities, neonatal seizures, and induced spasms in a mouse model of tuberous sclerosis complex. Epilepsia. 2020;61:879–891. doi: 10.1111/epi.16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing N., Moy B., Friedman J.L., Galindo R., Wong M. Longitudinal analysis of developmental changes in electroencephalography patterns and sleep-wake states of the neonatal mouse. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial D., Puighermanal E., Chazalon M., Valjent E., Schiffmann S.N., de Kerchove d'Exaerde A. Mammalian target of rapamycin-RhoA Signaling impairments in direct striatal projection neurons induce altered behaviors and striatal physiology in mice. Biol. Psychiatr. 2020;88:945–954. doi: 10.1016/j.biopsych.2020.05.029. [DOI] [PubMed] [Google Scholar]
- Ribeiro S. Sleep and plasticity. Pflügers Archiv. 2012;463:111–120. doi: 10.1007/s00424-011-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D.S., Nusinowitz S., Azimi A.M., Martínez A., Soriano E., Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2001;31:929–941. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- Roffwarg H.P., Muzio J.N., Dement W.C. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Rogers E.J., Jada R., Schragenheim-Rozales K., Sah M., Cortes M., Florence M., Levy N.S., Moss R., Walikonis R.S., Palty R., Shalgi R., Lichtman D., Kavushansky A., Gerges N.Z., Kahn I., Umanah G.K.E., Levy A.P. An IQSEC2 mutation associated with intellectual disability and autism results in decreased Surface AMPA receptors. Front. Mol. Neurosci. 2019;12:43. doi: 10.3389/fnmol.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Han S., Tai C., Westenbroek R.E., Hunker A., Scheuer T., Catterall W.A. Dissecting the phenotypes of Dravet syndrome by gene deletion. Brain J. Neurol. 2015;138:2219–2233. doi: 10.1093/brain/awv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacai H., Sakoori K., Konno K., Nagahama K., Suzuki H., Watanabe T., Watanabe M., Uesaka N., Kano M. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat. Commun. 2020;11:5140. doi: 10.1038/s41467-020-18861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Puelles C., Calleja-Felipe M., Ouro A., Bougamra G., Arroyo A., Diez I., Erramuzpe A., Cortés J., Martínez-Hernández J., Luján R., Navarrete M., Venero C., Chan A., Morales M., Esteban J.A., Knafo S. PTEN activity defines an Axis for plasticity at cortico-amygdala Synapses and influences Social behavior. Cereb. Cortex N. Y. N. 2020;30:505–524. doi: 10.1093/cercor/bhz103. 1991. [DOI] [PubMed] [Google Scholar]
- Scandurra V., Emberti Gialloreti L., Barbanera F., Scordo M.R., Pierini A., Canitano R. Neurodevelopmental disorders and adaptive functions: a Study of children with autism Spectrum disorders (ASD) and/or attention deficit and hyperactivity disorder (ADHD) Front. Psychiatr. 2019;10 doi: 10.3389/fpsyt.2019.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S., Castillo P.E., Jo T., Mukherjee K., Geppert M., Wang Y., Schmitz F., Malenka R.C., Südhof T.C. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Shaffery J., Roffwarg H. The ontogenetic hypothesis of rapid eye movement sleep function revisited. Curr. Adv. Sleep Biol. 2009:177–216. [Google Scholar]
- Shaffery J.P., Sinton C.M., Bissette G., Roffwarg H.P., Marks G.A. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/S0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- Shang Y., Wang H., Mercaldo V., Li X., Chen T., Zhuo M. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J. Neurochem. 2009;111:635–646. doi: 10.1111/j.1471-4159.2009.06314.x. [DOI] [PubMed] [Google Scholar]
- Shen E.Y., Jiang Y., Javidfar B., Kassim B., Loh Y.-H.E., Ma Q., Mitchell A.C., Pothula V., Stewart A.F., Ernst P., Yao W.-D., Martin G., Shen L., Jakovcevski M., Akbarian S. Neuronal deletion of Kmt2a/mll1 histone methyltransferase in ventral Striatum is associated with defective spike-timing-dependent striatal Synaptic plasticity, altered response to dopaminergic drugs, and increased anxiety. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41:3103–3113. doi: 10.1038/npp.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M., Horii T., Shoji H., Morita S., Kimura M., Terawaki N., Miyakawa T., Hatada I. Arid1b haploinsufficiency causes abnormal brain gene expression and autism-related behaviors in mice. Int. J. Mol. Sci. 2017;18:E1872. doi: 10.3390/ijms18091872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W., Kweon H., Kang R., Kim D., Kim K., Kang M., Kim S.Y., Hwang S.N., Kim J.Y., Yang E., Kim H., Kim E. Scn2a haploinsufficiency in mice suppresses hippocampal neuronal excitability, excitatory Synaptic drive, and long-term potentiation, and Spatial learning and memory. Front. Mol. Neurosci. 2019;12:145. doi: 10.3389/fnmol.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.M. The neurobiology of sleep. Semin. Neurol. 2009;29:277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.L., Jung E.-M., Jeon B.T., Kim W.-Y. Arid1b haploinsufficiency in parvalbumin- or somatostatin-expressing interneurons leads to distinct ASD-like and ID-like behavior. Sci. Rep. 2020;10:7834. doi: 10.1038/s41598-020-64066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E.P., Zhou Y.-D., Zhang G., Jin Z., Stoppel D.C., Anderson M.P. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci. Transl. Med. 2011;3:103ra97. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed H.E., Kouser M., Xuan Z., Reimers J.M., Ochoa C.F., Gupta N., Liu S., Powell C.M. Autism-associated insertion mutation (InsG) of Shank3 exon 21 causes impaired Synaptic transmission and behavioral deficits. J. Neurosci. 2015;35:9648–9665. doi: 10.1523/JNEUROSCI.3125-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J.R., Wang X., Perfitt T.L., Parrish W.P., Shonesy B.C., Marks C.R., Mortlock D.P., Nakagawa T., Sutcliffe J.S., Colbran R.J. A novel human CAMK2A mutation disrupts dendritic morphology and Synaptic transmission, and causes ASD-related behaviors. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:2216–2233. doi: 10.1523/JNEUROSCI.2068-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel L.J., Kazdoba T.M., Schaffler M.D., Preza A.R., Heynen A., Crawley J.N., Bear M.F. R-baclofen reverses cognitive deficits and improves Social interactions in two lines of 16p11.2 deletion mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018;43:513–524. doi: 10.1038/npp.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.J., Ammanuel S., Kipnis P.A., Araki Y., Huganir R.L., Kadam S.D. Low-dose perampanel rescues cortical gamma dysregulation associated with parvalbumin interneuron GluA2 upregulation in epileptic Syngap1+/- mice. Biol. Psychiatr. 2020;87:829–842. doi: 10.1016/j.biopsych.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu G., Liu Y., Standley S., Ji A., Tunuguntla R., Wang Y., Claus C., Luo Y., Baudry M., Bi X. UBE3A regulates Synaptic plasticity and learning and memory by controlling SK2 channel endocytosis. Cell Rep. 2015;12:449–461. doi: 10.1016/j.celrep.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Zhou H., Cichon J., Yang G. Experience and sleep-dependent synaptic plasticity: from structure to activity. Philos. Trans. R. Soc. B Biol. Sci. 2020;375 doi: 10.1098/rstb.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K., Blundell J., Etherton M.R., Hammer R.E., Liu X., Powell C.M., Südhof T.C. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C., Chang C.-W., Yu G.-Q., Lopez I., Yu X., Wang X., Guo W., Mucke L. Tau reduction prevents key features of autism in mouse models. Neuron. 2020;106:421–437. doi: 10.1016/j.neuron.2020.01.038. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Gertner M.J., Zhou J., Parada L.F., Bennett M.V.L., Zukin R.S. Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4738–4743. doi: 10.1073/pnas.1222803110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Gudsnuk K., Kuo S.-H., Cotrina M.L., Rosoklija G., Sosunov A., Sonders M.S., Kanter E., Castagna C., Yamamoto A., Yue Z., Arancio O., Peterson B.S., Champagne F., Dwork A.J., Goldman J., Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Terzic B., Wang I.-T.J., Sarmiento N., Sizov K., Cui Y., Takano H., Marsh E.D., Zhou Z., Coulter D.A. Altered NMDAR signaling underlies autistic-like features in mouse models of CDKL5 deficiency disorder. Nat. Commun. 2019;10:2655. doi: 10.1038/s41467-019-10689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatavarty V., Torrado Pacheco A., Groves Kuhnle C., Lin H., Koundinya P., Miska N.J., Hengen K.B., Wagner F.F., Van Hooser S.D., Turrigiano G.G. Autism-associated Shank3 is essential for homeostatic compensation in rodent V1. Neuron. 2020;106:769–777. doi: 10.1016/j.neuron.2020.02.033. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye R., Wright N., Zaidman-Zait A., Bedford R., Zwaigenbaum L., Kerns C.M., Duku E., Mirenda P., Bennett T., Georgiades S., Smith I.M., Vaillancourt T., Pickles A., Szatmari P., Elsabbagh M., Pathways Team. Investigating longitudinal associations between parent reported sleep in early childhood and teacher reported executive functioning in school-aged children with autism. 2021. Sleep zsab122. [DOI] [PMC free article] [PubMed]
- Testa-Silva G., Loebel A., Giugliano M., de Kock C.P.J., Mansvelder H.D., Meredith R.M. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb. Cortex N. Y. N. 2012;22:1333–1342. doi: 10.1093/cercor/bhr224. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.M., Schwartz M.D., Saxe M.D., Kilduff T.S. Sleep/wake physiology and quantitative electroencephalogram analysis of the neuroligin-3 knockout rat model of autism Spectrum disorder. Sleep. 2017;40 doi: 10.1093/sleep/zsx138. [DOI] [PubMed] [Google Scholar]
- Tian D., Stoppel L.J., Heynen A.J., Lindemann L., Jaeschke G., Mills A.A., Bear M.F. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 2015;18:182–184. doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M., Unda B.K., Kwan V., Holzapfel N.T., White S.H., Chalil L., Woodbury-Smith M., Ho K.S., Harward E., Murtaza N., Dave B., Pellecchia G., D'Abate L., Nalpathamkalam T., Lamoureux S., Wei J., Speevak M., Stavropoulos J., Hope K.J., Doble B.W., Nielsen J., Wassman E.R., Scherer S.W., Singh K.K. OTUD7A regulates neurodevelopmental phenotypes in the 15q13.3 microdeletion Syndrome. Am. J. Hum. Genet. 2018;102:278–295. doi: 10.1016/j.ajhg.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung D.C., Iacono G., Méziane H., Blanchard E., Papon M.-A., Selten M., van Rhijn J.-R., Montjean R., Rucci J., Martin S., Fleet A., Birling M.-C., Marouillat S., Roepman R., Selloum M., Lux A., Thépault R.-A., Hamel P., Mittal K., Vincent J.B., Dorseuil O., Stunnenberg H.G., Billuart P., Nadif Kasri N., Hérault Y., Laumonnier F. Ptchd1 deficiency induces excitatory synaptic and cognitive dysfunctions in mouse. Mol. Psychiatr. 2018;23:1356–1367. doi: 10.1038/mp.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Hof M., Tisseur C., van Berckelear-Onnes I., van Nieuwenhuyzen A., Daniels A.M., Deen M., Hoek H.W., Ester W.A. Age at autism spectrum disorder diagnosis: a systematic review and meta-analysis from 2012 to 2019. Autism Int. J. Res. Pract. 2021;25:862–873. doi: 10.1177/1362361320971107. [DOI] [PubMed] [Google Scholar]
- Verhoeff M.E., Blanken L.M.E., Kocevska D., Mileva-Seitz V.R., Jaddoe V.W.V., White T., Verhulst F., Luijk M.P.C.M., Tiemeier H. The bidirectional association between sleep problems and autism spectrum disorder: a population-based cohort study. Mol. Autism. 2018;9:8. doi: 10.1186/s13229-018-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulto-van Silfhout A.T., Rajamanickam S., Jensik P.J., Vergult S., de Rocker N., Newhall K.J., Raghavan R., Reardon S.N., Jarrett K., McIntyre T., Bulinski J., Ownby S.L., Huggenvik J.I., McKnight G.S., Rose G.M., Cai X., Willaert A., Zweier C., Endele S., de Ligt J., van Bon B.W.M., Lugtenberg D., de Vries P.F., Veltman J.A., van Bokhoven H., Brunner H.G., Rauch A., de Brouwer A.P.M., Carvill G.L., Hoischen A., Mefford H.C., Eichler E.E., Vissers L.E.L.M., Menten B., Collard M.W., de Vries B.B.A. Mutations affecting the SAND domain of DEAF1 cause intellectual disability with severe speech impairment and behavioral problems. Am. J. Hum. Genet. 2014;94:649–661. doi: 10.1016/j.ajhg.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lv X., Wu Y., Xu T., Jiao M., Yang R., Li X., Chen M., Yan Y., Chen C., Dong W., Yang W., Zhuo M., Chen T., Luo J., Qiu S. Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons. Nat. Commun. 2018;9:2267. doi: 10.1038/s41467-018-04672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Pang K., Han K., Adamski C.J., Wang W., He L., Lai J.K., Bondar V.V., Duman J.G., Richman R., Tolias K.F., Barth P., Palzkill T., Liu Z., Holder J.L., Zoghbi H.Y. An autism-linked missense mutation in SHANK3 reveals the modularity of Shank3 function. Mol. Psychiatr. 2020;25:2534–2555. doi: 10.1038/s41380-018-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Bey A.L., Katz B.M., Badea A., Kim N., David L.K., Duffney L.J., Kumar S., Mague S.D., Hulbert S.W., Dutta N., Hayrapetyan V., Yu C., Gaidis E., Zhao S., Ding J.-D., Xu Q., Chung L., Rodriguiz R.M., Wang F., Weinberg R.J., Wetsel W.C., Dzirasa K., Yin H., Jiang Y.-H. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016;7 doi: 10.1038/ncomms11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., McCoy P.A., Rodriguiz R.M., Pan Y., Je H.S., Roberts A.C., Kim C.J., Berrios J., Colvin J.S., Bousquet-Moore D., Lorenzo I., Wu G., Weinberg R.J., Ehlers M.D., Philpot B.D., Beaudet A.L., Wetsel W.C., Jiang Y.-H. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M.F., Wimmer R.D., Schmitt L.I., Feng G., Halassa M.M. Thalamic reticular impairment underlies attention deficit in Ptchd1Y/− mice. Nature. 2016;532:58–63. doi: 10.1038/nature17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderski W., Wang L., Krokhotin A., Walsh J.J., Li H., Shoji H., Ghosh S., George R.D., Miller E.L., Elias L., Gillespie M.A., Son E.Y., Staahl B.T., Baek S.T., Stanley V., Moncada C., Shipony Z., Linker S.B., Marchetto M.C.N., Gage F.H., Chen D., Sultan T., Zaki M.S., Ranish J.A., Miyakawa T., Luo L., Malenka R.C., Crabtree G.R., Gleeson J.G. Loss of the neural-specific BAF subunit ACTL6B relieves repression of early response genes and causes recessive autism. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10055–10066. doi: 10.1073/pnas.1908238117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T.N., Hubel D.H. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T.N., Hubel D.H. SINGLE-CELL responses in striate cortex of kittens deprived OF VISION IN one eye. J. Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Williams M.R., DeSpenza T., Li M., Gulledge A.T., Luikart B.W. Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J. Neurosci. Off. J. Soc. Neurosci. 2015;35:943–959. doi: 10.1523/JNEUROSCI.3144-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintler T., Schoch H., Frank M.G., Peixoto L. Sleep, brain development, and autism spectrum disorders: insights from animal models. J. Neurosci. Res. 2020 doi: 10.1002/jnr.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor J.P., DeLorey T.M., Homanics G.E., Edgar D.M. Sleep states and sleep electroencephalographic spectral power in mice lacking the beta 3 subunit of the GABA(A) receptor. Brain Res. 2002;955:221–228. doi: 10.1016/s0006-8993(02)03467-4. [DOI] [PubMed] [Google Scholar]
- Won H., Lee H.-R., Gee H.Y., Mah W., Kim J.-I., Lee J., Ha S., Chung C., Jung E.S., Cho Y.S., Park S.-G., Lee J.-S., Lee K., Kim D., Bae Y.C., Kaang B.-K., Lee M.G., Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Wood L., Gray N.W., Zhou Z., Greenberg M.E., Shepherd G.M.G. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L., Shepherd G.M.G. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in a mutant mouse model of Rett syndrome. Neurobiol. Dis. 2010;38:281–287. doi: 10.1016/j.nbd.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak D.S., Green J.T., Levin S.I., Meisler M.H. Inactivation of sodium channel Scn8A (Na-sub(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav. Neurosci. 2006;120:229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Workman A.D., Charvet C.J., Clancy B., Darlington R.B., Finlay B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-H., Qu W.-M., Bian M.-J., Huang F., Fei J., Urade Y., Huang Z.-L. Essential roles of GABA transporter-1 in controlling rapid eye movement sleep and in increased slow wave activity after sleep deprivation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Lewis F.C., Sarvi M.S., Foley G.M., Crawley J.N. 16p11.2 Deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn. Mem. Cold Spring Harb. N. 2015;22:622–632. doi: 10.1101/lm.039602.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Walder-Christensen K.K., Kim N., Wu D., Lorenzo D.N., Badea A., Jiang Y.-H., Yin H.H., Wetsel W.C., Bennett V. ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc. Natl. Acad. Sci. U.S.A. 2019;116:15262–15271. doi: 10.1073/pnas.1904348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K., Riday T.T., Condon K.H., Roberts A.C., Bernardo D.R., Prakash R., Weinberg R.J., Ehlers M.D., Philpot B.D. Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook C., Kim K., Kim D., Kang H., Kim S.-G., Kim E., Kim S.Y. A TBR1-K228E mutation induces Tbr1 upregulation, altered cortical distribution of interneurons, increased inhibitory Synaptic transmission, and autistic-like behavioral deficits in mice. Front. Mol. Neurosci. 2019;12:241. doi: 10.3389/fnmol.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Chen L.Y., Liu X., Maxeiner S., Lee S.-J., Gokce O., Südhof T.C. Neuroligins sculpt cerebellar purkinje-cell circuits by differential control of distinct classes of Synapses. Neuron. 2015;87:781–796. doi: 10.1016/j.neuron.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Guo D., Han L., Rensing N., Satoh A., Wong M. Hypothalamic orexin and mechanistic target of rapamycin activation mediate Sleep dysfunction in a mouse model of tuberous Sclerosis complex. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Yu F., Zhu J., Han S., Chen J., Wu X., Chen Y., Shen T., Liao J., Guo W., Yang X., Wang R., Qian Y., Yang J., Cheng L., Zhao Y., Hui C.-C., Li J., Peng G., He S., Jing N., Tang K. Imbalance of excitatory/inhibitory neuron differentiation in neurodevelopmental disorders with an NR2F1 point mutation. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.03.085. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Lai C.S.W., Bai Y., Li W., Zhao R., Yang G., Frank M.G., Gan W.-B. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat. Commun. 2020;11:4819. doi: 10.1038/s41467-020-18592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K., Lindly O.J., Chavez A.E. Timeliness of autism Spectrum disorder diagnosis and use of services among U.S. Elementary School–aged children. Psychiatr. Serv. 2016;68:33–40. doi: 10.1176/appi.ps.201500549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L., Bauman M.L., Stone W.L., Yirmiya N., Estes A., Hansen R.L., McPartland J.C., Natowicz M.R., Choueiri R., Fein D., Kasari C., Pierce K., Buie T., Carter A., Davis P.A., Granpeesheh D., Mailloux Z., Newschaffer C., Robins D., Roley S.S., Wagner S., Wetherby A. Early identification of autism Spectrum disorder: recommendations for practice and research. Pediatrics. 2015;136(Suppl. 1):S10–S40. doi: 10.1542/peds.2014-3667C. [DOI] [PMC free article] [PubMed] [Google Scholar]