Abstract
Since the publication of the first epidemiological study to establish the connection between long‐term exposure to atmospheric pollution and effects on human health, major efforts have been dedicated to estimate the attributable mortality burden, especially in the context of the Global Burden of Disease (GBD). In this work, we review the estimates of excess mortality attributable to outdoor air pollution at the global scale, by comparing studies available in the literature. We find large differences between the estimates, which are related to the exposure response functions as well as the number of health outcomes included in the calculations, aspects where further improvements are necessary. Furthermore, we show that despite the considerable advancements in our understanding of health impacts of air pollution and the consequent improvement in the accuracy of the global estimates, their precision has not increased in the last decades. We offer recommendations for future measurements and research directions, which will help to improve our understanding and quantification of air pollution‐health relationships.
Keywords: health impact, outdoor air pollution, global estimates
Key Points
Large differences are present in the published estimates of excess mortality attributable to outdoor air pollution at the global scale
The differences are mostly due to the exposure response functions as well as the number of health outcomes included in the calculations
Although the accuracy of the global estimates has improved, their precision has not increased in the last decades
1. Introduction
Air pollution has been recognized as a major human health risk since ancient history (Fowler et al., 2020). After the Industrial Revolution air pollution has grown in both intensity and geographical extent, reaching critical levels; many large industrial areas and cities in Europe and North America have experienced episodes of marked air pollution. However, the limited evidence on its impact on human health at the time, meant that the significance of the hazard went unappreciated. Severe air pollution episodes in Donora, USA in the late 1940s and the infamous Great London smog of 1952 were among the first instances of extreme air pollution to be statistically associated with negative health outcomes. By the 1980s, the link between air pollution and pulmonary disorders became widely acknowledged, but quantification of this relationship remained rare. In the 1990s, large cohort studies in North America reported surprisingly large health effects even at low levels of exposure (Dockery et al., 1993). The importance of air quality for human health has since been undisputed.
A large body of long‐term exposure epidemiological studies relating air pollution exposure to human health outcomes has contributed enormously over the last two decades toward the development of quantitative methods to link chronic and acute exposures to these health outcomes. This, together with the development of networks of high precision measurements of air pollution, which allowed epidemiological investigations on a large scale, the development of global datasets of population distribution as well as the availability of reports of cause‐specific disease rates by national statistical offices, made it possible to estimate the health burden resulting from exposure to air pollution on national, regional, and global scales.
Both short and long‐term exposure to ambient air pollution causes increased morbidity and excess mortality from multiple health endpoints (Manisalidis et al., 2020), though the latter has comparatively much larger impacts on public health, being significant even at very low exposure levels (Beverland et al., 2012; US EPA National Center for Environmental Assessment et al., 2020). The chronic exposure to ambient PM2.5 (particulate matter with an aerodynamic diameter of less than 2.5 μm) and ozone are associated with reduced life‐expectancy, loss of healthy life years and excess mortality from cardiovascular as well as respiratory diseases (R. T. Burnett et al., 2014; J. Lelieveld et al., 2015; Murray et al., 2020; Pope et al., 2009; Prabhakaran et al., 2020). Recent studies have also implicated ambient PM2.5 exposure in adverse birth outcomes (Balakrishnan et al., 2018; Heft‐Neal et al., 2018; Ritz et al., 2006) and mortality and morbidity from numerous other non‐communicable diseases including diabetes, neurological disorders and cancers among others (R. Burnett et al., 2018; Hahad et al., 2021; J. Lelieveld et al., 2020; Peters et al., 2019; Turner et al., 2020; Wong et al., 2016).
In 2019, 74% of total deaths globally resulted from non‐communicable diseases (NCD), a significant increase from 54% in 1990. Of all deaths from non‐communicable diseases in 2019, about 20% may be attributed to environmental risk factors (including, ambient air pollution, household air pollution, lead and radon exposure, extremes of temperature, unsafe water, sanitation, and hand washing). The exposure to ambient air pollution was found to be the single largest one (responsible for roughly 50% of the deaths from all environmental risk factors). More than 50% of these deaths from ambient air pollution exposure occur in China and South Asia, and about 20% of the total global air pollution related deaths occur in high‐income countries in Europe and North America. Given the now‐extensive evidence of health risks even at very low concentrations of ambient PM2.5, this signifies the urgency of adequate air quality management also in countries with relatively low levels of air pollution exposure (Papadogeorgou et al., 2019; Pappin et al., 2019; Shi et al., 2016; Yu et al., 2020). Recent studies indicate that excess mortality from ambient air pollution exposure has increased over the last decade (Butt et al., 2017; S. Chowdhury et al., 2020; Cohen et al., 2017), with further increases expected in the future (S. Chowdhury et al., 2018; Silva et al., 2017).
Recognizing the importance of the problem, partially due to aging of the population for whom NCD risks are higher, the United Nations Sustainable Development Goals (SDGs) have called for mitigation of mortality caused by exposure to outdoor PM2.5 by 2030. Achieving this goal will require accurate assessment of the mortality from exposure to present and past levels of ambient air pollution so to evaluate of the effectiveness of air pollution control policies to avert the health impacts. Therefore, accurate and precise estimation of mortality attributable to air pollution are extremely important to support and evaluate air pollution reduction policies.
The Global Burden of Disease (GBD) Studies (Cohen et al., 2017; Lim et al., 2012; Murray et al., 2020; Stanaway et al., 2018) provide a consistent and comparative description of the burden of diseases and injuries from multiple risk factors including exposure to air pollution, which can help national‐level decision‐making and planning processes. Beginning with the 2015 data, the GBD is updated annually, allowing progress in mitigating the effect of air pollution (and other impacts) to be assessed and measured by all countries. With every update, the GBD incorporates the latest evidence, data and methods, thus refining the estimates of the burden of disease, changing the previous estimates, and making their comparison difficult. Dedicated studies have found that the data curated from evidence presented by the GBD are valuable for planning and prioritizing health policy, facilitate accountability, and monitor progress and trends over time between countries (Boogaard et al., 2019; Lundkvist et al., 2021; State of Global Air 2020, 2020). Apart from the GBD studies, over the last decade multiple global and regional studies have quantified the health impacts of ambient air pollution exposure, also linking the latter to source sectors (Balakrishnan et al., 2019; S. Chowdhury et al., 2018, 2020; Dedoussi et al., 2020; Goodkind et al., 2019; J. Lelieveld et al., 2015, J. Lelieveld, Klingmüller, Pozzer, Burnett, et al., 2019; Lelieveld, Klingmüller, Pozzer, Pöschl, et al., 2019; J. Lelieveld et al., 2020). Most such health impact assessment studies report the excess deaths associated with exposure to ambient air pollution, which may be defined as a steady‐state difference in number of deaths between exposed and unexposed populations, that is, the number of deaths that would not have occurred with absent exposure over a defined period of time (R. Burnett et al., 2018; Hammitt et al., 2020). In addition, some studies also report age or time dependent criteria, for example, disability‐adjusted life years (DALYs) and the years of life lost (J. Lelieveld et al., 2020; Owusu & Sarkodie, 2020).
Estimation of excess deaths attributable to ambient air pollution requires inputs on the spatial and temporal distribution of ambient air pollution exposure; an exposure response function that relates exposure to ambient air pollution and the relative risk (or hazard ratio, which represents instantaneous risk over the study time period); population counts and population age‐distribution; background disease‐specific death rates; and the counterfactual exposure below which no excess risk of excess mortality from air pollution exposure exists. An attributable fraction (AF), which indicates the contribution of a risk factor (here, ambient air pollution) to mortality from a disease is estimated from the relative risk corresponding to the exposure. Excess deaths are estimated as a product of AF and baseline death rates and population counts in a grid or an administrative unit. Such global health impact assessment studies published over the last decade report large disparities in excess deaths from ambient air pollution exposure, for example, Cohen et al. (2017) estimated 4.2 (95% confidence interval 3.7–4.8) million excess deaths due to air pollution exposure for the year 2015 while R. Burnett et al. (2018) estimated 8.9 (7.5–10.3) million excess deaths for the same year. Such differences among the published estimates, can arise from the sources of the input parameters, which may nevertheless confuse or mislead decision makers and others who are not experts in the field.
To the best of our knowledge, no comprehensive reviews to date have examined the disparities in excess deaths from exposure to ambient air pollution at global level reported by published works. Various studies have been published considering only specific aspects of the global estimates. For example, the impact of different ERF has been previously investigated by R. Burnett and Cohen (2020), while the differences between two estimates (GBD 2013 and GBD 2015) were analyzed in detail in Ostro et al. (2018). Although comprehensive views have been presented in Evangelopoulos et al. (2020) and Fuller et al. (2022), these works consider only a subset of GBD studies, leaving out numerous studies from the comparison.
Here, we systematically review the published studies reporting global excess mortality from chronic exposure to ambient air pollution. The aim is to analyze the methods, data and metrics applied, which may help explain the differences between results and conclusions in the literature. We focus on studies that report global excess deaths from chronic exposure to ambient PM2.5 and ambient ozone, together termed the exposure to ambient air pollution. We acknowledge that besides PM2.5 and O3, chronic exposure to other criterion air pollutants like nitrogen dioxide (NO2) and sulfur dioxide (SO2) are also hazardous to health (Anenberg et al., 2018; S. Chowdhury et al., 2021; Greenberg et al., 2016), however we do not assess their impacts in the current review. We attempt to compare the identified studies by comparing the input variables used by the authors to estimate the excess deaths. We have only included studies that report excess deaths globally since 2004.
2. Literature Research
We conducted a literature search in January 2022 using the database in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science (https://apps.webofknowledge.com/). We limited our search to journal articles published in English from January 2004 to December 2021 focusing on all studies that report excess mortality from ambient air pollution exposure. To identify the research articles we used the following keywords: “air pollution” AND “excess death or mortality, premature” AND “global.” In addition, we included studies suggested by experts and we investigated the references of the identified papers, to identify possible additional literature studies.
In Tables 1 and 2, the literature with global estimates of mortality attributable to air pollution is presented. A total of 31 studies is included in this work, with the first work published in 2005 (Cohen et al., 2005). The studies estimate excess mortality attributable to air pollution globally for different years, ranging from 1990 to 2020. In this sense particular attention has to be given to publications related to the GBD; these, may not only be referred to as milestones in this area, but also place deaths attributable to air pollution in relation to all other causes thus enhancing its significance in a broader context.
Table 1.
Mortality Estimates From Fine Particulate Exposure
| Study | Study characteristics | Excess deaths (95% CL) | ERF used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year reported | Population data | Affected population | BMR | PM2.5 data | Causes | TMREL | |||
| Cohen et al. (2005) a | 2000 | UN (2002) | 0–5, >30 | Cohen et al. (2005) | GMAPS (2004) | Cardiopulmonary, LC | 7.5 | 0.8 (0.2–1.2) | LI (Arden Pope, 2002) |
| Anenberg et al. (2010) | 2000 | Landscan (2008) | >30 | WHO (2004a, 2004b) | MOZART‐2 (Horowitz, 2006) | Cardiopulmonary, LC | 5.8 | 3.72 (2.74–4.7) | LI (HEI, 2009) |
| Lim et al. (2012) | 1990 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2010) | IHD, stroke, COPD, LC, ALRI | 5.8–8.8 | 2.91 (2.54–3.29) | IER (R. T. Burnett et al., 2014) |
| Lim et al. (2012) | 2010 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2010) | IHD, stroke, COPD, LC, ALRI | 5.8–8.8 | 3.22 (2.83–3.62) | IER (R. T. Burnett et al., 2014) |
| Rao et al. (2012) | 2005 | UN (2002) | >30 years | WHO (2004b); WHO (2004a) | TM5 (Krol et al., 2005) | Cardiopulmonary, LC | 7.5–50 | 2.7 (0.72–4.23) | LI (Arden Pope, 2002) |
| J. Lelieveld et al. (2013) | 2005 | GPW3 (2005) | >30 years | WHO (2012) | EMAC (Jöckel et al., 2010) | CVD, LC | Pre‐Industrial | 2.2 | LI (HEI, 2009) |
| Age UN (2002) | |||||||||
| Y. Fang et al. (2013) | 2000 | GPW3 (2005) | >30 years | WHO (2003) | AM3 (Donner et al., 2011) | Cardiopulmonary, LC | PI (1860) | 1.6 (1.3–1.9) | LI (HEI, 2009) |
| Age UN (2002) | |||||||||
| Silva et al. (2013) | 2000 | Landscan (2008) | >30 years | WHO (2010) | ACCMIP (Lamarque et al., 2013) | Cardiopulmonary, LC | PI (1850) | 2.11 (1.3–3) | LI (HEI, 2009) |
| Age (UN, 2002) | |||||||||
| Evans et al. (2013) | 2004 | GPW3 (2005) | >30 years | WHO (2004b) | GWR (van Donkelaar et al., 2010) | Cardiopulmonary, IHD, LC | 5.8 | 3.3 | LI (HEI, 2009) |
| Age (UN, 2002) | |||||||||
| J. Lelieveld et al. (2015) | 2010 | GPW3 (2005) | 0–5, >30 years | WHO (2012) | EMAC (Jöckel et al., 2010) | IHD, stroke, COPD, LC, ALRI | 5.8 | 3.15 (1.52–4.6) | IER (R. T. Burnett et al., 2014) |
| Age (UN, 2002) | |||||||||
| Apte et al. (2015) | 2010 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | GWR (Brauer et al., 2012) | IHD, stroke, COPD, LC, ALRI | 5.8 | 3.24 (3.24–3.24) | IER (R. T. Burnett et al., 2014) |
| Forouzanfar et al. (2015) a | 1990 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2010) | IHD, stroke, COPD, LC, ALRI | 5.9–8.7 | 2.9 (2.7–3) | IER (Forouzanfar et al., 2015) |
| IHME age | |||||||||
| Forouzanfar et al. (2015) a | 2013 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2010) | IHD, stroke, COPD, LC, ALRI | 5.9–8.7 | 2.2 (2.1–2.3) | IER (Forouzanfar et al., 2015) |
| IHME age | |||||||||
| Silva, West, et al. (2016) | 2000 | Landscan (2008) | >25 years | GBD (IHME) | ACCMIP (Lamarque et al., 2013) | IHD, stroke, COPD, LC | PI (1850) | 0.1.7 (1.3–2.1) | IER (R. T. Burnett et al., 2014) |
| Age UN (2002) | |||||||||
| Forouzanfar et al. (2016) a | 2005 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2016) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 3.9 (3.4–4.4) | IER (Forouzanfar et al., 2015) |
| IHME age | |||||||||
| J. Lelieveld (2017) | 2015 | GPW3 (2005) | 0–5, >30 years, | WHO | EMAC (Jöckel et al., 2010) | IHD, stroke, COPD, LC, ALRI | 2.4 | 4.04 (2–6.03) | IER (R. T. Burnett et al., 2014) |
| Age UN (2002) | |||||||||
| Butt et al. (2017) | 2009 | GPW3 (2005) | >25 years | GBD (IHME) | HadGEM‐3 (Turnock et al., 2015) | IHD, stroke, COPD, LC, ALRI | 5.8 | 2.6 (1.87–3.57) | IER (Forouzanfar et al., 2015) |
| Age UN (2002) | |||||||||
| Zhang et al. (2017) | 2007 | Landscan (2008) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 5.8 | 3.45 (2.38–4.14) | IER (Forouzanfar et al., 2015) |
| Age UN (2002) | |||||||||
| Cohen et al. (2017) a | 1990 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 3.5 (3–4) | IER (Forouzanfar et al., 2016) |
| IHME age | |||||||||
| Cohen et al. (2017) a | 2015 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 4.2 (3.7–4.8) | IER (Forouzanfar et al., 2016) |
| IHME age | |||||||||
| Stanaway et al. (2018) a | 2007 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 2.42 (2.08–2.76) | IER (Stanaway et al., 2018) |
| IHME age | T2‐DM | ||||||||
| Stanaway et al. (2018) a | 2017 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 2.94 (2.5–3.36) | IER (Stanaway et al., 2018) |
| IHME age | T2‐DM | ||||||||
| Liang et al. (2018) | 2010 | Landscan (2008) | >25 years | GBD (IHME) | TF‐HTAP2 (HTAP, 2022) | IHD, stroke, COPD, LC | 5.8–8.8 | 2.8 (0.5–4.6) | IER (R. T. Burnett et al., 2014) |
| Age UN (2002) | |||||||||
| R. Burnett et al. (2018) | 2015 | GPW4 (2016) | >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | NCDs, LRI | 2.4 | 8.9 (7.5–10.3) | GEMM (R. Burnett et al., 2018) |
| IHME age | |||||||||
| R. Burnett et al. (2018) | 2015 | GPW4 (2016) | >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | IHD, stroke, COPD, LC, ALRI | 2.4 | 6.9 (4.9–8.5) | GEMM (R. Burnett et al., 2018) |
| IHME age | |||||||||
| Crippa et al. (2019) | 2010 | GPW4 (2016) | 0‐5,>25 years | WHO (2015) | TM5‐FASST (Van Dingenen et al., 2018) | IHD, stroke, COPD, LC, ALRI | 5.8 | 2.1 (1.1–3.3) | IER (R. T. Burnett et al., 2014) |
| Age UN (2002) | |||||||||
| J. Lelieveld, Klingmüller, Pozzer, Burnett, et al. (2019) | 2015 | UN (2002) | 0–5, >25 years | WHO (2015) | EMAC (Jöckel et al., 2010) | NCD, LRI | 2.4 | 8.5 (6.8–10) | GEMM (R. Burnett et al., 2018) |
| Age UN (2002) | |||||||||
| J. Lelieveld et al. (2020) | 2015 | UN (UN, 2002) | 0–5, >25 years | WHO (2015) | EMAC (Jöckel et al., 2010) | NCD, LRI | 2.4 | 8.5 (6.8–10) | GEMM (R. Burnett et al., 2018) |
| Age UN (2002) | |||||||||
| S. Chowdhury et al. (2020) | 2000 | UN(UN, 2002) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | NCDs, LRI | 2.4 | 6.87 (5.57–8.15) | GEMM (R. Burnett et al., 2018) |
| Age UN (2002) | |||||||||
| S. Chowdhury et al. (2020) | 2015 | UN(UN, 2002) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | NCDs, LRI | 2.4 | 8.89 (7.29–11.04) | GEMM (R. Burnett et al., 2018) |
| Age UN (2002) | |||||||||
| H. Yin et al. (2020) | 2016 | GPWv4 | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | NCDs, LRI | 2.4–5.9 | 8.42 (6.5–10.5) | GEMM (R. Burnett et al., 2018) |
| Age UN (2002) | |||||||||
| Murray et al. (2020) a | 2019 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 4.14 (3.45–4.8) | MRBRT (Murray et al., 2020) |
| IHME age | T2‐DM, neonatal diseases | ||||||||
| Murray et al. (2020) a | 2010 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | IHD, stroke, COPD, LC, ALRI | 2.4–5.9 | 3.36 (2.72–3.99) | MRBRT (Murray et al., 2020) |
| IHME age | T2‐DM, neonatal diseases | ||||||||
| Vohra et al. (2021) | 2012 | GPW4 (2016) | >14 years | GBD (IHME) | GEOS‐Chem (Bey et al., 2001) | All cause | Non‐fossil fuel | 10.2 (−47.1–17.0) | LI (Vodonos et al., 2018) |
| S. Chowdhury et al. (2022) | 2015 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | EMAC (Jöckel et al., 2010) | IHD, stroke, COPD, LC, ALRI | 0 | 4.23 (3.0–6.14) | MRBRT (Murray et al., 2020) |
| IHME age | T2‐DM, neonatal diseases | ||||||||
| R. T. Burnett et al. (2022) | 2019 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | IHD, stroke, COPD, LC, ALRI | 0 | 8.2 (5.9–10.0) | FUSION (R. T. Burnett et al., 2022) |
| IHME age | T2‐DM, neonatal diseases | ||||||||
Denotes GBD publications, excess deaths in million.
Table 2.
Mortality Estimates From Ozone Exposure
| Study | Study characteristics | Excess Deaths (95%CL) | ERF used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year reported | Population data | Affected population | BMR | O3 data | Causes | TMREL | |||
| Anenberg et al. (2010) | 2000 | Landscan (2008) | >30 | WHO (2004b); WHO (2004a) | MOZART‐2 (Horowitz, 2006) | Respiratory | 33.3 | 700 (400–10000) | LI (Jerrett et al., 2009) |
| Lim et al. (2012) a | 1990 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | TM5 model | COPD | 33.3–41.9 | 143 (48–252) | LI (Jerrett et al., 2009) |
| Lim et al. (2012) a | 2010 | GPW3 (2005) | 0–5, >25 years | GBD (IHME) | TM5 model | COPD | 33.3–41.9 | 152 (52–267) | LI (Jerrett et al., 2009) |
| J. Lelieveld et al. (2013) | 2005 | GPW3 (2005) | >30 years | WHO (2012) | EMAC (Jöckel et al., 2010) | COPD | PI | 800 | LI (Jerrett et al., 2009) |
| Age UN (2002) | |||||||||
| Y. Fang et al. (2013) | 2000 | GPW3 (2005) | >30 years | WHO (2003) | AM3 (Donner et al., 2011) | Respiratory | PI | 375 (129–592) | LI (Jerrett et al., 2009) |
| Age UN (2002) | |||||||||
| Silva et al. (2013) | 2000 | Landscan (2008) | >30 years | WHO (2010) | ACCMIP (Lamarque et al., 2013) | Respiratory | PI | 470 (180–900) | LI (Jerrett et al., 2009) |
| Age UN (2002) | |||||||||
| J. Lelieveld et al. (2015) | 2010 | GPW3 (2005) | 0–5, >30 years | WHO (2012) | EMAC (Jöckel et al., 2010) | COPD | 33.3–41.9 | 142 (90–208) | LL (Jerrett et al., 2009) |
| Age UN (2002) | |||||||||
| Forouzanfar et al. (2015) a | 1990 | GPW3 (2005), IHME | 0–5, >25 years | GBD (IHME) | TM5‐FASST (Brauer et al., 2016) | COPD | 33.3–41.0 | 133 (105–162) | LI (Jerrett et al., 2009) |
| IHME age | |||||||||
| Forouzanfar et al. (2015) a | 2013 | GPW3 (2005), IHME | 0–5, >25 years | GBD (IHME) | TM5‐FASST (Brauer et al., 2016) | COPD | 33.3–41.0 | 217 (161–272) | LI (Jerrett et al., 2009) |
| IHME age | |||||||||
| Silva, West, et al. (2016) | 2000 | Landscan (2008) | >25 years | GBD (IHME) | ACCMIP (Lamarque et al., 2013) | Respiratory | PI | 382 (121–728) | LI (Jerrett et al., 2009) |
| Age (UN, 2002) | |||||||||
| Forouzanfar et al. (2016) a | 2005 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | TM5‐FASST (Brauer et al., 2016) | COPD | 33.3–41.9 | 207 (77–353) | LI updated (Jerrett et al., 2009) |
| IHME age | |||||||||
| J. Lelieveld (2017) | 2015 | GPW3 (2005) | 0–5, >30 years | WHO | EMAC (Jöckel et al., 2010) | COPD | 33.3–41.9 | 298 (148–448) | LL (Cohen et al., 2017) |
| UN age (UN, 2002) | |||||||||
| Cohen et al. (2017) a | 2015 | GPW4 (2016) | 0‐5, >25 years | GBD (IHME) | TM5‐FASST (Brauer et al., 2016) | COPD | 33.3–41.9 | 254 (97–422) | LI updated (Jerrett et al., 2009) |
| IHME age | |||||||||
| Malley et al. (2017) | 2010 | GPW3 (2005) | >30 years | GBD (IHME) | GEOS‐Chem (6mDM1h) | Respiratory | 33.3 | 547 (198–897) | LI updated (Jerrett et al., 2009) |
| Age UN (2002) | |||||||||
| Malley et al. (2017) | 2010 | GPW3 (2005) | >30 years | GBD (IHME) | GEOS‐Chem (ADMA8) | Respiratory | 26.7 | 1234 (851–1616) | LI updated (Turner et al., 2016) |
| Age UN (2002) | |||||||||
| Stanaway et al. (2018) a | 2007 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | TOAR (Schultz et al., 2017) | COPD | 29.1–35.7 | 392 (146–638) | LI (Turner et al., 2016) |
| IHME age | |||||||||
| Stanaway et al. (2018) a | 2017 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | TOAR (Schultz et al., 2017) | COPD | 29.1–35.7 | 472 (177–768) | LI (Turner et al., 2016) |
| IHME age | |||||||||
| Liang et al. (2018) | 2010 | Landscan (2008) | >25 years | GBD (IHME) | TF‐HTAP2 (HTAP, 2022) | COPD | 290 (30–600) | LI (Jerrett et al., 2009) | |
| Age UN (2002) | |||||||||
| J. Lelieveld, Klingmüller, Pozzer, Burnett, et al. (2019) | 2015 | UN (2002) | 0–5, >25 years | WHO (2015) | EMAC (Jöckel et al., 2010) | COPD | 29.1–35.7 | 283 (88–504) | LL (Cohen et al., 2017) |
| Age UN (2002) | |||||||||
| J. Lelieveld et al. (2020) | 2015 | UN (2002) | 0–5, >25 years | WHO (2015) | EMAC (Jöckel et al., 2010) | COPD | 29.1–35.7 | 283 (88–504) | LI (Cohen et al., 2017) |
| Age UN (2002) | |||||||||
| S. Chowdhury et al. (2020) | 2000 | UN (2002) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | Respiratory | 26.7–31.1 | 1110 (790–1420) | LI (Turner et al., 2016) |
| UN (2002) | |||||||||
| S. Chowdhury et al. (2020) | 2015 | UN (2002) | 0–5, >25 years | GBD (IHME) | GWR (Shaddick et al., 2018) | Respiratory | 26.7–31.1 | 1300 (930–1680) | LI (Turner et al., 2016) |
| UN (2002) | |||||||||
| Murray et al. (2020 a | 2010 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | COPD | 29.1–35.7 | 314 (148–493) | MRBRT (Cohen et al., 2017) |
| IHME age | |||||||||
| Murray et al. (2020) a | 2019 | GPW4 (2016) | 0–5, >25 years | GBD (IHME) | GWR (van Donkelaar et al., 2019) | COPD | 29.1–35.7 | 365 (174–564) | MRBRT (Cohen et al., 2017) |
| IHME age | |||||||||
Denotes GBD publications, excess deaths in thousands.
3. Methodology for Estimating Excess Mortality
The calculation of excess mortality attributable to long‐term exposure to air pollution in a certain location (M(x, y), with x, y representing the geographical coordinates) follows a well‐established procedure (Anenberg et al., 2010):
| (1) |
where j represents the disease (e.g., lung cancer, ischemic heart disease, and so on), k the age class, “Mort” the mortality registered in the location and “AF” the attributable fraction, that is, the fraction of “Mort” that can be attributed to the exposure to the pollutant X. Clearly, different sources of data can be used, and, as the mortality is normally registered at national level, a traditional assumption is to scale it to the population data, by using the baseline mortality rate (BMR, i.e., Mort j,k /Pop j,k ) at national levels and the population (Pop) distribution at high resolution, so that
| (2) |
Although the M(x, y) estimates can be derived at country, regional or local levels, the general approach for global calculation is to distribute these parameters on a global grid, so to avoid errors in the exposure due to co‐location of pollution and population. The global estimate (M) is then obtained by the sum of M(x, y) for each location in the world (i.e., M = ∑ x,y M(x, y)).
The population distribution (Pop k ) and the disease‐related Mortality (Mort j,k ) are obtained from official national statistical agencies. With the notable exception of the early GBD work (Cohen et al., 2005) where only monitoring stations were used in the estimates, pollution concentrations are derived on a global scale, by either remotely sensed observations (Evans et al., 2013), model results (J. Lelieveld et al., 2015), or a combination of the two, in part also including measurement station data (van Donkelaar et al., 2019), with different resolution (from 10 × 10 km [Evans et al., 2013] to ∼100 × 100 km [J. Lelieveld et al., 2013]). Although satellite remote sensing aerosol products are available at relatively fine spatial resolution, temporal coverage is not comprehensive (e.g., due to cloud presence and fixed time daily overpasses), and they need information from model simulations to estimate vertical concentration profiles to derive surface level exposure. On the other hand, model simulations, despite the coarser resolution, and provided that they reproduce the observations, allow studies on the attribution of pollution sources; these, therefore, have an important role by providing a basis for emission control strategies.
3.1. Exposure Response Function (ERF)
The attributable fraction (AF) can be estimated for each age class k and disease j as:
| (3) |
with RR j,k (X) being the relative risk (or hazard ratio) for the pollutant X. The ERF is a mathematical function that describes the relative risk dependency on the pollutant abundance (i.e., RR j,k (X)). The ERF is an important term in the mortality calculation, as it quantifies and associates the increase in pollution with the increase in relative risk for different age categories and disease. The ERF has been investigated in several studies based on a multitude of epidemiological studies that focused on long‐term exposure effects. As different naming conventions have been used over the years, here we applied the one used in the work of Ostro et al. (2004).
The initial efforts to develop an ERF based on epidemiological data were reported in Arden Pope (2002) and Ostro et al. (2004), where a log‐linear (LI) function was adopted:
where X is the concentration of the pollutants (PM2.5 or O3) and X 0 is the threshold where the pollutant is considered as not being harmful (i.e., RR j,k (X) = 1 for X < X 0). This threshold is also named Theoretical Minimum Risk Exposure Level (TMREL). The unknown parameters (α j,k and β j,k , where only the last one is effectively used) are estimated by regression methods based on a collection of epidemiological study data. This function is still widely used in estimates of health effects from pollutants.
Soon after a log‐log (LL) function was proposed as an ERF, to avoid unrealistically high values at high pollution concentrations (Cohen et al., 2005; Ostro et al., 2004):
A value of 1 was added to the X terms in the formula to ensure that the log function is defined at X = 0. Both the LI and the LL functions have been applied to different pollutants, such as ozone and PM2.5.
One major limitation of the aforementioned ERFs for PM2.5, has been the use of cohort studies limited to areas with relatively low concentrations (typically with an annual average below 35 μgm−3), which may not be representative for highly polluted regions, for example, in South and East Asia. To remedy this limitation, the Integrated Exposure‐Response (IER) function was developed for PM2.5 (R. T. Burnett et al., 2014), under the assumption that the toxicity is a function of mass concentration alone and that large intake over a short time period is equivalent to continuous inhalation for longer time periods, if the total dose remains the same. As cohort studies were not available at that time in low‐ and middle‐income countries or with high levels of ambient PM2.5 exposure, rather than extrapolating the risk at higher exposure ranges, the IER was constrained. This was done through the inclusion of studies performed at higher ranges of exposure, including those estimating RR due to household air pollution, passive smoking and active smoking along with the studies performed at lower ranges of ambient PM2.5 exposure in developed countries. IER is expressed as
with RR = 1 for X < X 0. The GBD updates the IER's parameters (α j,k , γ j,k and δ j,k ) on a yearly basis with inclusion of new epidemiological evidence. Furthermore also additional outcomes are added once scientific evidence is present: while the IER traditionally includes 5 diseases (stroke (CEV), ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), lung cancer (LC) and acute lower respiratory tract infections (ALRI)), in the 2017 update of GBD, new parameters were developed for type‐2 diabetes mellitus.
To relax some assumptions such as the inclusion of household air pollution in the development of the IER, R. Burnett et al. (2018) developed the Global Exposure Mortality Model (GEMM) by only including results from 15 studies that examined the relationship between long term exposure to ambient PM2.5 and excess mortality. A study on the association between ambient PM2.5 exposure and excess mortality among Chinese men was included as a part of these 15 studies. The average long term ambient PM2.5 exposure among the participants in the Chinese men study (P. Yin et al., 2017) was estimated to be 84 μgm−3, which was considered as a realistic high end of PM2.5 exposure. Along with the information from these 15 cohort studies, 26 cohort studies for which subject‐level information was not available were also included (R. Burnett & Cohen, 2020; R. Burnett et al., 2018). Furthermore, the GEMM was constructed for a broad group of mortality causes, incorporating all non‐communicable diseases plus lower respiratory tract infections (NCD + ALRI). The GEMM has the following form
with Z = max (0, X − 2.4), X being PM2.5 and with θ j,k , α j,k μ j,k and ν j,k as parameters to be fitted with epidemiological studies results. Furthermore, the GEMM was constrained for all‐natural cause mortality (non communicable diseases), albeit with large uncertainties at high pollutant concentrations. The large differences in the results between GEMM and the previous ERFs have been acknowledged (R. Burnett & Cohen, 2020), see also Section 4.1, and it is expected that future results of these methods will converge, either through inclusion of additional studies or by improving further the shape of the function, especially at lower‐level exposures.
Furthermore, the cohort of Chinese men (P. Yin et al., 2017) with exposures up to 84 μgm−3 skewed the GEMM curve, necessitating the inclusion of additional cohorts with higher levels of exposure (R. Burnett & Cohen, 2020). A new ERF, the MRBRT (meta‐regression–Bayesian regularized trimmed), which was introduced in the most recent GBD update (Murray et al., 2020) addresses this issue by including studies of household air pollution, second hand smoking as well as multiple studies performed at high ambient exposure settings (T. Li et al., 2018; Yang et al., 2018; Yusuf et al., 2020):
where X and X CF represent the range of exposure characterized by the effect size. In contrast to the IER, the MRBRT and does not include active smoking.
One additional constrain in all epidemiological studies is the fact that they provide changes in mortality on a unit change of pollutant. Vodonos et al. (2018) to better match the epidemiological data, suggested an LL ERF with a varying β j,k depending on pollution level. While in general the ERFs model the response (and the coefficients) on the RR scale, the coefficient estimation of Vodonos et al. (2018) for the LL model are hence on the derivative scale.
This was followed by R. T. Burnett et al. (2022), who suggested an additional ERF (so called Fusion), build also in the derivative space:
with θ based on the concentration distribution of the cohorts and γ, ρ, and μ being estimated.
All these parameters in the ERFs are in principle constrained based on existing epidemiological studies, and their accuracy and precision strongly depends on the availability of such information. Any additional accuracy and/or precision given by a more comprehensive function (with more parameters) may diminish when appropriate data is lacking (here epidemiological studies). The IER, GEMM, and MRBRT are only built and used for estimating the impacts of PM2.5 exposure, while the LL and LI are generally used for ozone. However, the most recent GBD used the MRBRT developed from five cohort studies for ozone.
3.2. Pollutants Associated With Mortality
3.2.1. PM2.5
PM2.5 are fine particles suspended in the air (called aerosols), less than a 30th of the diameter of a human hair, not visible by the naked eye. These particles can be emitted directly (primary PM) or may be formed in the atmosphere through chemical reactions (secondary PM). They can be emitted from both natural (e.g., aeolian dust, sea salt, volcanic eruptions) and human‐made sources (e.g., transportation, power plants, industrial activities). Although larger particles (PM10) can also be harmful to human health, PM2.5 penetrate deeper, reaching the alveolar sacs; these fine particles have relatively large surface area per volume and mass and can reach the vascular system directly having more far‐reaching health implications than PM10. Data on the effects of exposure to ultrafine particles, which may have additional health impacts through their ability to migrate into the blood stream, are scarce. A large body of literature over the course of the last two decades has established exposure to PM2.5 as a robust metric for excess mortality and morbidity from cardiopulmonary, cardiovascular, lung cancer and other diseases.
PM2.5 is reported in micrograms per cubic meter and can be measured with high confidence by well maintained and calibrated ground‐based measurement sites. However, monitoring density is very low in most low‐ and middle‐income countries, noting that about 60% of the countries, accounting for 18% of the global population, have no PM2.5 (air quality) monitoring at all (Martin et al., 2019; Shaddick et al., 2018). Therefore, alternative methods for PM2.5 exposure assessment have gained importance. Global atmospheric chemistry models (informed by emission inventories) validated against observations, can be used for assessing exposure to PM2.5 (Brauer et al., 2012, 2016; S. Chowdhury et al., 2020, 2022; J. Lelieveld et al., 2015, 2020; Lelieveld, Klingmüller, Pozzer, Burnett, et al., 2019) but are often hamstrung by the coarse horizontal resolution. Recent exposure assessment studies have applied advanced techniques to utilize the desirable temporal resolution from global atmospheric models, accuracy of ground based measurements and high spatial resolution of satellite retrievals to produce hybrid products of PM2.5 exposure (Shaddick et al., 2018; van Donkelaar et al., 2010, 2019). Such a hybrid model has been validated against available PM2.5 in‐situ observations (Hammer et al., 2020) for annual averages, obtaining a very high correlation (R 2 = 0.92) proving its value for obtaining pollutant exposure in areas where ground monitors are sparse or do not exist.
3.2.2. Ozone
Tropospheric ozone is photochemically formed in the presence of carbon monoxide, volatile organic compounds, nitrogen oxides, and sunlight. It has adverse impacts on respiratory health in human beings and negatively affects vegetation and crop productivity. The precursors of tropospheric ozone to a large degree come from anthropogenic sources such as transportation, power plants, industrial activities and burning of solid fuels in households. Tropospheric ozone is also a greenhouse gas that contributes to climate change. Until recently, atmospheric chemistry model results alone were used to estimate ozone exposure (S. Chowdhury et al., 2020; Malley et al., 2017). However, the recent GBD uses a hybrid approach to combine the ground‐based measurements from the Tropospheric Ozone Assessment Report (TOAR, Tarasick et al., 2019) with atmospheric model simulations to build a 0.1° × 0.1° resolution global data set (DeLang et al., 2021; Murray et al., 2020).
Tropospheric ozone is measured in mixing ratios units of nmol/mol (10−9 mol/mol), or, equivalently, parts per billion (ppb). In many publications the general term concentration is used, “not distinguish between mixing ratio metrics or true concentrations metrics such as μgm−3” (Lefohn et al., 2018), which is to be considered incorrect (Sander, 2000).
Unlike PM2.5 exposure, which is generally expressed in the form of annual population‐weighted means, exposure to ozone is represented by appropriate metrics as described in the TOAR (Lefohn et al., 2018). Until the GBD 2019 release (Murray et al., 2020), which adopted the MRBRT, all studies used the LI/LL function for estimating the RR from tropospheric ozone exposure; normally, the studies that used the coefficient (β) from Jerrett et al. (2009) applied the highest 3‐ or 6‐month averages of the daily maximum 1‐hr ozone concentration over a full year as the ozone exposure metric. The studies that used the more recent coefficients from Turner et al. (2016) adopted the annual average daily maximum 8‐hr ozone concentration for each grid cell, consistent with the analysis from which the relative risk estimates were derived from these two cohort studies. The most recent update of the GBD used “the highest 6‐month average of the daily maximum 8‐hr ozone concentration” (Murray et al., 2020), where mixing ratio is meant in place of concentration.
3.2.3. Counterfactual Level
All the ERFs presented above were fitted across the entire spectrum of exposure from the highest level to a level at which no excess risks from exposure to a pollutant are assumed (X 0). Since the GBD 2015 update (Cohen et al., 2005; Forouzanfar et al., 2015), X 0 for PM2.5 has ranged between 2.4 and 5.9 μgm−3. Over the years the value of X 0 for PM2.5 was reduced from 7.5 μgm−3 (Cohen et al., 2005), but still no firm threshold for PM2.5 has been identified below which no damage to health is observed (WHO, 2013). The most recent MRBRT is structured to allow its users to select an X 0 of 0 μgm−3. Recently, Weichenthal et al. (2022) investigated the health outcomes of air pollution in low exposure environments, revealing a much larger impact than previously estimated, with a possible addition of 1.5 million attributable deaths for PM2.5 below 5 μg/m3.
The X 0 for tropospheric ozone exposure ranged between 26.7 and 41.9 nmol/mol depending on the exposure metric applied and the ERF used. The recent GBD distributes X 0 between 29.1 and 35.7 ppb.
4. Results
4.1. Fine Particulate Matter
Figures 1 and 2, depict the global‐total excess deaths estimated in the studies tabulated in Table 1. The global estimates tend to cluster in two groups, one with excess deaths above 6 million/year, and another with excess deaths between 2 and 4 million/year. The lowest number of excess deaths estimated among the studies reviewed here is 0.8 million/year from Cohen et al. (2005), which was the first burden of disease study performed, and included the two end points‐cardiopulmonary diseases and lung cancer. This work included impacts of fine particulates only in large population centers, therefore leaving out most of world's population; furthermore, it used counterfactual of 7.5 and, due to the lack of studies at higher concentrations, the risk level was limited at PM2.5 equal to 50 μgr/m3. More recently, a comparably low value has been estimated by Silva, Adelman, et al. (2016), where only 1.7 million excess deaths were estimated for the year 2000. The highest number of excess deaths was estimated by Vohra et al. (2021) at 10.2 million/year, for all‐cause mortality, using a LI ERF built with coefficients obtained from a meta‐analysis that pools 53 studies examining the effects of chronic exposure to fossil fuel related PM2.5 on health. This comparatively high estimate can be attributed to (a) the use of LI ERF, the shape of which does not limit the magnitude of relative risk at higher concentrations, unlike in GEMM, IERs and MRBRTs, and (b) the estimation of excess deaths from all‐causes for the population above 14 years old. It must be stressed that the uncertainties associated with this estimate are very high, with a 95% confidence level between −47.1 and 17.0 million/year, only associated with the AF uncertainties. This will be discussed in Section 4.3.
Figure 1.

Graphical representation of estimates of global excess mortality attributable to atmospheric fine particulate (PM2.5) air pollution. The symbols show the average estimates, while error bars represent the 95% confidence levels. The color code denotes the ERF used in the study (yellow: LI, green: IER, orange: GEMM, blue: MRBRT, gray: LI with β from Vodonos et al. [2018], red: FUSION). The dashed lines represent calculations for all‐cause mortality. The circles are estimates from the GBD. The x‐axis indicates the publication year. Data are from Table 1.
Figure 2.

Colors and symbols as Figure 1. The x‐axis indicates the years for which the estimates were derived.
The choice of ERF (color‐coded in Figure 1), and the number of the health outcomes investigated are the most significant factors resulting in the large disparities among studies, with the GEMM being used in the studies which estimated an excess mortality higher than 8 million/year for all NCD plus ALRI, while LI, LL, and IER present similar results over the years. Interestingly, R. Burnett et al. (2018), using the GEMM function and the same population and BMR distribution, estimated higher numbers for NCD plus ALRI (8.9 million/year), compared to sum of LC, IHD, CEV, COPD, and ALRI (6.9 million/year), suggesting that PM2.5 may increase the risk of deaths from additional causes beyond these 5. Nevertheless, even including only the LC, IHD, CEV, COPD and ALRI as cause of death, the GEMM function gives higher results than similar work with other ERFs. This was already pointed out in the work of R. T. Burnett et al. (2022), who suggested that the main difference is due to the inclusion of cohort studies of second‐hand smoke and household pollution with lower hazard ratio estimates compared to those obtained from cohort studies of outdoor air pollution.
In addition, not only the ERF function but also its shape can significantly change the estimates. In fact, as shown by Weichenthal et al. (2022), most of the world population is exposed to relatively low level of pollution, so that the concavity of the ERF in this range can drastically change the global estimates.
We anticipate a slightly smaller, though not inconsequential, effect of other factors (i.e., source of PM2.5 data, population data, baseline death rates, choice of counterfactual concentration and study year) on the estimated total excess deaths (see Equation 2). To investigate this, we redraw Figure 1 by the study year for which the excess deaths were estimated (Figure 2). The estimates based on the GEMM and the work of Vodonos et al. (2018) present larger differences than the one using LI, LL, IER and MR‐BRT, independently of the estimated year. This indicates the largest influence of the ERF selection compared to the other input data on the overall results, as already noticed before. Nevertheless, once the ERF is fixed, other factors do influence the results significantly, with noticeable differences between the estimates. For example, the GBD studies (Anenberg et al., 2010; Cohen et al., 2017; Forouzanfar et al., 2015; Lim et al., 2012; Murray et al., 2020; Stanaway et al., 2018) and the study of S. Chowdhury et al. (2020), that estimated the excess mortality for two study years using similar data sources for all factors, found a considerable impact of epidemiological and demographic transitions between the two study years on changes in excess mortality. Similar to our results, Ostro et al. (2018) found that the GBD estimates between 2013 and 2015 increased by 23% by updating the ERF coefficients of the IER function alone, and by only 8% by updating the exposure data (i.e., population and pollutants distribution); the combination of both changes increased the estimates by 35%.
Furthermore, the source and spatial resolution of PM2.5 exposure data can result in minor disparities among the estimates, although (Kushta et al., 2018) found only a minor impact of changes in horizontal resolution of exposure data on excess deaths compared to the large influence of the choice of ERF and health endpoints investigated (S. Chowdhury et al., 2020, 2022).
Finally, the large majority of studies used the same population data, and, it can be assumed that differences is such data are generally modest although they might vary between different versions of the data set (GPW3, 2005; GPW4, 2016). A similar assumption can be made for the BMR, where data from the same source (GBD) have been used for all studies since 2010 (Forouzanfar et al., 2015, 2016; Lozano et al., 2012; Murray et al., 2020; Stanaway et al., 2018). While these data can be considered of the highest available quality, this approach limits the differences among the various estimates.
4.2. Ozone
The global estimates of excess mortality due to long term ozone exposure are reported in Table 2 and presented graphically in Figure 3. The largest value estimated is about 1.3 million/year (S. Chowdhury et al., 2020) for excess deaths from all respiratory diseases, while the minimum estimated is 142 thousand/year from COPD (J. Lelieveld et al., 2015). The estimates presented show that the PM2.5 exposure causes roughly 12 ± 8 times higher excess mortality than that due to ozone exposure, that is, fine particulate related mortality is roughly an order of magnitude larger than from ozone. Nevertheless, this ratio varies widely, ranging from 2.7 (J. Lelieveld et al., 2013) to 30.0 (J. Lelieveld et al., 2020). Importantly, in the global estimates presented, the excess mortality is calculated either for only one disease (COPD) or for all respiratory diseases, with a factor of 3 difference between the two approaches, even with coefficients derived from similar cohort studies, pollution levels and population distribution. In Figure 3, the estimates are graphically represented, with causes of death depicted in different colors.
Figure 3.

Graphical representation of estimates of global excess mortality attributable to ozone (O3) pollution. The symbols are the average estimates, while error bars represent the 95% confidence level. The color code denotes the cause of death used in the study (yellow: all respiratory diseases, green: COPD). All studies used a LI ERF, with the exception of the dashed ones, which adopted an LL ERF. The circles are estimates from the GBD. The x‐axis indicates the publication year. Data are from Table 2.
In contrast to PM2.5 the estimates of excess mortality are less variable with time, with a general consensus of ∼350 (CL 95%:150–550) thousands excess deaths/year for COPD as a cause of death.
4.3. Uncertainties
In the studies considered, uncertainties of mortality estimates are typically represented by 95% confidence levels. To analyze the sources of errors associated with Equation 2, the confidence levels have been converted to normalized standard error (NSE, i.e., standard error divided by the estimated value, also known as relative standard deviation), to obtain a uniform metric for uncertainty comparison, by assuming a normal distribution of the uncertainties. Following Equation 2, the uncertainties associated with excess mortality arise from the multiplication terms, that is, the attributable fraction (AF j,k (X (x, y))), the baseline mortality (BMR j,k ), and the population distribution (POP k (x, y)).
As the population data is also used to globally distribute the baseline mortality rates, it is essential to have a reliable population spatial distribution map. Most of the studies use similar data for population from the Gridded Population of the World data set (GPW3, 2005; GPW4, 2016), regardless of the availability of various gridded population datasets (Leyk et al., 2019), even at higher spatial resolutions. These gridded population datasets strongly differ in the methodology used for population distribution, such as areal weighting method, dasymetric mapping, statistical modeling, or a hybrid approach (Leyk et al., 2019). GPW, different to other global gridded datasets, does not use any modeling for population distribution, and it assumes a uniform distribution in the target areas. This approach, although computationally very efficient, means that this data set is suited for policy‐making efforts that do not require fine spatial resolution (Doxsey‐Whitfield et al., 2015). It has been shown, in fact, that on a local level, the GPW is associated with discrepancies compared to other (probably more accurate) datasets of regional/local population distribution (e.g., Bai et al., 2018; X. Yin et al., 2021). It is difficult to assign an uncertainty to the population distribution and size, as this depends on the errors in the original (census) data, areal aggregation and the ancillary data used for the distribution (Leyk et al., 2019). Furthermore, as GPW does not adopt any statistical method to distribute the population, traditional metrics such as standard deviations or standard errors are not available. We therefore adopted as an indication of the uncertainties, the confidence level range that the GBD provides for its population data at country levels. Although this does not represent the error of the GPW data itself (and the uncertainties in the population distribution), it can be assumed to be of the same magnitude. The GBD, based on more than 1200 censuses and 700 population registries, estimated a maximum NSE of ∼15% for Afghanistan, a value of below 1% for many countries, and an average NSE of ∼4% (at country level).
Similar to the population, the baseline mortality rate is subject to statistical and methodological uncertainties. The numbers of deaths and causes of mortality are available at global scale on country level from the Global Health Estimates (GHE) of the World Health Organization (WHO), which are used to produce the GBD estimates. The WHO relies on information provided by member states, using vital registration data contained in the International Classification of Disease (ICD) (Roth et al., 2020). Also here the uncertainties are difficult to assess, as they mostly depend on data source reliability which in turn is influenced, for example, by the attribution of causes of death and practices in the reporting countries, which may contain large uncertainties, especially in low and middle income countries. GHE also assesses the data quality of the mortality data set (Mathers, 2005) and 95%CL for the country mortality rate is available for the GBD. As expected, the NSE depends on the country, age group, and burden of disease from different health outcomes. Based on the health outcomes included in the global estimates, the maximum NSE (at country level and for all age classes) for the BMR are 72%, 42%, 40%, 186%, 55%, and 36% for LC, IHD, CEV, COPD, ALRI and NCD, respectively. The lowest NSE is below 2% for all of the health outcomes listed. On average (at country level and all age classes), NSE values of 13%, 10%, 12%, 20%, 14%, and 6% have been calculated for LC, IHD, CEV, COPD, ALRI, and NCD, respectively. The uncertainties at country levels for the BMRs are therefore somewhat larger than for the population, although this varies for different countries. Another source of uncertainties for BMR is its spatial distribution: as BMR is produced by dividing the mortality at the country level (cause by specific diseases and for a specific age class) with the total population of the same country (i.e., BMR j,k = Mort j,k /Pop j,k ), errors in population estimation also directly affect the BMR. Similarly, as the BMR is constant at country level, its distribution does not reflect socio‐economic differences between rural and urban population, which could be significant (Brochu et al., 2011; Zimmer & Kwong, 2004).
As the final term in Equation 2, the attributable fraction is affected by two fundamental uncertainties. The first one is associated with the pollutant distribution X (x, y), while the second relates to the statistical uncertainties in the formulation of the ERF. It is usually assumed that the pollutants' distributions contain no errors, as they are empirically based on observations, although this is not correct, because observations are subject to measurement errors. Furthermore, global data set of pollutants are to some degree augmented by numerical modeling which intrinsically brings additional uncertainties in the estimation. Uncertainties in X (x, y) could also arise through an overly coarse spatial resolution of the data set to effectively resolve pollutants' spatio‐temporal gradients, resulting in an underestimation of the effective pollutant concentration. Here we note that secondary pollutants that do not have a very short lifetime, that is, most of PM2.5 and O 3, do not exhibit a strong spatial gradients as they are mixed during formation in the atmosphere, while short‐lived pollutants (with lifetime in order of hours) such as NO2 (Liu et al., 2016) are subject to strong gradients that need to be represented at relatively high resolution. Furthermore, the process used for deriving X (x, y) could be inadequate for certain locations or areas, or the original data set could simply lack the necessary information (e.g., satellite observations masked by cloud presence, lack of in‐situ measurements). Each pollutant's distribution data set has therefore a unique fingerprint, and the error evaluation requires tedious and hard to reproduce evaluation work. A lower limit for the NSE for this parameter can be taken from the work of van Donkelaar et al. (2021), who suggested an NSE in the range of ∼3% at the global level, but with higher values at regional scales (up to 32% in the southern Sub‐Saharan Africa region). The expected NSE for data from the global circulation model alone, however, should be higher and at least of the order of 10% (Crippa et al., 2019; Pozzer et al., 2021).
Finally, the uncertainties from the formulation of ERFs must be taken into account. These uncertainties are associated with (a) the number of cohort studies used as well as the functional shape assumed, (b) the use of cohort studies only from specific regions (namely U.S./Europe/China), and (c) the different aerosol properties when only the PM2.5 metric is used (e.g., composition and distribution, see Sections 5.1 and 5.2). The ERFs also intrinsically include the uncertainties in the low concentration threshold, below which no effect from pollution is expected, as these thresholds are mathematically derived once the ERFs are estimated (i.e., pollutant concentration that gives hazard ratios equal or below 1). Due to their mathematical nature, all the ERFs in the literature present a confidence level interval from which the NSE can be calculated. In addition, the NSE depends on the pollutant level and increases at higher concentrations. The reason is the lack of cohort studies at high concentration levels, which does not constrain the ERF. To estimate the uncertainties associated with the AF coefficient, the NSE values have been estimated at 32 μgm−3 for PM2.5 and 63.4 ppb for O3, representing the population weighted annual average exposure for these pollutants for the year 2016 (Brauer et al., 2016); these values have been corroborated by more recent analysis for fine particulate matter (van Donkelaar et al., 2021). Here, we focus on the NSE for the GEMM formulation, as it applies the largest number of cohort studies, therefore representing a lower limit for the NSE. The GEMM AF presents a maximum NSE between all age classes of 7%, 4%, 9%, 8%, 18%, and 3% for LC, IHD, CEV, COPD, ALRI and NCD, respectively. For ozone, the LI ERF with a coefficient from Cohen et al. (2017) has an NSE of 41% for COPD. It must however be stressed that, in contrast to the GEMM and IER, the NSE of the AF associated to LI and LL decreases with increasing pollutant concentrations. This is due to the particular construction of the LI function, where the NSE grows at a slower pace than the average estimates. Of particular interest are the uncertainties associated with the recent “Penalized spline mode” estimation (Vodonos et al., 2018): this estimation of the RR derivative presents low uncertainties at 15 μgm−3 (with an NSE of ∼3%), but much larger NSE values for higher concentrations (with NSE higher than 100%), reflecting also the large uncertainties associated with the lack of cohort studies at PM2.5 concentrations above 40 μgm−3. These uncertainties are reflected in the large NSE value (about 160%) in the global estimates obtained by Vohra et al. (2021), where this ERF was adopted even though no other source of uncertainties was included in the confidence level assessment. The very large statistical uncertainties for this ERF (Vodonos et al., 2018) are biologically implausible as they imply a possible beneficial effect of air pollution at high concentrations.
In summary, the largest uncertainties arise from the AF (combination of ERF and pollutant concentration uncertainties) and the BMR, both of which have NSEs in the range of ∼10% at the global scale and potentially higher in some regions, especially those where epidemiological studies are lacking, while the population data have significantly lower uncertainties.
Not all studies published so far have explicitly stated the different sources of uncertainties. In most cases it is somewhat unclear how these have been estimated which could be by direct estimation or via Monte‐Carlo approach. A direct estimation of the errors via propagation of uncertainties is challenging from the mathematical point of view: as most of the datasets used tend to correlate with each other and are thus not statistically independent (e.g., population density is correlated with pollution concentration), the resulting uncertainty propagation function contains also non‐negligible second‐order terms.
In Figure 4, the NSE for the different studies is presented. Despite the growing number of epidemiological studies, as well as improvements in availability of the ancillary data, in the last decades the reported relative uncertainties have been more or less constant or have even slightly increased, with the GBD reports systematically suggesting a NSE of at least ∼8%, and with all other studies indicating higher uncertainty. These reported uncertainties are often independent from the health outcome investigated, as NCD estimates present similar NSE as the estimates based on the sum of the typical five disease categories (R. Burnett et al., 2018).
Figure 4.

Normalized Standard Error (NSE) in (%) for PM2.5 mortality estimated by the studies included in this review. The x‐axis shows the publication year. Colors and symbols are the same as in Figure 1.
In the last decades, tremendous progress has been made in quantifying the relationship between air pollution and the associated health impacts. The knowledge in the global estimates has been improving largely by including an increasing number of different health outcomes as well as improved estimations of pollution levels around the world. Nevertheless, the precision of the estimations has not improved significantly, with a relative uncertainty which is at least of 10% and without any substantial reduction in the last decades.
4.4. Source Apportionment
An accurate and precise estimation of the health burden due to outdoor air pollution is essential in the context of policy making, as it can motivate and evaluate environmental policies. To further optimize the regulation and aim at the most important pollutants, it is important also to know the sources of atmospheric pollution, that is, to know which and how each anthropogenic activity (or natural emissions) contributes to air pollution.
Source apportionment is a technique that informs about the source sectors (e.g., transportation, industry, residential, natural) that contribute toward a pollutant of interest. Source apportionment techniques help to explicitly identify region specific sources of pollution and also help in designing region specific action plans and assessing effectiveness of abatement measures. There are two methods (Hopke et al., 2020; J. Lelieveld et al., 2015; Thunis et al., 2019) for assessing sources of air pollutants: (a) top‐down techniques that use in‐situ measurement data and trace the share of sources contributing at a measurement site, and (b) bottom‐up techniques that associate specific activity data with emission estimates and then develop and use chemical transport models or chemistry general circulation models to arrive at concentrations. These methods, though distinct, when operated in conjunction can serve as an important tool for identifying major sources of PM2.5 and thus support air quality management decisions. Both of these approaches help in reconstructing the atmospheric concentration of pollutants associated with the different emission sources. However, while top‐down techniques can only inform about sources of a pollutant of interest at a single point in space at a given time, bottom‐up studies can inform about sources by broader geography and at multiple points in space at a given time.
Table 3 lists the five global studies that associated the exposure to ambient PM2.5 and related excess deaths to anthropogenic and natural sources (S. Chowdhury et al., 2022; J. Lelieveld et al., 2015; J. Lelieveld, Klingmüller, Pozzer, Burnett, et al., 2019; McDuffie et al., 2021; Weagle et al., 2018). Studies that attribute only anthropogenic PM2.5 without considering the natural sources (Crippa et al., 2019; Silva, Adelman, et al., 2016; Vohra et al., 2021) were excluded from the table. Anthropogenic sources include contributions from activities such as the burning of fossil and bio fuels, waste incineration and agricultural activities, while natural sources includes for example, biomass burning (wildfires), natural vegetation emissions, as well as dust and sea salt particles. These five global studies report 62%–79% and 21%–38% of the total ambient PM2.5 exposure to be of anthropogenic and natural origin, respectively. Such large range in the reported contributions may be associated with the input data set, emission inventories and model configuration used in these studies. It should be noted that these studies generally apportion the exposure to multiple anthropogenic sources (industry, residential, power generation, agriculture, waste incineration, transportation, shipping and other minor sources) and natural sources (desert dust, sea salt), however to simplify the representation in this review, we lump the anthropogenic and natural sources reported in these studies. The global studies also inform about the sources by countries and regions, however, well designed regional source apportionment studies (Reddington et al., 2019; Thunis et al., 2018; Upadhyay et al., 2018) are better adept for such information.
Table 3.
Global Source Apportionment Studies
| Study | Study characteristics | Source contribution | |||
|---|---|---|---|---|---|
| Year reported | Model or observation | Emission inventory | Anthropogenic sources | Natural and other sources | |
| J. Lelieveld et al. (2015) | 2010 | EMAC (Jöckel et al., 2010) | EDGAR (Centre et al., 2010) | 77 | 23 |
| Weagle et al. (2018) | 2014 | GEOS‐Chem (Bey et al., 2001), SPARTAN (Snider et al., 2016) | EDGAR (Crippa et al., 2018), MIX (M. Li et al., 2017) | 71 | 29 |
| J. Lelieveld, Klingmüller, Pozzer, Burnett, et al. (2019) | 2015 | EMAC (Jöckel et al., 2010) | EDGAR (Crippa et al., 2018) | 63 | 37 |
| McDuffie et al. (2021) | 2019 | GEOS‐Chem (Bey et al., 2001) | CEDS (McDuffie et al., 2020) | 79 | 21 |
| S. Chowdhury et al. (2022) | 2015 | EMAC (Jöckel et al., 2010) | CEDS (Hoesly et al., 2018) | 62 (73) a | 38 (27) a |
The numbers in parenthesis indicate the source contributions considering anthropogenic organic aerosols to be twice more toxic compared to other aerosols.
5. Outlook
The uncertainties thus far discussed are the so‐called “statistical or aleatoric uncertainties,” which are derived from the uncertainties associated with the original data and/or mathematical elaboration of such data, and define the precision of the mortality estimates. An additional source of uncertainty should be acknowledged, that is, the “systematic or epistemic uncertainties,” which represent the accuracy of the estimates. These uncertainties arise from our incomplete understanding of the processes involved and the consequent potential misrepresentation in mortality estimates. For example, additional health outcomes are included in the estimates when enough epidemiological studies are available to quantify their relationship with air pollution, hence increasing our accuracy. Epidemiological studies are fundamental for estimating exposure‐response functions, but conversely are the main cause of uncertainty in global estimates of mortality attributable to outdoor pollution. The impact at lowest/highest extremes of PM2.5 concentration distribution remains largely uncertain, either by the strong sensitivity (at low concentrations) or by lack of data (at high concentrations).
Nevertheless, cohort studies rely on the data available from observations, which may only partially characterize the pollutants (especially true for the complex aerosol phase). In this context, we point to possible future research directions and the different methodologies that can be used to pursue links between aerosol components and health outcomes, including sophisticated machine learning techniques, which could possibly help to achieve new insights and to significantly reduce the uncertainties associated with mortality estimates (Hüllermeier & Waegeman, 2021).
5.1. Aerosol Particle Distribution
Particulate matter in the atmosphere is composed of aerosols of different sizes, ranging from a few nanometers to many micrometers in diameter, of which only the smaller ones are considered relevant for health. The aerosol distribution is therefore not only described by the aerosol composition, but also by the number of particles found at a certain size (i.e., aerosol diameter). In Figure 5, two aerosol size distributions, with the same particle composition and density, are presented. Although both have the same total mass of PM2.5, their distribution is rather different. In one case (left), the aerosol distribution reveals a large number of particles of small sizes, while in the other case (right), less particles are present but with larger sizes, more typical for a physico‐chemically “aged” aerosol. Both distributions show the same PM2.5 mass of 32 μgm−3 and therefore would have the same estimated health impact according to common practice. Nevertheless, it has been shown that smaller particles have a greater potential to penetrate deeply into the lungs (Hong & Jee, 2020; Schraufnagel, 2020; Schraufnagel et al., 2019), potentially having larger health effects. This has been investigated in detail by the Health Effects Institute Review Panel on Ultrafine Particles, which concluded (on Ultrafine Particles, 2013) that, for the time being, compelling evidence that ultrafine particle (UFP) exposure (i.e., exposure to particle with an aerodynamic diameter below 0.1 μm) can account in substantial ways to the adverse effects that have been associated with other ambient pollutants such as PM2.5 is lacking. Nevertheless, in the same study it is mentioned that health effects of UFP cannot be ruled out, due “to underlying deficiencies in exposure data, to numerous challenges in comparing and synthesizing results of existing studies, and to the inherent complexity of the task that scientists have set out to accomplish.” The importance of the aerosol size on health impacts should therefore be investigated in greater detail in epidemiological studies, which also pose the challenge of the development a ultrafine particle exposure assessment across larger areas as well as detailed multipollutant concentration exposure to evaluate independent effects.
Figure 5.
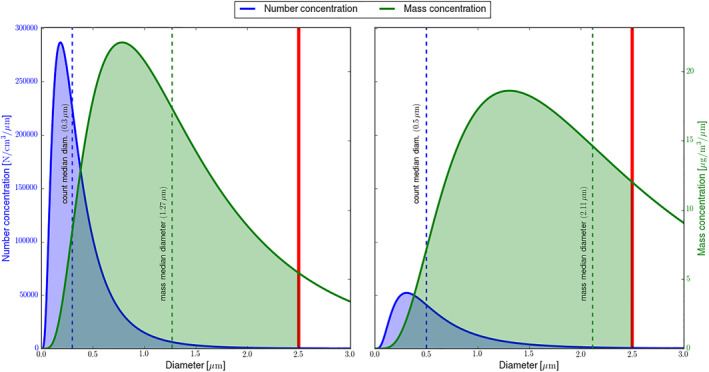
Two aerosol distributions with the same PM2.5 mass. The blue curve represents the number density of an aerosol distribution, the green curve represents the mass density of the distribution in the same panel. The PM2.5 is estimated as the integral of the green curve between zero and the 2.5 μm, the last limit depicted with the red line. The median for the number and mass are also presented. Please note the different vertical axis for the green and blue curves. The aerosol density is identical in both distributions. The total mass of PM2.5 is 32 μgm−3, equivalent to the global population weighted annual average exposure for the year 2016 (Brauer et al., 2016; van Donkelaar et al., 2021).
A possible outcome is to augment the exposure‐response function with the aerosol size, or, alternatively, the aerosol number concentration, which is dominated by the smaller particles, resulting in a 2‐variable function. Input data of fine particulates would consequently need to be extended, by including information on the aerosol size distribution.
5.2. Aerosol Composition
Although the exposure‐response functions consider PM2.5 mass, its composition can vary greatly between different regions (S. Chowdhury et al., 2022; Weagle et al., 2018). It is important, therefore, to investigate the impact of different substances present in aerosols on human health, Evidence suggests that anthropogenic secondary aerosols (such as sulfates and nitrates), black carbon and primary organic aerosols, are main causes of respiratory and cardiovascular diseases, due to the induced oxidative stress and inflammatory response (Bates et al., 2019; S. Chowdhury et al., 2022; Daellenbach et al., 2020; Huang et al., 2012; Lippmann et al., 2013; Niranjan & Thakur, 2017; Park et al., 2018; Weichenthal et al., 2016). However, no definitive conclusions have thus far been reached, and more research in this direction is indeed needed to clarify the health burden of aerosol components as advocated already in many publications (Kelly & Fussell, 2012; J. Lelieveld et al., 2015; Rohr & Wyzga, 2012).
On the other hand, the ability of PM2.5 to generate Reactive Oxygen Species (ROS) generation in epithelial fluid has been proposed as a metric to calculate the toxicity of PM2.5 particles (Crobeddu et al., 2017; WHO, 2007). Notable progress has been made in the last years in connecting fine particulate composition to ROS (T. Fang et al., 2019; S. Lelieveld et al., 2021), based on mechanistic approaches. If a clear link between health status and ROS could be established (Auten & Davis, 2009; Shields et al., 2021), then the impact of different aerosol components could be assessed, as it is possible to correlate the different components with ROS production (P. H. Chowdhury et al., 2019; Zhou et al., 2019) in the human body. This seems to be a promising way forward, and may clarify the processes that lead pollution to have adverse health effects, improving our understanding. Similarly to the particle‐distribution issue, to have a global estimates based on differential toxicity for different aerosol components requires the development of a global exposure distribution for each of them, which is challenging and associated with large uncertainties. Therefore, any progress in this direction would not directly translate in an improvement of the precision in the global estimates.
It must be stressed that a possible link between fine particulate composition and health outcomes is very important for supporting policy makers decisions. In fact, Pai et al. (2022) showed that most countries are not able to meet the new WHO air quality guidelines for PM2.5 of 5 μgm−3 even without any anthropogenic emissions. It is therefore important to know what is the realistic exposure reduction scenario with the largest health advantage.
6. Conclusions
In this work, we reviewed and compared the global estimates of excess mortality attributable to outdoor air pollution in the recent literature (past two decades). The methodologies adopted by different studies have been explained, and we have described the state‐of‐the‐art in excess mortality estimates, based on a combination of recent developments in epidemiology and atmospheric chemistry. It has been shown that the mortality attributable to PM2.5 is generally estimated to be about a factor of 10 higher than that attributable to O3.
The comparison between the different estimates reveals that the formulation of exposure response functions is responsible for the main differences between recent studies, followed by the type and number of health outcomes that are included in the calculations.
We showed that in the last decades a large number of publications considerably improved our understanding or the air pollution‐health link, therefore strongly increasing the accuracy of our global estimates. Nevertheless, the precision associated with global estimates of excess mortality attributable to air pollution has not improved, amounting to at least 10% of the estimated mean values. The GBD reports, in particular, can be considered to present the up to date estimations, due to their regular improvements in all the data needed for the estimates.
Potential research directions which could improve our understanding of the relationship between fine particulate matter and human health include improved formulation of the ERF based not only on PM2.5 mass, but rather on size distribution, as well a possible mechanistic link of air pollution composition to ROS in the epithelial fluid. This would require more sophisticated measurement networks, where not only the gravimetric mass of the fine particulate matter is measured but also its composition, the size distribution, and number concentration. The detailed measurements thus obtained could then be used in epidemiological studies to further improve the ERFs. An obvious final recommendation is to improve and extend measurement networks and perform dedicated epidemiological studies in low‐ and middle‐income countries, which have so far been inadequately represented in global burden of disease studies.
Finally, despite the methodological differences present in the literature and here investigated, all studies point to a very large health burden from ambient air pollution, which remains to be one of the most important causes of death worldwide.
Conflict of Interest
One of the co‐author is a member of the editorial board of GeoHealth. The other authors declare no conflicts of interest relevant to this study.
Acknowledgments
The authors acknowledge the contributions of Rick Burnett, Aaron Cohen, Matthias Kohl and Sara Bacer in the manuscript preparation. SD acknowledges support from IIT Delhi for the Institute Chair Fellowship and from SERB, Government of India under SUPRA scheme (SPR/2020/000212). SCA acknowledges support from NASA (Grant 80NSSC21K0511). Open Access funding enabled and organized by Projekt DEAL.
Pozzer, A. , Anenberg, S. C. , Dey, S. , Haines, A. , Lelieveld, J. , & Chowdhury, S. (2023). Mortality attributable to ambient air pollution: A review of global estimates. GeoHealth, 7, e2022GH000711. 10.1029/2022GH000711
Data Availability Statement
Only published data was used in this work. Reference to each data can be found in Tables 1, 2, 3.
References
- Anenberg, S. C. , Henze, D. K. , Tinney, V. , Kinney, P. L. , Raich, W. , Fann, N. , et al. (2018). Estimates of the global burden of ambient PM2.5, ozone, and NO2 on asthma incidence and emergency room visits. Environmental Health Perspectives, 126(10), 107004. 10.1289/ehp3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anenberg, S. C. , Horowitz, L. W. , Tong, D. Q. , & West, J. J. (2010). An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environmental Health Perspectives, 118(9), 1189–1195. 10.1289/ehp.0901220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte, J. S. , Marshall, J. D. , Cohen, A. J. , & Brauer, M. (2015). Addressing global mortality from ambient PM2.5 . Environmental Science & Technology, 49(13), 8057–8066. 10.1021/acs.est.5b01236 [DOI] [PubMed] [Google Scholar]
- Arden Pope, III, C. (2002). Lung cancer, cardiopulmonary mortality, and long‐term exposure to fine particulate air pollution. JAMA, 287(9), 1132–1141. 10.1001/jama.287.9.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten, R. L. , & Davis, J. M. (2009). Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatric Research, 66(2), 121–127. 10.1203/pdr.0b013e3181a9eafb [DOI] [PubMed] [Google Scholar]
- Bai, Z. , Wang, J. , Wang, M. , Gao, M. , & Sun, J. (2018). Accuracy assessment of multi‐source gridded population distribution datasets in China. Sustainability, 10(5), 1363. 10.3390/su10051363 [DOI] [Google Scholar]
- Balakrishnan, K. , Dey, S. , Gupta, T. , Dhaliwal, R. S. , Brauer, M. , Cohen, A. J. , et al. (2019). The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The Global Burden of Disease Study 2017. The Lancet Planetary Health, 3(1), e26–e39. 10.1016/S2542-5196(18)30261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan, K. , Ghosh, S. , Thangavel, G. , Sambandam, S. , Mukhopadhyay, K. , Puttaswamy, N. , et al. (2018). Exposures to fine particulate matter (PM2.5) and birthweight in a rural‐urban, mother‐child cohort in Tamil Nadu, India. Environmental Research, 161, 524–531. 10.1016/j.envres.2017.11.050 [DOI] [PubMed] [Google Scholar]
- Bates, J. , Fang, T. , Verma, V. , Zeng, L. , Weber, R. J. , Tolbert, P. E. , et al. (2019). Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environmental Science & Technology, 53(8), 4003–4019. 10.1021/acs.est.8b03430 [DOI] [PubMed] [Google Scholar]
- Beverland, I. J. , Cohen, G. R. , Heal, M. R. , Carder, M. , Yap, C. , Robertson, C. , et al. (2012). A comparison of short‐term and long‐term air pollution exposure associations with mortality in two cohorts in Scotland. Environmental Health Perspectives, 120(9), 1280–1285. 10.1289/ehp.1104509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey, I. , Jacob, D. J. , Yantosca, R. M. , Logan, J. A. , Field, B. D. , Fiore, A. M. , et al. (2001). Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation. Journal of Geophysical Research, 106(D19), 23073–23095. 10.1029/2001JD000807 [DOI] [Google Scholar]
- Boogaard, H. , Walker, K. , & Cohen, A. J. (2019). Air pollution: The emergence of a major global health risk factor. International Health, 11(6), 417–421. 10.1093/inthealth/ihz078 [DOI] [PubMed] [Google Scholar]
- Brauer, M. , Amann, M. , Burnett, R. T. , Cohen, A. , Dentener, F. , Ezzati, M. , et al. (2012). Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environmental Science & Technology, 46(2), 652–660. 10.1021/es2025752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer, M. , Freedman, G. , Frostad, J. , Van Donkelaar, A. , Martin, R. V. , Dentener, F. , et al. (2016). Ambient air pollution exposure estimation for the global burden of disease 2013. Environmental Science & Technology, 50(1), 79–88. 10.1021/acs.est.5b03709 [DOI] [PubMed] [Google Scholar]
- Brochu, P. J. , Yanosky, J. D. , Paciorek, C. J. , Schwartz, J. , Chen, J. T. , Herrick, R. F. , & Suh, H. H. (2011). Particulate air pollution and socioeconomic position in rural and urban areas of the Northeastern United States. American Journal of Public Health, 101(S1), S224–S230. 10.2105/ajph.2011.300232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, R. , Chen, H. , Szyszkowicz, M. , Fann, N. , Hubbell, B. , Pope, C. A. , et al. (2018). Global estimates of mortality associated with long‐term exposure to outdoor fine particulate matter. Proceedings of the National Academy of Sciences of the United States of America, 115(38), 9592–9597. 10.1073/pnas.1803222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, R. , & Cohen, A. (2020). Relative risk functions for estimating excess mortality attributable to outdoor PM2.5 air pollution: Evolution and state‐of‐the‐art. Atmosphere, 11(6), 589. 10.3390/atmos11060589 [DOI] [Google Scholar]
- Burnett, R. T. , Pope, C. A., III , Ezzati, M. , Olives, C. , Lim, S. S. , Mehta, S. , et al. (2014). An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environmental Health Perspectives, 122(4), 397–403. 10.1289/ehp.1307049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, R. T. , Spadaro, J. V. , Garcia, G. R. , & Pope, C. A. (2022). Designing health impact functions to assess marginal changes in outdoor fine particulate matter. Environmental Research, 204, 112245. 10.1016/j.envres.2021.112245 [DOI] [PubMed] [Google Scholar]
- Butt, E. W. , Turnock, S. T. , Rigby, R. , Reddington, C. L. , Yoshioka, M. , Johnson, J. S. , et al. (2017). Global and regional trends in particulate air pollution and attributable health burden over the past 50 years. Environmental Research Letters, 12(10), 104017. 10.1088/1748-9326/aa87be [DOI] [Google Scholar]
- Centre, J. R. , for Environment, I. , Sustainability, Aardenne, J. , Dentener, F. , & Van Dingenen, R. (2010). Climate and air quality impacts of combined climate change and air pollution policy scenarios. Publications Office. 10.2788/33719 [DOI] [Google Scholar]
- Chowdhury, P. H. , He, Q. , Carmieli, R. , Li, C. , Rudich, Y. , & Pardo, M. (2019). Connecting the oxidative potential of secondary organic aerosols with reactive oxygen species in exposed lung cells. Environmental Science & Technology, 53(23), 13949–13958. 10.1021/acs.est.9b04449 [DOI] [PubMed] [Google Scholar]
- Chowdhury, S. , Dey, S. , & Smith, K. R. (2018). Ambient PM2.5 exposure and expected premature mortality to 2100 in India under climate change scenarios. Nature Communications, 9(318), 1–10. 10.1038/s41467-017-02755-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, S. , Haines, A. , Klingmüller, K. , Kumar, V. , Pozzer, A. , Venkataraman, C. , et al. (2021). Global and national assessment of the incidence of asthma in children and adolescents from major sources of ambient NO2 . Environmental Research Letters, 16(3), 035020. 10.1088/1748-9326/abe909 [DOI] [Google Scholar]
- Chowdhury, S. , Pozzer, A. , Dey, S. , Klingmueller, K. , & Lelieveld, J. (2020). Changing risk factors that contribute to premature mortality from ambient air pollution between 2000 and 2015. Environmental Research Letters, 15(7), 074010. 10.1088/1748-9326/ab8334 [DOI] [Google Scholar]
- Chowdhury, S. , Pozzer, A. , Haines, A. , Klingmüller, K. , Münzel, T. , Paasonen, P. , et al. (2022). Global health burden of ambient PM2.5 and the contribution of anthropogenic black carbon and organic aerosols. Environment International, 159, 107020. 10.1016/j.envint.2021.107020 [DOI] [PubMed] [Google Scholar]
- Cohen, A. J. , Anderson, H. R. , Ostro, B. , Pandey, K. D. , Krzyzanowski, M. , Künzli, N. , et al. (2005). The global burden of disease due to outdoor air pollution. Journal of Toxicology and Environmental Health, Part A, 68(13–14), 1301–1307. 10.1080/15287390590936166 [DOI] [PubMed] [Google Scholar]
- Cohen, A. J. , Brauer, M. , Burnett, R. , Anderson, H. R. , Frostad, J. , Estep, K. , et al. (2017). Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet, 389(10082), 1907–1918. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa, M. , Guizzardi, D. , Muntean, M. , Schaaf, E. , Dentener, F. , van Aardenne, J. A. , et al. (2018). Gridded emissions of air pollutants for the period 1970–2012 within EDGAR v4.3.2. Earth System Science Data, 10(4), 1987–2013. 10.5194/essd-10-1987-2018 [DOI] [Google Scholar]
- Crippa, M. , Janssens‐Maenhout, G. , Guizzardi, D. , Van Dingenen, R. , & Dentener, F. (2019). Contribution and uncertainty of sectorial and regional emissions to regional and global PM2.5 health impacts. Atmospheric Chemistry and Physics, 19(7), 5165–5186. 10.5194/acp-19-5165-2019 [DOI] [Google Scholar]
- Crobeddu, B. , Aragao‐Santiago, L. , Bui, L.‐C. , Boland, S. , & Squiban, A. B. (2017). Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environmental Pollution, 230, 125–133. 10.1016/j.envpol.2017.06.051 [DOI] [PubMed] [Google Scholar]
- Daellenbach, K. R. , Uzu, G. , Jiang, J. , Cassagnes, L.‐E. , Leni, Z. , Vlachou, A. , et al. (2020). Sources of particulate‐matter air pollution and its oxidative potential in Europe. Nature, 587(7834), 414–419. 10.1038/s41586-020-2902-8 [DOI] [PubMed] [Google Scholar]
- Dedoussi, I. C. , Eastham, S. D. , Monier, E. , & Barrett, S. R. H. (2020). Premature mortality related to United States cross‐state air pollution. Nature, 578(7794), 261–265. 10.1038/s41586-020-1983-8 [DOI] [PubMed] [Google Scholar]
- DeLang, M. N. , Becker, J. S. , Chang, K.‐L. , Serre, M. L. , Cooper, O. R. , Schultz, M. G. , et al. (2021). Mapping yearly fine resolution global surface ozone through the Bayesian maximum entropy data fusion of observations and model output for 1990–2017. Environmental Science & Technology, 55(8), 4389–4398. 10.1021/acs.est.0c07742 [DOI] [PubMed] [Google Scholar]
- Dockery, D. W. , Pope, C. A. , Xu, X. , Spengler, J. D. , Ware, J. H. , Fay, M. E. , et al. (1993). An association between air pollution and mortality in six US cities. New England Journal of Medicine, 329(24), 1753–1759. 10.1056/nejm199312093292401 [DOI] [PubMed] [Google Scholar]
- Donner, L. J. , Wyman, B. L. , Hemler, R. S. , Horowitz, L. W. , Ming, Y. , Zhao, M. , et al. (2011). The dynamical core, physical parameterizations, and basic simulation characteristics of the atmospheric component AM3 of the GFDL global coupled model CM3. Journal of Climate, 24(13), 3484–3519. 10.1175/2011JCLI3955.1 [DOI] [Google Scholar]
- Doxsey‐Whitfield, E. , MacManus, K. , Adamo, S. B. , Pistolesi, L. , Squires, J. , Borkovska, O. , & Baptista, S. R. (2015). Taking advantage of the improved availability of census data: A first look at the gridded population of the world, version 4. Papers in Applied Geography, 1(3), 226–234. 10.1080/23754931.2015.1014272 [DOI] [Google Scholar]
- Evangelopoulos, D. , Perez‐Velasco, R. , Walton, H. , Gumy, S. , Williams, M. , Kelly, F. J. , & Künzli, N. (2020). The role of burden of disease assessment in tracking progress towards achieving WHO global air quality guidelines. International Journal of Public Health, 65(8), 1455–1465. 10.1007/s00038-020-01479-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. , van Donkelaar, A. , Martin, R. V. , Burnett, R. , Rainham, D. G. , Birkett, N. J. , & Krewski, D. (2013). Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environmental Research, 120, 33–42. 10.1016/j.envres.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Fang, T. , Lakey, P. S. , Weber, R. J. , & Shiraiwa, M. (2019). Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid. Environmental Science & Technology, 53(21), 12784–12792. 10.1021/acs.est.9b03823 [DOI] [PubMed] [Google Scholar]
- Fang, Y. , Naik, V. , Horowitz, L. W. , & Mauzerall, D. L. (2013). Air pollution and associated human mortality: The role of air pollutant emissions, climate change and methane concentration increases from the preindustrial period to present. Atmospheric Chemistry and Physics, 13(3), 1377–1394. 10.5194/acp-13-1377-2013 [DOI] [Google Scholar]
- Forouzanfar, M. H. , Afshin, A. , Alexander, L. T. , Anderson, H. R. , Bhutta, Z. A. , Biryukov, S. , et al. (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1659–1724. 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar, M. H. , Alexander, L. , Anderson, H. R. , Bachman, V. F. , Biryukov, S. , Brauer, M. , et al. (2015). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet, 386(10010), 2287–2323. 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, D. , Brimblecombe, P. , Burrows, J. , Heal, M. R. , Grennfelt, P. , Stevenson, D. S. , et al. (2020). A chronology of global air quality. Philosophical Transactions of the Royal Society A, 378(2183), 20190314. 10.1098/rsta.2019.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, R. , Landrigan, P. J. , Balakrishnan, K. , Bathan, G. , Bose‐O’Reilly, S. , Brauer, M. , et al. (2022). Pollution and health: A progress update. The Lancet Planetary Health, 6(6), e535–e547. 10.1016/s2542-5196(22)00090-0 [DOI] [PubMed] [Google Scholar]
- GMAPS . (2004). GMAPS—Air pollution in world cities 2000, PM10 concentrations. Development Research Group and the Environment Department, World Bank. Retrieved from https://microdata.worldbank.org/index.php/catalog/424 [Google Scholar]
- Goodkind, A. L. , Tessum, C. W. , Coggins, J. S. , Hill, J. D. , & Marshall, J. D. (2019). Fine‐scale damage estimates of particulate matter air pollution reveal opportunities for location‐specific mitigation of emissions. Proceedings of the National Academy of Sciences of the United States of America, 116(18), 8775–8780. 10.1073/pnas.1816102116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GPW3 . (2005). Gridded Population of the World (GPW), v3. SEDAC. Retrieved from https://sedac.ciesin.columbia.edu/data/collection/gpw-v3 [Google Scholar]
- GPW4 . (2016). Gridded Population of the World (GPW), v4. SEDAC. Retrieved from https://sedac.ciesin.columbia.edu/data/collection/gpw-v4 [Google Scholar]
- Greenberg, N. , Carel, R. S. , Derazne, E. , Bibi, H. , Shpriz, M. , Tzur, D. , & Portnov, B. A. (2016). Different effects of long‐term exposures to SO2 and NO2 air pollutants on asthma severity in young adults. Journal of Toxicology and Environmental Health, Part A, 79(8), 342–351. 10.1080/15287394.2016.1153548 [DOI] [PubMed] [Google Scholar]
- Hahad, O. , Frenis, K. , Kuntic, M. , Daiber, A. , & Münzel, T. (2021). Accelerated aging and age‐related diseases (CVD and neurological) due to air pollution and traffic noise exposure. International Journal of Molecular Sciences, 22(5), 2419. 10.3390/ijms22052419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, M. S. , van Donkelaar, A. , Li, C. , Lyapustin, A. , Sayer, A. M. , Hsu, N. C. , et al. (2020). Global estimates and long‐term trends of fine particulate matter concentrations (1998–2018). Environmental Science & Technology, 54(13), 7879–7890. 10.1021/acs.est.0c01764 [DOI] [PubMed] [Google Scholar]
- Hammitt, J. K. , Morfeld, P. , Tuomisto, J. T. , & Erren, T. C. (2020). Premature deaths, statistical lives, and years of life lost: Identification, quantification, and valuation of mortality risks. Risk Analysis, 40(4), 674–695. 10.1111/risa.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heft‐Neal, S. , Burney, J. , Bendavid, E. , & Burke, M. (2018). Robust relationship between air quality and infant mortality in Africa. Nature, 559(7713), 254–258. 10.1038/s41586-018-0263-3 [DOI] [PubMed] [Google Scholar]
- HEI . (2009). Extended follow‐up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Health Effects Institute. Retrieved from https://www.healtheffects.org/publication/extended-follow-and-spatial-analysis-american-cancer-society-study-linking-particulate [PubMed] [Google Scholar]
- Hoesly, R. M. , Smith, S. J. , Feng, L. , Klimont, Z. , Janssens‐Maenhout, G. , Pitkanen, T. , et al. (2018). Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the Community Emissions Data System (CEDS). Geoscientific Model Development, 11(1), 369–408. 10.5194/gmd-11-369-2018 [DOI] [Google Scholar]
- Hong, G. , & Jee, Y.‐K. (2020). Special issue on ultrafine particles: Where are they from and how do they affect us? Experimental & Molecular Medicine, 52(3), 309–310. 10.1038/s12276-020-0395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopke, P. K. , Dai, Q. , Li, L. , & Feng, Y. (2020). Global review of recent source apportionments for airborne particulate matter. Science of the Total Environment, 740, 140091. 10.1016/j.scitotenv.2020.140091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz, L. W. (2006). Past, present, and future concentrations of tropospheric ozone and aerosols: Methodology, ozone evaluation, and sensitivity to aerosol wet removal. Journal of Geophysical Research, 111(D22), D22211. 10.1029/2005JD006937 [DOI] [Google Scholar]
- HTAP . (2022). Hemispheric transport of air pollution. UNECE. Retrieved from https://unece.org/hemispheric-transport-air-pollution [Google Scholar]
- Huang, W. , Zhu, T. , Pan, X. , Hu, M. , Lu, S.‐E. , Lin, Y. , et al. (2012). Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: Interactions of systemic inflammation, overweight, and gender. American Journal of Epidemiology, 176(2), 117–126. 10.1093/aje/kwr511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüllermeier, E. , & Waegeman, W. (2021). Aleatoric and epistemic uncertainty in machine learning: An introduction to concepts and methods. Machine Learning, 110(3), 457–506. 10.1007/s10994-021-05946-3 [DOI] [Google Scholar]
- Jerrett, M. , Burnett, R. T. , Pope, C. A., III , Ito, K. , Thurston, G. , Krewski, D. , et al. (2009). Long‐term ozone exposure and mortality. New England Journal of Medicine, 360(11), 1085–1095. 10.1056/nejmoa0803894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöckel, P. , Kerkweg, A. , Pozzer, A. , Sander, R. , Tost, H. , Riede, H. , et al. (2010). Development cycle 2 of the modular Earth submodel system (MESSy2). Geoscientific Model Development, 3(2), 717–752. 10.5194/gmd-3-717-2010 [DOI] [Google Scholar]
- Kelly, F. J. , & Fussell, J. C. (2012). Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmospheric Environment, 60, 504–526. 10.1016/j.atmosenv.2012.06.039 [DOI] [Google Scholar]
- Krol, M. , Houweling, S. , Bregman, B. , van den Broek, M. , Segers, A. , van Velthoven, P. , et al. (2005). The two‐way nested global chemistry‐transport zoom model TM5: Algorithm and applications. Atmospheric Chemistry and Physics, 5(2), 417–432. 10.5194/acp-5-417-2005 [DOI] [Google Scholar]
- Kushta, J. , Pozzer, A. , & Lelieveld, J. (2018). Uncertainties in estimates of mortality attributable to ambient PM2.5 in Europe. Environmental Research Letters, 13(6), 064029. 10.1088/1748-9326/aabf29 [DOI] [Google Scholar]
- Lamarque, J.‐F. , Shindell, D. T. , Josse, B. , Young, P. J. , Cionni, I. , Eyring, V. , et al. (2013). The Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP): Overview and description of models, simulations and climate diagnostics. Geoscientific Model Development, 6(1), 179–206. 10.5194/gmd-6-179-2013 [DOI] [Google Scholar]
- Landscan . (2008). Home. LandScanTM. Retrieved from https://landscan.ornl.gov [Google Scholar]
- Lefohn, A. S. , Malley, C. S. , Smith, L. , Wells, B. , Hazucha, M. , Simon, H. , et al. (2018). Tropospheric ozone assessment report: Global ozone metrics for climate change, human health, and crop/ecosystem research. Elementa: Science of the Anthropocene, 6, 27. 10.1525/elementa.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld, J. (2017). Clean air in the Anthropocene. Faraday Discussions, 200, 693–703. 10.1039/C7FD90032E [DOI] [PubMed] [Google Scholar]
- Lelieveld, J. , Barlas, C. , Giannadaki, D. , & Pozzer, A. (2013). Model calculated global, regional and megacity premature mortality due to air pollution. Atmospheric Chemistry and Physics, 13(14), 7023–7037. 10.5194/acp-13-7023-2013 [DOI] [Google Scholar]
- Lelieveld, J. , Evans, J. S. , Fnais, M. , Giannadaki, D. , & Pozzer, A. (2015). The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature, 525(7569), 367–371. 10.1038/nature15371 [DOI] [PubMed] [Google Scholar]
- Lelieveld, J. , Klingmüller, K. , Pozzer, A. , Burnett, R. T. , Haines, A. , & Ramanathan, V. (2019). Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proceedings of the National Academy of Sciences of the United States of America, 116(15), 7192–7197. 10.1073/pnas.1819989116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld, J. , Klingmüller, K. , Pozzer, A. , Pöschl, U. , Fnais, M. , Daiber, A. , & Münzel, T. (2019). Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. European Heart Journal, 40(20), 1590–1596. 10.1093/eurheartj/ehz135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld, J. , Pozzer, A. , Pöschl, U. , Fnais, M. , Haines, A. , & Münzel, T. (2020). Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovascular Research, 116(11), 1910–1917. 10.1093/cvr/cvaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld, S. , Wilson, J. , Dovrou, E. , Mishra, A. , Lakey, P. S. , Shiraiwa, M. , et al. (2021). Hydroxyl radical production by air pollutants in epithelial lining fluid governed by interconversion and scavenging of reactive oxygen species. Environmental Science & Technology, 55(20), 14069–14079. 10.1021/acs.est.1c03875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyk, S. , Gaughan, A. E. , Adamo, S. B. , de Sherbinin, A. , Balk, D. , Freire, S. , et al. (2019). The spatial allocation of population: A review of large‐scale gridded population data products and their fitness for use. Earth System Science Data, 11(3), 1385–1409. 10.5194/essd-11-1385-2019 [DOI] [Google Scholar]
- Li, M. , Zhang, Q. , Kurokawa, J.‐I. , Woo, J.‐H. , He, K. , Lu, Z. , et al. (2017). MIX: A mosaic Asian anthropogenic emission inventory under the international collaboration framework of the MICS‐Asia and HTAP. Atmospheric Chemistry and Physics, 17(2), 935–963. 10.5194/acp-17-935-2017 [DOI] [Google Scholar]
- Li, T. , Zhang, Y. , Wang, J. , Xu, D. , Yin, Z. , Chen, H. , et al. (2018). All‐cause mortality risk associated with long‐term exposure to ambient PM2.5 in China: A cohort study. The Lancet Public Health, 3(10), e470–e477. 10.1016/S2468-2667(18)30144-0 [DOI] [PubMed] [Google Scholar]
- Liang, C.‐K. , West, J. J. , Silva, R. A. , Bian, H. , Chin, M. , Davila, Y. , et al. (2018). HTAP2 multi‐model estimates of premature human mortality due to intercontinental transport of air pollution and emission sectors. Atmospheric Chemistry and Physics, 18(14), 10497–10520. 10.5194/acp-18-10497-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S. S. , Vos, T. , Flaxman, A. D. , Danaei, G. , Shibuya, K. , Adair‐Rohani, H. , et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet, 380(9859), 2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann, M. , Chen, L. C. , Gordon, T. , Ito, K. , & Thurston, G. D. (2013). National Particle Component Toxicity (NPACT) initiative: Integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Research Report (Health Effects Institute), 177, 5–13. [PubMed] [Google Scholar]
- Liu, F. , Beirle, S. , Zhang, Q. , Dörner, S. , He, K. , & Wagner, T. (2016). NOX lifetimes and emissions of cities and power plants in polluted background estimated by satellite observations. Atmospheric Chemistry and Physics, 16(8), 5283–5298. 10.5194/acp-16-5283-2016 [DOI] [Google Scholar]
- Lozano, R. , Naghavi, M. , Foreman, K. , Lim, S. , Shibuya, K. , Aboyans, V. , et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet, 380(9859), 2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist, A. , El‐Khatib, Z. , Kalra, N. , Pantoja, T. , Leach‐Kemon, K. , Gapp, C. , & Kuchenmüller, T. (2021). Policy‐makers’ views on translating burden of disease estimates in health policies: Bridging the gap through data visualization. Archives of Public Health, 79(1), 1–11. 10.1186/s13690-021-00537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley, C. S. , Henze, D. K. , Kuylenstierna, J. C. , Vallack, H. W. , Davila, Y. , Anenberg, S. C. , et al. (2017). Updated global estimates of respiratory mortality in adults ≥30 years of age attributable to long‐term ozone exposure. Environmental Health Perspectives, 125(8), 087021. 10.1289/ehp1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisalidis, I. , Stavropoulou, E. , Stavropoulos, A. , & Bezirtzoglou, E. (2020). Environmental and health impacts of air pollution: A review. Frontiers in Public Health, 8, 14. 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, R. V. , Brauer, M. , van Donkelaar, A. , Shaddick, G. , Narain, U. , & Dey, S. (2019). No one knows which city has the highest concentration of fine particulate matter. Atmospheric Environment: X, 3, 100040. 10.1016/j.aeaoa.2019.100040 [DOI] [Google Scholar]
- Mathers, C. D. (2005). Uncertainty and data availability for the global burden of disease estimates.
- McDuffie, E. E. , Martin, R. V. , Spadaro, J. V. , Burnett, R. , Smith, S. J. , O’Rourke, P. , et al. (2021). Source sector and fuel contributions to ambient PM2.5 and attributable mortality across multiple spatial scales. Nature Communications, 12(3594), 1–12. 10.1038/s41467-021-23853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie, E. E. , Smith, S. J. , O’Rourke, P. , Tibrewal, K. , Venkataraman, C. , Marais, E. A. , et al. (2020). A global anthropogenic emission inventory of atmospheric pollutants from sector‐ and fuel‐specific sources (1970–2017): An application of the Community Emissions Data System (CEDS). Earth System Science Data, 12(4), 3413–3442. 10.5194/essd-12-3413-2020 [DOI] [Google Scholar]
- Murray, C. J. L. , Aravkin, A. Y. , Zheng, P. , Abbafati, C. , Abbas, K. M. , Abbasi‐Kangevari, M. , et al. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet, 396(10258), 1223–1249. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan, R. , & Thakur, A. K. (2017). The toxicological mechanisms of environmental soot (black carbon) and carbon black: Focus on oxidative stress and inflammatory pathways. Frontiers in Immunology, 8, 763. 10.3389/fimmu.2017.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- on Ultrafine Particles, H. E. I. R. P. (2013). Understanding the health effects of ambient ultrafine particles. Retrieved from https://www.healtheffects.org/publication/understanding-health-effects-ambient-ultrafine-particles
- Ostro, B. , Spadaro, J. V. , Gumy, S. , Mudu, P. , Awe, Y. , Forastiere, F. , & Peters, A. (2018). Assessing the recent estimates of the global burden of disease for ambient air pollution: Methodological changes and implications for low‐and middle‐income countries. Environmental Research, 166, 713–725. 10.1016/j.envres.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Ostro, B. , World Health Organization, & Occupational and Environmental Health Team . (2004). Outdoor air pollution: Assessing the environmental burden of disease at national and local levels. World Health Organization. [Google Scholar]
- Owusu, P. A. , & Sarkodie, S. A. (2020). Global estimation of mortality, disability‐adjusted life years and welfare cost from exposure to ambient air pollution. Science of the Total Environment, 742, 140636. 10.1016/j.scitotenv.2020.140636 [DOI] [PubMed] [Google Scholar]
- Pai, S. J. , Carter, T. S. , Heald, C. L. , & Kroll, J. H. (2022). Updated world health organization air quality guidelines highlight the importance of non‐anthropogenic PM2.5 . Environmental Science & Technology Letters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadogeorgou, G. , Kioumourtzoglou, M.‐A. , Braun, D. , & Zanobetti, A. (2019). Low levels of air pollution and health: Effect estimates, methodological challenges, and future directions. Current environmental health reports, 6(3), 105–115. 10.1007/s40572-019-00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappin, A. J. , Christidis, T. , Pinault, L. L. , Crouse, D. L. , Brook, J. R. , Erickson, A. , et al. (2019). Examining the shape of the association between low levels of fine particulate matter and mortality across three cycles of the Canadian Census Health and Environment Cohort. Environmental Health Perspectives, 127(10), 107008. 10.1289/EHP5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. , Joo, H. S. , Lee, K. , Jang, M. , Kim, S. D. , Kim, I. , et al. (2018). Differential toxicities of fine particulate matters from various sources. Scientific Reports, 8(1), 17007. 10.1038/s41598-018-35398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R. , Ee, N. , Peters, J. , Booth, A. , Mudway, I. , & Anstey, K. J. (2019). Air pollution and dementia: A systematic review. Journal of Alzheimer's Disease, 70(s1), 145–163. 10.3233/JAD-180631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, C. A. , Ezzati, M. , & Dockery, D. W. (2009). Fine‐particulate air pollution and life expectancy in the United States. New England Journal of Medicine, 360(4), 376–386. 10.1056/NEJMsa0805646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer, A. , Reifenberg, S. , Kumar, V. , Franco, B. , Taraborrelli, D. , Gromov, S. , et al. (2021). Simulation of organics in the atmosphere: Evaluation of EMACV2.54 with the Mainz Organic Mechanism (MOM) coupled to the ORACLE (v1.0) submodel. Geoscientific Model Development Discussions, 15(6), 2673–2710. 10.5194/gmd-2021-295 [DOI] [Google Scholar]
- Prabhakaran, D. , Mandal, S. , Krishna, B. , Magsumbol, M. , Singh, K. , Tandon, N. , et al. (2020). Exposure to particulate matter is associated with elevated blood pressure and incident hypertension in urban India. Hypertension. Retrieved from https://www.ahajournals.org/doi/full/10.1161/HYPERTENSIONAHA.120.15373 [DOI] [PMC free article] [PubMed]
- Rao, S. , Chirkov, V. , Dentener, F. , Van Dingenen, R. , Pachauri, S. , Purohit, P. , et al. (2012). Environmental modeling and methods for estimation of the global health impacts of air pollution. Environmental Modeling & Assessment, 17(6), 613–622. 10.1007/s10666-012-9317-3 [DOI] [Google Scholar]
- Reddington, C. L. , Conibear, L. , Knote, C. , Silver, B. J. , Li, Y. J. , Chan, C. K. , et al. (2019). Exploring the impacts of anthropogenic emission sectors on PM2.5 and human health in South and East Asia. Atmospheric Chemistry and Physics, 19(18), 11887–11910. 10.5194/acp-19-11887-2019 [DOI] [Google Scholar]
- Ritz, B. , Wilhelm, M. , & Zhao, Y. (2006). Air pollution and infant death in southern California, 1989–2000. Pediatrics, 118(2), 493–502. 10.1542/peds.2006-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr, A. C. , & Wyzga, R. E. (2012). Attributing health effects to individual particulate matter constituents. Atmospheric Environment, 62, 130–152. 10.1016/j.atmosenv.2012.07.036 [DOI] [Google Scholar]
- Roth, G. A. , Mensah, G. A. , Johnson, C. O. , Addolorato, G. , Ammirati, E. , Baddour, L. M. , et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. Journal of the American College of Cardiology, 76(25), 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, R. (2000). The volume of the solution is 1 kg: Pleading for scientific writing. Eos, Transactions American Geophysical Union, 81(1), 6–6. 10.1029/00eo00007 [DOI] [Google Scholar]
- Schraufnagel, D. E. (2020). The health effects of ultrafine particles. Experimental & Molecular Medicine, 52(3), 311–317. 10.1038/s12276-020-0403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel, D. E. , Balmes, J. R. , Cowl, C. T. , De Matteis, S. , Jung, S.‐H. , Mortimer, K. , et al. (2019). Air pollution and noncommunicable diseases: A review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The damaging effects of air pollution. Chest, 155(2), 409–416. 10.1016/j.chest.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, M. G. , Schröder, S. , Lyapina, O. , Cooper, O. R. , Galbally, I. , Petropavlovskikh, I. , et al. (2017). Tropospheric ozone assessment report: Database and metrics data of global surface ozone observations. Elementa: Science of the Anthropocene, 5. [Google Scholar]
- Shaddick, G. , Thomas, M. L. , Green, A. , Brauer, M. , van Donkelaar, A. , Burnett, R. , et al. (2018). Data integration model for air quality: A hierarchical approach to the global estimation of exposures to ambient air pollution. Journal of the Royal Statistical Society C, 67(1), 231–253. 10.1111/rssc.12227 [DOI] [Google Scholar]
- Shi, L. , Zanobetti, A. , Kloog, I. , Coull, B. A. , Koutrakis, P. , Melly, S. J. , & Schwartz, J. D. (2016). Low‐concentration PM2.5 and mortality: Estimating acute and chronic effects in a population‐based study. Environmental Health Perspectives, 124(1), 46–52. 10.1289/ehp.1409111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, H. J. , Traa, A. , & Van Raamsdonk, J. M. (2021). Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Frontiers in Cell and Developmental Biology, 9, 181. 10.3389/fcell.2021.628157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, R. A. , Adelman, Z. , Fry, M. M. , & West, J. J. (2016). The impact of individual anthropogenic emissions sectors on the global burden of human mortality due to ambient air pollution. Environmental Health Perspectives, 124(11), 1776–1784. 10.1289/EHP177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, R. A. , West, J. J. , Lamarque, J.‐F. , Shindell, D. T. , Collins, W. J. , Dalsoren, S. , et al. (2016). The effect of future ambient air pollution on human premature mortality to 2100 using output from the ACCMIP model ensemble. Atmospheric Chemistry and Physics, 16(15), 9847–9862. 10.5194/acp-16-9847-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, R. A. , West, J. J. , Lamarque, J.‐F. , Shindell, D. T. , Collins, W. J. , Faluvegi, G. , et al. (2017). Future global mortality from changes in air pollution attributable to climate change. Nature Climate Change, 7(9), 647–651. 10.1038/nclimate3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, R. A. , West, J. J. , Zhang, Y. , Anenberg, S. C. , Lamarque, J.‐F. , Shindell, D. T. , et al. (2013). Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environmental Research Letters, 8(3), 034005. 10.1088/1748-9326/8/3/034005 [DOI] [Google Scholar]
- Snider, G. , Weagle, C. L. , Murdymootoo, K. K. , Ring, A. , Ritchie, Y. , Stone, E. , et al. (2016). Variation in global chemical composition of PM2.5 . Atmospheric Chemistry and Physics, 16(15), 9629–9653. 10.5194/acp-16-9629-2016 [DOI] [Google Scholar]
- Stanaway, J. D. , Afshin, A. , Gakidou, E. , Lim, S. S. , Abate, D. , Abate, K. H. , et al. (2018). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1923–1994. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of Global Air 2020 . (2020). State of Global Air 2020. A global report card on air pollution exposures and their impacts on human health. Retrieved from https://www.stateofglobalair.org
- Tarasick, D. , Galbally, I. E. , Cooper, O. R. , Schultz, M. G. , Ancellet, G. , Leblanc, T. , et al. (2019). Tropospheric Ozone Assessment Report: Tropospheric ozone from 1877 to 2016, observed levels, trends and uncertainties. Elementa: Science of the Anthropocene, 7. 10.1525/elementa.376 [DOI] [Google Scholar]
- Thunis, P. , Clappier, A. , Tarrason, L. , Cuvelier, C. , Monteiro, A. , Pisoni, E. , et al. (2019). Source apportionment to support air quality planning: Strengths and weaknesses of existing approaches. Environment International, 130, 104825. 10.1016/j.envint.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunis, P. , Degraeuwe, B. , Pisoni, E. , Trombetti, M. , Peduzzi, E. , Belis, C. A. , et al. (2018). PM2.5 source allocation in European cities: A SHERPA modelling study. Atmospheric Environment, 187, 93–106. 10.1016/j.atmosenv.2018.05.062 [DOI] [Google Scholar]
- Turner, M. C. , Andersen, Z. J. , Baccarelli, A. , Diver, W. R. , Gapstur, S. M. , Pope, C. A. , et al. (2020). Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA: A Cancer Journal for Clinicians, 70(6), 460–479. 10.3322/caac.21632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, M. C. , Jerrett, M. , Pope, C. A., III , Krewski, D. , Gapstur, S. M. , Diver, W. R. , et al. (2016). Long‐term ozone exposure and mortality in a large prospective study. American Journal of Respiratory and Critical Care Medicine, 193(10), 1134–1142. 10.1164/rccm.201508-1633oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock, S. T. , Spracklen, D. V. , Carslaw, K. S. , Mann, G. W. , Woodhouse, M. T. , Forster, P. M. , et al. (2015). Modelled and observed changes in aerosols and surface solar radiation over Europe between 1960 and 2009. Atmospheric Chemistry and Physics, 15(16), 9477–9500. 10.5194/acp-15-9477-2015 [DOI] [Google Scholar]
- UN . (2002). World population prospects. Department of Economic and Social Affairs Population Division, United Nations. Retrieved from https://population.un.org/wpp [Google Scholar]
- Upadhyay, A. , Dey, S. , Chowdhury, S. , & Goyal, P. (2018). Expected health benefits from mitigation of emissions from major anthropogenic PM2.5 sources in India: Statistics at state level. Environmental Pollution, 242, 1817–1826. 10.1016/j.envpol.2018.07.085 [DOI] [PubMed] [Google Scholar]
- US EPA National Center for Environmental Assessment, Research Triangle Park Nc, Environmental Media Assessment Group, & Sacks, J. (2020). Integrated Science Assessment (ISA) for Particulate Matter (Final Report, December 2009). Retrieved from https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=216546
- Van Dingenen, R. , Dentener, F. , Crippa, M. , Leitao, J. , Marmer, E. , Rao, S. , et al. (2018). TM5‐FASST: A global atmospheric source–receptor model for rapid impact analysis of emission changes on air quality and short‐lived climate pollutants. Atmospheric Chemistry and Physics, 18(21), 16173–16211. 10.5194/acp-18-16173-2018 [DOI] [Google Scholar]
- van Donkelaar, A. , Hammer, M. S. , Bindle, L. , Brauer, M. , Brook, J. R. , Garay, M. J. , et al. (2021). Monthly global estimates of fine particulate matter and their uncertainty. Environmental Science & Technology, 55(22), 15287–15300. 10.1021/acs.est.1c05309 [DOI] [PubMed] [Google Scholar]
- van Donkelaar, A. , Martin, R. V. , Brauer, M. , Hsu, N. C. , Kahn, R. A. , Levy, R. C. , et al. (2016). Global estimates of fine particulate matter using a combined geophysical‐statistical method with information from satellites, models, and monitors. Environmental Science & Technology, 50(7), 3762–3772. 10.1021/acs.est.5b05833 [DOI] [PubMed] [Google Scholar]
- van Donkelaar, A. , Martin, R. V. , Brauer, M. , Kahn, R. , Levy, R. , Verduzco, C. , & Villeneuve, P. J. (2010). Global estimates of ambient fine particulate matter concentrations from satellite‐based aerosol optical depth: Development and application. Environmental Health Perspectives, 118(6), 847–855. 10.1289/ehp.0901623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar, A. , Martin, R. V. , Li, C. , & Burnett, R. T. (2019). Regional estimates of chemical composition of fine particulate matter using a combined geoscience‐statistical method with information from satellites, models, and monitors. Environmental Science & Technology, 53(5), 2595–2611. 10.1021/acs.est.8b06392 [DOI] [PubMed] [Google Scholar]
- Vodonos, A. , Awad, Y. A. , & Schwartz, J. (2018). The concentration‐response between long‐term PM2.5 exposure and mortality; A meta‐regression approach. Environmental Research, 166, 677–689. 10.1016/j.envres.2018.06.021 [DOI] [PubMed] [Google Scholar]
- Vohra, K. , Vodonos, A. , Schwartz, J. , Marais, E. A. , Sulprizio, M. P. , & Mickley, L. J. (2021). Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS‐Chem. Environmental Research, 195, 110754. 10.1016/j.envres.2021.110754 [DOI] [PubMed] [Google Scholar]
- Weagle, C. L. , Snider, G. , Li, C. , van Donkelaar, A. , Philip, S. , Bissonnette, P. , et al. (2018). Global sources of fine particulate matter: Interpretation of PM2.5 chemical composition observed by SPARTAN using a global chemical transport model. Environmental Science & Technology, 52(20), 11670–11681. 10.1021/acs.est.8b01658 [DOI] [PubMed] [Google Scholar]
- Weichenthal, S. , Lavigne, E. , Evans, G. , Pollitt, K. , & Burnett, R. T. (2016). Ambient PM2.5 and risk of emergency room visits for myocardial infarction: Impact of regional PM2.5 oxidative potential: A case‐crossover study. Environmental Health, 15(1), 1–9. 10.1186/s12940-016-0129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal, S. , Pinault, L. , Christidis, T. , Burnett, R. T. , Brook, J. R. , Chu, Y. , et al. (2022). How low can you go? Air pollution affects mortality at very low levels. Science Advances, 8(39), eabo3381. 10.1126/sciadv.abo3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2003). Global Burden of Disease (GBD) 2000: Version 3 estimates. World Health Organization. Retrieved from https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000_v3/en [Google Scholar]
- WHO . (2004a). The global burden of disease: 2004 update. World Health Organization. Retrieved from https://www.who.int/healthinfo/global_burden_disease/2004_report_update/en [Google Scholar]
- WHO . (2004b). The world health report 2004—Changing history. World Health Organization. Retrieved from https://www.who.int/whr/2004/en [Google Scholar]
- WHO . (2007). Health relevance of particulate matter from various sources: Report on a who workshop, Bonn, Germany 26–27 March 2007 (Technical Report). WHO Regional Office for Europe. [Google Scholar]
- WHO . (2010). World health statistics 2008. World Health Organization. Retrieved from https://www.who.int/whosis/whostat/2008/en [Google Scholar]
- WHO . (2012). World health statistics 2012. World Health Organization. Retrieved from https://apps.who.int/iris/handle/10665/44844 [Google Scholar]
- WHO . (2013). Health effects of particulate matter: Policy implications for countries in eastern Europe, Caucasus and central Asia.
- WHO . (2015). World health statistics 2015. World Health Organization. Retrieved from https://apps.who.int/iris/handle/10665/170250 [Google Scholar]
- Wong, C. M. , Tsang, H. , Lai, H. K. , Thomas, G. N. , Lam, K. B. , Chan, K. P. , et al. (2016). Cancer mortality risks from long‐term exposure to ambient fine particle. Cancer Epidemiology, Biomarkers & Prevention, 25(5), 839–845. 10.1158/1055-9965.EPI-15-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Tang, R. , Qiu, H. , Lai, P.‐C. , Wong, P. , Thach, T.‐Q. , et al. (2018). Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environment International, 117, 99–106. 10.1016/j.envint.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Yin, H. , Brauer, M. , Zhang, J. J. , Cai, W. , Navrud, S. , Burnett, R. , et al. (2020). Global economic cost of deaths attributable to ambient air pollution: Disproportionate burden on the ageing population. medRxiv. 10.1101/2020.04.28.20083576 [DOI] [Google Scholar]
- Yin, P. , Brauer, M. , Cohen, A. , Burnett, R. T. , Liu, J. , Liu, Y. , et al. (2017). Long‐term fine particulate matter exposure and nonaccidental and cause‐specific mortality in a large national cohort of Chinese men. Environmental Health Perspectives, 125(11), 117002. 10.1289/ehp1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X. , Li, P. , Feng, Z. , Yang, Y. , You, Z. , & Xiao, C. (2021). Which gridded population data product is better? Evidences from mainland southeast Asia (MSEA). ISPRS International Journal of Geo‐Information, 10(10), 681. 10.3390/ijgi10100681 [DOI] [Google Scholar]
- Yu, W. , Guo, Y. , Shi, L. , & Li, S. (2020). The association between long‐term exposure to low‐level PM2.5 and mortality in the state of Queensland, Australia: A modelling study with the difference‐in‐differences approach. PLoS Medicine, 17(6), e1003141. 10.1371/journal.pmed.1003141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf, S. , Joseph, P. , Rangarajan, S. , Islam, S. , Mente, A. , Hystad, P. , et al. (2020). Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high‐income, middle‐income, and low‐income countries (PURE): A prospective cohort study. Lancet, 395(10226), 795–808. 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Jiang, X. , Tong, D. , Davis, S. J. , Zhao, H. , Geng, G. , et al. (2017). Transboundary health impacts of transported global air pollution and international trade. Nature, 543(7647), 705–709. 10.1038/nature21712 [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Elser, M. , Huang, R.‐J. , Krapf, M. , Fröhlich, R. , Bhattu, D. , et al. (2019). Predominance of secondary organic aerosol to particle‐bound reactive oxygen species activity in fine ambient aerosol. Atmospheric Chemistry and Physics, 19(23), 14703–14720. 10.5194/acp-19-14703-2019 [DOI] [Google Scholar]
- Zimmer, Z. , & Kwong, J. (2004). Socioeconomic status and health among older adults in rural and urban China. Journal of Aging and Health, 16(1), 44–70. 10.1177/0898264303260440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Only published data was used in this work. Reference to each data can be found in Tables 1, 2, 3.


