Abstract
Study Design
A systematic review and meta-analysis
Objective
The purpose of this systematic review and meta-analysis was to compare the efficacy of lateral lumbar interbody fusion (LLIF) combined with posterior spinal fusion (PSF) with that of conventional PSF in the treatment of adult spinal deformity (ASD).
Methods
A comprehensive literature search was performed for relevant studies in PubMed, EMBASE, Web of Science, and the Cochrane Library. Spinopelvic parameters, surgical data, complications, and clinical outcomes at the last follow-up were compared between patients with ASD who underwent LLIF combined with PSF (LLIF+PSF group) and those who underwent conventional PSF (only-PSF group).
Results
Ten studies, comprising 621 patients with ASD (313 in the LLIF+PSF group and 308 in the only-PSF group), were included. The level of evidence was III for 7 studies and IV for 3 studies. There was no significant difference in the improvement in the visual analog scale score, systemic complication rate, and revision rate between groups. In the LLIF+PSF group, we noted a superior restoration of lumbar lordosis (weighted mean difference [WMD], 9.77; 95% confidence interval [CI] 7.10 to 12.44, P < .001), pelvic tilt (WMD, −2.50; 95% CI −4.25 to −.75, P = .005), sagittal vertical axis (WMD, −21.92; 95% CI −30.73 to −13.11, P < .001), and C7 plumb line-center sacral vertical line (WMD, −4.03; 95% CI −7.52 to −.54, P = .024); a lower estimated blood loss (WMD, −719.99; 95% CI −1105.02 to −334.96, P < .001) while a prolonged operating time (WMD, 104.89; 95% CI 49.36 to 160.43, P < .001); lower incidence of pseudarthrosis (risk ratio [RR], .26; 95% CI .08 to .79, P = .017) while higher incidence of neurologic deficits (RR, 2.04; 95% CI 1.27 to 3.25, P = .003); and a better improvement in Oswestry Disability Index score (WMD, −7.04; 95% CI −10.155 to −3.93, P < .001) and Scoliosis Research Society-22 total score (WMD, .27; 95% CI .11 to .42, P = .001). The level of evidence in this systematic review and meta-analysis was II.
Conclusion
Compared with conventional PSF, LLIF combined with PSF was associated with superior restoration of sagittal and coronal alignment, lower incidence of pseudarthrosis, better improvement in quality of life, and less surgical invasiveness in the treatment of ASD, albeit at the cost of prolonged surgical times and substantially high incidence of lower extremity symptoms. Surgeons should weigh the advantages and disadvantages of this procedure, and inform patients about its side effects.
Keywords: lateral lumbar interbody fusion, adult spinal deformity, posterior spinal fusion, spinopelvic parameters, surgical invasiveness
Introduction
With prolonged life expectancy, the prevalence of adult spinal deformity (ASD) is up to 68% in the elderly population. 1 Adult spinal deformity is associated with degeneration of the lumbar disc and facet joints, leading to malalignment in the sagittal and coronal planes.2,3 Patients with ASD commonly complain of axial low back pain, radiculopathy, disability, and poor health-related quality of life (HRQoL).4,5 The primary goals of surgical treatment are to harmoniously restore balance, alleviate pain, and improve the overall quality of life. 6
Posterior spinal fusion (PSF) has traditionally been performed for ASD, often with multilevel decompression, interbody fusion, and even radical osteotomy. 4 Although effective, the posterior approach enables less access to the anterior column, which might compromise its ability to obtain adequate spinal realignment and fusion. 7 Also, as a procedure with major surgical invasiveness and a high morbidity rate, PSF is often limited by patient age and medical comorbidities.8,9 Since ASD mostly affects the elderly population, searching for less invasive treatment strategies is of paramount importance.
Since first reported by Ozgur et al, lateral lumbar interbody fusion (LLIF) has gained popularity as a less invasive technique for various spinal degenerative diseases.10-12 LLIF allows for a large bone graft area, high fusion rate, less bleeding, and lower complication rate through the lateral surgical approach.13,14 When combined with posterior instrumentation and fusion, surgeons are convinced that LLIF could obtain promising clinical results and reduce surgical invasiveness in ASD.15-18
Some investigations have been performed to directly compare LLIF combined with PSF to conventional PSF for ASD. However, the efficacy of LLIF in ASD treatment remains controversial and has not yet been systematically confirmed.
The purpose of this systematic review and meta-analysis was to compare spinopelvic parameters, surgical data, complications, and clinical outcomes between LLIF combined with PSF and conventional PSF for the treatment of ASD.
Materials and Methods
This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered with PROSPERO (ID: CRD42021290684).19,20
Search Strategy
PubMed, EMBASE, Web of Science, and Cochrane Library databases were searched using the following terms: (((lateral) AND (interbody fusion)) AND ((posterior) OR (transforaminal))) AND (((spinal deformity) OR (scoliosis)) OR (kyphosis)).
The literature search was updated on October 30, 2021. Two reviewers (H.Y. and J.L.) independently screened the titles and abstracts, and any differences were settled by discussion with a third reviewer (B.H.).
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) adult patients (≥18 years) diagnosed with ASD, which was defined as the presence of at least 1 of the following indicators: Cobb angle ≥20° in the coronal plane, sagittal vertical axis (SVA) ≥ 50 mm, pelvic tilt (PT) ≥ 25°, lumbar lordosis (LL) < 20°, or thoracic kyphosis (TK) ≥ 60°; (2) studies in which the intervention was LLIF combined with open PSF (anterior/posterior/transforaminal lumbar interbody fusion could be performed in the lumbosacral region if necessary), with or without any facetectomies; studies in which LLIF was combined with percutaneous PSF and facetectomies could also be included; (3) studies comparing patients who solely underwent PSF (with or without any interbody fusion and posterior osteotomies); and (4) studies with the following outcomes: postoperative spinopelvic parameters, surgical data, complications, and clinical outcomes.
The exclusion criteria were as follows: (1) studies that included fewer than 10 patients; (2) studies that solely reported the outcomes of PSF combined with 3-column osteotomies (3CO) or percutaneous PSF without any facetectomies; (3) reviews, case reports, biomechanical studies, and cadaveric research; (4) studies with no available full text; (5) duplicate publications; and (6) articles not published in English.
Assessment of Study Quality
Study quality was assessed independently by 2 reviewers (H.Y. and J.L.) using the Newcastle–Ottawa scale (NOS) recommended for retrospective studies by Cochrane Handbook version 5.2.0.21,22 The level of evidence rating was assigned according to published guidelines.
Outcomes
Spinopelvic parameters included LL, TK, pelvic incidence (PI), PI-LL, PT, SVA, coronal Cobb angle, and C7 plumb line-center sacral vertical line (C7PL-CSVL), and were assessed preoperatively and at the last follow-up. Surgical data included estimated blood loss (EBL) and operating time (ORT). Complications were assessed during the perioperative period and at follow-up, including mechanical complications, surgical complications, systemic complications, and revision. Systemic complications included cardiopulmonary events (e.g., myocardial infarction or adult respiratory distress syndrome), deep vein thrombosis/pulmonary embolism, gastrointestinal events (e.g., ileus or stress ulcers), urinary events (e.g., acute renal failure or urinary retention), and central nervous system events (e.g., stroke or delirium). Clinical outcomes were the visual analog scale (VAS) score for leg and back pain, Oswestry Disability Index (ODI) score, and Scoliosis Research Society-22 (SRS-22) total score preoperatively and at the last follow-up.
Data Extraction
Data extraction was performed independently by 2 reviewers (H.Y. and J.L.). We also recorded demographic information, including age, sex, sample size, lumbar interbody fusion (LIF) technique (cranial to and at the L5-S1 level), number of posterior fixed segments, follow-up duration, and lordotic angle of LLIF cages. The data for 17 outcomes were extracted for analysis. Continuous outcomes included LL, TK, PI-LL, PT, SVA, coronal Cobb angle, C7PL-CSVL, EBL, ORT, VAS score for leg pain, VAS score for back pain, ODI score, and SRS-22 total score. Dichotomous outcomes included mechanical complications, surgical complications, systemic complications, and revision.
Data Analysis
All statistical analyses were performed using the Stata version 15.1. Outcomes reported in at least 2 studies were analyzed. For continuous outcomes, the weighted mean difference (WMD) was used to estimate the effect. The effect measure of dichotomous outcomes is displayed as a risk ratio (RR). The mean and standard deviation values of continuous outcomes or the counts and percentages of dichotomous outcomes for comparisons of data points are also displayed. The statistical heterogeneity among studies was evaluated using the I-square test and Cochran’s Q test. If the I2 value was less than 50% and the P-value was greater than .10, a fixed-effects model was used. If the I2 value was greater than 50% or the P-value was less than .10, a sensitivity analysis was applied to assess the impact of each study, and subgroup analysis was performed if necessary. If a source of potential heterogeneity could not be found, a random-effects model was used.
Assessment of Publication Bias
Potential publication bias was assessed by applying Egger’s test at a P-value less than .10 level of significance. 23 If publication bias was indicated, we further evaluated the number of missing studies by applying the “trim and fill” method and recalculated the pooled WMD or RR with the addition of those missing studies. 24
Results
Study Selection
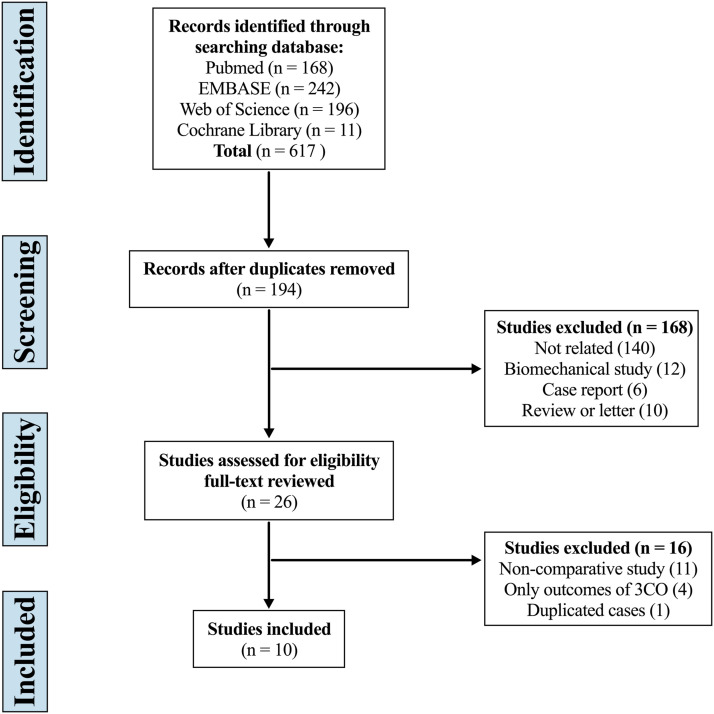
The systematic search yielded 617 articles, of which 423 were duplicates, 168 were excluded by screening the title and abstract, and 16 were considered improper after full-text review. Eventually, 10 studies were included in this systematic review and meta-analysis (Figure 1).25-34
Figure 1.
Flow diagram depicting the literature review, search strategy, and selection process.
Assessment of Study Quality and Publication Bias
The quality of the included studies was assessed using the NOS (Table 1). Of the 10 studies included, 6 were of high quality with scores of 8–9, and 4 were of moderate quality with scores of 7. The level of evidence was III for 7 studies and IV for 3 studies. The level of evidence in this systematic review and meta-analysis was II. Publication bias was not detected for any variable.
Table 1.
Quality Assessment of Studies According to Newcastle–Ottawa Scale.
| Author | Year | Selection | Comparability | Exposure | Total score |
|---|---|---|---|---|---|
| Tormenti | 2010 | 3 | 2 | 2 | 7 |
| Baghdadi | 2014 | 4 | 2 | 2 | 8 |
| Strom | 2016 | 3 | 2 | 2 | 7 |
| Theologis | 2016 | 3 | 2 | 2 | 7 |
| Nakashima | 2018 | 3 | 2 | 3 | 8 |
| Park | 2018 | 3 | 2 | 3 | 8 |
| Iwamae | 2020 | 4 | 2 | 2 | 8 |
| Matsukura | 2021 | 4 | 2 | 3 | 9 |
| Yamato | 2021 | 3 | 2 | 2 | 7 |
| Yamamoto | 2021 | 4 | 2 | 2 | 8 |
Characteristics of Included Studies
Ten studies, comprising 621 patients with ASD, were included. Of these patients, 313 underwent LLIF combined with PSF (LLIF+PSF group), and 308 patients were treated with conventional PSF (only-PSF group). Study and patient characteristics of the included studies are presented in Table 2. There were no significant differences at baseline between the 2 groups in the patients’ age (68.23 ± 7.65 years vs 68.09 ± 7.68 years, P = .998), male-to-female ratio (.24 vs .22, P = .517), number of posterior fixed segments (8.62 ± 2.28 vs 7.96 ± 2.88, P = .175), LL (21.27° ± 16.94° vs 23.28° ± 14.01°, P = .156), TK (21.05° ± 15.44° vs 21.46° ± 17.60°, P = .922), PI-LL (31.91° ± 17.04° vs 29.45° ± 15.03°, P = .109), PT (30.01° ± 11.19° vs 28.59° ± 9.73°, P = .104), PI (50.52° ± 11.34° vs 51.65° ± 10.43°, P = .408), SVA (86.51 ± 56.01 mm vs 84.00 ± 49.17 mm, P = .301), C7PL-CSVL (25.22 ± 19.3 mm vs 26.06 ± 25.10 mm, P = .980), VAS score for leg pain (6.12 ± 2.89 vs 6.36 ± 2.68, P = .451) and back pain (7.43 ± 1.82 vs 7.23 ± 2.37, P = .441), ODI score (44.06 ± 17.65 vs 41.52 ± 17.50, P = .205), and SRS-22 total score (2.53 ± .58 vs 2.62 ± .57, P = .290). However, the preoperative coronal Cobb angle was significantly greater in the LLIF+PSF group than in the only-PSF group (37.94° ± 17.16° vs 32.20° ± 14.37°, P = .021). The length of follow-up weighted by the sample size of each study was 25.99 ± 4.20 months in the LLIF+PSF group and 29.02 ± 7.20 months in the only-PSF group.
Table 2.
Characteristics of the Included Studies.
| Author | Year | Design | Level of evidence | Group | Sample size | LIF technique cranial to L5-S1 | LIF technique at L5-S1 | 3CO (%) | Age (years) | Sex (M/F) | Fixed segments | Mean FU (months) | LLIF cage lordotic angle (°) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tormenti | 2010 | Retrospective | IV | LLIF+PSF | 8 | XLIF | PLIF/TLIF | NM | 60.0 ± 4.2 | NM | 9.3 ± 2.3 | 10.5 | NM |
| Only-PSF | 4 | PLIF/TLIF | PLIF/TLIF | NM | 61.0 ± 6.6 | NM | 8.3 ± 3.3 | 11.5 | |||||
| Baghdadi | 2014 | Retrospective | III | LLIF+PSF | 33 | LLIF | ALIF | No | 66.0 ± 8.0 | 7/26 | 10.0 ± 3.0 | 21.6 ± 9.1 | NM |
| Only-PSF | 33 | NM | NM | No | 67.0 ± 9.0 | 7/26 | 7.0 ± 5.0 | 32.4 ± 22.1 | |||||
| Strom | 2016 | Retrospective | IV | LLIF+PSF | 32 | LLIF | TLIF | No | 66.0 ± 8.0 | 6/26 | 10.0 ± 4.0 | 28.0 ± 18.0 | 10 |
| Only-PSF | 43 | NM | TLIF | No | 68.0 ± 8.0 | 10/33 | 11.0 ± 4.0 | 27.0 ± 17.0 | |||||
| Theologis | 2016 | Retrospective | III | LLIF+PSF | 16 | LLIF | ALIF/TLIF | NM | 64.0 ± 10.0 | 4/12 | 10.8 ± 3.3 | ≥24.0 | NM |
| Only-PSF | 16 | TLIF | ALIF/TLIF | NM | 62.1 ± 10.9 | 2/14 | 9.1 ± 5.8 | ≥24.0 | |||||
| Nakashima | 2018 | Retrospective | III | LLIF+PSF | 27 | XLIF | NM | No | 74.2 ± 6.7 | 11/16 | 5.8 ± 2.5 | ≥24.0 | 10 |
| Only-PSF | 19 | PLIF | NM | No | 70.2 ± 5.9 | 9/10 | 5.5 ± 2.5 | ≥24.0 | |||||
| Park | 2018 | Retrospective | IV | LLIF+PSF | 48 | LLIF | PLIF | No | 69.2 ± 7.0 | 7/41 | 4.7 ± 1.3 | 33.6 ± 7.0 | NM |
| Only-PSF | 43 | PLIF | PLIF | No | 72.0 ± 4.9 | 7/36 | 4.7 ± 1.7 | 37.6 ± 7.6 | |||||
| Iwamae | 2020 | Retrospective | III | LLIF+PSF | 14 | LLIF | PLIF | No | 69.4 ± 11.2 | 2/12 | 8.2 ± 0.3 | 39.6 ± 7.9 | 10 |
| Only-PSF | 17 | PLIF | PLIF | No | 61.8 ± 9.7 | 0/17 | 8.2 ± 0.6 | 59.3 ± 25.3 | |||||
| Matsukura | 2021 | Retrospective | III | LLIF+PSF | 21 | OLIF/XLIF | PLIF/TLIF | No | 74.0 ± 7.6 | 2/19 | 8.4 ± 1.9 | 24.2 | NM |
| Only-PSF | 21 | PLIF/TLIF | PLIF/TLIF | No | 73.2 ± 7.3 | 2/19 | 7.7 ± 1.5 | 24.2 | |||||
| Yamato | 2021 | Retrospective | III | LLIF+PSF | 75 | LLIF | PLIF | No | 70.2 ± 6.8 | 10/65 | 8.4 ± 2.5 | ≥24.0 | 6 or 10 |
| Only-PSF | 63 | PLIF | PLIF | 11.1% | 69.5 ± 7.3 | 9/54 | 8.0 ± 1.9 | ≥24.0 | |||||
| Yamamoto | 2021 | Retrospective | III | LLIF+PSF | 39 | OLIF/XLIF | TLIF | 7.7% | 68.5 ± 7.0 | 2/37 | 11.1 ± 1.7 | ≥24.0 | NM |
| Only-PSF | 49 | TLIF | TLIF | 20.4% | 68.0 ± 7.2 | 2/47 | 10.6 ± 2.5 | ≥24.0 | |||||
LLIF indicates lateral lumbar interbody fusion; LIF, lumbar interbody fusion; XLIF, extreme lateral interbody fusion; OLIF, oblique lateral interbody fusion; PSF, posterior spinal fusion; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal interbody fusion; ALIF, anterior lumbar interbody fusion; 3CO, three-column osteotomy; FU, follow-up; NM, not mentioned.
Spinopelvic Parameters
Lumbar lordosis
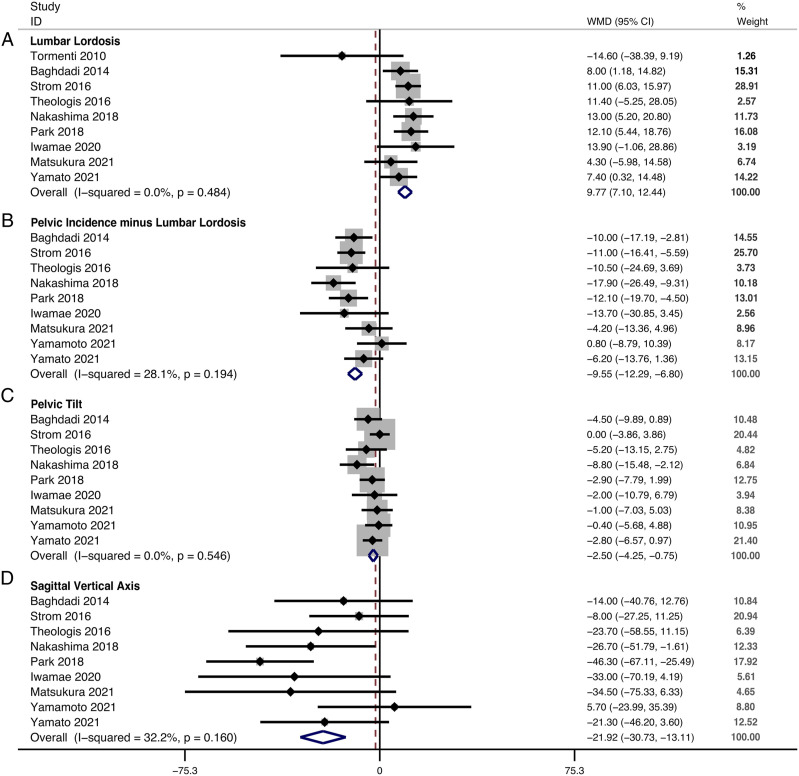
The restoration of LL could be obtained in 9 studies. The change in LL between the last follow-up and baseline was 24.06° ± 19.02° in the LLIF+PSF group and 15.27° ± 15.60° in the only-PSF group. The pooled results revealed a significantly greater restoration of LL in the LLIF+PSF than in the only-PSF group (WMD, 9.77; 95% confidence interval [CI] 7.10 to 12.44, P < .001), with no substantial heterogeneity among studies (I2 = .0%, P = .484) (Figure 2A). At the last follow-up, LL was 44.99° ± 11.26° in the LLIF+PSF group and 39.18° ± 12.11° in the only-PSF group.
Figure 2.
Forest plot of the restoration of spinopelvic parameters. (A) lumbar lordosis; (B) pelvic incidence minus lumbar lordosis; (C) pelvic tilt; (D) sagittal vertical axis.
Pelvic incidence minus lumbar lordosis
The restoration of PI-LL could be obtained in 9 studies. The change in PI-LL between the last follow-up and baseline was −27.61° ± 19.86° in the LLIF+PSF group and −18.30° ± 16.12° in the only-PSF group. The pooled results revealed a significantly greater restoration of PI-LL in the LLIF+PSF than in the only-PSF group (WMD, −9.55; 95% CI −12.29 to −6.80, P < .001), with no significant heterogeneity among studies (I2 = 28.1%, P = .194) (Figure 2B). At the last follow-up, PI-LL was 4.74° ± 12.99° in the LLIF+PSF group and 11.56° ± 13.58° in the only-PSF group.
Pelvic tilt
The restoration of PT could be obtained in 9 studies. The change in PT between the last follow-up and baseline was −8.74° ± 11.99° in the LLIF+PSF group and −5.70° ± 10.17° in the only-PSF group. The pooled results revealed a significantly greater restoration of PT in the LLIF+PSF than in the only-PSF group (WMD, −2.50; 95% CI −4.25, −.75, P = .005), with no substantial heterogeneity among studies (I2 = .0%, P = .546) (Figure 2C). At the last follow-up, PT was 21.54° ± 9.89° in the LLIF+PSF group and 22.87° ± 8.17° in the only-PSF group.
Sagittal vertical axis
The restoration of SVA could be obtained in 9 studies. The change of SVA between the last follow-up and baseline was −54.33 ± 57.89 mm in the LLIF+PSF group and −33.66 ± 51.13 mm in the only-PSF group. The pooled results revealed a significantly greater restoration of SVA in the LLIF+PSF than in the only-PSF group (WMD, −21.92; 95% CI −30.73 to −13.11, P < .001), with no significant heterogeneity among studies (I2 = 32.2%, P = .160) (Figure 2D). At the last follow-up, the SVA was 33.62 ± 41.81 mm in the LLIF+PSF group and 48.46 ± 44.08 mm in the only-PSF only group.
Thoracic kyphosis
The restoration of TK could be obtained in 5 studies. The change in TK between the last follow-up and baseline was 14.84° ± 18.40° in the LLIF+PSF group and 13.68° ± 18.04° in the only-PSF group. The pooled results revealed no significant difference in TK restoration between groups (WMD, 2.07; 95% CI −1.65 to 5.79, P = .276), with no substantial heterogeneity among studies (I2 = .0%, P = .932). At the last follow-up, TK was 35.20° ± 17.70° in the LLIF+PSF group and 34.55° ± 15.70° in the only-PSF group.
Coronal cobb angle
The correction of coronal Cobb angle could be obtained in 8 studies, and significant heterogeneity was detected among them (I2 = 71.9%, P = .001). The change in the coronal Cobb angle between the last follow-up and baseline was −24.09° ± 17.70° in the LLIF+PSF group and −16.85° ± 15.15° in the only-PSF group. The pooled results revealed a significantly greater correction in the coronal Cobb angle in the LLIF+PSF than in the only-PSF group (WMD, −7.09; 95% CI −12.33 to −1.86, P = .008) (Supplemental materials Figure 1). At the last follow-up, the coronal Cobb angle was 13.61 ± 9.99° in the LLIF+PSF group and 14.33° ± 10.29° in the only-PSF group.
C7 plumb line-center sacral vertical line
The restoration of C7PL-CSVL could be obtained in 4 studies. The change in C7PL-CSVL between the last follow-up and baseline was −12.21 ± 16.78 mm in the LLIF+PSF group and −8.07 ± 19.60 mm in the only-PSF group. The pooled results revealed a significantly greater restoration of C7PL-CSVL in the LLIF+PSF than in the only-PSF group (WMD, −4.03; 95% CI −7.52 to −.54, P = .024), with no substantial heterogeneity among studies (I2 = .0%, P = .446) (Supplemental materials Figure 2). At the last follow-up, the C7PL-CSVL was 13.15 ± 13.40 mm in the LLIF+PSF group and 17.49 ± 14.10 mm in the only-PSF group.
Surgical Data
Estimated blood loss
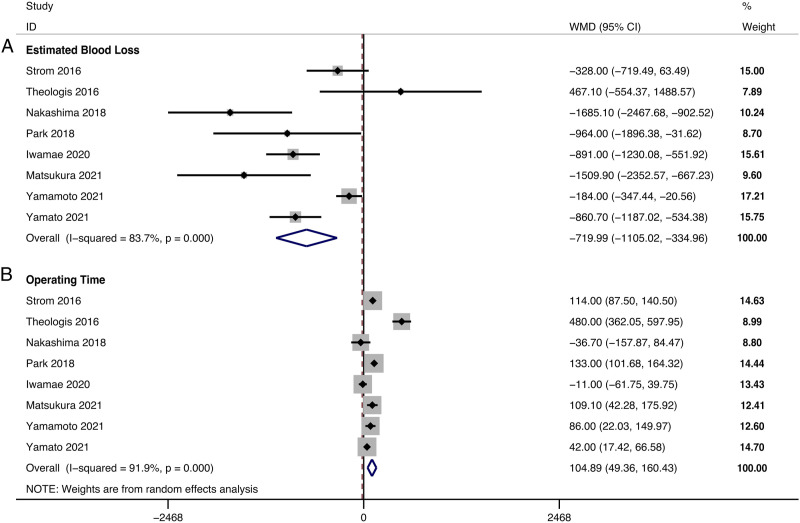
EBL could be obtained in 8 studies, and significant heterogeneity was detected (I2 = 83.7%, P < .001). The EBL was 1095.49 ± 736.13 mL in the LLIF+PSF group and 1862.57 ± 1386.69 mL in the only-PSF group. The pooled results revealed significantly reduced EBL in the LLIF+PSF group compared with that in the only-PSF group (WMD, −719.99; 95% CI −1105.02 to −334.96, P < .001) (Figure 3A).
Figure 3.
Forest plot of the surgical data. (A) estimated blood loss; (B) operating time.
Operating time
ORT could be obtained in 8 studies, and significant heterogeneity was detected (I2 = 91.9%, P < .001). The ORT was 486.06 ± 113.79 min in the LLIF+PSF group and 381.06 ± 112.83 min in the only-PSF group. The pooled results revealed significantly prolonged ORT in the LLIF+PSF than in the only-PSF group (WMD, 104.89; 95% CI 49.36 to 160.43, P < .001) (Figure 3B).
Complications
The results of various complications are summarized in Table 3.
Table 3.
Summary of Various Complications.
| Outcome | Number of included studies | Heterogeneity | Incidence (events/Total) | R, %R (95% CI) | P effect | ||
|---|---|---|---|---|---|---|---|
| I2, % | P | LLIF+PSF | Only-PSF, % | ||||
| Mechanical complications | |||||||
| Pseudarthrosis | 3 | 0.0 | .556 | 3.7 (3/81) | 17.4 (16/92) | .26 (.08, .79) | .017 |
| PJK/PJF | 8 | 0.0 | .830 | 9.7 (26/267) | 12.0 (32/267) | .90 (.57, 1.43) | .662 |
| Implant failure | 7 | 0.0 | .830 | 9.4 (23/245) | 7.5 (18/241) | 1.41 (.81, 2.44) | .220 |
| Surgical complications | |||||||
| Neurologic deficits | 8 | 0.0 | .820 | 16.4 (44/268) | 8.2 (22/269) | 2.04 (1.27, 3.25) | .003 |
| Dural tears | 4 | 0.0 | .611 | 1.2 (1/87) | 14.4 (14/97) | .16 (.04, .67) | .012 |
| Epidural hematoma | 4 | 0.0 | .399 | 0.6 (1/176) | 4.8 (8/166) | .27 (.08, .96) | .043 |
| Surgical site infection | 7 | 40.6 | .121 | 8.0 (21/263) | 7.8 (21/268) | 1.01 (.58, 1.75) | .971 |
| Systemic complications | |||||||
| Overall | 6 | 0.0 | .514 | 17.6 (35/199) | 22.2 (42/189) | .79 (.53, 1.17) | .232 |
| Cardiopulmonary | 3 | 5.5 | .347 | 3.3 (4/123) | 2.5 (3/122) | 1.29 (.33, 5.13) | .713 |
| DVT/PE | 6 | 0.0 | .719 | 2.5 (5/199) | 3.7 (7/189) | .72 (.26, 2.00) | .529 |
| Gastrointestinal | 4 | 0.0 | .717 | 6.9 (9/131) | 9.5 (12/126) | .71 (.31, 1.63) | .418 |
| Urinary | 2 | 0.0 | .760 | 4.7 (5/107) | 6.6 (7/106) | .73 (.24, 2.21) | .576 |
| CNS events | 3 | 0.0 | .434 | 9.5 (12/127) | 10.3 (13/126) | .84 (.41, 1.73) | .642 |
| Revision | 9 | 20.2 | .264 | 13.4 (38/284) | 17.0 (49/288) | .85 (.59, 1.22) | .377 |
LLIF indicates lateral lumbar interbody fusion; PSF, posterior spinal fusion; PJK/PJF, proximal junctional kyphosis/proximal junctional failure; DVT/PE, deep vein thrombosis/pulmonary embolism; CNS, central nervous system; RR, risk ratio.
Mechanical complications
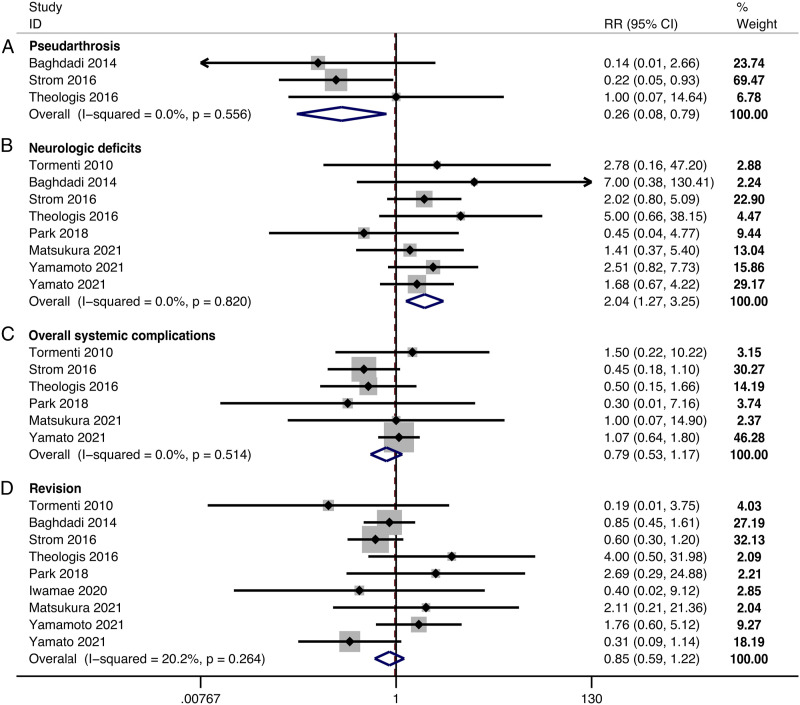
The incidence of pseudarthrosis was significantly lower in the LLIF+PSF than in the only-PSF group (RR, .26; 95% CI .08 to .79, P = .017) (Figure 4A). There were no significant differences in the incidence of proximal junctional kyphosis/failure (PJK/PJF) (RR, .90; 95% CI .57 to 1.43, P = .662) (Supplemental materials Figure 3) and implant failure (RR, 1.41; 95% CI, .81 to 2.44, P = .220) (Supplemental materials Figure 4) between groups.
Figure 4.
Forest plot of the complications and revision. (A) pseudarthrosis; (B) neurologic deficits; (C) overall systemic complications; (D) revision.
Surgical complications
The pooled results revealed a significantly higher incidence of neurologic deficits (RR, 2.04; 95% CI 1.27 to 3.25, P = .003) (Figure 4B) and a lower incidence of dural tear (RR, .16; 95% CI .04 to .67, P = .012) (Supplemental materials Figure 5) and epidural hematoma (RR, .27; 95% CI .08 to .96, P = .043) (Supplemental materials Figure 6) in the LLIF+PSF than in the only-PSF group. There was no significant difference in the incidence of surgical site infection between groups (RR, 1.01; 95% CI .58 to 1.75, P = .971).
Systemic complications
There were no significant differences between groups in the incidence of overall systemic complications (RR, .79; 95% CI .53 to 1.17, P = .232) (Figure 4C), cardiopulmonary events (RR, 1.29; 95% CI .33 to 5.13, P = .713), deep vein thrombosis/pulmonary embolism (RR, .72; 95% CI .26 to 2.00, P = .529), gastrointestinal events (RR, .71; 95% CI .31 to 1.63, P = .418), urinary events (RR, .73; 95% CI .24 to 2.21, P = .576), and central nervous system events (RR, .84; 95% CI .41 to 1.73, P = .642).
Revision
There was no significant difference in the incidence of revision between groups (RR, .85; 95% CI .59 to 1.22, P = .377) (Figure 4D).
Clinical Outcomes
Visual analog scale score
VAS score for leg pain
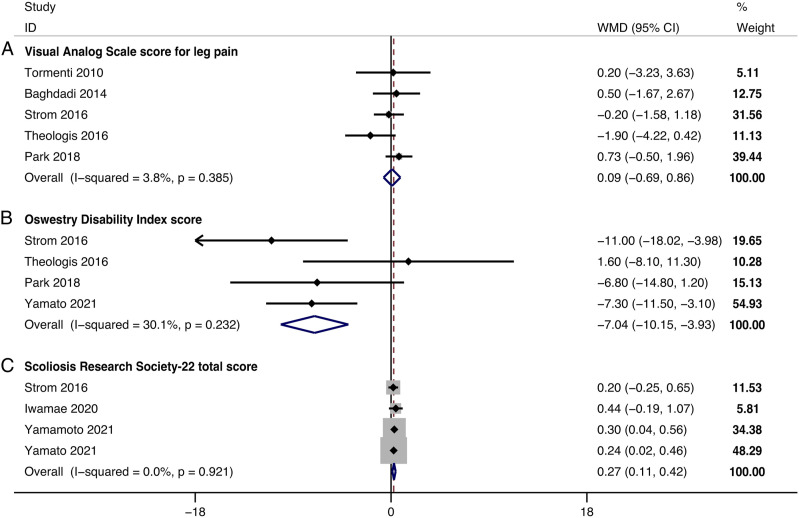
The improvement in the VAS score for leg pain could be obtained in 5 studies. The change in VAS score for leg pain between the last follow-up and baseline was −3.35 ± 3.30 in the LLIF+PSF group and −3.12 ± 3.30 in the only-PSF group. The pooled results revealed no significant difference in the improvement in the VAS score for leg pain between groups (WMD, .09; 95% CI −.69 .86, P = .825), with no significant heterogeneity detected among studies (I2 = 3.8%, P = .385) (Figure 5A). At the last follow-up, the VAS score for leg pain was 2.57 ± 2.92 in the LLIF+PSF group and 3.30 ± 3.28 in the only-PSF group.
Figure 5.
Forest plot of the improvement in the clinical outcomes. (A) Visual Analog Scale score for leg pain; (B) Oswestry Disability Index score; (C) Scoliosis Research Society-22 total score.
VAS score for back pain
The improvement in the VAS score for back pain could be obtained in 5 studies, with significant heterogeneity detected among them (I2 = 60.3%, P = .039). When the study by Baghdadi et al was omitted, heterogeneity was not significant (I2 = 42.1%, P = .159). The change in VAS score for back pain between the last follow-up and baseline was −3.89 ± 2.83 in the LLIF+PSF group and −3.63 ± 2.70 in the only-PSF group. The pooled results revealed no significant difference in the improvement in the VAS score for back pain between groups (WMD, −.23; 95% CI −1.02 to .55, P = .563) (Supplemental materials Figure 7). At the last follow-up, the VAS score for back pain was 3.27 ± 2.98 in the LLIF+PSF group and 3.28 ± 3.12 in the only-PSF group.
Oswestry disability index score
The improvement in the ODI score could be obtained in 4 studies. The change in ODI score between the last follow-up and baseline was −18.72 ± 16.10 in the LLIF+PSF group and −12.17 ± 14.35 in the only-PSF group. The pooled results revealed a significantly greater improvement in ODI score in the LLIF+PSF than in the only-PSF group (WMD, −7.04; 95% CI −10.15 to −3.93, P < .001), with no significant heterogeneity detected among studies (I2 = 30.1%, P = .232) (Figure 5B). At the last follow-up, the ODI score was 25.63 ± 14.02 in the LLIF+PSF group and 28.35 ± 16.26 in the only-PSF group.
Scoliosis research society-22 total score
The improvement in the SRS-22 total score could be obtained in 4 studies. The change in SRS-22 total score between the last follow-up and baseline was 1.19 ± .80 in the LLIF+PSF group and .89 ± .75 in the only-PSF group. The pooled results revealed a significantly greater improvement in the SRS-22 total score in the LLIF+PSF than in the only-PSF group (WMD, .27; 95% CI .11 to .42, P = .001), with no substantial heterogeneity detected among studies (I2 = .0%, P = .921) (Figure 5C). At the last follow-up, the SRS-22 total score was 3.70 ± .83 in the LLIF+PSF group and 3.56 ± .85 in the only-PSF group.
Sensitivity Analyses
The sensitivity analyses indicated that the omission of any study would not significantly affect the results, which verified the stability of the data and rationality of the analyses.
Discussion
With the advantages of reduced blood loss, increased fusion rate, and indirect neural decompression, LLIF has been proposed as an optimal surgical technique for various lumbar degenerative diseases.31,35 In recent years, in the combination of posterior instrumentation and facetectomies, LLIF has been adopted as a less invasive approach for the treatment of ASD. 6
This systematic review and meta-analysis directly compared the outcomes and complications of LLIF combined with PSF to those of PSF only for ASD. There was no significant difference in the incidence of systemic complications and revision between patients receiving LLIF+PSF or only-PSF. Nevertheless, a superior restoration of sagittal and coronal alignment, less blood loss, lower incidence of pseudarthrosis, and better improvement of HRQoL were observed in patients who underwent LLIF.
Spinopelvic Parameters
Restoration of LL and sagittal balance, which has been shown to correlate with better HRQoL, is the primary goal of ASD surgery.36,37 The results of the current study revealed that LLIF significantly restored more LL, PI-LL, and PT at the last follow-up. As of common knowledge, most of the LL (over 2/3) are located at the lumbosacral region, especially at L5-S1. 38 Due to the location of the iliac crest that obstructs lateral access, LLIF is not suitable for the L5-S1 level. 39 We found that most of the included studies performed L5-S1 interbody fusion through posterior LIF (PLIF), transforaminal LIF (TLIF), or anterior LIF (ALIF), and the selection of the procedure was matched between groups (Table 2). Some previous studies have reported that the addition of L5-S1 interbody fusion would not significantly benefit the restoration of LL in patients with ASD who underwent long fusion to the sacrum and sacropelvic fixation.40-42 Hence, the significantly greater spinopelvic correction in the LLIF+PSF group is more likely attributed to the more segmental lordosis obtained at the LLIF levels cranial to L5-S1. In addition, these superior acquisitions in regional spinopelvic parameters affected the global sagittal balance, which was reflected by the significantly greater restoration of SVA.
Coronal realignment has a smaller effect on clinical outcome but is important for relieving neurological symptoms related to the foraminal collapse.27,43 To date, there is evidence suggesting that less invasive lateral procedures are effective in correcting scoliosis and global coronal malalignment.15,16,44 Consistent with previous studies, this study suggested that LLIF+PSF can significantly correct more coronal Cobb angle and C7PL-CSVL at the last follow-up, compared with only-PSF. This result may be partially explained by the greater preoperative coronal Cobb angle in the LLIF+PSF group (37.94° ± 17.16°) than in the only-PSF group (32.20° ± 14.37°). However, the 2 approaches achieved an equivalent coronal Cobb angle (13.61° ± 9.99° vs14.33° ± 10.29°, P = .628) at the last follow-up, indicating that both LLIF+PSF and only-PSF yield a satisfactory coronal deformity correction.
It has been suggested that spinal malalignment in patients with ASD is mainly secondary to disc degeneration and collapse. Therefore, restoration of the disc height is the most reasonable approach.29,45 Generally, LLIF has been shown to provide anterior- and middle-column release and lift the interbody space using a large footprint cage.31,34 The cage is commonly located inside the anterior one-third of the interbody space, supporting the lateral rims of the endplate and achieving a firm contact with the upper and lower vertebrae. 46 Another advantage of LLIF is that it allows using cages with a greater lordotic angle, which enable greater segmental lordosis. 47 In this study, 10° lordotic cages were commonly used in the LLIF+PSF group (Table 2). These advantages may facilitate LLIF to maintain the disc and neural foramina height, and prevent subsidence and subsequent loss of deformity correction.7,31,34
Surgical Invasiveness
Complications after spinal surgery increase with age and surgical invasiveness, and most patients who undergo surgery for ASD are in their 60s to 70s. 48 Therefore, surgical invasiveness is a major concern for both patients and surgeons. EBL is 1 of the main factors to assess surgical invasiveness. 34 In the current study, the EBL was 1862.57 ± 1386.69 mL in the only-PSF group, an approximate degree of blood loss correlated with an increased perioperative complication rate in spinal fusion. 28 However, the EBL was 1095.49 ± 736.13 mL in the LLIF+PSF group, demonstrating a reduction in EBL of 719.99 mL compared with the only-PSF group. This advantage of LLIF could be attributed to the lateral access to the intervertebral discs and indirect decompression, which allows surgeons to perform manipulations without passing the epidural space, thereby avoiding bleeding from the epidural venous plexus.49,50
It is well known that surgical invasiveness and EBL increase with the performance of higher-grade osteotomy. 51 In some cases with severe and rigid spinal deformity, radical osteotomy is mandatory for optimal correction. However, as LLIF could provide more spinal realignments, some studies have suggested that LLIF combined with PSF may decrease the osteotomy grade needed by the only-PSF approach.18,46,52 Nakashima et al 29 reported that the average increase in local lordotic angle obtained by single-level LLIF is equivalent to that obtained by single-level posterior grade II osteotomy. When combined with posterior column osteotomy (PCO) or anterior column realignment (ACR) technique, LLIF could yield a sufficient correction with less blood loss even in cases of severe sagittal and coronal imbalance previously requiring 3CO.52,53 Therefore, LLIF may additionally minimize surgical invasiveness by avoiding the radical spinal osteotomy. Nevertheless, in the current study, most patients in the only-PSF group did not undergo 3CO, which may have caused the correction effect to be overshadowed. As studies comparing the efficacy of LLIF with additional PCO/ACR to one-stage posterior 3CO are limited, this concern needs to be investigated in future studies. From our perspective on the surgical concept of ASD, surgeons should choose a suitable procedure or a combination of procedures for optimal correction and less invasiveness.
Mechanical Complications
In the current study, the incidence of pseudarthrosis was significantly lower in the LLIF+PSF than in the only-PSF group. Our results coincided with those of a recent multicenter study reporting that the incidence of pseudarthrosis is 14.7% when using the posterior-only approach and only 7.6% when using the combined anterior-posterior approach for ASD surgery. 54 Among the risk factors for the occurrence of pseudarthrosis, biomechanical factors such as osteoporosis, pelvic fixation, lack of circumferential fusion, and sharply angulated 3CO, play a major role.54,55 However, LLIF may provide anterior column support and more solid interbody fusion by using a large footprint cage. Compared with PLIF or TLIF, LLIF exhibits more stable mechanical properties, as shown in several biomechanical studies.56,57 In addition, as mentioned above, combining LLIF with PCO provides sufficient spinal realignment. Not as angular as 3CO, the correction characteristic of PCO was rounded; therefore, hyperacutely countered rods could be avoided, and rod fatigue strength was distributed. 58 Owing to these biomechanical advantages of LLIF, the fact that the incidence of pseudarthrosis was lower in the LLIF+PSF group was expected, although the number of included studies was limited.
PJK/PJF have a multifactorial etiology related to surgical and patient factors, including upper instrumented vertebra in the lower thoracic region, long fusion to the sacrum, correction of SVA >5 cm, combined anterior-posterior approach, age at surgery >55 years, and low bone mineral density.59,60 However, for patients with ASD who undergo PSF with or without LLIF, most of these factors are unavoidable. In the current study, the incidence of PJK/PJF was similar between groups (9.7% vs 12.0%), indicating that the combination of LLIF did not affect the development of this complication. Disruption of the posterior muscular tension band is also a risk factor for PJK/PJF, but percutaneous techniques can alleviate it. 59 A recent study by Chan et al 61 reported that LLIF combined with percutaneous pedicle screw fixation significantly reduces the incidence of PJK, compared with the open approach. Nevertheless, most patients in the included studies, regardless of being in the LLIF+PSF or only-PSF group, underwent conventional open pedicle screw fixation, which was associated with iatrogenic injury to the paraspinal musculature and posterior tension band. We consider that this may be a factor skewing the incidence of PJK/PJF in this study.
Surgical Complications
Owing to the minimal epidural manipulations through the lateral approach, the finding that the incidence of both dural tear (1.2% vs 14.4%, P = .012) and epidural hematoma (.6% vs 4.8%, P = .043) was significantly lower in the LLIF+PSF group was not unexpected. However, the incidence of neurologic deficits in the LLIF+PSF group was twice as high as that in the only-PSF group (16.4% vs 8.2%, P = .003). The neurologic complications related to LLIF, including anterior thigh pain, thigh numbness, and hip flexion weakness, are a predominant concern among surgeons.30,62 The significantly higher incidence of neurologic deficits can be explained because LLIF requires dissection of the psoas major, which may cause muscle trauma and lumbosacral plexus irritation.4,63 Since most of these lower extremity symptoms are resolved spontaneously within 6–12 months after surgery, many authors have pointed out that these neurologic deficits should not be considered as complications but side effects of psoas manipulation during LLIF.64-66 Thus, an incidence of neurologic complications ranging from .7% to 78.8% has been reported by previous studies, which indicates significant heterogeneity. 6 Using triggered electromyography and limiting the psoas retraction time during the procedure may be feasible to mitigate the postoperative neurologic deficits. 67
Revision
With the advantages of both biomechanics and surgical approaches, the revision rate was also reasonably lower in the LLIF+PSF than in the only-PSF group (13.3% vs 17.0%), although the difference was not statistically significant. Six of the included studies reported the indications for revision in detail (Table 4).25-27,30,31,33 When data from these 6 studies were pooled, the revision rate in the LLIF+PSF group became significantly lower than that in the only-PSF group (RR, .64; 95% CI .42 to .98, P = .040). Among the revision surgeries, 68.9% were due to mechanical complications in the only-PSF group while only 48.0% in the LLIF+PSF group. However, surgical site infection was the leading cause of revision in the LLIF+PSF group, which may be due to the two-stage strategy.
Table 4.
Summary of Indications for Revision in the 6 Studies.
| Indication | LLIF+PSF group | Only-PSF group |
|---|---|---|
| n (%) | n (%) | |
| Mechanical complications | ||
| PJK/PJF | 6 (24.0%) | 12 (26.7%) |
| Implant failure | 4 (16.0%) | 4 (8.9%) |
| Pseudoarthrosis | 2 (8.0%) | 15 (33.3%) |
| Subtotal | 12 (48.0%) | 31 (68.9%) |
| Surgical complications | ||
| Neurological dificits | 4 (16.0%) | 4 (8.9%) |
| Surgical site infection | 8 (32.0%) | 10 (22.2%) |
| Screw reinsertion | 1 (4.0%) | 0 (.0%) |
| Subtotal | 13 (52.0%) | 14 (31.1%) |
| Total | 25 (100.0%) | 45 (100.0%) |
| Revision rate | 11.9% | 20.7% |
PJK indicates proximal junctional kyphosis; PJF, proximal junctional failure; LLIF, lateral lumbar interbody fusion; PSF, posterior spinal fusion.
Systemic Complications
Systemic complications are not uncommon after surgery for ASD, and studies that focus on this domain are limited. In a cohort of 448 patients with ASD, Soroceanu et al 68 reported that 26.8% of patients had at least 1 systemic complication. Similarly, in another cohort of 131 patients with adult degenerative scoliosis, Zhang et al 69 reported that the systemic complication incidence was 25.2% after PSF, and patients who smoked or had cardiovascular comorbidities were at high risk. In the current study, the systemic complication rate was 22.2% in the only-PSF group, which is consistent with the rate observed in previous studies. Although the difference was not statistically significant, the LLIF+PSF group presented with fewer systemic complications than did the only-PSF group (17.6% vs 22.2%). This finding may be attributed to the less EBL and staged-surgery strategy. 70 It has been reported that blood loss is a risk factor for systemic complications in spinal fusion surgery, whereas operating time is not. 71 Although the ORT was significantly longer when using LLIF combined with PSF, which was commonly performed in a two-staged manner, this strategy reduced surgical invasiveness and risks of the posterior procedure. This advantage may overweigh the prolonged ORT to benefit the management of complications. Thus, although not statistically significant, the LLIF procedure may have the potential to slightly reduce surgical and systemic complications at the cost of substantially high incidence of lower extremity symptoms. Surgeons should weigh the advantages and disadvantages of this procedure, and inform patients about its side effects.
Clinical Outcomes
The similar improvement in VAS score for leg (−3.35 ± 3.30 vs −3.12 ± 3.30, P = .825) and back pain (−3.89 ± 2.83 vs −3.63 ± 2.70, P = .563) between groups revealed that the indirect decompression by LLIF was as effective as the direct decompression to resolve the pain caused by ASD. Better restoration of normal radiographic alignment, solid spinal fusion, and a lower revision rate for mechanical complications were found in the LLIF+PSF group. These superior performances have been associated with a better HRQoL, which might explain the more significant improvement in ODI score and SRS-22 total score observed in the LLIF+PSF than in the only-PSF group.36,72,73 Additionally, the change in HRQoL parameters in the LLIF+PSF group reached the minimum clinically important difference for patients with ASD (14.96 for ODI score and .94 for SRS-22 total score), which was arguably not the case in the only-PSF group, further indicating that LLIF+PSF is more potent in improving the HRQoL for these patients. 74
Limitations
This study has several limitations. First, although the number of posterior fixed segments was similar between groups, the number of interbody fusions was higher in the LLIF+PSF group (3.78 vs 1.82). In addition, only 3 studies reported the incidence of pseudarthrosis, and data on the fusion rate according to definite assessment criteria (e.g., the method by Fraser et al 75 for interbody fusion status assessment or the method by Lenke et al 76 for posterolateral fusion status assessment) could not be obtained. Therefore, the reliability of the pseudarthrosis rate may be impacted, and we could not determine the lower pseudarthrosis rate for LLIF per se or the more circumferential fusion levels achieved by it. Second, most studies lacked data on comorbidities, which could have influenced some of the outcomes analyzed. Third, despite definite inclusion and exclusion criteria, the patients with ASD treated in each group were relatively similar on average, but individually variable. Thus, the variability in surgical techniques (i.e., PLIF or TLIF in the only-PSF group, number or grade of posterior osteotomies, and type of interbody cage [straight or lordotic]) should be addressed, although these data were not available for comparative analysis. Additionally, although some studies applied propensity score matching to construct a randomized experimental-like situation, no randomized controlled study was included at a higher level of methodological quality. 77 Further multicenter randomized controlled trials should be performed to obtain more convincing conclusions.
Conclusion
Compared with conventional PSF, LLIF combined with PSF was associated with superior restoration of sagittal and coronal alignment, lower incidence of pseudarthrosis, better improvement in HRQoL, and less surgical invasiveness in the treatment of ASD, albeit at the cost of prolonged surgical times and substantially high incidence of lower extremity symptoms. Surgeons should weigh the advantages and disadvantages of this procedure, and inform patients about its side effects.
Supplemental Material
Supplemental Material, sj-pptx-1-gsj-10.1177_21925682221089876 for What Are the Benefits of Lateral Lumbar Interbody Fusion on the Treatment of Adult Spinal Deformity: A Systematic Review and Meta-Analysis Deformity by Honghao Yang, Jingwei Liu, Yong Hai, and Bo Han in Global Spine Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Honghao Yang https://orcid.org/0000-0001-5300-1283
References
- 1.Ames CP, Scheer JK, Lafage V, et al. Adult spinal deformity: epidemiology, health impact, evaluation, and management. Spine Deform. 2016;4(4):310-322. doi: 10.1016/j.jspd.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Pritchett JW, Bortel DT. Degenerative symptomatic lumbar scoliosis. Spine. 1993;18(6):700-703. doi: 10.1097/00007632-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925-948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 4.Phan K, Huo YR, Hogan JA, et al. Minimally invasive surgery in adult degenerative scoliosis: a systematic review and meta-analysis of decompression, anterior/lateral and posterior lumbar approaches. J Spine Surg. 2016;2(2):89-104. doi: 10.21037/jss.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellisé F, Vila-Casademunt A, Vila-Casademunt A, et al. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J. 2015;24(1):3-11. doi: 10.1007/s00586-014-3542-1. [DOI] [PubMed] [Google Scholar]
- 6.Batheja D, Dhamija B, Ghodke A, Anand SS, Balain BS. Lateral lumbar interbody fusion in adult spine deformity - A review of literature. J Clin Orthop Trauma . 2021;22:101597. doi: 10.1016/j.jcot.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae J, Theologis AA, Strom R, et al. Comparative analysis of 3 surgical strategies for adult spinal deformity with mild to moderate sagittal imbalance. J Neurosurg Spine. 2018;28(1):40-49. doi: 10.3171/2017.5.Spine161370. [DOI] [PubMed] [Google Scholar]
- 8.Bhagat S, Vozar V, Lutchman L, Crawford RJ, Rai AS. Morbidity and mortality in adult spinal deformity surgery: Norwich spinal unit experience. Eur Spine J. 2013;22(suppl 1):S42-S46. doi: 10.1007/s00586-012-2627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara R, Takeshita K, Takahashi J, et al. The complication trends of adult spinal deformity surgery in Japan - the Japanese scoliosis society morbidity and mortality survey from 2012 to 2017. J Orthop Sci. 2021;26(4):533-537. doi: 10.1016/j.jos.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kwon B, Kim DH. Lateral lumbar interbody fusion: indications, outcomes, and complications. J Am Acad Orthop Surg. 2016;24(2):96-105. doi: 10.5435/jaaos-d-14-00208. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal N, Faramand A, Alan N, et al. Lateral lumbar interbody fusion in the elderly: a 10-year experience. J Neurosurg Spine. 2018;29(5):525-529. doi: 10.3171/2018.3.Spine171147. [DOI] [PubMed] [Google Scholar]
- 13.Xu DS, Walker CT, Godzik J, Turner JD, Smith W, Uribe JS. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: a literature review. Ann Transl Med. 2018;6(6):104. doi: 10.21037/atm.2018.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hai Y, Liu J, Liu Y, et al. Expert consensus on clinical application of lateral lumbar interbody fusion: results from a modified delphi study. Global Spine J. 2021;219256822110126. doi: 10.1177/21925682211012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tempel ZJ, Gandhoke GS, Bonfield CM, Okonkwo DO, Kanter AS. Radiographic and clinical outcomes following combined lateral lumbar interbody fusion and posterior segmental stabilization in patients with adult degenerative scoliosis. Neurosurg Focus. 2014;36(5):E11. doi: 10.3171/2014.3.Focus13368. [DOI] [PubMed] [Google Scholar]
- 16.Katz AD, Singh H, Greenwood M, Cote M, Moss IL. Clinical and radiographic evaluation of multilevel lateral lumbar interbody fusion in adult degenerative scoliosis. Clin Spine Surg. 2019;32(8):E386-e396. doi: 10.1097/bsd.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 17.Barone G, Scaramuzzo L, Zagra A, Giudici F, Perna A, Proietti L. Adult spinal deformity: effectiveness of interbody lordotic cages to restore disc angle and spino-pelvic parameters through completely mini-invasive trans-psoas and hybrid approach. Eur Spine J. 2017;26(suppl 4):457-463. doi: 10.1007/s00586-017-5136-1. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Xu Z, Li F, Chen Q. Does lateral lumbar interbody fusion decrease the grading of Lenke-Silva classification and determine the optimal fusion level in severe adult degenerative scoliosis? World Neurosurg. 2020;139:e335-e344. doi: 10.1016/j.wneu.2020.03.215. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies S. The importance of PROSPERO to the national institute for health research. Syst Rev. 2012;1:5. doi: 10.1186/2046-4053-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85(1):1-3. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28(3):E7. doi: 10.3171/2010.1.Focus09263. [DOI] [PubMed] [Google Scholar]
- 26.Baghdadi YM, Larson AN, Dekutoski MB, et al. agittal balance and spinopelvic parameters after lateral lumbar interbody fusion for degenerative scoliosis: a case-control study. Spine. 2014;39(3):E166-E173. doi: 10.1097/brs.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strom RG, Bae J, Mizutani J, Valone F, 3rd, Ames CP, Deviren V. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J Neurosurg Spine. 2016;25(6):697-705. doi: 10.3171/2016.4.Spine16157. [DOI] [PubMed] [Google Scholar]
- 28.Theologis AA, Mundis GM, Jr., Nguyen S, et al. Utility of multilevel lateral interbody fusion of the thoracolumbar coronal curve apex in adult deformity surgery in combination with open posterior instrumentation and L5-S1 interbody fusion: a case-matched evaluation of 32 patients. J Neurosurg Spine. 2017;26(2):208-219. doi: 10.3171/2016.8.Spine151543. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima H, Kanemura T, Satake K, et al. Comparative radiographic outcomes of lateral and posterior lumbar interbody fusion in the treatment of degenerative lumbar kyphosis. Asian Spine J. 2019;13(3):395-402. doi: 10.31616/asj.2018.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park HY, Ha KY, Kim YH, et al. Minimally invasive lateral lumbar interbody fusion for adult spinal deformity: clinical and radiological efficacy with minimum two years follow-up. Spine. 2018;43(14):E813-E821. doi: 10.1097/brs.0000000000002507. [DOI] [PubMed] [Google Scholar]
- 31.Iwamae M, Matsumura A, Namikawa T, et al. Surgical outcomes of multilevel posterior lumbar interbody fusion versus lateral lumbar interbody fusion for the correction of adult spinal deformity: a comparative clinical study. Asian Spine J. 2020;14(4):421-429. doi: 10.31616/asj.2019.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto T, Yagi M, Suzuki S, et al. Limited cost benefit of lateral interbody fusion for adult spinal deformity surgery. Spine. 2021;46(1):48-53. doi: 10.1097/brs.0000000000003703. [DOI] [PubMed] [Google Scholar]
- 33.Yamato Y, Hasegawa T, Yoshida G, et al. Planned two-stage surgery using lateral lumbar interbody fusion and posterior corrective fusion: a retrospective study of perioperative complications. Eur Spine J. 2021;30(8):2368-2376. doi: 10.1007/s00586-021-06879-0. [DOI] [PubMed] [Google Scholar]
- 34.Matsukura Y, Yoshii T, Morishita S, et al. Comparison of lateral lumbar interbody fusion and posterior lumbar interbody fusion as corrective surgery for patients with adult spinal deformity-a propensity score matching analysis. J Clin Med. 2021;10:4737. doi: 10.3390/jcm10204737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohba T, Ebata S, Haro H. Comparison of serum markers for muscle damage, surgical blood loss, postoperative recovery, and surgical site pain after extreme lateral interbody fusion with percutaneous pedicle screws or traditional open posterior lumbar interbody fusion. BMC Musculoskelet Disord. 2017;18(1):415. doi: 10.1186/s12891-017-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyrölä K, Repo J, Mecklin JP, Ylinen J, Kautiainen H, Häkkinen A. Spinopelvic changes based on the simplified SRS-schwab adult spinal deformity classification: relationships with disability and health-related quality of life in adult patients with prolonged degenerative spinal disorders. Spine. 2018;43(7):497-502. doi: 10.1097/brs.0000000000002370. [DOI] [PubMed] [Google Scholar]
- 37.Takemoto M, Boissière L, Vital JM, et al. Are sagittal spinopelvic radiographic parameters significantly associated with quality of life of adult spinal deformity patients? Multivariate linear regression analyses for pre-operative and short-term post-operative health-related quality of life. Eur Spine J. 2017;26(8):2176-2186. doi: 10.1007/s00586-016-4872-y. [DOI] [PubMed] [Google Scholar]
- 38.Yamato Y, Sato Y, Togawa D, et al. Differences in the geometrical spinal shape in the sagittal plane according to age and magnitude of pelvic incidence in healthy elderly individuals. J Orthop Sci. 2020;25(4):557-564. doi: 10.1016/j.jos.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2-18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lara NJ, Chung AS, Lockwood D, Revella J, Crandall D, Chang MS. Does interbody support at L5-S1 matter in long fusions to the Pelvis?: a 5-year analysis. Spine. 2021;46(15):1014-1019. doi: 10.1097/brs.0000000000003937. [DOI] [PubMed] [Google Scholar]
- 41.Annis P, Brodke DS, Spiker WR, Daubs MD, Lawrence BD. The fate of L5-S1 with low-dose BMP-2 and pelvic fixation, with or without interbody fusion, in adult deformity surgery. Spine. 2015;40(11):E634-E639. doi: 10.1097/brs.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 42.Rahman RK, Buchowski JM, Stephens B, Dorward IG, Koester LA, Bridwell KH. Comparison of TLIF with rhBMP-2 versus no TLIF and higher posterolateral rhBMP-2 dose at L5-S1 for long fusions to the sacrum with sacropelvic fixation in patients with primary adult deformity. Spine. 2013;38(26):2264-2271. doi: 10.1097/brs.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 43.Buell TJ, Smith JS, Shaffrey CI, et al. Multicenter assessment of surgical outcomes in adult spinal deformity patients with severe global coronal malalignment: determination of target coronal realignment threshold. J Neurosurg Spine. 2020;1-14. doi: 10.3171/2020.7.Spine20606. [DOI] [PubMed] [Google Scholar]
- 44.Choi SW, Ames C, Berven S, Chou D, Tay B, Deviren V. Contribution of lateral interbody fusion in staged correction of adult degenerative scoliosis. J Korean Neurosurg Soc. 2018;61(6):716-722. doi: 10.3340/jkns.2017.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youssef JA, Orndorff DO, Patty CA, et al. Current status of adult spinal deformity. Global Spine J. 2013;3(1):51-62. doi: 10.1055/s-0032-1326950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KY, Lee JH, Kang KC, et al. Minimally invasive multilevel lateral lumbar interbody fusion with posterior column osteotomy compared with pedicle subtraction osteotomy for adult spinal deformity. Spine J. 2020;20(6):925-933. doi: 10.1016/j.spinee.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Anand N, Cohen RB, Cohen J, Kahndehroo B, Kahwaty S, Baron E. The Influence of lordotic cages on creating sagittal balance in the CMIS treatment of adult spinal deformity. Int J Spine Surg. 2017;11(3):23. doi: 10.14444/4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carreon LY, Puno RM, Dimar JR, 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85(11):2089-2092. doi: 10.2106/00004623-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Lui DF, Butler JS, Yu HM, et al. Neurologic injury in complex adult spinal deformity surgery: staged multilevel oblique lumbar interbody fusion (MOLIF) using hyperlordotic tantalum cages and posterior fusion versus pedicle subtraction osteotomy (PSO). Spine. 2019;44(16):E939-e949. doi: 10.1097/brs.0000000000003034. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010;35(26 suppl l):S331-S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 51.Schwab F, Blondel B, Chay E, et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery. 2014;74(1):112-120; discussion 120. doi: 10.1227/NEU.0000000000000182o. [DOI] [PubMed] [Google Scholar]
- 52.Sakuma T, Kotani T, Akazawa T, et al. Efficacy of lateral lumbar interbody fusion combined with posterior spinal fusion compared with three-column osteotomy for adult spinal deformity with severe lumbar sagittal deformity. Eur J Orthop Surg Traumatol. 2021. doi: 10.1007/s00590-021-03068-z. [DOI] [PubMed] [Google Scholar]
- 53.Leveque JC, Yanamadala V, Buchlak QD, Sethi RK. Correction of severe spinopelvic mismatch: decreased blood loss with lateral hyperlordotic interbody grafts as compared with pedicle subtraction osteotomy. Neurosurg Focus. 2017;43(2):E15. doi: 10.3171/2017.5.Focus17195. [DOI] [PubMed] [Google Scholar]
- 54.Marques MF, Fiere V, Obeid I, et al. Pseudarthrosis in adult spine deformity surgery: risk factors and treatment options. Eur Spine J. 2021;30(11):3225-3232. doi: 10.1007/s00586-021-06861-w. [DOI] [PubMed] [Google Scholar]
- 55.How NE, Street JT, Dvorak MF, et al. Pseudarthrosis in adult and pediatric spinal deformity surgery: a systematic review of the literature and meta-analysis of incidence, characteristics, and risk factors. Neurosurg Rev. 2019;42(2):319-336. doi: 10.1007/s10143-018-0951-3. [DOI] [PubMed] [Google Scholar]
- 56.Peck JH, Kavlock KD, Showalter BL, Ferrell BM, Peck DG, Dmitriev AE. Mechanical performance of lumbar intervertebral body fusion devices: an analysis of data submitted to the food and drug administration. J Biomech. 2018;78:87-93. doi: 10.1016/j.jbiomech.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Metzger MF, Robinson ST, Maldonado RC, Rawlinson J, Liu J, Acosta FL. Biomechanical analysis of lateral interbody fusion strategies for adjacent segment degeneration in the lumbar spine. Spine J. 2017;17(7):1004-1011. doi: 10.1016/j.spinee.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Tang JA, Leasure JM, Smith JS, Buckley JM, Kondrashov D, Ames CP. Effect of severity of rod contour on posterior rod failure in the setting of lumbar pedicle subtraction osteotomy (PSO): a biomechanical study. Neurosurgery. 2013;72(2):276-283; discussion 283. doi: 10.1227/NEU.0b013e31827ba066. [DOI] [PubMed] [Google Scholar]
- 59.Anand N, Agrawal A, Ravinsky R, Khanderhoo B, Kahwaty S, Chung A. The prevalence of proximal junctional kyphosis (PJK) and proximal junctional failure (PJF) in patients undergoing circumferential minimally invasive surgical (cMIS) correction for adult spinal deformity: long-term 2- to 13-year follow-up. Spine Deform. 2021;9(5):1433-1441. doi: 10.1007/s43390-021-00319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu FY, Wang T, Yang SD, Wang H, Yang DL, Ding WY. Incidence and risk factors for proximal junctional kyphosis: a meta-analysis. Eur Spine J. 2016;25(8):2376-2383. doi: 10.1007/s00586-016-4534-0. [DOI] [PubMed] [Google Scholar]
- 61.Chan AK, Eastlack RK, Fessler RG, et al. Two- and three-year outcomes of minimally invasive and hybrid correction of adult spinal deformity. J Neurosurg Spine . 2021;1-14. doi: 10.3171/2021.7.Spine21138. [DOI] [PubMed] [Google Scholar]
- 62.Hijji FY, Narain AS, Bohl DD, et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J. 2017;17(10):1412-1419. doi: 10.1016/j.spinee.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 63.Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J. 2014;14(5):749-758. doi: 10.1016/j.spinee.2013.06.066. [DOI] [PubMed] [Google Scholar]
- 64.Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011;15(1):11-18. doi: 10.3171/2011.2.Spine10374. [DOI] [PubMed] [Google Scholar]
- 65.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24(4):242-250. doi: 10.1097/BSD.0b013e3181ecf995. [DOI] [PubMed] [Google Scholar]
- 66.Cahill KS, Martinez JL, Wang MY, Vanni S, Levi AD. Motor nerve injuries following the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;17(3):227-231. doi: 10.3171/2012.5.Spine1288. [DOI] [PubMed] [Google Scholar]
- 67.Uribe JS, Isaacs RE, Youssef JA, et al. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J. 2015;24(suppl 3):378-385. doi: 10.1007/s00586-015-3871-8. [DOI] [PubMed] [Google Scholar]
- 68.Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery: incidence, risk factors, and clinical impact. Spine. 2016;41(22):1718-1723. doi: 10.1097/brs.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 69.Zhang XN, Sun XY, Meng XL, Hai Y. Risk factors for medical complications after long-level internal fixation in the treatment of adult degenerative scoliosis. Int Orthop. 2018;42(11):2603-2612. doi: 10.1007/s00264-018-3927-6. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida G, Hasegawa T, Yamato Y, et al. Predicting perioperative complications in adult spinal deformity surgery using a simple sliding scale. Spine. 2018;43(8):562-570. doi: 10.1097/brs.0000000000002411. [DOI] [PubMed] [Google Scholar]
- 71.Cho KJ, Suk SI, Park SR, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine. 2007;32(20):2232-2237. doi: 10.1097/BRS.0b013e31814b2d3c. [DOI] [PubMed] [Google Scholar]
- 72.O'Neill KR, Lenke LG, Bridwell KH, Neuman BJ, Kim HJ, Archer KR. Factors associated with long-term patient-reported outcomes after three-column osteotomies. Spine J. 2015;15(11):2312-2318. doi: 10.1016/j.spinee.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 73.Bourghli A, Boissiere L, Larrieu D, et al. Lack of improvement in health-related quality of life (HRQOL) scores 6 months after surgery for adult spinal deformity (ASD) predicts high revision rate in the second postoperative year. Eur Spine J. 2017;26(8):2160-2166. doi: 10.1007/s00586-017-5068-9. [DOI] [PubMed] [Google Scholar]
- 74.Yuksel S, Ayhan S, Nabiyev V, et al. Minimum clinically important difference of the health-related quality of life scales in adult spinal deformity calculated by latent class analysis: is it appropriate to use the same values for surgical and nonsurgical patients? Spine J. 2019;19(1):71-78. doi: 10.1016/j.spinee.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28(10):997-1001. doi: 10.1097/01.Brs.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 76.Lenke LG, Bridwell KH, Bullis D, Betz RR, Baldus C, Schoenecker PL. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5(4):433-442. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637-1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pptx-1-gsj-10.1177_21925682221089876 for What Are the Benefits of Lateral Lumbar Interbody Fusion on the Treatment of Adult Spinal Deformity: A Systematic Review and Meta-Analysis Deformity by Honghao Yang, Jingwei Liu, Yong Hai, and Bo Han in Global Spine Journal