Abstract
Background
Racial health disparities across orthopaedic surgery subspecialties, including spine surgery, are well established. However, the underlying causes of these disparities, particularly relating to social determinants of health, are not fully understood.
Questions/purposes
(1) Is there a racial difference in 90-day mortality, readmission, and complication rates (“safety outcomes”) among Medicare beneficiaries after spine surgery? (2) To what degree does the Centers for Disease Control and Prevention Social Vulnerability Index (SVI), a community-level marker of social determinants of health, account for racial disparities in safety outcomes?
Methods
To examine racial differences in 90-day mortality, readmission, and complications after spine surgery, we retrospectively identified all 419,533 Medicare beneficiaries aged 65 or older who underwent inpatient spine surgery from 2015 to 2019; we excluded 181,588 patients with endstage renal disease or Social Security disability insurance entitlements, who were on Medicare HMO, or who had missing SVI data. Because of the nearly universal coverage of those age 65 or older, Medicare data offer a large cohort that is broadly generalizable, provides improved precision for relatively rare safety outcomes, and is free of confounding from differential insurance access across races. The Master Beneficiary Summary File includes enrollees’ self-reported race based on a restrictive list of mutually exclusive options. Even though this does not fully capture the entirety of racial diversity, it is self-reported by patients. Identification of spine surgery was based on five Diagnosis Related Groups labeled “cervical fusion,” “fusion, except cervical,” “anterior-posterior combined fusion,” “complex fusion,” and “back or neck, except fusion.” Although heterogeneous, these cohorts do not reflect inherently different biology that would lead us to expect differences in safety outcomes by race. We report specific types of complications that did and did not involve readmission. Although complications vary in severity, we report them as composite measures while being cognizant of the inherent limitations of making inferences based on aggregate measures. The SVI was chosen as the mediating variable because it aggregates important social determinants of health and has been shown to be a marker of high risk of poor public health response to external stressors. Patients were categorized into three groups based on a ranking of the four SVI themes: socioeconomic status, household composition, minority status and language, and housing and transportation. We report the “average race effects” among Black patients compared with White patients using nearest-neighbor Mahalanobis matching by age, gender, comorbidities, and spine surgery type. Mahalanobis matching provided the best balance among propensity-type matching methods. Before matching, Black patients in Medicare undergoing spine surgery were disproportionately younger with more comorbidities and were less likely to undergo cervical fusion. To estimate the contribution of the SVI on racial disparities in safety outcomes, we report the average race effect between models with and without the addition of the four SVI themes.
Results
After matching on age, gender, comorbidities, and spine surgery type, Black patients were on average more likely than White patients to be readmitted (difference of 1.5% [95% CI 0.9% to 2.1%]; p < 0.001) and have complications with (difference of 1.2% [95% CI 0.5% to 1.9%]; p = 0.002) or without readmission (difference of 3.6% [95% CI 2.9% to 4.3%]; p < 0.001). Adding the SVI to the model attenuated these differences, explaining 17% to 49% of the racial differences in safety, depending on the outcome. An observed higher rate of 90-day mortality among Black patients was explained entirely by matching using non-SVI patient demographics (difference of 0.00% [95% CI -0.3% to 0.3%]; p = 0.99). However, even after adjusting for the SVI, Black patients had more readmissions and complications.
Conclusion
Social disadvantage explains up to nearly 50% of the disparities in safety outcomes between Black and White Medicare patients after spine surgery. This argument highlights an important contribution of socioeconomic circumstances and societal barriers to achieving equal outcomes. But even after accounting for the SVI, there remained persistently unequal safety outcomes among Black patients compared with White patients, suggesting that other unmeasured factors contribute to the disparities. This is consistent with evidence documenting Black patients’ disadvantages within a system of seemingly equal access and resources. Research on racial health disparities in orthopaedics should account for the SVI to avoid suggesting that race causes any observed differences in complications among patients when other factors related to social deprivation are more likely to be determinative. Focused social policies aiming to rectify structural disadvantages faced by disadvantaged communities may lead to a meaningful reduction in racial health disparities.
Level of Evidence
Level III, therapeutic study.
Introduction
People of color in the United States experience worse health outcomes after medical care [8, 28], and this applies to orthopaedic care as well [9, 40]. Black patients are at an increased risk of having delayed surgery, complications, readmissions, and mortality in the setting of numerous orthopaedic procedures, including spine surgery [3, 4, 15, 16, 33, 37, 43].
Research on the underlying causes of racial disparities is incomplete. Contributing factors include implicit racial bias, overt racism, healthcare segregation [36], and social determinants of health, which may be endemic in certain communities [41]. Elucidating the contributions of each of these factors is necessary to address racial disparities.
Social determinants of health represent the complex conditions of an individual’s lived environment, including social structure and economic systems that affect health [38]. This has not be explored in depth in musculoskeletal research, but it does appear to affect disease burden and outcomes [22]. The Centers for Disease Control and Prevention developed the Social Vulnerability Index (SVI) as a metric of community-level markers of high risk of poor public health in the face of external stressors in US communities [11]. The SVI integrates data on factors such as poverty, employment, education, and income; household composition; minority status and English-language proficiency; and housing and transportation disadvantages. Understanding the degree to which SVI explains racial disparities in outcomes after orthopaedic surgery can dispel assumptions about the causality of race on outcomes, encourage future research on the social determinants of health, and inform social policies that seek to mitigate racial disparities [2].
Examining the interplay of SVI and racial disparities in relatively uncommon orthopaedic safety outcomes requires a large study population to be sufficiently powered to detect differences and to be generalizable. We chose spine surgery as a substrate because of the intensity of these surgical procedures; these are marked by relatively high resource use and morbidity profiles [23, 34]. We believe the findings could be translatable to other high-intensity inpatient orthopaedic interventions, including total joint arthroplasty and trauma. Though diverse spine procedures were included in this study, we believe the methodology balances generalizability with the risks of losing insight by pooling a relatively heterogeneous study population.
We asked: (1) Is there a racial difference in 90-day mortality, readmission, and complication rates (“safety outcomes”) among Medicare beneficiaries after spine surgery? (2) To what degree does the Centers for Disease Control and Prevention SVI, a community-level marker of social determinants of health, account for racial disparities in safety outcomes?
Patients and Methods
Study Design and Setting
This is a retrospective, comparative study drawn from a large, federally maintained database. We analyzed Medicare claims from October 2015 through October 2019, including fee-for-service beneficiaries 65 years or older with a hospital Diagnosis Related Group (DRG) code for spinal surgery. Because of its nearly universal coverage of those age 65 or older, Medicare data offer a large cohort that is broadly generalizable, provides improved precision for relatively uncommon safety outcomes, and is free of confounding due to differential insurance access across race. Additionally, it is one of the most comprehensive databases within a patient demographic (patients aged 65 or older), it requires patients to self-report their racial or ethnic identity, and it reliably reports mortality, readmission, and complications.
Patients
Based on the grouping of DRG codes from The Center for Medicare and Medicaid Innovation (CMMI), this study included cohorts labeled as “cervical fusion,” “fusion, except cervical,” “anterior-posterior combined fusion,” “complex fusion,” and “back or neck surgery, except fusion.” Although a heterogeneous mix of spinal pathology and procedures, these cohorts do not reflect any inherently different biology that would lead us to expect differences in safety outcomes by race. We excluded beneficiaries in the End-stage Renal Disease (ESRD), Social Security Disability Insurance (SSDI), and Medicare-HMO (for example, Medicare Advantage) programs as well as patients with missing zip code, SVI, or discharge year data.
After excluding 30% (181,588 of 601,121) of claims, most of which were for patients younger than 65 and/or in ESRD/SSDI entitlement programs, a total of 419,533 eligible Medicare beneficiaries met the inclusion criteria for analysis (Fig. 1). Most patients were White (90% [376,385 of 419,533]), 5% (22,878 of 419,533) of patients were Black, and 5% were grouped as Other (20,270 of 419,533). These findings align with the racial composition of the Medicare population.
Fig. 1.
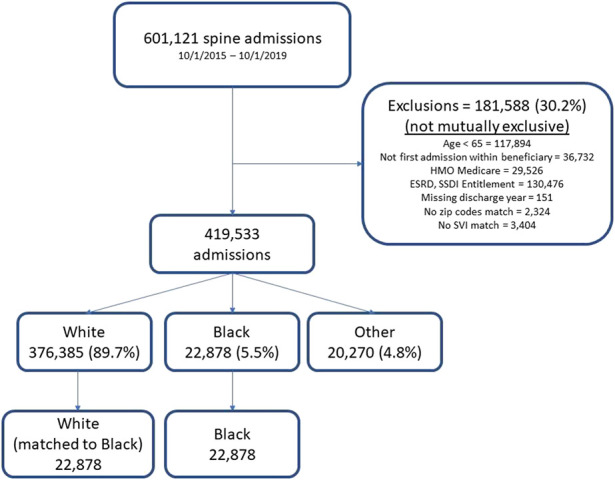
Flow chart of study population as exclusions were applied, racial breakdown of patients, and final sample size after matching.
Patients’ Data at Baseline
White patients in the cohort were older, disproportionately men, had fewer comorbidities, and were less likely to undergo inpatient cervical fusions than Black patients (Table 1).
Table 1.
Characteristics of Medicare spine surgery procedures by race
| White (n = 376,385) | Black (n = 22,878) | p value (Black vs White) | Other (n = 20,270) | p value (Other vs White) | |
| Age in years | 74 ± 6 | 73 ± 5 | < 0.001 | 72 ± 6 | < 0.001 |
| Age group in years 65-69 70-74 75-79 80-84 85+ |
28 (104,414) 32 (121,584) 24 (88,330) 12 (43,317) 5 (18,740) |
36 (8310)) 32 (7347) 20 (4580) 9 (1968) 3 (673) |
< 0.001 | 40 (8006) 32 (6481) 16 (3293) 8 (1620) 4 (870) |
< 0.001 |
| Men | 47 (175,292) | 42 (9585) | < 0.001 | 54 (10,898) | < 0.001 |
| Charlson comorbidity index 0 1 2+ |
46 (173,044) 27 (99,774) 28 (103,567) |
32 (7420) 28 (6302) 40 (9156) |
< 0.001 | 44 (8897) 27 (5543) 29 (5830) |
< 0.001 |
| Spine cohort Back or neck surgery nonfusion Cervical fusion AP fusion Complex fusion Lumbar fusion |
14 (52,344) 21 (78,914) 17 (65,202) 4 (16,353) 44 (163,569) |
14 (3123) 30 (6901) 15 (3348) 3 (745) 38 (8761) |
< 0.001 | 15 (2957) 23 (4746) 19 (3752) 4 (884) 39 (7931) |
< 0.001 |
Data presented as mean ± SD or % (n).
Independent Variable
The Master Beneficiary Summary File (MBSF) includes patients’ self-identification for race based on a mutually exclusive list of options. Race, the primary independent variable, was coded as Black (Black or African American), White (White or Caucasian), and a combined Other (includes other, Asian, Hispanic ethnicity, and North American native). The relatively small number of patients for the specific racial groups within the Other category precluded sufficient statistical power for a meaningful analysis. Furthermore, the comparison between Black and White patients may represent the most extreme difference in racial disparities given the preponderance of evidence showing health disparities between these groups. Though broadly categorizing race into Black and White lacks nuance given the racial diversity within each group, the data were limited by the options available for patient self-report during Medicare enrollment. The fact that patients selected their racial classification ensures that it at least broadly applies to them. A separate variable for ethnicity was not available; Hispanic and North American native ethnicities were included as distinct options within the race variable and were mutually exclusive of other options.
Mediating Variables: Social Vulnerability Index and the Four Themes
We examined differences in safety outcomes by race and the mediating effect of social disadvantage, as measured by the SVI, which is a composite measure of community disadvantage based on 15 factors reported at the United States Census Tract level [11]. It includes four themes that represent social disadvantages: socioeconomic (poverty, work, education, and income), household composition and disability, minority and language (English language proficiency), as well as housing and transportation. Examples of contributing factors include proportions of the population that are living below the poverty line, unemployed, uninsured, disabled, adults without a high school diploma, single-parent households, racial minorities, living in group quarters, and households with no vehicle available. The SVI is assigned to individuals based on ZIP Codes. We classified patients into low (1st through 33rd percentile), medium (34th through 66th percentile), and high (67th through 99th percentile) levels of disadvantage based on their ranking for each of the four SVI themes. The SVI has been validated in studies of medical diagnoses as well as studies of natural disasters as a factor that is associated with complications and mortality, though its use in orthopaedic research is limited [7, 11, 14, 17, 31].
Covariates
We collected patient demographics, including age (grouped into 5-year increments), gender, diagnosis codes from the ICD-10 included with the index admission, and spine procedure cohort. The Charlson comorbidity index (CCI) was calculated based on ICD-10 codes [32].
Safety-related Outcomes
We linked our cohort to the MBSF to obtain data on mortality and date of death. In addition, successive claims for the same patients were linked over time to identify beneficiaries who had any (all-cause) readmission. To facilitate a time-to-event analysis, the days between the date of the index hospital discharge and the date of death and/or the first inpatient readmission were calculated for each beneficiary.
We linked each spine admission to all subsequent inpatient claims (from the Inpatient Research Identifiable File) and Part B (office-based and provider) claims from the Carrier Claims File through 90 days after discharge to identify postoperative complications. Patients with admissions during the last quarter of 2019 were excluded to ensure a minimum 90-day follow-up period for all patients. Postoperative complications were based on ICD-10 codes that specifically identified a perioperative complication. Complication codes were grouped into broader categories (such as infection) and by whether they involved a subsequent inpatient admission (Supplementary Table 1; http://links.lww.com/CORR/A873). We separately reported complications that did or did not require a readmission, which served as a gauge of complication severity. Complications were not otherwise weighted by severity in the composite measures, introducing the limitations inherent to inferences based on aggregate measures. All-cause readmissions were included in the composite measure for inpatient complications. Death was treated independently and not counted in the composite complication measures.
Statistical Analysis
We report unadjusted rates of 90-day mortality, all-cause readmission, and complications across racial groups. Using claims spanning the analysis period, we then performed a time-to-event survival analysis based on Cox proportional hazard regression for mortality and readmission, including variables for race, age, gender, CCI, spine cohort, and the four SVI themes.
Because of differences in demographics and the potential for selection bias, we conducted a 1:1 matched cohort analysis for each outcome using Mahalanobis distance to match each Black patient to a White patient most similar in age, gender, comorbidity, and spine cohort. Mahalanobis distance matching is based on the covariance matrix without calipers or replacement and, similar to propensity score matching, explicitly addresses treatment bias in observational studies [1, 20]. Mahalanobis matching yielded better covariate balance than propensity score matching. We calculated the standardized differences (all near 0) and variance ratios (all near 1) for each factor in the matched cohort. Balance plots were examined to assess the post-match balance between the cohorts (Supplementary Fig. 1; http://links.lww.com/CORR/A874). For these analyses, we focused only on the evaluation of Black and White racial disparities. To understand the contribution of the SVI to racial disparities, we examined differences in the “average race effect among Black patients” using sequential models that did or did not include the four SVI themes. The term “average race effect among Black patients” is analogous to the “average treatment effect among the treated” from interventional trials. It compares the difference in mean safety outcomes between Black patients versus the counterfactual among Black patients as if they were treated in the health system as White. We calculated the influence of the SVI on racial disparities as a proportion of change in the average race effect among Black patients between the models with and without SVI as a matching covariate. All analyses were conducted using Stata-MP version 17.1 (StataCorp LLC) within Medicare’s Virtual Research Data Center [12].
Results
Racial Disparities in Safety-related Outcomes and the Influence of SVI
Black patients experienced higher rates of adverse safety events across all measures than White patients (Table 2). The differences between Black and White patients, as well as the contributions from demographic matching with and without SVI, are presented as follows.
Table 2.
Average race effect among Black patients for 90-day mortality, readmission, and complications in a matched cohort with and without inclusion of the SVI, 2015-2019a
| Observed unadjusted, % | Matched without SVI | Matched with SVI | ||||||||
| White | Black | Difference, ppt (95% CI) | p value | Difference, ppt (95% CI) | p value | % explained by non-SVI demographics | Difference, ppt (95% CI) | p value | % explained by SVI | |
| Mortality | 1.8 | 2.4 | 0.6 (0.4 to 0.8) | < 0.001 | 0.0 (-0.3 to 0.3) | 0.99 | 100% | -0.2 (-0.4 to 0.1) | 0.30 | |
| Readmission | 13 | 16 | 3.0 (2.4 to 3.5) | < 0.001 | 1.5 (0.9 to 2.1) | < 0.001 | 49% | 1.2 (0.5 to 1.8) | 0.001 | 23% |
| Complication with readmission | 25 | 27 | 2.3 (1.5 to 3.0) | < 0.001 | 1.2 (0.5 to 1.9) | 0.002 | 48% | 0.6 (-0.2 to 1.4) | 0.15 | 49% |
| Complication without readmission | 20 | 24 | 4.0 (3.3 to 4.8) | < 0.001 | 3.6 (2.9 to 4.3) | < 0.001 | 10% | 3.0 (2.2 to 3.8) | < 0.001 | 17% |
aBased on nearest-neighbor Mahalanobis matching on age, sex, comorbidity, and spine cohort and including bias adjustment for age and Charlson comorbidity and exact matching on spine cohort. Rounding may have affected the ppt difference results; ppt = percentage point difference based on average race effect among Black patients.
Mortality
Black patients had a higher observed 90-day mortality (2.4%) than White patients (1.8%) (Table 2) and higher adjusted mortality over the entire study period (HR 1.1 [95% confidence interval (CI) 1.1 to 1.2]; p < 0.001) (Fig. 2).
Fig. 2.
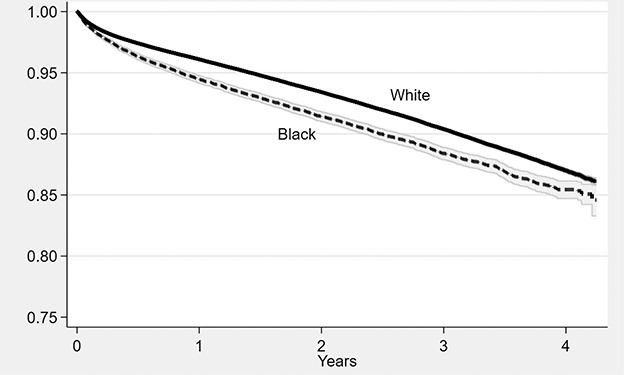
Unadjusted mortality after spine surgery among Medicare beneficiaries, by race. Shaded areas represent 95% CIs based on Kaplan-Meier survival plots.
The average race effect among Black patients for 90-day mortality was 0.00 (95% CI -0.3 to 0.3) percentage points (ppt) after matching for age, gender, CCI, and spine cohort, but not the SVI (Table 2). This suggests that the observed difference in 90-day mortality was entirely explained by differences in demographics. The effect was slightly reversed to -0.2 ppt (95% CI -0.4 to 0.1; p = 0.30) when adding the SVI themes to the model. Thus, matching on the SVI did not account for a difference in mortality between Black and White patients. High socioeconomic SVI was associated with an increased risk of overall mortality (HR 1.18 [95% CI 1.14 to 1.23]; p < 0.001) (Supplementary Table 2; http://links.lww.com/CORR/A875).
Readmission
Black patients had a higher 90-day readmission (16%) compared with White patients (13%), a 3.0 ppt difference (95% CI 2.4 to 3.5; p < 0.001) (Table 2). Matching on demographics but not the SVI attenuated the average race effect to 1.5 ppt (95% CI 0.9 to 2.1; p < 0.001), explaining 49% of the racial difference in readmission. Further matching on SVI yielded an average race effect of 1.2 ppt (95% CI 0.5 to 1.8), explaining an additional 23% of racial disparity. We tracked cumulative readmission over the entire study period (Fig. 3).
Fig. 3.
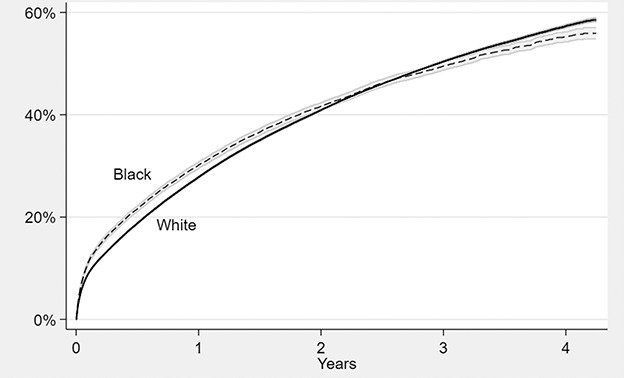
Unadjusted cumulative all-cause readmission rate after spine surgery among Medicare beneficiaries, by race. Shaded areas represent 95% CIs based on Kaplan-Meier failure plots.
The likelihood of 90-day readmission was increased among patients living in communities with high socioeconomic SVI (OR 1.08 [95% CI 1.05 to 1.12]; p < 0.001) and high minority and language SVI (OR 1.08 [95% CI 1.04 to 1.11]; p < 0.001) (Supplementary Table 2; http://links.lww.com/CORR/A875).
Complications
Black patients in the matched analysis had a greater likelihood of having complications that involved (1.2 ppt [95% CI 0.5 to 1.9]; p = 0.002) or did not involve readmission (3.6 ppt [95% CI 2.9 to 4.3]; p < 0.001) (Table 2). Adding the SVI to the models attenuated the average race effect to 0.6 ppt for complications with readmission (95% CI -0.2 to 1.4; p = 0.15) and 3.0 ppt without readmission (95% CI 2.2 to 3.8; p < 0.001). Thus, the SVI accounted for 49% and 17%, respectively, of the racial health disparities in complications that involved or did not involve readmission. The minority and language theme had the greatest influence on complications with (OR 1.07 [95% CI 1.04 to 1.10]; p < 0.001) or without readmission (OR 1.26 [95% CI 1.21 to 1.31]; p < 0.001) (Supplementary Table 2; http://links.lww.com/CORR/A875).
We further examined racial disparities in specific types of complications. Adjusted 90-day complication rates linked with readmission that were higher for Black patients than for White patients included infection (0.5 ppt [95% CI 0.2 to 0.8]; p < 0.001), pulmonary embolism (0.6 ppt [95% CI 0.4 to 0.9]; p < 0.001), and deep venous thrombosis (1.2 ppt [95% CI 0.9 to 1.4]; p < 0.001) (Table 3). Complications not involving readmission that were higher among Black patients than among White patients included infection (0.4 ppt [95% CI 0.1 to 0.6]; p = 0.003), musculoskeletal complications (0.6 ppt [95% CI 0.04 to 1.1]; p = 0.04), deep venous thrombosis (0.6 ppt [95% CI 0.3 to 1.0]; p < 0.001), and cerebrovascular complications (1.6 ppt [95% CI 1.3 to 2.0]; p < 0.001) (Table 4). Black-White disparities in musculoskeletal complications and deep venous thromboses were most explained by the SVI at 43% and 37%, respectively.
Table 3.
Average race effect among Black patients for select complications involving a readmission in a matched cohort with and without inclusion of the SVI, 2015-2019a
| Observed unadjusted, % | Matched without SVI | Matched with SVI | ||||||||
| White | Black | Difference, ppt (95% CI) | p value | Difference, ppt (95% CI) | p value | % explained by non-SVI demographics | Difference, ppt (95% CI) | p value | % disparities explained by SVI | |
| Respiratory | 1.1 | 1.3 | 0.2 (0.04 to 0.4) | 0.013 | 0.03 (-0.2 to 0.2) | 0.75 | 86 | 0.08 (-0.1 to 0.3) | 0.46 | |
| Infection | 1.6 | 2.2 | 0.7 (0.4 to 0.9) | < 0.001 | 0.5 (0.2 to 0.7) | < 0.001 | 27 | 0.5 (0.2 to 0.8) | < 0.001 | 0% |
| Pulmonary embolism | 1.1 | 1.7 | 0.7 (0.5 to 0.8) | < 0.001 | 0.5 (0.3 to 0.7) | < 0.001 | 19 | 0.6 (0.4 to 0.9) | < 0.001 | 0% |
| Deep venous thrombosis | 1.6 | 2.8 | 1.2 (1.0 – 1.5) | < 0.001 | 1.1 (0.8 to 1.4) | 0.002 | 11 | 1.2 (0.9 to 1.4) | < 0.001 | 0% |
| Cerebrovascular | 0.6 | 0.7 | 0.2 (0.08 to 0.3) | < 0.001 | 0.1 (-0.02 to 0.3) | < 0.001 | 37 | 0.1 (-0.02 to 0.3) | 0.08 | 0% |
aBased on nearest-neighbor Mahalanobis matching on age, sex, comorbidity, and spine cohort and including bias adjustment for age and Charlson comorbidity and exact matching on spine cohort. Rounding may have affected the ppt difference results; ppt = percentage point difference based on average race effect among Black patients.
Table 4.
Average race effect among Black patients for select complications not involving a readmission in a matched cohort with and without inclusion of the SVI, 2015-2019a
| Observed unadjusted, % | Matched without SVI | Matched with SVI | ||||||||
| White | Black | Difference, ppt (95% CI) | p value | Difference, ppt (95% CI) | p value | % explained by non-SVI demographics | Difference, ppt (95% CI) | p value | % disparities explained by SVI | |
| Musculoskeletal | 8.7 | 9.6 | 0.9 (0.3 to 1.5) | 0.002 | 1.0 (0.5 to 1.5) | < 0.001 | 0 | 0.6 (0.04 to 1.1) | 0.04 | 43 |
| Deep venous thrombosis | 2.3 | 3.3 | 1.0 (0.8 to 1.3) | < 0.001 | 1.0 (0.7 to 1.3) | < 0.001 | 4 | 0.6 (0.3 to 1.0) | < 0.001 | 37 |
| Vascular | 1.7 | 2.2 | 0.6 (0.3 to 0.8) | < 0.001 | 0.5 (0.2 to 0.7) | < 0.001 | 18 | 0.4 (0.1 to 0.7) | 0.004 | 13 |
| Infection | 1.3 | 1.7 | 0.4 (0.3 to 0.6) | < 0.001 | 0.4 (0.2 to 0.6) | 0.001 | 12 | 0.4 (0.1 to 0.6) | 0.003 | 5 |
| Cerebrovascular | 2.1 | 4.0 | 2.0 (1.7 to 2.3) | < 0.001 | 1.7 (1.4 to 2.0) | < 0.001 | 14 | 1.6 (1.3 to 2.0) | < 0.001 | 5 |
| Cardiac | 1.0 | 1.3 | 0.3 (0.2 to 0.5) | 0.001 | 0.2 (0.05 to 0.4) | 0.01 | 25 | 0.4 (0.2 to 0.6) | < 0.001 | 0 |
| Pulmonary embolism | 0.9 | 1.1 | 0.2 (0.1 to 0.4) | 0.005 | 0.2 (-0.02 to 0.3) | 0.08 | 27 | 0.2 (-0.03 to 0.4) | 0.09 | 0 |
aBased on nearest-neighbor Mahalanobis matching on age, sex, comorbidity, and spine cohort and including bias adjustment for age and Charlson comorbidity and exact matching on spine cohort. Rounding may have affected the ppt difference results; ppt = percentage point difference based on average race effect among Black patients.
Discussion
People from racial minority groups experience worse outcomes in many metrics across orthopaedic surgery subspecialties, but the reasons for racial health disparities are unclear [3, 4, 15, 16, 33, 37, 43]. Given minimal biological evidence for this association, there are likely structural and social explanations [25, 39]. In our analysis of Medicare patients undergoing spine surgery, we found a higher adjusted rate of readmission and postoperative complications among Black patients than among White patients. Interestingly, demographic differences such as age and comorbidity explained the observed racial difference in mortality. Among readmission and complications, SVI, a marker of social community disadvantage, explained 17% to 49% of the disparities. This suggests that race is not an inherent predictor of poor outcomes; instead, there are underlying societal factors that contribute to worse safety outcomes. Efforts to decrease racial health disparities should address the needs of underserved communities, including employment, education, housing, and transportation. Future research on race and outcomes should account for SVI to avoid misleading conclusions that race itself is responsible for patient outcomes.
Limitations
Our study has limitations. As with any observational study design, the potential for unobserved confounding prohibits direct causal inferences about the effect of the SVI on disparities. The study population included only patients with Medicare insurance. This increases the internal validity of our findings by reducing possible confounders of access to insurance and age but limits the generalizability of the findings to a broader population. Social factors may affect the health outcomes of older and younger patients differently. Patients with private insurance have a decreased risk of adverse safety outcomes compared with those who have Medicare insurance, so our population may skew toward higher rates of safety events than the overall population [15]. Relying on claims data prevents a detailed analysis of clinical factors influencing safety and does not include patient pain or functional status. Although the five spine surgery cohorts that we matched on represent a heterogeneous mix of spinal pathology and procedures, we do not believe that subgroups of spinal procedures or diagnoses within these cohorts reflect any inherently different biology that would lead us to expect differences in safety outcomes by race.
Inferences were only made between Black and White patients. Dichotomizing race into Black and White results in a loss of nuance about the racial diversity within each group [24]. However, race was based on patient self-identification from a limited set of predefined options at the time of Medicare enrollment. The general effects of racial disparities in this study are evident despite the heterogeneity within groups, although the reader should be aware that the implications for a given patient are more nuanced than can be assumed from their membership in the Black or White race. Although SVI has been validated elsewhere [7, 11, 14, 17, 31] and captures social disadvantages, its use in orthopaedic research is limited. Our most refined assessment of disadvantaged communities was via the ZIP Code and its translation to the SVI. Our findings might be prone to unobserved confounding, although we adjusted for these to the fullest extent possible by using other factors in our statistical models and with a matched cohort approach.
Discussion of Key Findings
The key finding from this study is that SVI explains up to 49% of the racial differences in postoperative safety outcomes. This finding supports the assertion of an Institute of Medicine report that socioeconomic status, racism, and culture each explain roughly one-third of healthcare disparities encountered in the United States, with genetic differences accounting for less than 10% [39]. Socioeconomic status and minority and disability status were the primary SVI themes associated with worse safety outcomes in our study. We believe that the design of this work, and the use of spine surgery as a substrate, allows translation to other aspects of orthopaedic care, such as total joint arthroplasty and fracture care in Medicare patients.
Previous studies have evaluated community disadvantage to explain racial health disparities in orthopaedic surgery, although none have quantified that contribution. Two studies found that patient-reported outcomes after arthroplasty were worse with increased community poverty, more so in Black patients than in White patients, suggesting amplified racial disparities among disadvantaged communities [18, 19]. Similarly, Dy et al. [15] reported increased risks of delayed surgery, reoperation, readmission, and mortality among Black patients undergoing treatment for hip fractures, even after controlling for the Area Deprivation Index, a neighborhood-level measure of socioeconomic disadvantage. Black patients more frequently experienced delayed surgery and readmission than White patients at every Area Deprivation Index level.
The present investigation supports community disadvantage, as measured by SVI, as an important contributor to racial health disparities in the United States. Addressing underlying factors that lead to social vulnerability, better stated as structural disadvantages for non-White people that lead to worse health outcomes, may reduce associated disparities. These factors include poverty, unemployment, education, insurance access, and adequate housing, particularly in some Black communities. Given these insights, a multiarmed approach by policymakers, healthcare administrators, and providers that addresses salient SVI themes, including poverty, employment, education, housing, and transportation, may improve the orthopaedic outcomes of Black people in the United States.
Racial disparities largely persist even after adjustment for socioeconomic status, as was seen in the present study as well as that of Barr [8]. This suggests that other unmeasured structural factors also drive racial healthcare disparities, with implicit bias, racism, and healthcare segregation as important candidates [36, 42]. Future studies on these topics could include examining racial disparities in outcomes of patients with patient-physician race concordance, where implicit bias and racism may be mitigated. If few disparities exist, then implicit bias and racism are likely to play some role in racial health disparities. If healthcare segregation is a prominent factor, study of racial disparities in outcomes of patients within the same medical facility may show decreased disparities. Elucidating patient perceptions of the healthcare system as a factor in racial disparities is more challenging given that patients must be individually asked about their trust in the system or likelihood to seek care, and that must be done with a sufficient sample size to find an association with health outcomes. Although difficult, such a study could be valuable by illustrating the need to earn the trust of racial minority patients.
Research on tangible strategies to improve health equity in orthopaedics is limited and shows mixed results. Equal access to insurance and care may decrease disparities [35]. In comparisons between racial minorities and White patients in universally insured populations, studies have shown similar mortality rates among patients with hip fractures [30], ACL reconstruction revision rates [27], and reoperation and complication rates after THA [29]. Nevertheless, in the Medicare-insured population, we could identify several important disparities. Therefore, greater access to health insurance alone may be insufficient. There is mixed evidence on the impact of state-level and national-level efforts by governments and orthopaedic societies to improve access to care to address racial health disparities in orthopaedics [5, 6, 10]. Given the findings of the present study, however, mixed results should not dissuade leaders from action but instead spur renewed and creative solutions to reduce social disadvantages and other potential underlying causes of racial health disparities.
In evaluating health disparities by race, it is important to avoid false conclusions regarding causality [26]. Historically, disparities have been sometimes attributed to “myths of racial biology, behavioral explanation predicated on racial stereotypes, and territorial stigmatization” [13]. In other words, people may attribute higher complication rates among non-White people to inherently different biology, higher-risk behaviors, or the concept that non-White-dominant neighborhoods may be to blame. Each of these has roots in racial stereotypes, and such conclusions are not substantiated by the work presented here [24, 25]. The use of the word “vulnerable,” as is used in SVI, is also problematic because it glosses over structural racism as a driver of disadvantages that Black communities face [21]. As a field, we must think critically about the context and framing of analyses involving race to avoid perpetuating systemic racism.
With this in mind, an additional implication of this study is that SVI, or a similar metric of community disadvantage, should be considered and adjusted for in future research on race. Identifying associations between race and outcomes lacks nuance and risks the reader assuming that a patient’s race is the reason for worse outcomes. This is a concerning premise that may lead to judgment being placed on particular races. If researchers account for community disadvantage, they may show that disadvantageous social factors are instead a key reason for worse outcomes in particular racial groups.
Conclusion
We found that community social disadvantage is an important driver of racial disparities in safety after spine surgery. Future research on racial health disparities in orthopaedics should account for community disadvantage, potentially preventing misleading assumptions about a patient’s inherent race as the driver of outcomes when in fact community disadvantage plays a substantial role. Furthermore, this finding can help focus efforts by orthopaedic practitioners, healthcare organizations, and policymakers to reduce racial health disparities by addressing elements of the SVI that are experienced by the communities they serve. Salient factors to be addressed include poverty, employment, education, housing, and transportation. However, even after controlling for patient demographics and community disadvantage, Black patients were still more likely to experience readmissions and complications. Therefore, additional factors that contribute to these disparities should also serve as the focus of future research. For example, finding associations between racial health disparities and patient-physician race concordance or patient trust in the healthcare system could implicate implicit bias/racism or patient perspectives on medical care, respectively, as driving factors of these disparities.
Footnotes
The institution of one or more of the authors (AJS, BIM) has received, during the study period, funding from the Agency for Healthcare Research and Quality (grant R01HS024714), National institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Department of Defense, and Orthopaedic Research Education Foundation.
One of the authors (AJS) certifies receipt of personal payments or benefits, during the study period, in an amount of less than USD 10,000 from Springer Nature, and in an amount of USD 100,001 to USD 1,000,000 from Wolters Kluwer Health.
One of the authors (BIM) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 100,001 to USD 1,000,000 from ownership in STATIX LLC, unrelated to this manuscript.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was not sought.
This work was performed at the Department of Orthopaedics, University of Utah, Salt Lake City, UT, USA.
Contributor Information
Kinjal D. Vasavada, Email: kinjal.vasavada@tufts.edu.
Megan E. Vanneman, Email: megan.vanneman@hsc.utah.edu.
Andrew J. Schoenfeld, Email: aschoenfeld@bwh.harvard.edu.
Brook I. Martin, Email: brook.martin@hsc.utah.edu.
References
- 1.Abadie A, Imbens GW. Large sample properties of matching estimators for average treatment effects. Econometrica. 2006;74:235-267. [Google Scholar]
- 2.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21:60-76. [DOI] [PubMed] [Google Scholar]
- 3.Adogwa O, Elsamadicy AA, Mehta AI, et al. Racial disparities in 30-day readmission rates after elective spine surgery: a single institutional experience. Spine (Phila Pa 1976). 2016;41:1677-1682. [DOI] [PubMed] [Google Scholar]
- 4.Aladdin DEH, Tangel V, Lui B, et al. Black race as a social determinant of health and outcomes after lumbar spinal fusion surgery: a multistate analysis, 2007 to 2014. Spine (Phila Pa 1976). 2020;45:701-711. [DOI] [PubMed] [Google Scholar]
- 5.Amen TB, Varady NH, Rajaee S, et al. Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the U.S.: a comprehensive analysis of trends from 2006 to 2015. J Bone Joint Surg Am. 2020;102:811-820. [DOI] [PubMed] [Google Scholar]
- 6.Aseltine RH Jr, Wang W, Benthien RA, et al. Reductions in race and ethnic disparities in hospital readmissions following total joint arthroplasty from 2005 to 2015. J Bone Joint Surg Am. 2019;101:2044-2050. [DOI] [PubMed] [Google Scholar]
- 7.Bakkensen LA, Fox-Lent C, Read LK, et al. Validating resilience and vulnerability indices in the context of natural disasters. Risk Anal. 2017;37:982-1004. [DOI] [PubMed] [Google Scholar]
- 8.Barr DA. Health Disparities in the United States: Social Class, Race, Ethnicity, and Health: JHU Press; 2014. [Google Scholar]
- 9.Bass AR, McHugh K, Fields K, et al. Higher total knee arthroplasty revision rates among united states blacks than whites: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2016;98:2103-2108. [DOI] [PubMed] [Google Scholar]
- 10.Best MJ, McFarland EG, Thakkar SC, et al. Racial disparities in the use of surgical procedures in the US. JAMA Surg. 2021;156:274-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry/Geospatial Research, Analysis, and Services Program. CDC/ATSDR Social Vulnerability Index. 2021. Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html. Accessed June 18, 2021.
- 12.Centers for Medicare and Medicaid Services. CCW Virtual Research Data Center (VRDC). Available at: https://resdac.org/cms-virtual-research-data-center-vrdc. Accessed June 14, 2021.
- 13.Chowkwanyun M, Reed AL., Jr. Racial health disparities and COVID-19 - caution and context. N Engl J Med. 2020;383:201-203. [DOI] [PubMed] [Google Scholar]
- 14.Delanois RE, Sax OC, Wilkie WA, et al. Social determinants of health in total hip arthroplasty: are they associated with costs, lengths of stay, and patient reported outcomes? J Arthroplasty. Published online February 18, 2022. DOI: 10.1016/j.arth.2022.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Dy CJ, Lane JM, Pan TJ, et al. Racial and socioeconomic disparities in hip fracture care. J Bone Joint Surg Am. 2016;98:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsamadicy AA, Adogwa O, Fialkoff J, et al. Race as an independent predictor of temporal delay in time to diagnosis and treatment in patients with cervical stenosis: a study of 133 patients with anterior cervical discectomy and fusion. World Neurosurgery. 2016;96:107-110. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan BE, Gregory EW, Hallisey EJ, et al. A social vulnerability index for disaster management. Journal of Homeland Security and Emergency Management. 2011;8:1-17. [Google Scholar]
- 18.Goodman SM, Mandl LA, Parks ML, et al. Disparities in TKA outcomes: census tract data show interactions between race and poverty. Clin Orthop Relat Res. 2016;474:1986-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman SM, Mehta B, Zhang M, et al. Disparities in total hip arthroplasty outcomes: census tract data show interactions between race and community deprivation. J Am Acad Orthop Surg. 2018;26:e457-e464. [DOI] [PubMed] [Google Scholar]
- 20.Guo S, Fraser MW. Propensity Score Analysis: Statistical Methods And Applications: SAGE Publications; 2014. [Google Scholar]
- 21.Joan CA. “Vulnerable” is a public health euphemism we need to stop using. Available at: https://theincidentaleconomist.com/wordpress/vulnerable-is-a-public-health-euphemism-we-need-to-stop-using/. Accessed May 2, 2022.
- 22.Karran EL, Grant AR, Moseley GL. Low back pain and the social determinants of health: a systematic review and narrative synthesis. Pain. 2020;161:2476-2493. [DOI] [PubMed] [Google Scholar]
- 23.Ko H, Brodke DS, Vanneman ME, et al. Is discretionary care associated with safety among medicare beneficiaries undergoing spine surgery? J Bone Joint Surg Am. 2022;104:246-254. [DOI] [PubMed] [Google Scholar]
- 24.Leopold SS. Editor's Spotlight/Take 5: Is there variation in procedural utilization for lumbar spine disorders between a fee-for-service and salaried healthcare system? Clin Orthop Relat Res. 2017;475:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leopold SS. Editorial: Beware of studies claiming that social factors are independently associated with biological complications of surgery. Clin Orthop Relat Res. 2019;477:1967-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopold SS, Beadling L, Calabro AM, et al. Editorial: The complexity of reporting race and ethnicity in orthopaedic research. Clin Orthop Relat Res. 2018;476:917-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro RA, Prentice HA, Inacio MCS, et al. The association between race/ethnicity and revision following ACL reconstruction in a universally insured cohort. J Bone Joint Surg Am. 2019;101:1546-1553. [DOI] [PubMed] [Google Scholar]
- 28.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666-668. [PMC free article] [PubMed] [Google Scholar]
- 29.Okike K, Chan PH, Prentice HA, et al. Association of race and ethnicity with total hip arthroplasty outcomes in a universally insured population. J Bone Joint Surg Am. 2019;101:1160-1167. [DOI] [PubMed] [Google Scholar]
- 30.Okike K, Chan PH, Prentice HA, et al. Association between race and ethnicity and hip fracture outcomes in a universally insured population. J Bone Joint Surg Am. 2018;100:1126-1131. [DOI] [PubMed] [Google Scholar]
- 31.Phelos HM, Deeb AP, Brown JB. Can social vulnerability indices predict county trauma fatality rates? J Trauma Acute Care Surg. 2021;91:399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [DOI] [PubMed] [Google Scholar]
- 33.Sanford Z, Taylor H, Fiorentino A, et al. Racial disparities in surgical outcomes after spine surgery: an ACS-NSQIP analysis. Global Spine J. 2019;9:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenfeld AJ, Harris MB, Liu H, et al. Variations in Medicare payments for episodes of spine surgery. Spine J. 2014;14:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenfeld AJ, Jiang W, Harris MB, et al. Association between race and postoperative outcomes in a universally insured population versus patients in the state of California. Ann Surg. 2017;266:267-273. [DOI] [PubMed] [Google Scholar]
- 36.Schoenfeld AJ, Sandhu T, Sturgeon DJ, et al. Examining healthcare segregation among racial and ethnic minorities receiving spine surgical procedures in the state of Florida. Spine (Phila Pa 1976). 2017;42:1917-1922. [DOI] [PubMed] [Google Scholar]
- 37.Seicean A, Seicean S, Neuhauser D, et al. The influence of race on short-term outcomes after laminectomy and/or fusion spine surgery. Spine (Phila Pa 1976). 2017;42:34-41. [DOI] [PubMed] [Google Scholar]
- 38.Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care . The National Academies Press; 2003. [PubMed] [Google Scholar]
- 40.White AA, 3rd, Hill JA, Mackel AM, et al. The relevance of culturally competent care in orthopaedics to outcomes and health care disparities. J Bone Joint Surg Am. 2007;89:1379-1384. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Regional Office for South-East A. Social determinants of health. WHO Regional Office for South-East Asia; 2008. [Google Scholar]
- 42.Yaya S, Yeboah H, Charles CH, et al. Ethnic and racial disparities in covid-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziedas A, Abed V, Swantek A, et al. Social determinants of health influence access to care and outcomes in patients undergoing anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2022;38:583-594. [DOI] [PubMed] [Google Scholar]


