Abstract
Study Objectives
Shift sleep onset earlier and extend school-night sleep duration of adolescents.
Methods
Forty-six adolescents (14.5–17.9 years; 24 females) with habitual short sleep (≤7 h) and late bedtimes (≥23:00) on school nights slept as usual for 2 weeks (baseline). Then, there were three weekends and two sets of five weekdays in between. Circadian phase (Dim Light Melatonin Onset, DLMO) was measured in the laboratory on the first and third weekend. On weekdays, the “Intervention” group gradually advanced school-night bedtime (1 h earlier than baseline during week 1; 2 h earlier than baseline during week 2). Individualized evening time management plans (“Sleep RouTeen”) were developed to facilitate earlier bedtimes. On the second weekend, Intervention participants received bright light (~6000 lux; 2.5 h) on both mornings. A control group completed the first and third weekend but not the second. They slept as usual and had no evening time management plan. Weekday sleep onset time and duration were derived from actigraphy.
Results
Dim light melatonin onset (DLMO) advanced more in the Intervention (0.6 ± 0.8 h) compared to the Control (−0.1 ± 0.8 h) group. By week 2, the Intervention group fell asleep 1.5 ± 0.7 h earlier and sleep duration increased by 1.2 ± 0.7 h; sleep did not systematically change in the Control group.
Conclusions
This multi-pronged circadian-based intervention effectively increased school-night sleep duration for adolescents reporting chronic sleep restriction. Adolescents with early circadian phases may only need a time management plan, whereas those with later phases probably need both time management and morning bright light.
Clinical Trials
Teen School-Night Sleep Extension: An Intervention Targeting the Circadian System (#NCT04087603): https://clinicaltrials.gov/ct2/show/NCT04087603
Keywords: sleep, delayed sleep onset, adolescence, bright light, DLMO, time management, circadian rhythms, phase shifts, school start time
Graphical Abstract
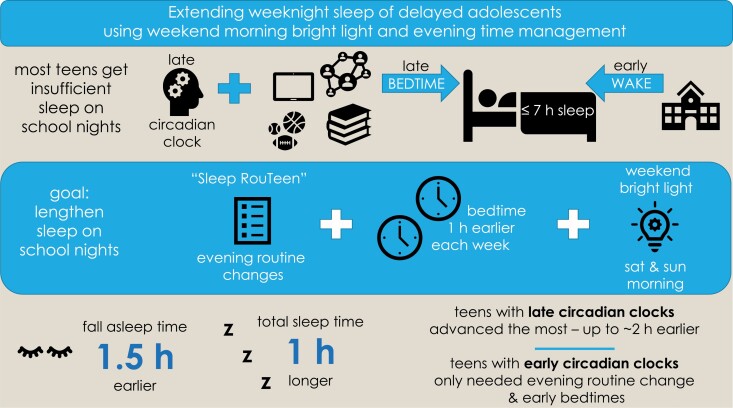
Graphical Abstract.
Statement of Significance.
This is the first test of an intervention that used a combination of short-term (2 days) morning bright light exposure, a gradual shift of bedtime to an earlier clock time, and a simple evening time-management strategy to increase sleep duration in adolescents who are chronically sleep deprived, getting less than 7 h of sleep on school nights. This combination of strategies appears effective and could be expanded and adapted to increase sleep duration in circadian-based sleep disorders, such as Delayed Sleep-Wake Phase Disorder which typically emerges during adolescence.
Introduction
The majority of adolescents obtain less than the current recommendation [1] of 8–10 h of sleep on school nights [2, 3]. In a comprehensive meta-analysis, Galland et al. [4] reported the pooled mean of nocturnal weekday total sleep time measured from actigraphy was 6.66 h in adolescents aged 15–18 years studied in the United States, Netherlands, and Australia. Despite getting insufficient sleep, and presumably carrying an increased pressure for sleep across the waking day, many adolescents do not fall asleep early. Bedtimes on school nights range from 10:30 to 11:30 pm [2, 4–11], and some report average bedtimes as late as midnight to 1:00 am [12–15].
Insufficient sleep during adolescence is the result of a combination of biological, psychosocial, and societal pressures that come together like a “Perfect Storm” as they compete with one another and impinge on sleep opportunity [16, 17]. Sleep regulatory mechanisms extend alertness late into the evening and night in adolescents. Laboratory studies show that adolescents of a more mature puberty stage have later onset and offset of melatonin secretion—markers of the central circadian clock—compared to less mature adolescents [18, 19] and similar findings of a circadian phase delay are reported in other pubertal animals [20–26]. Homeostatic sleep pressure also accumulates more slowly across a waking period in post-pubertal adolescents compared to their pre-pubertal peers [27], making it easier for adolescents to stay awake late as they get older. Finally, adolescents in their late teens stay awake later after the onset of their biological night (marked by the dim light melatonin onset [DLMO]) compared to younger adolescents [28]. Thus, not only are their circadian systems later, but they fall asleep later into their biological night.
In addition to biology favoring evening alertness, parental influence over bedtimes lessen [7, 9, 29, 30], and adolescents are able to and often choose to stay awake to engage in other activities besides sleep. After-school activities, including part-time work, homework, sports, and socializing contribute to delayed bedtimes on school nights [7, 31–34]. Spending time on screens, such as cell phones or tablets, can displace sleep, as well as stimulate alertness making sleep more difficult [35–37].
Unfortunately, late school-night sleep onset times are juxtaposed with early rise times on school-day mornings, making it difficult for adolescents to obtain sufficient sleep [38, 39]. Chronic partial sleep deprivation is especially common for adolescents who endorse late sleep timing, such as those who endorse an evening chronotype [40]. The conflict between biologically driven late sleep onset times and society-enforced early wake times also results in circadian misalignment (mistimed sleep relative to the circadian system) on school nights [41] and social jetlag [42, 43]. Insufficient and mis-timed sleep in adolescents is associated with a number of poor outcomes, including heightened anxiety [44], depressed mood [2, 9, 45, 46], poor emotion regulation [47], daytime sleepiness [41, 48–52], and poor academic performance [9, 53–55].
While efforts to delay school start times for middle and high schools are slowly gaining traction [38, 56–58], additional strategies are needed, particularly for adolescents who have difficulty falling asleep early enough to get the recommended amount of sleep on school nights. Shifting the circadian system earlier should facilitate adolescents’ ability to fall asleep earlier. The Phase Response Curve (PRC) to bright light for adolescents (14–17 years) shows that the largest phase advance shifts (advances move events earlier in time) are produced when bright light begins about 7–11 h after the DLMO (~1.5–3.5 h after habitual sleep midpoint) [59]. We found that appropriately timed bright light (2.5 h; ~6000 lux) on both mornings of one weekend can advance circadian phase of late and short-sleeping adolescents by about 1 h [60].
Previous studies examined changes to sleep behavior in response to morning bright light treatment over 4–6 weeks in adolescents who had difficulty going to bed and waking up early [61] or who were diagnosed with Delayed Sleep-Wake Phase Disorder (DSWPD) [62]. Gradisar et al. showed shortened self-reported sleep onset latency, earlier sleep onset, and increased total sleep time in adolescent patients diagnosed with DSWPD after Cognitive Behavioral Therapy (CBT) combined with morning bright light (0.5–2.0 h; 1000 lux from a light box or natural sunlight) administered over 3–6 weeks [62]. Kaplan et al. also reported shifting sleep onset about 50 min earlier and increasing nocturnal sleep by about 43 min in response to a passive light therapy (3-ms light flashes every 20 s in the final 2 h of habitual sleep) combined with CBT [61]. This latter study also showed that adolescents needed CBT to change their sleep behavior as it did not change in response to passive light therapy alone [61]. Unfortunately, circadian phase was not measured in either of these previous studies. Therefore, it remains unclear whether the circadian system shifted and whether change in circadian phase was the mechanism of action that changed sleep onset and sleep duration in these cohorts of late-sleeping adolescents. Moreover, morning bright light treatment occurred every day for several weeks in these previous studies. This may not be necessary as we reported circadian phase advances after 2 days of morning bright light [60].
The overall aim of this study was to advance sleep onset and therefore extend school-night sleep in adolescents who habitually reported late bedtimes and short sleep on school nights. A 2-week intervention targeted the circadian system two ways: (1) by gradually advancing bedtime (the beginning of the dark episode) over two school weeks; and (2) by using bright light exposure from light boxes on both mornings of the weekend occurring in the middle of the two school weeks. The intervention also included a behavioral component that targeted after-school and evening time use to facilitate the gradual advance of bedtime over 2 weeks. A “Sleep RouTeen” time-management plan was individually tailored to the teen with motivational supports during the 2 weeks. We hypothesized that the advance of the circadian clock, as measured by the DLMO, would be larger in the group assigned to the intervention compared to a control group that completed the exact same protocol, but was not given instruction about their sleep and time use, and was not exposed to bright light boxes. We also hypothesized that participants who were randomized to the Intervention group would be able to fall asleep earlier and therefore obtain more sleep on school nights by the end of the intervention compared to the control group. Daily subjective ratings of mood and alertness were also examined and predicted to improve in response to the intervention.
Methods
Participants
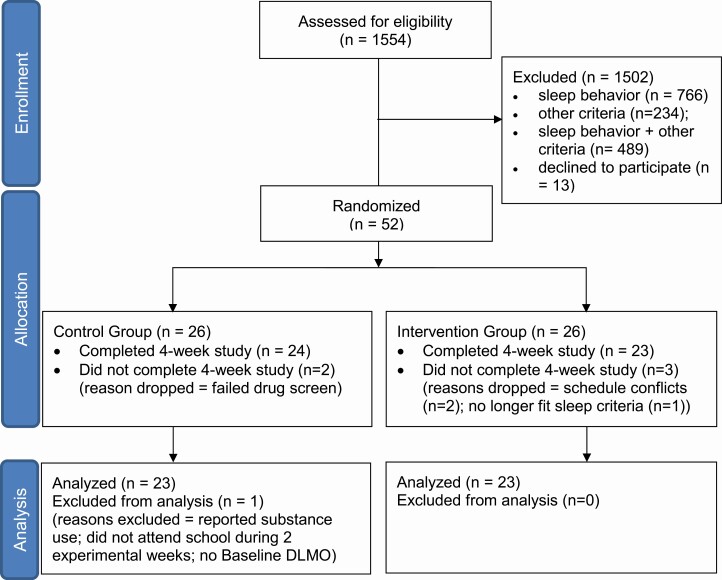
Figure 1 is a CONSORT diagram illustrating participant flow through the study. The intervention was designed to increase school-night sleep duration in adolescents who are chronically sleep restricted on school nights due to late bedtimes; therefore, we targeted healthy adolescents (14.0–17.9 years) who reported this behavioral sleep pattern and were enrolled in high school. Sleep behavior inclusion criteria were the following: average sleep duration ≤ 7 h on school nights, average school-night bedtime ≥ 23:00, average non-school night bedtime ≥ midnight, and average sleep duration ≥ 1 h longer on non-school nights than school days. The last criterion indicated that some recovery was needed from insufficient sleep on school nights [63]. Sleep behavior criteria were assessed from pre-study sleep logs collected before randomization (15 ± 3 nights), and then confirmed with wrist actigraphy during a 2-week baseline. Exclusion criteria, assessed by self- and parent/guardian-report were a history of a developmental disorder, psychotic disorder, bipolar disorder, neurological disorder, psychopathology, metabolic disorder, chronic medical condition, infectious illness, or sleep disorder (restless legs syndrome, periodic limb movement disorder, obstructive sleep apnea, or narcolepsy). Depressive symptoms can co-occur with late and short sleep [64–67]; therefore, elevated depressive symptoms (scores > 16 on the Center for Epidemiological Studies-Depression (CES-D) scale) [68] were not exclusionary. Participants were excluded, however, if they indicated a history of suicidal ideation. Participants were medication-free, except for two females who were taking an oral contraceptive. Participants did not work night shifts or travel beyond three time zones in the month before starting the study, and were not color blind or deficient as measured by the Ishihara Color Blindness test.
Figure 1.
CONSORT diagram illustrating participant flow of the study.
Participants were randomized to the Intervention group or the Control group using block randomization. Blocks of up to three participants completed the study at the same time. Blocks of participants were randomly assigned to the Intervention or Control group using a computer-generated random number list. We chose to run participants assigned to the same group together in the laboratory to avoid participants discussing the study protocol and differences in study instructions, which could identify group assignment. The words “control” and “intervention” were not used in any documents that participants read, and research staff did not use these words when speaking with participants or their families. Instead, one group was called “Team Mario” and the other group was called “Team Luigi.” Participants were paid the same dollar amount regardless of group assignment.
Table 1 shows participant demographics by group assignment. Circadian phase preference was measured with the Morningness Questionnaire of Smith et al. [69], which ranges in score from 13 (eveningness) to 55 (morningness). Mid-sleep on free days (corrected for weekend oversleep) and social jetlag (difference between midpoint of sleep on free days and non-free days) was assessed using the Munich Chronotype Questionnaire [70]. Participants self-reported their race/ancestry by choosing one of the following: White/Caucasian, Asian/Asian-American, Native American/Alaskan Native, Native Hawaiian/Pacific Islander, Black/African-American, multiracial, or specified another race/ancestry. They also self-reported their ethnicity by choosing either Not Hispanic/Latino or Hispanic/Latino. Adolescent reports of race and ethnicity were confirmed by a parent using the same response categories.
Table 1.
Participant demographics for analytic sample
| Control | Intervention | |
|---|---|---|
| N | 23 | 23 |
| Age (years) | 16.5 ± 0.8 | 16.0 ± 0.8 |
| (14.5–17.9) | (15.2–17.9) | |
| Sex (N) | ||
| Females | 13 | 11 |
| Males | 10 | 12 |
| Ancestry (N) | ||
| African-American | 6 | 12 |
| White | 14 | 6 |
| Multiple | 3 | 2 |
| Other | 0 | 3 |
| Ethnicity (N) | ||
| Non-Hispanic | 12 | 17 |
| Hispanic | 11 | 6 |
| High school grade (N) | ||
| 9th | 3 | 1 |
| 10th | 9 | 9 |
| 11th | 8 | 10 |
| 12th | 3 | 3 |
| Leave home for school (clock time) | ||
| Mean ± SD | 07:10 ± 00:40 | 07:03 ± 00:23 |
| (earliest–latest) | (05:50–07:50) | (06:30–07:45) |
| School start (clock time) | ||
| Mean ± SD | 07:47 ± 00:39 | 07:52 ± 00:20 |
| (earliest–latest) | (06:30–09:30) | (07:00–08:25) |
| Morningness scorea | 30.9 ± 4.6 | 32.4 ± 6.3 |
| Mid-sleep on free days (MSFsc)b,c | 05:08 ± 01:14 | 04:48 ± 01:37 |
| Mid-sleep on school daysb | 03:12 ± 00:26 | 03:06 ± 00:30 |
| Social jetlag (h)b,c,d | 3.1 ± 0.9 | 2.9 ± 1.2 |
| Depressive symptomse | 8.5 ± 5.4 | 11.3 ± 5.9 |
aSmith Morningness Questionnaire.
bMunich ChronoType Questionnaire (MCTQ).
cOnly those without alarm use on free days were included (N = 18 in Control group; N = 21 in Intervention group).
dSocial jet lag = the difference between mid-sleep on free days and mid-sleep on school days.
eCenter for Epidemiologic Studies Depression (CES-D) Scale.
The study was approved by the Rush University Medical Center’s Institutional Review Board, in compliance with the Declaration of Helsinki. A parent of the participant provided written consent for the child to participate in the study, and the adolescent cosigned the consent form to acknowledge assent. The study was registered on clinicaltrials.gov (NCT#04087603).
Study protocol
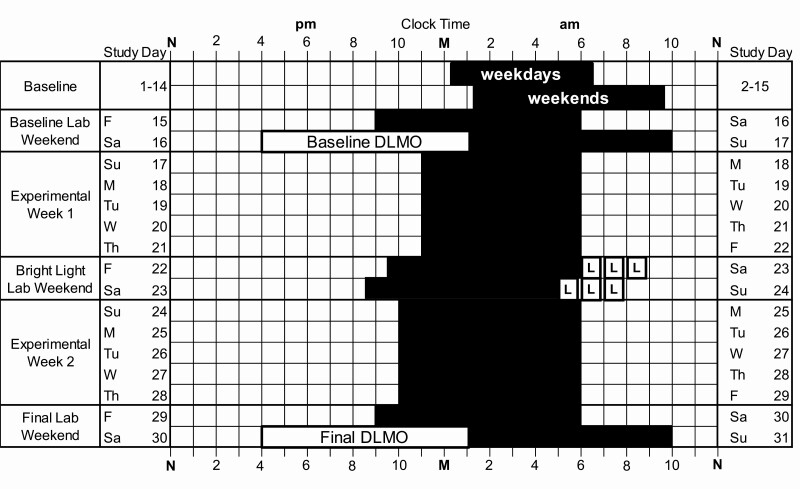
Adolescents completed the 1-month protocol illustrated in Figure 2 during the U.S. school year between January 2017 and May 2019. All participants slept at home on their usual sleep schedule for the first 2 weeks of the study (baseline) and then lived in the lab for a weekend (days 15–17; Baseline Lab Weekend). On Friday night (day 15), participants were given a 9-h sleep opportunity timed to end at their habitual school-day wake-up time. The 9-h duration was designed to provide an opportunity for sufficient sleep, and the fixed school-day wake time was designed to maintain a similar wake time to the previous week before assessing circadian phase in the laboratory. On Saturday (day 16), participants completed a circadian phase assessment (see below) to determine their Baseline DLMO, the most reliable marker of the central circadian clock [71, 72]. Participants ended their baseline phase assessment at their average baseline non-school night bedtime (to the nearest hour or half-hour clock time) and were put to bed within 15 min of the last saliva sample. On Sunday morning (day 17), participants were awakened 9 h later.
Figure 2.
Example protocol for intervention participants. M, midnight; N, noon. Black rectangles illustrate dark/sleep. Participants had two baseline weeks of usual sleep at home (days 1–14). Average baseline sleep onset and wake-up times on weekday and weekend nights are shown. Then, participants lived in the laboratory for a weekend (Baseline Lab Weekend). On Friday–Saturday (study days 15–16), a 9-h sleep opportunity was timed to end at the individual’s average school-day wake-up time. Dim light melatonin onset (DLMO) was measured on Saturday (day 16) via serial saliva sampling every 30 min starting at 1600 and ending at the individual’s average weekend bedtime (± 30 min). Participants were given a 9-h sleep opportunity in the laboratory after the DLMO phase assessment. During Experimental Week 1 (days 17–21), participants in the intervention group were instructed to go to bed and try to fall asleep 1 h earlier than their average baseline school-night sleep onset. Individualized behavioral goals (“Sleep RouTeen”) were identified for each participant to help them manage their time in the evening and facilitate earlier bedtimes during this week. Then, intervention participants lived in the laboratory for another weekend (Bright Light Lab Weekend, days 22–24) to receive 2.5 h intermittent bright light (three 50-min exposures with 10-min breaks between) from light boxes (~6000 lux) on both mornings to advance circadian rhythms. During Experimental Week 2 (days 24–28), assigned bedtime was shifted an additional 1 h earlier than Experimental Week 1 and the “Sleep RouTeen” behavioral goals continued to be reinforced. A Final Lab Weekend occurred on days 29–31 and was the same as the Baseline Lab Weekend. Control participants completed the same protocol as the intervention participants, except they continued to sleep as usual at home for both Experimental Weeks, did not receive the “Sleep RouTeen” behavioral goals, and did not participate in the Bright Light Lab Weekend.
Participants in the Intervention group completed a brief (~20-min) meeting with a clinical psychologist (JAC) or study coordinator (SLV) to discuss and set their “Sleep RouTeen” time management goals (see below) before leaving the laboratory on Sunday (day 17). These time management goals were designed to facilitate earlier sleep onset times as the Intervention group was asked to gradually advance the time that they got into bed to try to fall asleep (assigned bedtime) over Experimental Week 1 (days 17–21) and Experimental Week 2 (days 24–28). During Experimental Week 1, intervention participants were instructed to go to bed and try to fall asleep 1 h before their average baseline school-night sleep onset time. During Experimental Week 2, they were instructed to go to bed and try to fall asleep 2 h before their average baseline school-night sleep onset time. Assigned bedtimes for both weeks were set to the nearest hour or half hour clock time. In the example shown in Figure 2, assigned bedtime is at 22:00 during Experimental Week 2, but assigned bedtimes varied between participants. Scheduled wake-up was set to the participant’s average school-day wake-up time (to the nearest :00, :15, or :30).
After Experimental Week 1, intervention participants completed the Bright Light Lab Weekend (Days 22–24 in Figure 2), where morning bright light and a gradual shift of sleep/dark was timed to phase advance rhythms over a weekend based on our previous work [60]. The weekend phase advancing protocol was timed for each individual using their average sleep times derived from wrist actigraphy for the first 11 baseline nights (actigraphy is described below). On Saturday morning (day 23), participants were awakened 2 h (± 30 min) after their average baseline mid-sleep time, which corresponds to the phase advancing portion of the adolescent PRC to bright light [59]. Two light boxes (EnergyLight, Philips Consumer Lifestyle, Drachten, The Netherlands; 5000 K; screen size of 33 × 47 cm) were turned on within 5 min of waking. The spectral power distribution of the EnergyLights is shown in Figure 2 of our previous report [60]. The two light boxes were positioned on both sides of the computer monitor, facing in toward the participant. The center of the light box screens was 37 ± 3 cm from participants’ eyes. Study staff measured illuminance at the level of both eyes with an illuminance meter (ExTech EasyView 33, Extech Instruments, Waltham MA) every 15–20 min during morning bright light. Illuminance measured in the angle of gaze was 5949 ± 157 lux. On Sunday morning (day 24), participants were awakened 1 h earlier than on Saturday morning (day 23). Figure 2 shows an example of an adolescent with a mid-sleep time of 04:00; therefore, Saturday morning wake-up time was 06:00 and Sunday morning wake-up time was 05:00 in this example. Scheduled times varied among participants; Saturday morning wake-up times ranged from 04:30 to 09:00. Participants did not have access to their cell phones during the morning light sessions; however, they were allowed to watch movies or TV shows, access social media, or complete homework on our desktop computers set up between the light boxes.
Participants in both groups (Intervention and Control) completed a Final Lab Weekend (days 29–31), which was identical to the Baseline Lab Weekend described above. During weekends in the laboratory, participants lived in private bedrooms. Overhead bedroom lights were controlled by study staff from a control room. Illuminance averaged 29.4 ± 9.5 lux at the angle of gaze when participants were awake and bright light boxes were off. Bedrooms were dark (0 lux) during scheduled sleep times.
DLMO circadian phase assessments
During the circadian phase assessments, salivary melatonin concentration was measured from approximately 2 mL of saliva collected every 30 min using Salivettes (Sarstedt, Nümbrecht, Germany). Participants remained awake in dim light (<5 lux) sitting in recliners, except when they needed to use the attached washroom (also <5 lux). They were not allowed to eat or drink in the 10 min before each sample and washroom trips were not allowed during this time. Saliva samples were centrifuged immediately after collection and frozen. These samples were later radioimmunoassayed (RIA) for melatonin concentration using commercially available kits (Bühlmann Laboratories AG, Schönenbuch, Switzerland) by SolidPhase, Inc (Portland, ME). Each individual’s samples were analyzed in the same batch. The manufacturer reports that the analytic sensitivity (limit of detection) of the assay is 0.9 pg/mL. Intra-assay coefficients of variation for low (daytime), medium (evening), and high (nighttime) levels of salivary melatonin are 20.1%, 4.1%, and 4.8%, respectively. The inter-assay coefficients of variation for low, medium, and high levels of salivary melatonin are 16.7%, 6.6%, and 8.4%, respectively. DLMO phase, expressed in 24-h clock time, was determined by linear interpolation across the time points before and after the melatonin concentration increased to and stayed above 4 pg/mL [18, 73]. DLMO phase shift was computed as the difference between Baseline and Final DLMO, with positive numbers indicating a phase advance.
Actigraphic sleep
Participants in both groups wore an actigraph (Actiwatch Spectrum, Philips Respironics, Inc., Bend Oregon, United States) on their non-dominant wrist and completed daily sleep logs to record the time they got into bed, the time they tried to fall asleep, wake time, and sleep disturbances. They also telephoned daily to a time-stamped voicemail messaging system at bedtime and wake time. Participants visited the lab every 2–3 days so that we could download the actigraphy data and review sleep logs with them; participants were questioned about any inconsistencies between the actogram and sleep logs.
Actigraphy data were collected in 1-min epochs. The low wake threshold and the sleep epochs sleep interval detection algorithm in Actiware 6 (version 6.0.9, Philips Respironics, Inc., Bend Oregon, United States) were used to determine sleep and wake. In a comprehensive validation study against PSG, Meltzer et al. [74] showed that the low sensitivity is the best threshold for healthy adolescents aged 13–18 years. Each sleep episode was manually inspected within a rest interval beginning 15 min before the participants’ reported try to fall asleep time and ending 15 min after their reported wake up time on their daily sleep log. The first of three consecutive 1-min epochs of sleep defined sleep onset and the first epoch after the last five consecutive 1-min epochs of sleep defined wake-up time [75]. The following variables were derived separately for weekdays and weekends: sleep onset time, wake-up time, total sleep time (total hours of sleep scored by the program between sleep onset and wake-up time), duration (total hours between sleep onset and wake-up time), and sleep efficiency (total sleep time divided by duration × 100). Sleep onset latency (minutes between self-reported try to fall asleep time from daily sleep logs and actigraphic sleep onset time) was also computed for weekdays and weekends.
Sleep RouTeen and motivational supports
For the Intervention group, research staff met one-on-one with participants to collaboratively develop an afternoon/evening time management plan we named “Sleep RouTeen” on Sunday, day 17 (Figure 2). The goal of the Sleep RouTeen was to help each teen organize their afternoon/evening schedule so that they could go to bed at the prescribed earlier time during Experimental Week 1 (1 h earlier than baseline) and Experimental Week 2 (2 h earlier than baseline). After reviewing their assigned bedtime, participants were asked about perceived barriers to going to bed by this time. Then, participants described their typical afternoon and evening with approximate time durations for each activity, and were asked what they could change to get to bed on time. Two specific goals were developed together with the teen and recorded on a personalized Sleep RouTeen score card that was reviewed with participants at follow-up visits (days 20 and 27 when actigraphy data were downloaded; see Figure 2). Examples of personal goals were: “set alarm on phone to stop socializing at 9:30 pm” and “use study hall time during school to work on homework.” This initial goal-setting meeting was about 20 min. Scripted motivational interviewing techniques to reinforce goals during follow-up visits on days 20 and 27 were implemented as necessary. The Sleep RouTeen goals were reviewed and adjusted as needed after Experimental Week 1 to accommodate the additional 1-h shift in assigned bedtime during Experimental Week 2. The Control group was not given a time management plan or any behavioral goals, but completed the same follow-up visits at the lab to download their actigaphy data on days 20 and 27. Staff gave Control participants study reminders, feedback, and encouragement on completing study procedures at home.
Daily sleepiness and mood
Throughout the study, participants in both groups rated their overall sleepiness and mood during the day before going to bed. Nine word pairs were anchored on a 1–10 point scale, and included the following: happy-sad, tense-relaxed, sleepy-alert, even tempered-mood swings, irritable-easy going, poor concentration-good concentration, tired-energetic, worried-care free, and calm-jittery. Each morning, participants were also asked to rate how difficult it was to wake up (very easy = 1 to very hard = 10) and how alert they felt (wide awake = 1 to very sleepy = 10). Participants were instructed to complete the bedtime scales within 10 min before going to bed and the morning scales within 10 min after waking each day.
Statistical analyses
Distributions of the main outcomes were first inspected to verify assumptions of normality and heterogeneity of variance between groups; these assumptions were met.
Hypothesis 1: The main outcomes to assess changes to circadian phase in the Intervention group compared to the Control group were Baseline DLMO and Final DLMO. A repeated measures analysis of variance tested our hypothesis that DLMO phase advances would be larger in the Intervention group compared to the Control group. Time was the with-in subjects factor (Baseline vs. Final DLMO) and group (Intervention vs. Control) was the between subjects factor.
Hypothesis 2: The main outcomes to test whether adolescents were able to fall asleep earlier and obtain more sleep during a school week were sleep onset time, total sleep time, and sleep duration (interval from sleep onset to final awakening) derived from actigraphy. Weeknight sleep onset, total sleep time, and sleep duration were averaged for each individual during Baseline (Sunday–Thursday nights only), Experimental Week 1 (days 17–21), and Experimental Week 2 (days 24–28). A repeated measures analysis of variance tested our hypothesis that sleep onset would shift earlier, and sleep duration and total sleep time would lengthen during the school week in the Intervention group compared to the Control group. Time was the repeated factor (baseline vs. Experimental Week 1 vs. Experimental Week 2) and Group was the between subjects factor (Intervention vs. Control).
Hypothesis 3: The main outcomes to test whether adolescents reported improvements in daily mood and alertness were derived from scales completed in the evening each weekday before going to bed (happy, tense, sleepy, even tempered, irritable, concentration, tired, worried, and calm) and each morning at waking (morning alertness, difficulty waking). Aggregated means for each participant were derived from weekdays during Baseline (Monday–Friday) and days 25–29 of Experimental Week 2. A repeated measures analysis of variance tested our hypothesis that daily mood and alertness would improve in the Intervention group compared to the Control group. Time was the repeated factor (Baseline vs. Experimental Week 2) and Group was the between subjects factor (Intervention vs. Control).
For all models, the interaction term was the critical statistic to test our hypotheses. For any significant interaction term, post hoc independent samples t-tests followed to determine group differences. Statistical significance was defined as p < .05 (two-tailed). Change scores from baseline for all outcomes were computed for ease of interpretation and graphing. Effect sizes (Cohen’s d) were computed on change from baseline scores to evaluate group differences; effects sizes were evaluated with the standard descriptors (small = 0.2, medium = 0.5, and large = 0.8). Sex differences in main outcomes at baseline and changes from baseline were tested, but not observed. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., United States).
Results
Participants
A total of 47 participants completed the study (see Figure 1). One male participant in the Control group was excluded from the analysis because there was no clear rise in his baseline melatonin profile. He reported using substances (alcohol and cannabidiol) during the study. He also did not attend school during Experimental Weeks 1 and 2, making his sleep unrepresentative of his usual school-week sleep for a valid comparison to the Intervention group. Five participants discontinued the study for various reasons noted in Figure 1. Therefore, a total of 23 participants (13 females) were included in the Control group and 23 participants (11 females) were included in the Intervention group for analysis (Table 1). The analytic sample (N = 46) and the excluded sample (n = 6) were similar with respect to age (16.5 ± 0.8 vs. 16.7 ± 1.5 years), morningness score (31.6 ± 5.5 vs. 32.0 ± 4.9), MSFsc (04:57 ± 01:24 vs. 05:06 ± 00:38), social jetlag (3.0 ± 1.1 vs. 2.3 ± 0.4 h), and depressive symptoms on the CES-D scale (9.8 ± 5.8 vs. 11.7 ± 7.1).
Two participants in the Intervention group completed the study during the transition from daylight savings time to standard time (November 2017). The time change occurred on Day 17 (Sunday morning of Baseline Lab Weekend). Baseline DLMOs for these two participants were collected in DST and were adjusted to standard time (CST) to compute phase shifts. Baseline sleep was also adjusted to CST for analysis to match the remainder of the days collected in CST.
Three participants in the Control group complete the study during the change from standard time to daylight savings time (March 2017). The transition from standard time to daylight savings time occurred on Day 10 of baseline (see Figure 2). We did not have to adjust DLMO data as both DLMOs were computed in daylight savings time and the baseline DLMO was computed 6 days after the change from standard time to daylight savings time. This should be enough time for the circadian system to adjust. For the analysis, sleep data collected during standard time (days 1–9) were adjusted to daylight savings time to match the remainder of the days collected in daylight savings time.
Circadian phase and phase shifts
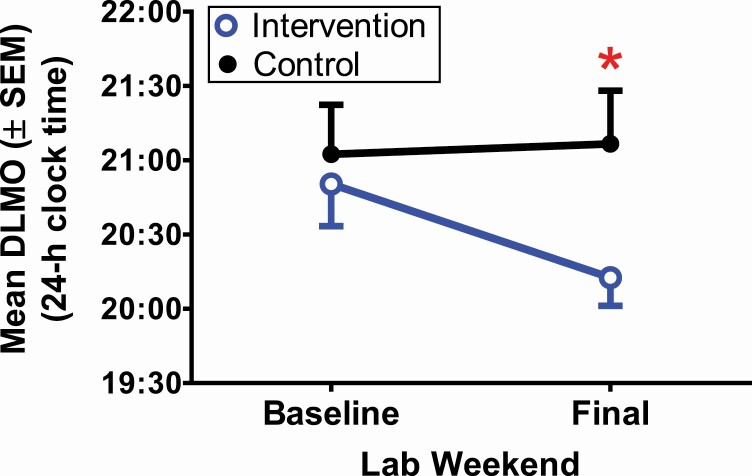
Figure 3 and Table 2 show DLMO and changes in DLMO after the two Experimental Weeks for both groups. DLMO did not differ between the Intervention and Control groups at baseline (t(44) = −0.18, p = .86). There was a significant time-by-group interaction (F(1,44) = 8.16, p = .007) such that DLMO advanced in the Intervention group, but did not systematically change, on average, in the Control group. The group effect size of DLMO phase shift was large (d = 0.9). By the end of the 2 weeks, the Final DLMO was 54 min earlier, on average, in the Intervention group compared to the Control group (t(44) = 2.2 p = .03).
Figure 3.
Circadian phase (DLMO) averaged for the intervention group (blue open circle) and control group (black closed circle). *p < .05.
Table 2.
DLMO, DLMO phase shift, and DLMO phase angles of entrainment (mean ± SD)
| Control | Intervention | |
|---|---|---|
| Baseline DLMO (day 16) | 21:06 ± 1:36 | 20:50 ± 1:22 |
| Final DLMO (day 30)* | 21:07 ± 1:43 | 20:13 ± 0:55 |
| DLMO Phase Shift (h)** | −0.1 ± 0.8 | 0.6 ± 0.8 |
| Baseline DLMO→Baseline sleep onset time (h) | 3.1 ± 1.4 | 3.2 ± 1.1 |
| Final DLMO→Experimental Week 2 sleep onset time (h) * | 3.1 ± 1.1 | 2.5 ± 0.9 |
| Baseline DLMO→Baseline wake-up time (h) | 9.2 ± 1.7 | 9.2 ± 1.4 |
| Final DLMO→Experimental Week 2 wake-up time (h) | 9.6 ± 1.8 | 9.9 ± 0.9 |
*p < .05.
** p < .01.
Morning bright light exposure was timed with respect to the individual’s midpoint of sleep during baseline. To verify that this approach timed bright light at the appropriate circadian phase, we computed post hoc the time interval between Baseline DLMO and wake-up time on Saturday morning (day 23), which is when the Intervention group was first exposed to the bright light boxes. Bright light started 7.3–10.8 h after Baseline DLMO (mean ± SD = 9.5 ± 1.0 h), which overlaps with the portion of the PRC that produces the largest phase advances according to our PRC to bright light constructed from adolescents [59].
Weekday sleep
Sleep measured from wrist actigraphy during Baseline reflects the entry criteria of the study, which aimed to include adolescents who fall asleep late (≥ 23:00) and obtain insufficient sleep (≤ 7 h) during the school week (Table 3). Average weekday sleep onsets, wake-up times, total sleep times, durations, sleep onset latencies, and sleep efficiencies did not differ between the Intervention and Control groups at baseline.
Table 3.
Weekday (Sundays–Thursdays) sleep from wrist actigraphy (mean ± SD)
| Control | Intervention | |
|---|---|---|
| N | 23 | 23 |
| Baseline (days 3–7 and 10–14) | ||
| Sleep onset time | 00:14 ± 00:51 | 00:13 ± 00:50 |
| Wake-up time | 06:35 ± 00:36 | 06:27 ± 00:44 |
| Total sleep time (h) | 5.3 ± 0.7 | 5.3 ± 0.7 |
| Duration (h) a | 6.4 ± 0.8 | 6.2 ± 0.8 |
| Sleep efficiency (%) | 75 ± 5 | 76 ± 6 |
| Sleep onset latency (min) b | 6.7 ± 9.9 | 8.5 ± 12 |
| Experimental Week 1 (days 17–21) | ||
| Sleep onset time | 00:01 ± 00:45 | 23:28 ± 00:52* |
| Wake-up time | 06:20 ± 00:39 | 06:05 ± 00:40 |
| Total sleep time (h) | 5.3 ± 0.7 | 5.6 ± 0.8 |
| Duration (h) a | 6.3 ± 0.9 | 6.6 ± 0.9 |
| Sleep efficiency (%) | 75 ± 6 | 76 ± 6 |
| Sleep onset latency (min) b | 6.3 ± 14.5 | 12.6 ± 14.1 |
| Experimental Week 2 (days 24–28) | ||
| Sleep onset time | 00:15 ± 01:01 | 22:41 ± 01:05*** |
| Wake-up time | 06:45 ± 01:09 | 06:09 ± 00:44* |
| Total sleep time (h) | 5.3 ± 0.8 | 6.3 ± 1.0** |
| Duration (h)a | 6.3 ± 1.1 | 7.5 ± 1.1** |
| Sleep efficiency (%) | 75 ± 6 | 75 ± 6 |
| Sleep onset latency (min)b | 8.9 ± 29.9 | 20 ± 19.1 |
Differences between Control and Intervention:
*p < .05;
** p < .01;
*** p < .001.
ainterval from sleep onset to final wake.
binterval from diary-reported try to fall asleep time to actigraphically scored sleep onset.
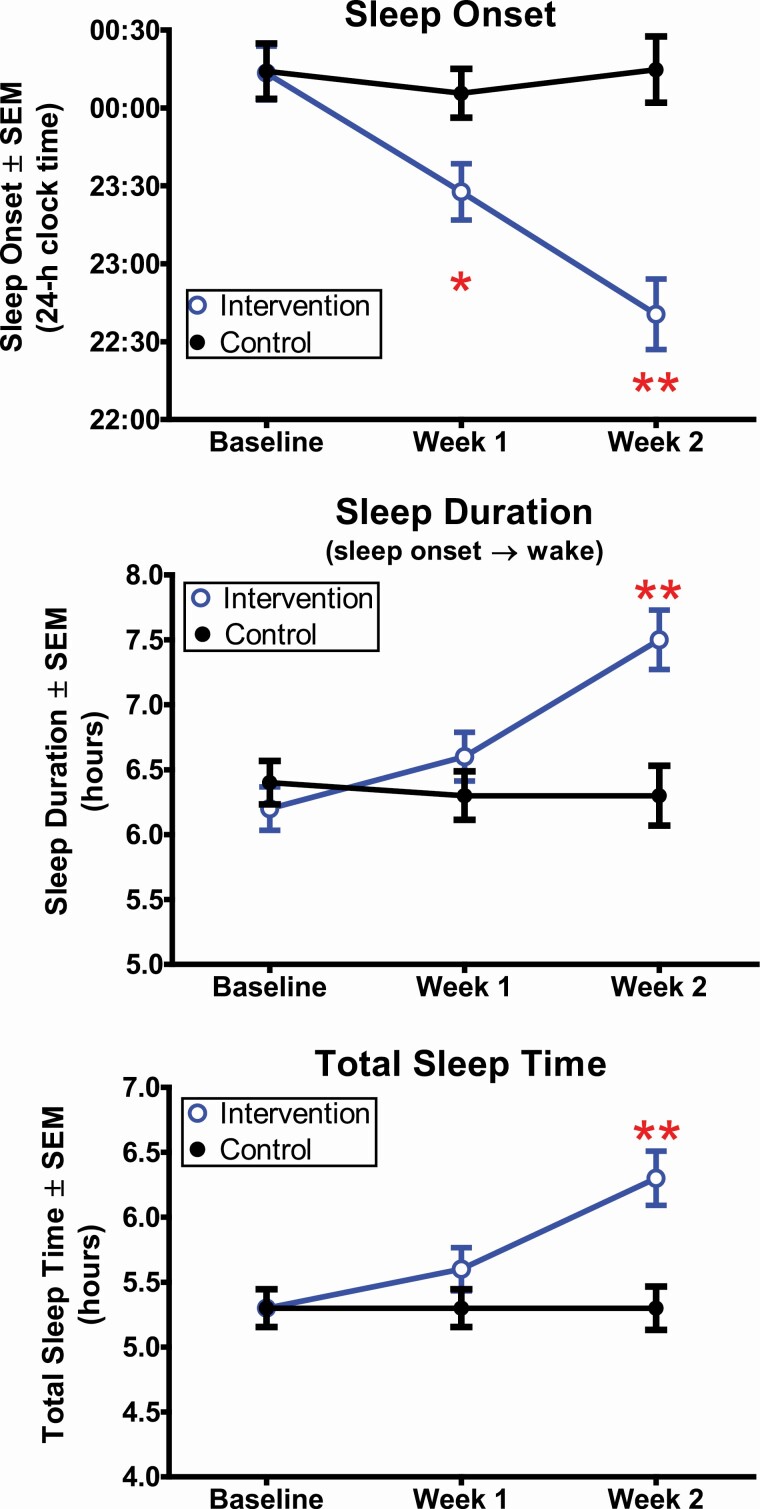
Figure 4 shows how weekday sleep of the Intervention group improved compared to the Control group. There was a significant time-by-group interaction for sleep onset time (F(2,88) = 23.2, p < .001). Participants in the Intervention group fell asleep 0.8 ± 0.5 h earlier during Experimental Week 1 and 1.5 ± 0.7 h earlier during Experimental Week 2 compared to their baseline. By contrast, sleep onset time for participants in the Control group did not systematically change, on average. Sleep onset time was significantly earlier in the Intervention group compared to the Control group during Experimental Weeks 1 (t(44) = −2.3, p = .03) and 2 (t(44) = −5.1, p < .001). The difference between groups in sleep onset shift from Baseline to Experimental Week 2 was large (d = 1.8). Assigned bedtime (prescribed time to turn lights out and try to fall asleep) for the Intervention group ranged from 22:00 to 01:00 for Experimental Week 1, and self-reported try to fall asleep time from daily sleep logs was 23:15 ± 00:52, on average. During Experimental Week 2, assigned bedtime ranged from 21:00 to 00:00 and participants reported trying to fall asleep at 22:21 ± 00:57, on average.
Figure 4.
Weekday sleep measured from wrist actigraphy for the Intervention group (blue open circles) and the control group (black closed circles) during Baseline, Experimental Week 1, and Experimental Week 2. *p < .05; **p < .001.
Sleep duration (F(2,88) = 15.9, p < .001) and total sleep time (F(2,88) = 17.2, p < 0.001) also showed a time-by-group interaction (Figure 4). The Intervention group showed a gradual increase in sleep duration and total sleep time across Experimental Weeks 1 and 2. By Week 2, sleep duration increased by 1.2 ± 0.7 h and total sleep time increased by 1.0 ± 0.6 compared to baseline. Sleep duration and total sleep time in the Intervention group were longer than in the Control group during Experimental Week 2 (duration: t(44) = 3.6, p = .001; total sleep time: t(44) = 3.6, p = 0.001). The difference between groups in sleep duration change (d = 1.6) and total sleep time change (d = 1.6) from Baseline to Experimental Week 2 were large. Changes to weekday sleep efficiency (F(2,88) = 0.5, p = .63) and sleep onset latency (F(2,88) = 1.2, p = .31) across the 2-week intervention did not differ between groups (Table 3).
Sleep onset time, wake-up time, total sleep time, sleep duration, sleep onset latency, and sleep efficiency for both groups during the weekends are provided in Supplementary Table S1 for descriptive purposes.
Individual responses to the intervention
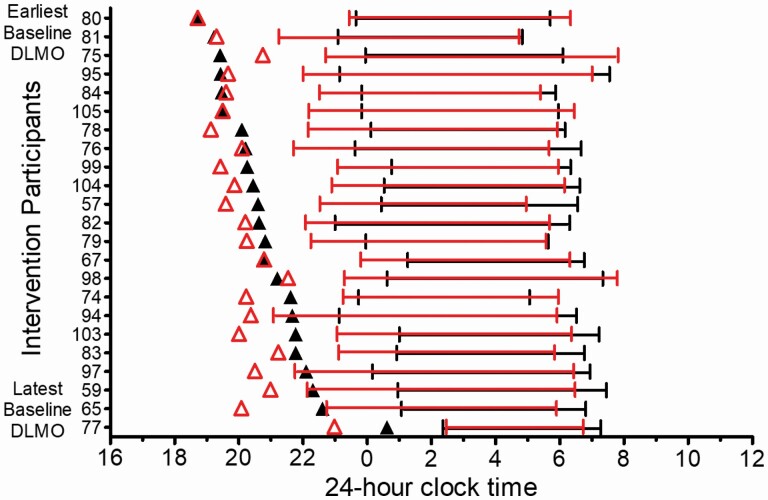
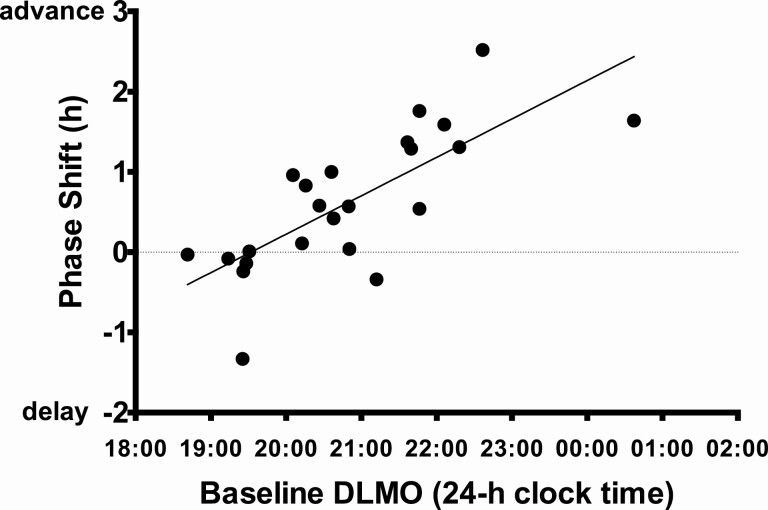
DLMO phase shifts and sleep onset shifts varied in responses to the 2-week intervention. Figure 5 illustrates DLMOs (triangles) and weekday sleep measured from actigraphy (lines) for each intervention participant with the earliest Baseline DLMO on the top and the latest Baseline DLMO on the bottom. Participants with the earliest Baseline DLMOs showed little change in DLMO timing and DLMO of one participant delayed (75 in Figure 5). Figure 6 illustrates that participants with the latest DLMOs showed large DLMO phase advances; baseline DLMO and phase shift of DLMO were correlated (r = .75, p < .001). All participants, except two (80 and 77 in Figure 5) fell asleep earlier during Experimental Week 2 (after the weekend of morning bright light exposure) compared to baseline. However, DLMO phase shift and sleep onset shift were not correlated (r = .20, p = .37). Assigned bedtime during Experimental Week 2 occurred 0.6–3.6 h after the time of the final DLMO.
Figure 5.
DLMOs (black triangles = baseline DLMO; red triangles = final DLMO) and weekday sleep timing (bars = sleep onset to wake-up times measured from actigraphy on Sunday—Thursday nights) for the Intervention group. Baseline sleep = black bars; Experimental Week 2 sleep = red bars. Participants are arranged on the y-axis with the earliest baseline DLMO on the top and the latest baseline DLMO on the bottom.
Figure 6.
Later baseline DLMOs were associated with larger phase advance shifts by the end of the 2-week intervention in the Intervention group. A linear regression line is fit to these data.
Mood ratings
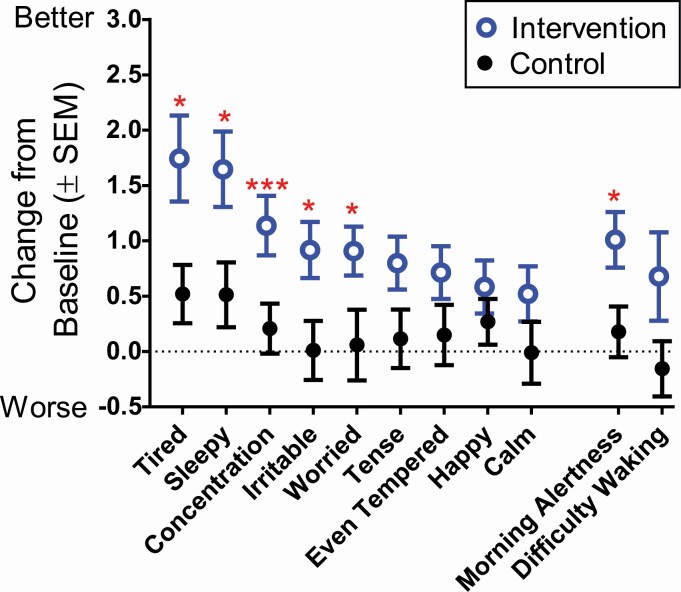
Daily subjective mood and sleepiness ratings during Experimental Week 2 are shown as mean changes from baseline in Figure 7. Baseline and Experimental Week 2 means, group-by-time interactions statistics, and effects sizes are summarized in Table 4. Compared to the Control group, the Intervention group showed greater improvements in daytime energy (were less tired), alertness (were less sleepy), concentration, irritability, and worry by Experimental Week 2 (Figure 7). The Control group showed no systematic change from baseline in these daytime measures. Ratings of morning alertness also showed greater improvement in the Intervention group compared to the Control group, but change in difficulty waking in the morning was not statistically significant (Figure 7 and Table 4).
Figure 7.
Change in weekday mood and sleepiness for the Intervention group (blue open symbols) and Control group (black closed symbols). Change from baseline = Experimental Week 2—Baseline; the inverse score was plotted for irritable, sleepy, tense, tired, and worried so that better subjective feelings are always positive numbers. Change from baseline group differences: *p < .05; **p < .01; ***p < .001.
Table 4.
Weekday subjective mood and sleepiness (means ± SD) during baseline and experimental week 2
| Baseline (study days 1–14) |
Experimental Week 2 (study days 25–29) |
Change from baseline | ||||
|---|---|---|---|---|---|---|
| Control (N = 23) |
Intervention (N = 23) |
Control (N = 23) |
Intervention (N = 23) |
Group × Time Interaction | Group effect size (d) | |
| Tired | 4.6 ± 1.3 | 5.3 ± 1.7 | 4.1 ± 1.6 | 3.6 ± 1.9 | F(1,44) = 6.8, p = .01 | 0.8 |
| Sleepy | 4.5 ± 1.4 | 5.1 ± 1.7 | 4.0 ± 1.6 | 3.5 ± 1.9 | F(1,44) = 6.4, p = .02 | 0.7 |
| Concentration | 7.3 ± 1.1 | 7.0 ± 1.7 | 7.1 ± 1.6 | 8.1 ± 1.6 | F(1,44) = 14.6, p < .001 | 1.1 |
| Irritable | 3.5 ± 1.1 | 3.6 ± 1.7 | 3.5 ± 1.7 | 2.7 ± 1.4 | F(1,44) = 6.1, p = .02 | 0.7 |
| Worried | 3.8 ± 1.3 | 4.1 ± 1.6 | 3.9 ± 1.8 | 3.2 ± 1.5 | F(1,44) = 6.2, p = .02 | 0.7 |
| Tense | 3.9 ± 1.4 | 3.9 ± 1.6 | 3.7 ± 1.8 | 3.1 ± 1.8 | F(1,44) = 3.7, p = .06 | 0.6 |
| Even tempered | 7.1 ± 1.5 | 7.6 ± 1.7 | 7.2 ± 1.6 | 8.3 ± 1.4 | F(1,44) = 2.4, p = .13 | 0.5 |
| Happy | 7.8 ± 1.3 | 7.5 ± 1.6 | 8.0 ± 1.2 | 8.1 ± 1.3 | F(1,44) = 1.0, p = .32 | 0.3 |
| Calm | 7.5 ± 1.4 | 7.7 ± 1.7 | 7.5 ± 1.5 | 8.2 ± 1.9 | F(1,44) = 2.0, p = .16 | 0.4 |
| Morning alertness | 5.3 ± 1.3 | 5.3 ± 1.4 | 5.4 ± 1.6 | 6.3 ± 1.7 | F(1,44) = 6.0, p = .02 | 0.7 |
| Difficulty waking | 4.7 ± 1.4 | 5.1 ± 1.4 | 4.9 ± 1.9 | 4.4 ± 2.0 | F(1,44) = 3.1, p = .08 | 0.5 |
Higher values indicate greater endorsement of the feeling.
In the Intervention group, improvements in ratings of worry were correlated with changes to sleep onset (r = 0.49, p = .02), sleep duration (r = 0.42, p = .04), and total sleep time (r = 0.47, p = .02) by Experimental Week 2. The remaining mood variables (tired, sleepy, concentration, irritable, and morning alertness) were not correlated with changes in sleep outcomes in the Intervention group. Mood changes were not correlated with changes in DLMO nor changes in phase angle of entrainment (DLMO to sleep onset or DLMO to wake-up time) in the Intervention group.
Discussion
We tested a combination of short-term (one-weekend) morning bright light exposure, a gradual advance of bedtime over two school weeks, and a simple evening time-management strategy (Sleep RouTeen) to facilitate earlier bedtimes and thus increase sleep duration in adolescents attending high school who were chronically getting less than 7 h of sleep on school nights. Baseline sleep duration averaged about 6 h on weekdays. The morning bright light on both mornings of one weekend and the gradual advance of the start of the dark episode (assigned bedtime) was designed to advance the central circadian clock, which in turn would make it easier for an adolescent to fall asleep at an earlier clock time. The individually tailored “Sleep RouTeen” and motivational supports provided simple and tangible behavioral goals that aimed to help the teens organize their after-school and evening time use and get into bed 1 h earlier than baseline during the first school week and 2 h earlier than baseline during the second school week. Overall, this combination of strategies was effective. In comparison to the control group, adolescents who were randomized to the Intervention group showed larger DLMO shifts in the advance (earlier) direction. Weekday sleep onset times also shifted earlier by 1.5 h, thereby extending sleep duration and total sleep time by about an hour on weeknights. After the intervention, adolescents reported increased alertness upon waking in the morning and were less tired and less sleepy during the day. They also reported an increased ability to concentrate during the day, and levels of irritability and worry were also decreased after the intervention.
Variation in response to our 2-week intervention (Figures 5 and 6) appears to be at least partly driven by circadian phase at baseline. Adolescents who started with an early circadian phase did not advance, but it is likely that they did not need to advance their DLMO (shift DLMO earlier) to be able to fall asleep earlier during the school week. Social or other external pressures, like homework, may have driven later bedtimes in these adolescents with early baseline DLMOs. Thus, it is more likely that the behavioral supports offered by their Sleep RouTeen and the study prescription of gradually shifting bedtime earlier over the two school weeks was adequate for most of them to get into bed and try to fall asleep early enough to extend their sleep duration during the school week. Adolescents with the latest initial circadian phases, however, showed the largest DLMO advances. In these adolescents with late DLMOs at baseline, it is likely that they needed both the behavioral supports of the Sleep RouTeen and the circadian-based intervention strategies to advance DLMO and facilitate earlier sleep onset and sleep duration extension during the school week. Indeed, without advancing the circadian clock, we would have asked some adolescents to initiate sleep during their “Forbidden Zone” for sleep (also called the wake maintenance zone). This zone occurs in the hours before the habitual sleep onset time [76, 77]. Moreover, the part of the forbidden zone in the 2 h before and 2 h after DLMO is more pronounced in adolescents compared to adults [78]. Therefore, for adolescents with a late sleep phenotype plus a delayed circadian clock, it is not enough to prescribe earlier bedtimes with behavioral supports; the circadian clock also needs to advance. In most cases, DLMO phase is unknown; therefore, in practice, taking a multi-pronged approach that combines morning bright light, a gradual advance of try to fall asleep time, and behavioral supports such as the Sleep RouTeen are likely needed to shift sleep onset earlier and extend sleep in adolescents.
One of the primary reasons we tested a weekend morning bright light protocol was feasibility; adolescents usually have more time on weekend mornings to complete a bright light exposure protocol compared to a school morning. Asking an adolescent to wake up even earlier than usual to fit bright light treatment into their school morning routine is not feasible. Kaplan et al. [61] came to a similar conclusion, which prompted them to test whether millisecond flashes of bright light exposure during the last 2 h of sleep could shift sleep onset earlier and increase total sleep time in adolescents who have difficulty going to bed and waking up early. The light was delivered over 4 weeks from light beacons close to the adolescent’s bed that generated 3-ms flashes of light (200–600 lux at the level of the cornea with eyelids closed) occurring 20 s apart. Adolescents included in this study had to report that they did not exclusively sleep on their front (prone position) to ensure that the light flash stimulus reached the eyes. Adolescents were also asked to go to bed 1 h earlier than their usual schedule and attend four 50-min in-person CBT sessions. Similar to the current study, self-reported sleep onset advanced (50.1 min) and total sleep time increased (43.3 min). Unfortunately, a physiological marker of the circadian system (e.g. DLMO) was not measured so it is unclear whether the changes in sleep were driven by changes to the circadian system, CBT, or both. The light source is also not commercially available yet, and therefore not easily accessible to most families. Light boxes, like those used in the current study, are commercially available. Nevertheless, our group and others have identified a critical need to develop and test feasible morning bright light delivery strategies for adolescents that do not put additional demands on their school-day mornings.
The weekend morning bright light exposure tested here was intended to be a one-time bolus of light to quickly reset rhythms (a “reset weekend”). This is in contrast to previous work in adolescents that tested sleep timing and duration changes in response to daily bright light exposure for 3–6 weeks [61, 62, 79]. Following the reset weekend, the expectation was that an early bedtime, early wake-up time and presumably early light exposure during the following school-week would help maintain the phase advance achieved after the reset weekend. Morning bright light on subsequent weekends (“booster weekends”) might be needed, but perhaps not to the extent that we tested here. The sleep schedule and morning bright light exposure could be more flexible during these booster weekends. For example, adolescents could go to bed an hour later than usual, but maintain school-day wake time on weekends. Short daytime naps could be offered as an alternative to sleeping later on the weekend, as a dark episode in the middle of the day should not impact circadian phase [80]. Bright light exposure from a bright light box, light visor, or outdoor light at an early wake-up time on the weekend may only be needed on one weekend morning and not both to keep the advanced circadian phase stable. We used 2.5 h of bright light (three 50-min exposures) because this duration on two weekend mornings was most effective in our previous study [60]; however, shorter durations of bright light may be sufficient on booster weekends. These long-term maintenance strategies need to be systematically tested, but we think it is reasonable to speculate that after this initial bolus of bright light on one weekend (reset weekend), maintenance of circadian phase could be implemented with some flexibility on subsequent weekends (booster weekends). A reset weekend may be needed again if sleep timing drifts later (e.g. after a school vacation), but could otherwise be used sparingly.
The current study was completed in a group of typically developing adolescents who reported late and insufficient sleep patterns but were without a diagnosed sleep disorder. Previous studies of adolescents diagnosed with Delayed Sleep-Wake Phase Disorder (DSWPD) have tested whether a combination of morning bright light treatment and behavioral strategies could advance sleep timing, reduce sleep onset latency, and increase total sleep time [62, 79]. Gradisar et al. [62] reported the first randomized clinical trial showing positive results in sleep outcomes in response to a combination of morning bright light and CBT in adolescents (11–18 years) diagnosed with DSWPD. These young patients were instructed to start light exposure (from natural sunlight or a broad spectrum light box, ~1000 lux) for at least 30 min starting at their natural wake-up time, and then to gradually shift their wake-up time and light exposure until the target time of 06:00. Once the target wake-up time was reached they were instructed to stop bright light treatment and maintain a regular wake-up time. Bedtime was not controlled; they were instructed to go to bed when they felt sleepy and to avoid naps. CBT sessions took place over 8 weeks (six 45–60-min sessions with adolescent and a parent). School-night sleep onset latency decreased by an average of 56 min, sleep onset time advanced by an average of 38 min, and total sleep time increased by 1 h, on average. Whether or not the positive changes in sleep were due to a circadian phase advance is unknown because there was no objective measure of circadian phase (e.g. DLMO). Nevertheless, the strategies tested in the current study may also benefit adolescents who are diagnosed with DSWPD, though modifications to behavioral supports may be needed to address symptoms of associated insomnia (i.e. Sleep RouTeen with CBT).
Clinical guidelines usually recommend starting bright light closer to spontaneous wake time to avoid the delay portion of an individual’s phase response curve [81, 82]. Our rationale for timing the start of the bright light 2 h after the mean baseline midpoint of sleep was to target the peak advance region of our PRC to bright light in adolescents [59]. The crossover point in our PRC—the time when phase delay shifts transition to advances—is when the bright light starts 5.7 h after the DLMO (see Figure 4 in [59]). In the current study, we noted that bright light on the first morning started 7.3–10.8 h after the DLMO, which is 1.6–5.1 h after the predicted crossover point of our adolescent PRC to light. Therefore, our approach of timing bright light still allows for some variability in individual PRCs. Moreover, as long as most of the 2.5 h of bright light falls on the advance portion of the PRC, a phase advance should occur (see Figure 2 in [83]). Therefore, we think it unlikely that our approach of timing the start of bright light with respect to the midpoint of sleep averaged over several days could inadvertently produce a phase delay shift. Of note, collecting sleep times, preferably from wrist actigraphy and sleep diaries, for at least a week to include school days and non-school days is needed to reliably estimate the midpoint of sleep and accurately time short-term (weekend) bright light exposure.
Data from adolescents [84] and adults [85] show that evening light exposure can attenuate phase advances in response to morning bright light given over 3 days. In a small study [84], we asked adolescents to keep a 10-h sleep schedule at home for a week. Then, they lived in the laboratory for a week and we kept half of the participants awake 4.5 h later than their baseline bedtime in room light (~140 lux) and the other half of participants were put to bed at their usual bedtime. After two nights, they completed a 3-day phase advancing protocol with morning bright light and a gradual advance of their sleep/dark episodes. As expected, participants who were not exposed to evening light advanced by about 2 h, on average. With 4.5 h of evening room light exposure, adolescents did not shift or shifted in the wrong direction (delayed) in response to the phase advancing protocol with morning bright light. These data suggest that evening light exposure hinders attempts to shift circadian phase earlier. In the current study, we did not instruct adolescents to limit evening light or wear eye glasses that block or significantly reduce blue light during the study because we were trying to simulate what they would naturally do at home. As we develop this intervention work, however, testing the added benefit of controlling illuminance and the spectral composition of evening light may be warranted.
Individual differences in circadian phase at baseline in the current study are noteworthy and speak to the need of a multi-pronged approach to change sleep/wake behavior. Despite reporting a similar behavioral phenotype of late sleep onset on school nights (≥ 23:00) and weekend nights (≥ 00:00), baseline DLMO ranged from 17:45 to 02:03 in the cohort of adolescents studied here. These data provide further evidence that adolescent sleep timing is not solely driven by a delayed circadian clock. As proposed in the Perfect Storm Model [16, 17], other factors, including the dynamics of the homeostatic sleep system, as well as psychosocial factors that displace sleep can also contribute to the delayed sleep/wake phenotype observed during adolescence. The unexpected early baseline circadian phases in adolescents who report late sleep timing mimics findings in adolescents and adults (16–64 years) with Delayed Sleep-Wake Phase Disorder (DSWPD), where about half of patients clinically diagnosed with DSWPD do not show evidence of a delay in circadian timing [86]. For those adolescents with a “non-circadian” delay in sleep timing, behavior modification, like the Sleep RouTeen, may be particularly important to help overcome the psychosocial pressures that displace sleep to a later time.
Our Sleep RouTeen approach aimed to accomplish two things: (1) to heighten the adolescent’s awareness of their own time use after school and in the evening and (2) provide individualized tangible goals to manage their time so that they could go to bed early enough during the school week. As adolescents transition into high school, parent-set bedtimes are rare, after-school activities and academic demands increase, part-time work is possible, and their social world—in person and online—expands. These increased obligations and opportunities make it difficult for many adolescents to get into bed early enough to obtain sufficient sleep on school nights, even if they want to and can fall asleep early. The advantages to our Sleep RouTeen approach were its simplicity and brevity. The initial one-on-one meeting to review after-school and evening time use, set achievable goals, and reflect on obstacles in achieving these goals was short (20 min) and the motivational supports during the subsequent 2 weeks were also brief (5–10 min). We reviewed Sleep RouTeen goals and provided motivational supports once per week when we downloaded participant’s actigraphy data; however, these brief motivational sessions could be completed over the phone or delivered via a telehealth platform.
For some adolescents, our Sleep RouTeen approach may be too unidimensional and would require additional components to address other domains that are associated with late and insufficient sleep. For example, the Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C) of Harvey et al. is a six-session series that targets psychosocial, behavioral, and cognitive processes that contribute to eveningness and a broad range of other sleep and circadian disorders that are associated with eveningness, such as insomnia [87, 88]. In their randomized-control trial of youth (10–18 years) who endorsed eveningness and late sleep onset times, the six-session TranS-C delivered during the school year increased total sleep time on weeknights by about 20–25 min, on average, and improved subjective alertness and sleep quality. There were no changes to school-night bedtime or sleep onset latency, and DLMO advanced by only 10 min (though TranS-C may have stabilized phase). The authors suggest that school-night bedtime may not have changed because of the “number of tasks that had to be completed—such as homework, sports, dinner—in the hours between school ending and bedtime.” [87] Our current study, however, shows that with tangible individually tailored time-use goals provided by the Sleep RouTeen, motivational supports in subsequent weeks, and a prescribed gradual advance of bedtime can help overcome these noted obstacles in the evening and may be considered within a broader approach such as TranS-C.
Despite an early assigned bedtime, sleep onset latency in the Intervention group did not differ from the Control group. Descriptively, however, sleep onset latency lengthened during the intervention from 8.5 to 20 min, on average, suggesting that some participants in the intervention may have experienced longer sleep latencies by the end of the study. A total of six participants out of 23 (26%) had sleep onset latencies longer than 30 min by Experimental Week 2. Two of these participants showed large DLMO phase advances (1.37 h and 1.64 h), but their sleep onset latencies were also consistently longer than average throughout the study (35–60 min for one participant and 23–38 min for the second participant). Four participants with long sleep onset latencies during Experimental Week 2 (34–75 min) showed no or small advances in DLMO (0 h, −0.14 h, 0.4 h, and 0.6 h). Therefore, longer sleep onset latencies during Experimental Week 2 may be driven by the adolescents trying to initiate sleep at an earlier time relative to their DLMO. Other unaccounted stressors or environmental factors may also be driving the increase in sleep onset latency for this subgroup of participants. Sleep onset latency data need to be interpreted with caution, however, because this variable was derived using self-reported try to fall asleep time and was not derived from investigator-confirmed lights out time in the laboratory or from polysomnography which may limit its precision.
Subjective ratings of alertness (both in the morning and across the day), ability to concentrate, irritability, and worry showed improvements in the Intervention group compare to the Control group after the 2-week intervention. These data are consistent with previous studies that extended sleep on school days in healthy adolescents [89] and adolescent who reported chronic insufficient sleep (5–7 h nightly) [90]. In the current study, improvements in subjective feelings of worry were associated with changes in sleep timing, total sleep time, and sleep duration, but not with DLMO phase shift. Improvements in the ability to concentrate, changes in irritability, and changes to subjective alertness ratings were not associated with changes to sleep onset, sleep duration, total sleep time, or DLMO. The mechanism for these changes in mood outcomes is unclear from the current analysis, but we speculate that improvement may be partly due to increasing sleep regularity in the Intervention group and not the Control group [91, 92]. It is also plausible that motivational elements of the Sleep RouTeen impacted these subjective mood measures independently of the sleep and circadian changes observed in the Intervention group. The Intervention group may have experienced more positive expectations compared to the Control group because the Intervention group completed the Bright Light Lab weekend and the Control group did not. Simply experiencing the bright light treatment may have driven positive expectations of the intervention. Future studies need to better address these potential expectancy effects.
Although the tested intervention shows promise to increase sleep duration in short and late-sleeping adolescents, limitations of the current study should be noted. First, there is a need to follow adolescents for longer than 2 weeks to learn whether the short-term benefits achieved are sustained. Long-term maintenance strategies, like “booster weekends” noted earlier need to be systematically tested to determine the most effective long-term strategy in adolescents. This work is ongoing in our laboratory. In this initial test of the intervention’s effectiveness, participants were paid to complete the month-long study (the Control and Intervention groups were paid the same dollar amount). It is unclear whether adolescents would complete this protocol without compensation. In a group of adolescents diagnosed with Delayed Sleep-Wake Phase Disorder, Micic et al. [93] reported that high levels of desire, confidence in their ability to, and commitment to change behaviors predicted compliance to daily 30–60 min of bright light treatment that lasted between 6 and 27 days. Although the current study protocol required only one weekend of morning bright light, additional motivators (e.g. parental involvement, peer support) may be needed.
Conclusions
The majority of adolescents get far less sleep than the current recommendation of 8–10 h per night on school nights, particularly for adolescent who start school too early. This is the result of several factors impinging on sleep opportunity. The biological propensity for alertness later in the evening permitted by maturing circadian and sleep-wake homeostatic systems, as well as social and academic pressures displace sleep to a later time. These biological and psychosocial factors compete with early school start times that force adolescents out of bed too early on school days. Data from the current study confirms that by shifting the circadian timing system earlier, prescribing a gradual advance of bedtime, and modifying after-school and evening time-use, adolescents are able to fall asleep early and increase their sleep duration during the school week. By doing so, adolescents report improvements in their daytime mood and alertness. This study lays the foundation to explore additional outcomes in response to phase advancing the circadian system and increasing sleep duration in late- and short-sleeping adolescents during the school week, and provides a starting point from which to expand and build.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Heart Lung and Blood Institute (R01 HL112756) to S.J.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute (NHLBI). NHLBI had no involvement in designing the study, data collection, data analysis, and interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. Thomas Molina, Vicky Tomaka, Chelsea Fournier, Samantha Evans, John Glines, Ali Norwood, Amelia Smith, Kyle Anthony, Taylor Recek, Kristopher Janevski, and Shelby Gilyard assisted with data collection. Lameese Akacem, PhD helped with initial study launch. We would also like to acknowledge the participants and their families for their dedication and cooperation while participating in this research project. The work described in this manuscript was performed at the Biological Rhythms Research Laboratory at Rush University Medical Center, Chicago, IL.
Contributor Information
Stephanie J Crowley, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Sabrina L Velez, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Logan G Killen, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Jamie A Cvengros, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Louis F Fogg, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Charmane I Eastman, Biological Rhythms Research Laboratory, Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago IL, USA.
Disclosure Statement
Financial Disclosures: none.
Non-financial Disclosures: none.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Paruthi S, et al. . Consensus Statement of the American Academy of Sleep Medicine on the recommended amount of sleep for healthy children: methodology and discussion. J Clin Sleep Med. 2016;12(11):1549–1561. doi: 10.5664/jcsm.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carskadon M, et al. . Contemporary sleep patterns of adolescents in the USA: results of the 2006 national sleep foundation sleep in america poll. J Sleep Res. 2006;15(Supplement 1):42. [Google Scholar]
- 3. Baiden P, et al. . The association between excessive screen-time behaviors and insufficient sleep among adolescents: findings from the 2017 youth risk behavior surveillance system. Psychiatry Res. 2019;281:112586. doi: 10.1016/j.psychres.2019.112586. [DOI] [PubMed] [Google Scholar]
- 4. Galland BC, et al. . Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep. 2018;41(4). doi: 10.1093/sleep/zsy017 [DOI] [PubMed] [Google Scholar]
- 5. Beijamini F, et al. . Influence of gender on psychomotor vigilance task performance by adolescents. Braz J Med Biol Res. 2008;41(8):734–738. doi: 10.1590/s0100-879x2008000800016 [DOI] [PubMed] [Google Scholar]
- 6. Giannotti F, et al. . Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x [DOI] [PubMed] [Google Scholar]
- 7. Loessl B, et al. . Are adolescents chronically sleep-deprived? An investigation of sleep habits of adolescents in the Southwest of Germany. Child Care Health Dev. 2008;34(5):549–556. doi: 10.1111/j.1365-2214.2008.00845.x [DOI] [PubMed] [Google Scholar]
- 8. O’Brien EM, et al. . Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3(3):113–133. doi: 10.1207/s15402010bsm0303_1 [DOI] [PubMed] [Google Scholar]
- 9. Wolfson AR, et al. . Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 10. Wolfson AR, et al. . Understanding adolescents’ sleep patterns and school performance: a critical appraisal. Sleep Med Rev. 2003;7(6):491–506. doi: 10.1016/s1087-0792(03)90003-7 [DOI] [PubMed] [Google Scholar]
- 11. Russo PM, et al. . Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16(2):163–169. doi: 10.1111/j.1365-2869.2007.00584.x [DOI] [PubMed] [Google Scholar]
- 12. Thorleifsdottir B, et al. . Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53(1):529–537. doi: 10.1016/s0022-3999(02)00444-0 [DOI] [PubMed] [Google Scholar]
- 13. Yang CK, et al. . Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115(1 Suppl):250–256. doi: 10.1542/peds.2004-0815G [DOI] [PubMed] [Google Scholar]
- 14. Gradisar M, et al. . Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–118. [DOI] [PubMed] [Google Scholar]
- 15. Kim J, et al. . Demographic and socioeconomic influences on sleep patterns among adolescent students. Int J Environ Res Public Health. 2020;17(12):4378. doi: 10.3390/ijerph17124378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. doi: 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crowley SJ, et al. . An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. doi: 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carskadon MA, et al. . An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12(3):278–289. doi: 10.1177/074873049701200309 [DOI] [PubMed] [Google Scholar]
- 19. Carskadon MA, et al. . Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. [DOI] [PubMed] [Google Scholar]
- 20. Hagenauer MH, et al. . Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. doi: 10.1159/000216538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tate BA, et al. . Maturational changes in sleep-wake timing: longitudinal studies of the circadian activity rhythm of a diurnal rodent. In: Carskadon MA, ed. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge: Cambridge University Press; 2002: 40–49. [Google Scholar]
- 22. Golub MS, et al. . Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, ed. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge: Cambridge University Press; 2002: 50–68. [Google Scholar]
- 23. Hummer DL, et al. . Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R586–R597. doi: 10.1152/ajpregu.00043.2006 [DOI] [PubMed] [Google Scholar]
- 24. McGinnis MY, et al. . Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92(5):1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinert D, et al. . Daily activity and body temperature rhythms do not change simultaneously with age in laboratory mice. Physiol Behav. 1999;66(4):605–612. doi: 10.1016/s0031-9384(98)00342-4 [DOI] [PubMed] [Google Scholar]
- 26. Melo PR, et al. . Circadian activity rhythm in pre-pubertal and pubertal marmosets (Callithrix jacchus) living in family groups. Physiol Behav. 2016;155:242–249. doi: 10.1016/j.physbeh.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 27. Jenni OG, et al. . Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. doi: 10.1093/sleep/28.11.1446 [DOI] [PubMed] [Google Scholar]
- 28. Crowley SJ, et al. . A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. doi: 10.1371/journal.pone.0112199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17(1):5–12. [PubMed] [Google Scholar]
- 30. Short MA, et al. . Time for bed: parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep. 2011;34(6):797–800. doi: 10.5665/SLEEP.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carskadon MA. Adolescent sleepiness: increased risk in a high-risk population. Alcohol Drugs Driv 1989–1990. 5/6(4/1):317–328. [Google Scholar]
- 32. Manber R, et al. . Changing sleep patterns in adolescents. Sleep Res. 1995;24:106. [Google Scholar]
- 33. Knutson KL, et al. . Sociodemographic and behavioral predictors of bed time and wake time among US adolescents aged 15 to 17 years. J Pediatr. 2009;154(3):426–430, 430 e421. doi: 10.1016/j.jpeds.2008.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 2004;27(1):101–104. [DOI] [PubMed] [Google Scholar]
- 35. Gradisar M, et al. . The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LeBourgeois MK, et al. . Digital media and sleep in childhood and adolescence. Pediatrics. 2017;140(Suppl 2):S92–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hale L, et al. . Youth screen media habits and sleep: sleep-friendly screen behavior recommendations for clinicians, educators, and parents. Child Adolesc Psychiatr Clin N Am. 2018;27(2):229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meltzer LJ, et al. . Changing school start times: impact on sleep in primary and secondary school students. Sleep. 2021;44(7). doi: 10.1093/sleep/zsab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meltzer LJ, et al. . COVID-19 instructional approaches (in-person, online, hybrid), school start times, and sleep in over 5,000 U.S. adolescents. Sleep. 2021;44(12). doi: 10.1093/sleep/zsab180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Owens JA, et al. . Drowsy driving, sleep duration, and chronotype in adolescents. J Pediatr. 2019;205:224–229. [DOI] [PubMed] [Google Scholar]
- 41. Carskadon MA, et al. . Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. [DOI] [PubMed] [Google Scholar]
- 42. Wittmann M, et al. . Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1):497–509. [DOI] [PubMed] [Google Scholar]
- 43. Roenneberg T, et al. . Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. [DOI] [PubMed] [Google Scholar]
- 44. Talbot LS, et al. . Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10(6):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gangwisch JE, et al. . Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep. 2010;33(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pallesen S, et al. . Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc. 2011;34(2):391–395. [DOI] [PubMed] [Google Scholar]
- 47. Palmer CA, et al. . Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- 48. Carskadon MA, et al. . Acute restriction of nocturnal sleep in children. Percept Mot Skills. 1981;53:103–112. [Google Scholar]
- 49. Randazzo AC, et al. . Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998;21(8):861–868. [PubMed] [Google Scholar]
- 50. Yang CM, et al. . A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. [DOI] [PubMed] [Google Scholar]
- 51. Fallone G, et al. . Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93(1):213–229. [DOI] [PubMed] [Google Scholar]
- 52. Taylor A, et al. . Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep Biol Rhythms. 2008;6:172–179. [Google Scholar]
- 53. Preckel F, et al. . Chronotype, cognitive abilities, and academic achievement: a meta-analytic investigation. Learn Individ Diff. 2011;21(5):483–492. [Google Scholar]
- 54. Lin WH, et al. . Unhealthy sleep practices, conduct problems, and daytime functioning during adolescence. J Youth Adolesc. 2015;44(2):431–446. [DOI] [PubMed] [Google Scholar]
- 55. Tonetti L, et al. . Association between circadian preference and academic achievement: a systematic review and meta-analysis. Chronobiol Int. 2015;32(6):792–801. [DOI] [PubMed] [Google Scholar]
- 56. Boergers J, et al. . Later school start time is associated with improved sleep and daytime functioning in adolescents. J Dev Behav Pediatr. 2014;35(1):11–17. [DOI] [PubMed] [Google Scholar]
- 57. Dunster GP, et al. . Sleepmore in Seattle: later school start times are associated with more sleep and better performance in high school students. Sci Adv. 2018;4(12):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ziporyn TD, et al. . Adolescent sleep health and school start times: setting the research agenda for California and beyond: a research summit summary: a research summit summary. Sleep Health. 2022;8(1):11–22. doi: 10.1016/j.sleh.2021.10.008 [DOI] [PubMed] [Google Scholar]
- 59. Crowley SJ, et al. . Human adolescent phase response curves to bright white light. J Biol Rhythms. 2017;32(4):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Misiunaite I, et al. . Circadian phase advances in response to weekend morning light in adolescents with short sleep and late bedtimes on school nights. Front Neurosci. 2020;14(13831):99. doi: 10.3389/fnins.2020.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaplan KA, et al. . Effect of light flashes vs sham therapy during sleep with adjunct cognitive behavioral therapy on sleep quality among adolescents: a randomized clinical trial. JAMA Netw Open. 2019;2(9):e1911944–e1911944. doi: 10.1001/jamanetworkopen.2019.11944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gradisar M, et al. . A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34(12):1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Owens J. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shirayama M, et al. . The psychological aspects of patients with delayed sleep phase syndrome (DSPS): a preliminary study. Jpn J Psychiat Neur. 1994;48:453–454. [Google Scholar]
- 65. Roberts RE, et al. . The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haraden DA, et al. . Internalizing symptoms and chronotype in youth: a longitudinal assessment of anxiety, depression and tripartite model. Psychiatry Res. 2019;272:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bauducco S, et al. . Chronotype, circadian rhythms and mood. Curr Opin Psychol. 2020;34:77–83. [DOI] [PubMed] [Google Scholar]
- 68. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1(3):385–401. [Google Scholar]
- 69. Smith CS, et al. . Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 70. Roenneberg T, et al. . A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 71. Klerman EB, et al. . Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17(2):181–193. [DOI] [PubMed] [Google Scholar]
- 72. Benloucif S, et al. . Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20(2):178–188. [DOI] [PubMed] [Google Scholar]
- 73. Crowley SJ, et al. . Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Med. 2016;20:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meltzer LJ, et al. . Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Acebo C, et al. . Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. [DOI] [PubMed] [Google Scholar]
- 76. Lavie P. Ultrashort sleep-waking schedule III. “Gates” and “forbidden zones” for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–425. [DOI] [PubMed] [Google Scholar]
- 77. Eastman CI, et al. . Sex and the forbidden zone. Sleep. 2017;40(Suppl 1):A264. [Google Scholar]
- 78. Crowley SJ, et al. . Sleep and the forbidden zone: trouble for teens. Sleep. 2018;41(Suppl):A96. [Google Scholar]
- 79. Richardson C, et al. . A randomised controlled trial of bright light therapy and morning activity for adolescents and young adults with Delayed Sleep-Wake Phase Disorder. Sleep Med. 2018;45:114–123. [DOI] [PubMed] [Google Scholar]
- 80. Buxton OM, et al. . Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R373–R382. [DOI] [PubMed] [Google Scholar]
- 81. Emens J, et al. . Effect of light and melatonin and other melatonin receptor agonists on human circadian physiology. Sleep Med Clin. 2015;10:435–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Auger RR, et al. . Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015;11(10):1199–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eastman CI, et al. . How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. [DOI] [PubMed] [Google Scholar]
- 84. Crowley SJ, et al. . Late bedtimes prevent circadian phase advances to morning bright light in adolescents. Chronobiol Int. 2018;35(12):1748–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burgess HJ. Evening ambient light exposure can reduce circadian phase advances to morning light independent of sleep deprivation. J Sleep Res. 2013;22(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murray JM, et al. . Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw002 [DOI] [PubMed] [Google Scholar]
- 87. Harvey AG, et al. . Modifying the impact of eveningness chronotype (“Night-Owls”) in youth: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2018;57(10):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gasperetti CE, et al. . The effect of the transdiagnostic sleep and circadian intervention (TranS-C) on actigraphic estimates of sleep and rest-activity rhythms in adolescents with an evening circadian preference. Sleep Health. 2022;8(2):191-194. doi: 10.1016/j.sleh.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 89. Beebe DW, et al. . Impact of multi-night experimentally-induced short sleep on adolescent performance in a simulated classroom. Sleep. 2017;40(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Van Dyk TR, et al. . Feasibility and emotional impact of experimentally extending sleep in short-sleeping adolescents. Sleep. 2017;40(9). doi: 10.1093/sleep/zsx123 [DOI] [PubMed] [Google Scholar]
- 91. Bei B, et al. . Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw067 [DOI] [PubMed] [Google Scholar]
- 92. Van Dyk TR, et al. . Feasibility and impact on daytime sleepiness of an experimental protocol inducing variable sleep duration in adolescents. PLoS One. 2019;14(6):e0218894. doi: 10.1371/journal.pone.0218894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Micic G, et al. . Readiness to change and commitment as predictors of therapy compliance in adolescents with Delayed Sleep-Wake Phase Disorder. Sleep Med. 2018;55:48–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.