Abstract
Background
Ischemic stroke is a leading cause of morbidity and mortality worldwide. One possible predictor is the use of biomarkers especially neurofilament light chain (NFL).
Objectives
To explore whether NFL could predict clinical outcome and hemorrhagic transformation in moderate to severe stroke.
Design
Single center prospective cohort study.
Methods
Fifty-one moderate to severe ischemic stroke patients were recruited. Blood NFL was obtained from patients at admission (First sample) and 24-96 hours later (Second sample). NFL was analyzed with the ultrasensitive single molecule array (Simoa). Later, we calculated incremental rate NFL (IRN) by changes in NFL per day from baseline. We evaluated National Institute of Health stroke scale (NIHSS), modified Rankins score (mRs), and the presence of hemorrhagic transformation (HT).
Results
IRN was found to be higher in patients with unfavorable outcome (7.12 vs 24.07, P = .04) as well as Second sample (49.06 vs 71.41, P = .011), while NFL First sample was not significant. IRN had a great correlation with mRS (r = .552, P < .001). Univariate logistic regression model showed OR of IRN and Second sample to be 1.081 (95% CI 1.016-1.149, P = .013) and 1.019 (1.002-1.037, P = .03), respectively. Multiple logistic regression model has shown to be significant. In receiver operating analysis, IRN, Second sample, combined IRN with NIHSS and combined Second sample with NIHSS showed AUC (.744, P = .004; 0.713, P = .01; 0.805, P < .001; 0.803, P < .001, respectively). For HT, First sample and Second sample had significant difference with HT (Z = 2.13, P = .033; Z = 2.487, P = .013, respectively).
Conclusion
NFL was found to correlate and predict clinical outcome. In addition, it was found to correlate with HT.
Keywords: Neurofilament light, simoa, ischemic stroke, hemorrhagic transformation, modified rankin scale
Introduction
Ischemic stroke remains one of the most prevalent neurological disorders and the second most common cause of mortality worldwide.1 Each year, 5.5 million deaths are reported globally. However, global age-standardized disability-adjusted life-years (DALYs) has decreased dramatically since 1990 due to scientific progress. The key successive factors include early detection by advanced imaging, early acute management strategies with alteplase and mechanical thrombectomy, and sustainable rehabilitation and prevention programs. Subsequently, future approaches include the development of a prediction model and the prevention of complications like hemorrhagic transformation.2 In recent years, biomarkers which represent neuronal loss, especially neurofilament, have emerged as potential predictors.3 The underlying value of using biomarkers is that they are less time-consuming and easier to obtain.4
Neurofilament is a scaffold protein situated within the cytoplasm of neurons and large axons. It is released upon neuronal tissue damage into the cerebral spinal fluid (CSF) and the bloodstream.5-7 Interestingly, it has been found to be highly specific to the nervous system in that other tissues do not contain this protein.8 However, it is not specific to any particular neurological disease. Neurofilament light chain (NFL) is a subunit of neurofilament. Certain neurological conditions with tissue damage, such as traumatic brain injuries, neurodegenerative diseases, multiple sclerosis, and stroke show abnormal concentration of NFL. Its level is also believed to be a direct marker of the severity of a given neurological disease.
For ischemic stroke, limited studies have been conducted regarding NFL. Most of the studies have been predicting clinical outcomes. One study using serum from the DAMDAS and CIRCULAR studies found that NFL level could predict recurrent ischemic stroke and prognosis at 3 months.9 Similar results have been found in a German and a Danish study.10 In contrast, a Finnish study reveals that NFL was only associated with infarct volume on brain imaging but not with prognosis.11 Another controversy in these studies is that the mean National Institutes of Health Stroke Scale (NIHSS) score at admission was low. In a previous study, a stroke with an NIHSS score of no more than 4 has a favorable outcome by its own means.12
Apart from prognosis, certain areas of complications regarding ischemic stroke have not been studied. Hemorrhagic transformation (HT) is a devastating consequence following an ischemic stroke. It has been known that HT follows the disruption of the blood-brain barrier (BBB).13 We hypothesize that BBB disruption should prompt a higher level of NFL excreted in the bloodstream. Hence, it could be a marker of HT.
The aim of the study is to analyze the association between NFL and the prognosis of moderate to severe strokes and to predict the occurrence of HT.
Method
Patients
This study is a single-center prospective observation study. Starting in April 2021, we invited patients admitted to the stroke intensive care unit of King Chulalongkorn Memorial Hospital, Thailand, to participate in this study. The inclusion criteria included those over the age of 18 years old with the NIHSS score at admission over 4. We excluded those who were pregnant and/or diagnosed with neurological disorders, including neurodegenerative disease, multiple sclerosis, amyotrophic lateral sclerosis, and stroke of any etiology within 1 year. This study is reported according to the STROBE check list for cohort studies. A consort diagram is illustrated in Supplementary Figure A. The medical history, neurological examination, NIHSS assessments, and risk factor history of all patients included in this study were analyzed thoroughly. NIHSS was reported as a score or stratified where applicable.14 Computed tomography (CT) scans were performed on each patient at admission, before discharge and when indicated. Intravenous recombinant tissue plasminogen activator (rtPA) and mechanical thrombectomy were given to all eligible patients without delay. All participants received an extensive search for stroke etiology (CT scan of the cerebral and neck vessels, magnetic resonance imaging when CT scan is controversial, echocardiogram, holter monitoring, blood samples including thrombophilia and stroke mimics). Embolic stroke of undetermined source (ESUS) is defined as a stroke that is not lacunar, no risk of embolism, absence of atherosclerosis causing >50% luminal narrowing of intracranial or extracranial vessels and no other specific cause is found. Stroke etiology was determined by a stroke specialist. Patients were given secondary prevention according to their etiology. Follow-up after discharge was at 2 weeks, 1 month, and 3 months. Modified Rankin Score (mRS) was assessed at discharge and follow-up at 3 months. The presence of hemorrhagic transformation and its subtypes were interpreted by radiologists.
Neurofilament collection and analysis
Blood samples were collected within 24 hours after admission to the hospital (First sample) and 1-4 days after the first blood collection (Second sample). Samples were collected using a tourniquet, 21-gauge needles, and universal precaution procedures. After placement into the ethylenediaminetetraacetic acid (EDTA) tube, they were immediately transferred to centrifugation at 2000 g for 10 minutes. Plasma samples were kept at −80°C. For analysis, samples were captured using the single molecule array platform (Simoa; Quanterix, Lexingtion, MA, USA) in capturing monoclonal antibody 47:3. The analytical sensitivity was 0.32 pg/mL. We reported NFL in pg/dL for both First sample and Second sample. Incremental rate of NFL (pg/dL x day), i.e., the increase in NFL levels divided by numbers of days apart, was determined for each subject. We used this value because changes in NFL were better predictors than a single measurement.15 All biochemical analysis were performed by a trained laboratory technician who was blinded to clinical data.
Variable outcomes
mRS at 3 months was used as a primary end point for functional outcome. A favorable functional outcome was defined as mRS ≤2, while mRS >2 was defined as an unfavorable outcome. Secondary outcomes included hemorrhagic transformation, which could be classified into 4 groups (HI-1, HI-2, PH-1, and PH-2) depending on the size of the hemorrhage.
Statistical analysis
Continuous baseline data were presented as mean (+/− SD) and median (interquartile range, IQR). Absolute count and percentages were used to present baseline categorical data. Normality was tested using the Shapiro-Wilk test. Mann-Whitey U test was used in the non-normal distribution variable and the Student T-test was used in the normal distribution variable. Chi-square test was used to compare nominal variable. The Spearman’s rank coefficient was used to assess the association between non-normally continuous distributed variables. Kruskal-Wallis Test was used to evaluate association between non-parametric continuous data and categorical data. A logistic regression model was used to predict the clinical outcome. A receiver operating characteristic (ROC) curve was established and calculated on the basis of prognosis of functional outcome and estimation of HT. Sample size estimation was calculated based on the power analysis. Our study had a power of over 80%. Statistical significance was defined as a P value of <.05. All analysis was done using IBM SPSS version 28 for Windows.
Ethics statement
In accordance with the Declaration of Helsinki and the International Conference on Harmonization in Good Clinical Practice, this study was approved by the institutional review board of the Faculty of Medicine, Chulalongkorn University on March 3, 2021 (COA 316/2021, IRB 917/63). Written informed consent was obtained from all participants or representatives.
Results
The clinical baseline characteristics of all groups of patients were presented in Supplementary Table A. In our cohort study, 51 patients were included. All patients completed follow-up at 3 months. The median age was 71 years (IQR, 59-82), 27 were male (52.9%), and the median NIHSS score at admission was 12 (IQR, 6-16). The median time interval from the first blood draw to the second (Second sample) was 2 days (IQR, 2.0-3.0). Clinical characteristics were stratified according to functional outcomes presented in Table 1.
Table 1.
Characteristics of individuals with regards to clinical outcomes.
| MRS ≤ 2 | MRS > 2 | P Value | |
|---|---|---|---|
| N | 20 | 31 | |
| Age (median; IQR) | 66 (55, 79) | 72 (62, 83) | P = .120 |
| Gender (Male) | 9 (45%) | 18 (58.1%) | P = .366 |
| NIHSS admission (median; IQR) | 6 (6, 12) | 13 (6, 27) | P < .001 |
| NIHSS discharge (median; IQR) | 2 (1,4) | 13 (4, 28) | P < .001 |
| NIHSS 3 months (median; IQR) | 1 (0, 2) | 9 (4,28) | P < .001 |
| MRS at discharge (median; IQR) | 2 (1, 2) | 4 (3,5) | P < .001 |
| Cardiovascular risk factors | |||
| Diabetes mellitus (%) | 4 (20%) | 12 (38.7%) | P = .164 |
| Hypertension (%) | 13 (65%) | 28 (90.3%) | P = .028 |
| Dyslipidemia (%) | 11 (55%) | 23 (74.2%) | P = .160 |
| Atrial fibrillation (%) | 8 (40%) | 15 (48.4%) | P = .561 |
| Smoking (%) | 6 (30%) | 6 (19.4%) | P = .386 |
| Chronic kidney disease (%) | 0 | 2 (6.5%) | P = .251 |
| Illicit drug use (%) | 1 (5%) | 1 (3.2) | P = .752 |
| Ischemic heart disease (%) | 3 (15%) | 5 (16.1%) | P = .915 |
| Intervention | |||
| Recombinant tissue plasminogen activator (%) | 7 (35%) | 6 (19.4%) | P = .215 |
| Mechanical thrombectomy (%) | 5 (25%) | 7 (22.6%) | P = .844 |
| Stroke subtype | |||
| Large arterial occlusion (%) | 5 (26.3%) | 10 (32.3%) | P = .582 |
| Cardioembolic (%) | 7 (35%) | 16 (51.6%) | P = .249 |
| Small vessel disease (%) | 8 (40%) | 2 (6.5%) | P = .04 |
| Other determined (%) | 0 | 1 (3.2%) | P = .422 |
| ESUS (%) | 0 | 1 (3.2%) | P = .422 |
| Hemorrhagic transformation | |||
| All subtypes (%) | 4 (20%) | 8 (31.5%) | P = .637 |
| Symptomatic HT | 1 (5%) | 7 (22.6%) | P = .07 |
| Hemorrhagic infarction 1 (%) | 1 (5%) | 1 (3.2%) | P = .752 |
| Hemorrhagic infarction 2 (%) | 1 (5%) | 1 (3.2%) | P = .752 |
| Petechial hemorrhage 1 (%) | 1 (5%) | 4 (12.9%) | P = .359 |
| Petechial hemorrhage 2 (%) | 1 (5%) | 2 (6.5%) | P = .831 |
| NFL value pg/Ml | |||
| NFL first sample pg/mL (median; IQR) | 21.39 (9.28, 44.42) | 26.69 (15.10, 64.39) | P = .177 |
| NFL second sample pg/mL (median; IQR) | 49.06 (25.22, 65.16) | 71.41 (47.58, 153.50) | P = .011 |
| Incremental rate NFL pg/dL × day (median; IQR) | 7.12 (1.09,11.93) | 24.07 (6.27, 40.30) | P = .004 |
Abbreviation: IQR, interquartile range; NFL, plasma neurofilament light, NIHSS, National Institutes of Health Stroke Scale; mRS, modified rankin score; ESUS, embolic stroke of undetermined source.
After univariate analysis, patients with good clinical outcomes (mRS ≤ 2) at 3 months had lower NIHSS scores at admission (6 vs 13, P < .001), NIHSS at discharge (2 vs 13, P < .001), and mRS at discharge (2 vs 4, P < .001). Regarding cardiovascular risk factors, patients with a history of hypertension (65% vs 90%, P = .028) were more prone to poor outcomes. Other cardiovascular risks were not statistically significant. Interventions were not different between the two groups. Small vessel stroke subtypes were associated with favorable outcomes (40% vs 6.5%, P = .04). Hemorrhagic transformation was also not different in either group. Patients with good outcome had lower NFL on Second sample (49.06 vs 71.41, P = .011), while NFL on First sample was not different (21.39 vs 26.69, P = .177). Interestingly, incremental rate NFL showed a statistical discretion between the two groups (7.12 vs 24.07, P = .004).
We evaluated the association between factors and mRS at 3 months (Table 2, Supplementary Table B). We found that the incremental rate of NFL had a relatively strong correlation (r = .552, P < .001). NFL on Second sample also had weaker correlation with an unfavorable outcome (rp = .360, P = .009). Other factors that were correlated with poor outcomes included NIHSS at admission (rp = .505, P < .001), NIHSS at discharge (rp = .769, P < .001) and mRS at discharge (rp = .818, P < .001).
Table 2.
Clinical characteristic correlation with mRS at 3 months.
| Correlation Efficient | P Value | |
|---|---|---|
| Age | .311 | .026 |
| NIHSS admission | .505 | <.001 |
| NIHSS discharge | .769 | <.001 |
| mRS discharge | .818 | <.001 |
| NFL first sample | .191 | .179 |
| NFL second sample | .360 | .009 |
| Incremental rate NFL | .552 | <.001 |
Abbreviation: NFL, plasma neurofilament light, NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin score.
A logistic regression model was implemented for the prediction of favorable outcomes (Table 3, Supplementary Table C). In the unadjusted model, incremental rate of NFL was indicated to be a good predictive factor (OR 1.081, 95%CI 1.016-1.149), while plasma NFL at First sample was not statistically significant. NFL at Second sample was a good predictor of clinical outcome (OR 1.019, 95% CI 1.002-1.037). NIHSS at admission was also a good predictor of clinical outcome (OR 1.296, 95% CI 1.102-1.524). After multiple logistic regression model, NFL Second sample and incremental rate of NFL remained good predictors (OR 1.026, 95% CI 1.002-1.051; OR 1.094, 95% CI 1.020-1.174, respectively). Factors involved in the adjustment model include age, sex, stratified NIHSS, smoking, HT, and dyslipidemia.
Table 3.
Unadjusted and adjusted logistic regression model.
| Unadjusted Logistic Regression Model | ||
|---|---|---|
| Odd Ratio (95% CI) | P Value | |
| NFL first sample | 1.012 (.995-1.029) | .159 |
| NFL second sample | 1.019 (1.002-1.037) | .03 |
| Incremental rate NFL | 1.081 (1.016-1.149) | .013 |
| Age | 1.032 (.988-1.077) | .156 |
| Sex | .591 (.190-1.836) | .363 |
| Hypertension | 5.026 (1.117-22.613) | .35 |
| Dyslipidemia | 2.352 (.713-7.755) | .16 |
| Atrial fibrillation | 1.406 (.45-4.391) | .557 |
| Recombinant tissue plasminogen activator | .446 (.124-1.603) | .216 |
| Mechanical thrombectomy | .842 (.235-3.264) | .842 |
| NIHSS admission | 1.296 (1.102-1.524) | .002 |
| Adjusted logistic regression model | ||
| NFL first sample | 1.012 (.989-1.035) | .301 |
| NFL second sample | 1.026 (1.002-1.051) | .035 |
| Incremental rate NFL | 1.094 (1.020-1.174) | .013 |
Abbreviation: IQR, interquartile range; NFL, plasma neurofilament light, NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin score.
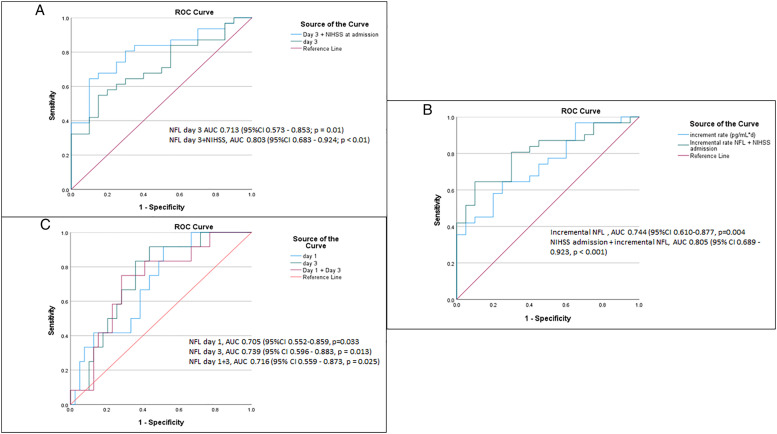
A receiver operating curve (ROC) was drawn to find the optimal point in distinguishing favorable and non-favorable outcomes. The area under the curve (AUC) of NFL Second sample was .713 (95% CI .573-.853; P = .01). We included a combination of factors that were statistically significant in the multiple logistic regression model to increase the AUC. We included NIHSS at admission in combination with NFL Second sample. The result showed an AUC of NFL Second sample with NIHSS admission of .803 (95% CI .683 - .924; P < .01) (Figure 1A). In addition, the sensitivity and specificity of the combined factor were 80.6% and 75%, respectively. We also used the incremental rate of the NFL to predict a favorable outcome. The AUC was .744 (95% CI .610-.877, P = .004). When combined with NIHSS at admission, AUC was .805 (95% CI .689-.923, P < .001) (Figure 1B). Therefore, it remained a good predictor.
Figure 1.
Receiver operating curve concerning (A) unfavorable outcome under consideration of NFL Second sample and NFL Second sample with NIHSS score. (B) Unfavorable outcome under consideration of incremental NFL and NIHSS plus incremental NFL. (C) Hemorrhagic transformation under consideration of NFL First sample, NFL Second sample, and NFL First sample + Second sample.
Hemorrhagic transformation prediction using NFL
In HT analysis, 12 patients (23.5%) developed hemorrhagic transformation. We defined HT into subgroups according to ECASS II.16 Mann-Whitney analysis for all HT revealed significant different for both NFL First sample and Second sample (Z = 2.13, P = .033; Z = 2.487, P = .013) (Table 4).
Table 4.
Plasma neurofilament light association with hemorrhagic transformation.
| Hemorrhagic Transformation | Test of Difference (Z-Score) | P Value |
|---|---|---|
| NFL first sample | 2.130 | .033 |
| NFL second sample | 2.487 | .013 |
| Incremental rate NFL | 1.71 | .087 |
Abbreviation: NFL, plasma neurofilament light.
In the ROC analysis between NFL and HT, AUC for NFL First sample was .705 (95% CI .552-.859, P = .033). The optimal cut-point was set at 20.3 pg/dL with a sensitivity of 83% and a specificity of 51.3%. NFL Second sample had a higher AUC of .739 (95% CI .596-.883, P = .013). The optimal cut off point was 55 pg/dL, which had a sensitivity of 91.1% and a specificity of 56.4%). Combined NFL did not improve the AUC (Figure 1C).
Discussion
To our knowledge, this is the first NFL cohort study to include patients who suffered from moderate to severe stroke. Our median NIHSS score was 12 at admission. As mentioned earlier, stroke with NIHSS score below 4 has a favorable outcome without the need for biomarker prediction. In previous studies, recruited patients had low NIHSS score and demonstrated a correlation with NFL.9,10,17 Therefore, the aim of this study is to demonstrate NFL correlation in higher NIHSS stroke.
NFL is an intermediate filament protein representing primary axonal injury and secondary neurodegeneration.18 It has been shown that NFL correlates with the degree of functional impairment. After the breakdown of the axon, NFL is released within the CSF and later leaks into the bloodstream.19 Studies have shown that CSF and blood NFL have a strong correlation. Since obtaining CSF NFL is invasive, blood NFL could represent CSF NFL.20 Until recently, detection of NFL in the blood is difficult due to their minute quantity. Nevertheless, Simoa has been shown to detect NFL as low as .29 pg/dL.21 Therefore, it is reasonable to use blood NFL as a predictor of stroke severity and prognosis.
A concerning issue is when NFL measurement should be obtained. A study showed that plasma NFL reaches its peak on day 7 after disease onset.9 However, accumulating data in recent years has shown controversial correlation between absolute NFL levels and disease outcome. In a review by Barro and Zetterberg, they proposed that the use of time course for NFL change might be a better predictor.15 The hypothesis was based on data that NFL levels varied between individuals according to underlying disease and lifestyle.22 In other words, individuals had their own baseline NFL levels and the absolute value might not represent the current disease. Therefore, in our study, we defined the rise in NFL as an incremental rate calculated by changes in NFL level divided by the number of days from the first blood obtained. The results were promising, with a positive correlation with the mRS score, a better AUC than NFL on Second sample alone, and a great predictor using the logistic regression model. As a result, our study has confirmed the hypothesis. We encourage physicians to use incremental rate of NFL rather than absolute value.
Regarding the test of difference, NIHSS score at admission, NIHSS at discharge, hypertension, small vessel etiology, incremental rate NFL, and plasma NFL at Second sample were statistically significant between groups. Plasma NFL on First sample was not different between the two groups. This could imply that NFL on First sample should only be used as a baseline, but not as a predictive factor. In the test of association, we found that these factors have a correlation with clinical outcomes. Moreover, incremental rate NFL was found to have the strongest correlation with mRS score. Hemorrhagic transformation did not correlate with the clinical outcome, presumably because not all types of HTs cause unfavorable outcomes.23
An unadjusted logistic regression model showed that NFL at Second sample and NIHSS score at admission could be used as predictors of outcome. In addition, after adjustment, incremental rate NFL and NFL on Second sample remained statistically significant. In adjunct to other previous studies, we could confirm that NFL could be used in all stroke severity to predict prognosis. Furthermore, in the ROC model, the AUC of incremental rate NFL and NFL at Second sample was favorably useful, with an AUC of .744 and .713, respectively. Therefore, it could be interpreted as an acceptable predictor. Furthermore, when combined with NIHSS at admission, the prediction model was better with an AUC of .805 and .803, respectively. It made the model more favorable.
Prediction of HT using NFL has never been mentioned in previous studies. Our hypothesis is that conditions associated with blood-brain barrier disruption should prompt a higher level of NFL released in the CSF and blood stream. Several studies have shown that the permeability of the blood-brain barrier does not affect the release of NFL from dying neurons. Moreover, if the integrity of the blood-brain barrier is disrupted, a higher NFL level should also be detected in the bloodstream.24 In our study, we used the classification according to ECASS II.16 The severity of HT depends on clinical changes, area of hemorrhage, and location of the hemorrhage. HTs are concerned with prolonging hospital stays and higher morbidity and mortality.25 Therefore, prediction of hemorrhage is crucial in early detection. Our results showed an association for both NFL First sample and Second sample. This is crucial as it could alert neurologist and neurosurgeon for early management to prevent the progression of HT or early intervention as appropriate by using the second sample. In ROC analysis, NFL First sample showed a fair prediction of AUC of .705, while NFL Second sample had an AUC of .739. Although they do not show promising sensitivity and specificity, we found that in our analysis, no other covariate factors were associated with HT. The optimal cut-point at First sample was 20.3 pg/dL with a sensitivity of over 80%, although the number of HT are low. This could raise alertness of the physician and prompt management.
It is of important to discuss the relationship between cardiovascular risk factors, HT and NFL. Several important cardiovascular risk factors have been associated with the occurrence of HT including hypertension, atrial fibrillation, diabetes mellitus and ischemic heart diseases.26 It is believed that these factors cause more disruption to the blood brain barrier’s integrity. Hypertension is an important predictor of HT. In a study done by Peterson et al found that uncontrolled hypertension above the limit could worsen the grades of HT and increase the likelihood of symptomatic HT.27 Another cause of HT that should be mentioned is the presence of arterial stiffness. In a study done by Acampa et al. found that patients who have higher arterial stiffness index were more prone to HT (OR: 1.9, 95% CI: 1.09-3.02, for every .2 increase of ASI, P < .01).28 The possible cause of arterial stiffness is associated with hypertension. In addition, in a recent study found that arterial stiffness increases multiple CSF biomarkers. However, NFL did not meet statistical significant.29 Leaving a question, whether NFL could be an independent factor to predict HT or not?
Our study has limitations in certain areas. First is the lack of external validation. Therefore, it is not ready to be applied in clinical practice. Second, we could not obtain NFL at more than two time points, in which case we could not evaluate the trend of the NFL’s rise. However, our study is novel and should prompt further research.
Conclusion
Blood NFL level is linked to the clinical outcome of moderate to severe ischemic stroke. We also demonstrate that the use of the incremental rate of the NFL might be a better predictor of prognostic factor. In addition, the NFL level is also associated with hemorrhagic transformation. Therefore, it might be useful in clinical settings.
Supplemental Material
Supplemental material for Neurofilament light is associated with clinical outcome and hemorrhagic transformation in moderate to severe ischemic stroke by Wanakorn Rattanawong, Tatchaporn Ongphichetmetha, Thiravat Hemachudha and Poosanu Thanapornsangsuth in Journal of Central Nervous System Disease
Acknowledgements
We would like to express our gratitude to The Chulalongkorn Stroke Center of Excellence, King Chulalongkorn Memorial Hospital for taking excellent care of all participants. We thank participants for their generous contribution, nurses and ward officers at the stroke unit and stroke intensive care unit for their enthusiasm towards our research.
Author Contributions: Wanakorn Rattanawong: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing-original draft, Writing-review & editing, Tatchaporn Ongphichetmetha: Conceptualization, Data curation, Investigation, Writing-review & editing, Thiravat Hemachudha: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing-original draft, Writing-review & editing, Poosanu Thanapornsangsuth: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing original draft, Writing-review & editing
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by The Health System Research Institute of Thailand (#64-027).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Wanakorn Rattanawong https://orcid.org/0000-0001-7277-9699
References
- 1.GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):439-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.König IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: A simple index works in patients from controlled clinical trials. Stroke. 2008;39(6):1821-1826. [DOI] [PubMed] [Google Scholar]
- 3.Maas MB, Furie KL. Molecular biomarkers in stroke diagnosis and prognosis. Biomark Med. 2009;3(4):363-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selleck MJ, Senthil M, Wall NR. Making meaningful clinical use of biomarkers. Biomark Insights. 2017;12:1177271917715236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125(Pt 14):3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varhaug KN, Torkildsen Ø, Myhr KM, Vedeler CA. Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol. 2019;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013-2018. doi: 10.1046/j.1471-4159.1996.67052013.x. [DOI] [PubMed] [Google Scholar]
- 8.Yuan A, Rao MV, Veerann0a, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9(4):a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91(14):e1338-e1347. [DOI] [PubMed] [Google Scholar]
- 10.Uphaus T, Bittner S, Gröschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. 2019;50(11):3077-3084. [DOI] [PubMed] [Google Scholar]
- 11.Onatsu J, Vanninen R, Jäkälä P, et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(8):2242-2249. [DOI] [PubMed] [Google Scholar]
- 12.Inoa V, Aron AW, Staff I, Fortunato G, Sansing LH. Lower NIH stroke scale scores are required to accurately predict a good prognosis in posterior circulation stroke. Cerebrovasc Dis. 2014;37(4):251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 suppl 1):S52-S57. [DOI] [PubMed] [Google Scholar]
- 14.Spilker J, Kongable G, Barch C, et al. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA stroke study group. J Neurosci Nurs. 1997;29(6):384-392. [DOI] [PubMed] [Google Scholar]
- 15.Barro C, Zetterberg H. Neurological symptoms and blood neurofilament light levels. Acta Neurol Scand. 2021;144(1):13-20. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. 1998;352(9136):1245-1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 17.De Marchis GM, Katan M, Barro C, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. 2018;25(3):562-568. doi: 10.1111/ene.13554. [DOI] [PubMed] [Google Scholar]
- 18.Pekny M, Wilhelmsson U, Stokowska A, Tatlisumak T, Jood K, Pekna M. Neurofilament light chain (NfL) in blood-a biomarker predicting unfavourable outcome in the acute phase and improvement in the late phase after stroke. Cells. 2021;10(6):1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Körtvelyessy P, Kuhle J, Düzel E, et al. Ratio and index of neurofilament light chain indicate its origin in guillain-barré syndrome. Ann Clin Transl Neurol. 2020;7(11):2213-2220. doi: 10.1002/acn3.51207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alagaratnam J, von Widekind S, De Francesco D, et al. Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ neurology open. 2021;3(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. [DOI] [PubMed] [Google Scholar]
- 22.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. doi: 10.1038/s41467-020-14612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.England TJ, Bath PM, Sare GM, et al. Asymptomatic hemorrhagic transformation of infarction and its relationship with functional outcome and stroke subtype: Assessment from the Tinzaparin in acute ischaemic stroke trial. Stroke. 2010;41(12):2834-2839. [DOI] [PubMed] [Google Scholar]
- 24.Uher T, McComb M, Galkin S, et al. Neurofilament levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler. 2021;27(2):220-231. doi: 10.1177/1352458520912379. [DOI] [PubMed] [Google Scholar]
- 25.van Kranendonk KR, Treurniet KM, Boers AMM, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg. 2019;11(5):464-468. [DOI] [PubMed] [Google Scholar]
- 26.Thomas SE, Plumber N, Venkatapathappa P, Gorantla V. A review of risk factors and predictors for hemorrhagic transformation in patients with acute ischemic stroke. Int J Vasc Med. 2021;2021:4244267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen NH, Silverman A, Strander SM, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51:914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acampa M, Camarri S, Lazzerini PE, et al. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int J Cardiol. 2017;243:466-470. [DOI] [PubMed] [Google Scholar]
- 29.Moore EE, Liu D, Li J, Schimmel SJ, et al. Association of aortic stiffness with biomarkers of neuroinflammation, synaptic dysfunction, and neurodegeneration. Neurology. 2021;97(4):e329-e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Neurofilament light is associated with clinical outcome and hemorrhagic transformation in moderate to severe ischemic stroke by Wanakorn Rattanawong, Tatchaporn Ongphichetmetha, Thiravat Hemachudha and Poosanu Thanapornsangsuth in Journal of Central Nervous System Disease