Abstract
Background
Past studies suggested that ginseng extracts and ginseng-derived molecules exerted significant regulatory effects on skin. However, no reports have described the effects of ginseng-derived nanoparticles (GDNPs) on skin cell proliferation and wound healing. In this study, we investigated whether GDNPs regulate the proliferation of skin cells and promote wound healing in a mouse model.
Methods
GDNPs were separated and purified via differential centrifugation and sucrose/D2O gradient ultracentrifugation. GDNP uptake, cell proliferation and cell cycle progression were measured by confocal microscopy, CCK-8 assay and flow cytometry, respectively. Cell migration and angiogenic effects were assessed by the wound scratch assay and tube formation assay, respectively. ELISA was used to detect extracellular matrix secretion. The relevant signaling pathway was confirmed by western blotting. The effects of GDNPs on skin wound healing were assessed by wound observation, HE staining, and western blotting.
Results
GDNPs possessed the essential features of exosomes, and they were accumulated by skin cells. Treatment with GDNPs notably enhanced the proliferation of HaCaT, BJ and HUVECs. GDNPs also enhanced the migration in HaCaT cells and HUVECs and angiogenesis in HUVECs. GDNPs increased the secretion of MMP-1, fibronectin-1, elastin-1, and COL1A1 in all three cell lines. GDNPs regulated cell proliferation through the ERK and AKT/ mTOR pathways. Furthermore, GDNPs facilitated skin wound healing and decreased inflammation in a mouse skin wound model.
Conclusion
GDNPs can promote skin wound healing through the ERK and AKT/mTOR pathways. GDNPs thus represent an alternative treatment for chronic skin wounds.
Keywords: cell cycle, cell proliferation, ginseng-derived nanoparticles, migration, wound healing
Graphical abstract
Abbreviations
- GDNPs
ginseng-derived nanoparticles
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- COL1A1
collagen type 1α
- mTOR
mechanistic target of rapamycin
- ERK
extracellular signal-regulated kinase
- S6K
P70S6K
- FBS
fetal bovine serum
1. Introduction
Among different vesicles secreted by mammalian cells, exosomes are defined as a series of small membranous nanovesicles 30–100 nm in diameter [1,2]. Exosomes have a lipid bilayer as well as protein and cytoplasmic components including mRNA and miRNA. Exosomes have important effects on cellular communication, and they transport bioactive cargo between cells [3]. Through changes in lipid content, as well as gene and protein expression in target cells, exosomes participate in various physiological and disease processes including stem cell function and tumor metastasis [[4], [5], [6], [7]]. Additionally, exosome-like nanoparticles from other species can provide new molecules and reprogram recipient cells, especially mammalian cells [8]. Recently, increasing numbers of plant exosome-like nanoparticles have been isolated and characterized. These nanoparticles have similar structures and morphologies as mammalian exosomes [9,10]. Meanwhile, increasing evidence indicates that plant-derived nanoparticles could be absorbed by the gastrointestinal tract, permitting them to mediate plant–mammalian cell communication. Broccoli-derived nanoparticles relieved colitis by stimulating DC cell AMP-activated protein kinase in mice [11]. Nanoparticles from grapes induced intestinal stem cells and relieved colitis in mice [12]. Nanoparticles from ginger protected mice from alcohol-induced liver injury through Nrf2 pathway [13]. Nanoparticles from grapes, ginger, grapefruit, and carrot, exert significant effects on anti-inflammatory pathways and maintain intestinal homeostasis [14]. Ginger-derived exoxomal microRNAs shaped the gastrointestinal microbiota in mice and humans [15]. Nanoparticles from ginseng changed macrophage polarization to inhibit tumor growth [16]. Wheat-derived nanovesicles promoted wound healing in vitro [17]. Furthermore, natural nontoxic nanovectors from plant nanoparticle-derived lipids have been considered a viable alternative modality for drug delivery [18,19].
As the largest organ, skin tissue possesses a variety of functions such as body thermoregulation, tactile sense, pain, pressure, and protective barrier effects against mechanical stress and pathogens [20]. Many conditions can lead to skin damage including surgery, trauma, burns, and diabetic complications [21]. Skin wound healing is a continuous and complex tissue regeneration process coordinated by many types of cells in the dermis and epidermis to rebuild the barrier [22]. This process involves a series of coordinated phases such as proliferation, inflammation, and remodeling [23]. After damage, keratinocytes move from the basal layer to the wound site, and then cells multiply to repair the epidermal layer. Meanwhile, fibroblasts migrate to the wound area and produce extracellular matrix (ECM), cytokines, and growth factors to rebuild the dermal layer [24]. Meanwhile, there are many signaling pathway involve in skin cells proliferation and repair. mTOR is a highly conserved protein kinase in mammals and a key central controller of cell metabolism and growth. The mTOR signaling pathway regulates various physiological functions including transcription, mRNA translation, cell cycle progression, autophagy, apoptosis, differentiation, motility, and metabolism. AKT can directly phosphorylate mTOR, which then phosphorylates S6K, a downstream substrate of mTOR that is crucial for cell growth. ERK1 and ERK2 are known to be critical for cell proliferation [25]. Although there are natural responses against skin damage, the processes of dermal restoration and epithelialization may be insufficient in severe pathological conditions. Thus, there is a need for alternative therapeutic strategies.
Panax ginseng Meyer has been used in eastern Asia for thousands of years. Ginseng possesses numerous benefits for health. Investigations uncovered the outstanding function of ginseng in diminishing the incidence rates of diabetes, hyperlipidemia, hypertension, tumors, irritation, and other psychological irregularities [26]. Past studies suggested that ginseng extracts and ginseng-derived molecules exerted significant regulatory effects on skin. Ginseng extracts attenuated ultraviolet-mediated inflammasome activation in keratinocytes [27]. Ginsenoside Rb1 enhanced keratinocyte migration and stimulated the cutaneous wound-healing process [28]. Extract from ginseng promoted dermal fibroblast proliferation and collagen synthesis [29]. Extracellular vesicles from ginseng facilitated anti-senescence activity in skin cells [30]. However, no reports have described the effects of ginseng-derived nanoparticles (GDNPs) on skin cell proliferation and wound healing. Here, we explored the effects and mechanisms of GDNPs in the proliferation and migration of keratinocytes, fibroblasts, and endothelial cells. We also investigated the effects of GDNPs on skin wound healing in mice. The results revealed that GDNPs activated the extracellular signal-regulated kinase (ERK) and AKT/mechanistic target of rapamycin (mTOR)/P70S6K (S6K) pathways, resulting in enhanced cell proliferation and wound healing. Meanwhile, GDNPs promoted skin wound healing and decreased inflammation
2. Materials and methods
2.1. GDNP isolation and purification
To isolate and purify nanoparticles from ginseng, ginseng was washed with water at room temperature to remove dirt. Then, the samples were cut vertically and slowly squeezed in PBS by a juice squeezer. The juice was mixed with a protease inhibitor cocktail and centrifuged at 400 × g for 20 min, 800 × g for 20 min, and 15,000 × g for 20 min to remove large particles. Then, the supernatant was ultra-centrifuged at 100,000 × g for 60 min, and the pellet was re-suspended in 20 mM Tris-HCl, subjected to a sucrose gradient (1 M sucrose/D2O and 2 M sucrose/D2O), and centrifuged at 150,000 × g for 180 min. The band between the 1-M and 2-M layers was harvested. After removing the sucrose, the pellet was re-suspended in 20 mM Tris-HCl. The protein concentration of GDNPs was analyzed using a BCA protein assay kit.
2.2. Characterization of GDNPs
GDNPs were prepared and observed using a transmission electron microscope (H-7650, Hitachi, Japan). The zeta potential and hydrodynamic size of the GDNPs were measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK).
2.3. In vitro and in vivo GDNP uptake assays
HaCaT cells, HUVECs, and BJ cells (1 × 105 cells/well) were cultured overnight in 12-well culture plates. GDNPs were labeled with DIO green fluorescent dye and co-cultured with the cells for 24 h. DIO was a lipophilic green fluorescent dye that usually be used to stain cell membranes and other lipid soluble biological structures. If the stained exosome were taken up by target cells, the cells will show green fluorescence under the fluorescence microscope [31]. DAPI was used to stain nuclei. Images were obtained using a Nikon confocal microscope.
ICR mice (6 weeks old) were purchased from Yisi Experimental Animal Technology Co, Ltd. (China, Changchun). The animals were anesthetized and shaved the back. Subsequently, 200 μL of Tris-HCl (negative control) or PKH26-labeled GDNPs were intradermally injected into the back skin using a syringe. PKH26 was a lipophilic red fluorescent dye and be used to label living cells and lipid soluble biological structures by binding to lipid molecules in membrane structures. PKH26 exhibited strong and stable fluorescence. It can be observed for up to several weeks in vivo [32]. Next day, skin tissues were harvested, frozen, and cryosectioned at a thickness of 10 μm. After staining with 10 μg/mL DAPI, the samples were observed and photographed using a fluorescence microscope (Olympus BX-53, Tokyo, Japan).
2.4. Cell cycle
After treatment by GDNPs for 24 h, HaCaT cells, HUVECs, and BJ cells were fixed with chilled 70% ethanol and stored at 4°C. Next day, the cells were stained with PI for 30 min before flow cytometry. Samples were analyzed by flow cytometry using FlowJo software.
2.5. Wound scratch assay
HUVECs and HaCaT cells (1 × 106 cells/well) were cultured in six-well plates. Next day, plates were scratched using a 1000-μL sterile pipette tip, and cell culture medium was replaced with fresh serum-free medium containing GDNPs. Cells were incubated for 48 h, and wound closure was observed and photographed using a microscope with a digital camera at 0, 24, and 48 h.
2.6. Tube formation assay
Plates (forty-eight–well) were coated with Matrigel® Basement Membrane Matrix, LDEV-free (Corning, Corning, NY, USA). Plates were incubated at 37°C for 1 h to allow polymerization. HUVECs (3 × 104 cells per well) were cultured in Matrigel Matrix-coated plates and treated with 20 μg/mL GDNPs. After 12 h, the tube-like structures were observed and photographed randomly.
2.7. Measurements of matrix metalloproteinase (MMP)-1, fibronectin-1, elastin-1, and collagen type 1α (COL1A1) secretion
HaCaT cells (1.2 × 105 cells/well), BJ cells (1.4 × 105 cells/well), and HUVECs (7 × 104 cells/well) were cultured in six-well plates. Next day, cell culture medium was replaced with fresh serum-free medium containing GDNPs and incubated for 48 h. Cell supernatant was collected, and then MMP-1, fibronectin-1, elastin-1, and COL1A1 concentrations were estimated by ELISA.
2.8. Western blotting
12%SDS-PAGE was used to separate cellular proteins under reducing conditions. The proteins were transferred to a PVDF membrane. Then, the membranes were incubated with specific primary (1:1000, overnight, 4°C) and secondary antibodies (1:1000, room temperature, 1 h). After washed with PBST, blots were developed with an Ultra High Sensitivity ECL system and were quantified by using ImageJ Software (v1.8.0).
2.9. Skin wound-healing assay
The skin wound model was constructed using 6-week-old mice. A round full-thickness skin excisional wound (4 mm in diameter) was created on the back of mice. The wound was covered with 20 μL of GDNPs (10 mg/mL), Tris-HCl, or a positive control (Kangfu Xinye, extracts of Periplaneta americana) every 24 h until the wound had healed. The wound closure rate of each group was compared. The mice were sacrificed, and wounded skin specimens were harvested on the 15th day after damage. The samples were placed in liquid nitrogen and lysed with lysis buffer for western blotting.
2.10. HE staining
The mice were sacrificed, and the complete wounded tissue was excised from the back. The paraffin-embedded tissue was subjected to HE staining using a standard procedure.
2.11. Statistical analysis
All data represent at least three independent experiments, and they are expressed as the mean ± SEM. Statistical comparisons were made by one-way analysis of variance (ANOVA) and Dunnett's post-hoc test.
3. Results
3.1. Isolation and identification of nanoparticles from ginseng
GDNPs were separated and purified from ginseng root juice by standard techniques (Fig. 1A). Isolated GDNPs were then examined according to their transmission electron microscopy findings (Fig. 1B), size distribution, and charge. The results indicated that the GDNPs were nanosized particles with a mean diameter of 215.2 nm (Fig. 1C). Zeta potential measurements illustrated that GDNPs have a negative zeta potential (mean, −34.7 mV, Fig. 1D). Due to ginsenosides was one of the important activity compositions in ginseng, we detected the ginsenosides contents of GDNPs by HPLC. As shown in Fig. S1 and Table 1, the GDNPs contains several kinds of ginsenoside such as Rg1, Re, Rh1, Rg2, Ro and Rd. The total content of ginsenoside was 15.786 μg/mg.
Fig. 1.
Identification and characterization of GDNPs. (A) Two bands were formed after sucrose/D2O gradient ultracentrifugation. (B) GDNPs from the 1-M/2-M interface were observed by electron microscopy. (C) The size distribution and (D) surface zeta potential of the particles were measured using a Zetasizer Nano ZS.
3.2. Uptake of GDNPs by skin cells in vivo and vitro
Next, we detected whether GDNPs could be accumulated by skin cells. Co-incubation of HaCaT cells, HUVECs, or BJ cells with DIO-labeled GDNPs demonstrated the uptake of GDNPs into all three cell lines (Fig. 2A). To investigate whether GDNPs could be absorbed into skin tissue in vivo, 3 mg of PKH26-labeled GDNPs were injected into mouse dorsal skin. After 24 h of treatment, we found that GDNPs were incorporated into cells from the dermal layers (Fig. 2B). These data suggested that GDNPs could be internalized into skin cells in vivo and vitro.
Fig. 2.
Uptake of GDNPs in skin cells and proliferation and cell cycle progression in skin cells treated with GDNPs. (A) GDNPs were stained with DIO and incubated with HaCaT cells, BJ cells, and HUVECs for 24 h. Nuclei were stained with DAPI before cells were analyzed. The scale bar was 100 μm. (B) Three milligrams of PKH26-labeled GDNPs were injected into the skin of back, and tissues were harvested after 24 h. Tris-HCl (20 mM) was used as negative control. Nuclei were stained with DAPI for counterstaining. Scale bars are 50 μm. (C) Cell proliferation analysis by the Cell Counting Kit-8 assay. After 48 h of GDNP treatment, the absorbance was detected at 450 nm. (D) After 24 h of GDNP treatment, the percentage of cells in each cell cycle phase was detected by flow cytometry.The results shown are a representative experiment of three independent experiments with similar results (∗p < 0.05,∗∗p < 0.01,∗∗∗p < 0.001 vs control).
3.3. Assessment of the role of GDNPs in cell proliferation and cell cycle progression
To study the effect of GDNPs on skin cells proliferation, HaCaT cells, HUVECs, and BJ cells were used to determine the proliferative ability of GDNPs. Firstly, the cytotoxic experiments were operated to confirm the optimum concentration of GDNPs. The toxic effect of GDNPs on keratinocytes, endothelial cells, and fibroblasts was measured by co-incubating the cells with different concentrations of GDNPs. As shown inFig. S2, GDNPs exhibited a negative effect on the cell growth at concentrates above 80 μg/mL. However, GDNPs induced a noteworthy increase in proliferation in all three cell lines at the concentrates of 5, 10 and 20 μg/mL (Fig. 2C and S2). Based on the results, we assessed the cell cycle to confirm the proliferative effect of GDNPs. The data revealed that treatment with GDNPs remarkably enhanced the number of cells in S phase compared with that in control-treated cells (Fig. 2D). The CCK-8 assay and cell cycle analysis revealed that treatment with GDNPs increased the proliferation of skin cells.
3.4. Evaluation of the role of GDNPs in cell migration
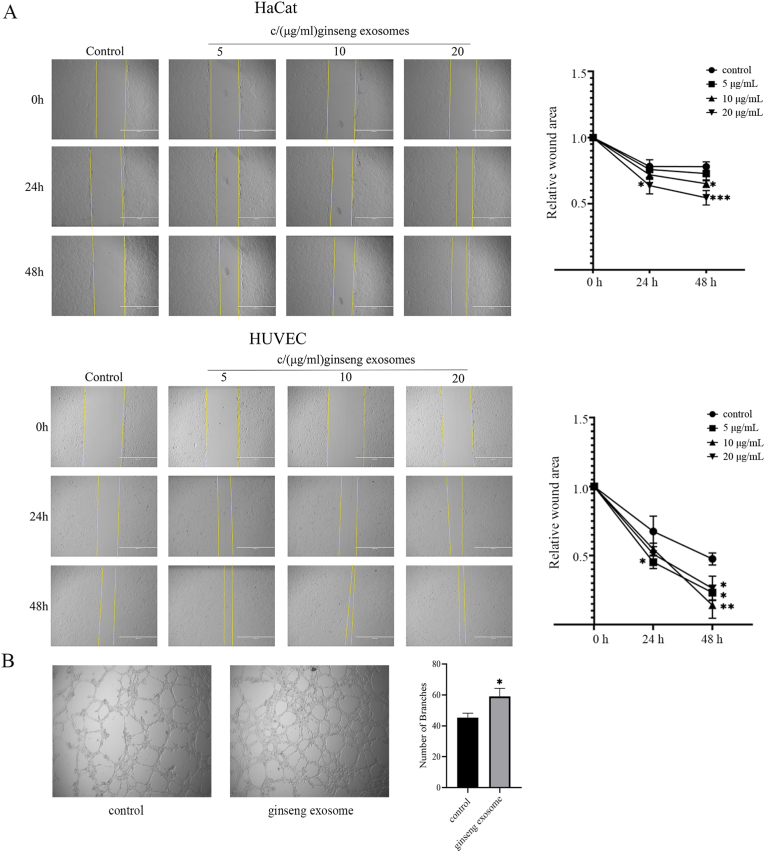
Scratch assay was used to confirm the role of GDNPs on cell migration. As presented in Fig. 3A, treatment with GDNPs at different concentrations remarkably reduced the scratch area in HaCaT cells and HUVECs after 24 and 48 h compared with the control findings. Meanwhile, the effect of GDNPs on cell migration was better in HUVECs than in HaCaT cells.
Fig. 3.
Wound scratch assay and tube formation assay in HaCaT and HUVEC cells treated with GDNPs. Representative photomicrographs of the wound edge in the scratch assay at 0, 24 and 48 h after treatment with different concentrations of GDNPs are presented. The migration rate is represented as the percent scratch closure. (C) Micrographs presenting the effect of GDNPs on tube formation by HUVECs. HUVECs (3 × 104/well) were incubated at 37°C for 12 h on the Matrigel substratum in the presence of the different concentrations of GDNPs. The bar graph quantifies tube formation in normal medium and medium containing 20 μg/mL GDNPs.The results shown are a representative experiment of three independent experiments with similar results (∗p < 0.05,∗∗p < 0.01,∗∗∗p < 0.001 vs control).
3.5. Angiogenic effect of GDNPs
Angiogenesis is considered a crucial procedure in the process of wound healing. We therefore investigated the angiogenic effect of GDNPs in HUVECs using “CORNING Matrigel Matrix.” As presented in Fig. 3B, GDNPs significantly increased tube-like structure formation by promoting the development of branches compared with the effects of the negative control. The results illustrated that GDNPs promote vascularization during wound healing.
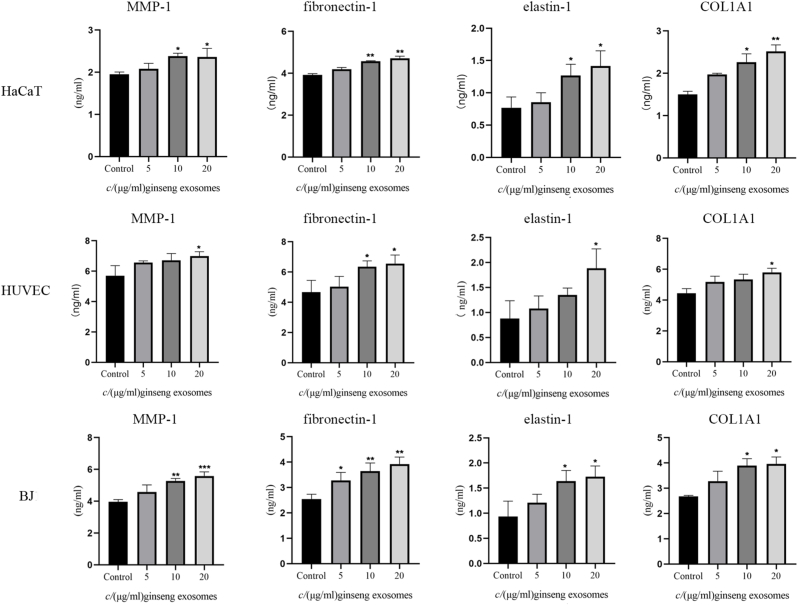
3.6. Effect of GDNPs on the expression of MMP1, fibronectin-1, elastin-1, and COL1A1
Various ECM components such as collagen, fibronectin, and MMPs are critical wound-healing mediators in skin cells. To further explore the effect of GDNPs on skin cells, we examined the secretion of MMP1, fibronectin-1, elastin-1, and COL1A1 in HaCaT cells, HUVECs, and BJ cells. As presented in Fig. 4, we found that ECM components involved in wound healing exhibited increased secretion after GDNP treatment in all three cell lines compared with the results in the negative control groups. In HaCaT cells, 10 and 20 μg/mL GDNPs significantly enhanced the secretion of MMP-1, fibronectin-1, elastin-1, and COL1A1. In HUVECs, 20 μg/mL GDNPs noteworthy increased the secretion of MMP-1, elastin-1, and COL1A1, and 10 and 20 μg/mL GDNPs promoted the secretion of fibronectin-1. In BJ cells, 10 and 20 μg/mL GDNPs remarkably enhanced the secretion of MMP-1, elastin-1, and COL1A1, and 5, 10, and 20 μg/mL GDNPs observably promoted the secretion of fibronectin-1. These results suggested that GDNPs strongly stimulate the secretion of various ECM components. The promotive effect of GDNPs on ECM component secretion was stronger in HaCaT and BJ cells than in HUVECs.
Fig. 4.
Relative soluble protein expression following GDNP treatment in HaCaT cells, BJ cells, and HUVECs. Skin cells were incubated with different concentration of GDNPs for 48 h, and the concentrations of matrix metalloproteinase (MMP-1), fibronectin-1, elastin-1, and collagen type 1α (COL1A1) were measured using ELISA kits. The data are presented as the mean ± SD of three independent experiments. ∗p < 0.05,∗∗p < 0.01,∗∗∗p < 0.001,significant difference versus the vehicle control group.
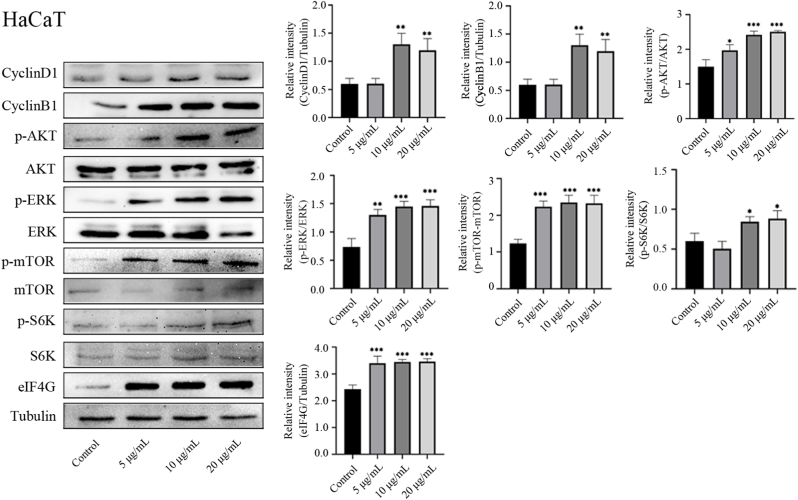
3.7. GDNPs regulate cell proliferation through the ERK and AKT/mTOR pathways
To investigate the specific mechanism by which GDNPs alter the proliferation and cell cycle progression of skin cells, we detected the activation of the ERK and AKT/mTOR signaling pathways. GDNPs significantly enhanced the expression of cyclins D1 and B1 in HaCaT cells, HUVECs, and BJ cells. Meanwhile GDNP treatment promoted the phosphorylation of AKT, ERK, mTOR, and S6K. GDNP treatment also upregulated the expression of eIF4G, another important downstream substrate of mTOR (Fig. 5, S3 and S4). These data demonstrated that GDNPs can promote skin cell proliferation by regulating ERK and AKT/mTOR pathway.
Fig. 5.
GDNPs regulate cell proliferation through the ERK and AKT/ mTOR pathways in HaCaT cells. HaCaT cells were incubated with different concentrations of GDNPs for 48 h. The protein expression of cyclin D1, cyclin B1, p-AKT, AKT, p-ERK, ERK, p-mTOR, mTOR, P70S6K (S6K), p-S6K, eIF4G, and tubulin was measured by western blotting. Tubulin was detected to confirm equal protein amounts in the cytoplasmic fractions.The results shown are a representative experiment of three independent experiments with similar results (∗p < 0.05,∗∗p < 0.01,∗∗∗p < 0.001 vs control).
3.8. GDNPs promoted skin wound healing and decreased inflammation
To investigate the therapeutic activity of GDNPs in the skin wound-healing process, we constructed an in vivo model. GDNPs applied on the wound decreased the wounded area. The observation period was extended to the 15th day (Fig. 6A). At this time point, the wounds treated by GDNPs had almost completely closed, and the effect was statistically significant compared with the result in the control group. Then, animals were sacrificed, and wounded tissues were cut into slices for HE staining. As shown in Fig. 6B, there were a large number of inflammatory cells, few fibroblasts and collagen fibers in the control group, and the formation of epidermal tissue was incomplete. The GDNPs and positive drug therapy have similar effects on skin wound healing, with fewer inflammatory cells and more collagen fibers that were arranged neatly and densely. Meanwhile, the epidermis formation of GDNPs group and positive group were better than that of the control group. Furthermore, GDNPs enhanced the expression of the wound healing molecular markers TGF-β and Ki-67 and decreased the expression of the inflammatory factors iNOS, COX-2, and NF-кB (Fig. 6C). These results suggested that GDNPs promoted skin wound healing and decreased inflammation.
Fig. 6.
The effect of topical application of GDNPs on skin wound closure in a mouse model. (A) Round full-thickness skin excisional wounds were made on the backs of ICR mice, followed by treatment with 10 mg/mL GDNPs or vehicle control (0.02 mL/mouse) every day until the wound had healed. (B) Representative HE sections of skin (10 × 10 and 10 × 40). Tissue morphology was analyzed after GDNP treatment. (C) The protein expression of TGF-β, Ki-67, NF-κB, iNOS, COX-2, and β-actin was measured by western blotting. (D) The quantitative data of panel (A).The results shown are a representative experiment of three independent experiments with similar results (∗p < 0.05,∗∗p < 0.01,∗∗∗p < 0.001 vs control).
4. Discussion
Cells communicate biomolecules to other cells through exosomes, which carry membrane molecules and luminal cargo. Regarding skin wound healing, exosomes from mesenchymal stem cells promote skin cell proliferation [33]. Exosomes from human mesenchymal stromal cell accelerate wound healing by modulating the biological properties of skin fibroblasts and keratinocytes [34]. Oral mucosal epithelial cell-derived exosomes accelerate wound healing [35]. Human trophoblasts-derived extracellular vesicles promote the regeneration of skin fibroblasts [36]. Past studies suggested that ginseng extracts and ginseng-derived molecules exerted significant regulatory effects on skin. One report claimed that extracellular vesicles from Panax ginseng could induce anti-senescence effects in human skin cells [29]. In this study, we isolated nanoparticles from ginseng root and detected their average diameter and zeta potential. Our findings illustrated GDNPs could be accumulated by skin cells in vivo and vitro. These findings indicate that GDNPs can exert effects on skin cells. Indeed, GDNPs remarkably promoted proliferation and cell cycle progression in three skin cell lines. Meanwhile, GDNPs induced the expression of cyclins B1 and D1, two cell cycle-related proteins. However, the proliferation of keratinocytes in the skin was a tightly controlled process. Out of control and excessive proliferation of keratinocytes will lead to skin diseases, such as psoriasis. The abnormal proliferation and differentiation of epidermal cells will activate the body's immune system [37]. Fortunately, our results showed the proliferation rate of HaCaT treated by GDNPs was only 120%. At the same time, pathological epidermal hyperplasia was not observed in animal experiments. GDNPs even reduced the inflammation in the skin wound. A longer term effects of GDNPs on keratinocytes and skin will be investigated in the future study.
Angiogenesis is an important procedure in the proliferative phase of the wound-healing process. In an experiment investigating the effect of GDNPs on endothelial cells, we found that GDNPs stimulated both migration and tube formation. Furthermore, GDNPs significantly promoted the expression of MMP1, fibronectin-1, elastin-1, and COL1A1 in different skin cell lines. Based on these results, we suggest that GDNPs have strong ability to enhance the growth of keratinocytes, fibroblasts, and endothelial cells.
We further investigated the mechanism by which GDNPs promoted wound healing. Wound healing is a sophisticated process including cell proliferation, cell cycle progression, cell migration, and the synthesis and secretion of ECM molecules involved in various signaling pathways [22]. AKT/mTOR signaling has a crucial effect on many aspects such as cell proliferation, protein translation, energy metabolism, and wound healing [38]. In normal wound healing, AKT/mTOR signaling is activated. However, this signaling pathway is weakened in wounds in patients with refractory diabetes [39]. In these patients, rapamycin and sirolimus (mTOR inhibitors) notably delayed normal wound healing, whereas insulin, polysaccharides, and other medicines that activate the AKT/mTOR pathway remarkably accelerated the healing of refractory wounds [40]. To confirm the effect of the AKT/mTOR pathway in GDNP-induced wound healing, we detected the expression and phosphorylation of AKT, mTOR, and the downstream molecules S6K and eIF4G in three skin cell lines after treatment with GDNPs. The results indicated that GDNPs increased AKT and mTOR phosphorylation, thereby enhancing the phosphorylation of S6K and expression of eIF4G.
Furthermore, previous researches claimed that ERK involve in the physiological processes required for cell growth, development, cell division, and wound healing. ERK takes a crucial part in calcium silicate-induced skin wound healing [41]. Dracorhodin perchlorate accelerates wound healing through ERK/P38 and AKT signaling in keratinocytes [42] . Kanglexin promotes diabetic wound healing via FGFR1/ERK signaling [43]. Our results suggested that GDNPs remarkably promoted the phosphorylation of ERK, and the stimulatory effect of GDNPs on wound healing is related to the ERK signaling pathway.
Skin wound healing is a conserved process in mammals that reconstructs homeostasis and barrier function in the skin. Various molecular and cellular pathways are tightly modulated and coordinated to restore the function of damaged skin. Ki-67 is a cell proliferation marker that is weakly expressed in resting cells and strongly expressed in cycling cells [44]. TGF-β1 secretion from fibroblasts was verified to induce epithelial mesenchymal migration and transition in keratinocytes, thereby promoting re-epithelialization during wound healing [45]. In our in vivo experiment, GDNPs were demonstrated to accelerate wound healing and enhance the expression of Ki-67 and TGF-β1.
Infection or injury can induce an inflammatory reaction via pathogen-associated molecular patterns or cytokines derived from platelet degranulation [46]. Macrophages both play an important effect in controlling wound healing and actively participate in restoring tissue homeostasis in the subsequent stages of the healing process. In the early stage of wound healing, M1 type macrophages (pro-inflammatory phenotype) release massive amounts of pro-inflammatory molecules and take a major part in the recruitment, proliferation, and activation of stromal and immune cells. In the later stage of wound healing, macrophages shift from the M1 type to the M2 type (anti-inflammatory phenotype). M2 type macrophages secrete suppressors of cytokine signaling and promote inflammatory resolution and new tissue formation [47]. In our study, we obtained tissue samples during the later stage of wound healing and detected inflammatory factors by western blotting. GDNPs markedly reduced the expression of NF-кB, COX-2, and iNOS, suggesting that GDNPs affect the polarization of macrophages. By promoting the polarization of M2 type macrophages, GDNPs promoted wound healing in the later stage. This will be examined in future research.
Chronic wounds are known to feature persistent inflammation that causes excessive tissue degradation and blocks the healing of damaged skin tissue. Using diabetes as an example, impaired wound healing results in remarkable reductions in cell proliferation, cell migration, and protein synthesis. These changes delay angiogenesis, re-epithelialization, and granulation tissue formation. The sequential polarization of M1 type and M2 type macrophages is perturbed, and M1 type macrophages are continuously activated [48,49]. Meanwhile, the AKT/mTOR signaling pathway involves in wound healing in diabetes, as various upstream and downstream factors of mTOR are downregulated in diabetic wounds [50]. These findings implied that GDNPs might improve the healing of chronic wounds such as those in patients with diabetes.
In the past reports, red ginseng extract, ginsenosides Rb1 and Rg3 enhanced the proliferation of human dermal papilla cells (hDPCs) and hair matrix keratinacyte, activate ERK and AKT signaling pathways in hDPCs, and inhibit the transcription of DHT-induced androgen receptors, promoted hair growth in C57BL/6 mice [51]. Other research demonstrated that hair regenerative mechanisms of red ginseng extract may be mediated by stimulating dermal papilla cell proliferation and enhancing skin functions [52]. In our results, the phosphorylation of ERK and AKT were increased in target cells. Meanwhile, GDNPS also seem to have a certain effect on hair growth. The relationship between hair growth and GDNPs will be explored in subsequent studies.
In conclusion, the potential application of GDNP treatment to promote skin wound healing was established using in vitro and in vivo wound-healing models. GDNPs increased cell proliferation and migration, wound healing-related gene expression, and vascularization through the ERK and AKT/mTOR pathways. In the in vivo experiment, GDNPs promoted mouse skin wound healing and decreased inflammation in the later stage of wound healing. These results support the development of GDNP therapy for the treatment of skin injuries.
Acknowledgments
This work was supported by the Technology Development Fund-Jilin Provincial Special project for Basic Research, China (202002054JC), National Natural Science Foundation of China (U19A2013) and the Science and Technology Project of Jilin Provincial Education Department (JJKH20220875KJ).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2022.07.005.
Contributor Information
Meichen Liu, Email: liumc0367@163.com.
Jiawen Wang, Email: wangjiawen1229@163.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
figs1.
figs2.
figs3.
figs4.
References
- 1.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Wurdinger T., Middeldorp J.M. Functional delivery of viral mirnas via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 4.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas S.L.N., Breakefield X.O., Weaver A.M. Extracellular vesicles: unique intercellular delivery vehicles. Trends in Cell Biology. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riazifar M., Pone E.J., Lotvall J., Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–154. doi: 10.1146/annurev-pharmtox-061616-030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for ctl cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M., Viennois E., Xu C., Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4 doi: 10.1080/21688370.2015.1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rome S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019;10:529–538. doi: 10.1039/c8fo02295j. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y., Gao J., He Y., Jiang L. Plant extracellular vesicles. Protoplasma. 2020;257:3–12. doi: 10.1007/s00709-019-01435-6. [DOI] [PubMed] [Google Scholar]
- 11.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., Samykutty A., Zhang L., Yan J., Miller D., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell amp-activated protein kinase. Molecular Therapy : The Journal of the American Society of Gene Therapy. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju S., Mu J., Dokland T., Zhuang X., Wang Q., Jiang H., Xiang X., Deng Z.B., Wang B., Zhang L., et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from dss-induced colitis. Mol Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang X., Deng Z.B., Mu J., Zhang L., Yan J., Miller D., Feng W., McClain C.J., Zhang H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu J., Zhuang X., Wang Q., Jiang H., Deng Z.B., Wang B., Zhang L., Kakar S., Jun Y., Miller D., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A., Hutchins E., Mu J., Deng Z., Luo C., et al. Plant-derived exosomal micrornas shape the gut microbiota. Cell Host Microbe. 2018;24:637–652 e638. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao M., Yan H., Han X., Weng L., Wei Q., Sun X., Lu W., Wei Q., Ye J., Cai X., et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin F., Kocak P., Gunes M.Y., Ozkan I., Yildirim E., Kala E.Y. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188:381–394. doi: 10.1007/s12010-018-2913-1. [DOI] [PubMed] [Google Scholar]
- 18.Teng Y., Mu J., Hu X., Samykutty A., Zhuang X., Deng Z., Zhang L., Cao P., Yan J., Miller D., et al. Grapefruit-derived nanovectors deliver mir-18a for treatment of liver metastasis of colon cancer by induction of m1 macrophages. Oncotarget. 2016;7:25683–25697. doi: 10.18632/oncotarget.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Zhuang X., Mu J., Deng Z.B., Jiang H., Zhang L., Xiang X., Wang B., Yan J., Miller D., et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Garcia D., Filipova A., Garza-Veloz I., Martinez-Fierro M.L. A beginner's introduction to skin stem cells and wound healing. Int J Mol Sci. 2021:22. doi: 10.3390/ijms222011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen C.K. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle) 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson H.N., Hardman M.J. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10 doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw T.J., Martin P. Wound repair. A showcase for cell plasticity and migration. Curr Opin Cell Biol. 2016;42:29–37. doi: 10.1016/j.ceb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Asati V., Mahapatra D.K., Bharti S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem. 2016 Feb 15;109:314–341. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Panax ginseng. Monograph. Altern Med Rev. 2009;14:172–176. [PubMed] [Google Scholar]
- 27.Ahn H., Han B.C., Hong E.J., An B.S., Lee E., Lee S.H., Lee G.S. Korean red ginseng attenuates ultraviolet-mediated inflammasome activation in keratinocytes. J Ginseng Res. 2021;45:456–463. doi: 10.1016/j.jgr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin K.O., Choe S.J., Uchida Y., Kim I., Jeong Y., Park K. Ginsenoside rb1 enhances keratinocyte migration by a sphingosine-1-phosphate-dependent mechanism. J Med Food. 2018;21:1129–1136. doi: 10.1089/jmf.2018.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee G.Y., Park K.G., Namgoong S., Han S.K., Jeong S.H., Dhong E.S., Kim W.K. Effects of panax ginseng extract on human dermal fibroblast proliferation and collagen synthesis. Int Wound J. 2016;13(Suppl 1):42–46. doi: 10.1111/iwj.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho E.G., Choi S.Y., Kim H., Choi E.J., Lee E.J., Park P.J., Ko J., Kim K.P., Baek H.S. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: an eco-friendly and sustainable way to use ginseng substances. Cells. 2021:10. doi: 10.3390/cells10030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettersson J., Lobov S., Novikova L.N. Labeling of olfactory ensheathing glial cells with fluorescent tracers for neurotransplantation. Brain Res Bull. 2010 Jan 15;81(1):125–132. doi: 10.1016/j.brainresbull.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser J.P., Bruinink A. Investigating cell-material interactions by monitoring and analysing cell migration. J Mater Sci Mater Med. 2004 Apr;15(4):429–435. doi: 10.1023/b:jmsm.0000021115.55254.a8. [DOI] [PubMed] [Google Scholar]
- 33.Kim S., Lee S.K., Kim H., Kim T.M. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tutuianu R., Rosca A.M., Iacomi D.M., Simionescu M., Titorencu I. Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22126239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjoqvist S., Ishikawa T., Shimura D., Kasai Y., Imafuku A., Bou-Ghannam S., Iwata T., Kanai N. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles. 2019;8 doi: 10.1080/20013078.2019.1565264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Go Y.Y., Lee C.M., Ju W.M., Chae S.W., Song J.J. Extracellular vesicles (secretomes) from human trophoblasts promote the regeneration of skin fibroblasts. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22136959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y.J., Xu Y.Y., Lan X.O., Liu X.Y., Zhang X.L., Gao X.H., Geng L. Shikonin induces apoptosis and suppresses growth in keratinocytes via CEBP-δ upregulation. Int Immunopharmacol. 2019 Jul;72:511–521. doi: 10.1016/j.intimp.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017;20:189–193. doi: 10.1016/j.cjtee.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxton R.A., Sabatini D.M. Mtor signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H., Cui W., Qiu W., Zhu M., Zhao R., Zeng D., Dong C., Wang X., Guo W., Xing W., et al. Impaired wound healing results from the dysfunction of the akt/mtor pathway in diabetic rats. J Dermatol Sci. 2015;79:241–251. doi: 10.1016/j.jdermsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Li B., Tang H., Bian X., Ma K., Chang J., Fu X., Zhang C. Calcium silicate accelerates cutaneous wound healing with enhanced re-epithelialization through egf/egfr/erk-mediated promotion of epidermal stem cell functions. Burns Trauma. 2021;9:tkab029. doi: 10.1093/burnst/tkab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C.C., Yang J.S., Chiu Y.J., Tsai F.J., Hsu Y.M., Yin M.C., Juan Y.N., Ho T.J., Chen H.P. Dracorhodin perchlorate enhances wound healing via beta-catenin, erk/p38, and akt signaling in human hacat keratinocytes. Exp Ther Med. 2021;22:822. doi: 10.3892/etm.2021.10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y., Wang X., Yang S., Song X., Sun N., Chen C., Zhang Y., Yao D., Huang J., Wang J., et al. Kanglexin accelerates diabetic wound healing by promoting angiogenesis via fgfr1/erk signaling. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110933. [DOI] [PubMed] [Google Scholar]
- 44.Remnant L., Kochanova N.Y., Reid C., Cisneros-Soberanis F., Earnshaw W.C. The intrinsically disorderly story of ki-67. Open Biol. 2021;11 doi: 10.1098/rsob.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiritsi D., Nystrom A. The role of tgfbeta in wound healing pathologies. Mech Ageing Dev. 2018;172:51–58. doi: 10.1016/j.mad.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Aitcheson S.M., Frentiu F.D., Hurn S.E., Edwards K., Murray R.Z. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26 doi: 10.3390/molecules26164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W., Qiu W., Ao X., Li W., He X., Ao L., Hu X., Li Z., Zhu M., Luo D., et al. Low-concentration dmso accelerates skin wound healing by akt/mtor-mediated cell proliferation and migration in diabetic mice. Br J Pharmacol. 2020;177:3327–3341. doi: 10.1111/bph.15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauck S., Zager P., Halfter N., Wandel E., Torregrossa M., Kakpenova A., Rother S., Ordieres M., Rathel S., Berg A., et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact Mater. 2021;6:4342–4359. doi: 10.1016/j.bioactmat.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei P., Zhong C., Yang X., Shu F., Xiao S., Gong T., Luo P., Li L., Chen Z., Zheng Y., et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via pi3k-akt-mtor-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park G.H., Park K.Y., Cho H.I., Lee S.M., Han J.S., Won C.H., Chang S.E., Lee M.W., Choi J.H., Moon K.C., et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J Med Food. 2015 Mar;18(3):354–362. doi: 10.1089/jmf.2013.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truong V.L., Jeong W.S. Hair growth-promoting mechanisms of red ginseng extract through stimulating dermal papilla cell proliferation and enhancing skin health. Prev Nutr Food Sci. 2021 Sep 30;26(3):275–284. doi: 10.3746/pnf.2021.26.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.