Abstract
Chronic kidney disease (CKD) affects about 10% of all populations worldwide, with about 2 million people requiring dialysis. Although patients with CKD are at high risk of cardiovascular disease and events, they are often underrepresented or excluded in clinical trials, leading to important knowledge gaps about how to treat these patients. KDIGO (Kidney Disease: Improving Global Outcomes) convened the fourth clinical Controversies Conference on the heart, kidney and vasculature in Dublin, Ireland, in February 2020, entitled Central and Peripheral Arterial Diseases in Chronic Kidney Disease. A global panel of multidisciplinary experts from the fields of nephrology, cardiology, neurology, surgery, radiology, vascular biology, epidemiology, and health economics attended. The objective was to identify key issues related to the optimal detection, management, and treatment of cerebrovascular diseases, central aortic disease, renovascular disease, and peripheral artery disease in the setting of CKD. This report outlines the common pathophysiology of these vascular processes in the setting of CKD, describes best practices for their diagnosis and management, summarizes areas of uncertainty, addresses ongoing controversial issues, and proposes a research agenda to address key gaps in knowledge that, when addressed, could improve patient care and outcomes.
Keywords: abdominal aortic aneurysm, acute kidney injury, aortic dissection, central aortic disease, cerebrovascular disease, chronic kidney disease, peripheral artery disease, renal artery stenosis, renovascular, stroke
In February 2020, Kidney Disease: Improving Global Outcomes (KDIGO) convened a clinical Controversies Conference in Dublin, Ireland, titled Central and Peripheral Arterial Diseases in Chronic Kidney Disease. The rationale for this conference was the recognition that although cardiovascular events account for a majority of deaths among persons with chronic kidney disease (CKD) and kidney failure, these individuals have a much higher risk of both atherosclerosis and arteriosclerosis. Atherosclerosis affects the intima of the vessels and is characterized by formation of lipid- and calcification-rich plaques that reduce the capability of arteries to transport blood to all parts of the body. The process of arteriosclerosis affects mainly the media, especially of larger vessels, and increases arterial stiffness, thereby reducing the elastic function of arteries.1 Furthermore, these noncardiac vascular diseases often cause substantial morbidity and have a major impact on quality of life in the CKD population. Despite these consequences, they have received less attention than heart disease. The objective of the conference was to identify key issues related to the optimal detection, management, and treatment of cerebrovascular diseases, central aortic disease, renovascular disease, and peripheral artery disease (PAD) in the setting of CKD. Conference details are posted on the KDIGO website: https://kdigo.org/conferences/central-peripheral-arterial-disease/.
ATHEROSCLEROTIC CEREBROVASCULAR DISEASE IN CKD
Epidemiology
There are strong associations between CKD and cerebrovascular disease that increase with declining kidney function.2,3 The incidence of stroke in CKD is 13.4 per 1000 person-years,4 rising to 25.3 per 1000 person-years in patients requiring dialysis,5 and it remains elevated at 6.0 per 1000 patient-years even after receipt of a kidney transplant.6 Risk appears to be greater for patients treated with hemodialysis, compared with those on peritoneal dialysis.7 Patients with proteinuria also represent a high-risk subgroup, with a previous meta-analysis showing that such individuals were at a 71% higher risk of stroke compared with those without proteinuria.8 Recent data suggest that proteinuria may be a better predictor of stroke risk in CKD than estimated glomerular filtration rate (eGFR), possibly as an indicator of microvascular disease.9 CKD is also associated with worse stroke outcomes, greater likelihood of institutionalization,10 and higher short- and long-term mortality.10,11
Pathophysiology
Multiple mechanisms for the high risk of stroke in CKD have been proposed,12 including shared risk factors (e.g., diabetes, hypertension), nontraditional risk factors arising as secondary consequences of kidney dysfunction (e.g., inflammation, abnormal calcium–phosphorus metabolism, accumulation of uremic toxins),13 enhanced apoptosis by decreased adenosine monophosphate (AMP) kinase phosphorylation, and increased activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway,14 dialysis-specific factors (e.g., cerebral “stunning,” dialysis-induced dysregulation of cerebral blood flow15), and other systemic conditions that cause stroke and CKD (e.g., systemic lupus erythematosus or Fabry’s disease).2,16
Hemodialysis-specific factors likely play a role, as hemodialysis has been shown to induce a significant reduction in global and regional cerebral blood flow.17 Intradialytic hemodynamic instability is associated with ischemic white matter changes18 as well as cognitive dysfunction.19
Acute stroke management in CKD
In an analysis of nearly 700,000 patients from the Get With The Guidelines–Stroke program, patients with CKD were less likely to receive “evidence-based therapies” compared with those without CKD,20 including thrombolysis and antiplatelet agents and preventive treatments, such as statin therapy and smoking cessation.
Most randomized controlled trials (RCTs) of i.v. thrombolysis excluded patients with advanced CKD, resulting in limited data on its safety and efficacy in this group. In a meta-analysis of 7 observational studies, CKD patients treated with thrombolysis had a higher risk of symptomatic intracranial hemorrhage and mortality than those without CKD.21 In contrast, in a post-hoc analysis of the ENCHANTED trial (Enhanced Control of Hypertension and Thrombolysis Stroke Study), patients with CKD who received thrombolysis had higher risk of mortality (attributable to nonvascular causes) but not of disability or intracranial hemorrhage.22 We concur with the American Heart Association/American Stroke Association guidelines recommending the use of thrombolysis in otherwise-eligible CKD patients without restriction, including hemodialysis patients with a normal partial thromboplastin time (PTT).23
Few thrombectomy trials have enrolled participants with advanced CKD,24,25 and limited observational data are inconsistent regarding outcomes.26,27 Despite the paucity of data, we would not withhold thrombectomy in CKD patients judged to be suitable candidates for interventional treatment.
Dialysis considerations in acute stroke.
Dialysis therapy is challenging in acute stroke. An increase in brain water content with intermittent hemodialysis, possibly related to osmotic shift due to acute urea reduction,28 may lead to increases in intracranial pressure (ICP); subclinical cerebral edema during intermittent hemodialysis has been observed even in hemodynamically stable patients.29 Blood pressure (BP) and volume fluctuations also have the potential to extend the penumbra in acute stroke, as global cerebral blood flow declines acutely by about 10% during hemodialysis.17 Systemic anticoagulation during hemodialysis may exacerbate hemorrhage.30 Unfortunately, there is no evidence to guide best clinical practice in this scenario.31,32 Figure 1 summarizes our dialysis prescription recommendations.
Figure 1 |. Suggestions for dialysis prescribing in acute stroke: Given are relevant factors to consider mainly for hemodialysis procedures in patients at high risk of neurologic deterioration from stroke extension or increasing cerebral edema (i.e., large infarcts, infarcts associated with high-grade intracranial or extracranial stenosis, or intracerebral hemorrhage [ICH]).
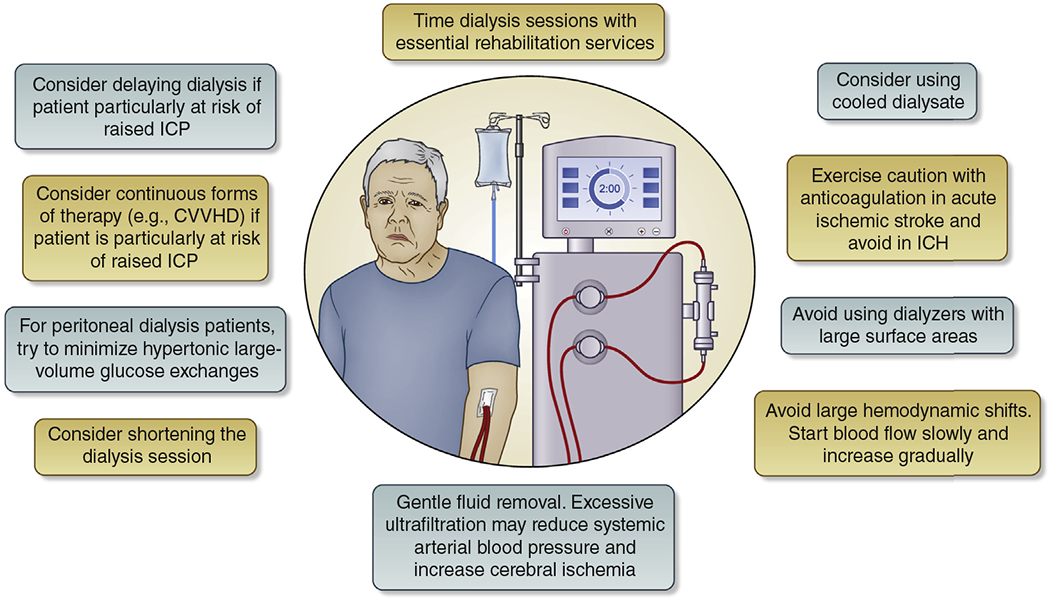
In addition, in peritoneal dialysis patients, hypertonic large-volume glucose exchanges should be minimized. CVVHD, continuous venovenous hemodialysis; ICP, intracranial pressure.
Preventive and long-term therapies after stroke
Antiplatelet therapies.
Patients with moderate-to-severe CKD were excluded from most clinical trials evaluating efficacy and safety of antiplatelet therapy.33 In a meta-analysis of 3 trials, antiplatelet therapy for primary prevention of stroke in patients with CKD increased the risk of major bleeding events without reducing major cardiovascular events or mortality.34 For secondary prevention in CKD, studies show a reduction in the risk of myocardial infarction, but not stroke, with antiplatelet therapy.35 However, despite a paucity of CKD-specific data, given the large benefits of aspirin in doses ranging from 50 to 325 mg/d demonstrated in the general population,36 its use for secondary prevention in patients with CKD should be considered.33,37,38
Dual pathway blockade.
A secondary analysis of the COMPASS (Cardiovascular OutcoMes for People using Anticoagulation StrategieS) trial revealed promising results for patients with CKD G1-G3b.39 COMPASS was a doubleblind RCT that compared low-dose rivaroxaban with or without aspirin in patients with a history of chronic coronary disease or PAD. The risk of stroke was reduced with dual blockade therapy. Although there was no excess relative risk of bleeding in patients with CKD, the absolute risk was higher. It may be reasonable to consider low-dose rivaroxaban and aspirin for the prevention of stroke in patients with an eGFR of 30–59 ml/min per 1.73 m2 and a prior history of coronary artery disease or PAD, after careful assessment of bleeding risk.
Lipid-lowering therapy.
The efficacy of statin therapy for the primary prevention of stroke in patients with CKD was demonstrated in the Study of Heart and Renal Protection (SHARP) trial, which demonstrated a 25% reduction in ischemic stroke in patients with CKD treated with a combination of simvastatin plus ezetimibe.40 However, in a meta-analysis of data from 28 trials, the efficacy of statins in CKD appeared to diminish with advancing kidney disease (P = 0.008 for trend), and there was little evidence of benefit for patients receiving dialysis (rate ratio: 0.94; 99% confidence interval: 0.79–1.11).41 According to a previous KDIGO guideline,42 all individuals over 50 years of age with CKD should be started on statin with or without ezetimibe therapy. Statins may be continued in patients on dialysis who are already taking them, but the guideline does not recommend starting statins in this age group.42
Antihypertensive therapy.
The KDIGO 2021 Clinical Practice Guideline on the Management of Blood Pressure in CKD has recommended a systolic BP target of <120 mm Hg in CKD for primary and secondary prevention when tolerated, using standardized office BP measurement.43 This recommendation was influenced by subgroup analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), in which targeting a systolic BP <120 mm Hg compared with <140 mm Hg reduced rates of major cardiovascular events and allcause death in patients with CKD.44
Carotid interventions.
The North American Symptomatic Carotid Endarterectomy Trial (NASCET) was the only large RCT of carotid interventions that reported results according to kidney function.45 Surgery was highly effective for patients with CKD with symptomatic high-grade stenosis, resulting in a risk reduction of 82.3% compared with 50.8% for patients without CKD. Rates of perioperative cardiac complications were higher in the CKD group, but perioperative death rates were not. We therefore agree with guidance from the Society for Vascular Surgery recommending carotid endarterectomy for symptomatic patients with CKD with high-grade stenosis.46
Subclinical cerebrovascular disease
There is a strong association between CKD and cerebral small vessel disease.47,48 Silent cerebral infarction (SCI) is prevalent in as many as 56.5% of asymptomatic patients in cross-sectional analyses.49,50 Several mechanisms for this association have been proposed, including the kidneys’ and brain’s shared anatomic and physiological susceptibility to hypertensive vascular injury,51 common vascular risk factors,48 and shared genetic susceptibility.52 The presence of SCI in CKD appears to have implications for future stroke risk, cognition, gait, and stability.50,53 The American Heart Association/American Stroke Association have issued a scientific statement with suggestions for the clinical care of patients with SCI in the general population,54 which we have used to develop modified consensus recommendations on management of SCI in CKD (Table 143,55).
Table 1 |.
Recommendations for the management of subclinical cerebrovascular disease in CKD
| Patient evaluation and investigations |
| • MRI is the preferred imaging modality as it has greater sensitivity than CT for diagnosis of silent cerebrovascular disease |
| • Assess common vascular risk factors: BP, diabetes, cholesterol, smoking |
| • Check pulse for AF, perform 12-lead electrocardiogram, and consider prolonged Holter monitoring |
| • Consider the following: carotid imaging when there are SCIs in the carotid territory and/or echocardiography when there is an embolic-appearing pattern of silent infarction |
| Management and treatment targets |
| • Consider treating with aspirin and statin therapy in those with SCI if no contraindication |
| • Target systolic BP control <120 mm Hg per KDIGO guideline43 |
| • It may be reasonable to consider the presence of an embolic pattern of SCI in carotid territory in clinical decision-making around carotid revascularization |
| • The presence of CMBs should not necessitate deprescribing or avoidance of prescribing antiplatelet or anticoagulant therapy, but the decision should be individualized, as these patients are at higher risk of ischemic stroke and ICH55 |
| • It is reasonable to administer thrombolysis to CKD patients with acute ischemic stroke and evidence of microbleeds if it is otherwise indicated |
AF, atrial fibrillation; BP, blood pressure; CKD, chronic kidney disease; CMB, cerebral microbleed; CT, computed tomography; ICH, intracerebral hemorrhage; KDIGO, Kidney Disease: Improving Global Outcomes; MRI, magnetic resonance imaging; SCI, silent cerebral infarct.
Conclusions and future research
There is a high incidence of stroke in patients with CKD that is mostly attributable to traditional risk factors. Inequities in stroke care exist at every step from presentation and diagnosis to treatment and prevention. The importance of admission to an acute stroke unit with expert, multidisciplinary care cannot be overstated. Tables 233,37,38,42,43,56 and 3 highlight key conference recommendations for primary and secondary prevention of stroke in CKD, and the critical research priorities.
Table 2 |.
Recommendations for the primary and secondary prevention of stroke in CKD
| Primary prevention | Secondary prevention | |
|---|---|---|
| Lifestyle modifications | Smoking cessation, weight restriction, and regular exercise should all be actively encouraged | Same as primary prevention |
| Antiplatelet therapy | There is currently insufficient evidence to support the use of antiplatelet therapy for primary prevention | Antiplatelet therapy for secondary prevention is uniformly recommended by NICE,33 KDIGO,37 and AHA/ASA38 |
| Anticoagulation | In general, anticoagulation is recommended for the primary prevention of stroke with AF in this group. This is a high-risk group in which risk-prediction tools such as CHA2DS2-VASc score may have limited utility. For those with eGFR >30 ml/min per 1.73 m2, first-line treatment should be with a DOAC. For those with eGFR 15–29 ml/min per 1.73 m2, the choice of agent should depend on the trajectory of their kidney function and should therefore be discussed with their nephrologist. For those with an eGFR <15 ml/min per 1.73 m2, the decision to anticoagulate and the choice of agent should be discussed with their nephrologist. | Same as primary prevention, but we would advise having an even lower threshold to anticoagulate. The AHA and ACC56 recommend using either a DOAC or warfarin in patients treated with dialysis, although long-term safety data on the former are lacking, and there is a risk of vascular calcification with the latter. Consider left atrial appendage occlusion devices in those with additional bleeding concerns, as they have been shown to be safe and effective in advanced CKD after the initial periprocedural period. |
| Dual blockade (antiplatelet + low-dose DOAC) | There may be a role for dual pathway blockade in CKD patients (eGFR: 30–59 ml/min per 1.73 m2) who have chronic coronary artery or peripheral artery disease | Dual pathway blockade could be considered for the secondary prevention of stroke, although there is no evidence to support its efficacy in this specific group. |
| Blood pressure control | Tight blood pressure control to systolic blood pressure <120 mm Hg is essential. RAS blockers are the antihypertensive agents of choice.43 | Same as primary prevention |
| Lipid-lowering therapy | Per KDIGO,42 if age >50 years and CKD present, treat with statin or statin/ezetimibe. In CKD patients treated with dialysis, do not start statins de novo, but continue if already taking. | We recommend statin therapy for all CKD patients who have had a stroke event. Per KDIGO guidelines,42 statins may be continued in dialysis patients who are already taking them, but they should not be started unless very high LDL-C levels (3.8 mmol/l, 145 mg/dl). |
| SGLT2 inhibitors | Consider for patients with diabetes and CKD with an eGFR >30 ml/min per 1.73 m2 | Consider for patients with diabetes and CKD with an eGFR >30 ml/min per 1.73 m2 |
| Carotid interventions | We would not recommend routine carotid revascularization for CKD patients with asymptomatic disease, although the decision may be individualized for high-risk plaques | Consider carotid revascularization in CKD patients not treated with dialysis with symptomatic moderate-severe stenosis, and in very high-risk dialysis patients with symptomatic disease |
| Dialysis-related interventions | Careful attention to blood pressure and volume control. Maintain hemoglobin values between 10 g/dl and 12 g/dl (100–120 g/l) | Same as primary prevention |
ACC, American College of Cardiology; AF, atrial fibrillation; AHA, American Heart Association; ASA, American Stroke Association; CKD, chronic kidney disease; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; KDIGO; Kidney Disease: Improving Global Outcomes; LDL-C; low-density lipoprotein cholesterol; NICE, National Institute for Health and Care Excellence; RAS, renin-angiotensin system; SGLT2, sodium-glucose cotransporter-2.
Table 3 |.
Research recommendations for cerebrovascular disease and CKD
| • Determine the stroke subtypes that occur most frequently in CKD and kidney failure, as this will give us better mechanistic and prognostic insights |
| • Develop more accurate bleeding risk prediction tools for patients requiring dialysis |
| • Investigate the contribution of nontraditional risk factors to the etiology of stroke in kidney disease |
| • Determine the optimal management strategy for silent cerebral infarcts in this setting |
| • Evaluate the efficacy and safety of i.v. thrombolysis and thrombectomy in CKD and kidney failure |
| • Determine the optimal dialysis modality and/or prescription in acute stroke |
| • Determine when anticoagulation should be used in kidney failure and which agent has the greatest safety and efficacy |
| • Evaluate the safety and efficacy of dual pathway blockade in advanced CKD and kidney failure |
| • Clarify the value, if any, of carotid interventions in kidney failure, which patients should be selected, and whether stenting or endarterectomy is superior |
| • Validate disability-scoring tools in kidney failure |
| • Determine predictors of medical and neuropsychiatric complications in CKD and kidney failure, and the best way to mitigate these |
| • Determine predictors of cognitive decline in patients with CKD and kidney failure |
CKD, chronic kidney disease.
CENTRAL AORTIC DISEASE AND CHRONIC KIDNEY DISEASE
Associations of CKD with occurrence of abdominal aortic aneurysm (AAA) and post-surgery outcomes
Preliminary evidence suggests that the prevalence of AAA is up to 30% higher among individuals with CKD than it is in the general population.57 In a recent, large observational study, an eGFR <75 ml/min per 1.73 m2 and a urine albumin–creatinine ratio (ACR) ≥10 mg/g (1 mg/mmol) were independently associated with a higher risk of incident AAA over a median follow-up of 13.9 years.58 CKD is also associated with AAA outcomes. Several observational studies have shown that CKD (eGFR <60 ml/min per 1.73 m2) before surgery is associated with greater post-operative eGFR decline59 and higher odds of eGFR loss >20% during long-term follow-up.60,61 Similarly, CKD has been associated with higher 30-day and long-term mortality, higher risk of cardiovascular events, and longer length of hospital stay after AAA surgery (Figure 2).62,63
Figure 2 |. Kaplan–Meier curves showing preoperative estimated glomerular filtration rate (eGFR) with morbidity and mortality after endovascular aneurysm repair for infrarenal abdominal aortic aneurysm.
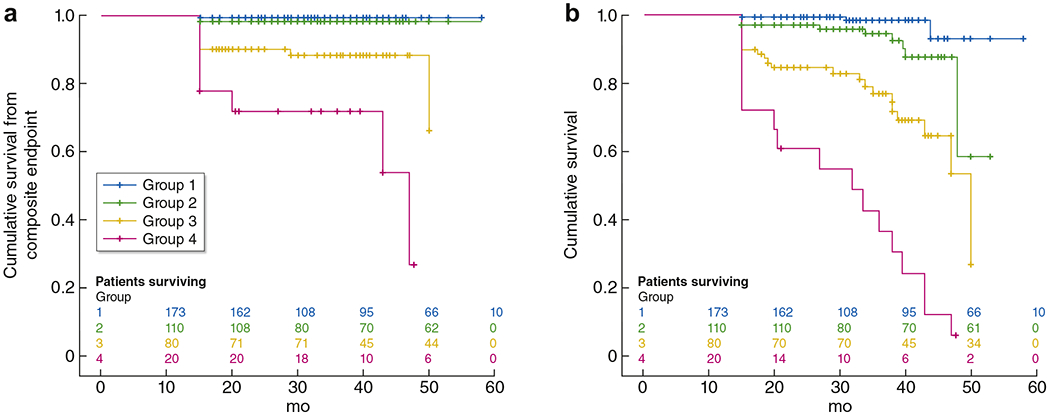
(a) Cumulative freedom for the combined cardiovascular endpoint consisting of death, nonfatal myocardial infarction, stroke, and peripheral vascular complications, logrank P < 0.001. (b) Cumulative survival: logrank P < 0.001 (n = 383; group 1: eGFR >90 ml/min per 1.73 m2; group 2: eGFR 60–89 ml/min per 1.73 m2; group 3: eGFR 30–59 ml/min per 1.73 m2; group 4: eGFR <30 ml/min per 1.73 m2). Reprinted from the Journal of Vascular Surgery, Volume 58, Saratzis A, Sarafidis P, Melas N, Saratzis N, Kitas G. Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair, Pages 879–885, Copyright © 2013, with permission from the Society of Vascular Surgery.63
Diagnostic evaluation and post-surgery follow-up of AAA in patients with CKD
Duplex ultrasonography, computed tomographic angiography, and magnetic resonance angiography are commonly used for diagnosis of AAA.64,65 Usually, a gray-scale ultrasound (B-mode imaging) is sufficient for the initial evaluation and follow-up of an AAA (size measurement and aneurysm extent); additional information (e.g., presence and extent of mural thrombus) can be obtained by color Doppler ultrasound.66 However, ultrasound alone is not sufficient for procedural planning, and computed tomographic angiography is commonly needed.64,65
Routine follow-up imaging is also required to monitor for complications after endovascular aneurysm repair (EVAR), such as endoleak, device migration, or continued aneurysm expansion requiring repeat intervention, whereas open repair does not require further imaging in the absence of clinical signs or symptoms. The traditional protocol for post-EVAR imaging includes computed tomographic angiography at 1 month, 6 months, 1 year, and then annually, paired with duplex ultrasound imaging.
Treatment considerations in patients with AAA and CKD
Open surgical repair and EVAR decrease AAA-specific mortality, but they both can have post-operative complications, including acute and chronic kidney dysfunction. Indications for elective and emergent AAA treatment do not differ based on the presence of CKD.67 However, CKD is associated with higher risk of post-surgery acute kidney injury (AKI), long-term eGFR decline, cardiovascular events, and mortality.
EVAR has superior short-term outcomes compared with open surgery and has become the treatment of choice for many patients.67 Given the lack of RCTs comparing EVAR with open surgery in patients with CKD, anatomic and other patient-related parameters should be considered when deciding on treatment. Depending on the anatomy, open AAA repair can be performed with suprarenal or infrarenal aortic clamping; the former is associated with higher morbidity and AKI rates68 due to longer kidney ischemia time. Endovascular repair for a standard infrarenal AAA can be performed with infrarenal or suprarenal fixation (e.g., use of bare stents or hooks to decrease the chance of device migration and endoleak).
AKI and eGFR decline after AAA surgery: incidence and impact on outcomes
In older studies, the incidence of AKI after elective AAA surgery ranged widely, due to variation in criteria for defining AKI.69 In recent years, however, studies that have used standardized criteria for AKI have reported an incidence of AKI after EVAR for infrarenal AAA of 9%–21% (Table 4).70–81 Open repair is associated with a 1.6- to 2-fold higher risk of AKI but better long-term preservation of kidney function59,80,82 than EVAR. Juxtarenal, suprarenal, or thoraco-abdominal aneurysms cannot be treated with standard “off the shelf” EVAR devices and require individualized fenestrated EVAR (fEVAR) or branched EVAR devices (Figure 3), which are associated with considerably higher rates of AKI.80 Studies also suggest that EVAR with suprarenal fixation is associated with greater long-term eGFR decline than EVAR with infrarenal fixation or open repair.59,83,84 AKI after AAA surgery is independently associated with faster loss of kidney function, as well as with cardiovascular events and mortality.61,73,74 It is unclear whether AKI is mechanistically involved in acceleration of cardiovascular disease or is a marker indicating that patients have a higher occult burden of cardiovascular disease.69 There is currently no single strategy proven to reduce the risk of AKI or long-term eGFR decline following open AAA surgery or EVAR. Prevention of adverse kidney outcomes post-AAA surgery has not been studied specifically in patients with CKD.69 Current guidance for patients at high risk for AKI is based on extrapolation from studies of cardiac catheterization and includes perioperative i.v. administration of crystalloid solutions for those with an eGFR <40 ml/min per 1.73 m2 or with a transplanted or solitary native kidney.85
Table 4 |.
Incidence of AKI in elective infrarenal EVAR using standardized AKI reporting criteria
| Reference | Type | Date | EVAR, n | AKI criteria | AKI incidence, % | AKI, n | AKI stage >2, n | Dialysis | Urine output available |
|---|---|---|---|---|---|---|---|---|---|
| Pirgakis et al.70 | Retrospective | 2014 | 87 | AKIN | 17 | 15 | None | 1 | No |
| Ueta et al.71 | Prospective | 2014 | 47 | AKIN | 14 | 6 | Stage 2: 1 | None | No |
| Pisimisis et al.72 | Retrospective | 2013 | 208 | RIFLE | 17 | 36 | NA | NA | No |
| Saratzis et al.73 | Prospective | 2015 | 149 | AKIN & KDIGO | 19 | 28 | Stage 2: 3 | None | Yes |
| Saratzis et al.74 | Retrospective | 2015 | 947 | KDIGO | 18 | 167 | Stage 2: 12; Stage 3: 2 | None | No |
| Saratzis et al.75 | Retrospective | 2016 | 484 | AKIN | 12 | 58 | NA | None | No |
| Obata et al.76 | Prospective | 2016 | 95 | AKIN | 9.4 | 9 | Stage 2: 1 | None | No |
| Lee et al.77 | Retrospective | 2017 | 78 | KDIGO | 14 | 11 | None | None | No |
| Saratzis et al.78 | Prospective (pilot randomized trial) | 2018 | 58 | KDIGO | 21 | 12 | None | None | Yes |
| Zabrocki et al.79 | Retrospective | 2018 | 91 | KDIGO | 13 | 12 | None | None | No |
| Saratzis et al.80 | Prospective multicenter | 2020 | 139 | KDIGO | 18 | 13 | None | None | Yes |
AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; EVAR, endovascular aneurysm repair; KDIGO, Kidney Disease: Improving Global Outcomes; NA, not available; RIFLE, risk, injury, failure, loss, end-stage renal disease.
Figure 3 |.
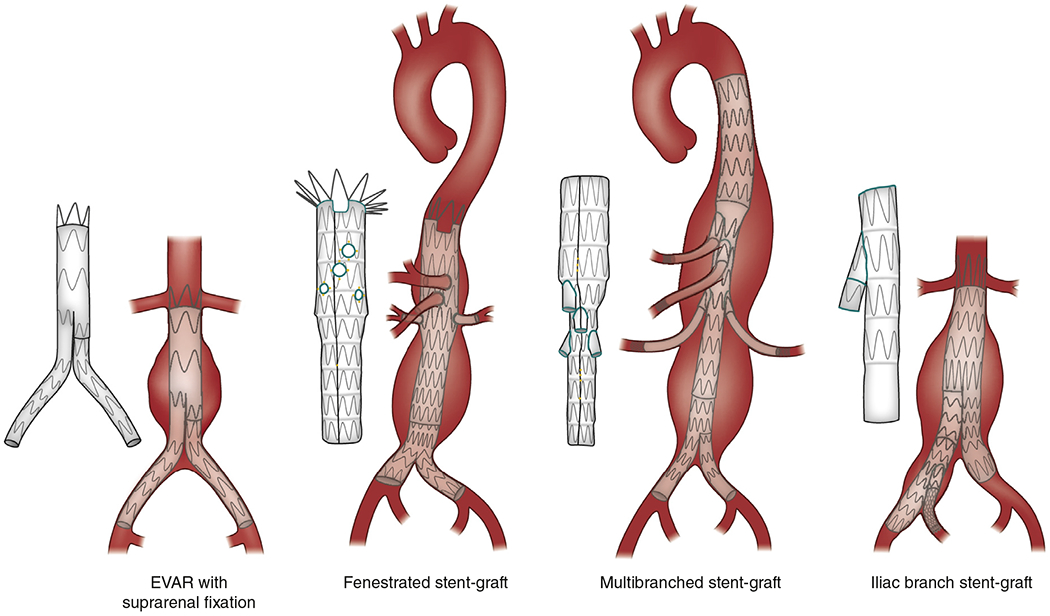
Types of endovascular aneurysm repair (EVAR), depending on the anatomy of the abdominal aneurysm (infrarenal aneurysms include a proximal aortic neck that provides an adequate landing zone for EVAR; juxtarenal aneurysms are adjacent to or include the lower margin of the renal arteries; suprarenal and thoraco-abdominal aneurysms also extend above the orifice of renal arteries).
Epidemiology and treatment of aortic dissection in patients with CKD
In previous reports, the prevalence of CKD was estimated as being 8.5%–10% of patients with acute aortic dissection.86,87 In a recent registry report of patients with acute aortic dissection type A (i.e., involving ascending aorta; n = 14,911) and type B (without ascending aorta involvement; n = 5622) in Germany, 19.3% of patients with type A and 20.4% of patients with type B dissections had CKD.88
In the German registry study, although CKD was not associated with mortality in patients with type A dissections, it was significantly more common among non-survivors of type B dissection than among survivors. Post-operative AKI occurred frequently, and 24.6% of patients with type A dissection, and 8.2% of patients with type B dissection, required kidney replacement therapy.88 A retrospective study of 478 patients with acute type B dissection undergoing intervention showed that 53% experienced AKI (27% stage 1, 15% stage 2, and 11% stage 3) that was associated with longer hospital stay and higher long-term mortality. Preoperative CKD was an independent predictor of AKI and mortality.87 In another analysis of 1034 patients with acute aortic dissections, AKI developed in 18% and was an independent mortality predictor.86
Currently, there are no data supporting differences in treatment of aortic dissection for patients with versus without CKD. Type A dissections are almost always treated surgically. Type B dissections are mostly treated with open surgical or endovascular repair, especially if visceral or renal arteries are compromised, leading to critical ischemia.89 Medical treatment with aggressive BP lowering is recommended for uncomplicated type B dissections and patients with a prohibitively high surgical risk profile. Patients with advanced CKD and advanced age or extensive preexisting comorbidities may be considered high risk and may be more likely to be treated with medical management.89
Conclusions and future research
Although the association of central aortic disease with CKD or AKI has been explored in recent years, there are still limitations of existing evidence and important areas for future research, as summarized in Table 5.
Table 5 |.
Gaps in knowledge and research recommendations for central aortic disease and CKD
| Limitations in existing evidence |
| • Studies on AKI incidence or long-term evolution of kidney function post-surgery for AAA may not adequately assess all involved factors (e.g., anatomic complexity, clamp time, clamp location, intentional occlusion of accessory renal arteries, etc.) |
| • Studies on long-term kidney function largely reflect treatment practices in the early era of EVAR (15–20 years ago) with regard to choice of fixation, procedural time, increased contrast volume, and complication rates |
| • Most evidence relates to outcomes after correction of infrarenal AAA and not of AAA involving the orifice of renal arteries or having more complicated anatomy |
| • Existing studies may not be representative of different parts of the world, as techniques and experience for AAA repair may vary substantially |
| • Studies on the associations of CKD and aortic dissection are scarce |
| Areas for future research |
| • Mechanisms through which CKD may affect development of AAA and aortic dissection |
| • Mechanisms through which AKI and long-term eGFR loss occur after open AAA repair and EVAR (especially with suprarenal fixation) |
| • Incidence of AKI and long-term eGFR decline with the use of fenestrated or physician-modified grafts for simple or complex aneurysm types |
| • Mechanisms through which AKI post-surgery for AAA affects cardiovascular events and all-cause mortality |
| • Prevalence of renal artery stenosis and its impact on outcomes in patients with AAA |
| • Effects of different diagnostic follow-up protocols for EVAR on long-term kidney function |
| • Randomized trials comparing the effects of different treatment modalities for AAA on kidney and cardiovascular outcomes in patients with CKD |
| • Randomized trials comparing the effect of AKI prevention strategies post-surgery for patients with CKD undergoing AAA or aortic dissection treatment |
| • Long-term evolution of kidney function post-surgery for type A and type B aortic dissections |
AAA, abdominal aortic aneurysm; AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EVAR, endovascular aneurysm repair.
ATHEROSCLEROTIC RENOVASCULAR DISEASE (ARVD)
While the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL)90 and Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL)91 trials failed to demonstrate benefits of renal artery stenting with respect to the key outcomes of kidney function, cardiovascular events, and mortality, at least in CORAL a consistent modest difference in systolic BP favoring the stent group was found. However, as a result, the medical community is now reluctant to contemplate revascularization for ARVD. Optimal medical therapy is therefore paramount, as is consideration of remaining uncertainty regarding revascularization.
What is the optimal medical therapy for patients with ARVD?
Patients with ARVD usually have polyvascular disease, and many are first identified during investigation of nonrenal vascular disease, including aortic, coronary, cerebral, and PAD, and heart failure. After an ARVD diagnosis, rates of vascular events are high, and annual mortality is many times higher than the risk of kidney failure requiring kidney replacement therapy.92 As a result, high-dose statin therapy (e.g., atorvastatin 80 mg daily) is essential, and guidelines recommend antiplatelet therapy (e.g., aspirin) as part of vasculoprotective treatment.
Control of BP to optimal targets (systolic BP <120 mm Hg when tolerated, using standardized office BP measurement) is the goal,43 but this may be achieved in only the minority of patients. Despite concerns regarding reduced glomerular filtration pressures associated with renin–angiotensin–aldosterone system (RAAS) blockers (i.e., angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) in patients with bilateral renal artery stenosis or with renal artery stenosis in a solitary functioning kidney, RAAS blockers are the most logical antihypertensive agents, given the role of RAAS upregulation in the ischemic kidney.93,94 RAAS blockers should be introduced in all patients with ARVD. Although a fall in eGFR may occur, we consider the recommendation to withdraw RAAS therapy if this occurs to be too cautious.95 However, major reductions in eGFR (e.g., > 4 ml/ min per year)96 in patients with high-grade (>75%) renal artery stenosis should prompt consideration of renal revascularization.
Can we identify kidneys with ARVD that have salvageable function?
The pathophysiology of the ischemic kidney beyond a high-grade renal artery stenosis has been evaluated in swine models and includes vascular rarefaction, RAAS-induced inflammation and oxidative stress, and eventual loss of functioning nephrons with fibrotic replacement.97,98 The human ARVD situation is often complicated by hypercholesterolemia, diabetes, smoking, hypertension, and genetic predisposition to vascular disease, and there is a limited correlation between renal artery stenosis severity and kidney dysfunction. At some point in the natural history of ARVD, the systemic inflammatory response triggered by chronic subclinical ischemia may take precedence over reduced blood flow as the driver of reduced kidney function. Identifying kidneys with renal artery stenosis that have yet to reach the “point of no return” (irreparable damage) is of critical importance. It is accepted that revascularization does not improve kidney function in the setting of atrophic kidneys or those with thin cortices. Proteinuria and a high Doppler ultrasound resistive index are additional markers of poor outcome because they are indicative of severely damaged renal parenchyma (Table 6). Additional markers of viability of kidney parenchyma will be needed to determine whether to intervene with future novel therapies (e.g., vascular endothelial growth factor [VEGF], endothelin antagonists, or stem cells) or with selected revascularization in appropriate patients.
Table 6 |.
Assessment of kidney parenchyma viability in a kidney with renal artery stenosis
| Nonviable | Likely to be viable | |
|---|---|---|
| Renal length (cm) | <7 cm | >8 cma |
| Cortical thickness | Loss of corticomedullary differentiation; no cortex | Cortex distinct, e.g., >0.5 cm |
| Proteinuriab | ACR >300 mg/g (30 mg/mmol) Equivalent to PCR >500 mg/g (50 mg/mmol) |
ACR <200 mg/g (20 mg/mmol) |
| Renal resistive index | >0.8 | <0.8 |
ACR, albumin-creatinine ratio; BMI, body mass index; PCR, protein-creatinine ratio.
Possibly consider kidney length-to-BMI ratio.
Proteinuria may be arising from an atrophic kidney.
What is the role of revascularization therapy in atherosclerotic renal artery stenosis?
Many patients enrolled in the ASTRAL90 and CORAL91 trials had lower-risk phenotypes, and some had physiologically insignificant renal artery stenosis. Patients with higher-risk features were often managed with revascularization outside of the RCT setting. Additionally, there are numerous reports of patients who appear to have had major clinical benefits from stenting. Key patient subgroups who were not well represented in these RCTs and for whom a positive response to revascularization may be more likely include those with high-grade bilateral renal artery stenosis or renal artery stenosis affecting a solitary kidney who present with AKI, marked reductions in eGFR with RAAS blockers, acutely decompensated heart failure, or the combination of progressively deteriorating CKD and uncontrolled arterial hypertension (Table 7).96 Percutaneous renal artery revascularization is the intervention of choice in the majority of patients selected for revascularization; open surgical revascularization should be reserved for patients with congenital disease, or with complex anatomy (e.g., multiple stenoses) and those refractory to or with multiple restenosis events after endovascular intervention.
Table 7 |.
Definite and possible indications for renal revascularization therapy in atherosclerotic high-grade renal artery stenosis
| KDIGO consensus: indications for renal revascularization therapy | Possible indications for revascularization therapy that require further evaluation |
|---|---|
| Flash pulmonary edema or acute decompensations of heart failure | Chronic heart failure |
| Progressive CKD in high-grade renal artery stenosis if bilateral or solitary kidney | Coexistence of progressive CKD and uncontrolled hypertension |
| Acute kidney injury due to acute renal artery occlusion or high-grade renal artery stenosis | High-grade renal artery stenosis supplying solitary kidney with viable renal parenchyma—to prevent atrophy |
| Intolerance of ACEi or ARB in high-grade renal artery stenosis when such therapy is necessary |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes.
Association of heart failure with ARVD
There is a clear link between ARVD and various heart failure phenotypes.99 Up to 30% of patients with chronic heart failure may have renal artery stenosis,99 and there are multiple case reports and observational case series describing the benefits of renal revascularization in patients suffering acute decompensations. The term “flash pulmonary edema” has become widespread to describe sudden onset (within minutes) of pulmonary edema in patients with no previous history of myocardial or coronary artery disease, and this presentation is highly suggestive of a renal artery stenosis etiology. Flash pulmonary edema is an accepted indication for renal revascularization in patients with high-grade renal artery stenosis.100 Revascularization may also be indicated for decompensated chronic heart failure,101 although clinical trial evidence for this approach is still lacking.
Conclusions and future research
Recognizing the heterogeneous nature of ARVD, clinicians evaluating patients for possible revascularization should consider renal artery stenosis severity, the viability of the kidney beyond the renal artery stenosis, and the presenting clinical syndrome. Key aspects of the clinical presentation that might signal a potential benefit of revascularization include AKI, RAAS-induced functional decline, acute heart failure, and progressive CKD with severe arterial hypertension. Areas in need of further research are enumerated in Table 8.
Table 8 |.
Gaps in knowledge and research recommendations for renovascular disease and CKD
| • Gain a better understanding of the pathogenesis and time course of ischemia and renal parenchymal injury in atherosclerotic renal artery stenosis |
| • Develop specific techniques for assessment of the physiologic relevance of renal artery stenosis (such as are those currently available for coronary artery stenoses) |
| • Determine optimal techniques to confirm kidney viability and non-viability prior to consideration of revascularization |
| • Determine the benefit of revascularization therapy in patients with renal artery stenosis and acute decompensated and progressive chronic heart failure |
| • Determine long-term outcomes after renal revascularization therapy. Is preventing kidney atrophy important regardless of clinical presentation? |
| • Further explore the relationship of proteinuria to outcomes after revascularization in atherosclerotic renal artery stenosis |
| • An RCT of medical therapy versus revascularization plus medical therapy is warranted for patients with renal artery stenosis and high-risk clinical presentations (Table 7) |
| • Encourage trials of novel therapies (e.g., stem cells, VEGF, endothelin inhibitors) in human ARVD |
ARVD, atherosclerotic renovascular disease; CKD, chronic kidney disease; RCT, randomized controlled trial; VEGF, vascular endothelial growth factor.
PAD IN CKD
Epidemiology of PAD in CKD
The prevalence of PAD is higher among persons with CKD than among those without, varying from 12% to 38% among people with CKD and those receiving dialysis.102,103 These variations can be attributed in part to variability in the methods used to ascertain PAD but also partially to differences in age, hypertension control, and diabetes of the patients in the distinct studies. Several studies have shown that the risk for severe PAD, including amputation, is markedly increased even in CKD G2 and G3. In a large population study, Bourrier et al.103 found high event rates in this population compared with those without CKD. In another large cohort from Germany, the amputation-free survival after 1 year was only about 80% among individuals with CKD G2-G3 and PAD,104 which may be due in part to underdiagnosis and undertreatment of these patients.
The Rose,105 Edinburgh,106 and San Diego107 claudication questionnaires are designed to detect symptomatic PAD but have not been validated in persons with CKD. The ankle-brachial index is a first-line diagnostic tool for PAD but has limitations among patients with CKD.100,108,109 Notably, the risk of amputation is also exceedingly high among patients receiving dialysis.104,110
Data regarding PAD in patients who have received a kidney transplant are sparse but suggest that transplant recipients have lower risk of PAD compared with those receiving maintenance dialysis.111 The presence of PAD is associated with poor allograft and overall mortality in transplant recipients.112
Key pathophysiology of PAD in CKD with diagnostic and therapeutic implications
Traditional risk factors such as diabetes and hypertension play an important role in the pathophysiology of PAD in CKD, and a history thereof may be a more important PAD risk determinant than age alone. However, vascular calcification, inflammation, oxidative stress, uremic toxins, and microvascular disease may also contribute to the elevated risk of PAD among patients with CKD.113
Vascular calcification can manifest as intima arterial calcification or medial arterial calcification (MAC).114 Atherosclerosis is the main cause of intima arterial calcification, whereas aging and cellular senescence play pivotal roles in MAC. CKD accelerates the aging process and increases the risk of MAC. Although atherosclerosis and intima arterial calcification are known to be central to the development and progression of PAD, the role of MAC is less clear. Nonetheless, it is clear that MAC can lead to false-normal or increased ankle-brachial index, even in the setting of severe PAD, which can pose diagnostic challenges.
Inflammation and oxidative stress contribute to the development of atherosclerotic disease in CKD. Some studies have shown that anti-inflammatory agents and antioxidants may reduce the risk of cardiovascular outcomes.115 Elevated levels of several uremic toxins, such as p-cresol, p-cresyl sulfate, and indoxyl sulfate, have been associated with increased risk of cardiovascular outcomes.116–118 To date, there are no established therapies to reduce these uremic toxins, except kidney replacement therapy. In this regard, gut microbiota may be an interesting potential therapeutic target,119 as they produce these uremic toxins and could be manipulated through dietary modifications, medication, or fecal transplantation.
Although PAD has been considered a large artery disease, recent studies have indicated that microvascular disease contributes to the progression of PAD and associated serious complications such as chronic limb-threatening ischemia and lower-extremity amputation.120–122 These observations raise further concern about using the ankle-brachial index as a diagnostic test for PAD, because it does not measure microvascular disease.
Challenges in diagnosing PAD in patients with CKD
Diagnostic testing for PAD is indicated in symptomatic patients but may also be appropriate in asymptomatic patients to ascertain the need to start a secondary preventive therapy, with statins or platelet inhibition, for example. Symptoms and signs of PAD are often not solicited by healthcare providers or volunteered by patients. Given that PAD is not often spontaneously discussed and has a high prevalence in the CKD population, a reasonable approach is to implement a systematic screening strategy using questionnaires107 to identify intermittent claudication in patients with CKD. Also, it is reasonable to perform routine foot examinations in patients on dialysis, given their high risk of critical limb ischemia and the feasibility of visual examination during hemodialysis.
The ankle-brachial index is the first-line diagnostic test for PAD. However, because MAC is common among patients with CKD and can lead to false negative results of ankle-brachial index screening, we advocate for simultaneous measurement of toe-brachial index in CKD populations.123 Regarding imaging modalities, duplex ultrasound is a gold standard diagnostic modality for PAD. Computed tomographic or magnetic resonance angiography may be helpful in planning leg revascularization procedures for selected patients.
Challenges and knowledge gaps relating to PAD treatment in CKD
Lifestyle modifications, including smoking cessation and supervised exercise programs, have proven benefits beyond the management of PAD124,125 and should be offered to all affected patients, including those with CKD. The benefit of antiplatelet agents in the setting of symptomatic PAD is unequivocal.126 In the COMPASS trial,39 the use of rivaroxaban plus aspirin was associated with a lower risk of major adverse cardiovascular events (MACE), compared with aspirin alone, among 6276 participants with a GFR <60 ml/min per 1.73 m2. Importantly, no increased risk of bleeding was noted among those with CKD.
Statin therapy decreases MACE among patients with CKD not treated with dialysis, including those with PAD.127 Further, the benefit of statins in improving amputation-free survival among persons with PAD has been documented in a large observational study of over 150,000 patients, and most patients with CKD over the age of 50 years would meet recommended criteria for statin therapy based on future CVD risk.128 Proprotein convertase subtilisin/kexin type 9 inhibitors further reduce the risk of MACE129 and major adverse limb events among individuals with established atherosclerotic disease who are already receiving treatment with a statin.130 Although likely to be safe and effective, use of proprotein convertase subtilisin/kexin type 9 inhibitors among patients with CKD needs further study.131
Patients with CKD and PAD are less frequently considered for limb revascularization132 and are at high risk of limb amputation.133 Although revascularization in persons with CKD is associated with increased risk of post-procedure complications,134,135 data suggest that it results in improved amputation-free survival and limb salvage at all severity stages of CKD.136
Conclusion and future research
A major obstacle in the development of appropriate treatment guidelines in persons with CKD and PAD is their exclusion from clinical trials. Nearly 45% of trials evaluating therapies for PAD have excluded persons with CKD.137 Most trials do not report subgroup analysis based on CKD status,137 and treatment strategies therefore need to be extrapolated from findings in the general population. As a result, a research agenda for the study of PAD in CKD is proposed in Table 9.138
Table 9 |.
Gaps in knowledge and research recommendations for PAD and CKD
| Knowledge gaps | Research recommendations |
|---|---|
|
Epidemiology • There is a paucity of epidemiologic studies examining PAD and outcomes (e.g., amputation) in patients with CKD or treated with dialysis, including kidney transplant recipients. |
• Investigators should be encouraged to report CKD subgroup analyses in PAD trials. |
| • Future studies should standardize reporting of PAD-related outcomes in CKD.138 There should also be inclusion of patient-reported outcomes and quality-of-life measures. | |
| • Future studies of PAD should be conducted in kidney transplant recipients. | |
| • Efforts should be made to identify PAD phenotypes and evaluate their impact. | |
| • Costs associated with PAD in CKD should be grounds for further study. | |
| Pathophysiology | |
| • To what extent does medial arterial calcification, which is most often observed in CKD, contribute to pathogenesis of PAD? | • Future studies should ascertain methods for quantifying intima arterial calcification and medial arterial calcification and assess their respective roles in the pathophysiology of PAD in CKD. Is there clinical value to their determinations? |
| Diagnosis | |
| • What is the incremental value of toe-brachial index in addition to ankle-brachial index for PAD diagnosis in CKD? | • Future studies should investigate the use of non-invasive tests to evaluate microcirculation. |
| • Are the general claudication questionnaires valid in CKD? | |
| • Is there utility for a risk-based screening approach? | |
| Treatment | |
| • What are the most promising agents (e.g., antioxidants; anti-inflammatories) or strategies (e.g., gut microbiota) in ameliorating risk of PAD in CKD? | • Research should investigate whether suppression of inflammation or oxidative stress would prevent the development and progression of PAD in the CKD population. |
| • Future studies should evaluate the treatment role of PCSK9 inhibitors among patients with CKD in reducing onset or progression of PAD. |
CKD, chronic kidney disease; PAD, peripheral artery disease; PCSK9, proprotein convertase subtilisin/kexin type 9.
CKD patients can be affected by atherosclerotic disease with various manifestations; therefore, the knowledge gaps and research needs outlined here, if addressed, could potentially improve patient care and outcomes.
ACKNOWLEDGMENTS
The conference was sponsored by Kidney Disease: Improving Global Outcomes (KDIGO) and supported in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Janssen, Lilly, and Vifor Fresenius Medical Care Renal Pharma.
DISCLOSURE
CWH declared receiving research support from the National Institutes of Health (NIH). KM declared receiving personal fees from Akebia, Fukuda Denshi, Healthy.io, and Kyowa Kirin; and research support from Kyowa Kirin and the NIH. MMS declared receiving speaker’s bureaus from AstraZeneca. CAH declared receiving consultant fees from Abbvie, Amgen, AstraZeneca, Bayer, Corvidia, DiaMedica, FibroGen, Janssen, the National Heart Lung and Blood Institute (NHLBI/NIH), NxStage, Pfizer, Relypsa, Sanifit, and University of Oxford; stock equity from Boston Scientific, Bristol-Myers Squibb (BMS), General Electric, Johnson & Johnson, and Merck; research support from Amgen, AstraZeneca, BMS, NHLBI/NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH, Relypsa, University of British Columbia; and author royalties from UpToDate. MJ declared receiving consultant fees from Astellas, AstraZeneca, Boehringer-Ingelheim, Fresenius Medical Care Asia Pacific, Mundipharma, and Vifor Fresenius Medical Care; speaker’s bureaus from Astellas, AstraZeneca, Mundipharma, and Vifor Fresenius Medical Care; research support from Amgen; and future research support from AstraZeneca. WCW declared receiving consultant fees from Akebia/Otsuka, AstraZeneca, Bayer, Janssen, Merck, Reata, and Relypsa; future consultant fees from Boehringer Ingelheim; and research support from the NIH. HR declared receiving personal fees from Corvia, Daiichi-Sankyo, DiaPlan, MedUpdate, NeoVasc, Novo Nordisk, Pluristem, and StreamedUp; and research support from Bard, Biotronik, BMS/Pfizer, and Pluristem. All the other authors declared no competing interests.
APPENDIX
Other Conference Participants
Zanfina Ademi, Tara I. Chang, Timothy W.I. Clark, Christopher J. Cooper, Michael H. Criqui, Áine de Bhailis, Marco De Carlo, Wolfram Döhner, Daniel T. Engelman, Gerry Fowkes, Darren Green, Allen D. Hamdan, Christian Heiss, Peter Huppert, Daniella Kadian-Dodov, Gregory Y.H. Lip, Jolanta Małyszko, Patrick B. Mark, Marius Miglinas, Patrick T. Murray, Christopher M. Reid, Paul J. Rochon, Josiah Ruturi, Athanasios Saratzis, Mark J. Sarnak, Cathy M. Shanahan, Laura Solá, Ulf Teichgräber, Stephen C. Textor, Kazunori Toyoda, Angela Yee-Moon Wang, and Christopher X. Wong.
Footnotes
See Appendix for list of Conference Participants.
REFERENCES
- 1.London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–1695. [DOI] [PubMed] [Google Scholar]
- 2.Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823–833. [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Katz R, Robinson-Cohen C, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Findlay MD, Dawson J, MacIsaac R, et al. Inequality in care and differences in outcome following stroke in people with ESRD. Kidney Int Rep. 2018;3:1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Findlay MD, Thomson PC, MacIsaac R, et al. Risk factors and outcome of stroke in renal transplant recipients. Clin Transplant. 2016;30:918–924. [DOI] [PubMed] [Google Scholar]
- 7.Findlay M, MacIsaac R, MacLeod MJ, et al. Renal replacement modality and stroke risk in end-stage renal disease—a national registry study. Nephrol Dial Transplant. 2018;33:1564–1571. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya T, Perkovic V, Verdon C, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53:417–425. [DOI] [PubMed] [Google Scholar]
- 9.Sandsmark DK, Messe SR, Zhang X, et al. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: chronic renal insufficiency cohort study. Stroke. 2015;46:2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Husseini N, Fonarow GC, Smith EE, et al. Renal dysfunction is associated with poststroke discharge disposition and in-hospital mortality: findings from Get With the Guidelines—Stroke. Stroke. 2017;48:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumai Y, Kamouchi M, Hata J, et al. Proteinuria and clinical outcomes after ischemic stroke. Neurology. 2012;78:1909–1915. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. 2019;50:229–239. [DOI] [PubMed] [Google Scholar]
- 13.Chelluboina B, Vemuganti R. Chronic kidney disease in the pathogenesis of acute ischemic stroke. J Cereb Blood Flow Metab. 2019;39:1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henaut L, Grissi M, Brazier F, et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci Rep. 2019;9:6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprick JD, Nocera JR, Hajjar I, et al. Cerebral blood flow regulation in end-stage kidney disease. Am J Physiol Renal Physiol. 2020;319:F782–F791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly D, Rothwell PM. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J Neurol Neurosurg Psychiatry. 2020;91:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polinder-Bos HA, Garcia DV, Kuipers J, et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol. 2018;29:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacEwen C, Sutherland S, Daly J, et al. Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol. 2017;28:2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findlay MD, Dawson J, Dickie DA, et al. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol. 2019;30:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ovbiagele B, Schwamm LH, Smith EE, et al. Patterns of care quality and prognosis among hospitalized ischemic stroke patients with chronic kidney disease. J Am Heart Assoc. 2014;3:e000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung JM, Kim HJ, Ahn H, et al. Chronic kidney disease and intravenous thrombolysis in acute stroke: a systematic review and meta-analysis. J Neurol Sci. 2015;358:345–350. [DOI] [PubMed] [Google Scholar]
- 22.Carr SJ, Wang X, Olavarria VV, et al. Influence of renal impairment on outcome for thrombolysis-treated acute ischemic stroke: ENCHANTED (Enhanced Control of Hypertension and Thrombolysis Stroke Study) post hoc analysis. Stroke. 2017;48:2605–2609. [DOI] [PubMed] [Google Scholar]
- 23.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 24.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 26.Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke. 2006;37:922–928. [DOI] [PubMed] [Google Scholar]
- 27.Laible M, Mohlenbruch MA, Pfaff J, et al. Influence of renal function on treatment results after stroke thrombectomy. Cerebrovasc Dis. 2017;44:351–358. [DOI] [PubMed] [Google Scholar]
- 28.Galons JP, Trouard T, Gmitro AF, et al. Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest. 1996;98:750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winney RJ, Kean DM, Best JJ, et al. Changes in brain water with haemodialysis. Lancet. 1986;2:1107–1108. [DOI] [PubMed] [Google Scholar]
- 30.Onoyama K, Ibayashi S, Nanishi F, et al. Cerebral hemorrhage in patients on maintenance hemodialysis. CT analysis of 25 cases. Eur Neurol. 1987;26:171–175. [DOI] [PubMed] [Google Scholar]
- 31.Davenport A. Changing the hemodialysis prescription for hemodialysis patients with subdural and intracranial hemorrhage. Hemodial Int. 2013;17(suppl 1):S22–S27. [DOI] [PubMed] [Google Scholar]
- 32.Davenport A Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int. 2008;12:307–312. [DOI] [PubMed] [Google Scholar]
- 33.National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- 34.Major RW, Oozeerally I, Dawson S. Aspirin and cardiovascular primary prevention in non-endstage chronic kidney disease: a meta-analysis. Atherosclerosis. 2016;251:177–182. [DOI] [PubMed] [Google Scholar]
- 35.Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013:CD008834. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 38.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 39.Fox KAA, Eikelboom JW, Shestakovska O, et al. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: from the COMPASS trial. J Am Coll Cardiol. 2019;73:2243–2250. [DOI] [PubMed] [Google Scholar]
- 40.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrington WG, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–839. [DOI] [PubMed] [Google Scholar]
- 42.Wanner C, Tonelli M. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–1309. [DOI] [PubMed] [Google Scholar]
- 43.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3 suppl):S1–S87. [DOI] [PubMed] [Google Scholar]
- 44.Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew A, Eliasziw M, Devereaux PJ, et al. Carotid endarterectomy benefits patients with CKD and symptomatic high-grade stenosis. J Am Soc Nephrol. 2010;21:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricotta JJ, Aburahma A, Ascher E, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–e31. [DOI] [PubMed] [Google Scholar]
- 47.Toyoda K. Cerebral small vessel disease and chronic kidney disease. J Stroke. 2015;17:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makin SD, Cook FA, Dennis MS, et al. Cerebral small vessel disease and renal function: systematic review and meta-analysis. Cerebrovasc Dis. 2015;39:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M, Hirawa N, Yatsu K, et al. Relationship between silent brain infarction and chronic kidney disease. Nephrol Dial Transplant. 2009;24:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naganuma T, Uchida J, Tsuchida K, et al. Silent cerebral infarction predicts vascular events in hemodialysis patients. Kidney Int. 2005;67:2434–2439. [DOI] [PubMed] [Google Scholar]
- 51.Ito S, Nagasawa T, Abe M, et al. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–121. [DOI] [PubMed] [Google Scholar]
- 52.Holliday EG, Traylor M, Malik R, et al. Polygenic overlap between kidney function and large artery atherosclerotic stroke. Stroke. 2014;45:3508–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao H, Araki Y, Takashima Y, et al. Chronic kidney disease and subclinical brain infarction increase the risk of vascular cognitive impairment: the Sefuri study. J Stroke Cerebrovasc Dis. 2017;26:420–424. [DOI] [PubMed] [Google Scholar]
- 54.Smith EE, Saposnik G, Biessels GJ, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. [DOI] [PubMed] [Google Scholar]
- 55.Wilson D, Ambler G, Lee KJ, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2019;18:653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 57.Chun KC, Teng KY, Chavez LA, et al. Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional Veterans Affairs health care system. Ann Vasc Surg. 2014;28:87–92. [DOI] [PubMed] [Google Scholar]
- 58.Matsushita K, Kwak L, Ballew SH, et al. Chronic kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis. 2018;279:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saratzis A, Bath MF, Harrison S, et al. Long-term renal function after endovascular aneurysm repair. Clin J Am Soc Nephrol. 2015;10:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charles ER, Lui D, Delf J, et al. Editor’s choice—the impact of endovascular aneurysm repair on long-term renal function based on hard renal outcomes. Eur J Vasc Endovasc Surg. 2019;58:328–333. [DOI] [PubMed] [Google Scholar]
- 61.Zabrocki L, Marquardt F, Albrecht K, et al. Permanent decline of renal function after infrarenal abdominal aortic aneurysm repair-frequency and risk factors. Ann Vasc Surg. 2018;47:272–278. [DOI] [PubMed] [Google Scholar]
- 62.Aranson NJ, Lancaster RT, Ergul EA, et al. Chronic kidney disease class predicts mortality after abdominal aortic aneurysm repair in propensity-matched cohorts from the Medicare population. Ann Surg. 2016;264:386–391. [DOI] [PubMed] [Google Scholar]
- 63.Saratzis A, Sarafidis P, Melas N, et al. Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2013;58:879–885. [DOI] [PubMed] [Google Scholar]
- 64.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. [DOI] [PubMed] [Google Scholar]
- 65.Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- 66.Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472–475. [DOI] [PubMed] [Google Scholar]
- 67.Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. [DOI] [PubMed] [Google Scholar]
- 68.Dariane C, Coscas R, Boulitrop C, et al. Acute kidney injury after open repair of intact abdominal aortic aneurysms. Ann Vasc Surg. 2017;39:294–300. [DOI] [PubMed] [Google Scholar]
- 69.Jhaveri KD, Saratzis AN, Wanchoo R, et al. Endovascular aneurysm repair (EVAR)- and transcatheter aortic valve replacement (TAVR)-associated acute kidney injury. Kidney Int. 2017;91:1312–1323. [DOI] [PubMed] [Google Scholar]
- 70.Pirgakis KM, Makris K, Dalainas I, et al. Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2014;28:1649–1658. [DOI] [PubMed] [Google Scholar]
- 71.Ueta K, Watanabe M, Iguchi N, et al. Early prediction of acute kidney injury biomarkers after endovascular stent graft repair of aortic aneurysm: a prospective observational study. J Intensive Care. 2014;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pisimisis GT, Bechara CF, Barshes NR, et al. Risk factors and impact of proximal fixation on acute and chronic renal dysfunction after endovascular aortic aneurysm repair using glomerular filtration rate criteria. Ann Vasc Surg. 2013;27:16–22. [DOI] [PubMed] [Google Scholar]
- 73.Saratzis A, Melas N, Mahmood A, et al. Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg. 2015;49:534–540. [DOI] [PubMed] [Google Scholar]
- 74.Saratzis A, Harrison S, Barratt J, et al. Intervention-associated acute kidney injury and long-term cardiovascular outcomes. Am J Nephrol. 2015;42:285–294. [DOI] [PubMed] [Google Scholar]
- 75.Saratzis A, Nduwayo S, Sarafidis P, et al. Renal function is the main predictor of acute kidney injury after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2016;31:52–59. [DOI] [PubMed] [Google Scholar]
- 76.Obata Y, Kamijo-Ikemori A, Ichikawa D, et al. Clinical usefulness of urinary liver-type fatty-acid-binding protein as a perioperative marker of acute kidney injury in patients undergoing endovascular or open-abdominal aortic aneurysm repair. J Anesth. 2016;30:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J, Park KM, Jung S, et al. Occurrences and results of acute kidney injury after endovascular aortic abdominal repair? Vasc Specialist Int. 2017;33:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saratzis A, Chiocchia V, Jiffry A, et al. Hydration and bicarbonate to prevent acute renal injury after endovascular aneurysm repair with suprarenal fixation: pilot/feasibility randomised controlled study (HYDRA pilot trial). Eur J Vasc Endovasc Surg. 2018;55:648–656. [DOI] [PubMed] [Google Scholar]
- 79.Zabrocki L, Marquardt F, Albrecht K, et al. Acute kidney injury after abdominal aortic aneurysm repair: current epidemiology and potential prevention. Int Urol Nephrol. 2018;50:331–337. [DOI] [PubMed] [Google Scholar]
- 80.Saratzis A, Joshi S, Benson RA, et al. Editor’s choice - acute kidney injury (AKI) in aortic intervention: findings from the Midlands Aortic Renal Injury (MARI) cohort study. Eur J Vasc Endovasc Surg. 2020;59:899–909. [DOI] [PubMed] [Google Scholar]
- 81.Reis PV, Morgado M, Valdoleiros I, et al. Complications of endovascular aneurysm repair: mortality, myocardial infarction and acute kidney injury. Turk J Anaesthesiol Reanim. 2018;46:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Adas Z, Shepard AD, Nypaver TJ, et al. Long-term decline in renal function is more significant after endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2018;68:739–748. [DOI] [PubMed] [Google Scholar]
- 83.Saratzis A, Sarafidis P, Melas N, et al. Comparison of the impact of open and endovascular abdominal aortic aneurysm repair on renal function. J Vasc Surg. 2014;60:597–603. [DOI] [PubMed] [Google Scholar]
- 84.Saratzis A, Sarafidis P, Melas N, et al. Suprarenal graft fixation in endovascular abdominal aortic aneurysm repair is associated with a decrease in renal function. J Vasc Surg. 2012;56:594–600. [DOI] [PubMed] [Google Scholar]
- 85.National Clinical Guideline Centre (UK). Acute Kidney Injury: Prevention, Detection and Management Up to the Point of Renal Replacement Therapy. London: Royal College of Physicians; 2013. [PubMed] [Google Scholar]
- 86.Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;130:S45–S50. [DOI] [PubMed] [Google Scholar]
- 87.Hoogmoed RC, Patel HJ, Kim KM, et al. Acute kidney injury in acute type B aortic dissection: outcomes over 20 years. Ann Thorac Surg. 2019;107:486–492. [DOI] [PubMed] [Google Scholar]
- 88.Reutersberg B, Salvermoser M, Trenner M, et al. Hospital incidence and in-hospital mortality of surgically and interventionally treated aortic dissections: secondary data analysis of the nationwide German diagnosis-related group statistics from 2006 to 2014. J Am Heart Assoc. 2019;8:e011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaji S. Acute medical management of aortic dissection. Gen Thorac Cardiovasc Surg. 2019;67:203–207. [DOI] [PubMed] [Google Scholar]
- 90.Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. [DOI] [PubMed] [Google Scholar]
- 91.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. [DOI] [PubMed] [Google Scholar]
- 93.Chrysochou C, Foley RN, Young JF, et al. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1403–1409. [DOI] [PubMed] [Google Scholar]
- 94.Hackam DG, Duong-Hua ML, Mamdani M, et al. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156:549–555. [DOI] [PubMed] [Google Scholar]
- 95.Clark AL, Kalra PR, Petrie MC, et al. Change in renal function associated with drug treatment in heart failure: national guidance. Heart. 2019;105:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ritchie J, Green D, Chrysochou C, et al. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63:186–197. [DOI] [PubMed] [Google Scholar]
- 97.Abumoawad A, Saad A, Ferguson CM, et al. Tissue hypoxia, inflammation, and loss of glomerular filtration rate in human atherosclerotic renovascular disease. Kidney Int. 2019;95:948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Silva R, Loh H, Rigby AS, et al. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100:273–279. [DOI] [PubMed] [Google Scholar]
- 100.Rooke TW, Hirsch AT, Misra S, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1555–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Green D, Ritchie JP, Chrysochou C, et al. Revascularization of atherosclerotic renal artery stenosis for chronic heart failure versus acute pulmonary oedema. Nephrology (Carlton). 2018;23:411–417. [DOI] [PubMed] [Google Scholar]
- 102.Garimella PS, Hart PD, O’Hare A, et al. Peripheral artery disease and CKD: a focus on peripheral artery disease as a critical component of CKD care. Am J Kidney Dis. 2012;60:641–654. [DOI] [PubMed] [Google Scholar]
- 103.Bourrier M, Ferguson TW, Embil JM, et al. Peripheral artery disease: its adverse consequences with and without CKD. Am J Kidney Dis. 2020;75:705–712. [DOI] [PubMed] [Google Scholar]
- 104.Luders F, Bunzemeier H, Engelbertz C, et al. CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol. 2016;11:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 106.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. [DOI] [PubMed] [Google Scholar]
- 107.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. [DOI] [PubMed] [Google Scholar]
- 108.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Intl Suppl. 2012;2:337–414. [Google Scholar]
- 109.Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:342–348. [DOI] [PubMed] [Google Scholar]
- 110.Butler CR, Schwarze ML, Katz R, et al. Lower extremity amputation and health care utilization in the last year of life among Medicare beneficiaries with ESRD. J Am Soc Nephrol. 2019;30:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snyder JJ, Kasiske BL, Maclean R. Peripheral arterial disease and renal transplantation. J Am Soc Nephrol. 2006;17:2056–2068. [DOI] [PubMed] [Google Scholar]
- 112.Patel SI, Chakkera HA, Wennberg PW, et al. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc Med. 2017;22:225–230. [DOI] [PubMed] [Google Scholar]
- 113.Arinze NV, Gregory A, Francis JM, et al. Unique aspects of peripheral artery disease in patients with chronic kidney disease. Vasc Med. 2019;24:251–260. [DOI] [PubMed] [Google Scholar]
- 114.Durham AL, Speer MY, Scatena M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 116.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. [DOI] [PubMed] [Google Scholar]
- 119.Biscetti F, Nardella E, Cecchini AL, et al. The role of the microbiota in the diabetic peripheral artery disease. Mediators Inflamm. 2019;4128682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beckman JA, Duncan MS, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang C, Kwak L, Ballew SH, et al. Retinal microvascular findings and risk of incident peripheral artery disease: an analysis from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2020;294:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsushita K, Ballew SH, Coresh J, et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017;5:718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsuyuki K, Kohno K, Ebine K, et al. Exercise-ankle brachial pressure index with one-minute treadmill walking in patients on maintenance hemodialysis. Ann Vasc Dis. 2013;6:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foley RN, Herzog CA, Collins AJ. Smoking and cardiovascular outcomes in dialysis patients: the United States Renal Data System Wave 2 Study. Kidney Int. 2003;63:1462–1467. [DOI] [PubMed] [Google Scholar]
- 125.Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleve Clin J Med. 2006;73(suppl 4):S8–S14. [DOI] [PubMed] [Google Scholar]
- 126.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.SHARP Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160:785–794. [DOI] [PubMed] [Google Scholar]
- 128.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 129.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 130.Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 131.Charytan DM, Sabatine MS, Pedersen TR, et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J Am Coll Cardiol. 2019;73:2961–2970. [DOI] [PubMed] [Google Scholar]
- 132.O’Hare AM, Bertenthal D, Sidawy AN, et al. Renal insufficiency and use of revascularization among a national cohort of men with advanced lower extremity peripheral arterial disease. Clin J Am Soc Nephrol. 2006;1:297–304. [DOI] [PubMed] [Google Scholar]
- 133.Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56:1524–1533. [DOI] [PubMed] [Google Scholar]
- 134.O’Hare AM, Feinglass J, Sidawy AN, et al. Impact of renal insufficiency on short-term morbidity and mortality after lower extremity revascularization: data from the Department of Veterans Affairs’ National Surgical Quality Improvement Program. J Am Soc Nephrol. 2003;14:1287–1295. [DOI] [PubMed] [Google Scholar]
- 135.Reddan DN, Marcus RJ, Owen WF Jr, et al. Long-term outcomes of revascularization for peripheral vascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001;38:57–63. [DOI] [PubMed] [Google Scholar]
- 136.Ortmann J, Gahl B, Diehm N, et al. Survival benefits of revascularization in patients with critical limb ischemia and renal insufficiency. J Vasc Surg. 2012;56:737–745. [DOI] [PubMed] [Google Scholar]
- 137.Wen Y, Potok OA, Wei X, et al. Exclusion of persons with kidney disease in trials of peripheral artery disease: a systematic review of randomized trials. Clin J Am Soc Nephrol. 2020;15:117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tong A, Craig JC, Nagler EV, et al. Composing a new song for trials: the standardized outcomes in nephrology (SONG) initiative. Nephrol Dial Transplant. 2017;32:1963–1966. [DOI] [PubMed] [Google Scholar]


