Abstract
Endothelial dysfunction represents a key mechanism underlying heart failure with preserved ejection fraction (HFpEF), diabetes mellitus (DM), and frailty. However, reliable biomarkers to monitor endothelial dysfunction in these patients are lacking. In this study, we evaluated the expression of a panel of circulating microRNAs (miRs) involved in the regulation of endothelial function in a population of frail older adults with HFpEF and DM treated for 3 months with empagliflozin, metformin, or insulin. We identified a distinctive pattern of miRs that were significantly regulated in HFpEF patients compared to healthy controls and to HFpEF patients treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin. Three miRs were significantly downregulated (miR-126, miR-342-3p, and miR-638) and two were significantly upregulated (miR-21 and miR-92) in HFpEF patients compared to healthy controls. Strikingly, two of these miRs (miR-21 and miR-92) were significantly reduced in HFpEF patients after the 3-month treatment with empagliflozin, whereas no significant differences in the profile of endothelial miRs were detected in patients treated with metformin or insulin. Taken together, our findings demonstrate for the first time that specific circulating miRs involved in the regulation of endothelial function are significantly regulated in frail HFpEF patients with DM and in response to SGLT2 inhibition.
SIGNIFICANCE STATEMENT
We have identified a novel microRNA signature functionally involved in the regulation of endothelial function that is significantly regulated in frail patients with HFpEF and diabetes. Moreover, the treatment with the SGLT2 inhibitor empagliflozin caused a modification of some of these microRNAs in a direction that was opposite to what observed in HFpEF patients, indicating a rescue of endothelial function. Our findings are relevant for clinical practice inasmuch as we were able to establish novel biomarkers of disease and response to therapy.
Introduction
Endothelial dysfunction is a pathogenically relevant mechanism underlying heart failure with preserved ejection fraction (HFpEF) and diabetes mellitus (DM) (Hadi and Suwaidi, 2007; Giamouzis et al., 2016; Gevaert et al., 2019; Knapp et al., 2019; Premer et al., 2019; Jankauskas et al., 2021; Mone et al., 2021a). HFpEF and DM are very common in older adults, increasing the risk of frailty, a systemic condition that leads to functional decline and adverse outcomes (Owan et al., 2006; Steinberg et al., 2012; Paulus and Tschope, 2013; Chioncel et al., 2017; McHugh et al., 2019; Jankauskas et al., 2021; Lejeune et al., 2021). The pathophysiology of frailty includes chronic inflammation, which is typical of aging (inflammaging), oxidative stress, insulin resistance, loss of anabolic hormones, and reduced tolerance to physical exercise with a reduction in muscle strength (Bandeen-Roche et al., 2015; Cruz-Jentoft and Sayer, 2019; Rusanova et al., 2019). Of note, we and others have shown that endothelial dysfunction plays a fundamental role also in the pathobiology of frailty (Alonso-Bouzon et al., 2014; Mansur et al., 2015; Amarasekera et al., 2021; Mone et al., 2021a, 2022a).
Empagliflozin is a relatively novel selective inhibitor of sodium glucose cotransporter 2 (SGLT2) that has been shown to reduce mortality and rehospitalization for HF (Zinman et al., 2015; Anker et al., 2021; Varzideh et al., 2021; Braunwald, 2022). Additional benefits of SGLT2 inhibitors include improved cardiovascular energetics, reduced vascular tone, decreased renal dysfunction, increased circulating levels of ketone bodies, and overall reduced systemic inflammation (Benetti et al., 2016; Prattichizzo et al., 2018; Wan et al., 2018; Oshima et al., 2019; Verma et al., 2019; Zhang et al., 2021; Jensen et al., 2021; Li et al., 2021; Sardu et al., 2021; Varzideh et al., 2021; Huang et al., 2022; Paolisso et al., 2022; Zhang et al., 2022). We have recently demonstrated that empagliflozin significantly improves cognitive impairment in frail older patients with diabetes with HFpEF (Mone et al., 2022c), also showing a correlation between physical and cognitive impairment (Mone et al., 2022a).
MicroRNAs (miRs) are small noncoding RNAs molecules of 18–24 nucleotides, which typically repress mRNAs by binding their 3′ untranslated region (Santulli, 2015; Stavast and Erkeland, 2019; Hu et al., 2021; Mirzaei et al., 2021; Mone et al., 2021b; Bielska et al., 2022; Karagiannopoulos et al., 2022; Mauro et al., 2022; Moisoiu et al., 2022; Qiu et al., 2022; Traber and Yu, 2022; Yaylim et al., 2022; Zeng et al., 2022). Substantial evidence has shown that miRs exert their activity in many biologic processes and several miRs have been proposed as biomarkers and potential targets of novel therapeutic strategies (Creemers et al., 2012; Wronska et al., 2015; Barwari et al., 2016; Zarone et al., 2017; Chen et al., 2018; Wong et al., 2018; Morelli et al., 2019; Kawasaki et al., 2020; Wang et al., 2020; Fonseca et al., 2021; Gambardella et al., 2021; Bonnet et al., 2022; Gambardella et al., 2022a,b; Kansakar et al., 2022; Varzideh et al., 2022). Several investigators have linked miRs to frailty pointing at their involvement in inflammation, endothelial dysfunction, and senescence (Quinn and O’Neill, 2011; Olivieri et al., 2012; Geiger and Dalgaard, 2017; Rusanova et al., 2019; Bu et al., 2021).
In this study, we aimed at assessing the effects of empagliflozin on the profile of circulating miRs involved in the regulation of endothelial function in frail older adults with DM and HFpEF treated with different antidiabetic regimens.
Materials and Methods
Study Design
We evaluated consecutive frail older adults with a confirmed diagnosis of DM and HFpEF, from October 2021 to December 2021. All subjects were recruited from the Sant’Angelo dei Lombardi Hospital, ASL (local health unit of the Italian Ministry of Health) Avellino, Italy. Inclusion criteria were age >65 years; a previous diagnosis of type 2 DM, frailty, and HFpEF; patients were excluded if they had experienced a previous stroke, acute myocardial infarction, or cardiac revascularization. As a control population, we enrolled age-matched subjects with no evidence of HFpEF or DM.
The patients fulfilling the above-mentioned eligibility criteria were divided into three interventional groups (empagliflozin: 10 mg; metformin: 500 mg; and insulin: basal-bolus regimen) and followed-up for three months.
All patients underwent clinical evaluation. Blood samples were taken at baseline and follow up. All patients received a transthoracic echocardiography assessment according to the American Society of Echocardiography recommendations (Lang et al., 2015). Every patient (or a legally authorized representative) signed a written informed consent. The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
Frailty Assessment
A physical frailty assessment was performed following previously described criteria (Mone et al., 2022b,d). A diagnosis of frailty was made with at least three of the following five points: 1) weight loss (unintentional loss of ≥4.5 kg in the past year), 2) weakness (handgrip strength in the lowest 20% quintile at baseline, adjusted for sex and body mass index), 3) exhaustion (poor endurance and energy), 4) slowness (walking speed under the lowest quintile adjusted for sex and height), and 5) low physical activity level (lowest quintile of kilocalories of physical activity during the past week).
miR Isolation, Quantification, and Normalization
We extracted miRs using the miRVana miRNA Isolation kit (ThermoFisher) according to the protocol provided by the manufacturer; reverse transcription was performed using the miRCURY LNA Universal RT microRNA PCR kit (Qiagen, Hilden, Germany); miR expression was analyzed by RT-qPCR. We analyzed a panel of miRs that had been previously reported to be involved in the regulation of endothelial dysfunction (Ni et al., 2011; Sabatel et al., 2011; Costa et al., 2013; Zhang et al., 2013; Santulli et al., 2014; Widmer et al., 2014; Kriegel et al., 2015; Ye et al., 2015; Chen et al., 2016; Santulli, 2016; Tang et al., 2017; Cheng et al., 2018; Wei et al., 2018; Gu et al., 2019; Hu and Dong, 2019; Xu et al., 2019; Du et al., 2020; Paterson et al., 2021). The RNA Spike-in kit (Qiagen) was used as an exogenous control of RNA extraction following the manufactureŕs instructions. To control yield, we used two synthetic RNA spike-ins (UniSp2 and UniSp5) in different concentrations; miR-320a and miR-423-5p were identified as the most stable miRs among all groups and were therefore used as endogenous normalizers. Relative gene expression was determined using the 2-ΔΔCT method.
Statistical Analysis
All data were analyzed using the Prism GraphPad software (Dotmatics, Boston, CA). Data are expressed as means ± S.D. or numbers and percentages. The differences in miR levels among groups were analyzed using two-tailed t tests or one-way ANOVA, followed by Bonferroni post hoc correction, as appropriate.
Results
We enrolled 51 frail older adults with HFpEF and DM. Twenty-one patients were excluded because they did not meet the eligibility criteria, refused to give consent, withdrew from the study, or did not have data from blood analyses at baseline or at follow up. Thus, 30 patients, divided into three treatment groups (empagliflozin, metformin, or insulin) successfully completed the 3-month follow up. Baseline characteristics of our population are reported in Table 1, whereas follow up data are in Table 2.
TABLE 1.
Baseline characteristics of the patients Data are means ± S.D. or n (%). “Control” refers to subjects who did not have any evidence of HFpEF or DM.
| Control | Empagliflozin | Metformin | Insulin | |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 |
| Age, y | 79.8 ± 8.9 | 81.6 ± 6.8 | 80.8 ± 6.9 | 81.8 ± 6.5 |
| Female sex, n (%) | 5 (50.0) | 6 (60.0) | 6 (60.0) | 5 (50.0) |
| BMI (kg/m2) | 25.6 ± 1.8 | 27.7 ± 1.4* | 27.6 ± 1.7* | 28.1 ± 1.5* |
| SBP (mmHg) | 118.8 ± 7.8 | 119.4 ± 7.2 | 119.8 ± 7.4 | 120.1 ± 7.3 |
| DBP (mmHg) | 76.3 ± 8.8 | 79.0 ± 7.0 | 79.3 ± 6.8 | 79.2 ± 6.9 |
| Heart rate (bpm) | 78.8 ± 11.1 | 87.3 ± 8.2 | 86.8 ± 8.5 | 87.3 ± 8.6 |
| EF (%) | 65.8 ± 7.3 | 55.4 ± 5.2* | 55.8 ± 5.4* | 55.2 ± 5.1* |
| Comorbidities, n (%) | ||||
| Hypertension | 4 (40.0) | 7 (70.0) | 6 (60.0) | 8 (80.0) |
| Dyslipidemia | 7 (70.0) | 8 (80.0) | 8 (80.0) | 7 (70.0) |
| COPD | 4 (40.0) | 4 (40.0) | 5 (50.0) | 6 (60.0) |
| CKD | 3 (30.0) | 5 (50.0) | 6 (60.0) | 7 (70.0) |
| Laboratory parameters | ||||
| Plasma glucose (mg/dl) | 103.5 ± 30.6 | 161.8 ± 39.1* | 163.7 ± 39.2* | 164.1 ± 39.0* |
| Cholesterol (mg/dl) | 202.9 ± 22.1 | 206.1 ± 20.2 | 205.9 ± 20.1 | 206.0 ± 19.8 |
| LDL-cholesterol (mg/dl) | 133.1 ± 16.1 | 132.3 ± 19.7 | 132.4 ± 19.5 | 132.5 ± 19.8 |
| HDL-cholesterol (mg/dl) | 35.1 ± 3.5 | 37.5 ± 3.4 | 36.9 ± 3.7 | 37.1 ± 3.4 |
| Creatinine (mg/dl) | 0.9 ± 0.3 | 1.2 ± 0.3* | 1.2 ± 0.4* | 1.3 ± 0.3* |
| HbA1c (mmol/mol) | — | 56 ± 6.4 | 55 ± 7.5 | 57 ± 5.3 |
| BNP (pg/ml) | — | 443.8 ± 24.7 | 445.1 ± 24.5 | 446.2 ± 25.0 |
BMI, body mass index; BNP, brain natriuretic peptide; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; EF, ejection fraction; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
*P < 0.05 versus control.
TABLE 2.
Follow up characteristics of the patients 3 months after starting the study Data are means ± S.D. or n (%). “Control” refers to subjects who did not have any evidence of HFpEF or DM.
| Control | Empagliflozin | Metformin | Insulin | |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 |
| BMI (kg/m2) | 25.4 ± 1.7 | 27.1 ± 1.1* | 27.3 ± 1.2* | 28.0 ± 1.3* |
| SBP (mmHg) | 117.9 ± 7.9 | 118.7 ± 6.8 | 118.6 ± 6.9 | 120.0 ± 7.1 |
| DBP (mmHg) | 76.2 ± 8.7 | 78.9 ± 6.4 | 79.0 ± 6.5 | 79.3 ± 6.8 |
| Heart rate (bpm) | 77.6 ± 10.3 | 87.0 ± 7.8* | 86.9 ± 8.1* | 87.2 ± 8.2* |
| EF (%) | 65.6 ± 7.4 | 56.2 ± 5.0* | 55.9 ± 5.2* | 55.1 ± 5.0* |
| Laboratory parameters | ||||
| Plasma glucose (mg/dl) | 100.2 ± 28.8 | 159.8 ± 37.8* | 162.9 ± 38.6* | 163.3 ± 38.8* |
| Cholesterol (mg/dl) | 201.5 ± 22.4 | 205.6 ± 20.0 | 205.5 ± 20.3 | 205.9 ± 19.9 |
| LDL-cholesterol (mg/dl) | 130.1 ± 16.5 | 131.8 ± 19.4 | 132.1 ± 19.3 | 132.3 ± 19.4 |
| HDL-cholesterol (mg/dl) | 36.1 ± 3.6 | 37.2 ± 3.2 | 36.8 ± 3.6 | 37.0 ± 3.3 |
| Creatinine (mg/dl) | 0.9 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| BNP (pg/ml) | — | 439.7 ± 23.8 | 444.5 ± 24.1 | 444.8 ± 24.6 |
BMI, body mass index; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; EF, ejection fraction; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
*P < 0.05 versus control.
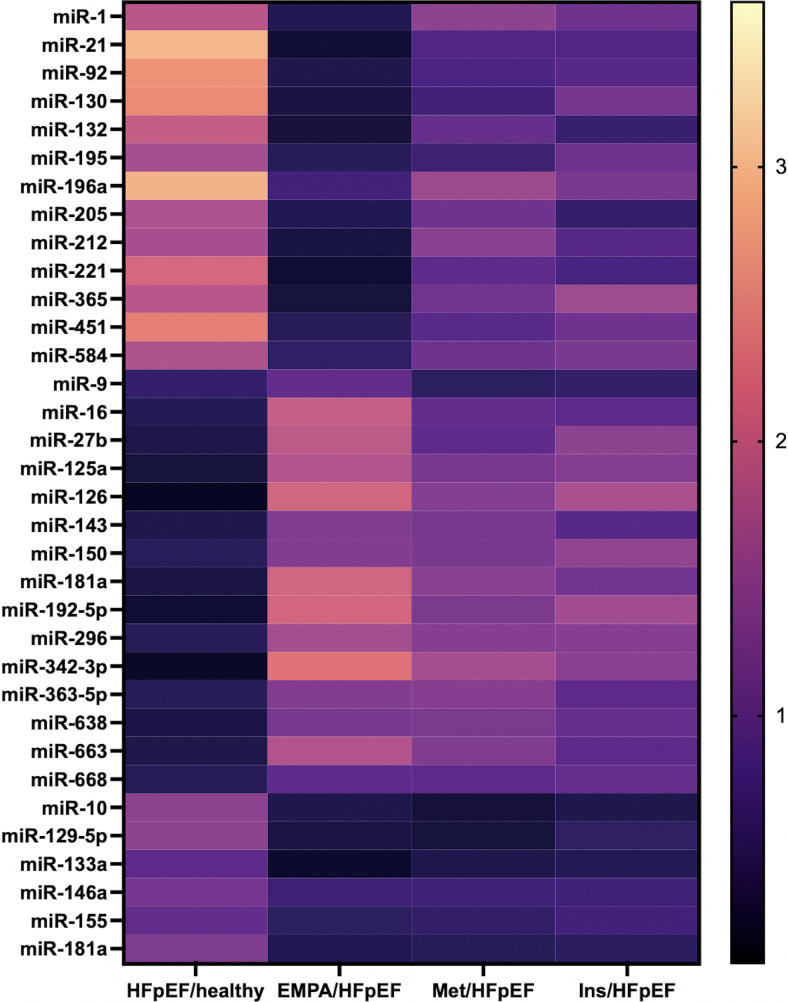
Interestingly, the evaluation of the miR signature of endothelial dysfunction revealed a unique pattern of miRs that were significantly regulated in HFpEF patients compared with healthy controls and in HFpEF patients pre and post treatment with the SGLT2 inhibitor empagliflozin (Fig. 1).
Fig. 1.
Heat-map illustrating the expression of circulating miRs in the indicated groups of patients. HFpEF, heart failure with preserved ejection fraction; Healthy, healthy control subjects; Empa, patients receiving empagliflozin; Met, patients receiving metformin; Ins, patients receiving insulin.
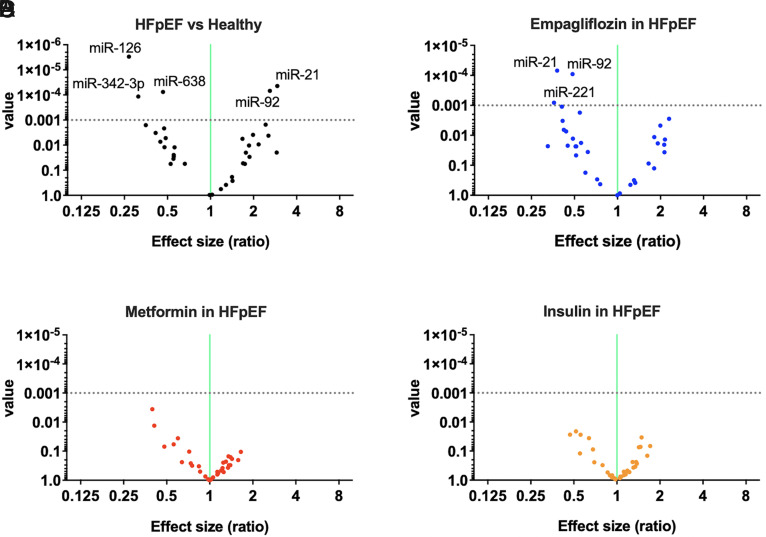
We were able to identify three circulating miRs that were significantly downregulated (miR-126, miR-342-3p, and miR-638) and two that were significantly upregulated (miR-21 and miR-92) in HFpEF patients compared with healthy controls (P < 0.001) (Fig. 2A). Intriguingly, circulating levels of two of these miRs (namely miR-21 and miR-92) were significantly (P < 0.001) reduced in HFpEF patients after the 3-month treatment with empagliflozin (Fig. 2B). Instead, no significant differences in the profile of endothelial miRs were detected in patients treated with metformin (Fig. 2C) or insulin (Fig. 2D).
Fig. 2.
Volcano plots depicting the miR analyses in the different groups. (A) HFpEF versus healthy controls; (B) effects of empagliflozin treatment in HFpEF patients; (C) effects of metformin treatment in HFpEF patients; and (D) effects of insulin Ctreatment in HFpEF patients. The horizontal dotted line represents a P value of 0.001; thus, the points in the plot above that line represent the differently expressed miRs with statistical significance.
Discussion
To the best of our knowledge, this is the first study investigating the effects of SGLT2 inhibitors on circulating miRs, with a significant relevance both in terms of mechanisms of action and clinical practice. Empagliflozin has been shown to have beneficial effects on cardiovascular outcomes, particularly on the rehospitalization rate for HF (Dave et al., 2020). Nevertheless, there are limited reports investigating the functional role of potential biomarkers to monitor the effects of SGLT2 inhibitors. In this sense, miRs have been widely used as biomarkers; however, limited data are available on the miR profile in frailty (Ipson et al., 2018; Carini et al., 2021). Besides, there are no studies investigating miRs in terms of endothelial dysfunction in HFpEF or frailty.
In our study, we identified five miRs as significantly regulated in HFpEF patients versus healthy control subjects, namely miR-21, miR-92 (upregulated), miR-126, miR-342-3p, and miR-638 (downregulated). Our findings are fully in agreement with previous reports. Indeed, miR-21 has been previously linked to inflammaging and age-related diseases: miR-21 has been proposed as a biomarker of systolic heart failure (Ben-Zvi et al., 2020) and its plasma levels have been linked to aging (Olivieri et al., 2012; Rusanova et al., 2019). Additionally, an increased expression of miR-21 in older adults has been shown to diminish the induction of transcription factor networks involved in memory cell generation (Kim et al., 2018).
Equally important, miR-92 is upregulated after vascular injury, both in vitro and in vivo (Deng et al., 2019), has been previously advocated as a biomarker of HF (Napoli et al., 2020), and its inhibition has been shown to have favorable effects in preventing detrimental cardiac remodeling (Bellera et al., 2014). Strikingly, both miRs were downregulated after empagliflozin treatment, strongly suggesting a rescue of endothelial dysfunction in HFpEF patients after a 3-month treatment with this SGLT2 inhibitor.
Consistent with our data, Cheng and collaborators had demonstrated that miR-342-3p is an indispensable modulator of angiogenic activation in endothelial cells, and deregulation of its expression mediates the vascular dysfunction caused by hyperinsulinemia (Cheng et al., 2018). Further studies are needed to determine the exact clinical relevance of miR-638 downregulation in HFpEF, which could also be compensatory, since previous studies, performed in the setting of hepatocellular carcinoma, suggested that this miR is promoting angiogenesis (Cheng et al., 2016; Yokota et al., 2021).
We observed decreased circulating levels of the master regulator of endothelial function, miR-126 (Liu and Olson, 2010; Santulli et al., 2014; Pei et al., 2020), in HFpEF patients, corroborating the view that endothelial dysfunction is playing an instrumental role in HFpEF. Consistently, previous analyses had evidenced lower levels of miR-126 in diabetic patients (Zampetaki et al., 2010).
Another miR that was found to be significantly downregulated after empagliflozin treatment is miR-221, which had been linked to muscle proliferation and sarcopenia both in elderly patients and aged mice (Hamrick et al., 2010; He et al., 2020; Roldan Gallardo and Quintar, 2021); the same miR had been also associated with DM and obesity (Lustig et al., 2014). Notably, we did not find evidence of any significant results in terms of endothelial miR network in patients treated with metformin and insulin.
In line with the present findings, most recently we demonstrated that empagliflozin improves endothelial function by reducing mitochondrial calcium overload and the generation of reactive oxygen species (Mone et al., 2022e) and that SGLT2 inhibition has a beneficial impact on quality of life.
In conclusion, our findings demonstrate for the first time that a specific profile of circulating miRs implied in the regulation of endothelial function are significantly regulated in frail HFpEF patients with DM and in response to empagliflozin treatment.
Acknowledgments
The authors thank Dr. X. Wang for insightful discussion.
Abbreviations
- BMI
body mass index
- BNP
brain natriuretic peptide
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- EF
ejection fraction
- Empa
empagliflozin
- HbA1c
glycated hemoglobin
- HDL
high-density lipoprotein
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- Ins
insulin
- LDL
low-density lipoprotein
- Met
metformin
- miR
miRNA (microRNA)
- SBP
systolic blood pressure
- SGLT2
sodium glucose cotransporter 2
Footnotes
The Santulli’s Laboratory is supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL146691, R01-HL164772, R01-HL159062, and T32-HL144456], National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK123259 and R01-DK033823] (to G.S.), by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. and J.S.S are supported by Postdoctoral Fellowships of the American Heart Association [Grants AHA-22POST995561 and AHA-21POST836407], respectively.
Author Contributions
Participated in research design: Mone, Lombardi, Frullone, Santulli.
Conducted experiments: Mone, Kansakar, Varzideh, Jankauskas, Pansini, De Gennaro, Famiglietti, Macina, Frullone.
Contributed new reagents or analytic tools: Kansakar, Varzideh, Jankauskas, Pansini, Marzocco, De Gennaro, Famiglietti, Macina, Frullone.
Performed data analysis: Mone, Santulli.
Wrote or contributed to the writing of the manuscript: Mone, Lombardi, Santulli.
References
- Alonso-Bouzón C, Carcaillon L, García-García FJ, Amor-Andrés MS, El Assar M, Rodríguez-Mañas L (2014) Association between endothelial dysfunction and frailty: the Toledo Study for Healthy Aging. Age (Dordr) 36:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasekera AT, Chang D, Schwarz P, Tan TC (2021) Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 50:725–732. [DOI] [PubMed] [Google Scholar]
- Anker SDButler JFilippatos GFerreira JPBocchi EBöhm MBrunner-La Rocca HPChoi DJChopra VChuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, Xue QL, Walston JD, Kasper JD (2015) Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 70:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwari T, Joshi A, Mayr M (2016) MicroRNAs in cardiovascular disease. J Am Coll Cardiol 68:2577–2584. [DOI] [PubMed] [Google Scholar]
- Bellera NBarba IRodriguez-Sinovas AFerret EAsín MAGonzalez-Alujas MTPérez-Rodon JEsteves MFonseca CToran N, et al. (2014) Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J Am Heart Assoc 3:e000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi I, Volinsky N, Grosman-Rimon L, Haviv I, Rozen G, Andria N, Asulin N, Margalit N, Marai I, Amir O (2020) Cardiac-peripheral transvenous gradients of microRNA expression in systolic heart failure patients. ESC Heart Fail 7:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti E, Mastrocola R, Vitarelli G, Cutrin JC, Nigro D, Chiazza F, Mayoux E, Collino M, Fantozzi R (2016) Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther 359:45–53. [DOI] [PubMed] [Google Scholar]
- Bielska ANiemira MBauer WSidorkiewicz ISzałkowska ASkwarska ARaczkowska JOstrowski DGugała KDobrzycki S, et al. (2022) Serum miRNA profile in diabetic patients with ischemic heart disease as a promising non-invasive biomarker. Front Endocrinol (Lausanne) 13:888948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet HBogard BHubé FIlieva MUchida SAriza-Mateos MASerganov APardini BNaccarati ASantulli G, et al. (2022) The non-coding RNA journal club: highlights on recent papers-11. Noncoding RNA 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E (2022) Gliflozins in the management of cardiovascular disease. N Engl J Med 386:2024–2034. [DOI] [PubMed] [Google Scholar]
- Bu Z, Huang A, Xue M, Li Q, Bai Y, Xu G (2021) Cognitive frailty as a predictor of adverse outcomes among older adults: a systematic review and meta-analysis. Brain Behav 11:e01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini G, Musazzi L, Bolzetta F, Cester A, Fiorentini C, Ieraci A, Maggi S, Popoli M, Veronese N, Barbon A (2021) The potential role of miRNAs in cognitive frailty. Front Aging Neurosci 13:763110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chen L, He H, Huang W, Zhang R, Li P, Meng Y, Jiang X (2016) Up-regulation of microRNA-16 in glioblastoma inhibits the function of endothelial cells and tumor angiogenesis by targeting Bmi-1. Anticancer Agents Med Chem 16:609–620. [DOI] [PubMed] [Google Scholar]
- Chen L, Sun H, Wang C, Yang Y, Zhang M, Wong G (2018) miRNA arm switching identifies novel tumour biomarkers. EBioMedicine 38:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Chen Y, Zhao P, Liu X, Dong J, Li J, Huang C, Wu R, Lv Y (2016) Downregulation of miRNA-638 promotes angiogenesis and growth of hepatocellular carcinoma by targeting VEGF. Oncotarget 7:30702–30711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Cui Y, Fan L, Mu X, Hua Y (2018) T2DM inhibition of endothelial miR-342-3p facilitates angiogenic dysfunction via repression of FGF11 signaling. Biochem Biophys Res Commun 503:71–78. [DOI] [PubMed] [Google Scholar]
- Chioncel OLainscak MSeferovic PMAnker SDCrespo-Leiro MGHarjola VPParissis JLaroche CPiepoli MFFonseca C, et al. (2017) Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 19:1574–1585. [DOI] [PubMed] [Google Scholar]
- Costa A, Afonso J, Osório C, Gomes AL, Caiado F, Valente J, Aguiar SI, Pinto F, Ramirez M, Dias S (2013) miR-363-5p regulates endothelial cell properties and their communication with hematopoietic precursor cells. J Hematol Oncol 6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers EE, Tijsen AJ, Pinto YM (2012) Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 110:483–495. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. Lancet 393:2636–2646. [DOI] [PubMed] [Google Scholar]
- Dave CV, Schneeweiss S, Wexler DJ, Brill G, Patorno E (2020) Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013-2018. Diabetes Care 43:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Zhang Y, Wang Y, Lu X, Jiang Q (2019) MicroRNA-92 regulates vascular smooth muscle cell function by targeting KLF4 during vascular restenosis and injury. Int J Clin Exp Pathol 12:4253–4262. [PMC free article] [PubMed] [Google Scholar]
- Du X, Hu N, Yu H, Hong L, Ran F, Huang D, Zhou M, Li C, Li X (2020) miR-150 regulates endothelial progenitor cell differentiation via Akt and promotes thrombus resolution. Stem Cell Res Ther 11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca A, Ramalhete SV, Mestre A, Pires das Neves R, Marreiros A, Castelo-Branco P, Roberto VP (2021) Identification of colorectal cancer associated biomarkers: an integrated analysis of miRNA expression. Aging (Albany NY) 13:21991–22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Coppola A, Izzo R, Fiorentino G, Trimarco B, Santulli G (2021) Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit Care 25:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Fiordelisi A, Sorriento D, Cerasuolo F, Buonaiuto A, Avvisato R, Pisani A, Varzideh F, Riccio E, Santulli G, et al. (2022a) Mitochondrial microRNAs are dysregulated in patients with Fabry disease. J Pharmacol Exp Ther DOI: 10.1124/jpet.122.001250 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella JKansakar USardu CMessina VJankauskas SMarfella RMaggi PWang XMone PPaolisso G, et al. (2022b) Exosomal miR-145 and miR-885 Regulate Thrombosis in COVID-19, JPET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J, Dalgaard LT (2017) Interplay of mitochondrial metabolism and microRNAs. Cell Mol Life Sci 74:631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert AB, Boen JRA, Segers VF, Van Craenenbroeck EM (2019) Heart failure With preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Front Physiol 10:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamouzis G, Schelbert EB, Butler J (2016) Growing evidence linking microvascular dysfunction with heart failure with preserved ejection fraction. J Am Heart Assoc 5:e003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Wang XQ, Lin MJ, Liang H, Fan SY, Wang L, Yan X, Liu W, Shen FX (2019) Molecular interplay between microRNA-130a and PTEN in palmitic acid-mediated impaired function of endothelial progenitor cells: effects of metformin. Int J Mol Med 43:2187–2198. [DOI] [PubMed] [Google Scholar]
- Hadi HA, Suwaidi JA (2007) Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3:853–876. [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, Zhou L, Isales CM, Mi QS (2010) The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun 400:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Zhang YL, Zhang Y, Feng B, Zheng Z, Wang D, Zhang S, Guo Q, Ye H (2020) Circulating microRNAs in plasma decrease in response to sarcopenia in the elderly. Front Genet 11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Dong ZL (2019) MicroRNA-212 promotes the recovery function and vascular regeneration of endothelial progenitor cells in mice with ischemic stroke through inactivation of the notch signaling pathway via downregulating MMP9 expression. J Cell Physiol 234:7090–7103. [DOI] [PubMed] [Google Scholar]
- Hu L, Wei S, Wu Y, Li S, Zhu P, Wang X (2021) MicroRNA regulation of the proliferation and apoptosis of Leydig cells in diabetes. Mol Med 27:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Ju F, Du L, Liu T, Zuo Y, Abbott GW, Hu Z (2022) Empagliflozin protects against pulmonary ischemia/reperfusion injury via an extracellular signal-regulated kinases 1 and 2-dependent mechanism. J Pharmacol Exp Ther 380:230–241. [DOI] [PubMed] [Google Scholar]
- Ipson BR, Fletcher MB, Espinoza SE, Fisher AL (2018) Identifying exosome-derived microRNAs as candidate biomarkers of frailty. J Frailty Aging 7:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A, Gambardella J, Santulli G (2021) Heart failure in diabetes. Metabolism 125:154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JOmar MKistorp CTuxen CGustafsson IKøber LGustafsson FFaber JMalik MEFosbøl EL, et al. (2021) Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 9:106–116. [DOI] [PubMed] [Google Scholar]
- Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G (2022) Functional role of microRNAs in regulating cardiomyocyte death. Cells 11:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannopoulos A, Esguerra JLS, Pedersen MG, Wendt A, Prasad RB, Eliasson L (2022) Human pancreatic islet miRNA-mRNA networks of altered miRNAs due to glycemic status. iScience 25:103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki HTakeuchi TRicciardiello FLombardi ABiganzoli EFornili MDe Bortoli DMesolella MCossu AMScrima M, et al. (2020) Definition of miRNA signatures of nodal metastasis in LCa: miR-449a targets notch genes and suppresses cell migration and invasion. Mol Ther Nucleic Acids 20:711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Hu B, Jadhav RR, Jin J, Zhang H, Cavanagh MM, Akondy RS, Ahmed R, Weyand CM, Goronzy JJ (2018) Activation of miR-21-regulated pathways in immune aging selects against signatures characteristic of memory T cells. Cell Rep 25:2148–2162.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M, Tu X, Wu R (2019) Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin 40:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel AJ, Baker MA, Liu Y, Liu P, Cowley AW Jr, Liang M (2015) Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 66:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RMBadano LPMor-Avi VAfilalo JArmstrong AErnande LFlachskampf FAFoster EGoldstein SAKuznetsova T, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- Lejeune S, Roy C, Slimani A, Pasquet A, Vancraeynest D, Vanoverschelde JL, Gerber BL, Beauloye C, Pouleur AC (2021) Diabetic phenotype and prognosis of patients with heart failure and preserved ejection fraction in a real life cohort. Cardiovasc Diabetol 20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DLiu YHidru THYang XWang YChen CLi KHCTang YWei YTse G, et al. (2021) Protective effects of sodium-glucose transporter 2 inhibitors on atrial fibrillation and Atrial flutter: a systematic review and meta- analysis of randomized placebo-controlled trials. Front Endocrinol (Lausanne) 12:619586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Olson EN (2010) MicroRNA regulatory networks in cardiovascular development. Dev Cell 18:510–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y, Barhod E, Ashwal-Fluss R, Gordin R, Shomron N, Baruch-Umansky K, Hemi R, Karasik A, Kanety H (2014) RNA-binding protein PTB and microRNA-221 coregulate AdipoR1 translation and adiponectin signaling. Diabetes 63:433–445. [DOI] [PubMed] [Google Scholar]
- Mansur HN, Lovisi JC, Colugnati FA, Raposo NR, Fernandes NM, Bastos MG (2015) Association of frailty with endothelial dysfunction and its possible impact on negative outcomes in Brazilian predialysis patients with chronic kidney disease. BMC Nephrol 16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro M, Berretta M, Palermo G, Cavalieri V, La Rocca G (2022) The multiplicity of Argonaute complexes in mammalian cells. J Pharmacol Exp Ther DOI: 10.1124/jpet.122.001158 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, Yancy CW, Green JB, Altman N, Hernandez AF (2019) Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol 73:602–611. [DOI] [PubMed] [Google Scholar]
- Mirzaei RBabakhani SAjorloo PAhmadi RHHosseini-Fard SRKeyvani HAhmadyousefi YTeimoori AZamani FKarampoor S, et al. (2021) The emerging role of exosomal miRNAs as a diagnostic and therapeutic biomarker in Mycobacterium tuberculosis infection. Mol Med 27:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisoiu TDragomir MPIancu SDSchallenberg SBirolo GFerrero GBurghelea DStefancu ACozan RGLicarete E, et al. (2022) Combined miRNA and SERS urine liquid biopsy for the point-of-care diagnosis and molecular stratification of bladder cancer. Mol Med 28:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, de Donato JA, Varzideh F, Kansakar U, Jankauskas SS, Pansini A, Santulli G (2022f) Functional role of miR-34a in diabetes and frailty. Front Aging 3:949924 DOI: 10.3389/fragi.2022.949924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone PGambardella JLombardi APansini ADe Gennaro SLeo ALFamiglietti MMarro AMorgante MFrullone S, et al. (2022a) Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc Diabetol 21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Gambardella J, Pansini A, de Donato A, Martinelli G, Boccalone E, Matarese A, Frullone S, Santulli G (2021a) Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells 10:2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Gambardella J, Pansini A, Martinelli G, Minicucci F, Mauro C, Santulli G (2022b) Cognitive dysfunction correlates with physical impairment in frail patients with acute myocardial infarction. Aging Clin Exp Res 34:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Gambardella J, Wang X, Jankauskas SS, Matarese A, Santulli G (2021b) miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding RNA 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, Frullone S, Santulli G (2022c) Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care 45:1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Pansini A, Frullone S, de Donato A, Buonincontri V, De Blasiis P, Marro A, Morgante M, De Luca A, Santulli G (2022d) Physical decline and cognitive impairment in frail hypertensive elders during COVID-19. Eur J Intern Med 99:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, Santulli G (2022e) SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients. Hypertension 79:1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli MB, Shu J, Sardu C, Matarese A, Santulli G (2019) Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int J Mol Sci 21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Benincasa G, Donatelli F, Ambrosio G (2020) Precision medicine in distinct heart failure phenotypes: focus on clinical epigenetics. Am Heart J 224:113–128. [DOI] [PubMed] [Google Scholar]
- Ni CW, Qiu H, Jo H (2011) MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol 300:H1762–H1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri FSpazzafumo LSantini GLazzarini RAlbertini MCRippo MRGaleazzi RAbbatecola AMMarcheselli FMonti D, et al. (2012) Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech Ageing Dev 133:675–685. [DOI] [PubMed] [Google Scholar]
- Oshima HMiki TKuno AMizuno MSato TTanno MYano TNakata KKimura YAbe K, et al. (2019) Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther 368:524–534. [DOI] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259. [DOI] [PubMed] [Google Scholar]
- Paolisso PBergamaschi LSantulli GGallinoro ECesaro AGragnano FSardu CMileva NFoà AArmillotta M, et al. (2022) Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol 21:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson MRJackson KLDona MSIFarrugia GEVisniauskas BWatson AMDJohnson CPrieto MCEvans RGCharchar FJ, et al. (2021) Deficiency of microRNA-181a results in transcriptome-wide cell-specific changes in the kidney and increases blood pressure. Hypertension 78:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271. [DOI] [PubMed] [Google Scholar]
- Pei CZ, Liu B, Li YT, Fang L, Zhang Y, Li YG, Meng S (2020) MicroRNA-126 protects against vascular injury by promoting homing and maintaining stemness of late outgrowth endothelial progenitor cells. Stem Cell Res Ther 11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A (2018) Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: is low-grade inflammation the neglected component? Diabetes Obes Metab 20:2515–2522. [DOI] [PubMed] [Google Scholar]
- Premer C, Kanelidis AJ, Hare JM, Schulman IH (2019) Rethinking endothelial dysfunction as a crucial target in fighting heart failure. Mayo Clin Proc Innov Qual Outcomes 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Zhang GF, Chai YN, Han XY, Zheng HT, Li XF, Duan F, Chen LY (2022) Ligustrazine attenuates liver fibrosis by targeting miR-145 mediated TGF-beta/Smad signaling in an animal model of biliary atresia. J Pharmacol Exp Ther 381:257–265. [DOI] [PubMed] [Google Scholar]
- Quinn SR, O’Neill LA (2011) A trio of microRNAs that control toll-like receptor signalling. Int Immunol 23:421–425. [DOI] [PubMed] [Google Scholar]
- Roldán Gallardo FF, Quintar AA (2021) The pathological growth of the prostate gland in atherogenic contexts. Exp Gerontol 148:111304. [DOI] [PubMed] [Google Scholar]
- Rusanova I, Fernández-Martínez J, Fernández-Ortiz M, Aranda-Martínez P, Escames G, García-García FJ, Mañas L, Acuña-Castroviejo D (2019) Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp Gerontol 124:110637. [DOI] [PubMed] [Google Scholar]
- Sabatel CMalvaux LBovy NDeroanne CLambert VGonzalez MLColige ARakic JMNoël AMartial JA, et al. (2011) MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One 6:e16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G (2015) microRNAs distinctively regulate vascular smooth muscle and endothelial cells: functional implications in angiogenesis, atherosclerosis, and in-stent restenosis. Adv Exp Med Biol 887:53–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G (2016) MicroRNAs and endothelial (Dys) function. J Cell Physiol 231:1638–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli GWronska AUryu KDiacovo TGGao MMarx SOKitajewski JChilton JMAkat KMTuschl T, et al. (2014) A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest 124:4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu CMassetti MTesta NMartino LDCastellano GTurriziani FSasso FCTorella MDe Feo MSantulli G, et al. (2021) Effects of sodium-glucose transporter 2 inhibitors (SGLT2-I) in patients with ischemic heart disease (IHD) treated by coronary artery bypass grafting via MiECC: inflammatory burden, and clinical outcomes at 5 years of follow-up. Front Pharmacol 12:777083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavast CJ, Erkeland SJ (2019) The non-canonical aspects of microRNAs: many roads to gene regulation. Cells 8:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC; Get With the Guidelines Scientific Advisory Committee and Investigators (2012) Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126:65–75. [DOI] [PubMed] [Google Scholar]
- Tang F, Yang TL, Zhang Z, Li XG, Zhong QQ, Zhao TT, Gong L (2017) MicroRNA-21 suppresses ox-LDL-induced human aortic endothelial cells injuries in atherosclerosis through enhancement of autophagic flux: involvement in promotion of lysosomal function. Exp Cell Res 359:374–383. [DOI] [PubMed] [Google Scholar]
- Traber GM, Yu AM (2022) RNAi based therapeutics and novel RNA bioengineering technologies. J Pharmacol Exp Ther DOI: 10.1124/jpet.122.001234 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varzideh F, Kansakar U, Donkor K, Wilson S, Jankauskas SS, Mone P, Wang X, Lombardi A, Santulli G (2022) Cardiac remodeling after myocardial infarction: functional contribution of microRNAs to inflammation and fibrosis. Front Cardiovasc Med 9:863238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varzideh F, Kansakar U, Santulli G (2021) SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother 7:e67–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SMazer CDYan ATMason TGarg VTeoh HZuo FQuan AFarkouh MEFitchett DH, et al. (2019) Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 140:1693–1702. [DOI] [PubMed] [Google Scholar]
- Wan N, Rahman A, Hitomi H, Nishiyama A (2018) The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol (Lausanne) 9:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Morelli MB, Matarese A, Sardu C, Santulli G (2020) Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail 7:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei QSun HSong SLiu YLiu PLivingston MJWang JLiang MMi QSHuo Y, et al. (2018) MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J Clin Invest 128:5448–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer RJ, Chung WY, Herrmann J, Jordan KL, Lerman LO, Lerman A (2014) The association between circulating microRNA levels and coronary endothelial function. PLoS One 9:e109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WKM, Sørensen AE, Joglekar MV, Hardikar AA, Dalgaard LT (2018) Non-coding RNA in pancreas and β-cell development. Noncoding RNA 4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wronska A, Kurkowska-Jastrzebska I, Santulli G (2015) Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 213:60–83. [DOI] [PubMed] [Google Scholar]
- Xu M, Duan Y, Xiao J (2019) Exercise improves the function of endothelial cells by microRNA. J Cardiovasc Transl Res 12:391–393. [DOI] [PubMed] [Google Scholar]
- Yaylim İ, Farooqi AA, Telkoparan-Akillilar P, Saso L (2022) Interplay between non-coding RNAs and NRF2 in different cancers: spotlight on miRNAs and long non-coding RNAs. J Pharmacol Exp Ther DOI: 10.1124/jpet.121.000921 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ye M, Li D, Yang J, Xie J, Yu F, Ma Y, Zhu X, Zhao J, Lv Z (2015) MicroRNA-130a targets MAP3K12 to modulate diabetic endothelial progenitor cell function. Cell Physiol Biochem 36:712–726. [DOI] [PubMed] [Google Scholar]
- Yokota YNoda TOkumura YKobayashi SIwagami YYamada DTomimaru YAkita HGotoh KTakeda Y, et al. (2021) Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci 112:1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki AKiechl SDrozdov IWilleit PMayr UProkopi MMayr AWeger SOberhollenzer FBonora E, et al. (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817. [DOI] [PubMed] [Google Scholar]
- Zarone MRMisso GGrimaldi AZappavigna SRusso MAmler EDi Martino MTAmodio NTagliaferri PTassone P, et al. (2017) Evidence of novel miR-34a-based therapeutic approaches for multiple myeloma treatment. Sci Rep 7:17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng QQi XMa JHu FWang XQin HLi MHuang SYang YLi Y, et al. (2022) Distinct miRNAs associated with various clinical presentations of SARS-CoV-2 infection. iScience 25:104309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Luo X, Meng H, Kang J, Qin G, Chen Y, Zhang X (2021) Sodium glucose cotransporter 2 inhibitors reduce the risk of heart failure hospitalization in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 11:604250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mao H, Chen JY, Wen S, Li D, Ye M, Lv Z (2013) Increased expression of microRNA-221 inhibits PAK1 in endothelial progenitor cells and impairs its function via c-Raf/MEK/ERK pathway. Biochem Biophys Res Commun 431:404–408. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Zhang H, Wang X (2022) Efficacy and safety of empagliflozin on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 13:836455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman BWanner CLachin JMFitchett DBluhmki EHantel SMattheus MDevins TJohansen OEWoerle HJ, et al. ; EMPA-REG OUTCOME Investigators (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128. [DOI] [PubMed] [Google Scholar]