Abstract
Background
Severe hyponatraemia can lead to serious neurological complications including coma, seizure and death. Hyponatraemia and the Syndrome of Inappropriate Antidiuretic Hormone (SIADH) has been previously described in cases of COVID-19, however there have been few reports post vaccination. We describe a case of severe hyponatraemia post second Pfizer BNT162b2 mRNA vaccination against COVID-19.
Case presentation
A 48-year-old previously well woman presented to the emergency department with severe headaches and confusion one day after she received her second Pfizer COVID-19 vaccination. She reported no more than 2.5 L fluid intake. Vital signs were normal. Laboratory investigation revealed serum sodium 113 mmol/L, potassium 3.4 mmol/L, urea 3.5 mmol/L and serum osmolality 266 mmol/kg. TSH, random cortisol and C-reactive protein levels were normal. She was found to be in urinary retention and developed marked polyuria post in dwelling catheter insertion. Following this she underwent spontaneous and rapid correction of serum sodium without intervention. Retrospective analysis showed an inappropriately high copeptin of 4.4 pmol/L.
Conclusions
It is important to be cautioned and aware of hyponatraemia as an immediate side effect of COVID-19 vaccination. The exact mechanism is unknown and further research is required to understand the acute endocrine effects which may arise in response to COVID-19 vaccination.
Keywords: Hyponatraemia, Urinary retention, COVID-19 vaccination, SIADH, Case report
Introduction
In response to the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic intensive vaccination programs have been commenced worldwide. In Australia, vaxzevria (AstraZeneca), Comirnaty (Pfizer), Spikevax (Moderna) and Nuvaxovid (Novavax) are approved vaccines. Comirnaty (BNT162b2, Pfizer-BioNtech) is a messenger ribonucleic acid (mRNA) based vaccine which codes for spike-proteins of the SARS-CoV-2 virus. These vaccines are highly effective in preventing severe illness with coronavirus disease 2019 (COVID-19) [14]. Most side effects have been self-limiting including fatigue, myalgia, arthralgia, fever, headache, chills and injection site local reaction [1]. In COVID-19 hospital admissions, hyponatraemia is associated with worse prognosis [3] and can be due to several mechanisms [6]. Two case reports have described significant hyponatraemia post mRNA based COVID-19 vaccinations, one due to a Syndrome of Inappropriate Antidiuretic Hormone (SIADH) [8] and the other secondary to hypophysitis [10]. Acute hyponatraemia can cause cerebral oedema and lead to neurological complications including coma, seizure and death [5]. We describe a case of severe hyponatraemia and depressed conscious state post second dose of BNT162b2 mRNA vaccination.
Case
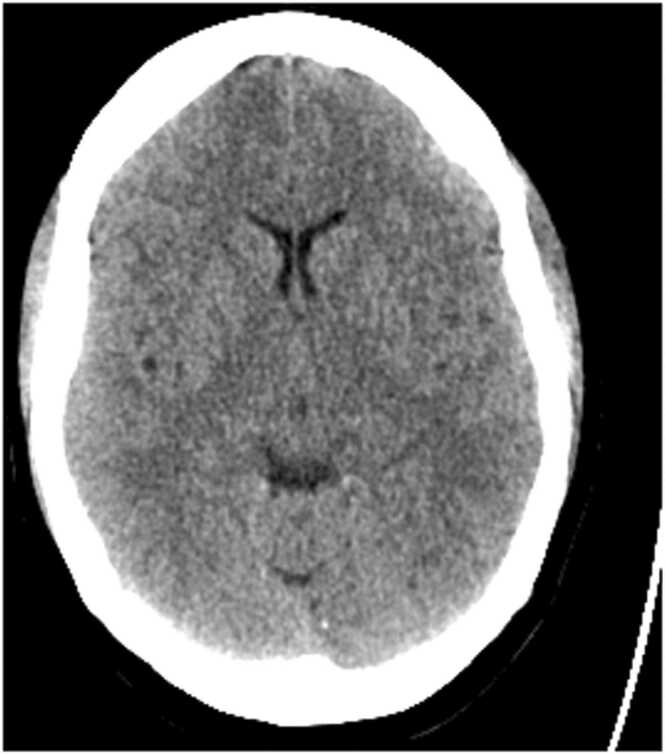
A 48-year-old previously well woman presented to the emergency department with severe headaches and depressed conscious state, Glasgow Coma Score (GCS) of 12 (E4V3M5), one day after she received her second Pfizer COVID-19 vaccination. She was not on prescribed medications or over the counter supplements. On examination, she was afebrile with normal oxygen saturation, blood pressure 125/76 mmHg, heart rate 70/min. An isolated right lateral gaze palsy and reduced GCS prompted a referral to the Stroke Team. Computed tomogram brain (CTB) and angiographic imaging studies did not reveal cerebrovascular accident, however cerebral oedema was noted (Fig. 1).
Fig. 1.
Non-contrast CTB demonstrating cerebral oedema.
Laboratory investigation revealed a serum sodium of 113 mmol/L, potassium of 3.4 mmol/L, urea 3.5 mmol/L, and serum osmolality of 266 mmol/kg. TSH, random cortisol, serum glucose, calcium and C-reactive protein levels were normal. Lumbar puncture revealed a clear, bland cerebrospinal fluid (CSF) with normal cell count and mildly elevate protein (0.63 g/L, reference range < 0.45 g/L)). Urine chemistry was not available on admission. Insertion of a urinary catheter revealed urinary retention of 1.4 L, with an urine osmolality of 81 mmol/L and a urine sodium of < 20 mmol/L.
The patient was commenced on empirical treatment for meningoencephalitis (benzylpenicillin, aciclovir) concurrently with normal saline (total of 350 ml at a rate of 100 ml/h) from presumptive polyuric hypovolemia. In ICU, GCS further decreased to 10 (E3V2M5), the patient became polyuric with an output up to 910 ml/h, and underwent rapid correction of serum sodium to 127 mmol/L four hours post baseline presentation of 113 mmol/L. The intravenous fluid therapy was altered to dextrose 5 % matched to the previous hours urine output plus 50 ml/h. Serum sodium continued to over correct to 137 mmol/L seven hours after presentation.
The patient’s neurological symptoms completely resolved 13 h post presentation with a serum sodium of 142 mmol/L the next morning. She recalled thirst and increased fluid intake on day of admission, but denied consumption of greater than 2.5 L/day. Antiviral therapy was ceased when CSF polymerase chain reaction (PCR) was negative for herpes, entero and adenoviruses. The patient was also negative for COVID-19, influenza A, influenza B and Respiratory Syncytial Virus. The clinical impression was she had developed acute hyponatraemia with spontaneous and rapid correction. The confusion was attributed to cerebral oedema due to severe hyponatraemia and possible seizure. The patient remained well after 4 days and was discharged without any manifestation of central pontine myelinolysis. Additional biochemical results became available which included an admission copeptin level of 4.4pmol/L which decreased further to 2.1 pmol/L four hours after the admission bloods.
Discussions and conclusion
We describe a case of severe hyponatraemia post BNT162b2 mRNA COVID vaccination. The possibility of a causal relationship between vaccine administration and the severe acute hyponatraemia was considered plausible given the close temporal relationship of these events. There was no evidence of excessive water intake, renal sodium loss or diuretic use, hypovolaemic disorders due to extrarenal sodium loss (e.g. diarrhoea, vomiting), or hypervolemia with excess total body water. Although fluid status was not well documented at presentation, a urea of 3.5 mmol/L was not consistent with fluid contraction. The patient was also euthyroid with an adequate serum cortisol (391nmol/L) and there was no evidence of infection (subsequent culture of CSF was negative). Given the clear temporal relationship between vaccination and illness in the absence of other causes, the mechanism was most likely transient SIADH secondary to Pfizer COVID-19 vaccination. This is supported by the inappropriately high copeptin in the context of severe symptomatic hyponatremia followed by reduction of copeptin level with subsequent aquaresis.
Copeptin (aka C-terminus Pro-Arginine Vasopressin) is the C-terminal glycoprotein moiety of pre-vasopressin and is a stable surrogate marker for arginine vasopressin/antidiuretic hormone (ADH) release [12]. The normal range of plasma copeptin levels in healthy normo-osmotic volunteers is 1.0–13.0–13.8 pmol/L [2], [9]. Low levels of copeptin < 3.9 pmol/L are associated with primary polydipsia (specificity 91 %, sensitivity 58 %) whereas elevated plasma copeptin levels > 84 pmol/L are typically indicative of hypovolaemic hyponatraemia (specificity 90 %, sensitivity 23 %) [11]. Apart from the specificity of marked low and high values, copeptin levels are not used to differentiate between SIADH and other causes of hyponatraemia [12]. The detectable plasma copeptin of 4.4pmol/L observed in the setting of severe hyponatraemia (serum sodium 113 mmol/L) is inappropriately high. We propose the COVID vaccine was associated with a transient aberrant ADH response. Restoration of the individual’s appropriate hypothalamic osmotic response then resulted in decreased ADH (copeptin) levels and resulting in an extreme diuresis which increased the serum sodium in a rebound manner. Plasma copeptin to urine sodium ratio has been shown to be a diagnostically superior method in the differentiation between SIADH and patients with sodium depletion [4]. Unfortunately, there was no urine biochemistry available at the time of admission and the subsequent urine sample was collected after intravenous fluid therapy was instituted and correction of the serum sodium.
Although not listed as a formal side effect of the BNT162b2 mRNA COVID vaccination, increased thirst has been reported. A study on self-reported symptoms following BNT162b2 mRNA COVID vaccination reported increased thirst in 1.2 % participants and 0.25 % experienced polyuria [7]. We propose in this case vaccination for COVID-19 may have triggered an inappropriate hypothalamic-pituitary ADH response exacerbated by overstimulation of thirst resulting in fluid retention and hyponatraemia. Similar to the case we described, a case of severe hyponatraemia and urinary retention has also been described in COVID-19 [13].
This case is instructive for several reasons. First, hyponatraemia should be considered when neurological symptoms occur after vaccination. Second, people receiving vaccination for COVID-19 should be cautioned to avoid excessive water consumption to minimise the risk of precipitating severe acute hyponatraemia in the event of an idiosyncratic vaccine reaction. Third, the case is a reminder for the importance of collecting paired urine and serum sodium levels prior to commencing intravenous therapy to assist with determining the aetiology of hyponatraemia. Further research is required to understand the acute endocrine effects, including potential transient SIADH, which may occur following COVID-19 vaccination.
CRediT authorship contribution statement
Dharma JFM: study design, data collections, data analysis, Writing – original draft, Writing – review & editing. Montalto S: study design, data collections. Johnson D.F: data analysis, Writing – original draft, Writing – review & editing. Chiang C: data analysis, Writing – original draft, Writing – review & editing. Fourlanos S: study design, data analysis, Writing – original draft, Writing – review & editing.
Funding Source
This research did not receive any funding source.
Ethical approval
There is no ethical approval required for this descriptive report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of Interests
The authors have no conflict of interest to declare.
References
- 1.Pfizer COVID-19 vaccine safety data – all participants; Available from: 〈https://ausvaxsafety.org.au〉.
- 2.Bhandari S.S., Loke I., Davies J.E., Squire I.B., Struck J., Ng L.L. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci. 2009;116(3):257–263. doi: 10.1042/CS20080140. [DOI] [PubMed] [Google Scholar]
- 3.De Carvalho H., Letellier T., Karakachoff M., Desvaux G., Caillon H., Papuchon E., et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol. 2021;34(4):991–998. doi: 10.1007/s40620-021-01036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenske W., Stork S., Blechschmidt A., Maier S.G., Morgenthaler N.G., Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab. 2009;94(1):123–129. doi: 10.1210/jc.2008-1426. [DOI] [PubMed] [Google Scholar]
- 5.Gankam Kengne F., Decaux G. Hyponatremia and the brain. Kidney Int Rep. 2018;3(1):24–35. doi: 10.1016/j.ekir.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghe G., Ilie M., Bungau S., Stoian A.M.P., Bacalbasa N., Diaconu C.C. Is there a relationship between COVID-19 and hyponatremia? Medicina. 2021;57(1):09. doi: 10.3390/medicina57010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner G., Ryser B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: case report. BMC Infect Dis. 2021;21(1):1000. doi: 10.1186/s12879-021-06690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgenthaler N.G., Struck J., Alonso C., Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 10.Murvelashvili N., Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Invest Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211043386. 23247096211043386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigro N., Winzeler B., Suter-Widmer I., Schuetz P., Arici B., Bally M., et al. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: 'the Co-MED study'. Clin Endocrinol. 2017;86(3):456–462. doi: 10.1111/cen.13243. [DOI] [PubMed] [Google Scholar]
- 12.Refardt J., Winzeler B., Christ-Crain M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin Endocrinol. 2019;91(1):22–32. doi: 10.1111/cen.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh A.O., Al-Shokri S.D., Ahmed A.O., Musa A.E., Mohamed M.F. Urinary retention and severe hyponatremia: an unusual presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(10) doi: 10.12890/2020_001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenforde M.W., Self W.H., Adams K., Gaglani M., Ginde A.A., McNeal T., et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Jama. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]