Abstract
Background
Post-acute COVID-19 syndrome (PACS) is a well-recognized, complex, systemic disease which is associated with substantial morbidity. There is a paucity of established interventions for the treatment of patients with this syndrome.
Objectives
To systematically review registered trials currently investigating therapeutic modalities for PACS.
Data sources
A search was conducted up to the 16 September, 2022, using the COVID-19 section of the WHO Internal Clinical Trials Registry Platform.
Study eligibility criteria, participants, and interventions
Interventional clinical trials of any sample size examining any therapeutic modality targeting persistent symptoms among individuals after diagnosis with COVID-19.
Methods
Data on trial characteristics and intervention characteristics were collected and summarized.
Results
After screening 17 125 trials, 388 trials, from 42 countries, were eligible. In total, we had 406 interventions, of which 368 were mono-therapeutic strategies, whereas 38 were intervention combinations. Among 824 primary outcomes identified, there were >300 different outcomes. Rehabilitation was the most employed class of intervention in 169 trials. We encountered 76 trials examining the pharmacological agents of various classes, with the most common agent being colchicine. Complementary and alternative medicine encompassed 64 trials exploring traditional Chinese medicine, Ayurveda, homeopathic medications, naturopathic medications, vitamins, dietary supplements, and botanicals. Psychotherapeutic and educational interventions were also employed in 12 and 4 trials, respectively. Other interventions, including transcranial direct current stimulation, transcutaneous auricular vagus nerve stimulation, general electrical stimulation, cranial electrotherapy stimulation, various stem cell interventions, and oxygen therapy interventions, were also employed.
Conclusion
We identified 388 registered trials, with a high degree of heterogeneity, exploring 144 unique mono-therapeutic interventions for PACS. Most studies target general alleviation of symptoms. There is a need for further high-quality and methodologically robust PACS treatment trials to be conducted with standardization of outcomes while following WHO's recommendation for uniform evaluation and treatment.
Keywords: Coronavirus, COVID-19, Long COVID, Post-acute COVID-19 syndrome, SARS-CoV-2
Introduction
Post-acute infection syndromes are commonly under-diagnosed and represent a burden on health care worldwide [1]. As the global COVID-19 pandemic comes to an end, a sizeable sub-set of recovering patients will continue to experience symptoms lasting weeks to months beyond the acute phase, as reported by several systematic reviews [[2], [3], [4], [5], [6], [7], [8], [9], [10]]. This conglomeration of symptoms has been denoted by different terms: ‘long COVID’, ‘long-haul COVID’, ‘chronic COVID’, ‘post-acute sequelae of SARS-CoV-2 infection’ or PASC, and ‘post-acute COVID-19 syndrome’ or PACS. The United Kingdom National Institute for Health and Care Excellence (NICE) has also further sub-categorized PACS into a sub-acute phase, defined as the persistence of symptoms 4–12 weeks after the onset of illness (on-going symptomatic COVID-19), and a chronic phase, in which symptoms persist for ≥12 weeks after the onset of the disease, with no alternative underlying disease (post-COVID-19 syndrome) [11]. On the other hand, the WHO has defined it as a condition characterized by symptoms that interfere with daily life and occur 3 months from the onset of acute symptoms of COVID-19, lasting for at least 2 months and cannot be explained by an alternative diagnosis [12].
More than 100 persistent symptoms have been associated with COVID-19 [13]. Fatigue, followed by dyspnoea, was the most reported symptom according to multiple systematic reviews [2,10,14]. To date, there have been no published systematic reviews examining registered interventional clinical trial entries evaluating different treatment modalities for PACS. In addition, there is a paucity of published studies on potential treatment options for PACS. Thus, we aimed to conduct a systematic review of registered interventional clinical trial entries examining therapeutic treatments for PACS. The purpose of this study is to provide an overview of therapies for PACS which are currently being investigated as well as the key limitations of current trials and gaps to guide future research.
Methods
Registry search
Trial registry entries were identified on September 16, 2022, using the COVID-19 section of the WHO Internal Clinical Trials Registry Platform (ICTRP), which includes trial entries from 17 different trial registries [15]. The WHO ICTRP necessitates that these registered trials meet specific criteria for content, quality, validity, accessibility, unambiguous identification, technical capacity, administration, governance, and requirements of the International Committee of Medical Journal Editors [16].
Trial selection
Eligibility criteria were assembled using the Patient Intervention Comparison Outcomes Study type framework [17]. The inclusion criteria were as follows:
Population: Any sample size of patients of any age diagnosed with COVID-19 who present with symptoms ≥4 weeks from the time of diagnosis or specifically mentioning PACS (or its equivalent);
Intervention/Comparator: Any intervention/comparator related to the treatment (not prevention) of PACS symptoms;
Outcomes: Any outcome;
Study type: Interventional clinical trials.
No restrictions were made on language, country, randomization, blinding, trial arms, or the trials' population characteristics. We excluded withdrawn or terminated trials, trials with unknown recruitment status, observational trials, COVID-19 diagnostic studies, non-clinical studies, and trials investigating the treatment of acute symptoms of COVID-19. For trial registry entries which were deemed eligible, data were extracted simultaneously by 12 reviewers (BAS, BNS, MSA, NAF, NEA, OAO, QAA, RMT, TM, TZA, WH, and YO) in duplicate into a shared Excel sheet. Any discrepancy was resolved in consultation with a senior reviewer (IMT). This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [18].
Data extraction and management
Data on registry source, recruitment country, recruitment status, site description, allocation status, phase, masking, intervention model, estimated enrolment, inclusion criteria, primary outcome(s), publication status, and intervention details were extracted as reported in the trial registries. The trial registry source was taken as linked in the ICTRP sheet. If not stated explicitly, we used the addresses of the facilities organizing the trial to identify recruitment countries. Trials with multiple recruitment countries were combined into ‘multiple countries’. Similarly, trials with phases 1 or 2 and 2 or 3 were combined into one category.
Two reviewers (BAS and NAF) merged overlapping primary outcomes and grouped them into appropriate outcome domains. None of the protocols reported composite primary outcomes. A consensus was achieved with the merging and organization of the primary outcomes. The taxonomy of outcome domains was based on the Williamson/Clarke revised version of the Cochrane Database of Systematic Reviews and the Core Outcome Measures in Effectiveness Trials database of core outcome sets, which is a taxonomy with 38 outcome domains in five core areas [19].
We explored the trials' inclusion criteria by noting their definition of PACS: mentioned a time reference of ≥4 weeks from acute COVID-19 infection or PCR (consistent with the NICE definition of PACS); mentioned a time reference of ≥12 weeks from acute COVID-19, with symptoms persisting for ≥8 weeks (consistent with the WHO definition of PACS); explicitly mentioned as patients with PACS (or its equivalent) without the definition of a time reference; or neither explicitly mentioned PACS (or its equivalent) nor defined a time reference but used the PACS term (or its equivalent) in the title or study description. In addition, the patients' hospitalization status during the acute COVID-19 phase was recorded. Furthermore, we determined whether the trials reported symptoms before COVID-19. Using each trial's scientific title and trial identification, a web search was performed to determine whether the trials' results were published.
The experimental arm(s) for each trial were used to identify all interventions under investigation for PACS. Each arm was recorded separately in the summary figure for trials with multiple experimental arms. For each intervention under investigation, we recorded the number of trials, total patients enrolled, countries, and specific clinical use as stated by the trial source. Because the trial registries did not record interventions in a standardized format, we organized all interventions into different classes and sorted them into seven different organ systems, as highlighted by our previous systematic review and meta-analysis of the prevalence of PACS symptoms [10]. The organ systems included the nervous, mental, gastrointestinal, pulmonary, cardiovascular, musculoskeletal, and other systems. Interventions targeting fatigue, hair loss, or other general indications (post-COVID-19 symptom relief, quality of life, etc.) were labelled as ‘other’.
WHO's definition of rehabilitation was used to organize the interventions under the rehabilitation class [20]. We utilized the WHO's Anatomical Therapeutic Chemical Classification System to classify drugs into intervention classes [21]. We used product information from the European Medicines Agency [22], U.S. Food and Drug Administration [23], or companies' websites to characterize some drugs which were not part of WHO's Anatomical Therapeutic Chemical algorithms. The mechanisms of action and approved clinical uses of drugs were extracted from the Food and Drug Administration or European Medicines Agency, where applicable. We also checked the Traditional Herbal Medicines Registration Scheme [24,25] to determine whether any of the herbal interventions acquired a Traditional Herbal Registration certification mark and the National Institute of Health National Center for Complementary and Integrative Health “Herbs at a Glance” [26] to determine whether any were mentioned; however, none were mentioned. Trials of Ayurveda, a natural system of medicine which originated in India; traditional Chinese medicine; traditional Korean medicine; herbals; homeopathy; and dietary supplement interventions were categorized under ‘complementary and alternative medicine’ [27].
Analysis
Descriptive statistics using Excel was used to analyse the extracted data. Using percentages, the trial characteristics were summarized.
Results
Trial search results
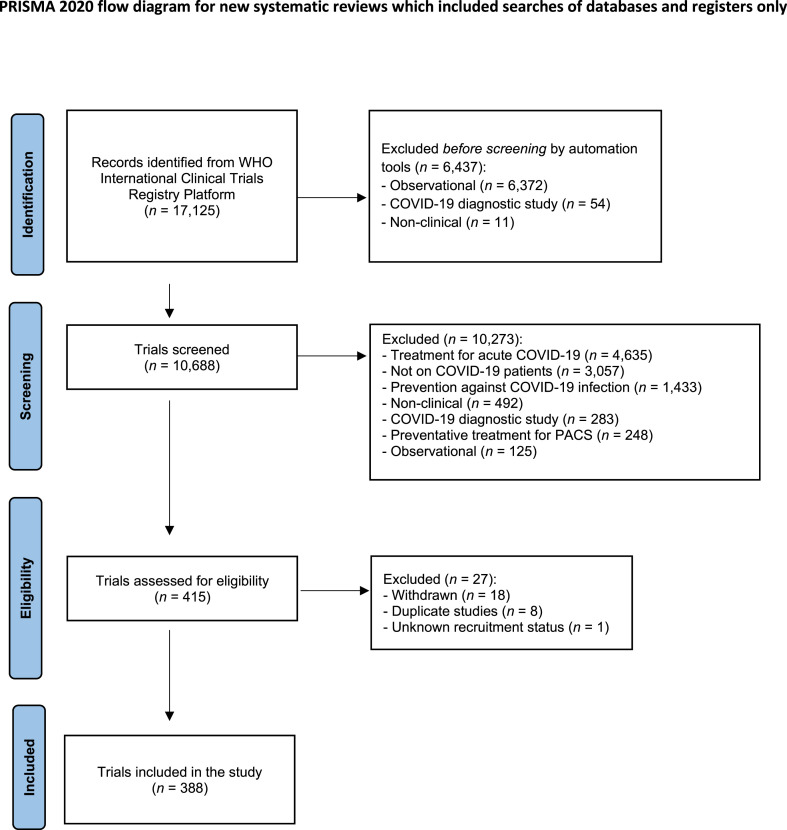
Of 17 125 trials identified, 10 688 registered trials were manually screened, and 388 trials, with 406 experimental arms, were included in our final analysis after de-duplication (Fig. 1 ). During the manual screening process, the most common reason for exclusion was trials investigating treatment strategies for acute COVID-19 infection (n = 4635). From the 388 trials, 48 trials (12.4%) were found to be published at the time of writing.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
General characteristics of the clinical trials
From the 17 WHO ICTRP record registries, 223 included trials (57.5%) were registered on ClinicalTrials.gov. The results indicate that on-going research on PACS treatment originated from 42 countries, with the United States (n = 60) and India (n = 57) harbouring the greatest number of trials. The most common recruitment regions for our included trials consisted of 114 trials (29.4%, n = 22 711) based in Europe, 98 trials (25.3%, n = 9492) in Asia, and 73 trials (18.8%, n = 6672) in North America (Table 1 ). There were four international multi-regional trials (NCT05002530, NCT05494424, NCT04652518, ACTRN12621000637842).
Table 1.
Characteristics of the clinical trials registered for the treatment of post-acute COVID-19 syndrome (n = 388)
| Trial characteristics | Total number of trials (n = 388), n (%) |
|---|---|
| Trial registry source | |
| ClinicalTrials.gov | 223 (57.5) |
| CTRI | 54 (13.9) |
| ReBEC | 23 (5.9) |
| ChiCTR | 23 (5.9) |
| IRCT | 20 (5.2) |
| ISRCTN | 14 (3.6) |
| German Clinical Trials Register | 14 (3.6) |
| Others | 17 (4.4) |
| Recruitment region | |
| Europe | 114 (29.4) |
| Asia | 98 (25.3) |
| North America | 73 (18.8) |
| Middle East | 55 (14.2) |
| South America | 40 (10.3) |
| Australia | 3 (0.8) |
| Africa | 1 (0.3) |
| Multiple regions | 4 (1.0) |
| Site description | |
| Single centre | 325 (83.8) |
| Multi-centre | 63 (16.2) |
| Recruitment status | |
| Recruiting | 165 (42.5) |
| Not yet recruiting | 120 (30.9) |
| Completed | 78 (20.1) |
| Active, not yet recruiting | 14 (3.6) |
| Enrolling by invitation | 10 (2.6) |
| N/A | 1 (0.3) |
| Allocation | |
| Randomized | 310 (79.9) |
| Not randomized | 25 (6.4) |
| N/A | 53 (13.7) |
| Phase | |
| Phase 0 | 12 (3.1) |
| Phase 1 | 9 (2.3) |
| Phase 2 | 48 (12.4) |
| Phase 3 | 27 (7.0) |
| Phase 4 | 19 (4.9) |
| Multiple phases | 26 (6.7) |
| N/A | 247 (63.7) |
| Trial arm | |
| Multi-arm | 332 (85.6) |
| Single arm | 56 (14.4) |
| Masking | |
| Open-label | 139 (35.8) |
| Single blind | 76 (19.6) |
| Double blind | 83 (21.4) |
| Triple blind | 29 (7.5) |
| Quadruple blind | 28 (7.2) |
| Not stated | 33 (8.5) |
| Trials' inclusion criteria for PACS definition | |
| Mentioned a time reference of ≥4 weeks from acute COVID-19 infection or PCR (consistent with the NICE definition of PACS) | 95 (24.5) |
| Mentioned a time reference of ≥12 weeks from acute COVID-19, with symptoms persisting for ≥8 weeks (consistent with WHO definition of PACS) | 87 (22.4) |
| Explicitly mentioned as patients with PACS (or its equivalent) without the definition of a time reference | 140 (36.1) |
| Neither explicitly mentioned PACS (or its equivalent) nor defined a time reference but has been stated in the title or study description | 66 (17.0) |
| Hospitalization status during acuteCOVID-19phase | |
| Hospitalized | 66 (17.0) |
| Non-hospitalized | 61 (15.7) |
| Mixed (hospitalized and non-hospitalized) | 22 (5.7) |
| Not stated | 239 (61.6) |
| Collected symptoms prior to COVID-19 | |
| Yes | 72 (18.6) |
| No | 316 (81.4) |
| Estimated enrolment | |
| Median (IQR) | 60 (40–101.5) |
| ≤50 | 139 (35.8) |
| 51–100 | 152 (39.2) |
| >100 | 97 (25.0) |
| Primary outcomes core areasa | |
| Mortality/survival | 3 (0.8) |
| Physiological/clinical | 522 (134.6) |
| Functioning | 282 (72.7) |
| Resource use | 4 (1.0) |
| Adverse events/effects | 13 (3.4) |
| Organ system targeteda | |
| Pulmonary system | 133 (34.3) |
| Nervous system | 70 (18) |
| Mental health | 47 (12.1) |
| Cardiovascular system | 26 (6.7) |
| Musculoskeletal system | 21 (5.4) |
| Gastrointestinal system | 5 (1.3) |
| Non-system specific | 178 (45.9) |
| Intervention typea | |
| Rehabilitation | 168 (43.3) |
| Pharmacotherapy | 77 (19.8) |
| Complementary and alternative medicine | 64 (16.5) |
| Psychotherapy | 12 (3.1) |
| Education | 4 (1.0) |
| Others | 43 (11.1) |
| Intervention combinations | 38 (9.8) |
ChiCTR, Chinese Clinical Trial Registry; CTRI, Clinical Trials Registry – India; IQR, interquartile range; IRCT, Iranian Registry of Clinical Trials; ISRCTN, International Standard Randomised Controlled Trial Number; N/A, not available; NICE, National Institute for Health and Care Excellence; PACS, post-acute COVID-19 syndrome; ReBEC, Brazilian Clinical Trials Registry.
Total percentages may exceed 100% because several trials had multiple primary outcomes, organ systems targeted or experimental interventions.
Only 78 trials (20.1%) had completed recruitment at the time of analysis (17 September, 2022). In the majority of the trials (238 trials, 61.3%), only one primary outcome was identified. Among 824 primary outcomes identified, there were >300 different outcomes. The trial characteristics, including patient populations, interventions, and outcome core areas, are detailed in Table 1. The top 20 primary outcomes identified according to their frequency are detailed in Table 2 . All primary outcomes identified, arranged according to Core Outcome Measures in Effectiveness Trials core outcome sets, can be found in Table S1.
Table 2.
The top 20 primary outcome measures in terms of frequency (N)
| Rank | Primary outcome measures | N |
|---|---|---|
| 1 | 6-minute walk test | 58 |
| 2 | Pulmonary function testing | 43 |
| 3 | Changes in symptoms of PACS | 24 |
| 4 | 36-Item Short-Form Survey | 23 |
| 5 | Cardiopulmonary exercise test/peak oxygen consumption (VO2 max) | 20 |
| 6 | European Quality of Life 5 Dimensions 5 Level Version | 19 |
| 7 | Visual Analogue Scale | 17 |
| 8 | Fatigue Severity Scale | 16 |
| 9 | Post-COVID-19 Functional Status Scale | 12 |
| 10 | Sniffin’ Sticks Test | 12 |
| 11 | Modified Medical Research Council Dyspnea Scale | 10 |
| 12 | Chalder Fatigue Scale (CFQ-11) | 9 |
| 13 | Fibrosis in high-resolution computed tomography of the lung | 9 |
| 14 | Incidence of treatment-emergent adverse effects | 9 |
| 15 | Inspiratory muscle strength or maximal inspiratory pressure | 9 |
| 16 | Borg Dyspnea Scale | 8 |
| 17 | Changes in blood oxygenation | 8 |
| 18 | Fatigue Assessment Scale | 8 |
| 19 | Handgrip strength dynamometer | 8 |
| 20 | Routine blood test | 7 |
| 20 | SGRQ | 7 |
PACS, post-acute COVID-19 syndrome; SGRQ, St. George's Respiratory Questionnaire.
Characteristics of trials' inclusion criteria
There were 239 trials (61.6%) that did not mention the hospitalization status of the included population resulting from the acute COVID-19 phase. Only 72 trials (18.6%) collected symptoms prior to COVID-19. Among the included trials, only 95 (24.5%) and 87 trials (22.4%) had their inclusion criteria consistent with the NICE and WHO definitions, respectively.
Sample size
The included trials had a median (interquartile range) enrolment of 60 participants (40–101.5), and the total number of participants planned for enrolment for all the included trials was 58 340. The smallest trial included only five patients. Of the four international multi-regional trials, one (NCT05002530) had the largest planned enrolment, with 10 000 participants.
Therapies being investigated
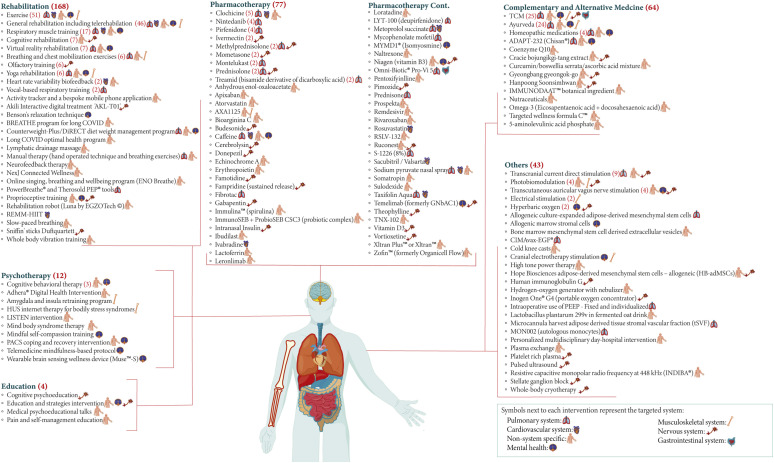
Out of the 406 interventions explored, 368 (n = 40 834) tested mono-therapeutic strategies, whereas 38 (n = 17 506) included a combination of interventions. There were 144 unique mono-therapeutic interventions in total. Among the trials indicated for non-system-specific symptoms, 108 targeted fatigue and 70 had broad indications (relief of PACS symptoms, improved quality of life, etc.). Out of the trials which tested mono-therapeutic interventions, 169 trials were on rehabilitation strategies, 76 trials were on pharmacotherapy, 64 trials were on complementary and alternative medicine, 12 trials were on psychotherapy, four trials were on education, and the rest were uncategorized.
Rehabilitation
Trials on rehabilitation most commonly employed exercises that included aerobic exercises, strength exercises, bicycling, swimming, etc. Various forms of rehabilitation, including tele-rehabilitation, cognitive rehabilitation, virtual reality rehabilitation, yoga rehabilitation, and even rehabilitation robots, were utilized. Inspiratory and/or expiratory muscle training as well as breathing exercises were also commonly used. Various applications and custom-made programmes, such as Akili Interactive; Breathing, Rest/recovery, Education, Activity management, Thinking/cognition, Healthy voice strategies, and Eating/nutrition programme; long COVID optimal health programme; etc., were designed to help ameliorate PACS symptoms.
Pharmacotherapy
There were 61 unique pharmacological interventions tested for PACS. The most commonly used agents were colchicine, nintedanib, pirfenidone, ivermectin, methylprednisolone, mometasone, montelukast, prednisolone, and treamid.
Complementary and alternative medicine
This category encompassed 25 trials on traditional Chinese medicine, which included multiple Chinese herbal products (decoctions, capsules, granules, etc.), Tai Chi, and different forms of acupuncture (needle, laser, or electro-acupuncture). There were 24 trials of Ayurvedic therapies, which mainly included herbal medicines such as ashwagandha. Other trials used homeopathic medications, naturopathic medications, vitamins, dietary supplements, and botanicals.
Psychotherapy
Different therapeutic approaches fell under this category, including cognitive behavioural therapy, amygdala and insula retraining programmes, mind-body syndrome therapy, etc.
Education
Only four educational interventions were encountered, which included cognitive psycho-education, education and strategies intervention, medical psycho-educational talks, and pain and self-management education.
Other interventions
Electrical therapy was commonly employed, with nine trials on transcranial direct current stimulation, four trials on transcutaneous auricular vagus nerve stimulation, two trials on general electrical stimulation, and one trial on cranial electrotherapy stimulation. Various stem cell interventions were used such as allogenic culture-expanded adipose-derived mesenchymal stem cells and allogenic marrow stromal cells. Oxygen therapy interventions, such as hyperbaric oxygen, a hydrogen-oxygen generator using a nebulizer, and a portable oxygen concentrator, were also used.
Each mono-therapeutic intervention, with its target organ system, can be found in Fig. 2 , whereas the combinations of interventions are detailed in Table S2. For full details, unique interventions under investigation, the number of registered clinical trials, estimated enrolment of all trials, countries, clinical use in the trial, mechanism of action, and approved clinical use can be found in Tables S3–S9.
Fig. 2.
Mono-therapeutic interventions were organized by treatment category with their target organ system (n = 368). BREATHE, Breathing, Rest/recovery, Education, Activity management, Thinking/cognition, Healthy voice strategies, and Eating/nutrition; DiRECT, Diabetes Remission Clinical Trial; ENO, English National Opera; Hb-adMSC, Hope Biosciences adipose-derived mesenchymal stem cell; HUS, Helsinki University Hospital; LISTEN, Long COVID PersonalIsed Self-managemenT support EvaluatioN; PACS, post-acute COVID-19 syndrome; PEEP, positive end-expiratory pressure; REMM-HIIT, REmotely Monitored, Mhealth supported High Intensity Interval Training; TCM, traditional Chinese medicine; tSVF, tissue stromal vascular fraction.
Discussion
Findings
COVID-19 has caused mortality and morbidity at an unprecedented scale globally, affecting >616 million people worldwide, of whom 10%–20% are thought to have PACS according to the WHO COVID-19 Dashboard [28]. The health-care demands of patients with PACS will continue to rise in the near future. Still, there are several deficiencies in the current work towards therapeutic modalities for PACS, and we believe that it does not match the substantial needs and burden caused by PACS worldwide. With only four international multi-centre trials, there is a definitive need for more trials addressing PACS on a larger scale.
Fatigue and dyspnoea were the two most reported symptoms by patients with PACS according to multiple systematic reviews [10,14,29]. This was concordant with the symptoms targeted by the experimental trials, with 108 trials specifically targeting fatigue and 133 trials targeting the pulmonary system. The majority of interventions targeting a single organ system were non-system specfic, with 70 trials using a holistic approach to target and alleviate PACS symptoms. This brings into question the reproducibility of such trials and the difficulty in measuring possible benefits later in the clinical setting.
Although there was a good number of trials investigating PACS treatment, most of the therapies investigated were re-purposed from similar conditions; for example, many of the rehabilitation interventions are currently used in the treatment of cancer-associated fatigue syndrome. In addition, most of the interventions were used to target multiple PACS symptoms concurrently. Although we encountered new interventional agents developed specifically for PACS (RSLV-132, AXA1125, etc.), they were very limited. In many instances, multiple trials proposed the same intervention for different symptoms.
It was not possible to classify interventions according to patients' clinical categories (such as being admitted to the intensive care unit or managed as out-patients or in-patients) because >60% did not indicate the hospitalization status of patients who were recruited for the trial in their inclusion and exclusion criteria. Regarding the definition of PACS employed by the included trials, considerable heterogeneity was exhibited. Moreover, no trial had a reference time of zero. Furthermore, 66 trials mentioning patients with PACS, with no time reference in their inclusion criteria, most simply referred to their patients as having a positive COVID-19 test result and then a negative test result. This raises the question of whether these patients recovered from COVID-19 and are now exhibiting PACS symptoms. Remarkably, in terms of the inclusion criteria of the four international multi-centre trials, 2 trials neither explicitly mentioned PACS (or its equivalent) nor defined a time reference, and one trial explicitly mentioned patients with PACS (or its equivalent) without defining a time reference.
Implications
The data presented are only as good as the data acquired from the registries; the ICTRP gathers records from 17 different trial registries, collecting information from all around the world. Despite its importance, there are still chances to improve the consistency and quality of the data provided [[30], [31], [32]]. Trials' protocols provide much more information and details on the methodology and primary outcome. We reviewed the trials' protocols whenever they were available; nonetheless, we had to rely on trial registry entries and not trial protocols for many of the trials.
With three-fourths of the trials enrolling ≤100 participants and more than one-third being open-label, the numerous interventions reported are likely to yield only preliminary evidence concerning safety and effectiveness against PACS. Additionally, with the bulk of the trials utilizing subjective and patient-reported scales, there is a high risk of outcome assessment biases [33]. The reported primary outcomes were highly heterogeneous among the included trials.
Considering the wide range of symptoms and the absence of conclusive diagnostic tests, it is challenging to identify patients with PACS consistently and systematically while assembling an adequate control group for comparison. With many of the registered trials designed expediently, the PACS trials inadequately defined their inclusion criteria. They failed to indicate severity status of the acute phase of COVID-19 in their population, creating a challenge to distinguish between, for example, PACS and post-intensive care syndrome. Furthermore, only a small number of trials reported acquiring history of symptoms prior to acute COVID-19, which may have resulted in the inclusion of patients without PACS in these trials. The patients may have been experiencing symptoms because of other chronic conditions or infectious diseases. Post-acute infection syndromes, which have already been recognized, present as indistinguishable symptom profiles independent of the infectious pathogen [1,34,35].
Because clinical trials are costly and time consuming, international collaborations are encouraged [36]. An example of such a partnership is the WHO COVID-19 Solidarity Therapeutics Trial, which is evaluating three treatment arms: artesunate, infliximab, and imatinib [37]. Additionally, the National Institute of Health created the REsearching COVID to Enhance Recovery initiative to learn, prevent, and treat PACS [38].
Strengths and limitations
Our study is the first comprehensive systematic review of registered interventional clinical trial entries investigating therapeutic interventions for PACS. In comparison with the extensive living networks to capture COVID-19 studies which still lack a dedicated section or filter for PACS treatment [39,40], we believe that our overview of investigated interventions, and the quantification of several methodological parameters has merits which are not provided elsewhere.
Even with double screening for inclusion as well as independent parallel screening and data extraction, several caveats are inherent to the methodology and study design of the included trials. The organization of interventions into different classes is, to some extent, arbitrary, because some drugs fit into more than one category. The trials in our review lacked uniform symptom terminology, standardized recording methods, accurate population identification, and grouping of multiple symptoms under umbrella terms. This limited our ability to filter trials and divide interventions according to their target organ system. In addition, some trials included may subsequently be withdrawn, terminated, or unable to reach the target enrolment.
Future directions
With the WHO describing the pandemic as causing more ‘mass trauma’ than World War II, we must keep pace with the evolving unmet needs of this pandemic. Although targeted re-purposing of existing treatments is critical in the early phases of a threat, there needs to be a focus on targeted novel therapies addressing the underlying pathophysiological mechanisms of PACS. Particularly, trials investigating complementary and alternative medicine are increasing in number but have low registration quality [41]. Therefore, it is important to improve the quality of reporting research protocols which are registered in strict accordance with guidelines for conducting and reporting clinical trials [42,43]. In addition, we support protocol sharing with trial registration to encourage routine public access for the benefit of patients and other users of clinical trial evidence [44].
Future trials should abide by the recommendations of Nasserie et al. [45] regarding areas for improvement in future research on PACS, whether in the conduct of studies or the reporting of various characteristics of symptoms for such conditions, including the use of a standardized definition for symptoms and time-zero, as well as using an objective measure of the severity and duration of symptoms. Furthermore, the primary outcomes assessed in trials are of essence and should have a meaningful clinical use that is of importance to patients [46,47]. Moreover, a core outcome set developed by an international Delphi consensus study for PACS in adults contained 12 outcomes and included survival, an outcome which was very scarcely reported among trials [48]. To facilitate homogeneous outcome reporting and enhance the strength of evidence in systematic reviews of upcoming trials, we recommend adherence to the core outcome set [48].
Although living mapping and living systematic review initiatives are vitally important for different sections for pharmacological, non-pharmacological, and preventative treatment, they should also aim to identify and differentiate PACS treatment and even PACS prevention studies from acute COVID-19 studies and present them in separate sections.
Conclusion
We identified 388 trials with a high degree of heterogeneity exploring 144 mono-therapeutic interventions for PACS. The definition of PACS illustrated by the trials was variable, and the primary outcomes were often non-system specific and not standardized. The data from these studies will guide future research and treatment. Given that the health-care demands of patients with PACS will keep increasing, there is a need for further high-quality and methodologically robust research on PACS treatment to be conducted with standardization of outcomes while following WHO's recommendations for uniform evaluation and treatment.
Author contribution
NAF and BAS contributed equally as first authors. RMT, TZA, and BNS contributed equally as second authors. IMT conceptualized the study. IMT, MSA, and OAO supervised the study. BAS and NAF were involved in project administration. BAS and NAF performed formal analysis. BAS, BNS, MSA, NAF, NEA, NO, OAO, QAA, RMT, TM, TZA, WH, and YO performed data curation. RMT performed visualization. BAS, BNS, NAF, RMT, and TZA wrote the original draft. EFB, KMA, MSA, and IMT reviewed and edited the manuscript.
Transparency declaration
The authors declare that they have no conflicts of interest.
Editor: L Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.01.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Choutka J., Jansari V., Hornig M., Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- 2.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SeyedAlinaghi S.A., Afsahi A.M., MohsseniPour M., Behnezhad F., Salehi M.A., Barzegary A., et al. Archives of Academic Emergency Medicine. Shaheed Beheshti University of Medical Sciences and Health Services; 2020. Late complications of COVID-19; a systematic review of current evidence: Vol. 9; p. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Oliveira Almeida K., Nogueira Alves I.G., de Queiroz R.S., de Castro M.R., Gomes V.A., Santos Fontoura F.C., et al. A systematic review on physical function, activities of daily living and health-related quality of life in COVID-19 survivors. Chronic Illn. 2022 doi: 10.1177/17423953221089309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almas T., Malik J., Alsubai A.K., Zaidi S.M., Iqbal R., Khan K., et al. Post-acute COVID-19 syndrome and its prolonged effects: an updated systematic review. Ann Med Surg. 2022;80 doi: 10.1016/j.amsu.2022.103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long Q., Li J., Hu X., Bai Y., Zheng Y., Gao Z. Follow-ups on persistent symptoms and pulmonary function among post-acute COVID-19 patients: a systematic review and meta-analysis. Front Med. 2021;8 doi: 10.3389/fmed.2021.702635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugazzaro S., Contri A., Esseroukh O., Kaleci S., Croci S., Massari M., et al. Rehabilitation interventions for post-acute COVID-19 syndrome: a systematic review. Int J Environ Res Public Health. 2022;19:5185. doi: 10.3390/ijerph19095185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera Martimbianco A.L., Pacheco R.L., Bagattini Â.M., Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik P., Patel K., Pinto C., Jaiswal R., Tirupathi R., Pillai S., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—a systematic review and meta-analysis. J Med Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkodaymi M.S., Omrani O.A., Fawzy N.A., Shaar B.A., Almamlouk R., Riaz M., et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (UK) 2020. COVID-19 rapid guideline: managing the long-term effects of COVID-19.https://www.nice.org.uk/guidance/ng188 [PubMed] [Google Scholar]
- 12.World Health Organization . 2021. A clinical case definition of post COVID-19 condition by a Delphi consensus.https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [Google Scholar]
- 13.Hayes L.D., Ingram J., Sculthorpe N.F. More than 100 persistent symptoms of SARS-CoV-2 (long COVID): a scoping review. Front Med. 2021;8 doi: 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joli J., Buck P., Zipfel S., Stengel A. Post-COVID-19 fatigue: a systematic review. Front Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.947973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Clinical Trials Registry Platform . 2022. Important information related to the COVID-19 outbreak.https://www.who.int/clinical-trials-registry-platform [Google Scholar]
- 16.International Clinical Trials Registry Platform . 2009. WHO registry criteria.https://www.who.int/clinical-trials-registry-platform/network/registry-criteria [Google Scholar]
- 17.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:1–6. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd S., Clarke M., Becker L., Mavergames C., Fish R., Williamson P.R. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. doi: 10.1016/j.jclinepi.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . 2021. Rehabilitation.https://www.who.int/news-room/fact-sheets/detail/rehabilitation [Google Scholar]
- 21.WHO Collaborating Centre for Drug Statistics Methodology . 2022. ATC/DDD index.https://www.whocc.no/atc_ddd_index/ [Google Scholar]
- 22.European Medicines Agency . 2022. Medicines.https://www.ema.europa.eu/en/medicines [Google Scholar]
- 23.U.S. Food & Drug Administration . 2022. Information by drug class.https://www.fda.gov/drugs/drug-safety-and-availability/information-drug-class [Google Scholar]
- 24.Royal Pharmaceutical Society of Great Britain . 2022. The traditional herbal medicine registration scheme.https://www.rpharms.com/resources/quick-reference-guides/herbal-medicine-registration-scheme#moreinfo [Google Scholar]
- 25.Gov.uk . 2022. Herbal medicines granted a traditional herbal registration.https://www.gov.uk/government/publications/herbal-medicines-granted-a-traditional-herbal-registration-thr/herbal-medicines-granted-a-traditional-herbal-registration [Google Scholar]
- 26.National Institute of Health (NIH) NC for C. IH (NCCIH) 2022. Herbs at a glance.https://www.nccih.nih.gov/health/herbsataglance [Google Scholar]
- 27.National Cancer Institute . 2021. Complementary and alternative medicine.https://www.cancer.gov/about-cancer/treatment/cam [Google Scholar]
- 28.World Health Organization . 2022. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 29.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.5281/zenodo.4669937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salholz-Hillel M., Grabitz P., Pugh-Jones M., Strech D., DeVito N.J. Results availability and timeliness of registered COVID-19 clinical trials: interim cross-sectional results from the DIRECCT study. BMJ Open. 2021;11 doi: 10.5281/zenodo.4669937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speich B., Gloy V.L., Klatte K., Gryaznov D., Heravi A.T., Ghosh N., et al. Reliability of trial information across registries for trials with multiple registrations. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tse T., Fain K.M., Zarin D.A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ. 2018;361:k1452. doi: 10.1136/bmj.k1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hróbjartsson A., Emanuelsson F., Skou Thomsen A.S., Hilden J., Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43:1272–1283. doi: 10.1093/ije/dyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannister B.A. Post-infectious disease syndrome. Postgrad Med J. 1988;64:559–567. doi: 10.1136/pgmj.64.753.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickie I., Davenport T., Wakefield D., Vollmer-Conna U., Cameron B., Vernon S.D., et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.BioWorld . 2022. EU boosts funding for COVID-19 epidemic, encourages clinical trial cooperation.https://www.bioworld.com/articles/433824-eu-boosts-funding-for-covid-19-epidemic-encourages-clinical-trial-cooperation?v=preview [Google Scholar]
- 37.WHO . 2022. COVID-19 solidarity therapeutics trial.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [Google Scholar]
- 38.National Institutes of Health . 2022. RECOVER: researching COVID to enhance recovery.https://recovercovid.org/ [Google Scholar]
- 39.WHO. Cochrane . 2022. The COVID-NMA initiative: a living mapping and living systematic review of Covid-19 trials.https://covid-nma.com/ [Google Scholar]
- 40.Infectious Diseases Data Observatory . 2022. A living systematic review of registered COVID-19 trials.https://www.iddo.org/covid-19/live-systematic-review-trials [Google Scholar]
- 41.Kuang Z., Li X., Cai J., Chen Y., Qiu X., Ni X. Calling for improved quality in the registration of traditional Chinese medicine during the public health emergency: a survey of trial registries for COVID-19, H1N1, and SARS. Trials. 2021;22:188. doi: 10.1186/s13063-021-05182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan A.W., Tetzlaff J.M., Gotzsche P.C., Altman D.G., Mann H., Berlin J.A., et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan A.W., Hróbjartsson A. Promoting public access to clinical trial protocols: challenges and recommendations. Trials. 2018;19:116. doi: 10.1186/s13063-018-2510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pletcher M.J., Pignone M. Evaluating the clinical utility of a biomarker. Circulation. 2011;123:1116–1124. doi: 10.1161/CIRCULATIONAHA.110.943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selleck M.J., Senthil M., Wall N.R. Making meaningful clinical use of biomarkers. Biomark Insights. 2017;12 doi: 10.1177/1177271917715236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munblit D., Nicholson T., Akrami A., Apfelbacher C., Chen J., de Groote W., et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir Med. 2022;10:715–724. doi: 10.1016/S2213-2600(22)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1