Objective:
The aim of this study was to estimate risks of myocardial infarction, ischemic stroke, and cardiovascular-related and all-cause mortality after Roux-en-Y gastric bypass (RYGB) for obesity compared with nonop-erated obese patients and matched nonobese population controls.
Background:
Few studies have assessed the influence of RYGB on fatal and non-fatal myocardial infarction and ischemic stroke, and the results vary between studies.
Method:
All patients aged 20 to 65 years with obesity diagnosis in the nationwide Swedish Patient Registry in 2001 to 2013 were included. These participants were divided into those who underwent RYGB within 2 years of obesity diagnosis (n = 28,204) and nonoperated (n = 40,827), and were matched for age, sex, and region with 2 nonobese population controls. Participants were followed until onset of outcome disease, death, or end of follow-up. Multivariable Cox regression provided hazard ratios (HR) with 95% confidence intervals (95% CI).
Results:
Compared with nonoperated patients with obesity, RYGB patients had a reduced risk of myocardial infarction [HR = 0.44 (95% CI 0.28-0.63)], similar risk of ischemic stroke [HR = 0.79 (95% CI 0.54–1.14)], and decreased risks of cardiovascular-related [HR = 0.47 (95% CI 0.35–0.65)] and all-cause mortality [HR = 0.66 (95% CI 0.54–0.81)] within the first 3 years of follow-up, but not later. Compared with nonobese population controls, RYGB patients had excess risks of ischemic stroke [HR = 1.57 (95% CI 1.08–2.29)], cardiovascular-related mortality [HR = 1.82 (95% CI 1.29–2.60)], and all-cause mortality [HR = 1.42 (95% CI 1.16–1.74)], but not of myocardial infarction [HR = 1.02 (95% CI 0.72–1.46)].
Conclusion:
RYGB for obesity might not decrease the risk of ischemic stroke, but seems to decrease the risk of myocardial infarction back to population levels.
Keywords: cardiovascular disease, gastric bypass, mortality, obesity, surgery for obesity
The prevalence of obesity [body mass index (BMI) ≥30] and severe obesity (BMI ≥35) has increased markedly in recent decades worldwide.1 Currently in Sweden, 20% of adults aged ≥20 years are estimated to be obese and nearly 6% have severe obesity.2
Obesity increases the risk of several morbidities and premature death,3 as it causes metabolic dysfunction and increases blood pressure and glucose and lipid levels, which are accompanied by hypertension and hyperlipidemia and eventually diabetes, sleep apnea, cancer, and cardiovascular disease.4–6 Despite successful prevention and treatment of these conditions,7,8 resulting in decreasing mortality owing to cardiovascular disease in many Western countries, coronary heart disease and stroke persist as the main causes of death worldwide,9 accounting for 2 3 deaths among obese individuals.4
Medical treatment for obesity and interventions targeting behavioral factors have had limited long-term success. In contrast, bariatric surgery, Roux-en-Y gastric bypass (RYGB) in particular, yields substantial and sustained weight loss, remission of diabetes and hypertension,10–12 and improved cardiac function.13,14 However, some relapses of obesity-related risk factors have been reported in parallel with accumulating weight gain over time after bariatric surgery.11
Studies have reported decreased risk of cardiovascular-related morbidity and mortality compared with medical treatment,10,15,16 in particular among patients with obesity and type 2 diabetes.17 However, there is a lack of studies on the risk of fatal and nonfatal myocardial infarction and ischemic stroke as separate events due to limited number of participants. In addition, the reported benefits of RYGB vary, perhaps depending on differences in sample size, follow-up time, and participants' characteristics.16 This highlights a need for large studies to assess both short- and long-term effects of RYGB. Therefore, the aim of this study was to estimate risk of myocardial infarction, ischemic stroke, and cardiovascular-related and all-cause mortality after RYGB, compared with both nonoper-ated obese patients and matched nonobese population controls.
Methods
Study Design
The Swedish healthcare system offers publicly financed medical care to all citizens at a low cost, including assessment at an obesity clinic, and subsequent surgical intervention if needed. Hospitalizations and hospital outpatient visits are recorded in the Swedish National Patient Registry, which includes complete data on principal and contributory discharge diagnoses for all hospitaliza-tions in Sweden since 1987 and for specialist outpatient visits since 2001.18 The Patient Registry has been validated in general with 85% to 95% accuracy, and for bariatric surgery specifically with 97% accuracy, compared with patient records.18,19 Main and contributory diagnoses are registered in the Patient Registry according to the International Classification of Diseases (ICD; ICD-9 1987–1996, ICD-10 from 1997 onwards).
In this prospective registry-based cohort study, we included all individuals 20to65 years of age who obtained a first recorded principal diagnosis of obesity (ICD-10 codes E65 or E66) in the Patient Registry between January 1, 2001 and December 31, 2013, generally at an outpatient obesity clinic, where all patients aged 18 years and above with a BMI of at least 35 kg/m2 are eligible assessment and treatment. The Patient Registry was also used to collect data on age, sex, discharge diagnoses, surgical procedures, and hospitalization dates. Information about socioeconomic variables was obtained from the Longitudinal integrated database for health insurance and labor market studies (LISA) (80% coverage). For each obese patient, two control participants, matched by year of birth, sex, and area of residence, and without a code representing obesity diagnosis or bariatric surgery, were randomly selected from Sweden’s Registry of the Total Population using the individual personal identity number assigned to all Swedish residents. Neither the Patient Registry nor the Registry of the Total Population contains data on an individual's height or weight. Still, because the controls from the Total Population Registry did not have an obesity diagnosis, for clarity, they are referred to as nonobese population controls throughout.
To avoid immortal time bias,20 we used landmark analysis, splitting up the follow-up time at a common, prespecified time point (landmark), where we set the study baseline at 2 years after an obesity diagnosis being recorded, considering events only if occurring after this landmark. All events that occurred before this landmark were considered as comorbidity.
Patients with obesity were divided into 2 groups, 1 RYGB group, and 1 group including nonoperated obese patients (eFigure 1, http://links.lww.com/SLA/D247). Through this procedure, we captured 81% of all individuals who underwent RYGB in Sweden during the study period. Obese patients in the nonoperated group who underwent any type of bariatric surgery during the follow-up time were censored at the date of surgery. RYGB was defined using the Swedish Classification of Operations and Major Procedures (NOMESCO) codes JDF10 or JDF11. Inclusion and exclusion criteria can be found in Figure 1.
Figure 1.
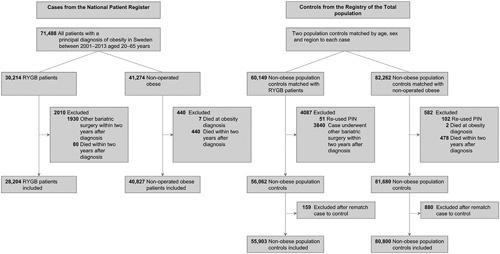
Flow diagram showing inclusion and exclusion criterias. IS indicates ischemic stroke; MI, myocardial infarction; PIN, personal identity number.
Outcomes and Comorbidities
Outcomes were obtained from the Patient Registry and the Cause of Death Registry according to the ICD-10. The Cause of Death Registry documents all deaths among Swedish residents. Outcomes were defined by the following ICD-10 codes: hospital-izations or death owing to myocardial infarction by I21; ischemic stroke by I63 and I64; and cardiovascular-related mortality by I00-I99 as the underlying cause of death.
Disorders present before or at the study baseline (2 years after obesity diagnosis) included the following diagnoses: obesity, myo-cardial infarction, ischemic stroke, diabetes, hypertension, sleep apnea, coronary heart disease, and malignancy (definitions in eTable 1, http://links.lww.com/SLA/D247).
Table 1.
Characteristics of Cohort
| Characteristics | RYGB Patients (n = 28,204) | Nonobese Population Controls (n = 55,903) | Nonoperated Obese Patients (n = 40,827) | Nonobese Population Controls (n = 80,800) |
|---|---|---|---|---|
| Age, y, mean (SD) | 40.8 (10.4) | 40.8 (10.4) | 43.1 (11.8) | 43.1 (11.7) |
| Sex, no. (%) | ||||
| Women | 21,295 (75.5) | 42,203 (75.5) | 27,983 (68.5) | 55,460 (68.6) |
| Outpatient, No (%) | 20,829 (92.3) | – | 33,737 (95.4) | – |
| Comorbidity, no. (%) | ||||
| Hypertension | 6731 (23.9) | 1853 (3.3) | 9540 (23.4) | 3392 (4.2) |
| Diabetes mellitus | 4151 (14.7) | 954 (1.7) | 6600 (16.2) | 1689 (2.1) |
| Sleep apnoea | 3459 (12.3) | 518 (0.9) | 6085 (14.9) | 756 (0.9) |
| Coronary heart disease | 806 (2.9) | 568 (1.0) | 2411 (5.9) | 1383 (1.7) |
| Malignancy | 759 (2.7) | 1734 (3.1) | 1900 (4.7) | 3063 (3.8) |
| Ischemic stroke* | 233 (0.8) | 228 (0.4) | 593 (1.5) | 470 (0.6) |
| Myocardial infarction* | 351 (1.2) | 231 (0.4) | 987 (2.4) | 644 (0.8) |
| Marital status†, no. (%) | ||||
| Single | 11,652 (41.3) | 23,872 (42.7) | 15,643 (38.3) | 31,263 (38.7) |
| Married/cohabiting | 12,011 (42.6) | 24,348 (43.6) | 17,563 (43.0) | 36,791 (45.5) |
| Divorced/widowed | 4292 (15.2) | 7179 (12.8) | 7031 (17.2) | 11,592 (14.3) |
| Country of birth, no. (%) | ||||
| Sweden | 23,771 (84.3) | 44,910 (80.3) | 32,823 (80.4) | 65,487 (81.0) |
| Education level‡, no. (%) | ||||
| ≤9 y | 4859 (17.2) | 6574 (11.8) | 8432 (20.7) | 11,654 (14.4) |
| 10–12 y | 17,327 (61.4) | 25,354 (45.4) | 22,002 (53.9) | 36,876 (45.6) |
| >12y | 5896 (20.9) | 23,160 (41.4) | 9987 (24.5) | 31,157 (38.6) |
Characteristics were derived at the study baseline, 2 years after obesity diagnosis for RYGB patients and nonoperated obese. Populations controls (matched for age, sex, and county of residence) were assigned the same start date as their matched case.
All individuals with a history of ischemic stroke or myocardial infarction at study baseline were excluded.
Missing data of marital status for 2497 (1.2%) individuals.
Missing data of education level for 2456 (1.2%) individuals.
Statistical Analysis
Participants were followed from the study baseline until any of the following events: hospitalization for myocardial infarction or ischemic stroke, death, reaching a maximum follow-up of 10 years, or end of the study (December 31, 2016), whichever occurred first. The follow-up time was restricted to a maximum 10 years to ensure more even follow-up times between groups, because nonoperated obese, compared to RYGB patients tended to have longer follow-up times. All participants had the possibility of at least 1 year of follow-up. Age- and sex-adjusted incidence and mortality rates, in total and for each year of follow-up, were calculated per 1000 person-years along with approximated 95% confidence intervals (CIs).21
We used multivariable Cox proportional hazards regression models to calculate hazard ratios (HR) with 95% CI, to estimate the relative risk of cardiovascular outcomes and mortality for RYGB and nonoperated obese patients compared with non-obese population controls, and for RYGB compared with nonoperated obese patients. Contrast matrices were used to compare HRs between groups. HRs were used to assess both short- (≤3 years from baseline) and long-term risk (>3–10years), because the HRs were not proportional during the follow-up period. Age and sex were included as cova-riates. Education was categorized into three groups (<9, 10–12, and >12 years of formal education) and was included in the models as an interaction term with the 2 obesity groups and the 2 control groups. We refrained from including preexisting comorbidities in the model because these have a mediating effect on the causal pathway between obesity and the outcomes rather than acting as confounders. Survival functions were created with multivariable Cox proportional hazard regression models (95% CI) and adjusted for age, education, and sex. The curves were set to represent 41-year-old women with intermediate (10–12 years) education. The proportionality assumptions were examined using methods based on weighted residuals,22 and all final models fulfilled the proportionality assumptions of proportional hazards.
Data management was performed using SAS version 9.4 (SAS Institute, Cary, NC) and the analyses were performed in R version 3.6.2 (The R Project for Statistical Computing, Vienna, Austria). All data were anonymized. The regional ethical review board in Gothenburg approved the study (DNR: 579–15).
Results
Participants
The study included 28,204 patients who underwent RYGB (90.3% laparoscopic surgery), mean age 40.8 years [standard deviation (SD) 10.4] with 75.5% women and 55,903 matched nonobese population controls, as well as 40,827 nonoperated obese patients, mean age 43.1 years (SD 11.8) with 68.5% women and 80,800 matched nonobese population controls. Baseline characteristics are shown in Table 1. The absolute majority of patients, or >90%, irrespective of whether they later underwent surgery or not, were registered in an outpatient setting. The prevalence of obesity-related comorbidity was fairly similar among RYGB and nonoperated obese patients, where diabetes (14.7% and 16.2%, respectively) and hypertension (23.9% and 23.4%, respectively) were the most common comorbidities. The prevalence of comorbidity among the nonobese population controls was low, the most common comorbidities were malignancy (3.1%– 3.8%) and hypertension (3.3%–4.2%).
Incidence of Study Outcomes
Table 2 shows the number and events, age at diagnosis, and follow-up time, along with overall age- and sex-adjusted incidence rates per 1000 person-years and their corresponding CIs in the 4 studied groups. The adjusted incidence rates by follow-up year, group, and outcome are shown in Figure 2 (incidence rates by RYGB patients and nonoperated obese patients vs their nonobese population controls can be found in eFigure 1, http://links.lww.com/SLA/D247 and eFigure 2, http://links.lww.com/SLA/D247). Descriptive information on number of events, person-years, and incidence rates by follow-up time and group can be found in eTable 2, http://link-s.lww.com/SLA/D247. Survival probabilities for all 4 groups and outcomes are shown in Figure 3 (survival probabilities by RYGB patients and nonoperated obese patients vs their nonobese population controls can be found in eFigure 3, http://links.lww.com/SLA/D247 and eFigure 4, http://links.lww.com/SLA/D247). The plotted adjusted survival curves represent women, aged 41 years, with an intermediate length of education (10–12 years).
Table 2.
Events and Adjusted Incidence Rates for All Outcomes
| Events | RYGB Patients | Nonobese Population Controls | Nonoperated Obese Patients | Nonobese Population Controls |
|---|---|---|---|---|
| Acute myocardial infarction, no. (%) | 97 (0.3) | 188 (0.3) | 518 (1.2) | 540 (0.7) |
| Age at acute myocardial infarction diagnosis, y (SD) | 52.8 (8.0) | 55.4 (8.3) | 57.1 (8.8) | 58.2 (8.4) |
| Follow–up time, median (IQR) | 4.0 (2.6–5.9) | 4.1 (2.6–5.9) | 4.7 (2.3–8.2) | 4.9 (2.4–8.5) |
| Cases per 1000 observation years (95% CI) | 0.9 (0.7–1.2) | 1.1 (0.9–1.3) | 2.2 (2.0–2.4) | 1.1 (1.0 –1.2) |
| Ischemic stroke, no. (%) | 134 (0.5) | 174 (0.3) | 486 (1.2) | 465 (0.6) |
| Age at ischemic stroke diagnosis, y (SD) | 51.1 (8.7) | 52.3 (9.6) | 56.7 (9.9) | 58.8 (9.0) |
| Follow–up time, median (IQR) | 4.0 (2.6–5.9) | 4.1 (2.6–5.9) | 4.8 (2.3–8.2) | 4.9 (2.4–8.5) |
| Cases per 1000 observation years (95% CI) | 1.2 (1.0–1.5) | 0.9 (0.8–1.1) | 2.1 (1.9–2.3) | 1.0 (0.9–1.1) |
| Cardiovascular-related mortality, no. (%) | 196 (0.7) | 154 (0.3) | 989 (2.4) | 615 (0.8) |
| Age at cardiovascular-related mortality, y (SD) | 53.3 (9.4) | 54.8 (8.4) | 59.0 (9.0) | 61.2 (7.6) |
| Follow–up time, median (IQR) | 4.1 (2.6–5.9) | 4.1 (2.6–5.9) | 4.8 (2.4–28.3) | 4.9 (2.4–8.6) |
| Cases per 1000 observation years (95% CI) | 2.1 (1.8–2.4) | 0.8 (0.7–1.0) | 4.0 (3.7–4.2) | 1.2 (1.1–1.3) |
| All-cause mortality, no. (%) | 505 (1.8) | 477 (0.9) | 1682 (4.1) | 1604 (2.0) |
| Age at all–cause mortality. years (SD) | 50.8 (10.3) | 53.1 (9.5) | 57.7 (10.0) | 59.3 (8.9) |
| Follow–up time, median (IQR) | 4.1 (2.6–5.9) | 4.1 (2.6–5.9) | 4.8 (2.4–8.3) | 4.9 (2.4–8.6) |
| Cases per 1000 observation years (95% CI) | 4.9 (4.5–5.4) | 2.4 (2.2–2.7) | 6.9 (6.6–7.2) | 3.2 (3.1–3.4) |
IQR indicates interquartile range.
Figure 2.
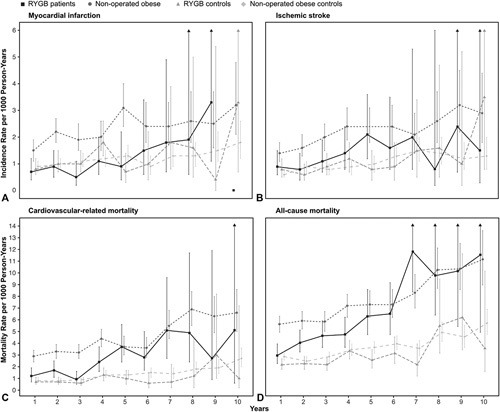
Yearly age-and sex-adjusted incidence and mortality rates by group for all outcomes. RYGB, Roux-en-Y gastric bypass. A, myocardial infarction, B, ischemic stroke, C) cardiovascular-related mortality, and D) all-cause mortality.
Figure 3.
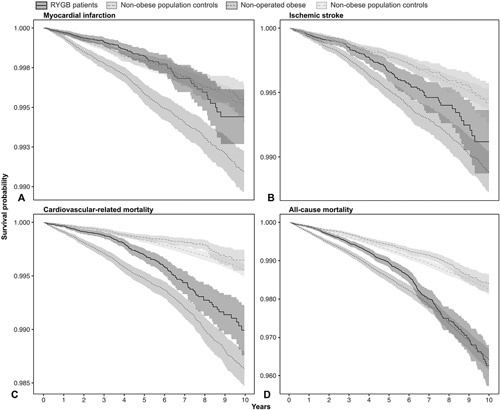
Age-, sex- and education-standardized survival probability curves by group for all outcomes. The survival curves were created with multivariable Cox proportional hazard regression models (95% CI) and adjusted for age, education and sex. The functions represent 41-year-old women with intermediate (10–12y) education. A, myocardial infarction, B, ischemic stroke, C, cardiovascular-related mortality, and D, all–cause mortality.
Figure 4.
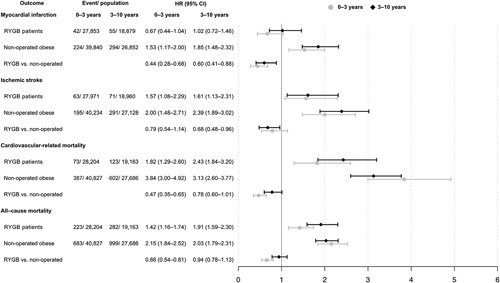
Hazard ratios for all outcomes, divided into 2 time periods. HRs for short- (≤3 years from baseline) and long-term risk (>3–10). Model adjusted for age, sex, and education level.
There was a steep increase in mortality rates with time throughout the study period among obese patients, irrespective of RYGB (Figure 3, eTable 2, http://links.lww.com/SLA/D247).
Risk of Study Outcomes
Figure 4 shows HRs with 95% CIs of the studied outcomes in RYGB patients and non-operated obese patients with their respective non-obese population controls as reference, and in RYGB patients compared with nonoperated obese patients as reference.
Compared with non-operated obese patients, RYGB patients had a reduced short- and long-term risk of myocardial infarction with HR (95% CI) of 0.44 (0.28–0.68) and 0.60 (0.41–0.88) respectively. In a sensitivity analysis, the model was further adjusted for preexisting coronary heart disease, and the results were similar to those of the main analysis (see eTable 3, http://links.lww.com/SLA/D247). For ischemic stroke, no clear short- but a borderline significant long-term difference were found: HR 95% CI of 0.79 (0.54–1.14) and 0.68 (0.48–0.96). RYGB patients had a decreased risk of cardiovascular-related and all-cause mortality compared with non-operated obese patients within the first 3 years of follow-up: HR (95% CI) of 0.47 (0.35–0.65) and 0.66 (0.54–0.81), respectively. However, this reduced risk was attenuated and no longer statistically significant during the final 3 to 10 years of follow-up, with HR (95% CI) of 0.78 (0.60–1.01) and 0.94 (0.78–1.13), respectively.
Compared with nonobese population controls, RYGB patients had an increased long-term risk of ischemic stroke (HR = 1.61,95% CI 1.13–2.31), cardiovascular-related mortality (HR = 2.43, 95% CI = 1.84–3.20), and all-cause mortality (HR = 1.91, 95% CI 1.59–2.30). However, both short- (HR = 0.67, 95% C 0.44–1.04) and long-term (HR = 1.02, 95% CI 0.72–1.46) risk of myocardial infarction was similar to that of nonobese population controls. An increased short-and long-term risk of all outcomes was found among nonoperated obese patients compared with nonobese population controls (Fig. 4).
Discussion
This study found that obese patients who had undergone RYGB had lower 10-year risk of myocardial infarction than nonoperated obese patients and similar risk to that of nonobese population controls; however, they had similar 10-year risk of ischemic stroke as non-operated controls. The results also indicated that RYGB reduced short-term, but not long term, cardiovascular-related and all-cause mortality compared with nonoperated obese, and that patients who underwent RYGB had an excess risk of mortality compared with nonobese population controls.
The negative impact of obesity on obesity-related comorbidity seemed to be attenuated by RYGB in this study, to the extent that the 10-year risk of myocardial infarction was similar to that of the general nonobese population. A previous study that compared RYGB patients with nonobese population controls found similar incidence rates and relative risk of myocardial infarction, with no difference in risk between RYGB patients and nonobese population controls.23 Other studies comparing RYGB patients with obese controls have found conflicting results, perhaps owing to a limited follow-up time and number of events. Two studies found a large reduction in risk of myocardial infarction following RYGB compared with obese con-trols,24,25 whereas another study found no difference up to 8 years following surgery.26 It should be noted that the RYGB patients in the present study had a lower prevalence of coronary heart disease at study baseline compared with non-operated obese patients, and double that of nonobese population controls. However, after adjustment for preexisting coronary heart disease, the risk reduction among the RYGB patients persisted. The results of the present study also showed that the increased risk of ischemic stroke compared with nonobese population controls persisted among patients who underwent RYGB, with a 3–year risk similar to that of non-operated obese, and a borderline significant reduced 10-year risk, indicating that RYGB might not affect the increased risk of ischemic stroke caused by obesity to the same extent as it does with myocardial infarction. Previous studies have reported conflicting results regarding the risk of stroke following RYGB; however, these studies had large differences in follow-up and definitions of stroke.23–26 Similar to our results, 1 study found that RYGB patients maintained an excess risk of ischemic stroke compared with nonobese population controls.23 In contrast, a Swedish study found a 34% reduced risk of fatal and nonfatal events combined; however, they did not have sufficient power to assess the risk of fatal and nonfatal stroke separately.25 Another study comparing RYGB patients with 2 control groups including severely obese patients who had undergone different types of non-bariatric surgery found a risk reduction for ischemic stroke in one control group, but not in the other.24 Another study found a 45% reduction in the risk of stroke, myocardial infarction, and heart failure as a composite event up to 8 years after surgery, but as separate events, whereas there was no difference in stroke.26 Taken together with our results, it appears that RYGB has a varying and somewhat unclear effect on the risk of ischemic stroke among obese patients. In addition, the studied populations in the present and previous studies were relatively young, as the risk of ischemic stroke increases with age future studies with longer follow-up might find more clear effects of the RYGB surgery.
One previous study found that by improving cholesterol, glucose, and blood pressure levels, half of the excess risk of coronary heart disease and two-thirds of the excess risk of stroke caused by increased BMI was reduced.6 In addition, hypertension is one of the strongest risk factors for stroke.27 RYGB has been shown to improve these risk factors11,26,28 and also lead to a remission of diabetes and hypertension.29,30 Hence, RYGB should theoretically reduce the risk of both myocardial infarction and ischemic stroke in the same matter. Unfortunately, we lacked information on weight status and lifestyle factors that increase the risk of myocardial infarction and ischemic stroke, that is, poor diet, physical inactivity, and tobacco smoking, often seen among individuals with obesity. RYGB patients who engage in behaviors related to weight loss and maintained weight loss, such as self-weighing, healthy eating behaviors,31 and physical activity32 have shown to have a more significant and maintained weight loss compared to those who do not engage in such behaviors. In the present study, patients with widely heterogeneous approaches were probably included, but our study provides no information on this, and accordingly, the overall risk measured in the present study represent an average of different lifestyle approaches.
In the present study, patients who underwent RYGB had a higher relative risk of cardiovascular-related and all-cause mortality throughout the study period, in comparison with nonobese population controls. Compared with nonoperated obese patients, those who received RYGB had a 53% reduced risk of cardiovascular-related mortality and 34% reduced relative risk of all-cause mortality during the first 3 years of follow-up; however, the risk was attenuated to nonsignificance during the final 3–10years of follow-up. Thus, the benefits of RYGB on cardiovascular-related mortality seem to be greatest during the first years of surgery.
Given that RYGB induces cardiovascular benefits through counteracting risk factors and lowers the risk of cardiovascular disease and heart failure,26,33,34 as well as the negative impact of elevated BMI on cardiovascular-related morbidity,6 alargerrisk reduction compared with nonoperated obese patients was expected. Previous studies have shown varying results in mortality after RYGB, potentially owing to different study designs, length, and completeness of follow-up, and distributions of age and comorbidity. RYGB may also increase mortality from other causes than mortality that is cardiovascular related and most common causes of death in individuals with obesity.9 It has previously been shown that deaths from external causes, including suicide, accidents and alcoholism, are higher among patients undergoing bariatric surgery than in obese controls,35 and nonobese population controls.36
Another possible explanation for these results regarding mortality is that there are subgroups of obese patients that benefit more from RYGB.37 A previous study with long-term follow-up compared operated and nonoperated obese individuals and found a large reduction in mortality limited to men within up to 14 years ofsurgery, but not in women.38 In that study, the prevalence of comorbidities was considerably higher and the mean age 10 years higher than that in the present study. Having type 2 diabetes before bariatric surgery has been associated with a marked risk reduction. A recent retrospective cohort study found that bariatric surgery among patients with obesity and type 2 diabetes compared with nonsurgical management was associated with 39% lower risk of major adverse cardiovascular events, including all-cause mortality, within up to 8 years of surgery among patients at a median age of 52.5 and 54.8 years, respectively.17 Another study found no difference in mortality up to 20 years after bariatric surgery among those younger than 43 years, but a significant 61% reduced risk among those aged 43 years and older.39 Yet another, recent nonrandomized case–control study found a 30% reduced risk of all-cause mortality among operated obese compared with that of nonoperated obese, whereas nonobese population controls had 62% lower risk compared with nonoperated obese during 24 years of follow-up.36 However, participants in that study were almost one decade older than those included in the present study.
Finally, health behaviors, weight regain, and relapse ofcomor-bidity among some RYGB patients could have an impact also for mortality.11 Additional studies with long-term follow-up are needed to identify subgroups that obtain the greatest benefits from RYGB regarding cardiovascular diseases and mortality, as well as to which patients might fare better with medical and behavioral targeted treatments.
Strengths and Limitations
Strengths of the present study include the nationwide coverage, accuracy, and completeness of Swedish registry data, as well as the fact that all Swedes are covered by the national health care system, which provides affordable access to relatively homogenous care.18,19 This provided a large study population including nearly all individuals undergoing RYGB during the study period as well as nonoperated patients generally identified through obesity outpatient clinics, a large number of valid myo-cardial infarction and ischemic stroke cases, and up to 10 years of complete follow-up.
This study also has limitations. The registries lack anthropo-metric data. However, detailed information on BMI and other measures for patients who underwent RYGB are available from the yearly reports of the Scandinavian Obesity Surgery Registry.40 Patients who have undergone RYGB are likely to achieve major weight loss after the surgery, whereas weight in nonoperated controls tends to remain stable. Two previous Swedish studies had information on baseline characteristics of operated and nonoperated obese patients with BMI between 40 and 42, respectively, at the start of the studies. They found a nearly 20-kg difference in weight between the operated versus nonoperated group up to 2 years of follow-up,34 and approximately 25-kg difference in weight up to 20 years of follow-up.10 There are no data on BMI for nonobese population controls, but the average BMI among adults in Sweden is estimated to be around 26,41 which is clearly lower than that among individuals who have an obesity diagnosis. There is a risk of detection bias of comorbidities because obese patients are more likely to receive hospital in- or outpatient care than individuals in the general population. However, all outcomes in the study require hospital inpatient care or are registered in the Patient Registry or Cause of Death Registry, which have nearly complete data. Finally, selection bias in the comparisons of the operated and non-operated obese patients may have influenced the results of this study. Because not all patients with an obesity diagnosis are eligible for surgery, the non-operated obese group might have poorer mental and physical health status and different lifestyle habits than the surgery group. There may also be other dimensions of patients’ characteristics that led to the decision to operate or not, including eligibility or contraindication of surgery and patients’ preference.42 Far from all individuals with obesity have an obesity diagnosis in the Patient registry, hence, the nonoperated obese group might have had worse health than undiagnosed obese individuals from the population. This should be considered when interpreting the results.
Conclusions
RYGB seems to be associated with a decreased risk of myocardial infarction, but not ischemic stroke within 10 years of follow-up. RYGB may transiently reduce the risk of cardiovascular-related and all-cause mortality during the initial 3 years after surgery but not thereafter.
Supplementary Material
Footnotes
Funding: This work was supported by grants from: the Swedish state under an Agreement Concerning Research and Education of Doctors (ALFGBG-717211); Swedish Heart-Lung Foundation (2018–0366, 2018–0589); The Swedish Research Council (2018–02527, 2019–00209, 2019–00193).
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
REFERENCES
- 1.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Hajifathalian K, Ezzati M, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorck L Capewell S, O'Flaherty Met al. Decline in coronary mortality in Sweden between 1986 and 2002: comparing contributions from primary and secondary prevention. PLoS One. 2015;10:e0124769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19 1 million participants. Lancet. 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Int Med. 2013;273:219–234. [DOI] [PubMed] [Google Scholar]
- 11.Sundbom M, Hedberg J, Marsk R, et al. Substantial decrease in comorbidity 5 years after gastric bypass: a population-based study from the Scandinavian Obesity Surgery Registry. Ann Surg. 2017;265:1166–1171. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. [DOI] [PubMed] [Google Scholar]
- 13.Kurnicka K, Domienik-Karlowicz J, Lichodziejewska B, et al. Improvement of left ventricular diastolic function and left heart morphology in young women with morbid obesity six months after bariatric surgery. Cardiol J. 2018;25:97–105. [DOI] [PubMed] [Google Scholar]
- 14.Ashrafian H, Le Roux CW, Darzi A, et al. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–2102. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso L, Rodrigues D, Gomes L, et al. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19:1223–1232. [DOI] [PubMed] [Google Scholar]
- 16.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253:484–487. [DOI] [PubMed] [Google Scholar]
- 17.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao W, Holmberg D, Näslund E, et al. Validation of obesity surgery data in the Swedish National Patient Registry and Scandinavian Obesity Registry (SOReg). Obes Surg. 2016;26:1750–1756. [DOI] [PubMed] [Google Scholar]
- 20.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125–130. [DOI] [PubMed] [Google Scholar]
- 21.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. [DOI] [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 23.Plecka Ostlund M, Marsk R, Rasmussen F, et al. Morbidity and mortality before and after bariatric surgery for morbid obesity compared with the general population. Br J Surg. 2011;98:811–816. [DOI] [PubMed] [Google Scholar]
- 24.Scott JD, Johnson BL, Blackhurst DW, et al. Does bariatric surgery reduce the risk of major cardiovascular events? A retrospective cohort study of morbidly obese surgical patients. Surg Obes Relat Dis. 2013;9:32–39. [DOI] [PubMed] [Google Scholar]
- 25.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 26.Benotti PN, Wood GC, Carey DJ, et al. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira SC, Neves JS, Souteiro P, et al. Impact of bariatric surgery on long-term cardiovascular risk: Comparative effectiveness of different surgical procedures. Obes Surg. 2020;30:673–680. [DOI] [PubMed] [Google Scholar]
- 29.Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension). Circulation. 2018;137:1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantziari S, Dayer A, Duvoisin C, et al. Long-term weight loss, metabolic outcomes, and quality of life at 10 years after Roux-en-Y gastric bypass are independent of patients' age at baseline. Obes Surg. 2020;30:1181–1188. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell JE, Christian NJ, Flum DR, et al. Postoperative behavioral variables and weight change 3 years after bariatric surgery. JAMA Surg. 2016;151: 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King WC, Hinerman AS, White GE, et al. Associations between physical activity and changes in weight across 7 years following ROUX-en-Y gastric bypass surgery: a multicenter prospective cohort study. Ann Surg. 2022;275:718–726. [DOI] [PubMed] [Google Scholar]
- 33.Persson CE, Björck L, Lagergren J, et al. Risk of heart failure in obese patients with and without bariatric surgery in Sweden—a registry-based study. J Card Fail. 2017;23:530–537. [DOI] [PubMed] [Google Scholar]
- 34.Sundstrom J, Bruze G, Ottosson J, et al. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gribsholt SB, Thomsen RW, Svensson E, et al. Overall and cause-specific mortality after Roux-en-Y gastric bypass surgery: a nationwide cohort study. Surg Obes Relat Dis. 2017;13:581–587. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson L, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. 2020;383:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telem DA, Talami M, Shroyer AL, et al. Long-term mortality rates (>8-year) improve as compared to the general and obese population following bariatric surgery. Surgl Endosc. 2015;29:529–536. [DOI] [PubMed] [Google Scholar]
- 38.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. [DOI] [PubMed] [Google Scholar]
- 39.Pontiroli AE, Ceriani V, Tagliabue E, et al. Bariatric surgery, compared to medical treatment, reduces morbidity at all ages but does not reduce mortality in patients aged <43 years, especially if diabetes mellitus is present: a post hoc analysis oftwo retrospective cohort studies. Acta Diabetol. 2020;57:323–333. [DOI] [PubMed] [Google Scholar]
- 40.Scandinavian Obesity Registry (SOReg). Annual Report SOReg 2014. Follow-up weight changes, change in comorbidity, long-term complications and quality indicators on the clinical level. Available at: https://www.ucr.uu.se/ soreg/in-english. Accessed May 15, 2020.
- 41.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Höskuldsdóttir G, Mossberg K, Wallenius V, et al. Design and baseline data in the BAriatic surgery SUbstitution and Nutrition study (BASUN): a 10-year prospective cohort study. BMC Endocr Disord. 2020;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]


