Abstract
Background and aims
High remnant cholesterol is associated with cardiovascular disease (CVD), but whether CVD risk attributable to remnant cholesterol vary between young and later adults remains unknown. The study aimed to assess age differences in the association between remnant cholesterol and CVD.
Methods
This prospective study included 95 663 participants without CVD and lipid-lowering treatment at baseline, including 16 254 young adults (age 18–39 years) and 79 409 later adults (age ≥40 years). Individuals were grouped by clinically meaningful remnant cholesterol concentrations of <0.50, 0.50–0.99, 1.00–1.49, and ≥1.50 mmol/L. Multivariable Cox regressions were performed to calculate hazard ratio (HR).
Results
During a median follow-up of 11.01 years, 164 incident CVD were identified in young adults, and 6081 cases in later adults. After multivariate adjustment, the association between remnant cholesterol and CVD was more pronounced in young adults than later adults (P for interaction=0.0019), the HR was 2.24 (95% confidence interval [CI], 1.45–3.47) for young individuals with remnant cholesterol ≥1.50 mmol/L, compared to individuals with remnant cholesterol <0.50 mmol/L, while the corresponding HR was 1.21 (95% CI, 1.13–1.29) for later adults. Furthermore, the population attributable risk percentage for remnant cholesterol < 1.50 vs ≥ 1.50 mmol/L was also higher in young (14.8%) than later adults (4.2%).
Conclusions
Despite a lower incidence risk of CVD among young adults compared later adults, the stronger association and greater attributable risk of remnant cholesterol among young adults highlight the importance of preventive efforts across the adult life course.
Keywords: Remnant cholesterol, Cardiovascular disease, Age difference, Risk factors, Primary prevention
Highlight
-
●
Step-wise increase in remnant cholesterol was associated with step-wise higher risk of CVD.
-
●
The association between remnant cholesterol and CVD was modified by age.
-
●
The association between remnant cholesterol and CVD was more pronounced in young adults than later adults.
-
●
High remnant cholesterol confers a higher relative excess risk in young versus later adults.
-
●
The findings highlight the importance of preventive efforts across the adult life course.
Introduction
Cardiovascular disease (CVD) is still the leading cause of mortality, morbidity and disability worldwide, accounting for one third of the overall mortality [1]. Identifying and managing risk factors for CVD are effective and economic strategies for the primary prevention of CVD [2]. Remnant cholesterol is the cholesterol content of the triglyceride-rich lipoproteins (TRLs), which in the fasting state comprise very-low-density lipoprotein and intermediate-density lipoprotein, and these two lipoproteins together with chylomicron remnants in the non-fasting state [3,4]. TRLs and the remnant cholesterol carried in these particles have the capacity to cross the arterial wall and are taken up by macrophages and smooth muscle cells [5]. Since triglycerides (TGs), but not cholesterol, can be easily metabolized in most cells, it has been hypothesized that cholesterol, not TGs is the harmful component in TRLs [6], accumulation in the arterial wall of the remnant cholesterol may play a causal role in atherosclerosis development, similar to low-density lipoprotein cholesterol (LDL-C) [7]. It thus may bring more attention to the potential of preventing CVD in subjects with high remnant cholesterol by focusing on the cholesterol content of remnants, instead of on TGs content.
Indeed, the relationships between remnant cholesterol and cardiovascular events have been confirmed in both observational and genetic studies [[7], [8], [9], [10], [11], [12]]. However, scarce information is available from Chinese prospective cohorts, because studies on the associations between remnant cholesterol with CVD have been mainly conducted in north European and U.S. population samples [[7], [8], [9], [10], [11], [12]]. Furthermore, previous studies have identified that the cardiovascular risk attributable to cholesterol may vary between younger and older individuals, young adult (aged < 40 years) exposures to elevated cholesterol was associated with increased CVD risk [13,14]. Nevertheless, the potential age-specific nature of the CVD risk associated with remnant cholesterol exposure has not been fully quantified and explored by the present data. Therefore, this study aimed to investigate the association of remnant cholesterol with risk of CVD and its subtypes across young (18–39 years of age) and later adults (≥40 years of age) in a large community-based Chinese cohort.
Material and methods
Study population
The Kailuan study is a prospective community-based cohort study. The detailed study design and procedures have been described previously [15,16]. At baseline of the study between June 2006 and October 2007, a total of 101 510 individuals with an age ranging from 18 to 98 years were enrolled and have completed questionnaires and health assessments every 2 years up to their death or to the end of the follow-up on December 31, 2017. In the present study, we excluded participants with missing data on lipids (including total cholesterol, TG, LDL-C, and high-density lipoprotein cholesterol, n=1440), receiving lipid-lowering treatment (n=958) and those with a history of stroke or myocardial infarction (MI) (n=3449) at baseline. Ultimately, a total of 95 663 participants were included in the study, of which 16 254 participants were young adults (18–39 years of age), and 79 409 were later adults (≥40 years of age) (Fig. S1). The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan Hospital and Beijing Tiantan Hospital.
Laboratory measurements
Fasting blood samples were collected from antecubital vein after an 8- to 12-h overnight fast and transferred to vacuum tubes containing EDTA. All the biochemical parameters were measured using Hitachi 747 auto-analyzer (Hitachi, Tokyo, Japan) in the central laboratory of Kailuan Hospital. Serum total cholesterol and TG were measured using the enzymatic colorimetric method, high-density lipoprotein cholesterol and LDL-C were measured by direct test method with the inter-assay coefficient of variation <10% (Mind Bioengineering Co.Ltd., Shanghai, China). LDL-C was calculated by the Friedewald equation (total cholesterol −HDL cholesterol−TG/2.2) when TGs were ≤ 4 mmol/L (n=90 160), otherwise LDL-C was measured directly (n=5503) [17]. Remnant cholesterol was calculated as total cholesterol minus LDL-C minus high-density lipoprotein cholesterol.
Assessments of outcomes
The outcome in the present study was the first occurrence of non-fatal CVD events. The types of CVD included stroke and MI. We defined CVD events as described previously [15,16]. The database of CVD diagnoses was obtained from the Municipal Social Insurance Institution and Hospital Discharge Register and was updated annually during the follow-up period. An expert panel collected and reviewed annual discharge records from 11 Kailuan hospitals to identify patients who were suspected of CVD. Incident stroke was diagnosis based on neurological signs, clinical symptoms, and neuroimaging tests, including computed tomography or magnetic resonance, according to the World Health Organization criteria, and classified into 3 types: cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage [18]. MI was diagnosed according to the criteria of the World Health Organization on the basis of clinical symptoms, changes in the serum concentrations of cardiac enzymes and biomarkers, and electrocardiographic results [16,19].
Assessment of covariates
Information on potential covariates was collected in 2006 and updated biennially thereafter, as detailed elsewhere [15,16]. In brief, sociodemographic characteristics, lifestyles, and clinical characteristics were collected via a standard questionnaire, including age, sex, education, income, smoking status, drinking status, physical activity levels, and medical history. Educational level was categorized as illiteracy or primary school, middle school, and high school or above. Income level was categorized as < 800 RMB/month and ≥800 RMB/month. Smoking and drinking status stratified into three levels: never, former, or current. Active physical activity was defined as more than 4 times per week and >20 min at a time. Body mass index was calculated by dividing body weight (kg) by the square of height (m). Blood pressure was measured in the seated position using a mercury sphygmomanometer, systolic blood pressure and diastolic blood pressure was calculated as the three average of three measurements. All the blood samples were analyzed using an auto-analyzer (Hitachi 747, Hitachi, Tokyo, Japan) on the day of the blood draw. The biochemical indicators tested included fasting blood glucose, serum lipids, serum creatinine, and high-sensitivity C-reactive protein. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [20]. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, any use of the antihypertensive drug, or self-reported history of hypertension. Diabetes was defined as fasting blood glucose ≥7.0 mmol/l, any use of glucose-lowering drugs, or any self-reported history of diabetes. Dyslipidemia was defined as total cholesterol ≥5.17 mmol/l, or any self-reported history or use of lipid-lowering drugs.
Statistical analysis
Young and later adults were divided into four categories based on remnant cholesterol concentrations using clinically meaningful cut-point values of <0.50 mmol/L, 0.50–0.99 mmol/L, 1.00–1.49 mmol/L, and ≥ 1.50 mmol/L in reference to the relevant literature, separately [21]. Continuous variables were described as mean ± standard deviation and were compared with analysis of variance, categorical variables were described as frequency with percentage and were compared with chi-square test. The person-year was determined from the date when the message was collect in the baseline survey (2006) to either the date of CVD onset, death, or end of the follow-up (December 31, 2017), which came first. The incidence rate was calculated by dividing the number of incident cases by the total follow-up duration (person-years).
The multivariable-adjusted hazard ratios (HRs) and 95% confidence interval (CIs) for CVD, stroke, and MI among young and later adults were determined by Cox proportional hazards regression models. Model 1 was adjusted for age and sex, Model 2 was further adjusted for body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive drugs, antidiabetic drugs, and high-sensitivity C-reactive protein. Proportion hazards assumption was satisfied by checking the Schoenfeld residual methods. P-values for trend were computed using remnant cholesterol categories as ordinal variables. The interaction between age and remnant cholesterol was also evaluated by Cox regression models. We also calculated the population attributable risk percentage (PAR%) [22,23] - an estimate of the percentage of incident CVD in this population during follow-up that hypothetically would not have occurred if all participants had been in the group of remnant cholesterol < 1.5 mmol/L, following a method previously suggested by Wacholder et al. [24] In addition, we used restricted cubic splines to examine the shape of the remnant cholesterol and outcomes with 5 knots (at the 5th, 25th, 50th, 75th, and 95th percentiles). The reference point for remnant cholesterol was 0.50 mmol/L, and the HR was adjusted for all the confounding variables above-mentioned.
To test the robustness of our findings, several sensitivity analyses were performed. First, competing risk model was applied to assess the association between remnant cholesterol and the outcomes considering non-CVD death as a competing risk event. Second, we excluded participants who used lipid-lowering agents during the follow-up. Third, to explore the potential impact of reverse causality, we excluded the outcome events that occurred within the first 2 years of the follow-up period. Forth, we examined whether LDL-C measurement method influenced the degree of association between remnant cholesterol and CVD. Moreover, to know the influence of more advanced age, the later adults were further divided into 2 groups by 65 years. We also examined the association between remnant cholesterol with the study outcomes among prespecified subgroup based on BMI, smoking status, drinking status, hypertension, diabetes, and dyslipidemia.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and R software version 3.6.1 (R Core Team, Vienna, Austria). All statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 95 663 eligible participants, 16 204 (16.99%) participants were aged from 18 to 39, and 79 409 participants were aged ≥ 40 years. Baseline characteristics according to remnant cholesterol categories are presented in Table 1. There were significant difference among the four remnant cholesterol categories in age, sex, education, income, body mass index, systolic blood pressure, diastolic blood pressure, smoking, drinking, physical activity, diabetes, dyslipidemia, antidiabetic agents, fasting blood glucose, total cholesterol, TG, high-density lipoprotein cholesterol, LDL-C, high-sensitivity C-reactive protein in both young and later adults.
Table 1.
Baseline characteristics according to remnant cholesterol (mmol/L) in young and later adults.
| Characteristics | Age < 40 years |
Age ≥ 40 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <0.50 (n=5679) | 0.50–0.99 (n=4285)< | 1.00–1.49 (n=2997) | ≥1.50 (n=3293) | P value | <0.50 (n=19843) | 0.50–0.99 (n=18488) | 1.00–1.49 (n=15733) | ≥1.50 (n=25345) | P value | |
| Age, years | 31.89 ± 5.26 | 32.01 ± 5.34 | 32.56 ± 5.01 | 33.40 ± 4.89 | <0.0001 | 55.47 ± 9.99 | 55.28 ± 9.72 | 55.56 ± 10.14 | 55.15 ± 9.35 | 0.0001 |
| Men, n (%) | 4203 (74.01) | 3292 (76.83) | 2150 (71.74) | 2463 (74.80) | <0.0001 | 16662 (83.97) | 15223 (82.34) | 12446 (79.11) | 19698 (77.72) | <0.0001 |
| High school or above, n (%) | 2908 (51.39) | 2130 (49.92) | 1338 (44.85) | 1266 (38.68) | <0.0001 | 2777 (14.58) | 2614 (14.47) | 2263 (14.98) | 3219 (13.45) | 0.0001 |
| Income >800 RMB/m, n (%) | 1162 (20.53) | 881 (20.67) | 588 (19.70) | 538 (16.43) | <0.0001 | 2247 (11.81) | 2371 (13.13) | 2126 (14.08) | 3112 (13.02) | <0.0001 |
| BMI, kg/m2 | 24.20 ± 3.74 | 24.67 ± 3.84 | 24.55 ± 3.83 | 25.24 ± 3.93 | <0.0001 | 24.74 ± 3.30 | 25.23 ± 3.40 | 25.09 ± 3.50 | 25.26 ± 3.42 | <0.0001 |
| SBP, mm Hg | 118.44 ± 15.30 | 118.82 ± 15.32 | 118.25 ± 15.94 | 120.51 ± 16.06 | <0.0001 | 133.47 ± 21.01 | 133.98 ± 21.41 | 132.02 ± 21.10 | 132.39 ± 20.60 | <0.0001 |
| DBP, mm Hg | 78.13 ± 10.34 | 78.66 ± 10.42 | 78.54 ± 10.70 | 80.02 ± 11.11 | <0.0001 | 84.47 ± 11.62 | 84.95 ± 11.87 | 83.72 ± 11.88 | 84.06 ± 11.65 | <0.0001 |
| Current smoker, n (%) | 1950 (34.42) | 1702 (39.82) | 1108 (37.06) | 1209 (36.83) | <0.0001 | 6676 (34.88) | 6904 (37.86) | 5165 (33.56) | 7105 (29.46) | <0.0001 |
| Current alcohol use, n (%) | 2578 (45.52) | 2161 (50.56) | 1405 (47.01) | 1456 (44.34) | <0.0001 | 6941 (36.24) | 6973 (38.22) | 5559 (36.10) | 7708 (31.95) | <0.0001 |
| Active physical activity, n (%) | 4943 (87.36) | 3763 (88.23) | 2697 (90.41) | 2956 (90.26) | <0.0001 | 17132 (90.23) | 16375 (90.72) | 13944 (92.40) | 22321 (93.68) | <0.0001 |
| Hypertension, n (%) | 11 (0.19) | 12 (0.28) | 7 (0.23) | 10 (0.30) | 0.7291 | 536 (2.70) | 590 (3.19) | 523 (3.32) | 851 (3.36) | 0.0004 |
| Diabetes Mellitus, n (%) | 113 (1.99) | 99 (2.31) | 92 (3.07) | 93 (2.82) | 0.0069 | 2202 (11.10) | 2529 (13.68) | 2120 (13.47) | 3252 (12.83) | <0.0001 |
| Dyslipidemia, n (%) | 49 (0.86) | 63 (1.47) | 51 (1.70) | 54 (1.64) | 0.0014 | 759 (3.82) | 934 (5.05) | 809 (5.14) | 1628 (6.42) | <0.0001 |
| Antihypertensive agents, n (%) | 9 (0.16) | 9 (0.21) | 4 (0.13) | 8 (0.24) | 0.7068 | 410 (2.07) | 436 (2.36) | 387 (2.46) | 649 (2.56) | 0.006 |
| Antidiabetic agents, n (%) | 94 (1.65) | 78 (1.82) | 75 (2.50) | 72 (2.19) | 0.0345 | 1859 (9.37) | 2179 (11.79) | 1806 (11.48) | 2832 (11.17) | <0.0001 |
| FBG, mmol/L | 5.08 ± 0.96 | 5.07 ± 0.94 | 5.11 ± 0.98 | 5.22 ± 1.22 | <0.0001 | 5.48 ± 1.57 | 5.49 ± 1.66 | 5.53 ± 1.69 | 5.62 ± 1.96 | <0.0001 |
| TC, mmol/L | 4.06 ± 0.98 | 4.62 ± 0.77 | 4.88 ± 0.73 | 5.63 ± 1.13 | <0.0001 | 4.27 ± 1.19 | 4.90 ± 0.87 | 4.99 ± 0.80 | 5.63 ± 1.10 | <0.0001 |
| TG, mmol/L | 1.34 ± 1.25 | 1.40 ± 0.92 | 1.57 ± 1.08 | 2.10 ± 1.85 | <0.0001 | 1.46 ± 1.30 | 1.43 ± 0.80 | 1.66 ± 1.05 | 2.07 ± 1.75 | <0.0001 |
| HDL-C, mmol/L | 1.48 ± 0.36 | 1.48 ± 0.31 | 1.49 ± 0.32 | 1.44 ± 0.34 | <0.0001 | 1.59 ± 0.43 | 1.58 ± 0.39 | 1.59 ± 0.41 | 1.51 ± 0.41 | <0.0001 |
| LDL-C, mmol/L | 2.66 ± 0.71 | 2.40 ± 0.70 | 2.16 ± 0.65 | 2.04 ± 0.72 | <0.0001 | 2.87 ± 0.94 | 2.58 ± 0.85 | 2.16 ± 0.73 | 1.85 ± 0.84 | <0.0001 |
| Hs-CRP, mg/dL | 1.53 ± 4.04 | 1.66 ± 4.54 | 1.79 ± 4.55 | 2.05 ± 5.24 | <0.0001 | 2.26 ± 5.81 | 2.14 ± 6.80 | 2.07 ± 4.96 | 3.24 ± 7.90 | <0.0001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Association between remnant cholesterol and the outcomes
During a median follow-up of 11.01 years (interquartile rage: 10.67–11.20), there were 164 incident cases of CVD (including 127 cases of stroke and 38 cases of MI) for young adults, and 6081 incident CVD (including 4832 stroke and 1398 MI) for participants later adults. The detailed number of CVD across the four remnant cholesterol is shown in Table 2. Participants with remnant cholesterol ≥ 1.50 mmol/L had the highest incidence rate of CVD, which was 1.60 (95% CI, 1.23–2.07) for young adults, and 8.26 (95% CI, 7.92–8.62) for later adults.
Table 2.
Association between remnant cholesterol and cardiovascular disease in young and later adults.
| Outcomes |
Remnant cholesterol, mmol/L |
Per 1 mmol/L increase |
P for trend |
Pinteraction |
|||
|---|---|---|---|---|---|---|---|
| <0.50 |
0.50–0.99 |
1.00–1.49 |
≥1.50 |
||||
| Age<40 years | |||||||
| Case, n (%) | 33(0.58) | 39(0.91) | 35(1.17) | 57(1.73) | |||
| Incidence rate, per 1000 person-y | 0.53(0.38–0.75) | 0.84(0.61–1.15) | 1.08(0.77–1.50) | 1.60(1.23–2.07) | |||
| Model 1 | Reference | 1.49(0.94–2.37) | 1.91(1.18–3.07) | 2.48(1.61–3.81) | 1.26(1.12–1.41) | <0.0001 | 0.0004 |
| Model 2 | Reference | 1.46(0.92–2.33) | 1.85(1.14–2.99) | 2.24(1.45–3.47) | 1.23(1.09–1.39) | <0.0001 | 0.0019 |
| Age≥40 years | |||||||
| Case, n (%) | 1454(7.33) | 1351(7.31) | 1131(7.19) | 2145(8.46) | |||
| Incidence rate, per 1000 person-y | 7.18(6.82–7.56) | 7.14(6.76–7.53) | 6.99(6.60–7.41) | 8.26(7.92–8.62) | |||
| Model 1 | Reference | 1.01(0.94–1.09) | 0.99(0.92–1.07) | 1.22(1.14–1.30) | 1.06(1.04–1.08) | <0.0001 | |
| Model 2 | Reference | 0.98(0.91–1.06) | 1.00(0.92–1.08) | 1.21(1.13–1.29) | 1.06(1.03–1.09) | <0.0001 | |
Model 1: adjusted for age and sex.
Model 2: further adjusted for body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive drugs, antidiabetic drugs, fasting blood glucose, and high sensitivity C-reactive protein.
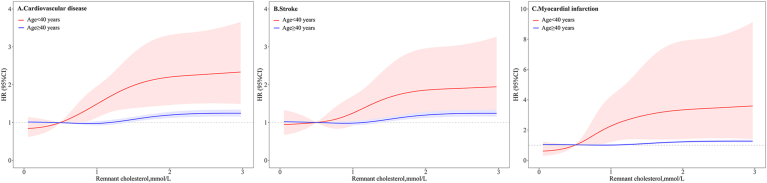
After multivariate adjustment, the risk of CVD was step-wise increased with elevated remnant cholesterol in both young and later adults (P for trend <0.0001). There was a significant interaction between age and remnant cholesterol on the risk of CVD (P for interaction=0.0019), the effect of remnant cholesterol on CVD appeared to be more pronounced among young adults than later adults. Young adults with remnant cholesterol ≥ 1.50 mmol/L had 2.24-fold (HR, 2.24; 95% CI, 1.45–3.47) higher risk of CVD compared to those with remnant cholesterol < 0.50 mmol/L, whereas the corresponding HR was 1.21 (95% CI, 1.13–1.29) for later adults (Fig. 1A). The PAR% (95% CI) of CVD in relation to high remnant cholesterol (≥1.50 mmol/L) was 14.8% (1.8%–27.2%) for young adults, and 4.2% (2.7%–5.7%) for later adults (Table 3). Multivariable-adjusted spline regression models showed an S-shaped association between remnant cholesterol and the risk of CVD (Fig. 2A).
Fig. 1.
Adjusted hazard ratio and 95% confidence interval for the association of remnant cholesterol with risk of cardiovascular disease, stroke and myocardial infarction.
Adjusted for age, sex, body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive drugs, antidiabetic drugs, and high sensitivity C
-reactive protein.
AbbreviationsCI, confidence interval.
Table 3.
PAR% and 95% confidence interval for CVD, stroke, and MI by remnant cholesterol of <1.50 vs. ≥1.50 mmol/L.
| Outcomes | Age<40 years |
Age≥40 years |
||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| CVD | 15.4 (3.5–26.9) | 14.8(1.8–27.2) | 4.4(2.9–5.8) | 4.2(2.7–5.7) |
| Stroke | 13.3(0.1–26.0) | 12.5(0.0–25.9) | 3.8(2.2–5.3) | 3.8(2.2–5.4) |
| MI | 21.1(0.0–44.5) | 20.2(5.8–33.8) | 6.7(3.4–10.0) | 5.4(1.8–9.0) |
Abbreviations: CVD, cardiovascular disease; MI, myocardial infarction; PAR%, population attributable risk percentage.
Model 1: adjusted for age and sex.
Model 2: further adjusted for body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive drugs, antidiabetic drugs, and high sensitivity C-reactive protein.
Fig. 2.
HRs and 95% CIs for the association between remnant cholesterol with risk of cardiovascular disease, stroke, and myocardial infarction by using restricted cubic spline regression with 5 knots with placed at the 5th, 25th, 50th, 75th, and 95th percentiles of remnant cholesterol.
The red line and area represented HR (95% CI) in young adults and blue line and area represented HR (95% CI) in later adults.
Adjusted for age, sex, body mass index, systolic blood pressure, diastolic blood pressure, education, income, smoking status, drinking status, physical activity, a history of hypertension, diabetes mellitus, and dyslipidemia, antihypertensive drugs, antidiabetic drugs, and high sensitivity C-reactive protein.
Abbreviations: HR, hazard ratio; CI, confidence interval.. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In the subtype analyses of CVD, similar results were yielded for stroke and MI, with higher HRs and PAR% presented in young than later adults (Table S1, Table 3, Fig. 1B-C, and Fig. 2B-C). However, the interaction between age and remnant cholesterol on the risk of MI was not statistically significant, which could be limited by the relatively small sample size of cases. The sensitivity analyses with competing risk model (Table S2), excluding participants taking lipid-lowering agents during the follow-up (n=1 024, Table S3), excluding the outcome events occurred within the first 2 years of the follow-up period (n=1 475, Table S4), and LDL-C measurement with different method (Table S5), all generated similar findings with the primary analysis. When the later adults were further divided into 2 groups by 65 years, there remained a significant interaction between age and remnant cholesterol in relation to the risk of CVD (P for interaction=0.0267), participants aged<65 years (HR, 2.33; 95 CI, 1.49–3.64 for participants aged <40 years; HR, 1.19; 95% CI, 1.10–1.29 for participants aged 40–64 years) had a higher risk of CVD than those aged over 65 years (HR, 1.14; 95% CI, 1.00–1.29) (Table S6). Subgroup analyses showed the association between remnant cholesterol and the risk of CVD were consistent across different subgroups (P for interaction>0.05 for all, Table S7).
Discussion
In this observational cohort of 95 663 Chinese individuals, we found that remnant cholesterol was associated with a stepwise increase in the risk of CVD. Notably, there was a significant interaction between age and remnant cholesterol on the risk of CVD, the association between remnant cholesterol and CVD was more pronounced in young adults than later adults. Similar patterns were observed for stroke and MI. The trend remained robust among multiple sensitivity analyses and the stratified analyses. These findings suggest that individuals, especially young adults with high remnant cholesterol warrant particular vigilance for the development of future CVD.
In line with the present results, previous studies have found associations between higher TG concentrations and higher risk of CVD [25]. High TGs are a marker of high remnant cholesterol concentrations, and the two are highly correlated, both as a result of the way we calculate remnant cholesterol are components of the same TRLs, chylomicron remnants, very low density lipoprotein, and intermediate density lipoprotein [3,4]. Results and conclusions from this study would therefore be similar if TG were studied instead of remnant cholesterol; however, given that it most likely is the cholesterol content of remnants that accumulates in atherosclerotic plaques, and not the TG, it is more clinically relevant to focus on remnant cholesterol. Also, because all cells can degrade TG, and TG per se probably do not cause atherosclerosis [6], there has been a tendency to disregard high TGs as an important risk factor for CVD. By focusing on the cholesterol content of remnants, instead of on the highly correlated TG content, this will hopefully bring more attention to the potential of preventing cardiovascular disease in individuals with high remnant cholesterol (and TG) concentrations [5,7,9].
Several epidemiology and genetic studies have sought to investigate the association between calculated remnant cholesterol and CVD. Findings from the Copenhagen General Population Study and the Copenhagen City Heart Study showed that non-fasting calculated remnant cholesterol was associated with gradually increased risk of atherosclerotic cardiovascular disease, ischemic heart disease, MI, ischemic stroke, and all-cause mortality in Danish population [4,9,12,21,[26], [27], [28]]. The Jackson Heart and Framingham Offspring Cohort studies found that remnant lipoproteins, which was strongly correlated to remnant cholesterol with an R [2] of 0.73, were predictive of incident coronary heart disease in primary prevention subjects in U.S. population [10]. The Atherosclerosis Risk In Communities study revealed that remnant-like particle cholesterol levels were predictive of CVD and associated with APOE variants [11]. Recently, a nested case-control study of 4662 individuals from the China Kadorrie Biobank demonstrated that remnant cholesterol concentrations (measured by nuclear magnetic resonance spectroscopy) were associated with 1.27-fold increased risk of MI and 1.20-fold increase risk of ischemic stroke [29]. As mentioned above, there is a lack of prospective studies comprehensively evaluating the relation of remnant cholesterol with incident CVD in Chinese population, which may have important clinical and public health implications.
It is noteworthy that the risk of CVD may attributable to cholesterol may vary between young and later adults. Pooled data from 6 U S. cohorts with observations spanning the life course from young to later life, the results showed that young adult exposures to elevated of serum LDL-C were stronger associated with CVD risks than later adult exposure [14]. Similarly, another Coronary Artery Risk Development in Young Adults study revealed that accumulated LDL-C at a younger age, compared with older age, resulted in a greater risk increase in CVD [30]. Although the different associations between LDL-C and CVD across age groups have been established, to our knowledge, relevant literature on effect of TRLs on CVD in young and later adults was insufficient. Copenhagen City Heart Study found that the associations between TGs and MI, ischemic heart disease, and death were more pronounced in individuals with age < 55 years vs older than 55 years [3]. Another multicenter case-control study conducted in Vienna focused on young individuals (≤40 years), the results showed that remnant cholesterol was strongly with premature MI [31]. In line with the findings, our analysis found that the risk of CVD was higher in young adults than later adults in the same remnant cholesterol categories, our finding extends these previous reports by quantifying a more contributable role of remnant cholesterol in the development of CVD in young adults, which makes the present findings novel.
The stronger association between remnant cholesterol and CVD in young adults than later adults is likely explained by the relatively high heritability of cholesterol, particularly in young adults [32,33]. Exposure to high remnant cholesterol in young adults is more likely driven by genetically determinants, and generically determined remnant cholesterol is an important cause of atherosclerosis early in life; whereas, in the elderly, pathologic process of atherosclerosis may be affected by some other dominant risk factors beyond cholesterol, such as blood pressure, blood glucose [34,35]. In addition, participants were more likely to be started on lipid-lowering medications as they grew older, the higher prevalence of lipid medication in later adults may have also contributed to the attenuated association between remnant cholesterol and CVD. However, when restricting our analysis to participants who never used lipid-lowering medications at baseline and during the follow-up period, we observed a similar pattern of associations. Considering the prevalence of dyslipidemia in young adults is relative high [36,37], our study together with these findings suggest that focusing on prevention of CVD in young adults is of growing importance in modern preventive cardiology.
The increased risk of CVD associated with remnant cholesterol could be attributed to the mechanisms related not only to atherosclerotic plaque formation but also to local inflammation. Remnant cholesterol can cause atherosclerosis by accumulation in the arterial wall, either directly in the cerebral vessels or in more distant vessels like the carotid artery or in the heart, where from blood clots can cause an embolism to the cerebral and cardiovascular arteries [21,38,39]. In addition, both observational and genetic studies have indicated the causal association between remnant cholesterol and low-grade inflammation, another hallmark of the atherosclerotic lesion [7,40]. High remnant cholesterol from the hydrolysis of TRLs could also induce the production of cytokines (tumor necrosis factor-α), interleukins, and pro-atherogenic adhesion molecules activating inflammation and the coagulation cascade through plasminogen activator inhibitor 1 [41,42]. All these processed may lead to plaque rupture and consequently result in the occurrence of CVD.
Our study has several strengths. The study was conducted in a large prospective community cohort with a high retention and standardized data collection protocols, which avoided bias in self-reported data. Furthermore, we evaluated the different effect of remnant cholesterol on CVD between young and later adults. The present study also has several potential limitations. Firstly, we did not measure remnant cholesterol directly, the indirect calculation of remnant cholesterol might have overestimated its value in comparison to direct measurement. However, the calculated remnant cholesterol is an affordable and inexpensive method that could provide valuable data for clinical management, and it is therefore widely available [4,9,21,26,28]. Secondly, because our study was observational, the causal relation of remnant cholesterol with CVD in young and later adults should be verified in further studies. Finally, although we have adjusted for all the potential covariates, we cannot exclude the possibility of residual or unmeasured confounding given the observational study design of the present analysis.
Conclusions
In conclusion, our study found that high remnant cholesterol was associated with increased risk of CVD in Chinese adults, and the association was stronger in young adults. The findings indicate that individuals with a higher remnant cholesterol level warrant particular vigilance in terms of subsequent risk of CVD. More future studies are needed to examine the potential benefit of remnant cholesterol lowering in individuals with elevated levels, especially in young adults.
Source of funding
This work was supported by National Natural Science Foundation of China (81870905, U20A20358), Beijing Municipal Science & Technology Commission (D171100003017002), Beijing Municipal Administration of Hospitals Incubating Program (PX2020021), and Beijing Excellent Talents Training Program (2018000021469G234).
Author contributions
Anxin Wang: conceptualization, resource, funding acquisition, methodology, formal analysis, investigation, writing-review and editing;
Xue Tian: conceptualization, methodology, formal analysis, investigation, writing original draft, writing-review and editing;
Yingting Zuo: methodology, formal analysis, writing-review and editing;
Shuohua Chen: resource, validation, writing-review and editing;
Xia Meng: resource, funding acquisition, writing-review and editing;
Pan Chen: methodology, writing-review and editing;
Hao Li: methodology, writing-review and editing;
Shouling Wu: conceptualization, investigation, resource, supervision, funding acquisition, writing-review and editing;
Yongjun Wang: conceptualization, investigation, resource, supervision, funding acquisition, writing-review and editing.
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group; and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2021.09.004.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
Data availability
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Vol. 392. Lancet; London, England): 2018. pp. 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leong D., Joseph P., McKee M., Anand S., Teo K., Schwalm J., et al. Reducing the global burden of cardiovascular disease, Part 2: prevention and treatment of cardiovascular disease. Circ Res. 2017;121:695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard B., Benn M., Schnohr P., Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen A., Frikke-Schmidt R., West A., Grande P., Nordestgaard B., Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 5.Castañer O., Pintó X., Subirana I., Amor A., Ros E., Hernáez Á., et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76:2712–2724. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet (London, England) 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 7.Varbo A., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 8.Varbo A., Freiberg J.J., Nordestgaard B.G. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem. 2015;61:533–543. doi: 10.1373/clinchem.2014.234146. [DOI] [PubMed] [Google Scholar]
- 9.Varbo A., Nordestgaard B. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85:550–559. doi: 10.1002/ana.25432. [DOI] [PubMed] [Google Scholar]
- 10.Joshi P., Khokhar A., Massaro J., Lirette S., Griswold M., Martin S., et al. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson heart and Framingham offspring cohort studies. Journal of the American Heart Association. 2016;5 doi: 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed A., Feofanova E., Yu B., Sun W., Virani S., Nambi V., et al. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72:156–169. doi: 10.1016/j.jacc.2018.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varbo A., Benn M., Tybjærg-Hansen A., Jørgensen A., Frikke-Schmidt R., Nordestgaard B. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 13.Gidding S., Robinson J. It is now time to focus on risk before age 40. J Am Coll Cardiol. 2019;74:342–345. doi: 10.1016/j.jacc.2019.04.064. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Vittinghoff E., Pletcher M., Allen N., Zeki Al Hazzouri A., Yaffe K., et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341. doi: 10.1016/j.jacc.2019.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Yuan Y., Zheng M., Pan A., Wang M., Zhao M., et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–2930. doi: 10.1016/j.jacc.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Jin C., Chen S., Vaidya A., Wu Y., Wu Z., Hu F.B., et al. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40:1565–1572. doi: 10.2337/dc17-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Stroke--1989 Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 19.Tunstall-Pedoe H., Kuulasmaa K., Amouyel P., Arveiler D., Rajakangas A., Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 20.Pottel H., Delanaye P., Schaeffner E., Dubourg L., Eriksen B.O., Melsom T., et al. Vol. 32. official publication of the European Dialysis and Transplant Association - European Renal Association; 2017. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C, Nephrology, dialysis, transplantation; pp. 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varbo A., Freiberg J., Nordestgaard B. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the copenhagen general population study. Clin Chem. 2018;64:219–230. doi: 10.1373/clinchem.2017.279463. [DOI] [PubMed] [Google Scholar]
- 22.Spiegelman D., Hertzmark E., Wand H. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Canc Causes Contr : CCC (Cancer Causes Control) 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 23.Hertzmark E., Wand H., Spiegelman D. 2021. The SAS PAR Macro. [Google Scholar]
- 24.Wacholder S., Benichou J., Heineman E., Hartge P., Hoover R. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 25.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K.K., Thompson A., et al. Major lipids, apolipoproteins, and risk of vascular disease. Jama. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varbo A., Nordestgaard B. Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol. 2014;25:266–273. doi: 10.1097/MOL.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 27.Jepsen A., Langsted A., Varbo A., Bang L., Kamstrup P., Nordestgaard B. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. 2016;62:593–604. doi: 10.1373/clinchem.2015.253757. [DOI] [PubMed] [Google Scholar]
- 28.Varbo A., Benn M., Tybjærg-Hansen A., Jørgensen A.B., Frikke-Schmidt R., Nordestgaard B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 29.Holmes M., Millwood I., Kartsonaki C., Hill M., Bennett D., Boxall R., et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domanski M., Tian X., Wu C., Reis J., Dey A., Gu Y., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 31.Goliasch G., Wiesbauer F., Blessberger H., Demyanets S., Wojta J., Huber K., et al. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. Journal of clinical lipidology. 2015;9 doi: 10.1016/j.jacl.2015.08.009. 801-806.e801. [DOI] [PubMed] [Google Scholar]
- 32.Pietiläinen K., Söderlund S., Rissanen A., Nakanishi S., Jauhiainen M., Taskinen M., et al. HDL subspecies in young adult twins: heritability and impact of overweight. Obesity. 2009;17:1208–1214. doi: 10.1038/oby.2008.675. [DOI] [PubMed] [Google Scholar]
- 33.Souren N.Y., Paulussen A.D., Loos R.J., Gielen M., Beunen G., Fagard R., et al. Anthropometry, carbohydrate and lipid metabolism in the east flanders prospective twin survey: heritabilities. Diabetologia. 2007;50:2107–2116. doi: 10.1007/s00125-007-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elder S.J., Lichtenstein A.H., Pittas A.G., Roberts S.B., Fuss P.J., Greenberg A.S., et al. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res. 2009;50:1917–1926. doi: 10.1194/jlr.P900033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 36.Tóth P.P., Potter D., Ming E.E. Prevalence of lipid abnormalities in the United States: the national health and nutrition examination survey 2003-2006. Journal of clinical lipidology. 2012;6:325–330. doi: 10.1016/j.jacl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Jeong S.M., Choi S., Kim K., Kim S.M., Lee G., Park S.Y., et al. Effect of change in total cholesterol levels on cardiovascular disease among young adults. Journal of the American Heart Association. 2018;7 doi: 10.1161/JAHA.118.008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordestgaard B.G., Wootton R., Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534–542. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- 39.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 40.Bernelot Moens S.J., Verweij S.L., Schnitzler J.G., Stiekema L.C.A., Bos M., Langsted A., et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler Thromb Vasc Biol. 2017;37:969–975. doi: 10.1161/ATVBAHA.116.308834. [DOI] [PubMed] [Google Scholar]
- 41.Shin H.K., Kim Y.K., Kim K.Y., Lee J.H., Hong K.W. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- 42.Olufadi R., Byrne C.D. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemostasis Thrombosis. 2006;35:281–291. doi: 10.1159/000093221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.