Abstract
Spinal cord injuries affect nearly five to ten individuals per million every year. Spinal cord injury causes damage to the nerves, muscles, and the tissue surrounding the spinal cord. Depending on the severity, spinal injuries are linked to degeneration of axons and myelin, resulting in neuronal impairment and skeletal muscle weakness and atrophy. The protection of neurons and promotion of myelin regeneration during spinal cord injury is important for recovery of function following spinal cord injury. Current treatments have little to no effect on spinal cord injury and neurogenic muscle loss. Clemastine, an Food and Drug Administration-approved antihistamine drug, reduces inflammation, protects cells, promotes remyelination, and preserves myelin integrity. Recent clinical evidence suggests that clemastine can decrease the loss of axons after spinal cord injury, stimulating the differentiation of oligodendrocyte progenitor cells into mature oligodendrocytes that are capable of myelination. While clemastine can aid not only in the remyelination and preservation of myelin sheath integrity, it also protects neurons. However, its role in neurogenic muscle loss remains unclear. This review discusses the pathophysiology of spinal cord injury, and the role of clemastine in the protection of neurons, myelin, and axons as well as attenuation of skeletal muscle loss following spinal cord injury.
Key Words: axonal damage, clemastine, myelination, neuronal death, oligodendrocytes, skeletal muscle, spinal cord injury
Introduction
Spinal cord injury (SCI) is a traumatic injury to the spinal cord. The incidents of SCI are drastically rising each year. The most recent estimate of the annual incidence of SCI is approximately 54 cases per one million people in the United States. According to the National Spinal Cord Injury Statistical Center, there are approximately 2.5 million individuals worldwide who have been afflicted by SCI (National Spinal Cord Injury Statistical Center, 2013). There is an economic burden associated with SCI and health-related detriments. In the United States alone, it has been estimated that health-related costs will exceed 9 billion dollars annually (Devivo, 2012). In Canada, there are estimated to be an occurrence of 85,556 persons involved in an SCI (Noonan et al., 2012), 51% of these cases are considered traumatic SCI whereas 49% are non-traumatic SCI. In areas of Western Europe figures reflect 16 per million cases of SCI. In Australia, it is reported around 15 per million incidences related to SCI. North America is associated with the highest rate of traumatic-related injuries at 18% percent of cases and 39 per million incident rates. In Western Europe, 8% of the incident are related to violent SCI and in Australia, the incident percentile is 2% (Chen et al., 2013).
The pathophysiology of acute SCI involves primary and secondary mechanisms of injury (Lasfargues et al., 1995; Anwar et al., 2016; Alizadeh et al., 2019). SCI results from damages to the nerves and tissues surrounding the afflicted area of the spinal column. SCI is highly correlated with traumatic injury and blunt force trauma detriments which can cause impartments to the central nervous system (CNS). Dorsal column impairments can drastically reduce sensory and motor functions depending on the amount of damage associated with the injury (Sekhon and Fehlings, 2001).
While the primary injury is caused by the initial traumatic event, the secondary injury is created by a series of biological and functional changes that can be debilitating and even life-threatening (Anwar et al., 2016; Ahuja et al., 2017). The majority of SCI are due to preventable causes such as road traffic crashes, falls, or violence. However, SCI often results in permanent disabilities because adult CNS neurons only exhibit limited axon regeneration (Lou et al., 2017). Individuals with SCI can sustain nerve damage, muscle atrophy, locomotor immobility, as well as autonomic and sensory dysfunction (Du et al., 2022; Sutor et al., 2022; Sydney-Smith et al., 2022; Torok et al., 2022). SCI can lead to the degeneration of myelinated axons in the spinal cord and has also been linked to the development of osteoporosis (Sutor et al., 2022). It can also diminish the quality of life an individual experiences, as it negatively impacts recipients’ abilities to function and accomplish simple activities of life (Morgan et al., 2021).
Myelin regeneration, protection of the damaged neurons, and preservation of axons are critical in allowing for recovery from SCI (Huntemer-Silveira et al., 2020). However, SCI is difficult to treat, and many patients deal with chronic pain and permanent damages after experiencing trauma to the spine. Patient care often requires long-term and multi-disciplinary treatment, and this coincided with societal burdens as well as a socioeconomic impact due to the high amount of medications and supplies required for proper upkeep after SCI (Peng et al., 2021). Currently, treatment options that promote functional recovery are limited for SCI patients and therapies remain unavailable (Du et al., 2022), which are significant problems. The remyelination properties of Clemastine, an FDA-approved antihistamine drug, have been studied in animal models and display promising results for potential treatment uses in SCI patient therapy (Turski et al., 2018). Understanding the disordered physiological processes associated with SCI can aid in determining the most adequate treatment options and how they will interact with the current pathophysiology.
As mentioned above, the underlying mechanism of SCI involves a two-step process that comprises primary mechanical damage followed by secondary injury (Lasfargues et al., 1995; Alizadeh et al., 2019). By utilizing properties and treatments that may aid in the prevention of these physiological properties will ultimately prevent further injury and neuronal damage. There are currently minimal therapy options for individuals suffering from SCI; therefore, it is critical to study the effects of Clemastine on remyelination and protection of axons and neurons after injury. Diminishing cell death after SCI is critical in promoting the recovery of patient’s spinal cord during the treatment process. Cell death can induce a cascade of biochemical and biomechanical effects (Beattie et al., 2002; Shi et al., 2021). Current animal model research suggests that necrotic and apoptotic mechanisms of cell death occur at different phases after SCI.
Glial cells, and astrocytes, in particular, are activated after SCI, and some of them rapidly proliferate to form glial scars which are thought to be one of the primary impediments to regeneration and repair in the injured spinal cord. The heterogeneous composition of the glial scar containing both beneficial and detrimental factors (Fehlings and Hawryluk, 2010; Cox et al., 2021), makes therapeutically targeting the glial scar a difficult task. However, recent studies suggest that the glial scar and scar-forming astrocytes may also play key roles in the recovery of SCI (Bradbury and Burnside, 2019; Yang et al., 2020). While glial scar may prevent recovery, demyelination to the axons causes further damage to the neurons surrounding the injury site (Xie et al., 2021). Remyelination supports axonal recovery and neuronal growth after SCI (Kwiecien, 2022). Therefore, the promotion of remyelination is critical during post-injury treatment. Successful SCI treatments require therapies that have the capability to permeate the blood-brain barrier of the CNS (Sydney-Smith et al., 2022). The use of Clemastine as a treatment can enhance oligodendrocyte differentiation and myelination (Liu et al., 2016; Du et al., 2022), and promotes remyelination of damaged, but preserved, axons (Green et al., 2017). Such remyelination may prevent neuronal as well as skeletal muscle loss in SCI. Preclinical data support this idea that Clemastine encourages oligodendrocyte precursor differentiation and remyelination without suppressing the immune system (Turski et al., 2018). These findings are critical in promoting successful treatment options utilizing Clemastine for individuals suffering from SCI. Due to motoneuron death after SCI, chronic muscle denervation occurs and alterations in muscle use to the striking muscle weakness and atrophy seen after human SCI (Giangregorio and McCartney, 2006; Thomas and Zijdewind, 2006; Biering-Sorensen et al., 2009). However, the clinical outcomes of SCI depend on the severity and location of the lesion and may include partial or complete loss of motor function below the level of injury. This review discusses gliosis, demyelination and axonal damage, neuronal impairment and neurogenic muscle loss after severe SCI and remyelination/regeneration after pharmacological intervention and protection of motor functions following injuries.
Search Strategy
Details of the strategy used for evidence are shown as follows:
| Search terms | Database | Date | Papers |
|---|---|---|---|
| SCI/myelination | Google scholar | 2015–2022 | 29300 |
| SCI/muscle damage | PubMed | 2010–2022 | 2277 |
| SCI/cell death | Google scholar | 2020–2022 | 140000 |
| Pathophysiology of SCI | Google scholar | 2020–2022 | 42400 |
| Glial scar SCI | Google scholar | 2018–2022 | 16900 |
| Demyelination and SCI | PubMed | 2011–2022 | 3925 |
| Remyelination | PubMed | 2015–2022 | 374 |
| Axonal recovery | PubMed | 2004–2022 | 1447 |
| Neuronal survival | PubMed | 1999–2022 | 57210 |
| Drug treatment SCI | Google scholar | 2018–2022 | 955000 |
| Clemastine myelination | PubMed | 2014–2022 | 36 |
Pathophysiology of Spinal Cord Injury
SCI is characterized by the loss of motor, sensory, and autonomic functions due to partial or whole damage to the spinal cord (Lasfargues et al., 1995; Chew and Sengelaub, 2021). SCI such as blunt force trauma can be detrimentally damaging to the spinal cord and result in paralysis, permanent nerve damage, and other life-long ailments (Legg Ditterline et al., 2020). In the United States, there are 30 to 50 SCI cases per million per year (Lasfargues et al., 1995; Otzel et al., 2021), and approximately 12,000 new SCI cases are reported each year (Lasfargues et al., 1995). SCI research on animal pathophysiology has indicated a two-step mechanism that begins with primary mechanical damage and is followed by a secondary injury composed of many destructive pathways (Lasfargues et al., 1995). These pathways are pivotal in degeneration of the injured cord and may be targeted for therapy. The primary mechanical damage occurs as a result of spinal cord contusion/compression. Flexion, extension, dislocation, or distraction forces exert stress on the spinal cord allowing for tears in the neural tissues and vascular structures. Gray matter can be permanently damaged if trauma in the spinal cord causes an interruption to the blood flow (Lasfargues et al., 1995). Hypoxia and ischemia local infarctions begin to occur due to SCI and neurons are at greater risk for irreparable damages (Lasfargues et al., 1995). The secondary damage begins minutes after the initial trauma and continues for hours or days after the primary injury transpires. Ischemia causes a lack of mechanical energy which can disrupt many regulatory pathophysiological mechanisms (Du et al., 2022). Other changes caused by SCI include hemorrhages, demyelination, edema, and the formation of cavities with axonal and neuronal necrosis (Yilmaz et al., 2014). Secondary injury can also prompt increased levels of inflammation, glutamate, excitotoxicity, oxidative damage, ischemia, free radical damage, protease and lipase activation, and lipid peroxidation within the cell membrane (Yilmaz et al., 2014). Demyelination and axonal degeneration occur during the secondary injury phase of SCI (Xie et al., 2021; Du et al., 2022). Studies have noted that the spinal cord is highly proficient in healing from injuries (Ryan et al., 2022; Verma et al., 2022). Surrounding neurons located within the descending pathways connecting the brain and spinal cord can also be afflicted by SCI and experience atrophy, apoptosis, or even necrosis (Otzel et al., 2021; Zhao et al., 2021). Skeletal muscle atrophy is a hallmark of severe SCI (Bigford et al., 2021; Otzel et al., 2021). Both acute and chronic traumatic incidents associated with SCI can be linked to the deposition of TAR DNA-binding protein 43 located in the brain. Over extended periods, neurotrauma can have detrimental and possibly irreversible effects on locomotive behavior and neurological function. In the post-injury stage, rats displayed an activation of pro-apoptotic genes as well as cytochrome-C release. There was also a high expression of neurofilaments during the early stage of initiation of the inflammatory signaling (Yip and Malaspina, 2012). Changes in the molecular signaling cascades that regulate muscle size are distinct after severe SCI, with muscle loss being more rapid than in other disuse conditions. SCI is also linked with other degenerative and pathological processes such as osteoporosis, specifically in women (Lasfargues et al., 1995). Osteoporosis triggers the accelerated breakdown of the osteoclasts as well as the hindrance of the rebuilding of the bone structure by osteoblasts (Invernizzi et al., 2020b; Peng et al., 2021). While osteoporosis and muscle atrophy are frequently cited complications occurring after an SCI (Chandrasekaran et al., 2020; Sutor et al., 2022), this review is focused on the effects of remyelination on the protection of neurons and muscle.
Cell Death Following Spinal Cord Injury
Neurological damages resulting from SCI trauma can be influenced by genetic and environmental factors such as the individual’s susceptibility to tolerate and heal from the initial incident. Injuries can progressively worsen or be permanent depending on the affliction of the trauma. Cell death, neurogenesis, and other molecular events hold liability for the formation of potentially irreversible damages within the post-injury phase. Atrophy, sensory loss, muscle weakening, and in some cases paralysis can all be linked to initial trauma stemming from damages to the injury site (Yip and Malaspina, 2012). SCI initiates neuronal and glial loss in turn promoting a prolonged period of secondary cell death. The deleterious substances produced in response to the initial injury stimulate the secondary damages. Reactive oxygen species and reactive nitrogen species are key contributors to the secondary cell death post-injury. Primary oxygen-free radical production acts as a highly reactive superoxide anion within the body. A superoxide anion is typically produced via cellular respiration by the mitochondria and is a by-product of oxidase enzymes within the cytoplasm. Under normal conditions, the body can employ natural antioxidants as a physiological defense system using the endogenous and exogenous antioxidants and enzymes. However, the rate of superoxide anion formation will exceed the capacity of the antioxidant defense system under pathological conditions. The super anions will destroy the antioxidant defense system and oxidative stress conditions occur (Bao et al., 2005).
Programmed cell death is a critical process that occurs after SCI (Shi et al., 2021). Programmed cell death is mediated by gene expression events and it is a critical mechanism used to eliminate unnecessary and damaged cells. Programmed cell death also acts as a form of defense against cytotoxicity, exogenous, and endogenous substances. Apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis, and paraptosis are all forms of programmed cell death (Shi et al., 2021). Cell death contributes to the body’s maintenance of cellular homeostasis. Necrosis is passive cell death resulting from the environmental perturbations or large abounds of inflammatory cellular contents. Apoptosis is the active form of cell death that is programmed to initiate autonomous cell dismantling without the release of cytoplasmic contents in the extracellular space (Shi et al., 2021).
Naturally occurring neuronal death is commonly observed in altricial species such as mice, rabbits, rats, as well as humans. Caspase-3 is the main convergence of intrinsic and extrinsic apoptotic pathways. Evidence suggests that among the variety of neurons and glia within the cerebellum the intervention of caspase-3 can aid in the regulation of neuronal death of post-mitotic cerebellar granule cells and Purkinje neurons. GABAergic interneurons also experience a comparable type of secondary cell death, but the intervention of caspase-3 is not evident. As a precursor to the pre-migratory stage of differentiation, it is observed that cerebellar granule cells undergo primary cell death. Glial cells also undergo a process of regulatory cell death, however, with the expression of caspase-3, there may be differentiation of the cells as opposed to death (Lossi et al., 2018).
Injury-induced apoptosis is observed in neurons, oligodendroglia, microglia, and neurons after SCI (Shi et al., 2021). In an animal study using rats with SCI, the apoptotic cells were detected and observed in the rim of the surviving tissue surrounding the center of the injured spinal cord. Apoptosis was also detected in oligodendrocytes in white matter tracts which displayed Wallerian degeneration, a substantial marker of chronic phase SCI. Results from various studies display that apoptosis contributes to the tissue damage post-SCI. Utilizing potential treatments to inhibit apoptosis in SCI may contribute to the reduction in tissue damage and lesion area expansion (Shi et al., 2021).
Recent studies in animal models have displayed multiple events in SCI, and their reactions to specific treatments. The initial trauma presented to the spinal cord tissue combined with long periods of secondary injury can produce a high volume of cellular and biomechanical cascades (Beattie et al., 2002; Otzel et al., 2021). While necrotic and apoptotic mechanisms of the cell death occur after injury, loss of motoneuron can result in the impairment of locomotor functioning as well as dendritic atrophy (Scholpa et al., 2019). Even surviving motoneurons display signs of functional deficits after injury of surrounding neurons (Chew and Sengelaub, 2021). Microglial activation and production of tumor necrosis factor-α have significant roles in acute SCI (Zhang et al., 2019; Fu et al., 2022). Synaptic stripping will occur after the loss of afferent synapses on a motor neuron after damage. This triggers rapid reactive responses recruiting activated microglial cells due to disconnect of the motor neuron from injury (Salvany et al., 2021). In one model, ninety minutes after a moderate contusion lesion, the dying neurons were surrounded by activated microglia. The microglial production of tumor necrosis factor-α appears to critically potentiate cell death mediated by glutamate receptors in atraumatic model of SCI (Beattie et al., 2002).
Glial Scar
The injury of the spinal cord often severs bulbospinal projections of the motor neurons inducing paralysis of the surrounding muscles below the injury site of the spinal cord (Allen et al., 2021; Jiang et al., 2021; Proietti et al., 2021). After SCI, three primary cell types: activated astrocytes, newly proliferated microglia, and oligodendrocyte progenitor cells, surround the lesion core, forming a dense borderline.
Glial scars form after severe damage occurs and reactive glia provide a barrier around the injury site to create a non-permissive microenvironment halting endogenous regeneration. Glial scars are comprised of two distinct cellular components which include the lesion core and the lesion border that surrounds the exterior of the core. The lesion core contains astrocytes, fibroblasts, pericytes, and ependymal cells as well as phagocytes and macrophages. Surrounding the glial scar are reactive astrocytes, NG2 glia, microglia, and other immune cells which form a barrier around the core preventing the spread of inflammation to healthy tissue. Without glial scar formation at the site of injury, there is no initiation of the preparative mechanisms associated with anti-inflammatory effects and thus there is a hindrance to neuronal growth and repair (Nicaise et al., 2022).
Scars are predominantly composed of astrocytes in lesions that do not affect the dura mater (Figure 1). Astrocytes are the most abundant glial cells in the CNS and aid in the regulation of blood flow, maintaining the blood-brain barrier integrity, modulating the plasticity and function of synapses, as well as keeping the balance of the neuronal microenvironment intact. They are characterized by a unique star shape and act as essential physiological insulation and support to the neurons (Yang et al., 2020). After the injury to the spinal cord, the astrocytes will proliferate, hypertrophy, and begin to migrate across the edge of the severed tissue. They will intertwine around the lesion center to begin the formation of the glial scar. Evidence has also shown that glial scars likely promote axonal regeneration. In the early stages of injury, glial scars act as a protective mechanism for the damaged tissue (Pang et al., 2021).
Figure 1.
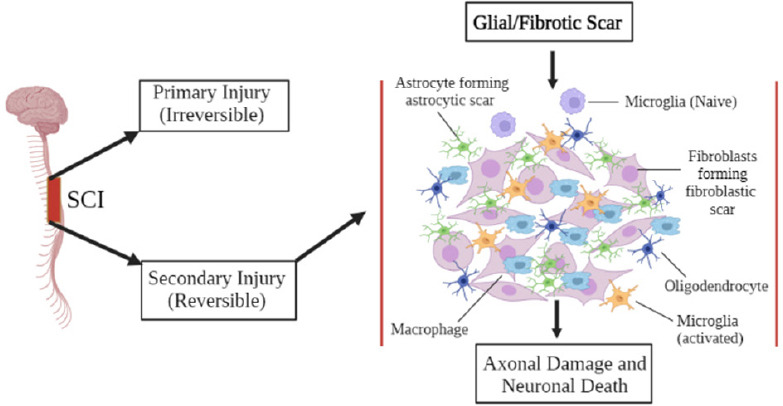
Glial scar formation after SCI.
The figure displays the two injury types, primary and secondary, conceived from a sectioning of SCI. Microglia are activated in the secondary injury process and induce inflammation at the lesion site, promoting the production of glial scar formation. Astrocytes begin forming astrocytic scar tissue and fibroblasts develop fibroblastic scars. Ultimately this leads to axonal damage and neuronal death if left untreated. SCI: Spinal cord injury.
The consequence of scar formation in the CNS is usually considered more detrimental than that in the peripheral organs because of impaired neural function recovery. The glial scar may be roughly divided into two main components - fibrous and glial (Leal-Filho, 2011). The fibrotic scar consists of extracellular matrix proteins such as fibronectin, collagen, and laminin that are secreted by infiltrating fibroblasts (Shearer and Fawcett, 2001). Fibroblasts in the lesion can be activated by a number of pro-fibrotic factors, including transforming growth factor-α and connective tissue growth factor. However, in severe lesions that lyse the meninges, the astroglia are mixed with the invading connective tissue components. The response to initial injury is referred to as reactive gliosis when the glial cell division occurs. This is typically restricted within the immediate penumbra which surrounds the lesions core (Faulkner et al., 2004). An increased production of intermediate filaments causes hypertoric reactivity of the astrocytes. Reactive astrocytes can become enlarged (thickened processes) and entangled around the dystrophic end balls of non-regenerating fibers. Thus, the production of reactive glia is often responsible for failed restoration and regeneration. The glial scar can develop a rubbery tenacious textured membrane over time (Pang et al., 2021; Wang et al., 2021).
Glial cells are highly plastic and are heavily influenced by their environmental surrounds. They can exhibit multiple functions and roles depending on the cellular settings (Yang et al., 2020). SCI lesion sites create an ion imbalance, free radical production, glutamate accumulation, reactive species, and an excessive production of reactive oxygen species. Excessive inflammatory responses are induced by the activation of infiltrating peripheral immune cells and colonized microglia. With the formation of glial scars in the early stages of injury, the scar can confine the inflammatory cells and other toxic molecules to control the environment of the damaged tissue area. This mechanism may aid in protecting the healthy spinal tissue from the surrounding inflammatory response (Pang et al., 2021).
Inhibition of neuronal and glial cell death after SCI is a fundamental approach for reducing glial scars. It is believed that axons with dystrophic endings do not lose their ability to regenerate. Even after four weeks of stagnation in the lesion environment, chronically injured spinal cord axons have been shown to regenerate into implanted peripheral nerve grafts (Houle, 1991). This study also showed the extent to which axons will retract after SCI. The hemisection of the cervical cord resulted in numerous dystrophic endbulbs forming. The persistence of the “growth cones” implies that cytoskeletal and membrane plasticity must be occurring in order to maintain the axon viability and stability (DeBrot and Yao, 2018; Allen et al., 2021).
Demyelination and Remyelination after Spinal Cord Injury
Myelin is a protective sheath surrounding axons. It facilitates the conduction of nerve impulses traveling along neuronal projections and allows for cell signaling. A multitude of oligodendrocyte processes covers a single axon to form the myelinated segments (Papastefanaki and Matsas, 2015; Hughes and Appel, 2019; Kuhn et al., 2019). Oligodendrocytes are the myelin-forming cells within the CNS that regulate the mechanical process of myelination and are sometimes aided by glial cells like microglia (Pukos et al., 2019; Cunha et al., 2020; Kalafatakis and Karagogeos, 2021). The nerve impulses jump from one node to another via voltage-gated channels. The myelinated glia is in constant communication with the axons and provides protection and support for axonal integrity.
When SCI occurs, the communication between myelinated glia and axons is disrupted, and axonal demyelination begins to arise in concurrence with a loss of neuronal function (Wu et al., 2021; Xie et al., 2021). Primary mechanical damages can occur immediately after the initial trauma, while secondary damage will transpire over a period of hours to days (Papastefanaki and Matsas, 2015). The initial injury side can induce a cascade of deleterious events and spread to surrounding tissues, creating a non-permissive regeneration environment leading to permanent loss of function (P Drasites et al., 2020).
Remyelination refers to the generation of new myelinating cells through a resident stem cell and/or oligodendrocyte progenitor cells that will replace lost oligodendrocytes, producing new myelin sheaths around the stripped axons (Xie et al., 2021). Remyelination is critical for the restoration of impulse condition as well as the formation and proper restoration of nodal and internodal regions of the axon (Papastefanaki and Matsas, 2015; Xie et al., 2021). Oligodendroglial cells promote the production of myelin through cell differentiation (Bacmeister et al., 2020; Fessel, 2022). This cell differentiation and maturation produces the sheath around the axon (Figure 2). Although myelination is predominately driven by oligodendrocytes, the other glial cells including astrocytes and microglia, also contribute to this process (Traiffort et al., 2020). For example, astrocytes provide lipids for myelin sheath production, and microglia-oligodendrocytes communication can help in myelin production. While mechanisms underlying astrocytic mediation of myelin sheath production and enhanced microglia-induced oligodendrocyte differentiation remain unclear, subpopulations of microglia and astrocytes may play roles in demyelination and remyelination after SCI. The utilization of a myelinating agent, Clemastine, may allow for the promotion of oligodendrocyte cells to remyelinate the sheath of the axon as shown in Figure 2. A recent study has shown that Clemastine treatment preserves myelin integrity, decreases loss of axons, and improves functional recovery in the rat SCI model (Du et al., 2022).
Figure 2.
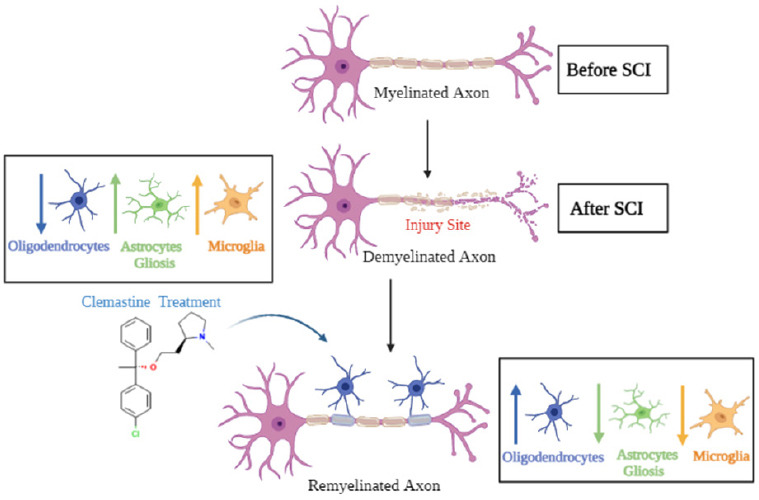
Demyelination and remyelination of a neuron.
This figure displays the contrast between a demyelinated axon and the breakdown of the myelin sheath with a properly remyelinated axon. The first neuron displays a properly myelinated axon prior to SCI. After SCI, the neuron is damaged and demyelination occurs alongside an increase in astrocyte gliosis and microglial activation, with a decrease in oligodendrocyte population. The utilization of clemastine may allow for the promotion of oligodendrocyte cells to remyelinate the sheath of the axon. Clemastine treatment may reduce gliosis and promote recovery of myelination by oligodendrocytes and survival of neurons. SCI: Spinal cord injury.
Axonal vulnerability can be avoided with remyelination, as it provides protection and metabolic support through oligodendrocytes and myelin layering. Spontaneous remyelination does not always guarantee a completely functional recovery. It is critical to avoid major amounts of demyelination in the early stages of injury to prevent permanent functionality damages. Demyelination results in damage and stripping of the protective layering of the myelin sheath which surrounds the neuronal axons (Wu et al., 2021). Damage to the sheath results in disruption of impulse signaling and can lead to neurological issues (Swanson, 2020).
Rodent and human studies on SCI suggest that demyelination of spared axons can occur in the surrounding tissues of the injury site. In a study conducted by James et al. (2011) rodents suffering from acute SCI experienced a complete conduction block in the ascending dorsal column axons in the timeframe of 2–4 weeks. The conduction becomes partially restored overtime; however, there was no evidence of further improvements at the chronic phase between 3–6 months post injury. The researchers identified a population of chronically demyelinated axons that were incapable of conducting any electrical activity (Papastefanaki and Matsas, 2015).
Neurogenic Muscle Loss after Spinal Cord Injury
Skeletal muscle makes up roughly 40% of the total human body mass, it is an endocrine organ and thus secretes myokines such as peptides, growth hormones, and cytokines which regulate the body to maintain homeostasis (P Drasites et al., 2020). SCI causes major disruptions to this organ system and without proper treatment; it can have lasting and possibly permanent effects on an individual’s health. Muscle atrophy is a secondary complication followed by initial SCI. It has detrimental effects on an individual and can lead to other complications such as immobility and loss in mechanical function. Movement occurs through muscle fiber operations, which is initiated by power and force to create contraction and relaxation of the muscle with the sliding filament theory. After SCI, there is a decrease in joint ability to maintain mechanical loading and a disruption in the neuro-muscular junction signaling. Damaged nerve endings and neurons can no longer send signaling to allow for muscle movements. This lack of mobility causes a decline in physical activity and increases muscle atrophy (P Drasites et al., 2020). Post-injury inflammation coincides with degradation of muscle fibers and progresses to cause immobility. Immobilized patients typically experience a decrease in tension and loading on joints and muscles which correlates with a decline in protein synthesis. Muscle atrophy can increase alongside inactivity (P Drasites et al., 2020).
SCI can cause rapid and severe loss of muscle mass below the site of injury. This degeneration of muscle tissue can be associated with serious risk factors and influence a lower percentage in the recovery rate among individuals with SCI (Giangregorio and McCartney, 2006; Jiang et al., 2021). SCI is also associated with involuntary movement and spasms in the muscle due to excitatory postsynaptic potentials (Jiang et al., 2021; Sangari et al., 2021). The development of spasticity is common after damage to the corticospinal axons (Sangari et al., 2021). Ubiquitin-proteasome systems relate to the degradation of intracellular proteins which contribute to the catabolism of skeletal muscles. Ubiquitin-proteasome systems-related biomarkers such as ring finger protein 1 (MuRF-1), muscle atrophy F-box protein (MAF-bx/atrogen-1), and E3 ubiquitin ligases can express signaling for atrophy in muscle tissues. Muscle breakdown and cell death can be identified with increased expression and activity of calpain relating to mitochondrial damages after SCI (P Drasites et al., 2020). Myopenia is also commonly diagnosed and linked with SCI (Invernizzi et al., 2020a; Bigford et al., 2021). Individuals who were six weeks post-SCI recovery had an average cross-sectional area of muscle 18% to 46% lower than in control subjects (Castro et al., 1999). A prospective study of these patients revealed that after twenty-four weeks post SCI there were further declines in the average gastrocnemius and soleus muscles of cross-sectional areas of 24% and 12% (Castro et al., 1999). Another prospective study employing a dual-energy-X-ray scan reported a 15% drop of lower limb lean mass in the first year after SCI (Wilmet et al., 1995).
Aging and the duration of injury are associated with a decrease in lean mass. Sublesional areas can experience muscle atrophy and degeneration of connective muscle fibers (Otzel et al., 2021; Zhao et al., 2021). A study conducted using monozygotic twins showed significantly lower trunk and lean leg mass in twins affected with SCI (Spungen et al., 2000). The neuromuscular junction is the source from which signals are received and transmitted to the muscles. These signals travel from the brain through the spinal cord to the motor neurons of the muscle to produce movement and motor function. However, after SCI the neuromuscular junction functions are drastically compromised. Muscle wasting and degradation occurs after an injury, and it can be categorized as denervation or disuse atrophy. Disuse atrophy is triggered by a loss in the utilization of the muscles causing shrinkage of the muscle fibers, whereas denervation atrophy is induced after damage to nerve endings occurs which breaks the communication signaling between the muscles and the brain. Studies have shown that without this signaling the neuromuscular junction disorders typically decrease nerve cell activity causing muscle weakness and withering. Atrophy is onset by a decline in muscle protein expression joined with an increase in the intramuscular proteolytic enzyme activity (P Drasites et al., 2020). Changes in muscle tissue combined with adaptations to preserved spinal circuitry may contribute to spastic motor behaviors and a loss in neuromuscular control after SCI. Musculoskeletal atrophy can be linked to central nervous system disruptions (P Drasites et al., 2020).
Individuals with SCI have 15% less muscle tissue in the fat-free soft tissue when compared to control subjects with no injury. A decrease in muscle mass can lead to a reduction in metabolic rate combined with an increase in fat storage (Monroe et al., 1998). SCI individuals typically have reduced energy expenditure due to limitations in mobility and may experience muscle atrophy due to this reduction in activity (Invernizzi et al., 2020a). Individuals may also experience a reduction in resting metabolic rate due to reduced peripheral sympathetic nervous system activity (Monroe et al., 1998).
Myelination Supports Axonal Recovery and Neuronal Survival Following Spinal Cord Injury
The myelin sheath is formed as a multilamellar membrane structure by the spiral wrapping and subsequent compaction of the oligodendroglial plasma membrane around CNS axons. Dynamic changes to axonal myelination occur throughout life or through remodeling of existing myelin sheaths. Oligodendrocytes are responsive to neuronal activity, which has been shown to induce changes to myelin sheaths, potentially to optimize conduction and neural circuit function. However, CNS injuries can induce multiple barriers to axonal growth. One barrier group linked to the injured neurons is the myelin-associated inhibitors (MAIs). MAIs are molecules located in the myelin of the CNS that regulate axonal growth (Geoffroy and Zheng, 2014). MAIs are correlated with axonal tracts, axon sprouting, and reproducibility presented in a variety of injury models. The regeneration of axons is limited by targeting these molecules. MAIs are likely to act as a barrier to regeneration and extrinsic axon growth modulators. Inhibitory signaling factors significantly decrease remyelination of axons (Grinspan, 2020). Bone morphogenetic proteins strongly inhibit oligodendrocyte progenitor differentiation as well as myelination. These proteins are highly expressed during pre-natal development of the dorsal CNS and help in the regulatory process of dorsal ventral patterning. In demyelinating diseases such as multiple sclerosis (MS), bone morphogenic proteins are expressed by immune cells invading the CNS. Bone morphogenic proteins in the CNS are associated with inflammation and increased demyelination of axons (Grinspan, 2020). Three prototypical inhibitors are Nogo, myelin-associated glycoprotein, and oligodendrocyte-myelin glycoprotein which signal through multiple neuronal receptors to exert growth inhibition (Vourc’h and Andres, 2004; Schwab, 2010; Geoffroy and Zheng, 2014). The targeting of myelin inhibition helps to modulate the compensatory sprouting of the uninjured axons. However, the effect on regeneration of these injured axons may be limited.
Regeneration and sprouting are separate terms used to define axonal growth. Regeneration is repair and axonal growth after an injury has occurred, while sprouting is the axonal growth of uninjured neurons (Geoffroy and Zheng, 2014). Modulating the sprouting and employing its naturally occurring form of restoration mechanics can be an additional measure for promoting functional repair after injury to the CNS (Geoffroy and Zheng, 2014). MAIs have shown potential inhibitory activity on the neurite growth in vitro (Alabed et al., 2007; Chen et al., 2020). MAIs utilize signaling through neuronal receptors and co-receptors to cause cytoskeleton rearrangements and neurite inhibition. The signaling pathways involve both Rho and Rho-associated kinase. Many MAIs are expressed by myelin and oligodendrocytes (Geoffroy and Zheng, 2014). While axonal regeneration in the mature CNS is limited by MAIs, neutralization of those inhibitors can help regeneration in vivo. Various treatment strategies have been developed overtime to counteract the effects of MAIs, and these treatments promote axonal regeneration in the damaged spinal cord and promote functional recovery through enhanced plasticity.
Drugs That Support Myelination in Spinal Cord Injury
The FDA currently approves only two drugs, miconazole and clobetasol, which demonstrate the ability to promote oligodendrocyte precursor cells maturation as well as enhance remyelination of axons. These drugs have been tested and provide evidence of increasing the number of new oligodendrocytes in mice brains (Wang et al., 2011; Najm et al., 2015). In a mouse model of MS, miconazole and clobetasol were administered and promoted the remyelination of the axons. These drugs can cross the blood-brain barrier and display a striking reversal of demyelination in the animal models. Both drugs appear to promote remyelination, also clobetasol works to suppress the immune system while miconazole appears to have no side effects on the immune system.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used in medical practice to help relieve pain and reduce inflammation after injury. NSAIDs such as ibuprofen subdue intracellular RhoA signal and improve significant axonal growth, function, and recovery post CNS injury (Xing et al., 2011). NSAIDs have demonstrated the ability to reduce the generation of amyloid-beta42 peptide via the inactivation of RhoA signal. This in turn supports RhoA-repressing functions of NSAIDs. Drug treatments such as indomethacin and ibuprofen with RhoA-inhibiting NSAIDs drastically reduce oligodendrocyte-related cell death. The RhoA-inhibiting NSAIDs therapeutic target has shown to promote remyelination and assist in the recovery of the CNS after SCI. Treatment studies utilizing these drugs resulted in a significant increase in axonal myelination as well as white matter tracts after traumatic contusions resulting from SCI. Axonal elongation prometon, glial survival, and remyelination of axons were all displayed with the NSAID and its inactivation of RhoA (Xing et al., 2011).
Results from a four-week in vivo study on rats with SCI assessed the myelin integrity and protein expression using transmission electron microscopy and immunofluorescence (Du et al., 2022). Clemastine treatment was shown to preserve myelin integrity as well as decrease the loss of axons and to improve functional recovery in the SCI rat model. While Clemastine does not fully restore all damages to the myelin sheath, it can be utilized as a therapeutic in combination with other forms of rehabilitation. This study confirmed that clemastine utilization enhanced oligodendrocyte differentiation and myelination. This enhancement helps to delay axonal degeneration and promote motor function recovery (Chen et al., 2022; Du et al., 2022). This therapeutic agent has been shown to work by blocking histamines created by the body to combat an allergic reaction. Histamines are an immune response used to block foreign pathogens from entering the body. They are produced by mast cells and basophils found within connective tissue and create inflammation. Antihistamines compete with histamine for cell receptor sites on effector cells. CF also blocks acetylcholine which alleviates fluids secreted during the initial anaphylactic reaction. Common brands of Clemastine include TAVIST and Agasten.
Clemastine fumarate, also known as (+) -2-[2-[(p-chloro-a-methyl-a-phenylbenzyl) oxy] ethyl]-1-methylpyrrolidine hydrogen fumarate, is categorized as a benzhydryl ether antihistaminic compound. It has a molecular weight of 460.0 (molecular formula C25H30CINO5) and is a synthetic ethanolamine with anticholinergic, sedative, and histamine H1 antagonistic properties. This drug acts as an antihistamine that blocks the H1 histamine receptor and prevents the associated symptoms that are caused by histamine activities. Specifically, it can reduce inflammation of the capillaries, bronchial and gastrointestinal smooth muscles including vasodilation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscles. This drug has been observed to aid in the prevention of histamine-induced pain and itching of mucous membranes (Administration). Clemastine fumarate,the fumaric acid salt of Clemastine, is also an antihistamine with antimuscarinic and moderate sedative properties. Its purpose is to treat allergic conditions such as rhinitis, urticaria, conjunctivitis, and in pruritic skin conditions and provide symptomatic relief. It has a role as a H1-receptor antagonist, an anti-allergic agent, a muscarinic antagonist, and an antipruritic drug as it contains the chemical makeup of Clemastine.
Clemastine Improves Myelination, Recovery of Neurons and Muscle
While Clemastine has antihistamine properties with anticholinergic and sedative side effects, it exhibits therapeutic potential in demyelinating diseases and SCI (Cree et al., 2018; Du et al., 2022). Patients who received an injection of Clemastine were observed to have peak antihistaminic activity 5 to 7 hours after the initial injection. The antihistaminic effects have been shown to persist for 10 to 12 hours after receiving the initial histamine injection. In some cases, the activity of the drug has been observed for up to 24 hours. With oral administration, it is absorbed from the gastrointestinal tract rapidly, and the peak plasma concentration can be attained in 2 to 4 hours. The major mode of excretion is via urinary emission (Administration).
A study (No author listed, 2022) conducted by Cristopher Turski and colleagues tested the effect of Clemastine in rat models with a myelination disorder (Turski et al., 2018). The rare inherited demyelinating disorder known as Pelizaeus Merzbachers diseases (PMD) manipulates the CNS causing detrimental neurological symptoms of ataxia, rotary nystagmus, pyramidal signs, seizures, extrapyramidal movement disorders, psychomotor delay, and intellectual disability. Individuals who inherit this X-linked recessive trait typically remain in life-long chronic care (Wilmet et al., 1995). New observations in the canine model of PMD suggest that there is a gradual spontaneous differentiation of oligodendrocyte progenitor cells in the spinal cord, but this is not present in the brain. This data correlates with clinical trials in animals, which monitored 2-year-old dogs who displayed more myelin microscopically in the spinal cord. These findings suggest that drugs such as Clemastine could help to promote differentiation of oligodendrocyte precursor cells (OPCs) earlier in the brain and spinal cord of PMD patients. Thus, clemastine could advance myelination and result in functionally significant neurologic improvement of PMD individuals. Other researchers reported that a cluster of antimuscarinic compounds improve oligodendrocyte differentiation and remyelination (Cully, 2014; Abiraman et al., 2015; Huntemer-Silveira et al., 2020). Clemastine is also one of the compounds noted to promote this differentiation, as it is an antihistamine and anticholinergic medication licensed in the United States for treatment of allergic conditions.
Clemastine is known to exhibit promotion of remyelination in lysolecithin-induced demyelination in a mouse model (Wilmet et al., 1995). A recent study attempted to determine if remyelination could be achieved through drug treatment utilizing clemastine (Green et al., 2017). Patients diagnosed with relapsing MS and chronic demyelinating optic neuropathy on stable immunomodulatory therapy participated in a double-blind, randomized, placebo-controlled crossover trial. Capsules of 5.36 mg clemastine fumarate were provided to the active treatment group while the untreated group received a placebo. The findings suggested that myelin repair was achievable via drug treatment for patients afflicted by chronic demyelinating disease such as MS. The evidence suggested in this study is among the first to display the remyelination effects of clemastine in chronic neurodegenerative conditions. Other preclinical data showed that clemastine encourages oligodendrocyte precursor differentiation and remyelination without suppressing the immune system (Mei et al., 2016; Kolahdouzan et al., 2019; Huntemer-Silveira et al., 2020). Myelin repair is demonstrated with clemastine as a drug treatment during chronic demyelination induced by MS in patients (Green et al., 2017). It also acts as a therapy to treat hypoxic brain injuries derived from white matter injury. Failed myelin regeneration during hypoxia can be linked to the hindrance of OPCs differentiation as a profound factor. Clemastine not only promotes oligodendrocyte differentiation and maturation it also allows for remyelination of axons and neuronal survival (Wu et al., 2021).
Clemastine is classified as a histamine H1 receptor blocker, which prevents the immune system from prompting an inflammatory reaction due to the onset of an injury. Clemastine preserves myelin sheath integrity through the promotion of oligodendrocyte development which allows for remyelination (Figure 3). Clinical research has proven clemastine treatments offer neurovegetative effects by increasing OPCs differentiation and allowing for remyelination of the neuronal axon sheath (Gruchot et al., 2019). One possible mechanism is that clemastine is known to modulate autophagy in an environment-dependent manner, as it can enhance autophagy by inhibiting mTOR (Li et al., 2021). Thus, clemastine could induce the upregulation of mTOR-mediated autophagy, which contributes to preventing the cellular senescence of OPCs. Examining the relationship between mTOR signaling, autophagy, and cellular senescence following SCI and treatment with clemastine would be interesting.
Figure 3.
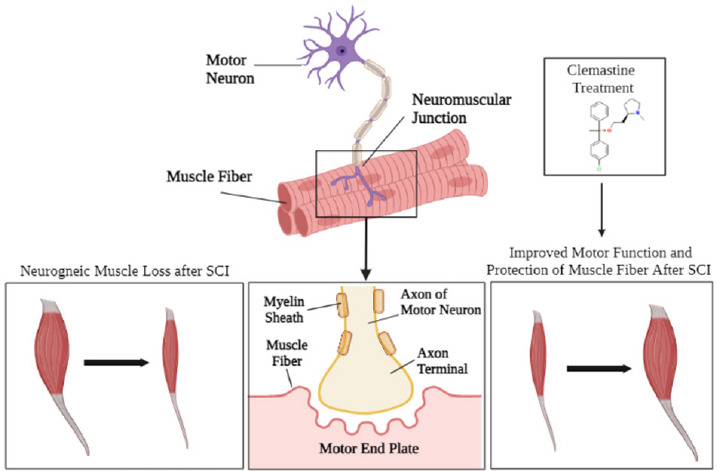
Muscle atrophy following SCI.
Before SCI occurs, the spinal cord is intact and the surrounding muscle fibers are undamaged. The motor neuron axons are intact and connected to the muscle fiber endings via the axon terminal. However, once injury occurs the axons are broken and cut due to the impact of the trauma. Neurogenic muscle loss occurs after SCI due to atrophy and damage to the motor neuron from demyelination. Clemastine treatment may promote remyelination and regeneration will begin to occur after the initial injury. The axons will begin remyelinating and muscle fibers are protected, and functionality begins to restore. SCI: Spinal cord injury.
Despite rehabilitative efforts over an extended period, certain neurological impairments stimulated by SCI may be irreversible and functionality can be permanently lost after injury. Depending on the severity of the injury, it is possible for gradual improvements to be made to locomotive function with the utilization of drug therapy and other forms of treatment such as timely progressive physical therapy coincided with adequate daily care (Yip and Malaspina, 2012). After SCI, many patients experience dramatic changes in their body composition which can lead to muscle atrophy and potentially an increase in fat mass due to cardiometabolic hindrance. Often, patients with SCI will become diagnosed with obesity and experience bone loss due to immobility and reduction in locomotor function (Fernndez and Ribeiro, 2019; McMillan et al., 2021). However, this diagnosis is reversible with proper treatment. In a separate study, lower limb muscles showed improvement in mobility and composition after SCI with the treatment of cardiovascular function with epidural stimulation (Legg Ditterline et al., 2020). Circuit resistance training was shown to aid in muscle maintenance and strengthening which promoted functional agility and mobility (Alves Rodrigues et al., 2021). Exercise can positively benefit patients with SCI as it allows for an increase in cardiovascular/muscular endurance, muscle strength, flexibility, stability, and maintenance of weight. Selective stimulation patterns during exercise can promote the workload and power output of paralyzed muscles. Overall, exercise-based treatment therapies paired with the Clemastine treatment can significantly help reverse the secondary injury damages occurring in SCI individuals. Exercise and physical therapy should be appropriately diagnosed and introduced to SCI patients overtime based on the individuals’ specific needs. While exercise does promote muscle tissue regeneration and growth, it should be slowly incorporated as a treatment option to deter from further damages to the initial injury of the spinal cord. Proper regimented and light exercises customized to the individual can aid in the regaining of functional agility and mobility alongside muscle strengthening (Alves Rodrigues et al., 2021).
Conclusions
SCI results in a loss of functional mobility, sensations, and neuromuscular and skeletal function. Injury can lead to apoptosis of the neurons and degradation of oligodendrocytes causing demyelination of the axon. Paralysis can also be an effect of SCI inducing immobility and the development of tetraplegia or quadriplegia. Fine motor skills degrade after paralysis therefore protection of neurons and skeletal muscle is critical to regain those locomotor functions. As the global rate of SCI-related incidents continues to rise, there is an urgent need for therapeutic treatment options. This review serves to evaluate Clemastine’s clinical relevance in relation to SCI patients and analyze the benefits of its use as a potential treatment. Although the detrimental effects of SCI can cause irreversible damages through the primary injury, treatment can be provided to patients in order to reverse the effects of secondary injury. Clemastine has been clinically proven to provide protection of axonal nerve endings after SCI as well as inducing remyelination through oligodendrocyte promotion and stimulating muscle fiber growth. Clemastine treatments negate cell death and the formation of glial scars while protecting further damages to the spinal cord and muscle tissue through remyelination and promoting the recovery of cognitive function. Clemastine could potentially serve as a positive drug treatment reinforcer alongside other clinically proven drugs and treatments to aid in the recovery from SCI. Although it has been clinically established and shown to support remyelination in damaged axons and deter cell death after injury, there are still more to be discovered about Clemastine’s potentially beneficial mechanisms. Clemastine is a relatively new form of drug treatment for SCI patients and research should continue to be conducted to formulate new findings and potential breakthroughs about this drug’s clinical relevance and therapeutic properties. With the utilization of these treatments, individuals suffering from SCI could potentially regain muscle function, and mobility, and have an overall improvement in their quality of life.
Acknowledgments:
We thank the Citadel Military College in Charleston for supporting Ali Myatich for her MS research program. We also thank Denise Matzelle (Department of Neurosurgery at the Medical University of South Carolina) for technical assistance. We also acknowledge BioRender.com for figures.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Funding: This work was supported in part by funding from the Veterans Administration (1IOBX001262, 1I01 BX004269) and South Carolina State Spinal Cord Injury Research Fund (SCIRF-2015P-01, SCIRF-2015P-04, SCIRF-2015-I-01, SCIRF #2016 I-03, and SCIRF #2018 I-01) (to AH). This work was also supported in part by funding from the National Institutes of Health (1R21NS118393-01) (to AH). Dr. Naren L. Banik is the recipient of a Research Career Scientist award (# IK6BX005964) from the Department of veterans Affairs.
References
- 1.Abiraman K, Pol SU, O'Bara MA, Chen GD, Khaku ZM, Wang J, Thorn D, Vedia BH, Ekwegbalu EC, Li JX, Salvi RJ, Sim FJ. Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J Neurosci. 2015;35:3676–3688. doi: 10.1523/JNEUROSCI.3510-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 3.Alabed YZ, Pool M, Ong Tone S, Fournier AE. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J Neurosci. 2007;27:1702–1711. doi: 10.1523/JNEUROSCI.5055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen LL, Nichols NL, Asa ZA, Emery AT, Ciesla MC, Santiago JV, Holland AE, Mitchell GS, Gonzalez-Rothi EJ. Phrenic motor neuron survival below cervical spinal cord hemisection. Exp Neurol. 2021;346:113832. doi: 10.1016/j.expneurol.2021.113832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves Rodrigues J, Torres Pereira E, Salgado Lopes J, MV DAFS, Resende NM, Fernandes DASS, Aidar FJ, Patrocinio DEOCE, Costa Moreira O. Effects of circuit resistance training on muscle power, functional agility, and bones'mineral content in people with spinal injury. J Sports Med Phys Fitness. 2021;61:505–511. doi: 10.23736/S0022-4707.20.11286-6. [DOI] [PubMed] [Google Scholar]
- 7.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacmeister CM, Barr HJ, McClain CR, Thornton MA, Nettles D, Welle CG, Hughes EG. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat Neurosci. 2020;23:819–831. doi: 10.1038/s41593-020-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- 10.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 11.Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve. 2009;40:499–519. doi: 10.1002/mus.21391. [DOI] [PubMed] [Google Scholar]
- 12.Bigford GE, Donovan A, Webster MT, Dietrich WD, Nash MS. Selective myostatin inhibition spares sublesional muscle mass and myopenia-related dysfunction after severe spinal cord contusion in mice. J Neurotrauma. 2021;38:3440–3455. doi: 10.1089/neu.2021.0061. [DOI] [PubMed] [Google Scholar]
- 13.Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. doi: 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekaran S, Davis J, Bersch I, Goldberg G, Gorgey AS. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen Res. 2020;15:1397–1407. doi: 10.4103/1673-5374.274326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Wang H, Xu H, Zhang Y, Sun H. Electroacupuncture promotes axonal regrowth by attenuating the myelin-associated inhibitors-induced RhoA/ROCK pathway in cerebral ischemia/reperfusion rats. Brain Res. 2020;1748:147075. doi: 10.1016/j.brainres.2020.147075. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Tang Y, Vogel LC, Devivo MJ. Causes of spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19:1–8. doi: 10.1310/sci1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Sheng J, Tang X, Zhao Y, Zhu S, Liu Q. Clemastine rescues chemotherapy-induced cognitive impairment by improving white matter integrity. Neuroscience. 2022;484:66–79. doi: 10.1016/j.neuroscience.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Chew C, Sengelaub DR. Exercise is neuroprotective on the morphology of somatic motoneurons following the death of neighboring motoneurons via androgen action at the target muscle. Dev Neurobiol. 2021;81:22–35. doi: 10.1002/dneu.22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox A, Capone M, Matzelle D, Vertegel A, Bredikhin M, Varma A, Haque A, Shields DC, Banik NL. Nanoparticle-based estrogen delivery to spinal cord injury site reduces local parenchymal destruction and improves functional recovery. J Neurotrauma. 2021;38:342–352. doi: 10.1089/neu.2020.7047. [DOI] [PubMed] [Google Scholar]
- 21.Cree BAC, Niu J, Hoi KK, Zhao C, Caganap SD, Henry RG, Dao DQ, Zollinger DR, Mei F, Shen YA, Franklin RJM, Ullian EM, Xiao L, Chan JR, Fancy SPJ. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141:85–98. doi: 10.1093/brain/awx312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cully M. Neurodegenerative diseases: Pillars of remyelination. Nat Rev Drug Discov. 2014;13:651. doi: 10.1038/nrd4416. [DOI] [PubMed] [Google Scholar]
- 23.Cunha MI, Su M, Cantuti-Castelvetri L, Muller SA, Schifferer M, Djannatian M, Alexopoulos I, van der Meer F, Winkler A, van Ham TJ, Schmid B, Lichtenthaler SF, Stadelmann C, Simons M. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J Exp Med. 2020;217:e20191390. doi: 10.1084/jem.20191390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBrot A, Yao L. The combination of induced pluripotent stem cells and bioscaffolds holds promise for spinal cord regeneration. Neural Regen Res. 2018;13:1677–1684. doi: 10.4103/1673-5374.238602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 26.Du W, Deng Y, Jiang R, Tong L, Li R, Jiang X. Clemastine enhances myelination, delays axonal loss and promotes functional recovery in spinal cord injury. Neurochem Res. 2022;47:503–515. doi: 10.1007/s11064-021-03465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehlings MG, Hawryluk GW. Scarring after spinal cord injury. J Neurosurg Spine. 2010;13:165–167. doi: 10.3171/2009.11.SPINE09862. [DOI] [PubMed] [Google Scholar]
- 29.Fernndez SSM, Ribeiro SML. Low appendicular lean mass index and associations with metabolic and demographic parameters in wheelchair athletes with spinal cord injury. J Neuromuscul Dis. 2019;6:517–525. doi: 10.3233/JND-190409. [DOI] [PubMed] [Google Scholar]
- 30.Fessel J. Reversing Alzheimer's disease dementia with clemastine, fingolimod or rolipram, plus anti-amyloid therapy. Alzheimers Dement (N Y) 2022;8:e12242. doi: 10.1002/trc2.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu D, Chen C, He L, Li J, Li A. Protective effect of mild hypothermia on spinal cord ischemia-induced delayed paralysis and spinal cord injury. Neurochem Res. 2022;47:1212–1225. doi: 10.1007/s11064-021-03515-7. [DOI] [PubMed] [Google Scholar]
- 32.Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol. 2014;27:31–38. doi: 10.1016/j.conb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Budingen HC, Henry RG, Hauser SL, Chan JR. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled double-blind, crossover trial. Lancet. 2017;390:2481–2489. doi: 10.1016/S0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- 35.Grinspan JB. Inhibitors of myelination and remyelination, bone morphogenetic proteins are upregulated in human neurological disease. Neurochem Res. 2020;45:656–662. doi: 10.1007/s11064-020-02980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruchot J, Weyers V, Gottle P, Forster M, Hartung HP, Kury P, Kremer D. The molecular basis for remyelination failure in multiple sclerosis. Cells. 2019;8:825. doi: 10.3390/cells8080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houle JD. Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve grafts. Exp Neurol. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- 38.Hughes AN, Appel B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat Commun. 2019;10:4125. doi: 10.1038/s41467-019-12059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huntemer-Silveira A, Patil N, Brickner MA, Parr AM. Strategies for oligodendrocyte and myelin repair in traumatic cns injury. Front Cell Neurosci. 2020;14:619707. doi: 10.3389/fncel.2020.619707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Invernizzi M, de Sire A, Carda S, Venetis K, Reno F, Cisari C, Fusco N. Bone muscle crosstalk in spinal cord injuries: pathophysiology and implications for patients'quality of life. Curr Osteoporos Rep. 2020a;18:422–431. doi: 10.1007/s11914-020-00601-7. [DOI] [PubMed] [Google Scholar]
- 41.Invernizzi M, de Sire A, Reno F, Cisari C, Runza L, Baricich A, Carda S, Fusco N. Spinal cord injury as a model of bone-muscle interactions: therapeutic implications from in vitro and in vivo studies. Front Endocrinol (Lausanne) 2020b;11:204. doi: 10.3389/fendo.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang MC, Birch DV, Heckman CJ, Tysseling VM. The involvement of CaV1.3 channels in prolonged root reflexes and its potential as a therapeutic target in spinal cord injury. Front Neural Circuits. 2021;15:642111. doi: 10.3389/fncir.2021.642111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalafatakis I, Karagogeos D. Oligodendrocytes and microglia: key players in myelin development, damage and repair. Biomolecules. 2021;11:1058. doi: 10.3390/biom11071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolahdouzan M, Futhey NC, Kieran NW, Healy LM. Novel molecular leads for the prevention of damage and the promotion of repair in neuroimmunological disease. Front Immunol. 2019;10:1657. doi: 10.3389/fimmu.2019.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8:1424. doi: 10.3390/cells8111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwiecien JM. Barriers to axonal regeneration after spinal cord injury: a current perspective. Neural Regen Res. 2022;17:85–86. doi: 10.4103/1673-5374.314299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62–68. doi: 10.1038/sc.1995.16. [DOI] [PubMed] [Google Scholar]
- 48.Leal-Filho MB. Spinal cord injury: from inflammation to glial scar. Surg Neurol Int. 2011;2:112. doi: 10.4103/2152-7806.83732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Legg Ditterline B, Harkema SJ, Willhite A, Stills S, Ugiliweneza B, Rejc E. Epidural stimulation for cardiovascular function increases lower limb lean mass in individuals with chronic motor complete spinal cord injury. Exp Physiol. 2020;105:1684–1691. doi: 10.1113/EP088876. [DOI] [PubMed] [Google Scholar]
- 50.Li ZY, Chen LH, Zhao XY, Chen H, Sun YY, Lu MH, Wang ZT, Chen M, Lu L, Huang W, Chen R, Xu DE, Xu RX, Ma QH. Clemastine attenuates AD-like pathology in an AD model mouse via enhancing mTOR-mediated autophagy. Exp Neurol. 2021;342:113742. doi: 10.1016/j.expneurol.2021.113742. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci. 2016;36:957–962. doi: 10.1523/JNEUROSCI.3608-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lossi L, Castagna C, Merighi A. Caspase-3 mediated cell death in the normal development of the mammalian cerebellum. Int J Mol Sci. 2018;19:3999. doi: 10.3390/ijms19123999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lou WP, Mateos A, Koch M, Klussman S, Yang C, Lu N, Kumar S, Limpert S, Göpferich M, Zschaetzsch M, Sliwinski C, Kenzelmann M, Seedorf M, Maillo C, Senis E, Grimm D, Puttagunta R, Mendez R, Liu K, Hassan BA, et al. Regulation of adult CNS axonal regeneration by the post-transcriptional regulator Cpeb1. Front Mol Neurosci. 2017;10:445. doi: 10.3389/fnmol.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMillan DW, Nash MS, Gater DR, Jr, Valderrabano RJ. Neurogenic obesity and skeletal pathology in spinal cord injury. Top Spinal Cord Inj Rehabil. 2021;27:57–67. doi: 10.46292/sci20-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mei F, Lehmann-Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S, Xiao L, Teuscher C, von Budingen HC, Wess J, Lawrence JJ, Green AJ, Fancy SP, Zamvil SS, Chan JR. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5:e18246. doi: 10.7554/eLife.18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68:1223–1227. doi: 10.1093/ajcn/68.6.1223. [DOI] [PubMed] [Google Scholar]
- 57.Morgan KA, Paton S, Patten A, Tucker S, Walker K. Community-based exercise goals of persons with spinal cord injury: interpreted using the International Classification of Functioning, Disability, and Health. J Spinal Cord Med. 2021;doi: 10.1080/10790268.2021.1970896 doi: 10.1080/10790268.2021.1970896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, Quick KL, Schlatzer DM, Tang H, Papoian R, Brimacombe KR, Shen M, Boxer MB, Jadhav A, Robinson AP, Podojil JR, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicaise AM, D'Angelo A, Ionescu RB, Krzak G, Willis CM, Pluchino S. The role of neural stem cells in regulating glial scar formation and repair. Cell Tissue Res. 2022;387:399–414. doi: 10.1007/s00441-021-03554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.No author listed. Clemastine-prescribing information, side effects and uses. 2022. [Accessed May 19, 2022]. Available at: https://www.drugs.com/search.php?searchterm=Clemastine .
- 61.Noonan VK, Fingas M, Farry A, Baxter D, Singh A, Fehlings MG, Dvorak MF. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology. 2012;38:219–226. doi: 10.1159/000336014. [DOI] [PubMed] [Google Scholar]
- 62.Otzel DM, Kok HJ, Graham ZA, Barton ER, Yarrow JF. Pharmacologic approaches to prevent skeletal muscle atrophy after spinal cord injury. Curr Opin Pharmacol. 2021;60:193–199. doi: 10.1016/j.coph.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.P Drasites K, Shams R, Zaman V, Matzelle D, C Shields D, P Garner D, J Sole C, Haque A, Banik NL. Pathophysiology, biomarkers, and therapeutic modalities associated with skeletal muscle loss following spinal cord injury. Brain Sci. 2020;10:933. doi: 10.3390/brainsci10120933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang QM, Chen SY, Xu QJ, Fu SP, Yang YC, Zou WH, Zhang M, Liu J, Wan WH, Peng JC, Zhang T. Neuroinflammation and scarring after spinal cord injury: therapeutic roles of MSCs on inflammation and glial scar. Front Immunol. 2021;12:751021. doi: 10.3389/fimmu.2021.751021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63:1101–1125. doi: 10.1002/glia.22809. [DOI] [PubMed] [Google Scholar]
- 66.Peng Y, Zhao W, Hu Y, Guo XE, Wang J, Hao K, He Z, Toro C, Bauman WA, Qin W. Administration of high-dose methylprednisolone worsens bone loss after acute spinal cord injury in rats. Neurotrauma Rep. 2021;2:592–602. doi: 10.1089/neur.2021.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Proietti D, Giordani L, De Bardi M, D'Ercole C, Lozanoska-Ochser B, Amadio S, Volonte C, Marinelli S, Muchir A, Bouche M, Borsellino G, Sacco A, Puri PL, Madaro L. Activation of skeletal muscle-resident glial cells upon nerve injury. JCI Insight. 2021;6:e143469. doi: 10.1172/jci.insight.143469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pukos N, Goodus MT, Sahinkaya FR, McTigue DM. Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia. 2019;67:2178–2202. doi: 10.1002/glia.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan CB, Choi JS, Al-Ali H, Lee JK. Myelin and non-myelin debris contribute to foamy macrophage formation after spinal cord injury. Neurobiol Dis. 2022;163:105608. doi: 10.1016/j.nbd.2021.105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salvany S, Casanovas A, Piedrafita L, Tarabal O, Hernandez S, Caldero J, Esquerda JE. Microglial recruitment and mechanisms involved in the disruption of afferent synaptic terminals on spinal cord motor neurons after acute peripheral nerve injury. Glia. 2021;69:1216–1240. doi: 10.1002/glia.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sangari S, Kirshblum S, Guest JD, Oudega M, Perez MA. Distinct patterns of spasticity and corticospinal connectivity following complete spinal cord injury. J Physiol. 2021;599:4441–4454. doi: 10.1113/JP281862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scholpa NE, Simmons EC, Tilley DG, Schnellmann RG. beta2-adrenergic receptor-mediated mitochondrial biogenesis improves skeletal muscle recovery following spinal cord injury. Exp Neurol. 2019;322:113064. doi: 10.1016/j.expneurol.2019.113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 74.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 75.Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface--a barrier to successful nerve regeneration? Cell Tissue Res. 2001;305:267–273. doi: 10.1007/s004410100384. [DOI] [PubMed] [Google Scholar]
- 76.Shi Z, Yuan S, Shi L, Li J, Ning G, Kong X, Feng S. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54:e12992. doi: 10.1111/cpr.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol (1985) 2000;88:1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- 78.Sutor TW, Kura J, Mattingly AJ, Otzel DM, Yarrow JF. The effects of exercise and activity-based physical therapy on bone after spinal cord injury. Int J Mol Sci. 2022;23:608. doi: 10.3390/ijms23020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swanson JW. Find out more about demyelinating disease like multiple sclerosis. Mayo Clinic. 2020. [Accessed August 18, 2022]. https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/expert-answers/demyelinating-disease/faq-20058521.
- 80.Sydney-Smith JD, Spejo AB, Warren PM, Moon LDF. Peripherally delivered adeno-associated viral vectors for spinal cord injury repair. Exp Neurol. 2022;348:113945. doi: 10.1016/j.expneurol.2021.113945. [DOI] [PubMed] [Google Scholar]
- 81.Thomas CK, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve. 2006;33:21–41. doi: 10.1002/mus.20400. [DOI] [PubMed] [Google Scholar]
- 82.Torok DG, Fekecs Z, Pajer K, Pinter S, Nogradi A. The use of a detailed video-based locomotor pattern analysis system to assess the functional reinnervation of denervated hind limb muscles. J Neurosci Methods. 2022;365:109398. doi: 10.1016/j.jneumeth.2021.109398. [DOI] [PubMed] [Google Scholar]
- 83.Traiffort E, Kassoussi A, Zahaf A, Laouarem Y. Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Front Cell Neurosci. 2020;14:79. doi: 10.3389/fncel.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turski CA, Turski GN, Chen B, Wang H, Heidari M, Li L, Noguchi KK, Westmark C, Duncan I, Ikonomidou C. Clemastine effects in rat models of a myelination disorder. Pediatr Res. 2018;83:1200–1206. doi: 10.1038/pr.2018.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verma N, Fazioli A, Matijasich P. Natural recovery and regeneration of the central nervous system. Regen Med. 2022;17:233–244. doi: 10.2217/rme-2021-0084. [DOI] [PubMed] [Google Scholar]
- 86.Vourc'h P, Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Brain Res Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Wang GY, Cheng ZJ, Yuan PW, Li HP, He XJ. Olfactory ensheathing cell transplantation alters the expression of chondroitin sulfate proteoglycans and promotes axonal regeneration after spinal cord injury. Neural Regen Res. 2021;16:1638–1644. doi: 10.4103/1673-5374.301023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L, Zhang Y, Tian J, Li H, Sun X. Conjugation polymer nanobelts: a novel fluorescent sensing platform for nucleic acid detection. Nucleic Acids Res. 2011;39:e37. doi: 10.1093/nar/gkq1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 90.Wu W, Zhang X, Zhou J, Yang H, Chen J, Zhao L, Zhong J, Lin WJ, Wang Z. Clemastine ameliorates perioperative neurocognitive disorder in aged mice caused by anesthesia and surgery. Front Pharmacol. 2021;12:738590. doi: 10.3389/fphar.2021.738590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie YY, Pan TT, Xu DE, Huang X, Tang Y, Huang W, Chen R, Lu L, Chi H, Ma QH. Clemastine ameliorates myelin deficits via preventing senescence of oligodendrocytes precursor cells in Alzheimer's disease model mouse. Front Cell Dev Biol. 2021;9:733945. doi: 10.3389/fcell.2021.733945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, Li S. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol. 2011;231:247–260. doi: 10.1016/j.expneurol.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang T, Dai Y, Chen G, Cui S. Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front Cell Neurosci. 2020;14:78. doi: 10.3389/fncel.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yilmaz T, Turan Y, Keleş A. Pathophysiology of the spinal cord injury. J Clin Exp Invest. 2014;5:131–136. [Google Scholar]
- 95.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Liu Z, Zhang W, Wu Q, Zhang Y, Liu Y, Guan Y, Chen X. Melatonin improves functional recovery in female rats after acute spinal cord injury by modulating polarization of spinal microglial/macrophages. J Neurosci Res. 2019;97:733–743. doi: 10.1002/jnr.24409. [DOI] [PubMed] [Google Scholar]
- 97.Zhao W, Peng Y, Hu Y, Guo XE, Li J, Cao J, Pan J, Feng JQ, Cardozo C, Jarvis J, Bauman WA, Qin W. Electrical stimulation of hindlimb skeletal muscle has beneficial effects on sublesional bone in a rat model of spinal cord injury. Bone. 2021;144:115825. doi: 10.1016/j.bone.2020.115825. [DOI] [PMC free article] [PubMed] [Google Scholar]


