Abstract
Proteins belonging to the LraI (for “lipoprotein receptor antigen”) family function as adhesins in several streptococci, as a virulence factor for endocarditis in at least one of these species, and potentially as metal transporters in many bacteria. We have identified and characterized the chromosomal locus containing the LraI family gene (designated sloC) from Streptococcus mutans, an agent of dental caries and endocarditis in humans. Northern blot analysis indicated that sloC is cotranscribed with three other genes. As with other LraI operons, the sloA and sloB genes apparently encode components of an ATP-binding cassette transport system. The product of the fourth gene, sloR, has homology to the metal-dependent regulator from Corynebacterium diphtheriae, DtxR. A potential binding site for SloR was identified upstream from the sloABCR operon and was conserved upstream from LraI operons in several other streptococci. Potential SloR homologs were identified in the unfinished genomic sequences from two of these, S. pneumoniae and S. pyogenes. Mutagenesis of sloC in S. mutans resulted in apparent loss of expression of the entire operon as assessed by Northern blot analysis. The sloC mutant was indistinguishable from its wild-type parent in a gnotobiotic rat model of caries but was significantly less virulent in a rat model of endocarditis. Virulence for endocarditis was restored by correction of the sloC mutation but not by provision of the sloC gene in trans, suggesting that virulence requires the expression of other genes in the sloC operon.
Examination of adherence mechanisms in the oral streptococci has led to the identification of a number of homologous proteins referred to collectively as the lipoprotein receptor antigen I (LraI) family (31). Although identified initially in oral streptococci, LraI members have since been discovered in other streptococci and in other genera. These proteins have several features in common. They are thought to be lipoproteins since they contain a consensus lipoprotein signal sequence and since the SsaB LraI member from Streptococcus sanguis and the SitC member from Staphylococcus epidermidis are fatty acylated in living cells (11, 20). The LraI genes are contained within operons apparently encoding ATP-binding cassette (ABC) transport systems. In gram-positive bacteria, these operons minimally contain genes for an ATP-binding protein (ATPB) an integral membrane protein (IMP), and the LraI lipoprotein. The LraI proteins have homology to the periplasmic substrate-binding proteins of gram-negative ABC transport systems and are thought to share the transport function of these proteins (1, 18, 23). Surprisingly, an LraI operon containing a putative lipoprotein gene has also been identified in the spirochete Treponema pallidum (26).
Adhesin functions have been demonstrated for LraI members from S. sanguis (SsaB [22]), S. gordonii (ScaA [35]), S. parasanguis (FimA [51]), and S. agalactiae (Lmb [56]) but not for the LraI member from S. crista (ScbA [12]), suggesting that adhesion is a common but not universal function of these proteins. Given the location of LraI genes within apparent ABC transport operons and the homology of these genes to a Mn import operon in a cyanobacterium (4), transport functions have been sought for some LraI members. Two LraI operons have been identified in S. pnemoniae, psaBCAD and adcCBA (17). Mutation of the adcC gene resulted in an increased Zn requirement for normal growth, and a psaA mutation caused an increased MnSO4 requirement, suggesting that Zn and Mn import are functions of these two operons. Similarly, mutation of the scaA gene in S. gordonii resulted in decreased 54Mn2+ uptake and impairment of growth in media containing low levels of Mn2+ (34). Finally, mutation of an LraI member in S. pyogenes, mtsA, caused reduced uptake of 55Fe and 65Zn, suggesting that the mtsABC operon encodes a transport system with specificity for multiple metals (30).
The study by Viscount et al. suggested that S. mutans possesses an LraI gene (62). In a Southern blot analysis using the fimA gene from S. parasanguis as a probe, a single hybridizing DNA fragment was detected in S. mutans. Also, an amplicon of the expected size was obtained when PCR was performed with oligonucleotide primers designed from conserved LraI gene sequences, although the efficiency of the amplification was poor. It was less clear whether the LraI member is expressed in S. mutans, since in three of four plaque isolates, an LraI protein could not be detected using antiserum raised against FimA. Characterization of an LraI member in S. mutans would be of interest for several reasons. First, S. mutans is one of several oral streptococci that can cause endocarditis. This disease is thought to occur when oral streptococci escape from the oral cavity into the bloodstream and adhere to previously damaged heart valves (13). In S. parasanguis, mutation of the fimA gene causes loss of virulence for endocarditis in a rat model (8). The mutant strain also binds less well to fibrin monolayers in vitro, suggesting that FimA may allow the bacterium to adhere to fibrin at the site of the infection. The FimA protein has also proven to be a promising vaccinogen for preventing S. parasanguis-induced endocarditis in the same model (62).
S. mutans is unique among the oral streptococci in its ability to cause both smooth-surface dental caries and endocarditis (25). The demonstrated role of LraI members in adherence and metal uptake in other oral streptococci and the efficacy of the FimA protein as a vaccinogen suggest that the LraI protein from S. mutans might represent an attractive target for an anticaries vaccine. Here we report the cloning and characterization of the operon containing the S. mutans LraI family member gene, designated sloC. We have evaluated the effect of an in-frame deletion in sloC on virulence. We have also discovered within this operon a gene potentially encoding a metal-dependent regulator.
MATERIALS AND METHODS
Bacterial strains and growth.
ATCC 25175 is the S. mutans type strain and was obtained from the American Type Culture Collection. V403 is a fructan-hyperproducing, cariogenic strain of S. mutans (32, 47, 48, 55) originally obtained as a human blood isolate (provided by R. Facklam, Centers for Disease Control and Prevention). S. mutans strains created in this study are described in the text. S. mutans was routinely grown in anaerobic atmosphere at 37°C in tryptic soy (TS) broth or brain heart infusion (BHI) broth (both from Difco Inc., Detroit, Mich.) supplemented with 1.5% agar for growth on plates. Genetic transformation was by the method of Lindler and Macrina (38). Erythromycin was included at 10 μg/ml for plasmid selection.
Animal models.
The gnotobiotic rat model of caries has been described previously (46, 47). Rats were divided into groups of six at 19 days of age and orally inoculated with overnight cultures of S. mutans strains grown in TS broth plus 10 μg of erythromycin per ml. Oral inoculation was repeated on days 20 and 21. The rats were provided a diet containing 5% sucrose (diet 305) throughout the experiment and sacrificed on experimental day 62. The mandibular jaws were removed from each rat for microbiological enumeration and caries assessment. Values were expressed as the mean ± standard error of the mean and were evaluated by analysis of variance. The rat model of endocarditis employed here has also been described previously (48). Briefly, a catheter was inserted through the carotid artery past the aortic valve to produce valve damage; the catheter was sutured in place in the carotid and remained in place throughout the remainder of the experiment. At 4 or 5 days later, streptococci grown in BHI broth plus 0.5% sucrose were harvested, washed in phosphate-buffered saline, and inoculated into the tail vein of catheterized rats. Then 2 days later, the rats were killed by CO2 inhalation. The heart was removed and correct catheter placement was assessed visually. The aortic valve and any apparent vegetations were removed, homogenized with phosphate-buffered saline, and plated on TS agar plates. Rats were judged to be infected if bacteria from the inoculum were recovered on the plates. Colony morphology, phase-contrast microscopy, and examination of HaeIII-digested DNA (32) were used to confirm that colonies obtained were derived from the inoculum. Rats in which correct catheter placement could not be verified at necropsy, which had no apparent vegetations, and from which no bacteria were recovered were dropped from the study. All other rats from which bacteria were not recovered were judged to be uninfected. Differences in infectivity were evaluated using Fisher's exact test, with significance set at P = 0.05.
Caries testing was carried out under University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC) protocol 98C04235, and endocarditis testing was performed under Virginia Commonwealth University IACUC protocol 9710-2476.
DNA methods.
Chromosomal DNA was isolated from S. mutans as follows. Cultures were grown overnight in 3 ml of BHI broth containing 20 mM dl-threonine (BHIT) and then diluted with 9 ml of BHIT. Growth for 1 h at 37°C was followed by the addition of solid glycine to 0.5% and continued growth at 37°C for 45 min. Cells were harvested by centrifugation, washed with H2O, and suspended in 0.36 ml of 25% glucose–10 mM Tris-Cl (pH 8.0)–1 mM EDTA. Then 50 μg of RNase A and 1 mg of lysozyme were added, and the suspension was incubated at 37°C for 30 min. A 100-μg portion of proteinase K was added, followed by the addition of Sarkosyl to a final concentration of 1.3%. Incubation was continued at 37°C for 1 h, and was followed by extraction with phenol-CHCl3 (1:1) and CHCl3 and ethanol precipitation. DNA pellets were suspended in 10 mM Tris-Cl (pH 8.0)–1 mM EDTA and quantitated by spectrophotometry. Southern blot signals were detected using the Genius digoxigenin system (Roche Molecular Biochemicals, Indianapolis, Ind.) with digoxigenin-dUTP incorporated into the probe by random-primer labeling. PCR was routinely performed in a GeneAmp 9600 thermal cycler (PE Biosystems, Foster City, Calif.) using Platinum PCR Supermix (Bethesda Research Laboratories, Gaithersburg, Md.) as specified by the manufacturer.
DNA sequence analysis.
Cycle sequencing was performed using custom oligonucleotide primers and the ABI Prism FS kit (PE Biosystems) as specified by the manufacturer. Electrophoresis was done with ABI373 and ABI377 automated sequencers. DNA sequences were assembled into contigs using Sequencher software (Genecodes Corp., Ann Arbor, Mich.). GenBank databases were searched using BLASTN, BLASTX, and BLASTP programs (2). Amino acid similarity values were determined using the GAP program from Genetics Computer Group (Madison, Wis.). Alignments were created using PILEUP and displayed using PRETTY, both from the Genetics Computer Group package. Inverted repeats were located using the Gene Inspector program (Textco Inc., Lebanon, N.H.). Preliminary sequence data were obtained from The Institute for Genomic Research website at http://www.tigr.org (S. pneumoniae) and the University of Oklahoma Streptococcal Genome Sequencing Project at http://www.genome.ou.edu (S. mutans and S. pyogenes).
RNA analysis.
RNA was isolated from S. mutans by the method of Lunsford (41). Following treatment with RQ1 DNase (Promega, Madison, Wis.), 20-μg samples were separated by electrophoresis through a 1.2% formaldehyde–morpholinepropanesulfonic acid (MOPS) agarose gel, transferred to a nylon membrane (Roche Molecular Biochemicals), and fixed by UV cross-linking. Lanes containing RNA molecular weight standards (Millennium markers; Ambion, Inc.; Austin, Tex.) were removed for staining with methylene blue (45). Hybridization of the remaining lanes was performed at 37°C in Ultrahyb fluid (Ambion, Inc.) with DNA probes labeled by PCR incorporation of digoxigenin-dUTP (Roche Molecular Biochemicals). Detection of hybridization by chemiluminescence was carried out as described for Southern blots.
Protein analysis.
Lysates of S. mutans were prepared, electrophoresed, and detected by Western blotting using rabbit anti-FimA antiserum as described previously (62). Densitometric analysis of digitized Western blot images was performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/).
DNA sequence accession number.
The GenBank accession number of the sloABCR nucleotide sequence is AF232688.
RESULTS
Cloning and nucleotide sequence analysis of an LraI operon from Streptococcus mutans.
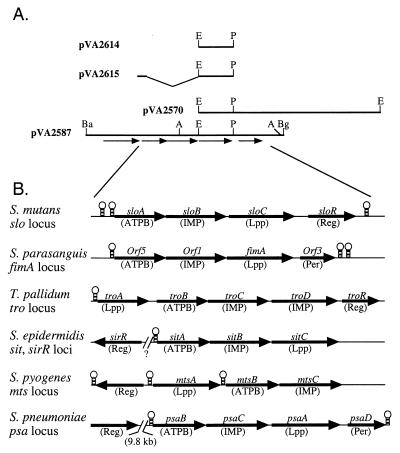
The PCR amplification of LraI family genes from oral streptococci using primers complementary to conserved regions within the 5′ and 3′ regions of these genes has been described previously (62). These conditions were used to amplify a ∼740-bp fragment from S. mutans strain ATCC 25175, presumably containing most of the S. mutans LraI family gene. This fragment was used as a probe in Southern blots of S. mutans chromosomal DNA. The results indicated that there was a single, 4.9-kb EcoRI fragment with strong homology to the probe. This fragment was cloned into pUC19 to create pVA2570 (Fig. 1A). Initial restriction mapping and DNA sequence analysis indicated that this fragment contained the entire sequence of an S. mutans LraI member in addition to downstream sequences but contained only a few base pairs of upstream sequence. Because we wanted to examine genes upstream from the LraI member gene, an overlapping 5.46-kb BamHI-BglII fragment was cloned into pUC19 to create pVA2587 (Fig. 1A). Preliminary sequence was obtained for the entire insert of pVA2587. Five open reading frames (ORFs) were found. The first had limited similarity to the mevalonate kinase gene from several archaea, for example, Pyrococcus horikoshii (accession number BAA30737). This ORF was followed by a hairpin loop that had features of rho-independent transcriptional terminators, although the loop was longer than usually seen in Escherichia coli terminators (15). This finding, as well as other data discussed below, suggested that the gene was not part of the LraI operon. The remaining ORFs appeared to form an operon and are shown in Fig. 1B. Another hairpin loop was found 95 bp downstream from the fifth ORF. No additional downstream ORFs were found. The DNA sequence of a 3,932-bp region, from the end of the mevalonate kinase-like gene to the end of the insert in pVA2587, was determined on both strands.
FIG. 1.
LraI loci in S. mutans and other bacteria. ORFs are indicated by arrows. (A) Maps of plasmids containing the slo region from S. mutans. Restriction sites are EcoRI (E), PvuII (P), BamHI (Ba), AlwNI (A), and BglII (Bg). (B) Comparison of the sloABCR operon to LraI-like operons in other bacteria. DNA sequences containing inverted repeats are depicted as hairpin loops. Gene names are indicated in italics. Putative functions for the ORFs are indicated in parentheses: Lpp, lipoprotein receptor; Reg, metal-dependent regulator; Per, peroxidase. Sequences and features are taken from accession number M26130 (S. parasanguis), accession number U55214 (T. pallidum), accession numbers X99127 and X99128 (Staphylococcus epidermidis), accession number AF180520 and the Streptococcal Genome Sequencing Project (S. pyogenes), and accession number AF055088 and the S. pneumoniae Genome Sequencing Project (S. pneumoniae).
Based on DNA sequence analysis and other information discussed below, the final four ORFs in pVA2587 were designated sloA, sloB, sloC, and sloR (for “S. mutans LraI operon”) (Fig. 1; Table 1). The sloA gene potentially encodes the ATPB component of an ABC transport system. A BLASTP search with the SloA sequence produced the highest matches to LraI operon members ScaC from S. gordonii (35), PsaB from S. pneumoniae (50), and the ORF5 product from S. parasanguis (19) (Table 1). Like these homologs, SloA contained sequences expected of this class of proteins, including a Walker A motif at positions 34 to 42 (GPNGAGKST; the consensus is GXXGXGKST), the ABC signature at positions 134 to 137 (LSGG; same as the consensus), a Walker B motif at positions 154 to 161 (YIEFLDEPF; the consensus is hhhhDEPT, where h is any hydrophobic amino acid), and a more recently described motif at positions 187 to 192 (LIIHHD; the consensus is hhhhH+/−, where +/− is a charged amino acid) (40, 63).
TABLE 1.
Characteristics of the predicted protein products of the slo operon
| Gene | Size of product, aaa (kDa) | Putative function | Three best GenBank matches (% aa identity) | Accession no. |
|---|---|---|---|---|
| sloA | 240 (26.9) | ATPB | ScaC, S. gordonii (65%) | AAA71945 |
| PsaB, S. pneumoniae (62%) | AAD09976 | |||
| ORF5, S. parasanguis (57%) | AAA53075 | |||
| sloB | 279 (30.0) | IMP | ScaB, S. gordonii (84%) | AAA71946 |
| ORF1, S. parasanguis (80%) | AAA53076 | |||
| MtsC, S. pyogenes (78%) | AAD56938 | |||
| sloC | 306 (34.3) | Lipoprotein receptor, adhesin (?) | ScbA, S. crista (76%) | AAC44133 |
| SsaB, S. sanguis (75%) | AAC98426 | |||
| ScaA, S. gordonii (74%) | AAA71947 | |||
| sloR | 217 (25.1) | Metal-dependent regulator | SirR, S. epidermidis (36%) | CAA67572 |
| SirR, M. tuberculosis (36%) | CAA15583 | |||
| AAF12080, D. radiodurans (31%) | AAF12080 |
aa, amino acids.
The sloB gene follows sloA, partially overlapping it (Fig. 1; Table 1). A BLASTP search with the SloB sequence suggested that SloB was the IMP component of an ATP transport system; the best database matches to SloB were the ScaB and ORF1 proteins from the LraI operons of S. gordonii and S. parasanguis, respectively, and the S. pyogenes MtsC protein, also encoded by an LraI family operon (30). SloB, like the other LraI IMPs listed in Table 1, was found to contain only weak homology to the “conserved EAA loop,” which is defined mostly on the basis of alignments of E. coli IMPs (6, 14). However, SloB exhibited a hydropathy profile expected of an IMP (18, 19). Analysis with the program TopPred2 (10) suggested that SloB contains nine transmembrane helixes. This is consistent with the model proposed by Bartsevich and Pakrasi (5) for the SloB homolog, MntB, a member of an ABC manganese importer in Synechocystis (4).
The third gene, sloC, potentially encodes the S. mutans LraI family lipoprotein. The SloC amino-terminal sequence matched the consensus for bacterial lipoprotein lipid attachment sites (PDOC00013 at http://www.expasy.ch/cgi-bin/prosite-search-ac?) (28). The protein product was expected to be 34 kDa prior to processing (Table 1) and would be shortened to 287 amino acids and 32 kDa if cleaved adjacent the cysteine residue at amino acid 20. SloC was most similar to ScbA from S. crista (12), SsaB from S. sanguis (21), and ScaA from S. gordonii (35).
The position of the putative lipoprotein gene downstream from the genes for an ATPB and IMP in S. mutans (Fig. 1B) was found to be shared by the S. parasanguis fimA operon (19), the S. pneumoniae adcCBA (18) and psaBCAD (50) operons, the S. gordonii scaA operon (35), and the Staphylococcus epidermidis sitABC operon (11). The intergenic spacing was similar for these operons in the streptococci. The ATPB gene (including its stop codon) and the IMP gene overlapped by 4 bp in the S. mutans, S. parasanguis, and S. pneumoniae psa operons and by 8 bp in the S. pneumoniae adc operon (18). The distance between the IMP gene and the lipoprotein gene ranged from 9 bp in the adc operon to 47 bp for S. mutans. Intergenic spacing in the Staphylococcus epidermidis operon differed somewhat from those listed above. The ATPB and IMP genes (sitA and sitB) are separated by 90 bp in GenBank accession number X99127. Our inspection indicated, however, that the sitB gene could begin 90 bp upstream from the reported start site, causing sitB to abut sitA. The additional 30 amino acids that would be encoded in the longer SitB protein had homology to the amino termini of other LraI IMPs (data not shown). The SitB protein has not been characterized, and it is not clear which start site is employed in Staphylococcus epidermidis. However, neither potential arrangement of sitB and sitA was observed in the other operons mentioned above. Moreover, the sitC gene, encoding the putative lipoprotein, was unusual in apparently overlapping sitB by 4 bp (11).
A gene encoding a putative peroxidase was found downstream of the lipoprotein gene in the psaA locus of S. pneumoniae (50), the fimA locus of S. parasanguis (19), the scaA locus of S. gordonii (35), the ssaB locus of S. sanguis (21), and the scbA gene of S. crista (12). In contrast, the gene downstream from sloC in S. mutans (sloR) was predicted to encode a protein with homology to the iron-dependent repressor DtxR from Corynebacterium diphtheriae (7) and other bacteria (Fig. 1B; Table 1). The degree of similarity between SloR and its closest GenBank homologs (listed in Table 1) was less than that observed for the other members of the sloABCR locus. This was not surprising, however, given the phylogenetic diversity of the bacteria from which these genes were isolated. Comparisons of the two SirR proteins and the Deinococcus radiodurans ORF product (64) to one another yielded identity values of 33 to 41% (data not shown), which are similar to the values shown in Table 1 for their relatedness to SloR. All four proteins were more similar to one another than to the archetypal protein of this class, DtxR. An alignment of SloR, SirR from Staphylococcus epidermidis (27), TroR from T. pallidum (26), and DtxR is shown in Fig. 2.
FIG. 2.
Alignments of SloR and homologous proteins. Amino acids present in two or more proteins at any position in the alignment are indicated in capital letters. Periods indicate gaps introduced to optimize alignments. Features of DtxR are indicated as follows: #, metal ion-binding site 2 of Ding et al. (16); ∗, helixes of the helix-turn-helix motif (54, 59); ∧, metal-binding site 1 of Goranson-Siekierke et al. (24); +, anion-binding sites in the work of Goranson-Siekierke et al. (24).
The ATPB, IMP, and lipoprotein genes are cotranscribed in many LraI operons (3, 17, 19, 26, 30, 50). The arrangement and homologies of the sloA to sloR genes, as well as other data presented below, suggested that these genes also formed an operon and that this operon could be regulated by binding of SloR to upstream DNA. The DtxR binding site has been identified as an interrupted inverted repeat (54, 59). SirR- (27) and TroR (26, 52)-binding sites containing inverted repeats have also been identified upstream from the LraI operons sitABC and troABCDR, respectively. To locate sequences that could serve as binding sites for SloR, DNA sequences upstream from sloA were compared to the above-mentioned sites and to available sequences upstream from other streptococcal LraI operons. This alignment is shown in Fig. 3A. Inverted repeats were evident in all of the sequences and are indicated by arrows in Fig. 3A and as hairpin loops in Fig. 1B. The length, spacing, and sequence of the repeats varied. A 22-bp segment containing part or all of both repeats and the intervening region was conserved in the sequences from the oral streptococci. The S. pyogenes inverted repeat was similar to the other streptococcal sequences, but the repeats in the remaining bacteria showed little resemblance to the streptococcal repeats or to one another. The distance between the repeats and the start of the adjacent protein also varied, ranging from 39 bp for the S. pneumoniae psaB gene to 20 bp for troA. The variation is even greater if the scaC gene in S. gordonii starts with the ATG codon abutting the inverted repeat, as proposed previously (35), rather than the downstream, in-frame ATG codon shown at the right of Fig. 3A.
FIG. 3.
(A) Potential binding sites for SloR and its homologs. Arrows indicate inverted repeats. Single underlines indicate sequences identical to the putative SloR-binding site. Double underlines indicate sequences shown to be protected by binding of TroR (troA sequence) and DtxR (tox sequence). The dashed underline indicates the sequence of a double-stranded oligonucleotide shown to bind to SirR in a gel retardation assay (27). Bold type indicates potential start codons. Sources of the sequences shown are as in the legend to Fig. 1, as well as accession number L11577 (scaC), Fig. 2 of reference 27 (sitA) and accession number V01536 (tox). (B) Regions upstream from sloA and from sloC in its chromosomal location and in pVA2615. Bold type indicates putative ribosomal-binding sites. Single underlines indicate the EcoRI site, and double underlines indicate start codons. Gaps introduced to optimize alignments are indicated by periods.
Creation and complementation of a sloC mutant.
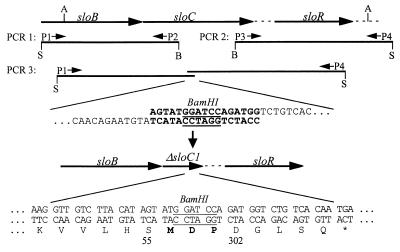
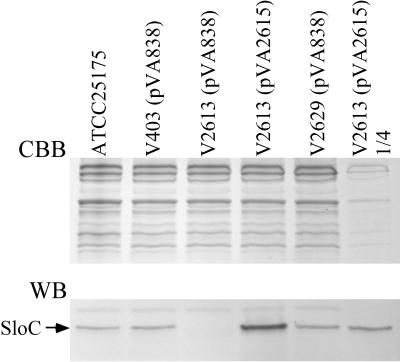
LraI gene products have been implicated in the adherence of S. parasanguis (51) and S. sanguis (22) to saliva-coated hydroxyapatite, a model for tooth adherence, and in the coaggregation of S. gordonii with Actinomyces naeslundii (33). The LraI gene from S. parasanguis, fimA, is also required for virulence in an animal model of endocarditis, possibly because of its contribution to fibrin binding, as demonstrated in vitro (8). Since S. mutans is a recognized agent of both dental caries and infective endocarditis, we were interested in determining whether elimination of the putative LraI lipoprotein SloC would affect caries formation or virulence for endocarditis in animal models. A PCR technique was used (29, 39) to create an in-frame deletion in the sloC gene, as illustrated in Fig. 4. The first two amplifications, using primers P1 to P4 (Table 2), produced ∼1-kb products containing either the 5′ or 3′ ends of the sloC gene and flanking DNA. The primers introduced either SalI or BamHI sites as indicated. The BamHI-containing ends of the two amplicons were complementary to one another, such that use of the two amplicons as a template in a third PCR with primers P1 and P4 resulted in amplification of a product equivalent to the ligation of the first two products at their BamHI sites. The result was the deletion of 741 bp of the sloC gene encoding 247 amino acids and its replacement with 9 bp of synthetic sequence encoding 3 amino acids and containing the BamHI site (which was inserted as a diagnostic aid for monitoring the procedure). It was hoped that the in-frame deletion would eliminate SloC expression without altering the expression of other genes in the putative operon. The deletion derivative of sloC was ligated into the suicide vector pVA891 (42). This construct was introduced into the chromosome of the cariogenic isolate, V403, by a Campbell-type recombination. One transformant which had integrated the mutagenic plasmid as expected was passaged in the absence of antibiotic selection and screened for loss of erythromycin resistance (Ermr) (conferred by the vector). Among 173 colonies examined, two were found to be Erms and were characterized by PCR and Southern blotting. Both strains contained the deleted version of sloC in place of the wild-type gene. One strain was chosen for further experiments and was designated V2613. Figure 5 shows a Western blot of ATCC 25175, V403, and V2613 reacted with polyclonal antiserum raised against the FimA protein of S. parasanguis, along with a gel stained with Coomassie brilliant blue to indicate the relative amount of protein loaded in each lane. Both wild-type S. mutans strains (ATCC 25175 and V403) contained a single strongly reactive band which migrated at ∼38 kDa, presumably SloC. Although the band appeared slightly larger than expected for SloC, it comigrated with FimA from S. parasanguis, which was predicted to be the same size as SloC (data not shown). This band was absent in the sloC mutant, V2613, confirming its identity as SloC.
FIG. 4.
Procedure for creation of an in-frame deletion in sloC. P1 to P4 are PCR primers (see Table 2). The restriction sites shown are AlwNI (A), SalI (S), and BamHI (B). The portions of amplicons resulting from PCR1 and PCR2 that are complementary to one another are indicated in bold, with the internal BamHI site underlined. The amino acid sequence surrounding the site of the deletion of the ΔsloC1 gene product is indicated at the bottom of the figure. Amino acids in bold are those introduced during mutagenesis. The BamHI site is again underlined. The numbering refers to the amino acid coordinates of the wild-type SloC protein.
TABLE 2.
PCR primers used in this study
| Designation | Sequencea |
|---|---|
| P1 | ggcgtcgACAAAATGCCTTGGTTAC |
| P2 | ccatctggatccATACTATGTAAGACAACC |
| P3 | agtatggatcCAGATGGTCTGTCAC |
| P4 | gccgtcgaCCCTTAATAAACATCTTTCG |
| P5 | TTATCGTGATCATCTTGC |
| P6 | CCTACAGCTCCTGCTACG |
| P7 | GCTTATTTGCCTGTACTTC |
| P8 | CACTTCATCATCGCATAG |
| P9 | TTTATGAACTCAGTGAACG |
| P10 | GTAAGCTTTCTTTTCAATGT |
| P11 | TTAGGAAtTCTCTAATATTAACTG |
| P12 | gggaattCTCCTTTTATAAGTAATATAACC |
Native S. mutans sequences are shown in capital letters, while nucleotides added or changed are shown in lowercase letters.
FIG. 5.
Western blot analysis of SloC expression. The top panel shows lysates from the strains indicated above separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue (CBB). The bottom panel shows a Western blot (WB) of identically treated lysates transferred to a nitrocellulose membrane and reacted with rabbit polyclonal anti-FimA antiserum. The position of SloC is indicated. The right-hand lane contains one-fourth the amount of protein loaded in all the other lanes.
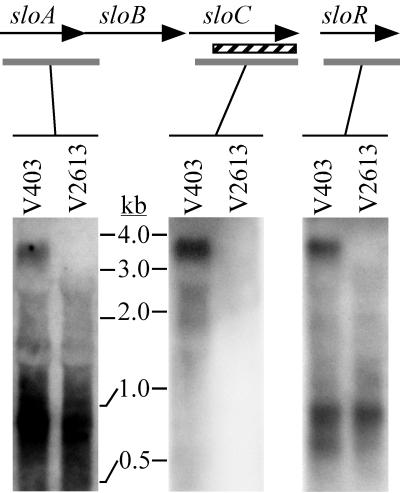
RNA samples from the parental strain (V403) and the sloC mutant (V2613) were examined by Northern blot analysis (Fig. 6). Probes specific for the sloA-sloB region, the sloC gene, and the sloR gene were synthesized by PCR using primers P5 with P6, P7 with P8, and P9 with P10, respectively. All bound to a transcript of 3.5 to 3.7 kb in V403, which compared favorably to the 3.6-kb length expected for a transcript extending from the inverted repeats shown in Fig. 3A to the inverted repeats following sloR. The sloC deletion in V2613 is 732 bp, and so a hybridizing transcript of 2.8 to 3 kb was expected in V2613. No such transcript was observed in any of the blots (Fig. 6). To detect possible strain-specific RNA degradation, a probe from the mevalonate kinase-like gene was reacted with the same RNA samples. Transcripts of 1 and 2.5 to 3 kb were observed with equal intensity in both V403 and V2613 (data not shown).
FIG. 6.
Northern blot analysis of the slo region from S. mutans V403 and V2613. The shaded boxes indicate the locations of the probes used for the blots beneath them. The hatched box indicates the portion of the sloC gene deleted in V2613. The RNA samples in the three panels were obtained from the same batches of cells, with the samples in the first panel being DNase treated and electrophoresed separately. The relative migration of RNA standards on both gels is indicated.
Because of our apparent failure to maintain expression of the rest of the operon while disrupting the sloC gene, another method for assessing the role of sloC in virulence was required. We therefore introduced sloC into V2613 in trans. The gene was cloned as an EcoRI-PvuII fragment into the E. coli-streptococcal shuttle vector pVA838 (43) to create pVA2614 (Fig. 1). However, introduction of pVA2614 into the sloC mutant, V2613, resulted in no SloC expression detectable by Western blotting (data not shown). Since the EcoRI-PvuII fragment contained only 8 bp of upstream sloC sequence and included neither the promoter nor the presumed ribosome-binding site (Fig. 3B), the lack of expression was not surprising. Rather than placing exogenous expression sequences upstream from sloC, native sequences were used. Figure 6 suggests that transcription of the chromosomal sloC gene originated upstream of sloA, in or near the region shown in Fig. 3A. PCR amplification using primers P11 and P12 (Table 2) was used to place a 0.22-kb fragment from the sloA-upstream region into the EcoRI site of pVA2614, creating pVA2615 (Fig. 1). The fragment contained the last 15 bp of the ORF preceding sloA (with 1 bp changed to create an EcoRI site), as well as the intervening sequence between this ORF and sloA, including the potential SloR-binding site shown in Fig. 3A. As shown in Fig. 3B, the first 7 bp upstream from sloC was retained in pVA2615 while the remaining sequence either was shared by the regions upstream from sloA and sloC (the next 12 bp) or was specific to sloA. The sequence and orientation of the inserted DNA were confirmed by restriction analysis and DNA sequence analysis. Introduction of pVA2615 into the sloC mutant, V2613, resulted in overproduction of SloC (Fig. 5). Thus, addition of sloA-upstream sequences provided all the necessary signals for expression of sloC. Densitometric analysis of Fig. 5 indicated that V2613(pVA2615) produced about four times as much SloC as did V403. This conclusion was also apparent from inspection of the lane in which one-fourth the amount of the V2613(pVA2615) lysate was loaded (Fig. 5).
Virulence testing of sloC and sloC-complemented mutants.
Three strains were tested in the gnotobiotic rat model of caries: the wild-type S. mutans strain (V403) and its sloC derivative (V2613), both containing the vector pVA838, and the sloC mutant complemented with the sloC-expressing plasmid, pVA2615. Table 3 shows the results of this study. The relative numbers of plaque bacteria recovered from the three groups of infected rats (six rats per group) are shown in Table 3. No significant differences were found among the three groups, suggesting that colonization and proliferation were not affected by the sloC mutation. Different media were used to assess the identity of the recovered bacteria. TS agar is a nonselective medium, mitis salivarius agar shows some selectivity for oral streptococci (25), and the addition of erythromycin selects for Ermr, which was conferred on the inoculum strains by their recombinant plasmids. The lack of a significant difference in the numbers of colonies obtained on the three different media in any given group indicates that most of the bacteria recovered were derived from the inocula and had not lost their plasmids. At least three representative isolates from each group were also examined by HaeIII digestion of genomic DNA, and all were indistinguishable from the inoculated strains. Thus, the plate counts reflected the relative abundance of the inoculated strains rather than of other oral bacteria. Caries formation was also evaluated on the buccal, sulcal, and proximal surfaces of the molars from each rat. Of all the values shown for the three groups of rats, only two were significantly different—the severity of extensive dentinal lesions on the sulcal surface was lower for the V2613 (pVA2615) group than for the V2613 (pVA838) group.
TABLE 3.
Virulence of S. mutans V403 and ΔsloC1 mutants for caries in gnotobiotic Fischer ratsa
| Infecting strain | Wt of rats (g) | No. of plaque bacteria recovered (104 CFU/ml)b on:
|
Mean caries scoresb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buccal
|
Sulcal
|
Proximal
|
||||||||||||||
| TSA | MS | MS + ERM | E | Ds | Dm | Dx | E | Ds | Dm | Dx | E | Ds | Dm | Dx | ||
| V403(pVA838) | 127 ± 5 | 453 ± 304 | 394 ± 264 | 310 ± 200 | 18.7 ± 0.7 | 16.3 ± 0.3 | 11.7 ± 0.6 | 8.5 ± 0.2 | 22.5 ± 0.4 | 19.3 ± 0.7 | 13.3 ± 0.4 | 7.8 ± 0.5 | 7.7 ± 0.3 | 0.3 ± 0.3 | 0.0 | 0.0 |
| V2613(pVA838) | 140 ± 11 | 103 ± 41 | 111 ± 41 | 104 ± 42 | 20.0 ± 0.9 | 17.3 ± 0.8 | 13.5 ± 1.2 | 10.5 ± 1.1 | 22.8 ± 0.3 | 20.7 ± 0.5 | 14.3 ± 0.7 | 9.3 ± 1.0 | 5.7 ± 1.5 | 1.8 ± 1.3 | 0.5 ± 0.5 | 0.0 |
| V2613(pVA2615) | 133 ± 10 | 106 ± 40 | 143 ± 54 | 80 ± 32 | 19.5 ± 0.5 | 17.0 ± 0.4 | 13.0 ± 0.5 | 9.0 ± 0.4 | 22.2 ± 0.3 | 20.8 ± 0.4 | 13.7 ± 0.6 | 6.7c ± 0.2 | 7.3 ± 0.4 | 2.7 ± 0.7 | 0.5 ± 0.2 | 0.0 |
Rats (six per group) were provided a diet containing 5% sucrose (diet 305) throughout the experiment and sacrificed on experimental day 62.
Mean caries scores are defined as the extent of caries activity on molar surfaces involving enamel (E), dentinal slight (Ds), dentinal moderate (Dm), and dentinal extensive (Dx) lesions. Plaque bacteria were enumerated on TS agar (TSA), mitis salivarius agar (MS), and mitis salivarius agar plus 10 μg of erythromycin per ml. (MS + ERM). All measurements are presented as mean ± standard error of the mean.
Significantly different from V2613(pVA838) (P < 0.05).
The same three strains were tested for virulence in a rat model of endocarditis. The sloC mutant, V2613, was less virulent than its wild-type parent, V403 (P = 0.04) (Table 4, experiment 1). Similar results were obtained previously when a fimA mutant of S. parasanguis was found to be less virulent than the wild type in the same rat model of endocarditis (8). Surprisingly, however, complementation of the sloC mutant with the sloC-bearing plasmid, pVA2615, did not restore virulence.
TABLE 4.
Virulence of S. mutans V403 and ΔsloC1 mutants in a rat model of endocarditis
| Strain | Genotype on chromosome (plasmid) | No. of rats infected/ total no. (% infected) |
|---|---|---|
| Expt 1 | ||
| V403(pVA838) | Wild type | 5/10 (50%) |
| V2613(pVA838) | ΔsloC1 | 0/7 (0%)a |
| V2613(pVA2615) | ΔsloC1 (sloC+) | 1/9 (11%) |
| Expt 2 | ||
| V403(pVA838) | Wild type | 4/10 (40%) |
| V2613(pVA838) | ΔsloC1 | 0/10 (0%) |
| V2629(pVA838) | sloC+ | 6/11 (55%)b |
P = 0.04 with respect to V403(pVA838).
P = 0.01 with respect to V2613(pVA838).
To exclude the possibility that an inadvertent mutation occurring at a secondary site was responsible for the loss of virulence in both the sloC mutant and the complemented mutant, an attempt was made to correct the sloC mutation in V2613. A 2.8-kb AlwNI fragment from pVA2587 was used as a source of wild-type sloBCR DNA (Fig. 1 and 4). This fragment was chosen because its large size would favor efficient homologous recombination and because it was almost identical to the fragment used to create the sloC mutant, except without the sloC deletion; AlwNI cuts 30 bp downstream from the P1 binding site and 11 bp upstream from the P4 binding site (Fig. 4). The purified 2.8-kb fragment was cotransformed into V2613 along with pVA838 at a 10:1 mass ratio. Colonies resistant to 10 μg of erythromycin per ml, and therefore incorporating pVA838, were screened for correction of the chromosomal sloC deletion to wild type by PCR. We reasoned that cells incorporating pVA838 would have a high probability of incorporating the chromosomal AlwNI fragment as well (37). The PCR analysis and a subsequent Southern blot analysis indicated that 1 of 35 transformants examined had incorporated the wild-type sloC gene into the chromosome in place of the deleted version (data not shown). This transformant was designated V2629. SloC expression in V2629 was measured by densitometric analysis of Western blots and found to be within 10% of that of the wild-type strain, V403 (Fig. 5).
The rat endocarditis assay was repeated with V403 (wild-type parent), V2613 (sloC mutant), and V2629 (V2613 with corrected sloC gene), all containing the shuttle vector, pVA838. Table 4, experiment 2, again suggested that the sloC mutation abolished virulence in S. mutans strain V403, although the difference did not reach the level of significance (P = 0.09). Restoration of the chromosomal sloC mutation in V2629 restored virulence (P = 0.01 with respect to V2613).
DISCUSSION
As suggested by the study by Viscount et al. (62), S. mutans has a single LraI lipoprotein gene, sloC, with substantial homology to fimA of S. parasanguis. Previous Western blot analyses using anti-FimA antiserum failed to detect SloC expression in several S. mutans strains, including the type strain, ATCC 25175 (62). Our detection of SloC expression in ATCC 25175 in the present study using the same anti-FimA antiserum may be due to different growth conditions. SloC expression was found to vary substantially according to the growth media used. Also, the abundance of the sloABCR transcript varied by growth phase, being greatest in cells harvested before reaching the stationary phase of growth (data not shown). Finally, although SloC is clearly related to FimA and the homologs listed in Table 1, these SloC homologs, as well as PsaA from S. pneumoniae (50), are all more similar to one another than they are to SloC (data not shown). This variance may result in loss of SloC detection by anti-FimA antiserum under conditions of low SloC expression.
The discovery of the sloR gene potentially encoding a metal-dependent regulator downstream from the sloC gene in S. mutans was initially surprising. LraI operons from other oral streptococci have not been reported to contain such a gene. To determine whether this gene arrangement was found in other S. mutans isolates, a PCR analysis was performed using primers specific for sloC and sloR. More than a dozen strains were tested, and all yielded amplicons of the expected size, indicating that sloC and sloR were arranged in these other strains in the same way as in V403 and ATCC 25175 (data not shown). Also, the ATCC 25175 sequence was searched against the unfinished genomic DNA sequence of S. mutans strain UA159, a clinical isolate from a caries-active child (49). The region from bp 291 (within the sloA gene) through the end of the submitted sequence was found to be 99% identical to the first 3,643 bp of one S. mutans contig. Thus, the linkage of the sloA to sloR genes also occurs in UA159.
Other bacteria are known to contain a dtxR-homologous gene in association with an LraI operon. In the spirochete T. pallidum, the troABCDR operon contains homologs of LraI operon members just upstream from a dtxR homolog, troR (26). In this case, the number and arrangement of the putative ABC transporter genes are different from those in S. mutans (Fig. 1B). Staphylococcus epidermidis also contains a gene for a metal-dependent regulator (sirR) in association with a putative ABC-type iron uptake system, sitABC (27). In this locus, the sirR gene is located upstream from the ABC transporter operon and is transcribed in the opposite orientation (Fig. 1B), although the exact length and sequence of the region separating the two are not reported (27). Finally, a recent publication reports the identification and characterization of an ABC metal import operon in S. pyogenes with homology to the sloA, sloB, and sloC genes (30). The sequence of the operon was obtained from the incomplete S. pyogenes genomic sequencing project. Our further analysis of the available DNA sequence, which has since been assembled into a single contig, indicated the presence of a gene encoding a SloR homolog. The change of a single nucleotide at position 338 in the ORF from a G to a T allows for a potential 215-amino-acid protein with homology to SloR (data not shown). As indicated in Fig. 1B, this ORF was located just upstream from the mtsABC genes and would be transcribed in the opposite orientation.
Even when no physical association exists between LraI family genes and a dtxR homolog, a functional relationship may occur. If the region upstream from sloA shown in Fig. 3A is a binding site for SloR, it would be expected that the homologous sequences in S. parasanguis, S. gordonii, S. pneumoniae, and S. pyogenes would also serve as binding sites for SLoR homologs in those bacteria. As mentioned above, a sloR homolog was identified from the unfinished genomic sequence of S. pyogenes just upstream from the sitABC locus. Furthermore, a similar analysis of the unfinished S. pneumoniae genomic sequence identified a sloR-homologous gene 9.8 kb upstream from the psaBCAD operon on the same contig, as indicated in Fig. 1B. In comparison with the proteins shown in Fig. 2, the SloR homologs identified in S. pneumoniae and S. pyogenes were much more similar to SloR, with amino acid identities of 58 and 56%, respectively (data not shown). It would therefore not be surprising to find that they bind to a DNA sequence similar to that bound by SloR.
The functions of the sloA, sloB, and sloC genes have not been determined, but their homologies suggest that they encode an ABC-type metal uptake system. Both the scaCBA and psaBCA operons are involved in Mn import (17, 34). Table 1 indicates that genes from these operons are among the most similar to the sloABC genes, suggesting that the S. mutans LraI operon may also import manganese. However, the S. pyogenes mtsABC genes, which are also homologous to the sloABC genes, apparently encode an import system with specificity for multiple metals (30). Initial experiments with the S. mutans sloC mutant (V2613) did not reveal growth or transformation defects in standard media (data not shown). The use of metal-free, defined media may reveal a requirement for one or more metals for growth or transformation of this mutant strain, as has been observed with psa, adc, and sca mutants (17, 34).
The function of sloR is also suggested by its similarity to other genes, especially dtxR, sirR, and troR. In C. diphtheriae, DtxR represses transcription of multiple genes in the presence of excess Fe2+ (61). DtxR binds to an inverted-repeat sequence upstream from these genes (Fig. 3A) in vitro when complexed with Fe2+ or certain other metals including Mn2+ (54, 58, 59). The SirR protein binds to the region upstream from the sitABC operon (Fig. 3A) in the presence of Fe2+ or Mn2+ in gel retardation assays (27). The sitABC transcript was shown to be less abundant in cells grown in the presence of Fe2+, and the SitC protein was less abundant in cells grown in excess Fe2+ or Mn2+, suggesting that SirR represses transcription of the sitABC operon in response to excess Fe2+ or Mn2+ (27). The TroR protein from T. pallidum has also been shown to bind an apparent operator sequence upstream from the troABCDR operon (Fig. 3A) in a metal-dependent manner, although in contrast to DtxR and SirR, this binding occurs in the presence of Mn2+ but not in the presence of Fe2+ or several other divalent cations (52). For SirR, TroR, SloR, and the SloR homolog in S. pyogenes, it seems reasonable to assume that the metal(s) that activates the repressor function would be identical to the metal(s) imported by the associated LraI genes. The result would thus be repression of the metal uptake system at times when an abundance of the metal exists. Evidence supporting this assumption has been presented for the T. pallidum (52) and S. epidermidis (27) systems.
The DtxR protein has been well characterized both physically and genetically. Figure 2 indicates amino acids shown previously to be important for DtxR structure and function. Although all three amino acids identified as contributing to metal binding site 1 of DtxR (24) were found in SloR as well as in the other two proteins, only one of three amino acids of the anion-binding site in the work of Goranson-Siekierke et al. (24) or of metal-binding site 2 in the work of Ding et al. (16) were conserved. It was shown by mutagenesis that aspartate was the only amino acid that could functionally substitute for the cysteine in metal-binding site 2 (position 105 of the alignment) in DtxR (60). However, this cysteine is replaced by glutamate in the other three proteins (Fig. 2). At all the positions specified above, SloR and SirR contained the same amino acid sequence when they differed from the DtxR sequence. These findings suggests that these two proteins are structurally and functionally more similar to one another than to DtxR. (TroR appears to be quite different from the other proteins, extending roughly two-thirds their length.)
The virulence tests reported here also shed light on the function of the S. mutans sloABCR operon. Strain V2613 was indistinguishable from its wild-type parent with regard to caries formation or the number of cells recovered from plaque in the rat model used (Table 3). This would suggest that the two functions attributed to LraI proteins in other oral streptococci, i.e., metal uptake and adherence, either are not performed by the slo operon or are not required in this model. Neither possibility can be excluded at this time. In S. pneumoniae and S. gordonii, a requirement for increased Mn2+ in an LraI member mutant is only evident when Mn2+ levels are reduced below about 1 μM (17, 34). A recent study found the level of Mn2+ in human saliva to be about 2 mg/liter (9), or 36 μM, suggesting that LraI-mediated Mn2+ uptake may be required only intermittently in the oral cavity. Also, the sucrose diet consumed by the rats and supplied ad libitum allowed for glucan production by the test strains, which is important for colonization and for caries formation in this animal model (55). This raises the possibility that exopolysaccharide production may substitute in S. mutans for the adherence function performed by LraI lipoproteins in some other oral streptococci. Alternatively, the use of gnotobiotic rats may have obscured a transport or adherence function for SloC that may be required in the environment of the human oral cavity, in which S. mutans must compete with and adhere to other oral bacteria on the tooth surface.
In contrast to the caries model, the rat model of endocarditis revealed a requirement for the slo operon for endocarditis virulence (Table 4). Strain V2613 produced no infections in either experiment. By creating an in-frame deletion in the sloC gene of V2613, we had hoped to maintain expression of the rest of the operon, allowing us to examine the role of sloC in virulence. Figure 6 suggests, however, that V2613 lacks expression of the entire sloABCR operon. The avirulence of strain V2613 could therefore be due to loss of expression of any of these genes. The restoration of virulence by correction of the sloC mutation (strain V2629) but not by provision of the sloC gene in trans [strain V2613(pVA2615)] (Table 4) suggests three possible scenarios. The first is that SloC functions as an adhesin and that lack of this activity is alone responsible for loss of virulence in V2613. The lack of virulence in strain V2613 (pVA2615) would be explained by the assumption that SloC is overexpressed in vivo in this strain as in vitro (Fig. 5) and that SloC overexpression is as detrimental for adherence as is loss of expression. It has been shown that the MsmE lipoprotein of S. mutans, which is part of an ABC sugar transport system, is secreted into the growth medium as well as being found in association with the cell (57). This may also occur with SloC, with overproduction resulting in increased extracellular secretion. The extracellular protein might then compete with cell-bound SloC for available sites on the heart valve, interfering with adherence. Alternatively, SloC may be required for metal uptake in addition to or instead of adherence. In this case, it would be expected that expression of SloC would not restore virulence without concomitant expression of the other members of the transport system, SloA and SloB. It would also be expected that S. mutans would require an Mn2+ uptake system if its Mn2+ requirement is similar to that of other oral streptococci. The concentration of Mn2+ in human serum is about 1 μg/liter (36), or 0.02 μM, and much of this Mn2+ is bound to serum proteins (53), which puts the level of free Mn2+ well below the 1 μM concentration required by LraI mutants of S. gordonii and S. pneumoniae (17). Finally, another possible explanation for our findings is that neither SloC expression nor metal uptake is required for virulence but SloR expression is required. Although this hypothesis cannot currently be excluded, the only study of which we are aware in which mutation of a dtxR homolog has been shown to affect virulence is in Mycobacterium tuberculosis, where virulence is attenuated not by loss of a dtxR homolog but by expression of an altered form of DtxR that is constitutively active (44).
ACKNOWLEDGMENTS
We gratefully acknowledge Cecily C. Harmon for her expertise with the rat caries model, Charlotte J. Hammond for help with microbiological analysis of plaque samples, and Hochong Smith Gilles and Leslie Muldowney Williams for assistance with the endocarditis model.
Support for this project was provided by NIH grants DE09081 and DE08182 to S. M. Michalek and DE04224 to F. L. Macrina. The S. mutans Genome Sequencing Project was funded by a USPHS/NIH grant from the Dental Institute to B. A. Roe, R. Y. Tian, H. G. Jia, Y. D. Qian, S. P. Linn, L. Song, R. E. McLaughlin, M. McShan, and J. Ferretti. The S. pyogenes Genome Sequencing Project was funded by a USPHS/NIH grant to B. A. Roe, S. P. Linn, L. Song, X. Yuan, S. Clifton, R. E. McLaughlin, M. McShan, and J. Ferretti. Sequencing of S. pneumoniae was accomplished with support from the National Institute of Allergy and Infectious Diseases and the Merck Genome Research Institute to The Institute for Genomic Research.
REFERENCES
- 1.Alloing G, Trombe M C, Claverys J P. The ami locus of the Gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of Gram-negative bacteria. Mol Microbiol. 1990;4:633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaeffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen R N, Lunsford R D, Kolenbrander P E. Determination of the transcript size and start site of the putative sca operon of Streptococcus gordonii ATCC 51656 (formerly strain PK488) Adv Exp Med Biol. 1997;418:657–660. doi: 10.1007/978-1-4899-1825-3_153. [DOI] [PubMed] [Google Scholar]
- 4.Bartsevich V V, Pakrasi H B. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 1995;14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartsevich V V, Pakrasi H B. Membrane topology of MntB, the transmembrane protein component of an ABC transporter system for manganese in the cyanobacterium Synechocystis sp strain PCC 6803. J Bacteriol. 1999;181:3591–3593. doi: 10.1128/jb.181.11.3591-3593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- 7.Boyd J, Oza M N, Murphy J R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci USA. 1990;87:5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnette-Curley D, Wells V, Viscount H, Munro C L, Fenno J C, Fives-Taylor P, Macrina F L. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect Immun. 1995;63:4669–4674. doi: 10.1128/iai.63.12.4669-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chicharro J L, Serrano V, Urena R, Gutierrez A M, Carvajal A, Fernandez-Hernando P, Lucia A. Trace elements and electrolytes in human resting mixed saliva after exercise. Br J Sports Med. 1999;33:204–207. doi: 10.1136/bjsm.33.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claros M G, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 11.Cockayne A, Hill P J, Powell N B, Bishop K, Sims C, Williams P. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998;66:3767–3774. doi: 10.1128/iai.66.8.3767-3774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correla F F, DiRienzo J M, McKay T L, Rosan B. scbA from Streptococcus crista CC5A: an atypical member of the lraI gene family. Infect Immun. 1996;64:2114–2121. doi: 10.1128/iai.64.6.2114-2121.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dajani A S. Prevention of bacterial endocarditis: highlights of the latest recommendations by the American Heart Association. Pediatr Infect Dis J. 1998;17:824–825. doi: 10.1097/00006454-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Dassa E, Hofnung M. Sequence of gene malG in E. coli K12: homologies between integral membrane components from binding protein-dependent transport systems. EMBO J. 1985;4:2287–2293. doi: 10.1002/j.1460-2075.1985.tb03928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d'Aubenton C Y, Brody E, Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990;216:835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, Zeng H, Schiering N, Ringe D, Murphy J R. Identification of the primary metal ion-activation sites of the diphtheria tox repressor by X-ray crystallography and site-directed mutational analysis. Nat Struct Biol. 1996;3:382–387. doi: 10.1038/nsb0496-382. [DOI] [PubMed] [Google Scholar]
- 17.Dintilhac A, Alloing G, Granadel C, Claverys J P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 18.Dintilhac A, Claverys J P. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res Microbiol. 1997;148:119–131. doi: 10.1016/S0923-2508(97)87643-7. [DOI] [PubMed] [Google Scholar]
- 19.Fenno J C, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 20.Ganeshkumar N, Arora N, Kolenbrander P E. Saliva-binding protein (SsaB) from Streptococcus sanguis 12 is a lipoprotein. J Bacteriol. 1993;175:572–574. doi: 10.1128/jb.175.2.572-574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganeshkumar N, Hannam P M, Kolenbrander P E, McBride B C. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect Immun. 1991;59:1093–1099. doi: 10.1128/iai.59.3.1093-1099.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganeshkumar N, Song M, McBride B C. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect Immun. 1988;56:1150–1157. doi: 10.1128/iai.56.5.1150-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilson E, Alloing G, Schmidt T, Claverys J P, Dudler R, Hofnung M. Evidence for high affinity binding-protein dependent transport systems in Gram-positive bacteria and in Mycoplasma. EMBO J. 1988;7:3971–3974. doi: 10.1002/j.1460-2075.1988.tb03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goranson-Siekierke J, Pohl E, Hol W G, Holmes R K. Anion-coordinating residues at binding site 1 are essential for the biological activity of the diphtheria toxin repressor. Infect Immun. 1999;67:1806–1811. doi: 10.1128/iai.67.4.1806-1811.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada S, Slade H D. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardham J M, Stamm L V, Porcella S F, Frye J G, Barnes N Y, Howell J K, Mueller S L, Radolf J D, Weinstock G M, Norris S J. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene. 1997;197:47–64. doi: 10.1016/s0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 27.Hill P J, Cockayne A, Landers P, Morrissey J A, Sims C M, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect Immun. 1998;66:4123–4129. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horton R M. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol Biotechnol. 1995;3:93–99. doi: 10.1007/BF02789105. [DOI] [PubMed] [Google Scholar]
- 30.Janulczyk R, Pallon J, Bjorck L. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol Microbiol. 1999;34:596–606. doi: 10.1046/j.1365-2958.1999.01626.x. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson H F. Cell surface protein receptors in oral streptococci. FEMS Microbiol Lett. 1994;121:133–140. doi: 10.1111/j.1574-6968.1994.tb07089.x. [DOI] [PubMed] [Google Scholar]
- 32.Kiska D L, Macrina F L. Genetic analysis of fructan-hyperproducing strains of Streptococcus mutans. Infect Immun. 1994;62:2679–2686. doi: 10.1128/iai.62.7.2679-2686.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolenbrander P E, Andersen R N. Characterization of Streptococcus gordonii (S. sanguis) PK488 adhesin-mediated coaggregation with Actinomyces naeslundii PK606. Infect Immun. 1990;58:3064–3072. doi: 10.1128/iai.58.9.3064-3072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolenbrander P E, Andersen R N, Baker R A, Jenkinson H F. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J Bacteriol. 1998;180:290–295. doi: 10.1128/jb.180.2.290-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolenbrander P E, Andersen R N, Ganeshkumar N. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect Immun. 1994;62:4469–4480. doi: 10.1128/iai.62.10.4469-4480.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krachler M, Rossipal E, Micetic-Turk D. Concentrations of trace elements in sera of newborns, young infants, and adults. Biol Trace Elem Res. 1999;68:121–135. doi: 10.1007/BF02784401. [DOI] [PubMed] [Google Scholar]
- 37.Kretschmer F J, Chang A C, Cohen S N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975;124:225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindler L E, Macrina F L. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J Bacteriol. 1986;166:658–665. doi: 10.1128/jb.166.2.658-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linton K J, Higgins C F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol Microbiol. 1998;28:5–13. doi: 10.1046/j.1365-2958.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 41.Lunsford R D. Recovery of RNA from oral streptococci. BioTechniques. 1995;18:412–414. [PubMed] [Google Scholar]
- 42.Macrina F L, Evans R P, Tobian J A, Hartley D L, Clewell D B, Jones K R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983;25:145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- 43.Macrina F L, Tobian J A, Jones K R, Evans R P, Clewell D B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982;19:345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- 44.Manabe Y C, Saviola B J, Sun L, Murphy J R, Bishai W R. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc Natl Acad Sci USA. 1999;96:12844–12848. doi: 10.1073/pnas.96.22.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 46.Michalek S M, McGhee J R, Navia J M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975;12:69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro C, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munro C L, Macrina F L. Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol Microbiol. 1993;8:133–142. doi: 10.1111/j.1365-2958.1993.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 49.Murchison H H, Barrett J F, Cardineau G A, Curtiss R., III Transformation of Streptococcus mutans with chromosomal and plasmid (pYA629) DNAs. Infect Immun. 1986;54:273–282. doi: 10.1128/iai.54.2.273-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Novak R, Braun J S, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol Microbiol. 1998;29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 51.Oligino L, Fives-Taylor P. Overexpression and purification of a fimbria-associated adhesin of Streptococcus parasanguis. Infect Immun. 1993;61:1016–1022. doi: 10.1128/iai.61.3.1016-1022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posey J E, Hardham J M, Norris S J, Gherardini F C. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheuhammer A M, Cherian M G. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840:163–169. doi: 10.1016/0304-4165(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt M P, Holmes R K. Analysis of diphtheria toxin repressor-operator interactions and characterization of a mutant repressor with decreased binding activity for divalent metals. Mol Microbiol. 1993;9:173–181. doi: 10.1111/j.1365-2958.1993.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder V A, Michalek S M, Macrina F L. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun. 1989;57:3560–3569. doi: 10.1128/iai.57.11.3560-3569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spellerberg B, Rozdzinski E, Martin S, Weber-Heynemann J, Schnitzler N, Lutticken R, Podbielski A. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect Immun. 1999;67:871–878. doi: 10.1128/iai.67.2.871-878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutcliffe I C, Tao L, Ferretti J J, Russell R R B. MsmE, a lipoprotein involved in sugar transport in Streptococcus mutans. J Bacteriol. 1993;175:1853–1855. doi: 10.1128/jb.175.6.1853-1855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao X, Boyd J, Murphy J R. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc Natl Acad Sci USA. 1992;89:5897–5901. doi: 10.1073/pnas.89.13.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao X, Murphy J R. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem. 1992;267:21761–21764. [PubMed] [Google Scholar]
- 60.Tao X, Murphy J R. Cysteine-102 is positioned in the metal binding activation site of the Corynebacterium diphtheriae regulatory element DtxR. Proc Natl Acad Sci USA. 1993;90:8524–8528. doi: 10.1073/pnas.90.18.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao X, Schiering N, Zeng H Y, Ringe D, Murphy J R. Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 62.Viscount H B, Munro C L, Burnette-Curley D, Peterson D L, Macrina F L. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect Immun. 1997;65:994–1002. doi: 10.1128/iai.65.3.994-1002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Minton K W, Fleischmann R D, Ketchum K A, Nelson K E, Salzberg S, Smith H O, Venter J C, Fraser C M. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]