Abstract
The role of gut-kidney crosstalk in the progression of diabetic nephropathy (DN) is receiving increasing concern. On one hand, the decline in renal function increases circulating uremic toxins and affects the composition and function of gut microbiota. On the other hand, intestinal dysbiosis destroys the epithelial barrier, leading to increased exposure to endotoxins, thereby exacerbating kidney damage by inducing systemic inflammation. Dietary inventions, such as higher fiber intake, prebiotics, probiotics, postbiotics, fecal microbial transplantation (FMT), and engineering bacteria and phages, are potential microbiota-based therapies for DN. Furthermore, novel diabetic agents, such as glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-dependent glucose transporter-2 (SGLT-2) inhibitors, may affect the progression of DN partly through gut microbiota. In the current review, we mainly summarize the evidence concerning the gut-kidney axis in the advancement of DN and discuss therapies targeting the gut microbiota, expecting to provide new insight into the clinical treatment of DN.
Keywords: diabetic nephropathy, gut microbiota, microbiota-derived metabolites, GLP-1 receptor agonist, DPP-4 inhibitor, SGLT-2 inhibitor
Introduction
The human gastrointestinal tract hosts a mass of microbiota, which encodes a genome 150-fold larger than human genes and is considered the “second” human genome. These microbiomes influence the physiological metabolism of the human body through interaction with the environment and the human genome and actively perform structural and histological functions [1]. The main functions of metabolism, immunity, and endocrine in the host are related to gut microbiota [2]. Under normal conditions, the gut microbiota is relatively stable, maintaining symbiotic and antagonistic relationships in the intestinal tract. When the composition of gut microbiota changes, in the case of changes in nutritional status, morbid state, and other factors, the balance of the microbiota breaks, namely, dysbiosis of the bacterial community [ 3, 4] . Dysbiosis of the gut microbiota can affect insulin resistance (IR), bile acid (BA) metabolism, inflammatory response and intestinal permeability, which is closely related to the occurrence and development of diabetes [ 5, 6] . Impaired intestinal permeability is one of the causes of systemic inflammation. This chronic low-grade inflammatory state is considered a hallmark of diabetes and its complications, such as diabetic nephropathy (DN) [7]. Therefore, maintaining the diversity and balance of the intestinal microbiota is critical in regulating the health maintenance and homeostasis of the host.
DN is a kind of chronic kidney disease (CKD) and a common complication of diabetes mellitus (DM), which is the leading cause of end-stage renal disease (ESRD) in most countries and regions [8]. Notably, both type 1 diabetes (T1D) and type 2 diabetes (T2D) develop DN to some extent, with T1D appearing with a greater possibility [9]. The symptoms of DN are characterized by increased excretory proteinuria, progressively decreased glomerular filtration rate (GFR), ongoing intrinsic tubular injury, or endothelial/microvascular injury [10]. In addition, maladaptive repair and unopposed inflammation may also occur with the progression of DN [10]. Furthermore, urinary tract infections (UTIs) are common in diabetic patients and may aggravate the damage to renal function, suggesting their possible involvement in the pathogenesis of DN [11]. However, the mechanism of DN has not been fully elucidated. A series of recent reports indicate that the endothelial-to-mesenchymal transition (EndMT) of glomerular endothelial cells plays a crucial role in DN, of which the role of lysine methyltransferase 5A (KMT5A) appears to be even more critical, as KMT5A is involved in the progression of DN by affecting inflammation, glycolytic processes, and endothelial injury [ 12‒ 14] . Apart from the above and currently known molecular signaling, microbiota-derived metabolites such as short-chain fatty acids (SCFAs), secondary bile acids (BAs), and uremic toxins may also be involved [ 15‒ 17] .
Therefore, to clarify the pathogenesis of DN, we will first summarize the gut-kidney axis theory, including the changes in the renal barrier and intestinal permeability and the composition of gut microbiota in the progression of DN. Then, the effects of microbiota-derived metabolites, such as SCFAs, secondary BAs and uremic toxins, on the advancement of DN will be discussed. Finally, the impacts and possibilities of currently available novel diabetic drugs on DN targeting the microbiota will be reviewed.
The Gut-kidney Axis in DN
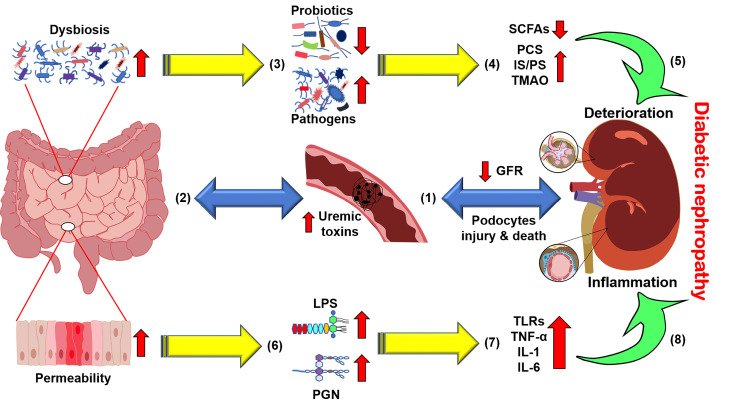
A growing body of evidence has indicated that the bidirectional crosstalk between the host and microbiota is pathophysiologically relevant in patients with CKD, the so-called gut-kidney axis [18] ( Figure 1). Changes in gut microbiota in DN are usually manifested by an increase in the proportion of multiple pathogenic bacteria and a decrease in probiotics [ 19‒ 22] . The gut-kidney axis theory illustrates that changes in the gastrointestinal tract or the kidney can affect the other side through the intestinal mucosa, microbiota, immune inflammation, and other aspects, resulting in adverse consequences [23]. These may be attributed to the severe impairment of renal function in the later stage, and the release of many nitrogen-containing products through the intestinal tract may cause the immense proliferation of intestinal pathogens which utilize these wastes, thereby aggravating the disorder of gut microbiota [ 24, 25] ( Figure 1). Meanwhile, the dysbiosis of microbiota and the impaired gut barrier function may also induce systemic inflammation through the endotoxins and metabolites of the microbiota, resulting in the deterioration of renal function ( Figure 1).
Figure 1 .
The gut-kidney axis in diabetic nephropathy (DN)
With the manifestation of renal dysfunction, (1) glomerular filtration rate (GFR) decreases with podocyte damage and death, and (2) uremic toxins accumulate in the circulation and destroy the homeostasis of the intestinal microbiota, namely, dysbiosis. Subsequently, (3) pathogens utilizing nitrogenous waste are increased at the expense of fewer probiotics. (4) Short-chain fatty acid (SCFA) production decreases, while the levels of typical uremic toxins, such as p-cresyl sulfate (PCS), indolyl sulfate (IS), phenyl sulfate (PS) and trimethylamine N-oxide (TMAO), are increased, and (5) ultimately deteriorates DN. In addition, (6) changes in the intestinal microenvironment result in increased intestinal permeability, leading to the leakage of bacterial components such as lipopolysaccharides. (7) These endotoxins bind to signaling molecules such as toll-like receptors (TLRs) to recruit inflammatory cytokines and (8) cause chronic systemic inflammation in the kidney.
Specifically, the kidney cannot entirely excrete numerous metabolic wastes in circulation, and as a result, they will accumulate. These wastes enter the intestinal lumen through the bowel wall, resulting in the imbalance of the intestinal microbiome and probiotic reduction. Consequently, the increase in toxic metabolites produced by intestinal microbes, such as indoxyl sulfate, p-cresol sulfate, and phenylacetylglutamine, accelerates the deterioration of renal function [26]. According to the experimental results of Vaziri et al. [24], patients with ESKD showed both a contraction of the bacterial family containing butyrate-producing enzymes and an expansion of the bacterial family producing indoles and p-cresyl. Just as uremia affects the composition and metabolism of gut microbes, critical uremic toxins are also derived from microbial metabolism [23]. Taken together, renal function damage is associated with microbiota disorders, and alterations in the gut microbiota, in turn, continue to aggravate renal impairment ( Figure 1).
Impairment of the renal barrier in DN
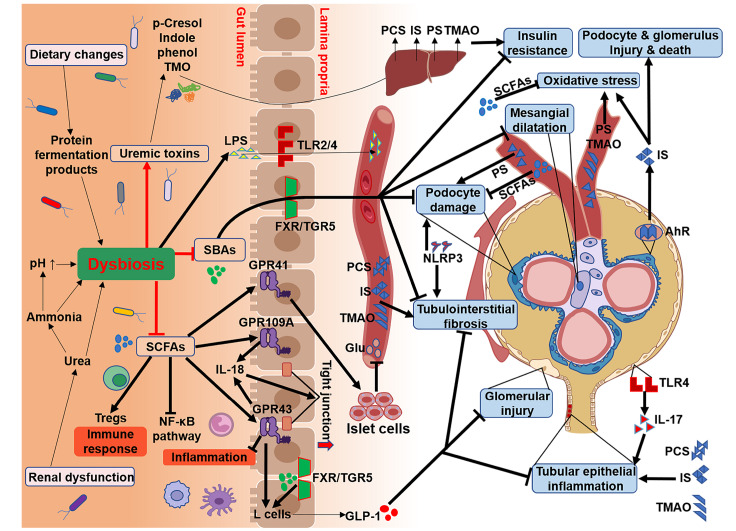
Podocytes are glomerular visceral epithelial cells that form the glomerular filtration barrier together with endothelial cells and the glomerular basement membrane (GBM) [27]. Podocytes are terminally differentiated cells with poor proliferative capacity and are highly vulnerable to injury. Excessive expression of Toll-like receptors (TLRs), such as TLR2/4, in DN patients could also cause podocyte injury and mesangial dilation, leading to kidney injury [15] ( Figure 2). Once podocytes are damaged, they fall off from the basement membrane and are excreted with urine, which could damage the filtration barrier and produce albuminuria; meanwhile, damaged podocytes fail to compensate for the basement membrane, leading to glomerulosclerosis, a hallmark of DN [28].
Figure 2 .
Gut microbiota-derived metabolites in the progression of DN
Dysbiosis caused by renal dysfunction and dietary changes results in metabolic disorders. The immunomodulatory and anti-inflammatory effects of SCFAs, through regulatory T-cell cell (Treg) involvement and inhibition of the nuclear factor-κB (NF-κB) pathway, are suppressed due to the reduction in SCFA-producing bacteria. The weakening of renal protection by SCFAs through G-protein-coupled receptors (GPRs), such as preventing tubulointerstitial fibrosis, glomerular damage, and tubule epithelial cell inflammation, undoubtedly promotes the progression of DN. Secondary bile acids (BAs), by binding to its receptor FXR/TGR5, can improve DN by reducing podocyte damage and mesangial expansion, which are also restrained by the disturbance of the intestinal microbiota. The precursors of uremic toxins produced by colonic fermentation are transported to the liver to synthesize circulating uremic toxins, including PCS, IS, PS and TMAO, which steadily deteriorate DN through direct toxic activity and oxidative stress or damage to podocytes and glomeruli by binding to receptor AHRs.
In particular, the NLRP3 inflammasome complex actuates the production of active proinflammatory cytokines, while the expression of NLRP3 inflammasome-activating markers in the kidney is associated with the severity of albuminuria in patients with renal dysfunction [29]. More importantly, the administration of NLRP3 inhibitor MCC950 could normalize the urinary albumin-to-creatinine ratio (UACR) and ameliorate podocyte injury and renal fibrosis in db/ db mice [30], suggesting the potential of NLRP3-targeted therapy in the treatment of DN ( Figure 2). Furthermore, NLRP3 is an intracellular sensor that initiates inflammatory responses by detecting a broad range of microbial motifs, such as bacterial translocation in both the urethra and intestines [31]. Bacteriuria or abnormal urinary tract microecology caused by decreased GFR in DN may trigger an NLRP3-mediated inflammatory cascade that exacerbates the disease, including proteinuria, podocyte injury, and renal fibrosis.
UTI is a common complication in diabetic patients, and DN patients are more prone to UTI due to impaired renal function. More recently, there has been increasing concern about discovering and measuring the relevance of the urinary microbiome to human health [ 32, 33] . The urinary microbiome is significantly changed in DN patients with UTIs, while there is little direct evidence showing that the abnormal urinary microbiome influences the progression of DN. However, some indirect links are being explored. For example, the most common pathogens of UTIs, uropathogenic E. coli (UPEC), and other symbiotic bacteria that colonize the urinary tract, such as Lactobacillus, Streptococcus, and Bifidobacteria, metabolize the extracellular glycosaminoglycan (GAG) layer of the uroepithelium of the host, acting as a natural barrier [34]. As a result, urinary tract waste and microorganisms leak into the circulation, leading to the occurrence and deterioration of DN. Even though the mechanisms remain to be clarified, such as the cause-and-effect relationship between the glomerular filtration barrier and UTIs and the interaction of bacteria with GBM, maintaining the integrity of the renal barrier and stable urine microbiota may be a breakthrough for the prospective treatment of DN.
Intestinal permeability changes in DN
The intestinal tract is a significant barrier separating the microorganisms in the intestinal lumen from the internal environment ( Figure 2). Numerous microbial systems interact with the internal environment through the intestinal wall so that they are isolated by the intestinal tract and confined to the lumen [35]. Alterations in the composition and function of the gut microbiota in DN patients lead to damage to the intestinal epithelial barrier and increased intestinal permeability [36]. Specifically, due to the decrease in renal filtration in DN patients, the reabsorption of ammonia increases from the hydrolysis of large amounts of urea, followed by the resynthesis of urea in the liver, which causes a marked decrease in transepithelial electrical resistance (TER) and loss of key tight junction proteins, such as claudin-1, occludin, and zonula occludens-1 (ZO-1) [37]. Ammonia can also be converted into ammonium hydroxide, thereby causing an increase in intestinal pH, aggravating the intestinal mucosal injury, and leading to epithelial barrier structure and function disorders [38] ( Figure 2). Furthermore, the breakdown of intestinal epithelial tight junctions causes the entry of microbial components into the underlying tissue compartment, triggering a local inflammatory process that in turn leads to the persistence of intestinal barrier damage [39]. Cresol, indolinyl molecules and other toxins are then transferred to the blood, which induces systemic inflammation, further contributing to the pathogenesis of DN [40] ( Figure 2).
Increased intestinal permeability could lead to sustained infiltration of lipopolysaccharide (LPS) into the portal vein, resulting in metabolic endotoxemia and increased levels of inflammatory cytokines that accelerate the progression of DN [41]. LPS is the surface antigen of gram-negative bacteria, which mediates host inflammation via TLR2- and TLR4-related pathways [42] ( Figure 2). Studies have revealed that TLR2 and TLR4 are involved in the continuous inflammatory response process of DN by inducing the release of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6, and the activation of the nuclear factor-κB (NF-κB)-mediated inflammatory cascade reaction [43] ( Figure 2). Upregulation of TLR4 expression in glomeruli of the early stage of DN could lead to elevated levels of IL-17 and urinary albumin, thereby exacerbating chronic inflammation of DN [44], which might be a good predictor of renal function decline in DN ( Figure 2).
Intestinal dysbiosis in DN
As mentioned above, patients with DN are associated with microbiota dysbiosis ( Table 1). The decrese in the relative abundance of probiotics such as Bifidobacterium, Lactobacillus and Prevotella and the increase in the number of pathogenic bacteria such as Coprobacillus and Desulfovibrio confirm the imbalance of gut microbiota in rodent models with DN [ 15, 19, 45] ( Table 1). Li et al. [21] found that Allobaculum and Anaerosporobacter aggravate renal function deterioration in DN, while Blautia may play a protective role in mice. Similar changes were found in mice with acute kidney injury (AKI) [ 46, 47] . In addition, changes in microbiota that are related to uremic toxins could also be detected in patients with renal disease, represented by the anaerobic gram-positive families Christensenellaceae, Lachnospiraceae and Ruminococcaceae [48]. Butyrate-producing bacteria, Roseburia and Faecalibacterium, are negatively associated with renal function in patients [ 49‒ 51] ( Table 1). A recent study showed that DN patients had reduced intestinal microbiome richness compared to healthy subjects; specifically, Coriobacteriaceae was enriched in DN subjects, while Prevotellaceae was the most abundant bacteria in healthy individuals [20]. The researchers also found that the level of Prevotella_9 could be an accurate predictor of diabetic individuals by analyzing the microbiological differences among the diabetic patients, DN patients, and healthy controls [20]. Compared to diabetic patients without renal complications, DN patients could be accurately distinguished by Escherichia-Shigella and Prevotella_9 variables, with the former notably increased and the latter significantly decreased [20]. Other studies have reported that Enterobacteriaceae are more abundant in the DN patients than in healthy individuals [ 24, 50] ( Table 1). In addition, the increased relative abundance of Enterobacteriaceae might also be associated with the inflammatory response, as they express effective immune stimulants, such as LPS and peptidoglycans (PGNs) [ 52, 53] ( Figure 2).
Table 1 Changes of gut microbiota composition in renal diseases
|
Subject |
Changes in gut microbiota |
Functional effects and/or relevance |
Reference |
||
|
Phylum level |
Family level |
Genus/species level |
|||
|
Mice with diabetic nephropathy |
Actinobacteria ↑ |
Unknown |
Prevotella ↓, Bifidobacterium ↓, Rikenella ↓, Ruminococcus ↓, Bacteroides acidifaciens ↓ |
Related to SCFAs production |
15 |
|
Rats with diabetic nephropathy |
Actinobacteria ↑, Firmicutes ↓, Proteobacteria ↓ |
S24-7 ↓ |
Turicibacter ↑, Coprobacillus ↑, Prevotella ↓, Clostridium ↓, Ruminococcus ↓, Oscillospira ↓ |
Associated with the progression of diabetic nephropathy |
19 |
|
14 T2DM patients with diabetic nephropathy |
Unknown |
Coriobacteriaceae ↑, Prevotellaceae ↓ |
Escherichia-Shigella ↑, Prevotella_9 ↓ |
Contributed to the physiopathological diagnosis of DN from DM |
20 |
|
Mice with diabetic nephropathy |
Firmicutes ↓ |
Unknown |
Anaerosporobacter ↑, Allobaculum ↑, Blautia ↓ |
Gut flora may contribute to the heterogeneity of the induced response |
21 |
|
Rats with diabetic nephropathy |
Unknown |
Coriobacteriaceae ↑, Erysipelotrichaceae ↑ |
Adlercreutzia ↑ |
Related to proteinuria in diabetic nephropathy |
22 |
|
Rats with diabetic nephropathy |
Unknown |
Peptostreptococcaceae ↑, Rikenellaceae ↓, Ruminococacceae ↓ |
Turicibacter ↑, Desulfovibrio ↑, SMB53 ↑, Clostridium ↓, Lactobacillus ↓ |
Related to the metabolic disorder of diabetic nephropathy |
45 |
|
24 patients with ESRD and uremic animals |
Actinobacteria ↑, Firmicutes ↑, Proteobacteria ↑ |
Brachybacterium ↑, Enterobacteriaceae ↑, Thiothrix ↑, Halomonadaceae ↑, Nesterenkonia ↑ Lactobacillaceae ↓, Prevotellaceae ↓ |
Unknown |
Changes in the biochemical environment caused by uremia and its therapeutic intervention |
24 |
|
Mice with bilateral I/R injury |
Unknown |
Enterobacteriaceae ↑, Ruminococacceae ↓ |
Lactobacilli ↓ |
The hallmarks of I/R induced dysbiosis, associated with SCFAs reduction and intestinal inflammation |
46 |
|
Mice with unilateral I/R injury |
Unknown |
Unknown |
Clostridium ↑, Ruminococcin ↑, Bifidobacterium ↓, TM7 ↓ |
Related to D-Serine/L-Serine level and tubular damage/tubular cell proliferation |
47 |
|
855 individuals with early renal function decline |
Unknown |
Christensenellaceae ↑, Ruminococcaceae ↑, Lachnospiraceae ↑ |
Unknown |
Related to circulating metabolites derived from bacterial protein fermentation and eGFR |
48 |
|
65 patients with CKD |
Unknown |
Unknown |
Roseburia spp. ↓, Faecalibacterium prausnitzii ↓ |
Butyrate-producing bacteria are negatively associated with renal function |
49 |
|
892 adults with kidney disease/52 patients with ESRD |
Proteobacteria↑,Verrucomicrobia↑, Actinobacteria↓ |
Enterobacteriaceae ↑, Streptococcaceae ↑, Prevotellaceae ↓ |
Streptococcus ↑, Prevotella ↓, Prevotella 9 ↓, Roseburia ↓, Faecalibacterium ↓, Clostridium ↓, Coprococcus ↓ |
Uremic toxin generation in adults with kidney disease at the expense of producing less butyrate |
50, 51 |
On the other hand, the murine model of DN showed a declining Firmicutes/ Bacteroides(F/B) ratio [21], and the level of Firmicutes was negatively correlated with the degree of kidney damage in DN, which seemed to conflict with the current view that increased F/B ratios indicated poorer health conditions [54]. The F/B ratio in healthy mammals is relatively stable, and an increase or a decrease in the F/B ratio may represent a disease state. However, whether it is a disease state can no longer be judged based simply on the ratio of F/B, and more data are needed to clarify the precise role of F/B. Although animal models cannot adequately represent disease progression in humans, these findings provide substantial evidence for the indispensable role of gut microbiota in DN.
Gut Microbiota-derived Metabolites in the Progression of DN
SCFAs
SCFAs stimulate the proliferation of epithelial cells and play an essential role in preventing and treating metabolic disorders, of which acetate, propionate and butyrate are the most abundant [ 55, 56] ( Figure 2). The major butyrate-producing bacteria in the gut belong to the phylum Firmicutes, particularly Faecalibacterium prausnitzii and Roseburia spp., as well as Clostridium leptum [ 49‒ 51] . In addition to butyrate, the production of other SCFAs is mediated by strains involved in carbohydrate fermentation processes, such as Bifidobacterium [57].
It has been reported that acetate and butyrate partially ameliorate T2D-induced renal injury by inhibiting oxidative stress, the NF-κB pathway, and the inflammatory response in mesangial cells. However, propionate showed only modest effects compared with the other SCFAs [ 58, 59] . In addition, butyrate has been shown to facilitate the immunosuppressive activity and expansion of regulatory T cells (Tregs) [60] ( Figure 2). Related clinical trials have revealed that Treg level in the peripheral blood is lower in patients with nephropathy than in diabetic patients without kidney damage [61]. Furthermore, fewer Treg counts were identified in DN patients and inversely correlated with the albumin-to-creatinine ratio (ACR), thereby being an indicator of renal injury [62].
SCFAs also act as signal transduction molecules to activate G-protein-coupled receptors (GPRs), including GPR41, GPR43, and GPR109A [ 15, 63] ( Figure 2). GPR41 can increase the intestinal transport rate and improve the survival and function of islet cells, which has obvious benefits for diabetes and DN [64]. The activation of GPR43 alleviates inflammation and stimulates the release of GLP-1 from L cells [65], which plays a crucial role in improving glomerular injury, tubulointerstitial fibrosis, and renal tubular epithelial inflammation and apoptosis [66]. In addition, the GLP-1/GLP-1 receptor complex has been found to partially ameliorate the renal function of early diabetes by reducing proximal renal tubule overresorption [67]. Furthermore, diabetic mice with higher fiber intake were less likely to develop DN, exhibiting less albuminuria, glomerular hypertrophy, podocyte injury, and interstitial fibrosis [15] ( Figure 2). One of the most important mechanisms of this finding was the expansion of SCFA-producing bacteria, such as Prevotella and Bifidobacteria [15].
It has also been reported that SCFAs prevente diabetic mice from nephropathy, but not in the absence of GPR43 or GPR109A [15]. In addition, SCFAs increase the production of IL-18 by activating GPR43 and GPR109A in intestinal epithelial cells (IECs) [68], and IL-18 is a crucial cytokine for repairing and maintaining epithelial integrity. Although evidence for the involvement of IL-18 in inflammatory processes has been reported, its role in barrier defense is also recognized [ 69, 70] ( Figure 2). Further studies are required to clarify the exact role of IL-18 in SCFA-mediated amelioration of DN. People with DN cannot consume foods rich in sugar and potassium, including fruits and vegetables which are fermented into SCFAs, provide a significant source of nutrients for normal colon bacteria, and are bound to affect the intestinal microbiome of DN patients. Therefore, gut microbiota dysbiosis and dietary factors could lead to abnormal SCFA production and inhibit the activation of GPRs, thus affecting the progression of DN ( Figure 2).
Secondary BAs
As microbial metabolites, secondary BAs are involved in kidney diseases [ 16, 71] ( Figure 2). The activation of BA receptors, such as the nuclear hormone receptor farnesoid X receptor (FXR) and G protein-coupled receptor TGR5, plays renal-protective roles and shows prospective therapeutic potential in preventing the progression of DN [72] ( Figure 2). The primary BAs are converted from precursor cholesterol in the liver and then enter the intestines where they are converted into secondary BAs by Firmicutes [73]. In addition, Lactobacillus, Bacteroides, and Roseburia also participate in the metabolic process of secondary BAs in the intestinal tract [74].
Animal studies have revealed that activation of FXR and TGR5 reduces nephritis, renal oxidative stress, and fibrosis [16] ( Figure 2). For example, treatment with the FXR/TGR5 dual agonist INT-767 ameliorated albuminuria and prevented podocyte damage, mesangial dilatation, and tubulointerstitial fibrosis in diabetic mice [72], suggesting the involvement of FXR and TGR5 signaling in the progression of DN ( Figure 2). Ding et al. [75] found that activation of TGR5 signaling induced intestinal release of GLP-1, indicating that BAs may be a promising target for DN treatment. Consistently, the TGR5-specific agonist INT-777 prevented oxidative stress and lipid accumulation in the kidneys of diabetic mice, reduced albuminuria, and improved renal podocyte injury and mesangial dilatation [76]. Furthermore, in diabetes, renal FXR and TGR5 expressions are downregulated along with their target genes which are also correlated with the severity of inflammation and renal fibrosis ( Figure 2). Therefore, secondary BAs and their receptors are involved in the pathogenesis of DN and may serve as therapeutic targets.
Uremic toxins
Metabolic abnormalities in patients with DN impair protein digestion and absorption, fueling the production of protein end-products, such as p-cresol, indole, phenol and TMA, by disordered gut microbiota [28] ( Figure 2). These precursors elevate hepatic production of p-cresyl sulfate (PCS), indolyl sulfate (IS), phenyl sulfate (PS) and trimethylamine N-oxide (TMAO) ( Figure 2), all of which are typical uremic toxins [ 22, 77‒ 79] . Furthermore, Anaerococcus hydrogenalis, Edwardsiella tarda and Escherichia fergusonii were identified to be involved in the formation of TMA [80], while Clostridium difficile and Escherichia coli were related to the generation of p-cresol and indole, respectively [ 81, 82] . Importantly, high levels of PCS and IS could lead to insulin resistance and renal tubulointerstitial fibrosis [ 83, 84] ( Figure 2). Moreover, due to the protein-binding toxin properties of PCS and IS, they can easily bind to albumin and cannot be removed adequately by traditional hemodialysis, leading to the deposition of these solutes in ESKD patients and possibly with increased toxicity [85]. Furthermore, Joossens et al. [86] demonstrated the presence of bacteria responsible for producing uremic toxins after identifying the fecal microbiota of patients with kidney disease. Specifically, Akkermansia, Bacteroides, Blautia, Enterococcus, Dialester, and Ruminococcus are related to increased uremic toxins, which seems to contradict the protective effect of Blautiaand Akkermansia, as they are associated with the source of acetate and propionate [ 21, 87] .
Under normal conditions, aryl hydrocarbon receptors (AHRs) are IS receptors that regulate the function of podocytes, while the chronic activation of AHRs by high-level IS could also lead to progressive injury of podocytes and glomeruli [88] ( Figure 2). In vivo and in vitro experiments confirmed that podocyte damage included cell morphology changes and increased the expressions of proinflammatory cytokines and chemokines [88]. As mentioned above, podocytes play a vital role in forming the glomerular filtration barrier and protein transport, indicating that uremic toxins may be involved in renal deterioration in patients with DN. More importantly, in a cohort of 40 patients with CKD, the researchers found that IS level is responsible for renal endothelial dysfunction, as IS level is positively related to a reduced rate of flow-mediated endothelium-dependent vasodilation. It was also found that IS restricted endothelial cell proliferation and nitric oxide production and induced oxidative stress and senescence in endothelial cells [89] ( Figure 2).
In addition, elevated level of hydrogen peroxide has been reported in the plasma of patients undergoing hemodialysis compared to that in healthy individuals, and PS-treated renal tubular cells were more susceptible to oxidative stress and showed higher cell mortality induced by hydrogen peroxide than normal cells [90] ( Figure 2). Meanwhile, PS caused proteinuria and podocyte injury in diabetic rats, and the level of PS was increased with the progression of diabetes but decreased in proteinuria-restricted rats [22]. In patients with microalbuminuria, the level of PS is significantly correlated with basic albuminuria and two-year progression of DN, suggesting that PS may be an early diagnostic marker and therapeutic target of DN [22] ( Figure 2).
On the other hand, TMAO can be eliminated by glomerular filtration, so hemodialysis can effectively remove TMAO from systemic circulation [91]. Elevated TMAO level is associated with an increased risk of death in CKD patients [92]. Sun et al. [93] found that increased levels of TMAO and its precursor choline were associated with enhanced phosphorylation of SMAD3 and TGF-β signaling, thereby aggravating renal collagen deposition and tubulointerstitial fibrosis in mice fed with a high-fat diet (HFD) ( Figure 2). In addition, increased TMAO level in a mouse model of diet-induced obesity was also found to be related to increased NADPH oxidase-4 and inflammatory cytokines compared with that in the control group, while supplementation with a TMA formation inhibitor ameliorated HFD-induced renal fibrosis and decreased the expressions of kidney injury molecule-1 (KIM-1) and proinflammatory cytokines in mice [93]. Xu et al. [94] demonstrated a significant increase in plasma TMAO level in patients with CKD, and the rise in plasma TMAO can be transferred to normal mice by transplanting fecal microorganisms from these patients. These studies suggest that high level of TMAO might be a pathogenic mediator of CKD progression. However, direct evidence pointing to the adverse effects of TMAO on DN has thus far been absent; further studies are needed to clarify the role and mechanism of TMAO in DN progression.
Gut Microbiota-targeted Therapies for DN
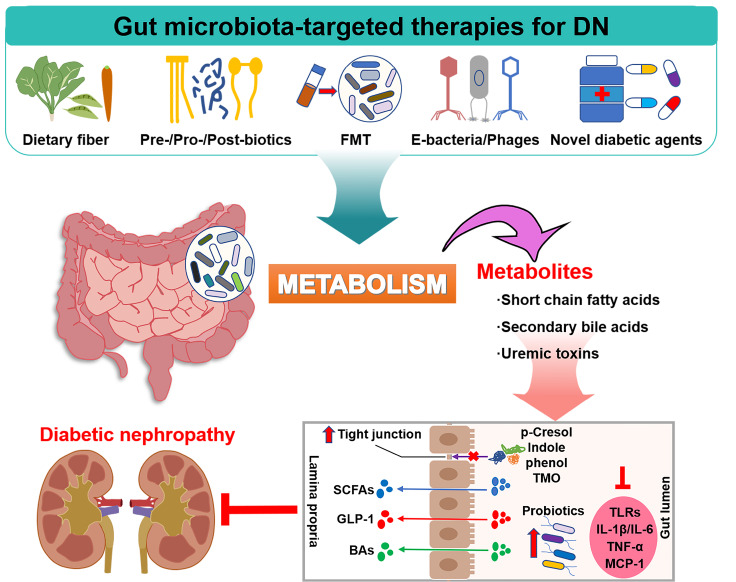
From a therapeutic perspective, due to the complex metabolic disorders of DN, it is more challenging to treat the disease before it progresses to ESRD than other kidney diseases. Meanwhile, alterations in the composition and function of gut microbiota could further exacerbate these disorders. Therefore, early correct and effective treatment has important clinical significance for reversing renal function damage, delaying the progression of diseases, and optimizing the gut microbiota concurrently ( Figure 3).
Figure 3 .
Gut microbiota-targeted therapies for DN
Dietary fiber, prebiotic, probiotic, and postbiotic intake could increase the expression of intestinal tight junctions, improve intestinal metabolic disorders, increase the release of SCFAs and secondary BAs, and further attenuate the production of inflammatory cytokines. In addition, fecal microbiota transplantation (FMT), engineered bacteria and phages could also reshape the gut microbiota structure and exert a similar ameliorative effect on DN. Novel diabetic agents, such as glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-dependent glucose transporter-2 (SGLT-2) inhibitors, have been found to have great potential for the clinical treatment of DN, the effects of which might be partly regulated through the gut microbiota.
Dietary therapy
Experiments in animals have suggested that the benefits of intermittent fasting against a variety of metabolic diseases may be partly related to improving gut microbiota composition [ 95, 96] . Dietary strategies rich in unsaturated fatty acids and dietary fiber are beneficial in combating metabolic endotoxemia and systemic inflammation [97]. Dietary polyphenols also boost the secretion of GLP-1 in the gut, which increases insulin level and lowers blood glucose level [98]. Furthermore, it can also facilitate the growth of certain beneficial bacteria, such as the prototypical example of Akkermansia muciniphila [99] with vascular protection.
Prebiotics, probiotics, and postbiotics
Prebiotics are a class of substances selectively utilized by gut microbes to benefit host health. Clinical studies have confirmed that prebiotics can ameliorate renal inflammation and fibrosis in diabetic patients, accompanied by enriching the species of Bacteroides fragilis and Clostridium and increasing the levels of SCFAs [ 100, 101] . Furthermore, a meta-analysis of 14 randomized controlled trials showed that prebiotic intake significantly reduced circulating PCS level in patients with nephropathy [78].
Probiotics also appear to be a potential treatment for DN. Recent studies showed that prophylactic supplementation with Lactobacillus casei Zhang ameliorated AKI and subsequent chronic kidney fibrosis [102], while Bifidobacterium animalissubsp. lactis LKM512 attenuated inflammation and IR in mice [103]. In addition, researchers also found that in a placebo-controlled trial involving 62 patients with stage 3‒5 kidney disease, Lactobacillus casei Zhang supplementation slowed kidney function decline with a favorable safety profile. In particular, Oscillospira may be a next-generation probiotic candidate, as this butyrate-producing genus has shown promising nephroprotective effects [104].
In addition to prebiotics and probiotics, postbiotics also have certain health benefits. Postbiotics include any substances released or produced by the metabolic activities of microorganisms and inactivated microbes themselves, which have beneficial effects on the host, directly or indirectly [105]. Although there is no direct evidence of postbiotics on nephroprotection, its immunomodulatory role and microbial-derived vitamins and SCFAs still suggest its potential in treating DN [106].
Prebiotics, probiotics, and postbiotics are therapeutically designed to restore the gut to its original predysmal state or a new balance, thereby eliminating or alleviating the underlying disease. However, little is known about probiotic persistence, colonization patterns, and biological host responses, and “personalized microbiome” bacteriotherapies proposed by Zmora et al. [107] may be a prospective solution.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) can reconstruct the abnormal intestinal microecology and cure intestinal microbiota disorders, such as ulcerative colitis and refractory or recurrent Clostridium difficile infections (CDI) [ 108, 109] . However, direct evidence of FMT in the development of DN is still lacking. Two FMT trials showed that the microbiota of leaner donors improved insulin sensitivity in patients with metabolic syndrome, accompanied by alterations in microbiota composition, suggesting that the advantageous effects of lean FMT on glucose metabolism are related to changes in intestinal microbiota [ 110, 111] . Another study showed that FMT from healthy donors alleviates tubulointerstitial injury by overcoming the disruption of cholesterol homeostasis, which shows the potential of FMT for the treatment of diabetes and DN [112]. Due to limited prospective safety data, the long-term effects on the host-microbial community after transplantation must be taken into account, which is also the reason why FMT is not widely used in clinical practice. More studies on the safety and efficacy of FMT are needed.
Engineering bacteria and phages
In addition to the therapies mentioned above, engineered bacteria and phages are also emerging as new therapies for treating metabolic diseases such as DN by targeting the gut microbiota. For example, daily feeding with engineered Lactobacillus gasseri significantly increases insulin level and improves glucose tolerance by secreting GLP-1(1‒37) in diabetic rats, the mechanism of which is reprogramming the intestinal cells into glucose-responsive insulin-secreting cells by engineered bacteria [113]. Furthermore, Han et al. [114] observed that engineered bacteria carrying microcapsules could stably release protein drugs and reduce blood glucose within two weeks in diabetic rats, showing the great potential of engineered bacteria for the treatment of DN.
Phages are viruses that attack bacteria in a host-specific manner and thereby dominate the gut viral community. The transfer of cecal virus communities from mice with a lean phenotype into mice with an obese phenotype resulted in reduced body weight gain and normalization of glycemic parameters, and these effects might be attributable to mutual antagonism between phage and host bacteria [115]. Evidence has suggested that phages can also promote biofilm formation, conferring population-level benefits to their bacterial hosts [116]. Therefore, more attention should be paid to the therapeutic role of phages in DN.
Novel diabetic agents
GLP-1 receptor agonists
GLP-1 is a peptide hormone released by intestinal L cells, which acts by binding to the GLP-1 receptor (GLP-1R) [117]. GLP-1/GLP-1R in the human glomerular arteriole plays a protective role in maintaining GFR [118]. GLP-1 level is decreased in diabetic patients, and GLP-1R agonists can restore the function of GLP-1 [ 119, 120] . The central agonists used in clinical practice are exenatide, liraglutide, dulaglutide, and lixisenatide ( Table 2).
Table 2 Effects of diabetic drugs on gut microbiota and renal outcomes
|
Agent |
Drug |
Effect on gut microbiota |
Effect on renal outcome |
Reference |
||
|
Phylum level |
Family level |
Genus/species level |
||||
|
GLP-1 receptor agonists |
Exenatide |
Unknown |
Unknown |
Unknown |
Reduce albuminuria and pathological renal injury |
121,122 |
|
Liraglutide |
Bacteroides↑, Verrucomicrobia↑, Proteobacteria↓, Actinobacteria↓ |
Rikenellaceae↑, S24-7↑, Erysipelotrichaceae↑, Lachnospiraceae↓, Peptostreptococcacea↓ |
Akkermansia↑, Romboutsia↑, Anaerotruncus↓, Klebsiella↓, Lachnospiraceae_UCG-001↓, Lachnospiraceae_NK4A136_group↓, Ruminiclostridium↓, Desulfovibrio ↓ |
Reduce the incidence of nephropathy and macroalbuminuria |
125,127,129,130 |
|
|
Dulaglutide |
Bacteroides↑, Firmicutes↓ |
Unknown |
Aerococcus↑, Akkermansia↑, Bacteroides↑, Barnesiella↑, Ruminococcus↑, Oscillibacter↑, Coprobacillus↓ |
Lower the incidence in new-onset large albuminuria and sustained decline of eGFR |
131,133 |
|
|
Lixisenatide |
Unknown |
Unknown |
Unknown |
Improve UACR |
134,135 |
|
|
DPP-4 inhibitors |
Linagliptin |
Bacteroides↑, Actinobacteria↓, Proteobacteria↓ |
Unknown |
Unknown |
Reduce proteinuria and improve renal injury |
140,142,143 |
|
Alogliptin |
Unknown |
Unknown |
Unknown |
Improve UACR and maintain eGFR |
144 |
|
|
Saxagliptin |
Firmicutes↑, Bacteroides ↓ |
Erysipelotrichaceae↑, Bacteroidaceae ↓ |
Lactobacillus↑, Allobaculum↑, Turicibacter↑, Prevotella ↓ |
Improve UACR |
147,148 |
|
|
Sitagliptin |
Bacteroides↑, Proteobacteria↑, Actinobacteria↑, Firmicutes↓, Tenericutes↓ |
Ruminococcaceae↑ |
Roseburia↑, Bifidobacterium↑, Blautia ↓ |
Reduce urinary albumin levels |
150,151 |
|
|
SGLT-2 intibitors |
Canagliflozin |
Actinobacteria↓, TM-7↓ |
Unknown |
Alistipes↑, Alloprevotella↑, Olsenella↑, Oscillospira↓, Bifidobacterium↓, Helicobacter↓, Mucispirillum↓ |
Reduce plasma uremic toxins and lower UACR |
155,157,158 |
|
Dapagliflozin |
Firmicutes↑, Proteobacteria,↑ Bacteroides ↓, Actinobacteria↓ |
Desulfovibrionaceae↑, Lactobacillaceae ↓ |
Oscillospira↓, Mucispirillum↓, Barnesiella↓ |
Prevent progression of kidney disease |
160−163 |
|
|
Empagliflozin |
Bacteroides↑, Firmicutes↓ |
Unknown |
Bacteroides↑, Bifidobacterium↑, Parabacteroides↑ |
Reduce the risk of nephropathy, proteinuria and serum creatinine |
133,164,165 |
|
|
Ertugliflozin |
Unknown |
Unknown |
Unknown |
Reduce the risk of renal composite endpoint and preserve eGFR and UACR |
167,168 |
As the first GLP-1R agonist, exenatide was approved by the US FDA in 2005. Animal models have shown that exenatide can reduce the excretion of oxidative stress markers and proteins in the urine and improve the histopathological changes of the kidney [ 121, 122] ( Table 2). Furthermore, exenatide alleviated fibrosis in the renal interstitium and inflammation in the glomeruli [123]. However, there has been no case about exenatide influencing gut microbiota, and renal dysfunction may affect its clearance [124].
Clinical results showed that liraglutide could improve DN symptoms and reduce the incidence of nephropathy and persistent macroalbuminuria in T2D patients [ 125‒ 127] ( Table 2). Results from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial supported the addition of liraglutide to the standard of care to reduce the risk of major cardiovascular events and all-cause mortality in patients with T2D and CKD. These results appeared to apply to all patients with CKD [128]. Furthermore, liraglutide treatment seemed to reverse microbiota dysbiosis in mice with disease states, with decreased Actinobacteria and Desulfovibrio and increased S24-7 [ 129, 130] ( Table 2). It is worth mentioning that liraglutide also increases the relative abundance of Akkermansia in db/ db mice [129], a new type of probiotic in the gut, suggesting the possibility of microbiota-modulating effects of liraglutide in the treatment of DN.
Results from dulaglutide and renal outcomes in T2D, an exploratory analysis of the Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND) trial, showed that diabetic patients treated with dulaglutide had a lower incidence of new-onset large albuminuria and a sustained decline in eGFR than the placebo group [131] ( Table 2). Dulaglutide also reduced the incidence of ischemic stroke and non-fatal stroke or all-cause death in patients with T2D compared with placebo [132]. In addition, an increase in the abundance of Akkermansia was found after dulaglutide treatment in mice, and coupled with its promoting effects on GLP-1 secretion, Akkermansia might be a potential target for GLP-1 receptor agonists [ 117, 133] ( Table 2).
The Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide) (ELIXA) trial revealed that lixisenatide slowed the progression of UACR in patients with large proteinuria and reduced the likelihood of significant proteinuria without increasing cardiovascular risk [ 134, 135] ( Table 2). However, no such change of an increased abundance of Akkermansia has been found in lixisenatide yet. In summary, GLP-1 receptor agonists might prevent and improve DN symptoms in mice and patients, possibly through the gut microbiota. However, more direct evidence linking the cause and effect of the microbiota in DN is urgently needed.
DPP-4 inhibitors
Under physiological conditions, GLP-1 has a half-life of approximately two minutes and is rapidly degraded by DPP-4 [136]. DPP-4 inhibitors can inhibit the activity of DPP-4 and increase the utilization of GLP-1. In addition, DPP-4 inhibitors were found to alleviate inflammation and oxidative stress in the kidneys of diabetic patients to delay glomerulosclerosis [ 137‒ 139] . The representative drugs of DPP-4 inhibitors include linagliptin, alogliptin, saxagliptin, and sitagliptin ( Table 2).
Tanaka et al. [140] found that linagliptin restrained the inflammatory response induced by free fatty acid (FFA)-binding albumin in proximal tubule cells and exhibited a significantly ameliorative effect on kidney injury in mice. In an earlier clinical trial, linagliptin also reduced proteinuria in adults with T2D [141]. However, linagliptin cannot reduce albuminuria but effectively improves glycemic control in the early stage of DN, indicating its primary hypoglycemic function and specific renal repair ability [142]. A decrease in the relative abundance of Actinobacteria was also observed after linagliptin treatment [143] ( Table 2).
In a long-term follow-up of 36 diabetic patients with kidney disease, alogliptin reversed the steady decline in eGFR and showed a downwards trend in UACR. However, this study did not provide evidence that alogliptin could improve renal function due to the sample size limitation [144] ( Table 2). In addition, alogliptin appeared to be more effective when combined with other drugs [ 145, 146] , although it has not been reported to affect the gut microbiota.
Saxagliptin treatment could improve UACR without affecting eGFR in patients with T2D who were followed for a median of 2.1 years on renal outcomes [147]. In addition, saxagliptin increased the F/B ratio and the relative abundance of Lactobacillus; simultaneously, the respective rise and fall of Allobaculum and Prevotella appeared to be at odds with therapeutic efficacy [148] ( Table 2).
Diabetic patients treated with sitagliptin showed a significant decrease in glycosylated hemoglobin and inflammation-related cytokines, suggesting a potent and rapid anti-inflammatory effect of sitagliptin [149]. A randomized clinical trial showed that sitagliptin improved glucose and chylomicron metabolism and reduced urinary albumin level in T2D patients [150] ( Table 2). In addition to its renal protective effects, sitagliptin also increased the levels of certain probiotics, Bifidobacterium, and butyrate-producing bacteria, Roseburia, even though another study reported that sitagliptin did not affect gut microbiota [ 151, 152] ( Table 2). Therefore, these DPP-4 inhibitors showed excellent hyperglycemic and urinary albumin-lowering effects. However, more subsequent clinical trials are expected to confirm whether they have better renal protection in the progression of DN through gut microbiota.
SGLT-2 inhibitors
SGLT-2 is found in the renal proximal convoluted tubules and mainly mediates the process of renal glucose reabsorption [153]. SGLT-2 inhibitors primarily act on the SGLT-2 receptor in the proximal tubule to increase glucose excretion to the urine to reduce blood glucose level and improve glomerular hyperperfusion and hyperfiltration, subsequently restoring renal function [154]. Currently listed SGLT-2 inhibitors include canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin ( Table 2).
In a randomized, double-blind trial, the relative risk of doubling creatinine levels or mortality from renal causes was 34% lower in the canagliflozin-treated group with a reduced relative risk of developing ESRD, demonstrating its clinical efficacy and safety in renal disease protection [155] ( Table 2). Consistent with these results, canagliflozin reduced the risk of cardiovascular and renal events in patients with T2D and proteinuria [156]. Similarly, a decrease in Actinobacteria was observed after canagliflozin treatment, along with a decline in TM-7 in mice. Furthermore, a reduction in the relative abundance of the opportunistic pathogen Mucispirillum was observed. Oscillospira also showed a decrease after canagliflozin intervention [ 157, 158] ( Table 2).
In a randomized trial with a sustained reduction of at least 50% in eGFR, ESRD, or death from renal or cardiovascular causes as the primary outcome, patients with kidney disease in the dapagliflozin-treated group had a significantly lower compound risk for renal endpoints [159]. In addition, in the DECLARE-TIMI 58 trial, dapagliflozin preserved renal function in most patients [160], which is undoubtedly a boon for DN patients ( Table 2). Among the changes in the gut microbiota, the increase of Firmicutes and Proteobacteria and the decrease of Actinobacteria were first observed at the phylum level after dapagliflozin intervention [161]. In addition, Oscillospira and Mucispirillum mentioned above were also declined [ 162, 163] , and these two bacteria may act as targets of SGLT-2 inhibitors ( Table 2).
Empagliflozin decreased UACR and urinary albumin level in T2D patients with complicated cardiovascular disease after short-/long-term therapy [ 164, 165] ( Table 2). Further findings of EMPA-REG OUTCOME supported that empagliflozin lowered the risk of clinically relevant renal events and might delay the progression of DN [166]. In terms of gut microbiota, it was found that the relative abundance of Bifidobacterium was increased after empagliflozin treatment in mice [133].
Analyses from the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes (VERTIS CV) trial showed that ertugliflozin led to a lower incidence of the exploratory renal composite outcome of a sustained reduction in eGFR, chronic renal dialysis/transplantation, or renal death compared with the placebo control [167]. In addition, both UACR and eGFR tended to be close to their baselines after ertugliflozin treatment [ 167, 168] ( Table 2). Therefore, based on the above clinical data, the nephroprotective effects of SGLT-2 have been gradually revealed. Its impact in DN treatment is worthy of expectation, especially in its association with gut microbes, even though ertugliflozin has not been reported to affect the gut microbiota.
In addition to the novel diabetes agents mentioned above, more and more effective DN therapy strategies are being developed [169], targeting multiple metabolic pathways including inhibitors of protein kinase C (PKC), advanced glycated end product (AGE), endothelin receptor (ETR), and transforming growth factor-β (TGF-β), and so on, which have shown promising prospects in the treatment of DN. More recently, the concept of machine learning (ML) has been proposed to describe and predict drug-microbiome interactions at the level of individual patient [170], which may become the mainstream treatment for DN and even ESRD in the future.
Conclusion and Prospect
Gut microbiota dysbiosis is closely related to metabolic diseases such as DN. Intestinal dysbacteriosis and kidney damage lead to loss of beneficial bacterial strains, accumulation of large amounts of uremic toxins, and increased incidence of UTIs, all of which contribute to the progression of DN. Gut microbiota-based therapy might be a promising strategy for preventing and treating DN in the future. Changes in dietary structure contribute to the improvement of intestinal microbiota dysbiosis. Microbiota transplantation is also expected to play a role in restoring the microflora ecology of DN under safe and standardized conditions. In addition, burgeoning hypoglycemic drugs, such as GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors targeting the gut microbiota, have shown reliable therapeutic effects on the renoprotection and modification of gut microbiota. However, most of the current data are limited to rodent models, and more reliable clinical trials are needed to elucidate pivotal pathways and specific strains in the pathogenesis of DN.
Supporting information
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Natural Science Foundation of China of Zhejiang Province (No. LY19C110001 to Y.N.), the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (No. LHDMZ22H050001), and the Key Project of Basic Scientific Research Operating Funds of Hangzhou Medical College (No. KYZD202002 to J.J.), and Innovative Research Team in University (No. IRT_17R97 to F.Z.).
References
- 1.Shine EE, Crawford JM. Molecules from the microbiome. Annu Rev Biochem. . 2021;90:789–815. doi: 10.1146/annurev-biochem-080320-115307. [DOI] [PubMed] [Google Scholar]
- 2.Amato KR, Arrieta MC, Azad MB, Bailey MT, Broussard JL, Bruggeling CE, Claud EC, et al. The human gut microbiome and health inequities. Proc Natl Acad Sci USA. . 2021;118:e2017947118. doi: 10.1073/pnas.2017947118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. . 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. . 2021;75:S67–S81. doi: 10.1016/j.jhep.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. . 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He F, Li Y. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. . 2020;13:73. doi: 10.1186/s13048-020-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. . 2019;25:805–813. doi: 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughton CK, Tripyla A, Hartnell S, Daly A, Herzig D, Wilinska ME, Czerlau C, et al. Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med. . 2021;27:1471–1476. doi: 10.1038/s41591-021-01453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis S, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes. Minerva Med. . 2018;109:218–228. doi: 10.23736/S0026-4806.17.05496-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WR, Parikh CR. Biomarkers of acute and chronic kidney disease. Annu Rev Physiol. . 2019;81:309–333. doi: 10.1146/annurev-physiol-020518-114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Tejeda AU, Sampieri CL, Suárez-Torres I, Morales-Romero J, Demeneghi-Marini VP, Hernández-Hernández ME, Rodríguez-Hernández A. Association of urinary activity of MMP-9 with renal impairment in Mexican patients with type 2 diabetes mellitus. Peer J. . 2018;6:e6067. doi: 10.7717/peerj.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Li X, Zhong Z, Zhou W, Zhou D, Zhu M, Miao C. KMT5A downregulation participated in high glucose-mediated endMT via upregulation of ENO1 expression in diabetic nephropathy. Int J Biol Sci. . 2021;17:4093–4107. doi: 10.7150/ijbs.62867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang T, Li X, Wang F, Lu L, Hou W, Zhu M, Miao C. The CREB/KMT5A complex regulates PTP1B to modulate high glucose-induced endothelial inflammatory factor levels in diabetic nephropathy. Cell Death Dis. . 2021;12:333. doi: 10.1038/s41419-021-03629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Zhong Z, Gu J, Nan K, Zhu M, Miao C. ets1 associates with KMT5A to participate in high glucose-mediated EndMT via upregulation of PFN2 expression in diabetic nephropathy. Mol Med. . 2021;27:74. doi: 10.1186/s10020-021-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y, Ma J, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid–mediated activation of G protein–coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. . 2020;31:1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman-Edelstein M, Weinstein T, Levi M. Bile acid receptors and the kidney. Curr Opin Nephrol Hypertension. . 2018;27:56–62. doi: 10.1097/MNH.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 17.Koppe L, Fouque D, Soulage CO. Metabolic abnormalities in diabetes and kidney disease: role of uremic toxins. Curr Diab Rep. . 2018;18:97. doi: 10.1007/s11892-018-1064-7. [DOI] [PubMed] [Google Scholar]
- 18.Lobel L, Cao YG, Fenn K, Glickman JN, Garrett WS. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science. . 2020;369:1518–1524. doi: 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng Y, Zheng S, Zhang C, Zhao C, He X, Xu W, Huang K. Mulberry leaf tea alleviates diabetic nephropathy by inhibiting PKC signaling and modulating intestinal flora. J Funct Foods. . 2018;46:118–127. doi: 10.1016/j.jff.2018.04.040. [DOI] [Google Scholar]
- 20.Tao S, Li L, Li L, Liu Y, Ren Q, Shi M, Liu J, et al. Understanding the gut–kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol. . 2019;56:581–592. doi: 10.1007/s00592-019-01316-7. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Su X, Gao Y, Lv C, Gao Z, Liu Y, Wang Y, et al. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta (BBA) - Mol Basis Dis. . 2020;1866:165764. doi: 10.1016/j.bbadis.2020.165764. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, Mise K, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. . 2019;10:1835. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evenepoel P, Poesen R, Meijers B. The gut–kidney axis. Pediatr Nephrol. . 2017;32:2005–2014. doi: 10.1007/s00467-016-3527-x. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. . 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Prado R, Esteras R, Perez-Gomez M, Gracia-Iguacel C, Gonzalez-Parra E, Sanz A, Ortiz A, et al. Nutrients turned into toxins: microbiota modulation of nutrient properties in chronic kidney disease. Nutrients. . 2017;9:489. doi: 10.3390/nu9050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song JY, Shen TC, Hou YC, Chang JF, Lu CL, Liu WC, Chen PJ, et al. Influence of resveratrol on the cardiovascular health effects of chronic kidney disease. Int J Mol Sci. . 2020;21:6294. doi: 10.3390/ijms21176294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HS, Suh JY, Kang BC, Lee E. Lipotoxicity dysregulates the immunoproteasome in podocytes and kidneys in type 2 diabetes. Am J Physiol-Renal Physiol. . 2021;320:F548–F558. doi: 10.1152/ajprenal.00509.2020. [DOI] [PubMed] [Google Scholar]
- 28.Mosterd CM, Kanbay M, van den Born BJH, van Raalte DH, Rampanelli E. Intestinal microbiota and diabetic kidney diseases: the role of microbiota and derived metabolites inmodulation of renal inflammation and disease progression. Best Pract Res Clin Endocrinol Metab. . 2021;35:101484. doi: 10.1016/j.beem.2021.101484. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Chen J, Li YY, Xia LL, Wu YG. Bruton’s tyrosine kinase regulates macrophage‑induced inflammation in the diabetic kidney via NLRP3 inflammasome activation. Int J Mol Med. . 2021;48:177. doi: 10.3892/ijmm.2021.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CX, Zhu XW, Li LL, Ma TK, Shi M, Yang Y, Fan QL. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab Syndr Obes. . 2019;12:1297–1309. doi: 10.2147/DMSO.S199802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. . 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adebayo AS, Ackermann G, Bowyer RCE, Wells PM, Humphreys G, Knight R, Spector TD, et al. The urinary tract microbiome in older women exhibits host genetic and environmental influences. Cell Host Microbe. . 2020;28:298–305. doi: 10.1016/j.chom.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Jones-Freeman B, Chonwerawong M, Marcelino VR, Deshpande AV, Forster SC, Starkey MR. The microbiome and host mucosal interactions in urinary tract diseases. Mucosal Immunol. . 2021;14:779–792. doi: 10.1038/s41385-020-00372-5. [DOI] [PubMed] [Google Scholar]
- 34.Neugent ML, Hulyalkar NV, Nguyen VH, Zimmern PE, Nisco NJ, Garsin DA. Advances in understanding the human urinary microbiome and its potential role in urinary tract infection. mBio. 2020, 11: e00218–00220 . [DOI] [PMC free article] [PubMed]
- 35.Cheru L, Saylor CF, Lo J. Gastrointestinal barrier breakdown and adipose tissue inflammation. Curr Obes Rep. . 2019;8:165–174. doi: 10.1007/s13679-019-00332-6. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmaco Ther. . 2017;93:412–419. doi: 10.1016/j.biopha.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. . 2013;37:1–6. doi: 10.1159/000345969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan I, Huang Z, Liang L, Li N, Ali Z, Ding L, Hong M, et al. Ammonia stress influences intestinal histomorphology, immune status and microbiota of Chinese striped-neck turtle ( Mauremys sinensis) . Ecotoxicol Environ Saf. . 2021;222:112471. doi: 10.1016/j.ecoenv.2021.112471. [DOI] [PubMed] [Google Scholar]
- 39.de Andrade LS, Ramos CI, Cuppari L. The cross-talk between the kidney and the gut: implications for chronic kidney disease. Nutrire. . 2017;42:27. doi: 10.1186/s41110-017-0054-x. [DOI] [Google Scholar]
- 40.Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, Jafari P, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. . 2017;91:435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 41.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. . 2016;67:483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Qi L, Feng Q, Zhang B, Li X, Liu C, Li W, et al. HIPK2 phosphorylates HDAC3 for NF-κB acetylation to ameliorate colitis-associated colorectal carcinoma and sepsis. Proc Natl Acad Sci USA. . 2021;118:e2021798118. doi: 10.1073/pnas.2021798118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X, Li S, Fu Y, Zhang N. Targeting gut microbiota as a possible therapy for mastitis. Eur J Clin Microbiol Infect Dis. . 2019;38:1409–1423. doi: 10.1007/s10096-019-03549-4. [DOI] [PubMed] [Google Scholar]
- 44.Yu R, Bo H, Villani V, Spencer PJ, Fu P. The inhibitory effect of rapamycin on Toll like receptor 4 and interleukin 17 in the early stage of rat diabetic nephropathy. Kidney Blood Press Res. . 2016;41:55–69. doi: 10.1159/000368547. [DOI] [PubMed] [Google Scholar]
- 45.Cai HD, Su SL, Guo JM, Duan JA. Effect of Salviae Miltiorrhizae Radix et Rhizoma on diversity of intestinal flora in diabetic nephropathy rats. Zhongguo Zhong Yao Za Zhi. 2021, 46: 426–435 . [DOI] [PubMed]
- 46.Yang J, Kim CJ, Go YS, Lee HY, Kim MG, Oh SW, Cho WY, et al. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. . 2020;98:932–946. doi: 10.1016/j.kint.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 47.Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, Miyake T, et al. Gut microbiota–derived D-serine protects against acute kidney injury. JCI Insight. . 2018;3 doi: 10.1172/jci.insight.97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, et al. Gut-microbiota-metabolite axis in early renal function decline. PLoS ONE. . 2015;10:e0134311. doi: 10.1371/journal.pone.0134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang S, Xie S, Lv D, Zhang Y, Deng J, Zeng L, Chen Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression . Antonie van Leeuwenhoek. . 2016;109:1389–1396. doi: 10.1007/s10482-016-0737-y. [DOI] [PubMed] [Google Scholar]
- 50.Stanford J, Charlton K, Stefoska-Needham A, Ibrahim R, Lambert K. The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrol. . 2020;21:1–23. doi: 10.1186/s12882-020-01805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang S, Xie S, Lv D, Wang P, He H, Zhang T, Zhou Y, et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep. . 2017;7:2870. doi: 10.1038/s41598-017-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. . 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. . 2021;117:154712. doi: 10.1016/j.metabol.2021.154712. [DOI] [PubMed] [Google Scholar]
- 54.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, et al. Age‐related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. . 2018;84:23–36. doi: 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neis EP, van Eijk HM, Lenaerts K, Olde Damink SW, Blaak EE, Dejong CH, Rensen SS. Distal versus proximal intestinal short-chain fatty acid release in man. Gut. . 2019;68:764–765. doi: 10.1136/gutjnl-2018-316161. [DOI] [PubMed] [Google Scholar]
- 56.Snelson M, Kellow NJ, Coughlan MT. Modulation of the gut microbiota by resistant starch as a treatment of chronic kidney diseases: evidence of efficacy and mechanistic insights. Adv Nutr. . 2019;10:303–320. doi: 10.1093/advances/nmy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. . 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang W, Man Y, Gao C, Zhou L, Gu J, Xu H, Wan Q, et al. Short-chain fatty acids ameliorate diabetic nephropathy via gPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxid Med Cell Longev. . 2020;2020:1–21. doi: 10.1155/2020/4074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, Xu Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp Clin Endocrinol Diabetes. . 2017;125:98–105. doi: 10.1055/s-0042-121493. [DOI] [PubMed] [Google Scholar]
- 60.Dang G, Wu W, Zhang H, Everaert N. A new paradigm for a new simple chemical: butyrate & immune regulation. Food Funct. . 2021;12:12181–12193. doi: 10.1039/D1FO02116H. [DOI] [PubMed] [Google Scholar]
- 61.Wang M, Chen F, Wang J, Zeng Z, Yang Q, Shao S. Th17 and Treg lymphocytes in obesity and type 2 diabetic patients. Clin Immunol. . 2018;197:77–85. doi: 10.1016/j.clim.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Abouzeid S, Sherif N. Role of alteration in Treg/Th17 cells’ balance in nephropathic patients with type 2 diabetes mellitus. Electron physician. . 2015;7:1613–1618. doi: 10.19082/1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. . 2016;6:37589. doi: 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides. . 2018;100:269–274. doi: 10.1016/j.peptides.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, Aoki R, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. . 2020;367:eaaw8429. doi: 10.1126/science.aaw8429. [DOI] [PubMed] [Google Scholar]
- 66.Skov J. Effects of GLP-1 in the kidney. Rev Endocr Metab Disord. . 2014;15:197–207. doi: 10.1007/s11154-014-9287-7. [DOI] [PubMed] [Google Scholar]
- 67.Vallon V, Docherty NG. Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4. Exp Physiol. . 2014;99:1140–1145. doi: 10.1113/expphysiol.2014.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang W, Zhou L, Guo H, Xu Y, Xu Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism. . 2017;68:20–30. doi: 10.1016/j.metabol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Chiang HY, Lu HH, Sudhakar JN, Chen YW, Shih NS, Weng YT, Shui-Wen J. IL-22 initiates an IL-18-dependent epithelial response circuit to enforce intestinal host defence. Nat Commun. . 2022;13:874. doi: 10.1038/s41467-022-28478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Zou J, Shi Z, Zhang B, Etienne-Mesmin L, Wang Y, Shi X, et al. IL-22–induced cell extrusion and IL-18–induced cell death prevent and cure rotavirus infection. Sci Immunol. . 2020;5:eabd2876. doi: 10.1126/sciimmunol.abd2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brock WJ, Beaudoin JJ, Slizgi JR, Su M, Jia W, Roth SE, Brouwer KL. Bile acids as potential biomarkers to assess liver impairment in polycystic kidney disease. Int J Toxicol. . 2018;37:144–154. doi: 10.1177/1091581818760746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang XX, Wang D, Luo Y, Myakala K, Dobrinskikh E, Rosenberg AZ, Levi J, et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol. . 2018;29:118–137. doi: 10.1681/ASN.2017020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arora A, Behl T, Sehgal A, Singh S, Sharma N, Bhatia S, Sobarzo-Sanchez E, et al. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. . 2021;273:119311. doi: 10.1016/j.lfs.2021.119311. [DOI] [PubMed] [Google Scholar]
- 74.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. . 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding L, Yang Q, Zhang E, Wang Y, Sun S, Yang Y, Tian T, et al. Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice. Acta Pharm Sin B. . 2021;11:1541–1554. doi: 10.1016/j.apsb.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. . 2016;27:1362–1378. doi: 10.1681/ASN.2014121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gryp T, Huys GR, Joossens M, Van Biesen W, Glorieux G, Vaneechoutte M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int J Mol Sci. . 2020;21:1986. doi: 10.3390/ijms21061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L, Shi J, Ma X, Shi D, Qu H. Effects of microbiota-driven therapy on circulating indoxyl sulfate and P-cresyl sulfate in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. . 2022;13:1267–1278. doi: 10.1093/advances/nmab149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu W, Romano KA, Li L, Buffa JA, Sangwan N, Prakash P, Tittle AN, et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe. . 2021;29:1199–1208.e5. doi: 10.1016/j.chom.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Dai M. Trimethylamine N-Oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflammation. . 2020;2020:1–15. doi: 10.1155/2020/4634172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Passmore IJ, Letertre MPM, Preston MD, Bianconi I, Harrison MA, Nasher F, Kaur H, et al. Para-cresol production by Clostridium difficileaffects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog. 2018, 14: e1007191 . [DOI] [PMC free article] [PubMed]
- 82.Tennoune N, Andriamihaja M, Blachier F. Production of indole and indole-related compounds by the intestinal microbiota and consequences for the host: the good, the bad, and the ugly. Microorganisms. . 2022;10:930. doi: 10.3390/microorganisms10050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang JF, Hsieh CY, Lu KC, Chen YW, Liang SS, Lin CC, Hung CF, et al. Therapeutic targeting of aristolochic acid induced uremic toxin retention, SMAD 2/3 and JNK/ERK pathways in tubulointerstitial fibrosis: nephroprotective role of propolis in chronic kidney disease. Toxins. . 2020;12:364. doi: 10.3390/toxins12060364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. . 2013;24:88–99. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A, Eloot S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins. . 2015;7:3933–3946. doi: 10.3390/toxins7103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joossens M, Faust K, Gryp T, Nguyen ATL, Wang J, Eloot S, Schepers E, et al. Gut microbiota dynamics and uraemic toxins: one size does not fit all. Gut. . 2019;68:2257.1–2260. doi: 10.1136/gutjnl-2018-317561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. . 2022;8:350–360. doi: 10.1016/j.aninu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W, Rosenberg AZ, et al. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS ONE. . 2014;9:e108448. doi: 10.1371/journal.pone.0108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu M, Kim YJ, Kang DH. Indoxyl sulfate–induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. . 2011;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edamatsu T, Fujieda A, Itoh Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE. . 2018;13:e0193342. doi: 10.1371/journal.pone.0193342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M, Lemoine S, et al. Elevation of trimethylamine-N-oxide in chronic kidney disease: Contribution of decreased glomerular filtration rate. Toxins. . 2019;11:635. doi: 10.3390/toxins11110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJ, Dullaart RP. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep. . 2017;7:13781–13789. doi: 10.1038/s41598-017-13739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun G, Yin Z, Liu N, Bian X, Yu R, Su X, Zhang B, et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem Biophys Res Commun. . 2017;493:964–970. doi: 10.1016/j.bbrc.2017.09.108. [DOI] [PubMed] [Google Scholar]
- 94.Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep. . 2017;7:1445. doi: 10.1038/s41598-017-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. . 2017;26:672–685.e4. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, Zhang W, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. . 2020;11:855. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bailey MA, Holscher HD. Microbiome-mediated effects of the mediterranean diet on inflammation. Adv Nutr. . 2018;9:193–206. doi: 10.1093/advances/nmy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avila JAD, Rodrigo García J, González Aguilar G, de la Rosa L. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules. . 2017;22:903. doi: 10.3390/molecules22060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anhê FF, Choi BS, Dyck JRB, Schertzer JD, Marette A. Host–microbe interplay in the cardiometabolic benefits of dietary polyphenols. Trends Endocrinol Metab. . 2019;30:384–395. doi: 10.1016/j.tem.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 100.He C, Shan Y, Song W. Targeting gut microbiota as a possible therapy for diabetes. Nutr Res. . 2015;35:361–367. doi: 10.1016/j.nutres.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 101.O’Connor S, Chouinard-Castonguay S, Gagnon C, Rudkowska I. Prebiotics in the management of components of the metabolic syndrome. Maturitas. . 2017;104:11–18. doi: 10.1016/j.maturitas.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Zhu H, Cao C, Wu Z, Zhang H, Sun Z, Wang M, Xu H, et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. . 2021;33:1926–1942.e8. doi: 10.1016/j.cmet.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 103.Ma L, Zheng A, Ni L, Wu L, Hu L, Zhao Y, Fu Z, et al. Bifidobacterium animalis subsp. lactis lkm512 attenuates obesity‐associated inflammation and insulin resistance through the modification of gut microbiota in high‐fat diet‐induced obese mice . Mol Nutr Food Res. . 2022;66:2100639. doi: 10.1002/mnfr.202100639. [DOI] [PubMed] [Google Scholar]
- 104.Yang J, Li Y, Wen Z, Liu W, Meng L, Huang H. Oscillospira—a candidate for the next-generation probiotics. Gut Microbes. . 2021;13:1987783. doi: 10.1080/19490976.2021.1987783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics—a step beyond pre- and probiotics. Nutrients. . 2020;12:2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EM, Sanders ME, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. . 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. . 2018;174:1388–1405. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 108.Reinshagen M, Stallmach A. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Z Gastroenterol. . 2017;55:779–780. doi: 10.1055/s-0043-109349. [DOI] [PubMed] [Google Scholar]
- 109.Juul FE, Garborg K, Bretthauer M, Skudal H, Øines MN, Wiig H, Rose Ø, et al. Fecal microbiota transplantation for primary Clostridium difficile infection . N Engl J Med. . 2018;378:2535–2536. doi: 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 110.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. . 2017;26:611–619. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. . 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 112.Hu ZB, Lu J, Chen PP, Lu CC, Zhang JX, Li XQ, Yuan BY, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. . 2020;10:2803–2816. doi: 10.7150/thno.40571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. . 2015;64:1794–1803. doi: 10.2337/db14-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han C, Zhang X, Pang G, Zhang Y, Pan H, Li L, Cui M, et al. Hydrogel microcapsules containing engineered bacteria for sustained production and release of protein drugs. Biomaterials. . 2022;287:121619. doi: 10.1016/j.biomaterials.2022.121619. [DOI] [PubMed] [Google Scholar]
- 115.Rasmussen TS, Mentzel CM, Kot W, Castro-Mejía JL, Zuffa S, Swann JR, Hansen LH, et al. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut. . 2020;69:2122–2130. doi: 10.1136/gutjnl-2019-320005. [DOI] [PubMed] [Google Scholar]
- 116.Obeng N, Pratama AA, Elsas JD. The significance of mutualistic phages for bacterial ecology and evolution. Trends Microbiol. . 2016;24:440–449. doi: 10.1016/j.tim.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 117.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim J, Han D, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. . 2021;6:563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 118.Hu J, Du Y. Managing chronic kidney disease in diabetes patients with the latest chemical therapies. Expert Rev Clin Pharmacol. . 2019;12:53–60. doi: 10.1080/17512433.2019.1552829. [DOI] [PubMed] [Google Scholar]
- 119.Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP‐1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta‐analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. . 2019;21:2576–2580. doi: 10.1111/dom.13847. [DOI] [PubMed] [Google Scholar]
- 120.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. . 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 121.Wang X, Zhang H, Zhang Q, Guan M, Sheng S, Mo W, Zou M, et al. Exenatide and renal outcomes in patients with type 2 diabetes and diabetic kidney disease. Am J Nephrol. . 2020;51:806–814. doi: 10.1159/000510255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Li Z, Huang X, Li F, Liu J, Li Z, Bai D. An experimental study of exenatide effects on renal injury in diabetic rats. Acta Cir Bras. . 2019;34 doi: 10.1590/s0102-865020190010000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W, Cui M, Wei Y, Kong X, Tang L, Xu D. Inhibition of the expression of TGF-β1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell Physiol Biochem. . 2012;30:749–757. doi: 10.1159/000341454. [DOI] [PubMed] [Google Scholar]
- 124.Tong L, Adler S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: information for primary care providers. Postgraduate Med. . 2018;130:381–393. doi: 10.1080/00325481.2018.1457397. [DOI] [PubMed] [Google Scholar]
- 125.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. . 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 126.Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, Bosch-Traberg H, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. . 2016;39:222–230. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 127.Zobel EH, von Scholten BJ, Goldman B, Persson F, Hansen TW, Rossing P. Pleiotropic effects of liraglutide in patients with type 2 diabetes and moderate renal impairment: individual effects of treatment. Diabetes Obes Metab. . 2019;21:1261–1265. doi: 10.1111/dom.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, von Scholten BJ, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. . 2018;138:2908–2918. doi: 10.1161/CIRCULATIONAHA.118.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Q, Cai B, Zhu L, Xin X, Wang X, An Z, Li S, et al. Liraglutide modulates gut microbiome and attenuates nonalcoholic fatty liver in db/db mice. Life Sci. . 2020;261:118457. doi: 10.1016/j.lfs.2020.118457. [DOI] [PubMed] [Google Scholar]
- 130.Madsen MSA, Holm JB, Pallejà A, Wismann P, Fabricius K, Rigbolt K, Mikkelsen M, et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. . 2019;9:15582. doi: 10.1038/s41598-019-52103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. . 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 132.Gerstein HC, Hart R, Colhoun HM, Diaz R, Lakshmanan M, Botros FT, Probstfield J, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. . 2020;8:106–114. doi: 10.1016/S2213-8587(19)30423-1. [DOI] [PubMed] [Google Scholar]
- 133.Hupa-Breier KL, Dywicki J, Hartleben B, Wellhöner F, Heidrich B, Taubert R, Mederacke YS, et al. Dulaglutide alone and in combination with empagliflozin attenuate inflammatory pathways and microbiome dysbiosis in a non-diabetic mouse model of NASH. Biomedicines. . 2021;9:353. doi: 10.3390/biomedicines9040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, Langkilde AM, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes Metab. . 2018;20:2532–2540. doi: 10.1111/dom.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muskiet MH, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJ, van Raalte DH. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. . 2018;6:859–869. doi: 10.1016/S2213-8587(18)30268-7. [DOI] [PubMed] [Google Scholar]
- 136.Ribeiro-Parenti L, Jarry AC, Cavin JB, Willemetz A, Le Beyec J, Sannier A, Benadda S, et al. Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nat Commun. . 2021;12:110. doi: 10.1038/s41467-020-20301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deacon CF. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of Type 2 diabetes. Front Endocrinol. . 2019;10:80. doi: 10.3389/fendo.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kubo A, Hidaka T, Nakayama M, Sasaki Y, Takagi M, Suzuki H, Suzuki Y. Protective effects of DPP-4 inhibitor on podocyte injury in glomerular diseases. BMC Nephrol. . 2020;21:402. doi: 10.1186/s12882-020-02060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. . 2020;16:642–653. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 140.Tanaka Y, Kume S, Chin-Kanasaki M, Araki H, Araki S, Ugi S, Sugaya T, et al. Renoprotective effect of DPP-4 inhibitors against free fatty acid-bound albumin-induced renal proximal tubular cell injury. Biochem Biophys Res Commun. . 2016;470:539–545. doi: 10.1016/j.bbrc.2016.01.109. [DOI] [PubMed] [Google Scholar]
- 141.Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. . 2013;36:3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, Schernthaner G, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. . 2017;19:1610–1619. doi: 10.1111/dom.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun YM, Qu W, Liao JB, Chen L, Cao YJ, Li HL. Jiangtangjing ameliorates type 2 diabetes through effects on the gut microbiota and cAMP/PKA pathway. Tradit Med Res. . 2022;7:7. doi: 10.53388/TMR20211112251. [DOI] [Google Scholar]
- 144.Sakai Y, Suzuki A, Mugishima K, Sumi Y, Otsuka Y, Otsuka T, Ohno D, et al. Effects of alogliptin in chronic kidney disease patients with type 2 diabetes. Intern Med. . 2014;53:195–203. doi: 10.2169/internalmedicine.53.1292. [DOI] [PubMed] [Google Scholar]
- 145.Kaku K, Katou M, Igeta M, Ohira T, Sano H. Efficacy and safety of pioglitazone added to alogliptin in Japanese patients with type 2 diabetes mellitus: a multicentre, randomized, double-blind, parallel-group, comparative study. Diabetes Obes Metab. . 2015;17:1198–1201. doi: 10.1111/dom.12555. [DOI] [PubMed] [Google Scholar]
- 146.Okajima F, Sugihara H, Emoto N. Efficacy and safety of miglitol- or repaglinide-based combination therapy with alogliptin for drug-naïve patients with type 2 diabetes: an open-label, single-center, parallel, randomized controlled pilot Study. J Nippon Med Sch. . 2021;88:71–79. doi: 10.1272/jnms.JNMS.2021_88-205. [DOI] [PubMed] [Google Scholar]
- 147.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im KA, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. . 2017;40:69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 148.Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. . 2016;6:33251. doi: 10.1038/srep33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. . 2012;97:3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tanimura-Inagaki K, Nagao M, Harada T, Sugihara H, Moritani S, Sasaki J, Kono S, et al. Sitagliptin improves plasma apolipoprotein profile in type 2 diabetes: a randomized clinical trial of sitagliptin effect on lipid and glucose metabolism (SLIM) study. Diabetes Res Clin Pract. . 2020;162:108119. doi: 10.1016/j.diabres.2020.108119. [DOI] [PubMed] [Google Scholar]
- 151.Yan X, Feng B, Li P, Tang Z, Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res. . 2016;2016:1–10. doi: 10.1155/2016/2093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smits MM, Fluitman KS, Herrema H, Davids M, Kramer MHH, Groen AK, Belzer C, et al. Liraglutide and sitagliptin have no effect on intestinal microbiota composition: A 12-week randomized placebo-controlled trial in adults with type 2 diabetes. Diabetes Metab. . 2021;47:101223. doi: 10.1016/j.diabet.2021.101223. [DOI] [PubMed] [Google Scholar]
- 153.Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium–glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. . 2019;68:248–257. doi: 10.2337/dbi18-0007. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez DE, Foresto RD, Ribeiro AB. SGLT-2 inhibitors in diabetes: a focus on renoprotection. Rev Assoc Med Bras. . 2020;66:s17–s24. doi: 10.1590/1806-9282.66.s1.17. [DOI] [PubMed] [Google Scholar]
- 155.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, Edwards R, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. . 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 156.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, Bajaj HS, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. . 2020;31:1128–1139. doi: 10.1681/ASN.2019111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mishima E, Fukuda S, Kanemitsu Y, Saigusa D, Mukawa C, Asaji K, Matsumoto Y, et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol-Renal Physiol. . 2018;315:F824–F833. doi: 10.1152/ajprenal.00314.2017. [DOI] [PubMed] [Google Scholar]
- 158.Wang X, Wang Z, Liu D, Jiang H, Cai C, Li G, Yu G. IDDF2021-ABS-0198 canagliflozin alleviates diabetic cardiovascular disease via lipid lowering, mitochondrial homeostasis, and gut microbiota regulation. Gut. 2021, 70: A58–A59
- 159.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. . 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 160.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. . 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 161.Yang M, Shi FH, Liu W, Zhang MC, Feng RL, Qian C, Liu W, et al. Dapagliflozin modulates the fecal microbiota in a type 2 diabetic rat model. Front Endocrinol. . 2020;11 doi: 10.3389/fendo.2020.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oh TJ, Sul WJ, Oh HN, Lee YK, Lim HL, Choi SH, Park KS, et al. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci Rep. . 2019;9:20300. doi: 10.1038/s41598-019-56684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. . 2018;17:62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kadowaki T, Nangaku M, Hantel S, Okamura T, Eynatten M, Wanner C, Koitka‐Weber A. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA‐REG OUTCOME ® trial . J Diabetes Investig. . 2019;10:760–770. doi: 10.1111/jdi.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. . 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 166.Butler J, Zannad F, Fitchett D, Zinman B, Koitka-Weber A, von Eynatten M, Zwiener I, et al. Empagliflozin improves kidney outcomes in patients with or without heart failure. Circ-Heart Fail. . 2019;12:e005875. doi: 10.1161/CIRCHEARTFAILURE.118.005875. [DOI] [PubMed] [Google Scholar]
- 167.Cherney DZ, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, Shih WJ, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. . 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu H, Sridhar VS, Lovblom LE, Lytvyn Y, Burger D, Burns K, Brinc D, et al. Markers of kidney injury, inflammation, and fibrosis Associated with ertugliflozin in patients with CKD and diabetes. Kidney Int Rep. . 2021;6:2095–2104. doi: 10.1016/j.ekir.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J, Xiang H, Lu Y, Wu T, Ji G. New progress in drugs treatment of diabetic kidney disease. Biomed Pharmacother. . 2021;141:111918. doi: 10.1016/j.biopha.2021.111918. [DOI] [PubMed] [Google Scholar]
- 170.McCoubrey LE, Gaisford S, Orlu M, Basit AW. Predicting drug-microbiome interactions with machine learning. Biotechnol Adv. . 2022;54:107797. doi: 10.1016/j.biotechadv.2021.107797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.