Abstract
Coffee is one of the most important cash crops in Yunnan Province, China. Yunnan is ranked as the biggest producer of high-quality coffee in China. During surveys of microfungi from coffee plantations in Yunnan, six fungal strains that resemble Nigrogranaceae were collected. Multi-gene analyses of a combined SSU-LSU-ITS-rpb2-tef1-α sequence data matrix were used to infer the phylogenetic position of the new species in Nigrograna while morphological characteristics were used to deduce the taxonomic position of the new species. Six fungal strains isolated from decaying branches of Coffeaarabica represent three new saprobic species in Nigrograna. The three new species, N.asexualis, N.coffeae, and N.puerensis, are described with full (macro and micro characteristics) descriptions, illustrations, and a phylogenetic tree that shows the phylogenetic position of new taxa.
Keywords: 3 new taxa, Coffeaarabica , Nigrogranaceae, phylogeny, saprobic fungi, taxonomy
Introduction
Coffee (Coffea L.) was first planted in Yunnan Province, China in 1982 (Zhang et al. 2014). To date, about 170 varieties of coffee (Global Biodiversity Information Facility database (GBIF), available at: https://www.gbif.org/species/2895315 (accessed on 07 November 2022)) are available in the world, of which Coffeaarabica L. is the most popular coffee accounting for 75% of the world’s production, while 25% is provided by C.canephora Pierre ex A. Froehner, and less than 1% by C.liberica W. Bull and other varieties (Sharma 2020). The coffee production in Yunnan Province is approximately 90% of China’s total coffee production (Neilson and Wang 2019), while Pu’er is the largest coffee planting area in Yunnan, in terms of the highest yield and the best quality (Li 2014).
Fungal diversity is highly uncertain; the current estimated numbers are between 1.5 to 12 million, of which about 150,000 species have been named and classified (Hawksworth and Lücking 2017; Hyde et al. 2020; Bhunjun et al. 2022). Fungi are important organisms in terrestrial and aquatic ecosystems that are involved in the decomposition and nutrient cycling of dead plant material (Hyde et al. 2020; Bhunjun et al. 2022; Phukhamsakda et al. 2022). Also, saprobic fungi play vital roles in soil food chains, decomposition of plant, and animal materials, and solubilization of phosphorous (Dighton 2003; Pandey et al. 2008). However, coffee saprobic fungi have been poorly investigated (Arias and Abarca 2014; Lu et al. 2022a). Coffee saprobic fungi are distributed in 15 orders, and among them, Pleosporales Luttr. is the most common order (Lu et al. 2022a).
Pleosporales, belonging to Dothideomycetes O.E. Erikss. & Winka, was first proposed by Luttrell (1955), and later it was formally established by Barr (1987). In 2021, it consists of 91 families and 614 genera as the largest order (Hongsanan et al. 2020a; Wijayawardene et al. 2022). They are distributed in terrestrial and aquatic habitats (Zhang et al. 2008; Jiang et al. 2021). The members of Pleosporales are characterized by perithecioid and ostiolar ascomata, with or without periphyses, presence of cellular pseudoparaphyses, bitunicate, with ocular chambers or apical ring asci, various shapes of ascospores, with pigmentation and septation, and sheath present or absent (Zhang et al. 2012; Tennakoon et al. 2021; Yang et al. 2022).
Nigrogranaceae Jaklitsch & Voglmayr (Pleosporales) was proposed as a new family by Jaklitsch and Voglmayr (2016) to accommodate Nigrograna Gruyter, Verkley & Crous as the type genus. Liu et al. (2017) estimated that the divergence time of Nigrogranaceae is around 79 (44–124) Mya in crown age and 131 (86–180) Mya in stem age. Nigrogranaceae is monotypic, and they exist as endophytic, human pathogenic, and saprobic lifestyles (Hongsanan et al. 2020b; Zhang et al. 2020; Boonmee et al. 2021). The sexual morph of Nigrogranaceae is characterized by globose and black, ostiolar, clavate, and fissitunicate ascomata, with a short stipe and asci with a knob-like base, fusoid to narrowly ellipsoid, septate, and smooth or faintly verruculose ascospores (Jaklitsch and Voglmayr 2016). The asexual morph is characterized by pycnidia similar to ascomata, filiform and branched conidiophores, ampulliform or lageniform phialides, rod-like to ellipsoid, and hyaline or sub-hyaline conidia (Jaklitsch and Voglmayr 2016).
Nigrograna was introduced by de Gruyter (2012) with N.mackinnonii (Borelli) Gruyter, Verkley & Crous (basionym: Pyrenochaetamackinnonii Borelli) as the type species. Pyrenochaetamackinnonii was reported from a mycetoma patient by Borelli (1976), but it was found to be remote from the generic type species P.nobilis De Not. (de Gruyter et al. 2010, 2013). Since it was not possible to determine which family in PleosporalesP.mackinnonii belongs to, only the new genus Nigrograna was introduced to accommodate P.mackinnonii and named as N.mackinnonii (de Gruyter 2012). Later, Nigrograna was used as a synonym of Biatriospora K.D. Hyde & Borse, as N.mackinnonii is phylogenetically closely related to the type species of Biatriospora (B.marina K.D. Hyde & Borse) (Ahmed et al. 2014), while Hongsanan et al. (2020a) treated Biatriospora and Nigrograna as two separate genera. In 2022, Nigrograna represents 20 epithets listed in Index Fungorum (2022), and the members have been reported as saprobic, human pathogenic, and endophytic worldwide (Kolařík 2018; Zhao et al. 2018), showing a wide range of hosts (marine and terrestrial habitats) (Hyde et al. 2017; Tibpromma et al. 2017; Dayarathne et al. 2020). The sexual morph of Nigrograna is characterized by globose to subglobose and black ascomata, with ostiolar, two-layered peridium, clavate and fissitunicate asci, fusoid to narrowly ellipsoid, straight or curved, septate, and smooth or verruculose ascospores (Jaklitsch and Voglmayr 2016; Zhang et al. 2020). Asexual morph is characterized by globose to subglobose or pyriform pycnidia, filiform and branched conidiophores, hyaline, phialidic, discrete conidiogenous cells, sub-hyaline, aseptate and ellipsoidal conidia (de Gruyter 2012; Jaklitsch and Voglmayr 2016).
In this study, three saprobic Nigrograna were collected from Coffeaarabica branches in Yunnan Province, China. One species was isolated as an asexual morph (N.asexualis), while the other two isolated as sexual morphs (N.coffeae, N.puerensis) are illustrated and described as new species based on morphology and multi-gene phylogenetic analyses and are compared with closely related taxa.
Materials and methods
Collection, morphology and isolation
Coffee branch samples were collected from coffee plantations in Pu’er and Xishuangbanna, Yunnan Province, China. Specimens were put in plastic bags and taken to the mycology laboratory at Qujing Normal University. The vertical sections of fruiting structures were made for microscope studies and photomicrography. Micro-morphological characteristics were observed using a Leica DM2500 compound microscope and photographed with a Leica DMC4500 camera fitted onto the microscope. Color codes in the manuscript followed colorhexa (https://www.colorhexa.com). The measurements were processed in Tarosoft (R) Image Frame Work v. 0.9.7, and photographic plates were made in Adobe Photoshop CC 2018. Single spore isolation was carried out following Senanayake et al. (2020). Herbarium specimens were deposited at Zhongkai University of Agriculture and Engineering (ZHKU), while the living cultures growing on potato dextrose agar (PDA) were deposited at the culture collection of Zhongkai University of Agriculture and Engineering (ZHKUCC). Faces of fungi (FoF) numbers and Index Fungorum (IF) numbers were obtained as explained in Jayasiri et al. (2015) and Index Fungorum (2022).
DNA extraction and PCR amplification
Genomic DNA was extracted from the fresh fungal mycelia which were grown on PDA for about two weeks, using Biospin Fungus Genomic DNA Extraction Kit–BSC14S1 (BioFlux, China) following the manufacturer’s instructions. Lu et al. (2021) was followed for the Polymerase Chain Reaction (PCR). Five partial gene regions were used in this study viz. the internal transcribed spacer (ITS) region was amplified with the primers ITS4 and ITS5 (White et al. 1990), the 18 s small subunit (SSU) region was amplified by primers NS1 and NS4 (White et al. 1990), the nuclear ribosomal 28 s large subunit (LSU) region was amplified by the primers LROR and LR5 (Vilgalys and Hester 1990), the partial RNA polymerase II subunit (rpb2) region was amplified with the primers RPB2-5F and RPB2-7cR (Liu et al. 1999), and the partial translation elongation factor 1-alpha (tef1-α) gene was amplified with the primers EF1-983F and 2218R (Rehner and Buckley 2005). Lu et al. (2022b) was followed for the amplification reactions of different primers. Amplified PCR products were sent to Sango Biotechnology Co., Ltd. (Shanghai, China) for sequencing. All sequences generated in this study were deposited in GenBank (Table 1).
Table 1.
Taxa names, strain numbers, and corresponding GenBank accession numbers of the taxa used in the phylogenetic analyses. Newly generated sequences in this study are indicated in bold. The type species are noted with T after the species name, while NA indicates the unavailability of data.
| Taxon | Strain numbers | ITS | LSU | rpb2 | SSU | tef1-α |
|---|---|---|---|---|---|---|
| Cyclothyriellarubronotata (Berk. & Broome) Jaklitsch & Voglmayr T | CBS 141486 | KX650544 | KX650519 | NA | KX650507 | KX650574 |
| Cyclothyriellarubronotata | CBS 419.85 | NA | GU349002 | GU301875 | NA | GU371728 |
| Nigrogranaantibiotica (M. Kolařík & A. Kubátová) M. Kolařík T | CCF 4378 | JX570932 | KF925327 | NA | KF925328 | JX570934 |
| Nigrogranaantibiotica | CCF 4998 | LT221894 | NA | LT221895 | NA | NA |
| Nigrogranaaquatica W. Dong, H. Zhang & K.D. Hyde T | MFLUCC 14-1178 | MF399065 | MF415392 | NA | MF415394 | MF498582 |
| Nigrogranaaquatica | MFLUCC 17-2318 | MT627705 | MN913705 | NA | NA | NA |
| Nigrogranaasexualis T | ZHKUCC 22-0214 | OP450965 | OP450971 | OP432241 | OP450979 | OP432245 |
| Nigrogranaasexualis | ZHKUCC 22-0215 | OP450966 | OP450972 | OP432242 | OP450980 | OP432246 |
| Nigrogranacangshanensis Z.L. Luo, H.Y. Su & K.D. Hyde T | MFLUCC 15-0253 | KY511063 | KY511064 | NA | KY511065 | NA |
| Nigrogranacarollii M. Kolařík T | CCF 4484 | LN626657 | LN626682 | LN626662 | LN626674 | LN626668 |
| Nigrogranachromolaenae Mapook & K.D. Hyde T | MFLUCC 17-1437 | MT214379 | MT214473 | NA | NA | MT235801 |
| Nigrogranacoffeae T | ZHKUCC 22-0210 | OP450967 | OP450973 | OP432243 | OP450981 | OP432247 |
| Nigrogranacoffeae | ZHKUCC 22-0211 | OP450968 | OP450974 | OP432244 | OP450982 | OP432248 |
| Nigrogranafuscidula (Sacc.) Jaklitsch & Voglmayr T | CBS 141556 | KX650550 | NA | NA | NA | KX650525 |
| Nigrogranafuscidula | CBS 141476 | KX650547 | NA | KX650576 | KX650509 | KX650522 |
| Nigrogranafuscidula | MF1a | KX650548 | NA | NA | NA | KX650523 |
| Nigrogranafuscidula | MF3 | KX650549 | NA | NA | NA | KX650524 |
| Nigrogranahydei J.F. Zhang, J.K. Liu & Z.Y. Liu T | GZCC 19-0050 | MN387225 | MN387227 | NA | NA | MN389249 |
| Nigrogranaimpatientis J.F. Zhang, J.K. Liu & Z.Y. Liu T | GZCC 19-0042 | MN387226 | MN387228 | NA | NA | MN389250 |
| Nigrogranajinghongensis Wanas. & K.D. Hyde T | KUMUCC 21-0035 | MZ493303 | MZ493317 | MZ508421 | MZ493289 | MZ508412 |
| Nigrogranajinghongensis | KUMUCC 21-0036 | MZ493304 | MZ493318 | MZ508422 | MZ493290 | MZ508413 |
| Nigrogranakunmingensis T.Y. Du & Tibpromma T | ZHKUCC 22-0242 | OP456214 | OP456379 | NA | OP456382 | OP471608 |
| Nigrogranakunmingensis | ZHKUCC 22-0243 | OP484334 | OP456380 | NA | OP456383 | OP471609 |
| Nigrogranalocuta-pollinis F. Liu & L. Cai T | CGMCC 3.18784 | MF939601 | MF939583 | MF939610 | NA | MF939613 |
| Nigrogranalocuta-pollinis | LC11690 | MF939603 | MF939584 | MF939611 | NA | MF939614 |
| Nigrogranamackinnonii T | CBS 674.75 | KF015654 | KF015612 | KF015703 | GQ387552 | KF407986 |
| Nigrogranamackinnonii | E5202H | JX264157 | KJ605422 | JX264156 | JX264155 | JX264154 |
| Nigrogranamackinnonii | E9303e | JN545759 | LN626681 | LN626666 | LN626678 | LN626673 |
| Nigrogranamagnoliae Wanas. T | MFLUCC 20-0020 | MT159628 | MT159622 | MT159611 | MT159634 | MT159605 |
| Nigrogranamagnoliae | GZCC 17-0057 | MF399066 | MF415393 | NA | MF415395 | MF498583 |
| Nigrogranamagnoliae | MFLUCC 20-0021 | MT159629 | MT159623 | MT159612 | MT159635 | MT159606 |
| Nigrogranamycophila Jaklitsch, Friebes & Voglmayr T | CBS 141478 | KX650553 | NA | NA | NA | KX650526 |
| Nigrogranamycophila | CBS 141483 | KX650555 | NA | KX650577 | KX650510 | KX650528 |
| Nigrogranamycophila | MF6 | KX650554 | NA | NA | NA | KX650527 |
| Nigrogrananorvegica Jaklitsch & Voglmayr T | CBS 141485 | KX650556 | NA | KX650578 | KX650511 | NA |
| Nigrogranaobliqua Jaklitsch & Voglmayr T | CBS 141477 | KX650560 | NA | KX650580 | NA | KX650531 |
| Nigrogranaobliqua | CBS 141475 | KX650558 | NA | KX650579 | KX650512 | KX650530 |
| Nigrogranaobliqua | MRP | KX650561 | NA | KX650581 | NA | KX650532 |
| Nigrogranaperuviensis (M. Kolařík & R. Gazis) M. Kolařík T | CCF 4485 | LN626658 | LN626683 | LN626665 | LN626677 | LN626671 |
| Nigrogranapuerensis T | ZHKUCC 22-0212 | OP450969 | OP450975 | NA | OP450983 | OP432249 |
| Nigrogranapuerensis | ZHKUCC 22-0213 | OP450970 | OP450976 | NA | OP450984 | OP432250 |
| Nigrogranarhizophorae Dayar., E.B.G. Jones & K.D. Hyde T | MFLUCC 18-0397 | MN047085 | NA | MN431489 | NA | MN077064 |
| Nigrogranarhizophorae | MFLU 19-1234 | NA | MN017845 | MN431490 | NA | MN077063 |
| Nigrogranasamueliana Devadatha, V.V. Sarma & E.B.G. Jones T | NFCCI-4383 | MK358817 | MK358812 | MK330939 | MK358810 | MK330937 |
| Nigrogranathymi Mapook, Camporesi & K.D. Hyde T | MFLUCC 14-1096 | KY775576 | KY775573 | NA | KY775574 | KY775578 |
| Nigrogranayasuniana M. Kolařík T | YU.101026 | HQ108005 | LN626684 | LN626664 | LN626676 | LN626670 |
| Occultibambusabambusae D.Q. Dai & K.D. Hyde T | MFLUCC 13-0855 | KU940123 | KU863112 | KU940170 | NA | KU940193 |
| Occultibambusafusispora Phookamsak, D.Q. Dai & K.D. Hyde | MFLUCC 11-0127 | MZ329036 | MZ325466 | MZ329032 | MZ329028 | MZ325469 |
| Occultibambusapustula D.Q. Dai & K.D. Hyde T | MFLUCC 11-0502 | KU940126 | KU863115 | NA | NA | NA |
| Paradictyoarthriniumdiffractum Matsush. | MFLUCC13-0466 | KP744455 | NA | KP744498 | NA | NA |
| Paradictyoarthriniumtectonicola Doilom & K.D. Hyde T | MFLUCC 13-0465 | KP744456 | NA | KP744500 | KP753961 | KX437763 |
| Seriascomadidymosporum Phookamsak, D.Q. Dai, Karun. & K.D. Hyde T | MFLUCC 11-0179 | KU940127 | KU940196 | KU863116 | NA | KU940173 |
| Seriascomahonghense H.B. Jiang, Phookamsak & K.D. Hyde T | KUMCC 21-0021 | MZ329039 | MZ325468 | MZ329035 | NA | MZ325470 |
| Versicolorisporiumtriseptatum Sat. Hatak., Kaz. Tanaka & Y. Harada T | HHUF 28815 | NR_119392 | NA | NG_042318 | NG_060995 | NA |
Phylogenetic analyses
Phylogenetic analyses of the aligned sequences referred to Dissanayake et al. (2020). Newly generated reverse and forward sequences were assembled with Geneious program (9.1.2) and the preliminary identification was done by the BLASTn search in NCBI (https://www.ncbi.nlm.nih.gov). Additional highly similar sequences were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) based on the BLASTn results and recent publications. Single-gene sequence alignments were made in MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/), edited in trimAl v1.2 (http://trimal.cgenomics.org), and multi-gene alignments were made by Sequence Matrix program (1.7.8) (Vaidya et al. 2011). The sequence datasets used to build the phylogenetic trees are shown in Table 1.
Phylogenetic analyses were conducted with maximum likelihood (ML) and Bayesian inference (BI) algorithms on the CIPRES Science Gateway portal (https://www.phylo.org/) (Miller et al. 2012). The ML tree was run with RAxML-HPC v.8 on XSEDE (Stamatakis 2014), and GTRGAMMA substitution model with 1000 bootstrap iterations. The BI tree was run with MrBayes on XSEDE (3.2.7a) (Ronquist et al. 2012). MrModeltest 2.2 (Nylander 2004) and PAUP v. 4.0b10 (Ronquist and Huelsenbeck 2003) were used to evaluate the best models of evolution, the evolutionary model of SYM+I+G substitution model was selected for LSU, HKY+I+G substitution model was selected for SSU, and GTR+I+G substitution model was selected for ITS, rpb2 and tef1-α. Six simultaneous Markov Chains were run for two million generations and trees were sampled at every 200th generation (resulting in 10,000 trees), and these chains stopped when all convergences met and the standard deviation fell below 0.01. All resulting trees were plotted using FigTree v. 1.4.0 (Rambaut 2014) and the layout of the trees was made by Microsoft Office PowerPoint 2020.
Results
Phylogenetic analyses
Three new species formed a distinct clade in Nigrograna with strong statistical support (N.coffeae and N.puerensisML = 100%, BIPP = 1.00, and N.asexualisML = 68%, BIPP = 0.97). Multi-locus data (SSU, LSU, ITS, rpb2 and tef1-α) composed of 54 strains (Table 1), and Cyclothyriellarubronotata strains CBS 141486 and CBS 419.85 were used as the outgroup taxa. A total of 4485 characters were fed to the phylogenetic analysis after alignment, 1–1047 (SSU), 1048–1956 (LSU), 1957–2477 (ITS), 2478–3510 (rpb2) and 3511–4485 (tef1-α). The topology of the phylogenetic tree generated by the ML method was highly similar to that by BI, and therefore it was chosen to represent the evolutionary history of Nigrograna.
The ML analysis of the combined dataset yielded a best-scoring tree with a final ML optimization likelihood value of -23091.568105. The alignment has 1495 distinct alignment patterns, with 33.58% completely undetermined characters and gaps. Parameters for the GTR + I + G model of the combined SSU, LSU, ITS, rpb2 and tef1-α were as follows: estimated base frequencies A = 0.247145, C = 0.250645, G = 0.263985, T = 0.238225; substitution rates AC = 1.810004, AG = 4.475190, AT = 1.758134, CG = 1.340389, CT = 10.583215, GT = 1.000; gamma distribution shape parameter α = 0.167006. The phylogenetic tree resulting from RAxML analysis is shown in Fig. 1.
Figure 1.
The maximum-likelihood phylogram of Nigrograna based on a combined SSU, LSU, ITS, rpb2 and tef1-α sequence dataset with Cyclothyriellarubronotata CBS 141486 and CBS 419.85 as the outgroup taxa (Dayarathne et al. 2020). The maximum-likelihood bootstrap values (ML ≥ 60%, left) and Bayesian Inference Posterior Probability values (BIPP ≥ 0.90, right) are shown above the nodes. Strains derived from the current study are in blue, while type strains are in bold.
Taxonomy
. Nigrograna coffeae
L. Lu & Tibpromma sp. nov.
EB69069B-B814-520B-BFD4-4E61265EAB85
Index Fungorum number: IF559425
Facesoffungi Number: FoF12765
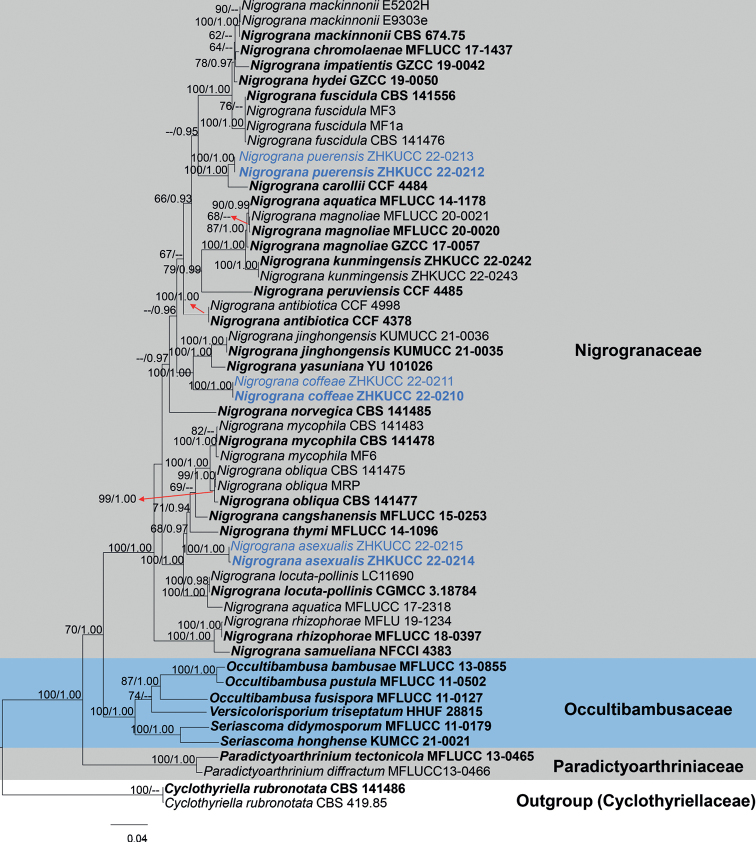
Figure 2.
Nigrogranacoffeae (ZHKU 22-0121, holotype) a, b ascomata on the host substrate c a vertical section through an ascoma d peridium e hamathecium f–k asci l germinated ascospore m culture on pda from above and reverse n–s ascospores (arrows indicate the septa). Scale bars: 50 μm (c); 10 μm (d–l); 5 μm (n–s).
Etymology.
Species epithet refers to the host genus “Coffea” where the fungus was isolated.
Holotype.
ZHKU 22-0121.
Description.
Saprobic on decaying branch of Coffeaarabica. Sexual morph: Ascomata 90–140 µm high, 140–200 μm wide (x̄ = 115 × 168 μm, n = 10), immersed, solitary, black spots on substrate, subglobose to oval, sometimes obpyriform, some with ostiolate. Peridium 10–15 µm wide, composed of 3–5 layers, hyaline to brown (#937463) cells of textura angularis. Hamathecium 1.5–3 μm wide, composed of numerous, hyaline, filamentous, septate, branched, pseudoparaphyses. Asci 50–70 × 7–11 μm (x̄ = 58 × 9 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate to cylindric-clavate, short stalked, some with club-shape pedicel, apically rounded, with a small ocular chamber. Ascospores 12–16 × 4–5 μm, (x̄ = 14.4 × 4.6 μm, n = 30), overlapping uni- to bi-seriately arranged, fusiform, straight or slightly curved, hyaline when immature and become pale brown (#e1af33) to dark-brown (#6e5031) when mature, mostly 1-septate, few 2 or 3-septate, constricted at each septum, with obviously guttulate. Asexual morph: Undetermined.
Culture characteristics.
Ascospores germinated on PDA within 24 h and germ tubes arising from both ends. Colonies on PDA, reaching 4.5 cm diam. after two months of incubation at room temperature (22–26 °C), initially white (#f2f3f4) becoming grey (#bbbeb2) to dark brown (#6e5031) at maturity, dense, circular, slightly raised, smooth surface, radially fimbriate at the edge, reverse dark green (#3a4543) to brown (#937463).
Material examined.
Pu’wen Town, Xishuangbanna, Yunnan Province, China, on a decaying branch of Coffeaarabica, (22°31'18"N, 101°2'44"E, 856.89 m), 15 September 2021, LiLu, JHPW16 (ZHKU 22-0121, holotype), ZHKUCC 22-0210 = ZHKUCC 22-0211. GenBank number; ITS: OP450967, LSU: OP450973, rpb2: OP432243, SSU: OP450981, tef1-α: OP432247 (ZHKUCC 22-0210, ex-type); ITS: OP450968, LSU: OP450974, rpb2: OP432244, SSU: OP450982, tef1-α: OP432248 (ZHKUCC 22-0211).
Notes.
Our phylogenetic analyses showed that Nigrogranacoffeae forms an independent clade (100% ML, 1.00 BIPP, Fig. 1), and is phylogenetically related to N.yasuniana and N.jinghongensis. Nigrogranayasuniana was reported as endophytes from Conceveibaguianensis Aubl. in Ecuador, but there were not enough morphological data, the comparison of base pairs in ITS showed 3.4% differences (15/433 bp), LSU showed 1.5% differences (12/812bp), SSU only showed 0.3% differences (3/1028 bp), rpb2 showed 14% differences (117/829 bp), and tef1-α showed 3.2% differences (31/954 bp) (Kolařík et al. 2017). Nigrogranajinghongensis was introduced as a saprobic fungus from woody litter in China, and our new isolate shares a similar size (12–16 × 4–5 μm vs 12–15 × 4–5.5 µm) and color (hyaline to dark brown vs yellowish-brown to brown) of ascospores with N.jinghongensis (Boonmee et al. 2021), but there are some significant differences in the size of the ascomata (90–140 µm high, 140–200 μm wide vs 300–400 µm high 220–300 μm wide) and the shape of ascospores (fusiform, straight or slightly curved vs ellipsoid) (Boonmee et al. 2021). Based on the sequence blast results, ITS, LSU and rpb2 gene sequences were similar to Nigrograna sp., with 97.5% (MZ270683), 98.4% (MK762716), and 86% (MZ508421) respectively, SSU was similar to N.mycophila with 99% (KX650510), and tef1-α was similar to N.yasuniana with 96.6% (LN626670). Therefore, we introduce our new isolate as a new species N.coffeae based on both morphological characteristics and phylogenetic analyses.
. Nigrograna puerensis
L. Lu & Tibpromma sp. nov.
1B95946C-F016-5EA6-988D-8E1715C78A93
Index Fungorum number: IF559426
Facesoffungi Number: FoF12766
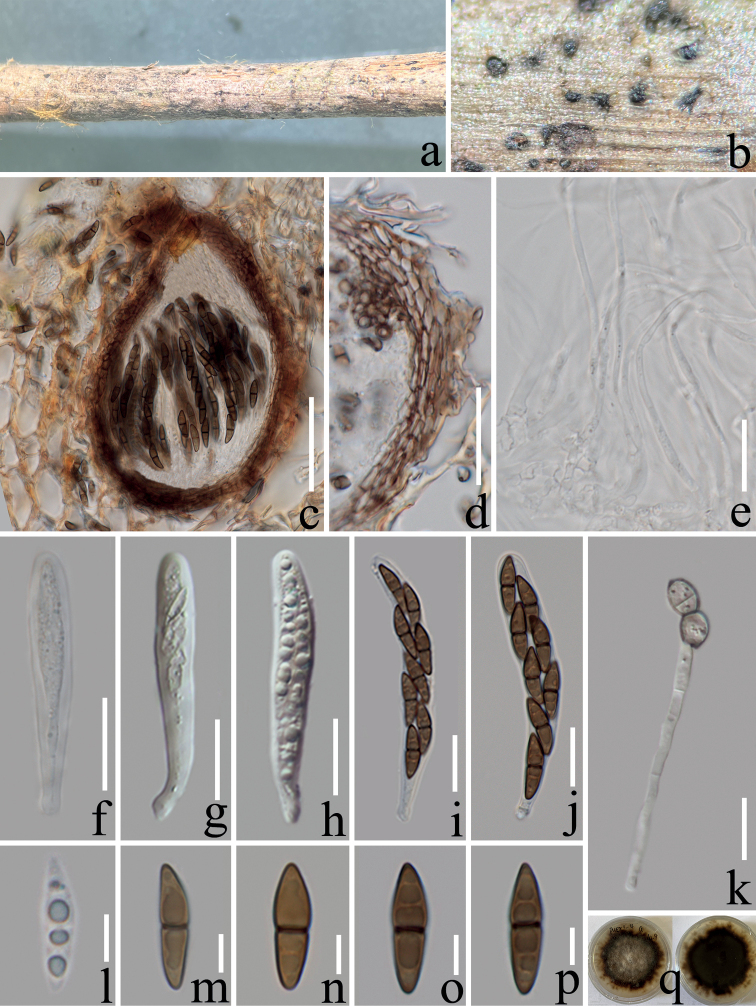
Figure 3.
Nigrogranapuerensis (ZHKU 22-0122, holotype) a, b ascomata observed on host substrate c a vertical section through an ascoma d peridium e hamathecium f–j asci k germinated ascospore l–p ascospores q culture on PDA from above and reverse. Scale bars: 50 μm (c); 30 μm (d); 15 μm (e–k); 5 μm (l–p).
Etymology.
The specific epithet “puerensis” refers to the location Pu’er City, where the type species was collected.
Holotype.
ZHKU 22-0122.
Description.
Saprobic on decaying branch of Coffeaarabica. Sexual morph: Ascomata 90–180 µm high, 90–150 μm wide (x̄ = 138 × 115 μm, n = 10), immersed, with only ostiolar necks visible on the host surface or erumpent, solitary, subglobose to ellipsoid, dark brown (#6e5031). Peridium 10–15 μm wide (x̄ = 13 μm, n = 15), outer layer consists of 2–3 layers of textura prismatica, brown (#937463) and thick-walled cells, inner layer hyaline with thin-walled cells. Hamathecium composed of numerous, 1.5–2 µm wide (x̄ = 1.8 μm, n = 20), filamentous, hyaline, septate, pseudoparaphyse. Asci 50–80 × 8–11 μm (x̄ = 66 × 9.5 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, short pedicellate, apically rounded, with poorly developed ocular chamber. Ascospores 15–18 × 4–5 μm, (x̄ = 16 × 4.5 μm, n = 30), uni- to bi-seriately arranged, fusoid, apical cell and basal cell acute, and apical cell slightly wider than basal cell, straight or slightly curved, 1-septate, constricted at septum, guttulate, hyaline to yellow-brownish (#daceb8) when young, brownish (#937463) when mature. Asexual morph: Undetermined.
Culture characteristics.
On PDA, colonies reached up to 4 cm diam. after two months at room temperature (22–26 °C). Colony dense, circular, slightly raised at the center, surface with white aerial mycelium, fluffy, with a serrate edge, grayish (#c9bfb3) to dark brown (#6e5031) from center to edge, reverse dark green (#3a4543) to dark brown (#6e5031).
Material examined.
Pu’er City, Yunnan Province, China, on a decaying branch of Coffeaarabica, (22°36'2"N, 101°0'59"E, 1016.43 m), 16 September 2021, LiLu, Puer 1-4 (ZHKU 22-0122, holotype), ZHKUCC 22-0212 = ZHKUCC 22-0213. GenBank number; ITS: OP450969, LSU: OP450975, SSU: OP450983, tef1-α: OP432249 (ZHKUCC 22-0212, ex-type); ITS: OP450970, LSU: OP450976, SSU: OP450984, tef1-α: OP432250 (ZHKUCC 22-0213).
Notes.
Nigrogranapuerensis clusters with N.carollii with significant statistical support from ML 100% and BIPP 1.00. In morphology, our new strains best fit Nigrograna by having immersed ascomata, clavate and short pedicellate asci, and pale to brown, fusoid to narrowly ellipsoid, and septate ascospores (Jaklitsch and Voglmayr 2016; Zhang et al. 2020). Blast search results of ITS, LSU and tef1-α sequence data revealed that our taxon (ZHKUCC 22-0212) is similar to N.mackinnonii (96% MZ270697, 99% KJ605422, and 95% LT797087 respectively), while the similarity of SSU sequence to N.carollii is as high as 99%. Based on nucleotide comparisons, our isolate (ZHKUCC 22-0212) differs from N.carollii (CCF 4484) by 9/490 bp (1.8%) in ITS, 2/222 bp (1%) in LSU, 2/1306 bp (0.2%) in SSU, and 10/530 bp (2%) in tef1-α. Unfortunately, for N.carollii, sufficient morphological data was not available to compare with our novel taxon which was isolated as an endophyte on living sapwood of wild Heveabrasiliensis Müll. Arg., and N.mackinnonii which was isolated as a human pathogen (de Gruyter 2012; Kolařík et al. 2017). In addition, the colony morphology of N.carollii on PDA is described as colonies plane, effuse, and light gray (Kolařík et al. 2017), while N.puerensis colony surface is seen as white aerial mycelium, fluffy, with a serrate edge, and grayish to dark brown from center to edge. Therefore, based on morphological and phylogenetic analyses, we introduce N.puerensis as a distinct new species.
. Nigrograna asexualis
L. Lu & Tibpromma sp. nov.
6C59D4C7-E1AF-5732-BDD6-02F00DCC1623
Index Fungorum number: IF559427
Facesoffungi Number: FoF12767
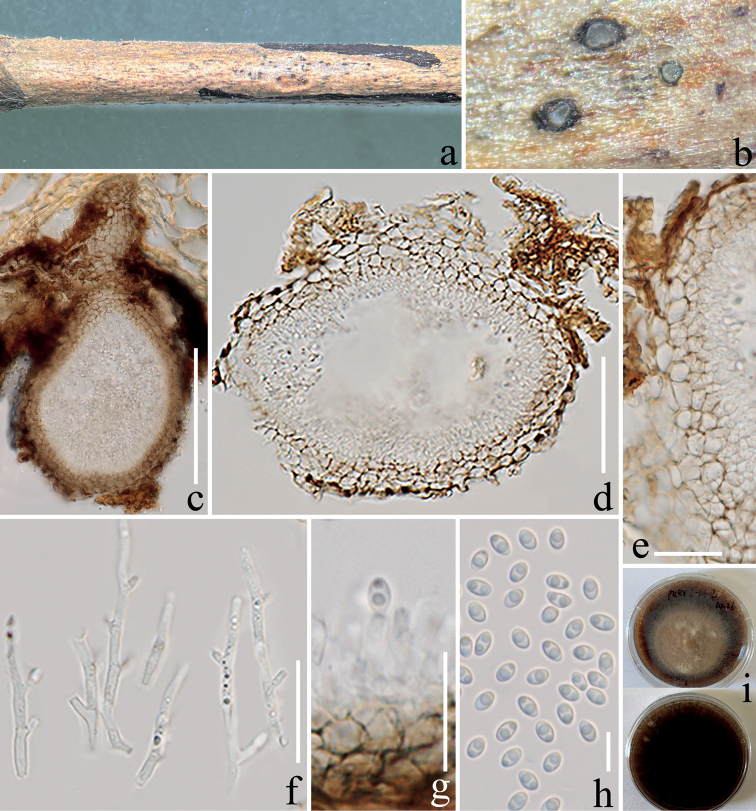
Figure 4.
Nigrogranaasexualis (ZHKU 22-0123, holotype) a, b conidiomata on the host substrate c, d vertical sections of a conidioma e peridium f, g conidiophores with phialides h conidia i culture on PDA from above and reverse. Scale bars: 100 μm (c); 50 μm (d); 15 μm (e); 30 μm (f); 20 μm (g); 10 μm (h).
Etymology.
The species epithet ‘asexualis’ refers to the asexual morph.
Holotype.
ZHKU 22-0123.
Description.
Saprobic on decaying branch of Coffeaarabica.Sexual morph: Undetermined. Asexual morph: Coelomycetous. Pycnidia 100–230 µm high, 120–180 µm wide (x̄ = 156 × 144 µm, n = 10), globose to subglobose, or pyriform, immersed, solitary, unilocular, dark brown, papillate ostiole, appearing as black spots on host surface. Pycnidial wall 11–16 µm wide (x̄ = 14 µm, n = 15), brown (#937463), the wall with pseudoparenchymatous cells. Conidiophores arising from the pycnidial wall, up to 46 µm long and 3–4.4 µm wide (x̄ = 3.4 µm, n = 25), filiform, septate, hyaline, simple to sparsely branched, with pegs along one or two sides and solitary phialides terminally. Phialides 3–6 × 1–2 µm (x̄ = 4.5 × 1.5 µm, n = 15), variable in shape, phialidic, discrete, ampulliform-lageniform-subcylindrical. Conidia 5–6.5 × 3–4 µm (x̄ = 5.5 × 3.7 µm, n = 30), ellipsoidal, unicellular, aseptate with 1–2 granules, subhyaline, smooth-walled.
Culture characteristics.
Conidium germinated on PDA within 24 h. Colonies growing on PDA reaching 5 cm diam. after two months at room temperature (22–26 °C). Colony dense, circular, surface sparsely hairy, radially striate, with a fimbriate edge, yellowish (#eabf83) to pale brown (#e1af33) at the center and dark brown (#6e5031) at the margin, reverse dark brown (#6e5031).
Material examined.
Pu’er City, Yunnan Province, China, on a decaying branch of Coffeaarabica, (22°36'2"N, 101°0'59"E, 1016.43 m), 16 September 2021, LiLu, Puer 1-14 (ZHKU 22-0123, holotype), ZHKUCC 22-0214 = ZHKUCC 22-0215. GenBank number; ITS: OP450965, LSU: OP450971, rpb2: OP432241, SSU: OP450979, tef1-α: OP432245 (ZHKUCC 22-0214, ex-type); ITS: OP450966, LSU: OP450972, rpb2: OP432242, SSU: OP450980, tef1-α: OP432246 (ZHKUCC 22-0215).
Notes.
In multi-gene phylogeny, Nigrogranaasexualis formed a separate (68% ML, 0.97 BIPP) and distinct clade within Nigrograna (Fig. 1). Morphologically, N.asexualis conforms to the morphological characteristics of Nigrograna by having hyaline or subhyaline, long and branched conidiophores, solitary phialides, and aseptate, ellipsoidal or cylindrical conidia (Jaklitsch and Voglmayr 2016; Dayarathne et al. 2020; Wanasinghe et al. 2020). Blast results of the sequences show that ITS is similar to N.fuscidula with 89% (MH856004), and SSU is similar to N.mycophila with 99.8% (KX650510). Nigrogranaasexualis is different from N.fuscidula and N.mycophila by its ellipsoidal conidia, but the similarities of these three species are hyaline, 1-celled, smooth-walled conidia forming on philipides (Jaklitsch and Voglmayr 2016). The LSU and rpb2 sequences of our strain blast results are similar to N.obliqua, and the similarities are 98.9% (KX650560) and 87% (KX650579) respectively, but N.obliqua lacks the asexual morph (Jaklitsch and Voglmayr 2016). The tef1-α sequence of our strain is 95.8% (MF939615) similar to N.locuta-pollinis, which was isolated from hive-stored pollen of Brassicacampestris L. that lacks morphology (Zhao et al. 2018). Therefore, we introduce N.asexualis as a distinct new species from coffee in China.
Discussion
Members of Nigrograna are distributed worldwide in soil, wood, and other plant debris (Mapook et al. 2020), and the hotspots of Nigrograna are reported as Central and South America, where the taxa are also found as human pathogens (Kolařík 2018; Puing et al. 2020). To date, five Nigrograna species viz. N.cangshanensis (decaying wood, Yunnan), N.jinghongensis (dead woody litter, Yunnan), N.kunmingensis (dead twigs of Gleditsiasinensis Lam., Yunnan), N.magnoliae (living branches of Magnoliadenudate Desr., Yunnan), and N.locuta-pollinis (hive-stored pollen, Hubei) have been isolated from different hosts in China (Tibpromma et al. 2017; Zhao et al. 2018; Wanasinghe et al. 2020; Boonmee et al. 2021; Zhou et al. 2022). In this study, three new saprobic fungi were isolated from decaying branches of Coffeaarabica in Yunnan Province, China, and this is the first report of Nigrograna species from coffee.
Species of Nigrograna are morphologically very similar and overlapping, hence can be interpreted as cryptic species. Therefore, it is difficult to delimit the species based only on their morphological characteristics (Jaklitsch and Voglmayr 2016; Zhang et al. 2020). In our research, we found that N.coffeae and N.puerensis have similar morphology, but in phylogeny, they are distributed differently within Nigrograna. This confirms the view of Jaklitsch and Voglmayr (2016) that the gene sequences are important and crucial for the identification of taxa at the genus and the species level.
Supplementary Material
Acknowledgements
Li Lu thanks Mae Fah Luang University for the award of a fee-less scholarship. The Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biological Resource and Food Engineering, Qujing Normal University is thanked for the facilities provided for the research work. Dr. Shaun Pennycook is thanked for his advice on new fungi names. Dai Dong-qin thanks the National Natural Science Foundation of China (No. NSFC 31760013, 31950410558), and High-Level Talent Recruitment Plan of Yunnan Provinces (“Young Talents” Program). Samantha C. Karunarathna thanks the National Natural Science Foundation of China grant number 32260004 for the support. Nakarin Suwannarach thanks Chiang Mai University, Thailand for financial support. Saowaluck Tibpromma thanks “the most cited article award” for allowing one free publication in MycoKeys.
Citation
Lu L, Karunarathna SC, Dai D-q, Jayawardena RS, Suwannarach N, Tibpromma S (2022) Three new species of Nigrograna (Dothideomycetes, Pleosporales) associated with Arabica coffee from Yunnan Province, China. MycoKeys 94: 51–71. https://doi.org/10.3897/mycokeys.94.95751
Contributor Information
Nakarin Suwannarach, Email: suwan.462@gmail.com.
Saowaluck Tibpromma, Email: saowaluckfai@gmail.com.
References
- Ahmed SA, van de Sande WWJ, Stevens DA, Fahal A, van Diepeningen AD, Menken SBJ, de Hoog GS. (2014) Revision of agents of black-grain eumycetoma in the order of Pleosporales. Persoonia 33: 141–154. 10.3767/003158514X684744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias RM, Abarca GH. (2014) Fungal diversity in coffee plantation systems and in a tropical montane cloud forest in Veracruz, Mexico. Agroforestry Systems 88: 921–933. 10.1007/s10457-014-9736-z [DOI] [Google Scholar]
- Barr ME. (1987) Prodromus to class Loculoascomycetes. Published by the Author, Amherst, Massachusetts; University of Massachusetts, U.S.A., 168 pp. [Google Scholar]
- Bhunjun CS, Niskanen T, Suwannarach N, Wannathes N, Chen YJ, McKenzie EH, Maharachchikumbura SS, Buyck B, Zhao CL, Fan YG, Zhang JY, Dissanayake AJ, Marasinghe DS, Jayawardena RS, Kumla J, Padamsee M, Chen YY, Liimatainen K, Ammirati JF, Phukhamsakda C, Liu JK, Phonrob W, Randrianjohany E, Hongsanan S, Cheewangkoon R, Bundhun D, Khuna S, Yu WJ, Deng LS, Lu YZ, Hyde KD, Lumyong S. (2022) The numbers of fungi: Are the most speciose genera truly diverse? Fungal Diversity 27: 1–76. 10.1007/s13225-022-00501-4 [DOI]
- Boonmee S, Wanasinghe DN, Calabon MS, Huanraluek N, Chandrasiri SK, Jones GE, Rossi W, Leonardi M, Singh SK, Rana S, Singh PN, Maurya DK, Lagashetti AC, Choudhary D, Dai YC, Zhao CL, Mu YH, Yuan HS, He SH, Phookamsak R, Jiang HB, Martín MP, Dueñas M, Telleria MT, Kałucka LK, Jagodziński AM, Liimatainen K, Pereira DS, Phillips AJL, Suwannarach N, Kumla J, Khuna S, Lumyong S, Potter TB, Shivas RG, Sparks AH, Vaghefi N, Abdel-Wahab MA, Abdel-Aziz FA, Li GJ, Lin WF, Singh U, Bhatt RP, Lee HB, Nguyen TTT, Kirk PM, Dutta AK, Acharya K, Sarma VV, Niranjan M, Rajeshkumar KC, Ashtekar N, Lad S, Wijayawardene NN, Bhat DJ, Xu RJ, Wijesinghe SN, Shen HW, Luo ZL, Zhang JY, Sysouphanthong P, Thongklang N, Bao DF, Aluthmuhandiram JVS, Abdollahzadeh J, Javadi A, Dovana F, Usman M, Khalid AN, Dissanayake AJ, Telagathoti A, Probst M, Peintner U, Garrido-Benavent I, Bóna L, Merényi Z, Boros L, Zoltán B, Stielow JB, Jiang N, Tian CM, Shams E, Dehghanizadeh F, Pordel A, Javan-Nikkhah M, Denchev TT, Denchev CM, Kemler M, Begerow D, Deng CY, Harrower E, Bozorov T, Kholmuradova T, Gafforov Y, Abdurazakov A, Xu JC, Mortimer PE, Ren GC, Jeewon R, Maharachchikumbura SSN, Phukhamsakda C, Mapook A, Hyde KD. (2021) Fungal diversity notes 1387–1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 111: 1–335. 10.1007/s13225-021-00489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli D. (1976) Pyrenochaetamackinnonii nova species agente de micetoma. Castellania 4: 227–234. [Google Scholar]
- Dayarathne MC, Jones E, Maharachchikumbura S, Devadatha B, Sarma V, Khongphinitbunjong K, Chomnunti P, Hyde KD. (2020) Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11: 1–188. 10.5943/mycosphere/11/1/1 [DOI] [Google Scholar]
- de Gruyter JH. (2012) Revised taxonomy of Phoma and allied genera. Wageningen University and Research, 1–181.
- de Gruyter J, Woudenberg JH, Aveskamp MM, Verkley GJ, Groenewald JZ, Crous PW. (2010) Systematic reappraisal of species in PhomasectionParaphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081. 10.3852/09-240 [DOI] [PubMed] [Google Scholar]
- de Gruyter J, Woudenberg JH, Aveskamp MM, Verkley GJ, Groenewald JZ, Crous PW. (2013) Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36. 10.3114/sim0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighton J. (2003) Fungi in ecosystem processes. Marcel Dekker, New York, 434 pp. 10.1201/9781315371528 [DOI] [Google Scholar]
- Dissanayake AJ, Bhunjun CS, Maharachchikumbura SSN, Liu JK. (2020) Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 11: 2652–2676. 10.5943/mycosphere/11/1/18 [DOI] [Google Scholar]
- Hawksworth DL, Lücking R. (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiology Spectrum 5(4). 10.1128/microbiolspec [DOI] [PubMed]
- Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali DS, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu Y, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Samarakoon MC, Chethana KWT, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Bhunjun CS, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Xu JC, Zheng JS, Liu G, Feng Y, Xie N. (2020a) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11: 1553–2107. 10.5943/mycosphere/11/1/13 [DOI] [Google Scholar]
- Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Lücking R, Boonmee S, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali DS, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Phukhamsakda C, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Thambugala KM, Dai DQ, Samarakoon MC, Chethana KWT, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Bhunjun CS, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Xu JC, Zheng JS, Liu G, Feng Y, Xie N. (2020b) Refined families of Dothideomycetes: Orders and families incertae sedis in Dothideomycetes. Fungal Diversity 105(1): 17–318. 10.1007/s13225-020-00462-6 [DOI] [Google Scholar]
- Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kušan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, De Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones GEB, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz Jr HL, Lumyong S, Maharachchikumbura SSN, Matočec N, Mckenzie EHC, Meśič A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su HY, Suetrong S, Tkalčec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE. (2017) Fungal diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Diversity 87: 1–235. 10.1007/s13225-017-0391-3 [DOI] [Google Scholar]
- Hyde KD, Jeewon R, Chen YJ, Bhunjun CS, Calabon MS, Jiang HB, Lin CG, Norphanphoun C, Sysouphanthong P, Pem D, Tibpromma S, Zhang Q, Doilom M, Jayawardena RS, Liu JK, Maharachchikumbura SSN, Phukhamsakda C, Phookamsak R, Al-Sadi AM, Thongklang N, Wang Y, Gaforov Y, Jones EBG, Lumyong S. (2020) The numbers of fungi: Is the descriptive curve flattening? Fungal Diversity 103(1): 219–271. 10.1007/s13225-020-00458-2 [DOI]
- Index Fungorum (2022) Index Fungorum. http://www.indexfungorum.org/Names/Names.asp [Retrieved 20 September 2022]
- Jaklitsch WM, Voglmayr H. (2016) Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology 85(1): 35–64. 10.1016/j.simyco.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang KA, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawarden NN, Zhao RL, Zhao Q, Kang JC, Promputtha I. (2015) The Faces of Fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Diversity 74: 3–18. 10.1007/s13225-015-0351-8 [DOI] [Google Scholar]
- Jiang HB, Phookamsak R, Hyde KD, Mortimer PE, Xu JC, Kakumyan P, Karunarathna SC, Kumla JA. (2021) A taxonomic appraisal of bambusicolous fungi in Occultibambusaceae (Pleosporales, Dothideomycetes) with new collections from Yunnan Province, China. Life 11(9): 932. 10.3390/life11090932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolařík M. (2018) New taxonomic combinations in endophytic representatives of the genus Nigrograna. Czech Mycology 70: 123–126. [Google Scholar]
- Kolařík M, Spakowicz DJ, Gazis R, Shaw J, Kubátová A, Nováková A, Chudíčková M, Forcina GC, Kang KW, Kelnarová I, Skaltsas D. (2017) Biatriospora (Ascomycota: Pleosporales) is an ecologically diverse genus including facultative marine fungi and endophytes with biotechnological potential. Plant Systematics and Evolution 303(1): 35–50. 10.1007/s00606-016-1350-2 [DOI] [Google Scholar]
- Li ZW. (2014) Issues and suggestions on building an international brand of “Pu’er Coffee”. Selected papers on rural agricultural reform, innovation and agricultural modernization 2: 719–721. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jeewon R, Phillips AJ, Maharachchikumbura SS, Ryberg M, Liu ZY, Zhao Q. (2017) Ranking higher taxa using divergence times: A case study in Dothideomycetes. Fungal Diversity 84(1): 75–99. 10.1007/s13225-017-0385-1 [DOI] [Google Scholar]
- Lu L, Tibpromma S, Karunarathna SC, Thiyagaraja V, Xu JC, Jayawardena RS, Lumyong S, Hyde KD. (2021) Taxonomic and phylogenic appraisal of a novel species and a new record of Stictidaceae from coffee in Yunnan Province, China. Phytotaxa 528(2): 111–124. 10.11646/phytotaxa.528.2.4 [DOI] [Google Scholar]
- Lu L, Tibpromma S, Karunarathna SC, Jayawardena RS, Lumyong S, Xu JC, Hyde KD. (2022a) Comprehensive review of fungi on coffee. pathogens 11: 411. 10.3390/pathogens11040411 [DOI] [PMC free article] [PubMed]
- Lu L, Karunarthna SC, Dai DQ, Xiong YR, Suwannarach N, Stephenson SL, Elgorban AM, Al-Rejaie S, Jayawardena RS, Tibpromma S. (2022b) Description of four novel species in Pleosporales associated with coffee in Yunnan, China. Journal of Fungi 8(10): 1113. 10.3390/jof8101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell ES. (1955) The ascostromatic Ascomycetes. Mycologia 47: 511–532. 10.1080/00275514.1955.12024473 [DOI] [Google Scholar]
- Mapook A, Hyde KD, McKenzie EH, Jones EB, Bhat DJ, Jeewon R, Stadler M, Samarakoon MC, Malaithong M, Tanunchai B, Buscot F. (2020) Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaenaodorata (Siam weed). Fungal Diversity 101(1): 1–175. 10.1007/s13225-020-00444-8 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2012) The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, 1–8. 10.1145/2335755.2335836 [DOI]
- Neilson J, Wang JHZ. (2019) China and the changing economic geography of coffee value chains. Singapore Journal of Tropical Geography 40(3): 429–451. 10.1111/sjtg.12279 [DOI] [Google Scholar]
- Nylander JAA. (2004) MrModeltest v2. Program distributed by the author Evolutionary Biology Centre, Uppsala University, Uppsala.
- Pandey A, Das N, Kumar B, Rinu K, Trivedi P. (2008) Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World Journal of Microbiology & Biotechnology 24(1): 97–102. 10.1007/s11274-007-9444-1 [DOI] [Google Scholar]
- Phukhamsakda C, Nilsson RH, Bhunjun CS, de Farias AR, Sun YR, Wijesinghe SN, Raza M, Bao DF, Lu L, Tibpromma S, Dong W, Tennakoon DS, Tian XG, Xiong YR, Karunarathna SC, Cai L, Luo ZL, Wang Y, Manawasinghe IS, Camporesi E, Kirk PM, Promputtha I, Kuo CH, Su HY, Doilom M, Li Y, Fu YP, Hyde KD. (2022) The numbers of fungi: Contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Diversity 28: 1–60. 10.1007/s13225-022-00502-3 [DOI] [Google Scholar]
- Puing AG, Couture‐Cossette A, Wang AX, Zygourakis CC, Cheng X, Stevens BA, Banaei N, Novoa RA, Ho DY, Subramanian AK. (2020) Simultaneous coccidioidomycosis and phaeohyphomycosis in a kidney transplant recipient: A case report and literature review. Transplant Infectious Disease 22(6): e13365. 10.1111/tid.13365 [DOI] [PubMed]
- Rambaut A. (2014) FigTree v1. 4.2, a graphical viewer of phylogenetic trees.
- Rehner SA, Buckley EA. (2005) Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. 10.1080/15572536.2006.11832842 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake IC, Rathnayaka AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, Hurdeal VG, Pem D, Dissanayake LS, Wijesinghe SN, Bundhun D, Nguyen TT, Goonasekara ID, Abeywickrama PD, Bhunjun CS, Jayawardena RS, Wanasinghe DN, Jeewon R, Bhat DJ, Xiang MM. (2020) Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 11: 2678–2754. 10.5943/mycosphere/11/1/20 [DOI] [Google Scholar]
- Sharma H. (2020) A detail chemistry of coffee and its analysis. In Coffee-Production and Research, 79 pp. 10.5772/intechopen.91725 [DOI]
- Stamatakis A. (2014) RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon DS, Kuo CH, Maharachchikumbura SS, Thambugala KM, Gentekaki E, Phillips AJ, Bhat DJ, Wanasinghe DN, de Silva NI, Promputtha I, Hyde KD. (2021) Taxonomic and phylogenetic contributions to Celtisformosana, Ficusampelas, F.septica, Macarangatanarius and Morusaustralis leaf litter inhabiting microfungi. Fungal Diversity 108: 1–215. 10.1007/s13225-021-00474-w [DOI] [Google Scholar]
- Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ. (2017) Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83: 1–261. 10.1007/s13225-017-0378-0 [DOI] [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. (2011) SequenceMatrix: Concatenation software for the fast assembly of multi‐gene datasets with character set and codon information. Cladistics 27(2): 171–180. 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe DN, Wijayawardene NN, Xu J, Cheewangkoon R, Mortimer PE. (2020) Taxonomic novelties in Magnolia-associated pleosporalean fungi in the Kunming Botanical Gardens (Yunnan, China). PLoS ONE 15(7): e0235855. 10.1371/journal.pone.0235855 [DOI] [PMC free article] [PubMed]
- White TJ, Bruns T, Lee SJWT, Taylor JL. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18: 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI] [Google Scholar]
- Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoðdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Kumla J, Leontyev DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M. (2022) Outline of fungi and fungus-like taxa–2021. Mycosphere 13: 53–453. 10.5943/mycosphere/13/1/2 [DOI] [Google Scholar]
- Yang EF, Tibpromma S, Karunarathna SC, Phookamsak R, Xu JC, Zhao ZX, Karunanayake C, Promputtha I. (2022) Taxonomy and phylogeny of novel and extant taxa in Pleosporales associated with Mangiferaindica from Yunnan, China (Series I). Journal of Fungi 8(2): 152. 10.3390/jof8020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JH, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD. (2008) Multi-locus phylogeny of Pleosporales: A taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 61(1): 111–119. 10.3114/sim.2009.64.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012) Pleosporales. Fungal Diversity 53(1): 1–221. 10.1007/s13225-011-0117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li J, Zhou H, Chen Z, Song G, Peng Z, Pereira AP, Silva MC, Varzea VM. (2014) Arabica coffee production in the Yunnan Province of China. Proceedings of the 24th International Conference on Coffee Science (ASIC).
- Zhang JF, Liu JK, Thambugala KM, Yang J, Meng ZH, Liu ZY. (2020) Two new species and a new record of Nigrograna (Nigrogranaceae, Pleosporales) from China and Thailand. Mycological Progress 19: 1365–1375. 10.1007/s11557-020-01633-0 [DOI] [Google Scholar]
- Zhao YZ, Zhang ZF, Cai L, Peng JW, Liu F. (2018) Four new filamentous fungal species from newly-collected and hive stored bee pollen. Mycosphere 9: 1089–1116. 10.5943/mycosphere/9/6/3 [DOI] [Google Scholar]
- Zhou LW, Wang XW, Liu SL, Li GJ, Deng CY, Rossi W, Leonardi M, Liimatainen K, Kekki T, Niskanen T, Smith ME, Ammirati J, Bojantchev D, Abdel-Wahab MA, Zhang M, Tian E, Lu YZ, Zhang JY, Ma J, Dutta AK, Acharya K, Du TY, Xu JZ, Kim JS, Lim YW, Gerlach A, Zeng NK, Han YX, Razaghi P, Raza M, Cai L, Calabon MS, Jones EBG, Saha R, Kumar TKA, Krishnapriya K, Thomas A, Kaliyaperumal M, Kezo K, Gunaseelan S, Singh SK, Singh PN, Lagashetti AC, Pawar KS, Jiang SH, Zhang C, Zhang H, Qing Y, Bau T, Ramirez NA, Niveiro N, Li MX, Yang ZL, Wu G, Tarafder E, Tennakoon DS, Kuo CH, da Silva TM, Souza-Motta CM, Bezerra JDP, He G, Ji XH, Suwannarach N, Kumla J, Lumyong S, Wannathes N, Rana S, Hyde KD. (2022) Fungal diversity notes: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity (in press).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.