Abstract
Many drugs are still prescribed off‐label to the pediatric population. Although off‐label drug use not supported by high level of evidence is potentially harmful, a comprehensive overview of the quality of the evidence pertaining off‐label drug use in children is lacking. The Dutch Pediatric Formulary (DPF) provides best evidence‐based dosing guidelines for drugs used in children. For each drug‐indication‐age group combination—together compiling one record—we scored the highest available level of evidence: labeled use, systematic review or meta‐analysis, randomized controlled trial (RCT), comparative research, noncomparative research, or consensus‐based expert opinions. For records based on selected guidelines, the original sources were not reviewed. These records were scored as guideline. A total of 774 drugs were analyzed comprising a total of 6,426 records. Of all off‐label records (n = 2,718), 14% were supported by high quality evidence (4% meta‐analysis or systematic reviews, 10% RCTs of high quality), 20% by comparative research, 14% by noncomparative research, 37% by consensus‐based expert opinions, and 15% by selected guidelines. Fifty‐eight percent of all records were authorized, increasing with age from 30% in preterm neonates (n = 110) up to 64% in adolescents (n = 1,630). Many have advocated that off‐label use is only justified when supported by a high level of evidence. We show that this prerequisite would seriously limit available drug treatment for children as the underlying evidence is low across ages and drug classes. Our data identify the drugs and therapeutic areas for which evidence is clearly missing and could drive the global research agenda.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Off‐label drug use in children is very prevalent, and many have advocated that off‐label use is only justified when supported by a high level of evidence. However, a comprehensive overview of the quality of the evidence pertaining off‐label drug use in children is lacking.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the highest level of evidence pertaining off‐label drug use in pediatrics?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study shows that the level of evidence for off‐label pediatric pharmacotherapy is low: for only 14% of off‐label records, high quality studies are available. Thirty‐seven percent of off‐label records are not supported by any clinical studies at all.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Performing high quality randomized controlled trials for the > 2,000 off‐label record is a very long pathway to close this information gap. Alternatively, modeling and simulation may be valuable approaches to strengthen the evidence base for off‐label use of drugs, especially in younger age groups.
Despite American and European legislation addressing unmet medical needs in children, many drugs are still used off‐label in the pediatric population. 1 , 2 Off‐label drug use is defined as the prescription of drugs outside of the indications, age groups, dosage, formulations, or routes of administration as authorized (or “labeled”) by medicines evaluation authorities. 3
Although the overall burden of ineffective and unsafe pharmacotherapy in children has never been established, the high prevalence of off‐label drug use is worrisome 4 as it is clearly related to an increased risk of adverse drug reactions (ADRs). 5 , 6 , 7 , 8 The risk of developing ADRs in adults is inversely related to the level of evidence supporting the off‐label prescriptions: when the off‐label drug use is supported by strong scientific evidence (i.e., at least one randomized controlled trial (RCT)), patients have a similar risk of developing ADRs as compared with authorized drugs. Conversely, patients are at higher risk of developing ADRs if off‐label drug use is supported by weak scientific evidence only. 9 Yet, off‐label prescribing is often the only option for a child when licensed drugs or alternatives supported by strong(er) evidence are not available.
In addition, the European Medicines Agency (EMA) concluded that the lack of proper labelling and the consequent lack of dosing recommendations also leads to medication handling errors, including dosing errors: a scenario EMA labeled “evidence of harm.” 10
As off‐label drug use supported by weak scientific evidence is thought to compromise efficacy and safety, knowledge on the level of evidence supporting pediatric pharmacotherapy is crucial to enhance rational use of drugs. 11 However, to the best of our knowledge, this has not yet been systematically assessed.
We therefore aimed to evaluate the highest level of evidence pertaining off‐label drug use in pediatrics. As secondary objectives, we explored the difference in available evidence between different drug classes and set up a priority list for research based on the World Health Organization (WHO) essential medicines list. 12
METHODS
Data source
The Dutch Pediatric Formulary (DPF) was launched in 2008 to provide dosing guidelines for all drugs—both authorized and off‐label—relevant to the pediatric population in the Netherlands. Recently, Germany, Austria, and Norway have also adopted this formulary in a country‐specific version.
Drugs are included in the formulary when a medical need is identified by pediatric professionals and/or when a drug is authorized for a pediatric age group. For every drug, a monograph is developed and maintained following a structured decision framework. As part of this process, for every drug, the authorization status is evaluated. A drug is considered to be authorized for pediatric use when the posology section of the Dutch Summary of Product Characteristics (SmPC; paragraphs 4.1 and 4.2) contains an explicit and unambiguous dose recommendation for pediatric age groups.
For off‐label use, a standardized PubMed search is performed by a senior pharmacist to retrieve available scientific information on efficacy, safety, and dose in the pediatric population. 13 The exact search query can be found in Table S1 . The available scientific evidence supporting a dose recommendation and related safety issues are documented in benefit–risk analysis documents and reviewed by a multidisciplinary editorial board. The assessments are repeated every 5 years or more frequent if warranted by emerging evidence. For selected drug groups, the DPF does not review original evidence as generally accepted and/or high‐quality drug dosing guidelines are available. This applies to the National Institutes of Health (NIH) Guideline for the use of Antiretroviral agents in pediatric HIV infection, 14 SKION (Foundation Children's Oncology Netherlands) guidelines for drugs used in pediatric oncology 15 and the Blau handbook for metabolic diseases. 16 In the past 12 years, pediatric dose recommendations for more than 750 drugs were established, and the repository continues to expand as it is constantly updated.
Data collection
Record definition
For each drug, indication and prespecified age group—together constituting one record—the highest level of evidence available was scored, using the documented studies in the benefit–risk analysis documents. Thus, each drug might have multiple records when it is prescribed for multiple indications and/or age groups (Figure 1 ).
Figure 1.
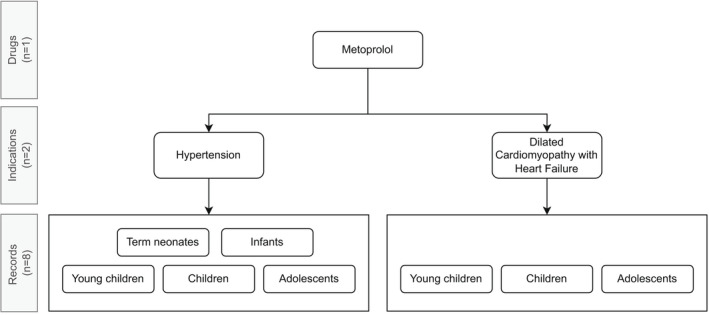
Example of the number of records per drug, taking metoprolol as an example.
Anatomic Therapeutic Chemical‐classification of drugs
Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) system to allow analysis on several levels of drug classes (Figure S1 ). 17 This international classification system assigns drugs to a group based on the organ or system on which they act and their therapeutic, pharmacological, and chemical properties. At ATC level 1, there are 14 major drug groups that are defined (A to V).
Definition of age groups
Age categories were defined according to the EMA classification system 18 (Table S2 ). Groups were defined as preterm neonates (< 37 weeks gestational age and < 28 days of postnatal age), term neonates (≥ 37 weeks gestational age and < 28 days of postnatal age), infants (1 month to 2 years), young children (2–6 years), children (6–12 years), and adolescents (12–18 years). Corresponding weight‐based categories were defined using Dutch Growth Chart data to enable appropriate scoring when only weight categories were listed in the SmPC or DPF. 19 Records for preterm and term neonates were only included when explicitly specified in the DPF. When the dose recommendation applied to only a part of the EMA classified age range (e.g., 4–6 years), the evidence level was scored for the entire EMA category (e.g., 2–6 years).
Scoring of the level of evidence
All records were scored using a predefined scoring system. An instruction protocol and a flowchart (Figure 2 ) were developed to facilitate uniform scoring among researchers. Each ATC group was allocated to one researcher (authors N.S., M.S., H.H., L.B., A.H., J.F., or J.H.) who scored all records within the ATC group.
Figure 2.
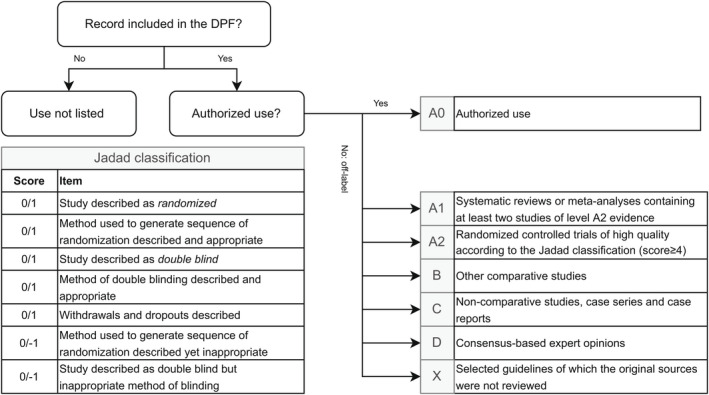
Scoring system of the level of evidence of records. Jadad classification adapted from Jadad et al. DPF, Dutch Pediatric Formulary.
First, the authorization status per record was evaluated. Next, if a record was off‐label, the listed literature from the benefit–risk analysis document was scored for the level of evidence, defined by the evidence‐based medicine methodology. 20 The quality of RCTs was evaluated using the Jadad classification 21 (Figure 2 ). When no published scientific evidence was available, the dose recommendation of the DPF was established based on clinical practice and expert opinion of the editorial board. These records were scored as consensus (D). Records for selected drug groups (HIV, oncology, and metabolic diseases) were scored as selected guideline (X) as the original sources were not reviewed. For off‐label records, levels A1 and A2 were considered high‐level evidence, whereas levels B, C, and D were considered low‐level evidence. 9
To ensure that no high‐quality evidence was missed due to a lag time between the latest update (< 5 years ago) and the current study, the initial PubMed DPF search was repeated for all drugs with a level B, C, or D classification. In addition, the European Public Assessment Reports (EPAR) as part of Articles 45 and 46 of the Pediatric Regulation were checked on additional clinical studies.
Data verification
To validate our scoring system, a senior pharmacist (author M.H.) verified the scored level of evidence for a sample of 10% of all drugs listed in the DPF. All drugs were sorted based on ATC code to assure every ATC group and thus each assessor was reflected in the sample. Every tenth drug based on ATC5 code was incorporated in the verification sample. The Intraclass Correlation Coefficient (ICC), two‐way mixed model was selected to assess conformity between two assessors. As suggested by Koo et al., 22 a score above 0.75 indicates good/excellent agreement and therefore a score of > 0.75 was considered acceptable for further analysis.
Data analysis
The number of drugs and records were evaluated in its entirety as well as per the ATC level 1 group. The percentage of authorized drugs by age group was also analyzed. As the level of evidence may vary substantially by indication, the level of evidence per record was analyzed (Figure 1 ). For instance, enalapril is authorized for the treatment of hypertension and symptomatic heart failure in children weighing more than 20 kg. 23 Yet, its use is off‐label for the treatment of proteinuria related to chronic kidney diseases. The latter would be missed when only analyzing our data on a drug level.
Data were collected from July 2020 to October 2020 and logged using Castor EDC version 2020.2.32. IBM SPSS Statistics version 25.0.0.1 and Microsoft Excel version 2016 were used for descriptive analysis of collected data.
Priority list
To assess the international relevance of our results, data were analyzed separately for drugs included on the WHO Essential Medicines for children list, seventh edition (2019). 12 The core list contains a list of minimum medicine needs for a basic healthcare system, listing the most efficacious, safe, and cost‐effective medicines for priority conditions. The complementary list, on the other hand, contains essential medicines for priority diseases, for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed. We included drugs listed on both lists, thereby including 250 drugs. As the WHO list does not specify indications per drug, indications were not taken into account. Therefore, the analysis on WHO essential medicines took place on a drug level in contrast to other analyses which were performed on the record level thus including age and indication.
Ethical approval and consent
As this study did not enroll patients, nor reviewed individual patient data, this review was not subject to institutional review board approval according to the Dutch Medical Research Involving Human Subjects Act (WMO).
Data sharing
To enable a more detailed presentation of our results by record, our full database is available in an open repository (https://doi.org/10.17026/dans‐27e‐s6yf).
RESULTS
General
Authorization status and level of evidence for off‐label records were determined for 774 drugs with 6,426 records, with a median of 2 indications per drug (range 1–15). Of the 774 drugs, 73 drugs contained dosing information for preterm neonates, 252 for term neonates, 558 for infants, 658 for young children, 727 for children, and 757 for adolescents. Consequently, the number of records varied between age groups with a smaller number of records available for preterm neonates (n = 110) and term neonates (n = 481) compared with infants (n = 1,230), young children (n = 1,425), children (n = 1,550), and adolescents (n = 1,630).
Categorized by ATC level 1, the number of drugs and records per ATC class varied (Table 1 ). ATC class “G” (genito‐urinary system and sex hormones) contained only 16 drugs with 95 records and ATC class “J” (anti‐infectives for systemic use) contained 130 drugs with 1,410 records. The number of indications and records also varied per group.
Table 1.
Number of drugs and records included in the DPF, sorted by ATC level 1 class
| ATC level 1 | Number of drugs | Number of indications per drug, median (range) | Number of records (number of indications × number of age groups) |
|---|---|---|---|
| A: Alimentary tract and metabolism (e.g., anti‐acids, anti‐emetics, constipation drugs, motility disorders, insulins, and drugs for metabolic diseases) | 109 | 1 (1–9) | 935 |
| B: Blood and blood forming organs (e.g., anti‐thrombotics, anti‐hemorrhagics, and anti‐anemic drugs) | 49 | 2 (1–12) | 523 |
| C: Cardiovascular system (e.g., cardiac, antihypertensive, diuretic, vaso‐dilating, vaso‐protecting drugs, beta‐blockers, calcium antagonist, and RAAS affecting drugs) | 59 | 1 (1–8) | 444 |
| D: Dermatologicals (e.g., topically applied anti‐inflammatory and anti‐infective drugs, and emollients) | 41 | 1 (1–7) | 189 |
| G: Genito‐urinary system and sex hormones (e.g., gynecological antimicrobial drugs, sex hormones and modulators of the genital tract, and urinary system drugs) | 16 | 2 (1–5) | 95 |
| H: Systemic hormonal preparations, excluding sex hormones and insulins (e.g., pituitary and hypothalamic hormones, corticosteroids for systemic use, thyroid drugs, pancreatic drugs, and calcium regulating drugs) | 20 | 2 (1–15) | 356 |
| J: Anti‐infectives for systemic use (e.g., antibacterial, anti‐viral, anti‐mycobacterial drugs, vaccines, and immunoglobulins) | 130 | 2 (1–15) | 1,410 |
| L: Antineoplastic and immunomodulating agents (e.g., oncolytic drugs, immunostimulants, and immunosuppressants) | 67 | 1 (1–9) | 541 |
| M: Musculo‐skeletal system (e.g., anti‐inflammatory drugs, anti‐rheumatoids, muscle relaxants, and drugs used in bone diseases) | 24 | 2 (1–6) | 189 |
| N: Nervous system (e.g., anesthetics, analgesics, anti‐epileptics, psycholeptics, and psycho‐analeptics) | 106 | 2 (1–6) | 731 |
| P: Antiparasitic products, insecticides, and repellents (e.g., antiprotozals, anthelmintics, ectoparasiticides, incl scabidides, and repellents) | 21 | 2 (1–13) | 284 |
| R: Respiratory system (e.g., drugs for nasal use, oropharyngeal drugs, drugs used in asthma/COPD, cough and cold drugs, and antihistamines) | 64 | 1 (1–6) | 283 |
| S: Sensory organs (e.g., drugs for ocular use, and drugs for auricular use) | 42 | 1 (1–4) | 275 |
| V: Various (e.g., allergens, antidotes, iron chelating agents, drugs for treatment of hyperkalemia and hyperphosphatemia, and detoxifying agents for antineoplastic treatment) | 26 | 1 (1–4) | 171 |
| Total | 774 | 2 (1–15) | 6,426 |
ATC, Anatomical Therapeutic Chemical; COPD, chronic obstructive pulmonary disease; DPF, Dutch Pediatric Formulary; RAAS, renin angiotensin aldosterone system.
Data verification
Data validation took place for 77 drugs, including 168 indications, with a total of 1,008 records. The inter‐rater reliability for these cases had an ICC of 0.869 (95% confidence interval 0.853–0.883). For 80.8% (n = 814) the level of evidence scored by the first observer was exactly similar to the level scored by the senior pharmacist. For 89.2% (n = 899), the level of evidence was exactly similar or deviated only by one level. Underestimation of the level of evidence (n = 106) occurred approximately as frequently as an overestimation (n = 88).
Authorized use
Considering the different indications per drug, 3,708 (58%) of all records reflected authorized use, varying from 33 (30%) for preterm neonates to 1,042 (64%) for adolescents (Figure 3 ).
Figure 3.
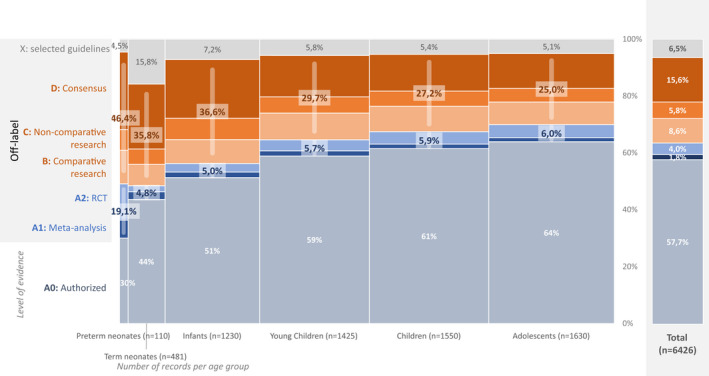
Highest level of evidence available per record. X‐axis: Group size (i.e., number of records) is indicated by the width of the bars and reported per age group (between brackets). Y‐axis: Level of evidence: A0: Authorized use; A1: systematic review or meta‐analysis with at least two studies of level A2; A2: randomized controlled trial with at least 4 points on the Jadad‐scale; B: comparative research with a maximum of 3 on the Jadad‐scale; C: noncomparative research; D: consensus or expert opinion; X: Selected guidelines (HIV, oncology, and metabolic diseases). Blue bars represent high level of evidence (level A1 and A2). Orange bars represent low level of evidence (B, C, and D). RCT, randomized controlled trial.
Off‐label
When analyzing off‐label records only (n = 2,718; Figure 3 depicts both authorized and off‐label records), only 376 (14%) were supported by high‐quality evidence: 118 (4%) by meta‐analysis or systematic review and 258 (10%) by RCT of high quality. Five hundred fifty‐four (20%) were supported by other comparative research, 371 (14%) by noncomparative research, 1,000 (37%) by consensus, and 417 (15%) by selected guidelines.
ATC level 1
The overall level of available evidence for the different ATC level 1 groups varied widely (Figure 4 ). Furthermore, there were large differences between age groups (Table S3 ).
Figure 4.
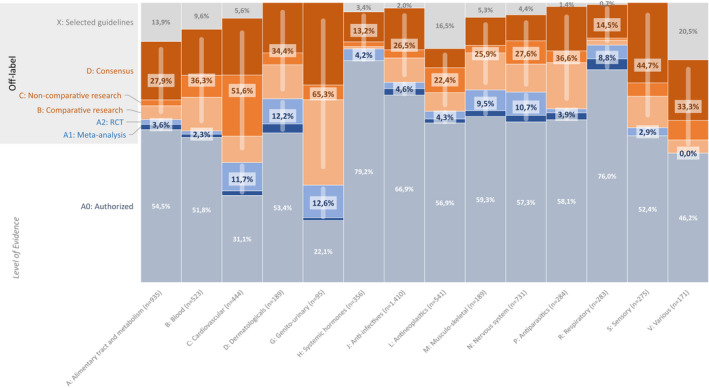
The level of evidence available regardless of age in each ATC level 1 group. The number of records for each ATC group is listed between brackets; ATC level 1: A: Alimentary tract and metabolism; B: Blood and blood forming organs; C: Cardiovascular system; D: Dermatologicals; G: Genito‐urinary system and sex hormones; H: Systemic hormonal preparations, excluding sex hormones and insulins; J: Anti‐infectives for systemic use; L: Antineoplastic and immunomodulating agents; M: Musculoskeletal system; N: Nervous system; P: Antiparasitic products, insecticides and repellents; R: Respiratory system; S: Sensory organs; V: Various. Y‐axis: Level of evidence: A0: Authorized use; A1: systematic review or meta‐analysis with at least two studies of level A2; A2: randomized controlled trial with at least 4 points on the Jadad scale; B: comparative research with a maximum of 3 on the Jadad scale; C: noncomparative research; D: consensus or expert opinion; X: Selected guidelines (HIV, oncology, and metabolic diseases). Blue bars represent high level of evidence (level A1 and A2). Orange bars represent low level of evidence (B, C, and D). ATC, Anatomical Therapeutic Chemical; RCT, randomized controlled trial.
Across ATC groups, off‐label records supported by high‐quality evidence ranged from 0.0% in various drugs (ATC group V) and 12.6% in genito‐urinary drugs (ATC group G). Off‐label records supported by low‐quality evidence ranged from only 13.2% in systemic hormones (ATC group H) to 63.3% in genito‐urinary drugs (Figure 4 ). The group of systemic hormonal preparations, excluding sex hormones and insulins (ATC group H), comprised the highest number of authorized records 282 (79%). Conversely, drugs for the genito‐urinary system and sex hormones (ATC group G) together with cardiovascular drugs (ATC group C) comprised the lowest percentage of authorized‐use records with only 21 (22%) and 138 (31%), respectively.
Priority list
For drugs listed on the WHO essential medicines list, 44–89% was authorized, vs. 34–79% for all DPF drugs. For all off‐label drugs, different levels of evidence were available dependent on age group (Table 2 ). Table 3 shows an overview of drugs on the WHO essential medicines for which at least one age group is supported by low‐quality evidence (B, C, or D) only, thus indicating global priorities for research.
Table 2.
Percentage of drugs with indicated level of evidence per age group
| Age group (total number of drugs in age group overall and on WHO essential medicines list) | Authorized (A0) | High‐level evidence (A1/A2) | Low‐level evidence (B/C/D) | Guideline | ||||
|---|---|---|---|---|---|---|---|---|
| DPF (%) | WHO (%) | DPF (%) | WHO (%) | DPF (%) | WHO (%) | DPF (%) | WHO (%) | |
| Preterm (n = 73; n = 50) | 34 | 44 | 22 | 18 | 38 | 30 | 8 | 8 |
| Term (n = 252; n = 123) | 48 | 54 | 6 | 6 | 34 | 30 | 12 | 10 |
| Infant (n = 558; n = 218) | 60 | 72 | 6 | 5 | 29 | 18 | 5 | 5 |
| Young child (n = 658; n = 233) | 71 | 83 | 5 | 5 | 21 | 10 | 3 | 1 |
| Child (n = 727; n = 246) | 75 | 87 | 4 | 2 | 19 | 10 | 3 | 1 |
| Adolescent (n = 757; n = 246) | 79 | 89 | 11 | 3 | 15 | 7 | 3 | 1 |
AO: Authorized use; A1: systematic review or meta‐analysis with at least two studies of level A2; A2: randomized controlled trial with at least 4 points on the Jadad‐scale; B: comparative research with a maximum of 3 on the Jadad‐scale; C: non‐comparative research; D: consensus or expert opinion; Guidelines (HIV, oncology, and metabolic diseases); DPF, Dutch Pediatric Formulary; WHO, World Health Organization.
Table 3.
Overview of drugs on WHO essentials medicines list for which at least one age group is only supported by low quality evidence (B, C, or D)
| Drug name | Preterm neonate | Term neonate | Infant | Young child | Child | Adolescent |
|---|---|---|---|---|---|---|
| Acetylsalicylic acid (cartazolate calcium) | — | — | B | B | D | D |
| Acyclovir | B | A0 | A0 | A0 | A0 | A0 |
| Adalimumab | — | — | C | A0 | A0 | A0 |
| Adrenalin (epinephrin) | — | D | A0 | A0 | A0 | A0 |
| Amoxicillin + clavulanic acid | — | D | A0 | A0 | A0 | A0 |
| Artesunate | — | D | D | D | D | D |
| Atropine ocular use | — | D | B | A0 | A0 | A0 |
| Azithromycin | — | D | A0 | A0 | A0 | A0 |
| Benzathine‐benzylpenicillin | — | B | A2 | A2 | A0 | A0 |
| Benzoylperoxide | — | — | — | — | D | A2 |
| Benzylpenicillin | B | A0 | A0 | A0 | A0 | A0 |
| Carbamazepine | C | C | A0 | A0 | A0 | A0 |
| Cefazolin | A2 | B | A0 | A0 | A0 | A0 |
| Ceftazidime | — | B | A0 | A0 | A0 | A0 |
| Chloroquine | — | D | D | A0 | A0 | A0 |
| Ciprofloxacin | D | D | A0 | A0 | A0 | A0 |
| Clindamycin | D | D | A0 | A0 | A0 | A0 |
| Colistin (penta‐Na‐mesilate) | C | A0 | A0 | A0 | A0 | A0 |
| Cyclopentolate | — | D | A0 | A0 | A0 | A0 |
| Deferoxamine | — | — | D | A0 | A0 | A0 |
| Dexamethasone | A2 | B | A0 | A0 | A0 | A0 |
| Dopamine | A1 | A2 | D | D | D | D |
| Enalapril | — | C | A0 | A0 | A0 | A0 |
| Enoxaparin | B | B | B | B | B | B |
| Erythromycin cutaneous use | — | — | — | — | D | A0 |
| Esketamine | — | — | B | A1 | A1 | A1 |
| Ethambutol | — | — | D | D | A0 | A0 |
| Flucytosine | — | D | D | D | D | D |
| Folic acid | — | D | B | B | A0 | A0 |
| Gentamycin | B | A0 | A0 | A0 | A0 | A0 |
| Haloperidol | — | — | C | C | A0 | A0 |
| Hydrochlorothiazide | C | A2 | A0 | A0 | A0 | A0 |
| Insulin intermediate acting | — | — | D | A0 | A0 | A0 |
| Insulin short acting | — | D | A0 | A0 | A0 | A0 |
| Itraconazole | — | — | B | B | B | B |
| Kinin | — | — | B | B | B | B |
| Lactulose | — | D | A0 | A0 | A0 | A0 |
| Levofloxacin | — | — | A2 | A2 | B | B |
| Lidocaine | B | B | A0 | A0 | A0 | A0 |
| Lidocaine auricular use | — | — | D | A2 | A2 | A2 |
| Mannitol | — | B | B | B | B | A0 |
| Mercaptopurine | — | Guideline | D | A0 | A0 | A0 |
| Meropenem | B | B | A0 | A0 | A0 | A0 |
| Methadone (hydrochloride) | — | — | C | C | C | C |
| Methotrexate | — | D | A0 | A0 | A0 | A0 |
| Miconazole cutaneous use | — | A2 | A2 | D | D | D |
| Mupirocin | — | — | B | A0 | A0 | A0 |
| Ofloxacin – ocular/auricular use | — | D | A2 | A2 | A2 | A2 |
| Omeprazole | — | D | B | A0 | A0 | A0 |
| Pancreatin | D | — | A0 | A0 | A0 | A0 |
| Paromomycin | — | — | C | C | C | C |
| Pentamidine | — | — | D | B | B | B |
| Phenytoin | — | — | B | A0 | A0 | A0 |
| Phytomenadione (vitamin K) | A0 | A0 | C | C | B | B |
| Piperacillin/tazobactam | — | — | B | A0 | A0 | A0 |
| Prednisolone | — | D | A0 | A0 | A0 | A0 |
| Propofol | A2 | C | A0 | A0 | A0 | A0 |
| Propranolol | — | D | A0 | B | A0 | A0 |
| Propylthiouracil | — | D | D | B | A0 | A0 |
| Pyridoxin | Guideline | Guideline | D | A0 | A0 | A0 |
| Pyrimethamine | — | D | A0 | A0 | A0 | A0 |
| Ranitidine | — | D | A0 | A0 | A0 | A0 |
| Retinol (vitamin A) | — | D | A1 | A1 | D | D |
| Senna | — | — | — | — | B | A0 |
| Silversulfadiazine | — | — | B | A0 | A0 | A0 |
| Sodium docusate | — | — | D | B | B | A0 |
| Sodium valproate | — | — | B | A0 | A0 | A0 |
| Sodium chloride 0.9% | D | A0 | A0 | A0 | A0 | A0 |
| Spironolactone | D | D | A0 | A0 | A0 | A0 |
| Sulfadiazine | — | B | B | B | B | B |
| Terbinafine cutaneous use | — | — | D | D | D | A0 |
| Thiamazole | — | — | D | A0 | A0 | A0 |
| Thiamine (vitamin B1) | — | — | C | C | D | D |
| Thiopental | — | — | C | A0 | A0 | A0 |
| Valganciclovir | — | C | A0 | A0 | A0 | A0 |
| Vancomycin oral use | — | — | D | D | D | A0 |
| Vecuronium | D | A0 | A0 | A0 | A0 | A0 |
| Xylometazoline | — | — | D | A0 | A0 | A0 |
| Zinc sulfate | — | D | D | D | D | D |
AO: Authorized use; A1: systematic review or meta‐analysis with at least two studies of level A2; A2: randomized controlled trial with at least 4 points on the Jadad scale; B: comparative research with a maximum of 3 on the Jadad scale; C: noncomparative research; D: consensus or expert opinion; X: Selected guidelines (HIV, oncology, and metabolic diseases); — Not included in DPF; WHO, World Health Organization.
DISCUSSION
Our study demonstrates that the underlying evidence pertaining off‐label drug use in children is low across ages and drug classes. Furthermore, we confirm that for a significant proportion of drugs and indications a pediatric market authorization is still lacking, especially in the younger age groups.
To the best of our knowledge, there are no similar studies providing such detailed and comprehensive (> 750 drugs) overview on the level of evidence pertaining off‐label drug use in the pediatric population—including drug, age group and indication for use. Although treatment guidelines and reviews establish levels of evidence of treatment modalities, they often do not clearly distinguish between authorized and off‐label used drugs or are usually limited by the number of drugs and/or indications. An example of such a smaller study supporting our findings is the study by Kaley et al. 24 concluding that for β‐blockers in various cardiac and vascular conditions in children, for most indications only moderate to low‐evidence is available. Interestingly, the situation for adult off‐label prescribing appears more favorable. In the intensive care setting, off‐label drug use was supported by high to moderate quality evidence, defined as level A1, A2 or B, in 64% of the cases, 25 compared with only 34% in our study. In adult oncology patients, 61% of off‐label prescriptions were supported by at least well designed non‐RCTs. 26
In line with Tan et al., 27 our study confirms the relatively low percentage of authorized drugs in the lower age groups, whereas increasing with age. The limited number of authorized drugs in children compared with adults, as well as the overall low level of evidence pertaining off‐label records and the observed differences between drug classes may be explained by the methodological and legislative challenges that arise when performing state‐of‐the‐art studies in the pediatric population.
Currently, a German, an Austrian, and a Norwegian equivalent, based on the Dutch Pediatric Formulary's database, have been established. Although pediatric drug authorizations seem to differ between countries to some extent, the (off‐label) dose recommendations, based on a thorough review of internationally published studies are adopted by all countries. We are therefore confident that the observed quality of evidence for off‐label use is representative for all other countries and may thus be important for countries outside of Europe.
Many advocate that off‐label drug use is only justified when supported by a high level of evidence, even in pediatrics. 3 , 9 , 28 , 29 , 30 , 31 Our data show that including this prerequisite in treatment guidelines or reimbursement schemes will seriously jeopardize drug treatment of children, as denying off‐label drug use may be more deleterious than the potentially increased risk of ADRs and lack of efficacy. 32 , 33 , 34 It is reassuring though that drugs listed on the WHO essential medicines list are more often licensed for children and the percentage of off‐label drugs supported by low‐quality evidence only seems lower compared with all drugs in the DPF, thus better satisfying the priority healthcare needs of the global pediatric population. Yet, drugs on the WHO list are selected with due regard to evidence of efficacy and safety, leading to overall better available evidence.
At the same time, our data clearly call for action to close the identified information gaps to improve pediatric pharmacotherapy. In this evaluation, we have focused on the traditional evidence‐based medicine approach, with systematic reviews or meta‐analyses of RCTs as the highest level of evidence, to evaluate the scientific strength pertaining off‐label prescribing. This approach has traditionally guided the development and market authorization of drugs and is still widely used to support pediatric treatment guidelines and thus rational prescribing. It is obvious that performing multiple high quality RCTs for the > 2,000 off‐label records we have identified would not be feasible for obvious financial, ethical, and practical reasons. Alternatively, we advocate the newer regulatory approach for pediatric drug development and the use of real‐world data (RWD) to generate real world evidence. 35 , 36 , 37 In the regulatory approach, extrapolation of efficacy data from other age groups can be considered when the disease mechanism is the same, 38 , 39 thereby reducing the need for challenging RCTs. Pharmacokinetic (PK) studies may still be needed to verify extrapolated doses from adults, as unexpected variation in the mechanisms involved in drug disposition may affect drug disposition in children of different ages. However, these studies may include less children or may be less complicated when modeling and simulation can support their design. 40 , 41 , 42 In addition, electronic health records host a pool of RWD generated by clinical practice, such as patient characteristics, laboratory findings, routinely collected blood concentrations, and pharmacodynamic outcomes. The usability of RWD, however, is still hindered by the diversity of systems that capture these data and most importantly the temporal relation to these data. Yet, these data may aid in closing the information gap without exposing children to the burden of clinical trials.
Our data identify information gaps and can aid to determine research priorities in addition to other important criteria related to medical need and risks of the off‐label use. These criteria include, but are not limited to, epidemiological data on frequency and severity of disease, drug characteristics and the availability of appropriate therapeutic alternatives. Most importantly, age should be considered as an important criterion. As PK parameters are subject to maturation and growth, and these changes are the most pronounced during the first 2 years of life, incorrect dosing based on changed PKs leading to toxicity or lack of efficacy is most likely to occur in this age group and less likely in older children or adolescents. Furthermore, criteria for research priorities may differ across different pediatric subspecialties (e.g., pediatric gastro‐enterology vs. infectious diseases) and also between healthcare systems as elucidated by our comparison with the WHO essential medicines list. We therefore have provided our dataset in a public repository (https://doi.org/10.17026/dans‐27e‐s6yf), enabling healthcare professionals, research groups, regulatory agencies, and other relevant authorities worldwide to include the already available quality of evidence as a criterion for defining research priorities. The EMA has drafted the “Need for pediatric medicines lists,” indicating research priorities. 43 Comparison with this list, however, is less appropriate as these listings, organized by drug class, are primarily based on pediatric authorization in member states and not on available evidence. Furthermore, these lists, drafted in the early 2000s, seem to be outdated for many drugs. For example, morphine and enalapril have been studied in the listed age categories. 44 , 45 The US Food and Drug Administration (FDA) adopted more a pragmatic approach to prioritize research in pediatric oncology based on molecular targets for which there is evidence and/or a biologic rationale. 46
To reduce the risk for therapy failure and toxicity related to off‐label use, the Benefit and Risk Assessment for Off‐label (BRAvO) use decision tool was recently developed to assess and balance benefits and risks of off‐label drug use in children. 47 This may help physicians and guideline committees to address information gaps in daily practice. Our data also call for a re‐appreciation of consensus and clinical expertise, although traditionally considered as low level of evidence. 48 Many drugs lacking solid scientific support have been used off‐label for many years in clinical practice. Physician experience and consensus among healthcare professionals should therefore be appreciated to complement limited scientific data and to identify and balance benefits and risks.
Our study has several limitations. First, our study was not intended to establish efficacy and safety of individual drugs in the pediatric population. This requires a meta‐analysis approach, including an in‐depth evaluation on study design, age‐appropriate dose leading to adult equivalent exposures, age‐appropriate outcomes, and adverse events. The traditional evidence‐based medicine interpretation of efficacy and safety usually does not address these clinical pharmacological principles either. In addition, many pediatric drug trials have serious flaws 49 As a meta‐analyses approach was not feasible, we used the well‐documented summaries of available evidence of the editorial process of the DPF. Despite this methodological limitation our data do provide important insight into the low level of evidence for pediatric pharmacotherapy. After all, off‐label drug use that has been studied by means of an RCT is still more likely to reflect the true effect of pharmacotherapy than a case series describing the drug. Second, we did not investigate the supporting evidence for the authorized records (licensed use). Especially for old drugs, authorization is not always based on high‐quality clinical studies either. But even for recent authorizations, pediatric dosing may fall short. For example, Völler et al. 50 has demonstrated that recently authorized midazolam doses for preterm neonates do not lead to adequate exposure. Therefore, our data may be even more significant, as the number of records supported by a low level of evidence may be underestimated. Third, the number of drugs evaluated for evidence was much lower for neonates than for the older age groups, as only the most frequently used drugs for neonatal care are included in the DPF. Fourth, the level of evidence of three therapeutic areas was not evaluated (i.e., oncology, HIV, and metabolic diseases) representing 15% of all records. As referenced dosing guidelines were already available, the DPF did not perform structured literature searches for these drugs, preventing duplicate efforts. Finally, the selection of drugs represents prescribing patterns in the four European countries where the DPF dosing guidelines are available. This may differ across other parts of the world, but because the number of drugs is so large and will overlap considerably, we do not expect a significant different overall trend for other countries.
CONCLUSION
Our data demonstrate that the overall level of evidence pertaining off‐label prescribing in the pediatric population is low. Because it is not feasible to study all off‐label drugs in children with a low level of evidence for all indications and all age groups, a tailored approach, including extrapolation of existing data and collection of missing PK data, safety, and/or efficacy data, could aid to close the information gap for pediatric prescribing. Our data identify the drugs and therapeutic areas for which clinical evidence is clearly missing and could therefore drive the global research agenda.
FUNDING
The Dutch Pediatric Formulary has been developed by Dutch Knowledge Center Pharmacotherapy for Children which is funded by a government grant by the Dutch Ministry of Health. This research was performed independent from the government grant.
CONFLICTS OF INTEREST
T.vdZ. is managing director of the Dutch Knowledge Center Pharmacotherapy for Children. S.dW. is medical director of Dutch Knowledge Center Pharmacotherapy for Children All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
T.M.vdZ., N.J.L.S., M.G.M., M.dH., and S.N.dW. wrote the manuscript. All authors designed the research. T.M.vdZ., N.J.L.S., M.F.T.S., H.J.H., L.J.C.B., J.E.M.vdH., J.J.M.F., A.A.L.H., I.H.G.H., and M.dH‐S. performed the research. T.M.vdZ. and N.J.L.S. analyzed the data.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was performed in close collaboration with the Dutch Knowledge Center Pharmacotherapy for Children, who shared their benefit–risk analysis documents for review of the level of evidence.
References
- 1. Regulation (EC) no 1901/2006 of the European Parliament and of the Council on Medicinal Products for Pediatric Use and amending Regulation (EEC) no 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) no 726/2004, EC/190 (2006).
- 2. Carmack, M. , Hwang, T. & Bourgeois, F.T. Pediatric drug policies supporting safe and effective use of therapeutics in children: a systematic analysis. Health Aff (Millwood) 39, 1799–1805 (2020). [DOI] [PubMed] [Google Scholar]
- 3. Frattarelli, D.A. et al. Off‐label use of drugs in children. Pediatrics 133, 563–567 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Weda, M . et al. Study on the off‐label use of medicinal products in the European Union <www.ec.europa.com> Updated March 14, 2017.
- 5. Bellis, J.R. et al. Adverse drug reactions and off‐label and unlicensed medicines in children: a nested case‐control study of inpatients in a pediatric hospital. BMC Med 11, 238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saiyed, M.M. , Lalwani, T. & Rana, D. Is off‐label use a risk factor for adverse drug reactions in pediatric patients? A prospective study in an Indian tertiary care hospital. Int. J. Risk Saf. Med. 27, 45–53 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Bellis, J.R. , Kirkham, J.J. , Nunn, A.J. & Pirmohamed, M. Adverse drug reactions and off‐label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital. Br. J. Clin. Pharmacol. 77, 545–553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elzagallaai, A.A. , Greff, M. & Rieder, M.J. Adverse drug reactions in children: the double‐edged sword of therapeutics. Clin. Pharmacol. Ther. 101, 725–735 (2017). [DOI] [PubMed] [Google Scholar]
- 9. Eguale, T. et al. Association of off‐label Drug use and Adverse Drug Events in an adult population. JAMA Intern. Med. 176, 55–63 (2016). [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency . Evidence of harm from off‐label or unlicensed medicines in children (2004) (EMEA/126327/2004).
- 11. World Health Organization . Essential Medicines and Health Products <https://www.who.int/activities/promoting‐rational‐use‐of‐medicines/> (2012).
- 12. World Health Organization . Model list of essential medicines for Children (2019).
- 13. van der Zanden, T. , De Wildt, S. , Liem, T. , Offringa, M. & de Hoog, M. Developing a pediatric formulary for the Netherlands. Arch. Dis. Child. 102, 357–361 (2017). [DOI] [PubMed] [Google Scholar]
- 14. Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV . Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection <https://clinicalinfo.hiv.gov/en/guidelines/pediatric‐arv>. Accessed June 20, 2022.
- 15. Foundation Children's Oncology Netherlands . Treatment guidelines <https://www.skion.nl/voor‐professionals/per‐ziekte‐beeld/>. Accessed June 2021.
- 16. Blau Hoffmann Leonard and Clarke . Physicians Guide to the Treatment and Follow‐Up of Metabolic Diseases (Springer, New York, 2006). [Google Scholar]
- 17. World Health Organization . Anatomical Therapeutic Chemical (ATC) Classification <https://www.whocc.no/atc/structure_and_principles/>. Accessed June 2020.
- 18. European Medicines Agency . ICH Topic E 11 R1 Clinical Investigation of Medicinal Products in the Paediatric Population 2017 (Revision 1).
- 19. Netherlands Organisation for Applied Scientific Research . Growth charts in PDF format. TNO <https://www.tno.nl/nl/aandachtsgebieden/gezond‐leven/roadmaps/youth/groeidiagrammen‐in‐pdf‐formaat/>. Accessed April 27, 2020.
- 20. Djulbegovic, B. & Guyatt, G.H. Progress in evidence‐based medicine: a quarter century on. Lancet 390, 415–423 (2017). [DOI] [PubMed] [Google Scholar]
- 21. Jadad, A.R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- 22. Koo, T.K. & Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Organon NV. SmPC Renitec tablet 5 mg (RVG 10575) 01‐06‐2021. Dutch Medicins Evaluations Board <https://www.geneesmiddeleninformatiebank.nl/smpc/h10575_smpc.pdf>. Accessed June 6, 2021.
- 24. Kaley, V.R. , Aregullin, E.O. , Samuel, B.P. & Vettukattil, J.J. Trends in the off‐label use of β‐blockers in pediatric patients. Pediatr. Int. 61, 1071–1080 (2019). [DOI] [PubMed] [Google Scholar]
- 25. Shoulders, B.R. , Smithburger, P.L. , Tchen, S. , Buckley, M. , Lat, I. & Kane‐Gill, S.L. Characterization of guideline evidence for off‐label medication use in the intensive care unit. Ann. Pharmacother. 51, 529–542 (2017). [DOI] [PubMed] [Google Scholar]
- 26. Herrero Fernandez, M. et al. The off‐label use of Antineoplastics in oncology is limited but has notable scientific support in a university hospital setting. Front. Pharmacol. 10, 1210 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan, E. , Cranswick, N.E. , Rayner, C.R. & Chapman, C.B. Dosing information for paediatric patients: are they really "therapeutic orphans"? Med. J. Aust. 179, 195–198 (2003). [DOI] [PubMed] [Google Scholar]
- 28. Ansani, N. et al. Designing a strategy to promote safe, innovative off‐label use of medications. Am. J. Med. Qual. 21, 255–261 (2006). [DOI] [PubMed] [Google Scholar]
- 29. Stafford, R.S. Regulating off‐label drug use‐‐rethinking the role of the FDA. N. Engl. J. Med. 358, 1427–1429 (2008). [DOI] [PubMed] [Google Scholar]
- 30. Largent, E.A. , Miller, F.G. & Pearson, S.D. Going off‐label without venturing off‐course: evidence and ethical off‐label prescribing. Arch. Intern. Med. 169, 1745–1747 (2009). [DOI] [PubMed] [Google Scholar]
- 31. Gazarian, M. , Kelly, M. , McPhee, J.R. , Graudins, L.V. , Ward, R.L. & Campbell, T.J. Off‐label use of medicines: consensus recommendations for evaluating appropriateness. Med. J. Aust. 185, 544–548 (2006). [DOI] [PubMed] [Google Scholar]
- 32. Cuzzolin, L. , Atzei, A. & Fanos, V. Off‐label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin. Drug Saf. 5, 703–718 (2006). [DOI] [PubMed] [Google Scholar]
- 33. Choonara, I. & Conroy, S. Unlicensed and off‐label drug use in children: implications for safety. Drug Saf. 25, 1–5 (2002). [DOI] [PubMed] [Google Scholar]
- 34. Mason, J. , Pirmohamed, M. & Nunn, T. Off‐label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur. J. Clin. Pharmacol. 68, 21–28 (2012). [DOI] [PubMed] [Google Scholar]
- 35. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) . General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products – Guidance for Industry (draft); FDA‐2013‐D‐1275 (2014).
- 36. Turner, M.A. , Portman, R.J. & Davis, J.M. Regulatory science in neonates: a framework that supports evidence‐based drug therapy. JAMA Pediatr. 171, 721–722 (2017). [DOI] [PubMed] [Google Scholar]
- 37. Van Driest, S.L. & Choi, L. Real‐world data for pediatric Pharmacometrics: can we upcycle clinical data for research use? Clin. Pharmacol. Ther. 106, 84–86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dunne, J. et al. Extrapolation of adult data and other data in pediatric drug‐development programs. Pediatrics 128, e1242–e1249 (2011). [DOI] [PubMed] [Google Scholar]
- 39. European Medicines Agency . Reflection paper on the use of extrapolation in the development of medicines for paediatrics (EMA/189724/2018) (2018).
- 40. van den Anker, J. , Reed, M.D. , Allegaert, K. & Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 58(Suppl 10), S10–S25 (2018). [DOI] [PubMed] [Google Scholar]
- 41. European Medicines Agency . Guideline on the role of pharmacokinetics in the development of medicinal products in the paediatric population. Vol. EMEA/CHMP/EWP/147013/2004 2006 <https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐role‐pharmacokinetics‐development‐medicinal‐products‐paediatric‐population_en.pdf>
- 42. Vinks, A.A. & Barrett, J.S. Model‐informed pediatric drug development: application of Pharmacometrics to define the right dose for children. J. Clin. Pharmacol. 61(Suppl 1), S52–S59 (2021). [DOI] [PubMed] [Google Scholar]
- 43. European Medicines Agency . Needs for paediatric medicines <https://www.ema.europa.eu/en/human‐regulatory/research‐development/paediatric‐medicines/needs‐paediatric‐medicines>. Accessed Aug 16, 2022.
- 44. Laeer, S. et al. Enalapril and enalaprilat pharmacokinetics in children with heart failure due to dilated cardiomyopathy and congestive heart failure after Administration of an Orodispersible Enalapril Minitablet (LENA‐Studies). Pharmaceutics 14(6), 1163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krekels, E.H. et al. Evidence‐based morphine dosing for postoperative neonates and infants. Clin. Pharmacokinet. 53, 553–563 (2014). [DOI] [PubMed] [Google Scholar]
- 46. Food and Drug Administration . Pediatric Oncology: Promoting the development of safe and effective new drugs and biologics to treat cancer in children. US Food and Drug Administration <https://www.fda.gov/about‐fda/oncology‐center‐excellence/pediatric‐oncology>. Updated 06/27/2022. Accessed Aug 16, 2022.
- 47. van der Zanden, T.M. et al. Benefit‐ risk assessment of off‐label drug use in children: the bravo framework. Clin. Pharmacol. Ther. 110(4), 952–965 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guyatt, G.H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abdel‐Rahman, S.M. , Reed, M.D. , Wells, T.G. & Kearns, G.L. Considerations in the rational design and conduct of phase I/II pediatric clinical trials: avoiding the problems and pitfalls. Clin. Pharmacol. Ther. 81, 483–494 (2007). [DOI] [PubMed] [Google Scholar]
- 50. Völler, S. et al. Recently registered midazolam doses for preterm neonates do not Lead to equal exposure: a population pharmacokinetic model. J. Clin. Pharmacol. 59, 1300–1308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


