Central Illustration
Key Words: immunotherapy, mass cytometry, monoclonal antibody, myocarditis, treatment
Abbreviations and Acronyms: CITE-seq, cellular indexing of transcriptomes and epitopes by sequencing; CTLA-4, cytotoxic T-lymphocyte antigen 4; CyTOF, time-of-flight mass cytometry; ICI, immune checkpoint inhibitor; IRAE, immune-related adverse event; PD-1, programmed death-1; scRNA-seq, single-cell RNA sequencing; scTCR-seq, single-cell T-cell receptor sequencing
Highlights
-
•
ICIs used in cancer therapy can cause serious cardiac immune-related side effects.
-
•
Single-cell multi-omics are powerful tools in understanding cell subsets/phenotypes.
-
•
Multi-omics technology can elucidate disease mechanisms in ICI-induced myocarditis.
Cancer progression can correlate with dysfunction of the immune system as malignant cells evade host immune detection through manipulation of immune checkpoint pathways.1 Immune checkpoints, including the PD-1 (programmed cell death-1) and CTLA-4 (cytotoxic T-lymphocyte antigen-4) pathways,1 represent important physiological dampeners that prevent T-cell autoimmunity against healthy “self” cells. In the past decade, immune checkpoint inhibitors (ICIs) directed against these checkpoints have been developed as mainstay cancer therapies, with current examples including ipilimumab (anti-CTLA-4), nivolumab/pembrolizumab (anti-PD-1), durvalumab/atezolizumab (anti–programmed death ligand-1), relatlimab (anti–lymphocyte activating-3), and combination therapies to treat melanoma, lung cancer, kidney cancer, and others.1 However, inherent effects of activating T cells can lead to autoimmune side effects in many organ systems, limiting the safe use of ICIs.1
Cardiovascular Toxicity From Immunotherapy
Because of their immune activating effects, ICIs can cause autoimmune side effects known as immune-related adverse events (IRAEs), including dermatitis, thyroiditis, colitis, pneumonitis, encephalitis, and myocarditis. In addition to myocarditis, cardiovascular IRAEs also include pericarditis, vasculitis, myocardial infarction, and arrhythmia.1
Among all IRAEs, myocarditis remains one of the most devastating, with high mortality rates of up to 50%.1 Onset of myocarditis usually occurs within 1 to 3 months of treatment initiation1; cases may be smoldering, minimally symptomatic, or fulminant. The clinical significance of ICI-induced myocarditis has provided motivation for investigations into its pathogenesis.
Introduction to Single-Cell Immune Profiling
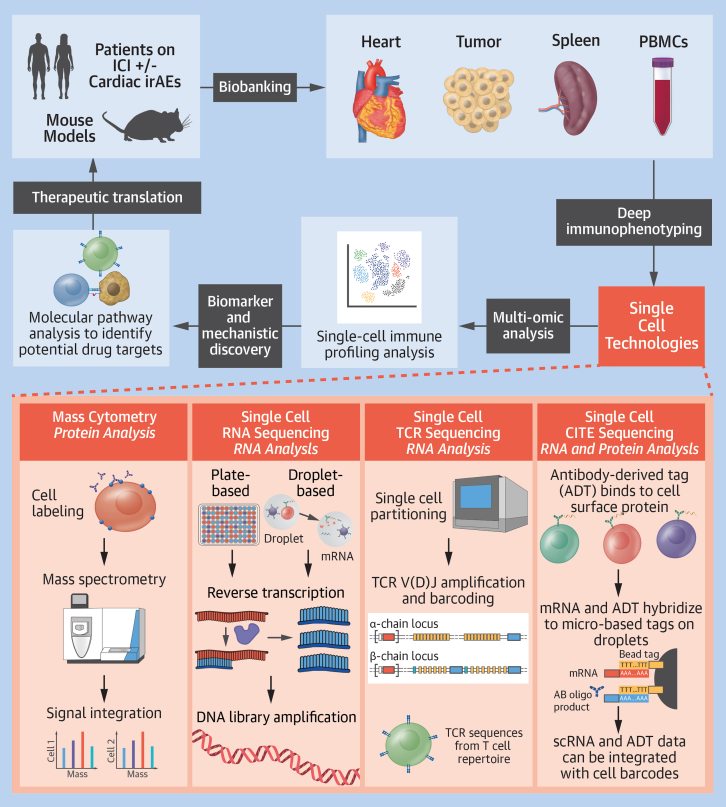
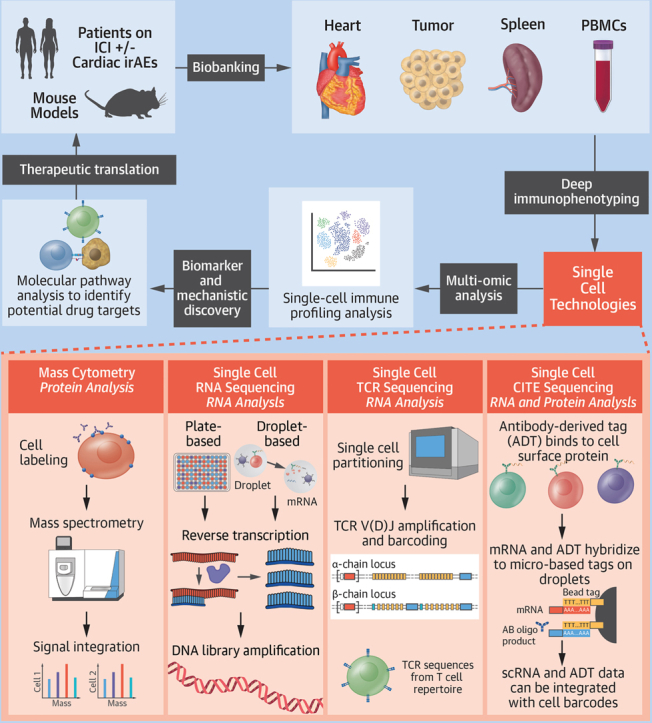
Single-cell profiling techniques such as time-of-flight mass cytometry (CyTOF), single-cell RNA sequencing (scRNA-seq), single-cell T-cell receptor sequencing (scTCR-seq), and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) are omics-based tools that offer unprecedented opportunities for new insights into the immunologic landscape and biological mechanisms of ICI-induced cardiotoxicities (Figure 1).
Figure 1.
Single-Cell Multiomics in Immunotherapy-Induced Cardiotoxicity
Bench-to-bedside work flow using multiomics on patient samples with immune checkpoint inhibitor (ICI)–induced cardiotoxicity and corresponding ICI-induced myocarditis mouse models. CITE = cellular indexing of transcriptomes and epitopes; irAE = immune-related adverse event; mRNA = messenger RNA; PBMC = peripheral blood mononuclear cell; scRNA = single-cell RNA; TCR = T-cell receptor.
CyTOF is a single-cell proteomic analysis technique evaluating targeted intracellular and cell surface proteins using flow cytometry with a mass spectrometry readout.2 By labeling cells with antibodies conjugated to polymers chelated with heavy metal isotopes and analyzing labeled cells according to mass, more than 50 markers can be simultaneously profiled, with minimal crosstalk between detector channels. This allows detailed phenotypic and functional characterization of individual cells within a sample.
scRNA-seq maps RNA transcripts to individual cells to allow high-resolution transcriptomic profiling of cellular heterogeneity present within a tissue, which is otherwise inaccessible with traditional methods such as bulk RNA sequencing. Commercial methods are generally droplet or microwell based. In particular, 10X Genomics Chromium is a well-established droplet-based platform that has enabled profiling of more than 10,000 high-quality cells per run.3 It captures single cells in oil droplets and uses barcoding technology to assign individual barcodes to single cells as well as individual messenger RNA molecules using unique molecular identifiers. BD Rhapsody is a microwell-based scRNA-seq method that can be gentler for sensitive cell types and can accommodate multiplexed samples of up to about 40,000 cells per microwell cartridge.4 Plate-based scRNA-seq methods such as Smart-seq3 rely on single-cell sorting methods such as fluorescence-activated cell sorting to select cells and sort into plates for scRNA-seq. Compared with higher throughput methods, plate-based protocols capture lower cell numbers. An advantage, however, is higher sensitivity for gene detection given the ability to sequence full-length messenger RNAs compared with droplet-based methods that capture only the 5′ or 3′ ends of messenger RNA molecules. Moreover, plate-based methods offer higher flexibility to capture a wider range of cell sizes, providing utility for cell populations that cannot fit through droplet-based microfluidic chips.3
scTCR-seq has transformed our understanding of the T-cell repertoire in cardioimmune diseases. T-cell receptors (TCRs) are highly polymorphic, and their expression on the surface of T cells allows recognition and defense against a wide variety of pathogens. scTCR-seq allows single-cell sequencing of paired alpha/beta TCR chains to characterize the breadth and depth of the antigen-specific T-cell repertoire in a disease state.2
Ab-seq uses oligonucleotide-labeled antibodies to stain cells for readout by high-throughput sequencing. Combining Ab-seq with single-cell transcriptomics—a technology called CITE-seq—provides opportunity to integrate proteomics with transcriptomics.2 scRNA-seq/CITE-seq data can be paired with TCR or B-cell receptor targeting to allow simultaneous immunophenotyping of the transcriptome, cell surface proteome, and TCR or B-cell receptor repertoire of single cells.
Compared with standard bulk RNA sequencing, which provides an average expression level for each transcript,3 single-cell profiling techniques provide the advantage of allowing the evaluation of associations between gene or protein expression within individual cells of a given sample. By using these single-cell profiling techniques, the relationship between the antitumor response, the influence of ICIs on immune checkpoints, and the pathobiology of the resulting autoimmune cardiac response can be elucidated.
Single-Cell Immune Profiling of Cardiac IRAEs
Studies using single-cell transcriptomics on ICI-induced cardiotoxicity are currently limited. However, emerging insights into the immunologic mechanism of ICI myocarditis may be gleaned from both bulk and early single-cell transcriptomic profiling studies.
Autopsy specimens from patients with ICI myocarditis have demonstrated cardiac infiltration primarily by T lymphocytes and macrophages, with notable absence of B cells and antibody deposits.1 Bulk TCR sequencing has demonstrated common high-frequency TCR sequences between cardiac and skeletal muscles. Interestingly, common TCR sequences between heart or skeletal muscle and tumor have also been found, suggesting potential tumor-expressed antigen triggers for ICI-induced myocarditis.5
A bulk RNA-seq study of 18 donor heart tissue samples from patients with giant cell myocarditis, lymphocytic myocarditis, and no myocarditis revealed significant up-regulation of proinflammatory genes (RNASET2, ITGAX, TNFAIP2, TNFRSF14, PTPN7, and HLA-DRA) in the giant cell myocarditis group compared with healthy control subjects. Moreover, down-regulation of cardiac contraction, conduction, and homeostasis genes (MYL2, CAMK2B, MLIP, and AKAP6) was also observed in the giant cell myocarditis group.6 Another bulk RNA-seq study using troponin I–directed autoimmune myocarditis mouse models (LMP7−/−) and endomyocardial biopsy samples from 2 patients with ICI myocarditis demonstrated cardiac-specific autoimmune signatures in myocarditis samples characterized by high immunoproteasome expression and interleukin-17 production.7 Bulk RNA-seq has additionally been used to compare transcriptomics in myocardial samples from 19 patients with ICI-associated myocarditis with those from patients with virus-induced myocarditis and dilated cardiomyopathy. Several key genes (CXCL9, CXCL11, GBP5, and GBP6) in the CD8 and interferon-gamma inflammasome pathway were found to be up-regulated.8 These findings implicate complex changes in multiple inflammatory and homeostatic pathways, although observations at single-cell resolution require further study.
A recent scRNA-seq study using an induced model of murine myocarditis showed that macrophages account for 60% of the cardiac immune cell population. Additionally, type 17 helper T cells with up-regulated Hif1a gene expression constituted the main T-cell population in the acute myocarditis phase. Hif1a expression level correlated with the extent of inflammation.9
In a recent study, CyTOF, scRNA-seq, TCR sequencing, and CITE-seq were performed on patients with ICI myocarditis and experimental PD-1-deficient mice with spontaneous myocarditis (MRL/Pdcd1−/−).10 Clonal cytotoxic terminally differentiated effector memory CD8+ cells were significantly increased in the blood of patients with ICI myocarditis compared with nonmyocarditis IRAE and non-IRAE groups. This immunologic signature corresponded to an analogous increase of clonal CD8+ effector memory T cells in the blood and hearts of PD-1-deficient mice with myocarditis. The clonally expanded effector CD8+ cells demonstrated a unique transcriptional profile consisting of proinflammatory and cytotoxicity markers (GZMB, GNLY, CST7, NKG7, KLRB1, and IL32), and myocardial-tropic chemokines CCL5, CCL4, and CCL4L2. In a recently published manuscript, scRNA-seq or TCR sequencing performed on Pdcd1−/−Ctla4+/− mice showed expansion of activated, clonal CD8+ T cells, with alpha-myosin found as an autoantigen.11 These findings suggest promising therapeutic targets to treat cardiac IRAEs.
There are several proposed mechanisms for myocardial damage in immune checkpoint–mediated cardiotoxicity. One theory is that T cells target a shared antigen between tumor and heart.5 Whole-transcriptome bulk RNA sequencing in 2 patients with ICI myocarditis and myositis has revealed muscle-specific transcripts (TMNI1, TMNT1, DES, TRDN, and MYH6) in tumor tissue, correlating with robust T-cell infiltration of both heart and skeletal muscle.5 Once activated and expanded, T cells may induce myocardial injury through direct cytotoxicity and/or increased presence of proinflammatory cytokines,12 initiating recruitment of additional immune cells. T cells associated with ICI myocarditis are clonally expanded in the peripheral blood, display an effector-type CD8+ phenotype, and exhibit a unique cytotoxic transcriptional profile with increased activation and cytotoxicity markers and proinflammatory chemokines (CCL5, CCL4, and CCL4L2), which may contribute to myocardial homing.10
Novel Treatments for Cardiac Immunotoxicity
Available therapies for ICI-associated myocarditis are predominantly immunosuppressive agents, including corticosteroids as first-line therapy.1 Consensus-based recommendations for corticosteroid use for ICI-associated myocarditis have been driven by general management of IRAEs and observational studies, and more targeted and mechanism-driven therapies are needed. Individual case reports and observational studies13 have been published describing use of immunomodulating agents, including alemtuzumab (a monoclonal antibody targeting CD52),13 abatacept (a CTLA-4 agonist),1 and tofacitinib (a JAK inhibitor).14
Few studies have used data from single-cell multiomics to guide treatment for IRAEs, with most focusing on noncardiac IRAEs. In a study using scRNA-seq in 15 patients with colitis secondary to CTLA-4 and PD-1 inhibitors and 10 control patients without colitis, activated CD8+ and CD4+ T cells were found in tissue from patients with colitis. The most intense activation occurred in CD8+ CD103+ T cells, with enrichment of the interferon-γ-JAK-STAT signaling pathway in bulk RNA sequencing.15 In patients who received JAK inhibition with tofacitinib, there was resolution of CD8+ T-cell activation and down-regulation of JAK-STAT signaling on RNAplex. As a corollary, other groups have reported successful use of the JAK inhibitor tofacitinib in treating ICI-associated myocarditis.14 This highlights the potential of single-cell multiomics to characterize the mechanisms underlying IRAEs and identify treatment strategies.
Limitations of Single-Cell Profiling
Although single-cell technologies have advanced our understanding of the immunologic landscape of cardioimmune diseases, limitations exist. Partial sampling and reduction of dimensions in scRNA-seq data analysis can cause bias for abundant genes, making it challenging to identify rare ones. Low-abundance transcripts may result from computational noise that masks biological variation. Batch-to-batch variation can further aggravate levels of technical noise and mask true differences in gene expression. There is also a lack of an established standardized protocol for cell-specific markers or statistical cutoffs when identifying cell types.
Although single-cell nuclei RNA sequencing may be run on frozen tissue and new 10X Genomics Chromium technology can now accommodate fixed tissue, many single-cell procedures require fresh samples, which may be difficult to acquire in large numbers. Multi-institutional biobanks and collaborations can address this problem. Finally, the cost of sample acquisition and processing is not insignificant. Methods of reducing these costs include multiplexing samples together by labeling individual cell samples with molecular tags and combining samples, providing additional solutions.
Conclusions
Single-cell profiling techniques can provide new insights into the immunologic landscape of ICI-mediated cardiotoxicity through the discovery of new cell populations and cell-specific transcriptional states. Although emerging studies using both bulk and single-cell techniques have provided early insight into ICI myocarditis, further studies are needed. Ongoing studies of single-cell multiomics of ICI myocarditis through elucidation of key cell subsets and molecular mechanisms will be critical for discovery of potential drug targets for treatment and prevention. More broadly, research on cardiotoxicities resulting from chimeric antigen receptor T cells and other immunotherapies may also benefit from these applications.
Funding Support and Author Disclosures
This work was supported by National Institutes of Health grant 1K08HL16140501; a Sarnoff Scholar Career Development Award; National Institute of General Medical Sciences grant 1RM1 GM131981-02; an American Heart Association Established Investigator Award; the Hoffmann/Schroepfer Foundation; the Additional Venture Foundation; the Joan and Sanford I. Weill Scholar Fund; an iAward grant from Sanofi US; National Heart, Lung, and Blood Institute grant R01HL13483004; and American Heart Association grant AW849785 (Transformative Award). Dr Wu has received funding from Sanofi. Dr Witteles sits on advisory boards for Pfizer, Alnylam, Ionis/Akcea, Eidos, and Intelia. Dr Neal has served in a consulting or an advisory role for AstraZeneca, Genentech/Roche, Exelixis, Jounce Therapeutics, Takeda Pharmaceuticals, Eli Lilly, Calithera Biosciences, Amgen, Iovance, Biotherapeutics, Blueprint Pharmaceuticals, Regeneron Pharmaceuticals, and Natera; and has received research funding from Genentech/Roche, Merck, Novartis, Boehringer Ingelheim, Exelixis, Nektar Therapeutics, Takeda Pharmaceuticals, Adaptimmune, GlaxoSmithKline, Janssen, and AbbVie. Dr Waliany has received consulting fees from AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Tomas G. Neilan, MD, MPH, served as the Guest Associate Editor for this paper. Kathryn J. Ruddy, MD, MPH, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Waliany S., Lee D., Witteles R.M., et al. Immune checkpoint inhibitor cardiotoxicity: understanding basic mechanisms and clinical characteristics and finding a cure. Annu Rev Pharmacol Toxicol. 2021;61:113–134. doi: 10.1146/ANNUREV-PHARMTOX-010919-023451. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Qu S., Zhang T., et al. Applications of single-cell omics in tumor immunology. Front Immunol. 2021;12 doi: 10.3389/FIMMU.2021.697412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M., Gu M., Liu L., Liu Y., Tian L. Single-cell RNA sequencing (scRNA-seq) in cardiac tissue: applications and limitations. Vasc Health Risk Manag. 2021;17:641. doi: 10.2147/VHRM.S288090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao C., Zhang M., Chen L. The comparison of two single-cell sequencing platforms: bd rhapsody and 10X Genomics Chromium. Curr Genomics. 2020;21(8):602. doi: 10.2174/1389202921999200625220812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amancherla K., Qin J., Wang Y., et al. RNA-sequencing reveals a distinct transcriptomic signature for giant cell myocarditis and identifies novel druggable targets. Circ Res. 2021;129(3):451–453. doi: 10.1161/CIRCRESAHA.121.319317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockstahler M., Fischer A., Goetzke C.C., et al. Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation. 2020;141(23):1885–1902. doi: 10.1161/CIRCULATIONAHA.119.043171. [DOI] [PubMed] [Google Scholar]
- 8.Finke D., Heckmann M.B., Salatzki J., et al. Comparative transcriptomics of immune checkpoint inhibitor myocarditis identifies guanylate binding protein 5 and 6 dysregulation. Cancers (Basel) 2021;13(10):2498. doi: 10.3390/CANCERS13102498/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua X., Hu G., Hu Q., et al. Single-cell RNA sequencing to dissect the immunological network of autoimmune myocarditis. Circulation. 2020;142(4):384–400. doi: 10.1161/CIRCULATIONAHA.119.043545. [DOI] [PubMed] [Google Scholar]
- 10.Zhu H., Galdos F.X., Lee D., et al. Identification of pathogenic immune cell subsets associated with checkpoint inhibitor-induced myocarditis. Circulation. 2022;146(4):316–335. doi: 10.1161/CIRCULATIONAHA.121.056730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelrod M.L., Meijers W.C., Screever E.M., et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611:818–826. doi: 10.1038/s41586-022-05432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baik A.H., Oluwole O.O., Johnson D.B., et al. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ Res. 2021;128:1780–1801. doi: 10.1161/CIRCRESAHA.120.315894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esfahani K., Buhlaiga N., Thébault P., Lapointe R., Johnson N.A., Miller W.H. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380(24):2375–2376. doi: 10.1056/nejmc1903064. [DOI] [PubMed] [Google Scholar]
- 14.Wang C., Lin J., Wang Y., et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: a comparative analysis of corticosteroid and tofacitinib treatment. Front Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasson S.C., Slevin S.M., Cheung V.T.F., et al. Interferon-gamma-producing CD8+ tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. 2021;161(4):1229–1244.e9. doi: 10.1053/j.gastro.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]