Highlights
-
•
Only 1 in 4 young adults have ideal cardiovascular health (CVH).
-
•
The CVH in young adults has not improved in last decade.
-
•
Race/ethnicity and sex differences exist in CVH among young adults.
-
•
47.3% individuals with ideal CVH were reclassified to a lower CVH category by the Life's Essential 8 score.
Keywords: Cardiovascular health, Life's essential 8, Sleep, Young adults
Abstract
Objective
This study assessed cardiovascular health (CVH) in young adults using the 2022 AHA Life's Essential 8 (LE8) score and compared it with the Life's Simple 7 (LS7) score.
Methods
Individuals aged 18 to 44 years without a history of cardiovascular disease in the National Health and Nutrition Examination Survey (NHANES) cycles were included. Data from 2007-2008 to 2017-2018 were combined to create 3 groups (2007-2010, 2011-2014, and 2015-2018) for analysis. The LE8 score and its components were computed in the overall population and stratified by sex and race/ethnicity. Trends for the LE8 score were analyzed using adjusted linear regression models.
Results
Among 12,197 young adults, representing an estimated 89.4 million individuals, from the NHANES 2007-2018, the CVH in the overall population and across all subgroups was stable (Ptrend >0.05). The blood lipid score improved across all subgroups (Ptrend:<0.05). The mean LE8 score was 69.2±0.3. Females (71.4±0.4) had better CVH compared with males (67.2±0.4). Non-Hispanic Black individuals (65.1± 0.3) had the lowest CVH compared with Non-Hispanic White individuals (69.9±0.5), Mexican American individuals (67.3±0.3), and other race individuals (71.2±0.4). Of the 46.1 million individuals categorized as intermediate CVH by the LS7 score, 8.1 million (17.6%) and 2.3 million (5.0%) were reclassified to poor and ideal CVH by the LE8 score, respectively. Of the 40.1 million individuals categorized as ideal CVH by the LS7 score, 18.9 million (47.1%) and 0.1 million (0.2%) were reclassified to poor CVH and intermediate CVH by the LE8 score, respectively.
Conclusion
Among US young adults, there has been no improvement in CVH over the last decade with notable sex and race/ethnicity-associated differences in the LE8 score. Nearly 1 in 4 young adults had ideal CVH using the LE8 score compared with 1 in 2 individuals using the LS7 score.
1. Introduction
Despite the overall improvement in cardiovascular mortality in the US, the proportion of young adults experiencing cardiovascular events has increased [1,2]. Prior studies have shown that the prevalence of ideal cardiovascular health (CVH) in young adults is abysmally low [3], [4], [5]. In addition to traditional risk factors for cardiovascular disease, poor sleep health has been associated with an increased risk of cardiovascular mortality [6], [7], [8], [9]. Inadequate sleep is reported by about 30% of young adults and may affect other determinants of CVH [5,10,11]. Though the association of sleep with cardiovascular outcomes has been known, it has only recently been formally included as a determinant of CVH [5].
In 2010, the American Heart Association (AHA) introduced the concept of ideal CVH to improve health at the individual and population levels through an emphasis on primordial prevention and introduced the Life's Simple 7 (LS7) score for CVH measurement [12]. The 2022 AHA Life's Essential 8 (LE8) score improves upon the existing framework established by the LS7 by optimizing the methods by which the 7 different components (physical activity, blood glucose, blood lipids, blood pressure, smoking, body mass index, and diet) are measured and by adding sleep as a new CVH determinant [5,12]. In addition to adding sleep as a factor, the LE8 score is graded on a scale of 0 to 100 which makes it inherently easier to understand, improves the quantification of CVH in an individual, and increases the sensitivity of measuring the changes in CVH over time at an individual and population level [13]. With the introduction of the LE8 score, the reclassification of CVH categories previously estimated using the LS7 score among young US adults is unknown.
This study utilized the National Health and Nutrition Examination Survey (NHANES) data from 2007-2018 to assess the CVH of young adults between 18 to 44 years using the LE8 score in the overall population and subgroups of sex and race/ethnicity. This study also compares the CVH scores as quantified by the LE8 score with the LS7 score.
2. Methods
This study utilized the NHANES data from 2007-2008 to 2017-2018. The National Center for Health Statistics (NCHS) and Center for Disease Control and Prevention (CDC) conduct the NHANES every 2 years to examine the nutritional and health status of a sample population, selected using a multistage probability sampling design, representing the civilian, non-institutionalized population of the United States [14], [15], [16], [17], [18]. Each individual in the survey underwent a home interview during which data on demographics, sleep, diet, physical activity, smoking, medical conditions such as diabetes mellitus and hypertension, and medication use were collected [14]. Individuals who consented to a clinical examination were invited to a mobile examination center where they underwent anthropometric measurements, detailed physical examination, blood sampling for laboratory testing, and vital signs measurement [14]. Written informed consent was obtained for each individual before the home interview and the physical examination [14]. Ethical oversight for this study was provided by the University of Alabama at Birmingham Institutional Review Board.
This study included individuals between 18 and 44 years from 6 consecutive NHANES cycles (2007-2008 to 2017-2018). Individuals who did not undergo a physical examination, pregnant or breastfeeding females, individuals with self-reported cardiovascular diseases (stroke, coronary heart disease, heart failure, angina, and “heart attack”) [13], and those with missing data in any of the components of the LE8 score were excluded. Self-identified age, sex, and race/ethnicity were used to sub-categorize individuals. Race/ethnicity was categorized into 4 groups: Non-Hispanic-White, Non-Hispanic Black, Mexican American, and other races/ethnicities. The other races/ethnicities group was a racially/ethnically diverse group that included Non-Hispanic Asian individuals (data available from the 2011-12 cycle), other Hispanic individuals, multi-racial/ethnic individuals, and those not self-identifying as any of the above-listed races/ethnicities.
The LE8 score is composed of 4 health factors [body mass index (BMI), blood glucose levels, blood lipid levels, and blood pressure] and 4 health behaviors (smoking status, physical activity, diet, and sleep) [5]. The definitions and levels of each component have been described in Supplementary Table 1 [5,13]. Each of these components is graded on a scale of 0 to 100. The LE8 score is the mean value of the 8 components.
The LS7 categories are composed of 7 components which include smoking status, diet, physical activity, blood pressure, HbA1C levels, total cholesterol, and BMI [12,[19], [20], [21]]. Each of these components is graded as 0 (poor), 1 (intermediate), and 2 (ideal) as reported in Supplementary Table 2 [12]. The LS7 is the sum of the scores of the 7 components.
The LE8 and LS7 scores vary in the definitions of the components. Specifically, the LE8 score uses non-HDL cholesterol compared with the total cholesterol used in the LS7 score for the blood lipids component [5,12]. Furthermore, the LE8 score introduced a negative scoring system for the smoking, blood pressure, and blood lipid levels components [5].
To record blood pressure, 3 measurements were taken in a seated position after 5 minutes of rest during the physical examination visit. The mean of the 3 values was used to calculate the systolic and diastolic blood pressures. In case of a missing value in the 3 measurements, the first value was considered as the systolic and diastolic blood pressures. The current use of anti-hypertensive medications was determined from the home interview questionnaire.
HbA1C (determined using high-performance liquid chromatography), fasting blood glucose (determined using hexokinase-based enzyme assay), and cholesterol (determined using an enzymatic assay) levels were obtained from the blood samples collected during the physical examination visit. Non-HDL cholesterol values were calculated by subtracting HDL cholesterol levels from the total cholesterol level. The use of lipid-lowering medications, insulin, and oral hypoglycemic agents was obtained from the home interview questionnaire.
The weight in kilograms and the height in meters (both measured during the physical examination) were used to calculate the BMI. Physical activity was determined using self-reported duration (frequency per week and duration per day) and self-reported intensity (moderate and vigorous) of recreational physical activity. Moderate physical activity was described as activity leading to a small increase in breathing and heart rate or light sweating. Vigorous physical activity was described as activity leading to a large increase in breathing and heart rate or heavy sweating.
Two 24-hour dietary recall interviews for each individual were conducted to determine their self-reported adherence to the recommendations of the Healthy Eating Index-2015 (HEI-2015). The HEI-2015 was scored from 0 to 100 utilizing the calorie-indexed values for the servings of total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, added sugars, and saturated fats consumed. The maximum scores and scoring descriptions for each component have been depicted in Supplementary Table 3.
The tobacco use questionnaire was used to assess the self-reported cigarette smoking status, use of inhaled nicotine delivery systems (e-cigarettes, hookahs, cigars, and pipes) in the previous 5 days, and duration since quitting cigarette smoking. Exposure to secondhand smoke indoors at home was assessed using the household smokers questionnaire.
The average self-reported duration of sleep per day was obtained from the sleep disorders questionnaire. Additionally, data on insurance status (yes/no), number of healthcare visits in the past year (none, 1 to 3, 4 or more), poverty income ratio (≥3.50, 1.30-3.49, <1.30), and educational status (≤12 years of education, some college education, and a college degree or higher) were used in this study [15,22].
The CVH determined by the LE8 score was categorized as ideal (≥80), intermediate (50-79), and poor (<50) [13]. Similarly, ideal, intermediate, and poor CVH were defined as an LS7 score of 0-4, 5-9, and 10-14, respectively [23], [24], [25].
All statistical analyses were performed on SAS 9.4 (Cary, NC). To account for the complex multistage sample selection design, the SURVEYFREQ and SURVEYMEANS procedures were used for data analyses in SAS as recommended by the NCHS. This study combined the NHANES cycles from 2007-2008 through 2017-2018 [16,22]. As recommended by the NHANES NCHS analytic guidelines, the sample weight for the physical examination sub-sample was used and was adjusted to account for combining 6 survey cycles. The analyses were carried out in the overall population and stratified by race/ethnicity, sex, and NHANES cycles. The overall and component scores were presented as mean (standard error) and median (interquartile range). As recommended by NHANES, the NHANES cycles were categorized into three 4-year groups (2007-2010, 2011-2014, and 2015-2018) to study the trends of the LE8 score and the component scores. For analyzing trends, a weight accounting for combining 2 cycles was constructed and used. Temporal trends in the LE8 score and its components were assessed using linear regression models with adjustment for race/ethnicity, age, insurance status, education level, number of healthcare visits per year, and income [[15], [16], [17],26]. Agreement between the LE8 score and LS7 score in CVH categorization was assessed using the weighted Cohen's kappa statistic. The statistical significance was set at a two-sided p-value of <0.05.
3. Results
The NHANES cycles from 2007-2018 included 70,190 individuals. The final study sample had 12,197 individuals after excluding 20,302 individuals aged >44 years, 33,610 individuals <18 years of age, 374 pregnant females, 208 breastfeeding females, 603 individuals who did not undergo a physical examination, 254 individuals with a history of cardiovascular disease, and 2,642 lacking complete data for the components of the LE8 scores were excluded. (Supplementary Figure 1) This represented 89.4 million individuals of the non-institutionalized, civilian US population. The median age of the participants was 30.6 (24.1, 37.5) years.
In the overall population of young adults aged 18-44 years, the mean CVH was 69.2 (0.3). The highest and lowest scoring components were blood sugar [91.2 (0.2)] and diet [35.7 (0.6)], respectively (Table 1).
Table 1.
Baseline Characteristics and Life's Essential 8 Scores of the Overall Population and Stratified by Sex in National Health and Nutrition Examination Survey 2007-2018
| Overall [n=12,197 (89,422,241)] |
Male [n=6,115 (46,314,839)] |
Female [n=6,082 (43,107,401)] |
||||
|---|---|---|---|---|---|---|
| Age* | 30.6 (24.1, 37.5) | 30.4 (24.1, 37.2) | 30.9 (24.2, 37.8) | |||
| Race | ||||||
| Non-Hispanic White | 59.4 (56.3-62.5) | 59.9 (56.7-63.0) | 58.9 (55.6-62.3) | |||
| Non-Hispanic Black | 11.8 (10.2-13.4) | 10.7 (9.2-12.2) | 13.0 (11.2-14.9) | |||
| Mexican American | 12.2 (10.3-14.1) | 13.0 (11.0-15.0) | 11.3 (9.4-13.2) | |||
| Other | 16.6 (15.0-18.1) | 16.4 (14.7-18.1) | 16.7 (15.1-18.4) | |||
| Insurance Status | ||||||
| Insured | 74.4 (72.8-76.0) | 71.5 (69.6-73.4) | 77.5 (75.9-79.2) | |||
| Uninsured | 25.6 (24.0-27.2) | 28.5 (26.6-30.4) | 22.5 (20.8-24.1) | |||
| Family Poverty Income Ratio | ||||||
| >=3.50 | 34.5 (32.3-36.7) | 36.5 (33.9-39.1) | 32.3 (30.0-34.6) | |||
| 1.30-3.49 | 34.4 (32.8-36.1) | 34.4 (32.3-36.5) | 34.5 (32.7-36.3) | |||
| <1.30 | 31.1 (29.1-33.0) | 29.1 (27.0-31.3) | 33.2 (31.1-35.2) | |||
| Number of Healthcare Visits | ||||||
| None | 22.9 (21.7-24.0) | 31.1 (29.3-32.8) | 14.1 (13.0-15.1) | |||
| 1 to 3 | 51.8 (50.5-53.0) | 50.5 (48.7-52.3) | 53.1 (51.5-54.7) | |||
| >=4 | 25.4 (24.3-26.4) | 18.4 (17.1-19.7) | 32.9 (31.4-34.3) | |||
| Median (IQR) | Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | Mean (SE) | |
|---|---|---|---|---|---|---|
| Essential 8 Score | 69.8 (58.6, 80.4) | 69.2 (0.3) | 67.8 (56.8, 77.9) | 67.2 (0.4) | 72.2 (60.9, 82.9) | 71.4 (0.4) |
| Physical Activity Score | 76.9 (0.0, 94.1) | 56.5 (0.8) | 84.9 (0.0, 94.6) | 59.6 (0.9) | 59.4 (0.0, 93.4) | 53.2 (1.0) |
| Blood Pressure Score | 83.8 (58.3, 91.9) | 82.7 (0.3) | 80.0 (46.5, 90.0) | 77.6 (0.5) | 86.5 (77.7, 93.3) | 88.2 (0.4) |
| Blood Lipids Score | 79.1 (40.7, 90.0) | 72.9 (0.4) | 54.7 (33.7, 88.1) | 67.5 (0.5) | 82.9 (46.6, 91.5) | 78.7 (0.5) |
| Blood Sugar Score | 75.3 (62.9, 87.6) | 91.2 (0.2) | 74.6 (61.9, 87.3) | 90.4 (0.3) | 76.0 (64.0, 88.0) | 92.1 (0.3) |
| Body Mass Index Score | 50.7 (22.3, 78.9) | 63.4 (0.6) | 48.9 (23.7, 76.4) | 63.4 (0.7) | 53.2 (20.4, 81.0) | 63.4 (0.7) |
| Smoking Score | 82.4 (10.8, 91.2) | 68.0 (0.7) | 80.3 (0.6, 90.2) | 63.4 (0.8) | 84.1 (37.5, 92.1) | 73.0 (0.8) |
| Sleep Score | 91.2 (54.9, 95.6) | 83.3 (0.4) | 91.2 (54.0, 95.6) | 83.1 (0.5) | 91.2 (56.2, 95.6) | 83.6 (0.4) |
| Diet Score | 19.7 (0.0, 46.1) | 35.7 (0.6) | 16.6 (0.0, 42.3) | 32.9 (0.7) | 23.4 (0.0, 50.1) | 38.7 (0.8) |
Median (interquartile range), mean (standard deviation), and frequency (percentage) have been used to describe data.
Median (interquartile range) has been used.
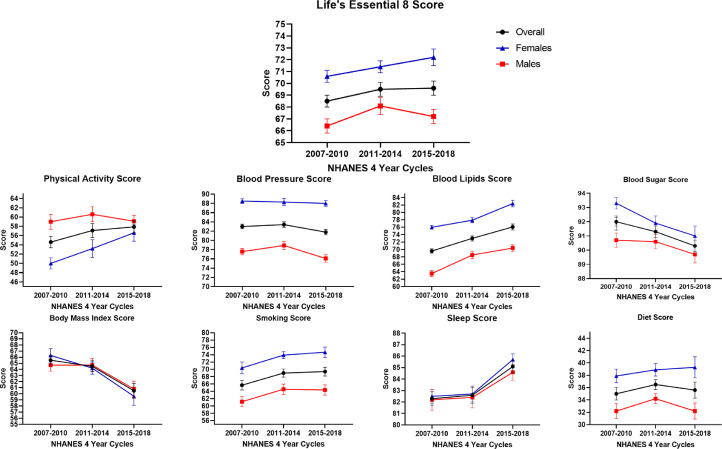
The mean LE8 score remained stable from 2007-2010 [68.5 (0.5)] to 2015-2018 [69.6 (0.6)]. An increasing score was noted in the sleep [82.3 (0.6) in 2007-2010 to 85.1 (0.5) in 2015-2018, Ptrend: <0.001] and blood lipid [69.6 (0.6) in 2007-2010 to 76.1 (0.8) in 2015-2018, Ptrend: <0.001] components of the score and decreasing score was noted in the blood pressure [83.0 (0.5) in 2007-2010 to 81.8 (0.6) in 2015-2018, Ptrend: 0.004], blood sugar [92.0 (0.3) in 2007-2010 to 90.3 (0.4) in 2015-2018, Ptrend: <0.001], and BMI [65.5 (0.8) in 2007-2010 to 60.2 (1.2) in 2015-2018, Ptrend: <0.001] components (Table 2).
Table 2.
Trend of Essential 8 Score and its Components Stratified by Sex in National Health and Nutrition Examination Survey 2007-18
| 2007-2010 [n=4,239 (88,558,753)] |
2011-2014 [n=4,239 (90,911,084)] |
2015-2018 [n=3,719 (88,796,885)] |
Ptrend | |
|---|---|---|---|---|
| Overall | ||||
| Essential 8 Score | 68.5 (0.5) | 69.6 (0.6) | 69.6 (0.6) | 0.89 |
| Physical Activity Score | 54.6 (1.2) | 57.1 (1.5) | 57.9 (1.3) | 0.32 |
| Blood Pressure Score | 83.0 (0.5) | 83.4 (0.6) | 81.8 (0.6) | 0.004 |
| Blood Lipids Score | 69.6 (0.6) | 73.0 (0.8) | 76.1 (0.8) | <0.001 |
| Blood Sugar Score | 92.0 (0.3) | 91.3 (0.4) | 90.3 (0.4) | <0.001 |
| Body Mass Index Score | 65.5 (0.8) | 64.5 (0.9) | 60.2 (1.2) | <0.001 |
| Smoking Score | 65.7 (1.3) | 69.0 (1.1) | 69.4 (1.2) | 0.73 |
| Sleep Score | 82.3 (0.6) | 82.6 (0.7) | 85.1 (0.5) | <0.001 |
| Diet Score | 35.0 (1.0) | 36.5 (0.8) | 35.6 (1.3) | 0.90 |
| Males | ||||
| Essential 8 Score | 66.4 (0.6) | 68.1 (0.7) | 67.2 (0.6) | 0.34 |
| Physical Activity Score | 59.0 (1.6) | 60.6 (1.6) | 59.1 (1.3) | 0.18 |
| Blood Pressure Score | 77.6 (0.7) | 78.9 (0.9) | 76.1 (0.8) | 0.01 |
| Blood Lipids Score | 63.5 (0.8) | 68.5 (1.0) | 70.4 (0.9) | <0.001 |
| Blood Sugar Score | 90.7 (0.5) | 90.6 (0.5) | 89.7 (0.6) | 0.03 |
| Body Mass Index Score | 64.7 (1.0) | 64.7 (1.1) | 60.8 (1.3) | 0.003 |
| Smoking Score | 61.2 (1.4) | 64.6 (1.4) | 64.4 (1.4) | 0.87 |
| Sleep Score | 82.2 (0.9) | 82.4 (0.9) | 84.6 (0.7) | 0.02 |
| Diet Score | 32.2 (1.2) | 34.2 (0.8) | 32.2 (1.3) | 0.46 |
| Females | ||||
| Essential 8 Score | 70.6 (0.5) | 71.4 (0.5) | 72.2 (0.7) | 0.50 |
| Physical Activity Score | 50.0 (1.2) | 53.2 (1.9) | 56.6 (1.8) | 0.002 |
| Blood Pressure Score | 88.5 (0.5) | 88.3 (0.8) | 88.0 (0.6) | 0.09 |
| Blood Lipids Score | 76.0 (0.8) | 77.9 (0.7) | 82.4 (0.9) | <0.001 |
| Blood Sugar Score | 93.3 (0.4) | 91.9 (0.5) | 91.0 (0.7) | <0.001 |
| Body Mass Index Score | 66.3 (1.1) | 64.2 (1.0) | 59.6 (1.5) | <0.001 |
| Smoking Score | 70.4 (1.6) | 73.9 (0.9) | 74.7 (1.4) | 0.76 |
| Sleep Score | 82.5 (0.6) | 82.7 (0.7) | 85.7 (0.5) | <0.001 |
| Diet Score | 37.9 (1.1) | 38.9 (1.0) | 39.3 (1.7) | 0.68 |
The Essential 8 score and its components have been presented as mean (standard error).
The mean LE8 score was 67.2 (0.4) in males and 71.4 (0.4) in females. Females had higher scores than males in every component of the LE8 score except physical activity [53.2 (1.0) compared with 59.6 (0.9) in males]. (Table 2).
An increasing score in the mean blood lipids score was observed in both males [63.5 (0.8) in 2007-2010 to 70.4 (0.9) in 2015-2018, Ptrend: <0.001] and females [76.0 (0.8) in 2007-2010 to 82.4 (0.9) in 2015-2018, Ptrend: <0.001]. (Table 2) In males, an increasing score for sleep [82.2 (0.9) in 2007-2010 to 84.6 (0.7) in 2015-2018, Ptrend: 0.02] was noted and decreasing scores for blood sugar [90.7 (0.5) in 2007-2010 to 89.7 (0.6) in 2015-2018, Ptrend: 0.03], blood pressure [77.6 (0.7) in 2007-2010 to 76.1 (0.8) in 2015-2018, Ptrend: 0.01] and BMI [64.7 (1.0) in 2007-2010 to 60.8 (1.3) in 2015-2018, Ptrend: 0.003] components were noted. In females, increasing scores for physical activity [50.0 (1.2) in 2007-2010 to 56.6 (1.8) in 2015-2018, Ptrend: 0.002] and sleep [82.5 (0.6) in 2007-2010 to 85.7 (0.5) in 2015-2018, Ptrend: <0.001] were noted and decreasing scores for the blood sugar [93.3 (0.4) in 2007-2010 to 91.0 (0.7) in 2015-2018, Ptrend: <0.001], and BMI [66.3 (1.1) in 2007-2010 to 59.6 (1.5) in 2015-2018, Ptrend: <0.001] components were noted (Fig. 1).
Fig. 1.
Trends of the Life's Essential 8 Score in the 18-44 Year Age Group, Overall and Stratified by Sex from NHANES Cycles 2007 to 2018.
This figure depicts the trends of the Life's Essential 8 (LE8) score and its components from 2007-2018. Two consecutive NHANES cycles were combined to create 3 groups (2007-2010, 2011-2014, and 2015-2018) for analyzing the trends of the LE8 score and its components. The overall population, males, and females have been depicted in black, red, and blue, respectively.
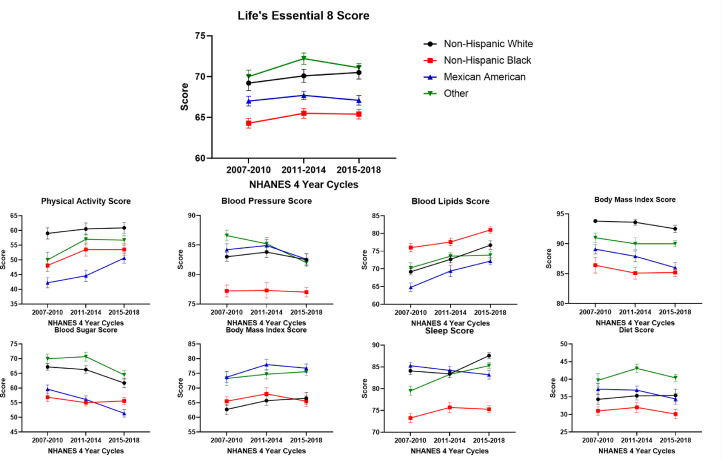
On the race-stratified analysis, the other race group [71.2 (0.4)] had the highest LE8 score and Non-Hispanic Black individuals [65.1 (0.3)] had the lowest LE8 score. Non-Hispanic Black individuals had the lowest scores for blood pressure [77.2 (0.6)], blood sugar [85.6 (0.6)], sleep [74.8 (0.6)], and diet [31.1 (0.7)]. (Table 3)
Table 3.
Essential 8 Score and its components Stratified by race/ethnicity in National Health and Nutrition Examination Survey 2007-18
| Non-Hispanic White [n= 4,521 (53,127,793)] |
Non-Hispanic Black [n=2,478 (10,577,294)] |
Mexican American [n=2,192 (10,888,071)] |
Other [n=3,006 (14,829,082)] |
|||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | |
| Essential 8 Score | 70.7 (58.8, 81.2) | 69.9 (0.5) | 65.5 (54.8, 75.5) | 65.1 (0.3) | 67.5 (56.9, 77.4) | 67.3 (0.3) | 72.2 (61.2, 81.9) | 71.2 (0.4) |
| Physical Activity Score | 83.2 (0.0, 94.5) | 60.1 (1.1) | 56.2 (0.0, 93.6) | 51.7 (1.2) | 21.6 (0.0, 92.8) | 45.8 (1.1) | 70.6 (0.0, 93.9) | 55.1 (1.2) |
| Blood Pressure Score | 83.9 (58.8, 91.9) | 83.1 (0.5) | 81.3 (46.3, 90.7) | 77.2 (0.6) | 84.3 (60.8, 92.2) | 83.9 (0.6) | 84.6 (61.4, 92.3) | 84.4 (0.5) |
| Blood Lipids Score | 73.5 (40.3, 89.9) | 72.7 (0.6) | 82.9 (46.0, 91.4) | 78.2 (0.6) | 55.6 (36.9, 88.4) | 68.9 (0.8) | 80 (40.5, 90.0) | 72.8 (0.7) |
| Blood Sugar Score | 76.5 (64.8, 88.3) | 93.3 (0.3) | 70.9 (55.1, 85.4) | 85.6 (0.6) | 72.9 (58.9, 86.5) | 87.6 (0.5) | 74.7 (62.0, 87.3) | 90.3 (0.4) |
| Body Mass Index Score | 53.7 (23.6, 80.2) | 65.2 (0.8) | 39 (15.2, 75.1) | 55.8 (0.7) | 38.2 (18.7, 67.6) | 55.6 (0.7) | 57.1 (27.0, 81.1) | 68.1 (0.9) |
| Smoking Score | 80.8 (0.0, 90.4) | 64.9 (1.1) | 82.7 (2.8, 91.3) | 66.3 (1.1) | 85 (55.3, 92.5) | 76.2 (1.0) | 84.8 (42.7, 92.4) | 74.6 (1.0) |
| Sleep Score | 91.6 (58.7, 95.8) | 84.9 (0.5) | 70.7 (39.5, 94.3) | 74.8 (0.6) | 91.3 (56.4, 95.6) | 84.2 (0.5) | 91.2 (54.1, 95.6) | 83.1 (0.5) |
| Diet Score | 18.5 (0.0, 46.0) | 35 (0.9) | 14.9 (0.0, 39.4) | 31.1 (0.7) | 21.1 (0.0, 45.4) | 36.2 (0.8) | 26.6 (3.1, 51.8) | 41.1 (0.8) |
Median (interquartile range) and mean (standard deviation) have been used to describe the Life's Essential 8 Score and its components.
The LE8 score remained stable from 2007-2010 to 2015-2018 across all race/ethnic groups. (Table 4) Blood LE8 lipid scores increased across all race/ethnicity groups. In Non-Hispanic White individuals, the sleep score increased from 84.1 (0.8) in 2007-2010 to 87.6 (0.7) in 2015-2018 (Ptrend: 0.003). However, a decreasing blood sugar score [93.8 (0.3) in 2007-2010 to 92.5 (0.6) in 2015-2018, Ptrend: 0.006] and the BMI [67.2 (1.1) in 2007-2010 to 61.7 (1.6) in 2015-2018, (Ptrend: <0.001)] score trends were noted. In Non-Hispanic Black individuals, the trend of the components of the LE8 score remained stable (other than blood LE8 lipid score). In Mexican American individuals, the physical activity score increased from 42.2 (1.7) in 2007-2010 to 50.6 (1.7) in 2015-2018 (Ptrend: 0.004). A decrease in the trends of the blood pressure [84.2 (1.0) in 2007-2010 to 82.6 (0.9) in 2015-2018, (Ptrend: 0.008)], blood sugar [89.1 (0.8) in 2007-2010 to 86.0 (0.9) in 2015-2018, (Ptrend: <0.001)] and the BMI [59.6 (1.4) in 2007-2010 to 51.4 (1.3) in 2015-2018, (Ptrend: <0.001)] scores were noted (Fig. 2).
Table 4.
Trend of Essential 8 Score and its Components Stratified by Race/Ethnicity in National Health and Nutrition Examination Survey 2007-18
| 2007-2010 [n=4,239 (88,558,753)] |
2011-2014 [n=4,239 (90,911,084)] |
2015-2018 [n=3,719 (88,796,885)] |
Ptrend | |
|---|---|---|---|---|
| Non-Hispanic White | ||||
| Essential 8 Score | 69.2 (0.9) | 70.1 (0.8) | 70.5 (0.8) | 0.88 |
| Physical Activity Score | 59.0 (1.9) | 60.5 (2.0) | 60.9 (1.9) | 0.96 |
| Blood Pressure Score | 83.0 (0.8) | 83.8 (0.9) | 82.5 (1.0) | 0.20 |
| Blood Lipids Score | 69.2 (0.9) | 72.7 (1.0) | 76.7 (1.2) | <0.001 |
| Blood Sugar Score | 93.8 (0.3) | 93.6 (0.5) | 92.5 (0.6) | 0.006 |
| Body Mass Index Score | 67.2 (1.1) | 66.3 (1.3) | 61.7 (1.6) | <0.001 |
| Smoking Score | 62.7 (1.8) | 65.7 (1.6) | 66.5 (2.0) | 0.65 |
| Sleep Score | 84.1 (0.8) | 83.4 (0.9) | 87.6 (0.7) | 0.003 |
| Diet Score | 34.3 (1.5) | 35.3 (1.2) | 35.4 (1.8) | 0.67 |
| Non-Hispanic Black | ||||
| Essential 8 Score | 64.3 (0.6) | 65.5 (0.6) | 65.4 (0.6) | 0.85 |
| Physical Activity Score | 48.1 (2.1) | 53.5 (2.2) | 53.5 (2.0) | 0.27 |
| Blood Pressure Score | 77.2 (1.0) | 77.3 (1.3) | 77.0 (0.8) | 0.57 |
| Blood Lipids Score | 76.0 (1.2) | 77.6 (1.1) | 81.0 (0.9) | 0.001 |
| Blood Sugar Score | 86.4 (1.3) | 85.1 (1.0) | 85.2 (0.7) | 0.25 |
| Body Mass Index Score | 56.9 (1.4) | 55.0 (1.1) | 55.6 (1.3) | 0.43 |
| Smoking Score | 65.5 (1.5) | 68.0 (2.1) | 65.5 (1.8) | 0.23 |
| Sleep Score | 73.3 (1.1) | 75.7 (1.2) | 75.3 (0.8) | 0.16 |
| Diet Score | 31.0 (1.2) | 32.0 (1.4) | 30.1 (1.3) | 0.38 |
| Mexican American | ||||
| Essential 8 Score | 67.0 (0.6) | 67.7 (0.5) | 67.1 (0.6) | 0.14 |
| Physical Activity Score | 42.2 (1.7) | 44.6 (1.9) | 50.6 (1.7) | 0.004 |
| Blood Pressure Score | 84.2 (1.0) | 84.9 (1.0) | 82.6 (0.9) | 0.008 |
| Blood Lipids Score | 64.8 (1.2) | 69.4 (1.6) | 72.2 (1.0) | 0.005 |
| Blood Sugar Score | 89.1 (0.8) | 87.9 (0.9) | 86.0 (0.9) | <0.001 |
| Body Mass Index Score | 59.6 (1.4) | 56.1 (1.2) | 51.4 (1.3) | <0.001 |
| Smoking Score | 73.7 (1.8) | 78.0 (1.8) | 76.8 (1.4) | 0.87 |
| Sleep Score | 85.3 (0.8) | 84.2 (0.9) | 83.2 (1.0) | 0.65 |
| Diet Score | 37.2 (1.6) | 36.9 (1.2) | 34.4 (1.5) | 0.10 |
| Other | ||||
| Essential 8 Score | 70.0 (0.8) | 72.2 (0.7) | 71.1 (0.5) | 0.90 |
| Physical Activity Score | 50.0 (2.6) | 57.0 (1.7) | 56.7 (1.7) | 0.09 |
| Blood Pressure Score | 86.6 (0.9) | 85.2 (1.1) | 82.0 (0.7) | <0.001 |
| Blood Lipids Score | 70.3 (1.4) | 73.6 (1.0) | 73.9 (1.1) | 0.16 |
| Blood Sugar Score | 91.0 (0.8) | 90.0 (1.0) | 90.0 (0.5) | 0.11 |
| Body Mass Index Score | 70.0 (1.6) | 70.7 (1.5) | 64.5 (1.5) | <0.001 |
| Smoking Score | 73.3 (2.4) | 74.7 (1.6) | 75.6 (1.3) | 0.74 |
| Sleep Score | 79.5 (1.1) | 83.3 (0.8) | 85.3 (0.7) | <0.001 |
| Diet Score | 39.7 (1.9) | 43.1 (1.2) | 40.4 (1.0) | 0.80 |
The Essential 8 score and its components have been presented as mean (standard error).
Fig. 2.
Trends of the Life's Essential 8 Score in the 18-44 Year Age Group Stratified by Race/Ethnicity from NHANES Cycles 2007 to 2018.
This figure depicts the trends of the Life's Essential 8 (LE8) score and its components from 2007-2018 stratified race/ethnicity. Two consecutive NHANES cycles were combined to create 3 groups (2007-2010, 2011-2014, and 2015-2018) for analyzing the trends of the LE8 score and its components. Non-Hispanic White, Non-Hispanic Black, Mexican American, and other races have been depicted in black, red, blue, and green, respectively.
A strong correlation between the LS7 score and the LE8 score was noted (0.79, P<0.001). After the categorization of the LS7 and LE8 scores into poor, intermediate, and ideal categories, there was moderate agreement between the LE8 and LS7 score categories with a weighted Kappa coefficient of 0.44 (P<0.001). Of the 1.7 million individuals categorized as poor CVH by the LS7 score, 1.6 million (94.1%) remained in the poor CVH category and 0.1 million (5.9%) were reclassified to intermediate CVH when classified by the LE8 score. Of the 46.1 million individuals categorized as intermediate CVH by the LS7 score, 35.6 million (77.2%) remained in the intermediate CVH category, 8.1 million (17.6%) were reclassified as poor CVH, and 2.3 million (5.0%) were reclassified to ideal CVH by the LE8 score. Of the 40.1 million individuals categorized as ideal CVH by the LS7 score, 21.1 million (52.6%) remained in the ideal CVH category, 18.9 million (47.1%) were reclassified as intermediate CVH, and 0.1 million (0.2%) were reclassified to poor CVH by the LE8 score (Central Illustration).
4. Discussion
This study of 12,197 young US adults, representing 89.4 million individuals of the non-institutionalized US population, showed that the CVH of the 18-44 years age group using the LE8 score has not shown any improvement from 2007 to 2018. Females had a higher mean score than males in all components of the LE8 score, except BMI and physical activity. However, an improvement in the physical activity score was noted in females from 2007 to 2018. The overall LE8 score was the lowest in the Non-Hispanic Black individuals compared with the other race/ethnic groups. On analyzing the trend of the LE8 score and its components, the sleep score increased and the blood pressure, blood glucose, and BMI scores decreased over the years in the overall population. The blood lipids score increased among the study cohort participants across the sex and race/ethnic subgroups. Nearly 1 in 4 young adults in the US were classified as having ideal CVH using the LE8 score compared with 1 in 2 individuals using the LS7 score. Approximately 19.0 million (47.3%) young adults with ideal CVH as per the LS7 score were reclassified as having intermediate or poor CVH as per the LE8 score.
The use of the LE8 score captures greater granularity in the overall CVH and each component. As opposed to the LS7 score, the LE8 score and its components are measured on a continuous scale from 0 to 100 [5]. Each component of the score has at least 5 levels which allows each level to have a narrow range of values. This increases the sensitivity of the score and prevents the clubbing of a wide range of values into a single category. The LS7 score does not quantify the metrics in a manner beyond traditional clinical categories whereas the LE8 score estimates CVH using the entire range of the respective CVH metric. Therefore, the blood pressure, BMI, blood glucose, and blood lipids categories in the LS7 score do not reflect the incremental increase in the risk of cardiovascular disease with the increase of these indices over and above the normal range [27], [28], [29], [30]. Although a moderate increase in the sleep score was noted, the CVH of more than half of the young adults was classified as intermediate by the LE8 score which was concordant with the LS7 score. About ∼20 million individuals with LS7-defined ideal CVH were reclassified as intermediate or poor CVH by the LE8 score. These individuals represent a previously unidentified section of the population who would benefit from primary preventative interventions.
This study noted sex differences in the overall LE8 score and its components. Females in early adulthood are more likely to seek help and follow up more frequently with a physician compared with males which may explain the higher scores in the blood pressure, blood lipids, and blood glucose categories [31,32]. The prevalence of hypertension in females is lower than that of males in the young adult age group which has been previously attributed to sex hormones and sex-associated differences in the renin-angiotensin system along with sex-associated differences in social determinants of health [33].
In race/ethnicity-based analysis, Non-Hispanic Black young adults had the lowest scores in blood pressure, blood sugar, sleep, and diet categories and the total LE8 score compared with other racial/ethnic groups. Social determinants of health include the non-medical factors that impact health. These factors may largely explain the lower scores observed in Non-Hispanic Black individuals. Non-Hispanic Black individuals are more likely to have lower socioeconomic status and attain lower education levels compared with Non-Hispanic White individuals [34,35]. Furthermore, Non-Hispanic Black individuals are more likely to reside in poorer neighborhoods and lack transportation which may limit access their access to healthy food and healthcare [34,35]. Lower scores in the blood pressure and blood sugar category have been previously attributed to poor dietary patterns, poor sleep, lower awareness, lower adherence to therapy, inadequate therapy, and lack of access to healthcare [15,[36], [37], [38], [39]]. The reduced duration of sleep in Non-Hispanic Black individuals could be attributed to a combination of environmental factors, socioeconomic status, and psychosocial factors [40], [41], [42], [43], [44]. The poor dietary pattern in Non-Hispanic Black individuals may be multi-factorial including socioeconomic status, neighborhood effect, cultural traditions, and targeted advertisements promoting unhealthy diets [45,46].
The multi-dimensionality of sleep and its strong association with other determinants of CVH has led to its inclusion in the LE8 score. Poor sleep health has been associated with obesity, hypertension, hyperlipidemia, and diabetes mellitus [38,39,[47], [48], [49]]. In addition to increasing the risk of cardiovascular disease through its association with the determinants of CVH, poor sleep independently has also been associated with a higher risk of coronary heart disease, stroke, and heart failure [47]. Racial/ethnic differences in sleep health may partly explain differences in the prevalence of cardiovascular diseases by race/ethnicity. Self-reported data showed that Non-Hispanic Black individuals have a lower sleep duration compared with Non-Hispanic White individuals [50], [51], [52], [53], [54]. Additionally, objective measurement of sleep using polysomnography and actigraphy-based studies showed that in addition to reduced duration of sleep, Non-Hispanic Black individuals had poor sleep quality characterized by shorter duration of sleep, lower sleep maintenance, higher fragmentation of sleep, and reduced short wave sleep duration [54], [55], [56], [57]. Racial/ethnic differences in sleep have been previously noted to persist even after controlling for socioeconomic status, other social determinants of health that affect sleep require further examination [52,53,[55], [56], [57]].
With young adults forming the majority of the demographic dividend of the population, poor CVH in young adults may devastatingly impact the economy not only by increasing healthcare expenditure due to cardiovascular morbidity and mortality but also loss of the workforce of the country. Regional differences in CVH, with the southeastern US having disproportionately low CVH and high cardiovascular mortality, may lead to the development of a geographic disparity in the economy [58,59]. The proportion of young adults experiencing a cardiovascular event has increased from 27% to 32% over the last two decades [1]. An estimated ∼90% of these patients have a modifiable risk factor during an index event, most commonly hypertension, dyslipidemia, and obesity [60]. Furthermore, an increasing trend in the poor control of these modifiable risk factors such as hypertension, diabetes mellitus, and dyslipidemia [16,18]. Control of these risk factors in young adults has been associated with a reduction of cardiovascular and all-cause mortality in later life [61], [62], [63]. Widespread use of the LE8 score enables the measurement of these risk factors and allows the trending of the CVH of an individual over time. This can help identify the specific categories which require improvement and therefore plan a directed intervention to improve CVH. However, due to the interrelated nature of the components of the score, improvement in the overall score should be the aim at an individual level. For example, inadequate sleep has been associated with obesity, hypertension, dyslipidemia, and type 2 diabetes mellitus [39]. At an individual level, aggressive control of the health factors (blood glucose, blood lipids, and blood pressure) should be promoted. Increased taxation of tobacco products, junk food, and sweetened beverages [64], incentivization of physical activity [65], improved access to healthcare, and subsidization of healthy food can help improve CVH at a population level.
5. Limitations
This study has several limitations. First, the serial cross-sectional nature of the NHANES cycles prohibits any causal inferences but provides robust population-level estimates of CVH. Additionally, changes in the CVH at an individual level could not be assessed longitudinally over time. Second, NHANES uses self-identified data for race/ethnicity and sex. Self-identified race/ethnicity is a construct combining social, cultural, and geographical factors that may not reflect genetic ancestry. This study could not compare the CVH of the Non-Hispanic Asian population with the other racial/ethnic groups due to the unavailability of data before the 2011-2012 cycles. Third, the data used for the calculation of the components of the LE8 score may be subject to measurement errors due to the reliance on self-reported data, such as for physical activity and smoking. Blood pressure measurement on a single day may be an insufficient measure of the 24-hour average blood pressure in an individual. Fourth, 2,642 individuals in the study population had missing components for the calculation of the LE8 score. This study excluded individuals with incomplete data to compute the scores. The weights assigned by NHANES to each individual can be highly variable due to oversampling of groups of interest. Therefore, the exclusion of individuals may contribute to the increased variance in population-level estimates.
6. Conclusions
An estimated one in four young US adults has ideal CVH estimated using the AHA LE8 score. The CVH status among young US adults has not demonstrated any overall improvement between 2007 and 2018, or when stratified by sex and race/ethnicity. Nearly 47% of young adults categorized as having ideal CVH as per the LS7 score were reclassified as having intermediate or poor CVH as per the LE8 score.
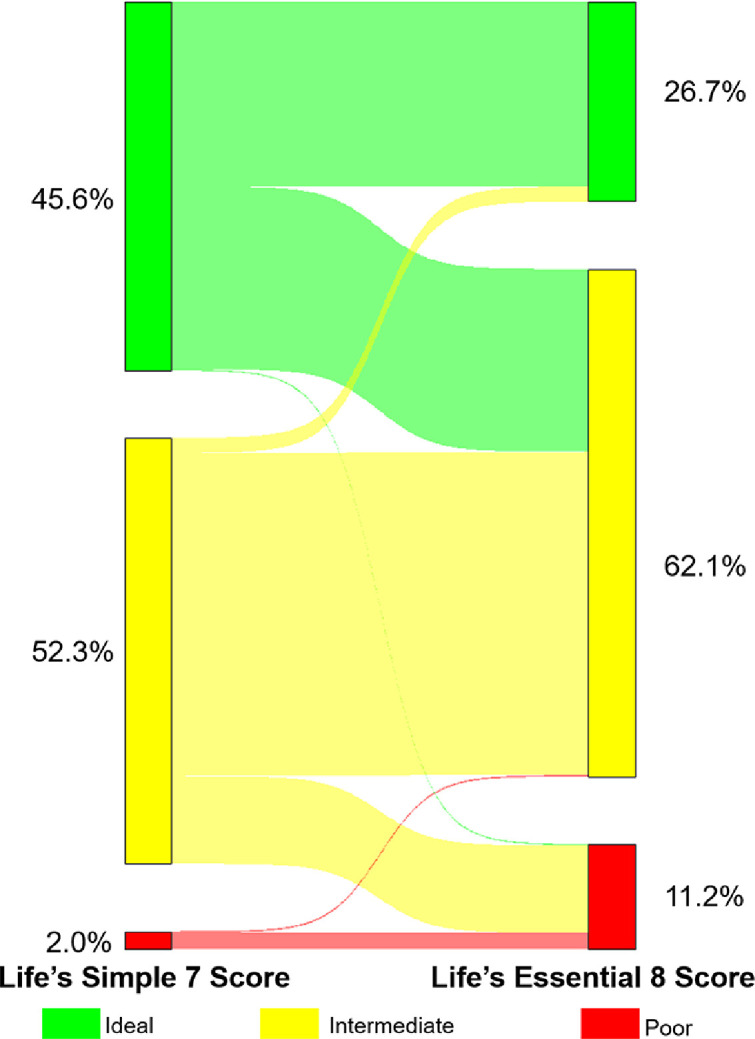
Central Illustration: Sankey Diagram Depicting Reclassification of Cardiovascular Health Categories Using the Life's Simple 7 and Life's Essential 8 Scores.
This figure depicts the reclassification of cardiovascular health (CVH) in young adults using the Life's Simple 7 (LS7) score and the Life's Essential 8 (LE8) Score. LS7 scores of 0-4, 5-9, and 10-14 were defined as poor, intermediate, and ideal CVH, respectively. LE8 scores of <50, 50-79, and ≥80 were defined as poor, intermediate, and ideal CVH, respectively. Of the 1.7 million individuals categorized as poor CVH by the LS7 score, 1.6 million (94.1%) remained in the poor CVH category and 0.1 million (5.9%) were reclassified to intermediate CVH when classified by the LE8 score. Of the 46.1 million individuals categorized as intermediate CVH by the LS7 score, 35.6 million (77.2%) remained in the intermediate CVH category, 8.1 million (17.6%) were reclassified as poor CVH, and 2.3 million (5.0%) were reclassified to ideal CVH by the LE8 score. Of the 40.1 million individuals categorized as ideal CVH by the LS7 score, 21.1 million (52.6%) remained in the ideal CVH category, 18.9 million (47.1%) were reclassified as intermediate CVH, and 0.1 million (0.2%) were reclassified to poor CVH by the LE8 score.
Author contributions
NSS, VP, and PA contributed to the conception, design, acquisition, analysis, interpretation, and critical revision of the manuscript. GA contributed to the conception, design, acquisition, interpretation, and critical revision of the manuscript. NP, IY, CB, CL, AP, RK, and PL contributed to the design, interpretation, and drafting of the manuscript.
Sources of funding
Dr. Pankaj Arora is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) awards R01HL160982, R01HL163852, R01HL163081, and K23HL146887, and by the Doris Duke Charitable Foundation COVID-19 Fund to Retain Clinician Scientists (Grant #2021255); UAB COVID-19 CARES Retention Program (CARES at UAB).
Disclosures
None of the other authors had any conflicts of interest or financial disclosures to declare.
Acknowledgments
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2022.100452.
Appendix. Supplementary materials
References
- 1.Arora S., Stouffer G.A., Kucharska-Newton A.M., et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Flaherty M., Buchan I., Capewell S. Contributions of treatment and lifestyle to declining CVD mortality: why have CVD mortality rates declined so much since the 1960s? Heart. 2013;99:159–162. doi: 10.1136/heartjnl-2012-302300. [DOI] [PubMed] [Google Scholar]
- 3.Shay C.M., Ning H., Allen N.B., et al. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003-2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonen M., Laitinen T.T., Magnussen C.G., et al. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: a Presidential Advisory From the American Heart Association. Circulation. 2022 doi: 10.1161/CIR.0000000000001078. 101161CIR0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Chen H., Li S., Pan L., Jia C. Association of Sleep Duration with the Morbidity and Mortality of Coronary Artery Disease: A Meta-analysis of Prospective Studies. Heart Lung Circ. 2015;24:1180–1190. doi: 10.1016/j.hlc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Nagai M., Hoshide S., Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayas N.T., White D.P., Manson J.E., et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Lao X.Q., Liu X., Deng H.B., et al. Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. J Clin Sleep Med. 2018;14:109–117. doi: 10.5664/jcsm.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Onge M.P., Grandner M.A., Brown D., et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the american heart association. Circulation. 2016;134:e367–ee86. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Wheaton A.G., Chapman D.P., Cunningham T.J., Lu H., Croft JB. Prevalence of Healthy Sleep Duration among Adults–United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Jones D.M., Ning H., Labarthe D., et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association's New "Life's Essential 8" Metrics: prevalence Estimates from the National Health and Nutrition Examination Survey (NHANES), 2013-2018. Circulation. 2022 doi: 10.1161/CIRCULATIONAHA.122.060911. [DOI] [PubMed] [Google Scholar]
- 14.Zipf G., Chiappa M., Porter K.S., Ostchega Y., Lewis B.G., Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. 2013;1:1–37. [PubMed] [Google Scholar]
- 15.Parcha V., Patel N., Kalra R., Arora G., Arora P. Prevalence, awareness, treatment, and poor control of hypertension among young american adults: race-stratified analysis of the national health and nutrition examination survey. Mayo Clin Proc. 2020;95:1390–1403. doi: 10.1016/j.mayocp.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Kalra R., Parcha V., Patel N., et al. Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005-2016. Eur J Prev Cardiol. 2021;28:304–312. doi: 10.1177/2047487320905190. [DOI] [PubMed] [Google Scholar]
- 17.Patel N., Bhargava A., Kalra R., et al. Trends in Lipid, Lipoproteins, and Statin Use Among U.S. Adults: Impact of 2013 Cholesterol Guidelines. J Am Coll Cardiol. 2019;74:2525–2528. doi: 10.1016/j.jacc.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Patel N., Kalra R., Bhargava A., Arora G., Arora P. Ideal cardiovascular health among American adults after the economic recession of 2008-2009: insights from NHANES. Am J Med. 2019;132:1182–1190. doi: 10.1016/j.amjmed.2019.06.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han L., You D., Ma W., et al. National trends in american heart association revised life's simple 7 metrics associated with risk of mortality among US adults. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q., Cogswell M.E., Flanders W.D., et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford E.S., Greenlund K.J., Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parcha V., Heindl B., Kalra R., et al. Insulin resistance and cardiometabolic risk profile among nondiabetic American young adults: insights from NHANES. J Clin Endocrinol Metab. 2022;107:e25–e37. doi: 10.1210/clinem/dgab645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folsom A.R., Olson N.C., Lutsey P.L., Roetker N.S., Cushman M. American Heart Association's Life's Simple 7 and incidence of venous thromboembolism. Am J Hematol. 2015;90:E92. doi: 10.1002/ajh.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folsom A.R., Shah A.M., Lutsey P.L., et al. American heart association's life's simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976. doi: 10.1016/j.amjmed.2015.03.027. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasbani N.R., Ligthart S., Brown M.R., et al. American Heart Association's Life's Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation. 2022;145:808–818. doi: 10.1161/CIRCULATIONAHA.121.053730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram D.D., Malec D.J., Makuc D.M., et al. National center for health statistics guidelines for analysis of trends. Vital Health Stat. 2018;2:1–71. [PubMed] [Google Scholar]
- 27.Katzmarzyk P.T., Reeder B.A., Elliott S., et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health. 2012;103:147–151. doi: 10.1007/BF03404221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawes C.M., Parag V., Bennett D.A., et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 29.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 30.Stamler J., Daviglus M.L., Garside D.B., Dyer A.R., Greenland P., Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Moran AE. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertension. 2017;70:736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galdas P.M., Cheater F., Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–623. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez L.A., Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31:1247–1254. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parcha V., Malla G., Suri S.S., et al. Geographic Variation in Racial Disparities in Health and Coronavirus Disease-2019 (COVID-19) Mortality. Mayo Clin Proc Innov Qual Outcomes. 2020;4:703–716. doi: 10.1016/j.mayocpiqo.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noonan A.S., Velasco-Mondragon H.E., Wagner F.A. Improving the health of African Americans in the USA: an overdue opportunity for social justice. Public Health Rev. 2016;37:12. doi: 10.1186/s40985-016-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman W.H., Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067–1072. doi: 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano Y., Gao Y., Johnson D.A., et al. Sleep characteristics and measures of glucose metabolism in blacks: the jackson heart study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calhoun D.A., Harding SM. Sleep and hypertension. Chest. 2010;138:434–443. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid S.M., Hallschmid M., Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 40.Jackson C.L., Redline S., Kawachi I., Williams M.A., Hu F.B. Racial disparities in short sleep duration by occupation and industry. Am J Epidemiol. 2013;178:1442–1451. doi: 10.1093/aje/kwt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson D.A., Jackson C.L., Williams N.J., Alcantara C. Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79–95. doi: 10.2147/NSS.S169312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan K.J., Knutson K.L., Pereira A.C., von Schantz M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med Rev. 2017;33:70–78. doi: 10.1016/j.smrv.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitaker K.M., Jacobs D.R., Jr., Kershaw K.N., et al. Racial Disparities in Cardiovascular Health Behaviors: the Coronary Artery Risk Development in Young Adults Study. Am J Prev Med. 2018;55:63–71. doi: 10.1016/j.amepre.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James S.A., LaCroix A.Z., Kleinbaum D.G., Strogatz DS. John Henryism and blood pressure differences among black men. II. The role of occupational stressors. J Behav Med. 1984;7:259–275. doi: 10.1007/BF00845359. [DOI] [PubMed] [Google Scholar]
- 45.Black C., Moon G., Baird J. Dietary inequalities: what is the evidence for the effect of the neighbourhood food environment? Health Place. 2014;27:229–242. doi: 10.1016/j.healthplace.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCullough M.L., Chantaprasopsuk S., Islami F., et al. Association of Socioeconomic and Geographic Factors With Diet Quality in US Adults. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson C.L., Redline S., Emmons K.M. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappuccio F.P., D'Elia L., Strazzullo P., Miller M.A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cappuccio F.P., Taggart F.M., Kandala N.B., et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hale L., Do D.P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krueger P.M., Friedman E.M. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandner M.A., Petrov M.E., Rattanaumpawan P., Jackson N., Platt A., Patel N.P. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9:897–905. doi: 10.5664/jcsm.2990. A-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whinnery J., Jackson N., Rattanaumpawan P., Grandner M.A. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37:601–611. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn S., Lobo J.M., Logan J.G., Kang H., Kwon Y., Sohn M.W. A scoping review of racial/ethnic disparities in sleep. Sleep Med. 2021;81:169–179. doi: 10.1016/j.sleep.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 55.Carnethon M.R., De Chavez P.J., Zee P.C., et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50–55. doi: 10.1016/j.sleep.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung J., Goodman M., Huang T., et al. Racial-ethnic Differences in Actigraphy, Questionnaire, and Polysomnography Indicators of Healthy Sleep: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2021 doi: 10.1093/aje/kwab232. [DOI] [PubMed] [Google Scholar]
- 57.Lauderdale D.S., Knutson K.L., Yan L.L., et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 58.Parcha V., Kalra R., Best A.F., et al. Geographic Inequalities in Cardiovascular Mortality in the United States: 1999 to 2018. Mayo Clin Proc. 2021;96:1218–1228. doi: 10.1016/j.mayocp.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 59.Parcha V., Kalra R., Suri S.S., et al. Geographic variation in cardiovascular health among American adults. Mayo Clin Proc. 2021;96:1770–1781. doi: 10.1016/j.mayocp.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yandrapalli S., Nabors C., Goyal A., Aronow W.S., Frishman W.H. Modifiable Risk Factors in Young Adults With First Myocardial Infarction. J Am Coll Cardiol. 2019;73:573–584. doi: 10.1016/j.jacc.2018.10.084. [DOI] [PubMed] [Google Scholar]
- 61.Dong C., Rundek T., Wright C.B., Anwar Z., Elkind M.S., Sacco R.L. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mok Y., Sang Y., Ballew S.H., et al. American Heart Association's Life's Simple 7 at Middle Age and Prognosis After Myocardial Infarction in Later Life. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perak A.M., Ning H., Khan S.S., et al. Associations of Late Adolescent or Young Adult Cardiovascular Health With Premature Cardiovascular Disease and Mortality. J Am Coll Cardiol. 2020;76:2695–2707. doi: 10.1016/j.jacc.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebreab S.Y., Davis S.K., Symanzik J., Mensah G.A., Gibbons G.H., Diez-Roux A.V. Geographic variations in cardiovascular health in the United States: contributions of state- and individual-level factors. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajat C., Hasan A., Subel S., Noach A. The impact of short-term incentives on physical activity in a UK behavioural incentives programme. NPJ Digit Med. 2019;2:91. doi: 10.1038/s41746-019-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.