Abstract
The shallow-water hydrothermal vent system of Kueishan Island has been described as one of the world's most acidic and sulfide-rich marine habitats. The only recorded metazoan species living in the direct vicinity of the vents is Xenograpsus testudinatus, a brachyuran crab endemic to marine sulfide-rich vent systems. Despite the toxicity of hydrogen sulfide, X. testudinatus occupies an ecological niche in a sulfide-rich habitat, with the underlying detoxification mechanism remaining unknown. Using laboratory and field-based experiments, we characterized the gills of X. testudinatus that are the major site of sulfide detoxification. Here sulfide is oxidized to thiosulfate or bound to hypotaurine to generate the less toxic thiotaurine. Biochemical and molecular analyses demonstrated that the accumulation of thiosulfate and hypotaurine is mediated by the sodium-independent sulfate anion transporter (SLC26A11) and taurine transporter (Taut), which are expressed in gill epithelia. Histological and metagenomic analyses of gill tissues demonstrated a distinct bacterial signature dominated by Epsilonproteobacteria. Our results suggest that thiotaurine synthesized in gills is used by sulfide-oxidizing endo-symbiotic bacteria, creating an effective sulfide-buffering system. This work identified physiological mechanisms involving host-microbe interactions that support life of a metazoan in one of the most extreme environments on our planet.
Keywords: shallow-water hydrothermal vent, sulfide detoxification, brachyuran crab, microbes, transporters, physiological adaptation
1. Background
Hydrogen sulfide is highly toxic for organisms due to its ability to inhibit cytochrome-c oxidase, a critical enzyme for mitochondrial respiration [1]. Therefore, metazoans living in sulfide-rich waters like those found in most hydrothermal vent systems evolved behavioural, morphological and physiological mechanisms to allow existence in these highly toxic marine habitats [2–6]. While some deep-sea hydrothermal vent species have sulfide detoxification mechanisms independent from microbial symbionts [7,8], many sessile species, including vesicomyid bivalves, mytilids and vestimentiferans, evolved symbioses with sulfide-oxidizing chemoautotrophic bacteria that support detoxification and provide nutrition to the host [9,10]. To date, many studies on sulfide detoxification mechanisms in marine species focused on those inhabiting deep-sea hydrothermal vent systems of the mid-ocean ridges [6,11]. However, volcanic vent systems can also be found in shallow waters that are among the most extreme marine habitats concerning sulfide toxicity. Shallow-water vent systems with moderate temperatures (<200°C), high concentrations of sulfide and CO2 were abundant in the primeval oceans, but due to the decrease in volcanic activity, such vent systems became rare in today's oceans [12]. One of these vent systems is found off the coast of Taiwan in the shallow-water at the eastern part of Kueishan Island (24°50′N, 121°57′E) [13]. Kueishan Island lies to the southern rifting end of the Okinawa Trough, a back-arc depression of the Ryukyu arc-trench system and has active volcanic features [14]. This underwater volcano has been described as one of the world's most acidic vents, discharging water that has a high content of elemental sulfur particles, a minimum pH of 1.52 [15], and a gas composition consisting of high concentrations of sulfide (H2S) (800 µM) and CO2 (<92%) [13]. Due to these hostile conditions, the biodiversity of this vent habitat is extremely low [16], with the brachyuran crab Xenograpsus testudinatus being the only recorded metazoan species in the direct vicinity of the vents. X. testudinatus is endemic to shallow-water (<200 m) sulfide-rich vent systems [17], sporadically feeding on dead zooplankton killed by the toxic vent discharges [18].
Sulfide oxidation to the less toxic thiosulfate has been demonstrated as a major detoxification mechanism in other invertebrates from sulfide-rich marine habitats [7,8] that host sulfide-oxidizing endosymbionts in their tissues [19,20]. In addition, some hydrothermal vent species utilize sulfide-binding factors like hypotaurine that bind sulfide to synthesize thiotaurine, which is less toxic to cellular respiration [9,10]. These unusual amino acids were first discovered in vent vestimentiferans and bivalves that carry thiotrophic endosymbionts, but are also observed in species without endosymbionts. Accordingly, many invertebrates from vents and seeps have high concentrations of hypotaurine and thiotaurine independent from their symbioses with thiotrophic symbionts [10,21]. This adaptive sulfide detoxification mechanism is prevalent in animals that cannot avoid sulfide exposure (e.g. vestimentiferans, polychaetes, vesicomyid clams and bathymodiolin mussels) or that feed on external bacteria and detritus (e.g. polychaetes and gastropods) in sulfide-laden waters [9,10,19,22,23].
In most species, sulfide oxidation capacities are associated with specialized tissues, including the epidermis, gills and hepatopancreas [19,24–27]. In some vent species, these tissues exhibit distinct morphological features, including large numbers of electron-dense organelles termed sulfide oxidizing bodies [28]. Furthermore, sulfide-oxidizing endosymbionts are well known to be found in specialized tissues of vent species [29]. Vent-dwelling organisms like the sessile tubeworm Riftia pachyptila or some bivalve species have intracellular symbionts that are located in specialized host cells, the bacteriocytes of the trophosome or gill [30–32]. The host provides all substrates necessary for chemosynthesis to the symbionts [33], and in return, the symbionts provide fixed organic carbon to nourish the gutless host [34]. A recent study reported a specific microbial signature consisting of sulfur-oxidizing Epsilonproteobacteria and Gammaproteobacteria in several tissues of X. testudinatus [20,35]. Epsilonproteobacteria are capable of autotrophic growth fueled by oxidation of reduced sulfur compounds, and Gammaproteobacteria are known to perform chemolithoautotrophic sulfur oxidization [36–38]. However, it remains unknown if a specific microbial signature in X. testudinatus is associated with their high tolerance to environmental sulfide and if these microbial residents may provide nutrition to their hosts in this food-limited marine environment.
Thus, the present work aims at identifying and characterizing the cellular mechanisms of sulfide detoxification that allow X. testudinatus to exist in sulfide-rich vent systems. By determining and localizing the microbial community in gill tissues, the present work supports the concept that host–microbiome interactions underlie the extraordinary sulfide tolerance of X. testudinatus, allowing this species to occupy a unique ecological niche in one of the most extreme marine habitats.
2. Material and methods
(a) . Study area and sample collection
Kueishan Island (121°57′ E; 24°50′N) is a volcanic island and the hydrothermal vents are only present near the eastern edge of Kueishan Island, including nine larger sulfur mounds and numerous smaller gas vents, distributed from the sublittoral to 20 m depth. The collection site (24°50.055′N; 121°57.716′E) is located at a cluster of vents discharging a sulfide-rich venting plume. Two perpendicular 100 m transect lines were set for the determination of sulfide gradients in this habitat. The north–south line was at a depth of 17 m, and the east–west line was at a depth descending from 6 to 19 m. The fieldwork was carried out from August 2005 to October 2019. Detailed information about field estimations and animal sampling is described in the electronic supplementary material.
(b) . Determination of sulfur compounds
The procedure for measuring sulfide, sulfite and thiosulfate followed previously described protocols [39]. The reacted products were analysed using a Waters 600E HPLC (Mildford, MA, USA) within 2.5 h. And the sulfate concentration in SW and haemolymph was respectively determined with a portable photometer and 96-well plate photometer. Detailed information about sulfur compound determinations is described in the electronic supplementary material.
(c) . Sulfide tolerance and exposure experiments
Tolerance of X. testudinatus and other marine species (including pebble crab (crabs), marine segmented worm (annelids), short-necked clam (molluscs), sea urchin (echinoderms) and sea bream (teleosts)) to environmental sulfide concentrations was investigated using a continuous flow-through system with dissolving rinsed Na2S·9H2O as described by [40]. Besides, X. Testudinatus was exposed to 1000 µM sulfide condition as described previously for 7 days. Detailed information treatment process is described in the electronic supplementary material.
(d) . Hypotaurine and thiotaurine measurements
Hypotaurine and thiotaurine were extracted from haemolymph and norvaline was added as an internal standard. The sample derivatives were injected in an ultra-performance liquid chromatography system (UPLC) (ACQUITY UPLC H-class System, Waters) equipped with a TUV detector and then identified and quantified by comparing sample data with retention times and peak areas of standards. Detailed information about hypotaurine and thiotaurine measurements is described in the electronic supplementary material.
(e) . Purification of total RNA and reverse-transcription
Purification of total RNA and RT-PCR were performed as previously described [3]. Detailed information about molecular sample preparation is described in the electronic supplementary material.
(f) . Molecular cloning
To amplify the partial cDNA fragment of SLC26A11 and Taut family genes, primers for slc26a11 and slc6a6 (encoded as Taut) were designed, as shown in electronic supplementary material, table S2. For PCR amplification, 1 µg of cDNA was used as a template. Detailed information about the cloning process is described in the electronic supplementary material.
(g) . Quantitative real-time quantitative PCR
Primers of slc26a11a, slc26a11b, taut1 and taut2 genes were designed as listed in electronic supplementary material, table S2. Quantitative real-time quantitative PCR (qRT-PCR) was performed on a Roche LightCycler System (Roche Applied Science, Mannheim, Germany) using the LightCycler 480 SYBR Green I Master (Roche), and the ribosomal protein L9 (rpl9) was used as the reference gene. Detailed information about the qRT-PCR is described in the electronic supplementary material.
(h) . Transmission electron microscope
The ultrathin sections (70 nm) were prepared from the 5th pair of gill arches and cut by Leica EM UC7, then mounted on nickel grids, and observed by a Tecnai G2 F20 S-TWIN field emission gun electron microscope (FEI Company, USA). Detailed information about the transmission electron microscope (TEM) process is described in the electronic supplementary material.
(i) . Fluorescence in situ hybridization and immunohistochemical staining
Horizontal sections (10 µm; vertical to vascular) from the 5th pair of gill arches were collected on poly-l-lysine-coated slides for experiments. For target gene RNA labelling, isolated plasmids from the targeted clones were used to synthesize antisense and sense RNA probes. For the H&E staining, sections were stained with haematoxylin and eosin. For immunohistochemical staining, the primary antibody for Na+/K+-ATPase (NKA), H-300, was used. For microbial communities visualization, 4 µm paraffin sections were prepared as previously described [35]. The oligonucleotide probes targeting general Eubacteria 16S rRNA (EUB338) and thiotrophic symbiont (BangT-642) were utilized. Detailed information about the fluorescence in situ hybridization (FISH) and immunohistochemical staining is described in the electronic supplementary material.
(j) . DNA extraction, amplicon sequencing, data analysis and microbiome quantification
DNA extraction was performed as previously described [35]. The DNA tagging PCR (5 cycles) using the total genomic DNA sample (from the 5th pair of gill arches) was used to tag the ends of 16S RNA gene V6-V8 amplicons. The operational taxonomic units (OTUs) were taxonomically classified according to the SILVA database. For the quantitative PCR assay, two primer sets were designed to target the Epsilonproteobacteria and Gammaproteobacteria (listed in electronic supplementary material, table S2). Detailed information about the microbiome analysis is described in the electronic supplementary material.
(k) . Protein purification and western blot analyses
Protein extractions and western blot analyses were performed as previously described [3]. Detailed information about the protein expression analysis is described in the electronic supplementary material.
(l) . Statistical analyses
Values are presented as the mean ± standard deviation (s.d.). Statistical analyses were performed using Sigma Stat 10.0 (Systat Software). Student's unpaired t-test was used to compare thiosulfate, hypotaurine and thiotaurine contents in haemolymph, RNA/protein expression in gill. One-way ANOVA followed by Tukey's post-hoc tests were used to compare thiosulfate contents in gills under different sulfide concentrations and the microbiome abundances in gills along with the time series. In all cases, significance was defined as p < 0.05.
3. Results and discussion
(a) . Environmental sulfide concentrations of the shallow-water hydrothermal vent system and sulfide tolerance of X. testudinatus
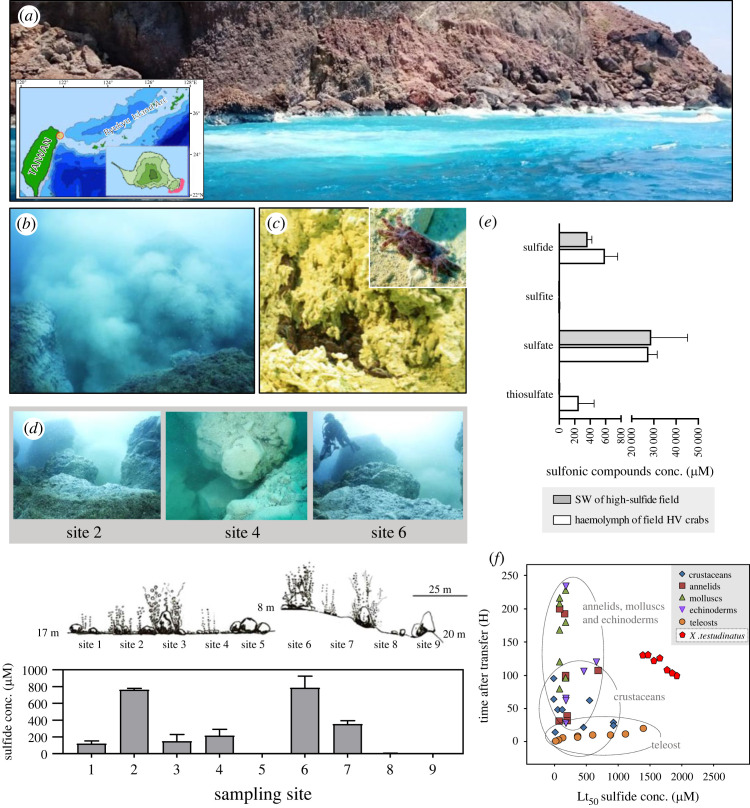
The shallow-water (<25 m) hydrothermal vent system of Kueishan Island (24°50′N, 121°57′E) is located at the western edge of the Pacific Ocean, off the coast of Taiwan [13] (figure 1a; electronic supplementary material, video S1). X. testudinatus is the only metazoan species in the direct vicinity of the vents usually found at high densities within sulfur-covered crevices (figure 1b and c; electronic supplementary material, video S2). Based on our observations, X. testudinatus do not congregate near the vent fluid directly above the vent opening but rather in the surrounding area that is less impacted by the heat from the vents. It has been suggested that the extremely high and fluctuating sulfide concentrations are the major factor for the very low biodiversity of macrofauna in this habitat [41,42]. In the present study, water sulfide concentrations of the two main hydrothermal vent areas, Site 2 and Site 6, were determined to be 762.90 ± 8.52 µM and 786.40 ± 79.52 µM, respectively (figure 1d). These hydrogen sulfide concentrations exceed those measured in hydrothermal vent fields of the deep-sea that typically range from 6–318 µM [26,43–45]. Our most recent data also revealed that the sulfide concentrations in the vent areas where X. testudinatus congregated fluctuated dramatically [35] with peak sulfide levels higher than 1000 µM after a dramatic geological activity. The haemolymph of X. testudinatus specimens collected from this area contained high concentrations of sulfide derivatives, including sulfate and thiosulfate (figure 1e), indicating that X. testudinatus utilizes sulfide oxidation as a detoxification mechanism. The oxidation of sulfide to thiosulfate is a widespread detoxification mechanism and has been demonstrated for other crustaceans from sulfide-rich marine sediments [46,47] and deep-sea vent systems [26]. The evolutionary advantage of generating thiosulfate as a detoxification metabolite lies in the fact that it has a more efficient stoichiometry than the synthesis of other sulfide derivates where 1 mol sulfide can be oxidized to thiosulfate with 1.5 mol oxygen [48].
Figure 1.
Landscape and physiochemical features of the hydrothermal vent system study area. (a) Picture of the surface seawater around the upper sublittoral hydrothermal vent region of the study site at Kueishan Island (24°50′N, 121°57′E). Kueishan Island lies at the southern rifting end of the Okinawa Trough (inserted panel). A large area of the sea surface has a white appearance because of sulfur particles from the volcanic vent discharge. (b) Underwater photographs of the vent system demonstrate the low abundance of metazoans in these sub-tropical waters. (c) A high density of X. testudinatus crabs which are endemic to sulfide-rich hydrothermal vent systems can be found in sulfide-rich crevices in the immediate vicinity of the vents. Active feeding behaviour can be observed where crabs scavenge sulfide-rich sediments and dead zooplankton that were killed by the toxic vent discharge. (d) sulfide concentrations were measured along two transects in the hydrothermal vent area of Kueishan Island. The schematic illustration shows the seafloor properties at the two sampling transects. The first transect included sampling sites 1–5 and was oriented in the north–south direction at 17 m depth. The second transect included sampling sites 6–9 with an east–west orientation and descended from 8 m to 20 m depth. Values are presented as mean ± s.d. (n = 4). (e) Content of sulfur compounds was measured in the seawater (SW) near Kueishan island (open bars) and the haemolymph of the native X. testudinatus (filled bars). Values represent the means ± s.d. (n = 3–7). (f) Sulfide tolerance as a function of exposure time of X. testudinatus (determined in the present study) compared to other marine species.
Since X. testudinatus is the only recorded metazoan species reported in this shallow-water hydrothermal vent system, it can be expected to exhibit an extraordinary sulfide tolerance compared to other marine invertebrates. The median lethal time (Lt50) of adult X. testudinatus exposed to up to 2000 µM sulfide at normoxic conditions was about 120 h which is high compared to other marine species, including organisms from other hydrothermal vent systems (figure 1f). The Lt50 value determined for X. testudinatus is higher than those of other hydrothermal vent species including the nematode Oncholaimus campylocercoides (108 h hypoxia and 500 µM sulfide [8]) and the annelid Capitella capitata (107 h hypoxia and 760 µM sulfide; [7]) (figure 1f); moreover, X. testudinatus appears to be more tolerant to sulfide than mactrid clams (Mulinia lateralis) that burrow into sediments containing sulfide concentrations that are lethal for most aquatic organisms [49]. X. testudinatus has substantial sulfide tolerance that is comparable to that of other vent crustaceans like the hydrothermal vent crab Bythograea thermydron. Bythograea thermydron has been demonstrated to have comparatively high sulfide tolerance with unchanged heart rates up to a sulfide concentration of 1400 µM [26]. Despite this high sulfide tolerance and a sulfide oxidation mechanism in place, little information is available regarding the cellular and molecular mechanisms of sulfide/thiosulfate handling in X. testudinatus and other deep-sea hydrothermal vent crustaceans [26].
(b) . Gill tissues and the hepatopancreas are major sites of sulfide detoxification
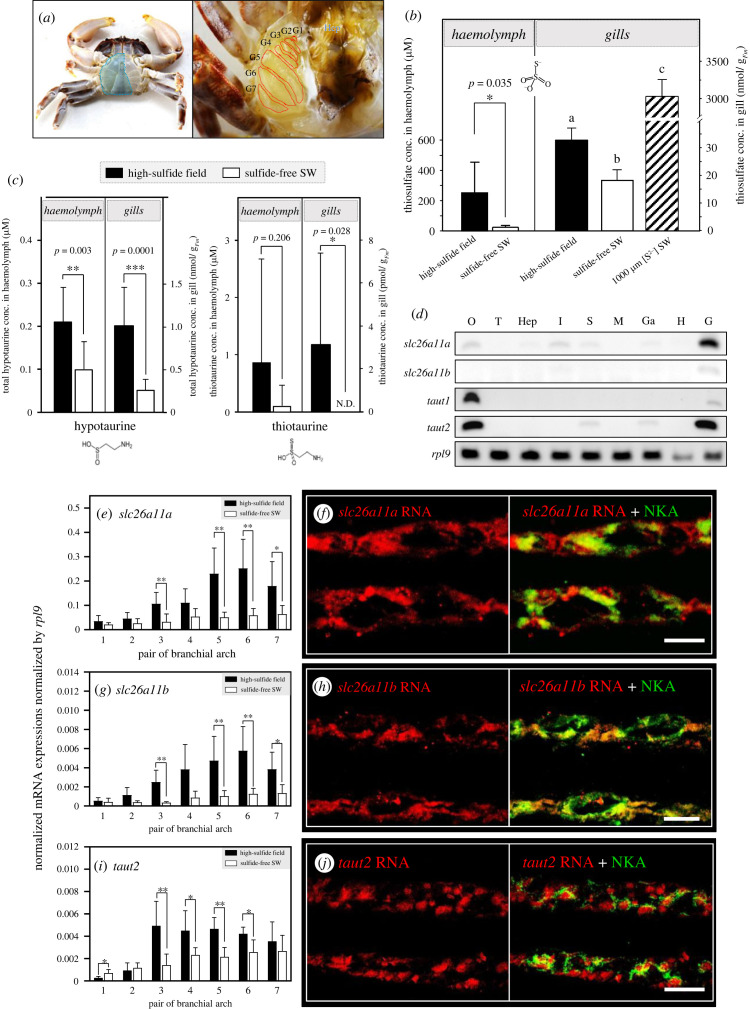
The gill organ of X. testudinatus consists of seven pairs of gill arches located within the branchial chamber (figure 2a) and represents together with the hepatopancreas, the major sites for sulfide detoxification indicated by high levels of thiosulfate (electronic supplementary material, figure S1). Animals collected from their natural habitats and those experimentally exposed to 1000 µM sulfide for 7 days exhibited a high thiosulfate concentration in these tissues and in the haemolymph. This suggests that these molecules are generated in the gills and the hepatopancreas and transported within the organism (figure 2b). Furthermore, hypotaurine and thiotaurine concentrations were measured in the haemolymph and gill tissues of X. testudinatus, and both amino acids were present in high concentrations in animals that were directly sampled from the native sulfidic environment (figure 2c). Some deep-sea vent vesicomyid bivalves, mytilids and vestimentiferans were shown to utilize such sulfide-binding compounds to decrease the potential affinity between sulfide and mitochondrial cytochrome c oxidase [23]. Hypotaurine, the precursor of taurine, is one such sulfide-binding compound. Upon sulfide binding, hypotaurine is converted to thiotaurine, which is less detrimental to cellular respiration. At vents and seeps, many invertebrates with or without thiotrophic symbionts have high levels of hypotaurine and thiotaurine [10,21]. This adaptation is prevalent in animals that are sessile and that cannot avoid sulfide exposure (e.g. vestimentiferans, polychaetes, vesicomyid clams and bathymodiolin mussels) or those that feed on external bacteria and detritus (e.g. polychaetes and gastropods) in sulfide-laden waters [9,10,19,22,23]. The present findings are also consistent with previous laboratory studies demonstrating that thiotaurine content increased during sulfide exposure of symbiont-bearing tissues (e.g. gills or foot) of deep-sea clams (vesicomyid), mussels (bathymodiolins), tube worms (vestimentiferans) [23] and shallow-living solemyid clams [50]. Although thiotaurine serves as a biomarker for deep-sea hydrothermal vent species [9], this is the first report that this molecule was detected in a crustacean species associated with sulfide detoxification mechanisms [51].
Figure 2.
(Overleaf.) Thiosulfate, hypotaurine and thiotaurine content and related transporter expression levels in X. testudinatus gill tissues. (a) Morphology of the branchial chamber of X. testudinatus. X. testudinatus has eight thoracic sternal segments and seven-gill pairs (aside to the hepatopacrease (Hep)) of which gill 1–3 (G1 to G3) are podobranchs, and G4 to G7 are arthrobranchs. (b) Thiosulfate contents were analysed in the haemolymph of X. testudinatus collected from the native high-sulfide field (black bar; n = 4), and sulfide-free SW acclimated counterpart (white bar; n = 5) (left panel). Whole gill tissue of X. testudinatus sampled from the high-sulfide field (black bar; n = 8), sulfide-free SW (white bar; n = 8), and 1000 µM [S2−] SW (slashed bar; n = 7) were used for thiosulfate concentration measurements (right panel). (c) Hypotaurine (left panel) and thiotaurine (right panel) contents were analysed in the haemolymph of X. testudinatus collected from the high-sulfide field (black bar), and sulfide-free SW acclimated counterparts (white bar). (N.D. represents non-detected; n = 10). Due to the distinction between haemolymph and gill sample types, the representative value units for the contents on the y-axis of panels (b) and (c) were displayed in different formats. (d) Semi-quantitative RT-PCR analysis of the solute carrier family (SLC) 26 member 11 (slc26a11a and slc26a11b) and solute carrier family (SLC) 6 member 6 (taut1 and taut2), was performed in gill (G), heart (H), ganglion (Ga), muscle (M), stomach (S), intestine (I), hepatopancreas (Hep), testis (T) and ovary (O) tissues of X. testudinatus. Ribosomal protein L9 (rpl9) was used as the internal control. Relative mRNA expression of slc26a11a (e) slc26a11b (g) and taut2 (i) among different pairs of gill arch under the native high-sulfide hydrothermal vent environment (black bar) and sulfide-free SW (white bar). mRNA expression of the candidate genes was normalized to the mean value from ribosomal protein L9 (rpl9) (n = 4–6). Values are expressed as mean ± s.d. *, Significant differences between high-sulfide and sulfide-free conditions. (Student's t-test, *: p < 0.05, **: p < 0.01, ***: p < 0.001). Different letters indicate significant differences among different treatment groups (one-way ANOVA, Tukey's pairwise comparisons). The same horizontal gill section (the 5th pair of gill arch) of X. testudinatus was double-labelled with (f) slc26a11a-specific, (h) slc26a11b-specific or (j) taut2-specific RNA probe for fluorescence in situ hybridization (red signal) and Na+/K+ ATPase (NKA, green signal) antibody for immunohistochemical staining. Scale bars correspond to 10 µm.
(c) . Sulfate and taurine transporters are stimulated in gill tissues during exposure to sulfide
Our transcriptomic and gene expression analyses identified the sodium-independent sulfate anion transporters (slc26a11a and slc26a11b) as well as the taurine transporter (taut2) that are expressed in gill tissues at a relatively high level (figure 2d). sulfide exposure stimulated expression levels of these sodium-independent sulfate anion transporters (figure 2e,g; electronic supplementary material, figure S2). In live-bearing Poecilia mexicana fish collected from river drainage, slc26a11 was associated with H2S tolerance in a population living near a sulfide spring [27]. In X. testudinatus the sulfate and taurine transporters were mainly expressed in epithelial principal and pilaster cells (figure 2f,h), with particularly high expression of SLC26A11 homologues in the 3rd, 5th, 6th and 7th pairs of gill arches. Na+/K+-ATPase (NKA)-rich cells of the posterior gills are the major sites for ion- and pH regulatory capacities [3,52], and indicates that X. testudinatus also utilizes NKA-rich cells of posterior gills for the shuttling of sulfide derivates and detoxification. These observations suggest that the SLC26A11 sulfate anion transporter plays a pivotal role in the sulfide/sulfate metabolism of animals including those from hydrothermal vent systems.
Regulation of hypotaurine transport across the cell membrane depends on the taurine transporter (Taut) SLC6A6 [53,54], which couples sodium and chloride transport with that of taurine and hypotaurine [55]. We found that X. testudinatus had high expression levels of taut2 in gill tissues (figure 2d) which is in accordance with observations made in the vent-endemic mussel Bathymodiolus septemdierum that also has particularly high expression levels of Taut in gill and gonad tissues [55]. Expression levels of Taut2 in gills of X. testudinaus were elevated in animals collected from the native sulfide-rich environment (figure 2i; electronic supplementary material, figure S2) compared to those kept under sulfide-free conditions. Furthermore, histological analyses using fluorescence in situ hybridization in posterior gills revealed that the taut2 mRNA signal is co-expressed with NKA-rich principal and pilaster cells (figure 2j). This colocalization pattern suggests that X. testudinatus may use the electrochemical sodium gradient generated by the NKA to energize cellular hypotaurine accumulation for the detoxification of sulfide to thiotaurine. Notably, increased slc26a11 and slc6a6 mRNA expression levels do not perfectly mirror statistically higher protein levels in this study. However, higher mRNA levels of these transporters under high sulfide conditions correlate with the accumulation of their putative substrates, which could still promote transporter activity and influence turnover rate through posttranslational modifications.
(d) . Structure of the gill microbiome depends on environmental sulfide concentrations
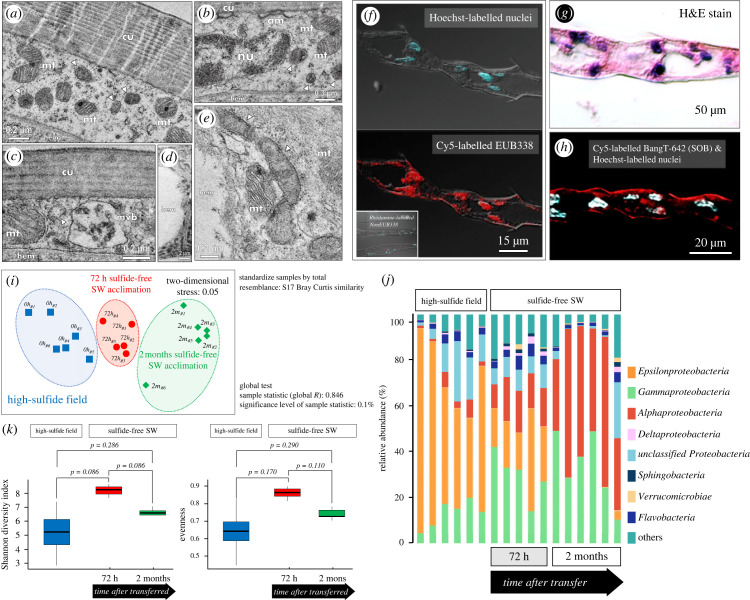
Thiotaurine has been proposed to play a dual role in vent-dwelling organisms. On one hand, it is used to detoxify sulfide compounds, and on the other hand, it can be used as an energy source by symbiotic sulfide-oxidizing bacteria [56]. Synthesis of thiotaurine from hypotaurine has been proposed to serve as a reversible non-toxic mechanism for sulfide storage in cells that can fuel endosymbionts during reduced sulfide availability [10]. For example, marine chemoautotrophic bacteria (e.g. Beggiatoa sp.) use sulfide and other reduced sulfur compounds for carbon fixation, with energetic efficiencies of 21–37% [57,58]. Furthermore, symbiotic sulfide-oxidizing bacteria have been shown to generate a large fraction of the energy requirements of their metazoan hosts in sulfidic habitats [59]. The present work, as well as a previous study [20], demonstrated a high abundance of Epsilonproteobacteria and Gammaproteobacteria within gill tissues of X. testudinatus crabs (figure 3, electronic supplementary material, figure S3). Epsilonproteobacteria are capable of autotrophic growth fueled by oxidation of reduced sulfur compounds, and Gammaproteobacteria are known to perform chemolithoautotrophic sulfur oxidization [36–38]. TEM (figure 3a–e) and fluorescence in situ hybridization (FISH) (figure 3f,h) analyses indicate the presence of symbiotic bacteria within gill tissues (figure 3g) of X. testudinatus. The symbiotic bacteria are not found on the surface of gill filaments/lamella but are located within epithelial cells (electronic supplementary material, figure S4). Ultrastructural analyses indicate the presence of coccoid-like symbiotic bacteria underlying the apical microvilli within principal cells of the gill (figure 3a–c). In addition, bacillus-like symbiotic bacteria clusters were observed in epithelial pilaster cell (figure 3d,e). Under fluorescence excitation light, crossed-sectioned gill tissue targeted with probes for bacteria (EUB338) displayed strong fluorescent signals within epithelial principal- and pilaster cells (figure 3f), suggesting the presence of endosymbionts within branchial cells. The morphology and tissue localization of microbial symbionts resemble those found in gill tissues of mytilid species from hydrothermal vent habitats [32]. In these bivalve species, coexistence of different bacteria species within one bacteriocyte was demonstrated [32]. Interestingly, it seems that X. testudinatus maintains different endosymbionts within at least two cell types of the gill, including principal cells that face the seawater as well as pilaster cells that are in contact with the haemolymph.
Figure 3.
Analysis of the microbial community in gill tissues of X. testudinatus. (a) Distinct spatial organization of bacteria in transmission electron micrographs of the 5th pair gill arch. (a–c) Principal cells contain intracellular coccoid-like symbiotic bacteria (indicated by arrowheads) underlying the apical microvilli (am)-anchored cuticle (cu). Principal cells display typical subcellular structures including nuclei (nu), multiple mitochondria (mt), and multivascular body (mvb). (d,e) Intracellular image of the epithelial pilaster cell with bacillus-like symbiotic bacteria clusters (indicated by arrowheads). (f–h) Fluorescence in situ hybridization (FISH) on gill cross-sections (H&E staining in (g)) using a domain-specific probe (Cy5-labelled EUB338) for bacteria and a Rhodamine-labelled NonEUB338 probe to the adjacent section as the negative control (f). A specific probe for sulfur-oxidizing bacteria (Cy5-labelled BangT-642) was also applied on another gill cross-section (h). (i,j) Two-dimensional scatter plot shows multidimensional scaling (MDS) analysis (i), and relative abundance of major bacterial classes normalized to the total bacterial community (j). Gills of X. testudinatus from the high-sulfide field (blue squares), 72 h sulfide-free SW acclimation (red circles), and two months sulfide-free SW-acclimation (green diamonds) were used in the experiment. The similarity index was calculated using the relative percentage of each bacteria genus in each sample. (k) Alpha diversity (intra- host diversity) was assessed by describing and comparing the microbial biodiversity metrics. The Shannon diversity index and evenness were presented for the high-sulfide field, 72 h sulfide-free SW acclimation, and two months of sulfide-free SW-acclimation, and compared using a pairwise wilcox test.
In the next step, 16s rRNA analyses were used to investigate the temporal differences in gill tissue microbial community structure between crabs living in the native sulfide-rich vent system and those kept under sulfide-free conditions. Beta-diversity analysis using nMDS with Bray–Curtis similarities identified a distinct commensal bacteria community composition after transferring X. testudinatus from the high-sulfide field to sulfide-free seawater in the laboratory (figure 3i). Significance of clustering was tested with ANOSIM (R = 0.846, p < 0.05). Epsilonproteobacteria were the most dominant bacterial class from the phylum Proteobacteria, accounting for 34.79% to 89.31% of total sequences in the gills of X. testudinatus from native sulfide-rich vent systems (six independent samples) (figure 3j). This result is similar to a previous report where 16S rRNA gene amplicon pyrosequencing and full-length LoopSeq sequencing using tissues from X. testudinatus demonstrated the presence Gamma- and Epsilonproteobacteriathat are usually associated with deep-sea hydrothermal systems [20,35,60,61]. Furthermore, the identified bacteria species are capable of autotrophic growth by oxidizing reduced sulfur compounds and a potential trophic symbiotic relationship was suggested [20,35]. Interestingly, after transfer from the native sulfide-rich vent system to the sulfide-free conditions for 72 h (five independent samples), Gammaproteobacteria became the most abundant class (14.27–42.33%) in gills. Crabs acclimated to sulfide-free seawater for two months had Gammaproteobacteria (10.62–49.20%) and Alphaproteobacteria (31.22–65.53%) as their dominantly associated bacterial classes with a relatively low fraction of Epsilonproteobacteria (0.04–3.88%) (figure 3j). Despite not reaching statistical significance due to a large degree of variation in the data, the alpha diversity analysis revealed that the microbial communities in the 5th pair of gill arches from the native sulfide-rich vent had lower microbial diversity (p = 0.086) and evenness (p = 0.170) than the samples from the acute 72 h sulfide-free treatment (figure 3k). When compared to 72 h of acute sulfide-free treatment, two months of exposure to sulfide-free SW resulted in a decrease in Shannon diversity index (p = 0.086) and evenness (p = 0.110) (figure 3k). In animals from the native sulfide-rich environment, the dominant groups within the proteobacteria classes were sulfur oxidizers, including Sulfurovum and Thiotrichaceae. After transfer to sulfide-free conditions, the relative abundance of Sulfurovum and Thiotrichaceae was decreased, with Vibrionaceae and Pseudoalteromonas becoming more dominant bacterial groups (electronic supplementary material, figure S3), which were described as potential symbiotic or pathogenic bacteria groups in aquatic organisms [62–64]. The relative expression levels of V6 to V8 hypervariable regions of Epsilonproteobacteria and Gammaproteobacteria, the key chemolithotrophic sulfur-oxidizing bacteria (SOB) at the deep-sea hydrothermal vent systems [65,66], 16S rRNA in the 5th pair of gill arch were further examined along a time course from several hours up to four months. We observed a decrease in 16S rRNA gene copy numbers for both Epsilonproteobacteria and Gammaproteobacteria at 6 h after transfer of the crabs from the native sulfide-rich vent system to sulfide-free conditions (electronic supplementary material, figure S5). After acclimation to sulfide-free seawater for more than 4 months, Epsilonproteobacteria were not detected in the 5th pair of gills, whereas Gammaproteobacteria relative abundance remained constant (figure 3j; electronic supplementary material, figure S5). FISH images utilizing a specific probe for thiotrophic symbionts further confirmed the absence of SOBs in gill epithelial cells (figure 3h). These findings further support the potential role of gill-associated microbial symbionts in handling sulfide and its metabolites in gill tissues of X. testudinatus. Based on our microbiome analyses and biochemical examination of sulfur compounds in gill tissues and/or haemolymph, we propose that the endosymbiotic bacteria in gills of X. testudinatus contribute to the breakdown of thiotaurine synthesized by the host. As such, reduced sulfur from the host cells could be used by the symbiotic bacteria as an energy source and in turn may provide the host with energy equivalents (summarized in figure 4). We cannot exclude the possibility that the gill microbiome is also affected by other environmental factors including pH and CO2. However, the strongest changes in abundance were detected for sulfide metabolizing microbial residents upon transfer from the native field to sulfide-free conditions suggesting that sulfide is the major factor for the differences observed in the microbial composition of the gills. Furthermore, very different microbial signatures were documented for organisms from sulfide-rich hydrothermal vent systems and CO2-enriched habitats [67,68]. This further underlines our conclusion that changes in the microbial signature of X. testudinatus observed in this work are predominantly related to environmental sulfide.
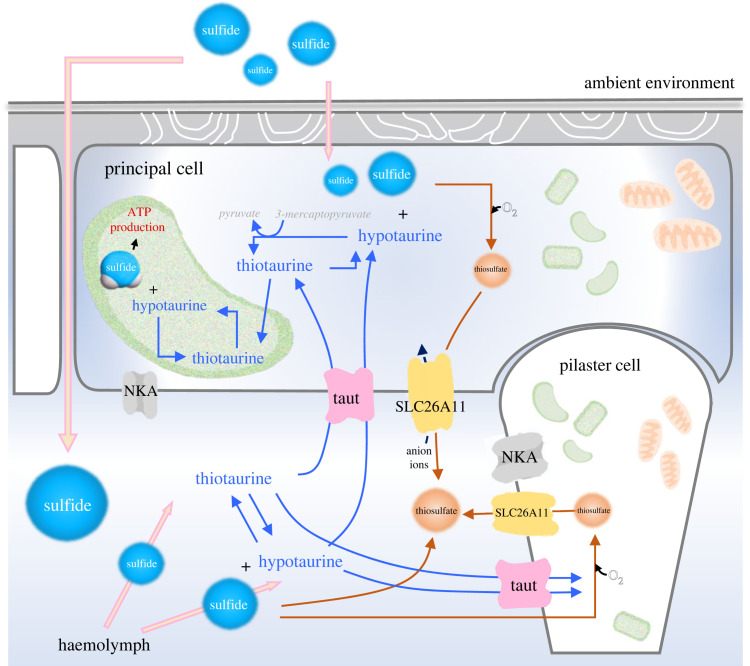
Figure 4.
Putative organization of sulfur metabolite exchange and chemoautotrophic symbiotic relationship in gills of X. testudinatus. Reduced sulfur compounds (thiosulfate or sulfate), and sulfide-binding amino acid (hyprotaurine) are generated from sulfide and transported by SLC26A11 and Taut to minimize sulfide accumulation in the haemolymph. In gill epithelial cells, hypotaurine reacts with diffused sulfide to form thiotaurine. Thiotaurine and reduced sulfur compounds (including thiosulfate) may thereafter be translocated to the sulfur-reducing bacteria, where they can be further oxidized to yield ATP and reduced metabolites. ATP and reduced metabolites may then be used in part to drive net CO2 fixation. Some fraction of the reduced carbon compounds synthesized by bacteria may be translocated to the animal host. The mitochondria and bacteria were shown in orange and green, respectively. Na+/K+-ATPase, NKA; SLC26A11, solute carrier family 26 member 11; Taut, taurine transporter.
4. Conclusion and perspectives
In the shallow-water hydrothermal vent ecosystem of Kueishan Island, weak predator–prey relationships and low trophic complexity were observed. This is mostly because of the low species variety in this extreme and nutrient-poor vent habitat, which results in the sporadic feeding behaviour of X. testudinatus on marine snow [69]. This work provides a line of evidence demonstrating that the shallow-water hydrothermal vent crab, X. testudinatus, has evolved biochemical and cellular pathways of sulfide detoxification mechanisms associated with endo-symbiotic bacteria in their gill tissues. Understanding inter-kingdom interactions in ancient marine vent systems require understanding the sulfide-transport and -binding detoxifying processes by which the metazoan host interacts with endosymbiotic bacteria in sulfide-rich vent habitats. This is of particular significance given that hydrothermal vent systems are regarded to be the cradle of life on Earth [70] and hence may provide insight into the origins of host–microbe interactions [71]. Metabolic substrates generated by symbiotic bacteria or the bacteria itself may provide important additional energy sources for this crab population (figure 4). This trophic interaction between host and microbes in crustacean holobionts may explain the high concentration of (n-7) monounsaturated fatty acids (MUFAs) in midgut tissues of X. testudinatus [72]. (n-7) MUFAs serve as trophic markers of bacterial origin and dominate the fatty acid profiles of many deep-sea hydrothermal vent species [73,74]. Accordingly, this work provides the first understanding of the cellular detoxification processes and physiology of host-microbe interactions that allowed a crustacean holobiont to thrive in ecological niches of the most extreme environments on our planet.
Data accessibility
The data that support the findings of this study are available in the electronic supplementary material [75].
Authors' contributions
P.-H.C.: data curation, investigation, resources, writing—original draft; M.Y.H.: data curation, investigation, methodology, writing—original draft, writing—review and editing; Y.-J.G.: data curation, investigation, methodology, writing—original draft; G.-C.W.: data curation, investigation, project administration, writing—original draft; S.-H.Y.: data curation, investigation, methodology, writing—original draft; K.T.: data curation, methodology; Y.-T.S.: data curation, investigation, resources; L.-Y.L.: data curation, investigation; C.C.: methodology, resources; K.-Y.T.: data curation, methodology; M.-C.W.: data curation, investigation, methodology, resources; C.-M.Z.: data curation, investigation, resources; B.-C.H.: data curation, methodology; C.-C.L.: data curation, methodology; S.-L.T.: data curation, investigation, methodology, supervision; M.-S.J.: data curation, investigation, methodology, resources, supervision, writing—original draft; C.-F.C.: funding acquisition, investigation, project administration, resources, supervision, writing—original draft, writing—review and editing; Y.-C.T.: data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was financially supported by the grant from the National Science and Technology Council, Taiwan, Republic of China (MOST 111-2621-M-019-003).
References
- 1.Dorman DC, Moulin FJ-M, McManus BE, Mahle KC, James RA, Struve MF. 2002. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci. 65, 18-25. ( 10.1093/toxsci/65.1.18) [DOI] [PubMed] [Google Scholar]
- 2.Goffredi S, Childress J, Desaulniers N, Lee R, Lallier F, Hammond D. 1997. Inorganic carbon acquisition by the hydrothermal vent tubeworm Riftia pachyptila depends upon high external PCO2 and upon proton-equivalent ion transport by the worm. J. Exp. Biol. 200, 883-896. ( 10.1242/jeb.200.5.883) [DOI] [PubMed] [Google Scholar]
- 3.Hu MY, Guh Y-J, Shao Y-T, Kuan P-L, Chen G-L, Lee J-R, Jeng M-S, Tseng Y-C. 2016. Strong ion regulatory abilities enable the crab Xenograpsus testudinatus to inhabit highly acidified marine vent systems. Front. Physiol 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMullin ER, Bergquist DC, Fisher CR. 2007. Metazoans in extreme environments: adaptations of hydrothermal vent and hydrocarbon seep fauna. Gravit. Space Biol. Bull. 13, 13-24. [PubMed] [Google Scholar]
- 5.Ramirez-Llodra E, Shank TM, German CR. 2007. Biodiversity and biogeography of hydrothermal vent species: thirty years of discovery and investigations. Oceanography 20, 30-41. ( 10.5670/oceanog.2007.78) [DOI] [Google Scholar]
- 6.Zierenberg RA, Adams MW, Arp AJ. 2000. Life in extreme environments: hydrothermal vents. Proc. Natl Acad. Sci. USA 97, 12 961-12 962. ( 10.1073/pnas.210395997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamenick I, Vismann B, Grieshaber M, Giere O. 1998. Ecophysiological differentiation of Capitella capitata (Polychaeta). Sibling species from different sulfidic habitats. Mar. Ecol. Prog. Ser. 175, 155-166. ( 10.3354/meps175155) [DOI] [Google Scholar]
- 8.Thiermann F, Vismann B, Giere O. 2000. Sulphide tolerance of the marine nematode Oncholaimus campylocercoides--a result of internal sulphur formation? Mar. Ecol. Prog. Ser. 193, 251-259. ( 10.3354/meps193251) [DOI] [Google Scholar]
- 9.Brand GL, Horak RV, Bris NL, Goffredi SK, Carney SL, Govenar B, Yancey PH. 2007. Hypotaurine and thiotaurine as indicators of sulfide exposure in bivalves and vestimentiferans from hydrothermal vents and cold seeps. Mar. Ecol. 28, 208-218. ( 10.1111/j.1439-0485.2006.00113.x) [DOI] [Google Scholar]
- 10.Yancey PH, Ishikawa J, Meyer B, Girguis PR, Lee RW. 2009. Thiotaurine and hypotaurine contents in hydrothermal-vent polychaetes without thiotrophic endosymbionts: correlation with sulfide exposure. J. Exp. Zool. A Ecol. Genet. Physiol. 311, 439-447. ( 10.1002/jez.541) [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Sun QL, Luan ZD, Lian C, Sun L. 2017. Comparative transcriptome analysis of Rimicaris sp. reveals novel molecular features associated with survival in deep-sea hydrothermal vent. Sci. Rep. 7, 1-16. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarasov V, Gebruk A, Mironov A, Moskalev L. 2005. Deep-sea and shallow-water hydrothermal vent communities: two different phenomena? Chem. Geol. 224, 5-39. ( 10.1016/j.chemgeo.2005.07.021) [DOI] [Google Scholar]
- 13.Han C, Ye Y, Pan Y, Qin H, Wu G, Chen C-TA. 2014. Spatial distribution pattern of seafloor hydrothermal vents to the southeastern Kueishan Tao offshore Taiwan Island. Acta Oceanol. Sin. 33, 37-44. ( 10.1007/s13131-014-0405-x) [DOI] [Google Scholar]
- 14.Chung SL, Wang SL, Shinjo R, Lee CS, Chen CH. 2000. Initiation of arc magmatism in an embryonic continental rifting zone of the southernmost part of Okinawa Trough. Terra Nova 12, 225-230. ( 10.1046/j.1365-3121.2000.00298.x) [DOI] [Google Scholar]
- 15.Chen C. 2005. Investigation into extremely acidic hydrothermal fluids off Kueishantao islet, Taiwan. Acta Oceanol. Sin. 24, 125-133. [Google Scholar]
- 16.Jeng M-S, Clark PF, Ng PK. 2004. The first zoea, megalopa, and first crab stage of the hydrothermal vent crab Xenograpsus testudinatus (Decapoda: Brachyura: Grapsoidea) and the systematic implications for the Varunidae. J. Crustacean Biol. 24, 188-212. ( 10.1651/C-2359) [DOI] [Google Scholar]
- 17.Ng NK, Huang J, Ho P-H. 2000. Description of a new species of hydrothermal crab, Xenograpsus testudinatus (Crustacea: Decapoda: Brachyura: Grapsidae) from Taiwan. Natl. Taiwan Mus. Publ. Ser. 10, 191-199. [Google Scholar]
- 18.Jeng MS, Ng NK, Ng PKL. 2004. Feeding behaviour: Hydrothermal vent crabs feast on sea ‘snow’. Nature 432, 969. ( 10.1038/432969a) [DOI] [PubMed] [Google Scholar]
- 19.Vetter R. 1985. Elemental sulfur in the gills of three species of clams containing chemoautotrophic symbiotic bacteria: a possible inorganic energy storage compound. Mar. Biol. 88, 33-42. ( 10.1007/BF00393041) [DOI] [Google Scholar]
- 20.Yang S-H, Chiang P-W, Hsu T-C, Kao S-J, Tang S-L. 2016. Bacterial community associated with organs of shallow hydrothermal vent crab Xenograpsus testudinatus near Kuishan Island, Taiwan. PLoS ONE 11, e0150597. ( 10.1371/journal.pone.0150597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruski A, Médioni AF, Prodon R, Colomines J. 2000. Thiotaurine is a biomarker of sulfide-based symbiosis in deep-sea bivalves. Limnol. Oceanogr. 45, 1860-1867. ( 10.4319/lo.2000.45.8.1860) [DOI] [Google Scholar]
- 22.Arp A, Childress J, Vetter R. 1987. The sulphide-binding protein in the blood of the vestimentiferan tube-worm, Riftia pachyptila, is the extracellular haemoglobin. J. Exp. Biol. 128, 139-158. ( 10.1242/jeb.128.1.139) [DOI] [Google Scholar]
- 23.Pruski AM, Fiala-Médioni A. 2003. Stimulatory effect of sulphide on thiotaurine synthesis in three hydrothermal-vent species from the East Pacific Rise. J. Exp. Biol. 206, 2923-2930. ( 10.1242/jeb.00513) [DOI] [PubMed] [Google Scholar]
- 24.Powell M, Somero G. 1985. Sulfide oxidation occurs in the animal tissue of the gutless clam, Solemya reidi. Biol. Bull. 169, 164-181. ( 10.2307/1541396) [DOI] [Google Scholar]
- 25.Leinberger J, Milke F, Christodoulou M, Poehlein A, Caraveo-Patiño J, Teske A, Brinkhoff T. 2022. Microbial epibiotic community of the deep-sea galatheid squat lobster Munidopsis alvisca. Sci. Rep. 12, 1-15. ( 10.1038/s41598-022-06666-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetter R, Wells M, Kurtsman AL, Somero G. 1987. Sulfide detoxification by the hydrothermal vent crab Bythograea thermydron and other decapod crustaceans. Physiol. Zool. 60, 121-137. ( 10.1086/physzool.60.1.30158634) [DOI] [Google Scholar]
- 27.Kelley JL, Arias-Rodriguez L, Patacsil Martin D, Yee M-C, Bustamante CD, Tobler M. 2016. Mechanisms underlying adaptation to life in hydrogen sulfide-rich environments. Mol. Biol. Evol. 33, 1419-1434. ( 10.1093/molbev/msw020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon J, Willsie JK, Tauscher A, Arp AJ. 2003. Epidermal ultrastructure and implications for sulfide tolerance in six species of deep-sea polychaetes. Invertebr. Biol. 122, 334-346. ( 10.1111/j.1744-7410.2003.tb00098.x) [DOI] [Google Scholar]
- 29.Powell M, Somero G. 1986. Adaptations to sulfide by hydrothermal vent animals: sites and mechanisms of detoxification and metabolism. Biol. Bull. 171, 274-290. ( 10.2307/1541923) [DOI] [Google Scholar]
- 30.Bright M, Sorgo A. 2003. Ultrastructural reinvestigation of the trophosome in adults of Riftia pachyptila (Annelida. Siboglinidae) . Invertebr. Biol. 122, 347-368. ( 10.1111/j.1744-7410.2003.tb00099.x) [DOI] [Google Scholar]
- 31.Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB. 1981. Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science 213, 340-342. ( 10.1126/science.213.4505.340) [DOI] [PubMed] [Google Scholar]
- 32.Distel DL, Lee H, Cavanaugh CM. 1995. Intracellular coexistence of methano-and thioautotrophic bacteria in a hydrothermal vent mussel. Proc. Natl Acad. Sci. USA 92, 9598-9602. ( 10.1073/pnas.92.21.9598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Childress J, Girguis PR. 2011. The metabolic demands of endosymbiotic chemoautotrophic metabolism on host physiological capacities. J. Exp. Biol. 214, 312-325. ( 10.1242/jeb.049023) [DOI] [PubMed] [Google Scholar]
- 34.Felbeck H, Jarchow J. 1998. Carbon release from purified chemoautotrophic bacterial symbionts of the hydrothermal vent tubeworm Riftia pachyptila. Physiol. Zool. 71, 294-302. ( 10.1086/515931) [DOI] [PubMed] [Google Scholar]
- 35.Chiu L, Wang M-C, Tseng K-Y, Wei C-L, Lin H-T, Yang S-H, Tseng Y-C. 2022. Shallow-water hydrothermal vent system as an extreme proxy for discovery of microbiome significance in a crustacean holobiont. Front. Mar. Sci. 9, 976255. ( 10.3389/fmars.2022.976255) [DOI] [Google Scholar]
- 36.Goffredi SK. 2010. Indigenous ectosymbiotic bacteria associated with diverse hydrothermal vent invertebrates. Environ. Microbiol. Rep. 2, 479-488. ( 10.1111/j.1758-2229.2010.00136.x) [DOI] [PubMed] [Google Scholar]
- 37.Hügler M, Petersen JM, Dubilier N, Imhoff JF, Sievert SM. 2011. Pathways of carbon and energy metabolism of the epibiotic community associated with the deep-sea hydrothermal vent shrimp Rimicaris exoculata. PLoS ONE 6, e16018. ( 10.1371/journal.pone.0016018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponsard J, et al. 2013. Inorganic carbon fixation by chemosynthetic ectosymbionts and nutritional transfers to the hydrothermal vent host-shrimp Rimicaris exoculata. ISME J. 7, 96. ( 10.1038/ismej.2012.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lallier FH. 1998. Determination of reduced sulfur compounds by high-performance liquid chromatography in hydrothermal seawater and body fluids from Riftia pachyptila. Analyst 123, 1289-1293. ( 10.1039/a800032h) [DOI] [Google Scholar]
- 40.Laudien J, Schiedek D, Brey T, Pörtner H-O, Arntz W. 2002. Survivorship of juvenile surf clams Donax serra (Bivalvia, Donacidae) exposed to severe hypoxia and hydrogen sulphide. J. Exp. Mar. Biol. Ecol. 271, 9-23. ( 10.1016/S0022-0981(02)00030-8) [DOI] [Google Scholar]
- 41.Goffredi SK, Barry JP. 2002. Species-specific variation in sulfide physiology between closely related Vesicomyid clams. Mar. Ecol. Prog. Ser. 225, 227-238. ( 10.3354/meps225227) [DOI] [Google Scholar]
- 42.Sahling H, Rickert D, Lee RW, Linke P, Suess E. 2002. Macrofaunal community structure and sulfide flux at gas hydrate deposits from the Cascadia convergent margin, NE Pacific. Mar. Ecol. Prog. Ser. 231, 121-138. ( 10.3354/meps231121) [DOI] [Google Scholar]
- 43.Fisher C, et al. 1988. Physiology, morphology, and biochemical composition of Riftia pachyptila at Rose Garden in 1985. Deep-Sea Res. Pt I 35, 1745-1758. ( 10.1016/0198-0149(88)90047-7) [DOI] [Google Scholar]
- 44.Johnson KS, Childress JJ, Hessler RR, Sakamoto-Arnold CM, Beehler CL. 1988. Chemical and biological interactions in the Rose Garden hydrothermal vent field, Galapagos spreading center. Deep-Sea Res. Pt I 35, 1723-1744. ( 10.1016/0198-0149(88)90046-5) [DOI] [Google Scholar]
- 45.Zielinski FU, Gennerich HH, Borowski C, Wenzhöfer F, Dubilier N. 2011. In situ measurements of hydrogen sulfide, oxygen, and temperature in diffuse fluids of an ultramafic-hosted hydrothermal vent field (Logatchev, 14°45′N, Mid-Atlantic Ridge): implications for chemosymbiotic bathymodiolin mussels. Geochem. Geophy. Geosys. 12, 1-21. ( 10.1029/2011GC003632) [DOI] [Google Scholar]
- 46.Butterworth K, Grieshaber M, Taylor A. 2004. Behavioural and physiological responses of the Norway lobster, Nephrops norvegicus (Crustacea: Decapoda), to sulphide exposure. Mar. Biol. 144, 1087-1095. ( 10.1007/s00227-003-1276-4) [DOI] [Google Scholar]
- 47.Jahn A, Gamenick I, Theede H. 1996. Physiological adaptations of Cyprideis torosa (Crustacea, Ostracoda) to hydrogen sulphide. Mar. Ecol. Prog. Ser. 142, 215-223. ( 10.3354/meps142215) [DOI] [Google Scholar]
- 48.Johns A, Taylor A, Atkinson R, Grieshaber M. 1997. Sulphide metabolism in thalassinidean crustacea. J. Mar. Biol. Assoc. UK 77, 127-144. ( 10.1017/S0025315400033828) [DOI] [Google Scholar]
- 49.Shumway SE, Scott TM, Shick JM. 1983. The effects of anoxia and hydrogen sulphide on survival, activity and metabolic rate in the coot clam, Mulinia lateralis (Say). J. Exp. Mar. Biol. Ecol. 71, 135-146. ( 10.1016/0022-0981(93)90069-Z) [DOI] [Google Scholar]
- 50.Joyner JL, Peyer SM, Lee RW. 2003. Possible roles of sulfur-containing amino acids in a chemoautotrophic bacterium-mollusc symbiosis. Biol. Bull. 205, 331-338. ( 10.2307/1543296) [DOI] [PubMed] [Google Scholar]
- 51.Achituv Y, Pedrotti M. 1999. Costs and gains of porcelain crab suspension feeding in different flow conditions. Mar. Ecol. Prog. Ser. 184, 161-169. ( 10.3354/meps184161) [DOI] [Google Scholar]
- 52.Freire CA, Onken H, McNamara JC. 2008. A structure–function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 272-304. ( 10.1016/j.cbpa.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 53.Thimgan MS, Berg JS, Stuart AE. 2006. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J. Exp. Biol. 209, 3383-3404. ( 10.1242/jeb.02328) [DOI] [PubMed] [Google Scholar]
- 54.Nishimura T, Higuchi K, Yoshida Y, Sugita-Fujisawa Y, Kojima K, Sugimoto M, Santo M, Tomi M, Nakashima E. 2018. Hypotaurine is a substrate of GABA transporter family members GAT2/Slc6a13 and TAUT/Slc6a6. Biol. Pharm. Bull. 41, 1523-1529. ( 10.1248/bpb.b18-00168) [DOI] [PubMed] [Google Scholar]
- 55.Inoue K, Tsukuda K, Koito T, Miyazaki Y, Hosoi M, Kado R, Miyazaki N, Toyohara H. 2008. Possible role of a taurine transporter in the deep-sea mussel Bathymodiolus septemdierum in adaptation to hydrothermal vents. FEBS Lett. 582, 1542-1546. ( 10.1016/j.febslet.2008.03.052) [DOI] [PubMed] [Google Scholar]
- 56.Somero G. 1987. Symbiotic exploitation of hydrogen sulfide. Physiology 2, 3-6. ( 10.1152/physiologyonline.1987.2.1.3) [DOI] [Google Scholar]
- 57.Jørgensen B. 1982. Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Phil. Trans. R. Soc. Lond. B 298, 543-561. ( 10.1098/rstb.1982.0096) [DOI] [PubMed] [Google Scholar]
- 58.Nelson DC, Jannasch HW. 1983. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient cultures. Arch. Microbiol. 136, 262-269. ( 10.1007/BF00425214) [DOI] [Google Scholar]
- 59.Bagarinao T. 1992. Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 24, 21-62. ( 10.1016/0166-445X(92)90015-F) [DOI] [Google Scholar]
- 60.Dick GJ, Tebo BM. 2010. Microbial diversity and biogeochemistry of the Guaymas Basin deep-sea hydrothermal plume. Environ. Microbiol. 12, 1334-1347. ( 10.1111/j.1462-2920.2010.02177.x) [DOI] [PubMed] [Google Scholar]
- 61.Dick GJ. 2019. The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally. Nat. Rev. Microbiol. 17, 271-283. ( 10.1038/s41579-019-0160-2) [DOI] [PubMed] [Google Scholar]
- 62.Faghri MA, Pennington CL, Cronholm LS, Atlas RM. 1984. Bacteria associated with crabs from cold waters with emphasis on the occurrence of potential human pathogens. Appl. Environ. Microbiol. 47, 1054-1061. ( 10.1128/aem.47.5.1054-1061.1984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joseph FS, Latha N, Ravichandran S, Devi AS, Sivasubramanian K. 2014. Shell disease in the freshwater crab, Barytelphusa cunicularis . Int. J. Fish Aquat. Stud. 1, 105-110. [Google Scholar]
- 64.Haditomo A, Prayitno SB. 2018. The bacterial diversity associated with bacterial diseases on Mud Crab (Scylla serrata Fab.) from Pemalang Coast, Indonesia. In J. Phys. Conf. Ser., pp. 012076: IOP Publishing. [Google Scholar]
- 65.Meier DV, Pjevac P, Bach W, Hourdez S, Girguis PR, Vidoudez C, Amann R, Meyerdierks A. 2017. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J. 11, 1545-1558. ( 10.1038/ismej.2017.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halary S, Riou V, Gaill F, Boudier T, Duperron S. 2008. 3D FISH for the quantification of methane-and sulphur-oxidizing endosymbionts in bacteriocytes of the hydrothermal vent mussel Bathymodiolus azoricus. ISME J. 2, 284-292. ( 10.1038/ismej.2008.3) [DOI] [PubMed] [Google Scholar]
- 67.Fonseca F, Cerqueira R, Fuentes J. 2019. Impact of ocean acidification on the intestinal microbiota of the marine sea bream (Sparus aurata L). Front. Physiol. 10, 1446. ( 10.3389/fphys.2019.01446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Botté ES, Nielsen S, Abdul Wahab MA, Webster J, Robbins S, Thomas T, Webster NS. 2019. Changes in the metabolic potential of the sponge microbiome under ocean acidification. Nat. Commun. 10, 1-10. ( 10.1038/s41467-019-12156-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang N-N, Lin L-H, Tu T-H, Jeng M-S, Chikaraishi Y, Wang P-L. 2018. Trophic structure and energy flow in a shallow-water hydrothermal vent: insights from a stable isotope approach. PLoS ONE 13, e0204753. ( 10.1371/journal.pone.0204753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson-Sathi S, Martin WF. 2016. The physiology and habitat of the last universal common ancestor. Nat Microbiol 1, 1-8. ( 10.1038/nmicrobiol.2016.116) [DOI] [PubMed] [Google Scholar]
- 71.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. 2012. Animal behavior and the microbiome. Science 338, 198-199. ( 10.1126/science.1227412) [DOI] [PubMed] [Google Scholar]
- 72.Hu MY-A, Hagen W, Jeng M-S, Saborowski R. 2012. Metabolic energy demand and food utilization of the hydrothermal vent crab Xenograpsus testudinatus (Crustacea: Brachyura). Aquat. Biol. 15, 11-25. ( 10.3354/ab00396) [DOI] [Google Scholar]
- 73.Phleger CF, Nelson MM, Groce AK, Cary SC, Coyne K, Gibson JA, Nichols PD. 2005. Lipid biomarkers of deep-sea hydrothermal vent polychaetes—Alvinella pompejana, A. caudata, Paralvinella grasslei and Hesiolyra bergii. Deep-Sea Res. Pt I 52, 2333-2352. ( 10.1016/j.dsr.2005.08.001) [DOI] [Google Scholar]
- 74.Phleger CF, Nelson MM, Groce AK, Cary SC, Coyne KJ, Nichols PD. 2005. Lipid composition of deep-sea hydrothermal vent tubeworm Riftia pachyptila, crabs Munidopsis subsquamosa and Bythograea thermydron, mussels Bathymodiolus sp. and limpets Lepetodrilus spp. Comp. Biol. Physiol. B Biochem. Mol. Biol. 141, 196-210. ( 10.1016/j.cbpc.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 75.Chou P-H, et al. 2023. Cellular mechanisms underlying extraordinary sulfide tolerance in a crustacean holobiont from hydrothermal vents. Figshare. ( 10.6084/m9.figshare.c.6328044) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the electronic supplementary material [75].