Abstract
Introduction
Ulcerative colitis (UC) is a chronic non-specific inflammatory bowel disease, and until now therapeutic agents for UC still cannot exert satisfied effects. Therefore, this study aimed to investigate the ameliorative effect of boswellic acid coated zinc nanoparticles (BAs-ZnNPs) on dextran sodium sulphate (DSS) induced-UC in rats.
Methods
Rats were divided into five groups; control, BAs-ZnNPs, DSS, DSS+BAs, and DSS + BAs-ZnNPs. The activity of alkaline phosphatase (ALP) was determined colorimetrically, while the concentration of IgM, IgG, TNF-α, IL-1β, and IL-8 were measured by ELISA. Levels of gene expression of NF-κB and COX-2 genes were evaluated by RT-qPCR, while the expression of protein levels of PI3K and STAT-3 were done by western blotting. Finally, histopathological examination of colon tissues of different groups of rats was done.
Results
The depicted ball-like structure of the BAs-ZnNPs in the TEM images ranging in size from 50 to 100 nm in diameter while their formation was confirmed by UV–visible spectroscopy with a sharp peak of maximum absorbance at 266 nm. Our results revealed that BAs-ZnNPs exerted an anti-inflammatory effect in the experimental model of colitis, demonstrated histologically and biochemically as shown by the improvement of ALP, IgM, IgG, and the gene expression levels of NF-κB and COX-2. Also, this beneficial effect was associated with the reduction in the expression of TNF-α, IL-1β, IL-8, PI3K, and STAT-3. Thus, this effect improves the altered immune response associated with the colonic inflammation.
Conclusion
BAs-ZnNPs can be proposed as a therapeutic candidate to attenuate UC. The potential underlying mechanism includes suppression of ALP, IgM, IgG, IL-1β, and IL-8 levels via regulation of NF-κB and COX-2 gene expression and STAT-3 and PI3K protein expression in a UC rat model.
Keywords: Ulcerative colitis, zinc nanoparticles, boswellic acids, nuclear factor-κB, cyclooxygenase-2, dextran sodium sulphate, phosphatidylinositol-3-kinase
Introduction
Ulcerative colitis (UC) is one of the two major subtypes of inflammatory bowel diseases (IBDs) and characterized by chronic inflammation, ulcers, and severe epithelial damage with disruption in colon homeostasis.1 According to the World Health Organization (WHO), the prevalence of UC is about 250 per 100,000, affecting both sexes equally and any age.2 Worldwide, IBDs cause essential morbidity and heavy productivity losses, and as a result of the increasing rate of incidence and prevalence of IBDs and the lack of a cure or effective long-term treatment options, there is a substantial financial burden to the healthcare systems.3
The current conventional therapeutic options (such as immunosuppressive agents, aminosalicylates, corticosteroids, and/or surgery) cause serious negative side effects and are characterized by poor long-term therapeutic efficacy, and a high short-term recurrence rate. Thus, there is an urgent need for developing new effective products with anti-inflammatory activity.4 In last years, herbal medicines have been used in UC treatment such as Boswellia serrate (Boswellia serrata).5 Frankincense, the extracted gum resin of B. serrata, has been used for centuries as a potential therapy for the treatment of several inflammatory diseases such as IBDs, diabetes, arthritis, asthma, or cancer,6 also its anti-inflammatory properties were confirmed.7 Frankincense is a multicomponent mixture containing boswellic acids (BAs) which are pentacyclic triterpenic acids and have been investigated intensively by modern medicine due to their potential therapeutic efficacy against several chronic inflammatory diseases.8
Zinc is an essential trace element for the living organisms, and is the second most abundant trace element in the human body which is essential for many physiological functions, such as growth, antioxidant activity, cellular signal pathways, and immunity. Moreover, zinc homeostatic imbalance is associated with many intestinal diseases.9 For decades, the nanomedicine field was found to improve treatment outcomes for millions of patients.10 Recently, zinc nanoparticles (Zn-NPs) are used widely in biomedicine, especially as anticancer and antibacterial, due to their protective and beneficial effects.11,12 Therefore, the present study aims to evaluate the ameliorative and anti-inflammatory effects of boswellic acid coated zinc nanoparticles (BA-ZnNPs) in DSS-induced colitis in rats.
Materials and methods
Chemicals
Various chemicals used in this study, such as boswellic acid, dimethylsulphoxide, zinc chloride (ZnCl2), and sodium hydroxide (NaOH), were procured from Sigma Aldrich Chemical Co., St. Louis, Mo. USA.
Experimental animals
Adult (20 weeks old) male albino rats weighing about 140 ± 20 gm were obtained from the Nile Pharmaceutical Co., Cairo, Egypt. The animals were kept under standard laboratory conditions of light/dark cycle (12/12 h), at a temperature of 25 ± 2°C and humidity of 60 ± 5%, and were housed in cages with free access to food and drinking water. Animals were fed on standard pellet diet and sterilized water was provided ad libitum. All animal procedures were carried out in accordance with the Ethics committee of The National Center for Radiation Research and Technology (89A/21).
Chemical studies
Synthesis of boswellic acid coated zinc nanoparticles (BAs-ZnNPs)
Firstly, BA was dissolved in 10 mM dimethylsulphoxide, and then the volume was completed to 100 ml. Secondly, zinc chloride (ZnCl2) was dissolved in deionized water to prepare a concentration of 10 mM. Then, 10 ml of boswellic acid was mixed with 2 ml of ZnCl2 and the pH of the solution was immediately adjusted to 10.0 by 1.0 M NaOH. Finally, the reaction mixture was maintained at room temperature for 30 min, and BAs-ZnNPs were condensed and purified by centrifugation at 15,000 r.p.m for 10 min and then washed with double-distilled water three times.
Characterization of BAs-ZnNPs
The prepared BAs-ZnNPs sample was characterized with microscopic and spectroscopic methods. The sample for transmission electron microscopy (TEM) of the synthesized BAs-ZnNPs was prepared to determine size and morphology. The micrographs were obtained on TEM (HRTEM TEOL JEM-2100, Japan). The sample of BAs-ZnNPs was analyzed for size distribution throughout dynamic light scattering (DLS) by photon correlation spectroscopy using a Zetasizer ZS instrument (Malvern Instruments Ltd., Malvern, UK). For Fourier transform infrared spectroscopy (FT-IR) measurements, the prepared BAs-ZnNPs sample was analyzed for functional groups using an FT-IR spectrometer (FT-IR, VERTEX 70/70v model).
Biochemical studies
Experimental design
Fifty male albino rats weighing (140 ± 20 gm) were equally divided randomly into the following five groups:
• Group 1 (Control): Untreated control group of healthy male rats.
• Group 2 (BAs-ZnNPs): Group of rats was orally administered with BAs-ZnNPs (1 mg/kg b.wt. five times per week for 6 weeks).
• Group 3 (DSS): Group of rats was administered orally with 3% (w/v) DSS in their drinking water for 7 days, according to Li et al.13
• Group 4 (DSS + BAs): Group of rats was administrated 3% DSS as in group 3 after the last dose of DSS, animals which were administered orally received the largest dose of BAs (500 mg/kg) five times per week for 6 weeks, according to Sami et al.14
• Group 5 (DSS + BAs-ZnNPs): Group of rats was administrated 3% DSS as in group 3 after the last dose of DSS, BAs-ZnNPs was administrated orally as in group 2.
Twenty-four hours after the last treatment, all rats were anesthetized using urethane, then scarified, and blood samples were obtained via heart puncture and collected in plain vacutainer tubes. Then, samples were left to coagulate and the serum was obtained after being centrifuged at 4000 r.p.m for 10 min for biochemical analysis. The colon was excised from animals in all groups. Part of the collected colons was homogenized (10% w/v) in phosphate-buffered saline (0.02 M sodium phosphate buffer with 0.15 M sodium chloride, pH 7.4) using a glass homogenizer, producing homogenates which were used for biochemical and molecular analysis and the other part was quickly rinsed in tap water and then kept in 10% formalin for histopathological examination.
Biochemical investigation
The level of alkaline phosphatase (ALP) was determined in the serum using colorimetric method (Bio-diagnostic kit), Cairo, Egypt. The levels of IgM, IgG in serum, TNF-α, IL-1β, and IL-8 in colon tissue were determined using enzyme-linked immunosorbent assay (ELISA) kit (Quantikine® ELISA Rat IgM, IgG, TNF-α, IL-1β and IL-8 kits).
Molecular investigation
Quantitative real-time polymerase chain reaction
Levels of gene expression of nuclear factor κB (NF- κB) and cyclooxygenase-2 (COX-2) genes were evaluated by using RT-qPCR. Firstly, total RNA was isolated from 50 mg colon using TRIzol reagent (Invitrogen), and then first-strand complementary DNA (cDNA) was synthesized using reverse transcriptase (Invitrogen) and 1 μg RNA template.
RT-qPCR was performed in a thermal cycler Step One Plusb (Applied Biosystems, USA) using the Sequence Detection Software (PE Biosystems, CA). The primers utilized for COX-2 and NF-kB with β-actin gene are represented in Table 1. A reaction mixture of a total volume of 25 μl consists of 2× SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each primer, and 2 μl of cDNA. PCR thermal cycling conditions included an initial step at 95°C for 5 min and 40 cycles at 95°C for 20 s, 60°C for 30 s, and 72°C for 20 s. The relative mRNA expression of the studied genes was calculated using the comparative threshold cycle method of Pfaffl.15
Table 1.
List of primers used for quantitative real-time polymerase chain reaction.
| Gene symbol | Primers sequence |
|---|---|
| NF-κβ | F: 5′-ACAACCCCTTCCAAGTTCCCT-3′ |
| R: 5′-TGGTCCCGTGAAATACACCT-3′ | |
| COX-2 | F: 5′-TGCGATGCTCTT CCGAGCTGTGCT3′ |
| R: 5′ TCA GGAAGTTCCTTATTTCCTTTC3′ | |
| β-Actin (rat) | F: 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
| R: 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Western blotting of STAT-3 and PI3K proteins
Phosphatidylinositol-3-kinase (PI3K) and Signal transducer and activator of transcription 3 (STAT-3) proteins were extracted from colon tissues using TRIzol reagent (Invitrogen), and protein concentrations were quantified according to Bradford.16 Twenty micrograms of proteins per lane was separated with 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were incubated at room temperature for 2 h with blocking solution (5% non-fat dried milk in 10 mM Tris–Cl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20) and then incubated overnight at 4°C with rabbit IgG PI3K or STAT-3 antibody (Dilution ratio 1:1000) (Cell Signaling Technologies, USA) and β-actin antibody (Proteintech, USA) as loading control. After washing three times in 10 mM Tris–Cl at pH 7.5 with 100 mM NaCl and 0.1% Tween 20, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG (Dilution ratio 1:2000) (Cell Signaling Technologies, USA) at room temperature for 2 h, and then the membranes were washed four times with the washing buffer. The membranes were developed and visualized by chemiluminescence using Amersham detection kit according to the manufacturer’s protocols and then exposed to X-ray film. Quantification of PI3K or STAT-3 proteins was carried out using a scanning laser densitometer analysis (Biomed Instrument Inc., USA).
Histopathological examination
Colon tissue specimens were collected from colon fixed in formalin 10% and trimmed off, washed, and dehydrated in ascending grades of alcohol. The dehydrated specimens were then cleared in xylene, embedded in paraffin blocks and sectioned at 4–6 μm thick. The obtained tissue sections were deparaffinized using xylol and stained using hematoxylin and eosin (H&E) for histopathological examination through the electric light microscope according to Bancroft et al.17 The parameters used for scoring of colonal ulcer based on a 0–3 described by Gaudio et al.18 with little modification: (1) Destruction of epithelium and glands: 0 = morphologically normal, 1 = focal destruction of the epithelial surface and/or focal crypt dropout, 2 = zonal destruction of the epithelial surface and/or zonal crypt loss, 3 = diffuse and/or mucosal ulceration involving submucosa and/or diffuse crypt loss; (2) Inflammatory cells infiltration: 0 = absence of infiltration, 1 = infiltrate at the subepithelial and lamina propria level or crypt bases, 2 = infiltration reaching muscularis mucosa, 3 = severe and extensive infiltration reaching submucosa and/or involving muscularis propria; (3) Hemorrhagic mucosa: 0 = absent, 1 = focal, 2 = zonal, 3 = diffuse.
Statistical analysis
Statistical analysis was performed using IBM SPSS software (version 23.0; IBM Corp., Armonk, NY, USA), and data were presented as means ± SE. Data were analyzed with one-way analysis of variance (ANOVA) followed by post hoc test (LSD) for multiple comparisons. The criterion for significance was p < 0.05.
Results
Characterization of BAs-ZnNPs
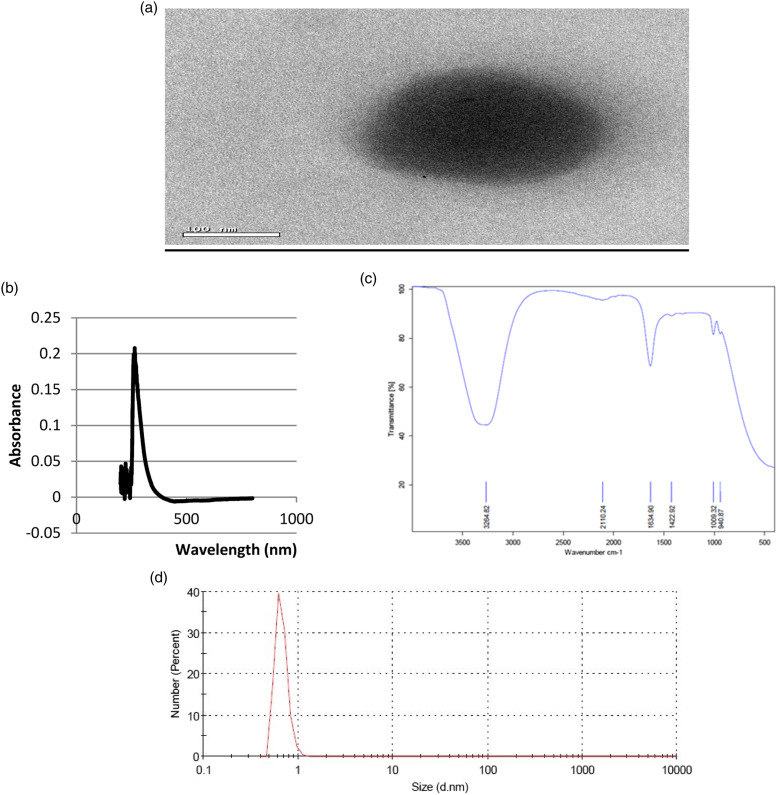
The prepared BAs-ZnNPs were characterized by using different techniques. Firstly, the size and morphology of the synthesized BAs-ZnNPs were characterized by transmission electron microscopy (TEM). TEM images of the prepared BAs-ZnNPs showed a uniform distribution and confirmed their spherical morphology. The depicted ball-like structure of the BAs-ZnNPs in the TEM images ranging in size from 50 to 100 nm in diameter (Figure 1(a)) with a thin film encapsulating the nanoballs was seen, confirming the presence of a polymeric layer covering the nanoballs. The formation of BAs-ZnNPs was confirmed by UV–visible spectroscopy with a sharp peak of maximum absorbance at 266 nm indicating the synthesis of BAs-ZnNPs, Figure 1(b). The FT-IR peaks of BAs-ZnNPs showed a strong peak at 3318.71 (hydroxyl group) and a peak at 1636.47 (amide I bond), and fingerprint signals at 978.55–607.42 arise from a single bond of carbon atom bound to hydrogen or nitrogen (Figure 1(c)). Finally, DLS measurement of the hydrodynamic effective diameter of the synthesized BAs-ZnNPs showed that the zeta average diameter was measured to be 127 ± 2.5 nm with 0.957 poly dispersity index (PDI) indicating the homogeneity and uniform dispersion of the synthesized BAs-ZnNPs (Figure 1(d)). The disagreement between the sizes observed by TEM and DLS was due to the fact that DLS measures the hydrodynamic volume while TEM analyzes the metallic core. Zeta potential was determined to be −0.555 mV. These results confirmed the presence of bioactive molecules and functional groups in BAs-ZnNPs preparations. It appeared that there is no chemical interaction interfered with the synthesis of BAs-ZnNPs and the prepared nanoparticles were capped by physical interaction with organic molecules.
Figure 1.
Characterization of BAs-ZnNPs. (a) Transmission electron microscopic image of BAs-ZnNPs; (b) UV absorbance of BAs-ZnNPs; (c) FTIR wavenumber of BAs-ZnNPs; (d) Dynamic light scattering of BAs-ZnNPs.
Effect of BAs-ZnNPs on immunoglobulins and inflammatory cytokines
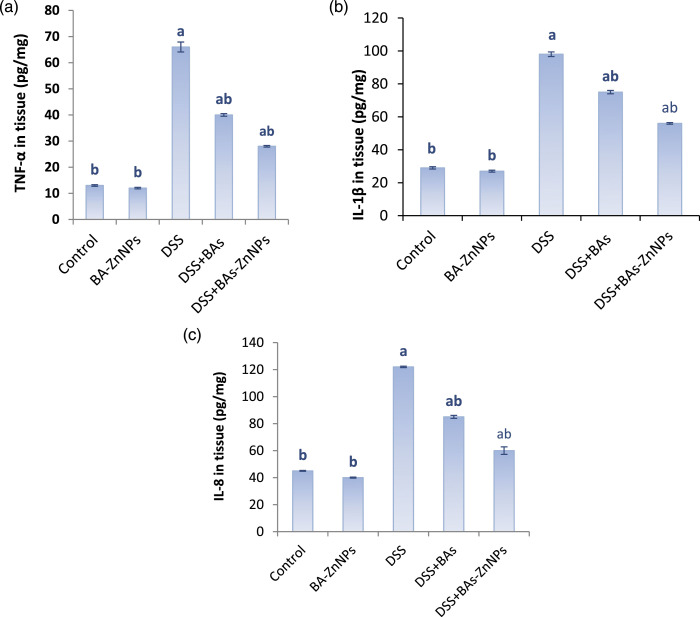
The effect of BAs-ZnNPs on immunoglobulins and inflammatory cytokines in the sera of different studied groups is evaluated as shown in Table 2 and Figure 2. It was found that DSS treatment induced ulcerative colitis throughout the significant abnormalities in ALP (292.2 ± 4.8 IU/L), IgM (41.1 ± 0.4 mg/dL), and IgG (152.9 ± 3.9 mg/dL), which ameliorated significantly after the administration of BAs-ZnNPs (177.5 ± 1.0 IU/L, 30 ± 0.1 mg/dL and 108 ± 1.5 mg/dL; respectively) more than the administration of BAs (240 ± 1.5 IU/L, 36 ± 0.6 mg/dL and 125 ± 1.3 mg/dL, respectively), as shown in Table 2. It is worthy to note that DSS intoxication promoted significant increase in the levels of colonic tissue inflammatory cytokines TNF-α (66 ± 1.9 pg/mg), IL-1β (98 ± 1.4 pg/mg), and IL-8 (122 ± 0.5 pg/mg), and by administration of BAs alone or combined with ZnNPs to DSS treated groups showed statistically significant decrease in the levels of TNF-α (44 ± 0.5 and 28 ± 0.3 pg/mg, respectively), IL-1β (75 ± 1.0 & 56 ± 0.5 pg/mg, respectively), and IL-8 (85 ± 1.1 and 60 ± 2.8 pg/mg, respectively), when compared with the DSS group but the effect of BAs-ZnNPs is greater and more significant (Figure 2).
Table 2.
Effect of BAs-ZnNPs on the levels of ALP, IgM, and IgG in serum of different studied groups.
| Groups | Parameters | ||
|---|---|---|---|
| ALP (IU/L) | IgM (mg/dL) | IgG (mg/dL) | |
| Control | 124.6 ± 2.1a | 28.3 ± 0.2a | 90.7 ± 1.1a |
| BAs-ZnNPs | 128.5 ± 0.3a | 27 ± 1.3a | 83.7 ± 2.3a |
| DSS | 292.2 ± 4.8b | 41.1 ± 0.4b | 152.9 ± 3.9b |
| DSS+BAs | 240 ± 1.5a,b | 36 ± 0.6a,b | 125 ± 1.3a,b |
| DSS+BAs-ZnNPs | 177.5 ± 1.0a,b | 30 ± 0.1a,b | 108 ± 1.5a,b |
Data are expressed as mean ± SE (n = 10).
asignificant compared to DSS group (p < 0.05).
bsignificant compared to control.
Figure 2.
Effect of BAs-ZnNPs on the colonic production of leukotriene TNF-α (a), IL-1β (b), and IL-8 (c) determined by ELISA. Data are expressed as mean ± SE (n = 10), a: significant compared to control, b: significant compared to DSS group (p < 0.05).
Effect of BAs-ZnNPs on DSS-induced alteration in inflammatory mediators (NF-κB and COX-2) gene expression
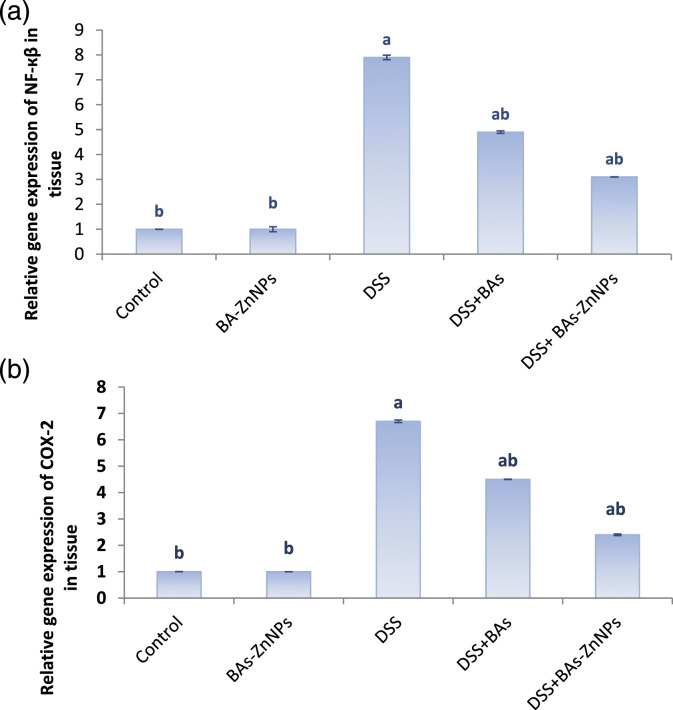
The current study was conducted to investigate how BAs-ZnNPs counteract the UC induced by DSS by investigating the expression of genes involved in the inflammatory response. Expression of NF-κB and COX-2 genes was analyzed by qPCR. The transcript levels of NF-κB and COX-2 genes were significantly increased with DSS in comparison with controls, but in DSS group treated with BAs-ZnNPs, a significant modulation was observed in comparison with DSS group as shown in Figure 3.
Figure 3.
Boswellic acid zinc nanoparticles alleviate DSS-induced ulcerative colitis. Quantitative real-time polymerase chain reaction was performed to detect the gene expression levels of (a) NF-ҡβ and (b) COX-2. Data are expressed as mean ± SE (n = 10). a: significant compared to control, b: significant compared to DSS group (p < 0.05).
Effect of BAs-ZnNPs on DSS-induced STAT-3 and PI3K protein expression
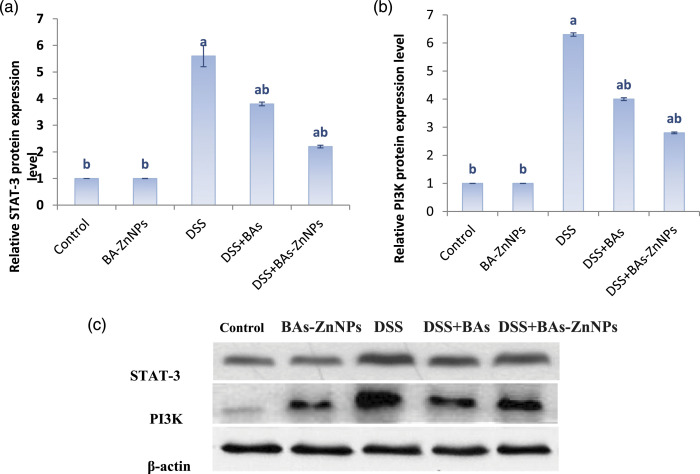
Immunoblot detection of STAT-3 and PI3K proteins in the colon of different treated groups and the statistical analyses are shown in Figure 4; this work observed a significant increase in STAT-3 and PI3K protein expression levels with DSS toxicity in comparison to the control group but BAs-ZnNPs significantly inhibited their expression against DSS-induced UC compared with DSS group.
Figure 4.
Effect of boswellic acid zinc nanoparticles on the expression of STAT-3/PI3K pathway-related proteins in DSS rats. Western blot assay was performed to detect the protein expression levels of (c, a) STAT-3 and (c, b) PI3K. Data are expressed as mean ± SE (n = 10). a: significant compared to control, b: significant compared to DSS group (p < 0.05).
Histopathological findings
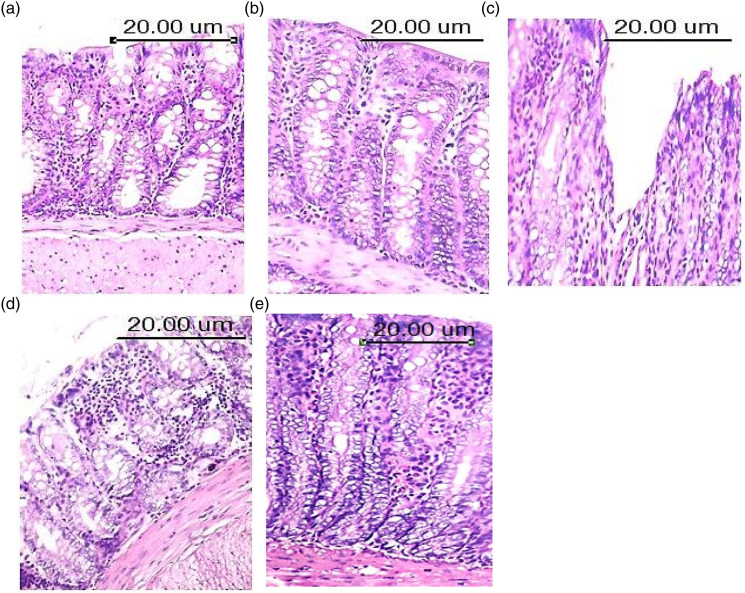
Tissue specimens of colon from control mice (Figure 5(a)) and BAs-ZnNPs treated mice (Figure 5(b)) showed normal histological structure of both villi and gland, which are characterized by intact epithelial lining with goblet cells in between score (0). By contrast, colon specimens from DSS-treated mice (Figure 5(c)) revealed severe damage of villi, which are characterized by desquamation of its epithelial lining and loss of villus architecture with disappearance of inter-villus space score (2). On the other hand, colon specimens from BAs (Figure 5(d)) and BAs-ZnNPs (Figure 5(e)) treated mice showed improvement of intestinal mucosa which appeared as epithelial shedding at the apices of some intestinal villi with normal lining of intestinal crypts.
Figure 5.
Photomicrograph of colon mucosa of male rats of; (a) Control group, showing normal histological structure of intestinal mucosa and the intestinal glands appeared intact and lined by high cuboidal epithelial cells score (0) arrow (H&E X200); (b) BAs-ZnNPs group, showing normal histological structure of both villi and gland with numerous numbers of intestinal glands score (0) arrow (H&E X200); (c) DSS group, showing loss of villus architecture with disappearance of inter-villus space and zonal destruction of the epithelial surface and zonal crypt loss score (2) arrow (H&E X200); (d): DSS + BAs group, showing few numbers of inflammatory cells were detected in both mucosa and submucosa score (1) and the intestinal glands showed intact epithelial lining with basal basophilic nuclei. Leukocytic infiltration of lamina propria by mononuclear leukocytes mainly lymphocytes and macrophages was detected score (1). (e): DSS + BA-ZnNPs group, showing epithelial shedding and leukocytic infiltration of lamina propria by mononuclear leukocytes score (1) arrow (H&E X200).
Discussion
The current study evaluated the protective and ameliorative effects of boswellic acid coated zinc nanoparticles on DSS-induced ulcerative colitis in rats. Several studies reported the beneficial effects of boswellic acid19,20 but to the best of our knowledge, this is the first report in the literature that addresses the protective effect of boswellic acid coated zinc nanoparticles to ameliorate DSS-induced ulcerative colitis in rats. Our prepared BAs-ZnNPs were characterized with microscopic and spectroscopic methods. The TEM has recently been developed as a direct imaging method for nanoscale dynamics enabling the direct tracking of the individual space-time trajectories of nanoscale objects in liquid.21 According to our results, the TEM images of the prepared BAs-ZnNPs showed a uniform distribution and confirmed their spherical morphology. The depicted ball-like structure of the BAs-ZnNPs in the TEM images ranging in size from 50 to 100 nm in diameter (Figure 1(a)). The size of nanoparticles is currently measured using an optical technique such as DLS, which is preferred in most studies regarding nanoparticles characterization especially if they are suspended in a fluid.22 Our results revealed that DLS measurement of the hydrodynamic effective diameter of the synthesized BAs-ZnNPs showed that the zeta average diameter was measured to be 127 ± 2.5 nm with 0.957 poly dispersity index (PDI) indicating the homogeneity and uniform dispersion of the synthesized BAs-ZnNPs (Figure 1(d)).
Results of the current study revealed that boswellic acid coated zinc nanoparticles (BAs-ZnNPs) have anti-inflammatory effects more than boswellic acids itself in DSS-induced UC rats, which make it a good treatment to be produced for human UC therapy. Boswellia serrata is one from herbal supplements which is widely advertised by dietary supplement manufacturers to treat UC.23 Also, irregular zinc homeostasis is correlated with the gastrointestinal diseases pathogenesis,24 and Liu et al.25 reported that the zinc level was decreased in the inflamed gut. The supplementation with zinc ameliorates intestinal permeability, intestinal barrier function, nitrogen absorption, and also increases immune responses.26 The integration of nanoparticles into natural products delivery system would be a major progression in the efforts to ameliorate their therapeutic effects.27 In the present study, it was observed that the healthy rats treated with BAs-ZnNPs showed a non-significant change in all parameters estimated in this study in respect to the control group, which indicates that there is no toxicity caused by BAs-ZnNPs on healthy cells. Anthoni et al.28 mentioned that BAs have not shown any deleterious side effects in any clinical studies.
It is well known that ulcerative colitis is an inflammatory disease characterized by substantial pro-inflammatory responses and several inflammatory pathways such as NF-κB, STATs, and MAPK are involved.29 We hypothesized that BAs-ZnNPs would inhibit NF-κB activation-mediated inflammation by down regulation of COX-2 level, as our results show that BAs-ZnNPs inhibit, both NF-κB-induced pro-inflammatory cytokines and COX-2-induced prostaglandin production causing inflammation. COX-2 is expressed at low levels in healthy mucosa and during UC remission but observed that its expression levels are significantly increased in active UC.30 The enhanced expression levels of COX-2 are a protective response in the recovery process, which improves the protection of intestinal mucosal cells, stimulates intestinal epithelial hyperplasia and intestinal blood flow, and promotes epithelial cells repair. COX-2 stimulates membrane phospholipids to release AA products, and produce a variety of PGs and leukotrienes as inflammatory mediators. The current study documented that BAs-ZnNPs treatment significantly reduced COX 2 protein expression levels in DSS group. Rashan et al.31 reported that NF-κB and several pro-inflammatory cytokines like TNF-α, IL-1β, IL-6 and inducible enzymes such as COX-2 are inhibited by boswellic acids in experimentally induced arthritis. Results of the present work revealed a significant decrease in the protein expression levels of STAT-3 in the DSS+BAs-ZnNPs group in comparison with DSS group.
Kunnumakkara et al.32 found that protein expressions of STAT-3 are closely related to inflammation, agents that can inhibit the activation of STAT-3 may have great potential in the treatment of cancer and other inflammatory diseases. They also elucidated that AKBA is a novel inhibitor of STAT-3, due to its ability to inhibit nuclear translocation of STAT-3, inhibit DNA binding activity of STAT-3 and inhibit IL-6-induced STAT-3 phosphorylation. Another inflammation marker is PI3K which increases significantly in this present study and agrees with Ge et al.33 who documented the significant increase in the expression level of PI3K in DSS treatment group compared to those noted in the control group. In the current study, authors observed that there is a significant down regulation in inflammation estimated groups treated with BAs-ZnNPs compared to the DSS group. The obtained data were in agreement with the study of Lv et al.34 who showed that AKBA mediated inhibition of PI3K; this protein is crucial for cell proliferation and survival. So, the BAs-ZnNPs had a positive inhibitory effect on the activation of the PI3K protein expression. The PI3K/Akt signaling pathway plays an important role in the pathogenesis of colitis, so that UC patients are treated with blocking the PI3K/Akt signaling pathway to inhibit the activation of NF-κB which reduces the release of cytokines, including IL-1β,34 IL-8,35 IL-613 and inducible enzymes such as COX-2.36 From the aforementioned results, we can deduce that BAs-ZnNPs reduce COX-2 expression in DSS-induced colitis by targeting NF-κB, TNF-α, IL-1β, and STAT-3 and finally could alleviate the inflammation.
There are some limitations that need to be addressed regarding the present study. First of all, the limited size of study and additional large scale studies are needed to confirm this finding. Secondly, other indicators such as weight change, disease index score, and colon length need to be examined in the further studies to prove the anti-UC effect of BA-ZnNPs. Third, safety study of BA-ZnNPs should be done, and histological analysis must be done for all the vital organs and compared with control healthy mice to validate our findings.
Conclusions
To the best of our knowledge, this is the first report in the literature that addresses the ameliorative and anti-inflammatory effects of BA-ZnNPs in DSS-induced colitis in rats. The present study demonstrated that the BAs-ZnNPs suppresses, more than boswellic acid alone, immunoglobulin M & G, ALP, IL-1β, and IL-8 levels via down regulation of TNF-α, STAT-3, and PI3K protein levels and down regulation of NF-κB and COX-2 gene levels in a UC rat model, indicating its potential anti-inflammatory properties. The current study indicates that BAs-ZnNPs may be a novel potential target for the therapeutic effects of UC. It seems the administration of BAs-ZnNPs might be adequate for UC patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: The current study was approved by the ethics committee of the National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt (The approval No; 89A/21).
Availability of data of material: All data generated or analyzed during this study are included in this published article.
ORCID iD
Riham Abdel-Hamid Haroun https://orcid.org/0000-0002-2382-0760
References
- 1.Ahmad A, Vaghasiya K, Kumar A, et al. (2021) Enema based therapy using liposomal formulation of low molecular weight heparin for treatment of active ulcerative colitis: new adjunct therapeutic opportunity. Materials Science & Engineering C 121: 111851. [DOI] [PubMed] [Google Scholar]
- 2.Gupta M, Mishra V, Gulati M, et al. (2022) Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology 30(2): 397–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeshi K, Ruscher R, Hunter L, et al. (2020) Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. Journal of Clinical Medicine 9(5): 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Wang T, Xu B, et al. (2020) Anthocyanin-containing purple potatoes ameliorate DSS-Induced colitis in Mice. Current Developments in Nutrition 29; 4(Suppl 2): 426. [DOI] [PubMed] [Google Scholar]
- 5.Ke F, Yadav PK, Ju LZ. (2012) Herbal medicine in the treatment of ulcerative colitis. Saudi Journal of Gastroenterology 18(1): 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomaa AA, Mohamed HS, Abd-Ellatief RB, et al. (2021) Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology 29(4): 1033–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui MZ. (2011) Boswellia serrata, a potential antiinflammatory agent: an overview. Indian Journal of Pharmaceutical Sciences 73(3): 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmiech M, Ulrich J, Lang SJ, et al. (2021) 11-Keto-α-Boswellic acid, a novel triterpenoid from Boswellia spp. With chemotaxonomic potential and antitumor activity against triple-negative breast cancer cells. Molecules 26(2): 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Y, Zhang B. (2022) The impact of zinc and zinc homeostasis on the intestinal mucosal barrier and intestinal diseases. Biomolecules 12(7): 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germain M, Caputo F, Metcalfe S, et al. (2020) Delivering the power of nanomedicine to patients today. Journal of Controlled Release 326: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Sonbaty S, Kandil EI, Haroun RAH. (2022) Assessment of the antitumor activity of green biosynthesized zinc nanoparticles as therapeutic agent against renal cancer in rats. Biological Trace Element Research. DOI: 10.1007/s12011-022-03126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadatmand M, Al-Awsi GRL, Alanazi AD, et al. (2021) Green synthesis of zinc nanoparticles using Lavandula angustifolia Vera. Extract by microwave method and its prophylactic effects on Toxoplasma gondii infection. Saudi Journal of Biological Sciences 28(11): 6454–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Chen H, Wang B, et al. (2017) ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Scientific Reports 7: 43126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sami MM, Ali EA, Galhom RA, et al. (2019) Boswellic acids ameliorate doxorubicin-induced nephrotoxicity in mice: a focus on antioxidant and antiapoptotic effects. Egyptian Journal of Basic and Applied Sciences 6(1): 10–24. [Google Scholar]
- 15.Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29(9): e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 17.Banchroft JD, Stevens A, Turner DR. (1996) Theory and Practice of Histological Techniques. 4th edition. New York, London, San Francisco, Tokyo: Churchil Livingstone. [Google Scholar]
- 18.Gaudio E, Taddei G, Vetuschi A, et al. (1999) Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Digestive Diseases and Sciences 44(7): 1458–1475. [DOI] [PubMed] [Google Scholar]
- 19.Tohamy HG, El-Kazaz SE, Alotaibi SS, et al. (2021) Ameliorative effects of boswellic acid on Fipronil-Induced toxicity: antioxidant state, apoptotic markers, and testicular steroidogenic expression in male rats. Animals (Basel) 11(5): 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caterino C, Aragosa F, Della Valle G, et al. (2021) Clinical efficacy of curcuvet and boswellic acid combined with conventional nutraceutical product: an aid to canine osteoarthritis. PLoS One 16(5): e0252279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang S, Kim JH, Lee M, et al. (2021) Real-space imaging of nanoparticle transport and interaction dynamics by graphene liquid cell TEM. Science Advances 7(49): eabi5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolae-Maranciuc A, Chicea D, Chicea LM. (2022) Ag nanoparticles for biomedical applications-synthesis and characterization-A review. International Journal of Molecular Sciences 23(10): 5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. (2011) Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clinical Pharmacokinetics 50(6): 349–369. [DOI] [PubMed] [Google Scholar]
- 24.Skrovanek S, DiGuilio K, Bailey R, et al. (2014) Zinc and gastrointestinal disease. World Journal of Gastrointestinal Pathophysiology 5(4): 496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JZ, Jellbauer S, Poe AJ, et al. (2012) Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host and Microbe 11(3): 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi W, Fukada T. (2019) Contribution of zinc and zinc transporters in the pathogenesis of inflammatory bowel diseases. Journal of Immunology Research 2019: 8396878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins R, Wu L, Zhang C, et al. (2015) Natural product-based nanomedicine: recent advances and issues. The International Journal of Nanomedicine 10: 6055–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthoni C, Laukoetter MG, Rijcken E, et al. (2006) Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 290(6): G1131–G1137. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Wang T, Fu W, et al. (2021) Role of Gut microbiota in the anti-colitic effects of anthocyanin-containing potatoes. Molecular Nutrition & Food Research 65: 2100152. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Soendergaard C, Bergenheim FH, et al. (2018) COX-2-PGE2 signaling impairs intestinal epithelial regeneration and associates with TNF inhibitor responsiveness in Ulcerative Colitis. EBioMedicine 36: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashan L, Hakkim FL, Idrees M, et al. (2019) Boswellia gum resin and essential oils: potential health benefits− An evidence based review. International Journal of Nutrition, Pharmacology, Neurological Diseases 9(2): 53. [Google Scholar]
- 32.Kunnumakkara AB, Nair AS, Sung B, et al. (2009) Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Molecular Cancer Research 7(1): 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Ge W, Wang HY, Zhao HM, et al. (2020) Effect of Sishen Pill on memory T cells from experimental Colitis induced by dextran sulfate sodium. Frontiers in Pharmacology 11: 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv M, Shao S, Zhang Q, et al. (2020) Acetyl-11-Keto-β-Boswellic acid exerts the anti-cancer effects via cell cycle arrest, apoptosis induction and autophagy suppression in non-small cell lung cancer cells. Oncotargets and Therapy 13: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muri J, Thut H, Feng Q, et al. (2020) Thioredoxin-1 distinctly promotes NF-κβ target DNA binding and NLRP3 inflammasome activation independently of Txnip. Elife 9: e53627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu A, Das AS, Sharma M, et al. (2017) STAT3 and NF-κβ are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema. Biochemistry and Biophysics Reports 12: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]