Abstract
Introduction
Balance impairments frequently occur after stroke. Achieving effective core trunk stability is the key to improving balance ability. However, there is still a lack of advanced well-defined rehabilitation protocols for balance improvement in patients with stroke. Intermittent theta-burst stimulation (iTBS) is a non-invasive brain activity modulation strategy that can produce long-term potentiation. The cerebellar vermis is a fundamental structure involved in balance and motor control. However, no study has demonstrated the therapeutic effect and potential mechanism of cerebellar vermis iTBS on balance after stroke.
Methods and analysis
This study will be a prospective single-centre double-blind randomised controlled clinical trial with a 3-week intervention and 3-week follow-up. Eligible participants will be randomly allocated to the experimental group or the control group in a 1:1 ratio. After routine conventional physical therapy, patients in the experimental group will receive cerebellar vermis iTBS, whereas patients in the control group will receive sham stimulation. The overall intervention period will be 5 days a week for 3 consecutive weeks. The outcomes will be measured at baseline (T0), 3 weeks postintervention (T1) and at the 3-week follow-up (T2). The primary outcomes are Berg Balance Scale and Trunk Impairment Scale scores. The secondary outcomes are balance test scores via the Balance Master system, muscle activation of the trunk and lower limbs via the surface electromyography recordings, cerebral cortex oxygen concentrations measured via the resting-state functional near-infrared spectroscopy, Fugl-Meyer Assessment of Lower Extremity and Barthel index scores.
Ethics and dissemination
This study was approved by the West China Hospital Clinical Trials and Biomedical Ethics Committee of Sichuan University. All participants will sign the informed consent form voluntarily. The results of this study will be published in peer-reviewed journals and disseminated at academic conferences.
Trial registration number
ChiCTR2200065369.
Keywords: stroke, protocols & guidelines, motor neurone disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our study comprehensively assesses the trunk control and balance function by clinical scales, balance tests via the Smart Equitest Balance Master System and surface electromyography measurements.
Resting-state functional near-infrared spectroscopy will be used to collect the concentration of oxygenated haemoglobin in the cerebral cortex.
This study lacks a long-term follow-up assessment.
Introduction
Stroke is the third most common cause of disability worldwide.1 The incidents, prevalence and disability-adjusted life-years of stroke have increased over the past two decades2 and are considered to place heavy economic burdens on society. Balance impairments frequently occur in patients with stroke, with a reported incidence ranging from 61% to 83%.3 The main manifestations are postural instability, weak trunk control and difficulty shifting weight,4which ultimately result in falls, poor mobility, decreased physical activity and reduced quality of life in patients.5 Therefore, improvement of balance function is a cardinal requirement in patients with stroke.
The trunk plays a fundamental role in trunk control, balance and mobility during sitting and transferring.6 The synchronised activity of trunk muscles is necessary for maintaining dynamic balance. In addition, proper trunk muscle control is essential for stabilising distal limbs.7 Muscle weakness of the lower limbs is associated with decreased standing balance control.8 Impaired trunk control and core muscle weakness attenuate balance and physical function in individuals after stroke.9 Therefore, achieving effective core trunk stability is crucial to improving balance ability after stroke.
The cerebellum, a central brain structure located in the posterior cranial fossa, works in concert with the cerebral cortex, brainstem and spinal cord and is involved in motor control.10 11 It consists of two lateral hemispheres and the cerebellar vermis. The cerebellar vermis is a fundamental structure involved in balance and motor processing,12 13 and is responsible for regulating the trunk, head, neck and proximal limb muscles to control posture and maintain balance.14 Balance dysfunction in cerebellar disorders is most likely caused by lesions of the medial zone of the cerebellum.15 At present, the main clinical interventions to improve the balance function in stroke rehabilitation are muscle strength training or balance training. The activation of the cerebellar vermis in the central nervous system through neuromodulation with non-invasive brain stimulation has great potential for enhancing balance function in patients with stroke.
Repetitive transcranial magnetic stimulation (rTMS) is a safe, reliable and standardised non-invasive brain activity modulation strategy to regulate cortical excitability and facilitate neural plasticity.16 Intermittent theta-burst stimulation (iTBS) is a novel form of rTMS that can produce long-term potentiation and is more rapid and efficacious than standard rTMS.17 Previously published studies revealed that iTBS over the cerebellar hemisphere could promote gait and balance recovery in patients with chronic ischaemic stroke.18 Similarly, our research group recently provided evidence that iTBS over the cerebellar hemisphere could promote upper limb spasticity, balance and walking performance recovery in patients with stroke.19–21 However, one of the results indicated that the difference in Berg Balance Scale (BBS) scores between the cerebellar iTBS group and the sham stimulation group was 1.58 points, which did not reach the minimal clinically important difference.22 Therefore, the identification of a more effective stimulation target for improving balance function after stroke is necessary. No study has demonstrated the therapeutic effect and potential mechanism of cerebellar vermis iTBS on balance in individuals with stroke. Our preliminary pilot study found that cerebellar vermis iTBS contributed to increasing the excitability of the bilateral supplementary motor areas (SMAs) during balance tasks in healthy adults.23
Objective
Since no clinical research verifying the effectiveness of cerebellar vermis iTBS stimulation has been reported, a randomised controlled double-blind trial will be conducted to determine the effects of cerebellar vermis iTBS on trunk control, muscle activation and balance function in patients with subacute ischaemic stroke. We hypothesise that cerebellar vermis iTBS can promote the activation of trunk and lower limb muscles and increase the excitability of SMAs to improve trunk control and balance function in patients with subacute ischaemic stroke.
Methods
Study design and setting
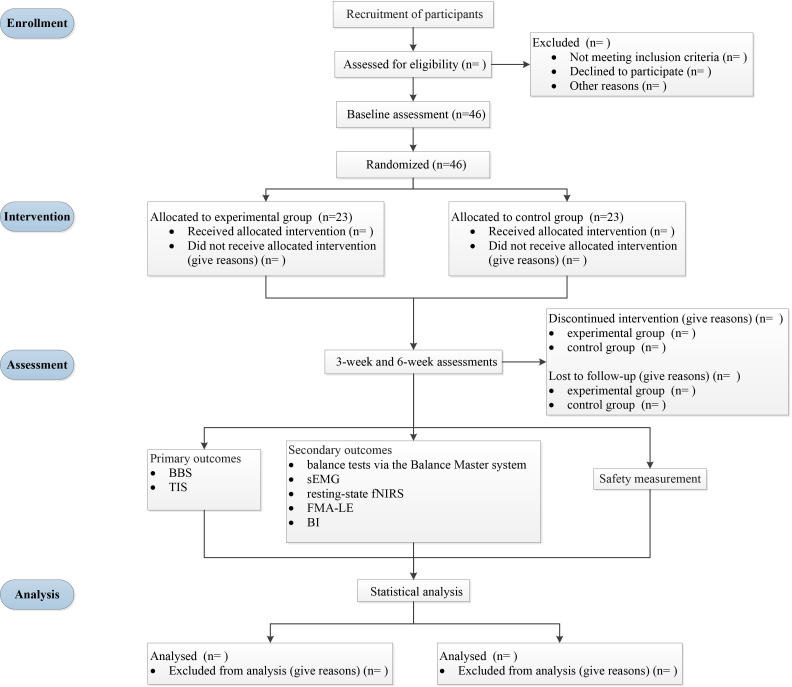
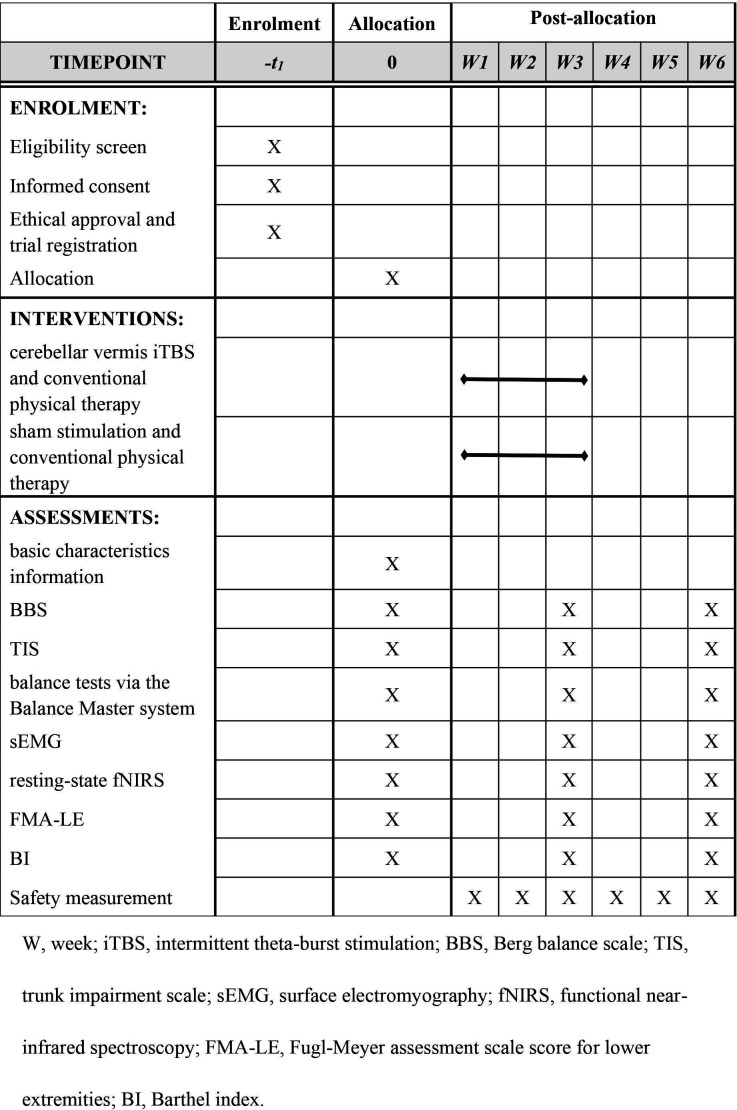
This study will be a prospective single-centre double-blind randomised controlled clinical trial with a 3-week intervention and 3-week follow-up. The protocol strictly follows the Standard Protocol Items: Recommendations for Intervention Trials 2013 Statement.24 Eligible participants will be randomly allocated to the experimental group or control group in a 1:1 ratio. After routine conventional physical therapy, patients assigned to the experimental group will receive cerebellar vermis iTBS, whereas patients assigned to the control group will receive sham stimulation. The overall intervention period will be 5 days a week for 3 consecutive weeks. The outcomes will be measured at baseline (T0), 3 weeks postintervention (T1) and at the 3-week follow-up (T2). The whole study will be performed at the Department of Rehabilitation Medicine of Sichuan University, West China Hospital (Chengdu, Sichuan Province, China). Figure 1 shows the flow diagram of the study design. We plan to start subject recruitment on the first of December 2022 and complete the trial in December 2025. Figure 2 illustrates the study schedule.
Figure 1.
The flow diagram of the study design. BBS, Berg Balance Scale; TIS, Trunk Impairment Scale; sEMG, surface electromyography; fNIRS, functional near-infrared spectroscopy; FMA-LE, Fugl-Meyer Assessment of Lower Extremity; BI, Barthel index.
Figure 2.
The schedule of enrolment, interventions and assessments.
Sample size calculation
The sample size calculation was conducted via G*power of 3.1.9.2 based on the result of the BBS score in our published study, which indicated an estimated effect size of f=0.380.20 Other parameters were set as follows: a significance level of α=0.05 (two tails), power (1–β)=90%, correlation among repeated measures=0.5, non-sphericity correction ε=1, number of measurements=3 and number of groups=2. Therefore, a sample size of n=40 was obtained. After allowing for a 15% dropout rate, a minimum total of 46 participants is needed.
Participants
Recruitment
The participants will be recruited from the Department of Rehabilitation Medicine of Sichuan University West China Hospital in Chengdu, Sichuan Province, China. After carefully screening the inclusion and exclusion criteria, voluntary participants will be required to provide written informed consent before the experiment.
Inclusion criteria
Participants will be considered for inclusion if they meet the following criteria:
A diagnosis of ischaemic stroke according to the Diagnostic criteria of cerebrovascular diseases in China (V. 2019).25
Aged between 18 and 65 years.
First-ever unilateral ischaemic stroke confirmed by imaging examination.
Participants with subacute stroke, the stroke onset ranging from 2 weeks to 6 months.26–28
Having motor deficit and balance dysfunction, with a Fugl-Meyer Assessment for Lower Extremities (FMA-LE) score <34 points and BBS score <56 points.20
Exclusion criteria
Participants will be excluded if they meet any of the following criteria:
Diagnosis of coexisting other neurological diseases.
Injury of cerebellum or brain stem.
Having contraindications for iTBS (eg, history of seizures, intracranial metallic implants, microprocessor implants in the body, tumours and pregnancy).
Cognitive impairment defined as a Mini-Mental State Examination (MMSE) score <27.
Treatment with benzodiazepines, baclofen, antiepileptics and antidepressants.
Interventions
All enrolled participants will receive one session of cerebellar vermis iTBS or sham stimulation before routine conventional physical therapy from Monday to Friday, with a total of 15 sessions. Patients in the experimental group will receive cerebellar vermis iTBS coupled with conventional physical therapy, and those in the control group will receive sham stimulation coupled with conventional physical therapy. The whole intervention period will last for a total of 3 consecutive weeks.
Cerebellar vermis iTBS stimulation
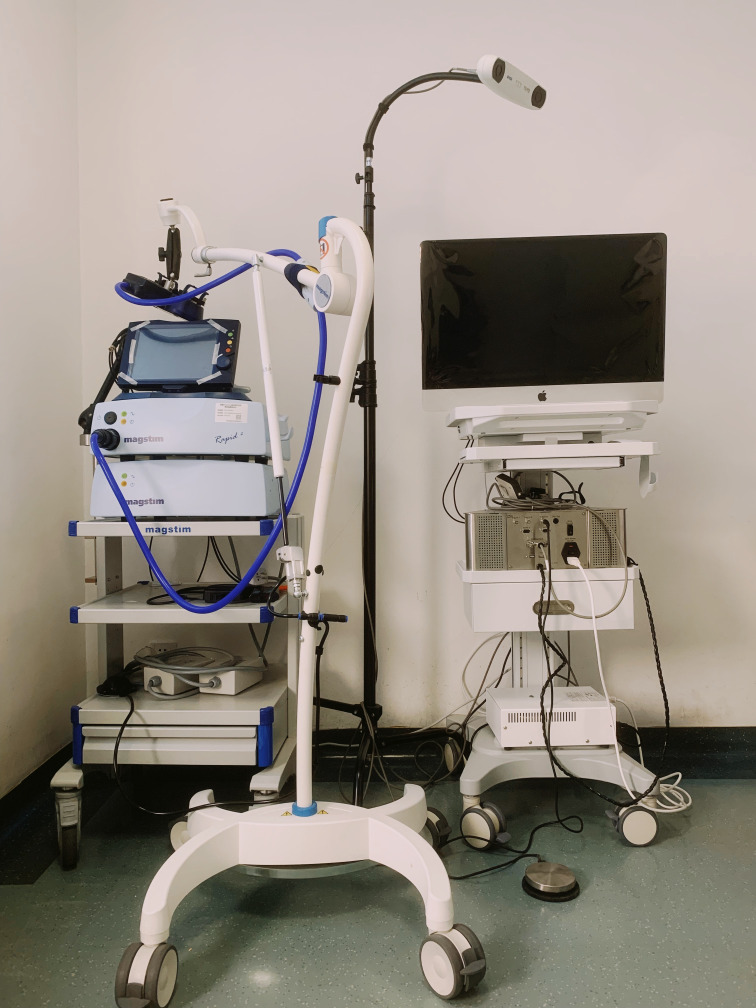
The stimulation protocol will strictly adhere to the safety guidelines and recommendations endorsed by the International Federation for Clinical Neurophysiology in 2021.29 We will use a Magstim Rapid2 stimulator (The Magstim Company Limited) connected to a 70 mm figure-of-8 Double Rapid2 Air Cooled Coil (P/N 3910–00) to stimulate the cerebellar vermis (figure 3). The centre of the coil will be placed tangentially to the target scalp area, and the coil current direction will point downwards. iTBS will be applied over the cerebellar vermis, 1 cm inferior to the inion.30 We will use a neuronavigation system (BrainSightt, Rogue Research Inc.) coupled with a Polaris Vicra infrared camera to ensure that cerebellar vermis iTBS is applied over the same spot for the same participant across different sessions (figure 3). The pattern of iTBS consists of 600 pulses containing 3 pulses at 50 Hz repeated at a rate of 5 Hz, with 20 trains of 10 bursts given at 8 s intervals.31 The standard stimulus intensity will be set at 80% of the active motor threshold, which is the lowest intensity evoking at least 5 out of 10 motor-evoked potentials (MEPs) with a peak-to-peak amplitude >200 µV in the abductor pollicis brevis muscle during 10% of the maximum voluntary contraction measured by a dynamometer.21 If the participant cannot elicit MEPs or cannot tolerate the preset standard stimulus intensity, the stimulator output intensity will be set to the participant’s maximum tolerated intensity.32
Figure 3.
The Magstim Rapid2 stimulator with a BrainSight neuronavigation system.
Sham stimulation
Participants in the control group will be treated identically to those in the experimental group, except the Magstim sham coil (P/N 3950–00) will be used to deliver the sham stimulation.33 The sham coil has the same external appearance, parameters and application methods for stimulating the sensation produced by the real coil without inducing a magnetic field. Therefore, it can sufficiently ensure that the patients remain blinded to the intervention.
Conventional physical therapy
After receiving cerebellar vermis iTBS or sham stimulation, all participants will receive conventional physical therapy, including limb positioning, balance exercise, trunk control, and postural and transfer training, with each session lasting 50 min during the intervention phase.
Criteria for discontinuing the allocated interventions
Interventions will be discontinued for participants if any of the following events occur:
Serious adverse events, such as epilepsy, severe headache, persistent tinnitus and syncope, occur during the stimulation.
Participants withdraw from the trial.
Participants are not compliant with the allocation and intervention plan.
Participants join in additional studies during the trial.
Group exposure for participants and outcome evaluators lead to the failure of blindness.
Improving adherence strategies
To improve the participant compliance, the researcher in charge of the trial will contact the participants regularly to clarify their rehabilitation progress and discuss the subsequent physical therapy programme. Additionally, patients who complete the entire procedure in accordance with the protocol will be provided with a subject fee and an additional free rehabilitation consultation. If a participant drops out, the specific reasons for withdrawal will be recorded.
Outcome measures
On the day of enrolment, the basic characteristics of the participants, including age, sex, type of stroke, lesion site, course of disease, degree of neurological deficit as assessed by the National Institutes of Health Stroke Scale and cognitive function as assessed by the MMSE, will be documented. The outcome assessments will be conducted at the treatment site at T0, T1 and T2. The primary outcomes are BBS and Trunk Impairment Scale (TIS) scores. The secondary outcomes are balance tests via the Balance Master system, muscle activation of the trunk and lower limbs via the surface electromyography (sEMG) recordings, cerebral cortex oxygen concentrations measured via the resting-state functional near-infrared spectroscopy (fNIRS), FMA-LE scores and Barthel Index scores. Each assessment will be performed by a professional clinician or by a qualified physical therapist who will be blinded to the experimental condition of the participant.
Primary outcomes
BBS
The BBS is a well-validated scale for assessing balance among individuals with neurological disease.34 It has high reliability and internal validity, with an intraclass correlation coefficient for intermeasure reliability and intrameasure reliability of 0.97 and 0.98, respectively.35 This scale is a 14-item measure with a total score of 56, and the score of each item ranges from 0 (poor balance) to 4 (good balance).36
Trunk Impairment Scale
The TIS is a scale designed to assess motor impairment of the trunk after stroke, demonstrating the most promising performance in psychometric properties with satisfactory reliability and validity.37 It is a 17-item scale used to evaluate static and dynamic sitting balance and trunk coordination for patients with stroke, with a total score ranging from 0 to 23 points.38 A higher score indicates better trunk control.
Secondary outcomes
Balance tests via the Balance Master system
The assessments of dynamic balance and postural control abilities will be performed by the Sensory Organisation Test (SOT), limits of stability (LOS) and rhythmic weight shift (RWS) via the Smart Equitest Balance Master System (NeuroCom Int., Clackamas, Oregon) (figure 4).
Figure 4.
The Smart Equitest Balance Master System.
Sensory organisation test
The SOT evaluates postural control when participants undergo different somatosensory, visual and vestibular feedback perturbations. During testing, inaccurate interference information is delivered to the patient’s eyes, feet and joints and is controlled through calibrated sway referencing of the support surface and/or visual surroundings. The participant is required to maintain balance to keep their centre of gravity (COG) as steady as possible. A composite equilibrium score is provided to characterise the participant’s overall level of performance through the six conditions described in table 1. During the SOT, each trial lasts for 20 s and is repeated three times.39 40
Table 1.
Sensory organisation test
| Condition | Vision | Surface | Surround | Interference |
| 1 | Eyes open | Stable | Fixed | Null |
| 2 | Eyes closed | Stable | Fixed | Vision |
| 3 | Eyes open | Stable | Unfixed | Vision |
| 4 | Eyes open | Unstable | Fixed | Somatosensation |
| 5 | Eyes closed | Unstable | Fixed | Somatosensation and vision |
| 6 | Eyes open | Unstable | Unfixed | Somatosensation and vision |
Limits of stability
The LOS quantifies the voluntary ability to shift the COG in eight different directions: forwards, forwards-right, right, backwards-right, backwards, backwards-left, left and forwards-left. When the test is performed, a real-time display of the participant’s COG position in relation to targets placed at the centre of the base of support and the stability limits is shown. Once the command is given, the participant must move their COG from a central position out towards one of the eight targets as quickly (up to 8 s) and accurately as possible.41
Rhythmic weight shift
The RWS evaluates a participant’s ability to perform rhythmic movements of their COG from left to right (lateral) and forwards to backwards (anterior/posterior) between two targets at three different speeds (slow, medium and fast).42 Movement velocity and directional control are measured for each direction and speed.
Surface electromyography recordings
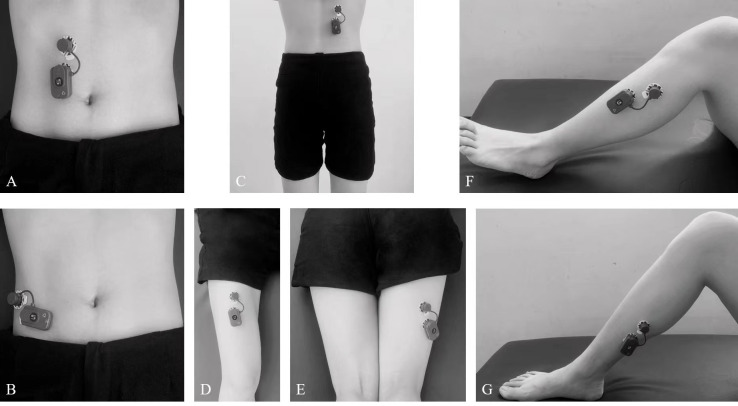
The sEMG recordings will be conducted in accordance with the Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles guidelines.43 A 20-channel wireless BTS-FREEEMG 300 (BTS Biomechanics, Italy) will be used to collect the sEMG signals of the following muscles: bilateral rectus abdominis, external oblique muscle, erector spinae (longissimus), rectus femoris, biceps femoris, tibialis anterior and soleus (table 2 and figure 5 illustrate the sensor locations on the individual muscles). Before starting, the skin will be cleaned using 75% alcohol and would be shaved if needed to ensure a maximum skin impedance below 5 kΩ. After skin preparation, the participant will be put into the starting posture, depending on the muscle at which the electrodes will be placed. A pair of pregelled electrodes certified for medical use and in compliance with the directive 93/42/EEC (amended by 2007/47/EC) will be placed on the belly of the target muscle with an interelectrode distance of 2 cm.44 When the electrodes are placed and fixed, a certified physical therapist will teach the patient to perform the maximum voluntary isometric contraction (MVIC) of the target muscle. For individual muscles, we will record three 3 s MVIC trials with a 2 min rest period between each trial.
Table 2.
The sensor locations on individual muscles for sEMG recordings*
| Muscle | Starting posture of participant | Electrode placement | |
| Location | Orientation | ||
| RA | Supine or standing | 2 cm superior and 2–4 cm lateral to the umbilicus | Vertical |
| EO | Supine or standing | At a 2-finger width above the anterior half of the iliac crest | In the direction of the line from the outside of the 5–12 ribs to the anterior half of the iliac crest |
| Longissimus | Prone with the lumbar vertebral columns slightly flexed | At a 2-finger width lateral from the proc. spin. of L1. | Vertical |
| RF | Sitting on a table with the knees in slight flexion and the upper body bend slightly backwards | At 50% on the line from the anterior spina iliaca superior to the superior part of the patella | In the direction of the line from the anterior spina iliaca superior to the superior part of the patella |
| BF | Lying on the belly with the face down with the thigh down on the table, the knees flexed (to less than 90 degrees), the thigh in a slight lateral rotation and the leg in a slight lateral rotation with respect to the thigh | At 50% on the line between the ischial tuberosity and the lateral epicondyle of the tibia | In the direction of the line between the ischial tuberosity and the lateral epicondyle of the tibia |
| TA | Supine or sitting | At one-third on the line between the tip of the fibula and the tip of the medial malleolus | In the direction of the line between the tip of the fibula and the tip of the medial malleolus |
| Soleus | Sitting with the knee flexed approximately 90 degrees and the heel/foot of the investigated leg on the floor | At two-third of the line between the medial condyle of the femur to the medial malleolus | In the direction of the line between the medial condyle to the medial malleolus |
*According to the SENIAM recommendations for sensor locations for muscles.
BF, biceps femoris; EO, external oblique muscle; RA, rectus abdominis; RF, rectus femoris; sEMG, surface electromyography; SENIAM, Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles; TA, tibialis anterior.
Figure 5.
The sensor locations on individual muscles for sEMG recording (A. rectus abdominis, B. external oblique muscle, C. rector spinae (longissimus), D. rectus femoris, E. biceps femoris, F. tibialis anterior, G. soleus). sEMG, surface electromyography.
sEMG signals will be sampled at 1000 Hz. Collected data will be synchronously transmitted to a BTS EMG-Analyzer (BTS Bioengineering) with the bandpass filtered from 20 Hz to 500 Hz. We will rectify and filter the recorded signal and extract the data of averaged electromyography (AEMG), root mean square (RMS), mean power frequency (MPF) and median frequency (MF) data for subsequent analyses.
Resting-state fNIRS
A multichannel fNIRS system with 24 sources and 24 detectors (NirScan, HuiChuang, China) will be used to record changes in oxygenated haemoglobin (HbO2), deoxygenated Hb and total Hb of the cerebral cortex when the participant is at rest. Relevant parameters will be set as follows: the wavelengths are between 730 nm and 850 nm, the source-detector distance is 3 cm, and the sampling frequency is over 11 Hz. The international 10/20 system is referenced for identifying optodes on the bilateral prefrontal and parietal lobes.45 Collected fNIRS data will be analysed by the NirSpark software package with the bandpass filtering from 0.01 Hz to 0.1 Hz. The mean HbO2 value of each channel will be extracted for statistical analyses.
Fugl-Meyer Assessment of Lower Extremity
The lower extremity function of patients with stroke will be assessed by FMA-LE, which has good inter-rater reliability and concurrent validity.46 The maximum score of this 17-item scale is 34 points. Each item is scored on a 3-point ordinal scale, with 0 points for inability, 1 point for partial ability and 2 points for full ability to perform the required movement.47
Barthel index
The Barthel index is a self-reported scale comprising of 10 items, including bathing, grooming, bladder management, bowel management, dressing, feeding, toilet use, transfers, ascending and descending stairs, and walking, to measure basic activities of daily living.48 The total scores vary from 0 (totally dependent) to 100 (independent). This scale has good clinimetric properties and excellent interrater reliability with standardised administration for patients with stroke.19 49
Safety measurements
Possible stimulation-related adverse events, such as headache, nausea, neck pain, seizure, mood changes, fatigue, tinnitus, dizziness, sleepiness and syncope, are listed in the informed consent form. An adverse reaction record will be used to monitor and provide detailed reports after each stimulation. In addition, any adverse events related to conventional physical therapy will also be recorded using the adverse event case report form (CRF).
Randomisation and blinding
The study will be a randomised, double-blind, sham-controlled trial. Enrolled participants will be randomly assigned based on the computer-generated random numbers that are concealed in opaque numbered envelopes and opened in numerical order by a neutral non-involved researcher. We plan to blind the participants and evaluators. If blinding fails, the participants will be removed. A sham coil will be used to ensure that the patients are blinded to the intervention. Outcome evaluations will be conducted by a professional clinician or by a qualified physical therapist who is blinded to the group assignment. An independent researcher will be designated to complete data analysis. Unblinding will be carried out after the data analysis is completed. In the case of serious adverse events occurring during interventions, emergency unblinding will also be implemented.
Data management and analysis
Data management
Data will be recorded on CRFs in a timely, complete and accurate manner. Two researchers will independently input data into Excel software and cross-check each other. Thus, electronic data will be stored and available to the relevant researcher only. The West China Hospital Clinical Trials and Biomedical Ethics Committee of Sichuan University are responsible for monitoring the safety and process of the study and have the right to terminate the trial if serious advent events occur. All procedures will comply with the confidentiality standards for medical data.
Statistical analysis
Statistical analyses will be performed using GraphPad Prism V.8.4.3 (GraphPad Software, La Jolla, California) based on the intention-to-treat principle. Missing data will be imputed using the last observation carried forwards approach. The Shapiro-Wilk test will be conducted to evaluate the normal distribution of the data. The level of significance is set at α=0.05. Continuous variables, ordinal variables and categorical variables will be presented as mean (±SD), medians (IQR) and number (percentage, %), respectively. Based on different types of data, the independent-samples t test, Mann-Whitney U test and χ2 test will be used to compare demographic and baseline data between groups. Two-way mixed measures analysis of variance with group as the between-individual factor and time as the within-individual factor will be performed for outcome measures analyses. Non-sphericity correction will be conducted using the Greenhouse-Geisser correction if necessary, and Tukey’s post hoc multiple comparison test will be applied.
Patient and public involvement
Patients and the public will not be involved in the study design, recruitment, implementation or reporting. However, the study results will be disseminated to the public through academic papers and conferences.
Ethical approval, trial registration and dissemination
The study was approved by the West China Hospital Clinical Trials and Biomedical Ethics Committee of Sichuan University on 19 May 2022 (ethics reference: 2022 (573)) and will be conducted in accordance with the Declaration of Helsinki.
This protocol was registered on 3 November 2022, in the Chinese Clinical Trial Registry. This trial is a subproject of the National Natural Science Foundation of China with the registration number ChiCTR2200061225. All participants will be fully informed of the study procedures and sign the informed consent form voluntarily before inclusion (see the online supplemental appendix). The private information of all participants will be kept confidential and securely placed in a locked cabinet and will only be accessible to researchers of the study. However, the results of this study will be published in peer-reviewed journals and disseminated at academic conferences.
bmjopen-2022-066356supp001.pdf (120.1KB, pdf)
Discussion
At present, no research has revealed the effect and potential mechanism of cerebellar vermis iTBS on balance in patients with subacute stroke. This prospective single-centre double-blind randomised controlled clinical trial with a 3-week intervention and 3-week follow-up is designed to confirm its effectiveness.
Our study will comprehensively assess trunk control and balance function by clinical scales, balance tests via the Smart Equitest Balance Master System and sEMG measurements. Additionally, we will also collect the concentration of HbO2 in the cerebral cortex via resting-state fNIRS. The integrated data results will sufficiently verify the research hypothesis.
For trunk control, the TIS scores can reveal motor impairment of the trunk in patients with stroke. The sEMG signal reflects the activation of muscles directly and contains information about movement intentions generated by the brain.50 AEMG represents the degree of muscle activation and the synchronisation of activated motor units. RMS quantifies the effort of the muscle. MPF and MF are frequency domain features and indicate muscle fatigue.51 52
For balance function, the BBS score reflects the overall performance of static and dynamic balance. Accurate integration of sensory information is critical to maintaining balance. The composite equilibrium score of the SOT characterises the impairments of individual sensory systems.53 The ability to voluntarily move the COG within the LOS is fundamental to mobility tasks. By the LOS test, reaction time, movement velocities and excursions are recorded to measure the voluntary ability to shift the COG without losing balance. Reaction time reflects the cognitive processing ability. Movement velocities indicate high-level central nervous system function. Excursions can be restricted by biomechanical deficits.54 Overall, limitations in the LOS are associated with instability during weight-shifting activities. RWS measures movement velocity and directional control during rhythmic movements. Patients with disrupted normal rhythmic movement control exhibit reduced velocities and/or poor directional control ability.55
For cortical activation, fNIRS is a widespread non-invasive measurement that provides real-time monitoring of haemodynamic signals to reflect changes in brain activation.56 Increased HbO2 is positively correlated with cortical excitability. In addition, balance function and postural stability are positively related to the changes in HbO2 signals in the bilateral SMAs in patients with stroke.57 Additionally, our previous work revealed that single-session cerebellar vermis iTBS can increase bilateral SMAs excitability during balance tasks in healthy adults.23
We hypothesise that cerebellar vermis iTBS can promote the activation of muscles in the trunk and lower limbs and increase the excitability of the SMAs to improve trunk control and balance function in patients after stroke. The cerebellar vermis plays an important role in postural tone, balance and locomotion through descending spinal pathways since the vermis receives vestibulocerebellar and proprioceptive spinocerebellar afferents.58 SMA contributes to anticipatory postural adjustments and postural stability during gait initiation.59 iTBS consists of high-frequency stimulation bursts that strongly modulate the neural activity of the cerebellar vermis. Studies with humans have shown that iTBS drives acute changes to motor behaviour and neuronal excitability.60 A possible mechanism has been reported by an animal study showing that iTBS can promote neural structural remodelling and functional recovery by enhancing neurogenesis and migration via the miR-551b-5p/BDNF/TrkB pathway.61 The first study of cerebellar vermis stimulation was reported in 1995, which investigated its effects on saccade metrics in a man via TMS.62 At present, researchers have reported that cerebellar vermis stimulation is a safe and well-tolerated brain stimulation technology with a potential therapeutic effect on schizophrenia.63 In addition, cerebellar vermis rTMS can induce a suppressive effect on pharyngeal motor cortical activity and swallowing behaviour.64 However, limited studies have reported that the cerebellar vermis plays an important role in postural response and balance stability.13 65 Therefore, we hope to identify the effectiveness of cerebellar vermis iTBS in trunk control and balance function for patients with subacute ischaemic stroke. Our results may provide valuable information for developing a novel treatment method for the rehabilitation of balance dysfunction after stroke.
Supplementary Material
Footnotes
Contributors: Conceptualisation, validation and original draft: YC. Recruitment: LH, H-HJ and Q-CW. Data collection: YC, WS and H-XT. Data analysis: C-FG and Q-FG. Manuscript review and editing: C-FG and QG. QG designed the trial and was responsible for the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by the National Natural Science Foundation of China grant number NSFC 82172540.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hankey GJ. Stroke. Lancet 2017;389:641–54. 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- 3.Komiya M, Maeda N, Narahara T, et al. Effect of 6-week balance exercise by real-time postural feedback system on walking ability for patients with chronic stroke: a pilot single-blind randomized controlled trial. Brain Sci 2021;11:1493. 10.3390/brainsci11111493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang C, Yang J, et al. Comparing the effects of short-term Liuzijue exercise and core stability training on balance function in patients recovering from stroke: a pilot randomized controlled trial. Front Neurol 2022;13:748754. 10.3389/fneur.2022.748754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Gao Q, He C-Q, et al. Effect of virtual reality on balance in individuals with Parkinson disease: a systematic review and meta-analysis of randomized controlled trials. Phys Ther 2020;100:933–45. 10.1093/ptj/pzaa042 [DOI] [PubMed] [Google Scholar]
- 6.Luque-Moreno C, Kiper P, Solís-Marcos I, et al. Virtual reality and physiotherapy in post-stroke functional re-education of the lower extremity: a controlled clinical trial on a new approach. J Pers Med 2021;11:1210. 10.3390/jpm11111210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesi G, Ballardini G, Barone L. Modified functional reach test: upper-body kinematics and muscular activity in chronic stroke survivors. Sensors 2021;22:230. 10.3390/s22010230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schröder J, Saeys W, Yperzeele L, et al. Time course and mechanisms underlying standing balance recovery early after stroke: design of a prospective cohort study with repeated measurements. Front Neurol 2022;13:781416. 10.3389/fneur.2022.781416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karthikbabu S, Verheyden G. Relationship between trunk control, core muscle strength and balance confidence in community-dwelling patients with chronic stroke. Top Stroke Rehabil 2021;28:88–95. 10.1080/10749357.2020.1783896 [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo E. Physiology of the cerebellum. Handb Clin Neurol 2018;154:85–108. 10.1016/B978-0-444-63956-1.00006-0 [DOI] [PubMed] [Google Scholar]
- 11.Roostaei T, Nazeri A, Sahraian MA, et al. The human cerebellum: a review of physiologic neuroanatomy. Neurol Clin 2014;32:859–69. 10.1016/j.ncl.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 12.De Bartolo P, Florenzano F, Burello L, et al. Activity-dependent structural plasticity of Purkinje cell spines in cerebellar vermis and hemisphere. Brain Struct Funct 2015;220:2895–904. 10.1007/s00429-014-0833-6 [DOI] [PubMed] [Google Scholar]
- 13.Lam CK, Tokuno CD, Staines WR, et al. The direction of the postural response to a vestibular perturbation is mediated by the cerebellar vermis. Exp Brain Res 2016;234:3689–97. 10.1007/s00221-016-4766-6 [DOI] [PubMed] [Google Scholar]
- 14.Marfeo A. Neuroanatomy through clinical cases: Inc. publishers 2010.
- 15.Ilg W, Giese MA, Gizewski ER, et al. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 2008;131:2913–27. 10.1093/brain/awn246 [DOI] [PubMed] [Google Scholar]
- 16.Ille S, Kelm A, Schroeder A, et al. Navigated repetitive transcranial magnetic stimulation improves the outcome of postsurgical paresis in glioma patients – a randomized, double-blinded trial. Brain Stimul 2021;14:780–7. 10.1016/j.brs.2021.04.026 [DOI] [PubMed] [Google Scholar]
- 17.Chen S-C, Yang L-Y, Adeel M, et al. Transcranial electrostimulation with special waveforms enhances upper-limb motor function in patients with chronic stroke: a pilot randomized controlled trial. J Neuroeng Rehabil 2021;18:106. 10.1186/s12984-021-00901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch G, Bonnì S, Casula EP, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke. JAMA Neurol 2019;76:170–8. 10.1001/jamaneurol.2018.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wei Q-C, Zhang M-Z, et al. Cerebellar intermittent theta-burst stimulation reduces upper limb spasticity after subacute stroke: a randomized controlled trial. Front Neural Circuits 2021;15:655502. 10.3389/fncir.2021.655502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao L-Y, Xie Y-J, Chen Y, et al. Cerebellar theta-burst stimulation combined with physiotherapy in subacute and chronic stroke patients: a pilot randomized controlled trial. Neurorehabil Neural Repair 2021;35:23–32. 10.1177/1545968320971735 [DOI] [PubMed] [Google Scholar]
- 21.Xie Y-J, Wei Q-C, Chen Y, et al. Cerebellar theta burst stimulation on walking function in stroke patients: a randomized clinical trial. Front Neurosci 2021;15:688569. 10.3389/fnins.2021.688569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badke MB, Sherman J, Boyne P, et al. Tongue-based biofeedback for balance in stroke: results of an 8-week pilot study. Arch Phys Med Rehabil 2011;92:1364–70. 10.1016/j.apmr.2011.03.030 [DOI] [PubMed] [Google Scholar]
- 23.Tan H-X, Wei Q-C, Chen Y, et al. The immediate effects of intermittent theta burst stimulation of the cerebellar vermis on cerebral cortical excitability during a balance task in healthy individuals: a pilot study. Front Hum Neurosci 2021;15. 10.3389/fnhum.2021.748241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinese Society of Neurology CSS . Diagnostic criteria of cerebrovascular diseases in China (version 2019). Chinese J Neurol 2019;52:710–5. [Google Scholar]
- 26.Hayward KS, Kramer SF, Dalton EJ, et al. Timing and dose of upper limb motor intervention after stroke: a systematic review. Stroke 2021;52:3706–17. 10.1161/STROKEAHA.121.034348 [DOI] [PubMed] [Google Scholar]
- 27.Soulard J, Huber C, Baillieul S, et al. Motor tract integrity predicts walking recovery: a diffusion MRI study in subacute stroke. Neurology 2020;94:e583–93. 10.1212/WNL.0000000000008755 [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable Taskforce. Int J Stroke 2017;12:444–50. 10.1177/1747493017711816 [DOI] [PubMed] [Google Scholar]
- 29.Rossi S, Antal A, Bestmann S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol 2021;132:269–306. 10.1016/j.clinph.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg S, Sinha VK, Tikka SK, et al. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res 2016;243:413–20. 10.1016/j.psychres.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 31.Huang Y-Z, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 32.Spampinato D, Ibáñez J, Spanoudakis M, et al. Cerebellar transcranial magnetic stimulation: the role of coil type from distinct manufacturers. Brain Stimul 2020;13:153–6. 10.1016/j.brs.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 33.Kumar N, Vishnubhatla S, Wadhawan AN, et al. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul 2020;13:840–9. 10.1016/j.brs.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 34.Meseguer-Henarejos A-B, Rubio-Aparicio M, López-Pina J-A, et al. Characteristics that affect score reliability in the Berg balance scale: a meta-analytic reliability generalization study. Eur J Phys Rehabil Med 2019;55:570–84. 10.23736/S1973-9087.19.05363-2 [DOI] [PubMed] [Google Scholar]
- 35.Hyun S-J, Lee J, Lee B-H. The effects of sit-to-stand training combined with real-time visual feedback on strength, balance, gait ability, and quality of life in patients with stroke: a randomized controlled trial. Int J Environ Res Public Health 2021;18:12229. 10.3390/ijerph182212229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou H-Y, Lo Y-C, Tsai Y-W, et al. Increased anxiety and depression symptoms in post-acute care patients with stroke during the COVID-19 pandemic. Int J Environ Res Public Health 2021;19:162. 10.3390/ijerph19010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Chau JPC, Zang Y, et al. Psychometric properties of the Chinese version of the trunk impairment scale in people with a stroke. Health Qual Life Outcomes 2021;19:85. 10.1186/s12955-021-01730-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thijs L, Voets E, Wiskerke E, et al. Technology-supported sitting balance therapy versus usual care in the chronic stage after stroke: a pilot randomized controlled trial. J Neuroeng Rehabil 2021;18:120. 10.1186/s12984-021-00910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin L, Liu K, Liu C, et al. Effect of kinesiology tape on muscle activation of lower extremity and ankle Kinesthesia in individuals with unilateral chronic ankle instability. Front Physiol 2021;12:786584. 10.3389/fphys.2021.786584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bultitude JH, Pidgeon DM, LeBlanc PR, et al. Two weeks of twice-daily prism adaptation treatment does not improve posture or gait in Parkinson’s disease: a double-blind randomized controlled trial. Trials 2021;22:846. 10.1186/s13063-021-05832-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolářová B, Janura M, Svoboda Z, et al. Postural control strategies and balance-related factors in individuals with traumatic transtibial amputations. Sensors 2021;21:7284. 10.3390/s21217284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi-Izquierdo M, Santos-Pérez S, Faraldo-García A, et al. Impact of obesity in elderly patients with postural instability. Aging Clin Exp Res 2016;28:423–8. 10.1007/s40520-015-0414-4 [DOI] [PubMed] [Google Scholar]
- 43.Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361–74. 10.1016/S1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, Chen L, Zhang L, et al. Effectiveness of a novel contralaterally controlled neuromuscular electrical stimulation for restoring lower limb motor performance and activities of daily living in stroke survivors: a randomized controlled trial. Neural Plast 2022;2022:5771634 10.1155/2022/5771634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W, Wu X, Zhang T, et al. Narrowband resting-state fNIRS functional connectivity in autism spectrum disorder. Front Hum Neurosci 2021;15:643410. 10.3389/fnhum.2021.643410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y-L, Lin G-H, Huang Y-J, et al. Refining 3 measures to construct an efficient functional assessment of stroke. Stroke 2017;48:1630–5. 10.1161/STROKEAHA.116.015516 [DOI] [PubMed] [Google Scholar]
- 47.Akazawa N, Kishi M, Hino T, et al. Increased intramuscular adipose tissue of the quadriceps is related to decreased activities of daily living in patients who have had a stroke. Nutrition 2021;90:111277. 10.1016/j.nut.2021.111277 [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Tsang RC, Zhou J, et al. Relationship of Barthel index and its short form with the modified Rankin scale in acute stroke patients. J Stroke Cerebrovasc Dis 2020;29:105033. 10.1016/j.jstrokecerebrovasdis.2020.105033 [DOI] [PubMed] [Google Scholar]
- 49.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: Clinimetric and clinical considerations. Clin Interv Aging 2013;8:201–11. 10.2147/CIA.S32405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qing Z, Lu Z, Cai Y, et al. Elements influencing sEMG-Based gesture decoding: muscle fatigue, forearm angle and acquisition time. Sensors 2021;21:7713. 10.3390/s21227713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei P, Zhang J, Wang B, et al. Surface electromyography and electroencephalogram-based gait phase recognition and correlations between cortical and locomotor muscle in the seven gait phases. Front Neurosci 2021;15:607905. 10.3389/fnins.2021.607905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhusuny A, Cook M, Khalil A, et al. The relationship between visual impairments and activity of the neck/shoulder muscles among surgeons during simulated surgical tasks. Surg Endosc 2022;36:5326–38. 10.1007/s00464-021-08913-0 [DOI] [PubMed] [Google Scholar]
- 53.Acuña SA, Tyler ME, Thelen DG. Individuals with chronic mild-to-moderate traumatic brain injury exhibit decreased neuromuscular complexity during gait. Neurorehabil Neural Repair 2022;15459683221081064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho GF, Becnel AR, Miske C, et al. Postural control impairment in patients with headaches—A systematic review and meta‐analysis. Headache 2022;62:241–70. 10.1111/head.14281 [DOI] [PubMed] [Google Scholar]
- 55.Chen C-L, Chen F-F, Lin C-H, et al. Effect of anterior ankle-foot orthoses on weight shift in persons with stroke. Arch Phys Med Rehabil 2015;96:1795–801. 10.1016/j.apmr.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita S, Tamashiro H, Okamoto T, et al. Association between imbalance of cortical brain activity and successful motor recovery in sub-acute stroke patients with upper limb hemiparesis. Neuroreport 2019;30:822–7. 10.1097/WNR.0000000000001283 [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto H, Mihara M, Hattori N, et al. Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuroimage 2014;85:547–54. 10.1016/j.neuroimage.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 58.Maiti B, Rawson KS, Tanenbaum AB, et al. Functional connectivity of vermis correlates with future gait impairments in Parkinson’s disease. Mov Disord 2021;36:2559–68. 10.1002/mds.28684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richard A, Van Hamme A, Drevelle X, et al. Contribution of the supplementary motor area and the cerebellum to the anticipatory postural adjustments and execution phases of human gait initiation. Neuroscience 2017;358:181–9. 10.1016/j.neuroscience.2017.06.047 [DOI] [PubMed] [Google Scholar]
- 60.Tang AD, Bennett W, Bindoff AD, et al. Subthreshold repetitive transcranial magnetic stimulation drives structural synaptic plasticity in the young and aged motor cortex. Brain Stimul 2021;14:1498–507. 10.1016/j.brs.2021.10.001 [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Zhou Y, Chen X, et al. Long-term iTBS promotes neural structural and functional recovery by enhancing neurogenesis and migration via miR-551b-5p/BDNF/TrkB pathway in a rat model of cerebral ischemia-reperfusion injury. Brain Res Bull 2022;184:46–55. 10.1016/j.brainresbull.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto M, Ohtsuka K. Transcranial magnetic stimulation over the posterior cerebellum during visually guided saccades in man. Brain 1995;118:1185–93. 10.1093/brain/118.5.1185 [DOI] [PubMed] [Google Scholar]
- 63.Escelsior A, Belvederi Murri M, Calcagno P, et al. Effectiveness of cerebellar circuitry modulation in schizophrenia. Journal of Nervous & Mental Disease 2019;207:977–86. 10.1097/NMD.0000000000001064 [DOI] [PubMed] [Google Scholar]
- 64.Sasegbon A, Niziolek N, Zhang M, et al. The effects of midline cerebellar rTMS on human pharyngeal cortical activity in the intact swallowing motor system. Cerebellum 2021;20:101–15. 10.1007/s12311-020-01191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colnaghi S, Honeine J-L, Sozzi S, et al. Body sway increases after functional inactivation of the cerebellar vermis by cTBS. Cerebellum 2017;16:1–14. 10.1007/s12311-015-0758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066356supp001.pdf (120.1KB, pdf)