Abstract
Insomnia can cause damage to function and other medical and mental illnesses, and it is also a risk factor for increasing medical care costs. Although simple behavior intervention is feasible in primary medical institutions, the lack of corresponding technical training has obviously restricted its use, patients' autonomy dependence is generally poor, and early missions have some difficulties. Relatively speaking, acupuncture in traditional therapy is more likely to be accepted, but the mechanism is still unclear. In this study, a model of insomnia was constructed using chlorophenylalanine (PCPA) in 6-week-old male SD rats. Electroacupuncture was used to stimulate Baihui (DU20), Shenmen (HT7), and Sanyinjiao (SP6), and the behavior, histopathology, cAMP/CREB/BDNF, PI3K/Akt pathways and the expression of sleep-related factors were observed. Our study showed that IL-1β, PGD2, MT, IL-10, IL-6, TNF-α, IFN-γ and CORT in rats could be regulated after electroacupuncture stimulation. The expression of TrkB, PI3K, Akt, P-TrkB, p-Akt, cAMP, CREB, and BDNF can also be up- or downregulated. Apoptosis-related Bax, Bad and Caspase-3, as well as the monoamine neurotransmitters 5-HT, DA, NE and EPI, were also modulated by electroacupuncture. Taken together, these data illustrate the potential of DU20, HT7 and SP6 as a multitargeted therapy for insomnia in rats. The novelty of the study lies in the description of the Traditional Chinese Medicine stimulation methods different from Chinese Herbs: electroacupuncture stimulates acupoints of sleep factors, cAMP/CREB/BDNF, PI3K/Akt pathways and the multipath and multitarget body response regulation mechanism of apoptosis.
Keywords: Electroacupuncture, Acupoints, Rat, Insomnia, Apoptosis, Mechanism
Electroacupuncture; acupoints; rat; insomnia; apoptosis; mechanism.
Insomnia is a common clinical disorder that is characterized by difficulty falling asleep or staying asleep, and it is accompanied by irritability or fatigue when awake. The prevalence rate of insomnia is approximately 10–20%, of which approximately 50% of cases are chronic [1]. Insomnia can lead to impaired functioning and other medical and psychiatric disorders, and it is a risk factor that increases healthcare costs. The causes of insomnia include genetic, environmental, behavioral, and physiological factors that ultimately lead to hyperexcitability. Effective treatments include behavioral, cognitive, and pharmacological interventions. Benzodiazepines, nonbenzodiazepines, antidepressants, and melatonin agonists are widely used in the treatment of insomnia. However, these treatments have negative impacts on patient cognitive function and can cause addiction and central nervous system depression [2, 3]. Although simple behavioral interventions are feasible in primary medical institutions, the lack of corresponding technical training significantly limits their use, patient adherence is generally poor, and early education is also difficult. Compared with other treatment methods, the use of acupuncture in traditional therapy is more acceptable, and related studies have also shown that acupuncture can improve insomnia symptoms, sleep time, and daytime function [4, 5]. Although previous studies have described the mechanisms by which acupuncture regulates immune cytokine production, oxidative defense systems, and neurotransmitters [6, 7, 8], insomnia involves a variety of complex mechanisms, and further research is needed to improve the acupuncture-insomnia regulatory system.
Acupuncture and moxibustion follow particular guidelines related to acupoint compatibility, and choosing different acupoints according to different theories is the basis of traditional Chinese medicine (TCM). TCM believes that the brain is the house of the primordial spirit and the point where all yang meets, and the brain is a key organ that regulates insomnia. The Baihui point first seen in the "Acupuncture and Moxibustion A and B Classic" is located in the depression in the middle of the top of the head, 5 inches away from the front hairline. It can harmonize the yin and yang balance of the whole body. At the same time, TCM believes that the lower part of Baihui is where the brain is located, and the brain is the "sea of marrow", which is also the "house of the primordial spirit", which governs the human body's mental activities such as thinking, consciousness, memory, etc., and there are 14 governor vessels where Baihui is located. The only meridian that is directly related to the brain collaterals. Therefore, Baihui is an important point for regulating the mind, which can treat various brain diseases. Thus, the Baihui point (DU20), which is located at the highest part of the brain, is the first choice. On the other hand, TCM believes that the heart controls the gods and is the viscera of insomnia onset. The ancient Chinese literature "Jingyue Quanshu" also clearly put forward the concept of "Shen's peace and sleep". The "Golden Mirror of Medicine" also clearly clarifies that acupuncture at the Shenmen point can soothe and calm the mind. In the development of TCM acupuncture, the soothing effect of Shenmen has become the "first-class main point" for the treatment of insomnia. Therefore, as an important acupoint for regulating consciousness, Shenmen (HT7) should also be selected to treat insomnia. TCM believes that the liver, spleen and kidney are closely related to qi, blood, and yin and yang of the whole body, so the Sanyinjiao (SP6) point, which is related to these three viscera, is the third selected acupoint. Therefore, according to the theory of TCM, this study will try to explain the mechanism by which acupuncture functions in the treatment of insomnia by stimulating the three acupoints mentioned above (DU20, HT7, and SP6; referred to as DHS).
Long-term insomnia increases the incidence of hypertension, coronary heart disease, Alzheimer's disease, anxiety, depression, neurosis, menopausal syndrome, chronic indigestion, anemia, atherosclerosis and other related diseases and seriously affects human lifespan. People with insomnia are five times more likely to suffer from depression and anxiety than the general population, have a multiplied risk of cardiovascular disease, and have a significantly higher risk of death. The medical resource consumption of insomnia patients is approximately 12 times that of those without insomnia, the incidence of insomnia in females is higher than that in males, and insomnia in females is 1.7 times higher than that in males in people over 45 years old [9, 10]. Insomnia has multiple regulatory mechanisms. 5-Hydroxytryptamine (5-HT) mainly exists in the cerebral cortex and synapses of mammals. It is a central sleep-promoting substance [11]. In addition, IL-1β is a sleep-promoting factor that can promote nonrapid eye movement sleep. The expression of IL-1β is in the form of night high and day low, which is positively correlated with the degree of sleep. Insufficient secretion of IL-1β reduces rebound after spontaneous sleep or sleep deprivation [12]. The PI3K/Akt pathways can promote the secretion of IL-1β. Recent studies have found that apoptosis occurs in the brains of sleep-deprived animals [12, 13], and brain apoptosis markers in rats are also positive, including BCL-2-related death-initiating protein, apoptotic protein precursor, and Caspase- 9, which start the pathway within the mitochondria, leading to cell apoptosis [14]. Because apoptosis is involved in the whole process of insomnia [15] and neuronal death caused by apoptosis is the main pathological feature of familial insomnia [16], medicinal plants that can regulate apoptosis also have the effect of regulating insomnia [17]. Therefore, in this study, our goal was to demonstrate the body response regulation mechanism of sleep factors, cAMP/CREB/BDNF, PI3K/Akt pathways and apoptosis of acupoints stimulated by electroacupuncture. To further improve the acupuncture-insomnia regulation system. The novelty of this study is based on the theory of TCM, the selected acupoints, and the modern systematic research methods, multipathway, multitarget elaboration of the mechanism of electroacupuncture in regulating insomnia. This study will add evidence for the effectiveness of TCM complementary therapy, acupuncture.
1. Materials and methods
1.1. Experimental reagents
Para-chlorophenylalanine (PCPA) was purchased from Shanghai Genye. The 0.9% NaCl solution was adjusted to a weakly basic pH (pH 7.0–8.0) with NaOH and HCl to prepare a PCPA suspension (5% Tween-80) for use in this study. 7,8-Dihydroxyflavone (7,8-DHF) was purchased from MCE, pentobarbital sodium was purchased from Sigma Merck, dimethyl sulfoxide (DMSO) was purchased from Solepipe, and PEG300 was purchased from MCE. Eosin was purchased from Solarbio, hematoxylin was purchased from Servicebio, nisin staining solution was purchased from Servicebio, neutral resin was purchased from Shanghai Sinopharm, the In Situ Cell Death Detection Kit, the DAB concentrated kit was purchased from Solarbio, hematoxylin was purchased from Servicebio, the Marker was purchased from Helix, and the BCA protein concentration assay kit was purchased from Solarbio. ELISA kits for interleukin-1β (IL-1β), prostaglandins D2 (PGD2), melatonin (MT), corticosterone (CORT), IL-6, IL-10, tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and brain-derived neurotrophic factor (BDNF) were purchased from Bioswamp. TRIzol was purchased from Ambion, and PrimeScript II Rtase was purchased from TAKARA. Antibodies against Bcl-2, Bax, Bad, Caspase-3, TrKB, PI3K, Akt, cAMP, CREB, BDNF, GAPDH, p-TrKB, p-Akt and p-CREB, as well as goat anti-rabbit IgG, were purchased from CST.
1.2. Rat insomnia model and treatment
Fifty six-week-old male SD rats were selected and housed under specific pathogen-free (SPF) conditions. All animals were obtained from Hunan Slaughter Jingda Laboratory Animal Co. The rats were adaptively reared for 7 days. The rats were divided into five groups for the experiments. The control group (n = 10) was randomly selected and injected intraperitoneally with 10 ml/kg saline, and the PCPA group (n = 10) was injected intraperitoneally with PCPA at a concentration of 60 mg/ml in a volume of 10 ml/kg once a day for two consecutive days. The PCPA + DHS group (n = 10) was treated as follows. On the second day after the model was established, the rats were fixed with a flat rat fixator, and 1-inch acupuncture needles (Hua Tuo brand) of 0.25 mm × 25 mm thickness were inserted into the rats at the Baihui (2 mm diagonally toward the front skin), bilateral Shen Men (3 mm directly), and bilateral Sanyinjiao (2–3 mm directly) positions. Then, the rats were connected to an electroacupuncture instrument after twisting the needles for 1 min with flat tonic and flat diarrhea. The waveform was sparse and dense, the frequency was 2/15 Hz, the electroacupuncture intensity was 1 mA, and the needles were maintained in their positions for 30 min. These procedures were repeated once per day for 5 days. On the 4th day, 30 min after the completion of acupuncture, the open field experiment was performed, and 30 min after the last acupuncture, the positive reflex experiment was performed. In the PCPA+7,8-DHF group (n = 10), the TrkB agonist 7,8-DHF was injected intraperitoneally at a concentration of 10 mg/ml on day 2 after the model was established. In the PCPA + DHS+7,8-DHF group (n = 10), the TrkB agonist 7,8-DHF was injected intraperitoneally at a concentration of 10 mg/ml on day 2 after the model was established, and needling was performed 6 h later (same procedure as in the PCPA + DHS group) once per day for 5 days [18].
1.3. Behavioral observations and experiments
Changes in the coat color, activity, response, defecation, diet, water intake and body mass of the rats in each group were observed once every 2 days. Open field experiments: On day 4, 30 min after the acupuncture intervention, the rats were acclimatized in a wooden box for 1 min, and the number of times the rats crossed the grid, the number of times the rats stood, the time spent in the central grid, the number of grooming behaviors and the number of feces in each group within 3 min were recorded. Between the two experiments, the inner wall and bottom surface of the field box were cleaned. Reversal reflex experiment: Thirty minutes after the last acupuncture, the rats in each group were injected intraperitoneally with sodium pentobarbital (40 mg/kg) and placed on a plate in a supine position. The sleep latency and sleep duration of the rats were observed and recorded. A lack of turning behavior for 1 min was considered to indicate the disappearance of the turning reflex. The sleep latency was measured from the time of injection to the disappearance of the righting reflex. The duration of sleep was measured from the time of injection to the time when the rats laid on their backs, automatically turned their bodies over, and did not turn over again for 1 min, which was considered to indicate the restoration of the righting reflex. The disappearance of the righting reflex was considered to indicate the moment when the rats fell asleep. The return of the flip-right reflex indicated awakening. After the behavioral observations and experiments, blood was obtained from the abdominal aorta after intraperitoneal anesthesia with 3% pentobarbital sodium, and hippocampal and brainstem tissues were isolated and preserved [19, 20].
1.4. Hematoxylin and eosin staining (HE)
Fixed rat hippocampus and brainstem tissues were placed flat side down at the bottom of a plastic embedding box for dehydrated immersion wax embedding. The tissue samples were then frozen at -20 °C and sectioned (3 μM) [21] when the tissue reached proper hardness. The tissue sections were dewaxed in water, washed with water for 2 min and stained with hematoxylin solution for 6 min. The hematoxylin solution was removed with running water for 2 min, and then, the sections were incubated with 1% hydrochloric acid alcohol for 3 s. After washing with water, the blue-promoting solution was added and incubated for 10 s to change the color to blue. After washing with water for 30 s, 0.5% eosin solution was added and incubated for 3 min. The sections were washed with water for 2 s followed by 80% ethanol for 30 s, 95% ethanol for 30 s, anhydrous ethanol for 2 s, xylene (I) for 3 s, and xylene (II) for 3 s. Then, the sections were sealed with neutral gum. The sections were imaged by microscopy, and images of the relevant parts of the sections were captured by the Leica Application Suite graphics system.
1.5. Nissl staining
The rat hippocampus and brainstem tissues were dewaxed, embedded in wax and sectioned (3 μM). After spreading and baking, the tissue sections were routinely dewaxed in water and stained for 5 min with Nissl staining solution. The sections were then washed, dried in an oven at 65 °C and sealed with a neutral resin. The sections were imaged with a microscope, and images of the relevant areas of the sections were captured by the Leica Application Suite graphics system. The nisomes stained dark blue, the nuclei were light blue, and the background was essentially colorless.
1.6. Transferase-mediated dUTP nick end labeling
The fixed rat hippocampal and brainstem tissues were dewaxed, embedded in wax, and then sectioned (3 μM). TUNEL staining was performed after spreading and baking the sections. The slides were first incubated at a constant temperature of 65 °C for 1 h, incubated in xylene I for 15 min and then incubated in xylene II for 15 min. Then, the dewaxed sections were incubated in 100% alcohol, 95% alcohol, 85% alcohol and 75% alcohol for 5 min and washed with tap water for 10 min. The sections were incubated with proteinase K working solution at 37 °C for 15 min. The sections were washed twice with PBS, and 50 μL of enzyme solution was added to 450 μL of labeling solution and mixed thoroughly. Then, 50 μL TUNEL reaction mix was added to the sections and incubated for 60 min at 37 °C in a wet box in the dark with a cover. The cells were washed 3 times with PBS, 50 μL DAB substrate was added, and the cells were incubated for 10 min at room temperature. The sections were washed 3 times with PBS, counterstained with hematoxylin, dehydrated and cleared. Finally, blocking, microscopic observation, and image acquisition were performed. The positive apoptotic cell nuclei stained brownish yellow or gray, and nonapoptotic cell nuclei stained blue.
1.7. Western blotting
The proteins were extracted, and the protein concentration of each group was determined using a BCA kit. Then, 20 μg of protein was added to gels (prepared with 12% isolate and 5% concentrate), and the proteins were separated by electrophoresis as follows: 80 V for 40 min for concentration and 120 V for 50 min for separation. Then, the proteins were transferred to membranes at 90 V for 50 min, and the membranes were blocked with 5% skim milk powder at room temperature overnight at 4 °C [22, 23]. Antibodies against Bcl-2 (1:1000), Bax (1:1000), Bad (1:1000), Caspase-3 (1:1000), TrKB (1:1000), p-TrKB (1:1000), PI3K (1:1000), Akt (1:1000), p-Akt (1:1000), cAMP (1:1000), CREB (1:1000), BDNF (1:1000) and GAPDH (1:1000) were added and incubated at room temperature for 1 h. After washing the membrane, goat anti-rabbit immunoglobulin G (IgG) (1:20,000) was added and incubated for 1 h at room temperature. The ECL luminescent solution was added and placed in a fully automated chemiluminescent analyzer for color development, and the grayscale values of the relevant bands were measured by TANON GIS software, with three replicates in each group.
1.8. Enzyme-linked immunosorbent assay (ELISA)
The levels of the sleep-promoting factors IL-1β, prostaglandin D2 (PGD2), and melatonin (MLT) as well as those of the wakefulness-promoting factor corticosterone (CORT) in the peripheral blood serum of rats were measured by ELISA kits. The levels of the inflammatory cytokines IL-6, IL-10, TNF-α, and IFN-γ in serum were also measured. The procedures were carried out in strict accordance with the instructions. The absorbance (OD) of each well was measured at 450 nm, and the absorbance of blank wells was adjusted to zero.
1.9. Quantitative reverse transcriptase PCR (qRT‒PCR)
One hundred milligrams of hippocampal and brainstem tissue samples from each group of rats were placed in 1 mL of TRIzol for the extraction of total RNA. cDNA was synthesized by reverse transcription, and PCR amplification was performed using cDNA as a template. The reaction procedure was as follows [24]: 95 °C for 3 min; 40 cycles of 95 °C for 5 s, 56 °C for 10 s, and 72 °C for 25 s. The CT values were obtained at the end of the reaction, and statistical analysis was performed using the 2-ΔΔCT method with GAPDH as an internal reference. The PCR primers were synthesized by Wuhan Tianyi Huayu Gene Technology Co., and the sequences are listed in Table 1.
Table 1.
Primer sequences.
| Primers | Sequence | Amplified fragment size (bp) |
|---|---|---|
| TRKB-F | CCAACCATCACATTTCTCG | 260 |
| TRKB-R | TGTCTCTCGTCCTTCCCATA | |
| cAMP-F | CACTGTCACTGCTATTGCTCC | 277 |
| cAMP-R | TTCACTACCCCCTGTTCCTT | |
| CREB-F | AGTGCCCAGCAACCAAGT | 113 |
| CREB-R | GGGAGGACGCCATAACAA | |
| BDNF-F | GAGCGTGTGTGACAGTATTAGC | 171 |
| BDNF-R | CCTTCCTTCGTGTAACCCA | |
| GAPDH-F | ACCCACTCCTCTACCTTCG | 108 |
| GAPDH-R | CACCACCCTGTTGCTGT |
1.10. Data statistical analysis
The experimental data were analyzed using SPSS 19.0 software, and the results are expressed as the mean ± standard deviation (mean ± SD). One-way ANOVA (one-way ANOVA) was used to compare data among multiple groups, and the LSD test was used for two-way comparisons between means. The difference was statistically significant at P < 0.05.
2. Results
2.1. Effects of DHS stimulation on the behavior of rats with insomnia
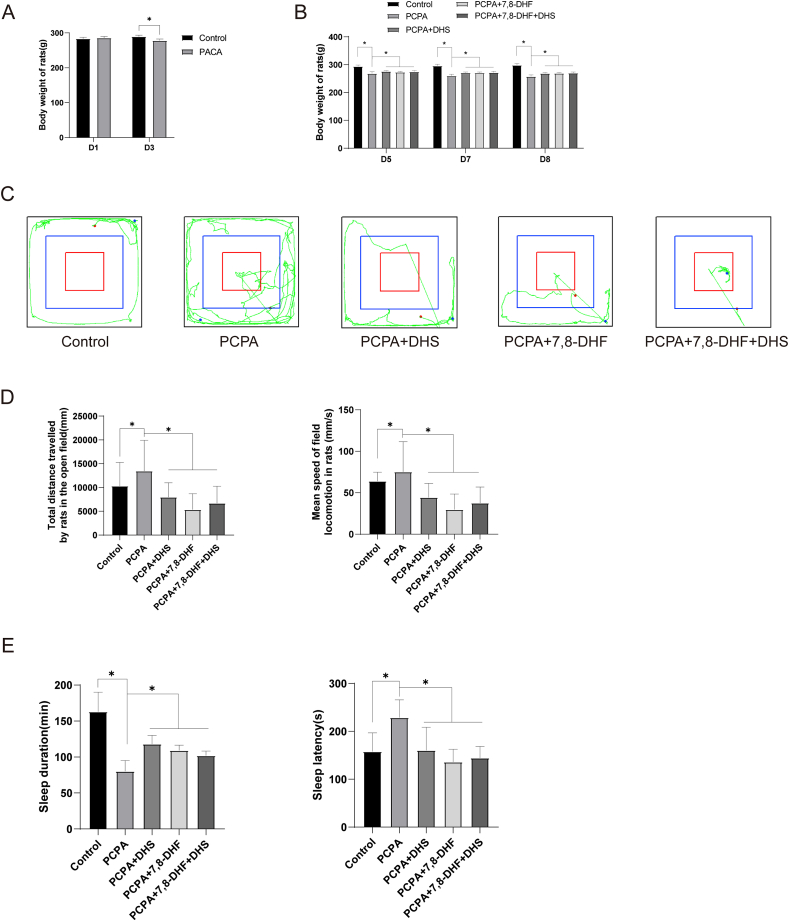
Evaluation of insomnia models by observing animal behavior has been widely used [19]. PCPA is a specific, potent, irreversible tryptophan hydroxylase (TPH) inhibitor that can reduce slow-wave sleep in animals [25, 26]. The behavior of rats was observed on the second day of PCPA injection, and we found that the rats had dull hair, alopecia, increased daytime activity, disrupted circadian rhythms, increased aggression, and significantly decreased body weight after the model was established, as shown in Figure 1A. After DHS or 7,8-DHF intervention, the rats’ diet, water intake, and defecation habits gradually returned to normal, and their body weight also recovered, as shown in Figure 1B. Similarly, compared with the PCPA group, the movement trajectories of the rats in the DHS or 7,8-DHF intervention groups were sparse, indicating that the anxiety of the rats in the open field was significantly reduced (Figure 1C, D). Then, we performed a sodium pentobarbital trigger reflex experiment (Figure 1E). After DHS or 7,8-DHF intervention, the sleep time and sleep latency of the rats were improved to some extent.
Figure 1.
Effects of DHS stimulation on rat behavior. Figure 1A, the body weight of the rats in the model group decreased significantly compared with that in the control group (P < 0.05). The general status of the rats was observed every 2 days after the model was established, and there were no significant changes in the rats in the model group. The rats in the PCPA + DHS group, PCPA+7,8-DHF group and PCPA+7,8-DHF + DHS group showed significant improvement in general status, and their diet, water intake and defecation habits gradually normalized. On days 5, 7 and 8, the weight of the rats in each group was observed (Figure 1B). Compared with the control group, the body weight of the rats in the model group decreased significantly (P < 0.05). Compared with the model group, the body weight of the rats in the PCPA + DHS, PCPA+7,8-DHF and PCPA+7,8-DHF + DHS groups was significantly increased (P < 0.05). Figure 1C shows the movement trajectory of each group of rats. Compared with the control group, the model group had more frequent movements and more intensive movement trajectories in the open field, indicating that the model rats had increased anxiety in the open field. In contrast, the motor trajectories were sparse in the DHS and 7,8-DHF intervention groups compared to the model group, indicating that rats experienced significantly less anxiety in the open field. By analyzing the indicators of motion in the open field (Figure 1D), compared with the control group, the total distance and mean speed in the model group were significantly higher (P < 0.05); compared with the model group, the total distance and mean speed in the PCPA + DHS, PCPA+7,8-DHF and PCPA+7,8-DHF + DHS groups were significantly lower (P < 0.05). We then performed a pentobarbital sodium flip-flop reflex experiment (Figure 1E). Compared with the control group, sleep duration was significantly lower (P < 0.05) and sleep latency was significantly higher (P < 0.05) in the model group. In contrast, after DHS and 7,8-DHF interventions, sleep duration increased (P < 0.05), and sleep latency decreased (P < 0.05).
2.2. Effects of DHS stimulation on histopathology and the expression of sleep-related factors in rats with insomnia
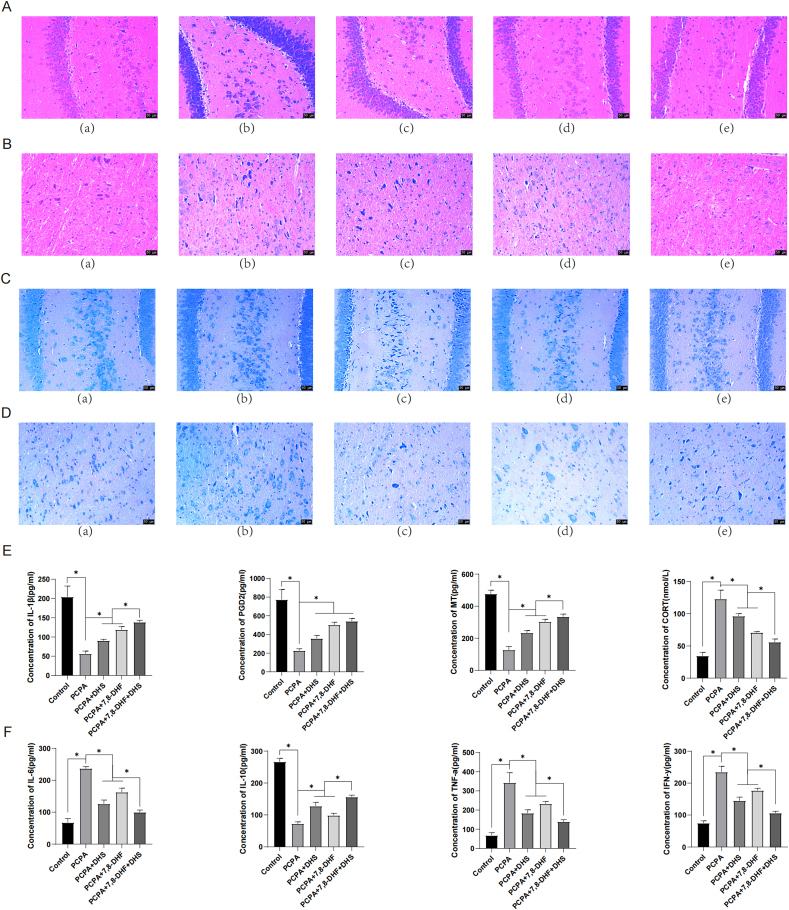
The histopathological observation of the hippocampus and brainstem of rats by HE staining showed that the nuclei of neurons in the brainstem and hippocampus of the PCPA group were darkly stained, the cytoplasm was dissolved, the volumes of a large number of vertebral cells were reduced, and the intercellular spacing was significantly increased, as shown in Figure 2A, B. After DHS or 7,8-DHF intervention, the neurons in the brainstem and hippocampus were well aligned, with reduced nuclear fixation and cytoplasmic lysis, and more well-arranged pyramidal cells with fewer vacuoles and normal intercellular spacing were observed. As shown in Figure 2C, D, Nissl staining revealed that the hippocampus and brainstem neurons in the PCPA group were enlarged, and the structure of the pyramidal cell layer was disordered. After DHS or 7,8-DHF intervention, most cells in the brainstem and hippocampus were normal in shape, with no significant reduction in the number of cell layers, an increase in cerebrospinal fluid, and only a small amount of neuronal loss and gliosis.
Figure 2.
Effects of DHS stimulation on histopathology and the expression of sleep-related factors in rats with insomnia. A and C show the hippocampus, and B and D show the brainstem. (a), (b), (c), (d), and (e) show the control, PCPA, PCPA + DHS, PCPA+7,8-DHF, and PCPA+7,8-DHF + DHS groups, respectively. Figure 2A and B show HE staining. The morphology of the hippocampus and brain stem cells in the control group was normal, the boundary between the molecular layer and the outer granular layer was clearer, and the arrangement of the vertebral cell layer was normal. In the PCPA group, the nuclei of the neurons in the brainstem and hippocampus were deeply stained, the cytoplasm was dissolved, the volumes of a large number of vertebral cells were reduced, and the intercellular spacing was significantly increased. After DHS or 7,8-DHF intervention, the neurons in the brainstem and hippocampus were well aligned, with reduced nuclear fixation and cytoplasmic lysis and more well-arranged pyramidal cells with fewer vacuoles and normal intercellular spacing. Figure 2C and D show the morphology of neurons in the hippocampus and brainstem tissues of the rats in each group as observed by Nissl staining. The hippocampus and brainstem cells in the control group were normal in shape, neatly arranged, and lacked neuronal damage or loss, and the cytoplasm and nucleus were clear. The hippocampal and brainstem neurons in the PCPA group were enlarged, and the structure of the vertebral cell layer was disordered. After DHS or 7,8-DHF intervention, most cells in the brainstem and hippocampus were normal in shape, with no significant reduction in the number of cell layers, an increase in cerebrospinal fluid, and only a small amount of neuronal loss and gliosis. Figure 2E and F show the levels of related factors in the peripheral blood of rats. Compared with the control group, the serum levels of IL-1β, PGD2, MT, and IL-10 in the PCPA group were significantly decreased (P < 0.05). The levels of IL-6, TNF-α, IFN-γ and CORT were significantly increased (P < 0.05). The serum levels of IL-1β, PGD2, MT and IL-10 were significantly increased after PCPA + DHS or PCPA+7,8-DHF intervention (P < 0.05), and the levels of IL-6, TNF-α, IFN-γ and CORT were significantly decreased (P < 0.05).
We then examined the levels of sleep-promoting and wake-promoting factors in the peripheral blood of the rats. As shown in Figure 2E, F, the serum levels of IL-1β, PGD2, MT, and IL-10 in the PCPA group were significantly decreased (P < 0.05), while the levels of IL-6, TNF-α, IFN-γ and CORT were significantly increased. The serum levels of IL-1β [27], PGD2, MT and IL-10 were significantly increased, while the levels of IL-6, TNF-α, IFN-γ and CORT were significantly decreased after PCPA + DHS or PCPA+7,8-DHF intervention. The effects of the combination of DHS and 7,8-DHF intervention were the best.
These results indicate that DHS stimulation can improve neuronal morphology in the hippocampus and brainstem and the levels of sleep-related factors in the serum of rats with insomnia.
2.3. DHS stimulation inhibits neuronal apoptosis in the hippocampus and brainstem tissues of rats
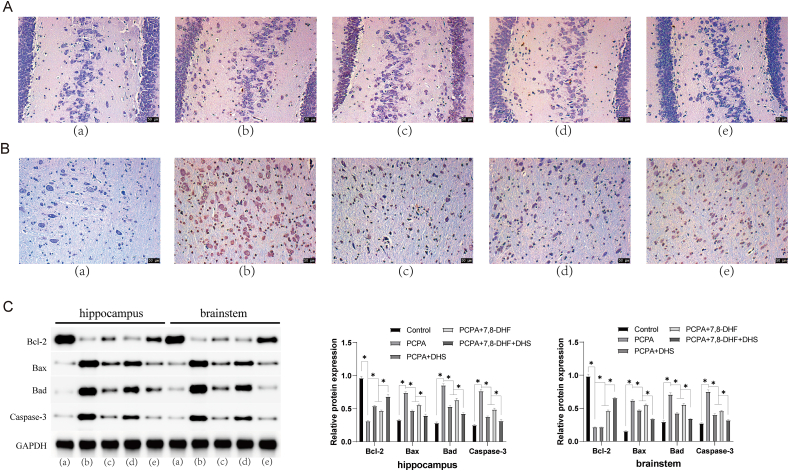
TUNEL staining is a common method to detect cell apoptosis staining. The principle is that when cells undergo apoptosis, some DNA endonucleases will be activated. These endonucleases cleave genomic DNA between nucleosomes, and apoptosis can be observed by labeling these DNA fragments [28]. We examined neuronal apoptosis in the hippocampus and brainstem of each group of rats by TUNEL staining. The number of stained cells in the hippocampus and brainstem neurons of the rats in the PCPA group was significantly increased, the staining was darker, and apoptosis was increased, as shown in Figure 3A and B. After DHS or 7,8-DHF intervention, the number of stained cells in hippocampal and brainstem neurons decreased, the staining was lighter, and apoptosis was decreased.
Figure 3.
DHS stimulation inhibits neuronal apoptosis in rat hippocampus and brainstem tissues. A shows the hippocampus, and B shows the brainstem. (a), (b), (c), (d), and (e) show the control, PCPA, PCPA + DHS, PCPA+7,8-DHF, and PCPA+7,8-DHF + DHS groups, respectively. Neuronal apoptosis in the hippocampus and brainstem of the rats in each group was examined by TUNEL staining. Compared with the control group, the number of stained cells in the hippocampus and brainstem neurons of the rats in the PCPA group was significantly increased, the staining was darker, and apoptosis was increased. Compared with the PCPA group, the hippocampal and brainstem neurons in the DHS and 7,8-DHF groups had fewer stained cells, lighter staining, and decreased apoptosis (Figure 3A, B). Western blotting was used to measure the expression of apoptotic proteins in the hippocampus and brainstem tissues of the rats in each group, as shown in Figure 3C. Compared with the control group, the expression of Bcl-2 in the hippocampus and brainstem of the model rats was significantly downregulated (p < 0.05), and the expression levels of Bax, Bad and Caspase-3 were significantly upregulated (P < 0.05). Compared with the PCPA group, the expression levels of Bcl-2 in the hippocampus and brainstem of rats subjected to DHS or 7,8-DHF intervention were significantly upregulated (P < 0.05), and the expression levels of Bax, Bad and Caspase-3 were significantly downregulated (P < 0.05).
Western blotting was used to measure the expression of apoptotic proteins in the hippocampus and brainstem tissues of the rats in each group (Figure 3C). DHS or 7,8-DHF intervention upregulated the expression of Bcl-2 and downregulated the expression of Bax, Bad and Caspase-3. This means that DHS can inhibit neuronal apoptosis in the hippocampus and brainstem tissues of rats.
2.4. Effects of DHS stimulation on the PI3K/Akt pathway
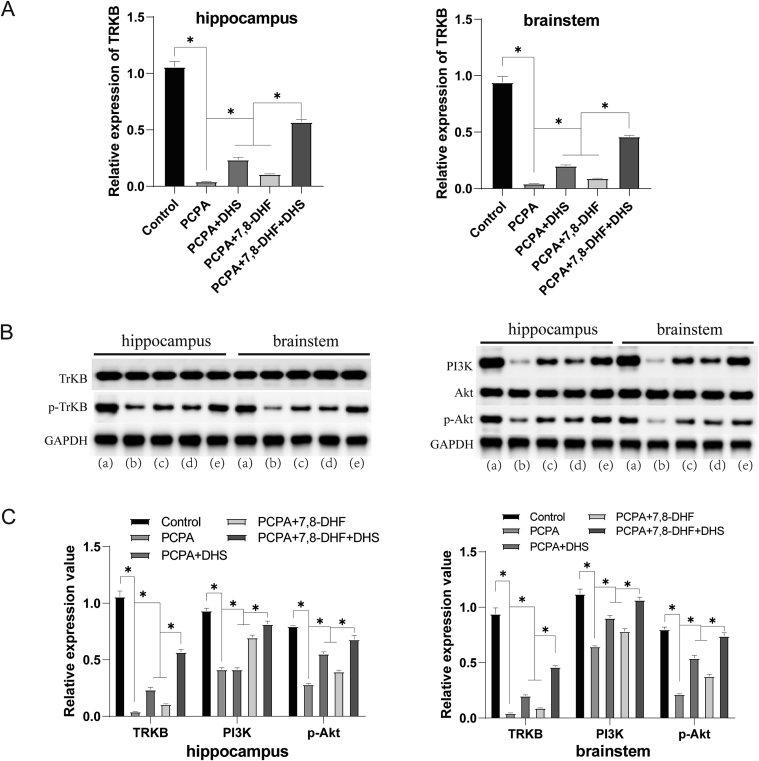
TrkB expression in rat hippocampus and brainstem tissues was measured by qRT‒PCR, and the expression of TrkB, PI3K, Akt, P-TrkB, and p-Akt was measured by Western blotting (Figure 4). Compared with the PCPA group, the mRNA expression of TrkB was increased in the DHS and 7,8-DHF intervention groups, and the protein expression levels of p-TrkB, PI3K and p-Akt were also increased. These results seem to indicate a regulatory effect of DHS stimulation on the TrKB and PI3K/Akt pathways.
Figure 4.
Effects of DHS stimulation on the PI3K/Akt pathway. (a), (b), (c), (d), and (e) show the control, PCPA, PCPA + DHS, PCPA+7,8-DHF, and PCPA+7,8-DHF + DHS groups, respectively. The mRNA expression of TrkB in rat hippocampus and brainstem tissues was measured by qRT‒PCR (Figure 4A). Compared with the control group, the mRNA expression of TrkB in the hippocampus and brainstem tissues of the PCPA group was significantly decreased (P < 0.05). The mRNA expression of TrkB in the DHS or 7,8-DHF group was significantly higher than that in the PCPA group (P < 0.05). The effect of the combined DHS and 7,8-DHF intervention was better than that of the single interventions (P < 0.05). The Western blotting results were consistent (Figure 4, B). Compared with the control group, the protein expression of p-TrkB in the PCPA group was significantly decreased (P < 0.05). The protein expression of p-TrkB in the DHS or 7,8-DHF group was significantly higher than that in the PCPA group (P < 0.05). Compared with the PCPA group, the relative protein expression levels of PI3K and p-Akt were significantly lower than those in the control group (P < 0.05). The relative protein expression of PI3K and p-Akt in the DHS or 7,8-DHF group was significantly higher than that in the PCPA group (P < 0.05). The effect of the combined DHS and 7,8-DHF intervention was better than that of the single interventions (P < 0.05).
2.5. Effects of DHS stimulation on the cAMP/CREB pathway
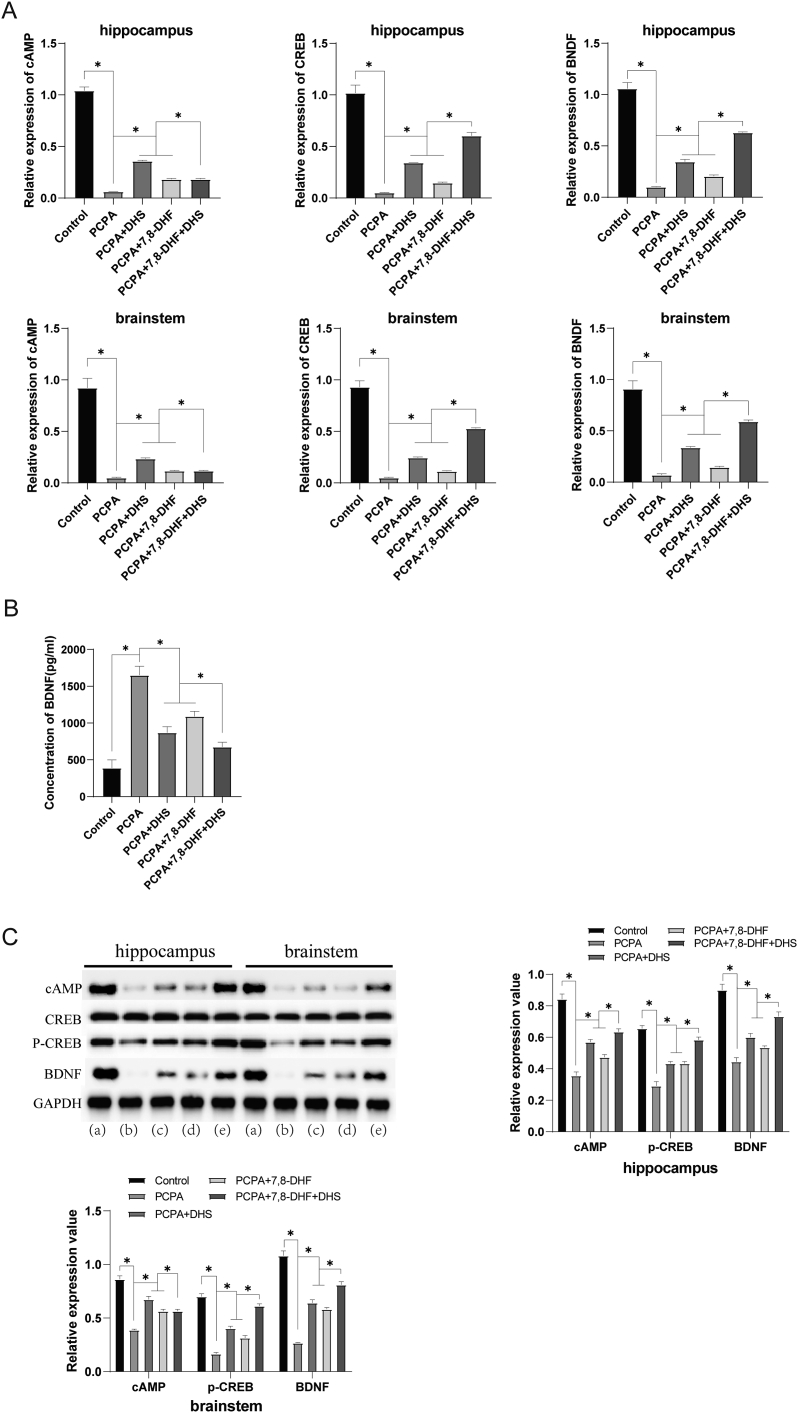
We then examined factors associated with the cAMP/CREB/BDNF pathway. As shown in Figure 5A, the mRNA expression levels of cAMP, CREB and BDNF were significantly upregulated in the DHS or 7,8-DHF group compared with the PCPA group. The level of BDNF in peripheral blood serum was measured by ELISA (Figure 5B), and the level of BDNF was also significantly increased after intervention. The same results were obtained using Western blotting. As shown in Figure 5C, cAMP, p-CREB and BDNF protein expression was upregulated by DHS or 7,8-DHF intervention. These results suggest that DHS stimulation may alleviate the mechanism underlying PCPA-induced insomnia in rats via the cAMP/CREB/BDNF pathway.
Figure 5.
Effects of DHS stimulation on the cAMP/CREB pathway. (a), (b), (c), (d), and (e) show the control, PCPA, PCPA + DHS, PCPA+7,8-DHF, and PCPA+7,8-DHF + DHS groups, respectively. Figure 5A shows that compared with the control group, the mRNA expression levels of cAMP, CREB and BDNF in the hippocampus and brainstem of the PCPA group were significantly downregulated (P < 0.05). Compared with the PCPA group, the mRNA expression levels of cAMP, CREB and BDNF were significantly upregulated in the DHS group and 7,8-DHF group (P < 0.05). The levels of BDNF in peripheral blood serum were measured by ELISA (Figure 5B). The serum BDNF level of the PCPA group was significantly lower than that of the control group (P < 0.05). The serum BDNF levels in the DHS group and 7,8-DHF group were significantly higher than those in the PCPA group (P < 0.05). Western blotting also revealed that DHS or 7,8-DHF intervention upregulated the protein expression of cAMP, p-CREB and BDNF (p < 0.05) (Figure 5C).
2.6. Effects of DHS stimulation on monoamine neurotransmitters
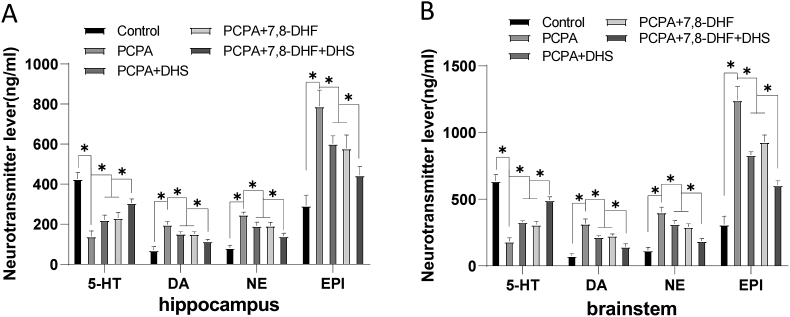
5-HT can promote the accumulation of hypnotic peptide substances in the hypothalamus and simultaneously act synergistically with neurotransmitters such as norepinephrine (NE) and dopamine (DA), induce the brain to enter a sleep state, and have a dual regulatory effect on the sleep-wake cycle [29]. Continuing to evaluate the effect of DHS stimulation on monoamine neurotransmitters, as shown in Figure 6, the DHS or 7,8-DHF intervention group had significantly higher levels of 5-HT and lower levels of DA, NE, and EPI than the PCPA group. This result indicates that DHS stimulation can effectively inhibit the excitability of the central nervous system and improve the sleep quality of rats with insomnia.
Figure 6.
Effects of DHS stimulation on monoamine neurotransmitters. Neurotransmitter lever of hippocampus (Figure 6A) and Neurotransmitter lever of brainstem (Figure 6B). Compared with the control group, the contents of 5-HT in the hippocampus and brainstem of the PCPA group were significantly decreased (P < 0.05), while the contents of DA, NE and EPI were significantly increased (P < 0.05). The level of 5-HT in the DHS or 7,8-DHF group was significantly increased (P < 0.05), and the levels of DA, NE and EPI were significantly decreased (P < 0.05).
3. Discussion
Insomnia leads to a variety of different behavioral manifestations [30], including jumping, bumping, irritability, mania, excitement, hair loss, listlessness and other behavioral abnormalities [19]. This effect may occur because psychiatric conditions are closely related to sleep disturbances [31]. Therefore, benzodiazepine receptor agonists and some antidepressants, antihistamines, antipsychotics, and melatonin are often used in the treatment of insomnia [32]. To elucidate the experimental evidence regarding the use of acupuncture to treat insomnia, this study evaluated the hair, daytime activity, circadian rhythm, aggression, body weight, diet, water intake, and defecation habits of rats with insomnia. We found that DHS stimulation had a corrective effect on undesirable traits. In addition, gene knockout experiments found that the deficiency of 5-HT reduced the awakening level of the central nervous system [29]. DA is an important regulatory factor for central nervous system neurons and synapses, which are mainly mediated by D1 and D2 receptors. Among them, dopamine D2 receptor (D2R) is an important receptor to maintain awakening. The D2R agonist has a two-way effect, that is, increased sleep at low doses and high-dose-induced awakening [33, 34]. Norepinephrine (NE) can associate awakening behaviors with other awakening paths by actively activating the cerebral cortex and edge systems [35, 36]. Our research has found that DHS can also reduce the release of the excitement monoamine neurotransmitters DA, NE, and EPI, which is similar to previous research on the treatment of depression rats [37]. However, we expanded the scope of the treatment of electroacrolescence to insomnia. Of course, perhaps the same diseases that affect monoclonal neurotransmitters, such as Alzheimer's disease, tinnitus, anxiety, and chronic fatigue, can also be treated with electroacupons. Related aspects still need more research.
Studies have shown that during insomnia, accompanied by the activation of small gel cells and hyperplasia and apoptosis of nerve cells [16], one of the important causes of a large loss of neurons is the large amount of nurnic cell apoptosis. Due to the irreplaceability of nerve cells, after the number of losses reaches a certain amount, organic damage to the brain occurs, which affects brain function. Although the specific mechanism of apoptosis still needs to be further clarified in the specific mechanism of insomnia, the importance of apoptosis in the insomnia process is self-evident. Our data show that DHS can increase the expression of BCL-2, reduce the expression of BAX, BAD, and Caspase-3, and reduce apoptosis in TUNEL chromatics. These results all prove that DHS can reduce apoptosis in insomnia rats. In addition, the PI3K/AKT pathway participates in the regulation of various cell functions, such as cell proliferation, differentiation, apoptosis, and glucose transfer. Previous studies have shown that starting AKT phosphate can reduce the expression of CASPASE 3 and BAX and stimulate BCL-2 expression, inhibiting the apoptosis of rats induced by the same cysteine [38]. This means that the PI3K/AKT pathway can directly inhibit the occurrence of apoptosis upstream. In addition, BDNF, as a member of the neurotic factor family, participates in the plasticity of multiple neurons in multiple brain areas. There is much evidence that experiencing psychological pressure will reduce the expression of BDNF, and a lack of neurotic nutritional support will lead to severe depression [39]. Our research found that DHS can increase the expression of BDNF, which may be another mechanism by which DHS regulates insomnia. The CAMP/Creb pathway is widely distributed in the central nervous system and is of great significance for the survival and growth of neurons and the formation of long-term enhancement of synapses [40]. The CAPM effect element promoter can be combined with CREB to initiate gene transcription, which promotes downstream proteins such as neurotipides, that is, early gene family proteins (such as C-FOS and C-JUN), and BDNF protein synthesis, which in turn regulates the differentiation, apoptosis and survival of neurons [41]. In other words, in addition to adjusting BDNF, we also proved that the upstream CAMP/CREB pathway can be adjusted by DHS, and nerve apoptosis can be adjusted by CAMP/CREB pathway [42]. Some immune function indicators respond to insomnia and damage immune function [43]. Cyclicals involved in regulating sleep-awakening rhythm are mainly tumor necrosis factor (TNF), interferon (IFN), collodated stimulus factor (CSF), leukocyte (IL), carbon-based toxin, and conversion growth factor (TGF-P). Among them, IL-1, IL-12, IL-8, TNF-α, IL-18 and other inflammatory reaction factors are related to sleep. Other inflammatory reactions, such as IL-10, IL-4, and IL-13, may affect slow wave sleep and even cause insomnia [44]. The HPA axis is a nerve endocrine system that regulates the stress reaction of the body. Long-term chronic stress can activate the almond core, thereby activating the HPA axis and causing the activation of the HPA axis, and its excessive activation has a close relationship with the development of insomnia. As the end product of the HPA axis, CORT is recognized as the HPA shaft activity indicator [45]. Morning IL-6 levels in the saliva correlate with sleep efficiency during the night and nighttime wakefulness, and IL-1β levels correlate with sleep duration and sleep onset latency; thus, both cytokines are indicators of sleep efficiency and quality [27]. While PGD2 is a potent endogenous hypnotic agent, its sleep-inducing mechanism has been well characterized [46]. MT has been used to improve sleep in patients with insomnia primarily because patients who take melatonin do not experience negative effects the next day or exhibit signs of addiction. The MT(1) and MT(2) melatonin receptors ramelteon are effective in increasing total sleep time and sleep efficiency, as well as reducing sleep latency, in patients with insomnia [47]. CORT can induce depression [48] and is involved in the occurrence and development of comorbidities of insomnia, anxiety and depression [49]. In short, we have proven that DHS can significantly increase the levels of IL-1β, PGD2, MT, and IL-10, which reduces the levels of IL-6, TNF-α, IFN-γ, and CORT. These data provide favorable support for the effectiveness of DHS in regulating insomnia.
4. Conclusion
TCM has always been considered to have a multicomponent and multitarget regulatory mechanism. However, the multitarget mechanism of other TCM regulation methods has rarely been studied in depth. In this study, under the guidance of TCM theory, Stimulation DU20, HT7, and SP6 were selected as treatment methods. We found that DHS stimulation has a clear effect in improving the characteristics and behavior of rats with insomnia, such as hair, activity, diet, drinking, and defecation. DHS can also increase the levels of insomnia IL-1β, PGD2, MT, and IL-10 and reduce the levels of sleep-related factors such as IL-6, TNF-α, IFN-γ, and CORT, and the pathology of rats can also be improved. In addition, DHS can adjust the apoptotic function of insomnia rats and brain stem tissues through the PI3K/AKT pathway and CAMP/CREB pathway and can also produce benign regulatory effects on mononamine neurotransmitters. In short, our research ranges from rat behavior, monoamine neurotransmitters, the PI3K/AKT and CAMP/Creb pathways, apoptosis, MT), BDNF, cytokines, and cytokines. We believe this provides strong evidence for the global application promotion of DHS. Treatment methods such as electroacupuncture or acupuncture have been recognized by most countries, including the United States. The next step should be to explore the technical specifications and training of more effective promotion and application in primary medical institutions to make more people benefit. At the same time, more clinical evidence should also be collected to increase evidence.
Declarations
Author contribution statement
Fang Cao, Yan Xu: Contributed reagents, materials, analysis tools or data; wrote the paper.
Min Zhang, Xiaohui Li: Performed the experiments.
Yizhen Chen: Conceived and designed the experiments.
Mujun Zhi, Yan Li: Analyzed and interpreted the data.
Funding statement
Fang Cao was supported by Natural Science Foundation of Henan Province [202300410251], Henan Province Traditional Chinese Medicine Scientific Research Special Project [2019JDZX2033].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
None.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Buysse D.J. Insomnia. JAMA. 2013;309(7):706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertisch S.M., Herzig S.J., Winkelman J.W., Buettner C. National use of prescription medications for insomnia. NHANES 1999-2010, Sleep. 2014;37(2):343–349. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz T.L., Goradia V. Managing insomnia: an overview of insomnia and pharmacologic treatment strategies in use and on the horizon. Drugs Context 2013. 2013 doi: 10.7573/dic.212257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu C., Zhao N., Liu Z., Yuan L.H., Xie C., Yang W.J., Yu X.T., Yu H., Chen Y.F. Acupuncture improves peri-menopausal insomnia: a randomized controlled trial. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx153. [DOI] [PubMed] [Google Scholar]
- 5.Bergdahl L., Broman J.E., Berman A.H., Haglund K., von Knorring L., Markström A. Sleep patterns in a randomized controlled trial of auricular acupuncture and cognitive behavioral therapy for insomnia. Compl. Ther. Clin. Pract. 2017;28:220–226. doi: 10.1016/j.ctcp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Hayhoe S. Insomnia: can acupuncture help. Pain Manag. 2017;7(1):49–57. doi: 10.2217/pmt-2016-0025. [DOI] [PubMed] [Google Scholar]
- 7.Tang L., You F., Hu X., Li Y.F. [Electroacupuncture improves insomnia by down-regulating peripheral benzodiazepine receptor expression in hippocampus, and up-regulating 5-HT, 5-HIAA, TNF-α and IL-1β contents in hypothalamus in insomnia rats] Zhen Ci Yan Jiu. 2019;44(8):560–565. doi: 10.13702/j.1000-0607.180610. [DOI] [PubMed] [Google Scholar]
- 8.Yeung W.F., Yu B.Y., Yuen J.W., Ho J., Chung K.F., Zhang Z.J., Mak D., Suen L.K., Ho L.M. Semi-individualized acupuncture for insomnia disorder and oxidative stress: a randomized, double-blind, sham-controlled trial. Nat. Sci. Sleep. 2021;13:1195–1207. doi: 10.2147/NSS.S318874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi W. 2016. Research on the Brain Magnetic Resonance of Primary Insomnia with Cognitive Dysfunction. [Google Scholar]
- 10.Ying L., Xiaoyang Z., Fei B., Aijun S. Research and analysis of clinical efficacy of acupuncture in the treatment of primary insomnia. Chinese acupuncture. 2018;38(7):793–797. doi: 10.13703/j.0255-2930.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Yongqin L., erhe L. Effect of acupuncture combined with acupoints on primary insomnia and its influence on neurotransmitter level. Journal of Cardiovascular and Cerebrovascular Diseases of Integrated Traditional Chinese and Western Medicine. 2020;18(2):359–362. [Google Scholar]
- 12.Zhai S., Tao S., Wu X., Zou L., Yang Y., Xie Y., Li T., Zhang D., Qu Y., Tao F. Associations of sleep insufficiency and chronotype with inflammatory cytokines in college students. Nat. Sci. Sleep. 2021;13:1675–1685. doi: 10.2147/NSS.S329894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H., Zhong R., Liu H., Zhang F., Li S., Le W. Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer's disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J. Alzheimers Dis. 2016;50(3):669–685. doi: 10.3233/JAD-150774. [DOI] [PubMed] [Google Scholar]
- 14.Somarajan B.I., Khanday M.A., Mallick B.N. Rapid eye movement sleep deprivation induces neuronal apoptosis by noradrenaline acting on Alpha1 adrenoceptor and by triggering mitochondrial intrinsic pathway. Front. Neurol. 2016;7:25. doi: 10.3389/fneur.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y., Mao Z.J., Ruan Y.P., Zhang X. Exploration of the anti-insomnia mechanism of Ganoderma by central-peripheral multi-level interaction network analysis. BMC Microbiol. 2021;21(1):296. doi: 10.1186/s12866-021-02361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorandeu A., Wingertsmann L., Chrétien F., Delisle M.B., Vital C., Parchi P., Montagna P., Lugaresi E., Ironside J.W., Budka H., Gambetti P., Gray F. Neuronal apoptosis in fatal familial insomnia. Brain Pathol. 1998;8(3):531–537. doi: 10.1111/j.1750-3639.1998.tb00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboy M.S., Marcarini J.C., Luiz R.C., Barros I.B., Ferreira D.T., Ribeiro L.R., Mantovani M.S. In vitro evaluation of the genotoxic activity and apoptosis induction of the extracts of roots and leaves from the medicinal plant Coccoloba mollis (Polygonaceae) J. Med. Food. 2010;13(3):503–508. doi: 10.1089/jmf.2009.0119. [DOI] [PubMed] [Google Scholar]
- 18.Jiang M., Chen X., Zhang L., Liu W., Yu X., Wang Z., Zheng M. Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol. Res. 2021;54(1):24. doi: 10.1186/s40659-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y.H., Sun Y., Wang J., Jiang H.Q., Cui N., Su B.Z., Yu Z.Y. [Establishment of rat heart-kidney insomnia model consistent with traditional Chinese medicine syndrome and its serum metabolomics] Zhongguo Zhongyao Zazhi. 2020;45(2):383–390. doi: 10.19540/j.cnki.cjcmm.20190524.502. [DOI] [PubMed] [Google Scholar]
- 20.Ipsiroglu O.S., Wind K., Hung Y.A., Berger M., Chan F., Yu W., Stockler S., Weinberg J. Prenatal alcohol exposure and sleep-wake behaviors: exploratory and naturalistic observations in the clinical setting and in an animal model. Sleep Med. 2019;54:101–112. doi: 10.1016/j.sleep.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil H., Eliwa H.A., El-Shiekh R.A., Al-Mokaddem A.K., Hassan M., Tawfek A.M., El-Maadawy W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.114141. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Y., Xue X.J., Liu G., Shen M.M., Chao C.Y., Zhang J., Guo Y.Q., Niu Q.Q., Yu Y.N., Song Y.T., Wang H.H., Wang S.X., Chen Y.J., Jiang L.H., Li P., Yin Y.L. Perillaldehyde improves cognitive function in vivo and in vitro by inhibiting neuronal damage via blocking TRPM2/NMDAR pathway. Chin. Med. 2021;16(1):136. doi: 10.1186/s13020-021-00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Y.F., Luo T., Lu Z.Y., Shen C.Y., Jiang J.G. Targets and underlying mechanisms related to the sedative and hypnotic activities of saponins from Rhodiola rosea L. (crassulaceae) Food Funct. 2021;12(21):10589–10601. doi: 10.1039/d1fo01178b. [DOI] [PubMed] [Google Scholar]
- 24.Yang P., Qin Y., Zhu Y., Li F., Xia S.S., Zhou B., Wang Q., Lu J., Li L., Huang H.Y. Chaihu-Longgu-Muli decoction relieves epileptic symptoms by improving autophagy in hippocampal neurons. J. Ethnopharmacol. 2020;259 doi: 10.1016/j.jep.2020.112990. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y., Li Q., DU H.Z., Shen C.X., Li A.P., Pei X.P., DU C.H., Qin X.M. Determination of five neurotransmitters in the rat brain for the study of the hypnotic effects of Ziziphi Spinosae Semen aqueous extract on insomnia rat model by UPLC-MS/MS. Chin. J. Nat. Med. 2019;17(7):551–560. doi: 10.1016/S1875-5364(19)30077-9. [DOI] [PubMed] [Google Scholar]
- 26.Murray N.M., Buchanan G.F., Richerson G.B. Insomnia caused by serotonin depletion is due to hypothermia. Sleep. 2015;38(12):1985–1993. doi: 10.5665/sleep.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaVoy E.C., Palmer C.A., So C., Alfano C.A. Bidirectional relationships between sleep and biomarkers of stress and immunity in youth. Int. J. Psychophysiol. 2020;158:331–339. doi: 10.1016/j.ijpsycho.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Loo D.T. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Methods Mol. Biol. 2011;682:3–13. doi: 10.1007/978-1-60327-409-8_1. [DOI] [PubMed] [Google Scholar]
- 29.Okaty B.W., Commons K.G., Dymecki S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019;20(7):397–424. doi: 10.1038/s41583-019-0151-3. [DOI] [PubMed] [Google Scholar]
- 30.Holman R.B., Elliott G.R., Barchas J.D. Neuroregulators and sleep mechanisms. Annu. Rev. Med. 1975;26:499–520. doi: 10.1146/annurev.me.26.020175.002435. [DOI] [PubMed] [Google Scholar]
- 31.Sutton E.L. Insomnia. Ann. Intern. Med. 2021;174(3):ITC33–ITC48. doi: 10.7326/AITC202103160. [DOI] [PubMed] [Google Scholar]
- 32.Riemann D., Baglioni C., Bassetti C., Bjorvatn B., Dolenc Groselj L., Ellis J.G., Espie C.A., Garcia-Borreguero D., Gjerstad M., Gonçalves M., Hertenstein E., Jansson-Fröjmark M., Jennum P.J., Leger D., Nissen C., Parrino L., Paunio T., Pevernagie D., Verbraecken J., Weeß H.G., Wichniak A., Zavalko I., Arnardottir E.S., Deleanu O.C., Strazisar B., Zoetmulder M., Spiegelhalder K. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 33.Odagaki Y., Kinoshita M., Ota T. Dopamine-induced functional activation of Gα(q) mediated by dopamine D(1)-like receptor in rat cerebral cortical membranes. J. Recept. Signal Transduct. Res. 2019;39(1):9–17. doi: 10.1080/10799893.2018.1562470. [DOI] [PubMed] [Google Scholar]
- 34.Kurup R.K., Kurup P.A. Hypothalamic digoxin, cerebral chemical dominance and myalgic encephalomyelitis. Int. J. Neurosci. 2003;113(5):683–701. doi: 10.1080/00207450390200026. [DOI] [PubMed] [Google Scholar]
- 35.Daniele T., de Bruin P., Rios E., de Bruin V. Effects of exercise on depressive behavior and striatal levels of norepinephrine, serotonin and their metabolites in sleep-deprived mice. Behav. Brain Res. 2017;332:16–22. doi: 10.1016/j.bbr.2017.05.062. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y.P., Cui X.Y., Liu Y.T., Cui S.Y., Zhang Y.H. Ginsenoside Rg1 promotes sleep in rats by modulating the noradrenergic system in the locus coeruleus and serotonergic system in the dorsal raphe nucleus. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;116 doi: 10.1016/j.biopha.2019.109009. [DOI] [PubMed] [Google Scholar]
- 37.Xuejiao X., Tianying L., Xin L., Yanying Q., Junlin L., Jiahua T., Xiuli H. Effect of acupuncture on expression of monoamine neurotransmitter related genes in rats with depression. Clinical Journal of Acupuncture and Moxibustion. 2022;38(5):42–47. [Google Scholar]
- 38.Ren H., Mu J., Ma J., Gong J., Li J., Wang J., Gao T., Zhu P., Zheng S., Xie J., Yuan B. Selenium inhibits homocysteine-induced endothelial dysfunction and apoptosis via activation of AKT. Cell. Physiol. Biochem. 2016;38(3):871–882. doi: 10.1159/000443041. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt K., Holsboer-Trachsler E., Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016;48(1-2):42–51. doi: 10.3109/07853890.2015.1131327. [DOI] [PubMed] [Google Scholar]
- 40.Teich A.F., Nicholls R.E., Puzzo D., Fiorito J., Purgatorio R., Fa M., Arancio O. Synaptic therapy in Alzheimer's disease: a CREB-centric approach. Neurotherapeutics. 2015;12(1):29–41. doi: 10.1007/s13311-014-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakhina V., Arey R.N., Kaletsky R., Kauffman A., Stein G., Keyes W., Xu D., Murphy C.T. Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron. 2015;85(2):330–345. doi: 10.1016/j.neuron.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong H., Cohen D.E., Cui L., Supinski A., Savas J.N., Mazzulli J.R., Yates J.R., 3rd, Bordone L., Guarente L., Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 2011;18(1):159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin M.R., Opp M.R., Sleep Health Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42(1):129–155. doi: 10.1038/npp.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krueger J.M., Obál F.J., Fang J., Kubota T., Taishi P. The role of cytokines in physiological sleep regulation. Ann. N. Y. Acad. Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 45.Liqin L., Yan L., Ruirui Z., Jianyou G. Effects of ginsenosides on behavior, HPA axis and BDNF in chronic stress depression model rats. Chinese Journal of Traditional Chinese Medicine. 2011;36(10):1342–1347. [Google Scholar]
- 46.Urade Y. [Molecular mechanisms of insomnia], Nihon rinsho. Jpn. J. Clin. Med. 2009;67(8):1489–1493. [PubMed] [Google Scholar]
- 47.Cardinali D.P., Srinivasan V., Brzezinski A., Brown G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012;52(4):365–375. doi: 10.1111/j.1600-079X.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- 48.Bai G., Qiao Y., Lo P.C., Song L., Yang Y., Duan L., Wei S., Li M., Huang S., Zhang B., Wang Q., Yang C. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 2022;283 doi: 10.1016/j.jep.2021.114717. [DOI] [PubMed] [Google Scholar]
- 49.Liu C., Zhao Y., Qin S., Wang X., Jiang Y., Wu W. Randomized controlled trial of acupuncture for anxiety and depression in patients with chronic insomnia. Ann. Transl. Med. 2021;9(18):1426. doi: 10.21037/atm-21-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.