Abstract
Lyme disease and human granulocytic ehrlichiosis (HGE) are tick-borne illnesses caused by Borrelia burgdorferi and the agent of HGE, respectively. We investigated the influence of dual infection with B. burgdorferi and the HGE agent on the course of murine Lyme arthritis and granulocytic ehrlichiosis. Coinfection resulted in increased levels of both pathogens and more severe Lyme arthritis compared with those in mice infected with B. burgdorferi alone. The increase in bacterial burden during dual infection was associated with enhanced acquisition of both organisms by larval ticks that were allowed to engorge upon infected mice. Coinfection also resulted in diminished interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha levels and elevated IL-6 levels in murine sera. During dual infection, IFN-γ receptor expression on macrophages was also reduced, implying a decrease in phagocyte activation. These results suggest that coinfection of mice with B. burgdorferi and the HGE agent modulates host immune responses, resulting in increased bacterial burden, Lyme arthritis, and pathogen transmission to the vector.
Borrelia burgdorferi, the spirochete that causes Lyme disease, is the most common arthropod-borne pathogen in the United States (35). Over the past 5 years, it has become apparent that the Ixodes scapularis ticks that harbor B. burgdorferi also transmit the agent of human granulocytic ehrlichiosis (HGE), among other pathogens (18, 20, 22, 23, 59, 70). The agent of HGE is a newly described obligate intracellular pathogen with a tropism for the neutrophil (7). Coinfection with B. burgdorferi and the HGE agent has been documented in humans (1, 7, 48, 71), ticks (20, 59, 70), and mice (49). However, the frequency of dual infection and its effect on the course of disease is not known. Laboratory mice can also be infected with B. burgdorferi (64, 65) or HGE bacteria (38, 39, 70), and murine models of Lyme borreliosis and granulocytic ehrlichiosis (11, 13, 16, 39) have facilitated studies on these pathogens.
The pathogenesis of Lyme arthritis has been studied in both humans and mice. In humans, B. burgdorferi infection commonly results in a pathognomonic skin rash named erythema migrans, and persistent infection can lead to the development of Lyme arthritis (36, 66, 67). Human Lyme arthritis is associated with CD4+-T-cell helper type 1 (Th1) responses to B. burgdorferi, including increased gamma interferon (IFN-γ) production by T cells in affected joints (34, 73, 74). The experimental murine model of Lyme arthritis provides some similarities with human joint disease (9). C3H/He mice, which are susceptible to the development of Lyme arthritis, generate high levels of IFN-γ, consistent with a murine Th1 phenotype (45). In contrast, BALB/c mice, which are relatively resistant to Lyme arthritis, develop higher levels of interleukin-4 (IL-4), indicative of a predominant Th2 response (43, 45). Moreover, neutralization of IFN-γ or IL-12 reduces Lyme arthritis in C3H/He mice and inhibition of IL-4 exacerbates disease in BALB/c mice, further demonstrating the importance of CD4+-T-cell differentiation in the genesis of joint inflammation (4, 55).
Antibodies to B. burgdorferi can also influence the course of Lyme disease. In humans, the development of high-titer BBK32, also known as P35, antibodies during early-stage Lyme disease is associated with a decreased risk of progression to Lyme arthritis (31–33). Similarly, passive transfer of B. burgdorferi immune sera (12, 29) can induce disease regression in mice, and outer surface protein C (OspC) (32), decorin-binding protein A (DbpA) (24), or BBK32 (28) antibodies can partially clear B. burgdorferi from an infected animal. Therefore, both the host humoral and cellular responses to B. burgdorferi can modify the course of spirochete infection and the severity of arthritis (41).
The first case of HGE was described in 1994 (19). The HGE agent is very similar to Ehrlichia equi and Ehrlichia phagocytophila and preferentially resides within the neutrophil (19). Fever, myalgia, thrombocytopenia, leukopenia, and anemia often mark infection (17, 22). Morulae containing the HGE agent are present in peripheral neutrophils of some patients in the early stages of infection (22). In addition morulae can be detected during the first weeks of murine infection with the HGE agent, partially resembling human illness (39, 70). In general, immunocompetent mice clear HGE bacteria from the bloodstream within several weeks, while HGE organisms reside within the polymorphonuclear leukocytes of severe combined immunodeficient (SCID) mice for several months (39), suggesting that acquired immune responses help control this pathogen. This is supported by observations that antibodies to HGE provide partial protection from infection (46, 69). Moreover, immunocompetent mice develop high levels of IFN-γ after challenge with the HGE agent (3, 54), and organism levels are elevated in mice deficient in IFN-γ (3), indicating that IFN-γ helps control ehrlichial propagation.
Dual infection involving B. burgdorferi and the HGE agent has been documented in humans and mice (7, 49, 52, 56). In addition, ticks may be colonized by both pathogens (49, 59). In a number of coinfection scenarios, the influence of one or both organisms on the host immune response has been associated with the inhibition or exacerbation of disease. For example, Santiago et al. reported that coinfection with Toxoplasma gondii and Leishmania major inhibited the tissue parasitism observed with L. major alone (61). Helmby et al. demonstrated higher levels of malaria parasitemia in mouse coinfection with Schistosoma mansoni and Plasmodium chabaudi, and increased disease was accompanied by lower tumor necrosis factor alpha (TNF-α) responses to P. chabaudi and reduced Th2 responses to S. mansoni (37). Higher mortality rates have also been demonstrated in rabbits coinfected with enteropathogenic Escherichia coli and the obligate intracellular bacterium Lawsonia intracellularis (62), and Marshall et al. showed that increased TNF-α production resulted in the death of mice coinfected with T. gondii and S. mansoni (53). To date, well-documented cases of acute infection with HGE bacteria and B. burgdorferi have been more infrequent than serologic evidence of exposure to both pathogens. B. burgdorferi and HGE bacteria can, however, be transmitted by the same tick bite (63), and coinfection could influence the host immune response and disease outcome. We have now investigated the effect of murine infection with B. burgdorferi and the HGE agent on infection, transmission, immune responses, and severity of Lyme arthritis.
MATERIALS AND METHODS
Mice, bacteria, and ticks.
Six-week-old C3H/HeN (C3H) and C3H/HeN-scid (C3H-scid) mice were obtained from the Frederick Cancer Research Center (Frederick, Md.). Mice were maintained in filter-framed cages and euthanized by CO2 inhalation. B. burgdorferi cN40, a clonal low-passage isolate with proven infectivity and pathogenicity which has been extensively characterized, was used throughout (11). The spirochetes were cultured in Barbour-Stoenner-Kelly II medium (8).
The HGE bacterial isolate NCH-1 was originally recovered from a patient in Massachusetts and has been used repeatedly for murine infection (70). HGE bacteria were maintained in vivo by infection of SCID mice previously shown to persistently harbor the infection (39, 70). These mice served as donors of blood to inoculate naive mice with the HGE agent. HGE bacteria were also maintained in culture by infection of the promyelocytic cell line HL-60 as previously described (33).
I. scapularis larval ticks were obtained from a colony at the Connecticut Agricultural Experiment Station and maintained in a humidified chamber until use. Mated adult female I. scapularis ticks were collected in the field, and the egg mass was laid in the laboratory. All tick rearing was performed in an incubator at 26°C with 85% relative humidity and a 12-h–12-h light-dark photoperiod regimen.
B. burgdorferi lysates were prepared from spirochetes cultured for 14 days. Spirochetes were pelleted at 13,000 × g, followed by three washes with phosphate-buffered saline (PBS). The pellet was resuspended in 500 μl of H2O and disrupted by sonication with three 10-s pulses. Insoluble material was pelleted at 16,000 × g for 1 min. The supernatant (B. burgdorferi lysate) was collected and stored at −20°C until use. HGE-infected HL-60 and uninfected HL-60 cell lysates were obtained by pelleting cells at 4,000 × g, followed by three washes with PBS. The cell pellet was resuspended in 500 μl of H2O and sonicated as indicated above. Insoluble material was removed by centrifugation, and the supernatants (HGE lysate and HL-60 lysate) were stored at −20°C until use. Protein concentrations were determined by the method of Bradford (Bio-Rad Laboratories, Hercules, Calif.) according to the manufacturer's instructions.
Infection of mice.
Mice were infected with either 10,000 spirochetes administered by intradermal injection according to established protocols (27) or 100 μl of HGE-infected C3H-scid blood by intraperitoneal injection (3). In coinfection studies, mice were infected with each organism using a separate inoculation. Mice were sacrificed at 1 week, 2 weeks, and 2 months following infection. Tissues were harvested and stored at −70°C until use.
DNA preparation and PCR.
DNA was prepared via the QIAmp tissue and blood kit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions. Mixtures for PCR amplification of B. burgdorferi-specific DNA consisted of 500 ng of total DNA in a 50-μl volume containing a final concentration of 1× PCR buffer, 0.8 μM ospA 283-303 (5′-GGT CAA ACC ACA CTT GAA GTT-3′), 0.8 μM ospA 615-598 (5′-GTC AGT GTC ATT AAG TTC-3′), 0.2 mM deoxynucleoside triphosphates, and 2.5 U of Taq polymerase. PCR was performed for 35 cycles under the following conditions: 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. A final extension of 3 min at 72°C was performed, and the samples were chilled at 4°C until gel analysis. For competitive PCR, B. burgdorferi-specific bbk50 (also known as p37) primers and a tested B. burgdorferi competitor DNA construct (30, 31) were used. The concentrations of competitor DNA are indicated on the figures.
PCR of HGE-specific DNA was done under similar conditions. The hge-44-specific primers 395-419 (5′-TCA AGA CCA AGG GGT ATT AGA GAT AG-3′) and 920-898 (5′-GCC ACT ATG GAA TTT TCTT CGG G-3′) were based on the sequence of hge-44 (42), a member of an Ehrlichia gene family that codes for immunodominant antigens (40, 42, 75, 76). Beta-actin primers (5′-AGC GGG AAA TCG TGC GTG-3′ and 5′-CAG GGT ACA TGG TGG TGC C-3′) were used in control PCRs to ensure that equivalent concentrations of DNA were utilized. PCR for HGE DNA in larval ticks was performed in a similar fashion. DNA was amplified from pools of five larval ticks that fed to repletion on uninfected, HGE-infected, or HGE- and B. burgdorferi-infected mice.
Morula determination.
Peripheral blood was collected in EDTA, and 4-μl portions were placed on slides. Duplicate blood smears were air dried, fixed with methanol, and stained with Diff-Quick reagents 1 and 2 (Baxter Healthcare Cooperation, Miami, Fla.). The percentage of morulae was determined by examining over 100 granulocytes for each blood smear.
Antibody ELISA.
Fifty nanograms of B. burgdorferi lysate in 100 μl of coating buffer (0.1 M sodium bicarbonate, pH 9.6) was added to 96-well enzyme-linked immunosorbent assay (ELISA) plates (ICN Biochemicals Inc., Costa Mesa, Calif.) and kept overnight at 4°C. Plates were washed three times with wash buffer (PBS with 0.05% Tween 20). Nonspecific sites were blocked with 200 μl of blocking buffer (10% fetal calf serum in PBS) for 2 h at room temperature. Blocking buffer was removed, and 50 μl of primary antibody (diluted 1:500 to 1:1,000) was incubated for 1 h at room temperature. The plates were washed five times with wash buffer, followed by the addition of biotinylated isotype-specific antibodies (Pharmingen, San Diego, Calif.) or anti-mouse immunoglobulin M (IgM) (Sigma Chemical Co., St. Louis, Mo.) at a 1:2,000 dilution in blocking buffer. Plates were then incubated at room temperature for 1 h, followed by six washes with washing buffer. Streptavidin-conjugated horseradish peroxidase was then added to the wells and left for 45 min, followed by six washes with washing buffer. The plates were tapped to remove excess solution, 100 μl of tetramethylbenzidine (TMB) solution (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was added, and the reaction was stopped with 100 μl of TMB (one-component) Stop solution (Kirkegaard and Perry).
Capture ELISA.
Cytokine antibodies and recombinant proteins were purchased from Pharmingen, and ELISA was performed according to the manufacturer's recommendations. Briefly, 100 μl of cytokine capture antibody (1 μg/ml) was applied to wells and left overnight at 4°C. Wells were washed twice with washing buffer, followed by the addition of blocking solution. After 2 h at room temperature, blocking solution was removed and 50 μl of either sera or supernatants and recombinant cytokine standards was added. All of the sera and supernatants were tested in duplicate. Plates were incubated at 37°C for 1 h. Plates were washed five times, followed by incubation for 1 h with 50 μl of biotinylated cytokine sandwich antibody. Plates were washed and incubated with 50 μl of streptavidin-horseradish peroxidase conjugate for 45 min. Following extensive washing, plates were incubated with TMB solution. Cytokine concentrations were extrapolated from the values obtained with known concentrations of recombinant cytokines. The assay results were linear over the range of concentrations that were detected. The lower limits of detection for the cytokines were as follows: IL-5, 0.5 pg/ml; IL-6, 0.4 pg/ml; IL-10, 0.3 pg/ml; IL-12, 0.5 pg/ml; IFN-γ, 0.5 pg/ml; and TNF-α, 0.5 pg/ml.
Tick acquisition and immunofluorescence assay (IFA).
Approximately 50 I. scapularis larvae were placed on three mice from each group. Ticks were allowed to feed to repletion and collected. After 7 days of resting in a humidified chamber, 10 ticks were homogenized individually in 10 μl of PBS. Serial dilutions of 1:10 and 1:100 were made, and 5-μl portions of the undiluted and diluted tick homogenates were spotted onto microwell slides. Slides were air dried and fixed in acetone for 5 min. Nonspecific sites were blocked for 1 h with 10% fetal calf serum in PBS, followed by incubation with a 1:50 dilution of an anti-B. burgdorferi fluorescein isothiocyanate (FITC)-conjugated antibody for 30 min at room temperature. Samples were washed three times with washing buffer (PBS, 0.05% Tween 20). Following air drying, samples were mounted with coverslips and visualized by fluorescence microscopy. The entire field was surveyed for spirochetes and counted.
In vitro stimulation of whole splenocytes.
Whole splenocytes were isolated as previously described (5). All assays were performed in duplicate in each study, and at least three separate experiments were performed. Briefly, spleens were mechanically disrupted, followed by depletion of red cells. For B. burgdorferi stimulation, either 10 μg of B. burgdorferi lysate or 4 μg of concanavalin A (ConA) was added to 106 cells/ml. For HGE stimulation, the same number of splenocytes were incubated either with 10 μg of lysate from HGE-infected HL-60 cells or uninfected HL-60 cells or with 4 μg of ConA. Supernatants were collected after 48 h of incubation for cytokine determination.
Histopathology of murine Lyme arthritis.
Knees and tibiotarsal joints were fixed with formalin, embedded in paraffin, and examined microscopically for evidence of disease. Arthritis in both the joints and knees of each mouse was assessed as described previously (27). Disease severity was scored on a scale of 0 (no disease), 1 (mild disease), 2 (moderate disease), and 3 (severe disease). The joints, tendons, or ligamentous sheaths were examined for the presence of fibrinoid exudation and necrosis and neutrophilic infiltration. The synovium was examined for hypertrophy and hyperplasia. Mild disease consisted of neutrophilic infiltration of one ligament, tendon sheath, or joint. Moderate disease was marked by neutrophilic infiltration in more than one tendon sheath and one joint and at least some evidence of fibrinoid exudation. Severe disease was marked by neutrophilic infiltration of two or more tendon sheaths or joints, with fibrinoid necrosis and synovial hypertrophy. All measurements were made in a double-blinded fashion.
Flow-cytometric analysis.
Splenocytes in single-cell suspensions at 106 cells in 100 μl were surface stained with a panel of antibodies labeled with either FITC, biotin, phycoerythrin (PE), or Cy-chrome (Pharmingen). CD4+ T cells, macrophages, and neutrophils were specifically labeled with CD4–Cy-chrome, Mac-3–PE, or Ly6-G–FITC, respectively, for 30 min at room temperature. CD4+ T cells were stained for CD25 and CD40 ligand. Macrophages were stained for either IFN-γ receptor (CD119α) or major histocompatibility complex (MHC) class II (I-Ek and I-Ak). Neutrophils were stained with Mac-1 and IFN-γ receptor. When necessary, a secondary staining was performed with streptavidin-PE or streptavidin-FITC after washing the samples. After the final incubation, the cells were washed and analyzed with a flow cytometer using the Cell Quest software package (Becton Dickinson, Franklin Lakes, N.J.).
RESULTS
Infection of mice with B. burgdorferi and the HGE agent.
Groups of five mice were challenged with both B. burgdorferi and the HGE agent to assess the influence of these two pathogens on the course of infection. As controls, mice were challenged with either HGE bacteria or B. burgdorferi alone. Experiments were repeated three to five times, and in all of the studies, animals were infected with known inocula of each organism, as described in Materials and Methods. Animals were sacrificed at 1, 2, and 8 weeks, time points that represent specific intervals during the course of murine Lyme borreliosis and HGE. B. burgdorferi-infected mice develop acute arthritis and carditis at 2 weeks, and by 8 weeks the disease usually resolves as the animals remain persistently infected (10). Mice challenged with HGE bacteria usually have morulae in the polymorphonuclear leukocytes at 1 to 2 weeks (39, 69). The HGE agent is then generally cleared from the bloodstream (39).
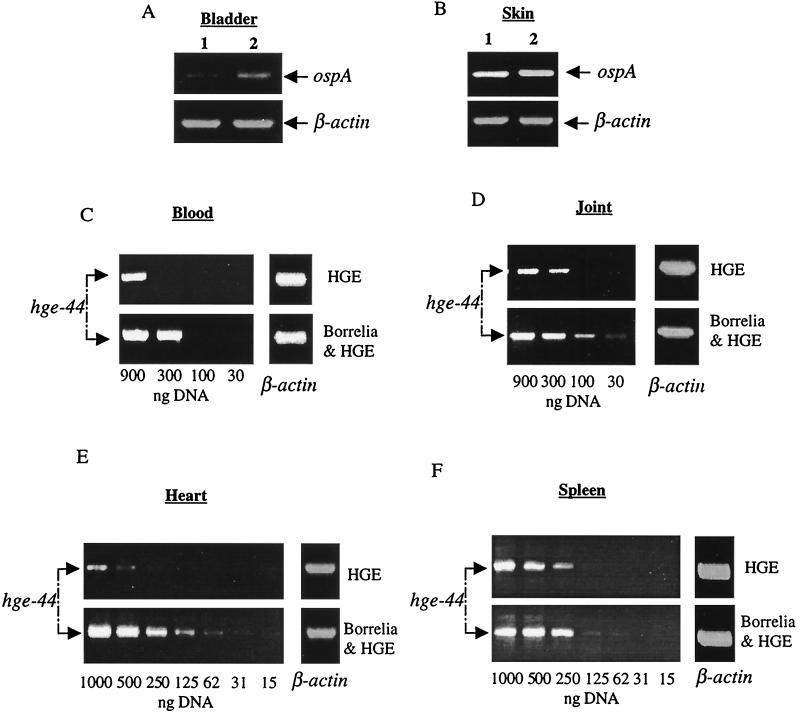
At 1 week, higher levels of B. burgdorferi DNA were detected in the bladders of the coinfected mice than in those of the B. burgdorferi-infected mice (Fig. 1A). Equivalent concentrations of B. burgdorferi DNA were present in the skin of B. burgdorferi-infected and coinfected mice (Fig. 1B). Spirochete DNA was not detected in the joints and hearts at this time point (not shown), consistent with the observation that arthritis and carditis are not yet apparent at this interval. Previous reports have demonstrated HGE bacteria in multiple organs, including the lungs, blood, spleen, and liver (22), and the heart and joints are two tissues associated with Lyme disease. Therefore, the presence of HGE bacteria in the blood, spleen, heart, and joints was assessed. At 1 week, the level of HGE bacteria, as demonstrated by PCR, was elevated in the blood of the coinfected mice compared to animals that were infected only with HGE bacteria (Fig. 1C). In addition, an increased number of neutrophils containing morulae with Ehrlichia were apparent in the blood of the coinfected mice compared with animals infected only with HGE bacteria (Table 1). Increased levels of the HGE agent were also detected in the joints, hearts, and spleens of the coinfected mice (Fig. 1D, E, and F). As a typical example, HGE levels in the heart in five separate experiments were examined by densitometry, and the DNA bands in the coinfected mice were 3.2 (mean) ± 0.7 (standard deviation) times as intense as the DNA bands in the HGE-infected mice (Student's t test, P < 0.02).
FIG. 1.
Levels of B. burgdorferi and the HGE agent after 1 week of coinfection. DNAs from specific tissues were pooled from five mice (from each group of animals) and analyzed by PCR with primers specific for either B. burgdorferi ospA or hge-44. Bladder (A) and skin (B) DNAs from singly B. burgdorferi-infected (lanes 1) and coinfected (lanes 2) mice were analyzed for ospA. Blood (C), joint (D), heart (E), and splenic (F) DNAs were analyzed by dilutional PCR for hge-44. The numbers represent concentrations of total DNA used in the PCR. PCR for β-actin was performed with 1 μg of total DNA to ensure that equal amounts of DNA were used. These data represent samples from one experiment with five mice in each group. Five separate experiments with different mice yielded similar results.
TABLE 1.
Coinfection increases the percentage of neutrophils with Ehrlichiaa
| Infectionb | % (mean ± SD) of neutrophils with morulaec at:
|
|
|---|---|---|
| 1 wk | 2 wk | |
| Uninfected (control) | 0 | 0 |
| HGE | 6.8 ± 1.9 | 0.3 ± 0.5 |
| HGE and B. burgdorferi | 17 ± 1.4d | 3.3 ± 1.7e |
Results from one of four separate experiments with similar results are shown.
Groups of five mice were either infected with HGE bacteria, coinfected with HGE bacteria and B. burgdorferi, or remained uninfected (control).
The percentage of neutrophils with morulae was assessed by analysis of 100 granulocytes in duplicate blood smears from each of the four mice in each infected group.
P < 0.0001 relative to HGE-infected mice.
P < 0.02 relative to the HGE-infected mice.
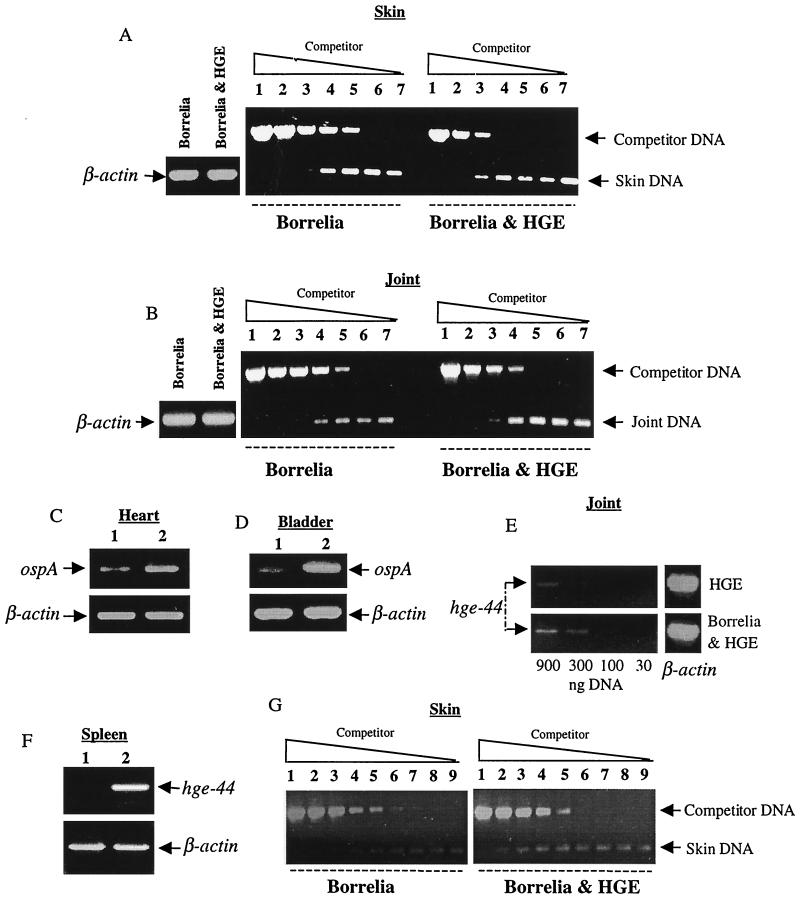
At 2 weeks, HGE bacteria were also more readily detected in the joints and spleens of the coinfected mice than in those of the HGE-infected mice (Fig. 2E and F). An elevated percentage of neutrophils with morulae was also detected in the blood of the coinfected mice (Table 1). At this time point, coinfected mice had approximately 100-fold more B. burgdorferi-specific DNA in the skin (Fig. 2A). Joints of coinfected mice had approximately 10-fold more B. burgdorferi DNA than joints of B. burgdorferi-infected mice (Fig. 2B). In addition, the hearts and bladders of the coinfected mice showed higher levels of spirochete DNA than those of the B. burgdorferi-infected mice (Fig. 2C and D). Histopathologic examination of the murine joints showed more severe arthritis in the coinfected mice than in the B. burgdorferi-infected mice at this peak phase of infection (Table 2). As expected, mice infected with HGE bacteria did not develop arthritis (not shown).
FIG. 2.
Coinfection elevates levels of the HGE agent and B. burgdorferi at 2 weeks. DNAs from various tissues of the five mice in each group were pooled and analyzed by PCR with primers specific for either B. burgdorferi ospA or hge-44. Skin (A) and joints (B) were analyzed by competitive PCR with primers specific for B. burgdorferi bbk50 (also known as p37) (28, 31). Lanes 1 through 7 contain 10-fold dilutions (87 to 0.000087 fg) of competitor DNA. Primers specific for ospA were used to amplify heart (C) and bladder (D) DNAs from singly B. burgdorferi-infected (lanes 1) and coinfected (lanes 2) mice. Joint DNA (E) was analyzed by dilutional PCR for hge-44. Splenic DNA (F) from mice either infected with HGE only (lane 1) or coinfected (lane 2) was analyzed for hge-44. Skin DNA (G) from mice infected for 2 months was analyzed by competitive PCR with primers specific for B. burgdorferi bbk50. Lanes 1 through 9 contain fourfold dilutions (0.137 to 0.000002 fg). PCR for β-actin was performed with 1 μg of total DNA to ensure that equal amounts of DNA were used. Five separate experiments were performed, with similar results. Results from one of these five representative experiments are presented.
TABLE 2.
Coinfection increases the severity of acute murine Lyme arthritis
| Infecting agent(s) | 2 wk (peak)
|
8 wk (late)
|
||||
|---|---|---|---|---|---|---|
| Diseasea:
|
Infectionb (no. infected/total) | Disease:
|
Infection (no. infected/total) | |||
| Severityc | Incidence | Severity | Incidence | |||
| B. burgdorferi | 0.8 ± 0.2 | 7/11 | 11/11 | 0.5 ± 0.3 | 4/11 | 11/11 |
| B. burgdorferi and HGE | 1.5 ± 0.3d | 11/13 | 13/13 | 0.7 ± 0.2 | 7/13 | 13/13 |
Disease was assessed in a blinded fashion in joint tissue acquired 2 weeks (peak) or 8 weeks (late) after challenge. Each group had five mice. Results from one of two similar experiments are shown.
A mouse was considered to be infected if spirochetes were isolated by culture or a skin specimen was PCR positive for Borrelia DNA.
Disease severity was scored on a scale from 0 to 3 as previously reported (26): 0, no arthritis; 1, mild arthritis; 2, moderate arthritis; and 3, severe arthritis. Values represent the mean ± SEM for the mice in each group.
P < 0.05 relative to the B. burgdorferi-infected mice.
At 8 weeks the HGE agent was not detected in the blood or spleens of any mice (not shown). B. burgdorferi was present in the skin of both the singly B. burgdorferi-infected and coinfected mice, and higher spirochete levels were evident in the dually infected animals (Fig. 2G). The joints of both groups of mice contained equivalent levels of spirochete DNA (not shown), and arthritis was resolving in both groups of animals at this interval (Table 2).
Effect of dual infection on the ability of ticks to acquire and transmit each pathogen.
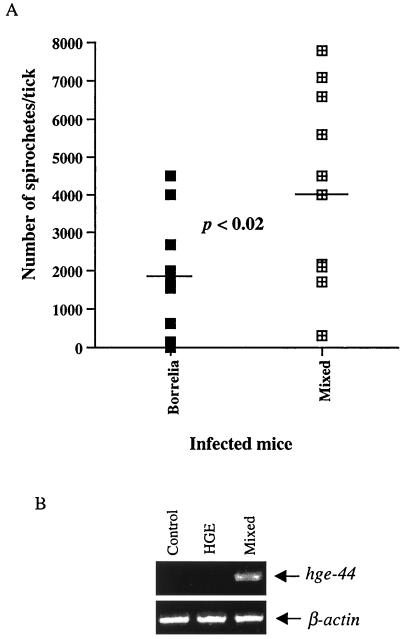
The influence of coinfection on transmission of Ehrlichia and B. burgdorferi to ticks was investigated because mixed infection alters the bacterial burden. Approximately 200 uninfected larvae were placed on groups of five infected mice at 1 week postinfection. Engorged larvae were collected, and pools of five ticks were assessed by IFA for spirochetes and by PCR for the HGE bacteria. IFA showed that 95% of the ticks that fed on B. burgdorferi-infected and coinfected mice contained spirochetes. However, ticks that engorged on coinfected mice contained significantly higher numbers of spirochetes (mean, 4,189) than ticks that fed on B. burgdorferi-infected mice (mean, 1,740) (Student's t test, P < 0.02) (Fig. 3A). At 1 week, HGE bacteria were detected by PCR in DNA from groups of five ticks that fed on coinfected mice but not in ticks fed on mice infected with the HGE agent (Fig. 3B). Three experiments yielded similar results. In addition, when individual ticks were examined by PCR, 50% of the ticks from the coinfection studies had evidence of HGE DNA, while HGE DNA was rarely detected (one tick) in ticks that fed on mice that were infected only with the HGE agent.
FIG. 3.
Coinfection increases acquisition of B. burgdorferi and HGE bacteria by larval ticks. (A) Two hundred larval ticks were fed on groups of four or five mice infected with either B. burgdorferi or B. burgdorferi and HGE bacteria. Engorged ticks were collected and analyzed by IFA with anti-B. burgdorferi specific antibody. Individual ticks were homogenized in 10 μl of 1× PBS, and 5-μl aliquots of undiluted sample and 1:10 and 1:100 dilutions were spotted onto slides. The graph shows the average number of spirochetes per tick from each group. (B) Five hundred nanograms of DNA from a pool of five larval ticks that fed on either control (uninfected) mice or HGE- or HGE- and B. burgdorferi-infected mice was evaluated by PCR with primers specific for hge-44. PCR for β-actin was also performed to ensure that equal amounts of DNA were used. Results of one of three experiments with similar results are shown.
Cytokine and antibody profiles during dual infection.
Cytokine and antibody responses are potential factors that may be associated with the increase in the bacterial burden observed in the coinfected animals. Antibody titers were first determined by ELISA with B. burgdorferi and HGE-44 extracts as substrates. HGE-44, an immunodominant Ehrlichia antigen, was used because it exhibits less cross-reactivity with anti-B. burgdorferi sera than HGE lysates (42). Three separate experiments, with five mice in each group, were performed, and the results were averaged. At 8 weeks, B. burgdorferi-specific antibody levels were not statistically different (Student's t test, P > 0.5) in sera from B. burgdorferi-infected (IgG1, 0.57 ± 0.02; IgG2a, 0.95 ± 0.05; IgG2b, 0.71 ± 0.04; and IgG3, 0.53 ± 0.06) and coinfected (IgG1, 0.58 ± 0.02; IgG2a, 0.94 ± 0.04; IgG2b, 0.68 ± 0.02; and IgG3, 0.49 ± 0.06) mice. Antibodies specific for HGE-44 were also comparable (Student's t test, P > 0.5) in the HGE-infected (IgG1, 0.43 ± 0.02; IgG2a, 1.04 ± 0.03; IgG2b, 0.60 ± 0.01; and IgG3, 0.56 ± 0.03) and coinfected (IgG1, 0.38 ± 0.01; IgG2a, 1.06 ± 0.03; IgG2b, 0.52 ± 0.07; and IgG3, 0.24 ± 0.04) mice, except for the IgG3 antibodies, which were at slightly lower levels (Student's t test, P < 0.05) in the coinfected mice.
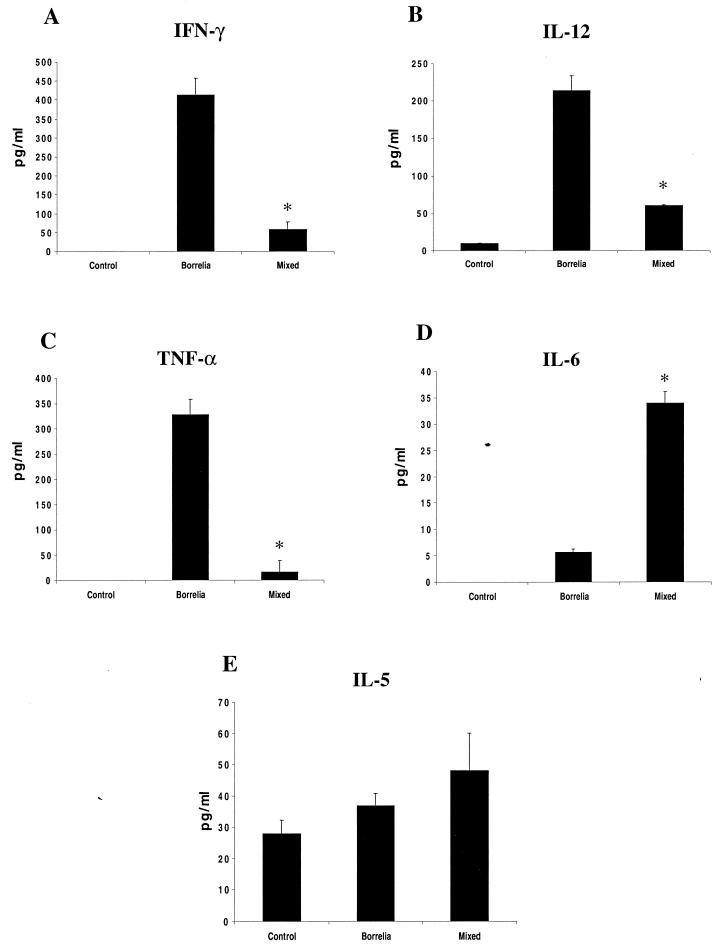
Lyme disease severity is partially dependent on the number of spirochetes that invade the joints and on the immune response generated during infection (4, 55, 72). Our results show that coinfection increased the bacterial burden. Therefore, we investigated the effect of dual infection on cytokines that are associated with Lyme arthritis. In each experiment, sera were pooled from five mice in each group. Three separate experiments were performed, and the means and standard deviations are presented. Sera were first analyzed for IL-12, IFN-γ, and TNF-α, cytokines that are known to be expressed during B. burgdorferi infection and that correlate with increased acute inflammation (4, 21, 34, 45, 68). IL-5 and IL-6, indicative of Th2 responses that have been associated with resistance to Lyme arthritis, were also analyzed (5, 44, 47, 57, 58). At 2 weeks postinfection, capture ELISA revealed diminished IFN-γ, IL-12, and TNF-α levels and increased IL-6 levels in the sera of the coinfected mice compared to B. burgdorferi-infected mice (Fig. 4). Three separate experiments showed that these results were statistically significant (TNF-α, P < 0.005; IFN-γ, P < 0.05; IL-12, P < 0.001; IL-6, P < 0.05 [Student's t test]). IL-5 levels were not appreciably different between the groups (IL-5, P > 0.5).
FIG. 4.
IL-12, IFN-γ, TNF-α, IL-5, and IL-6 levels in murine sera. Sera were pooled from groups of five control (uninfected), B. burgdorferi-infected, or B. burgdorferi- and HGE-infected mice. IFN-γ (A), IL-12 (B), TNF-α (C), IL-6 (D), and IL-5 (E) levels were analyzed by capture ELISA. Results from one of three comparable experiments are presented. ∗, P < 0.05 using Student's t test, as stated in the text. Error bars indicate standard deviations.
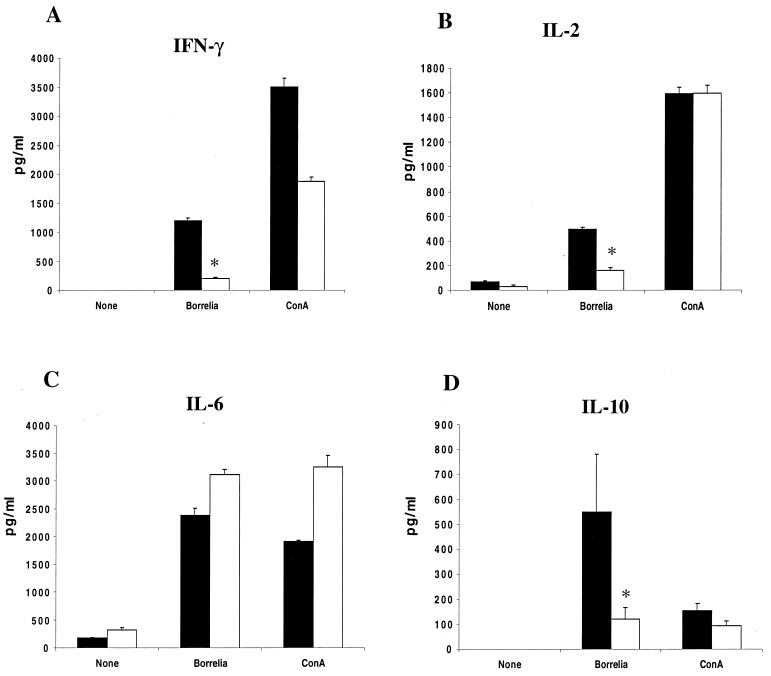
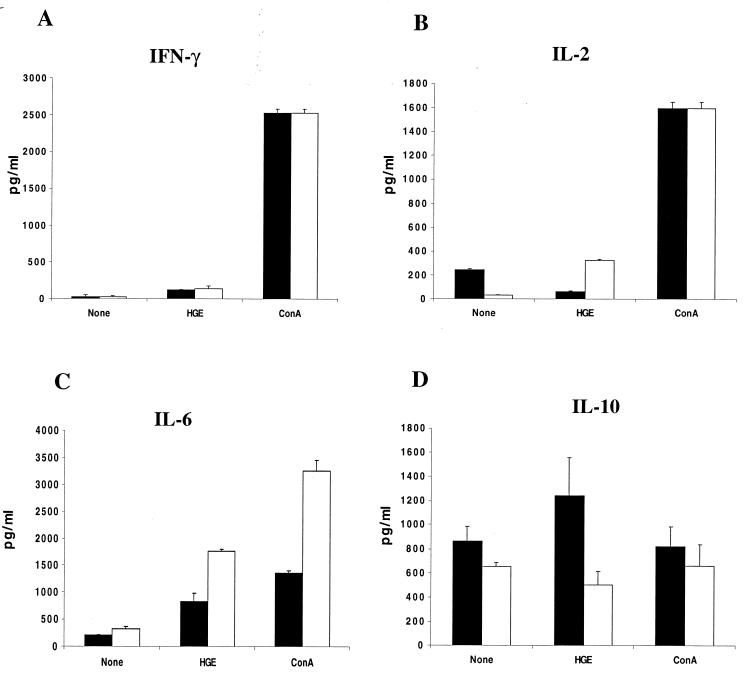
Restimulation assays were then performed to examine responses directed towards either B. burgdorferi or Ehrlichia antigens. At 2 weeks, splenocytes from infected mice were stimulated in vitro with either B. burgdorferi or HGE lysates. Four separate studies with groups of five mice are presented. The medium supernatants were assessed after 48 h of stimulation for cytokine production. In addition to IFN-γ and IL-6, which were evaluated in the sera, IL-2 and IL-10 were assessed as indicators of T-cell activation. B. burgdorferi lysates elicited lower levels of IFN-γ (P < 0.05), IL-2 (P < 0.005), and IL-10 (P < 0.05) (Fig. 5A, B, and D) and similar levels of IL-6 (P > 0.5) (Fig. 5C) in coinfected mice. HGE lysates elicited similar levels of IFN-γ (P > 0.05), IL-2 (P > 0.5), IL-6 (P > 0.1), and IL-10 (P > 0.5) in the HGE-infected and coinfected mice (Fig. 6).
FIG. 5.
IFN-γ, IL-2, IL-10, and IL-6 responses to B. burgdorferi during coinfection. Splenocytes (106 cells/ml) were pooled from groups of three mice and stimulated in vitro with either 10 μg of B. burgdorferi lysates or 4 μg of ConA per ml for 48 h. Culture supernatants were collected and analyzed for IFN-γ (A), IL-2 (B), IL-6 (C), and IL-10 (D) by capture ELISA. Black and white bars, supernatants from B. burgdorferi-infected and coinfected mice, respectively. Results from one of three similar experiments are presented. ∗, P < 0.05 using Student's t test, as stated in the text. Error bars indicate standard deviations.
FIG. 6.
IFN-γ, IL-2, IL-10, and IL-6 responses to HGE during coinfection. Splenocytes (106 cells/ml) were pooled from groups of three mice and stimulated with either 10 μg of HGE lysate or 4 μg of ConA per ml for 48 h. Culture supernatants were analyzed for the presence of IFN-γ (A), IL-2 (B), IL-6 (C), and IL-10 (D) by capture ELISA. Black and white bars, supernatants from HGE-infected and coinfected mice, respectively. Results from one of three similar experiments are presented. Error bars indicate standard deviations.
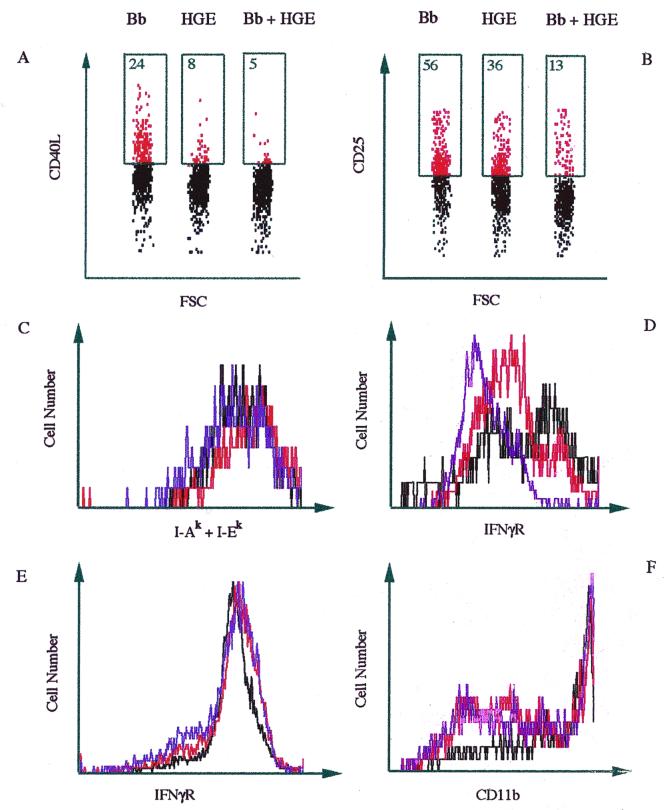
The reduced levels of IL-2 produced by splenocytes of coinfected mice upon stimulation with B. burgdorferi lysates suggested that these CD4+ T cells may not exhibit the same degree of activation as cells from mice infected with B. burgdorferi alone. The expression of T-cell activation markers such as CD40 ligand and IL-2 receptor (CD25) was therefore investigated by fluorescence-activated cell sorter analysis. One experiment using five mice in each infection group is presented in Fig. 7. CD4+ T cells from coinfected mice showed a reduction in expression of the CD40 ligand (Fig. 7A). Twenty-four percent of the cells from B. burgdorferi-infected mice showed expression of CD40 ligand, while HGE-infected and coinfected mice showed 8 and 5% expression, respectively (Fig. 7A). Results from three separate experiments demonstrated that the average reduction in CD40 ligand in mixed infection was 84% ± 9% compared with B. burgdorferi infection and 59% ± 19% compared with Ehrlichia infection. CD25 was also reduced with coinfection (Fig. 7B). Fifty-six percent of the CD4+ T cells from B. burgdorferi-infected mice expressed CD25, while HGE-infected and coinfected mice showed 36 and 13% expression, respectively (Fig. 7B). Three different experiments showed that the average reduction in CD25 in mixed infection was 65% ± 13% compared with B. burgdorferi infection and 35% ± 30% compared with Ehrlichia infection. We also evaluated the activation status of the neutrophils and macrophages by examining levels of the IFN-γ receptor, MHC class II, and CD11b (Mac-1). Macrophages from the coinfected mice demonstrated reduced expression of IFN-γ receptor compared to those from the singly infected mice (Fig. 7D). From three experiments, the average reduction in IFN-γ receptor in mixed infection was 72% ± 32% compared with B. burgdorferi infection and 63% ± 42% compared with Ehrlichia infection. Expression of MHC class II was not affected (Fig. 7C). Neutrophils from coinfected mice also showed levels of IFN-γ receptor (Fig. 7E) and CD11b (Fig. 7F) similar to those of neutrophils from either B. burgdorferi- or HGE-infected mice.
FIG. 7.
Decreased activation of CD4+ T cells and macrophages during coinfection. CD4+ T cells were stained with anti-CD4 and analyzed for the presence of CD40 ligand (A) or CD25 (B). Numbers within the boxes represent the percent expression. Bb, B. burgdorferi. Macrophages were stained with anti-Mac-3 and analyzed for the presence of MHC class II (I-Ak + I-Ek) (C) and IFN-γ receptor (IFNγR) (CD119α) (D). Neutrophils were stained with anti-Ly6-G and analyzed for the presence of IFN-γ receptor (CD119a) (E) and CD11b (F). Cells were collected and pooled from spleens of three HGE-infected (red lines), B. burgdorferi-infected (black lines), or coinfected (blue lines) mice. Results from one of three experiments with comparable results are shown.
DISCUSSION
Infection with more than one pathogen, including P. chaubaudi and S. mansoni (37), S. mansoni and T. gondii (53), and Candida albicans and E. coli (2), can result in altered host responses and disease. We now show that coinfection with B. burgdorferi and the HGE agent, two organisms that can be transmitted by the same I. scapularis ticks, results in elevated bacterial burden, modified immune responses, increased Lyme arthritis, and enhanced pathogen transmission from the host back to the vector. Dual infection may therefore assist in the persistence of these microbes, which are ecologically linked and influence the clinical outcome of human infection.
Our results revealed that coinfection with B. burgdorferi and the HGE agent enhances B. burgdorferi pathogenesis. We observed that in coinfection the spirochete burden was markedly elevated at the peak phase of disease (2 weeks) and was accompanied by an increase in the severity of acute murine Lyme arthritis. Paradoxically, this was associated with reduced levels of IL-12, TNF-α, and IFN-γ, Th1-type cytokines that are generally associated with the development of more severe disease, during coinfection. However, IL-6, a cytokine shown to direct Th2 responses (60) and to help decrease Lyme arthritis in C57BL/6 (B6) mice (5), was increased in the sera during dual infection. The relative influence of B. burgdorferi numbers and specific cytokine responses may contribute to the disease outcome, depending on the situation (4, 14, 15, 55, 74). For example, bacterial numbers can directly affect disease, because resistance to Lyme arthritis in BALB/c mice can be overcome by infecting the animals with higher numbers of spirochetes (51) and the lack of pathogenesis of long-term-passage cN40 spirochetes (N40-75) is directly correlated with reduced bacterial burden in the joint tissues (6). On the other hand, inhibition of IL-12 in immunocompetent mice results in decreased joint inflammation, even when B. burgdorferi levels are elevated (4). During dual infection, the increased bacterial burden and elevated IL-6 levels in sera could have been more important factors than the reduction in the levels of IFN-γ, IL-12, and TNF-α in the sera.
Antibodies have been shown to influence B. burgdorferi and HGE bacterial clearance and the course of Lyme arthritis (12, 24, 25, 29, 32, 46, 69). Our data show that CD4+ T cells from coinfected mice had reduced levels of expression of CD40 ligand, an important costimulatory signal for B-cell activation, suggesting that antibody responses may be diminished. However, consistent with previous observations that protective antibodies to B. burgdorferi can arise in the absence of T-cell help (25, 26), antibody titers to these pathogens were not affected by coinfection, except for the slight decrease in IgG3 antibodies to HGE-44 during coinfection. Therefore, humoral responses are not likely to play a dominant role in affecting the course of disease during coinfection.
Dual infection also resulted in the decreased activation of macrophages, cells that are important in innate immune responses and bacterial clearance. In particular, expression of the IFN-γ receptor was lower on macrophages. Since antigen presentation and antimicrobial activity can be induced by IFN-γ, the reduction in expression of the IFN-γ receptor suggests that activation of these phagocytic cells may be impaired during coinfection, resulting in increased numbers of each pathogen.
Our results also demonstrated that coinfection influences levels of the HGE agent. Recent reports demonstrate that IFN-γ may be involved in the control of HGE infection, because mice begin to clear Ehrlichia after IFN-γ levels become readily detectable (3, 54). Moreover, HGE infection of IFN-γ-deficient mice resulted in elevated levels of Ehrlichia (3). The reduced levels of IFN-γ and the IFN-γ receptor in coinfection may create a favorable environment for survival of the HGE agent, thereby resulting in an increased percentage of neutrophils with morulae during coinfection.
Enhanced larval acquisition of both pathogens was also observed. At 1 week of infection, ticks that fed on coinfected mice acquired larger numbers of spirochetes than ticks that engorged on B. burgdorferi-infected mice. Surprisingly, at 1 week of infection, the HGE agent was more readily detected in larvae that fed on coinfected mice and not in larvae that engorged on mice infected with HGE alone. Levin and Fish have recently shown that nymphal ticks infected with either Ehrlichia or B. burgdorferi can acquire the second pathogen from infected mice (50). Nymphal procurement of a second pathogen is a different phenomenon from larval acquisition of both pathogens from coinfected mice and suggests that the interplay between vector-host and pathogen may be dependent upon the infection status of the host, developmental stage of the tick, and other multifactorial influences. Indeed, the HGE agent must be able to complete the transition from mouse to tick in the absence of B. burgdorferi, because in the natural environment both mice and ticks can harbor HGE bacteria without concomitant B. burgdorferi infection. The present studies demonstrate, however, that the natural transmission of each pathogen in the vector-host-vector life cycle is facilitated by coinfection.
We have demonstrated that coinfection can influence the B. burgdorferi and Ehrlichia pathogen burden, the pathogenesis of Lyme arthritis, and transmission of the HGE agent and B. burgdorferi from the murine host to the tick vector. Coinfection influences the host in several ways, and a single response is not directly responsible for enhancing disease. These findings may have implications for human Lyme disease, which can range from acute cutaneous disease with erythema migrans to persistent infection with cardiac, musculoskeletal, and neurologic involvement, and for HGE, which may be self-limited or severe. In particular, the increase in joint inflammation in the murine studies suggests that coinfection may influence the severity of human Lyme arthritis. Epidemiological studies have previously suggested that concurrent Lyme disease and babesiosis (a disease caused by a protozoan that is transmitted by I. scapularis) result in increased Lyme disease severity, providing one clinically relevant example of this phenomenon (46a). Our present murine studies now suggest that B. burgdorferi and the agent of HGE transmission and pathogenicity increase during coinfection, and this may be one explanation for the similar natural reservoirs, vectors, and geographic distributions of these pathogens and for differences in the severity of disease.
ACKNOWLEDGMENTS
We thank Deborah Beck for technical assistance.
This work was supported by grants from the National Institutes of Health, the Arthritis Foundation, and the American Heart Association and by a gift from SmithKline Beecham Biologicals. Erol Fikrig is a recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
REFERENCES
- 1.Ahkee S, Ramirez J. A case of concurrent Lyme meningitis with ehrlichiosis. Scand J Infect Dis. 1996;28:527–528. doi: 10.3109/00365549609037953. [DOI] [PubMed] [Google Scholar]
- 2.Akagawa G, Abe S, Yamaguchi H. Mortality of Candida albicans-infected mice is facilitated by superinfection of Escherichia coli or administration of its lipopolysaccharide. J Infect Dis. 1995;171:1539–1544. doi: 10.1093/infdis/171.6.1539. [DOI] [PubMed] [Google Scholar]
- 3.Akkoyunlu M, Fikrig E. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect Immun. 2000;68:1827–1833. doi: 10.1128/iai.68.4.1827-1833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anguita J, Rincon M, Samanta S, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J Infect Dis. 1998;178:1512–1515. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 6.Anguita J, Samanta S, Revilla B, Suk K, Das S, Barthold S W, Fikrig E. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect Immun. 2000;68:1222–1230. doi: 10.1128/iai.68.3.1222-1230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 8.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 9.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25(Suppl. 1):S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 13.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 14.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunnell J E, Magnarelli L A, Dumler J S. Infection of laboratory mice with the human granulocytic ehrlichiosis agent does not induce antibodies to diagnostically significant Borrelia burgdorferi antigens. J Clin Microbiol. 1999;37:2077–2079. doi: 10.1128/jcm.37.6.2077-2079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunnell J E, Trigiani E R, Srinivas S R, Dumler J S. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J Infect Dis. 1999;180:546–550. doi: 10.1086/314902. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y F, Novosel V, Chang C F, Kim J B, Shin S J, Lein D H. Detection of human granulocytic ehrlichiosis agent and Borrelia burgdorferi in ticks by polymerase chain reaction. J Vet Diagn Investig. 1998;10:56–59. doi: 10.1177/104063879801000110. [DOI] [PubMed] [Google Scholar]
- 19.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels T J, Falco R C, Schwartz I, Varde S, Robbins R G. Deer ticks (Ixodes scapularis) and the agents of Lyme disease and human granulocytic ehrlichiosis in a New York City park. Emerg Infect Dis. 1997;3:353–355. doi: 10.3201/eid0303.970312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 23.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 24.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fikrig E, Barthold S W, Chen M, Chang C H, Flavell R A. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J Immunol. 1997;159:5682–5686. [PubMed] [Google Scholar]
- 26.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 27.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992;60:773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, 3rd, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. . (Erratum, 9:775, 1998.) [DOI] [PubMed] [Google Scholar]
- 29.Fikrig E, Bockenstedt L K, Barthold S W, Chen M, Tao H, Ali-Salaam P, Telford S R, Flavell R A. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis. 1994;169:568–574. doi: 10.1093/infdis/169.3.568. [DOI] [PubMed] [Google Scholar]
- 30.Fikrig E, Feng W, Barthold S W, Telford S R, Flavell R A. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J Immunol. 2000;164:5344–5351. doi: 10.4049/jimmunol.164.10.5344. [DOI] [PubMed] [Google Scholar]
- 31.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. . (Erratum, 335:361.) . [DOI] [PubMed] [Google Scholar]
- 34.Gross D M, Steere A C, Huber B T. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–1028. [PubMed] [Google Scholar]
- 35.Gubler D J. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis. 1998;4:442–450. doi: 10.3201/eid0403.980326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habicht G S, Beck G, Benach J L. Lyme disease. Sci Am. 1987;257:78–83. doi: 10.1038/scientificamerican0787-78. [DOI] [PubMed] [Google Scholar]
- 37.Helmby H, Kullberg M, Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect Immun. 1998;66:5167–5174. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodzic E, Fish D, Maretzki C M, De Silva A M, Feng S, Barthold S W. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. 1998;36:3574–3578. doi: 10.1128/jcm.36.12.3574-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodzic E, Ijdo J W, Feng S, Katavolos P, Sun W, Maretzki C H, Fish D, Fikrig E, Telford S R, Barthold S W. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177:737–745. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh T, DiPietrantonio A M, Horowitz H W, Dumler J S, Aguero-Rosenfeld M E, Wormser G P, Wu J M. Changes in expression of the 44-kilodalton outer surface membrane antigen (p44 kD) for monitoring progression of infection and antimicrobial susceptibility of the human granulocytic ehrlichiosis (HGE) agent in HL-60 cells. Biochem Biophys Res Commun. 1999;257:351–355. doi: 10.1006/bbrc.1999.0457. [DOI] [PubMed] [Google Scholar]
- 41.Hu L T, Klempner M S. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J Clin Immunol. 1997;17:354–365. doi: 10.1023/a:1027308122565. [DOI] [PubMed] [Google Scholar]
- 42.Ijdo J W, Sun W, Zhang Y, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol. 1995;155:4604–4612. [PubMed] [Google Scholar]
- 45.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 46.Kim H Y, Rikihisa Y. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J Clin Microbiol. 1998;36:3278–3284. doi: 10.1128/jcm.36.11.3278-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Krause P J, Telford S R, 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 47.Lamkhioued B, Gounni A S, Aldebert D, Delaporte E, Prin L, Capron A, Capron M. Synthesis of type 1 (IFN gamma) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Ann N Y Acad Sci. 1996;796:203–208. doi: 10.1111/j.1749-6632.1996.tb32582.x. [DOI] [PubMed] [Google Scholar]
- 48.Lebech A M, Hansen K, Pancholi P, Sloan L M, Magera J M, Persing D H. Immunoserologic evidence of human granulocytic ehrlichiosis in Danish patients with Lyme neuroborreliosis. Scand J Infect Dis. 1998;30:173–176. doi: 10.1080/003655498750003582. [DOI] [PubMed] [Google Scholar]
- 49.Levin L M, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–208. doi: 10.3201/eid0502.990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levin L M, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnarelli L A, Dumler J S, Anderson J F, Johnson R C, Fikrig E. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J Clin Microbiol. 1995;33:3054–3057. doi: 10.1128/jcm.33.11.3054-3057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall A J, Brunet L R, van Gessel Y, Alcaraz A, Bliss S K, Pearce E J, Denkers E Y. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- 54.Martin M E, Bunnell J E, Dumler J S. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J Infect Dis. 2000;181:374–378. doi: 10.1086/315206. [DOI] [PubMed] [Google Scholar]
- 55.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzella F M, Roman A, Perez A. A case of concurrent presentation of human ehrlichiosis and Lyme disease in Connecticut. Conn Med. 1996;60:515–519. [PubMed] [Google Scholar]
- 57.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 58.Mosmann T R, Schumacher J H, Fiorentino D F, Leverah J, Moore K W, Bond M W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- 59.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford S R, 3rd, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 60.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santiago H C, Oliveira M A, Bambirra E A, Faria A M, Afonso L C, Vieira L Q, Gazzinelli R T. Coinfection with Toxoplasma gondii inhibits antigen-specific Th2 immune responses, tissue inflammation, and parasitism in BALB/c mice infected with Leishmania major. Infect Immun. 1999;67:4939–4944. doi: 10.1128/iai.67.9.4939-4944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schauer D B, McCathey S N, Daft B M, Jha S S, Tatterson L E, Taylor N S, Fox J G. Proliferative enterocolitis associated with dual infection with enteropathogenic Escherichia coli and Lawsonia intracellularis in rabbits. J Clin Microbiol. 1998;36:1700–1703. doi: 10.1128/jcm.36.6.1700-1703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz I, Fish D, Daniels T J. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- 64.Simon M M, Schaible U E, Wallich R, Kramer M D. A mouse model for Borrelia burgdorferi infection: approach to a vaccine against Lyme disease. Immunol Today. 1991;12:11–16. doi: 10.1016/0167-5699(91)90106-4. [DOI] [PubMed] [Google Scholar]
- 65.Stanek G, Burger I, Hirschl A, Wewalka G, Radda A. Borrelia transfer by ticks during their life cycle. Studies on laboratory animals. Zentbl Bakteriol Mikrobiol Hyg. 1986;263:29–33. doi: 10.1016/s0176-6724(86)80098-0. [DOI] [PubMed] [Google Scholar]
- 66.Steere A C, Bartenhagen N H, Craft J E, Hutchinson G J, Newman J H, Pachner A R, Rahn D W, Sigal L H, Taylor E, Malawista S E. Clinical manifestations of Lyme disease. Zentbl Bakteriol Mikrobiol Hyg. 1986;263:201–205. doi: 10.1016/s0176-6724(86)80123-7. [DOI] [PubMed] [Google Scholar]
- 67.Steere A C, Gibofsky A, Patarroyo M E, Winchester R J, Hardin J A, Malawista S E. Chronic Lyme arthritis: clinical and immunologic differentiation from rheumatoid arthritis. Ann Intern Med. 1979;90:896–901. doi: 10.7326/0003-4819-90-6-896. [DOI] [PubMed] [Google Scholar]
- 68.Straubinger R K, Straubinger A F, Summers B A, Erb H N, Harter L, Appel M J. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect Immun. 1998;66:247–258. doi: 10.1128/iai.66.1.247-258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun W, Ijdo J W, Telford S R, Hodzic E, Zhang Y, Barthold S W, Fikrig E. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Investig. 1997;100:3014–3018. doi: 10.1172/JCI119855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Telford S R, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wormser G P, Horowitz H W, Nowakowski J, McKenna D, Dumler J S, Varde S, Schwartz I, Carbonaro C, Aguero-Rosenfeld M. Positive Lyme disease serology in patients with clinical and laboratory evidence of human granulocytic ehrlichiosis. Am J Clin Pathol. 1997;107:142–147. doi: 10.1093/ajcp/107.2.142. [DOI] [PubMed] [Google Scholar]
- 72.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthrit Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 74.Yssel H, Shanafelt M C, Soderberg C, Schneider P V, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 76.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]