In humans, T lymphocytes bearing a Vγ9Vδ2 antigen receptor (TCR) exhibit strong cytotoxic activity against cells infected by a wide variety of intracellular pathogens, from bacteria (4, 5, 13, 15, 17, 19, 25, 28) to complex eukaryotic parasites (1, 12). It is now well established that the involvement of human γδ T cells in antiinfectious immunity depends on their TCR-dependent activation by small, protease-resistant ligands containing critical phosphate residues (phosphoantigens). Peripheral Vγ9Vδ2 T cells are subjected to an intense postnatal amplification, most probably due to recurrent encounter with these widespread molecules. Such antigens have been isolated from the mycobacteria Plasmodium falciparum and Francisella tularensis (2, 7, 25, 33), and it is suspected that they exist in several other species (15, 18). Thus, it is clear that the phosphoantigens responsible for γδ T-cell activation are broadly distributed in living organisms. It has been shown that the γδ T-cell response is directed towards cells that contain live bacteria (14) as well as towards live parasites (34), which means that the presence of the recognized ligand depends on an active parasitical metabolism rather than on degradation by-products within the host cell. Finally, the absence of a requirement for classical major histocompatibility complex molecules in the activation of Vγ9Vδ2 T cells reveals a mode of antigen recognition totally different from that of αβ T cells, which enables a particularly rapid response.
Isopentenyl pyrophosphate (IPP) was described as the first structurally identified natural ligand for human γδ T lymphocytes (33). IPP is an essential precursor in the synthesis of isoprenoids (vitamins and steroids, etc.) and is generally synthesized through a mevalonate-dependent pathway (6). This ubiquitous mevalonate pathway begins with the condensation of three molecules of acetyl coenzyme A, leading to mevalonic acid (see Fig. 1). IPP is very widespread in organisms, from bacteria to fungi and higher eukaryotes. Thus, the significance of the Vγ9Vδ2 T-cell response to IPP in humans raises the question of how its production in healthy human cells does not lead to strong γδ T-cell-mediated autoimmunity. It has been suggested that the differential concentration of intracellular IPP—higher in infected cells, as the metabolism of the pathogen is intense—could account for γδ T-cell discrimination between infected and healthy cells (8). It has also been proposed that the differential subcellular sequestration of IPP could allow the same kind of distinction, with the IPP produced by the host cell remaining in the cytoplasm whereas that of parasitical origin being released inside the phagosome (8).
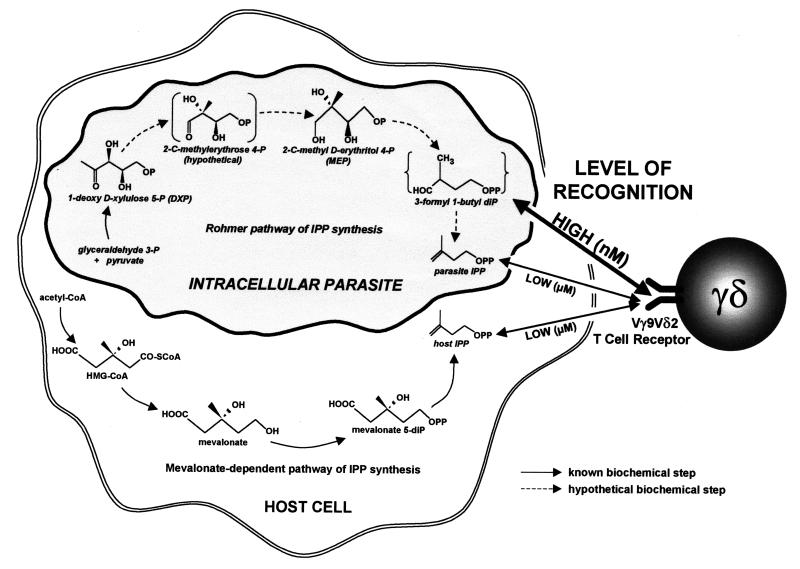
FIG. 1.
Differential features of metabolic routes leading to production of phosphoantigenic signals for γδ T lymphocytes. CoA, coenzyme A; OP, phosphate; OPP, pyrophosphate.
Very recent studies have given new clues to the understanding of the basis of Vγ9Vδ2 T lymphocyte activation by infected cells and their discrimination from noninfected cells.
It had already been demonstrated that some species produce IPP independently of mevalonate through another essential biochemical pathway (23, 26, 30; for a review, see reference 11), often referred to as the Rohmer pathway. This pathway begins with the transketolization of pyruvate and glyceraldehyde 3-phosphate (27), which is catalyzed by deoxy d-xylulose 5-phosphate (DXP) synthase (Fig. 1). DXP is then converted through several yet-uncharacterized steps into 2-C-methyl d-erythritol 4-phosphate (9) by DXP reductoisomerase. Both DXP synthase and DXP reductoisomerase are highly conserved in evolution (16, 21, 22, 24, 29, 31, 32). Finally, 2-C-methyl d-erythritol 4-phosphate is transformed into IPP (10) through yet-unidentified intermediates involving a second phosphorylation step (Fig. 1), most likely catalyzed by the isopentenyl monophosphate kinase (IPK) cloned from Escherichia coli and peppermint (20). Another recent work establishes that only the bacterial strains in which IPP synthesis depends on the Rohmer pathway elicit γδ T-cell proliferation in vitro (15). First, the investigators show that in extracts from such bacteria, the IPP concentration does not reach the minimum required to activate γδ T cells (15). Then, they demonstrate that the stimulatory activity of these extracts should rather be attributed to one (or more) of the IPP precursors from the Rohmer pathway (15). Moreover, the recent elucidation of the structure of 3-formyl-1-butyl pyrophosphate, the moiety common to γδ-stimulating mycobacterial antigens, has most probably identified the natural ligand of Vγ9Vδ2 T cells in bacterial and parasitical infections (3). The origin of this 5-carbon, pyrophosphate-bearing metabolite can be attributed to several pathways: 3-formyl-1-butyl pyrophosphate could correspond to an IPP precursor expected in the last steps of the Rohmer pathway (3) (Fig. 1). It could also result from the phosphorylation of an IPP precursor by IPK, as the in vivo substrate specificity of this novel enzyme remains to be fully established (20). A recent publication by Jomaa et al. (16) also demonstrates that the second enzyme of the Rohmer pathway is conserved and fully functional in the eukaryotic parasite P. falciparum. As it is now well known that γδ T cells account for the strong immunological response observed in malarial infections (1), it is likely that malarial ligands for γδ T cells also rely on the Rohmer pathway of IPP synthesis.
Taken together, these lines of evidence shed a new light on the way human Vγ9Vδ2 T lymphocytes discriminate between infected cells and healthy cells.
γδ T cells recognize phosphoantigens in a rapid and direct fashion, which requires a high degree of specificity in order to control the safety of the response. Both the parasite and the host cell produce IPP through distinctive pathways of biosynthesis involving different phosphorylated precursors. By precisely discriminating these precursors, the γδ T-cell response is focused on the parasite. Some of these parasitic precursors are able to elicit an immune response at nanomolar concentrations, whereas the γδ T-cell response requires micromolar concentrations of the metabolic product IPP. It has been established that at the concentrations reached by the diverse natural phosphoantigens in bacterial extracts (15) and thus most certainly in living cells, only the Rohmer pathway metabolites and not IPP itself can elicit a γδ T-cell response. On the whole, it seems that the Vγ9Vδ2 T lymphocyte response to phosphoantigenic molecules in antiinfectious immunity obeys both qualitative and quantitative rules. These rules involve the discrimination of different metabolic routes and of different levels of antigen concentration. Therefore, targeting of the Vγ9Vδ2 T-cell response to phosphoantigen thresholds attained solely in proliferating pathogens significantly lowers the risk of autoimmunity. These new results also explain the current observation that γδ T lymphocytes exhibit a specific although broad reactivity to many intracellular pathogenic species distant in evolution. In fact, γδ T cells may have evolved to target a distinctive and vital metabolic route shared by these pathogens, regardless of their nature.
ACKNOWLEDGMENTS
This work was supported by institutional grants from INSERM, Programme APEX, Association pour la Recherche sur le Cancer, la Fondation pour la Recherche Médicale, la Région Midi-Pyrénées, and by grants from la Ligue Nationale Contre le Cancer to H.S.
REFERENCES
- 1.Behr C, Dubois P. Preferential expansion of Vγ9Vδ2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol. 1992;4:361–366. doi: 10.1093/intimm/4.3.361. [DOI] [PubMed] [Google Scholar]
- 2.Behr C, Poupot R, Peyrat M A, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie J J. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–2896. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmant C, Espinosa E, Poupot R, Peyrat M-A, Guiraud M, Poquet Y, Bonneville M, Fournie J-J. 3-Formyl-1-butyl pyrophosphate, a novel mycobacterial metabolite-activating human γδ T cells. J Biol Chem. 1999;274:32079–32084. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 4.Bender A, Heckl-Ostreicher B, Grondal E J, Kabelitz D. Clonal specificity of human γδ T cells: Vγ9+ T-cell clones frequently recognize Plasmodium falciparum merozoites, Mycobacterium tuberculosis, and group-A streptococci. Int Arch Allergy Immunol. 1993;100:12–18. doi: 10.1159/000236381. [DOI] [PubMed] [Google Scholar]
- 5.Bertotto A, Gerli R, Spinozzi F, Muscat C, Scalise F, Castellucci G, Sposito M, Candio F, Vaccaro R. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 6.Beytia E D, Porter J W. Biochemistry of polyisoprenoid biosynthesis. Annu Rev Biochem. 1976;45:113–142. doi: 10.1146/annurev.bi.45.070176.000553. [DOI] [PubMed] [Google Scholar]
- 7.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 8.De Libero G. Sentinel function of broadly reactive human γδ T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 9.Duvold T, Bravo J-M, Pale-Grosdemange C, Rohmer M. Biosynthesis of 2-C-methyl-d-erythritol, a putative C5 intermediate in the mevalonate independent pathway for isoprenoid biosynthesis. Tetrahedron Lett. 1997;38:4769–4772. [Google Scholar]
- 10.Duvold T, Calf P, Bravo J-M, Rohmer M. Incorporation of 2-C-methyl-d-erythritol, a putative isoprenoid precursor in the mevalonate-independent pathway, into ubiquinone and meanquinone of Escherichia coli. Tetrahedron Lett. 1997;38:6181–6184. [Google Scholar]
- 11.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk M H, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 12.Goerlich R, Hacker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparum merozoites primarily stimulate the Vγ9 subset of human γδ T cells. Eur J Immunol. 1991;21:2613–2616. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, et al. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Investig. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havlir D V, Ellner J J, Chervenak K A, Boom W H. Selective expansion of human γδ T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Investig. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jomaa H, Feurle J, Luhs K, Kunzmann V, Tony H P, Herderich M, Wilhelm M. Vγ9/Vδ2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-d-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–378. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 16.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler H K, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 17.Jouen-Beades F, Paris E, Dieulois C, Lemeland J F, Barre-Dezelus V, Marret S, Humbert G, Leroy J, Tron F. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julia M R, Serra P, Matamoros N, Raga S, Martinez P. Small cytoplasmic antigens from Pseudomonas aeruginosa stimulate γδ T lymphocytes. Scand J Immunol. 1998;48:672–678. doi: 10.1046/j.1365-3083.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 19.Kabelitz D, Bender A, Prospero T, Wesselborg S, Janssen O, Pechhold K. The primary response of human γδ+ T cells to Mycobacterium tuberculosis is restricted to Vγ9-bearing cells. J Exp Med. 1991;173:1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange B M, Croteau R. Isopentenyl diphosphate biosynthesis via a mevalonate-independent pathway: isopentenyl monophosphate kinase catalyzes the terminal enzymatic step. Proc Natl Acad Sci USA. 1999;96:13714–13719. doi: 10.1073/pnas.96.24.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange B M, Croteau R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: cloning and heterologous expression of 1-deoxy-d-xylulose-5-phosphate reductoisomerase from peppermint. Arch Biochem Biophys. 1999;365:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- 22.Lange B M, Wildung M R, McCaskill D, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenthaler H K, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- 24.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat M A, Bonneville M, Fournie J J, Sjostedt A. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 28.Russo D M, Armitage R J, Barral-Netto M, Barral A, Grabstein K H, Reed S G. Antigen-reactive γδ T cells in human leishmaniasis. J Immunol. 1993;151:3712–3718. [PubMed] [Google Scholar]
- 29.Schwender J, Muller C, Zeidler J, Lichtenthaler H K. Cloning and heterologous expression of a cDNA encoding 1-deoxy-d-xylulose-5-phosphate reductoisomerase of Arabidopsis thaliana. FEBS Lett. 1999;455:140–144. doi: 10.1016/s0014-5793(99)00849-2. [DOI] [PubMed] [Google Scholar]
- 30.Schwender J, Seemann M, Lichtenthaler H K, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprenger G A, Schorken U, Wiegert T, Grolle S, de Graaf A A, Taylor S V, Begley T P, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka Y, Morita C T, Nieves E, Brenner M B, Bloom B R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 34.Waterfall M, Black A, Riley E. γδ+ T cells preferentially respond to live rather than killed malaria parasites. Infect Immun. 1998;66:2393–2398. doi: 10.1128/iai.66.5.2393-2398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]