Immunotherapy with CD19-directed chimeric antigen receptor (CAR) T cells (tisagenlecleucel, axicabtagene and lisocabtagene) has revolutionized the treatment of de novo and transformed relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), inducing long-term complete response (CR) in about 40% of cases.1-3
The majority of patients candidate to CD19 directed CAR T cells for R/R DLBCL have been previously exposed to many anti-tumor agents, including chemotherapy drugs, conjugated monoclonal antibodies and radiation, administered to achieve CR during the course of the disease or as a bridging therapy to CAR T-cell infusion. Chemo-radio-therapy is well known to exert a genotoxic effect favoring the occurrence of secondary tumors, including myelodys-plastic syndrome (MDS) and acute myeloid leukemia (AML).4 Myeloid malignancies have been so far occasionally reported after CD19-directed CAR T-cell therapy of B-cell acute lymphoblastic leukemia (B-ALL). They include the lineage switch of MLL-rearranged B-ALL into a clonally related AML under the immune pressure of anti-CD19 CAR T cells5 or the development of a clonally unrelated new myeloid neoplasm.6 On the other hand, no information on the development of myeloid neoplasms in DLBCL patients treated with CD19-directed CAR T cells is to our knowledge available.
Here, we present the first case of AML developing soon after CAR T-cell infusion in an R/R DLBCL patient.
A 69-year-old women was diagnosed with a follicular lymphoma, grade 2 in 2016. A positron emission tomography/computerized tomography (PET/CT) scan showed involvement of almost all superficial, thoracic and abdominal lymph nodes (LN), the presence of two subcutaneous nodules and a pleural effusion, while a bone marrow (BM) biopsy showed no involvement of the BM. The blood cell count (BCC) was: white blood cells (WBC) 17.690/mm3 (neuthrophils 83%), hemoglobin (Hb) 14 g/dL platelets 459.000/mm3. She received six cycles of benda-mustine plus rituximab, achieving a near CR (persistence of a few neck LN). Three months later, she relapsed in all nodal and extranodal sites initially involved by the disease. A new LN biopsy revealed DLBCL transformed from follicular lymphoma, without MYC, BCL2 and BCL6 rearrangements. Therefore, the patient underwent six cycles of R-COMP plus prophylaxis for central nervous system-CNS involvement, achieving a CR that only lasted 3 months. Then, she received one cycle of R-DHAOX (rituxi-mab, dexamethasone, cytarabine and oxaliplatin) followed by collection of hematopoietic stem cells (HSC) and auto-logous stem cell transplantation (ASCT) using FEAM (fote-mustine, etoposide, cytarabine and melphalan) as conditioning regimen. This resulted into a CR but, 5 months later, the PET/CT scan showed reappearance of the disease in all nodal and extranodal sites. The patient started with lenalinomide (10 mg/day for 3 weeks) plus prednisone, achieving CR after five cycles. The BCC in November 2018 was: WBC 7.000/ mm3 (neuthrophils 78%), Hb 11.7 g/dL, platelets 115.000/mm3. She proceeded with additional four cycles of lenalidomide plus prednisone that were stopped because of severe pancytopenia. The BM biopsy, performed 1 month after lenalidomide discontinuation, showed an hypocellular marrow consistent with previous myelotoxic therapy but no neoplastic infiltration. However, disease progression in all nodal and extranodal sites was documented at PET/TC scan. A submandibular LN biopsy (June 2019) showed development of DLBCL expressing all B-cell markers, including CD19. The patient started again lenalidomide plus steroids achieving a CR that lasted about 1.5 years. In March 2021, PET/CT showed again disease progression (Figure 1A). Thus, the patient was regarded as eligible for CD19-directed CAR T cells and leukapheresis was performed. The blood cell count was: WBC 2.380/mm3 (neuthrophils 60%), Hb 11.2 g/dL, platelets 69.000/mm3. The BM biopsy showed hypocellular marrow that was interpreted as due to previous therapies. As bridging therapy to CAR T cells, she received HAM regimen (mitoxantrone and cytarabine) followed by reinfusion of autologous HSC achieving partial remission. In the following months, several episodes of cytomegalovirus (CMV) reactivation prevented to proceed with CAR T-cell infusion and therefore in July 2021 we decided to control the disease through a total lymphoid radiation (TLI) for 5 days. In particular, all the main nodal station received 15 Gy in ten fractions. A simultaneous integrated boost (2 Gy up to 20 Gy) was delivered to the active disease.
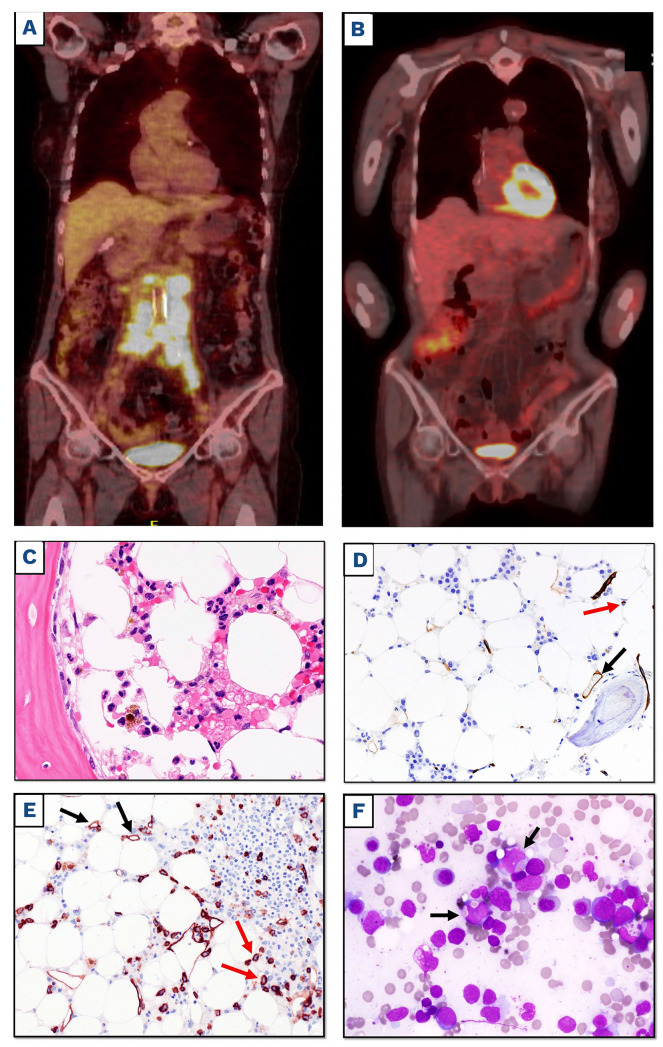
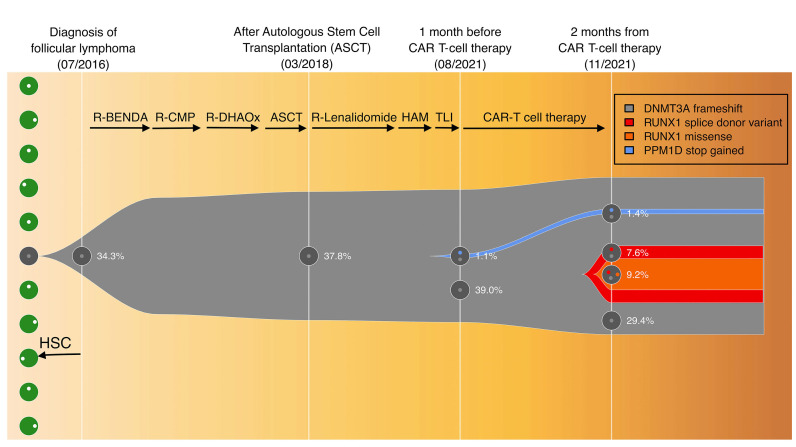
In August 2021, the BCC was: WBC 4.310/mm3 (neuthrophils 56%), Hb 10.6 g/dL, platelets 80.000/mm3 and the BM biopsy showed hypocellular marrow without clear MDS or neoplastic infiltrates (Figure 1C) with a normal percentage of CD34+ cells (Figure 1D). Therefore, she received infusion of CD19-directed CAR T cells after lymphocyte depletion with fludarabine and cyclophosphamide. No cytokine release syndrome (CRS) or neurotoxicity was recorded. CD19 CAR T-cell expansion was detectable by flow-cytometry from day 7 to 21. PET/CT scan performed at 1 and 3 months after CAR T cells showed CR (Figure 1B). Due to persistent severe pancytopenia resulting from lymphodepleting therapy (WBC 450/mm3, Hb 8 g/dL, platelets 6.000/mm3 on 27/10/21) lasting 60 days after CAR T-cell infusion and requiring transfusion support, a new BM biopsy and aspirate was performed that showed hypocellular marrow infiltrated by 40-50% blast cells with myelomonocytic appearance expressing CD34 (Figure 1E), myeloperoxidase and CD68, and frequently exhibiting erythrophagocytosis (Figure 1F). No normal or neoplastic B cells were detected in the BM by flow cytometry and immunohistochemistry. Cytogenetic analysis showed a complex karyotype: 45,xx,-7, del(11)(p15) [8]/46,idem, t(2;19) (p12;q13.3)[6] /46,XX[3]. Targeted sequencing detected mutations of the following genes: DNMT3A V626GfsTer4 (variant allele frequency [VAF] 46.2%), RUNX1 splicing-site mutation (VAF 16.8%) and missense mutation N136K (VAF 9.2%), and PPM1D S453* (VAF 1.4%). Targeted sequencing of stored DNA from the BM samples taken before CAR T-cell therapy showed the presence of the PPM1D mutation but not RUNX1 mutations (Figure 2). We also retrospectively analyzed the kariotype from a BM aspirate taken in March 2021, which already showed the following karyotype: 45,XX,-7, del(11)(p15)[8]/46,XX[12]. Given the diagnosis of AML, while in CR for DLBCL, we decided to start the patient on 5-Azacitidine plus venetoclax a bridge to a potential allogenic SCT. However, venetoclax was stopped after the first cycle due to persistent pancytopenia and a BM evaluation performed after two cycles of 5-Azacitidine showed AML persistence. After four cycles of 5-Azacitidine pancytopenia continues while the patient is still in complete remission for DLBCL at 6 months from CAR T-cell therapy.
Figure 1.
Positron emission tomography/computerized tomography scan and bone marrow examination before and afer chimeric antigen receptor T-cell infusion. (A and B) Positron emission tomography/computerized tomography (FDG-PET/CT) scan coronal maximum intensity projection (MIP) images before leukapheresis with avid uptake of abdominal lymph nodes (A) and 3 months after CD19-directed chimeric antigen receptor (CAR) T-cell therapy (B), showing metabolic complete response of diffuse large B-cell lymphoma (DLBCL). (C) Bone marrow (BM) biopsy taken before CAR T-cell infusion showing an hypocellular marrow without leukemic infiltration (hematoxylin and eosin staining; magnification x400). (D) The same sample as (C) showing only rare CD34+ cells within the normal range (red arrow); vessel endothelial cells serve as positive control (black arrow) (immunoperoxidase, diaminobenzidine; magnification x200). (E) BM biopsy taken 2 months after CAR T-cell infusion showing hypocellular marrow infiltrated by CD34+ leukemic cells (red arrows); vessel endothelial cells serve as positive control (black arrows) (immunoperoxi-dase, diaminobenzidine; magnification x200). (F) BM smear 2 months after CAR T-cell infusion showing myeloid blasts exhibiting erythrophagocytosis (black arrows) (May-Grunwald-Giemsa staining, magnification x400).
Our patient showed persistent severe pancytopenia after CAR T-cell therapy. Pancytopenia of various degree frequently occurs in the first 1-3 months after infusion of CD19-directed CAR T cells and may be ascribed to several conditions, including bridging and/or lymphodepletive therapy administered before CAR T cells, cytokine release by CAR T cells, BM involvement by lymphoma or leukemia, BM failure related to drugs other than CAR T cells and/or infectious complications (e.g., CMV).7- 8 Among these causes, BM pancytopenia due to BM involvement by lymphoma was unlikely in our patient because it was never documented during the previous 5 years of the disease. Similarly, no infectious complications could be recorded after CAR T cells. Instead, myelotoxicity due to bridging chemoradiotherapy (HAM regimen and TLI) could have played a role in the persistent pancytopenia of our patient. In order to discriminate between these possibilities, we performed a BM biopsy 60 days after CAR T-cell therapy, that surprisingly revealed AML with complex karyotype, including monosomy 7. This diagnosis was in a way unexpected since a BM biopsy taken just before CAR T-cell infusion showed no evidence of MDS/AML.
Figure 2.
Clonal evolution from clonal hematopoiesis to therapy related acute myeloid leukemia. The fish plot shows the inferred clonal evolution pattern based on targeted sequencing. The phylogenetic trees visualize the estimated order of mutation acquisition and the proportion of subclones with a different combination of mutations at each time point. The PPM1D mutation has been depicted as presumptively subclonal within the DNMT3A-mutant preleukemic clone, but its low variant allele frequency (VAF) would be compatible also with the existence of a separate PPM1D-mutant chromatin immunoprecipitation clone independent from (i.e., outside of) the DNMT3A-mutant preleukemic clone. RUNX1 missense mutation has been presumptuously depicted as subclonal within the RUNX1 splice site mutant leukemic clone. R-BENDA: rituximab, bendamustine; R-CMP: rituximab, cyclofosfamide, non-pegylated liposomal doxorubicin, prednisone; R-DHAOX: rituximab, high dose ARAc, oxaliplatin; ASCT, preceded by fotemustine, etoposide, ARAc and melphalan; R-lenalidomide: rituximab-lenalidomide; HAM: high dose ARA-C and mitoxantrone; TLI: total lymphonodal irradiation.
The pathogenesis of AML in our case remains unclear. Clonal expansion of CAR T cells due to unintentional lentiviral vector mediated insertion of the CAR transgene in the TET2 or ABL gene has been reported.9,10 However, the possibility that AML could have developed following unintentional insertion of the CAR transgene into AML-associated genes in a contaminating HSC during manufacturing was excluded by flow cytometry, showing CAR expression only on T lymphocytes inside all peripheral blood population, thereby excluding the presence of any possible circulating CAR-positive myeloid cells. According to the World Health Organization classification of myeloid neoplasms, the leukemia in our patient was consistent with a therapy-related AML (t-AML), due to the previous history of chemo-radiotherapy and the complex karyotype. t-MDS/AML has been ascribed for a long time to DNA mutations and chromosome breakage induced in HSC by ionizing radiation and/or genotoxic drugs (e.g., alkylating agents or topoisomerase 2 inhibitors). Instead, it is now clearly emerging that these stressors of hematopoiesis can promote expansion of pre-existing mutant clones driven by clonal hematopoiesis (CH).11 CH has been reported in 48% of patients receiving CAR T-cell therapy for B-cell non-Hodgkin lymphomas or multiple myeloma but, unlike CH found in patients pre-ASCT,12 it was not associated with a worse survival, including increased risk of death from t-MDS/AML.13 Mutations of DNA damage response genes TP53 and PPM1D, typically observed in therapy-related CH,14 were not detected in our patient since TP53 was germline while the PPM1D mutation, despite developing (at a VAF 1.1%) before leukemia onset, remained at a similar low frequency (VAF 1.4%) at leukemia onset without seeding the tumor clone (which had RUNX1 mutations at higher VAF up to 16.8%). In contrast, CH in our patient was mainly driven by DNMT3A that was already present in 2016, at the time of lymphoma diagnosis. DNMT3A driven CH has been shown to be by itself predictor for AML development.11,14 In contrast, RUNX1 mutations were detected for the first time in the BM sample taken 2 months after CAR T cells, pointing to RUNX1 mutations as drivers in promoting t-AML in our patient,15 probably through cooperation with deletion of monosomy 7. As there was no evidence of AML in the BM taken just before CAR T-cell infusion, we hypothesize that AML may have developed as consequence of the immunosuppression related to lymphodepletion pre-CAR T-cell infusion, although we cannot exclude that it could be related to previous multiple genotoxic treatments (e.g., anthracyclines, radiotherapy or ASCT). However, studies of similar cases are required to better clarify this issue. For the time being, we recommend CD34 immunos-taining as well as next-generation sequencing and cytogenetic analysis in the BM of heavily pretreated DLBCL patients before CAR T therapy, especially if they show evidence of cytopenia than can be erroneously interpreted as BM hypoplasia related to previous therapy. Considering the higher risk to develop t-AML, such patients should be carefully evaluated in order to decide if they could be excluded from CAR T-cell therapy favoring immunotherapy approaches not requiring lymphodepletion, as bispecific or drug-conjugated antibodies.
Acknowledgments
We thank Prof Stefano Lazzi for performing FISH analysis on lymph node biopsy.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 4.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo G, Wang HW, Talleur AC, et al. Diagnostic approach to the evaluation of myeloid malignancies following CAR T-cell therapy in B-cell acute lymphoblastic leukemia. J Immunother Cancer. 2020;8(2):e001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643-1650. [DOI] [PubMed] [Google Scholar]
- 8.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraietta JA, Nobles CL, Sammons MA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558(7709):307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NN, Qin H, Yates B, et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3(15):2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter MJ, Shen D, Ding L, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366(12):1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husby S, Favero F, Nielsen C, et al. Clinical impact of clonal hematopoiesis in patients with lymphoma undergoing ASCT: a national population-based cohort study. Leukemia. 2020;34(12):3256-3268. [DOI] [PubMed] [Google Scholar]
- 13.Miller PG, Sperling AS, Brea EJ, et al. Clonal hematopoiesis in patients receiving chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5(15):2982-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations of AML1 are common in therapy-related myelodysplasia following therapy with alkylating agents and are significantly associated with deletion or loss of chromosome arm 7q and with subsequent leukemic transformation. Blood. 2004;104(5):1474-1481. [DOI] [PubMed] [Google Scholar]