Abstract
Objective
To develop a clinical practice guideline to support the management of chronic pain, including low back, osteoarthritic, and neuropathic pain in primary care.
Methods
The guideline was developed with an emphasis on best available evidence and shared decision-making principles. Ten health professionals (4 generalist family physicians, 1 pain management–focused family physician, 1 anesthesiologist, 1 physical therapist, 1 pharmacist, 1 nurse practitioner, and 1 psychologist), a patient representative, and a nonvoting pharmacist and guideline methodologist comprised the Guideline Committee. Member selection was based on profession, practice setting, and lack of financial conflicts of interest. The guideline process was iterative in identification of key questions, evidence review, and development of guideline recommendations. Three systematic reviews, including a total of 285 randomized controlled trials, were completed. Randomized controlled trials were included only if they reported a responder analysis (eg, how many patients achieved a 30% or greater reduction in pain). The committee directed an Evidence Team (composed of evidence experts) to address an additional 11 complementary questions. Key recommendations were derived through committee consensus. The guideline and shared decision-making tools underwent extensive review by clinicians and patients before publication.
Recommendations
Physical activity is recommended as the foundation for managing osteoarthritis and chronic low back pain; evidence of benefit is unclear for neuropathic pain. Cognitive-behavioural therapy or mindfulness-based stress reduction are also suggested as options for managing chronic pain. Treatments for which there is clear, unclear, or no benefit are outlined for each condition. Treatments for which harms likely outweigh benefits for all or most conditions studied include opioids and cannabinoids.
Conclusion
This guideline for the management of chronic pain, including osteoarthritis, low back pain, and neuropathic pain, highlights best available evidence including both benefits and harms for a number of treatment interventions. A strong recommendation for exercise as the primary treatment for chronic osteoarthritic and low back pain is made based on demonstrated long-term evidence of benefit. This information is intended to assist with, not dictate, shared decision making with patients.
Chronic pain is one of the most complex and difficult conditions to treat. The Canadian Pain Task Force estimates that in Canada 7.6 million people (1 in 5) live with chronic pain.1 The total direct and indirect cost of chronic pain in 2019 was estimated to be $38.2 billion to $40.3 billion.1 However, even the best available treatments for chronic pain provide, at most, limited improvement for most people. Messages around pain management have been inconsistent and frequently extrapolated from acute pain and palliative care settings.2 While opioids were once heavily promoted for the management of chronic pain, the promised benefits in chronic pain management have not materialized. Additionally, an increase in prescription opioid abuse, overdoses, and death has been observed.2 Despite the prevalence of chronic pain and the subsequent search for effective therapies, an optimal approach in primary care management remains elusive.
Task forces have been assembled to address the growing issue of chronic pain.1,3 The absence of a one-size-fits-all solution is reflected in recommendations for individualized treatment and self-management of pain.4 Decisions may be based on numerous factors including patient experiences with different therapies, acceptability of side effects, accessibility, cost, and coverage. Self-management requires evidence-based education tools to help inform patient decisions.
This guideline will focus on the highest-quality evidence for common conservative therapeutic interventions in low back pain, osteoarthritis, and neuropathic pain. Interventions that are accessible to most urban and rural primary care clinicians were prioritized.
METHODS
Committee membership and roles
As with previous PEER guidelines,5,6 we followed the principles of the Institute of Medicine’s Clinical Practice Guidelines We Can Trust, the Guidelines International Network, and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.7-9
The Guideline Committee consisted of 10 health professionals (4 generalist family physicians [C.S.K., S.M., J.Y., A.G.S.], 1 pain management–focused family physician [L.M.], 1 anesthesiologist [P.M.], 1 physical therapist [K.E.], 1 pharmacist [J.M.], 1 nurse practitioner [C.R.], and 1 psychologist [M.C.T.]), a patient representative, and a nonvoting pharmacist and guideline methodologist (A.J.L.). Member selection was based on profession, expertise, practice setting, and lack of financial conflicts of interest. The separate Evidence Team was composed of evidence and methodology experts. There were 2 overlapping members: the Guideline Committee Chair (C.S.K.) and the guideline methodologist (A.J.L.). The Evidence Team (G.M.A., S.S.M., J.P., S.G., B.T., A.J.L., M.R.K., A.P., J.K., A.D.T., J.T., R.D.T., T.N., D.P., J.W., J.F., N.D., L.S., K.C., J.P.M., C.S.K.) completed the systematic reviews and rapid evidence reviews for use by the Guideline Committee to create recommendations. All members of the Guideline Committee and the Evidence Team reported no financial conflicts of interest. (The full disclosure of competing interests appears in Appendix 1, available from CFPlus.*)
Evidence review
The Evidence Team completed 3 systematic reviews including 285 randomized controlled trials (RCTs) between March 2020 and May 2021.10-12 These reviews focused on therapies for chronic pain (defined as pain for at least 12 weeks, which is consistent with the current ICD-11 definition of chronic pain).13 Conditions were those commonly seen in primary care: osteoarthritis, low back pain (including sciatica and other radicular pain), and neuropathic pain. Methods have been previously published10-12; however, in brief, each systematic review included RCTs that compared an intervention with placebo or a control group and reported a responder analysis for chronic pain.14 The responder analysis could include, for example, the proportion of patients who achieved a 30% or greater improvement in their pain. Safety outcomes were captured where possible to provide information on adverse effects for each intervention. For each systematic review, there were some interventions for which responder analyses were not identified (osteoarthritis: platelet-rich plasma injections, rubefacients, counseling, cannabinoids, tricyclic antidepressants [TCAs]; low back pain: acetaminophen, cannabinoids, muscle relaxants, selective serotonin reuptake inhibitors, TCAs, topical nonsteroidal anti-inflammatory drugs [NSAIDs], anticonvulsants; neuropathic pain: exercise, lidocaine).
Through an iterative process the committee identified and prioritized 11 supplemental questions, some of which included interventions for which no responder analyses had been identified in the systematic reviews. These questions were intended to augment information provided in the systematic reviews and were answered using a rapid review process. Similar to the systematic reviews, the quality of evidence in the rapid reviews was evaluated using GRADE methodology. Questions included the following:
In primary care, can interventions in the acute pain period prevent progression to chronic pain?
Is exercise effective for chronic neuropathic pain?
How can we encourage people with chronic pain, including low back pain or osteoarthritis, to increase their physical activity?
What is the most effective type of exercise for chronic pain?
Are psychological strategies effective in chronic pain management?
Are cannabinoids effective for treating chronic non-cancer pain (osteoarthritis, low back pain, neuropathic pain)?
Are TCAs effective in the treatment of osteoarthritis and chronic low back pain?
Does combination pharmacologic therapy improve pain outcomes more than monotherapy for patients with chronic pain?
Are topical TCAs, nitrates, ketamine, muscle relaxants, or combinations of these effective in chronic pain?
In patients with chronic pain taking long-term opioids, does pain or function improve if the opioid dose is reduced or tapered off?
Will diet-induced weight loss reduce osteoarthritic knee pain in overweight and obese adults?
Details of the rapid reviews, including methodology and findings, are available in Appendix 1.*
Guideline process
The guideline process was iterative in identifying key questions, reviewing evidence, and developing recommendations. Based on the evidence provided by the Evidence Team, the Guideline Committee made treatment recommendations using the GRADE framework.15 Considerations in making recommendations included treatment efficacy and safety, data quality, cost, patient preferences and values, equity, feasibility, and acceptability. Owing to the nature of chronic pain and the vast differences in patient preferences and values surrounding treatment, all recommendations were formed with shared decision making in mind. As outlined by GRADE, strong recommendations were prefaced by the words “we recommend” and weak recommendations by “we suggest.”
The committee considered the development of practice points in the absence of evidence, with each member submitting practice points based on clinical experience. Clinicians with expertise in chronic pain were also asked to submit recommendations. Final practice points were identified through an iterative voting process.
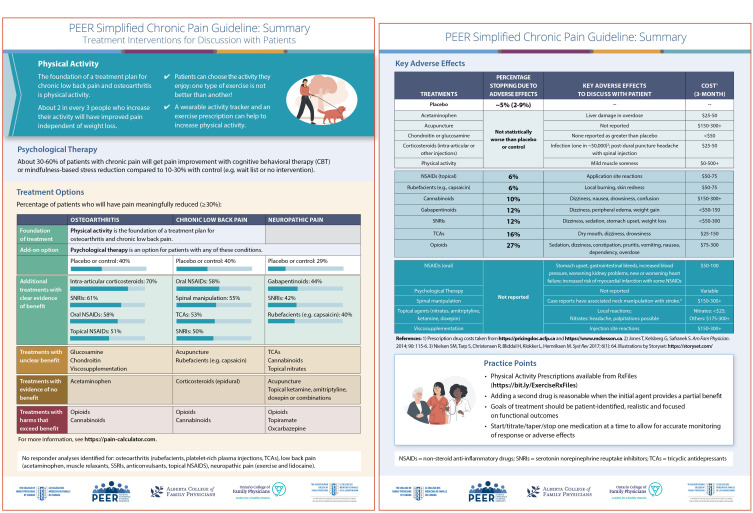
A 2-page summary (Figure 1*) and a patient handout (Appendix 2*) (both available from CFPlus*) were developed to simplify the recommendations and assist with shared, informed decision making between provider and patient.
Figure 1.
PEER simplified chronic pain guideline: Summary. Treatment interventions for discussion with patients
The guideline and related tools underwent extensive review by clinicians and patients before publication. This feedback and how it was considered by the authors is in Appendix 3.*
RECOMMENDATIONS
All recommendations are summarized in Box 1.6 Individual recommendation statements are provided below, followed by supporting evidence. Evidence regarding harms is reported on the 2-page summary (Figure 1*). A summary of the quality of evidence for all recommendations is reported in Table 1.10-12 Full details, including data on specific interventions and dosing studied, are available in Appendix 1.*
Box 1. Summary of recommendations for management of chronic pain (low back pain, osteoarthritis, and neuropathic pain) in primary care.
We strongly recommend discussion of physical activity as the foundation for managing osteoarthritis and chronic low back pain
We recommend any type of exercise, based on patient preference, as they are all likely similarly effective
We suggest that the goal of exercise be pain management, independent of weight loss
In patients who request assistance to increase their physical activity, we recommend the use of wearable activity trackers with an exercise prescription
We suggest CBT or mindfulness-based stress reduction be offered to patients to help manage chronic pain, when access to services allows
We recommend the use of shared decision making (including the use of decision aids) to inform additional treatment options beyond physical activity for patients with chronic osteoarthritic, low back, or neuropathic pain
- We recommend treatments with evidence of benefit be considered and discussed first as options
-
-Osteoarthritis: intra-articular corticosteroids, SNRIs, oral NSAIDs, topical NSAIDs
-
-Chronic low back pain: oral NSAIDs, SNRIs, spinal manipulation, TCAs
-
-Neuropathic pain: gabapentinoids, SNRIs, rubefacients
-
-
- We suggest that the following treatments with no or unclear benefit could be discussed with patients when interventions with clear evidence of benefit have already been considered
-
-Osteoarthritis
-
—Unclear: glucosamine, chondroitin, viscosupplementation
-
—No benefit: acetaminophen
-
—
-
-Chronic low back pain
-
—Unclear: acupuncture, rubefacients
-
—No benefit: corticosteroid injections
-
—
-
-Neuropathic pain
-
—Unclear: TCAs, cannabinoids, topical nitrate spray on affected area
-
—No benefit: acupuncture, topical ketamine, topical amitriptyline, topical doxepin, topical combinations
-
—
-
-
- We suggest that treatments where the harms likely exceed the benefits be avoided in most patients
-
-Osteoarthritis: opioids, cannabinoids
-
-Chronic low back pain: opioids, cannabinoids
-
-Neuropathic pain: opioids, topiramate, oxcarbazepine
-
-
We suggest that the addition of another medication could be discussed if the original medication has only provided partial benefit
For patients with chronic pain without opioid use disorder who are interested in tapering their long-term opioids, we suggest discussion of slow dose reductions, supported by CBT where possible. Best evidence suggests potential harm in patients who are not interested in reducing or stopping opioids. If opioid use disorder is suspected, please refer to the PEER simplified guideline on the management of opioid use disorder6
CBT—cognitive-behavioural therapy, NSAID—nonsteroidal anti-inflammatory drug, SNRI—serotonin-norepinephrine reuptake inhibitor, TCA—tricyclic antidepressant.
Table 1.
GRADE quality-of-evidence table for all recommendations
| TOPIC | FINAL GRADE RATING |
|---|---|
| Exercise for osteoarthritis10 | Low |
| Exercise for chronic low back pain11 | Moderate |
| TCAs for low back pain | Moderate |
| Topical agents (non-nitrate) | Moderate |
| Topical agents (nitrates) | Low |
| Psychological treatments | Low |
| Best exercise type | Low |
| Tapering opioids | Very low |
| Cannabinoids | Very low |
| Assisting to exercise | Very low |
| Drug combinations | Very low |
| Weight loss for osteoarthritis | Low |
| Intra-articular steroids for osteoarthritis10 | Very low |
| SNRIs for osteoarthritis10 | Moderate |
| Oral NSAIDs for osteoarthritis10 | Moderate |
| Topical NSAIDs for osteoarthritis10 | Low |
| Glucosamine for osteoarthritis10 | Very low |
| Chondroitin for osteoarthritis10 | Moderate |
| Viscosupplementation for osteoarthritis10 | Very low |
| Opioids for osteoarthritis10 | Very low |
| Acetaminophen for osteoarthritis10 | Low |
| Oral NSAIDs for back pain11 | Moderate |
| SNRIs for back pain11 | Moderate |
| Spinal manipulation for back pain11 | Low |
| Acupuncture for back pain11 | Very low |
| Rubefacients for back pain11 | Low |
| Corticosteroid injections for back pain11 | Very low |
| Opioids for low back pain11 | Very low |
| Anticonvulsants for neuropathic pain12 | Moderate |
| SNRIs for neuropathic pain12 | Moderate |
| Rubefacients for neuropathic pain12 | Low |
| TCAs for neuropathic pain12 | Very low |
| Opioids for neuropathic pain12 | Low |
GRADE—Grading of Recommendations Assessment, Development and Evaluation; NSAID—nonsteroidal anti-inflammatory drug; SNRI—serotonin-norepinephrine reuptake inhibitor; TCA—tricyclic antidepressant.
There is no specific pathway or hierarchy for individual treatment options. Interventions where benefits likely exceed harms would generally be prioritized more highly. A lack of response to one treatment does not necessarily mean that a patient must move to the next category. It is suggested that these recommendations be implemented in conjunction with resources and programs that are available in local jurisdictions.
There is a focus on shared decision making throughout the guideline. Previous systematic reviews suggest that decision aids increase patient knowledge, accuracy of risk perception, and congruency between values and care choices.16
Physical activity
Recommendations. We recommend discussion of physical activity as the foundation for managing osteoarthritis and chronic low back pain.
We recommend any type of physical activity, based on patient preference, as they are all likely similarly effective.
We suggest that the goal of physical activity be pain management, independent of weight loss.
For patients who request assistance to increase their physical activity, we recommend the use of wearable activity trackers with an exercise prescription.
There is no specific recommendation for physical activity for chronic neuropathic pain, as the evidence of benefit is unclear.
Systematic reviews of chronic osteoarthritis10 and low back pain11 revealed exercise to be the most effective intervention for patients to attain meaningful pain relief (eg, approximately 30% reduction in pain). For osteoarthritis, meta-analysis of 11 RCTs with 1367 patients found that more patients attained meaningful pain relief with exercise compared with control (risk ratio [RR]=2.36; 95% CI 1.79 to 3.12). The most common type of exercise was physiotherapy-guided exercise programs. For low back pain, meta-analysis of 18 RCTs with 2561 patients found that more patients attained meaningful pain relief with exercise compared with control (RR=1.71; 95% CI 1.37 to 2.15). Exercise interventions were most commonly physiotherapy-guided exercise programs, but also included yoga, Pilates, tai chi, and Nordic walking. In both osteoarthritis and low back pain, exercise benefit persisted beyond 12 weeks. Adverse effects were inconsistently reported. Withdrawal due to adverse effects was not increased compared with control.
No exercise trials met the inclusion criteria for the systematic review for chronic neuropathic pain.12 The supplemental evidence review (Appendix 1*) concluded that exercise in chronic neuropathic pain results in a small potential reduction in pain scores and inconsistent improvements in quality-of-life measures, but the differences were generally not statistically significant and were of borderline clinical significance.
Types of activity. The supplemental evidence review identified low- to moderate-quality evidence that little difference exists between different types of exercises for improvement of pain and function in the management of osteoarthritis and chronic low back pain (Appendix 1*). For chronic low back pain, focus on motor control and core stabilization might offer a small, likely clinically unimportant, benefit for pain and function over the benefit of other exercises (eg, <8-point additional benefit on a 0- to 100-point scale). Strengthening exercises, yoga, and aerobic exercises are all likely similarly effective for osteoarthritis.
Motivating patients to be physically active. Three systematic reviews and 2 additional RCTs addressed this topic (Appendix 1*). Wearable activity trackers increased physical activity levels on top of counseling and education by increasing daily step count (by about 1500 steps) and time spent in moderate to vigorous exercise (by about 16 min/day). Many of the trials included goal setting with or without additional counseling. Providing patients with a written, stepwise, goal-oriented exercise program has been demonstrated to increase physical activity levels. Physical activity prescriptions, combined with patient-specific goals and monitoring, may increase physical activity levels in all patients by up to about 1200 steps daily at about 1 year, with an additional 1 person becoming active for every 10 prescribed activity compared with general advice alone.
Psychological interventions
Recommendation. We suggest the discussion of cognitive-behavioural therapy (CBT) or mindfulness-based stress reduction as options for patients to help manage chronic pain, when access to services allows.
Five RCTs addressed the efficacy of psychological strategies to treat chronic pain (Appendix 1*). Cognitive-behavioural therapy and mindfulness-based stress reduction provide clinically meaningful reduction in pain for patients with chronic low back pain (about 30% to 60% at 18 to 52 weeks) and patients with neuropathic pain (about 60% at 12 weeks) compared with control (about 20% to 30%). For osteoarthritic pain, one small trial suggested that Internet-based pain coping skills training (based on CBT principles) provides pain improvement for 26% of patients (vs 9% with control) in the short term (8 weeks) (Appendix 1*).
Osteoarthritis
Recommendations. We recommend the use of shared decision making (including the use of decision aids) when considering treatment options beyond physical activity for patients with chronic osteoarthritis.
We recommend that treatments with evidence of benefit be considered and discussed first as options: intra-articular corticosteroids, serotonin-norepinephrine reuptake inhibitors (SNRIs), oral NSAIDs, topical NSAIDs.
- We suggest that the following treatments with no or unclear evidence of benefit could be discussed with patients when interventions with clear evidence of benefit have already been considered.
-
-Unclear: glucosamine, chondroitin, viscosupplementation.
-
-No benefit: acetaminophen.
-
-
We suggest that treatments where the harms likely exceed the benefits be avoided in most patients: opioids, cannabinoids.
Beyond exercise, there are 4 interventions with consistent evidence of efficacy in osteoarthritis compared with control (evidence from 6 to 43 RCTs including 706 to 28,699 patients).10 They include intra-articular corticosteroids (RR=1.74; 95% CI 1.15 to 2.62), SNRIs (RR=1.53; 95% CI 1.25 to 1.87), oral NSAIDs (RR=1.44; 95% CI 1.36 to 1.52), and topical NSAIDs (RR=1.27; 95% CI 1.16 to 1.38). The RCTs assessing topical NSAIDs primarily focused on patients with hand and knee osteoarthritis.
The efficacy of other interventions is less clear. Although glucosamine, chondroitin, and viscosupplementation all had benefit in the overall analysis, their efficacy was not different from placebo in publicly funded trials, raising questions regarding the true magnitude of effects (if any).
Low-quality evidence (2 RCTs) suggests that acetaminophen is no more effective than placebo. Withdrawal due to adverse effects was also not greater than placebo. Considering risks and benefits and allowing for individual response, the committee determined that a trial of acetaminophen may be a reasonable option for patients who have contraindications to other medications, cost barriers, or personal preferences for acetaminophen over other interventions with evidence of benefit.
Interventions where harms likely exceed benefits include opioids and cannabinoids. Opioids demonstrated the smallest absolute effect versus control (RR=1.16; 95% CI 1.02 to 1.32). In addition, subgroup analysis based on duration of treatment did not show statistically significantly more responders than placebo beyond 4 weeks’ duration, suggesting that the short-term benefit may not persist. Opioids also demonstrated the highest risk of adverse effects, including a number needed to harm (NNH) of 8 to 10 for withdrawal due to adverse effects. No included trials assessed long-term adverse effects including opioid misuse, opioid use disorder, and overdose. These data are consistent with other reviews17 and the Osteoarthritis Research Society International guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis, which make a strong recommendation against opioid use for osteoarthritis.18
Our previously completed systematic review10 did not identify any RCTs of cannabinoids in osteoarthritic pain that reported responder analysis. Given the prevalence of cannabinoid use and inquiries regarding its benefit in primary care, the committee requested that the Evidence Team review current data on cannabinoids. The Evidence Team identified 1 RCT of cannabinoids in osteoarthritic pain that did not demonstrate any benefit over placebo on pain outcomes. The evidence review on cannabinoids also demonstrated, consistent with previous guidelines, a high rate of adverse effects associated with cannabinoids including dizziness (NNH=5), cognitive disturbance (NNH=4 to 7), sedation (NNH=5), dysphoria (NNH=8), and confusion (NNH=15).5 Based on this evidence, the committee suggested that for most patients, harms will likely exceed benefits for cannabinoid use in osteoarthritis. This recommendation varies from a recent rapid clinical guideline that made a weak recommendation for cannabinoids in all chronic pain.19 The systematic review that informed that guideline included only 1 RCT with patients with osteoarthritis, which did not find evidence of benefit in pain outcomes.20
Low back pain
Recommendations. We recommend the use of shared decision making (including the use of decision aids) when considering treatment options beyond physical activity for patients with chronic low back pain.
We recommend that treatments with evidence of benefit be considered and discussed first as options: oral NSAIDs, SNRIs, spinal manipulation, TCAs.
- We suggest that the following treatments with no or unclear evidence of benefit could be discussed with patients when interventions with clear evidence of benefit have already been considered.
-
-Unclear: acupuncture, rubefacients.
-
-No benefit: corticosteroid injections (epidural injections).
-
-
We suggest that treatments where the harms likely exceed the benefits be avoided in most patients: opioids, cannabinoids.
Beyond exercise, 4 treatments have consistent evidence of efficacy compared with control.11 This includes spinal manipulation therapy (RR=1.54; 95% CI 1.11 to 2.12), oral NSAIDs (RR=1.44; 95% CI 1.17 to 1.78), SNRIs (duloxetine) (RR=1.25; 95% CI 1.13 to 1.38), and TCAs. The first 3 were identified in the original systematic review (evidence from 4 to 5 RCTs including 686 to 1637 patients).11 No trials reporting responder analysis for TCAs in low back pain were identified in the systematic review. Given their use in primary care, the committee requested the Evidence Team review all existing RCT evidence as a supplemental question (Appendix 1*). The RCTs identified in 1 high-quality systematic review suggest TCAs provide a meaningful reduction in pain on a 100-point scale for patients with chronic low back pain (RR=-11.17; 95% CI -21.35 to -1.00) and for those with sciatica (RR=-16.99; 95% CI -29.25 to -4.72), both of which reach the definition of a minimum clinically important difference used in the review of a 10-point improvement.
The efficacy of other interventions is less clear.11 Although acupuncture benefit was statistically significant in the overall analysis, subgroup analysis demonstrated that benefit was no longer significant in studies that were longer, larger, and at low risk of bias. Similarly, rubefacients (a substance that irritates the skin causing redness, such as capsaicin) demonstrated statistically significant benefit in the overall analysis, but no study assessed outcomes beyond 3 weeks’ duration.
Very low-quality evidence suggests that epidural corticosteroid injections are no better than control. Meta-analysis of 10 RCTs with 1152 patients demonstrated no significant benefit compared with control (RR=1.07; 95% CI 0.87 to 1.30). None of the included trials assessed facet corticosteroid injections.
Interventions where harms likely exceed benefits include opioids and cannabinoids. Six trials of opioids found a significant benefit for pain compared with control (RR=1.26; 95% CI 1.02 to 1.55). The longest trial was 12 weeks in duration. Opioids demonstrated the highest withdrawal due to adverse effects (RR=4.41; 95% CI 3.30 to 5.91). In addition, individual adverse events, including nausea (NNH=6), dizziness (NNH=7), somnolence (NNH=8), constipation (NNH=9), and vomiting (NNH=9) were more frequently reported among patients using opioids. Similar to osteoarthritis, no trial assessed long-term adverse effects including opioid misuse, opioid use disorder, and overdose. The American College of Physicians guideline on chronic low back pain suggests that “clinicians should only consider opioids as an option in patients who have failed the aforementioned treatments and only if the potential benefits outweigh the risks for individual patients and after a discussion of known risks and realistic benefits with patients.”21 Based on available evidence, the committee believed the harms will likely exceed the benefits for most patients. Regarding cannabinoids, evidence from the supplemental review (Appendix 1*) identified 1 RCT in low back pain, with no evidence of benefit over placebo for most outcomes. In the absence of evidence of benefit, and with known harms,5 the committee suggested that for most patients with low back pain, harms will likely exceed benefits for cannabinoid use.
Neuropathic pain
Recommendations. We recommend the use of shared decision making (including the use of decision aids) when considering treatment options for patients with chronic neuropathic pain.
We recommend that treatments with evidence of benefit be considered and discussed first as options: gabapentinoids, SNRIs, rubefacients.
- We suggest that the following treatments with no or unclear benefit could be discussed with patients when interventions with clear evidence of benefit have already been considered.
-
-Unclear: TCAs, cannabinoids, topical nitrate spray on affected area.
-
-No benefit: acupuncture, topical ketamine, topical amitriptyline, topical doxepin, topical combinations.
-
-
We suggest that treatments where the harms likely exceed the benefits be avoided in most patients: opioids, topiramate, oxcarbazepine.
Four interventions have consistent evidence of efficacy compared with control (evidence from 8 to 27 RCTs including 2344 to 6069 patients). These include gabapentin (RR=1.60; 95% CI 1.42 to 1.81), pregabalin (RR=1.56; 95% CI 1.45 to 1.67), SNRIs (RR=1.45; 95% CI 1.33 to 1.59), and rubefacients (RR=1.40; 95% CI 1.26 to 1.55).12
The efficacy of other interventions is less clear.12 Very low-quality evidence from 2 RCTs demonstrated benefit with TCAs compared with placebo (RR=3.00; 95% CI 2.05 to 4.38). However, the trials were small; short in duration (6 to 8 weeks); had unclear sources of funding; had unclear descriptions of randomization, allocation concealment, and blinding; and had high heterogeneity (I2=88%).
The 2018 simplified guideline for prescribing medical cannabinoids in primary care5 addressed cannabinoid use in neuropathic pain, recommending against medical cannabinoids as first- or second-line therapy owing to limited benefits and high risk of harms. The guideline suggested that clinicians could consider medical cannabinoids for refractory neuropathic pain, with multiple considerations including a reasonable therapeutic trial of 3 or more prescribed analgesics first. An updated review of cannabinoids for neuropathic pain was explored as a supplementary question for this guideline (Appendix 1*). It was again noted that when including all types of neuropathic pain, cannabinoids provide meaningful (≥30% pain reduction) relief in chronic neuropathic pain for about 39% to 40% of participants versus about 30% of those receiving placebo. Most cannabinoids studied were pharmaceutically derived cannabinoids. The RCTs were at considerable risk of bias with concerns regarding blinding, primarily enrolling patients with a history of cannabis use, small size, short duration, and selective reporting. In addition, trials were not typically reflective of the neuropathic pain types seen most commonly in primary care.
Topical nitrate spray was noted in the supplemental review of topical preparations for neuropathic pain (Appendix 1*) to have evidence from 2 small RCTs in diabetic neuropathy. Nitroglycerin spray (0.4 mg) applied topically to the affected area at bedtime resulted in statistically significant pain decrease by 2.5 to 3.0 points on a 0- to 10-point visual analogue scale compared with placebo, which decreased pain by 0.5 to 0.6 points.
Low-quality evidence suggests acupuncture, topical ketamine, topical amitriptyline, topical doxepin, and topical combinations are no better than control (Appendix 1*). For example, 3 RCTs demonstrated no significant benefit of acupuncture over placebo (RR=1.81; 95% CI 0.55 to 5.98).
Interventions where the harms likely exceed benefit in most patients include opioids, topiramate, and oxcarbazepine.12 No benefit was seen with oxcarbazepine above placebo, and withdrawals due to adverse effects were the highest of any intervention assessed for neuropathic pain (NNH=6). One trial demonstrated significantly more responders with topiramate (RR=1.42; 95% CI 1.05 to 1.91); however, it also demonstrated an increased number of patients withdrawing owing to adverse effects (NNH=7).
Six trials assessed opioids and found a significantly higher number of patients achieving meaningful pain relief (RR=1.37; 95% CI 1.19 to 1.57).12 Withdrawals due to adverse effects occurred more frequently in the opioid group (NNH=12). Adverse effects occurring in more than 10% of patients included somnolence or fatigue, pruritus, nausea, vomiting, constipation, and dizziness. No trial assessed long-term adverse effects including opioid misuse, opioid use disorder, and overdose. While earlier guidelines recommended opioids as second-line treatment for neuropathic pain,22 more recent guidelines positioned them as fifth-line options owing to concerns about long-term efficacy and considerable side effects.23 Based on available evidence, the committee believed that the harms will likely outweigh the benefits for most patients.
Additional rapid review questions
Does combination pharmacologic therapy improve pain outcomes more than monotherapy for patients with low back pain, neuropathic pain, or osteoarthritic pain? (Appendix 1*)
Recommendation: We suggest that the addition of another medication can be discussed if the initial medication has only provided partial benefit.
Many RCTs have studied combination therapy for low back pain or neuropathic pain. However, the number of available studies for any one combination is limited. The current evidence is insufficient to make any specific recommendations about which combination to select. Although there is inadequate evidence to suggest which combinations may be superior, the committee agreed it would be reasonable to trial an additional medication (combination) if patients achieve some benefit from the initial medication.
In patients with chronic pain taking long-term opioids, does pain or function improve if the dose is reduced or tapered off? (Appendix 1*)
Recommendation: For patients with chronic pain without opioid use disorder who are interested in tapering their long-term opioids, we suggest discussion of slow dose reductions, supported by CBT where possible. Evidence suggests potential harm in patients who are not interested in reducing or stopping opioids. If opioid use disorder is suspected, please refer to the PEER simplified guideline on the management of opioid use disorder.6
Conducted RCTs have been unable to demonstrate statistically significant reductions in opioid use compared with control despite interventions specifically aimed at opioid reduction. Many studies reported high dropout rates. In a number of trials, both groups saw a modest decline in opioid use that was associated with stable, and at times slightly improved, outcomes. However, observational data suggest a possible link between tapering and risk of overdose, mental health crises, and suicide. Tapering decisions must be discussed with patients, and if tapering will commence it should be done slowly (eg, 5% to 10% every 2 to 4 weeks).
What is the effect of diet-induced weight loss on osteoarthritis-related knee pain? (Appendix 1*)
Recommendation: We suggest that the goal of exercise be pain management, independent of weight loss.
Observational data suggest that obesity may be a risk factor for developing osteoarthritis; however, trials reporting diet-induced weight loss alone (eg, 5% weight loss) demonstrate limited, likely clinically insignificant, improvements in osteoarthritic pain (about 5 points on a 100-point pain scale).
In primary care, can interventions in the acute pain period prevent progression to chronic pain? (Appendix 1*). Despite poorly managed acute pain being often cited as a risk factor for the development of chronic pain, there is currently no high-quality evidence supporting interventions in the acute period that successfully modify this outcome.
Practice points and resources
There are many aspects of chronic pain management for which there is no high-level evidence to guide us. In acknowledging this, the Guideline Committee, through an iterative process, created a list of practice points to assist clinicians in providing care for patients with chronic pain. Ultimately, it was noted that there was considerable variation in practice and approaches to pain, further confirming that the management of pain should be individualized and based on shared decision making. Practice points that rose to the top and were identified as priorities by the committee are listed in Box 2. In addition, the committee worked to identify a short list of resources that could be recommended to patients (Box 3).
Box 2. Practice points for managing chronic pain in primary care.
Goals of treatment should be identified by patients, realistic, and focused on functional outcomes
Initiate, titrate, taper, or discontinue 1 medication at a time to allow for more accurate monitoring of response or adverse effects
Lower doses of medications generally provide most of the benefit with the lowest risk of adverse effects
Consider stopping any pharmacologic interventions that have not demonstrated clear benefit by 8 to 12 weeks. Deprescribing may also be a form of chronic pain management
Pain scales are controversial, as they may be more reflective of emotional and psychological factors than actual pain. The focus should remain on coping with pain and impact on daily function and activities
Consider the use of a simple tool such as the POMI to identify patients currently taking opioid therapy who might have opioid use disorder
Consider screening for past trauma including adverse childhood experiences or posttraumatic stress disorder. This may assist in building therapeutic rapport and help understand the patient’s coping mechanisms
POMI—Prescription Opioid Misuse Index.
Box 3. Practical resources for clinicians and patients managing chronic pain in primary care.
Understanding and rethinking chronic pain for patients (by Professor Lorimer Moseley and Dave Moen): https://www.tamethebeast.org/
Sample exercise prescription (by RxFiles): https://www.rxfiles.ca/RxFiles/uploads/documents/Exercise-RxFiles-Rx.pdf
For a list of additional resources for health professionals and patients (by RxFiles): https://www.rxfiles.ca/RxFiles/uploads/documents/PainLinks.pdf
For general exercise videos for people with pain (by Pain BC): https://painbc.ca/gentle-movement-at-home
For exercise videos for people with specific pain types (by Dr Andrea Furlan): https://www.youtube.com/channel/UCXnFys9ZXBE0uyDhKHUi-dA
These recommendations are based on the opinions of the committee and current trends in practice.
DISCUSSION
Strengths of this guideline include a focus on shared decision making and the development of tools to help patients and clinicians make informed decisions. Decision aids have been demonstrated to help increase patient knowledge, accuracy of risk perception, and congruency between values and care choices.16 Numerous groups, including the Canadian Pain Task Force, suggest that educational materials be made available to both people living with pain and health professionals to increase understanding of evidence-informed approaches.1,4 Similarly, the National Institute for Health and Care Excellence guideline on multimorbidity recommends that clinicians should “review medicines and other treatments taking into account evidence of likely benefits and harms for the individual patient and outcomes important to the person.”24
The inclusion of best available evidence, including 3 systematic reviews of 285 RCTs,10-12 allows for an evidence-based discussion of benefit (in many cases approximately 30% improvement) and potential harms. A focus on responder analysis highlights clinically important patient outcomes and may help clarify patient expectations with treatments. It is possible that trials were more likely to report responder analysis when such analysis showed positive results, which would lead to overestimation of benefit for some interventions. For all interventions, we recorded withdrawal due to adverse effects as reported in the RCTs. This likely underestimates the incidence of adverse effects, as adverse effects are not always well reported, and many patients at increased risk of adverse effects, such as those with pre-existing conditions, may have been excluded from trials. When translating evidence from all interventions to a 2-page summary, we normalized the control rate for all interventions in order to indirectly compare event rates. This approach may exaggerate the effectiveness of some interventions and minimize the effectiveness of others.
Recommendations regarding medications that should be avoided in most patients are consistent with previous Canadian guidelines on cannabinoids,5 although they are less favourable with regard to the use of cannabinoids than recently published international guidelines.19 Similarly, our recommendations regarding the initiation of opioids in primary care are much less favourable than recommendations in previous Canadian guidelines.25 Our hesitancy toward both of these interventions is based on the absence of high-quality evidence of long-term benefit in chronic pain conditions and known risk of harms.
Limitations
A possible limitation of this guideline is lack of guidance for managing the substantial complexities associated with some chronic pain patients. As clinicians we recognize that while guidelines address common management issues in succinct and clear ways, applying these approaches to the unique challenges of particularly complex individual patients can be inadequate. Our guideline supports key features like activity, counseling, and shared decision making—which are needed in virtually all patients. However, there is little high-quality evidence to address the management of patients who, despite multiple interventions, are not coping well. In addition, the complexity of an opioid use disorder was not specifically addressed in this guideline; however, diagnosis and management of opioid use disorder have been outlined in a previous guideline.6 We attempted to collate practical points from the committee and experts in the field; however, we found considerable variation in practice and recommendations, highlighting the absence of a clear consensus on how to manage complex chronic pain.
It is important to recognize that this guideline is not intended to outline a stepwise approach to the management of chronic pain, but rather should be considered a tool to discuss available options. A shared discussion is important in that many components of chronic pain management do not have high-level evidence to guide us. For most patients, the use of interventions where harms exceed benefits should be avoided and should not be the default when patients have not responded to other interventions.
Conclusion
The highest-quality evidence suggests that many interventions have similar limited benefit beyond that of placebo. Thus, decisions among them may be based on a number of other factors, including patients’ past experiences with different therapies, acceptability of side effects, accessibility, cost, and coverage. All discussions of treatment should involve the patient’s preferences and values. An online decision aid is available.26 It is important, however, to not allow a comprehensive list of plausible interventions to distract providers and patients from the intervention with the evidence of greatest benefit—namely exercise—specifically in osteoarthritis and low back pain.
We hope that future RCTs will prioritize responder analyses, investigate primary care interventions that could be used to help prevent the progression from acute to chronic pain, and identify new or combination treatments that improve patient outcomes. In addition, future RCTs should explore how to assist patients with past trauma, as emotions, suffering, and pain may be closely linked.27
Supplementary Material
Acknowledgment
We thank the College of Family Physicians of Canada, the Ontario College of Family Physicians, the Alberta College of Family Physicians, and the Saskatchewan College of Family Physicians for their financial support. In addition, we thank Odelia Moses for her administrative support, Catherine Barrington for her assistance in compiling the supporting documents including the appendix, and the patients living with chronic pain who reviewed this guideline.
Editor’s key points
▸ Chronic pain is one of the most complex and difficult conditions to treat. This guideline was developed to assist clinicians and patients with managing chronic low back, osteoarthritic, and neuropathic pain. The best available evidence for common conservative therapeutic interventions was assessed, with an emphasis on shared decision-making principles. Interventions accessible to most urban and rural primary care clinicians were prioritized.
▸ Physical activity is recommended as the most effective intervention for managing osteoarthritis and chronic low back pain. Cognitive-behavioural therapy or mindfulness-based stress reduction are also suggested as options for these conditions, as well as for neuropathic pain. Other treatments with evidence of benefit are outlined for each condition. Various treatments for which there is unclear or no benefit could be discussed with patients after treatments with clear benefit have been considered. The harms of opioids and cannabinoids likely outweigh benefits and it is suggested they be avoided in most patients and in most conditions.
▸ A 2-page summary and a patient handout are included to simplify the recommendations and assist with shared, informed decision making between provider and patient.
Footnotes
The full disclosure of competing interests, details of the rapid reviews, the 2-page summary, a patient handout, the peer review feedback, details of the specific interventions and dosing studied, and the supplemental evidence review are available from https://www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
Members of the Evidence Team (Dr G. Michael Allan, Danielle Perry, Dr Samantha S. Moe, Dr Joey Ton, Dr Michael R. Kolber, Dr Jessica Kirkwood, Betsy Thomas, Dr Scott Garrison, Dr Jamison Falk, Dr Nicolas Dugré, Logan Sept, Dr Ricky D. Turgeon, Dr Allison Paige, Dr Jen Potter, Tony Nickonchuk, Dr Anthony D. Train, Dr Justin Weresch, Dr Karenn Chan, Dr Adrienne J. Lindblad, Dr James P. McCormack, and Dr Christina S. Korownyk) completed the systematic and rapid reviews. The Guideline Committee (Dr Christina S. Korownyk, Dr Lori Montgomery, Dr Jennifer Young, Dr Simon Moore, Dr Alexander G. Singer, Dr Peter MacDougall, Dr Sean Darling, Kira Ellis, Jacqueline Myers, Candice Rochford, Dr Marie-Christine Taillefer, and Dr Adrienne J. Lindblad) formulated the recommendations based on the review of evidence. All authors contributed to the recommendations and to preparing the manuscript for submission.
Competing interests
None of the authors has a financial conflict of interest to declare. The full disclosure is available from CFPlus.*
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to https://www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à https://www.cfp.ca dans la table des matières du numéro de mars 2022 à la page e63.
References
- 1.The Canadian Pain Task Force . An action plan for pain in Canada. Ottawa, ON: Health Canada; 2021. Available from: https://www.canada.ca/content/dam/hc-sc/documents/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2021-rapport/report-rapport-2021-eng.pdf. Accessed 2021 Sep 13. [Google Scholar]
- 2.Ballantyne JC, Sullivan MD. Intensity of chronic pain—the wrong metric? N Engl J Med 2015;373(22):2098-9. [DOI] [PubMed] [Google Scholar]
- 3.Pain Management Best Practices Inter-Agency Task Force . Pain management best practices. Inter-Agency Task Force report. Updates, gaps, inconsistencies, and recommendations. Washington, DC: US Department of Health and Human Services; 2019. Available from: https://www.hhs.gov/sites/default/files/pain-mgmt-best-practices-draft-final-report-05062019.pdf. Accessed 2021 Sep 26. [Google Scholar]
- 4.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education . Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 5.Allan GM, Ramji J, Perry D, Ton J, Beahm NP, Crisp N, et al. . Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician 2018;64:111-20 (Eng), e64-75 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 6.Korownyk C, Perry D, Ton J, Kolber MR, Garrison S, Thomas B, et al. . Managing opioid use disorder in primary care. PEER simplified guideline. Can Fam Physician 2019;65:321-30 (Eng), e173-84 (Fr). Erratum in: Can Fam Physician 2019;65:687. [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Graham R, Mancher M, Miller Wolma D, Greenfield S, Steinberg EP, editors. Clinical practice guidelines we can trust. Washington, DC: National Academies Press (US); 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209539/. Accessed 2019 Jan 19. [PubMed] [Google Scholar]
- 8.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P, et al. . Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med 2012;156(7):525-31. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ton J, Perry D, Thomas B, Allan GM, Lindblad AJ, McCormack J, et al. . PEER umbrella systematic review of systematic reviews. Management of osteoarthritis in primary care. Can Fam Physician 2020;66:e89-98. Available from: https://www.cfp.ca/content/cfp/66/3/e89.full.pdf. Accessed 2022 Jan 30. [PMC free article] [PubMed] [Google Scholar]
- 11.Kolber MR, Ton J, Thomas B, Kirkwood J, Moe S, Dugré N, et al. . PEER systematic review of randomized controlled trials. Management of chronic low back pain in primary care. Can Fam Physician 2021;67:e20-30. Available from: https://www.cfp.ca/content/cfp/67/1/e20.full.pdf. Accessed 2022 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falk J, Thomas B, Kirkwood J, Korownyk CS, Lindblad AJ, Ton J, et al. . PEER systematic review of randomized controlled trials. Management of chronic neuropathic pain in primary care. Can Fam Physician 2021;67:e130-40. Available from: https://www.cfp.ca/content/cfp/67/5/e130.full.pdf. Accessed 2022 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. . Chronic pain as a symptom or a disease: the IASP classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019;160(1):19-27. [DOI] [PubMed] [Google Scholar]
- 14.Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI] [PubMed] [Google Scholar]
- 15.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64(4):401-6. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 16.Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. . Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou R, Hartung D, Turner J, Blazina I, Chan B, Levander X, et al. . Opioid treatments for chronic pain. Rockville, MD: Agency for Healthcare Research and Quality; 2020. [PubMed] [Google Scholar]
- 18.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. . OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27(11):1578-89. Epub 2019 Jul 3. [DOI] [PubMed] [Google Scholar]
- 19.Busse JW, Vankrunkelsven P, Zeng L, Heen AF, Merglen A, Campbell F, et al. . Medical cannabis or cannabinoids for chronic pain: a clinical practice guideline. BMJ 2021;374:n2040. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Hong PJ, May C, Rehman Y, Oparin Y, Hong CJ, et al. . Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ 2021;374:n1034. [DOI] [PubMed] [Google Scholar]
- 21.Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians; Denberg TD, et al. . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166(7):514-30. Epub 2017 Feb 14. [DOI] [PubMed] [Google Scholar]
- 22.Mu A, Weinberg E, Moulin DE, Clarke H. Pharmacologic management of chronic neuropathic pain. Review of the Canadian Pain Society consensus statement. Can Fam Physician 2017;63:844-52. [PMC free article] [PubMed] [Google Scholar]
- 23.Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. . A comprehensive algorithm for management of neuropathic pain. Pain Med 2019;20(Suppl 1):S2-12. Epub 2019 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Guideline Centre . Multimorbidity: assessment, prioritisation and management of care for people with commonly occurring multimorbidity. London, UK: National Institute for Health and Care Excellence; 2016. [PubMed] [Google Scholar]
- 25.Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. . Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189(18):E659-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PEER . Comparing treatment options for pain: the C-TOP tool. Edmonton, AB: Alberta College of Family Physicians; 2022. Available from: https://pain-calculator.com/. Accessed 2022 Jan 4. [Google Scholar]
- 27.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. . Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013;136(Pt 9):2751-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.