Abstract
Background:
Social experiences influence susceptibility to substance use disorder. The adolescent period is associated with the development of social reward and is exceptionally sensitive to disruptions to reward-associated behaviors by social experience. Social isolation during adolescence alters anxiety- and reward-related behaviors in adult males, but less is known about females. The medial amygdala (meA) is a likely candidate for the modulation of social influence on drug reward because it regulates social reward, develops during adolescence, and is sensitive to social stress. However, little is known regarding how meA responds to drugs of abuse.
Methods:
We used adolescent social isolation coupled with RNA-sequencing to better understand the molecular mechanisms underlying meA regulation of social influence on reward.
Results:
We show that social isolation in adolescence, a well-established preclinical model for addiction susceptibility, enhances preference for cocaine in male but not female mice and alters cocaine-induced protein and transcriptional profiles within the adult meA particularly in males. To determine if transcriptional mechanisms within meA are important for these behavioral effects, we manipulated Crystallin mu (Crym) expression, a sex-specific key driver gene identified through differential gene expression and co-expression network analyses, specifically in the meA. Overexpression of Crym, but not another key driver that did not meet our sex-specific criteria, recapitulated the behavioral and transcriptional effects of adolescent social isolation.
Conclusions:
These results demonstrate that meA is essential for modulating the sex-specific effects of social experience on drug reward, and establish Crym as a critical mediator of sex-specific behavioral and transcriptional plasticity.
Keywords: RNA-sequencing, stress, cocaine, adolescent social isolation, anxiety, gene expression, gene co-expression network analysis
INTRODUCTION:
Social experience has profound effects on reward-associated behaviors including susceptibility to substance use disorders (SUDs). Humans with strong social connections are less likely to develop such disorders and display better treatment outcomes (1, 2). In contrast, adults and adolescents with SUDs have fewer and less stable social connections (3, 4) and individuals reporting greater loneliness are more likely to use drugs of abuse (5). While these findings suggest an effect of SUDs on social relationships, evidence shows that early disruptions in social attachment increase risk for SUDs in adulthood (6), suggesting that social disruptions earlier in life influence lifetime risk for SUD.
In rodents, social deprivation also influences self-administration of and preference for various drugs of abuse (7) and these effects are particularly potent if isolation takes place during adolescence (8). Adolescence is an especially sensitive period for programming reward-associated behaviors by social experience (9, 10). During adolescence, there is a highly conserved shift in the salience of social reward (11) and a re-organization of social structure away from family and towards peers (12). This restructuring of social organization is necessary for social species to develop the behavioral strategies essential for survival in adulthood (13). While critical to normative development, evidence suggests that the quality of social interactions during adolescence matter. For example, negative or lack (social isolation) of peer interactions in adolescence are strong predictors of neuropsychiatric disorders throughout life (14–18).
Given that adolescence is a secondary period of sexual differentiation and maturation (19), it is unsurprising that effects of social experience are different between sexes in humans (14, 20–22). However, less is known regarding how social experience influences sex-specific reward-related behaviors in rodents (8, 23, 24). In male rodents, adolescent social isolation (SI) in particular has long been used as a preclinical model for understanding neurotransmitter systems, brain regions, and candidate genes and proteins that may contribute to susceptibility to substance use and anxiety disorders (8). Few studies have investigated the sex-specific impact of adolescent SI, and investigations into underlying molecular mechanisms are especially lacking.
Medial amygdala (meA), part of the extended reward circuitry, is a key regulator of social reward including parental, copulation and aggressive behaviors (25–28). meA undergoes developmental changes in size, synaptic pruning, cell number and gene expression during adolescence (29, 30) and is sensitive to adolescent (31, 32) and adult (33) social stress at the cellular and molecular levels in males. Thus, meA is a promising target that is likely programmed by social experience, shaping subsequent reward-related behaviors in adulthood. We hypothesized that sexually-divergent effects of SI on drug-seeking/reward may be mediated in part via transcriptional changes in meA.
We tested this hypothesis by analyzing cocaine-induced behavior and transcription in meA after SI in male and female mice. Our findings revealed that, like in humans, adolescent SI induces different, in many cases opposite, changes in adult reward-related behavior in males vs. females. Further, sex-specific isolation-induced transcriptional changes in meA mirror the behavioral adaptations observed after SI. Through a series of bioinformatic approaches, we identified crystallin mu (Crym), a thyroid hormone-binding protein, as a key mediator in meA of this sex-specific behavioral and transcriptional regulation. Manipulation of Crym expression specifically in the meA replicates behavioral and transcriptional effects of SI, suggesting that thyroid hormone signaling helps shape effects of social experience on reward. Together, these studies identify a molecular mechanism by which sex-specific effects of social experience are programmed at the level of the meA to establish cocaine-related behaviors later in life.
RESULTS:
Adolescent SI affects reward-related behavior in males but not females
In humans (15, 17, 18) and rodents (9), social experience during adolescence influences drug-taking behaviors in adulthood. Therefore, we used cocaine conditioned place preference (CPP; 7.5 mg/kg) to determine if adolescent SI alters cocaine reward sensitivity in adult male and female mice (Figure 1B; Suppl Figure 1A). Animals were isolated from postnatal day P22-P42 and rehoused with their original cagemates at P42 (Figure 1A) until behavioral testing (Figure 1B–D). Two-way ANOVA revealed an interaction of sex and SI (Figure 1B; F(1, 143)=8.98; p<0.01). Tukey post-hoc analysis indicated that, as predicted, SI in males increased cocaine CPP over control males (p=0.04), an effect not seen in females (p=0.3). SI induced a gain of sex difference in cocaine CPP in SI animals (SI males vs. females; p<0.01), and a sex-specific effect on the proportion of animals that formed an aversion for cocaine (SI males = 0% vs. SI females = ~15%; Suppl Figure 1C; p=0.002). Together, these data suggest that SI induces a male-specific increase in aspects of cocaine reward.
Figure 1: Adolescent SI Enhances Preference for Cocaine in Males but not Females and Disrupts Sex Differences in Anxiety-Related Behaviors.
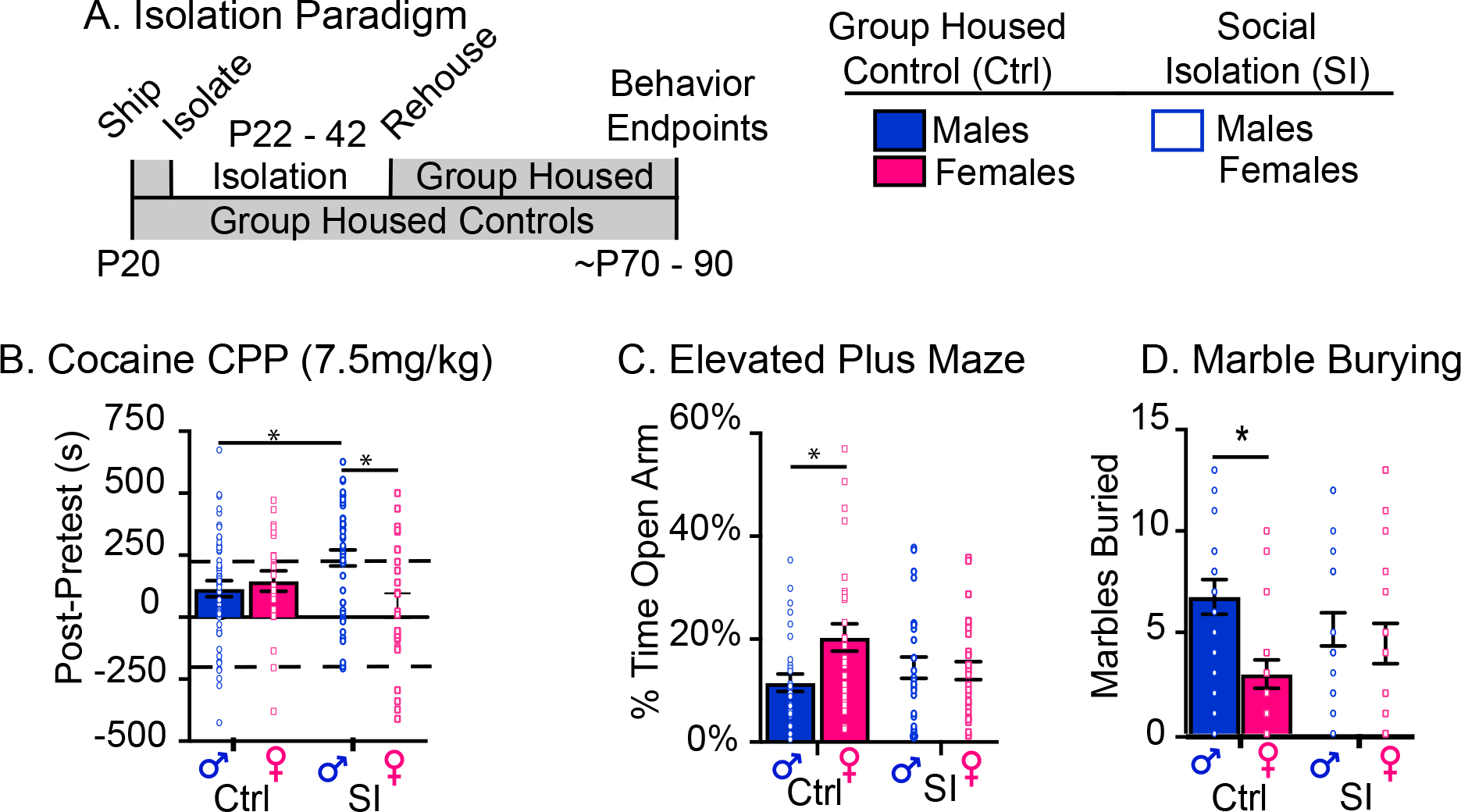
(A) Schematic of isolation paradigm. (B) Adolescent SI increases cocaine CPP in males, but decreases it in females, resulting in a gain of sex difference in cocaine-elicited behavior. Data points above and below the dashed lines indicate animals who formed a preference or aversion for cocaine. (C-D) Adolescent SI abolishes known sex differences in elevated plus maze (C) and marble burying (D). Post-hoc significant effects indicated as: *p<0.05; **p<0.001: error bars indicate SEM.
The male-specific impact of SI on cocaine CPP was unexpected given that adolescence is a period of enhanced vulnerability for SUDs across sexes (34). In adolescent girls, social stress increases rates of other neuropsychiatric disorders including depression and anxiety (14, 21). Therefore, we tested the hypothesis that adolescent SI exerts long-term effects on anxiety-related behaviors in male and female mice. Differences in anxiety-related behaviors were assessed by use of elevated plus maze (EPM), marble burying (MB) (Figure 1C–D) and open field (OF) (Suppl Fig 1C). Rather than increasing anxiety-related behaviors as predicted, SI abolished known sex differences (35–37) in EPM and MB (Figure 1B–C; Supp Figure 1B; EPM: Sex × SI F(1, 105)=5.3, p=0.02; Tukey post-hoc: control males vs. females p=0.02; total marbles buried: Kruskal-Wallis H(3, 78)=10.2, p=0.02), but had no effect on OF (Suppl Fig 1C). These behavioral effects were driven by opposite consequences of SI on males vs. females; generally SI in males decreases measures of anxiety (time in open arm of EPM and decreased marbles buried), whereas females displayed an increase in those endpoints. These data suggest that SI abolishes sex-specific behaviors through opposite, albeit relatively subtle, effects in both males and females.
Adolescent SI disrupts cocaine-induced repression of FOS and alters transcription in males but not female meA.
meA is a likely candidate for mediating sex-specific effects of social influence on cocaine reward, although this has not to date been tested directly. meA is a known sexually dimorphic brain region (38) which undergoes permanent developmental changes during adolescence (29), is disrupted by adolescent social stress (31, 32) and is critical for regulating sex-specific social behaviors (25, 26, 28, 39). While meA has been studied extensively in stress and natural reward (25–27, 39), very little is known about its role in drug reward (40, 41). Therefore, we tested the hypothesis that meA is responsive to cocaine and sensitive to reprogramming by SI in males and females by measuring FOS in meA 1 hr after a saline or cocaine injection (Figure 2A – E). By looking at FOS at this time point (used for CPP), we sought to better understand cellular processes contributing to sex-specific behavioral effects of SI. Similar to effects of SI on CPP, SI disrupts meA response to cocaine in males only (Kruskal-Wallace H(3, 78)=10.2, p=0.02). Specifically, in group-housed control males cocaine represses FOS in meA (p<0.05), an effect not observed after SI or in females. Rather, SI represses FOS expression at baseline when compared to group-housed control males (p<0.05). These results are specific to meA as SI has few sex-specific effects on FOS in other reward-associated brain regions (42). Importantly, no effects were observed in female meA suggesting that 1) male meA is more sensitive to cocaine than females and 2) SI disrupts this male-specific sensitivity.
Figure 2: Baseline and Cocaine-Induced FOS Expression in meA is Disrupted by Adolescent SI in Males but not Females.
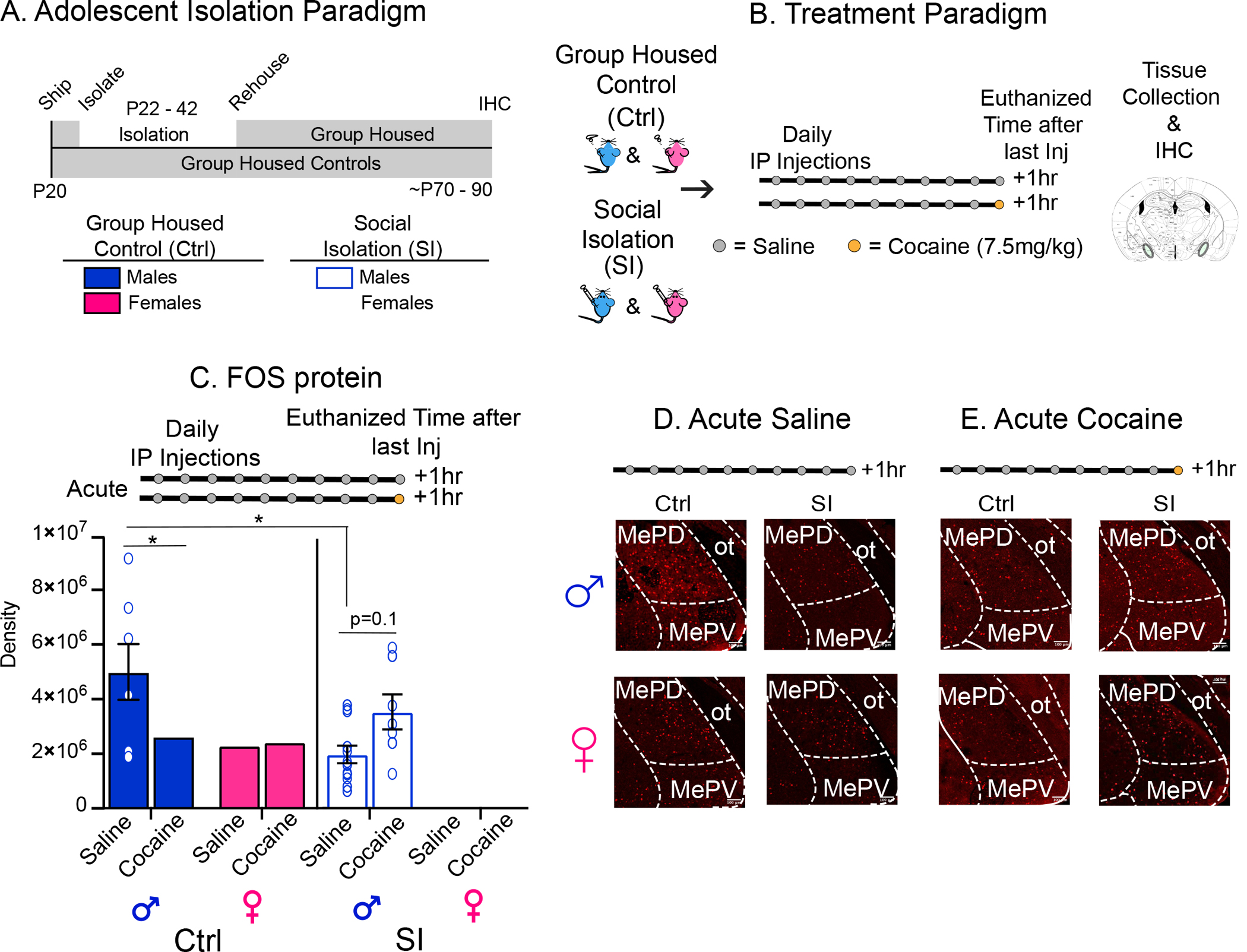
(A-B) Schematic of isolation (A) and treatment paradigms (B) for immunohistochemistry. (C-D) SI has sex-specific effects on FOS expression in meA at baseline and after acute cocaine in males but not females. Quantification of FOS in control (Ctrl) and SI males and females 1 hr after an injection of cocaine or saline (C). Representative images of FOS immunohistochemistry in Ctrl and SI males and females after an injection of saline (D) or cocaine (E). SI disrupts the expected FOS changes at baseline (saline) and after an injection of cocaine in males but not females. Post-hoc significant effects indicated as: *p<0.05; **p<0.001: error bars indicate SEM.
We next hypothesized that meA undergoes lasting sex-specific transcriptional reprogramming in response to adolescent SI that underlies the observed behavioral effects on cocaine reward. Using bulk RNAseq, we analyzed transcriptome-wide gene expression changes in meA of control males and females and SI males and females after acute (1 hr after first dose) or chronic (24 hr after 10th dose) exposures to cocaine (7.5 mg/kg) or saline (Figure 3C–F; Suppl Figure 2; Suppl Table 1). We chose these two dosing paradigms to model sex-specific behavioral effects of adolescent SI on cocaine CPP and self-administration (24) (see Supplemental Methods).
Figure 3: Adolescent SI Enhances the Transcriptional Response to Cocaine in SI Males but not Females and Disrupts Sex-Specific Transcription in meA.
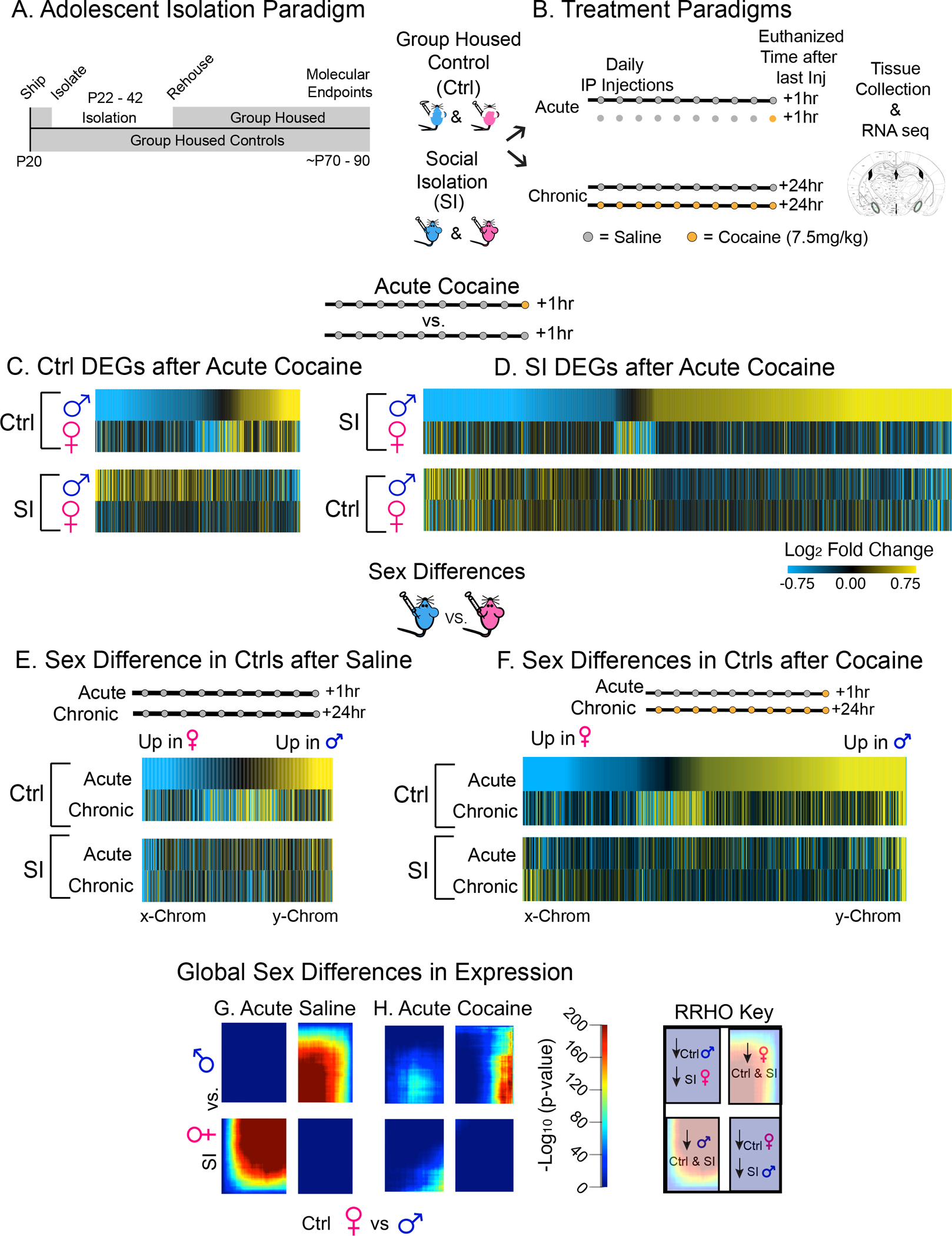
(A-B) Schematic of isolation (A) and treatment (B) paradigms for RNAseq. (C-H) Adolescent SI has sex-specific effects on cocaine-induced transcription. Union heatmaps of DEGs in Ctrl (C) and SI (D) males and females after acute cocaine, each compared to their own saline control. One dose of cocaine induces a robust and opposite transcriptional response in SI males but not females and there is very little overlap of DEGs regulated by cocaine in both Ctrl and SI males vs. females. (E-F). Union heatmaps of sex differences in DEGs in control animals after acute and chronic sal (E) or cocaine (F) are lost after SI suggesting that SI disrupts sex-specific gene expression in the meA both at basline and after cocaine. (G-H) Genes on the x- and-y chromosomes are highlighted on these heatmaps to verify that sex assigned at birth was reflected in the chromosomal sex and to confirm that SI had little to no effect on sex chromosome genes. Rank rank hypergeometric overlap (RRHO) of sex-specific expression after an injection of saline (G) or cocaine (H) reveals that global sex differences in transcriptional response to cocaine are disrupted by SI, suggesting an interaction between SI disruption of sex-specific and cocaine-induced transcription in the meA underlying the behavioral impact of adolescent SI.
We focused first on differentially-expressed genes (DEGs) in control (Figure 3C) and SI (Figure 3D) males and females after acute cocaine (Figure 3C–D). We considered genes to be differentially expressed if they met the following criteria: nominal p-value <0.05 and a 30% change in expression. We have successfully employed these criteria in numerous previous studies (42–45). In support of this approach, our analysis replicated known sex differences (e.g., Avp, Cyp19a1, Greb1, and Gal) and cocaine-induced differences (Fosb, Nr41a, and Egr1) in expression of several genes identified through both candidate gene and single-cell RNAseq studies (39, 46, 47).
Union heatmaps of meA DEGs regulated by cocaine in control males and females (Figure 3C) and SI males and females (Figure 3D) revealed that SI induced opposite transcriptional responses to acute cocaine in males but not females (Figure 3C–D). Additionally, SI-specific union heat maps of DEGs affected by acute cocaine (Figure 3D) or repeated cocaine (Suppl Figure 2C–D) reveal a robust transcriptional response to cocaine in SI males but not females (~twice as many transcripts are regulated by cocaine in SI vs. group-housed control males) suggesting that, even at the transcriptional level, SI induces hypersensitivity to cocaine in males. Similar to effects of SI on sex-specific behaviors, we observed a loss of sex differences specifically in DEGs in SI animals when compared to group-housed controls after saline or cocaine (Figure 3E–F). Together, these data suggest an interaction between sex differences in gene expression and transcriptional responses to cocaine in meA which are disrupted by adolescent social experience and point to transcriptional mechanisms underlying the behavioral impact of SI.
To better understand this interaction at the transcriptome-wide level, we used rank rank hypergeometric overlap (RRHO) to determine if cocaine-induced sex-specific transcription is disrupted by SI (Figure 3G–H). RRHO is a threshold-free method of comparing directional changes in expression across the entire transcriptome (48). Comparing sex differences in expression patterns in control females vs. males to those in SI females vs. males after acute saline (Figure 3G) or cocaine (Figure 3H) revealed considerable overlap of sex-specific expression patterns under saline conditions which are lost after cocaine. Interestingly, these effects are reversed after chronic cocaine where a loss of sex differences is observed in saline but not cocaine animals (Supplemental Figure 2E, F). This analysis shows that SI alters the global sex-specific transcriptional response to one dose of cocaine in meA, an effect that may contribute to the behavioral impact of SI on cocaine CPP.
SI disrupts sex-specific global co-expression in meA
We next used gene co-expression network analysis (Figure 4; Suppl Figure 4) coupled with pattern analysis (Suppl Fig 3), a dimensional reduction technique previously used by our laboratory (49), to: 1) determine if the SI-induced disruption of transcriptional patterns influences sex-specific global gene co-expression (Suppl Fig 4) and 2) identify key driver genes responsible for transcriptional and behavioral effects of adolescent SI (Figure 4).
Figure 4: Identification of Crym as a Sex-Specific Key Driver Gene Using Co-Expression Network Analysis.
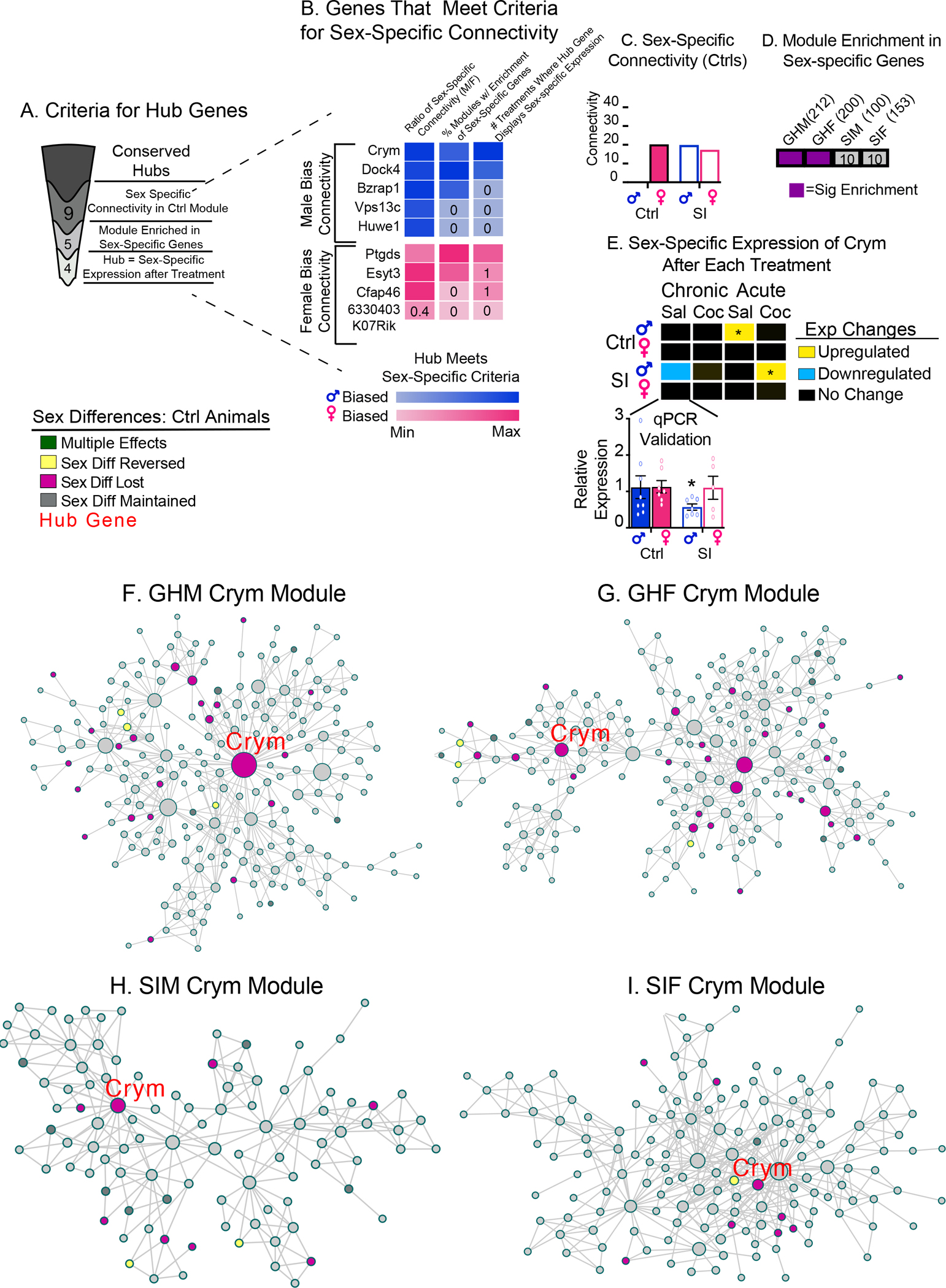
(A) Schematic of criteria used to identify sex-specific key-driver genes. The number in each segment indicates the number of key drivers meeting each criterion. (B) List of 10 genes with sex-specific connectivity within a module. Key drivers are separated by the sex-specific bias in connectivity (blue = male biased; pink = female biased). Darker pink/blue indicates maximum value for each criterion and lighter blue/pink indicates the lowest value for each criterion. Criterion 1: Male (blue) or female (pink) biased connectivity (>2 times connections of key driver with the genes in the modules) of the key driver in Ctrl animals is reversed or lost in SI animals. The number in each box indicates ratio of connections for the key driver in males vs. females. Criterion 2: The key driver’s module is enriched in sex- or SI-specific genes across groups (control or SI males or females; colors indicate percentage groups with enrichment; maximum = 100%). The number in each box indicates the percentage of groups with enrichment. Criterion 3: The key driver gene displays sex- or SI-specific expression under different treatment conditions (acute/chronic saline or cocaine; maximum 4). The number in each box indicates the number of treatment groups with sex- or SI-specific expression. (C-E) Crym meets all criteria as a sex-specific key driver. Crym displays male-biased sex-specific connectivity (C) within the respective modules for group-housed control (Ctrl) mice (closed bars). These differences are diminished in modules for SI mice (open bars). (D) Enrichment of genes with sex differences in expression (purple) or SI-only effects (bold outline) is observed in 50% of the modules containing Crym (Ctrl M and F). (E) Sex- or SI-specific expression changes for Crym under each treatment group which were validated by qPCR in a separate cohort of animals. Crym exhibits sex- or SI-specific expression changes under 3 different treatments (chronic saline, acute saline, and acute cocaine) when data are compared to the same baseline (*different from female controls given chronic saline). (F-I) Arachne plots of Crym modules in all groups. In each case, Crym is a key driver. Genes with sex-specific expression in control animals are color-coded. *p<0.05; **p<0.001.
We used multiscale embedded gene co-expression analysis (MEGENA) (50) which identifies modules of co-expressed genes and establishes a hierarchical organization among the modules - meaning rather than identifying modules consisting of a small number of genes as is done in other co-expression analyses, the entire transcriptome is analyzed for global co-expression. These large co-expression modules (maximum ~5,000 genes) are referred to as “parent” modules and are plotted as inner rings of sunburst plots (Suppl Figure 4A – D) and used to determine how sex-specific global co-expression is disrupted by SI (Suppl Figure 3). Next these parent modules are further analyzed for more strongly co-expressed sub-modules (outer rings of sunburst plots) which we interrogated for key driver genes related to transcriptional and behavioral effects of adolescent SI (Figure 4).
Co-expression modules were identified for each of the four groups (control males/females, SI males/females) by collapsing data from all four treatments (acute/chronic cocaine and saline). This was done to ensure robust statistical power and identify relationships that hold across multiple variables. We first asked if the SI-induced transcriptional disruption of sex-specific gene expression alters global transcriptomic “structure” by comparing parent modules across the 4 groups. Importantly, the larger the parent co-expression module the more co-expression that can be interpreted as increased transcriptomic structure. We found that sex differences in transcription are reflected in global transcriptomes of control males and females (Suppl Figure 4 A, B). In control animals, males had more parent modules composed of fewer genes (Suppl Figure 4A,E,F) than females (Suppl Figure 4B,E,F), an effect that was dramatically reversed after SI: males have fewer parent modules than SI females (Suppl Figure 4C–E), and the size of SI male parent modules is closer to that of control females (Suppl Figure 4F). The most striking effect was in SI females (Suppl Figure 4D–F) where the number of modules is vastly greater than the other groups and the size of the modules is significantly smaller (p<0.05). Enrichment of sex- and SI-specific DEGs identified via pattern analysis (see Supplemental Methods, Suppl Fig 3, Suppl Table 2) was also disrupted in SI animals, with males having more enrichment of sex-specific genes than females (Suppl Figure 4A–D). Together, these data reveal that, similar to sex-specific disruption of behavior and transcription, adolescent SI has opposite effects on global gene co-expression networks in meA, with larger parent modules observed in males and smaller parent modules observed in females.
Identification of sex-specific key driver genes underlying sex-specific effects of SI
The finding that transcriptional effects of SI closely mirror behavioral effects of SI suggests that transcriptional mechanisms control sex-specific behaviors after SI. We integrated co-expression and sex-specific pattern and enrichment analyses to identify key driver genes predicted to mediate these behavioral responses using four criteria chosen to reflect the sex-specific behavioral and transcriptional effects observed (Figure 4A): 1) key driver is conserved in all 4 groups (control males/females and SI males/females); 2) connectivity of key driver within the module is greater in control males or females but not in SI animals (representing a loss of sex differences); 3) co-expression modules with key driver are enriched in sex-specific DEGs across multiple groups (group-housed controls or SI; representing genes affected by both cocaine and SI in a sex-specific manner); and 4) key driver itself exhibits sex-specific expression after at least 1 treatment (acute/chronic saline/cocaine; representing sex-specific effects of cocaine or SI on key driver). Of 60 key drivers conserved across all 4 groups, only 4 met all four criteria (Figure 4B). Because SI had more potent effects on males, we identified key drivers with male-biased connectivity. Of the 2 male-biased key drivers that met all criteria, Crym displayed the greater male-biased connectivity in controls (Figure 4B–I). Additionally, of the 4 key drivers, only Crym is a transcriptional regulator (51) and we hypothesized that transcriptional changes within meA drive sex-specific behavioral effects of SI. Crym is a thyroid hormone-binding protein (52, 53) that is neuroprotective in striatum (54) and a marker of opioid-activated dopamine receptor 1-expressing neurons in nucleus accumbens (55).
Crym overexpression in adult meA mirrors sex-specific behavioral and transcriptional effects of SI
SI induces a sex difference in Crym expression at baseline (24 hr after chronic saline) which we validated in a separate cohort of animals using qPCR (SI males < SI females) and is stimulus-dependent specifically in males: it is induced in meA by acute saline (control males) or cocaine (SI males) (Figure 4E). Therefore, based on patterns of Crym expression in control and SI mice, we hypothesized that Crym overexpression in meA would recapitulate effects of SI on cocaine-related behaviors particularly in males. AAV vectors (AAV2-hsyn-Crym-GFP or GFP alone as a control vector) were injected bilaterally into meA of adult males and females and behavioral testing was conducted ~3 weeks later when transgene expression is maximal (Figure 5A). Targeting was confirmed by immunohistochemistry (Figure 5B) and qPCR (Figure 5C). As expected, there were no differences in cocaine CPP between GFP control males and females. However, Crym overexpression increased cocaine CPP specifically in males compared to GFP controls (p=0.05), suggesting that cocaine induction of Crym observed in SI males controls cocaine CPP (Figure 5D; Suppl Figure 5B).
Figure 5: Crym Overexpression in Adult meA Recapitulates Behavioral Effects of Adolescent SI.
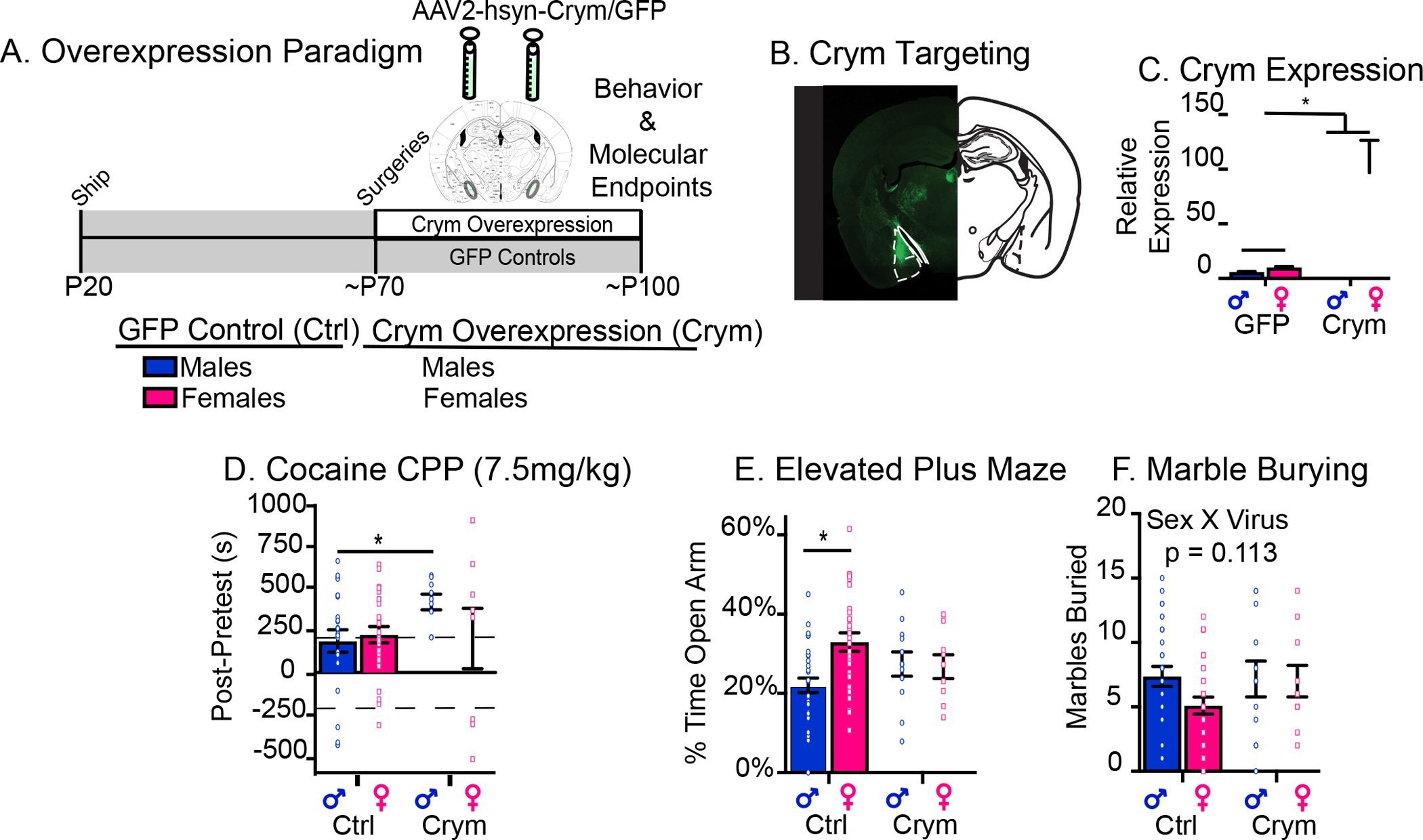
(A) Schematic of experimental design. (B-C) Viral placement and expression were confirmed in meA after behavioral testing by immunohistochemistry (B) and qPCR (C). (D) Similar to adolescent SI, Crym overexpression in meA increases cocaine CPP in males but not females. Data points above and below the dashed lines indicate animals who formed a preference or aversion for cocaine. (E-F) Also similar to adolescent SI, Crym overexpression in meA abolishes known sex differences in elevated plus maze (D) and marble burying (E). Post-hoc significant effects indicated as *p<0.05; **p<0.001. Error bars – SEM.
Baseline sex differences in Crym expression after SI (Figure 4E) suggest that other sex-specific behaviors may be modulated by Crym overexpression. Therefore, we tested anxiety-related behaviors after Crym overexpression (Figure 5E–F; Suppl Fig 5C–D). As with SI, predicted sex differences in EPM (Figure 5D; Sex × Virus F(1,66=5.11; p=0.029) were observed in GFP controls but not after Crym overexpression (Tukey post hoc: GFP control males vs. females; p<0.05). Two-way ANOVA revealed no significant effects of Crym overexpression on marbles buried (Figure 5F; Sex × Virus F(1,69)=1.07; p=0.11) or OF (Suppl Figure 5D; Sex × Virus F(1,69)=0.17; p=0.71). However, similar to SI, Crym overexpression increased the proportion of females that buried marbles to male levels (Suppl Figure 5C; Chi-squared: GFP control males (χ2=1.2; p=0.27), GFP control females (χ2=9.97; p<0.01), Crym males (χ2=1.33; p=0.25), and Crym females (χ2=0.0; p=1.0). These data indicate that Crym signaling in meA regulates reward- and anxiety-related behaviors especially in males.
To validate our bioinformatic approach for identifying sex-specific key driver genes, we overexpressed another gene of interest, Lhx8, in meA that does not meet all criteria for a sex-specific key-driver gene (Suppl Figure 6A – H). No effects on cocaine CPP are observed (Suppl Figure 7B–C) and sex differences are maintained in EPM (Suppl Figure 7D) and MB (Suppl Figure 7E–F), thus validating our network analysis and selection criteria.
To determine if Crym action is specific to meA, we overexpressed Crym in nucleus accumbens, a region where Crym expression is unaffected by SI or cocaine (42) (Suppl Figure 8) and found no effects on OF (Suppl Figure 8B), MB (Suppl Figure 8C–D) or cocaine CPP (Suppl Figure 8E–F). These findings provide regional specificity for the actions of Crym in meA in regulating sex-specific behavior.
Crym overexpression in adult meA mimics transcriptional effects of adolescent SI
We next tested the hypothesis that Crym in meA acts through transcriptional mechanisms to affect behavior. Using RNAseq of meA with qPCR-confirmed Crym overexpression (Figure 5C), we first compared effects of Crym overexpression on DEGs in males vs. females. Union heatmaps of all DEGs altered by Crym overexpression across sexes (compared to GFP controls) revealed that Crym overexpression induces more DEGs in males than females (Figure 6A) along with a male-specific repression of DEGs (Figure 6A–B) and a blunting of overlap of transcriptome-wide patterns (indicated by cooler colors; Figure 6C). Finally, as with SI, sex differences in DEGs in control males and females are lost after Crym overexpression (Figure D–E & G; Suppl Table 3). However, these effects do not disrupt global sex-specific patterns of expression, as RRHO revealed that Crym overexpression had no effect on sex differences transcriptome-wide (Figure 6F). These data mirror the transcriptional effects observed after SI (Figure 3; Suppl Figure 2), suggesting that altered Crym signaling in meA—through viral manipulation or in response to SI—controls sex-specific behavior through meA transcription.
Figure 6: Crym Overexpression Robust Effects on Transcription Specifically in Males But Does Not Disrupt Global Sex Differences in Transcription.
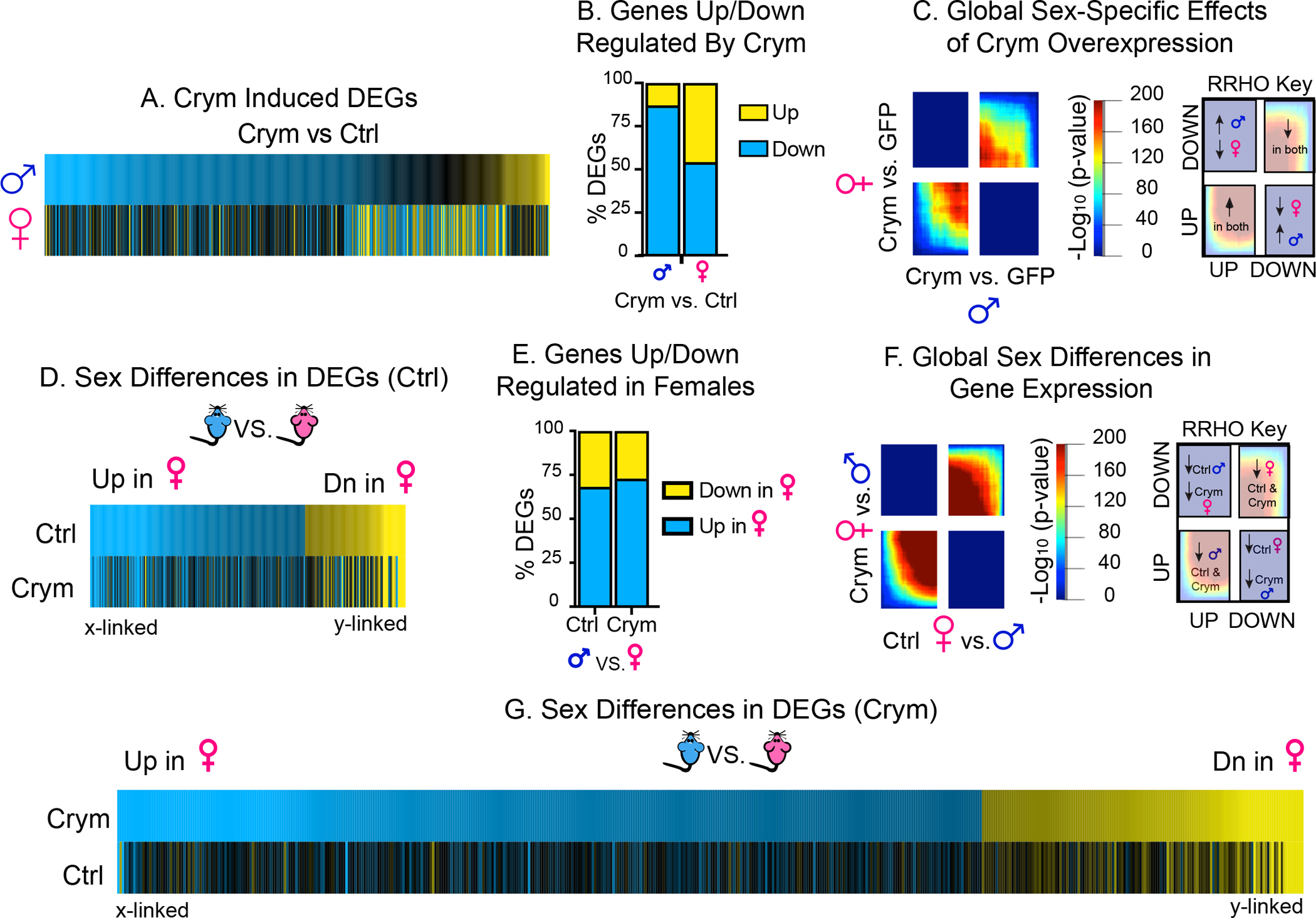
(A-C) Crym overexpression in meA affects more transcripts in males than females. Union heatmaps of genes altered by Crym overexpression in meA of males and females compared to their GFP counterparts reveals regulation of a greater number of transcripts in males vs. females (A). Crym overexpression downregulates a majority of transcripts in males but not females (B). RRHO of Crym effects in males vs. females reveals a blunting of the overlap of global transcriptional patterns after Crym overexpression (C). (D-G) Sex differences in gene expression are disrupted by Crym overexpression. Heatmaps of all sex differences in DEGs in GFP control (Ctrl) reveal that expected sex differences in expression are lost after Crym overexpression (D). However, Crym does not affect the number of genes up- or downregulated in males vs. females (E) nor does it impact sex differences in global transcriptional patterns analyzed by RRHO (F). Finally, heatmaps of sex differences in DEGs after Crym overexpression indicate that Crym increases the number of sex-specific DEGs in meA which are not observed in Ctrl animals (G). Together these data reveal that, similar to SI, Crym overexpression disrupts sex-specific gene expression and induces more transcriptional effects in males vs. females. x-linked = genes on X chromosome; y-linked = genes on Y chromosome.
To link the transcriptional changes induced by Crym to those altered by cocaine in SI animals, we compared expression changes of DEGs (Figure 7A) and transcriptome-wide patterns (Figure 7C) regulated by Crym in males and females to the control and SI-induced response to acute cocaine in both sexes (Figure 7B–C). We observed key sex-specific patterns in Crym-regulated genes after acute cocaine, which may contribute to the observed behavioral phenotypes. First, Crym overexpression reflects cocaine-induced transcriptional profiles, both DEGs (Figure 7A–B) and transcriptome-wide (Figure 7C) in control males, suggesting that Crym overexpression alone (without cocaine exposure) induces a transcriptional profile that resembles a cocaine-induced transcriptome in male meA. We also found that SI disrupts expression of those Crym-regulated genes, both DEGs (Figure 7A–B) and transcriptome-wide (Figure 7C), upon exposure to cocaine. Instead, SI expression of Crym-regulated genes reflects those affected by a saline injection (Suppl Figure 9A–C), suggesting that SI reprograms those transcripts to differentially respond to stimuli. Finally, these effects were not observed in females (Figure 7A–C; Suppl Figure 9A–C), suggesting that Crym-induced transcriptional changes are important for regulating sex-specific responses to cocaine. These data provide important insight into potential gene targets governing the opposite behavioral phenotypes induced by Crym overexpression and adolescent SI.
Figure 7: Genes Regulated by Crym Overexpression are Responsive to Acute Saline and Cocaine in a Sex-Specific Manner.
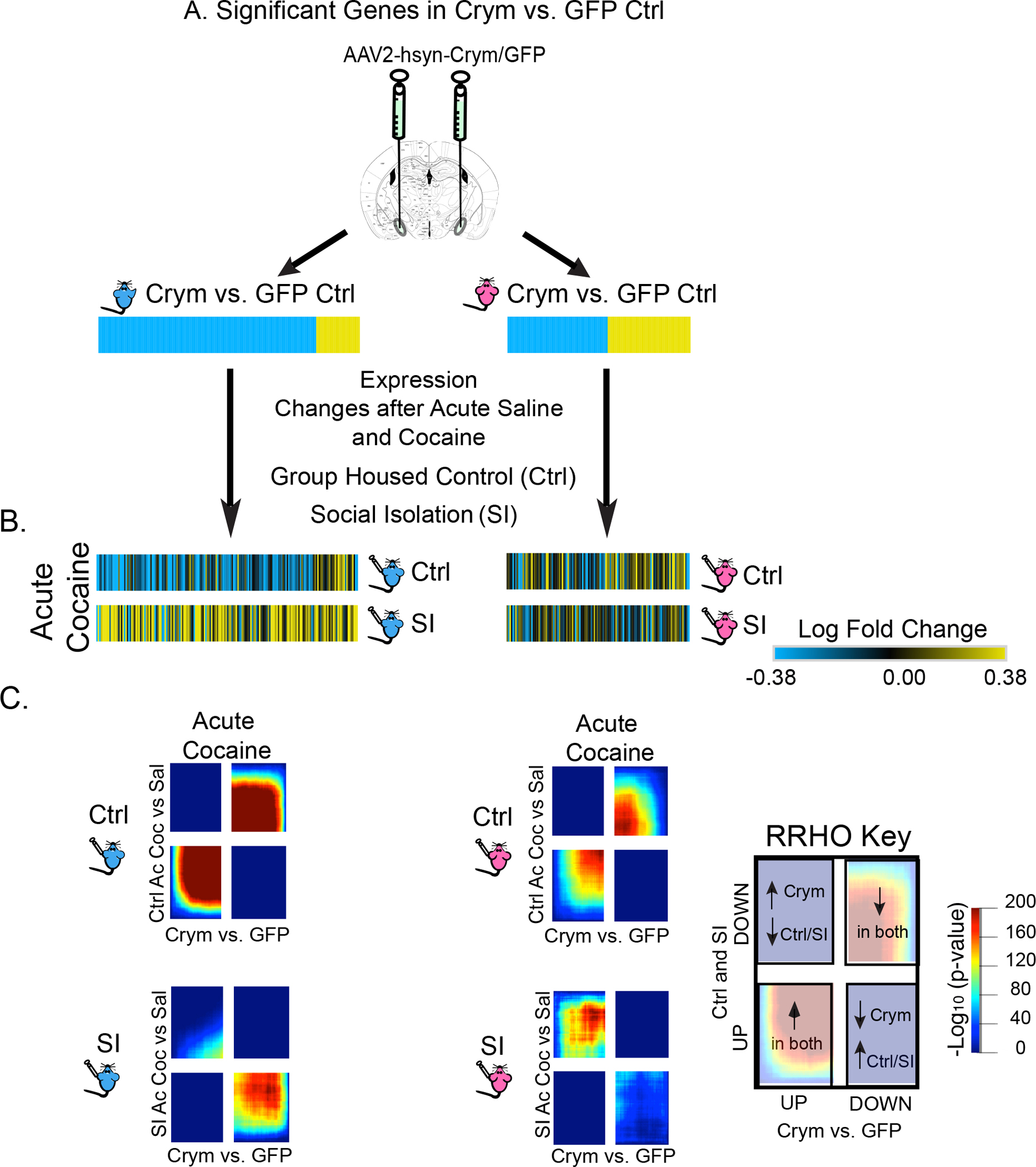
(A) Genes altered by Crym overexpression in meA of males (left) and females (right). (B-C) Expression profiles of genes altered by Crym overexpression in group-housed control (Ctrl) and SI males and females after acute cocaine (B). After acute cocaine, SI males show opposite regulation of Crym-regulated DEGs (B) and transcriptome-wide patterns (C) when comparted to Ctrl males. This effect was not observed in females (C).
DISCUSSION:
Here we integrated an adolescent manipulation with long-term behavioral and transcriptional outcomes, and employed dimensional-reducing bioinformatic techniques and co-expression network analysis to identify meA, and Crym signaling specifically, as a novel mediator of social influence on behavioral responses to cocaine. We establish that transcriptional changes induced in meA by adolescent SI are meaningful for sex-specific behavior by recapitulating behavioral and transcriptional effects of SI via manipulation of Crym, a key driver gene derived from our network analysis, specifically within meA. This work highlights the power of an integrative, multi-systems approach for studying experience-dependent programming of sex-specific behavior and transcription.
Adolescent SI influences cocaine reward through meA transcriptional regulation
Social reward undergoes vast changes during adolescence with peer-peer interactions becoming more rewarding than parental-offspring interactions, suggesting that social experience mediates reward-related behaviors long-term (11). In humans and rodents, negative or reduced peer interactions in adolescence influence susceptibility to neuropsychiatric disorders in adulthood (15). In rodents, adolescent SI is a long-used model for studying how social experience influences anxiety- and drug-related responses. However, few studies have included females and most have not resocialized animals before testing. Since SI itself is stressful, not rehousing animals before testing makes it difficult to determine if its long-term effects are due to the stress of isolation itself or if the adolescent period is a sensitive window for life-long effects (8). Our findings show that adolescent SI causes opposite long-term behavioral effects in males and females and enhances preference for cocaine in males but not females.
We then leverage sex-specific behavioral effects of adolescent SI to investigate underlying transcriptional mechanisms within meA, a brain region understudied in drug abuse research. We focused on meA because of its known sex differences in size (38), transcription (39), development (29), mechanisms of sexual differentiation (56–58), importance in sex-specific natural reward (26, 28, 39) and sensitivity to adolescent stress (31, 32). Additionally, while evidence suggests that meA responds to drugs of abuse in both sexes (40, 41), little is known about underlying molecular mechanisms involved. We show that transcriptional patterns induced by SI parallel behavioral changes observed in males and females. First, consistent with behavioral effects of SI on cocaine CPP, SI males display a robust transcriptional response to cocaine, suggesting increased sensitivity to cocaine at the transcriptional level as well. Second, SI resulted in a loss of sex differences in gene expression similar to the effects observed on anxiety-related behaviors. These data suggest that, similar to behavioral observations, adolescent SI reprograms transcriptional profiles within meA and points to a transcriptional mechanism that regulates such behaviors. Finally, we did not observe sex differences in cocaine CPP in our control animals, but identified very different transcriptional profiles induced by cocaine in males vs. females, suggesting that, as with other behavioral phenotypes (45, 59, 60), latent sex differences in transcription may be important for regulating similar behavioral responses to cocaine. Using adolescent SI, a stressor that disrupts sex-specific behavioral phenotypes and transcription, we uncovered a novel mechanism, namely, Crym regulation of transcription, underlying sex differences in drug-induced transcription and behavior. Given the known sex differences in vulnerability to SUDs in humans (61), these data will help identify potential therapeutic targets for addiction in both sexes.
Crym overexpression in meA recapitulates sex-specific behavioral and transcriptional effects of adolescent SI
We identified a molecular mechanism underlying sex-specific effects of SI on cocaine reward and validated the power of our approach by showing that the observed transcriptional changes induced within meA by adolescent SI are important for driving sex-specific behavior by manipulating a sex-specific key driver gene, Crym, derived from our integrated bioinformatic techniques. We show that, similar to SI, Crym overexpression in meA of adult naïve males and females disrupts sex-specific behavior and transcription (Figure 3). By contrast, overexpression of Lhx8, a key driver that does not meet our criteria for driving sex-specific effects after adolescent SI (Figure 4; Suppl Figure 6) in meA had no effect on behavior (Suppl Figure 7), and Crym overexpression in nucleus accumbens, a region where Crym is not regulated by cocaine or SI (42), has no detectable behavioral effects (Suppl Figure 8).
Crym is an intracellular thyroid hormone-binding protein that prevents thyroid hormone from interacting with its receptors (53) and blocks thyroid hormone-mediated transcription (62). Thyroid hormone has recently been proposed as a potential regulator of sex-specific critical windows of neuronal development in the visual system (63). Our data expand on this hypothesis by showing that Crym overexpression not only influences sex-specific behaviors (Figure 5) but recapitulates transcriptional changes induced in meA by adolescent SI (Figures 6). Finally, we show that those genes altered by Crym overexpression are sensitive to cocaine in control males but not females and that this sex difference is also disrupted by SI (Figure 7). Very little is known about Crym or thyroid hormone regulation of cocaine reward; our data provide evidence of region- and sex-specificity that deserves follow-up. Our study implicates Crym signaling, and thyroid hormone signaling indirectly, in meA as a critical mediator of how social experience influences drug reward particularly in males.
Conclusions
Social experience during adolescence programs meA to shape sex-specific behaviors that maximize reproductive success in adulthood. While anxiety- and reward-associated behaviors can be viewed through the lens of psychiatric disorders, sex differences in these behaviors also likely reflect differences in behavioral strategies used by males and females to find a mate. Our data suggest that adolescent SI disrupts such behaviors in part through programming the meA transcriptome. Because adolescent animals integrate information about the environment to fine-tune behaviors associated with reproductive strategies, we hypothesize that SI signals information about mate availability, social hierarchy, resource availability and the likelihood of communal nesting, all of which are influenced by metabolic factors. Therefore, it is compelling that Crym, and associated thyroid hormone signaling, is important for reprogramming transcriptional responses for metabolically costly efforts (e.g., copulation). We hypothesize that Crym may program sex-specific transcriptional responses to acute stimuli in meA as a mechanism for driving sex-specific behaviors necessary for reproductive success. This programming may also induce changes that control sex-specific risk for psychiatric disorders including addiction and anxiety when environmental mismatches take place in adulthood. Our data thereby provide a model for studying sex-dependent and -independent mechanisms of vulnerability to psychiatric disorders. Finally, given the social impact of COVID-19, these data are valuable in understanding the lasting impact on adolescents exposed to socially-isolating conditions for viral mitigation, and provide hope for novel therapeutic interventions in the post-pandemic era.
METHODS (Abridged):
Detailed descriptions of experimental design and approaches are provided in Supplemental Methods. All methods used for social isolation, injections, tissue collection, RNA isolation, and bioinformatic approaches were published recently (42). Anxiety-related testing (44) and cocaine CPP (64) were performed as published. All behaviors were analyzed using a 2-way ANOVA or Kruskall-Wallis non-parametric test depending on significance of a Levene’s test for homogeneity of variance followed by appropriate post hoc testing in SPSS Statisical Software, V25 (IBM, Atmonk, NY). Viral-mediated transgene overexpression and validation were conducted as published (43).
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | et | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Chicken anti-GFP | Aves Lab | GFP-10-20 | |
| Donkey anti-chicken Alexa Fluor 488 | Jackson ImmunoReserach | 70354155 | ||
| Bacterial or Viral Strain | AAV2 | |||
| Biological Sample | N/A | |||
| Cell Line | N/A | |||
| Chemical Compound or Drug | N/A | |||
| Commercial Assay Or Kit | TruSeq Stranded mRNA HT Sample Prep Kit | Illumina | RS-122-2101 | |
| Deposited Data; Public Database | Accession #: GSE146472 | |||
| Genetic Reagent | N/A | |||
| Organism/Strain | Mouse/ C57BL6 males and females | |||
| Peptide, Recombinant Protein | N/A | |||
| Recombinant DNA | N/A | |||
| Sequence-Based Reagent | N/A | |||
| Software; Algorithm | SPSS | IBM | ||
| Pattern Analysis | https://gist.github.com/aartrama/c94bf11c0be40dfb0093c9be4bd0c53d | |||
| Multiscale embedded gene co-expression network analysis | https://github.com/songw01/MEGENA | |||
| Prism Graph Pad | Microsoft | |||
| Transfected Construct | pAAV-hsyn-Crym-eYFP | Constructs generated by GeneScript; Plasmids sucbcloned and packaged at Duke Univeristy Viral Core | ||
| pAAV-hsyn-LHX8-eYFP | Constructs generated by GeneScript; Plasmids sucbcloned and packaged at Duke Univeristy Viral Core | |||
| pAAV-hsyn-eYFP | Packaged at Duke Univeristy Viral Core |
Significance Statement:
Social experience is known to influence substance use disorder in humans and addiction related behaviors in rodents but few have examined mechanisms regulating such influence. Social experience in adolescence is especially important in programming reward phenotypes and adolescent social isolation (SI) in particular has been used as preclinical model for susceptibility to addiction for decades. Here we show that adolescent SI enhances cocaine-associated behaviors in males but not females. These effects are reflected in the transcriptome of medial amygdala (meA), a sexually dimorphic brain region that develops during adolescence. We then harnessed multidimensional transcriptomic and behavioral data to identify a transcriptional mechanism mediated by crystallin mu, a thyroid hormone-binding protein, as a sex-specific key driver of the behavioral effects of SI.
ACKNOWLEDGMENTS AND FINANCIAL DISCLOSURES
This work was funded by grants from the National Institutes of Health: P01DA0047233 (EJN), R01DA007359 (EJN), R01MH051399 (EJN), K99DA042100 (DMW), U01AG046170 (BZ) and RF1AG054014 (BZ). The authors reported no biomedical financial interests or potential conflicts of interest. We would like to thank Dr. Erin Kendall Braun for their thoughtful comments in editing the manuscript and Dr. Andrew Wolfe for technical and intellectual support in the experimental design. A previous version of this manuscript was uploaded to bioRxiv as a preprint (doi: https://doi.org/10.1101/2020.02.18.955187).
Footnotes
AUTHOR CONFLICT OF INTEREST:
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data and materials availability:
All data have been uploaded to the NCBI Gene Expression Omnibus (GEO) depository accession number: GSE146472.
REFERENCES:
- 1.Stone AL, Becker LG, Huber AM, Catalano RF (2012): Review of risk and protective factors of substance use and problem use in emerging adulthood. Addict Behav. 37:747–775. [DOI] [PubMed] [Google Scholar]
- 2.Wills TA, Vaccaro D, McNamara G (1992): The role of life events, family support, and competence in adolescent substance use: a test of vulnerability and protective factors. Am J Community Psychol. 20:349–374. [DOI] [PubMed] [Google Scholar]
- 3.Power C, Estaugh V (1990): The role of family formation and dissolution in shaping drinking behaviour in early adulthood. Br J Addict. 85:521–530. [DOI] [PubMed] [Google Scholar]
- 4.Wilsnack SC, Klassen AD, Schur BE, Wilsnack RW (1991): Predicting onset and chronicity of women’s problem drinking: a five-year longitudinal analysis. Am J Public Health. 81:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutkind S, Gorfinkel LR, Hasin DS (2022): Prospective effects of loneliness on frequency of alcohol and marijuana use. Addict Behav. 124:107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairbairn CE, Briley DA, Kang D, Fraley RC, Hankin BL, Ariss T (2018): A meta-analysis of longitudinal associations between substance use and interpersonal attachment security. Psychol Bull. 144:532–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozarth MA, Murray A, Wise RA (1989): Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 33:903–907. [DOI] [PubMed] [Google Scholar]
- 8.Walker DM, Cunningham AM, Gregory JK, Nestler EJ (2019): Long-Term Behavioral Effects of Post-weaning Social Isolation in Males and Females. Front Behav Neurosci. 13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendersky CJ, Milian AA, Andrus MD, De La Torre U, Walker DM (2021): Long-Term Impacts of Post-weaning Social Isolation on Nucleus Accumbens Function. Front Psychiatry. 12:745406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sisk CL (2016): Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Curr Opin Neurobiol. 38:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear LP (2000): The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 24:417–463. [DOI] [PubMed] [Google Scholar]
- 12.Larson RW, Richards, Maryse H,Moneta, Giovanni Holmbeck, Grayson Duckett, Elena (1996): Changes in adolescents’ daily interactions with their families from ages 10 to 18: Disengagement and transformation. Developmental Psychology,. 32:744–754. [Google Scholar]
- 13.Gopnik A, O’Grady S, Lucas CG, Griffiths TL, Wente A, Bridgers S, et al. (2017): Changes in cognitive flexibility and hypothesis search across human life history from childhood to adolescence to adulthood. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loades ME, Chatburn E, Higson-Sweeney N, Reynolds S, Shafran R, Brigden A, et al. (2020): Rapid Systematic Review: The Impact of Social Isolation and Loneliness on the Mental Health of Children and Adolescents in the Context of COVID-19. J Am Acad Child Adolesc Psychiatry. 59:1218–1239 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mates D, Allison KR (1992): Sources of stress and coping responses of high school students. Adolescence. 27:461–474. [PubMed] [Google Scholar]
- 16.Thapar A, Collishaw S, Pine DS, Thapar AK (2012): Depression in adolescence. Lancet. 379:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner EF, Myers MG, McIninch JL (1999): Stress-coping and temptation-coping as predictors of adolescent substance use. Addict Behav. 24:769–779. [DOI] [PubMed] [Google Scholar]
- 18.Wills TA (1986): Stress and coping in early adolescence: relationships to substance use in urban school samples. Health Psychol. 5:503–529. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KM, Sisk CL (2016): The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev. 70:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE (1998): Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol 107:128–140. [DOI] [PubMed] [Google Scholar]
- 21.Hankin BL, Young JF, Abela JR, Smolen A, Jenness JL, Gulley LD, et al. (2015): Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. J Abnorm Psychol. 124:803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson TL, Smith LW, Smith TL, Yager J, Grant I (1992): Symptoms of illness in late adulthood are related to childhood social deprivation and misfortune in men but not in women. J Behav Med. 15:113–125. [DOI] [PubMed] [Google Scholar]
- 23.Begni V, Zampar S, Longo L, Riva MA (2020): Sex Differences in the Enduring Effects of Social Deprivation during Adolescence in Rats: Implications for Psychiatric Disorders. Neuroscience. 437:11–22. [DOI] [PubMed] [Google Scholar]
- 24.Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA (2019): Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res. 359:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergan JF, Ben-Shaul Y, Dulac C (2014): Sex-specific processing of social cues in the medial amygdala. Elife. 3:e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, et al. (2017): Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell. 171:1176–1190 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SM, Marcotulli D, Shen A, Zweifel LS (2019): Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat Neurosci. 22:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM (2015): Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL (2012): Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 1460:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz KM, Molenda-Figueira HA, Sisk CL (2009): Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 55:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke BM, Chowanadisai W, Breedlove SM (2000): Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 117:107–113. [DOI] [PubMed] [Google Scholar]
- 32.Hodges TE, Louth EL, Bailey CDC, McCormick CM (2019): Adolescent social instability stress alters markers of synaptic plasticity and dendritic structure in the medial amygdala and lateral septum in male rats. Brain Struct Funct. 224:643–659. [DOI] [PubMed] [Google Scholar]
- 33.Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP (2009): Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 162:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan CJ, Andersen SL (2017): Sensitive periods of substance abuse: Early risk for the transition to dependence. Dev Cogn Neurosci. 25:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer J (1975): Rodent sex differences in emotional and related behavior. Behav Biol. 14:451–479. [DOI] [PubMed] [Google Scholar]
- 36.Goel N, Bale TL (2009): Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 21:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voikar V, Koks S, Vasar E, Rauvala H (2001): Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 72:271–281. [DOI] [PubMed] [Google Scholar]
- 38.Hines M, Allen LS, Gorski RA (1992): Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 579:321–326. [DOI] [PubMed] [Google Scholar]
- 39.Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, et al. (2019): Sexually Dimorphic Control of Parenting Behavior by the Medial Amygdala. Cell. 176:1206–1221 e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knapska E, Radwanska K, Werka T, Kaczmarek L (2007): Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 87:1113–1173. [DOI] [PubMed] [Google Scholar]
- 41.Rudzinskas SA, Williams KM, Mong JA, Holder MK (2019): Sex, Drugs, and the Medial Amygdala: A Model of Enhanced Sexual Motivation in the Female Rat. Front Behav Neurosci. 13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker DM, Zhou X, Cunningham AM, Lipschultz AP, Ramakrishnan A, Cates HM, et al. (2021): Sex-Specific Transcriptional Changes in Response to Adolescent Social Stress in the Brain’s Reward Circuitry. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. (2016): Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron. 90:969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, et al. (2020): Sex-Specific Role for the Long Non-coding RNA LINC00473 in Depression. Neuron. 106:912–926 e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med. 23:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, et al. (2012): Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 7:e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA, 3rd, Bauman AJ, et al. (2020): A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv. 6:eaba4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML (2018): Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep. 8:9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song WM, Zhang B (2015): Multiscale Embedded Gene Co-expression Network Analysis. PLoS Comput Biol. 11:e1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohkubo Y, Sekido T, Nishio SI, Sekido K, Kitahara J, Suzuki S, et al. (2019): Loss of mu-crystallin causes PPARgamma activation and obesity in high-fat diet-fed mice. Biochem Biophys Res Commun. 508:914–920. [DOI] [PubMed] [Google Scholar]
- 52.Vie MP, Evrard C, Osty J, Breton-Gilet A, Blanchet P, Pomerance M, et al. (1997): Purification, molecular cloning, and functional expression of the human nicodinamide-adenine dinucleotide phosphate-regulated thyroid hormone-binding protein. Mol Endocrinol. 11:1728–1736. [DOI] [PubMed] [Google Scholar]
- 53.Hallen A, Cooper AJ, Jamie JF, Haynes PA, Willows RD (2011): Mammalian forebrain ketimine reductase identified as mu-crystallin; potential regulation by thyroid hormones. J Neurochem. 118:379–387. [DOI] [PubMed] [Google Scholar]
- 54.Francelle L, Galvan L, Gaillard MC, Guillermier M, Houitte D, Bonvento G, et al. (2015): Loss of the thyroid hormone-binding protein Crym renders striatal neurons more vulnerable to mutant huntingtin in Huntington’s disease. Hum Mol Genet. 24:1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J, Mitra RD (2018): Single-Cell RNA-Seq Uncovers a Robust Transcriptional Response to Morphine by Glia. Cell Rep. 24:3619–3629 e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, McCarthy MM (2017): Activation of Both CB1 and CB2 Endocannabinoid Receptors Is Critical for Masculinization of the Developing Medial Amygdala and Juvenile Social Play Behavior. eNeuro. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM (2010): Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci U S A. 107:20535–20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL (2006): Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 66:578–590. [DOI] [PubMed] [Google Scholar]
- 59.Barko K, Paden W, Cahill KM, Seney ML, Logan RW (2019): Sex-Specific Effects of Stress on Mood-Related Gene Expression. Mol Neuropsychiatry. 5:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker JB, Koob GF (2016): Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazcano I, Hernandez-Puga G, Robles JP, Orozco A (2019): Alternative ligands for thyroid hormone receptors. Mol Cell Endocrinol. 493:110448. [DOI] [PubMed] [Google Scholar]
- 63.Batista G, Hensch TK (2019): Critical Period Regulation by Thyroid Hormones: Potential Mechanisms and Sex-Specific Aspects. Front Mol Neurosci. 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cates HM, Lardner CK, Bagot RC, Neve RL, Nestler EJ (2019): Fosb Induction in Nucleus Accumbens by Cocaine Is Regulated by E2F3a. eNeuro. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been uploaded to the NCBI Gene Expression Omnibus (GEO) depository accession number: GSE146472.


