Abstract
Campylobacter jejuni strain 81-176 contains two, previously undescribed plasmids, each of which is approximately 35 kb in size. Although one of the plasmids, termed pTet, carries a tetO gene, conjugative transfer of tetracycline resistance to another strain of C. jejuni could not be demonstrated. Partial sequence analysis of the second plasmid, pVir, revealed the presence of four open reading frames which encode proteins with significant sequence similarity to Helicobacter pylori proteins, including one encoded by the cag pathogenicity island. All four of these plasmid-encoded proteins show some level of homology to components of type IV secretion systems. Mutation of one of these plasmid genes, comB3, reduced both adherence to and invasion of INT407 cells to approximately one-third that seen with wild-type strain 81-176. Mutation of comB3 also reduced the natural transformation frequency. A mutation in a second plasmid gene, a virB11 homolog, resulted in a 6-fold reduction in adherence and an 11-fold reduction in invasion compared to the wild type. The isogenic virB11 mutant of strain 81-176 also demonstrated significantly reduced virulence in the ferret diarrheal disease model. The virB11 homolog was detected on plasmids in 6 out of 58 fresh clinical isolates of C. jejuni, suggesting that plasmids are involved in the virulence of a subset of C. jejuni pathogens.
Although Campylobacter jejuni is one of the major causes of bacterial diarrhea worldwide (51, 59), the details of its molecular pathogenesis are not well understood. The clinical symptoms of campylobacter infection can range from a mild, watery diarrhea to a dysentery-like illness with fecal blood and leukocytes (2). Although there are reports of numerous cytotoxins, only the cytolethal distending toxin, which arrests eukaryotic cells at the G2 phase of the cell cycle (64), has been characterized in detail. There are numerous reports that C. jejuni strains can invade intestinal epithelial cells in vitro (20, 21, 24, 29, 30, 38), although levels of invasion by different strains vary considerably (20, 28, 38, 52). Strain 81-176, originally isolated from a diarrheal outbreak associated with raw-milk consumption (31), is one of the best-characterized strains of C. jejuni. This strain has been shown to cause an inflammatory diarrhea in two human feeding studies (8; D. Tribble, unpublished data) and to cause disease in experimental models using primates (40) and ferrets (19, 67). Further, C. jejuni strain 81-176 invades INT407 cells at levels higher than those of most other C. jejuni strains (28, 38).
Plasmids have been found in between 19 and 53% of C. jejuni strains (5, 9–11, 41, 53–58), and many of these have been reported to be R plasmids that are transmissible among Campylobacter spp. but not to Escherichia coli (53–58, 60). Despite the importance of plasmids to virulence in numerous other pathogens, it is generally believed that plasmids play no role in Campylobacter pathogenicity. This paradigm is based on the rather low level at which plasmids have been reported and on an early study which compared the plasmid content and relative virulence of different C. jejuni strains in a guinea pig model of disease (55). In previous studies with 81-176, we have observed the presence of two cryptic plasmids (R. Yao and P. Guerry, unpublished data). Herein, we show that one of these plasmids is an R factor encoding tetracycline resistance and that partial sequence analysis of the second plasmid revealed open reading frames (ORFs) encoding predicted proteins which display strong similarity to Helicobacter pylori proteins, one of which is encoded by the cag pathogenicity island. Moreover, we report that mutation of two of these genes, which encode homologs of type IV secretion systems, affect the virulence of C. jejuni 81-176.
MATERIALS AND METHODS
Strains and plasmids.
C. jejuni 81-176 has been described previously (8, 31). The isolate used in the present studies was isolated from a patient in the human feeding studies described by Black et al. (8). E. coli DH5α was used as the host for cloning experiments, and pBluescript was used as the cloning vector. C. jejuni VC83 Strr Nalr was made by selection of the previously described VC83 Strr mutant (26) onto Mueller-Hinton (MH) agar supplemented with 50 μg of naladixic acid per ml. Fresh clinical isolates from patients with diarrhea were obtained through the Armed Forces Research Institute in Bangkok, Thailand. Isolates were picked from campylobacter selective media directly onto blood agar plates for hybridization analysis (see below) without additional in vitro laboratory passage.
Bacterial growth conditions.
Bacterial strains were maintained at −80°C in brucella broth (Difco) supplemented with 40% glycerol. C. jejuni was grown routinely on MH agar under microaerobic conditions at 37°C. Cultures for adherence and invasion assays were grown in MH biphasic cultures, consisting of MH agar overlaid with MH broth in tissue culture flasks. Antibiotics were added when appropriate to the following concentrations: 20 μg of trimethoprim per ml, 50 μg of kanamycin per ml, and 20 μg of tetracycline per ml.
DNA manipulations.
Plasmids were purified from C. jejuni 81-176 by using mini-Qiagen columns (Qiagen, Chatsworth, Calif.) as directed by the manufacturer. Restriction enzymes and DNA-modifying enzymes were purchased from New England Biolabs (Beverly, Mass.). Double-stranded plasmid clones were sequenced using AmpliTaq DNA polymerase FS (Perkin-Elmer–Applied Biosystems, Foster City, Calif.) on an ABI 373XL DNA sequencer, and sequences were analyzed using Sequencer 3.1.1 (Gene Codes Corp., Ann Arbor, Mich.) and MacVector (Oxford Molecular Systems, Oxford, United Kingdom).
Conjugative transfer of C. jejuni plasmids.
Attempts at conjugative transfer of plasmids from 81-176 into C. jejuni VC83 Strr Nalr were made by the method of Taylor et al. (57). Essentially, overnight cultures of donor and recipient cells were mixed in ratios ranging from 1:1 to 1:10, spotted on MH agar, and incubated for 24 h at 37°C. Cultures were subsequently resuspended in 1 ml of MH broth, diluted, and plated onto the appropriate selective medium. For crosses with wild-type 81-176 into VC83 Strr Nalr, the selective medium contained 20 μg of tetracycline per ml, 20 μg of streptomycin per ml, and 50 μg of nalidixic acid per ml; for crosses in which we attempted to mobilize pRY111 from 81-176 into VC83 Strr Nalr, the selective medium was MH agar with 20 μg of chloramphenicol per ml, 20 μg of streptomycin per ml, and 50 μg of nalidixic acid per ml.
Site-specific mutagenesis.
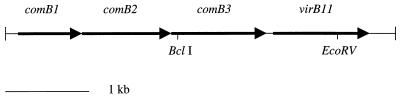
A 6-kb BglII fragment (indicated by the arrow in Fig. 1) was cloned from the 81-176 pVir plasmid into pBluescript, generating pDB100. The kanamycin resistance (Kmr) cassette from pILL600 (32) was used for all mutations. The orientation of the Kmr cassette within the target gene was confirmed by either PCR or sequence analysis to be in the same orientation as the genes are transcribed, an orientation which is nonpolar (43; A. L. McVeigh and P. Guerry, unpublished data). In the case of the virB11 mutation, the Kmr cassette was inserted into a unique EcoRV site within an EcoRI subclone of pDB100, generating pDB102. An EcoRI fragment containing the oriT from the campylobacter suicide vector, pGK2003 (25), was treated with Klenow polymerase (New England Biolabs) to generate a blunt-ended DNA fragment, which was subsequently cloned into the unique SmaI site in the multiple-cloning site of pDB102 to generate pDB103. This plasmid was conjugatively mobilized by RK212.2 (22) into C. jejuni 81-176, and transconjugants were selected on MH agar supplemented with 10 μg of trimethoprim per ml and 50 μg of kanamycin per ml. In a similar fashion, the Kmr cassette was inserted into a unique BclI site in the comB3 gene of pDB100 to generate pDB106, which was used to naturally transform 81-176 (26, 62). All putative C. jejuni mutants were characterized by PCR using primers within the target gene to confirm that the plasmids had integrated via a double crossover.
FIG. 1.
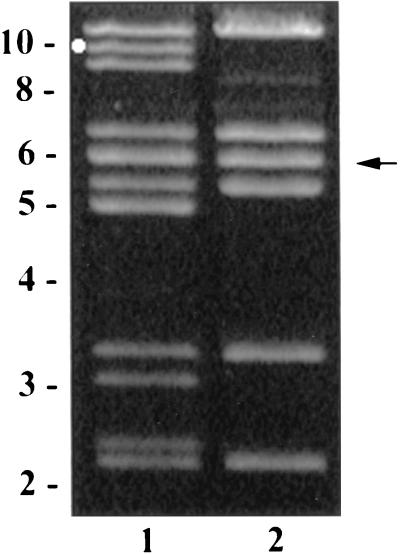
BglII digestion pattern of plasmids isolated from C. jejuni. Lanes: 1, 81-176; 2, DB179, a TetS derivative of 81-176. The circle marks the fragment which hybridized to a tetO probe, and the arrow indicates the 6-kb BglII fragment cloned in pDB100. The positions of size markers in kilobase pairs are indicated to the left.
Motility assays.
The motility of C. jejuni strains was determined by stabbing the culture onto MH broth supplemented with 0.4% agar and observing the zone of motility after a 24-h incubation at 37°C (25).
Adherence and invasion assays.
Adherence and invasion assays were done essentially as previously described (28, 38, 67). Briefly, mid-log-phase bacteria were added to a semiconfluent monolayer of approximately 2 × 105 INT407 cells at a multiplicity of infection of ca. 10 in 1 ml of culture medium per well. Infected monolayers were incubated for 2 h at 37°C in a 5% CO2–95% air atmosphere. For determination of adherence, the cells were washed four times in Hanks' balanced salt solution with strong agitation for 2 min, the monolayer was lysed with 0.01% Triton X-100 for 30 min at room temperature on an orbital shaker, and total bacteria were enumerated by the plate count method. For determination of invasion, the monolayer was washed twice with Hanks' balanced salt solution, and fresh prewarmed medium containing gentamicin at 100 μg/ml was added to kill extracellular bacteria. After a 2-h incubation, the monolayer was washed twice with Hanks' balanced salt solution and lysed with 0.01% Triton X-100 as above. Following serial dilution in phosphate-buffered saline, released intracellular bacteria were enumerated by the colony count method on MH agar cultured under microaerobic conditions. Invasion ability was expressed as the percentage of the inoculum surviving the gentamicin treatment, and adherent bacteria were expressed as the total number of bacteria counted without antibiotic treatment. Assays were repeated at least three times.
Invasion assays in the presence of biochemical inhibitors.
Inhibitors of eukaryotic cell processes were added to the monolayer 1 h prior to the addition of bacteria and were maintained throughout the 2-h invasion period, as described previously (28, 38, 67). Mid-log-phase bacteria were grown in MH biphasic medium and added at multiplicity of infection of ca. 20. Following the invasion period, the infected monolayer was washed three times with Earle's balanced salt solution and incubated for another 2 h in fresh culture medium containing 100 μg of gentamicin per ml to kill extracellular bacteria. Subsequently, the infected monolayers were washed and lysed, and the internalized bacteria were enumerated by the plate count method. Control studies were conducted to verify that at the concentrations employed, each inhibitor did not affect epithelial cell viability or bacterial viability over the assay period, as measured by trypan blue staining and the viable plate count method, respectively. Salmonella enterica serovar Typhi was used as a microfilament (MF)-dependent invasion control. All invasion inhibition assays were repeated on three separate occasions. Invasion efficiency (i.e., the percentage of the inoculum internalized) is presented as the mean ± standard error of the mean for all assays. The relative percent invasiveness was determined as recovery in the presence of inhibitors divided by recovery in the absence of inhibitors, multiplied by 100.
Ferret experiments.
The experiments reported herein were conducted according to the principles set forth in the ‘Guide for the care and Use of Laboratory Animals’, Institute of Laboratory Animals Resources, National Research Council, DHHS Publication No. (NIH) 86-23 (1985). Ferret feeding experiments were done as previously described (19, 67). Briefly, 6-week-old female ferrets (Marshall Farms) which were shown to be free of campylobacter were used. The animals were provided with Marshall Farms ferret chow and water ad libitum and were observed for 1 week prior to challenge for any signs of distress and diarrhea; on several days during this week, rectal swabs from each animal were cultured to confirm that the animals were campylobacter free.
Overnight biphasic cultures of bacteria were concentrated and used as the inoculum as described by Doig et al. (19). Viable counts were determined by serial dilution onto MH agar. Ferrets were anaesthetized with acepromazine-ketamine intramuscularly and fed 10.0 ml of bacterial culture via a pediatric intubation tube. At 1 h postchallenge, the animals were given 2.8 ml of tincture of opium per kg intraperitoneally to reduce peristalsis. After infection, the animals were monitored three times daily for signs of diarrhea.
Natural transformation of C. jejuni.
The biphasic natural transformation procedure was used as previously described (62). C. jejuni strains were grown overnight on plates and resuspended in MH broth to an optical density at 600 nm of 1.0. Aliquots of 200 μl of each strain were grown for an additional 2 h at 37°C in biphasic cultures in tubes (26). DNA (1 μg) from a streptomycin-resistant mutant of 81-176 (26) was added to the cultures, and the incubation continued for 4 h at 37°C. The cultures were serially diluted and plated in duplicate to MH agar containing 20 μg of streptomycin per ml. The results were expressed as the number of transformants per microgram of Smr DNA. Negative controls were strains treated identically without the addition of DNA.
DNA hybridizations.
Primers tetO F (5′-GGCGTTTTGTTTATGTGCG-3′) and tetO R (5′-ATGGACAACCCGACAGAAGC-3′) (35) were used to amplify a 559-bp product with 250 ng of total DNA from C. jejuni 81-176 as the template. The PCR conditions used were as follows: an initial melting temperature of 95°C for 2 min; 35 cycles of 95°C for 2 min, 52°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 2 min. Primers virB11 F (5′-GAACAGGAAGTGGAAAAACTAGC-3′) and virB11 R (5′-TTCCGCATTGGGCTATATG-3′) were used to amplify a 708-bp fragment within the virB11 gene with 250 ng of total DNA from C. jejuni 81-176 as the template. The PCR conditions were as follows: an initial melting temperature of 95°C for 2 min; 35 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 2 min; and a final extension at 72°C for 2 min. The PCR products were gel purified using a Qiaex II kit (Qiagen) and randomly primed in the presence of [32P]dCTP (NEN Life Science Products, Boston, Mass.). Hybridizations were performed in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–5× Denhardt's solution–0.1% sodium dodecyl sulfate–100 μg of calf thymus DNA per ml (42) at 60°C for 18 h and were followed by four 30-min washes with 0.5× SSC at 60°C. Southern blots or colony lifts were exposed to X ray film for 18 to 24 h.
Nucleotide sequence accession number.
The sequences of the genes described in this paper have been submitted to GenBank under accession number AF226280.
RESULTS
Plasmid content of 81-176.
Agarose gel electrophoretic analyses of plasmid DNA isolated from 81-176 revealed the presence of two plasmids (data not shown). An antibiogram determined that 81-176 is resistant to tetracycline (Tetr). When individual colonies of 81-176 were picked from MH agar to MH supplemented with tetracycline, 20 of 172 colonies (12%) had spontaneously lost tetracycline resistance. Comparison of the BglII digestion patterns of plasmids from 81-176 and a TetS derivative, DB179 (Fig. 1) indicates that DB179 has lost one of the two plasmids. Restriction analyses with several different enzymes, similar to that in Fig. 1, indicated that the plasmids were each approximately 35 kb in size. PCR primers specific for the Campylobacter tetO gene (35) were used to amplify a 559-bp product with 81-176 DNA as template. Partial DNA sequence analysis of this PCR product confirmed that it contained part of the tetO gene (data not shown). Southern hybridization using this PCR product as probe indicated that a BglII fragment of approximately 10 kb from one of the plasmids, corresponding to the one indicated by the circle in Fig. 1, lane 1, contained tetO. The R factor has been termed pTet and the cryptic plasmid has been called pVir, for reasons described below. Attempts to transfer pTet or pVir which had been tagged with a kanamycin resistance gene (see below) conjugally from 81-176 to C. jejuni VC83 Nalr Strr were uniformly unsuccessful (data not shown). Similarly, attempts to mobilize a nonconjugative chloramphenicol resistance shuttle plasmid, pRY111 (66), from 81-176 to VC83 Nalr Strr were unsuccessful.
DNA sequence analysis of part of plasmid pVir.
A 6-kb BglII fragment of pVir (arrow in Fig. 1), was cloned into pBluescript to generate pDB100. DNA sequencing of part of that fragment revealed the presence of four ORFs (Fig. 2). ORF1 through ORF3 are overlapping and appear to be organized in an operon. ORF4 begins 207 bp downstream of the 3′ end of ORF3, and there are no other ORFs present for >400 bp on either strand downstream of ORF4 (data not shown). ORF1 through ORF3 encode proteins that display significant identity to the products of the comB1, comB2, and comB3 genes, respectively, of H. pylori P1, which are involved in competence for natural transformation and DNA uptake in H. pylori (27). Orthologs of the comB1 through comB3 genes have also been found in both of the completed H. pylori genome sequences (Table 1). In H. pylori J99, the genes are complete and encode products with predicted molecular masses of 25.9, 40.9, and 42.8 kDa for ComB1, ComB2, and ComB3, respectively (3, 18). However, in H. pylori 26695, both the comB2 and comB3 genes appear not to be expressed, since they carry a frameshift mutation, and are represented by HP0039/HP0040 (ComB2) and HP0041/HP0042 (ComB3) (61). H. pylori ComB2 and ComB3, but not ComB1, possess typical N-terminal signal sequences. In addition, ComB1 and ComB3 are predicted to contain an α-helical transmembrane region (27). The C. jejuni ComB1 protein also lacks a leader sequence and is predicted to be associated with the inner membrane. C. jejuni ComB2 and ComB3 proteins have signal sequences and are predicted to be localized to the periplasmic space or the outer membrane. These three predicted C. jejuni proteins also have weaker homology to components of type IV secretion systems which are involved in transfer of various macromolecules. Type IV secretion systems are involved in transfer of the Ti plasmid DNA and proteins from Agrobacterium to plant cells (6, 14, 49), transfer of conjugative plasmids in E. coli (23, 69), transport of proteins required for intracellular survival of Legionella pneumophila (7, 12, 47), Brucella suis (36), and Rickettsia prowazekii (4), and transport of pertussis toxin across the outer membrane of Bordetella pertussis (63, 65). Thus, ComB1 shows between 16 to 23% identity and 32 to 34% similarity to VirB8 proteins from B. suis (36) and R. prowazekii (4); ComB2 shows 18% identity and 30% similarity to LvhB9 from a newly described type IV secretion system of L. pneumophila (47); and ComB3 shows 22% identity and 33% similarity to TrbI of plasmid RK2 (33) and 12% identity and 27% similarity to LvhB10 of L. pneumophila (47).
FIG. 2.
Schematic of the ORFs identified by sequence analysis of the pDB100 plasmid. The restriction sites mark the position of insertion of the Kmr cassette in the mutants.
TABLE 1.
Homology of pVir-encoded proteins to H. pylori proteins
| ORF | Protein | Length (aa)a | Mass (kDa) | pI | Genomic H. pylori orthologb | Length (aa) | % Identity | % Similarityc |
|---|---|---|---|---|---|---|---|---|
| ORF1 | ComB1 | 225 | 25.9 | 8.10 | HP0038 | 245 | 31 | 60 |
| JHP34d | 247 | 31 | 60 | |||||
| ORF2 | ComB2 | 356 | 40.9 | 8.77 | HP0039/HP0040 | 40/247 | 30e | 49e |
| JHP35 | 328 | 30 | 50 | |||||
| ORF3 | ComB3 | 378 | 42.8 | 7.95 | HP0041/HP0042 | 123/233 | 33e | 58e |
| JHP36 | 376 | 31 | 56 | |||||
| ORF4 | VirB11 | 317 | 36.4 | 5.79 | HP1421 | 304 | 35 | 59 |
| JHP1316 | 304 | 35 | 59 |
aa, amino acids.
The HP and JHP proteins represent the orthologs from H. pylori strains 26695 (59) and J99 (3), respectively.
The percent relatedness between orthologs is calculated as the number of identical or identical and conserved residues from an optimal alignment as a percentage of the length of the C. jejuni protein.
The C. jejuni ComB1 protein is also related to the H. pylori J99 protein JHP921, which is located in that strain's hypervariable plasticity zone (3). The percent identity and similarity to this ortholog are 36 and 62%, respectively.
Due to the splitting of the orthologs in H. pylori 26695 by a frameshift mutation, the percent identity and similarity were calculated by aligning each of the predicted portions and combining the totals.
ORF4 of pDB100 (Fig. 2) encodes a protein whose highest identity match by BLASTP analysis is to the H. pylori protein JHP1316/HP1421 (Table 1). This H. pylori protein is a member of a paralogous family that also contains a protein encoded by a gene that maps in the cag pathogenicity island (ORF11, HP0525, and JHP474) (1, 3, 39, 61). This paralogous family has significant identity to VirB11 of the Agrobacterium type IV secretion system (6, 49). The H. pylori VirB11-like paralogous family (HP0525 and HP1421) has been proposed to function in a type IV secretion system required for virulence (1, 16, 37, 44). The predicted protein encoded by ORF4 of pDB100 is soluble and contains a consensus ATP-GTP binding domain at amino acids 150 to 157 (GGTGSGKT), like other VirB11 homologs (49, 65).
Effect of mutation of comB3 and virB11 on adherence and invasion in vitro.
Site-specific insertional mutants were constructed in the C. jejuni homologs of comB3 and virB11 using a nonpolar (43) kanamycin resistance cassette (32) as described in Materials and Methods. Growth of the mutants on motility agar demonstrated that both mutants were unperturbed in flagellar function and were fully motile (data not shown). The mutants were then compared to wild-type C. jejuni 81-176 in in vitro adherence and invasion assays as previously described (28, 38, 67), and the results are summarized in Table 2. The comB3 mutant adhered to and invaded INT407 cells at levels that were approximately one-third those of the wild-type strain. However, the effects were more pronounced with the virB11 mutant, whose adherence and invasion levels were only 17.1% (P < 0.001) and 8.5% (P < 0.001) those of the wild-type strain, respectively. Strain DB179, which lacks the pTet plasmid, adhered to and invaded the INT407 cells at levels equivalent to wild-type levels. In contrast, C. jejuni strain NCTC 11168, whose complete genome has been sequenced by the Sanger Centre, and E. coli DH5α invaded INT407 cells at levels of 0.035% ± 0.02% and 0.08% ± 0.02%, respectively.
TABLE 2.
Adherence and invasion of 81-176 and derivatives
| Strain | Adherence
|
Invasion
|
||
|---|---|---|---|---|
| % Adherence | % of wild-type value | % Invasion | % of wild-type value | |
| 81-176 | 7.6 ± 0.87 | 100 | 4.67 ± 0.86 | 100 |
| DB179 | 9.4 ± 0.81 | 123 | 5.6 ± 0.85 | 120 |
| 81-176 comB3::Km | 2.7 ± 1.0a | 29 | 1.3 ± 0.49b | 28 |
| 81-176 virB11::Km | 1.34 ± 0.41b | 13 | 0.39 ± 0.27b | 9 |
P < 0.01 compared to strain 81-176.
P < 0.01 compared to strain 81-176.
Involvement of MT versus MF in the residual invasion of 81-176 virB11::Km.
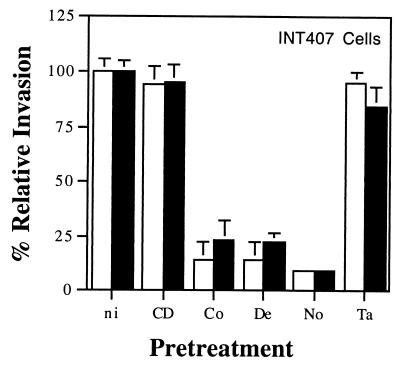
Strain 81-176 is internalized into eukaryotic cells by a microtubule (MT)-dependent mechanism (28, 38). We assessed different inhibitors of MF polymerization (cytochalasin D), MT polymerization (colchicine, demecolcine, and nocodazole), or MT depolymerization (taxol) to examine the cytoskeletal requirements for the residual invasion of INT407 cells seen in the virB11::Km mutant of 81-176. Compounds that affect MF or MT polymerization were individually used to pretreat INT407 cell monolayers before and during the invasion period, as described in Materials and Methods. Cytochalasin D pretreatment resulted in reduction of the MF-dependent invasion by the control S. enterica serovar Typhi strain from 19.6% ± 0.7% to 0.86% ± 0.79% (95.7% reduction). In contrast, the invasion ability of C. jejuni 81-176 was not inhibited by either 1 or 2 μM cytochalasin D, as previously demonstrated (28). Mutant C. jejuni 81-176 virB11::Km had a 91% decrease in invasion efficiency compared to the wild-type parent strain, 81-176 in the absence of inhibitors, and the remaining invasion ability was unaffected by cytochalasin D (Fig. 3). When host cells in concomitant studies were pretreated to depolymerize MTs with any of three inhibitors, the control S. enterica serovar Typhi strain was not reduced in its entry ability (data not shown). However, C. jejuni 81-176 and mutant virB11::Km were typically reduced more than 75% in their ability to invade INT407 cells by this latter treatment (Fig. 3). Taxol pretreatment of the monolayer showed no marked effect on the invasion ability of these Campylobacter strains. Thus, mutant 81-176 virB11::Km exhibited the same cytoskeletal requirements for invasion ability as the parent 81-176 strain (i.e., MT dependence).
FIG. 3.
Effect of various inhibitors on internalization into INT407 cells of C. jejuni 81-176 (□) and the mutant virB11::Km strain (■). At 1 h prior to the addition of bacteria to the monolayer, the epithelial cells were incubated either with no inhibitor (ni) or with 1 μM cytochalasin D (CD), 10 μM colchicine (Co), 1 μM demecolcine (De), 20 μM nocodazole (No), or 50 μM taxol (Ta). Each inhibitor was maintained throughout the 2-h invasion period. The relative percent invasiveness was determined as 100 multiplied by recovery in the presence of inhibitors divided by recovery in the absence of inhibitors (i.e., 100% relative invasiveness). S. enterica serovar Typhi Ty2W served as an MF-dependent invasive control. No other inhibitor showed a significant effect on S. enterica serovar Typhi internalization. Results are presented as the mean of at least three separate experiments and standard error, shown as bars above or below the mean.
Effect of the virB11 mutation on virulence in the ferret.
To determine the effect of the virB11 mutation on virulence in vivo, the mutant was compared to wild-type 81-176 in the ferret diarrhea model (19, 67). Groups of ferrets were fed a high dose of bacteria (ranging from 9 × 109 to 8 × 1010 CFU/ml) and a lower dose (ranging from 9 × 108 to 8 × 109 CFU/ml), and their symptoms were monitored. The results, shown in Table 3, indicate that eight of eight animals fed the high dose of 81-176 developed mucous diarrhea but that only four of eight animals fed the same dose of the virB11 mutant became ill (P < 0.05). In contrast, only one out of nine animals fed similar doses of NCTC 11168 developed any diarrheal symptoms, which is consistent with the inability of this strain to adhere to or invade INT407 cells in vitro. At the lower dose, 50% of the animals fed C. jejuni 81-176 developed diarrhea, compared to none of the animals fed the virB11 mutant (P < 0.05). Furthermore, at this low dose, none of the eight animals fed NCTC 11168 became ill. We have previously demonstrated that insertion of the Kmr cassette into a gene unrelated to virulence (arylsulfatase) does not affect disease in the ferret model (67, 68).
TABLE 3.
Virulence of C. jejuni strains in ferrets
| Strain | No. of animals with diarrhea/total no. given:
|
|
|---|---|---|
| Low dosea | High dosea | |
| 81-176 | 4/8 | 8/8 |
| 81-176 virB11::Km | 0/8b | 4/8b |
| NCTC 11168 | 0/8b | 1/9c |
The low dose of bacteria ranged from 9 × 108 to 8 × 109 CFU and the high dose ranged from 9 × 109 to 8 × 1010 CFU in two separate experiments.
P < 0.05 compared to strain 81-176 by Fisher's exact test.
P < 0.005 compared to strain 81-176 by Fisher's exact test.
Effect of plasmid mutations on competence for natural transformation.
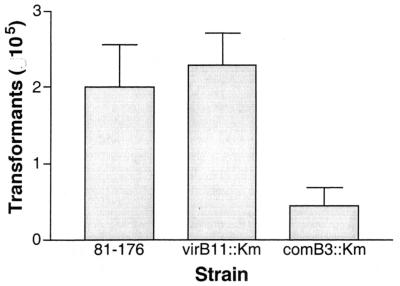
The comB3 gene product plays a role in natural transformation in H. pylori (27), where isogenic mutants demonstrate a marked reduction in the efficiency of transformation. We examined the C. jejuni 81-176 comB3 mutant to determine whether this gene product played a similar role in the natural transformation of C. jejuni. The transformation frequency for a chromosomally located Strr marker (26) was determined for the wild type and the two mutants. As seen in Fig. 4, there was no difference in transformation frequency between the wild-type strain and the virB11 mutant. However, similar to the phenotype observed with the H. pylori mutant, the C. jejuni 81-176 comB3 mutant carried a transformation-defective phenotype, which resulted in an 80% reduction in the natural transformation efficiency compared to the level for wild-type C. jejuni 81-176.
FIG. 4.
Relative transformation frequency of strain 81-176 and mutants. DNA (1 μg) from a streptomycin-resistant mutant of 81-176 (26) was used to transform C. jejuni strains. Results are expressed as the total number of transformants per microgram of DNA and represent the mean and standard deviation of three independent experiments.
Presence of the virB11 gene in other strains of C. jejuni.
None of the four genes from the pVir plasmid described here was found to be present in the genome of C. jejuni NCTC 11168, which has been sequenced by the Sanger Centre (http://microbios1.mds.qmw.ac.uk/campylobacter/). Furthermore, DNA hybridization studies failed to detect the presence of a virB11 homolog in nine laboratory strains of C. jejuni unrelated to 81-176 (data not shown). These probe-negative strains included A3249, which, like 81-176, caused diarrheal disease when fed to human volunteers, although the symptoms were less severe than those caused by 81-176 (8). Given that these C. jejuni strains had been passaged extensively under laboratory conditions, we used colony blot hybridization to examine the frequency of the virB11 gene in freshly isolated Campylobacter strains obtained from U.S. military personnel with diarrheal disease in Thailand. The strains screened included 58 fresh clinical isolates of C. jejuni and 4 of C. coli. These results indicated that 10.3% (6 of 58) of the C. jejuni strains and none of the C. coli strains were positive for the virB11 gene probe. Furthermore, the virB11 gene probe hybridized to a large plasmid in all six of these isolates (data not shown).
DISCUSSION
The perception that plasmids are not involved in pathogenesis of Campylobacter spp. is based on a limited number of early studies indicating that a relatively low proportion of different strains contained plasmids and one study in which plasmid content was not associated with disease in a guinea pig model (55). However, the relevance of this model, which measures abortion of pregnant guinea pigs following intraperitoneal injection (15, 50, 55), to diarrheal disease is arguable. The experimental observation that strain 81-176 contains plasmids, coupled with its relatively high levels of both internalization into intestinal epithelial cells in vitro (28, 38) and virulence in ferrets (19, 67) and human volunteers (8), led us to reevaluate the role of plasmids in the pathogenesis of this well-characterized strain of C. jejuni. The version of 81-176 with which we routinely work is an isolate from the human feeding studies conducted by Black et al. (8). We have also examined an isolate obtained prior to the human feeding study and one from a primate feeding study (40), and the plasmid complement appears identical in all three strains. However, repeated attempts to transfer the tetracycline resistance plasmid (pTet) or a kanamycin-tagged version of pVir conjugatively from 81-176 to another C. jejuni strain or to mobilize a shuttle plasmid using either resident plasmid have been uniformly unsuccessful. Nonetheless, the high frequency of spontaneous loss of tetracycline resistance in the absence of selective pressure suggests that there must be some genetic transfer system, perhaps natural transformation, operating to maintain this plasmid in a population of 81-176 cells.
The partial sequence of the pVir plasmid identified four genes, whose products all had identifiable orthologs in the closely related pathogen H. pylori, and one of these is found in the cag pathogenicity island. The cag pathogenicity island is a region of approximately 37 kb which distinguishes virulent type I strains of H. pylori from the less virulent type II strains (13). Type I strains are characterized by their ability to induce numerous cellular changes following attachment to eukaryotic cells in vitro, including effacement of microvilli, cup and pedestal formation, cytoskeletal rearrangements, synthesis of interleukin-8, and morphological changes typical of those induced by exposure to growth factors (44–46). Among the proteins encoded by the cag pathogenicity island are the 145-kDa CagA protein and several homologs of type IV secretion systems (1). It has been recently shown that insertion of the 145-kDa CagA protein into the host cell is a key step in induction of the observed host cellular changes, and it has been shown that the type IV secretion genes encode proteins responsible for this transfer process (37, 44, 48).
The pVir plasmid contains an apparent operon of three comB genes in a tandem physical arrangement identical to that seen in H. pylori isolates. Mutation of the comB3 gene significantly impaired the natural transformation ability of C. jejuni 81-176. Since strain 81-176 shows a higher transformation frequency than many other C. jejuni strains examined (P. Guerry, unpublished observations), it may be that the presence of the membrane-associated ComB3 protein enhances the level of natural transformation, perhaps by affecting surface changes that promote DNA binding. This apparent association of competence and virulence, while probably indirect, needs to be studied in more detail.
Although the level of homology for ORF1 (comB1) and ORF2 (comB2) is low, all four genes encode proteins with homology to proteins of type IV secretion systems, specifically VirB8, VirB9, VirB10, and VirB11, all of which are involved in formation of a channel or gate though which DNA and/or proteins are transferred. Mutation of either the comB3 (virB10) or virB11 genes in C. jejuni 81-176 resulted in a statistically significant reduction of adherence and internalization in vitro. However, the levels of invasion of both mutants still remained higher than those of both the negative control, E. coli DH5α, and another strain of C. jejuni, NCTC 11168. The internalization of the virB11 mutant displays the same pattern of inhibition by host cytoskeletal inhibitors as that of 81-176, suggesting that the residual invasion in the mutant is not likely to be due to the presence of a second invasion system. It is important to note that the invasion efficiency for wild-type strain 81-176 observed in this study was approximately double that reported previously (28, 38, 67). Invasion efficiency is most directly affected by the number of host cells in the monolayer, the number of bacteria added (i.e., multiplicity of infection), and the volume of medium in which the assay is conducted. In the present studies, all manipulations were the same as previously reported (28, 38, 67), except that the host cell number was inadvertently doubled. This resulted in, as expected, a doubling in the number of bacteria entering the monolayer. However, the percent reduction in invasion of the mutant 81-176 strains remained the same, even when the host cell concentration was lowered by twofold to the level used previously (28). Differences in invasion efficiency (i.e., the percentage of the inoculum internalized after a set time) of severalfold can occur easily unless the host cell and bacterial concentrations are carefully controlled; this phenomenon may largely explain laboratory-to-laboratory variation in observed invasion efficiencies for the same strain. Unlike determination of the average number of bacteria internalized per host cell (obtained by dividing the total number of internalized bacteria by the number of host cells per assay well), invasion efficiency is a relative number, which can vary when the experimental conditions are altered.
Reduction of adherence and invasion in vitro has previously been shown to correlate with reduced virulence in vivo in the ferret diarrhea model (67). This correlation is supported in this study, since the isogenic virB11 mutant, which displayed significantly reduced levels of adherence and invasion in vitro, also was shown to cause significantly less severe symptoms in vivo. However, the present data do not specify if the primary defect is in adherence, invasion, or other undefined factors. Although we cannot exclude the possibility that virulence plasmids in most strains of C. jejuni are lost rapidly upon subculture, the remarkable stability of pVir in strain 81-176 in the absence of selective pressure would suggest otherwise. Rather, the data suggest that there are different mechanisms by which different strains of C. jejuni can cause disease. This hypothesis is consistent with reports of distinct clinical presentations of campylobacter enteritis, with differences observed in virulence among isolates, including NCTC 11168, in the ferret model (D. H. Burr, unpublished data) and in in vitro invasion assays (38), and with the observation that only approximately 10% of the fresh clinical isolates of C. jejuni tested were positive for the virB11 gene probe. Genetic differences among strains may also explain some of the apparent discrepancies observed in different laboratories in terms of the MT versus MF dependency of campylobacter invasion (17, 28, 29, 38). Taken together, all of these data suggest that there may be distinct mechanisms of virulence among different strains of C. jejuni, analogous to the situation seen with diarrheagenic E. coli strains (34). The identification of this putative type IV secretion system in strain 81-176 should help elucidate the mechanism of virulence of this class of pathogenic C. jejuni.
ACKNOWLEDGMENTS
Ruijin Yao and Haiying Niu contributed to early studies on the plasmid complement of 81-176. We are grateful to David Tribble and Lorrin Pang for their help in analyses of the isolates from Thailand; to Lanfong Lee, Dave Rollins, and Carl Harding for their help with the ferret experiments; and to Isabelle Walker and Rob Anthony for technical assistance.
This work was supported by Naval Medical Research and Development Command Work Unit 3M161102BS13AN1291 and Interagency Agreement FDA 224-93-2444.
REFERENCES
- 1.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Allos B, Blaser M J. Campylobacter jejuni and the expanding spectrum of related infections. Clin Infect Dis. 1995;20:1092–1101. doi: 10.1093/clinids/20.5.1092. [DOI] [PubMed] [Google Scholar]
- 3.Alm R A, Ling L-S L, Moir D, King B L, Brown E D, Doig P, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jian Q, Taylor D E, Vovis G F, Trust T J. Genomic sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:160–189. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 4.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 5.Austen R A, Trust T J. Detection of plasmids in the related group of the genus Campylobacter. FEMS Microbiol Lett. 1980;8:201–204. [Google Scholar]
- 6.Berger B A, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 8.Black R E, Levine M M, Clements M L, Huges T P, Blaser M J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 9.Bopp C A, Birkness K A, Wachsmuth I K, Barrett T J. In vitro antimicrobial susceptibility, plasmid analysis, and serotyping of epidemic-associated Campylobacter jejuni. J Clin Microbiol. 1985;21:4–7. doi: 10.1128/jcm.21.1.4-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradbury W C, Munroe D L G. Occurrence of plasmid and antibiotic resistance among Campylobacter jejuni and Campylobacter coli isolated from healthy and diarrheic animals. J Clin Microbiol. 1985;22:339–346. doi: 10.1128/jcm.22.3.339-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradbury W C, Murray A M, Hennessy J N, Penner J L. Occurrence of plasmid DNA in serologically defined strains of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1983;40:460–463. doi: 10.1128/iai.40.2.460-463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 13.Censini S, Lange C, Ziang Z Y, Crabtree J E, Ghiara P, Brodovsky P M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie P J. Agrobacterium tumefaciens T complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coid C R, Sullivan A M, Dore C J. Variations in the virulence, for pregnant guinea pigs, of campylobacters isolated from man. J Med Microbiol. 1986;23:187–189. doi: 10.1099/00222615-23-2-187. [DOI] [PubMed] [Google Scholar]
- 16.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 17.DeMelo M A, Gabbiani G, Pechere J C. Cellular events and intracellular survival of Campylobacter jejuni during infection of Hep-2 cells. Infect Immun. 1989;57:2214–2222. doi: 10.1128/iai.57.7.2214-2222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doig P, de Jonge B L, Alm R A, Brown E D, Uria-Nickelsen M, Noonan B, Mills S D, Tummino P, Carmel G, Guild B C, Moir D T, Vovis G F, Trust T J. Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol Mol Biol Rev. 1999;63:675–707. doi: 10.1128/mmbr.63.3.675-707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doig P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 20.Everest P H, Goosens H, Butzler J P, Lloyd D, Knutton S, Ketley J M. Differentiated Caco-2 cells as a model for enteric infection by Campylobacter jejuni and Campylobacter coli. J Med Microbiol. 1992;37:319–325. doi: 10.1099/00222615-37-5-319. [DOI] [PubMed] [Google Scholar]
- 21.Fauchere J L, Rosenau A, Veron M, Moyen E N, Richard S, Pfister A. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect Immun. 1986;54:283–287. doi: 10.1128/iai.54.2.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth N, Ippen-Ihler K, Skurry R. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2377–2401. [Google Scholar]
- 24.Grant C C R, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerry P, Alm R A, Power M E, Logan S M, Trust T J. The role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerry P, Pope P M, Burr D H, Leifer J, Joseph S W, Bourgeois A L. Development and characterization of recA mutants of Campylobacter jejuni for inclusion in attenuated vaccines. Infect Immun. 1994;62:426–432. doi: 10.1128/iai.62.2.426-432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofreuter D, Odenbreti S, Henke G, Haas R. Natural competence for DNA transformation in Helicobacter pylori: identification and genetic characterization of the comB locus. Mol Microbiol. 1998;28:1027–1038. doi: 10.1046/j.1365-2958.1998.00879.x. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Kopecko D J. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konkel M E, Hayes S F, Joens L A, Cieplak W. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cells cultures. Microb Pathog. 1992;13:357–370. doi: 10.1016/0882-4010(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 30.Konkel M E, Joens L A. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect Immun. 1989;57:2984–2990. doi: 10.1128/iai.57.10.2984-2990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korlath J A, Osterholm M T, Judy A, Forgang J C, Robinson R A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 32.Labigne-Roussel A, Couroux P, Tompkins L S. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessl M, Balzer D, Pansegrau W, Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Exp Med. 1992;136:1173–1184. [PubMed] [Google Scholar]
- 34.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 35.Manavathu E K, Fernandez C L, Cooperman B S, Taylor D E. Molecular studies on the mechanism of tetracycline resistance mediated by Tet(O) Antimicrob Agents Chemother. 1988;34:71–77. doi: 10.1128/aac.34.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Callaghan D, Cazevielle C, Allardet-Servent A, Boschiroli M L, Bourg G, Foulongne, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Pt1 type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 37.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–5000. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 38.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porwollik S, Noonan B, O'Toole P W. Molecular characterization of a flagellar export locus of Helicobacter pylori. Infect Immun. 1999;67:2060–2070. doi: 10.1128/iai.67.5.2060-2070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell R G, Blaser M J, Sarmiento I, Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989;57:1438–1444. doi: 10.1128/iai.57.5.1438-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagara H, Mochizuke A, Okamura N, Nakaya R. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli with special reference to plasmid profiles of Japanese clinical isolates. Antimicrob Agents Chemother. 1987;31:713–719. doi: 10.1128/aac.31.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal E D, Cha J, Lo J, Falkow S, Tompkins L S. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal G, Russo J R, Shuman H A. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol Microbiol. 1999;34:799–809. doi: 10.1046/j.1365-2958.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 48.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens K M, Roush C, Nester E. Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence. J Bacteriol. 1995;177:27–33. doi: 10.1128/jb.177.1.27-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SultanDosa A B, Bryner J H, Foley J W. Pathogenicity of Campylobacter jejuni and Campylobacter coli in the pregnant guinea pig model. Am J Vet Res. 1983;44:2175–2178. [PubMed] [Google Scholar]
- 51.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 52.Tay S T, Devi S, Puthucheary S, Kautner I. In vitro demonstration of the invasive ability of campylobacters. Zentbl Bakteriol. 1996;283:306–313. doi: 10.1016/s0934-8840(96)80064-8. [DOI] [PubMed] [Google Scholar]
- 53.Taylor D E. Incidence of plasmid DNA in strains of Campylobacter jejuni isolated from stool specimens at 37°C and 43°C. J Infect Dis. 1983;47:965–966. doi: 10.1093/infdis/147.5.965. [DOI] [PubMed] [Google Scholar]
- 54.Taylor D E. Genetics of Campylobacter and Helicobacter. Annu Rev Microbiol. 1992;46:35–64. doi: 10.1146/annurev.mi.46.100192.000343. [DOI] [PubMed] [Google Scholar]
- 55.Taylor D E, Bryner J H. Plasmid content and pathogenicity of Campylobacter jejuni and Campylobacter coli strains in the pregnant guinea pig model. 1984. Am J Vet Res. 1984;45:2201–2202. [PubMed] [Google Scholar]
- 56.Taylor D E, Chang N, Garner R S, Sherburne R, Mueller L. Incidence of antibiotic resistance and characterization of plasmids in Campylobacter jejuni strains isolated from clinical sources in Alberta, Canada. Can J Microbiol. 1986;32:28–32. doi: 10.1139/m86-006. [DOI] [PubMed] [Google Scholar]
- 57.Taylor D E, DeGrandis S A, Karmali M A, Fleming P C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981;19:831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor D E, Garner R S, Allan B J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983;24:930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 60.Tenover F C, Williams S, Gordon K P, Nolan C, Plorde J I. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985;27:37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- 67.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]
- 68.Yao R, Guerry P. Molecular cloning and site-specific mutagenesis of a gene involved in arylsulfatase production in Campylobacter jejuni. J Bacteriol. 1996;178:3335–3338. doi: 10.1128/jb.178.11.3335-3338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziegelin G, Pansegrau W, Strack B, Balzer D, Kroger M, Kruft V, Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1:303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]