Summary
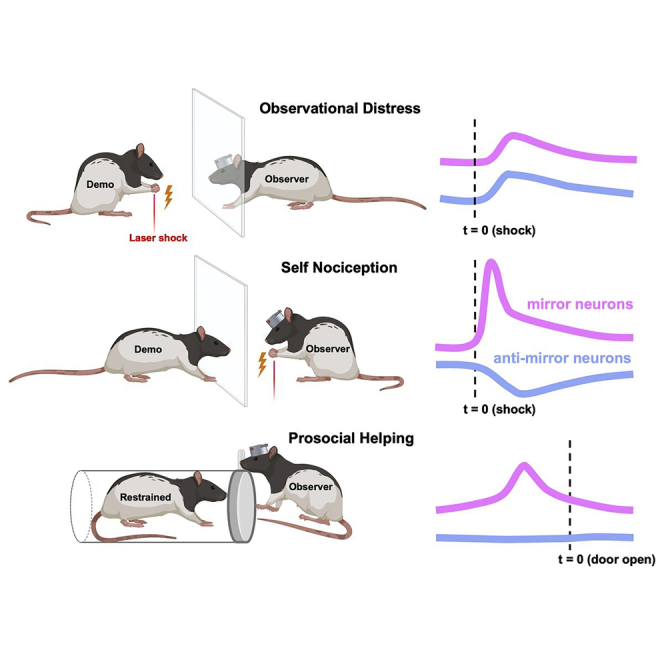
Although empathic emotion is closely related to prosocial behavior, neuronal substrate that accounts for empathy-associated prosocial action remains poorly understood. We recorded neurons in the anterior cingulate cortex (ACC) and insular cortex (InC) in rats when they observed another rat in pain. We discovered neurons with anti-mirror properties in the ACC and InC, in addition to those with mirror properties. ACC neurons show higher coupling between activation of self-in-pain and others-in-pain, whereas the InC has a higher ratio of neurons with anti-mirror properties. During others-in-pain, ACC neurons activated more when actively nose-poking toward others and InC neurons activated more when freezing. To further illustrate prosocial function, we examined neuronal activities in the helping behavior test. Both ACC and InC neurons showed specific activation to rat rescuing which is contributed by mirror, but not anti-mirror neurons. Our work indicates the functional involvement of mirror neuron system in prosocial behaviors.
Subject areas: Behavioral neuroscience, Molecular neuroscience, Cellular neuroscience
Graphical abstract
Highlights
-
•
Affective anti-mirror neurons have higher ratio in InC than ACC
-
•
Mirror and anti-mirror neurons associated with socially active and passive behaviors
-
•
InC and ACC but not MI showed helping-specific behaviors
-
•
Mirror neurons activated before helping-specific behaviors
Behavioral neuroscience; Molecular neuroscience; Cellular neuroscience
Introduction
Emotional contagion, the ability to experience the distress of others, is closely associated with prosocial behavior that benefits others.1,2,3 Neuroimaging studies in humans have suggested involvement of the anterior cingulate cortex (ACC) and insular cortex (InC) in pain empathy.4,5,6,7,8,9,10,11,12 An increasing number of studies show that rodents are able to share emotional states of others by showing various forms of empathy-like reactions, including emotional contagion,13,14,15,16 observational fear17,18 and prosocial helping.19,20,21,22 Neuroscience studies in rats have further identified emotional mirror neurons of pain in the ACC23 and the causal contribution of InC in helping behaviors.24 Emotional mirror neurons in rats are analogous to the mirror neuron system in primates, which explains their ability to understand observed motor actions.25,26,27
The mirror neuron system has also been proposed to participate in understanding mental states of others.28,29,30 Anti-mirror neurons have been reported in the motor system, neurons which increase their firring rate when monkeys executed a particular action, but decrease their firring rate below baseline when observing someone else performing this action.31,32 Anti-mirror neurons may disambiguate our own actions from those of others when understanding others. However, a potential analog of neurons with anti-mirror properties has not been addressed in an affective empathy assay. Furthermore, the neuronal logic that connects the mirror neuron system to prosocial actions remains unclear. According to the empathy-altruism hypothesis, empathy for others in distress arouses emotion sharing and motivates prosocial behavior.1,2,33 It is unknown whether generation of empathy-associated reactions that initiate active prosocial help shares the same mechanism that produces empathy-associated reactions without any prosocial behavior, e.g., emotional contagion.
A brain-wide survey of neural activity in the helping behavior test has identified InC and ACC as the brain regions consistently participating in the response to a trapped rat in different social contexts.34 In addition, observational fear before self-experience of the adverse event was shown to depend primarily on an ACC-amygdala circuit,35 whereas increased synchrony of the InC and amygdala was associated with elevated emotional contagion.36 We hypothesized that mirror and anti-mirror neurons provide a neuronal substrate for empathy-associated, socially active and passive reactions. We tested this hypothesis by recording neuronal activities in the ACC and InC of rats during an observational distress test following a helping behavior test.19 We also recorded neuronal activities in the primary motor cortex (MI) as a control for general correlation of neuronal activities with arousal, movements, or changes in state. An investigation of prosocial behavior depends on neural circuits overlapping with those of emotional contagion could deepen our understanding of circuit evolution between contagion and prosociality in social animals.
Results
Empathy-related behaviors identified in an observational distress test
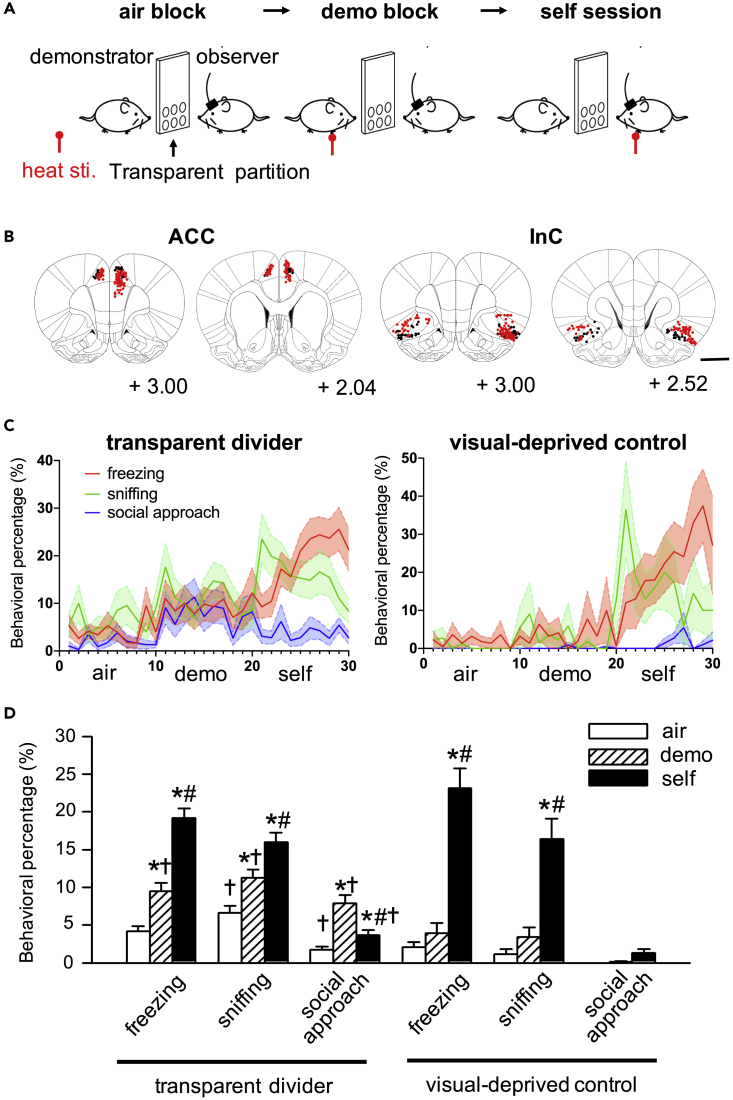
To examine neuronal correlates of emotional contagion, rats (n = 41) were tested in an observational distress test (Figure 1A) after implantation of 16-channel microelectrodes in the ACC or InC (Figure 1B). In this test, each observer rat observed an unfamiliar demonstrator rat experiencing pain on the forepaws, caused by infra-red laser heat pulses (Figure 1A, “demo” block). Observers were able to witness the demonstrator through visual, auditory, and olfactory sensations through a perforated, transparent divider, but were prevented from physical contact. Two control blocks were designed (Figure 1A): the “air” block, in which heat pulses were directed upward at no target, to capture baseline behaviors before the “demo” block, and a “self” block, in which heat pulses targeted the observer, to confirm state matching of their own pain after the “demo” block. The 3 types of blocks in the test were implemented in the order of 10 “air” blocks, 10 “demo” blocks, and 10 “self” blocks, to avoid prior knowledge of laser heat pulses during the “air” blocks and nonspecific fear generalization during the “demo” blocks. Among 7 classified behaviors (Figure S1; see STAR Methods), observers displayed significantly higher rates of freezing, sniffing, or social approaches as the first reaction after laser heat pulses during “demo” blocks than in “air” blocks (Figures 1C and 1D, Figure S1; p< 0.016, at a significance threshold of α = 0.05 after Bonferroni correction for the 3 pairs of comparisons). These reactions were all initiated at least 2.5 s after laser pulses (2.8 ± 0.5s for freezing, 3.4 ± 0.4s for sniffing, 3.5 ± 0.5s for social approach) and lasted for more than 4.0 s (Video S1; 5.8 ± 0.8s for freezing, 5.0 ± 0.6s for sniffing, 6.6 ± 0.8s for social approach). Notably, compared with “air” blocks, social approaches increased in the “demo” blocks, but did not increase in “self” blocks, indicating that this behavior is specific to demonstrator distress, but not self-distress (Figures 1C and 1D). These observations suggested candidate behaviors to capture reactions of emotional contagion in this paradigm.
Figure 1.
Identification of empathy-related behaviors in the observational distress test
(A) Diagram of the observational distress test. As a control experiment, a group of rats experienced the task with a solid, opaque divider (opaque control group) instead of a perforated, transparent divider (transparent group).
(B) Recording sites of ACC and InC electrodes from the transparent group (red dots) and opaque control group (black dots). Numbers at the bottom right corner of each brain coronal section, distance to bregma in mm along the anterior-posterior axis (Paxinos and Watson, 2007); scale bar, 2 mm
(C) Averaged percentages of freezing (red), sniffing (black), and social approach (blue) behaviors in observer rats through trials of “air”, “demo”, and “self” blocks from the transparent and opaque control groups.
(D) Percentages of freezing, sniffing and social approach are significantly higher during the “demo” block (oblique bars) than the percentages during the “air” block (open bars) in the transparent group, but not the opaque control group (mean ± SEM from the 10 trials in a block). ∗p< 0.016, compare to values in the “air” block. #p< 0.016, compare to values in the “demo” block in the same group. ✝p< 0.016, compare to values in the opaque control group (one sided Mann-Whitney U test at a significance level of α = 0.05, with Bonferroni correction). f, freezing; n, sniffing; a, social approach; w, walking; h, heading; r, resting; l, licking.
See also Figure S1.
The text “Laser” appears in the left corner when the demonstrator’s forepaw was hit at time 617.8271s. The demonstrator lifted and licked its forepaw and the observer then shifted its body and head position, pushed its nose against a hole in the partition. The activity of a simultaneously recorded InC unit is shown in the bottom panel.
Although behaviors that increased during “demo” and “self” blocks may simply reflect rat reactions to nonspecific social cues, we further confirmed emotional reactions with a control group of electrode-implanted rats (n = 11 rats) in conditions in which the divider was solid and opaque to disrupt multimodal transmission of sensory modality for the cognitive representation of another’s pain during the test, where visual sensation has been reported as an important factor for rodents to recognize pain of others.13 Notably, increases of freezing and sniffing during “demo” blocks were absent in this opaque control experiment, whereas increased social approach was absent in both “demo” and “self” blocks (Figure 1C and 1D). During “self” blocks, the ratio of freezing gradually increased during a block, whereas the ratio of sniffing decreased through a block (Figure 1C), suggesting that sniffing behaviors are less likely to be fear-related. These results suggest that freezing, as a passive reaction, indicated emotional state matching through emotional contagion, whereas a social approach, as a socially active reaction, may reflect a rat’s concern for conspecifics in pain.
Observational-distress-related mirror and anti-mirror neurons in the ACC and InC
We next sought to characterize neuronal responses related to observational distress in the test. In total, 806 neurons were recorded from the ACC or InC in 41 rats in single-unit recordings. Among them, 596 neurons were recorded from 30 rats tested with a transparent divider and the remaining 210 neurons were recorded from 11 rats tested with an opaque divider. Many ACC and InC neurons changed their firing rates in both “demo” blocks and “self” blocks (Figure 2A, example neurons; Figure 2B, ensemble neuronal activity and Table 1, numbers and percentages of all recorded neurons). For both the ACC and InC, there were significantly higher percentages of neurons that changed their firing rates after laser pulses during “demo” blocks than during “air” blocks (ACC: 14.7 ± 0.46% of “demo” versus 9.1 ± 0.33% of “air”, n = 19 rats, p = 0.045, Mann-Whitney U test; InC: 13.7 ± 0.34% versus 8.6 ± 0.26% before “self”, n = 19 rats, p = 0.02, Mann-Whitney U test). In contrast, in the opaque condition, percentages of neurons that changed their firing rates did not differ significantly between “demo” and “air” blocks (Table 1), suggesting that the observed changes of neuronal firing rates likely relate to demonstrator distress.
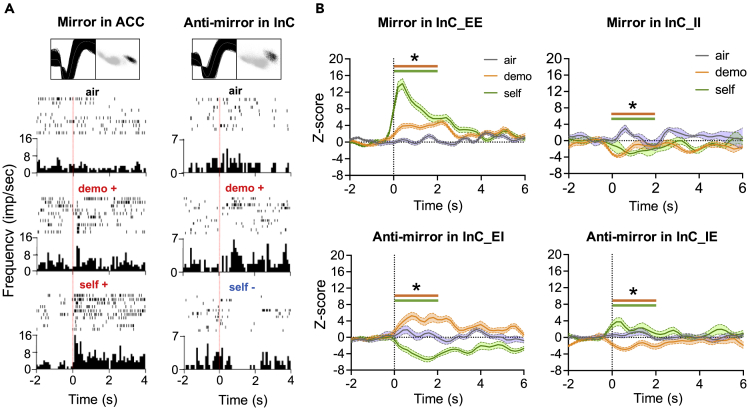
Figure 2.
Mirror and anti-mirror neurons in the limbic system
(A) Rasters and histograms (bottom panels) with superposed waveforms and corresponding PCA space (top panels) of the example mirror (left) and anti-mirror (right) neurons. Dashed line, time = 0 at the heat pause; bin = 100 ms.
(B) Normalized ensemble activities (z-scores) of mirror (EE and II) and anti-mirror (IE and EI) neurons. Bin size = 200 ms. Data are presented as mean ± SEM ∗p< 0.05, Mann-Whitney U-test compared to air.
See also Figures S2–S5.
Table 1.
Number and percentage of responsive neurons recorded in anterior cingulate cortex (ACC) and insular cortex (InC)
| Sessions | Air |
Demo |
self |
total | |||
|---|---|---|---|---|---|---|---|
| excitation | inhibition | Excitation | Inhibition | excitation | inhibition | ||
| ACC | |||||||
| transparent | 31 (9.1%) | 26 (7.6%) | 50 (14.7%) | 18 (5.3%) | 147 (43.2%) | 21 (6.2%) | 340 |
| opaque control | 10 (10.6%) | 6 (6.4%) | 9 (9.6%) | 2 (2.1%) | 39 (41.5%) | 2 (2.1%) | 94 |
| InC | |||||||
| transparent | 22 (8.6%) | 14 (5.5%) | 35 (13.7%) | 16 (6.3%) | 111 (43.3%) | 35 (13.7%) | 256 |
| opaque control | 21 (18.1%) | 8 (6.9%) | 19 (16.4%) | 5 (4.3%) | 44 (37.9%) | 13 (11.2%) | 116 |
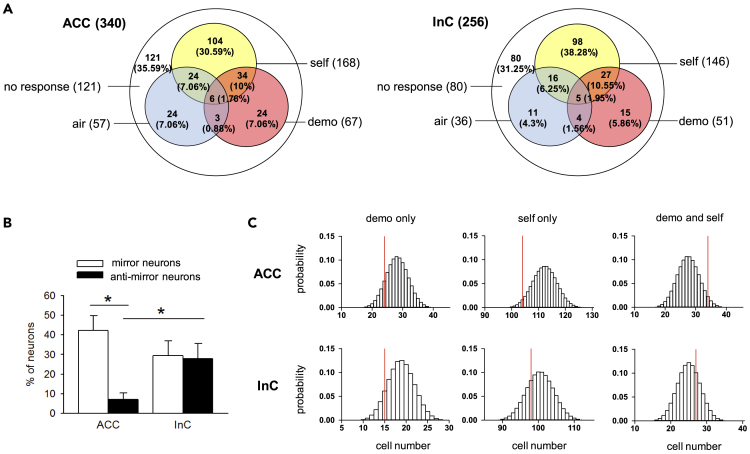
Mirror properties of mirror or anti-mirror neurons require consistent responses, not only when observing actions or reactions of others, but also when an animal itself performs the same action or experiences the same event. Among neurons that are activated in “demo” blocks, 41.2 ± 7.3% and 26.7 ± 7.1% of neurons in the ACC and InC, respectively, also activated during “self” blocks (“EE mirror neurons”; Figure 3A), similar to the percentage of ACC neurons that responded to pain, as reported in our previous studies.37,38 In addition, there were neurons that were inhibited in both “demo” and “self” blocks (“II mirror neurons”; 2.6 ± 1.8% in ACC and 2.8 ± 2% in InC). Of interest, a significantly higher percentage of InC neurons than of ACC neurons were inhibited after noxious laser-heat pulses to the forepaws in “self” blocks, but activated in “demo” blocks (“EI anti-mirror neurons”; 2.6 ± 1.9% in the ACC and 13.5 ± 4.4% in the InC, 19 rats each, p = 0.05, Mann-Whitney U test), and so were neurons that were activated in “self” blocks, but inhibited in “demo” blocks (“IE mirror neurons”; 6.1 ± 3.4% in ACC and 14.6 ± 6.1% in InC). Control datasets generated by bootstrapping all trials from all types of block showed lower ratios of statistically classified mirror (9.55% of EE and 0.05% of II; the actual data of EE and II mirror neurons in ACC and InC are all above 100.0% bootstrapped datasets) and anti-mirror neurons (0.67% of EI and 0.67% of IE; the actual data of EI and IE anti-mirror neurons in ACC and InC are all above 100.0% bootstrapped datasets), indicating that observed dynamics of mirror and anti-mirror neurons are very unlikely to have been generated by a random process. This statistical classification of the 4 types of neuronal ensembles (2 types of mirror activities and 2 types of anti-mirror activities) enabled us to examine more closely the functions of the ACC and InC in the observational distress test.
Figure 3.
Mirror property of the ACC and InC represented in population-level organization
(A) Venn diagram of “air”-, “demo”-, and “self”-responsive neurons in the ACC (left) and InC (right).
(B) The percentage of mirror (open bar) and anti-mirror (solid bar) neurons in the ACC and InC. n = 19 rats; ∗p< 0.05, Mann-Whitney U test.
(C) Observed cell numbers (red lines) of “demo”- (left), “self”- (middle), and “demo”+ “self”- respond (right) neurons in the ACC (top) and InC (bottom) compared with the distributions of corresponding shuffled datasets (open bars).
Distinct behavioral correlation of mirror and anti-mirror neurons in the ACC and InC during the observational distress test
To gain insights into the organizational principle that reflects the functional assignment of a neuron to reveal its mirror property, we created a group of control datasets in which classified neuronal responses, i.e., increased firing rate, decreased firing rate, or no change, from a type of block, i.e., “air”, “demo”, or “self”, were shuffled. The result showed that ACC neurons had stronger “demo”-“self” coupling compared to their shuffled datasets (above 94.7% shuffled datasets, although the actual position in the null distribution does not exceed 95%), whereas the “demo”-“self” coupling of InC neurons is close to the median of its shuffled datasets (above 67.8% shuffled datasets) (Figure 3C). This finding suggests that the functional assignment of neuronal responses during “demo” and “self” blocks is nearly random in the InC, but positively coupled in the ACC.
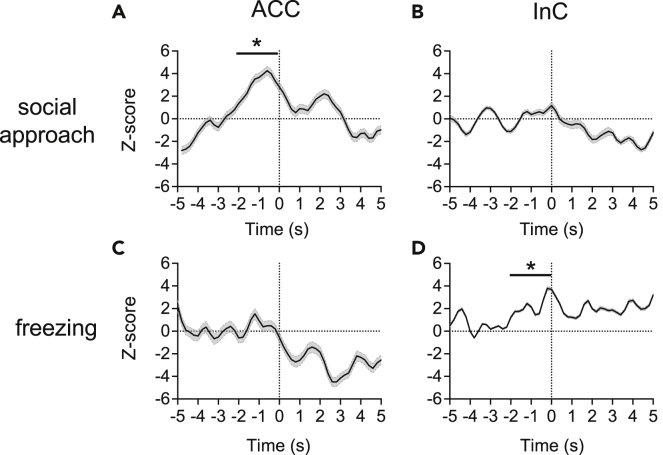
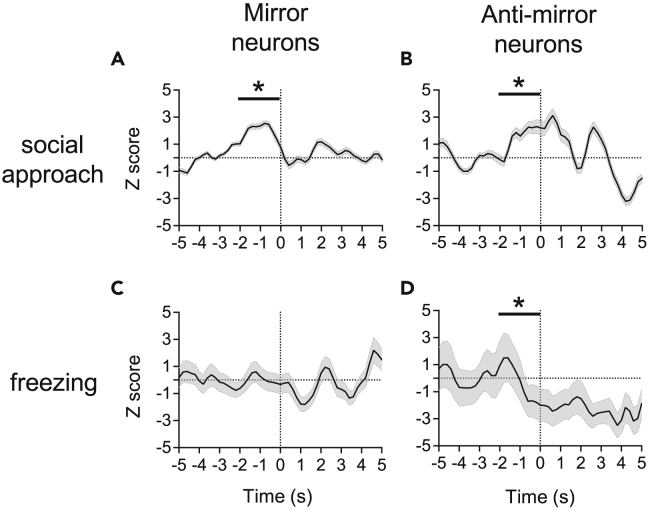
To investigate potential behavioral associations of the mirror neuron system, we examined ensemble activities before social approach and freezing behaviors in the observational distress test. Among the ensembles (Figures 4, 5, S6, andS7), we found an activity enhancement of ACC ensemble before social approach behavior during “demo” blocks (Figure 4A), in which both ACC-InC mirror and anti-mirror ensembles showed similar enhancement (Figures 5A and 5B). Such enhancement was not seen in the InC ensemble (Figure 4B). On the other hand, before freezing, we found an activity enhancement of the InC ensemble (Figure 4D) with a decrease in the activity of the ACC-InC anti-mirror ensemble (Figure 5D). No change of activities before freezing was observed in either the ACC ensemble (Figure 4C) or the ACC-InC mirror ensemble (Figure 5C). In summary, behavior-specific pre-movement neuronal correlates are region-specific, whereas mirror and anti-mirror ensemble activities showed a similar pattern of enhancement before social approaches, in which ensemble patterns were rather distinct before freezing. These observations motivated us to further investigate their roles in empathy-based prosocial help.
Figure 4.
Average ensemble activity of ACC and InC neurons increased specifically in relation to social approach or freezing behaviors
Average ensemble unit (ACC, n = 15 rats in (A and C); InC, n = 11 rats in (B and D) activity during the demo period in the observational distress test. Repetitive freezing or social approach behaviors occurred at time zero (dashed line). Bin size = 200 ms. Data are presented as mean ± SEM ∗p< 0.05, one-sample Wilcoxon signed-rank test.
See also Figures S6 and S7.
Figure 5.
Average ensemble activity of observational-distress-related neurons increased specifically in relation to social approach or freezing behaviors
Mirror neurons (A, n = 32 units) and anti-mirror (B, n = 7 units) showed significant correlation before and during social approach behavior. Anti-mirror neurons (D, n = 10 units) showed correlation before and during freezing behavior, whereas mirror neurons (C, n = 37 units) did not. Values in the Y axis are normalized Z-scores calculated from baseline activities 2 to 5 s before the onset of social approach or freezing behavior. Data are presented as mean ± SEM ∗p< 0.05, one-sample Wilcoxon signed-rank test.
See also Figures S6 and S7.
We next tested how different neuronal ensembles encode social approaches versus freezing, following investigation of mirror properties in neuronal ensembles. Although the mirror property remained at the ensemble level for both ACC EE and InC EE neurons, the anti-mirror property remained at the ensemble level only for InC EI and InC IE neurons (Figure 2B, InC, EE: Uair-demo = 100, p = 0.005, Uair-self = 100, p< 0.001; II: Uair-demo = 3, p< 0.001, Uair-self = 2, p< 0.001; EI: Uair-demo = 98, p< 0.001, Uair-self = 0, p< 0.001; IE: Uair-demo = 0, p< 0.001, Uair-self = 98, p< 0.001, Mann-Whitney U-test; Figure S2, ACC, EE: Uair-demo = 100, p< 0.001, Uair-self = 100, p< 0.001; II: Uair-demo = 12, p< 0.005, Uair-self = 3, p< 0.001; EI: Uair-demo = 20, p = 0.025, Uair-self = 16, p = 0.13; IE: Uair-demo = 0, p = 0.005, Uair-self = 66, p = 0.241, Mann-Whitney U-test), reflecting the diverse response dynamics of ACC anti-mirror neurons and II mirror neurons in both ACC and InC. Furthermore, by combining EE and II neurons into a mirror ensemble and combining EI and IE neurons into an anti-mirror ensemble, we observed that both ACC and InC mirror ensembles retained robust mirror activities, but the anti-mirror properties of both ACC and InC anti-mirror ensembles were impaired (Figure S3, Mirror in ACC, Uair-demo = 100, p< 0.001, Uair-self = 100, p< 0.001; Mirror in InC, Uair-demo = 96, p< 0.001, Uair-self = 100, p< 0.001; Anti-mirror in ACC, Uair-demo = 3, p< 0.005, Uair-self = 52, p =0.909; Anti-mirror in InC, Uair-demo = 37, p = 0.344, Uair-self = 31, p = 0.162; Mann-Whitney U-test). This observation is consistent with ACC-InC mirror ensemble activity from pooling together ACC and InC mirror neurons and ACC-InC anti-mirror ensemble activity from pooling together ACC and InC anti-mirror neurons (Figure S4, Mirror in ACC and InC, Uair-demo = 100, p< 0.001, Uair-self = 100, p< 0.001; Anti-mirror in ACC and InC, Uair-demo =0, p< 0.001, Uair-self = 34, p = 0.241, Mann-Whitney U-test). By pooling all neurons in the ACC and InC into ACC and InC ensembles, respectively, both ACC and InC ensembles only remained responses in “self” blocks (Figure S5, ACC, Uair-demo = 75, p = 0.06, Uair-self = 100, p< 0.001; InC, Uair-demo = 1, p< 0.001, Uair-self = 100, p< 0.001, Mann-Whitney U-test). In summary, these observations suggest that self-other differentiation, observational distress, and self-nociceptive pain are more strongly associated with states of individual neuronal activities, local ensemble activities, and cross-region neural activity, respectively.
Activation of mirror neurons prior to helping behavior
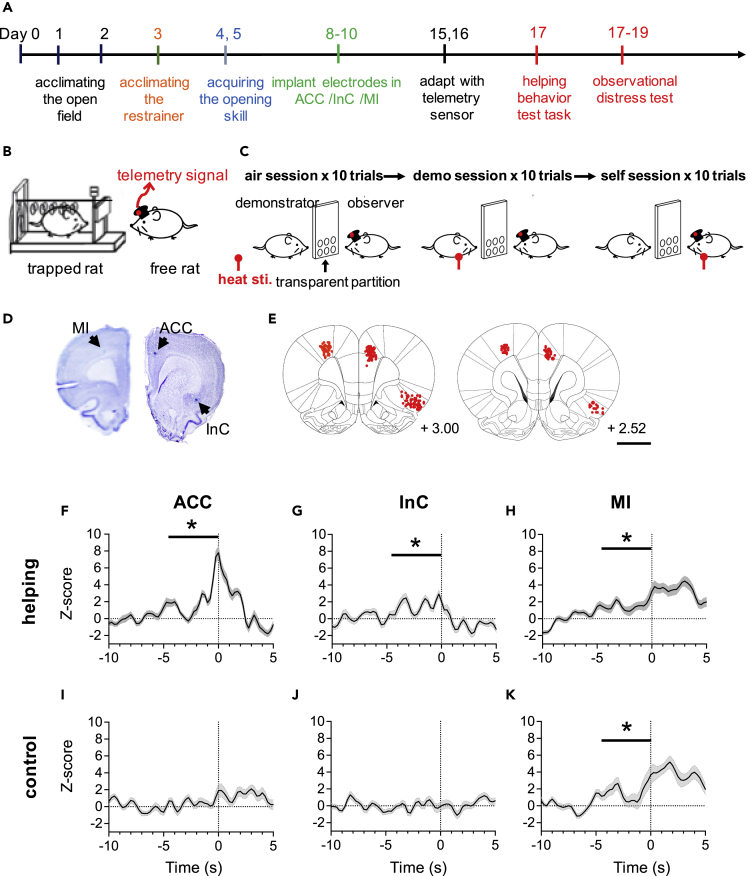
To investigate the neuronal substrate of empathy-associated prosocial action, we prepared rats that were trained in the helping behavior test followed by the observational distress test (Figures 6A–6C) after implantation of 16-channel microelectrodes in the ACC, InC, or MI (Figures 6D and 6E). In a session of the helping behavior test, each rat conducted 30 trials with 10-trial blocks of three different conditions in random order, in which the restrainer contained a conspecific rat, a toy rat, or was empty. Eight of the 12 rats opened the restrainer more often when a conspecific was in the restrainer, with the percentages of 77.3 ± 4.6%, 21.9 ± 3.6%, and 16.7 ± 4.4% during conditions with a conspecific rat (“rat”), a toy rat (“toy”), and empty (“empty”), respectively. In contrast, gate-opening behaviors in the other 4 rats were nonspecific, with percentages of gate opening being 33.8 ± 5.5% for a conspecific, 30.8 ± 7.1% for a toy rat, and 6.7 ± 4.0% for an empty restrainer. The 8 rats that showed conspecific-specific rescuing behaviors also showed shorter latencies to open the door (67.1 ± 6.3s) than the 4 rats that showed nonspecific door opening behaviors (93.2 ± 14.1s; p< 0.001. The observational distress test was performed after the helping behavior test to compare activities of neurons that were putatively the same in these tasks (79 ACC neurons from 8 rats, 56 InC neurons from 7 rats, and 77 MI neurons from 9 rats).
Figure 6.
Activity of neurons in the ACC and InC increased before helping behavior
(A) Experimental protocol for data collection.
(B and C) Diagram of the apparatus for the helping behavior test and the observational distress test, respectively. Scale bar = 2 mm
(D) Photomicrographs show coronal sections of the brain marked with iron deposits (arrows) in the ACC, InC and MI.
(E) Composite recording sites for all ACC, InC and MI. More details are shown in individual method sections. Average ensemble unit (ACC, n = 8 rats in F and I; InC, n = 7 rats in G and J; MI, n = 9 rats in H and K) activity around opening the restrainer (time = 0) in trials with a conspecific rat (helping) and empty or toy trials (control). Values in the Yaxis are normalized Z-scores calculated from individual rats. Repetitive gate-opening behaviors occurred at time zero. Bin sizes = 250 ms. Data are presented as mean ± SEM ∗p< 0.05, one-sample Wilcoxon signed-rank test.
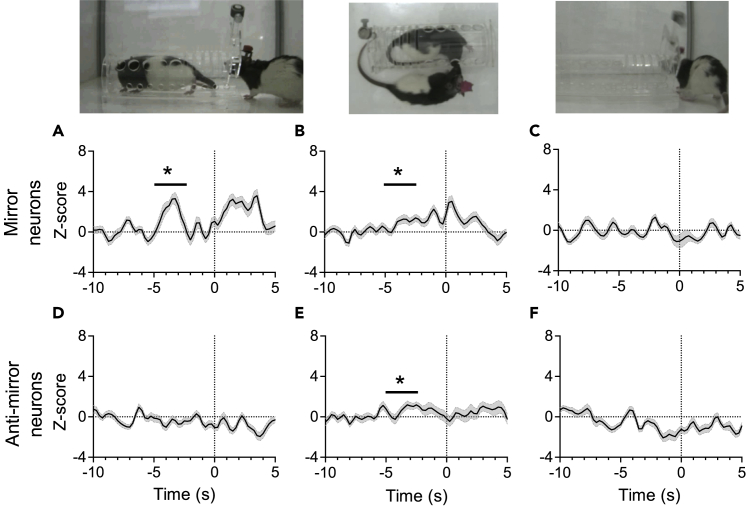
We analyzed ensemble activities before door opening and nose interaction behavior in the helping behavior test (Figures 6 and 7). Although the MI ensemble activated before the door opening in both rescuing (“rat”) and control (“toy” and “empty”) trials, the ACC and InC ensembles activated before door opening only in rescuing trials, but not in control trials (Figures 6F–6K and Video S2). In addition, the ACC-InC mirror ensemble activated both before rescuing and before nose interaction behaviors (Figures 7A and 7B), whereas the ACC-InC anti-mirror ensemble activated before nose interaction behaviors, but not before the door opening (Figures 7D and 7E). These ensembles did not show a change in activity levels in control trials when the rats opened a chamber with a toy or an empty chamber (Figures 7C and 7F). These findings associate observational-distress-related neurons in rats with their prosocial action of rescuing a conspecific rat from restraining stress.
Figure 7.
Average ensemble activity of mirror neurons increased specifically in relation to helping and nose interaction behaviors
Photographs, illustration of rat helping behavior (left), nose interaction (middle), and opening an empty restrainer (control open; right). n = 7, 7 and 6 mirror neurons in rescuing (A), nose interaction (B), and control open (C), respectively; n = 7, 7 and 7 anti-mirror neurons in rescuing (D), nose interaction (E), and control open (F), respectively; Values in the Yaxis are normalized Z-scores calculated from baseline activities 5 to 10 s before the onset of gate-opening behaviors. Bin size = 250 ms. Data are presented as mean ± SEM ∗p< 0.05, one-sample Wilcoxon signed-rank test.
Superimposed waveforms (left panel) and PC1-PC2 plot (right panel) were shown in the opening scene. A mirror neuron in InC activa. It shows no change before door opening at t = 96 in the toy trial.
Discussion
In this study, we addressed mirror and anti-mirror properties of neurons in the ACC and InC using affective empathy assays in rats, and proposed a neural substrate of empathy-associated prosocial behaviors by illustrating how the mirror neuron system is related to prosocial actions. Specifically, we found that mirror and anti-mirror ensembles showed similar activation patterns, activating before empathy-associated, socially active reactions, i.e., social approaches, prosocial help, and nose interactions, whereas activation patterns of these ensembles were more distinct before rats showed empathy-associated, socially passive reactions, i.e., freezing. These neuronal ensembles are organized into a brain circuit in which the InC maintains a balanced ratio between mirror and anti-mirror neurons, whereas the ACC contains a higher ratio of mirror neurons because of its coupling organization of pain transmission from self and others. Our finding that prosocial behavior depends on neural circuits overlapping with those of emotional contagion provides evidence of circuit evolution between contagion and prosociality in social animals.39
Our study surveyed neural associations with emotional contagion and helping behavior at different scales. These results suggest that self-nociceptive pain, contagious distress, and self-other differentiation are more strongly associated with neuronal states at cross-region activity, local ensembles, and individual neuronal activities, respectively, which would be of interest for future theory developments. Coupling between neural dynamics across these encoding scales could be a mechanism to accomplish multimodel integration of perception, emotion, and cognition. A particular behavioral reaction can further be initiated from dynamics of neural states in cross-circuit coupling34 through mechanics that couple kinetic and potential energy of each scale-specific neural state.
Empathy-related behaviors in affective tasks
In the present study, we used laser heat pulses to deliver short, fast stimuli with precise control, which is useful for inducing pain in freely moving rats40 as well as limiting uncertainty in further behavioral representation and neuronal correlation. Application of laser heat pulses to paw pads of rats elicited not only typical nociceptive behavior, e.g., licking the targeted pads, but also a set of behaviors that showed both when the rat felt pain and the demonstrator rat received the nociceptive stimulus, but not when the “air” control, e.g., freezing, sniffing, and social approaches. The latter behaviors may reflect emotional, exploratory, or social reactions in our affective empathy assay.
Freezing is a well-documented behavioral index of fear.41 Freezing in observer rats suggests that they may also sense a stressful state and share the fear with the stimulated rat. This representation is consistent with vicarious freezing demonstrated from paradigms of emotional contagion and affective transfer for subjects with16,18,42 or without prior experience with pain stimulation.43 Sniffing with whisking is a rhythmic orofacial motor activity that enables rodents to localize and track objects in their environments.44 Therefore, increased sniffing by observer rats in our test indicated that they showed more concern for the environmental context, which is consistent with previous reports. The gradual decrease of sniffing behavior through trials in a given block further supports this interpretation of sniffing as a general exploratory behavior.
Nose-poking behavior in rodents has been taken as a goal-directed action in social or non-social behavioral tests.45 We defined “social approach” in our test as nose pokes into holes of the divider or putting a paw on the plexiglass panel between the demonstrator and observer. Of interest, social approach was more frequent when a companion rat received heat pulses than when the observer rat itself experienced them. In addition, because visual cues help to convey information in rodents,13 social approaches when others were in pain became less frequent when the companion rat was prevented from observing the demonstrator. These findings indicate that social approach behavior of rats observed in the observational distress test is a reaction to distress of others. Whether prosocial behavior in the helping behavior test is motivated by empathy has been extensively discussed.3,46,47,48 Observing distress of others can serve as a negative reinforcer for rats49 and can determine their social learning.17,50,51,52 Overall, our data provide further evidence suggesting that rodents express behaviors similar to human empathy.53
Limitations of the study
Although well-defined behaviors in the helping behavior test allowed us to specify neuronal interpretation, the ratio of about one-third of rats showing stable conspecies-spesific door opening suggests a room for further improving the generalization of the current helping behavior test in laboratory rodents. In addition, the ratio of about two-fifth of these learned rats largely reducing their door opening events after electrode implantation indicates a challenge of using an invasive neuronal recording approach to investigate neuronal substrate in the current helping behavior test. These emphasize an aim of future improvement of the helping behavior test in laboratory rodents.
Limbic circuit for social orientation of empathy-associated reactions
Although empathy is the ability to experience the distress of others, differentiation between self and other is a necessary condition of empathy because the concept of empathy only holds in an awareness of being in a social condition. In the motor system, mirror neurons have been hypothesized to function in understanding actions of others.54,55 Although the functional roles and actual encoding of motor mirror neurons has been debated,56,57 the evidence of motor mirror neurons mapping observer’s and acting animal’s goals instead of effector moves or motor end-state58 support a strong involvement of cognitive inference in mirror neuron formation. Potential mechanisms to reveal self-other differentiation have been addressed in computational research and practice of artificial empathy agents where the existence of an acute mirror neuron system was acquired for correct sensorimotor mapping.59 The discovery of anti-mirror neurons led to the hypothesis that the role of these neurons is to avoid generation of actions when observing actions of others.31,60 In monkeys, the medial frontal cortex is thought to be involved in differentiating self from others and of providing neural signals that are essential for social motor learning.61 A subset of neurons in the human medial frontal and temporal cortices is also excited when executing actions and inhibited when observing actions.32
Successful prosocial help requires both empathic recognition of others’ distress and a clear self-other differentiation to correctly identify the source of distress, which suggests potential coordination between separate networks in the brain to execute prosocial help. Both brain imaging results in humans6,7,8 and electrophysiological data in rodents17,62 report empathy-related neuronal activities in the ACC and InC,5,23,34 which is consistent with findings of our study. The ACC and InC function in decision making in both rats63 and humans.64,65 Previous work has implicated the ACC in the cognition component of empathetic pain66,67 and the InC in affective information processing.68,69,70 The InC is involved in predicting the valence of both self and others70 and in evaluation and experiencing of emotion and interoceptive awareness.12,71,72 Nociceptive information is also transmitted to the ACC from the InC.73 Furthermore, pharmacological inactivation of the InC blocked hyperalgesia induced by cohabitation with a mouse in chronic pain, indicative of alteration of emotional contagion.69 Moreover, chemogenetic activation of the InC reversed the deficit of a heroin-induced decrease in helping.74,75,76 We hypothesized that the limbic cortex is a well-organized, distributed system that includes separate, empathy-directed mirror systems to drive diverse empathy-related reactions along the social axis, which may motivate important future studies. In view of the evidence for atypical empathy expression in autism,77,78 our findings may also have important implications for disease-relevant issues.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Long-Evans Rat | LASC, Taiwan | http://www.biolasco.com.tw/index.php/tw/ |
| Software and algorithms | ||
| Offline Sorter | Plexon | https://plexon.com/products/offline-sorter/ |
| SortClient | Plexon | |
| Neuroexplorer | Plexon | https://plexon.com/products/neuroexplorer/ |
| MATLAB | The Mathworks, Inc. | https://www.mathworks.com/products/matlab.html |
| SPSS | IBM | https://www.ibm.com/spss |
| Excel | Microsoft Office | https://www.microsoft.com |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ray X. Lee (raylee@mit.edu).
Materials availability
This study did not generate any new reagents or animal lines.
Experimental model and subject details
Animals
Experiments were conducted on adult, female Long-Evans rats weighing 200-300 g, age 9-20 weeks. We used only female rats because a significantly greater proportion of female than male rats exhibit prosocial behavior in the conspecific helping behavior test.19 Rats were housed individually in the animal facility in the Life Science Building at National Taiwan University, Taipei. They were maintained under a 12-h dark/light cycle (lights off at 6:00 p.m.) at 22°C with food and water available ad libitum. Animal care and experimental procedures followed Codes for Experimental Use of Animals of the Council of Agriculture, Taiwan, based on the Animal Protection Law, Taiwan, and were approved by the Institute Animal Care and Use Committee of National Taiwan University.
For the observational distress test, 41 rats were implanted and were tested in a cage in which the observing and demonstrating rats were segregated either by a transparent or an opaque divider. Sixteen had bilateral ACC implants, 11 of which were in the transparent group and 5 in the opaque group. Seventeen had bilateral InC implants, 11 of which were in the transparent group and 6 in the opaque group. Eight rats had implants only in the right ACC and InC.
In order to test involvement of observational distress responsive neurons in prosocial acts, a separate group of rats was trained to perform a helping behavior test followed by the observational distress test. A total of 12 rats completed the 2 experimental tasks with good recording quality. Three rats had implants in the ACC and InC, five rats had implants in the ACC and MI, and four rats had implants in the InC and MI.
Method details
Surgery and recording method
A rat was initially anesthetized with sodium pentobarbital (50 mg/kg, ip). Ketamine hydrochloride (50 mg/kg, i.m.) was supplemented as necessary to maintain the rat in deep anesthesia so that it had no flexor reflex throughout the surgery period. The core temperature of the rat was maintained at 37.5 °C with a feedback-controlled heating pad. The rat was mounted on a stereotaxic instrument. A midline incision was made on the skull. After retracting the skin and cleaning the soft tissue, small craniotomies were made for placing intra-cortical microelectrodes into the ACC, InC, or MI (Figures 1B, 6D, and 6E).
Two 8-channel microwire array electrodes were implanted in each rat. The array electrode was built in-house. Detailed procedures for constructing the array electrode have been described elsewhere.79 Briefly, 8 stainless-steel wires individually insulated with Teflon (50 μm OD) were implanted in a line with equal inter-electrode distances with a total span of 2.5 mm. Small longitudinal holes were opened in the fronto-parietal bone for the ACC or InC. Coordinates of the ACC were 1.5-3.5 mm anterior to and 0.6-0.8 mm lateral to the bregma, and 1.6-2.0 mm deep in the cortex. Coordinates of the InC were 1.5-3.5 mm anterior to and 3.0-5.0 mm lateral to the bregma, and 4.5-5.0 mm below the surface of the cortex. Coordinates of the MI were 1-3 mm anterior to and 3 mm lateral to the bregma, and 1.6-2.0 mm deep in the cortex. Once MI electrodes were in the target site, electrical stimulation was employed to ascertain their motor fields. Electrical pulses were delivered from a constant current stimulator (AM system, model 2100) consisting of a train of 7 square-wave pulses, each 0.2 ms in duration, 300 Hz in 100 ms train duration. Intensities of the test electrical stimulation ranged from 30 to 300 μA. This stimulation evoked muscle movements in whiskers (22.2%), neck (33.3%), or upper limbs (33.3%). No overt body movement could be discerned in the remaining 11% of stimulation sites.
A pair of stainless-steel screws (1 mm OD) was placed in the skull bilaterally, 2 mm posterior and 2 mm lateral to the bregma for EEG recording. The ground electrode was a stainless-steel screw located over the top of the cerebellum (mid-occipital bone). In addition, several stainless-steel screws were placed in the frontal and parietal bones for anchoring. A pair of seven-stranded stainless-steel wires (793,200, A-M systems) was inserted into the neck muscles for EMG recording. After implantation, holes in the skull and the implanted electrodes were sealed and secured with dental cement.
Data acquisition
Neuronal activity was recorded using a Multi-channel Neuronal Acquisition Processor system (MNAP, Plexon, Dallas, TX). Electrical signals were passed from the headset to an amplifier and band-pass filtered (spike signals: 154–13k Hz, gain: 10,000–32,000; EEG and EMG filters: 0.7–170 Hz, gain: 5,000 or 10,000) displayed on an oscilloscope and an audio monitor (Grass AM8). Real-time spike sorting was controlled with SortClient (Plexon), and the sampling rate of individual channels was 40 kHz. Synchronized video signals were acquired with CinePlex (Plexon).
Observational distress test
Rats were separated into two groups, 60 in the transparent group and 22 in the opaque group. In the transparent group, after recovery for 5 days, the observer rat was tethered with a recording wire and habituated next to a naive demonstrator rat for 3 days, 30 min each day. Each rat was placed in an equal-sized (27 × 21 × 17 cm) plexiglas compartment. The two compartments were separated by a transparent, perforated plexiglas divider (Figure 1A). The two rats stood on two independent wire meshes. Each was supported by a separate sponge padding to avoid the influence of vibration from the other chamber. In the opaque control group, all habituation protocols were the same except that the plexiglas divider between the two compartments was opaque.
On the test day, 7 days after surgery, observer rats were placed again in the plexiglas chamber 30 min for habituation with lights on during the light cycle. We used infra-red heat pulse irradiation generated by a CO2 laser (Model CI40 CO2 laser, 10.6 μm wavelength, Blue Sky Tech, Taipei) as the noxious stimulus applied to forepaws of the rats. The laser-pulse duration was 36 ms and the intensity was 4–5 W, corresponding to power levels ranging from 136 to 175 mJ. This intensity range was chosen based on preliminary trials and our previous findings37,38,80 so as to induce paw-lifting behavior 90-95% of the time that a rat’s paw was hit. The noxious heat pulse was directed either to the glabrous skin of the forepaw of one of the rats (50% to the left and 50% to the right, randomized in order), or to the air around the chambers. The laser was directed by an experimenter sitting beside the setup when rats were resting. Noxious heat pulses were administered when rats were immobile. The sequence of the protocol was ten times into the air (“air”), ten times to the demonstrator rat (“demo”), and ten times to the observer rat (“self”) (Figure 1A). There were no missed trials during the “demo” and “self” blocks. Inter-trial intervals were longer than 20 s. Significance levels to reject null hypotheses were set at α = 0.05 with Bonferroni correction for multiple comparisons, e.g., p< 0.016 for 3 pairs of “transparent demo vs. opaque demo”, “transparent demo vs. transparent self”, and “transparent demo vs. transparent air.”
Conspecific helping behavior test
To test behavioral functions of observational-distress-responsive neurons, another group of rats performed a conspecific helping behavior test followed by the observational distress test (Figures 6A–6C). At the commencement of the helping behavior test, all subjects were trained to open the gate of a restraining trap. Briefly, rats were acclimated to an arena (50 × 50 × 43 cm) for 1 h each during the first two days. Afterward rats were fasted overnight and the next day, they were placed in the arena containing an opened restrainer (25 × 8.75 × 7.5 cm) with rat chow inside. Rats were trained to open the gate during the following two days. Rats that could open the gate more than ten times in two successive days without help from the experimenter were defined as “openers.” Under this training protocol, 33 rats of 120 achieved opener status in 2 days and they received microelectrode implantation (Figures 6D and 6E).
After recovery from surgery for 5 days, implanted rats were fitted with a telemetry sensor (TBSI, W016020H07K1A) and habituated to the testing arena for the helping behavior test. On the testing day for the conspecific helping behavior test, we recorded behavior by digital video and neuronal activity by telemetry of the freely moving rat in the arena. In an experimental session, the implanted rat was acclimated to the test arena for 30 min and then a restrainer with a trapped rat was placed in the center of the arena. Control sessions included testing an implanted rat with the restrainer either empty or occupied by a toy rat. Each session comprised three types of trials and ten trials per type (30 in total) in a randomized sequence.
Among the 33 opener rats bearing electrodes, 26 had good quality unit recordings. Among these 26 rats, 6 lost their gate-opening skill after the surgery and 4 others did not open the trap during the rescuing session. Therefore, 16 rats completed the 2 experimental tasks with good recording quality. Because recorded units were very likely to be unstable after the “self” blocks of the observational distress test, rats were tested in the conspecific helping behavior test before being tested in the observational distress test. Even so, only 12 of 16 rats were able to have putatively stable units during the 2 tasks, according to waveforms in PC space (within the 95% confidence interval) and inter-spike interval (ISI) distributions during resting as baseline recording after habituation in the behavioral test setups (p< 0.05, Kolmogorov-Smirnov test).
Histology
After completion of the experiments, positions of microelectrode tips were marked with 3 × 10 s 5-μA positive DC current to deposit ferric ions while rats were under deep anesthesia. Animals were perfused intra-cardially with saline followed by 4% formalin containing 1% potassium ferrocyanide to mark the point of iron deposition (Figure 6D). Brains were removed and placed in the same perfusion fluid for 7 days. Serial frozen sections were cut at 50 μm with a sliding microtome. Sections were counter-stained with cresyl violet. Positions of electrode tips were ascertained with camera lucida drawings under a stereomicroscope.
Quantification and statistical analyses
Statistical details can be found within the results section. All groups of data are reported as mean ± SEM Statistical analyses described below were performed using MATLAB build-in functions and SPSS.
Behavioral scoring
Eight types of behavior of observer and demonstrator rats were analyzed in observational distress test. They were freezing, sniffing, social approaching, heading, walking, resting, whisking, and licking. Definitions of the behaviors are as follows. Freezing is defined as lack of movement in a standing position, other than respiration.41 Sniffing is defined as movement of the nose or whiskers. Social approaching occurs when a rat pokes its nose into a hole in the partition or puts its paw on the plexiglas partition between the demonstrator and observer. Walking is self-evident. Heading occurs when the rat swings its head up and down, or left and right. Resting is defined as a lack of movement in a rat that put its head on its forepaws with an electroencephalogram showing that it was awake (a power peak within the 4–12 Hz theta band). Licking occurs when a rat licks its forepaws. Behaviors were scored in 1-s time bins for 20 s after laser applied. Mean percentage of each behavior in self-block was compared to air-block and demo-block with one sided Mann-Whitney U test at a significance level of α = 0.05, with Bonferroni correction (Figure 1 and S1).
Neuronal response to observed pain
For the neuronal response statistics, In the observational distress test, for each neuron and each session, we assessed responsiveness by comparing the firing rate during a baseline period (0.5 s–1.5 s before stimulus onset) and the firing rate during the experimental condition (0 s–3 s after stimulus onset) on a trial-by-trial basis with two-tailed Mann-Whitney U tests. We examined 3-s periods because most social approach behavior occurred within that period. The statistical significance threshold for the Mann-Whitney U test across trials was set at p = 0.05. Sustained excitatory responses were identified if the number of spikes increased significantly in the ten trials tested in a session. Sustained inhibitory responses were defined if the number of spikes decreased significantly. A transient excitatory response was counted if the unit activity exceeded the 99% confidence interval (Z value ≥2.33) in two consecutive bins (bin size, 100 ms) in the experimental condition (0 s–3 s after stimulus onset) in the normalized peri-event histogram and if compared with baseline activity, the maximum firing rate increased in more than 50% of the trials. No attempt was made to identify transient inhibitory responses (Table 1). We performed Mann-Whitney U-test comparing post laser heat applied of self-block/demo-block to air-block (Figure 2 and S2–S5). To evaluate the uncertainty of cell classification, we created 10,000 shuffled datasets in which the “air”, “demo”, and “self” samples for each “air”-“demo”-“self” combination were randomly selected without replacement from the original dataset of “air”, “demo”, and “self” samples, respectively. We also created 100,000 bootstrapping datasets with 10 trials for a block where each trial was randomly picked with replacement from the original, pooled dataset of “air”, “demo”, and “self” samples. Observed percentages of cell classification from recorded data were compared with those from shuffled datasets as positions in the distributions (Figure 3).
Behavior correlated unit activity
A total of 17 rats showing social approach behavior more than 10 times were used to correlate behavior with unit activity. Among them, in 7 rats implanted with both ACC and InC arrays, in addition to the aforementioned 3 sessions, a new session was added to increase numbers of social approach behaviors. Following the 3 sessions, we replaced the demonstrator rat with a naive rat in the demonstrator’s compartment and recorded the neuronal activity of the observer for another 20 min. The beginning of the social approach behavior was used as time 0 to obtain a peri-event histogram of single-unit activity. Average ensemble activity was obtained by averaging the peri-event histograms, excluding inhibitory responses. In the “demo” blocks, freezing and social approach behaviors were initiated 4.0–11.6 s and 3.3–12.8 s (Q1–Q3), after laser heat pulses, respectively. Accordingly, we focused on neuronal activities from 2 s before to 4 s after heat pulses in subsequent analyses. To capture activity changes in freely behaving rats, we normalized neuronal activity by Z score scaling with respect to the mean firing rate 2 to 0 s before the heat pulse in all ten trials.
Neurons responsive to pain of both others and self were correlated with prosocial-related behaviors in the helping behavior test. Average ensemble activity was obtained by averaging peri-event histograms. Baseline activities from 5 to 10 s prior to every behavior were used to transform the data into normalized Z values.
For the statistic of ensemble neuronal responding to either social approach and freezing in observational distress test and helping, nose interaction, and control open in helping behavior test, we performed one-sample Wilcoxon signed-rank test compare 2 s before and after the particular behavior (Figures 4, 5, 6, 7, S6, and S7). Significance values are reported as follows: ∗p<0.05 and ∗∗p<0.01. For the figures, all data are given as the mean ± SEM. Detail information of the statistic test is embedded in figure legends.
Acknowledgments
We thank Prof. Peggy Mason for many helpful suggestions and Steven D. Aird for editing the manuscript. This work was funded by grants NSC 98-2321-B-002-031, NSC 99-2321-B-002-012, and NSC 100-2321-B-002-006 from National Science Council, Taiwan, a grant NHRI-EX103-10104NI from National Health Research Institutes, a grant NTU-ERP-104R892102 from National Taiwan University, and a grant 16J10077 from KAKENHI.
Author contributions
Conceptualization, K.C.L., C.T.Y., and R.X.L.; Methodology, C.T.Y., K.C.L., Y.C., R.X.L., and W.Y.W.; Investigation, W.Y.W.; Visualization, W.Y.W. and R.X.L.; Writing – Original Draft, W.Y.W.; Writing – Review and Editing, C.T.Y., K.C.L., and R.X.L.; Funding Acquisition, C.T.Y., K.C.L., and R.X.L. Each corresponding author’s specific contributions have been described in the author contributions section. The two corresponding authors R.X.L. and C.T.Y. have the role of supervision at later and earlier stages of the project, respectively.
Declaration of interests
The authors declare no competing interests.
Published: January 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105865.
Contributor Information
Ray X. Lee, Email: raylee@mit.edu.
Chen-Tung Yen, Email: ctyen@ntu.edu.tw.
Supplemental information
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Batson C.D. 2009. The Social Neuroscience of Empathy. [Google Scholar]
- 2.de Waal F.B.M., Preston S.D. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 2017;18:498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- 3.Decety J., Bartal I.B.A., Uzefovsky F., Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150077. doi: 10.1098/rstb.2015.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Y., Duncan N.W., de Greck M., Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Jackson P.L., Rainville P., Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Morrison I., Lloyd D., di Pellegrino G., Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cognit. Affect Behav. Neurosci. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- 7.Singer T., Seymour B., O'Doherty J., Kaube H., Dolan R.J., Frith C.D. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535303/5661/1157. [DOI] [PubMed] [Google Scholar]
- 8.Zaki J., Ochsner K.N., Hanelin J., Wager T.D., Mackey S.C. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc. Neurosci. 2007;2:276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mischkowski D., Crocker J., Way B.M. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc. Cognit. Affect Neurosci. 2016;11:1345–1353. doi: 10.1093/scan/nsw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian Keysers V.G. Elsevier; 2018. Neuronal Correlates of Empathy. [Google Scholar]
- 11.Cerniglia L., Bartolomeo L., Capobianco M., Lo Russo S.L.M., Festucci F., Tambelli R., Adriani W., Cimino S. Intersections and divergences between empathizing and mentalizing: development, recent advancements by neuroimaging and the future of animal modeling. Front. Behav.Neurosci. 2019;13:212. doi: 10.3389/fnbeh.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamm C., Decety J., Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Langford D.J., Crager S.E., Shehzad Z., Smith S.B., Sotocinal S.G., Levenstadt J.S., Chanda M.L., Levitin D.J., Mogil J.S. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 14.Smith M.L., Hostetler C.M., Heinricher M.M., Ryabinin A.E. Social transfer of pain in mice. Sci. Adv. 2016;2:e1600855. doi: 10.1126/sciadv.1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin L.J., Hathaway G., Isbester K., Mirali S., Acland E.L., Niederstrasser N., Slepian P.M., Trost Z., Bartz J.A., Sapolsky R.M., et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr. Biol. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Meyza K., Knapska E. What can rodents teach us about empathy? Curr.Opin. Psychol. 2018;24:15–20. doi: 10.1016/j.copsyc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Jeon D., Kim S., Chetana M., Jo D., Ruley H.E., Lin S.Y., Rabah D., Kinet J.P., Shin H.S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keum S., Shin H.S. Neural basis of observational fear learning: a potential model of affective empathy. Neuron. 2019;104:78–86. doi: 10.1016/j.neuron.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ami Bartal I., Decety J., Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato N., Tan L., Tate K., Okada M. Erratum to: rats demonstrate helping behavior toward a soaked conspecific. Anim. Cognit. 2015;18:1049. doi: 10.1007/s10071-015-0906-9. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Ami Bartal I., Shan H., Molasky N.M.R., Murray T.M., Williams J.Z., Decety J., Mason P. Anxiolytic treatment impairs helping behavior in rats. Front. Psychol. 2016;7:850. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice G.E., Gainer P. Altruism" in the albino rat. J. Comp. Physiol. Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 23.Carrillo M., Han Y., Migliorati F., Liu M., Gazzola V., Keysers C. Emotional mirror neurons in the rat's anterior cingulate cortex. Curr.Biol. 2019;29:1301–1312.e6. doi: 10.1016/j.cub.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox S.S., Kearns A.M., Woods S.K., Brown B.J., Brown S.J., Reichel C.M. The role of the anterior insular during targeted helping behavior in male rats. Sci. Rep. 2022;12:3315. doi: 10.1038/s41598-022-07365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzolatti G., Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 26.Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 27.Rizzolatti G., Fadiga L., Gallese V., Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 28.Rizzolatti G., Fabbri-Destro M. The mirror system and its role in social cognition. Curr.Opin.Neurobiol. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Rizzolatti G., Fogassi L., Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/3509006035090060. [DOI] [PubMed] [Google Scholar]
- 30.Heyes C., Catmur C. What happened to mirror neurons? Perspect. Psychol. Sci. 2022;17:153–168. doi: 10.1177/1745691621990638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraskov A., Dancause N., Quallo M.M., Shepherd S., Lemon R.N. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 2009;64:922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batson C.D., Shaw L.L. Evidence for altruism: toward a pluralism of prosocial motives. Psychol. Inq. 1991;2:107–122. [Google Scholar]
- 34.Ben-Ami Bartal I., Breton J.M., Sheng H., Long K.L., Chen S., Halliday A., Kenney J.W., Wheeler A.L., Frankland P., Shilyansky C., et al. Neural correlates of ingroup bias for prosociality in rats. Elife. 2021;10:e65582. doi: 10.7554/eLife.65582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terranova J.I., Yokose J., Osanai H., Marks W.D., Yamamoto J., Ogawa S.K., Kitamura T. Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron. 2022;110:1416–1431.e13. doi: 10.1016/j.neuron.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J., Jeong Y. Elevated emotional contagion in a mouse model of Alzheimer's disease is associated with increased synchronization in the insula and amygdala. Sci. Rep. 2017;7:46262. doi: 10.1038/srep46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo C.C., Yen C.T. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J. Neurophysiol. 2005;94:1825–1836. doi: 10.1152/jn.00294. [DOI] [PubMed] [Google Scholar]
- 38.Kuo C.C., Chiou R.J., Liang K.C., Yen C.T. Differential involvement of the anterior cingulate and primary sensorimotor cortices in sensory and affective functions of pain. J. Neurophysiol. 2009;101:1201–1210. doi: 10.1152/jn.90347. [DOI] [PubMed] [Google Scholar]
- 39.Keysers C., Knapska E., Moita M.A., Gazzola V. Emotional contagion and prosocial behavior in rodents. Trends Cognit. Sci. 2022;26:688–706. doi: 10.1016/j.tics.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Lu P.L., Hsu S.S., Tsai M.L., Jaw F.S., Wang A.B., Yen C.T. Temporal and spatial temperature distribution in the glabrous skin of rats induced by short-pulse CO2 laser. J. Biomed. Opt. 2012;17:117002. doi: 10.1117/1.jbo.17.11.117002. [DOI] [PubMed] [Google Scholar]
- 41.Fanselow M.S. Conditioned and unconditional components of post-shock freezing. Pavlovian J. Biol. Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 42.Keum S., Shin H.S. Rodent models for studying empathy. Neurobiol. Learn. Mem. 2016;135:22–26. doi: 10.1016/j.nlm.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Kim A., Keum S., Shin H.S. Observational fear behavior in rodents as a model for empathy. Gene Brain Behav. 2019;18:e12521. doi: 10.1111/gbb.12521. [DOI] [PubMed] [Google Scholar]
- 44.Deschênes M., Moore J., Kleinfeld D. Sniffing and whisking in rodents. Curr.Opin.Neurobiol. 2012;22:243–250. doi: 10.1016/j.conb.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Márquez C., Rennie S.M., Costa D.F., Moita M.A. Prosocial choice in rats depends on food-seeking behavior displayed by recipients. Curr. Biol. 2015;25:1736–1745. doi: 10.1016/j.cub.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Silberberg A., Allouch C., Sandfort S., Kearns D., Karpel H., Slotnick B. Desire for social contact, not empathy, may explain "rescue" behavior in rats. Anim. Cognit. 2014;17:609–618. doi: 10.1007/s10071-013-0692-1. [DOI] [PubMed] [Google Scholar]
- 47.Vasconcelos M., Hollis K., Nowbahari E., Kacelnik A. Pro-sociality without empathy. Biol. Lett. 2012;8:910–912. doi: 10.1098/rsbl.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox S.S., Reichel C.M. Rats display empathic behavior independent of the opportunity for social interaction. Neuropsychopharmacology. 2020;45:1097–1104. doi: 10.1038/s41386-019-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez-Lallement J., Attah A.T., Soyman E., Pinhal C.M., Gazzola V., Keysers C. Harm to others acts as a negative reinforcer in rats. Curr. Biol. 2020;30:949–961.e7. doi: 10.1016/j.cub.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Atsak P., Orre M., Bakker P., Cerliani L., Roozendaal B., Gazzola V., Moita M., Keysers C. Experience modulates vicarious freezing in rats: a model for empathy. PLoS One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knapska E., Mikosz M., Werka T., Maren S. Social modulation of learning in rats. Learn. Mem. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruchey A.K., Jones C.E., Monfils M.H. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav. Brain Res. 2010;214:80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sivaselvachandran S., Acland E.L., Abdallah S., Martin L.J. Behavioral and mechanistic insight into rodent empathy. Neurosci. Biobehav. Rev. 2018;91:130–137. doi: 10.1016/j.neubiorev.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Gallese V., Keysers C., Rizzolatti G. A unifying view of the basis of social cognition. Trends Cognit. Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Umiltà M.A., Kohler E., Gallese V., Fogassi L., Fadiga L., Keysers C., Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 56.Gallese V., Gernsbacher M.A., Heyes C., Hickok G., Iacoboni M. Mirror neuron forum. Perspect. Psychol. Sci. 2011;6:369–407. doi: 10.1177/1745691611413392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albertini D., Lanzilotto M., Maranesi M., Bonini L. Largely shared neural codes for biological and nonbiological observed movements but not for executed actions in monkey premotor areas. J. Neurophysiol. 2021;126:906–912. doi: 10.1152/jn.00296.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umiltà M.A., Escola L., Intskirveli I., Grammont F., Rochat M., Caruana F., Jezzini A., Gallese V., Rizzolatti G. When pliers become fingers in the monkey motor system. Proc. Natl. Acad. Sci. USA. 2008;105:2209–2213. doi: 10.1073/pnas.0705985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casile A., Caggiano V., Ferrari P.F. The mirror neuron system: a fresh view. Neuroscientist. 2011;17:524–538. doi: 10.1177/1073858410392239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigneswaran G., Philipp R., Lemon R.N., Kraskov A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol. 2013;23:236–243. doi: 10.1016/j.cub.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida K., Saito N., Iriki A., Isoda M. Representation of others' action by neurons in monkey medial frontal cortex. Curr. Biol. 2011;21:249–253. doi: 10.1016/j.cub.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Schneider K.N., Sciarillo X.A., Nudelman J.L., Cheer J.F., Roesch M.R. Anterior cingulate cortex signals attention in a social paradigm that manipulates reward and shock. Curr. Biol. 2020;30:3724–3735.e2. doi: 10.1016/j.cub.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sul J.H., Kim H., Huh N., Lee D., Jung M.W. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sterzer P., Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct.Funct. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- 65.Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 66.Johansen J.P., Fields H.L., Manning B.H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamm C., Batson C.D., Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J. Cognit. Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 68.Lamm C., Singer T. The role of anterior insular cortex in social emotions. Brain Struct.Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- 69.Zaniboni C.R., Pelarin V., Baptista-de-Souza D., Canto-de-Souza A. Empathy for pain: insula inactivation and systemic treatment with midazolam reverses the hyperalgesia induced by cohabitation with a pair in chronic pain condition. Front. Behav.Neurosci. 2018;12:278. doi: 10.3389/fnbeh.2018.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gogolla N. The insular cortex. Curr. Biol. 2017;27:R580–R586. doi: 10.1016/j.cub.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Craig A.D.B. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 72.Berret E., Kintscher M., Palchaudhuri S., Tang W., Osypenko D., Kochubey O., Schneggenburger R. Insular cortex processes aversive somatosensory information and is crucial for threat learning. Science. 2019;364:eaaw0474. doi: 10.1126/science.aaw0474. [DOI] [PubMed] [Google Scholar]
- 73.Tan L.L., Pelzer P., Heinl C., Tang W., Gangadharan V., Flor H., Sprengel R., Kuner T., Kuner R. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat. Neurosci. 2017;20:1591–1601. doi: 10.1038/nn.4645. [DOI] [PubMed] [Google Scholar]
- 74.Tomek S.E., Stegmann G.M., Olive M.F. Effects of heroin on rat prosocial behavior. Addiction Biol. 2019;24:676–684. doi: 10.1111/adb.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomek S.E., Stegmann G.M., Leyrer-Jackson J.M., Piña J., Olive M.F. Restoration of prosocial behavior in rats after heroin self-administration via chemogenetic activation of the anterior insular cortex. Soc. Neurosci. 2020;15:408–419. doi: 10.1080/17470919.2020.1746394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 77.Fan Y.T., Decety J., Yang C.Y., Liu J.L., Cheng Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry. 2010;51:981–988. doi: 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 78.Chan M.M.Y., Han Y.M.Y. Differential mirror neuron system (MNS) activation during action observation with and without social-emotional components in autism: a meta-analysis of neuroimaging studies. Mol. Autism. 2020;11:72. doi: 10.1186/s13229-020-00374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai M.L., Yen C.T. A simple method for fabricating horizontal and vertical microwire arrays. J. Neurosci. Methods. 2003;131:107–110. doi: 10.1016/s0165-0270(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 80.Wu W.Y., Liu C.Y., Tsai M.L., Yen C.T. Nocifensive behavior-related laser heat-evoked component in the rostral agranular insular cortex revealed using morphine analgesia. Physiol. Behav. 2016;154:129–134. doi: 10.1016/j.physbeh.2015.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The text “Laser” appears in the left corner when the demonstrator’s forepaw was hit at time 617.8271s. The demonstrator lifted and licked its forepaw and the observer then shifted its body and head position, pushed its nose against a hole in the partition. The activity of a simultaneously recorded InC unit is shown in the bottom panel.
Superimposed waveforms (left panel) and PC1-PC2 plot (right panel) were shown in the opening scene. A mirror neuron in InC activa. It shows no change before door opening at t = 96 in the toy trial.
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.