Abstract
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a variant antigen expressed on the surface of infected erythrocytes. Each parasite genome contains about 40 PfEMP1 genes, but only 1 PfEMP1 gene is expressed at a given time. PfEMP1 serves as a parasite-sequestering ligand to endothelial cells and enables the parasites to avoid splenic passage. PfEMP1 antibodies may protect from disease by inhibiting sequestration, thus facilitating the destruction of infected erythrocytes in the spleen. In this study, we have measured antibodies in Ghanaian children to a conserved region of PfEMP1 by enzyme-linked immunosorbent assay and antibodies to variant molecules on erythrocytes infected with field isolates of P. falciparum by flow cytometry. Based on close clinical monitoring, the children were grouped into those who did (susceptible) and those who did not (protected) have malaria during the season. The prevalences of antibodies to both the conserved PfEMP1 peptide and the variant epitopes were greater than 50%, and the levels of immunoglobulin G (IgG) correlated with age. The levels of antibodies to both the conserved peptide and the variant epitopes were higher in protected than in susceptible children. After correcting for the effect of age, the levels of IgG to variant antigens on a Sudanese and a Ghanaian parasite isolate remained significantly higher in protected than in susceptible children. Thus, the levels of IgG to variant antigens expressed on the surface of infected erythrocytes correlated with protection from clinical malaria. In contrast, the levels of IgG to a peptide derived from a conserved part of PfEMP1 did not correlate with protection from malaria.
Antibodies directed against the variant antigen Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) have been suggested to be a key element of malaria immunity (7, 21, 22). PfEMP1 is encoded by about 40 var genes (3, 27, 32) and mediates sequestration of the parasite to endothelial cells of blood vessels within various host organs including the brain and the placenta (2). Sequestration is probably a strategy evolved by the parasite to avoid filtration through and killing in the spleen (12). Sequestration will thus tend to increase parasite multiplication rates. Furthermore, the process is thought to contribute to the pathogenesis of severe malaria because the accumulation of parasites provokes a strong inflammatory response that can be harmful to the host (4). Antibodies to PfEMP1 can block the adhesion of mature parasitized erythrocytes to specific receptors (28), and individuals in malaria-endemic areas acquire antibodies that block parasite adhesion (17, 25, 26). Such antibodies may contribute to protection against malaria by reducing tissue-specific sequestration and inflammation and by reducing the parasite burden as nonbinding parasites are removed in the spleen. These protective mechanisms are not mutually exclusive, but the first will tend to reduce the number of severe infections whereas the second will tend to reduce the number of fever episodes, which occur as the parasite density increases above the fever threshold. It has been shown that development of malaria severe enough to warrant hospital admission is associated with lack of antibody reactivity to the variant antigens expressed by the parasite isolate causing the disease (7). The present study was designed to test whether antibodies to variant antigens are involved in protection of children from febrile malaria episodes. Ghanaian children have asymptomatic parasitemia controlled at relatively low densities most of the time. The first symptom of malaria in these children usually is fever, which occurs when parasite densities exceed the fever threshold because of insufficient control of parasite multiplication. In this study children were closely monitored clinically and parasitologically during the malaria season and subsequently divided into two groups consisting of those who did and those who did not develop malaria. We show that the levels in plasma of antibodies to variant antigens expressed on some parasite isolates before the malaria season were associated with protection against febrile malaria episodes.
MATERIALS AND METHODS
Study area, study population, and clinical surveillance.
The study was conducted in Dodowa, a semirural town outside Accra, Ghana. Malaria transmission is perennial but peaks during or immediately after the major rainy season and is lowest during the preceding dry season. The estimated number of infective bites per year is around 20, and about 80% of these are received during the major malaria season. Most infections (98%) are due to P. falciparum (1). Dodowa can thus be described as an area of hyperendemic, seasonal malaria transmission. In the present study, we studied a random subpopulation consisting of 118 sicklecell trait-negative children (age range, 3 to 15 years) drawn from a larger cohort of 300 children described in detail previously (13). The children in the study cohort were monitored by active and passive case detection between April and November 1994 (13). Heparinized venous blood samples were obtained in April 1994 (preseason) and November 1994 (postseason). Plasma samples were stored at −20°C until analysis. Control plasma samples were obtained from healthy Danish adults who had never lived in a malaria-endemic area. A pool of plasma obtained from adults living in Dodowa and selected for high antibody reactivity to variant surface antigens was used as positive control. Informed consent was obtained from all studied individuals and/or their parents. The Ghanaian Ministry of Health approved the study.
Antibody Measurements. (i) Antibody reactivity to variant antigen expressed on the surface of infected erythrocytes.
The levels of antibodies to five different parasite isolates were measured by flow cytometry (29). We used primary isolates L73 and L50, obtained from two asymptomatic children within the Ghanaian cohort; the Busua isolate, obtained from a Danish patient who contracted malaria while in another region in Ghana; and 2D3 and G12, obtained from Sudanese malaria patients. The parasite isolates were maintained in culture for up to 3 weeks before being assayed by standard procedures with slight modifications (11, 33). On the day of the assay, erythrocytes infected with mature blood stages were purified by exposure to a strong magnetic field, resulting in material having >75% parasitemia (29). Aliquots of 2 × 105 purified late-stage-infected erythrocytes labeled-with ethidium bromide were sequentially exposed to 20 μl of plasma previously adsorbed with 106 uninfected type O erythrocytes, 0.5 μl of goat anti-human immunoglobulin G (IgG) (Dako, Glostrup, Denmark), and 4 μl of fluorescein isothiocyanate-conjugated rabbit anti-goat IgG (Dako). Samples were washed twice in phosphate-buffered saline between each incubation step and analyzed on a Coulter EPICS XL-MCL flow cytometer (Coulter Electronics, Luton, United Kingdom) using WinMDI software (http://facs.scripps.edu/software.html). For each plasma sample, the mean late-stage-infected erythrocyte fluorescein isothiocyanate fluorescence index was recorded. Nonspecific labeling was evaluated by analysis of uninfected erythrocytes. A positive control pool from six residents of Dodowa was titrated and included with each parasite isolate assayed. For quantification, mean fluorescence index units were transformed to antibody units by using standard curves generated from the titrated plasma pool, for which the highest value was arbitrarily assigned 1,000 units. The lower limit of positivity was determined as values greater than the mean obtained with the plasma of 12 unexposed Danish donors plus 2 standard deviations. It has previously been shown that the main reactivity measured in this assay is directed against large (200- to 300-kDa) polymorphic surface-expressed molecules (29) and depends on expression of PfEMP1 (24).
(ii) Antibody reactivity to the synthetic peptide.
A conserved linear peptide from the published sequence of the var-1 gene of the Malayan Camp (MC) isolate (32) was obtained from Schaefer Co. (Copenhagen, Denmark). The amino acid sequence was DIGDIVRGKDLY (MCvar-1 amino acids 183 to 194). The purity of the peptide was greater than 95%. Antibody reactivity was measured by an enzyme-linked immunosorbent assay as previously described (31). To account for day-to-day variation, the results were calculated as relative optical density at 492 nm (OD): (ODsample − ODbackground)/(ODpositive control − ODbackground). A pool of plasma samples obtained from six adults living in Dodowa previously shown to react strongly with the peptide was used as positive control. The lower limit of positivity was determined as the mean relative OD of the plasma of 31 unexposed Danish donors plus 2 standard deviations.
Statistical analysis.
Statistical analysis was done using the Sigma Stat software package (Jandel Scientific, San Rafael, Calif.). The χ2 test was used to compare proportions of antibody responders in protected and unprotected children, while the Mann-Whitney tests for paired and unpaired data were used to compare the antibody levels between groups. Spearman's rank correlation test was used to correlate antibody responses in pre- and post-malaria transmission season samples and to assess associations between antibody levels and age. Two-way analysis of variance was used to compare antibody responses stratified by age groups among the malaria-susceptible or -protected children. RSEPT (http://www.ci.tuwien.ac.at/R/bin/windows/windows/Rsept.zip) statistical software was used to perform multiple-regression analysis to correct for the confounding effects of age. Differences were considered statistically significant if P < 0.05.
RESULTS
P. falciparum infections in the study cohort.
All 118 children in the cohort were parasitemic at one or more of the monthly parasite screenings done to determine the parasite point prevalence, which fluctuated around 50% throughout the 8-month duration of the clinical surveillance (13). Based on this surveillance, which included a combination of active- and passive-case detection (13) we divided the children into two groups. During the period of surveillance, 27 children had at least one episode with fever in the presence of asexual parasitemia of >5,000/μl, and these children were considered susceptible to malaria (group 1); 73 children who did not have episodes of measured or reported fever in the presence of asexual parasitemia were considered to be clinically protected (group 2). A group of 18 children who had episodes of measured or reported fevers in the presence of asexual parasitemia of <5,000/μl were excluded from the analysis, since it is unclear whether fever episodes in the presence of low-grade parasitemia were due to malaria, when asymptomatic parasitemia is common.
IgG recognition of the variant antigens and the conserved peptide sequence.
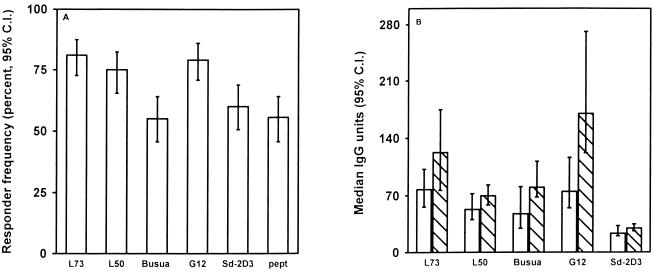
In the Ghanaian children the prevalence of IgG to variant antigens of parasite isolates from Ghana (L73, L50, and Busua) or Sudan (G12 and 2D3) and the prevalence of IgG to the PfEMP1 peptide from the relatively conserved Duffy binding-like region 1 (DBL1) were higher than 50% (Fig. 1A). This shows that the majority of the children had antibodies to both variant antigens on the surface of infected erythrocytes and to the peptide from the relatively conserved part of PfEMP1.
FIG. 1.
(A) Prevalence and 95% confidence interval (C.I.) of IgG antibody to variant antigens on the surface of erythrocytes infected with Ghanaian parasite isolates (L73, L50, and Busua) or Sudanese isolates (Sd-2D3 and G12) and to a peptide corresponding to a conserved part of PfEMP1 in premalaria season plasma from Ghanaian children. (B) Levels (median units and 95% confidence interval) of IgG to variant antigens on parasites from Ghana or Sudan in premalaria season plasma samples from Ghanaian children. Open bars indicate malaria-susceptible individuals, and hatched bars represent individuals protected from malaria.
Responses to the isolates correlated significantly [rs, 0.6 to 0.8; P(rs) < 0.001 for all correlations (data not shown)] both for isolates from the same region and for isolates from different regions. The reactivity to the conserved PfEMP1 peptide and the reactivity to the variant antigens expressed by the different parasite isolates also correlated [rs, 0.4 to 0.53; P(rs) < 0.001 for all comparisons [data not shown]).
Antibody levels and protection from malaria.
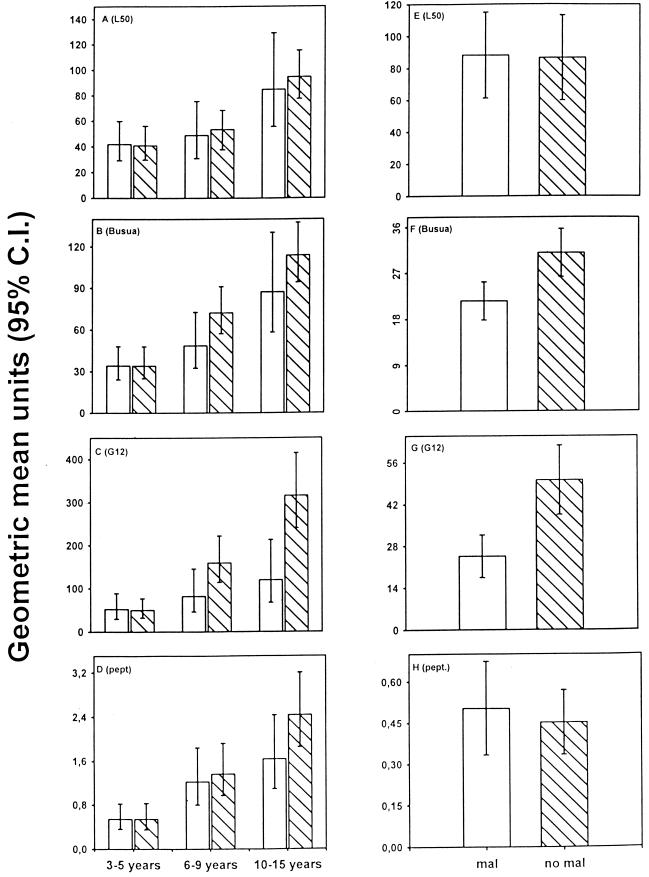
For all parasite isolates tested, the preseason levels of antibodies in the protected (group 2) children were higher than in the susceptible (group 1) children (Fig. 1B). However, the responses increased with age [rs, < 0.6 ; P(rs) < 0.001], and since the protected children were older than the susceptible children, comparisons between antibody levels in the two groups of children had to be corrected for age (Fig. 2A to D). Figure 2E to H shows the age-corrected levels of antibodies. Of the parasite isolates tested, the antibody levels against Busua and G12 were significantly higher in the protected children than in the susceptible children (P = 0.046 and P = 0.006, respectively). After age correction, the mean level of reactivity for G12 was approximately twice as high in the protected children as in the susceptible children (Fig. 2G). The difference between susceptible and protected children was most pronounced in the age groups from 6 to 9 years and from 10 to 15 years (Fig. 2C). This is consistent with clinical and parasitological data on the cohort, indicating that most of the protection from febrile disease is acquired after 7 years of age. The age-corrected levels for the Ghanaian isolate L50 (Fig. 2A and E), which did not differ significantly between the groups, are shown for comparison.
FIG. 2.
(A to D) Age-stratified (3 to 5, 6 to 9, and 10 to 15 years) plasma antibody levels to variant antigens on the surface of erythrocytes infected with parasite isolates L50, Busua, or G12 and to the conserved PfEMP1 peptide in Ghanaian children classified as susceptible (open bars labeled mal) to malaria or protected (hatched bars labeled no mal). (E to H) Age-adjusted IgG levels in susceptible and protected children.
Correlation of responses at different measurements in the same individual.
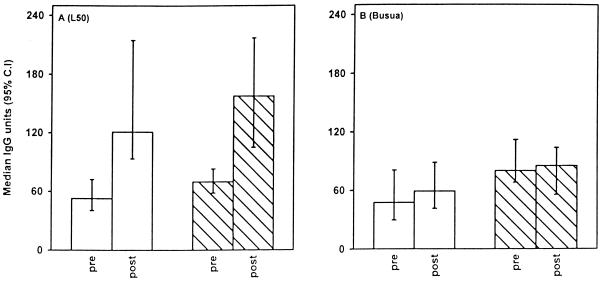
The bulk of transmission, about 20 infective bites per person, is concentrated in the major malaria transmission season. During this period, most individuals are likely to be challenged by “new” parasites, i.e., parasites that are antigenically different from those carried in many individuals with asymptomatic parasitemia outside the transmission season. To test whether new antigenic challenges would boost antibody levels, we compared the reactivity to two isolates (L50 and Busua) in matched samples collected at the beginning and end of the transmission season. In both protected and susceptible children, the median levels of antibodies against both isolates increased (Fig. 3) (L50, P < 0.001; Busua, P = 0.03) during the malaria season.
FIG. 3.
IgG levels in plasma to variant antigens on erythrocytes infected with parasites isolate L50 (A) or Busua (B) in pre- and post-malaria transmission samples from Ghanaian children, who had (open bars) or did not have (hatched bars) malaria.
DISCUSSION
Malaria immunity is slowly acquired by individuals living in areas of stable malaria transmission (9). Immunity is partial, since adults living in areas of highly endemic infection often have low-density parasitemia (5) and occasionally experience malaria fevers, which occur when the parasite densities increase over about 1,000 to 2,000 parasites/μl (23). These and other data strongly suggest that the immunoeffector mechanisms responsible for protection are mediated by antibodies (10) directed against the erythrocytic stages of the parasite. It has been debated whether the targets of these antigens are conserved proteins expressed on the surface of merozoites (15) or variant antigens on the surface of infected erythrocytes (21, 30).
The main finding of this study is that Ghanaian children with high levels in plasma of antibody to variant antigens during the premalaria season were less likely to contract malaria during the subsequent malaria season than were children with low levels of such antibodies. This association was found for two (G12 and Busua) of the five isolates tested. For one of the isolates, the P value was so low that it is unlikely that the association occurred by chance due to multiple comparisons. The most likely explanation for these observations is that during the malaria season the children were exposed to parasites expressing PfEMP1 variants that serologically cross-react with G12 and Busua and that those with high levels of antibodies against these parasites were protected from disease. However, the possibility that the levels of antibodies to G12 and Busua were associated with the levels of antibodies to other variant or conserved malaria antigens, which in turn mediated the protective effect, cannot be excluded in this type of immunoepidemiological study. In any case, our data are in concordance with those of other studies that have indicated the importance of antibodies to variant antigens for protection against both severe (7) and uncomplicated (20, 22) malaria. In the present study, the strongest correlate of protection was antibody reactivity to variant surface antigens expressed by the Sudanese parasite isolate G12. A similar study conducted in the Sudan showed correlation between protection and the presence of antibodies to the Ghanaian parasite isolate L73 (20). The reason why antibodies to the serotype expressed by the Ghanaian parasite were associated with protection in Sudan while antibodies to the serotype expressed by the Sudanese parasite were associated with protection in Ghana is obscure. It could be a coincidence, but these parasites possibly expressed “suitably common” serotypes. That is, the parasites were common enough to allow a large number of the cohort members in Sudan and Ghana to be exposed to them during the follow-up but not so common that all individuals already had developed immunity to them before the study. Alternatively, the presence of antibodies against an isolate derived from the other side of the African continent may be an indicator that the donor had a broad range of PfEMP1 antibodies and therefore was protected against challenge by parasites carrying a broad range of antigenic types.
For all the assays the median level of reactivity was higher in protected than in susceptible children, but age was a confounding factor, and after age correction the antibody levels to the variant antigens on three of the isolates and the conserved PfEMP1 peptide were comparable between the two groups. It is not surprising that an association between protection and antibody reactivity was not detectable for all isolates. Even if antibody to an isolate conveys protection, associations between susceptibility to malaria and antibody levels can be detected only if a sizeable fraction of children during the follow-up are challenged with new parasites carrying variants that serologically overlap with the isolates used for the assays.
A limited number of parasite-derived antigens are expressed on the surface of erythrocytes infected by late developmental stages of P. falciparum. The best characterized of these is the variant molecule PfEMP1. The PfEMP1 repertoire is very diverse, and little is known about which parts of the native 200- to 300-kDa molecule are accessible to antibodies when it is expressed on the surface of erythrocytes. This has made it difficult to base measurements of antibody responses to the variable parts of the molecule on recombinant products or synthetic peptides. Instead, most studies have used agglutination assays (6, 7, 19, 22) or flow cytometry-based measurements (18, 20, 24). The flow cytometry assay used in this study detects isolate specific antibodies of a similar molecular weight to PfEMP1 (29), and plasma from malaria-immune individuals does not react in the assay if the parasite isolate used does not express PfEMP1 (24). Furthermore, there is a strong correlation between the ability of individual plasmas to recognize parasite isolates in agglutination assays and in the flow cytometry-based method (29). Therefore, we believe that most of the antibodies detected by the flow cytometry assay used in this study are directed against PfEMP1, but we cannot rule out the possibility that some are directed against other surface-expressed parasite molecules such as the rifins (8, 16). Our data show that the levels of antibody to variant antigens generally were higher in postseason samples than in preseason samples. This seasonal effect was seen in samples from both protected and susceptible children. The effect was more pronounced for the levels of antibody to the L50 isolate than for those to the Busua isolate, which may reflect the fact that the antigenic type carried by L50 was the more common of the two and that individuals are therefore more likely to be exposed to parasites cross-reactive with L50 during the transmission.
We did not find any correlation between protection and antibody response to the conserved PfEMP1 peptide epitope originating from the DBL1 domain of PfEMP1. In a study from Sudan (31), higher levels of IgG to the same peptide epitope were found in asymptomatically infected individuals than in those with malaria, but in that study it was impossible to discriminate between the effects of age and protection. Later studies indicated that the conserved DBL1 region used in the present study is not accessible for antibodies on the surface of infected erythrocytes (T. Staalsoe, unpublished results).
In conclusion, we found an association between the levels of IgG to variant antigens on infected erythrocytes and protection from malaria in Ghanaian children, but not for the antibody levels to the conserved peptide from the DBL1 domain of PfEMP1. The data indicate that antibodies against a broadening range of variant antigens are important for protection against febrile malaria episodes in Ghanaian children who are in the process of acquiring malaria immunity. The data also support the notion that antibodies against variant antigens are involved in controlling parasite multiplication and maintaining parasitemia at levels below the fever threshold. Our data do not exclude the possibility that antibodies against conserved epitopes play a role in malaria immunity. Indeed, we have previously found an association between the levels of antibodies against glutamine-rich protein and protection using the same cohort of children (14). By reducing the parasite multiplication rate, such antibodies may extend the period available for the immune system to produce antibodies against new PfEMP1 variants before they cause symptoms.
ACKNOWLEDGMENTS
Ben Abuakwa, Gitte Pedersen, and Anne Corfitz are thanked for technical assistance. We are grateful to the children of Dodowa for donating blood samples for analysis.
The study received financial support from the Danish International Development Agency and the Fifth Framework Programme of The European Commission.
REFERENCES
- 1.Afari E A, Appawu M, Dunyo S, Baffoe-Wilmot A, Nkrumah F K. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr J Health Sci. 1995;2:312–316. [PubMed] [Google Scholar]
- 2.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 4.Berendt A R, Turner G D H, Newbold C I. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 5.Bruce-Chwatt L J. A longitudinal survey of natural malaria infection in a group of West African adults. W Afr Med J. 1963;12:141–173. [PubMed] [Google Scholar]
- 6.Bull P C, Kortok M, Kai O, Ndungu F, Ross A, Lowe B S, Newbold C I, Marsh K. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis. 2000;182:252–259. doi: 10.1086/315652. . (Erratum, 182:641.) [DOI] [PubMed] [Google Scholar]
- 7.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, Lanzer M, Saul A. Stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 9.Christophers S R. The mechanism of immunity against malaria in communities living under hyper-endemic conditions. Indian J Med Res. 1924;12:273–294. [Google Scholar]
- 10.Cohen S, McGregor I A, Carrington S. Gammaglobulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 11.Cranmer S L, Magowan C, Liang J, Coppel R L, Cooke B M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1997;91:363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 12.David P H, Hommel M, Miller L H, Udeinya I J, Oligino L D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci USA. 1983;80:5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodoo D, Theander T G, Kurtzhals J A L, Koram K, Riley E, Akanmori B D, Nkrumah F K, Hviid L. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with clinical protection from malaria. Infect Immun. 1999;67:2131–2137. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodoo D, Theisen M, Kurtzhals J A, Akanmori B D, Koram K A, Jepsen S, Nkrumah F K, Theander T G, Hviid L. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis. 2000;181:1202–1205. doi: 10.1086/315341. [DOI] [PubMed] [Google Scholar]
- 15.Druilhe P, Perignon J-L. A hypothesis about the chronicity of malaria infection. Parasitol Today. 1997;13:353–357. doi: 10.1016/s0169-4758(97)01095-8. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez V, Hommel M, Chen Q J, Hagblom P, Wahlgren M. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190:1393–1403. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried M, Nosten F, Brockman A, Brabin B T, Duffy P E. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 18.Giha H A, Staalsoe T, Dodoo D, Elhassan I M, Roper C, Satti G M, Arnot D E, Theander T G, Hviid L. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun. 1999;67:4092–4098. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giha H A, Staalsoe T, Dodoo D, Elhassan I M, Roper C, Satti G M H, Arnot D E, Hviid L, Theander T G. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology. 1999;119:7–17. doi: 10.1017/s0031182099004485. [DOI] [PubMed] [Google Scholar]
- 20.Giha H A, Staalsoe T, Dodoo D, Roper C, Satti G M, Arnot D E, Hviid L, Theander T G. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett. 2000;71:117–126. doi: 10.1016/s0165-2478(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 21.Marsh K, Howard R J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 22.Marsh K, Otoo L, Hayes R J, Carson D C, Greenwood B M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 23.Miller M J. Observations on the natural history of malaria in the semi-resistant West African. Trans R Soc Trop Med Hyg. 1958;52:152–168. doi: 10.1016/0035-9203(58)90036-1. [DOI] [PubMed] [Google Scholar]
- 24.Piper K P, Roberts D J, Day K P. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp Parasitol. 1999;91:161–169. doi: 10.1006/expr.1998.4368. [DOI] [PubMed] [Google Scholar]
- 25.Ricke C H, Staalsoe T, Koram K, Akanmori B D, Riley E M, Theander T G, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 26.Singh B, Ho M, Looareesuwan S, Mathai E, Warrell D A, Hommel M. Plasmodium falciparum: inhibition/reversal of cytoadherence of Thai isolates to melanoma cells by local immune sera. Clin Exp Immunol. 1988;72:145–150. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith J D, Craig A G, Kriek N, Hudson-Taylor D, Kyes S, Fagen T, Pinches R, Baruch D I, Newbold C I, Miller L H. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci USA. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staalsoe T, Giha H A, Dodoo D, Theander T G, Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry. 1999;35:329–336. doi: 10.1002/(sici)1097-0320(19990401)35:4<329::aid-cyto5>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Staalsoe T, Hviid L. The role of variant-specific immunity in asymptomatic infections: maintaining a fine balance. Parasitol Today. 1998;14:177–178. doi: 10.1016/s0169-4758(98)01228-9. [DOI] [PubMed] [Google Scholar]
- 31.Staalsø T, Khalil E A G, Elhassan I M, Zijlstra E E, Elhassan A M, Giha H A, Theander T G, Jakobsen P H. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) Immunol Lett. 1998;60:121–126. doi: 10.1016/s0165-2478(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 32.Su X, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 33.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]