Abstract
To quantify complement depletion by pneumolysin during Streptococcus pneumoniae bacteremia, cirrhotic and control rats were infected intravenously with one of three isogenic mutant strains of S. pneumoniae expressing different forms of pneumolysin. Outcome measures included clearance of the organisms from the bloodstream, alterations in 50% serum hemolytic complement (CH50) activity and complement C3 levels during infection, and serum opsonic capacity at 18 h postinfection. Cirrhotic rats had significantly lower CH50 and C3 levels than control rats, both before and after infection. However, initial complement levels did not predict bacterial load after 18 h of infection. Changes in CH50 and C3 levels over the 18-h period correlated with numbers of H+C+ but not H+C− or PLY− organisms in the bloodstream at 18 h postinfection. The sera of cirrhotic rats infected with the H+C+ strain had significantly decreased levels of C3 and showed significantly lower opsonizing activity for S. pneumoniae than sera from H+C+-infected control rats. These studies suggest that under limiting concentrations of complement, the expression of pneumolysin by pneumococci has a significant, negative effect on serum complement levels and reduces serum opsonic activity.
Type-specific antibody formation is an important host defense mechanism against infections caused by Streptococcus pneumoniae (the pneumococcus). However, effective opsonization of pneumococci by either immunoglobulin M (IgM) or IgG requires host complement components, making complement essential for recovery from pneumococcal disease (reviewed in reference 6). Patients with complement system defects, therefore, are highly susceptible to pneumococcal infections (1, 10, 14, 28). Complement's importance in host resistance to pneumococcal bacteremia was demonstrated in an experimental model in which cobra venom factor was used to induce severe complement depletion of guinea pigs. The animals with depleted complement levels displayed reduced bloodstream clearance and increased mortality after intravenous infection, even with small numbers of S. pneumoniae (11).
Pneumolysin, a 53-kilodalton protein produced by all clinical isolates of S. pneumoniae, is a key pneumococcal virulence factor (25). Pneumolysin is cytotoxic to a number of different cell types, including monocytes (13), neutrophils (15), endothelial cells (27), and alveolar epithelial cells (26). In addition, pneumolysin released during pneumococcal autolysis activates complement at a distance from the organisms, an activity thought to contribute to virulence by reducing serum opsonic activity (21).
Patients with alcoholic cirrhosis have depressed levels of several complement components and reduced serum complement activity, making them particularly susceptible to bacterial infections (10). S. pneumoniae is the most common gram-positive organism isolated from the bloodstream of cirrhotic patients (31), and cirrhosis is one of the most common underlying diseases associated with a high risk of mortality from pneumococcal bacteremia (19). Using a rat model of carbon tetrachloride-induced cirrhosis that is histologically indistinguishable from alcoholic cirrhosis in humans (20), workers in our laboratory have shown that the complement-activating activity of pneumolysin uniquely reduces pneumococcal bloodstream clearance and increases mortality from pneumococcal bacteremia in the cirrhotic host (2). In the present study, we quantified complement depletion during pneumococcal infection of cirrhotic and control rats to demonstrate that pneumolysin-induced reduction of complement levels prevents effective opsonophagocytosis of pneumococci by the hypocomplementemic, cirrhotic host.
MATERIALS AND METHODS
Experimentally induced cirrhosis.
Cirrhosis was induced in outbred male Sprague-Dawley rats (Charles River, Kingston, N.Y.) by weekly intragastric instillation of carbon tetrachloride (CCl4), as described previously (20, 24). All cirrhotic rats had been treated with CCl4 for at least 8 weeks, had visible ascites fluid for at least 2 consecutive weeks, and remained untreated for 1 week before being used in experiments. Phosphate-buffered saline (PBS) was instilled into age-matched control rats. This protocol was approved by the Animal Research Committee, Veterans Affairs Medical Center, Omaha, Nebr.
S. pneumoniae mutant strains.
Three isogenic mutant strains of S. pneumoniae (provided originally by Mary K. Johnson, Tulane University) were used in all of the animal infection studies. All the strains were produced from the serotype 3 WU2 parent organism, in which the pneumolysin gene was excised from the chromosome and reexpressed on plasmid pVA838 as described previously (15–17, 21). The reconstructed mutant strain, designated H+C+, expresses wild-type pneumolysin (with both hemolytic and complement-activating activities) and produces the same high level of pathology associated with the WU2 parental strain (16). The strain that produces pneumolysin with hemolytic but not complement-activating activity is designated H+C−. The strain designated PLY− carries the pVA838 plasmid lacking the pneumolysin gene, so it does not produce pneumolysin. We have shown previously that these mutant strains have similar growth characteristics in vitro and that they retain their plasmids and the pneumolysin gene during at least 18 h of growth in the rat bloodstream (2).
Bacteria were grown to logarithmic phase in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) containing 5% heat-inactivated rabbit serum (GIBCO, Grand Island, N.Y.) and 10 μg of erythromycin (Abbott Labs, North Chicago, Ill.) per ml, as described previously (2). The bacteria were collected by centrifugation and washed twice with sterile PBS. The final bacterial suspensions were adjusted spectrophotometrically at 540 nm to produce the desired concentration, which was then confirmed retrospectively by colony counts, with the results expressed in CFU per milliliter. For the in vitro opsonization studies, log-phase cultures of a genetically unaltered type 3 S. pneumoniae (type strain ATCC 6303; American Type Culture Collection, Rockville, Md.) were used. These organisms were grown as described above, except that erythromycin was not added to the medium.
Induction of experimental bacteremia.
Cirrhotic and control rats were infected intravenously via their tail veins under light ether anesthesia with 0.2 ml of inoculum containing one of the mutant organisms. Blood was drawn 3 to 5 min later from a different site on the tail to confirm that each rat received approximately the same number of organisms per milliliter of blood. The range was 1 × 107 to 5 × 107 organisms/ml, with 94% of the rats receiving 1 × 107 to 4 × 107 organisms/ml. Additional blood samples were collected by cardiac puncture for quantitative culture at 18 h postinfection. Serial 10-fold dilutions of the blood were made in PBS and plated onto sheep blood agar (Remel, Lenexa, Kans.) plates to determine the number of CFU per milliliter of blood at each time point. The remainder of the blood was allowed to clot for exactly 1 h in an ice bath before the serum was withdrawn and frozen immediately in small aliquots at −80°C.
Serum CH50 assay.
Total hemolytic complement activity, as measured by a 50% hemolytic complement (CH50) assay, was measured in serum samples collected 2 days before infection, as well as 2 and 18 h postinfection, using a modification of a previously published method (23). All steps except for the incubations described below were performed in an ice bath. The erythrocyte-antibody suspension was first prepared by mixing an equal volume of an appropriately titrated hemolysin solution with standardized sheep red blood cells. The mixture was incubated in a 37°C water bath for 15 min and then stored at 4°C until used in the assay.
Six serum dilutions were utilized for each serum sample tested. Veronal-buffered saline containing Ca+2 and Mg+2, erythrocyte-antibody, and various serum dilutions were added sequentially (cirrhotic serum dilution range, 1/10 to 1/150; control serum dilution range, 1/150 to 1/200). All samples were incubated for 2 h at 21°C with mild agitation. They then were centrifuged at 300 × g for 10 min at 4°C to pellet any remaining red cells. The absorbance of the supernatant from each tube was determined at 541 nm, and the CH50 value for each sample was calculated as described previously (25).
Quantitation of serum C3 levels by immunofixation.
Levels of complement C3 in sera collected from rats 2 days before infection and at 2 and 18 h postinfection were quantified by immunofixation. Serum samples were thawed on ice and diluted 1/10 in a sterile 0.85% saline solution. Two microliters of each diluted sample was loaded onto an agarose gel (Helena Laboratories, Beaumont, Tex.), which was electrophoresed according to the manufacturer's directions, except that the electrophoresis was performed at 4°C. Control sera (one sample pooled from normal rats and one sample pooled from rats treated with complement-depleting cobra venom factor [Sigma]) also were included on each gel. The gels were electrophoresed at 120 V for 1 h before being treated for 1 h with goat anti-rat C3 polyclonal antiserum (ICN Biomedical Research Products, Costa Mesa, Calif.) diluted with 1/2 volume of saline. The gels then were washed, pressed with a saline-soaked filter paper to remove unprecipitated proteins, dried, and stained with acid-blue stain (Helena Laboratories). Finally, the gels were rinsed and dried and the density of the C3 bands was determined with a densitometer (Molecular Dynamics, Sunnyvale, Calif.). Results are expressed in arbitrary units and were standardized according to the value obtained on that day for the normal control serum in order to control for minor day-to-day variations.
In vitro phagocytosis assay.
An in vitro phagocytosis assay was performed as described previously (8) to quantify the opsonic activities of serum samples collected from cirrhotic and control rats 18 h after they were infected with one of the isogenic mutant strains. Briefly, a logarithmic-phase culture of type 3 S. pneumoniae (ATCC 6303) was fluorochrome labeled by incubation with 1 mg of fluorescein isothiocyanate (Sigma) per ml in PBS for 1 h at 4°C. The washed culture was suspended in Hank's balanced salts solution containing 0.1% gelatin (GHBSS Bio-Rad Laboratories, Richmond, Calif.) at a concentration of 4.0 × 108 CFU/ml. One hundred-microliter aliquots of the bacterial suspension were incubated for 30 min in a 37°C shaking water bath with 300 μl of GHBSS and 100-μl samples of serum from individual rats to allow preopsonization of the organisms. Two hundred microliters of a suspension containing 1 × 106 human neutrophils isolated as described previously (8) was then added to each tube. The tubes were incubated for 15 min at 37°C with gentle, end-over-end rotation, and the reaction was stopped by the addition of 2.0 ml of cold (4°C) PBS. Unassociated organisms were removed by three cycles of differential centrifugation, and the final neutrophil pellet was suspended in 1.0 ml of fixative (1% paraformaldehyde in 0.85% saline) for analysis of fluorescence using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.) Results shown herein are the percent positive cells (percentage of cells ingesting bacteria); the mean fluorescence of ingesting cells (FL1), indicating the relative number of bacteria taken up per cell); and a phagocytic index (PI [calculated as the arithmetic product of the two previously mentioned parameters]), indicating total phagocytic capacity.
Statistical analysis.
Because the data were not normally distributed with equal variances, even after log transformation, nonparametric statistics were used for all calculations. These were performed with the SYSTAT 8.0 statistical package. The Mann-Whitney U test was used to determine significance for differences between cirrhotic and control rats, as well as between changes in complement levels before and 18 h after infection. The Kruskal-Wallis test was used to determine significant differences in results obtained with the three mutant strains. Spearman's rank order correlation coefficient (rs) was employed to analyze relationships between variables.
RESULTS
Bloodstream clearance studies.
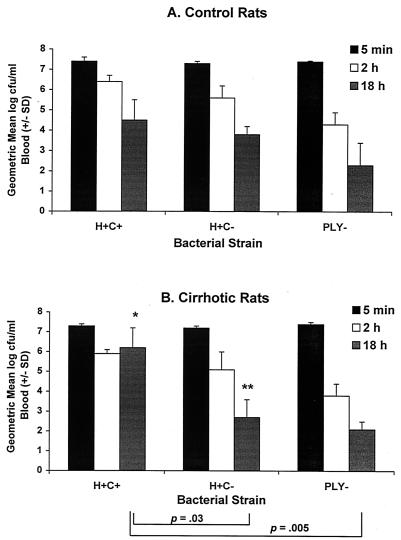
All rats (n = 10 or 13 for the H+C+ strain and 6 for the H+C− and PLY− strains) were infected intravenously with the respective bacterial strains to a concentration of 1 × 107 to 5 × 107 CFU/ml of peripheral blood. Control rats cleared a mean of 2.9, 3.6, and 5.1 log units, respectively, of the H+C+, H+C−, and PLY−bacteria from their bloodstreams by 18 h postinfection (Fig. 1A). These values did not differ statistically among strains. Cirrhotic rats cleared 1.1, 4.5, and 5.3 log units of the organisms, respectively, from their bloodstreams, with the clearance for the H+C+ strain being significantly lower than that for either the H+C− strain or the PLY− strain, which did not differ statistically from one another (Fig. 1B). The cirrhotic rats also had significantly more organisms than did control rats in their bloodstreams 18 h after infection with the H+C+ strain (P = 0.008), whereas they had significantly fewer organisms than did control rats 18 h after infection with the H+C− strain (P = 0.02).
FIG. 1.
Clearance of mutant bacterial strains from the bloodstreams of control (A) and cirrhotic (B) rats. Control and cirrhotic rats (n = 10 and 13, respectively, for rats infected with the H+C+ strain and 6 for all other groups) were infected intravenously (i.v.) with one of the isogenic mutant strains at 1 × 107 to 5 × 107 CFU/ml of peripheral blood, as verified within the first 5 min postinfection. Plate counts also were performed on blood collected at 2 and 18 h postinfection to determine clearance. Data presented are means ± standard deviations (SD [error bars]). Significant differences in clearance of the various strains by cirrhotic rats, as determined by the Kruskal-Wallace test, are indicated by brackets. ∗, value is significantly higher for cirrhotic rats than for control rats (P = 0.008); ∗∗, value is significantly lower for cirrhotic rats than for control rats (P = 0.02), as determined by the Mann-Whitney U test.
Serum complement levels and bloodstream clearance.
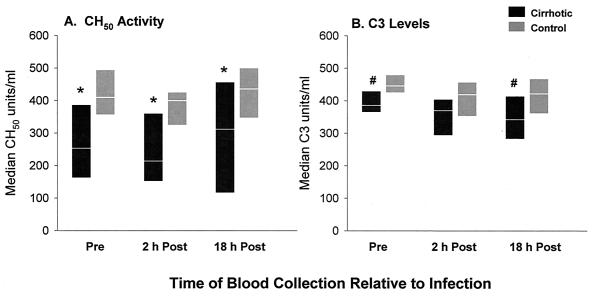
Total CH50 activity and C3 levels were measured in sera from the same rats used in the clearance studies. Sera were collected 2 days before infection and again at 2 and 18 h postinfection. Cirrhotic rats, examined as a group without regard for the infecting bacterial strain, had significantly lower serum CH50 activity levels than did control rats at all three time points (P range, 0.04 to 0.0001 [Fig. 2A]). C3 levels also were significantly lower for cirrhotic rats than for controls before infection and at 18 h postinfection (P = 0.02 and 0.006, respectively [Fig. 2B]). The sera from these animals had to be pooled for these comparisons in order to reach statistical significance, although the trend was evident for rats in each of the infection groups (see Fig. 3).
FIG. 2.
Comparison of serum CH50 activity levels (A) and serum C3 levels (B) in cirrhotic and control rats. All rats (n = 21 or 25/time point) were infected i.v. with one of the isogenic mutant strains at 1 × 107 to 5 × 107 CFU/ml of their peripheral blood. Data shown are medians, with the range from the 25th to the 75th percentile shown for each time point. ∗, value significantly lower in cirrhotic versus control rats (P = 0.0001, 0.001, and 0.04 before infection, 2 h postinfection, and 18 h postinfection, respectively). #, value significantly lower in cirrhotic versus control rats (P = 0.02 and 0.006 before infection and 18 h postinfection, respectively).
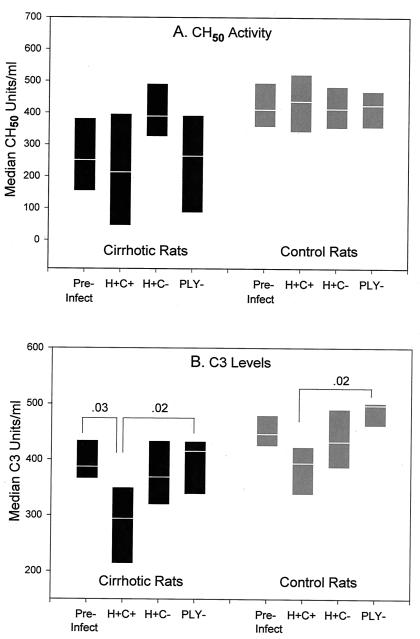
FIG. 3.
Comparative alterations in CH50 activity levels (A) and C3 levels (B) during infection with isogenic mutant strains. All rats were bled 2 days before infection (pre-infect) and again 18 h postinfection with 1 × 107 to 5 × 107 CFU of one of the isogenic mutant strains per ml of peripheral blood. Postinfection groups are labeled with the organism strain designation (n = 9 cirrhotic and 13 control animals for the H+C+ strain and 6 each for the H+C− and PLY− strains). Serum CH50 activity levels and C3 levels shown are medians, with the range from the 25th to the 75th percentile indicated. Significant differences (indicated by bars) between pre- and postinfection values were calculated by the Mann-Whitney U test. Differences among 18-h values for rats in the same treatment group infected with different strains were calculated by the Kruskal-Wallis test.
There were no statistically significant relationships established between preinfection serum CH50 activity or C3 levels and 18-h-postinfection bacteremia counts of all the mutant strains considered together (rs = −0.13 and −0.11, respectively). This also was true when cirrhotic and control rats infected with all mutant strains were analyzed separately (rs range, −0.03 to −0.37) or when rats from either treatment group infected with each bacterial strain were analyzed individually (rs range, −0.24 to 0.22). In addition, there were no statistically significant correlations between 18-h-postinfection serum CH50 or C3 levels and 18-h-postinfection bacteremia levels of any of the three bacterial strains considered separately in control rats (rs = −0.66 to 0.49). Neither did the 18-h-postinfection CH50 and C3 levels correlate with the number of H+C− or PLY− bacteria in the bloodstreams of cirrhotic rats at 18 h postinfection (rs range, −0.43 to 0.03). However, for cirrhotic rats infected with the H+C+ strain, the bacteremia levels at 18 h postinfection correlated significantly with both CH50 activity (rs = −0.75) and C3 (rs = −0.71) levels in the rats' sera 18 h after infection (P < 0.02 for both).
Effect of pneumolysin on serum complement levels.
The mean serum CH50 activity level remained relatively stable or even nonsignificantly increased for cirrhotic and control rats during the 18 h of infection with each of the pneumococcal strains (Fig. 3A). Mean C3 levels decreased significantly for cirrhotic rats infected with the H+C+ strain (P = 0.03 [Fig. 3B]). There also appeared to be a slight reduction in mean C3 levels for control rats, but the difference did not reach statistical significance (P = 0.1). Serum C3 levels were not reduced significantly in either cirrhotic or control rats infected with the H+C− or PLY− strains. Eighteen hours after infection, both cirrhotic and control rats infected with the H+C+ strain had significantly lower mean serum C3 levels than the rats in their respective treatment group infected with the PLY− strain (p = 0.02 for both). Rats in both treatment groups infected with the H+C− strain had intermediate C3 levels.
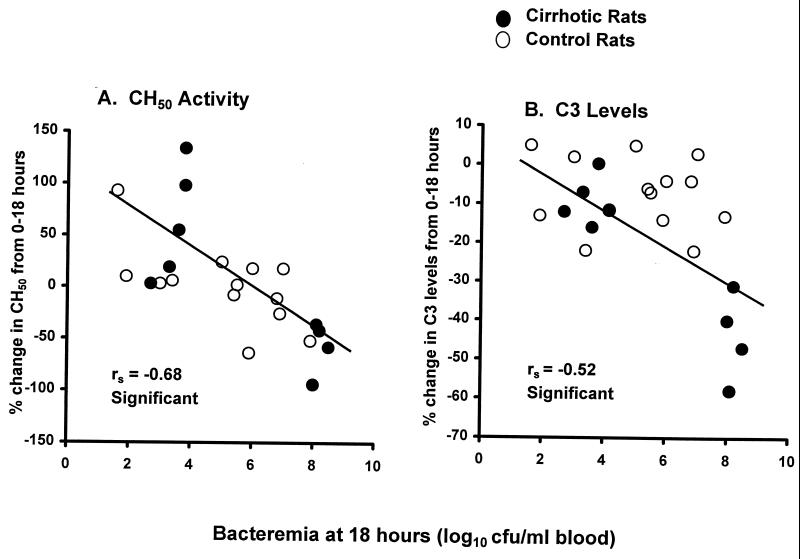
Spearman's rank order coefficients also were calculated for the 18-h-postinfection bacteremia levels of each strain and the percent change in complement values during infection. For rats infected with the H+C+ strain, there was a significant negative correlation between both the percent change in CH50 activity levels and the percent change in C3 levels and the number of organisms in the bloodstream of individual rats (rs = −0.68 for CH50 and −0.52 for C3 levels [Fig. 4]). There were no significant correlations, however, between the percent changes in CH50 activity or C3 levels and the numbers of H+C− or PLY− bacteria in the rats' bloodstreams (data not shown).
FIG. 4.
Correlation between bacteremia counts at 18 h postinfection and the percent change in serum CH50 activity levels (A) and C3 levels (B) of rats infected with the H+C+ strain. Each graph includes values for 9 cirrhotic and 13 control rats infected with 1 × 107 to 5 × 107 CFU of the H+C+ strain per ml of blood. Correlations between variables were determined by calculation of Spearman's rank order coefficient (rs).
In vitro opsonophagocytosis assay.
When sera from rats infected with the H+C+ strain were used in the opsonophagocytosis assay, all three uptake parameters for neutrophils incubated with S. pneumoniae preopsonized with sera from cirrhotic rats were significantly lower than those for neutrophils incubated with sera from control rats (P was from 0.02 to 0.03 [Table 1]). By contrast, none of the uptake parameters differed significantly when uptake for neutrophils incubated with sera from cirrhotic rats was compared with that for neutrophils incubated with sera from control rats infected with the H+C− or PLY− strain.
TABLE 1.
Phagocytosis of type 3 S. pneumoniae in vitro by human neutrophilsa
| Strain | Rat group | % Positive cells | FL1 | PI |
|---|---|---|---|---|
| H+C+ | Cirrhotic | 45 (36–55)b | 505 (480–518)b | 22,770 (16,848–28,490)b |
| Control | 66 (59–70) | 527 (502–542) | 35,836 (30,385–37,240) | |
| H+C− | Cirrhotic | 53 (42–60) | 510 (493–530) | 26,863 (21,168–32,160) |
| Control | 61 (51–60) | 504 (490–528) | 30,594 (25,245–35,035) | |
| PLY− | Cirrhotic | 61 (58–62)c | 545 (533–547)c | 33,213 (30,914–33,914)c |
| Control | 69 (54–73) | 529 (498–533) | 36,883 (26,676–38,909) |
Values are medians of results for 10 or 13 animals/group for the H+C+ strain and 6 animals/group for the remaining strains. Values in parentheses are ranges (25th to 75th percentile).
Uptake parameter was significantly lower when organisms were opsonized with serum from cirrhotic versus control rats infected with same organism (P = 0.002, 0.03, and 0.002 for percent positive, FL1, and PI, respectively, as determined by Mann-Whitney U test).
Uptake parameter was significantly higher when organisms were opsonized with serum from cirrhotic rats infected with the PLY− strain versus serum from cirrhotic rats infected with the H+C+ strain or those infected with the H+C− strain (P = 0.05, 0.007, and 0.05 for percent positive, FL1, and PI, respectively, as determined by the Kruskal-Wallis test).
Uptake also was compared for neutrophils incubated with sera from rats within the cirrhotic or control groups infected with different bacterial strains. The neutrophils took up significantly more type 3 S. pneumoniae when the organisms were preopsonized with sera from cirrhotic rats infected with the PLY− strain than they did when the organisms were preopsonized with sera from cirrhotic rats infected with either the H+C+ or the H+C− strain (P = 0.05, 0.007, and 0.05 for percent positive, FL1, and PI, respectively [Table 1]). There were no significant differences in the uptake parameters for organisms preopsonized with sera from control rats infected with the different bacterial strains (P > 0.3 for each parameter).
DISCUSSION
Cirrhosis has been documented as an important predisposing factor for pneumococcal bacteremia, setting the stage for a more rapid disease course and increased mortality (18, 19). Hypocomplementemic cirrhotic patients with depressed C3 levels were shown to have a particularly high incidence of infection (10). Patients with bacteremic pneumococcal infections who had very low serum C3 and/or C4 levels also were more likely to die from their infections than patients with normal complement levels (7). Increased susceptibility to pneumococcal disease due to depressed levels of complement also has been demonstrated in animal models. Guinea pigs and mice with levels of complement depleted by injection of cobra venom factor exhibited decreased pneumococcal bloodstream clearance and higher fatality rates than animals who had not received cobra venom factor injections (4, 5, 30). Preopsonization of the pneumococci with nonimmune serum containing complement reversed this effect for guinea pigs, at least early during infection (11). Cobra venom factor treatment of rabbits likewise increased their susceptibility to a normally nonvirulent type 25 pneumococcal strain (9).
In an earlier study (2), workers from our laboratory reported that pneumolysin's complement-activating activity contributed inordinately to the virulence of S. pneumoniae in hypocomplementemic cirrhotic rats. Pneumolysin has been shown to activate the classical complement pathway in vitro (22). Continuous activation of this pathway during S. pneumoniae infection is thought to consume complement at a distance from the surface of intact organisms, reducing available opsonins necessary for their uptake and killing by phagocytes. We therefore hypothesized that the complement-activating activity of pneumolysin could deplete complement components to critically low levels during infection of the cirrhotic host, helping to explain their increased susceptibility to fatal pneumococcal bacteremia. To test this hypothesis, we monitored the CH50 activity levels, C3 levels, and opsonophagocytic activity levels of sera from cirrhotic and control rats infected intravenously with S. pneumoniae producing wild-type pneumolysin, pneumolysin without complement-activating activity, or no pneumolysin.
Consistent with the results of our previous study (2), control rats cleared nearly as many of the H+C+ bacteria as they did H+C− bacteria from their bloodstreams during the first 18 h of infection. This supports the 1997 study by Benton et al., who reported that abolishing the complement-activating activity of pneumolysin did not have a major effect on virulence of type 2 pneumococci in two strains of mice with intact complement systems (3). Also consistent with the results of our previous study, cirrhotic rats cleared the H+C− and PLY− bacteria from their blood at least as well as control rats. By contrast, cirrhotic rats cleared significantly fewer of the H+C+ bacteria than did control rats. Cirrhotic rats also cleared significantly fewer of the H+C+ organisms than of either the H+C− or PLY− organisms. These results support the hypothesis that complement activation by pneumolysin contributes to the high virulence of pneumococci in the cirrhotic host. Most studies on pneumococcal infections during complement deficiency have utilized cobra venom factor-treated animals that had much lower levels of complement activity than the majority of our cirrhotic rats. Our study suggests that even partial reduction in complement availability is detrimental to host defense against pneumococcal infection.
Although the preinfection serum CH50 activity levels and C3 levels of cirrhotic rats were both significantly reduced compared to those of the controls, initial complement levels did not predict bloodstream clearance of any of the bacterial strains. This was somewhat suprising, given that C3 has been shown to play its major protective role within the first few hours of systemic pneumococcal infection (29). There also were no statistically significant correlations found for control rats between serum complement levels at 18 h postinfection and the numbers of any of the bacterial organisms remaining in their bloodstreams. This indicates that in the normal host complement levels are sufficient for control of relatively large numbers of pneumococci producing pneumolysin with complement-activating activity. By contrast, at 18 h postinfection, both the serum CH50 activity levels and the C3 levels of cirrhotic rats correlated with bacteremia levels of the H+C+ strain but not the H+C− or the PLY− strain. This underscores the importance of complement activation by pneumolysin during infection in the cirrhotic host with diminished complement synthetic capacity. However, the relationship between complement levels and extent of bacteremia is complex, making it unclear whether complement levels fell due to the increase in number of bacteria producing pneumolysin or, alternatively, the net bacterial growth rate increased due to the decline in available complement.
To determine whether pneumolysin's complement-activating property significantly reduced the rats' serum complement levels during infection, comparisons were made between CH50 activity levels and C3 levels before and after infection with each of the bacterial strains. The CH50 activity level was not reduced significantly during infection with any of the strains. However, levels of C3, the principal mediator of pneumococcal clearance in the nonimmune host (12), were significantly reduced, but only in cirrhotic rats during infection with the H+C+ strain. In addition, C3 levels were significantly lower in both cirrhotic and control rats when they were infected with the H+C+ compared to the PLY− strain. This demonstrates that pneumolysin does reduce complement levels within the host during infection. The severity of the reduction was greater in cirrhotic rats than in control rats. This may be due to their lower initial complement levels, exacerbated during infection, perhaps, by the reduced complement synthetic capacity within their diseased livers.
To further elucidate the effect of complement activation by pneumolysin on reduction of complement levels during infection, correlations were drawn between the 18-h-postinfection bloodstream levels of each bacterial strain and the percent change in complement levels of individual rats during the same 18-h period. To maximize the number of samples for these calculations, cirrhotic and control rats infected with the same strain were considered as a group. Again, bacterial numbers in the blood correlated significantly with decreases in CH50 activity levels and C3 levels only when the rats were infected with the H+C+ strain. These results suggest that the complement-activating activity of pneumolysin depresses host complement levels during infection, reducing available opsonins necessary for efficient phagocytosis and removal of the organisms.
To quantify the detrimental effect of pneumolysin-induced complement depletion on host phagocytic defense, the opsonic capacity of sera collected 18 h after infection was measured. Sera from cirrhotic rats infected with the H+C+ strain had significantly depressed opsonic capacity in comparison to sera from H+C+-infected control rats. Although the critical level of C3 needed for effective opsonization in this assay has not been determined, these results are consistent with the significantly lower serum C3 levels in cirrhotic versus control rats infected with the H+C+ strain. Sera from cirrhotic rats infected with the H+C− strain had intermediate opsonic capacity, which also was significantly lower than that for sera from cirrhotic rats infected with the PLY− strain. However, its biological significance is in question because the cirrhotic rats cleared the H+C− strain from their bloodstreams as well as they did the PLY− strain. It also is possible that pneumolysin has an additional detrimental effect on serum opsonic capacity aside from its complement-activating activity. Alternatively, cirrhosis may be associated with other immune defects that compound the effects of their hypocomplementemia.
The opsonophagocytosis assay was not performed with sera collected before infection, but we have shown previously that there is no difference in opsonizing capacity between sera from uninfected cirrhotic rats and those from control rats (8). Therefore, differences in the opsonizing capacity of cirrhotic versus control rat serum became apparent only after growth of the pneumococci and subsequent release of pneumolysin.
In conclusion, these studies suggest that pneumolysin's complement-activating activity exerts only minimal influence on host defense in a host with an intact complement-generating system. However, complement activation by pneumolysin may be particularly detrimental in the hypocomplementemic cirrhotic host by reducing inherently low complement stores to a level that prevents effective phagocytosis and bloodstream clearance.
ACKNOWLEDGMENTS
We thank Mary Snitily, Mei Yue, Kristina Haase, and Jill Gorny for technical assistance.
These studies were conducted with the support of Merit Review funds (awarded to L. C. Preheim and M. J. Gentry-Nielsen) from the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Adams H G, Jordan C. Infections in the alcoholic. Med Clin N Am. 1984;68:179–200. doi: 10.1016/s0025-7125(16)31249-4. [DOI] [PubMed] [Google Scholar]
- 2.Alcantara R B, Preheim L C, Gentry M J. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect Immun. 1999;67:2862–2866. doi: 10.1128/iai.67.6.2862-2866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton K A, Paton J C, Briles D E. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 4.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown E J, Hosea S W, Frank M M. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis. 1983;5:S797–S805. doi: 10.1093/clinids/5.supplement_4.s797. [DOI] [PubMed] [Google Scholar]
- 6.Bruyn G A W, Zegers B J M, van Furth R. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin Infect Dis. 1992;14:251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 7.Dee T H, Schiffman G, Sottile M I, Rytel M W. Immunological studies in pneumococcal disease. J Lab Clin Med. 1977;89:1198–1207. [PubMed] [Google Scholar]
- 8.Gentry M J, Snitiliy M U, Prehiem L C. Phagocytosis of Streptococcus pneumoniae measures in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J Infect Dis. 1995;171:350–355. doi: 10.1093/infdis/171.2.350. [DOI] [PubMed] [Google Scholar]
- 9.Guckian J C, Christensen G D, Fine D P. The role of opsonins in recovery from experimental pneumococcal pneumonia. J Infect Dis. 1980;142:175–190. doi: 10.1093/infdis/142.2.175. [DOI] [PubMed] [Google Scholar]
- 10.Homann C, Varming K, Hogasen K, Mollnes T E, Graudal N, Thomsen A C, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544–549. doi: 10.1136/gut.40.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosea S W, Brown E J, Frank M M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980;142:903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- 12.Hostetter M K. Opsonic and nonopsonic interactions of C3 with Streptococcus pneumoniae. Microb Drug Resist. 1999;5:85–89. doi: 10.1089/mdr.1999.5.85. [DOI] [PubMed] [Google Scholar]
- 13.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janoff E N, Rubins J B. Invasive pneumococcal disease in the immunocompromised host. Microb Drug Resist. 1997;3:215–232. doi: 10.1089/mdr.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 15.Johnson M K, Boese-Marrazzo D, Pierce W A., Jr Effects of pneumolysin on human polymorphonuclear leukocytes and platelets. Infect Immun. 1981;34:171–176. doi: 10.1128/iai.34.1.171-176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson M K, Hobden J A, O'Callaghan R J, Hill J M. Confirmation of the role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1992;12:1221–1225. doi: 10.3109/02713689208999547. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M K, Callegan M C, Engel L S, O'Callaghan R J, Hill J M, Hobden J A, Bolnois G J, Andrew P W, Mitchell T J. Growth and virulence of a complement-activation-negative mutant of Streptococcus pneumoniae in the rabbit cornea. Curr Eye Res. 1995;14:281–284. doi: 10.3109/02713689509033527. [DOI] [PubMed] [Google Scholar]
- 18.Kramer M R, Rudensky B, Hadas-Halperin J, Isacsohn M, Melzer E. Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature. Isr J Med Sci. 1987;23:174–180. [PubMed] [Google Scholar]
- 19.Kuikka A, Syrjanen J, Renkonen O-V, Valtonen V V. Pneumococcal bactereamia during a recent decade. J Infect. 1992;24:157–168. doi: 10.1016/0163-4453(92)92850-i. [DOI] [PubMed] [Google Scholar]
- 20.Mellencamp M A, Preheim L C. Pneumococcal pneumonia in a rat model of cirrhosis: effects of cirrhosis on pulmonary defense mechanisms against Streptococcus pneumoniae. J Infect Dis. 1991;163:102–108. doi: 10.1093/infdis/163.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell T J, Andrew P W. Biological properties of pneumolysin. Microb Drug Resist. 1997;3:19–26. doi: 10.1089/mdr.1997.3.19. [DOI] [PubMed] [Google Scholar]
- 22.Paton J C, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock J E, Tomar R H. Manual of laboratory immunology. Philadelphia, Pa: Lea and Febiger; 1980. pp. 207–213. [Google Scholar]
- 24.Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- 25.Rubins J B, Janoff E N. Pneumolysin: a multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 26.Rubins J B, Duane P G, Clawson D, Charboneau D, Young J, Niewoehner D E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993;61:1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubins J B, Mitchell T J, Andrew P W, Niewoehner D E. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect Immun. 1994;62:3829–3836. doi: 10.1128/iai.62.9.3829-3836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith F E, Palmer D L. Alcoholism, infection and altered host defenses: a review of clinical and experimental observations. J Chron Dis. 1976;29:35–49. doi: 10.1016/0021-9681(76)90066-7. [DOI] [PubMed] [Google Scholar]
- 29.Winkelstein J A. Complement and the host's defense against the pneumococcus. Crit Rev Microbiol. 1984;11:187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]
- 30.Winkelstein J A, Smith M R, Shin H S. The role of C3 as an opsonin in the early stages of infection. Proc Soc Exp Biol Med. 1975;149:397–401. doi: 10.3181/00379727-149-38815. [DOI] [PubMed] [Google Scholar]
- 31.Wyke R J. Bacterial infections complicating liver disease. Bailliere's Clin Gastroenterol. 1989;3:187–210. doi: 10.1016/0950-3528(89)90052-3. [DOI] [PubMed] [Google Scholar]