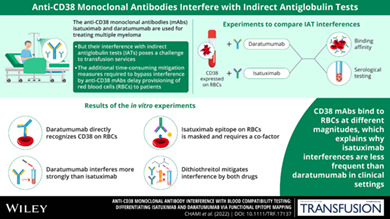
Abstract
Background
There are two FDA‐approved anti‐CD38 monoclonal antibodies for treatment of multiple myeloma: isatuximab and daratumumab. Owing to expression of CD38 on reagent red blood cells (RBCs), these antibodies interfere with indirect antiglobulin tests (IATs). We sought to understand differences in such interference by performing binding experiments.
Study Design and Methods
In vitro experiments to compare the binding to RBCs of isatuximab and daratumumab alone or in the presence of a mouse anti‐human CD38 antibody (HB‐7 or AT13/5) or a nicotinamide adenine dinucleotide‐analog CD38 inhibitor were performed and quantified by flow cytometry, imaging, mass spectrometry, surface plasmon resonance, and LigandTracer technologies. Serologic testing was performed on plasma samples spiked with isatuximab or daratumumab.
Results
CD38 expressed on RBCs can be directly bound by daratumumab, whereas isatuximab requires a co‐factor, such as HB‐7, AT13/5, or a CD38 inhibitor, suggesting that the isatuximab epitope on RBCs is masked in vitro. Daratumumab samples more frequently showed interference and had stronger reactions than isatuximab samples. Dithiothreitol treatment was equally effective in mitigating the interference caused by either drug.
Discussion
Both isatuximab and daratumumab interfere with IATs but at different magnitudes, reflecting distinct binding to CD38 on RBCs. From the binding studies, we conclude that the isatuximab epitope on RBCs is masked in vitro and binding requires a certain CD38 conformation or co‐factor. This circumstance may explain why interference is seen only in a subset of patients receiving isatuximab when compared with interference seen in most patients on daratumumab therapy.
1. INTRODUCTION
Multiple myeloma (MM) is the second most common hematologic malignancy in the world and remains incurable. 1 , 2 Monoclonal antibodies (mAbs), specifically immunoglobulin G1 (IgG1) antibodies, have proven effective in treating MM, particularly when combined with immunomodulatory drugs and proteasome inhibitors. 3 , 4 Two anti‐CD38 mAbs, isatuximab and daratumumab, are currently FDA‐approved for the treatment of MM. Isatuximab is approved in several countries in combination with dexamethasone plus either pomalidomide or carfilzomib in adult patients with relapsed/refractory MM who have received prior therapies. 5 Daratumumab is approved for use in MM as monotherapy and in various combination regimens including with melphalan, immunomodulatory drugs, and proteasome inhibitors. 6
Red blood cell (RBC) transfusions are an important part of care in patients with MM, who often present with anemia. Three standard pre‐transfusion laboratory tests are required prior to issuing RBCs, namely, blood type determination, antibody screening (indirect antiglobulin test [IAT]) for unexpected RBC alloantibodies in plasma, and determination of compatibility between donor RBCs and patient plasma by RBC crossmatching. 7 In pre‐transfusion testing, presence of hemagglutination is considered a positive reaction, regardless of method (i.e., manual or automated) or technology (e.g., gel column or solid phase) used. Blood transfusion services face unique challenges when testing samples from patients receiving daratumumab or isatuximab. Anti‐CD38 mAbs can bind to CD38 weakly expressed on the surface of RBCs, and cause agglutination in the presence of anti‐human globulin reagent. As these drugs can cause hemagglutination regardless of the presence of RBC alloantibodies or incompatibility between donor RBCs and patient plasma, they interfere with the interpretation of antibody screen and crossmatch tests. The additional, time‐consuming, mitigation measures required to bypass interference by anti‐CD38 mAbs delay provisioning of RBCs to patients. 8 , 9 , 10
Daratumumab binds to CD38 molecules expressed on reagent RBCs, causing panagglutination in IATs and incompatible crossmatches, in a dose‐ and interval‐dependent manner, for 2–6 months after the last infusion. 11 , 12 These reactions occur regardless of testing method (i.e., saline, low ionic strength saline [LISS], or polyethylene glycol). 9 Several test modifications to prevent binding to RBCs and eliminate interference have been evaluated, including treating reagent RBCs with dithiothreitol (DTT), which denatures CD38 and negates daratumumab interference. 8 , 9 , 11 Interference was also observed in plasma spiked with other humanized anti‐CD38 mAbs, suggesting that false positive IATs are not unique to daratumumab. 9 The ICARIA‐MM 13 and IKEMA 14 Phase 3 clinical trial data suggested that isatuximab causes less interference than daratumumab.
Here, we report results of in vitro studies comparing RBC binding characteristics of isatuximab and daratumumab as well as results of IATs based on drug spiking studies done independently in three different immunohematology laboratories (IHL) in France (IHL1), Japan (IHL2) and the USA (IHL3). Together, they inform on differences in CD38 binding and RBC agglutination between isatuximab and daratumumab.
2. MATERIALS AND METHODS
Full methodologic details regarding immunostaining reagents, confocal imaging, and tandem mass spectrometry are provided in the SUPPLEMENTARY INFORMATION.
2.1. Flow cytometry analysis
CD38‐expressing human cell lines, MOLP‐8, LP‐1, RPMI‐8266, NCI‐H929, SUDHL‐8 (MM), and Daudi (lymphoma; ATCC, Manassas, VA, USA & DSMZ, Braunschweig, Germany) were used for binding tests. Human RBCs were isolated from healthy donors' samples obtained from the Massachusetts General Hospital by density gradient centrifugation with Ficoll‐Paque (Cytiva, Marlborough, MA). Obtained RBCs were washed twice with phosphate buffered saline (PBS) before in vitro immuno‐staining.
RBCs were incubated with isatuximab, daratumumab, or human IgG (hIgG) at 0–100 μg/ml (in duplicated wells) in binding buffer (PBS + 1% BSA) for 30 min at room temperature (RT). After washing three times with washing buffer (PBS + 0.1% BSA), RBCs were incubated with goat anti‐hIgG‐allophycocyanin (APC) for 30 min at RT, then washed three times. Surface APC signals from bound hIgG, isatuximab, or daratumumab were detected by flow cytometry (Flow Cytometry Canto II, Becton Dickinson, Franklin Lakes, NJ). The median fluorescent intensity (MFI) was obtained using FlowJo software (FlowJo LLC, Ashland, OR). For treatments of cell lines, isatuximab, daratumumab, and hIgG1 were tested from 0–30 μg/ml (triplicates or duplicates), and detected with goat‐anti‐hIgG‐ fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch Laboratories, West Grove, PA).
For induced isatuximab binding tests, RBCs were pre‐incubated with mouse anti‐human CD38 antibody (HB‐7, AT13/5, iso‐control or HB‐7‐PE) at 1 μg/ml or 1 μM ara‐F‐nicotinamide adenine dinucleotide (NAD; a covalent inhibitor of the NAD‐glycohydrolase [NADase] activity of CD38) 15 in binding buffer (PBS + 1% BSA) at RT for 20 min. Isatuximab, daratumumab, or hIgG1 (0–100 μg/ml) were added and incubated for 30 min. After washing three times, RBCs were incubated with goat anti‐hIgG‐APC with or without goat anti‐mouse IgG [mIgG]‐FITC at RT for 30 min. After washing three times, fluorescence signals from surface bound isatuximab, daratumumab, or mouse antibodies on RBCs were detected by flow cytometry and analyzed by using FlowJo software.
2.2. Cell‐based affinity kinetics
JJN3 (DSMZ, Germany) is a human MM cell line with low expression of CD38 and was used to generate JJN3‐CD38+ cells (overexpressing CD38). Both cell lines were grown medium supplemented with 10% fetal bovine serum (Gibco) and maintained in a 5% CO2 humidified atmosphere at 37°C.
The binding kinetics of isatuximab or daratumumab with cell surface CD38 was measured using LigandTracer (LT) Green (Ridgeview Instruments AB, Vänge, Sweden). Approximately, 1E6 JJN3 and JJN3‐CD38+ cells were attached to the top and bottom spots of a petri dish, respectively, at a distance of about 5 mm from the rim, using a Biomolecular anchor molecule, BAM (SUNBRIGHT OE‐040CS, NOF Corporation), as previously described. 16
Isatuximab and daratumumab were labeled with DyLight dye 488 using DyLight Antibody Labeling Kits (Thermo Fisher Scientific, Waltham, MA) following the manufacturer's instructions.
After a stable fluorescence baseline was established, DyLight dye 488‐labeled isatuximab or daratumumab was added stepwise to a final concentration of 2, 6, and 10 nM, respectively, to record binding association. Subsequently, the solution in the petri dish was removed and replaced with 3 ml fresh medium containing unlabeled isatuximab (12 nM) or daratumumab (10 nM). The dissociation of bound isatuximab or daratumumab from CD38 was then recorded using a 15 seconds (s) detection time and 10 s detection delay.
Binding traces were analyzed with the evaluation software TraceDrawer 1.8.1 (Ridgeview Instruments). Data were fit to a 1:1 binding model to obtain binding kinetics parameters k on and k off, and equilibrium dissociation constant K D.
2.3. Surface plasmon resonance (SPR)
SPR experiments were performed on Biacore S200 (Cytiva, Sweden) at 22°C using 1X PBS‐P (Cytiva, part#28–9950‐84) as the running buffer. A dextran‐coated carboxymethylated (CM5) series S chip (Cytiva, part# BR100530) was used to covalently immobilize anti‐hIgG fragment crystallizable (Fc) antibody (Cytiva, part# BR‐1008‐39) using amine coupling chemistry. Anti‐hIgG Fc antibody was prepared by diluting 2 mg/ml antibody stock with 10 mM sodium acetate pH 5.0 (Acetate 5.0; Cytiva, part#BR100351) to a final concentration of 25 μg/ml. Final immobilization levels to both reference and active flow cells were about 10,000 Response Unit (RU) each. hIgG1 isotype control (Clone ET901; BioLegend Part# 403502) and isatuximab or daratumumab (each at 2 μg/ml prepared using running buffer) were captured to reference flow cell and active flow cell, respectively, by injecting for 16 seconds (s) at a flow rate of 10 μl/min. Dose response of recombinant human CD38 protein (rhCD38, Sino Biological Inc. Wayne, PA) (9‐point, 2‐fold serial dilutions from 32 μg/ml including 0 μg/ml; prepared using running buffer) was conducted by injecting rhCD38 at each concentration over both flow cells for 180 s followed by flowing running buffer for 300 s at a flow rate of 30 μl/min. Surface regeneration was performed by injecting Glycine 2.0 (10 mM glycine, pH 2) for 60 s at a flow rate of 30 μl/min.
Binding signals from active surface (with isatuximab or daratumumab captured) were subtracted from those of the reference surface (with IgG1 isotype control captured) and from buffer blank to correct for bulk effect and any possible non‐specific binding. The experimental data were processed using Biacore S200 Evaluation software v1.1 to obtain association rate constant (k a), dissociation rate constant (k d), and equilibrium dissociation constant (K D). The kinetic parameters (k a and k d) were determined by fitting binding curves to 1:1 binding model. K D value was calculated from the ratio of k d/k a.
The dose response of CD38 in the presence of mouse anti‐human CD38 antibody HB‐7 was performed as described above except that a fixed concentration (2 μg/ml) of HB‐7 was added to rhCD38 samples.
The dose response of CD38 in the presence of 1 μM ara‐F‐NAD was conducted using Biacore A‐B‐A injection mode (Figure S1). Serial dilutions of rhCD38 were prepared as described above using a running buffer containing 1 μM ara‐F‐NAD. Dose response of rhCD38 was conducted by injecting Solution A (1 μM ara‐F‐NAD in running buffer) for 180 s followed by injecting Solution B (rhCD38 + 1 μM ara‐F‐NAD) for 180 s. Finally, Solution A was injected for 300 s. Surface regeneration was performed by injecting Glycine 2.0 for 60 s. All injections were conducted at a flow rate of 30 μl/min over both flow cells.
2.4. Immunohematology testing
Three separate IHLs performed serological testing, each laboratory independently selecting the sample preparation, testing method, and drug concentrations employed for the spiking studies. Plasma samples of healthy blood donors (IHL1) or patients (IHL2 and IHL3) were collected in accordance with good laboratory practice and antibody screening was performed using RBCs without isatuximab or daratumumab to determine possible pre‐existing RBC alloantibodies. De‐identified samples with negative results were spiked with several concentrations of isatuximab or daratumumab and tested using routine immunohematology serologic testing methods as described below (at IHL3, patient samples were pooled before performing spiking studies). As CD38 expression on RBCs varies between blood donors, reagent RBCs used for isatuximab and daratumumab testing were identical for each method and expressed a broad range of RBC antigens. One reagent RBC used for any testing was counted as one test. Results were graded negative (0) or positive (reaction grades W+ to 4+).
Treatment with DTT was according to the method described by Chapuy et al. 8 , 11
2.4.1. IHL1
Before adding isatuximab or daratumumab, for the initial antibody screen using untreated RBCs, IATs were performed on 14 samples using DG Gel Coombs cards (Grifols, Spain) on the ERYTRA gel‐based analyzer system according to manufacturer's instructions, and using a panel of RBCs (Grifols, Spain). Samples were spiked to obtain increasing concentrations of daratumumab or isatuximab (50, 150, 300, 600, and 1000 μg/ml). For antibody screening (manual technique), a panel of 3 RBCs (provided by INTS, France) were used with the following Rhesus (Rh) phenotypes: D‐C‐E‐c + e + (rr); D + C + E‐c‐e + (R1R1); and D + C‐E + c + e‐ (R2R2).
2.4.2. IHL2
Plasma was spiked with 0.1, 0.5, 1.0, 5.0, 10, and 200 μg/ml of isatuximab. IATs were performed by test tube method using polyethylene glycol (PEG) enhancement (FUJIFILM Wako, Japan), anti‐IgG mAbs (Bio‐Rad Laboratories, Hercules, CA) and a panel of 3 RBCs known to express a broad range of RBC antigens (Panoscreen by Immucor, Norcross, GA) incubated at 37°C for 15 min according to manufacturer's instructions. Samples spiked with 10 and 200 μg/ml of isatuximab were tested in parallel with RBCs treated with 0.01 M or 0.2 M of DTT (Kanto Chemical, Japan) using PEG tube testing. RBC alloantibodies were obtained from Bio‐Rad Laboratories (Hercules, CA).
2.4.3. IHL3
Samples were prepared by spiking pooled plasma with 0.1, 1, 10, 18, 313, 574, 745, and 915 μg/ml of daratumumab or 0.1, 1, 10, 108, 297, 372, 487, 601, and 945 μg/ml of isatuximab.
Gel screen was performed with MTS™ anti‐IgG gel card and 0.8% Selectogen® reagent RBCs on the ORTHO ProVue™ analyzer, per manufacturer instructions (Ortho Clinical Diagnostics, Pompano Beach, FL). Solid phase testing was performed using the Capture‐R® Ready‐Screen (3) system on the Galileo Echo® analyzer per manufacturer instructions (Immucor, Norcross, GA). Manual (tube) testing was performed by adding 2 drops of spiked plasma, 1 drop of reagent RBCs (Ortho Clinical Diagnostics, Pompano Beach, FL or Immucor, Norcross, GA), mixing, and then adding 2 drops of either PEG (Immucor, Norcross, GA) or LISS (Immucor, Norcross, GA) as enhancing agents. Samples were mixed and incubated at 37°C for 15 min. PEG‐enhanced samples were then washed 3–4 times with buffered saline (Azer Scientific, Morgantown, PA) before adding 2 drops of anti‐human globulin anti‐IgG (Ortho Clinical Diagnostics, Pompano Beach, FL), mixing, centrifuging, and then evaluating for agglutination. After the 15 min incubation, LISS‐enhanced samples were centrifuged and evaluated for agglutination before washing. The subsequent steps as described for PEG tests.
2.5. Isatuximab‐treated patients and IAT interference
The Phase 3, randomized, multicenter ICARIA‐MM (NCT02990338), 13 and IKEMA (NCT03275285) 14 studies were conducted at 102 and 69 sites, respectively. Following standard testing methods at individual sites, patients randomly assigned to the isatuximab arms of each study had blood typing, complete blood phenotyping, and antibody screening performed prior to study treatment administration on Cycle 1 Day 1. The antibody screen was repeated on Cycle 2 Day 1 (or at the next blood sampling if the test was omitted at this visit). IAT results recorded during the isatuximab on‐treatment period (including those performed prior to any transfusion during study treatment) and transfusion details were entered into an electronic case report form. Analyses of baseline characteristics and efficacy were conducted in the randomized and safety populations, respectively: as these analyses were not preplanned, they are considered exploratory.
3. RESULTS
3.1. Isatuximab has similar affinity to daratumumab for CD38 expressed by tumor cells, but higher affinity for rhCD38
The binding affinity of isatuximab and daratumumab to cellular CD38 was evaluated by flow cytometry using a panel of MM and B‐cell lymphoma cell lines with different levels of surface CD38. The binding curves obtained for isatuximab and daratumumab were overlapping for each cell line, indicating similar ability of both antibodies to bind CD38 expressed on the tumor cells (Figure 1A). LigandTracer experiments using CD38‐overexpressing JJN3 cells and parental JJN3 (negative control) confirmed that both isatuximab and daratumumab have almost identical binding kinetics and affinity (Figure 1B, Table 1).
FIGURE 1.
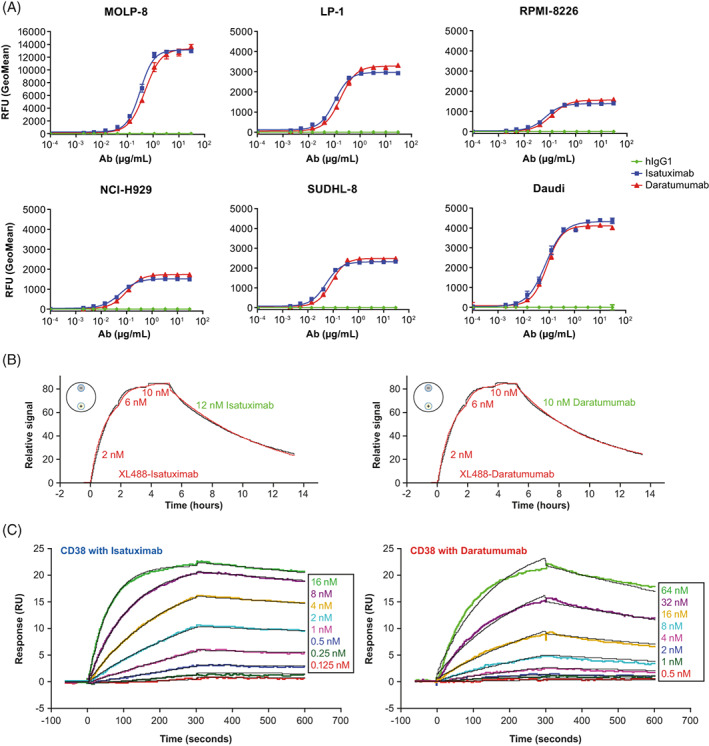
Isatuximab and daratumumab binding to CD38 expressed on cancer cell lines and rhCD38. (A) Detection of surface‐bound anti‐CD38 antibodies on MM cells (MOLP‐8, LP‐1, RPMI‐8226, NCI‐H929) or B‐cell lymphoma (SUDHL‐8, Daudi) by flow‐cytometric analysis. Cells (0.2 E6) were treated with hIgG1 (green), isatuximab (blue), or daratumumab (red) at concentrations ranging from 0–30 μg/ml. Surface‐bound antibody was detected with FITC‐Goat F(ab’)2 anti‐hIgG. (B) Determination of binding kinetics and binding affinity using LT. LT experiments were conducted with cells attached to the detection spots (small circles with “+” for JJN3‐CD38+ cells and “‐” for JJN3 parental cells) on a petri dish (schematically shown as a large circle on the top left of each time traces). Association and dissociation time traces (black, background and reference subtracted) are fitted with a 1:1 kinetic model (red). Concentrations of anti‐CD38 monoclonal antibody labeled with XL488 (XL488‐Isatuximab or XL488‐Daratumumab in red) added for association and unlabeled antibody (isatuximab or daratumumab in green) added for dissociation are shown in the figures. (C) Determination of binding kinetics and binding affinity using SPR. SPR experiments were conducted with isatuximab and daratumumab captured by anti‐Fc antibody immobilized on CM5 sensor chip. rhCD38 at varied concentrations (0.125–16.0 nM for isatuximab and 0.5–64 nM for daratumumab) was injected over the sensor surface. Association and dissociation time traces (colored, background, and reference subtracted) are fitted with a 1:1 kinetic binding model (black). K D values are calculated based on k a and k d from the fits of the binding kinetics. Ab, antibody; hIgG1, human immunoglobulin G1; ka, association rate constant; kd, dissociation rate constant; K D, equilibrium dissociation constant; LT, LigandTracer; rhCD38, recombinant human CD38; SPR, surface plasmon resonance; RFU, relative fluorescence unit; RU, response unit [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Binding kinetics and affinity of isatuximab and daratumumab with rhCD38 and CD38‐expressing MM cells
| Method | Neg control | Anti‐CD38 mAb | Sample | K a (M−1s−1) | K d (s−1) | K D (M) |
|---|---|---|---|---|---|---|
| SPR | hIgG1 isotype | Isatuximab | rhCD38 | 1.14E+06 | 3.06E−04 | 3.12E−10 |
| rhCD38/ara‐F‐NAD inhibitor | 1.36E+06 | 4.30E−05 | 3.16E−11 | |||
| rhCD38/HB‐7 | 3.32E+05 | <1.0E−06 | <3.01E−12 | |||
| Daratumumab | rhCD38 | 8.80E+04 | 8.41E−04 | 9.56E−09 | ||
| LT | Parental JJN3 | Isatuximab | JJN3.CD38 | 1.23E+05 | 4.19E−05 | 3.40E−10 |
| Daratumumab | 1.29E+05 | 4.73E−05 | 3.67E−10 |
Abbreviations: ara‐F‐NAD, ara‐F‐nicotinamide adenine dinucleotide; hIgG1, human immunoglobulin G1; ka, association rate constant; kd, dissociation rate constant; K D, equilibrium dissociation constant; LT, LigandTracer; M, molar; mAb, monoclonal antibody; neg, negative; rhCD38, recombinant human CD38; s, seconds, SPR, surface plasmon resonance.
SPR data showed that isatuximab binds to rhCD38 with about 30‐fold higher affinity than daratumumab (K D, 3.12 E−10 M vs 9.56 E−9 M; Figure 1C). The higher affinity of isatuximab was driven by its faster association rate and slower dissociation rate (Table 1).
3.2. Isatuximab does not bind CD38 on RBCs in vitro, unlike daratumumab
PBS‐washed RBC samples from 12 different healthy donors were exposed to isatuximab or daratumumab at concentrations ranging from 0–10 μg/ml. Antibody binding was quantified by flow cytometry. Isatuximab binding signal was not detectable in any of the samples tested while daratumumab induced a dose‐dependent increase of the binding signal in samples evaluated (Figure 2).
FIGURE 2.
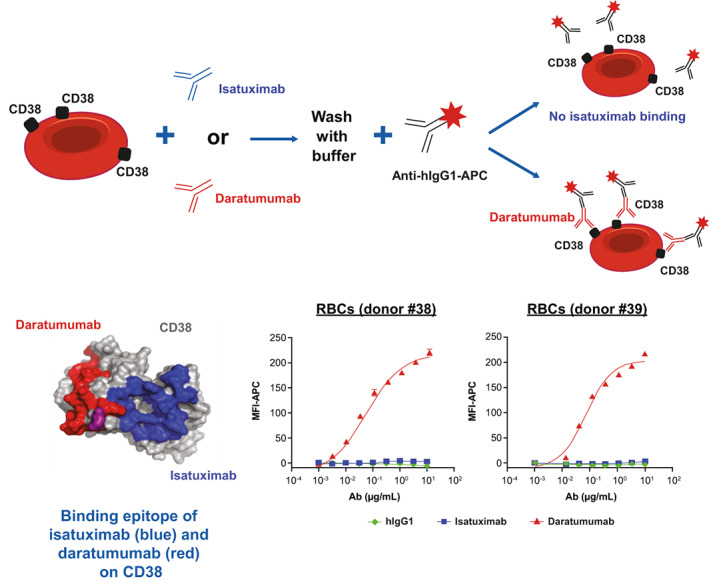
In vitro binding of isatuximab and daratumumab to RBCs. RBCs from healthy donors were treated with hIgG1 (green), isatuximab (blue), or daratumumab (red) at concentrations ranging from 0 to 10 μg/ml. Surface‐bound anti‐CD38 antibody was detected with goat anti‐hIgG‐APC by flow cytometry. Ab, antibody; APC, allophycocyanin; hIgG1, human immunoglobulin G1; MFI, median fluorescent intensity; RBC, red blood cell [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Isatuximab epitope is masked on RBCs in vitro
Given the similar binding of isatuximab and daratumumab to CD38 expressed, higher affinity of isatuximab over daratumumab to rhCD38, and their differential binding to PBS‐washed RBCs, we hypothesized that daratumumab binding to RBCs was specific and that the isatuximab epitope on RBC was masked.
In competition experiments using a mouse anti‐human CD38 antibody (clone HB‐7) that shares epitope sequences with daratumumab 17 , 18 (Figure 3A), pre‐treatment with increasing concentrations of daratumumab resulted in a dose‐dependent decrease of fluorescence signal from HB‐7 binding to CD38 on RBCs. In contrast, binding signal of HB‐7 was not reduced from pre‐treatment with isatuximab or control human IgG1 (hIgG1; Figure 3B). These findings indicate that daratumumab specifically binds to RBCs through CD38 and HB‐7 can displace RBC‐bound daratumumab.
FIGURE 3.
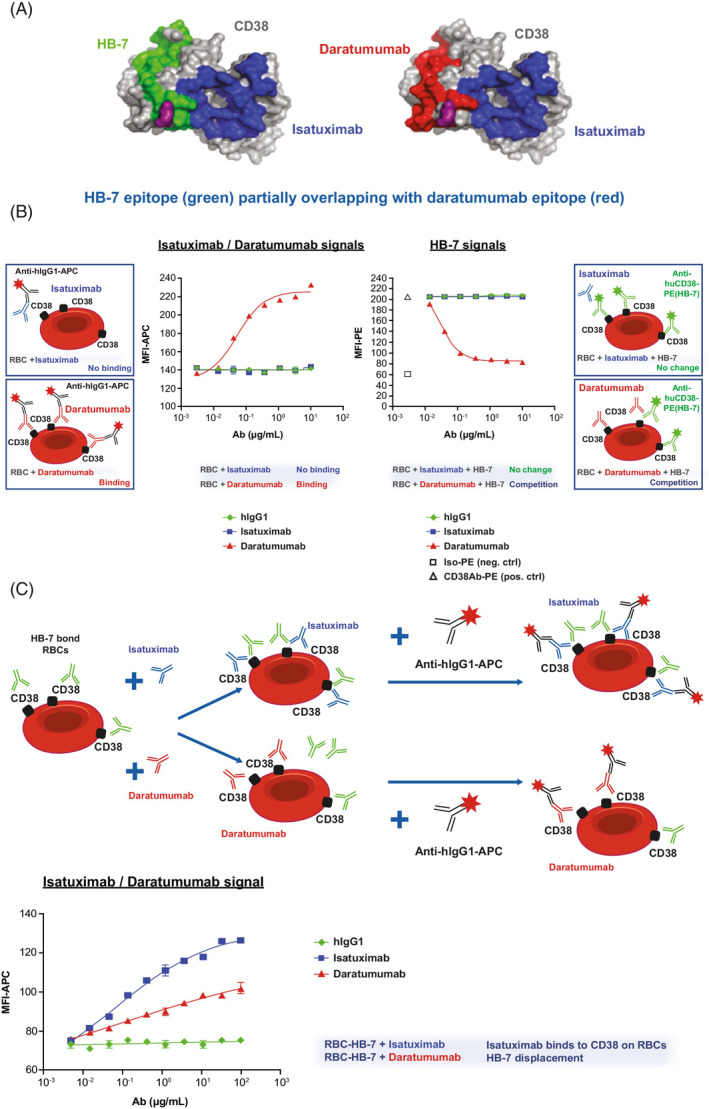
Daratumumab binding to CD38 on RBCs can be displaced by HB‐7 and pre‐treatment with HB‐7 induces binding of isatuximab to RBCs. (A) Representation of CD38 antigenic determinants of isatuximab (blue), daratumumab (red), and HB‐7 (green). E233 (purple) is a residue shared by all three epitopes. Protein Data Bank 4CMH (i.e., crystal structure of CD38 with CD38‐targeting antibody isatuximab) was used to generate CD38 surface. (B) Detection of APC signals from hIgG1 (green), isatuximab (blue), or daratumumab (red) binding to CD38 expressed on RBCs in vitro (left) or the ability of HB‐7 (PE signal) to bind CD38 expressed on RBCs in vitro in the presence of hIgG1 (green), isatuximab (blue), or daratumumab (red) (right) by flow cytometry. (C) RBCs were pre‐treated with mouse HB‐7 Ab before incubation with hIgG1 (green), isatuximab (blue) or daratumumab (red) at 0–100 μg/ml. Binding of isatuximab and daratumumab to CD38/HB‐7 complex on RBCs was detected with APC conjugated goat anti‐hIgG by flow cytometry. APC, allophycocyanin; ctrl, control; hIgG1, human immunoglobulin G1; MFI, median fluorescent intensity; PE, phycoerythrin; RBC, red blood cell [Color figure can be viewed at wileyonlinelibrary.com]
In RBCs pre‐treated with HB‐7, flow cytometry analysis revealed that, isatuximab bound to CD38 complex in a dose‐dependent manner without displacing HB‐7 (Figure 3C).
Microscopic imaging and quantification confirmed these findings, as the fluorescent isatuximab signal was only visualized on RBCs pre‐treated with HB‐7 (Figure 4). Mass spectrometry experiments also demonstrated that HB‐7 induced isatuximab to bind CD38 on RBCs (Figure S2). Additionally, another mouse anti‐human CD38 antibody (clone AT13/5) and the ara‐F‐NAD inhibitor 19 both induced isatuximab binding to CD38 on RBCs isolated from healthy donors (Figure 5A). Collectively, these data suggest that HB‐7, AT13/5, or ara‐F‐NAD inhibitor binding to RBCs leads to a CD38 conformational change that exposes the isatuximab binding epitope and allows isatuximab binding.
FIGURE 4.
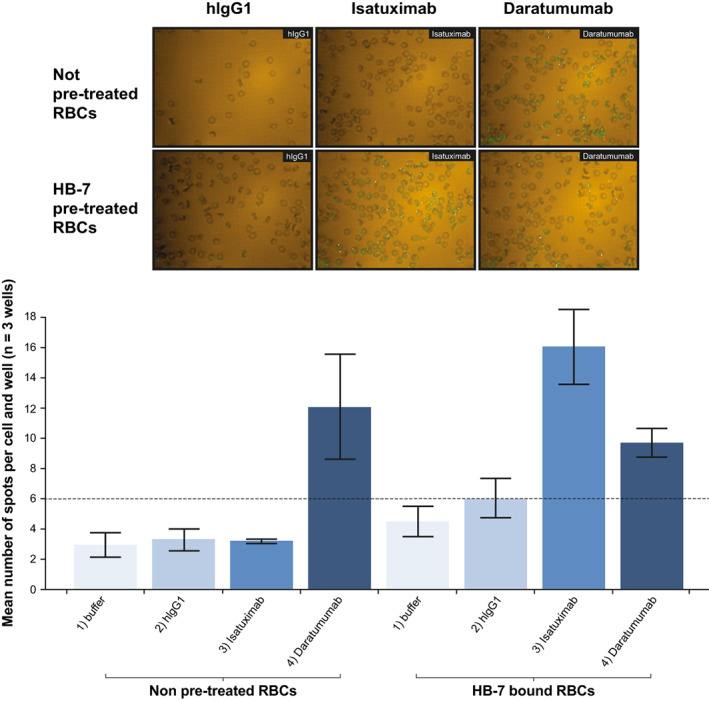
Confocal imaging analysis confirms HB‐7‐induced isatuximab binding to HB‐7‐bound RBCs. Binding of isatuximab and daratumumab (10 μg/ml) to RBCs in the absence or presence of HB‐7 (1 μg/ml) was evaluated by image‐based analysis. Green dots showing binding signals (AF488) on cell surface were visualized by microscope (60xW) (top panel of images) and number of positive spots were quantified (bottom graph). hIgG1, human immunoglobulin G1; RBC, red blood cell [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
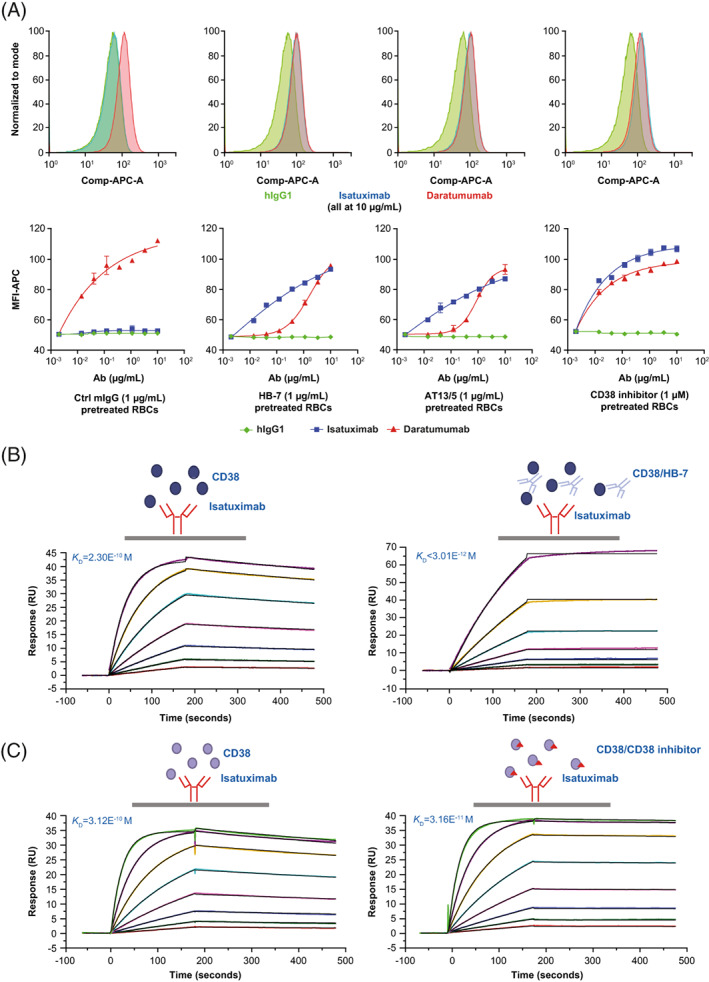
In vitro assay, mouse anti‐human CD38 antibodies (HB‐7 or AT13/5) and CD38 inhibitor (ara‐F‐NAD) can induce isatuximab binding to CD38 on RBCs and increase isatuximab binding affinity for rhCD38. (A) Induced isatuximab binding to RBCs. Pre‐treatment of RBCs with mIgG, HB‐7, AT13/5, or CD38 inhibitor at indicated concentrations and detection of hIgG1 (green), isatuximab (blue), or daratumumab (red) by flow cytometry. Top: flow cytometry histograms; Bottom: dose curves. (B–C) Isatuximab binding kinetics and binding affinity to rhCD38 in the presence of HB‐7 and CD38 inhibitor were measured by SPR. Ab, antibody; APC, allophycocyanin; ara‐F‐NAD, ara‐F‐nicotinamide adenine dinucleotide; Comp, compensated; hIgG1, human immunoglobulin G1; K D, equilibrium dissociation constant; MFI, mean fluorescent intensity; mIgG, mouse immunoglobulin G; RBC, red blood cell; rhCD38, recombinant human CD38; SPR, surface plasmon resonance; RU, response unit; s, seconds [Color figure can be viewed at wileyonlinelibrary.com]
SPR experiments using rhCD38 confirmed that, once HB‐7 or ara‐F‐NAD inhibitor is bound to CD38, the affinity of isatuximab for CD38 increases, with binding affinities (K D) changing from 3.12E−10 M to <3.01E−12 M and to 3.16E−11 M, respectively (Figure 5B,C, and Table 1).
3.4. Immunohematology testing shows less frequent and weaker interference with isatuximab than with daratumumab
Table 2 shows that, at IHL1, IATs done on all 14 isatuximab and 14 daratumumab‐spiked samples using the gel method‐induced panagglutination with untreated RBCs at all concentrations tested (50–1000 μg/ml). Most daratumumab‐spiked samples (81%) showed 2+ reactivity, with 19% showing 1+ reactivity. Reaction strengths obtained with isatuximab‐spiked samples (17% of samples showed W+ reactions, 47% showed 1+ reactions and 36% showed 2+ reactions) were weaker than those observed with daratumumab‐spiked samples. Treatment of RBCs with 0.2 M DTT abrogated both isatuximab‐ and daratumumab‐induced panagglutination using the gel method, while allowing the detection of clinically relevant antibodies, including anti‐D and anti‐Fya (data not shown).
TABLE 2.
Immunohematology testing assessing level of interference from isatuximab and daratumumab. Table shows the results of standard IATs performed independently in three different IHLs with plasma samples spiked with different concentrations of isatuximab or daratumumab and as well as with RBCs treated with 0.2 M DTT
| Blood bank | Anti‐CD38 mAb | Concentration | IAT Method | Agglutination grade | DTT 0.2 M a |
|---|---|---|---|---|---|
| IHL1 | Isatuximab | 50, 150, 300, 600, and 1000 μg/ml | Gel |
W+: 17% 1+: 47% 2+: 36% |
Negative |
| Daratumumab | 50, 150, 300, 600, and 1000 μg/ml | Gel |
1+: 19% 2+: 81% |
Negative | |
| IHL2 | Isatuximab | 0.1, 0.5, 1.0, 5.0, 10, and 200 μg/ml | Tube PEG |
0: 7% W+: 17% 1+: 74% 2+: 2% |
Negative a |
| IHL3 | Isatuximab | 0.1, 1, 10, 108, 297, 372, 487, 601, and 945 μg/ml | Gel |
1+: 94% 2+: 6% |
Negative |
| Daratumumab | 0.1, 1, 10, 18, 313, 574, 745, and 915 μg/ml | Gel |
1+: 56% 2+: 44% |
Negative | |
| Isatuximab | 0.1, 1, 10, 108, 297, 372, 487, 601, and 945 μg/ml | Tube PEG |
0: 52% W+: 46% 1+: 2% |
Negative | |
| Daratumumab | 0.1, 1, 10, 18, 313, 574, 745, and 915 μg/ml | Tube PEG |
0: 17% W+: 74% 1+: 9% |
Negative | |
| Isatuximab | 0.1, 1, 10, 108, 297, 372, 487, 601, and 945 μg/ml | Tube LISS |
0: 72% W+: 28% |
Not tested | |
| Daratumumab | 0.1, 1, 10, 18, 313, 574, 745, and 915 μg/ml | Tube LISS |
0: 31% W+: 69% |
Not tested | |
| Isatuximab | 0.1, 1, 10, 108, 297, 372, 487, 601, and 945 μg/ml | Solid phase |
0: 78% 1+: 15% 2+: 7% |
Not tested | |
| Daratumumab | 0.1, 1, 10, 18, 313, 574, 745, and 915 μg/ml | Solid phase |
0: 11% 1+: 26% 2+: 52% 3+: 11% |
Not tested |
Abbreviations: DTT, dithiothreitol; IAT, indirect antiglobulin test; IHL, immunohematology laboratory; mAb, monoclonal antibody; RBC, red blood cell.
DTT testing was only performed for selected concentrations. Similar results obtained with DTT 0.01 M at IHL2.
Using PEG IAT tube tests, IHL2 tested 37 samples spiked with isatuximab 0.1, 0.5, 1.0, 5.0, 10, and 200 μg/ml. Most samples spiked with isatuximab (74%) showed 1+ agglutination grade, while 17% and 2% showed W+ and + 2 agglutination grades, respectively. All samples spiked with 0.1 μg/ml did not agglutinate. RBCs pre‐treated with DTT at 0.2 M or 0.01 M were negative in PEG IAT tube tests using 10 and 200 μg/ml of isatuximab. PEG IATs of samples containing various RBC alloantibodies and spiked with 10 μg/ml of isatuximab were tested against untreated and 0.01 M DTT‐treated RBCs. While DTT treatment predictably abrogated the false positive reactivity with panel cells lacking the cognate antigens, the alloantibody reactivity directed against selected antigens was unaffected; strength of antigen‐specific agglutination was noted at the same level, or only one grade lower, when compared with the strength of agglutination seen with untreated RBCs (Table S1).
At IHL3, gel 2‐cell screen was performed on nine samples spiked with isatuximab and eight samples spiked with daratumumab. For the isatuximab samples, most reactions (94%) showed 1+ reactivity, across all concentrations tested, except one 2+ reaction (6%; 1 μg/ml). Daratumumab samples showed 1+ (56%) and 2+ (44%) reactivity. Most tube IAT reactions with the isatuximab samples were negative (52% and 72% with PEG and LISS, respectively), which was higher than daratumumab samples (17% and 31% negative reactions with PEG and LISS testing, respectively). Most positive reactions by tube methods were only weakly positive (W+) with both isatuximab and daratumumab. Using the solid phase method, samples spiked with isatuximab were often negative, with only 22% of wells showing positivity (15% and 7% showed 1+ and 2+ agglutination reaction, respectively). Using the same method, 86% of wells tested positive with samples spiked with daratumumab, with up to 3+ reactivity (23% of wells showed 1+, 52% showed +2 and 11% showed 3+ reactivity). For all methods (gel, solid phase, and tube testing), pattern and strength of reactions were not dependent on the concentrations of isatuximab or daratumumab. DTT (0.2 M) treatment of reagent RBCs abolished all reactivity caused by isatuximab or daratumumab by both gel and tube (PEG) test methods.
3.5. Isatuximab interference in the clinical setting
Results of two Phase 3 studies showed that patients treated with isatuximab developed a positive IAT test on treatment following a negative test at baseline. 13 , 14 In the earlier ICARIA‐MM study, antibody screen was performed during the treatment period in 99 patients (65.1%) in the isatuximab plus pomalidomide and dexamethasone arm, and 67 of these patients (67.7%) had a positive test. However, 20 patients (29.9%) did not have an IAT at baseline. 13 More rigorous testing at baseline was performed in the IKEMA study, with 163 of 177 patients in the isatuximab plus carfilzomib and dexamethasone arm having a negative IAT test confirmed at baseline (92.1%) and 150 of these patients (i.e., 84.7% of the intent to treat population) followed up during treatment. Ninety five of these patients demonstrated a positive test while on‐treatment yielding a rate of IAT interference of 63.3% (Table 3) Of note, even if the 13 patients who did not have on‐treatment follow‐up were conservatively assumed to have positive results, the resulting incidence of IAT interference would be 66.2%, or roughly 2/3 of patients.
TABLE 3.
Indirect antiglobulin test by status during on‐treatment period according to baseline status in the IKEMA study (Safety population) a
| IKEMA (Isa‐Kd) (n = 177) | |
|---|---|
| Number of patients with negative IAT at baseline | 163 (92.1%) |
| All tests negative during the on‐treatment period | 55 (33.7%) |
| At least one positive test during the on‐treatment period | 95 (58.3%) |
| No tests done during the on‐treatment period | 13 (8.0%) |
| Observed rate of IAT interference | 63.3% |
Abbreviations: d, dexamethasone; IAT, indirect antiglobulin test; Isa, isatuximab; K, carfilzomib.
Includes patients from the intent‐to‐treat population who actually received at least one dose or part of a dose of the study treatments.
In these two clinical studies, no racial‐, ethnic‐, or sex‐specific predilection for the development of a positive IAT were detected (Table S2). The development of a positive test neither correlated with response to treatment nor translated into an increase in hemolytic events or anemia (data not shown). No transfusion‐related adverse events were reported. 13 , 14
4. DISCUSSION
Results from the in vitro binding experiments presented here indicate that both isatuximab and daratumumab bind CD38 expressed on MM cells with almost identical binding kinetics and affinity. However, contrary to daratumumab, incubation of PBS‐washed RBCs with isatuximab did not result in detectable binding by flow cytometry, microscopic imaging, or tandem–mass spectrometry. This suggests a mechanism by which native CD38 on RBCs escapes isatuximab recognition in the absence of human plasma (in vitro). The lack of binding could be due to epitope masking because of either a CD38 conformation particular to RBCs or the presence of surface proteins that precludes isatuximab binding. To gain insights into isatuximab epitope masking on RBCs, we evaluated whether binding of mouse anti‐human CD38 antibodies (clone HB‐7 and AT13/5) with non‐overlapping epitopes with isatuximab's epitope could facilitate the access and binding of isatuximab to CD38. We demonstrated that isatuximab can bind CD38 previously bound to HB‐7 or AT13/5 antibodies. The extent of isatuximab binding to HB‐7 or AT13/5‐CD38 complex on RBCs is similar to that of daratumumab to untreated RBCs. The likely explanation for epitope unmasking upon HB‐7 or AT13/5 binding is conformational change on the surface of CD38 that allows isatuximab binding. Similar results and conclusion were also obtained using a covalent NAD‐analog that inhibits CD38 ectoenzymatic activity.
Patients with MM receiving anti‐CD38 mAbs frequently demonstrate interference in pretransfusion testing. As the American Association of Blood Banks (AABB) states that positive IATs can persist for up to 6 months following discontinuation of anti‐CD38 treatment, 6 , 9 it is reasonable to suggest a 6‐month wash out period for other drugs in this class (i.e., isatuximab). 8 , 10 , 11 , 20
Of note, there were differences in the strength of RBC agglutination caused by different anti‐CD38 molecules, with laboratory studies showing most isatuximab samples cause overall less frequent and weaker agglutination than daratumumab samples. Moreover, the collective results of 10 published blood bank studies of 88 patient samples after daratumumab therapy showed that all patients demonstrated IAT interference. 2 , 8 , 9 , 10 , 12 , 21 , 22 , 23 , 24 , 25 Isatuximab caused interference in 67.7% patients in the Phase 3 ICARIA‐MM 13 study and in 63.3% patients in the Phase 3 IKEMA study. 14
Investigators have reported similar patterns of agglutination when IAT is performed on samples containing other experimental anti‐CD38 mAbs. 9 , 26 Four anti‐CD38 mAbs (including daratumumab) caused agglutination in a dose‐dependent manner and this interference was inhibited by neutralizing agents (anti‐idiotype antibody and recombinant soluble CD38 extracellular domain). 9 TAK‐079, a human IgG1, non‐agonist anti‐CD38 antibody and daratumumab bound with similar affinity to distinct amino acids of CD38 immobilized on a silicon chip and to endogenously expressed CD38 on a clonal cell line, but bound differently to components of human blood . 26 Daratumumab bound 610% more intensively to a subpopulation of RBCs and 622% more intensively to a subpopulation of platelets than TAK‐079. 26 Conversely, TAK‐079 bound 181% more potently to CD38 expressed on blood B lymphocytes and 259% more potently to blood T lymphocytes. 26
The anti‐CD38 interference with blood bank serologic tests presents a challenge for transfusion services worldwide. Anti‐CD38 therapy interferes with pre‐transfusion compatibility tests, which delays issuance of RBC units to patients. To mitigate this problem, the AABB recommends that hospitals have procedures in place to inform the transfusion service when a patient is due to start treatment with daratumumab or isatuximab. 27 Prior to treatment, baseline blood typing and screen should be performed as well as RBC phenotyping or genotyping. Following treatment initiation, ABO/Rh typing may be performed normally, with antibody screening and identification using DTT‐treated cells to eliminate interference. For crossmatching in patients with a negative antibody screen using DTT‐treated reagent cells, an electronic or immediate‐spin crossmatch with ABO/Rh‐compatible, K‐matched (or K‐negative) units may be performed. For patients with known alloantibodies, phenotypically or genotypically matched RBCs may be provided, or K‐matched/K‐negative RBCs negative for the antigens the patient is sensitized to, if all other common clinically significant alloantibodies have been ruled out by testing with DTT‐treated RBCs. 10 , 28
Other methods of avoiding anti‐CD38 interference include the use of trypsin, a proteolytic enzyme that decreases daratumumab binding to CD38‐expressing cells, but it is not as extensively used as DTT. 29 Anti‐idiotype antibody and soluble recombinant CD38 are also reported to prevent binding of daratumumab with CD38‐expressing reagent RBCs; 9 however, although the cost of reagent is minimal, this method is expensive and, like DTT, encumbered by an absence of resources not routinely available at many hospital blood banks. 9 , 16 , 30 , 31
In conclusion, the current study shows that both isatuximab and daratumumab interfere with IATs but at different magnitudes in vitro and in vivo, reflecting distinct binding to CD38. In in vitro binding studies, CD38 expressed by RBCs can be directly recognized by daratumumab, whereas isatuximab requires a co‐factor, such as a mouse anti‐CD38 antibody (HB‐7 or AT13/5) or a CD38 inhibitor, suggesting that the isatuximab epitope on RBCs is masked. The masking of isatuximab epitope on RBCs may explain the observation from two Phase 3 clinical trials showing that IAT interference with isatuximab is not universal but only observed in two‐thirds of the treated patients. Future studies are essential to characterize the impact of human plasma on isatuximab binding to RBCs expressing CD38.
CONFLICT OF INTEREST
Btissam Chami: No relevant financial relationships to disclose. Makoto Okuda: No relevant financial relationships to disclose. Morvarid Moayeri: Institution support—Sanofi. France Pirenne: No relevant financial relationships to disclose. Yoko Hidaka: No relevant financial relationships to disclose. Ashok Nambiar: Institution support—Sanofi. Zhili Song: Employed by Sanofi; may hold stock and/or stock options in the company. Olivier Bedel: Employed by Sanofi at the time of study. Bailin Zhang: Employed by Sanofi; may hold stock and/or stock options in the company. Joern Hopke: Employed by Sanofi; may hold stock and/or stock options in the company. Gejing Deng: Employed by Sanofi; may hold stock and/or stock options in the company. Chen Zhu: Employed by Sanofi; may hold stock and/or stock options in the company. Sandrine Macé: Employed by Sanofi; may hold stock and/or stock options in the company. Marielle Chiron: Employed by Sanofi; may hold stock and/or stock options in the company. Francisco Adrian: Employed by Sanofi at the time of study. Taro Fukao: Employed by Sanofi; may hold stock and/or stock options in the company. Frank Basile: Employed by Sanofi at the time of study. Thomas Martin: Research funding—Sanofi, Seattle Genetics, Amgen, Janssen, GSK.
Supporting information
Appendix S1 Supplementary Information
ACKNOWLEDGMENTS
This study was sponsored by Sanofi. The authors thank the study centers and investigators for their contributions to the ICARIA and IKEMA studies. The authors thank Bruno Leroux (l'Établissement Français du Sang, Île‐de‐France) and Nyam Kamsu‐Kom and Helgi van de Velde (Sanofi) for their contributions to this manuscript. Medical writing support was provided by Camile Semighini Grubor, PhD of Elevate Medical Affairs, contracted by Sanofi Genzyme for publication support services.
Chami B, Okuda M, Moayeri M, Pirenne F, Hidaka Y, Nambiar A, et al. Anti‐CD38 monoclonal antibody interference with blood compatibility testing: Differentiating isatuximab and daratumumab via functional epitope mapping. Transfusion. 2022;62(11):2334–2348. 10.1111/trf.17137
Funding information Sanofi; Sanofi Genzyme
REFERENCES
- 1. Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol. 2016;43(6):676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin MH, Liu FY, Wang HM, Cho HC, Lo SC. Interference of daratumumab with pretransfusion testing, mimicking a high‐titer, low avidity like antibody. Asian J Transfus Sci. 2017;11(2):209–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal‐associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399–408. [DOI] [PubMed] [Google Scholar]
- 4. Martin TG, Corzo K, Chiron M, Velde HV, Abbadessa G, Campana F, et al. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cell. 2019;8(12):1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarclisa (isatuximab‐irfc) injection for intravenous use . Prescribing information. sanofi‐aventis U.S. LLC. Available from: https://products.sanofi.us/Sarclisa/sarclisa.pdf. Accessed July 21, 2022.
- 6. DARZALEX (daratumumab) injection for intravenous use . Prescribing information. Janssen Biotech Inc. Available from: https://www.janssenlabels.com/package‐insert/product‐monograph/prescribing‐information/DARZALEX‐pi.pdf Accessed July 21, 2022.
- 7. Alquist C, Harm S. Transfusion‐service‐related activities: Pretransfusion testing and storage, monitoring, processing, distribution, and inventory management of blood components. In: Cohn C, Delaney M, Johnson S, Katz L, editors. Bethesda, MD, USA: AABB Technical Manual. 20th ed. Digital; 2020. p. 816. [Google Scholar]
- 8. Chapuy CI, Nicholson RT, Aguad MD, Chapuy B, Laubach JP, Richardson PG, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion. 2015;55(6 Pt 2):1545–54. [DOI] [PubMed] [Google Scholar]
- 9. Oostendorp M, Lammerts van Bueren JJ, Doshi P, Khan I, Ahmadi T, Parren PW, et al. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion. 2015;55(6 Pt 2):1555–62. [DOI] [PubMed] [Google Scholar]
- 10. Chari A, Arinsburg S, Jagannath S, Satta T, Treadwell I, Catamero D, et al. Blood transfusion management and transfusion‐related outcomes in daratumumab‐treated patients with relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(1):44–51. [DOI] [PubMed] [Google Scholar]
- 11. Chapuy CI, Aguad MD, Nicholson RT, AuBuchon JP, Cohn CS, Delaney M, et al. International validation of a dithiothreitol (DTT)‐based method to resolve the daratumumab interference with blood compatibility testing. Transfusion. 2016;56(12):2964–72. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan HC, Gerner‐Smidt C, Nooka AK, Arthur CM, Thompson L, Mener A, et al. Daratumumab (anti‐CD38) induces loss of CD38 on red blood cells. Blood. 2017;129(22):3033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attal M, Richardson PG, Rajkumar SV, San‐Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low‐dose dexamethasone versus pomalidomide and low‐dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA‐MM): a randomised, multicentre, open‐label, phase 3 study. Lancet. 2019;394(10214):2096–107. [DOI] [PubMed] [Google Scholar]
- 14. Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open‐label, randomised phase 3 trial. Lancet. 2021;397(10292):2361–71. [DOI] [PubMed] [Google Scholar]
- 15. Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, van Schooten W. The pharmacology of CD38/NADase: an emerging target in cancer and diseases of aging. Trends Pharmacol Sci. 2018;39(4):424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bondza S, Foy E, Brooks J, Andersson K, Robinson J, Richalet P, et al. Real‐time characterization of antibody binding to receptors on living immune cells. Front Immunol. 2017;8:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8. [DOI] [PubMed] [Google Scholar]
- 18. Wong SW, Comenzo RL. CD38 monoclonal antibody therapies for multiple myeloma. Clin Lymphoma Myeloma Leuk. 2015;15(11):635–45. [DOI] [PubMed] [Google Scholar]
- 19. Sauve AA, Schramm VL. Mechanism‐based inhibitors of CD38: a mammalian cyclic ADP‐ribose synthetase. Biochemistry. 2002;41(26):8455–63. [DOI] [PubMed] [Google Scholar]
- 20. Lancman G, Arinsburg S, Jhang J, Cho HJ, Jagannath S, Madduri D, et al. Blood transfusion management for patients treated with anti‐CD38 monoclonal antibodies. Front Immunol. 2018;9:2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bub CB, Reis IND, Aravechia MG, Santos LD, Bastos EP, Kutner JM, et al. Transfusion management for patients taking an anti‐CD38 monoclonal antibody. Rev Bras Hematol Hemoter. 2018;40(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carreno‐Tarragona G, Cedena T, Montejano L, Alonso R, Miras F, Valeri A, et al. Papain‐treated panels are a simple method for the identification of alloantibodies in multiple myeloma patients treated with anti‐CD38‐based therapies. Transfus Med. 2019;29(3):193–6. [DOI] [PubMed] [Google Scholar]
- 23. Deneys V, Thiry C, Frelik A, Debry C, Martin B, Doyen C. Daratumumab: Therapeutic asset, biological trap! Transfus Clin Biol. 2018;25(1):2–7. [DOI] [PubMed] [Google Scholar]
- 24. Setia R, Dogra M, Sachdeva P, Handoo A, Choudhary D, Agarwal A. Daratumumab (Anti‐CD38) interference with serological testing: an emerging challenge for blood banks in developing countries. Glob J Transfus Med. 2017;2:163–5. [Google Scholar]
- 25. Subramaniyan R, Satheshkumar R, Pereira KR. Role of daratumumab in transfusion medicine: A must know entity. Rev Bras Hematol Hemoter. 2017;39(4):375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fedyk ER, Idamakanti N, Chen J, Estevam J, Hernandez V, Wagoner M, et al. The binding of CD38 therapeutics to red blood cells and platelets subverts depletion of target cells. Blood. 2019;134(Suppl 1):3136. [Google Scholar]
- 27. Regan DM, Markowitz MA. Mitigating the anti‐CD38 interference with serologic testing. AABB Association Bulletin 16‐02. 2016. Available from: https://www.aabb.org/docs/default‐source/default‐document‐library/resources/association‐bulletins/ab16‐02.pdf
- 28. Hannon J, Caruk B, Clarke G. Serological findings related to treatment with a human monoclonal antibody (daratumumab) in patients with advanced plasma cell myeloma (abstract). Transfusion. 2014;54(2S):162A. [Google Scholar]
- 29. Brierley CK, Staves J, Roberts C, Johnson H, Vyas P, Goodnough LT, et al. The effects of monoclonal anti‐CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59(7):2248–54. [DOI] [PubMed] [Google Scholar]
- 30. Mei Z, Wool GD. Impact of novel monoclonal antibody therapeutics on blood bank pretransfusion testing. Hematol Oncol Clin North Am. 2019;33(5):797–811. [DOI] [PubMed] [Google Scholar]
- 31. Jones AD, Moayeri M, Nambiar A. Impact of new myeloma agents on the transfusion laboratory. Pathology. 2021;53(3):427–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information


