Abstract
Daily variations in ambient fine particulate matter (PM2.5) could contribute to the morbidity of anxiety disorders in children and adolescents, but has not yet been studied longitudinally at a daily level. We tested this association using repeated weekly measures of anxiety symptom severity in a group of 23 adolescents with generalized anxiety disorder. After estimating ambient PM2.5 concentrations using a validated model, we found that increased concentrations were significantly associated with increased anxiety symptom severity and frequency two, three, and four days later. PM2.5 may be a novel, modifiable exposure that could inform population level interventions to decrease psychiatric morbidity.
1. Background
Anxiety disorders are a cause of major morbidity in children and adolescents, often interfering with development and education (Institute of Medicine (U.S, 2009; Erskine et al., 2015), but also carry an increased long-term risk of mood disorders, substance use disorders, and suicide well into adulthood (Weissman et al., 1999; Pine et al., 1998; Fergusson and Woodward, 2002; Kim-Cohen et al., 2003; Caspi et al., 1996; Bittner et al., 2007; Woodward and Fergusson, 2001; Beesdo et al., 2007; Asselmann et al., 2014). Although our understanding of the role of genetic, family environment, temperament, cognition and psychosocial milieu in the development of anxiety disorders has increased (Kieling et al., 2011; Reiss, 2013), few modifiable risk factors have been identified at a population level (Guloksuz et al., 2018). One potential such contributor to psychiatric morbidity of anxiety disorders is outdoor air pollution, specifically ambient particulate matter with an aerodynamic diameter smaller than 2.5 μm (PM2.5). Importantly, PM2.5 may lead to anxiety disorders by inducing oxidative stress and inflammation in the central nervous system, including microglial activation and toxicity in the brain (Block and Calderon-Garciduenas, 2009; Block et al., 2012; Calderon-Garciduenas et al., 2002; Costa et al., 2014; Allen et al., 2017).
Epidemiologic studies have used emergency department (ED) utilization data within daily time series and case-crossover studies to show that short term increases in ambient air pollution are associated with the exacerbation of psychiatric disorders (Kim et al., 2019; Oudin et al., 2018; Qiu et al., 2019), stress-related disorders (Kim et al., 2019), depressive disorders (Szyszkowicz et al., 2009, 2016; Wang et al., 2018; Cho et al., 2014), suicide attempts (Szyszkowicz et al., 2010), and completed suicide (Bakian et al., 2015). Though several studies have linked recent (30–180 days) PM2.5 with increased anxiety and depression symptom severity and frequency in adults (Power et al., 2015; Pun et al., 2017), acute (1–3 days) exposures have not yet been studied outside of ED utilization data. PM2.5 has also been associated with anxiety and related symptoms in pediatric populations, including psychiatric ED visits (Leeb et al., 2020). In a sample of children and adolescents presenting to the emergency department for psychiatric complaints, increased daily PM2.5 was related to a higher likelihood of visits related to anxiety and suicidal ideation. Moreover, this was especially true for children living in areas of high community material deprivation who are more susceptible to the effects of PM2.5 (Brokamp et al., 2019).
Here, we sought to determine the relationship between acute ambient PM2.5 exposures and repeated measures of anxiety symptom severity.
2. Material and methods
2.1. Study population
The study population was composed of 23 adolescents, aged 12–17 years with a primary diagnosis of generalized anxiety disorder (GAD), from the Cincinnati, OH, USA area enrolled in the placebo arm of a randomized controlled trial performed between 2015 and 2018 (Strawn et al., 2020). Adolescents included in the trial met DSM-IV-TR criteria for GAD, had a Pediatric Anxiety Rating Scale (PARS) score ≥15, and a Clinical Global Impression—Severity of Illness (CGI-S) score ≥4, as previously described, did not have any significant co-morbidities, and were not taking any psychotropic medications (Strawn et al., 2020).
2.2. Assessment of anxiety severity
The Pediatric Anxiety Rating Scale (PARS), is a clinician-administered, validated instrument used to assess pediatric anxiety severity in clinical trials of anxious youth (The Pediatric Anxiety Rating Scale, 2002). A 50-item checklist was rated by a board-certified child and adolescent psychiatrist with established reliability on this measure followed by a global assessment of seven dimensions of severity using a 0 to 5 scale. Of the seven dimensions of PARS assessed, we used the clinical trials scoring which sums five (symptom frequency, severity of distress, avoidance, interference at home, interference out of home), for a possible score range of 0–25 (Walkup et al., 2001). Anxiety symptom severity was assessed serially (about once a week) for each patient over the course of eight weeks.
2.3. Exposure and confounder assessment
Participant geocoded residential addresses were used to estimate daily ambient concentrations of average PM2.5 via a previously validated spatiotemporal exposure assessment model (Brokamp, 2022). The model was derived using meteorologic data, industrial PM2.5 emissions data, and spatiotemporal PM2.5 interpolation measures all calibrated with ground based PM2.5 monitoring data. Within the study region the prediction model was highly accurate, with a cross validated R2 of 0.92 and a median absolute error of 1.00 μg/m3. The geocoded addresses were also used to derive daily average air temperature and relative humidity from the North American Regional Reanalysis (NARR) database (Mesinger et al., 2006).
2.4. Statistical analysis
To identify the relationship between daily PARS and exposures to PM2.5 over time, we utilized a distributed lag nonlinear model (DLNM) framework. DLNMs, generally, use past and current values as predictors, referred to as “lagged” values to help identify temporal windows when the relationship between an exposure and outcome occurs (Gasparrini et al., 2010). We considered the binary logarithm of daily PM2.5 estimated exposures from the day of each PARS assessment and the previous six days. The relationship was modeled using a natural cubic spline, with 3 degrees of freedom, for both the dose-response and lag-response relationship. We considered a non-linear dose-response relationship because supralinear relationships, in which the risk of adverse health outcomes increases at a greater rate at lower exposure concentrations than when compared to higher concentrations, has been previously observed for PM2.5 and other health outcomes(Xie et al., 2015). To adjust for confounding by other temporal factors related to both PARS and PM2.5, we included natural cubic splines, with 3 degrees of freedom, of air temperature, relative humidity, and day of the year. We excluded day of the week as a temporal confounder because we did not observe any significant differences of PM2.5 concentrations or PARS scores across different days of the week. We used a fixed effects regression model by including subject-specific intercepts, as in the case time series design (Gasparrini, 2021), to avoid confounding by characteristics that do not vary throughout the study period (e.g., socioeconomic status). Sensitive windows were identified by plotting predicted changes in PARS at each lag day for a hypothetical change from the 25th percentile to the 50th and 75th percentiles of PM2.5 concentrations. All statistical computing and analyses were done using R (Team, 2020), specifically the dlnm (Gasparrini et al., 2017) package.
3. Results
Study participants (n = 23) were 74% female with a mean age of 15.0 (range (Kieling et al., 2011; Calderon-Garciduenas et al., 2002)). Each participant contributed a median of 6 PARS scores, for a total of 123 within the eight weeks (range (Erskine et al., 2015; Caspi et al., 1996)), mostly occurring one week apart. The average PARS score for all participants was 16 (standard deviation: 3.89). We estimated average PM2.5 for each PARS score assessment on each of the seven days leading up to (and including) the day the score was ascertained, for a total follow up of 861 person-days. We calculated a mean 24-h average PM2.5 of 8.35 μg/m3 (minimum: 2.22, 25th percentile: 5.93, median: 7.96, 75th percentile: 10.46, maximum: 26.38 μg/m3).
The estimated non-linear associations between PM2.5 and PARS scores from our adjusted regression model are shown in the figure. Fig. 1A shows that a change in PM2.5 concentration from the 25th percentile to the median (5.93 μg/m3 versus 7.96 μg/m3) was significantly associated with increased PARS scores two (0.57, 95% CI: 0.06, 1.07), three (0.82, 95% CI: 0.18, 1.45), and four (0.62, 95% CI: 0.11, 1.14) days later. Fig. 1B shows the estimated change in PARS score according to a range of PM2.5 concentrations (as compared to the 25th percentile, 5.93 μg/m3) for a three-day lag period. Here, there is visual evidence of a supralinear dose-response curve, with increases at lower concentrations being associated with larger changes in PARS scores compared to similar increases at higher concentrations. When considering a greater change in exposure from the 25th percentile to the 75th percentile (5.93 μg/m3 versus 10.46 μg/m3), the average PARS scored significantly increased three (1.24, 95% CI: 0.17, 2.31) and four (0.97, 95% CI: 0.10, 1.83) days later.
Fig. 1A.
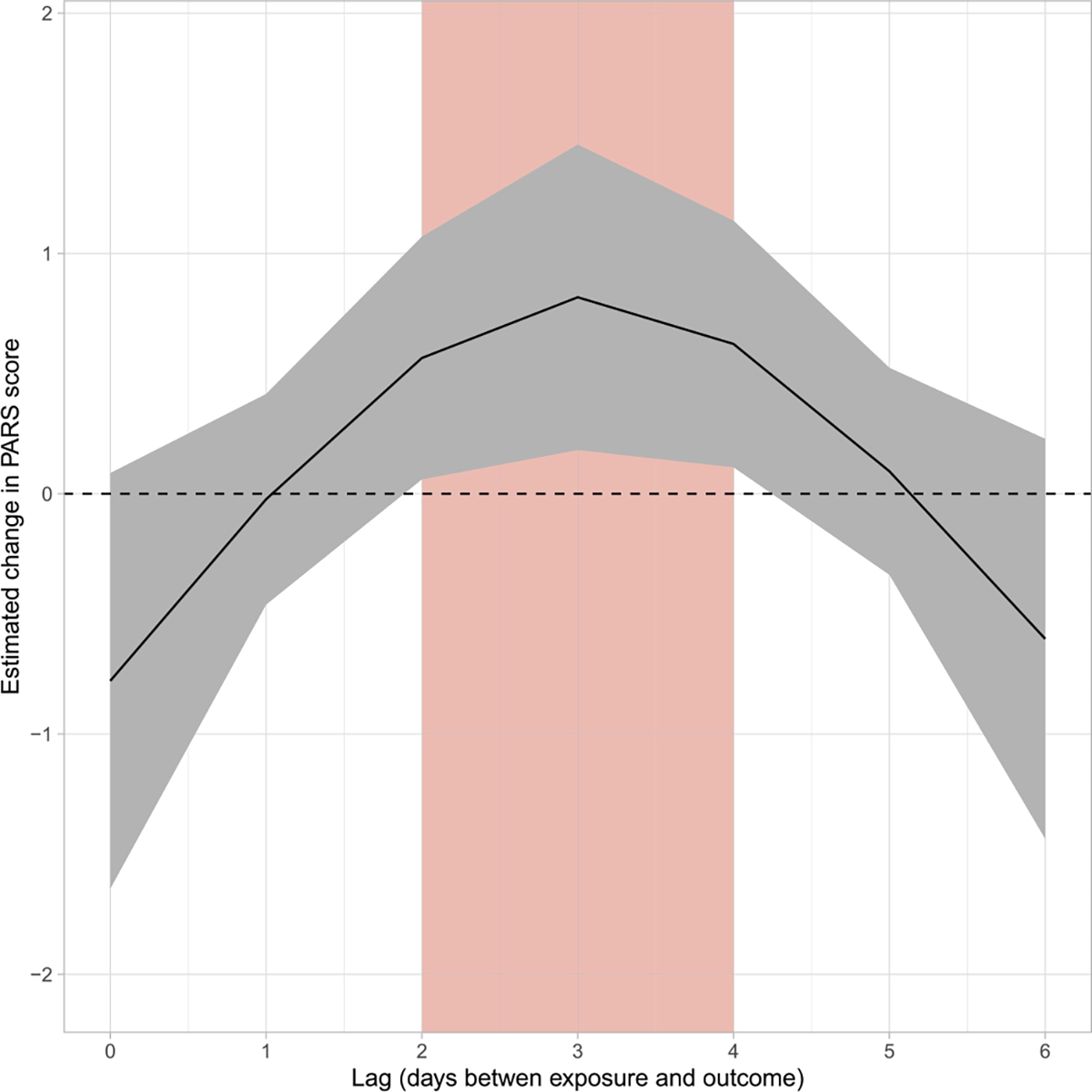
Estimated change in PARS score by lag associated with an increase in PM2.5 from the 25th percentile to the median (5.93 versus 7.96 μg/m3). The red, shaded rectangle denotes lag periods where the estimated change in PARS score was significantly different from zero.
Fig. 1B.
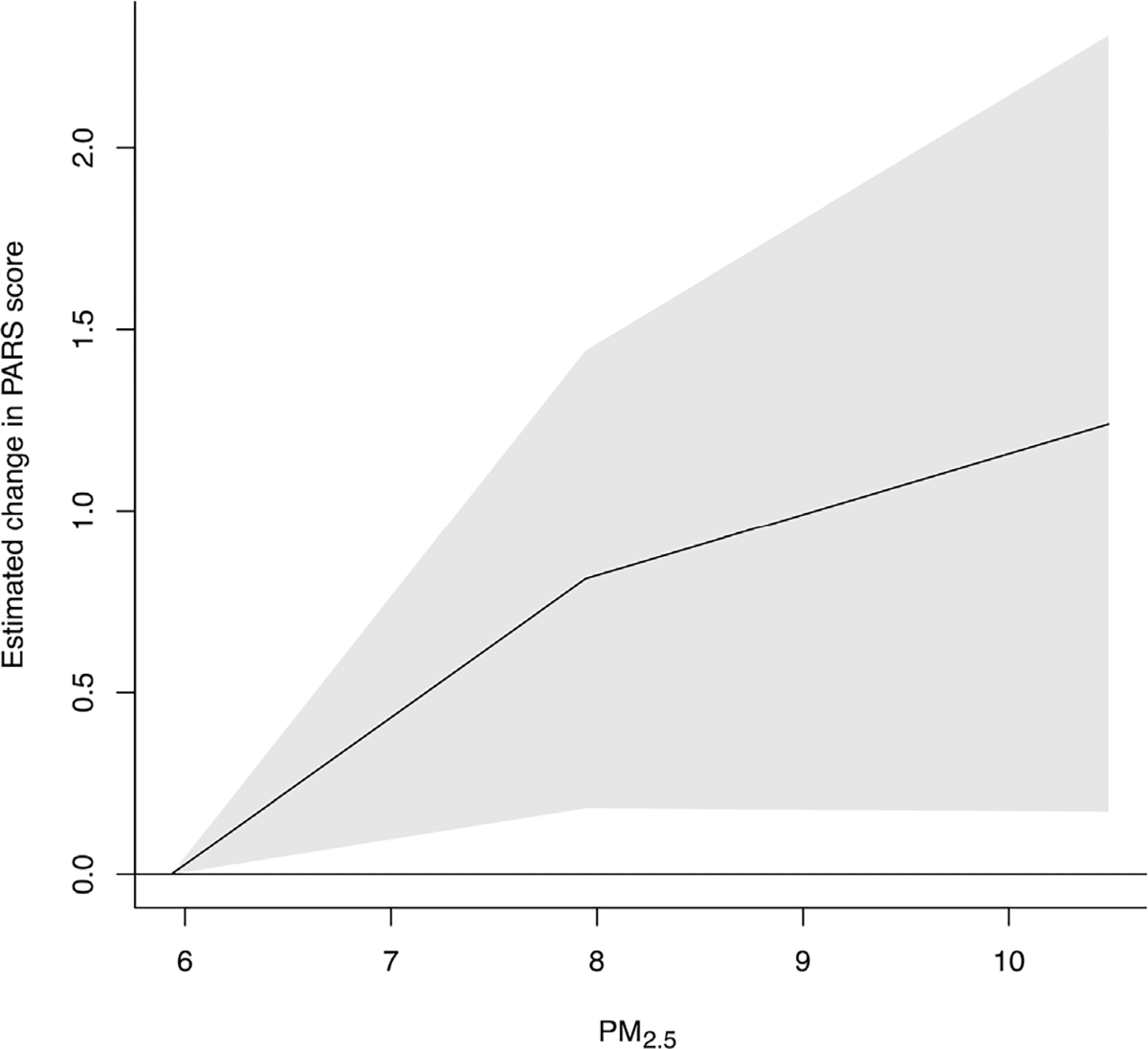
Dose-response curve for the estimated change in PARS score three days after an increase in PM2.5 concentrations relative to the 25th percentile (5.93 μg/m3). The range of the x-axis is from the 25th to the 75th percentile of PM2.5 concentrations.
4. Discussion
The association between PM2.5 and PARS occurred independently of both (1) characteristics that do not vary for an individual within the follow-up period, such as socioeconomic status, race, or sex and (2) measured exposures that do vary for an individual within the follow-up period and could confound the association, such as temperature and humidity. While a 1.24-point increase in PARS score may seem like a relatively small individual-level change, because nearly everyone is exposed to ambient PM2.5, it can have a large impact at a population-level.
One advantage of our study was the use of the Pediatric Anxiety Risk Score (PARS) because it is a clinician-administered, structured instrument that is often considered the gold standard for assessing anxiety severity in children and adolescents. Unlike other instruments, such as the Generalized Anxiety Disorder 7-item (GAD-7) (Mossman et al., 2017), PARS has more dimensionality and captures impairment. However, the PARS captures symptom severity during the previous seven days and may not be precise enough to capture daily temporal relationships. Future studies could work to develop an instrument for assessing anxiety symptom severity that may better capture daily fluctuations in anxiety symptoms useful for studying associations with exposures that have high temporal variation. It is important to consider that this study was conducted in adolescents with severe anxiety and may not be generalizable to populations in which severe anxiety symptoms are less common or non-existent.
The finding that the CNS may be particularly sensitive to daily fluctuating levels of PM2.5 also avails the possibility of tailoring interventions for the treatment and prevention of anxiety beyond standard treatments (e.g., cognitive behavioral therapy and selective serotonin reuptake inhibitors [SSRIs]). Reducing air pollution exposure can be achieved through primary interventions and policy changes. Notably, the associations we found here occurred when studying PM2.5 exposures that did not exceed the Environmental Protection Agency’s National Ambient Air Quality Standard for daily PM2.5 of 35 μg/m3. As the climate changes and wildfires increase in frequency and severity, short term periods of very high PM2.5 exposure exceeding these standards are expected to also increase in frequency and severity(Liu et al., 2016; Di Virgilio et al., 2019; Neumann et al., 2021). In conclusion, fine particulate matter may be a novel, modifiable influence that could be used in the future at a population level to intervene and protect adolescents from the effects of psychiatric disease morbidity.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Allen JL, et al. , 2017. Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr. Environ. Health Rep. 4 (2), 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselmann E, et al. , 2014. Associations of fearful spells and panic attacks with incident anxiety, depressive, and substance use disorders: a 10-year prospective-longitudinal community study of adolescents and young adults. J. Psychiatr. Res. 55, 8–14. [DOI] [PubMed] [Google Scholar]
- Bakian AV, et al. , 2015. Acute air pollution exposure and risk of suicide completion. Am. J. Epidemiol. 181 (5), 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, et al. , 2007. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatr. 64 (8), 903–912. [DOI] [PubMed] [Google Scholar]
- Bittner A, et al. , 2007. What do childhood anxiety disorders predict? JCPP (J. Child Psychol. Psychiatry) 48 (12), 1174–1183. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32 (9), 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, et al. , 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33 (5), 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, 2022. A high resolution spatiotemporal fine particulate matter exposure assessment model for the contiguous United States. Environmental Advances 7, 100155. [Google Scholar]
- Brokamp C, et al. , 2019. Pediatric psychiatric emergency department utilization and fine particulate matter: a case-crossover study. Environ. Health Perspect. 127 (9), 97006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2002. Air pollution and brain damage. Toxicol. Pathol. 30 (3), 373–389. [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. , 1996. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch. Gen. Psychiatr. 53 (11), 1033–1039. [DOI] [PubMed] [Google Scholar]
- Cho J, et al. , 2014. Air pollution as a risk factor for depressive episode in patients with cardiovascular disease, diabetes mellitus, or asthma. J. Affect. Disord. 157, 45–51. [DOI] [PubMed] [Google Scholar]
- Costa LG, et al. , 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed Res. Int. 2014, 736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio G, et al. , 2019. Climate change increases the potential for extreme wildfires. Geophys. Res. Lett. 46 (14), 8517–8526. [Google Scholar]
- Erskine HE, et al. , 2015. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol. Med. 45 (7), 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, 2002. Mental health, educational, and social role outcomes of adolescents with depression. Arch. Gen. Psychiatr. 59 (3), 225–231. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, 2021. The case time series design. Epidemiology 32 (6), 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG, 2010. Distributed lag non-linear models. Stat. Med. 29 (21), 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, et al. , 2017. A penalized framework for distributed lag non-linear models. Biometrics 73 (3), 938–948. [DOI] [PubMed] [Google Scholar]
- Guloksuz S, van Os J, Rutten BPF, 2018. The exposome paradigm and the complexities of environmental research in psychiatry. JAMA Psychiatr. 75 (10), 985–986. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (U.S, 2009. Committee on prevention of mental disorders and substance abuse among children youth and young adults: research advances and promising interventions. In: Preventing Mental, Emotional, and Behavioral Disorders Among Young People : Progress and Possibilities. National Academies Press. xxix, Washington, D.C., p. 562 et al. [Google Scholar]
- Kieling C, et al. , 2011. Child and adolescent mental health worldwide: evidence for action. Lancet 378 (9801), 1515–1525. [DOI] [PubMed] [Google Scholar]
- Kim SH, et al. , 2019. Association between ambient PM2.5 and emergency department visits for psychiatric emergency diseases. Am. J. Emerg. Med. 37 (9), 1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, et al. , 2003. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch. Gen. Psychiatr. 60 (7), 709–717. [DOI] [PubMed] [Google Scholar]
- Leeb RT, et al. , 2020. Mental health-related emergency department visits among children aged <18 Years during the COVID-19 pandemic - United States, january 1-october 17, 2020. MMWR Morb. Mortal. Wkly. Rep. 69 (45), 1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, et al. , 2016. Particulate air pollution from wildfires in the Western US under climate change. Climatic Change 138 (3), 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesinger F, et al. , 2006. North American regional Reanalysis. Bull. Am. Meteorol. Soc. 87 (3), 343–360. [Google Scholar]
- Mossman SA, et al. , 2017. The Generalized Anxiety Disorder 7-item scale in adolescents with generalized anxiety disorder: signal detection and validation. Ann. Clin. Psychiatr. 29 (4), 227–234A. [PMC free article] [PubMed] [Google Scholar]
- Neumann JE, et al. , 2021. Estimating PM2.5-related premature mortality and morbidity associated with future wildfire emissions in the western US. Environ. Res. Lett. 16 (3), 035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, et al. , 2018. The association between daily concentrations of air pollution and visits to a psychiatric emergency unit: a case-crossover study. Environ. Health 17 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, et al. , 1998. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatr. 55 (1), 56–64. [DOI] [PubMed] [Google Scholar]
- Power MC, et al. , 2015. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ 350, h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, Suh H, 2017. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the NSHAP study. Environ. Health Perspect. 125 (3), 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, et al. , 2019. Attributable risk of hospital admissions for overall and specific mental disorders due to particulate matter pollution: a time-series study in Chengdu, China. Environ. Res. 170, 230–237. [DOI] [PubMed] [Google Scholar]
- Reiss F, 2013. Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc. Sci. Med. 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Strawn JR, et al. , 2020. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J. Clin. Psychiatr. 81 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz M, Rowe BH, Colman I, 2009. Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health 22 (4), 355–362. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M, et al. , 2010. Air pollution and emergency department visits for suicide attempts in vancouver, Canada. Environ. Health Insights 4, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz M, et al. , 2016. Air pollution and emergency department visits for depression: a multicity case-crossover study. Environ. Health Insights 10, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C., 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- The pediatric anxiety rating scale (PARS): development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry 41 (9), 2002, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Walkup JT, et al. , 2001. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N. Engl. J. Med. 344 (17), 1279–1285. [DOI] [PubMed] [Google Scholar]
- Wang F, et al. , 2018. Ambient concentrations of particulate matter and hospitalization for depression in 26 Chinese cities: a case-crossover study. Environ. Int. 114, 115–122. [DOI] [PubMed] [Google Scholar]
- Weissman MM, et al. , 1999. Depressed adolescents grown up. JAMA 281 (18), 1707–1713. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM, 2001. Life course outcomes of young people with anxiety disorders in adolescence. J. Am. Acad. Child Adolesc. Psychiatry 40 (9), 1086–1093. [DOI] [PubMed] [Google Scholar]
- Xie W, et al. , 2015. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 101 (4), 257–263. [DOI] [PubMed] [Google Scholar]


