Abstract
The lymphatic vasculature forms an organized network that covers the whole body and is involved in fluid homeostasis, metabolite clearance, and immune surveillance. The recent identification of functional lymphatic vessels in the meninges of the brain and the spinal cord has provided novel insights into neurophysiology. They emerge as major pathways for fluid exchange. The abundance of immune cells in lymphatic vessels and meninges also suggests that lymphatic vessels are actively involved in neuroimmunity. The lymphatic system, through its role in the clearance of neurotoxic proteins, autoimmune cell infiltration, and the transmission of pro-inflammatory signals, participates in the pathogenesis of a variety of neurological disorders, including neurodegenerative and neuroinflammatory diseases and traumatic injury. Vascular endothelial growth factor C is the master regulator of lymphangiogenesis, a process that is critical for the maintenance of central nervous system homeostasis. In this review, we summarize current knowledge and recent advances relating to the anatomical features and immunological functions of the lymphatic system of the central nervous system and highlight its potential as a therapeutic target for neurological disorders and central nervous system repair.
Keywords: central nervous system, central nervous system injury, glymphatic system, lymphatic vessels, meninges, neurodegenerative disorders, neuroinflammatory diseases, vascular endothelial growth factor C
Introduction
Lymphatic vessels (LVs) are present throughout most body tissues and organs and have critical functions in mammalian physiology. They act as a drainage system, help maintain fluid homeostasis, and participate in immune responses. In mice, LV development begins at approximately embryonic day 9.5 (E9.5) mainly from a subpopulation of venous endothelial cells (ECs) (Wigle and Oliver, 1999; Wigle et al., 2002). LVs grow via sprouting from pre-existing LVs. The lymphatic vasculature absorbs the interstitial fluid (ISF) through initial lymphatics and drains lymph to collecting lymphatics and lymph nodes (LNs). Phasic contractions within the collecting lymphatics allow for unidirectional lymph propulsion (Oliver et al., 2020) in an arterial pulse-dependent manner. Lymph is ultimately drained into the right and left subclavian veins at the confluence of the internal jugular and subclavian veins (Alitalo, 2011). Genetic determinants, blood flow, and the extracellular matrix can all affect the fate of lymphatic endothelial cells (LECs), thereby contributing to the plasticity and organ-specificity of the lymphatic vasculature (Ma and Oliver, 2017). Recent progress in the understanding of how lymphatics develop has revealed the crucial role of the homeobox transcription factor prospero-related homeobox 1 (PROX1) and the receptor tyrosine kinase vascular endothelial growth factor receptor 3 (VEGFR-3 or FLT4) in lymphangiogenesis (Wigle and Oliver, 1999; Wigle et al., 2002; Srinivasan et al., 2014). Additionally, surface markers of LECs, including lymphatic endothelial hyaluronan receptor 1 (LYVE-1), chemokine (C-C motif) ligand 21 (CCL21), and the membrane glycoprotein podoplanin (PDPN), have also been identified (Petrova and Koh, 2020).
Until recently, the brain was considered to be an immune-privileged organ mainly owing to the absence of LVs. As early as 1787, Paolo Mascagni, an Italian physician, described the presence of LVs in human dura mater in his anatomical record (Bucchieri et al., 2015). During the next two centuries, there were scattered reports supporting the existence of meningeal lymphatics (Lecco, 1953; Andres et al., 1987). Using histological methods, Földi et al. (1966) described LVs surrounding the cranial nerves and blood vessels around the dura. A paradigm shift occurred when the presence of LVs in meninges was confirmed through advanced methods (Louveau et al., 2015a). Moreover, these vessels have now been suggested to be key contributors to immune-related neurological disorders. A previous study has already described the functional role of LVs in the meninges surrounding the brain and the spinal cord (Da Mesquita et al., 2018a). As a drainage system for the CNS, meningeal LVs (MLVs) have specific characteristics and execute unique functions (Petrova and Koh, 2018). Studies investigating lymphatic drainage have led to a better understanding of brain and spinal cord homeostasis. Additionally, they have contributed to elucidating the mechanisms governing the movement of fluids, immune cells, and macromolecules into and out of the CNS, as well as the etiology of neurological disorders. Here, we further discuss their implications in the identification of novel therapeutic targets for neurological disorders, particularly neurodegenerative and autoimmune diseases and traumatic injury.
Literature Search Strategy
In this non-systematic review, the PubMed database was searched for both original research articles and review articles published between January 2002 and April 2022. To cover studies that discussed the lymphatic system relating to the CNS, the following search terms were used: (“lymphatic system” OR “lymphatic vessel” OR “glymphatic system”) AND (“central nervous system” OR “brain” OR “meninges” OR “spinal cord”). After reviewing the title, abstract, and keywords, articles that were potentially related to anatomical, immune-related, and functional studies of the lymphatic system for the CNS were included. Several older publications (before 2002) were also included in consideration of their relevance to the field. Data extraction focused on information about the implication of the lymphatic system in neurological diseases.
Anatomical and Functional Features of Lymphatic Pathways for the Central Nervous System
The existence of functional lymphatic vasculature in the CNS is normally determined using lymphatic endothelium-specific markers. Important studies by Louveau et al. (2015b) and Aspelund et al. (2015) described vessel-like structures that expressed LEC-specific markers in the meningeal layer of the mouse dura mater. Meanwhile, Mezey et al (2021) identified similar structures in the autopsy of human meningeal specimens. LYVE-1, PDPN, and VEGFR-3 were employed as markers to characterize the distribution of potential lymphatic vasculature in postmortem human brain tissue. Besides meninges, LVs were also found along or within the walls of vessels in various brain regions and the subarachnoid space. Markers of lymphatic endothelium were also detected among the fibers and fascicles of cranial nerves (Mezey et al., 2021).
The lymphatic system of the brain
The cerebrospinal fluid (CSF) is secreted by the choroid plexus in lateral cerebral ventricles and circulates from the lateral ventricles to the third and fourth cerebral ventricles, finally entering the subarachnoid space and cistern to be absorbed into the blood by the arachnoid villi (Damkier et al., 2013). This process raises two significant questions, namely, how does the exchange of material occur between the CSF and ISF, and where does the drainage fluid end up, veins or LVs?
Studies have sought to describe the mechanisms underlying CSF-ISF exchange and ISF drainage from the CNS. The perivascular space (PVS) has long been suggested to be the conduit for the movement of CSF into or out of the brain (Rennels et al., 1985; Mathiisen et al., 2010). Iliff et al. (2012) were the first to propose the ‘glymphatic system’ to describe a paravascular pathway for fluid drainage involving glial cells. They found that subarachnoid CSF rapidly enters the brain parenchyma along the PSVs of penetrating arteries while perivascular ISF exits the brain via paravenous drainage pathways. Subsequently, imaging using intravital two-photon microscopy showed that perivascular CSF entered the brain via the periarterial space between smooth muscle cells (SMCs) and astrocyte end-feet. The employed tracers were observed to move inward along penetrating arteries and arterioles (maximally along large ventral perforating arteries of the basal ganglia and thalamus) to reach terminal capillary beds throughout the brain and outward along capillaries and parenchymal venules (primarily along medial internal cerebral and lateral–ventral caudal rhinal veins; Figure 1). The natural patterns of regional influx and tracer flow vary according to developmental stage (Munk et al., 2019). Besides, the astroglial aquaporin 4 (AQP4) water channel, which forms the subpial and subependymal glial limiting membrane, facilitates CSF-ISF exchange and promotes CSF flux into the brain parenchyma through the para-arterial space, and bulk ISF solute clearance from the parenchyma to the paravenous space (Iliff et al., 2012).
Figure 1.
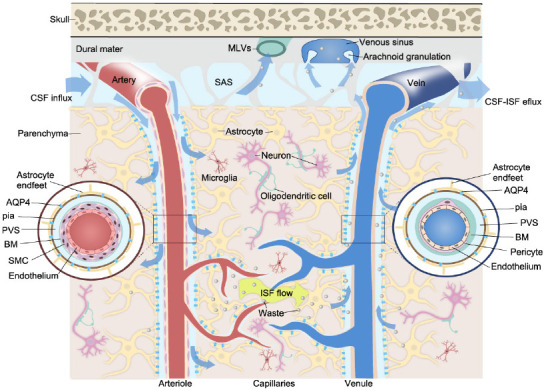
Schematic illustration of the CSF-ISF flow in the glymphatic system.
CSF flows in the PVS and moves inward along penetrating arteries and arterioles, and outward along capillaries and parenchymal venules. The PVS around the arteriole and venule forms based on the presence of different vascular unit components (inserts). The PVS surrounds vascular SMCs or the basement membrane and is bounded by perivascular astrocyte end-feet. AQP4 channels on the astrocyte end-feet allow fluid to pass between the PVS and brain parenchyma in both directions. Metabolic waste and macromolecules are drained to the SAS and are further absorbed via arachnoid granulations or MLVs. Created with Vectornator. AQP4: Aquaporin 4; BM: basement membrane; CSF: cerebrospinal fluid; ISF: interstitial fluid; MLVs: meningeal lymphatic vessels; PVS: perivascular space; SAS: subarachnoid space; SMC: smooth muscle cell.
The subarachnoid CSF is believed to leave the cranium (i) via arachnoid granulation absorption, (ii) along the internal carotid artery, and (iii) along the perineural spaces of the olfactory and trigeminal nerves. Specifically, a dense lymphatic network has been identified within the nasal submucosa, with evidence showing that the CSF can drain to the deep cervical lymph nodes (dCLNs) (Kida et al., 1993; Goldmann et al., 2006; Papadopoulos et al., 2020). Approximately 15% to 30% of subarachnoid CSF is cleared across the cribriform plate, which projects into the nasal submucosa following the olfactory tracts (Bradbury and Cole, 1980). The existence of CNS-draining LNs has led to the premise that CSF drainage flow requires LVs. CSF and ISF efflux to the dCLNs has been detected across mammalian species ranging from mice to humans, implying that it may be critical for the maintenance of fluid homeostasis (Bradbury and Westrop, 1983; Boulton et al., 1998; Antila et al., 2017; Xue et al., 2020). Macromolecules were also cleared from the CNS parenchyma via MLVs (Aspelund et al., 2015; Louveau et al., 2015b). The MLV represents an additional exit pathway for fluid from the CNS into the systemic circulation.
Aspelund et al. (2015) sought to identify the exact location of MLVs in the CNS using PROX1-GFP and Vegfr3+/LacZ reporter mice. The authors observed that MLVs were located in the dura matter at the base of the skull, mainly around the dural sinuses and middle meningeal arteries (Figure 2A and B). The mouse meninges are very small, which makes it hard to separate the meningeal layers, and may explain why the precise location of the MLVs in mice went undetected for so long. Also, MLVs develop postnatally, first appearing around the foramina in the basal parts of the skull and spinal canal, and then undergoing dynamic changes and sprouting along blood vessels and cranial and spinal nerves to various parts of the meninges surrounding the CNS. Antila et al. (2017) provided a detailed description of the anatomy of MLV development in mice. The authors reported that perisinusoidal MLVs developed from LVs located alongside the jugular veins and cranial nerves (IX and X). Like peripheral LVs, MLVs depend on VEGFR-3 signaling for their development. For example, K14-VEGFR-3-Ig transgenic mice display MLV aplasia, while VEGFR-3 signaling interruption affects CSF drainage functions. The expression of VEGF-C signals enwraps the blood vessels. The binding of VEGF-C to VEGFR-3 propels blood vessel growth and guides LV development and maintenance. Furthermore, Aspelund et al. (2015) reported that embryos lacking both VEGF-C alleles do not survive and that VEGF-C is essential for MLV development. Unlike with peripheral LVs, physical environmental properties might also affect the development and inhibit the sprouting of the meningeal lymphatic vasculature.
Figure 2.
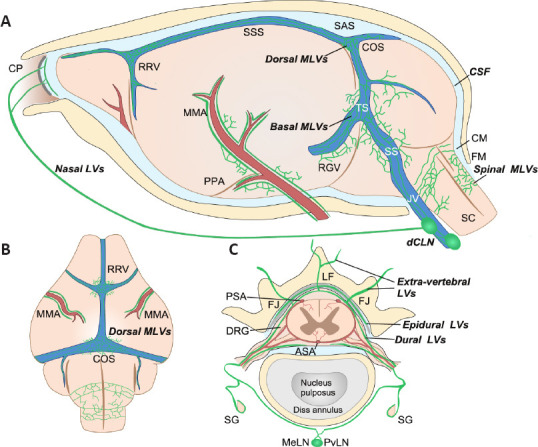
Schematic illustration of the anatomical position of meningeal lymphatic vessels around the mouse brain and spinal cord.
(A) On the lateral side of the mouse brain, MLVs run along the artery (red) and venous sinus (blue). Basal MLVs running along the TS and SS have been identified as the main route for CSF uptake and drainage. Parasinusoidal MLVs develop from LVs along the PPA, the JV, and cranial nerves. Spinal MLVs covering the FM connect to MLVs in the cervical spinal canal. (B) On the dorsal side of the mouse brain, dorsal MLVs run along the RRV, COS, and MMA. (C) Spinal MLVs (or vertebral LVs) are present in the epidural space around the spinal cord and the dura mater. On the dorsal side of the spinal canal, they connect at the midline and extend to the CM. However, they do not form a circuit on the ventral side. Spinal LVs further connect with extra-vertebral peripheral LVs at the ventral border of the LF or the level of the transverse FJ. They cover the dura mater of DRG and contact SG. Longitudinal connecting LVs between vertebral units also exist (not shown). Created with Vectornator. ASA: Anterior spinal artery; CM: cisterna magna; COS: the confluence of the sinuses; CP: cribriform plate; dCLNs: deep cervical lymph nodes; DRG: dorsal root ganglia; FJ: facet joint; FM: foramen magnum; JV: jugular vein; LF: ligamentum flavum; MeLN: mediastinal lymph node; MMA: middle meningeal artery; PPA: pterygopalatine artery; PSA: posterior spinal artery; PvLN: posterior vertebral lymph node; RGV: retroglenoid vein; RRV: rostral rhinal vein; SC: spinal cord; SG: sympathetic ganglia; SS: sigmoid sinus; SSS: superior sagittal sinus; TS: transverse sinus.
These studies have contributed to the unraveling of the fluid exchange pathways of the CNS, from the glymphatic pathway to draining LNs. These pathways are collectively referred to as the lymphatic system of the brain. However, many processes, including fluid entry into the MLVs and PVS extension into the subarachnoid space, take place in the meninges (Coles et al., 2017). Meanwhile, arguments and controversies remain on the cellular characteristics and developmental pattern of the lymphatic system (Bucchieri et al., 2015; Mestre et al., 2020). For instance, RNA-seq data has revealed that meningeal and peripheral LECs have distinct gene expression profiles (Louveau et al., 2018). LEC-specific gene set enrichment analysis, which included the Lyve1, Pdpn, and Prox1 genes, did not identify differences between the two sets of LECs. However, alterations in multiple pathways related to the extracellular matrix, focal adhesion, angiogenesis, and response to endogenous and exogenous stimuli were observed in meningeal LECs when compared with peripheral LECs. In particular, genes involved in the regulation of lymphatic development and cell stiffness were differentially expressed in the meningeal LECs (Louveau et al., 2018). Meanwhile, LECs with unique phenotypes have been identified in different CNS locations; however, their role in the lymphatic system remains unclear. Izen et al. (2018) utilized whole-mount imaging to depict the postnatal MLV developmental pattern in mice. They found that from birth to postnatal day 13 (P13), MLVs extended alongside dural blood vessels, while between P13 and P20, they extended along with dural sinuses towards the olfactory bulb. Finally, the pituitary was found to be enriched with LVs, which likely contacted the lymphatic network in the nasal mucosa (Elham et al., 2020).
The lymphatic system around the spinal cord
In the early postnatal period, MLVs are observed almost simultaneously in the cervical, thoracic, and lumbar regions of the spinal cord (Figure 2C; Antila et al., 2017). Furthermore, the MLVs exit the spinal canal along the blood vessels and spinal nerves. Spinal MLVs have site-specific functional properties. For example, the LV pattern in spinal meninges was shown to change along the ventral-dorsal and cranial-caudal axes of the spine (Antila et al., 2017). As the LVs around the spinal cord have a delicate structure and are embedded deep within the spinal vertebral canal, they are difficult to visualize; consequently, little is known about them. To visualize the complex 3D morphology of LVs inside the spinal vertebral canal, Jacob et al. (2019) used the iDISCO+ tissue preparation technique, which preserves intact vertebral bone structures for whole-mount immunostaining. They identified a dense lymphatic vasculature network that appeared to be predominantly restricted to the intervertebral spaces. The architecture of the vertebral LVs (vLVs) was found to be conserved along the vertebral column, and the vLVs were seen to be connected to the peripheral lymphatic system at intervertebral foramina along ventral nerve rami. Additionally, vLVs covering the dura matter of DRGs were observed to interact with the autonomous nervous system. Meanwhile, how vLVs connect to the peripheral lymphatic system differs among the cervical, thoracic, and lumbar vertebral levels (Jacob et al., 2019).
Advances in the Understanding of Central Nervous System Fluid Dynamics
In the CNS, several pathways are involved in the maintenance of CSF homeostasis. In the traditional routes, the molecular contents of the CSF are cleared via arachnoid granulations into the dural venous sinus, through the cribriform plate into the nasal mucosa, and via transporters and receptors present on the apical side of the choroid plexus epithelium (Weller et al., 2018). Research on the PVS or the glymphatic system forms the basis for understanding fluid management in the brain. Recent studies demonstrated that soluble tracers in the CSF can enter the brain via pial-glial basement membranes located between the layer of pia mater and astrocytes around cortical arteries; subsequently, the tracers drain out of the brain parenchyma along intramural peri-arterial drainage pathways (Albargothy et al., 2018; Owasil et al., 2020; Rasschaert et al., 2020). Carare et al. (2008) demonstrated that capillary and artery basement membranes act as ‘lymphatics of the brain’ for the drainage of fluid and solutes. Meanwhile, solute clearance along SMC basement membranes in the tunica media of arteries is referred to as intramural periarterial drainage. Albargothy et al. (2018) found that amyloid-β (Aβ) tracer and collagen IV co-localized in SMC capillary basement membranes, which indicate that Aβ was drained within the capillary basement membrane, thus questioning the function of the PVS for Aβ drainage. These observations indicate the need for an in-depth investigation of the function of arterial perivascular compartments.
The relative contribution of MLVs to CSF-ISF clearance among multiple post-glymphatic pathways needs further clarification using large-mammal models. The signal enhancement of tracers infused into ventricles can be observed using noninvasive imaging. An average initial signal increase at 15.9 ± 3.4 minutes has been reported in mandibular LNs (the subsequent draining LN of the dCLNs), significantly earlier than that for the venous signal (25.3 ± 2.8 minutes; Antila et al., 2017). This delay suggested that the active uptake of tracers occurred more rapidly via the lymphatic transport system. Additionally, signal enhancement was considerably higher in draining LNs than in saphenous veins (Antila et al., 2017). Through the intracerebroventricular administration of Evans blue dye, it was found that MLVs, and not nasal mucosa LVs, are the primary route for CSF drainage into the dCLN during the first 2 hours after administration (Louveau et al., 2015b). One study showed that the conditional ablation of MLVs nearly eliminated the drainage of intracerebroventricularly-injected microspheres to dCLNs (Antila et al., 2017). Furthermore, MLVs in the basal part of the skull (basal MLVs) showed a distinct structure from that previously reported for dorsal MLVs (Ahn et al., 2019). Basal MLVs, which run along the petrosquamosal and sigmoid sinuses, have larger diameters, a greater number of protruding capillary branches with blunt ends, and looser intercellular junctions than dorsal MLVs. They present collecting lymphatic-like structures, including more zipper-type junctions and lymphatic valves. However, the lack of SMC coverage makes them more like pre-collecting MLVs. The structure of basal MLVs and their proximity to the subarachnoid space render basal MLVs the ideal route for the uptake and drainage of CSF solute and macromolecules.
The spinal cord has an additional CSF flow pathway. Molecular tracers injected into one side of the spinal cord parenchyma at the thoracolumbar level were shown to accumulate in the ipsilateral paravertebral LV and mediastinal LN (Jacob et al., 2019). In vivo imaging revealed the CSF outflow routes in the spine. Tracers infused intracerebroventricularly indicated that the CSF flowed from the brain ventricles to the sacral spine within the central canal and accumulated in intervertebral regions. Moreover, the CSF outflow occurred along cranial nerves to extracranial LVs. On the dorsal side, tracers were drained into sacral LNs, while on the ventral side, collecting LVs along the axis of the spine drained tracers toward caudal mesenteric LNs. Tracers from both sides were ultimately drained to iliac LNs. Some of the LVs were also drained to the inguinal LNs. That CLNs still accounted for a large proportion of spinal CSF drainage suggested that these LN groups might play a key role in maintaining spinal cord homeostasis as well as in the occurrence of spinal cord disorders (Ma et al., 2019).
The Lymphatic System and the Immune-privileged Status of the Central Nervous System
For years, the brain was thought to be an immune-privileged organ owing to the absence of a classical lymphatic drainage system in the parenchyma and the existence of the blood-brain barrier (BBB). In the CNS, antigens or antigen-presenting cells reach the CLNs via the cribriform plate, MLVs, or the rostral migratory stream and activate an immune response in peripheral LNs (Mohammad et al., 2014; Louveau et al., 2017). Networks rich in dendritic cells (DCs) and resident macrophages were reported in the vascular tissues and meninges of rats (McMenamin, 1999). Meanwhile, Rustenhoven et al. (2021) demonstrated that the dural sinuses are a crucial interface for capturing and presenting CNS-derived antigens. Immune cells, such as T, B, and DCs, were found to be present in MLVs, indicating that the meningeal lymphatics participate in the drainage of immune cells from the CNS (Aspelund et al., 2015; Louveau et al., 2015b). The recognition of antigens drained through MLVs by antigen-presenting cells in the dural sinuses is an integral part of neuroimmune surveillance. Meningeal T cells are transported to the dCLN via a CCR7/CCL21-dependent pathway (Louveau et al., 2018). Resident microglia mainly induce a local adaptive immune response via the activation of infiltrating lymphocytes; consequently, immune surveillance is performed by long-lived macrophages in perivascular or subarachnoid spaces and meningeal DCs, which have access to the CSF-filled space (Papadopoulos et al., 2020). Activated T lymphocytes can also enter the CNS through the BBB, superficial brain barriers (pia mater and glia limitans), and the blood-CSF barrier (choroid plexus) (Engelhardt et al., 2016, 2017). Hence, the role of MLVs in neuroinflammatory and autoimmune diseases becomes a major research direction in the future.
Signal transmission may account for the possible association between the draining of CSF by MLVs and the pathological immune environment. Esposito et al. (2019) proposed the existence of a “brain-to-CLN” signaling pathway in a rat model of stroke. The authors found that dynamic crosstalk between the CNS and systemic responses were channeled through the MLVs. The injured brain secreted VEGF-C into the CSF, thereby activating the proliferation of LECs and pro-inflammatory macrophages in CLNs. Neuroinflammation-related components in the CSF were also observed to induce lymphangiogenesis via VEGF-C/VEGFR-3 signaling (Hsu et al., 2019). Importantly, this signaling pathway from the CNS to the peripheral immune system was shown to be bidirectional, namely, the activated MLVs displayed a unique transcriptional signature and contributed to the invasion of the brain by autoreactive T cells (Louveau et al., 2018). However, the mechanism underlying how MLVs and LECs modulate the phenotype of immune cells remains incompletely understood. Finally, Liu et al. (2020c) proposed that lymphoangiocrine signals might, themselves, exert significant effects on the immune microenvironment of the CNS.
Targeting the Lymphatic System in Neurological Disorders
The lymphatic system of the CNS not only plays a key role in the maintenance of tissue homeostasis by recycling ISF but is also important in immune surveillance regulation and immune-cell recirculation. In the CNS, lymphatic vasculature dysfunction results in the disruption of the ISF balance, which has been implicated in multiple neurological disorders. The impairment of lymphatic system function can have a significant impact on neurological disease outcomes (Aspelund et al., 2015; Tarasoff-Conway et al., 2015; Engelhardt et al., 2017; Ma et al., 2017). In the following sections, we describe and provide further insight regarding the function and role of the lymphatic system of the CNS in neurological disorders (Figure 3). The regulation of lymphatic function might be a promising therapeutic target to prevent neurological disorders or delay their progression (Table 1).
Figure 3.
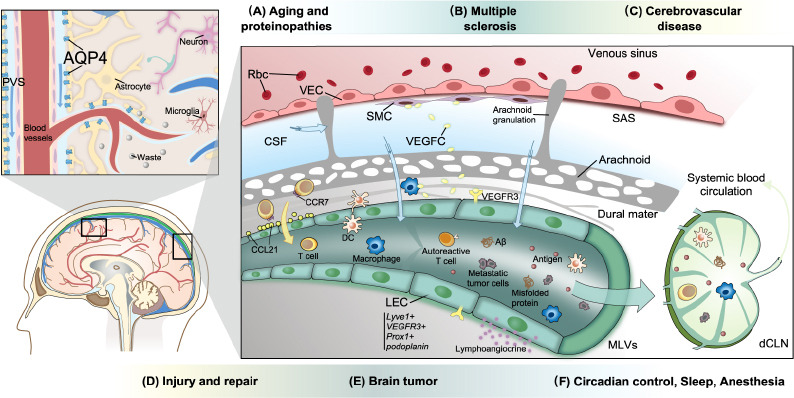
The role of the lymphatic system in neurological diseases.
The expression of AQP4 and MLV-mediated drainage play critical roles in the maintenance of CNS homeostasis. VEGF-C secreted by myeloid cells and SMCs promotes MLV development via VEGFR-3 present on LECs. Antigens and meningeal immune cells (lymphocytes, DCs, macrophages, and neutrophils, among others) cross the lymphatic vessel wall and are transported to the draining lymph nodes, where they activate inflammatory responses. Meningeal T cells are transported to the dCLNs via a CCR7/CCL21-dependent pathway. Mural LECs in leptomeninges or in the brain parenchyma, which have large vacuoles or inclusions, do not form tubular structures. (A) The clearance of neurotoxic proteins, such as Aβ, tau, and α-synuclein, via the glymphatic system and MLVs, is impaired in the aging brain. The abnormal accumulation of neurotoxic proteins leads to degenerative diseases. (B) Autoreactive encephalitogenic T cells are activated and invade the brain parenchyma via lymphatic pathways. (C) Enlargement of the PVS, impairment of the glymphatic pathway, and disruption of the BBB are characteristic of cerebrovascular diseases, such as SVD. (D) Brain-to-CLN signaling via VEGFR-3 is involved in the systemic inflammatory response after stroke and brain injury. Meanwhile, the loss of perivascular AQP4 polarization and the disruption of lymphatic drainage resulting from an increase in ICP in TBI both lead to impaired clearance. Moreover, lymphoangiocrine signals produced by LECs might help injury repair. (E) Glioblastoma and melanoma cells can promote lymphangiogenesis. Tumor cells can metastasize via MLVs. Meanwhile, tumor antigen from the brain reaches dCLNs and activates intracranial immunosurveillance. (F) Clearance rates are different during sleep, wakefulness, and under anesthesia. CSF influx exhibits a pattern of circadian control. Created with Vectornator. AQP4: Aquaporin 4; CCR7: C-C chemokine receptor 7; CCL21: C-C chemokine ligand 21; CSF: cerebrospinal fluid; DC: dendritic cell; dCLN: deep cervical lymph node; ICP: intracranial pressure; LEC: lymphatic endothelial cell; MLVs: meningeal lymphatic vessels; PVS: perivascular space; Rbc: red blood cell; SAS: subarachnoid space; SMC: smooth muscle cell; VEC: vascular endothelial cell; VEGF-C: vascular endothelial growth factor C; VEGFR-3: vascular endothelial growth receptor 3.
Table 1.
Interventions targeting the lymphatic system in neurodegenerative disorders and central nervous system injury
| Disease model | Treatment | Outcome | Reference |
|---|---|---|---|
| Aged mice | AAVs encoding VEGF-C; hydrogel-encapsulated VEGF-C peptide | Increased LV diameter; increased CSF tracer drainage; improved cognitive behavior. | Da Mesquita et al., 2018 |
| APP/PS1 transgenic mice, AD mice | Recombinant VEGF-C | Higher levels of dural lymphangiogenesis; decreased levels of soluble amyloid-β; restored spatial cognition. | Wen et al., 2018 |
| 5×FAD mice | AAVs encoding VEGF-C | Improved clearance of Aβ by monoclonal antibodies. | Da Mesquita et al., 2021 |
| EAE | Visudyne treatment with laser activation | Ablation of meningeal lymphatics-mediated drainage; delayed pathological process. | Louveau et al., 2018 |
| EAE | VEGFR-3 tyrosine kinase inhibitor (MAZ51) | Inhibited lymphangiogenesis; decreased the level of autoimmune antigen drainage; reduced EAE severity. | Hsu et al., 2019 |
| TBI | AAVs encoding VEGF-C | Rejuvenated lymphatics; mitigated TBI-induced inflammation. | Bolte et al., 2020 |
| Experimental SAH in mice | Visudyne treatment | Worsened brain perfusion, inflammation, apoptosis, and neurological impairments in early brain injury. | Liu et al., 2021 |
| Focal cerebral ischemia | VEGFR-3 tyrosine kinase inhibitor (MAZ51); lymphadenectomy | Reduced inflammatory process and brain injury. | Esposito et al., 2019 |
| Focal demyelination spinal cord injury | AAVs encoding VEGF-C | Increase in vLV area; amplification of the cytotoxic effect. | Jacob et al., 2019 |
AAV: Adeno-associated viral vector; CSF: cerebrospinal fluid; EAE: experimental autoimmune encephalomyelitis; SAH: subarachnoid hemorrhage; TBI: traumatic brain injury; VEGF-C: vascular endothelial growth factor C; VEGFR: vascular endothelial growth factor receptor; vLVs: vertebral lymphatic vessels.
Neurodegenerative disorders
The pathology of aging or proteinopathic neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), glaucoma, frontotemporal dementia, and amyotrophic lateral sclerosis, is characterized by the insufficient clearance of neurotoxic proteins, including Aβ and tau (Boland et al., 2018).
The impairment of extracellular clearance by the lymphatic system of the brain has emerged as a key pathogenic mechanism in aging. Aged mice exhibited regression of dorsal MLVs and hyperplasia of basal MLVs. Their basal MLVs increased in size and became highly branched, leading to fewer lymphatic valves and zipper-type junctions, and more button-type junctions. Moreover, basal MLVs from aged mice showed a dysmorphic distribution of type IV collagen and reduced fluid transport. The observed lymphatic hyperplasia was regarded as a compensatory mechanism for the impaired function of the lymphatic system (Ahn et al., 2019). Additionally, lymphatic outflow from the CSF to blood and draining LNs was slower in aged mice (Antila et al., 2017). RNA-seq analysis of LECs revealed that genes related to the lymphangiogenic signaling pathway were differentially expressed in aged mice (Da Mesquita et al., 2018b).
Glymphatic system impairment exacerbated the pathogenic accumulation of Aβ and tau (Xu et al., 2015; Harrison et al., 2020). Wang et al. (2020) also identified an ocular glymphatic clearance route for Aβ. Intra-axonal Aβ was cleared from the retina and vitreous via the perivenous space and subsequently drained to cervical LVs. The fluid and waste clearance function of the pathway was dependent on the AQP4 channel and translaminar pressure. The AQP4 channel has been frequently targeted given its crucial role in pathological protein clearance (Valenza et al., 2019). AQP4 was demonstrated to function in the clearance of interstitial Aβ, suggestive of an association between brain lymphatics and AD progression (Tarasoff-Conway et al., 2015; Mader and Brimberg, 2019). Impaired expression and function of AQP4 were demonstrated to be biomarkers for AD. Moreover, single nucleotide polymorphisms in the AQP4 gene were correlated with Aβ neuropathology, disease progression, and cognitive decline (Chandra et al., 2021). In aged mice, a radiotracer clearance assay showed a reduction in CSF-ISF exchange efficiency, Aβ clearance rate, vessel wall pulsatility, and perivascular AQP4 polarization (Kress et al., 2014). Meanwhile, patients with AD displayed a significant decrease in CSF AQP4 levels, which was correlated with diminished levels of Aβ in the CSF (Arighi et al., 2019). Additionally, the expression of AQP4 was reported to be reduced in aging human brains, while a marked loss of perivascular AQP4 localization in the frontal cortex was observed in individuals with AD (Zeppenfeld et al., 2017).
Da Mesquita et al. (2018b) reported that MLV ablation promoted Aβ deposition in the meninges and aggravated Aβ accumulation in the brain parenchyma, while Wang et al. (2019a) showed that the ligation of dCLN also exacerbated AD-like phenotypes in mice. However, a study on post-mortem meningeal tissue from patients with Alzheimer’s dementia did not detect Aβ deposition in MLVs, implying that MLVs were not Aβ-deposition sites (Goodman et al., 2018). An observational cohort study utilizing a novel MRI method showed that the glymphatic pathway and MLVs were impaired in aging human brains. The clearance of downstream putative MLVs was significantly related to the glymphatic system (Zhou et al., 2020). Using dynamic contrast-enhanced magnetic resonance imaging, Ding et al. (2021) demonstrated that patients with idiopathic PD or atypical Parkinsonian disorders exhibited reduced flow in the cerebral lymphatic system. The same method was used to measure the uptake of gadobutrol medium by MLVs and thereby quantify MLV flow. The signal intensity in the MLVs along the superior sagittal and sigmoid sinuses was significantly reduced in patients with idiopathic PD, and their dCLN perfusion was also delayed. Moreover, MLVs were observed to participate in the clearance of other abnormally accumulated proteins, such as tau and α-synuclein (Patel et al., 2019; Zou et al., 2019; Ding et al., 2021), suggestive of their clinical diagnostic value and their ability to restore lymphatic function in the treatment of neurodegenerative diseases.
Treatments for the lymphatic system are being investigated based on these mechanisms. Thrombolytic therapy, phosphodiesterase III inhibitors, anti-Aβ immunotherapy, and AQP4 activation have all been adopted to improve the glymphatic transfer of neurotoxic proteins to the circulation (Sun et al., 2018). Nevertheless, therapies that involve lymphatic intervention are still in their infancy. Seven days after the injection of VEGFR-3-specific recombinant VEGF-C into the cisterna magna, mice exhibited an increase in MLV diameter (Louveau et al., 2015b). A similar phenotype was observed in aged mice treated with AAV1-CMV-mVEGF-C. Moreover, 1 month after AAV1/CMV/mVEGF-C treatment, aged mice that displayed impaired lymphatic function showed a significant increase in CSF tracer drainage into the dCLNs. Consequently, the rate of tracer influx into the brain parenchyma was also increased. The same therapeutic effect was obtained following the transcranial delivery of hydrogel-encapsulated VEGF-C peptide (Da Mesquita et al., 2018b). However, in AD mice with no apparent MLV dysfunction at a young age, the mVEGF-C treatment was inefficient, suggesting an upper limit for VEGF-C in lymphatic function. Recombinant VEGF-C was shown to promote tube formation in human LECs in vitro, while the in vivo injection of 200 μg/mL recombinant human VEGF-C (rhVEGF-C) into the cisterna magna, every 2 days, four times in total, induced dural lymphangiogenesis, reduced the levels of soluble amyloid-β, and restored spatial cognition in AD model mice (Wen et al., 2018). Recently, the modulation of meningeal lymphatic function was shown to influence the outcome of passive immunotherapy in AD (Da Mesquita et al., 2021). The ablation of MLVs in a mouse model of amyloid deposition resulted in a decrease in the effectiveness of anti-Aβ monoclonal antibody therapy, whereas treatment with VEGF-C improved Aβ clearance. Additionally, the authors suggested that activation of the microglial inflammatory response might underlie the loss of treatment efficacy resulting from MLV ablation.
Neuroinflammatory diseases
In pathological states, including virus infections and autoimmune diseases, antigens from the CNS are drained to peripheral immune organs and activate lymphocytes, which then invade the CNS. Multiple sclerosis is an autoimmune neurological disease characterized by the infiltration of autoreactive T cells into the brain and subsequent neuronal damage (Liblau et al., 2013). CLNs have long been suggested as a site for activation of encephalitogenic T cells (Phillips et al., 1997). In an experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis, the excision of CNS-draining LNs ameliorated chronic-relapsing EAE within the spinal cord (van Zwam et al., 2009). Fluorescent tracer injection and multiphoton intravital imaging revealed that MLVs could uptake and transport CSF constituents. The meningeal lymphatic subarachnoid extensions along the transverse sinus, superior sagittal sinus, and cerebellar ring presented multiple entry sites for fluids. The dCLNs and supraclavicular LNs were confirmed to be the major CNS-draining LNs. Importantly, CCR7+ T cells have a close relationship with CCL21+ LECs, suggesting that the CCR7-CCL21 pathway plays a primary role in meningeal T-cell migration. Additionally, MLVs were shown to serve as a route for the drainage of immune cells and macromolecules from the CNS. A transcriptomic analysis further highlighted the unique properties of meningeal LECs relative to peripheral ones, which might explain the limited lymphangiogenic ability of meningeal LECs under inflammatory stimulation. Moreover, the ablation of meningeal lymphatics using Visudyne (laser-activated) delayed the pathological process of EAE in mice. The transcriptomic profile of antigen-specific T cells isolated from dCLNs revealed that impaired lymphatic drainage prevented the formation of encephalitogenic T cells in dCLNs (Louveau et al., 2018).
Meanwhile, lymphatics near the cribriform plate and nasal mucosa are essential in multiple sclerosis. The nasal region is not considered a major exit route for CNS immune cells; instead, it is closely associated with chronic inflammation and later stages of disease development (Louveau et al., 2018). Neuroinflammation-induced lymphangiogenesis was reported to be dependent on VEGF-C/VEGFR-3 signaling. During EAE, VEGF-C is significantly downregulated. Newly formed LVs carried CD11b- and CD11c-positive immune cells. LECs near the cribriform plate also expressed CCR7+ and were accompanied by increased numbers of CCR7+ CD11c+ cells. These observations confirmed that lymphangiogenesis occurred primarily in the cribriform plate. Accordingly, treatment with the VEGFR-3 tyrosine kinase inhibitor MAZ51 (10 mg/kg, once daily) reduced EAE severity by inhibiting lymphangiogenesis and decreasing the amount of autoimmune antigen drainage to the LNs (Hsu et al., 2019).
Neuromyelitis optica spectrum disorder is a neuroinflammatory, demyelinating disease of the CNS that most commonly affects the optic nerve and spinal cord. The presence of AQP4 IgG antibodies (AQP4-IgG) in serum represents the key determinant of this disease. AQP4-IgG is highly cytotoxic to astrocytes (Wu et al., 2019). Employing contrast-enhanced magnetic resonance imaging (Wang et al., 2021) observed that meningeal lymphatic flow was impaired in patients with acute neuromyelitis optica spectrum disorder. The contrast-enhanced magnetic resonance imaging parameters of mLVs in the superior sagittal sinus were correlated with disease severity and were suggested to have potential as disease predictors.
CNS injuries
Mice subjected to traumatic brain injury (TBI) exhibit the loss of perivascular AQP4 polarization and impaired interstitial solute clearance. Aqp4 knockout mice showed exacerbated post-traumatic neuroinflammation and cognitive deficits after TBI (Iliff et al., 2014). MRI revealed altered glymphatic clearance rates in limbic and olfactory bulbs after repetitive mild TBI (Christensen et al., 2020). Chronic glymphatic system dysfunction associated with repetitive brain trauma has been implicated in the subsequent development of chronic traumatic encephalopathy (Sullan et al., 2018). In mice subjected to TBI, treatment with omega-3 polyunsaturated fatty acids restored glymphatic clearance and reduced Aβ accumulation, thereby attenuating TBI-induced neurological impairment (Zhang et al., 2020).
After TBI, brain-to-CLN signaling largely depends on lymphatics. The suppression of glymphatic pathway activity prevented the delivery of TBI biomarkers (S100β, GFAP, and NSE) from the glymphatic system and dCLN to blood (Plog et al., 2015). The uptake of CSF by MLVs can also be largely impaired in TBI (Bolte et al., 2020). After 1 week of injury, mice with TBI exhibited increases in LYVE-1+ percent area coverage, the number of capillary loops and sprouts, and LV diameter, and these parameters changed dynamically over time. After TBI, the disruption of lymphatic drainage may result from an increase in intracranial pressure (ICP). Pre-existing MLV dysfunction in aged mice also exacerbated post-injury inflammation and cognitive decline. Viral-mediated delivery of VEGF-C rejuvenated lymphatics and mitigated TBI-induced inflammation in aged mice. Additionally, aged mice with TBI that received VEGF-C treatment had significantly lower levels of Iba1, which were more similar to the levels of Iba1 immunoreactivity in young mice subjected to TBI. These results indicated that LV enhancement can mitigate TBI-driven inflammation (Bolte et al., 2020). In a mouse model of subarachnoid hemorrhage, the Visudyne-mediated ablation of MLVs led to impaired brain perfusion and neurological function, as well as the aggravation of inflammation and apoptosis in early brain injury (Liu et al., 2021). In K14-VEGFR-3-Ig transgenic mice subjected to TBI, CD4+ T-cell infiltration in the perilesional cortex and hippocampus was reduced, suggesting that MLVs are indispensable for the establishment of a proper neuroimmune response after TBI (Wojciechowski et al., 2020).
However, compared to its beneficial effects in TBI, the brain-to-CLN signaling via VEGFR-3 participated in systemic inflammation in other diseases. In a rat model of stroke, the rapid appearance of dye that had originated from the CSF or brain parenchyma in CLNs suggested that inflammatory signals had been sent from the brain to peripheral LNs (Esposito et al., 2019). After cerebral ischemia, LEC proliferation, phosphorylated VEGFR-3 in CLNs, and VEGF-C upregulation were all observed. Moreover, activated lymphatic endothelium could upregulate pro-inflammatory macrophage responses in CLNs. After a stroke, the blockade of VEGFR-3 via the intracerebroventricular injection of MAZ51 or lymphadenectomy mitigated both the inflammatory process and brain injury (Esposito et al., 2019). In mice models of photothrombosis-induced stroke, meningeal lymphangiogenesis was seen in the alymphatic zone, lateral to the sagittal sinus. Additionally, the impairment of MLVs in mutant mice with defective VEGFR signaling worsened the outcome of stroke in the animals (Yanev et al., 2020). Meanwhile, in a lysolecithin-induced focal demyelination spinal cord injury model, LV diameter and area were only significantly enhanced in mice treated with VEGF-C and lysolecithin together, while the VEGF-C-induced increase in vertebral LV area amplified the cytotoxic effect resulting from lysolecithin-induced injury (Jacob et al., 2019). The physiological role of LVs in injury and inflammation, and whether they primarily reduce ICP through draining or promote the spread of inflammation, remain to be investigated.
Other neurological diseases
The fluid drainage and waste clearance functions of lymphatics are also associated with edema and hydrocephalus (Simka et al., 2018; Reeves et al., 2020). Congenital impairment of glymphatic function or AQP4 loss are critical pathogenic mechanisms in idiopathic normal pressure hydrocephalus (Hasan-Olive et al., 2019; Jacobsen et al., 2020). Furthermore, the location of MLVs renders them suitable as clearance pathways in subarachnoid hemorrhage and subdural hematomas (Pu et al., 2019; Liu et al., 2020b). Small vessel disease (SVD) is one of the most common etiologies of dementia in the elderly and can further deteriorate to stroke, disability, and cognitive impairment (Brown et al., 2018). The function of the lymphatic system is impaired in SVD. Enlarged PVS visible in MRI and protein deposits are hallmark features of this condition (Mestre et al., 2017). PVS enlargement can be seen in SVDs such as cerebral amyloid angiopathy and hypertensive arteriopathy (Martinez-Ramirez et al., 2018; Duperron et al., 2019). A large population-based cohort study found that a high burden of dilated PVS in basal ganglia and the hippocampus was associated with an increased risk of stroke and intracerebral hemorrhage (Duperron et al., 2019). Moreover, in hypertension, perivascular inflammation, pericyte dysfunction, BBB disruption, arterial stiffness, and decreased cerebrovascular pulsatility were reported to contribute to the obstruction and stagnation of fluid drainage (Mestre et al., 2017; Brown et al., 2018; Riba-Llena et al., 2018).
Dorsal MLVs presented significantly increased diameter and density in mice injected with glioma and melanoma cells, indicating that brain tumors can promote lymphangiogenesis. MLVs can serve as a tunnel through which tumor cells can metastasize toward dCLNs. Meanwhile, dorsal MLVs were shown to be the main route for the migration of DCs and other immune cells to dCLNs and the establishment of immune responses to brain tumors. Visudyne-mediated MLV ablation impaired the efficacy of anti-PD-1/CTLA-4 immunotherapy while overexpressing VEGF-C in tumor cells exerted the opposite effect in a CCL21/CCR7 signaling-dependent manner (Hu et al., 2020). The effect of treatments targeting the PD-1 checkpoint is also influenced by MLVs. The efficacy of monoclonal antibodies targeting PD-1, such as nivolumab, still can be enhanced in the treatment of glioblastomas (Wang et al., 2019b). For example, Song et al. (2020) revealed that the combination of anti-PD-1 antibody and VEGF could significantly suppress tumor growth and improve survival. Moreover, VEGF-C promoted antigen drainage and stimulated lasting and strong T-cell mediated immune responses. Finally, VEGF-C induced MLV lymphangiogenesis, leading to the generation of an anti-tumor environment that facilitated T-cell infiltration, priming, and recruitment (Song et al., 2020).
The glymphatic system is also implicated in various diseases. One study confirmed that glymphatic system impairment due to a reduction in AQP4 expression exacerbated hepatic encephalopathy (Hadjihambi et al., 2019). Hydrodynamic disorders, such as the retention of sodium and water in the body, that result from liver abnormalities, can lead to glymphatic system dysfunction (Gallina et al., 2019). Accordingly, cognitive dysfunction caused by substances that can injure the liver, such as alcohol, might be related to glymphatic system impairment (de la Monte and Kril, 2014). Indeed, studies have shown that alcohol can have a dose-dependent effect on glymphatic system function (Lundgaard et al., 2018; Cheng et al., 2019). Multiple studies have demonstrated the risk of glymphatic system impairment during depression. For example, mice treated with chronic unpredictable mild stress exhibited AQP4 downregulation, Aβ accumulation, and reduced arterial pulsation (Wei et al., 2019; Liu et al., 2020a).
Sleep, wakefulness, and anesthesia are all closely related to the function of the glymphatic system. During wakefulness, the intensity of tracer signals in the periarterial spaces, subpial regions, and brain parenchyma was reported to be significantly lower than that during the sleep state. Additionally, ketamine/xylazine-induced anesthesia was shown to increase CSF influx. These results confirmed that sleep and anesthesia can stimulate lymphatic drainage and enhance waste clearance (Xie et al., 2013). Several studies have demonstrated that sleep deprivation can suppress CSF transport, which is associated with the accumulation of neurotoxic macromolecules and the progression of neurodegenerative disease (Achariyar et al., 2016; Shokri-Kojori et al., 2018; Piantino et al., 2019). The effects of sleep and anesthesia on glymphatic system function may be attributable to interstitial space enlargement. For example, in sleeping and anesthetized mice, the volume and tortuosity of the interstitial space were significantly increased (Xie et al., 2013). Meanwhile, Benveniste et al. (2017) discovered that the additional administration of dexmedetomidine when anesthetizing the rats with isoflurane induced a more efficient glymphatic clearance . Diabetes-related sleep disturbance and cognitive dysfunction might also be caused by glymphatic system impairment, as evidenced by the fact that the diameter of PVS is reduced and waste clearance is impaired in type-2 diabetes mellitus (Jiang et al., 2017; Brzecka et al., 2021). The recording of the diurnal variation in the glymphatic system of mice led to the finding that CSF influx was under circadian control. The amount of CSF tracer influx into the brain during the day was approximately 53% more than that during the night, peaking at around mid-day when the mice were mostly asleep. The AQP4 intensity around the vasculature was also significantly higher during the day (Hablitz et al., 2020). This circadian control of the glymphatic system might also explain neural diseases caused by circadian misalignment (Depner et al., 2014). These studies have expanded the understanding of how circadian rhythms form and function and have also provided novel ideas regarding the occurrence of migraine, AD, and multiple other neurological disorders.
Discussion
MLVs and the perivascular drainage pathway together form a gradually elaborated lymphatic system for the CNS. The in-depth study of anatomical features has revealed the role of MLVs in CSF-ISF exchange, metabolite regulation, and immune cell migration. This harmoniously working ‘plumbing system’ helps maintain the delicate internal environment required for neuronal function. The lymphatic system-related accumulation of metabolites and toxic substances, as well as the imbalance and impairment of the immune response, can lead to the occurrence of several neurological conditions. Accordingly, the lymphatic system for the CNS has emerged as a novel therapeutic target for multiple neurological disorders.
The precise location of MLVs within the meningeal layers remains unclear. Their localization and contact with the subarachnoid space largely reflect the place where the absorption of macromolecule and immune cell occurs (Raper et al., 2016). Pathology data for human and nonhuman primates have indicated that at least some lymphatics are entirely contained within the dura (Absinta et al., 2017). Experimental/dynamic mathematical model combinations are still required to quantify the relative contribution of each CSF influx and efflux pathway to drainage. We propose the development of high-resolution intravital imaging tools to precisely visualize the structure of the meningeal lymphatic drainage system for the CNS. Meanwhile, given that meningeal LECs have gene expression patterns that are distinct from those of peripheral LECs (Louveau et al., 2018), additional research using genetic tools is required to determine the unique biological functions of meningeal LECs in the CNS. VEGF-C/VEGFR-3 signaling regulation controls LEC fate and ultimately affects LV development at the molecular level.
Although much is already known about the anatomy of perivascular fluid pathways and MLVs, relevant research interest is currently focused on their potential as therapeutic targets (Sun et al., 2018; Mestre et al., 2020). Targeted interventions can effectively influence the pathophysiology of neurological disorders, especially neurodegenerative diseases associated with the deposition of toxic substances (Rasmussen et al., 2018; Wen et al., 2018). Whether lymphatics can invade the brain parenchyma after an injury is still unknown. The observed co-expression of LYVE-1 and PROX1, and the assembly into putative pre-vessel structures by LYVE-1-expressing cells, have suggested that, in the adult rat spinal cord, mild lymphangiogenesis occurs after spinal cord contusion injury (Kaser-Eichberger et al., 2016). However, those phenomena need further elucidation regarding vessel structure and signatures. In zebrafish ischemic stroke models, MLVs were observed to rapidly grow into the injured brain parenchyma to drain the excess ISF and guide neovascular regeneration. The newborn LVs resulting from the response to ischemia assisted in tissue regeneration to a great extent. Moreover, once cerebrovascular regeneration was complete, the ingrown LVs were cleared via apoptosis, suggesting that they played only a transient role during injury (Chen et al., 2019). Although not yet confirmed in mammals, this finding revealed that the function of LVs consists of more than just the alleviation of edema during CNS injury. The expression of lymphatic markers was also detected in damaged mouse brain tissues after penetrating TBI (Meng et al., 2021). Recently, lymphoangiocrine signals secreted by LECs were identified as an essential component in cardiac repair (Liu et al., 2020c). The functions of LECs in CNS damage repair also merit further investigation.
Conclusion
Current evidence indicates that a strong relationship exists between the lymphatic system of the CNS and neurodegenerative, neuroinflammatory, and CNS injury disorders. These observations suggest that targeting the lymphatic system may represent a feasible therapeutic strategy for post-injury CNS repair. Notably, however, any invasive procedure can upset the delicate balance of the brain lymphatic system and interfere with the judgment of the actual physiological and pathological conditions of fluid flow, making experiments difficult to perform. The enhancement of lymphatic function via VEGF-C delivery has shown promising effects in promoting the clearance of neurotoxic substances and treating neurodegenerative disease. Lymphangiogenesis has also shown benefits in reducing ICP. VEGF-C intervention seemed to be mainly effective in aged or injured mice with pre-existing lymphatic function impairment. In autoimmune diseases or stroke, the ablation of the lymphatic pathway could also mitigate inflammation.
Footnotes
Funding: This work was supported by the Key Program of the National Natural Science Foundation of China, No. 82030071 and the Science and Technology Major Project of Changsha, No. kh2103008 (both to JZH).
Conflicts of interest: The authors declare that they have no competing interests.
C-Editor: Zhao M, S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11:74. doi: 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 4.Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system:tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136:139–152. doi: 10.1007/s00401-018-1862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 6.Andres KH, von Düring M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- 7.Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645–3667. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arighi A, Di Cristofori A, Fenoglio C, Borsa S, D'Anca M, Fumagalli GG, Locatelli M, Carrabba G, Pietroboni AM, Ghezzi L, Carandini T, Colombi A, Scarioni M, De Riz MA, Serpente M, Rampini PM, Scarpini E, Galimberti D. Cerebrospinal fluid level of aquaporin4:a new window on glymphatic system involvement in neurodegenerative disease? J Alzheimers Dis. 2019;69:663–669. doi: 10.3233/JAD-190119. [DOI] [PubMed] [Google Scholar]
- 9.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology. 2017;127:976–988. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, Mallucci GR, Kroemer G, Levine B, Eskelinen EL, Mochel F, Spedding M, Louis C, Martin OR, Millan MJ. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2018;17:660–688. doi: 10.1038/nrd.2018.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, Ennerfelt HE, Shapiro D, Nguyen BH, Frost EL, Lammert CR, Kipnis J, Lukens JR. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 2020;11:4524. doi: 10.1038/s41467-020-18113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulton M, Flessner M, Armstrong D, Hay J, Johnston M. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol. 1998;274:R88–96. doi: 10.1152/ajpregu.1998.274.1.R88. [DOI] [PubMed] [Google Scholar]
- 14.Bradbury MW, Cole DF. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol. 1980;299:353–365. doi: 10.1113/jphysiol.1980.sp013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114:1462–1473. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brzecka A, Madetko N, Nikolenko VN, Ashraf GM, Ejma M, Leszek J, Daroszewski C, Sarul K, Mikhaleva LM, Somasundaram SG, Kirkland CE, Bachurin SO, Aliev G. Sleep disturbances and cognitive impairment in the course of type 2 diabetes-a possible link. Curr Neuropharmacol. 2021;19:78–91. doi: 10.2174/1570159X18666200309101750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucchieri F, Farina F, Zummo G, Cappello F. Lymphatic vessels of the dura mater:a new discovery? J Anat. 2015;227:702–703. doi: 10.1111/joa.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries:significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 20.Chandra A, Farrell C, Wilson H, Dervenoulas G, De Natale ER, Politis M. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer's disease spectrum. Neurobiol Aging. 2021;97:1–9. doi: 10.1016/j.neurobiolaging.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, He J, Ni R, Yang Q, Zhang Y, Luo L. Cerebrovascular injuries induce lymphatic invasion into brain parenchyma to guide vascular regeneration in zebrafish. Dev Cell. 2019;49:697–710. doi: 10.1016/j.devcel.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y, Liu X, Ma X, Garcia R, Belfield K, Haorah J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free Radic Biol Med. 2019;143:115–126. doi: 10.1016/j.freeradbiomed.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Christensen J, Wright DK, Yamakawa GR, Shultz SR, Mychasiuk R. Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci Rep. 2020;10:6254. doi: 10.1038/s41598-020-63022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coles JA, Myburgh E, Brewer JM, McMenamin PG. Where are we?The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog Neurobiol. 2017;156:107–148. doi: 10.1016/j.pneurobio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system:a new player in neurophysiology. Neuron. 2018a;100:375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018b;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, Jiang H, Kodira CD, de Lima KA, Herz J, Louveau A, Goldman DH, Salvador AF, Onengut-Gumuscu S, Farber E, Dabhi N, Kennedy T, Milam MG, Baker W, Smirnov I, et al. Meningeal lymphatics affect microglia responses and anti-Aβimmunotherapy. Nature. 2021;593:255–260. doi: 10.1038/s41586-021-03489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93:1847–1892. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- 29.de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, Zhang ZX, Cao QC, Wang W, Li JY, Wu E, Tang BS, Ma MM, Teng JF, Wang XJ. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson's disease. Nat Med. 2021;27:411–418. doi: 10.1038/s41591-020-01198-1. [DOI] [PubMed] [Google Scholar]
- 32.Duperron MG, Tzourio C, Schilling S, Zhu YC, Soumaré A, Mazoyer B, Debette S. High dilated perivascular space burden:a new MRI marker for risk of intracerebral hemorrhage. Neurobiol Aging. 2019;84:158–165. doi: 10.1016/j.neurobiolaging.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Elham E, Wumaier R, Wang C, Luo X, Chen T, Zhong N. Anatomic evidence shows that lymphatic drainage exists in the pituitary to loop the cerebral lymphatic circulation. Med Hypotheses. 2020;143:109898. doi: 10.1016/j.mehy.2020.109898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt B, Carare RO, Bechmann I, Flügel A, Laman JD, Weller RO. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016;132:317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esposito E, Ahn BJ, Shi J, Nakamura Y, Park JH, Mandeville ET, Yu Z, Chan SJ, Desai R, Hayakawa A, Ji X, Lo EH, Hayakawa K. Brain-to-cervical lymph node signaling after stroke. Nat Commun. 2019;10:5306. doi: 10.1038/s41467-019-13324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Földi M, Gellért A, Kozma M, Poberai M, Zoltán OT, Csanda E. New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat (Basel) 1966;64:498–505. doi: 10.1159/000142849. [DOI] [PubMed] [Google Scholar]
- 38.Gallina P, Gallo O, Nicoletti C, Romanelli RG. A hydrodynamic hypothesis for the pathogenesis of glymphatic system impairment in hepatic encephalopathy. J Hepatol. 2019;71:228–229. doi: 10.1016/j.jhep.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 40.Goodman JR, Adham ZO, Woltjer RL, Lund AW, Iliff JJ. Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer's dementia subjects. Brain Behav Immun. 2018;73:34–40. doi: 10.1016/j.bbi.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411. doi: 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadjihambi A, Harrison IF, Costas-Rodríguez M, Vanhaecke F, Arias N, Gallego-Durán R, Mastitskaya S, Hosford PS, Olde Damink SWM, Davies N, Habtesion A, Lythgoe MF, Gourine AV, Jalan R. Impaired brain glymphatic flow in experimental hepatic encephalopathy. J Hepatol. 2019;70:40–49. doi: 10.1016/j.jhep.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O'Neill MJ, Wells JA, Lythgoe MF. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143:2576–2593. doi: 10.1093/brain/awaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK. Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia. 2019;67:91–100. doi: 10.1002/glia.23528. [DOI] [PubMed] [Google Scholar]
- 45.Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, Karpus WJ, Sandor M, Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. 2019;10:229. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, He T, Ning W, Kong Y, Huo Y, He A, Liu B, Zhang J, Adams R, He Y, Tang F, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020;30:229–243. doi: 10.1038/s41422-020-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izen RM, Yamazaki T, Nishinaka-Arai Y, Hong YK, Mukouyama YS. Postnatal development of lymphatic vasculature in the brain meninges. Dev Dyn. 2018;247:741–753. doi: 10.1002/dvdy.24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacob L, Boisserand LSB, Geraldo LHM, de Brito Neto J, Mathivet T, Antila S, Barka B, Xu Y, Thomas JM, Pestel J, Aigrot MS, Song E, Nurmi H, Lee S, Alitalo K, Renier N, Eichmann A, Thomas JL. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. 2019;10:4594. doi: 10.1038/s41467-019-12568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsen HH, Sandell T, Jørstad ØK, Moe MC, Ringstad G, Eide PK. In vivo evidence for impaired glymphatic function in the visual pathway of patients with normal pressure hydrocephalus. Invest Ophthalmol Vis Sci. 2020;61:24. doi: 10.1167/iovs.61.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326–1337. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaser-Eichberger A, Schroedl F, Bieler L, Trost A, Bogner B, Runge C, Tempfer H, Zaunmair P, Kreutzer C, Traweger A, Reitsamer HA, Couillard-Despres S. Expression of lymphatic markers in the adult rat spinal cord. Front Cell Neurosci. 2016;10:23. doi: 10.3389/fncel.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 55.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecco V. Probable modification of the lymphatic fissures of the walls of the venous sinuses of the dura mater. Arch Ital Otol Rinol Laringol. 1953;64:287–296. [PubMed] [Google Scholar]
- 57.Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F. Neurons as targets for T cells in the nervous system. Trends Neurosci. 2013;36:315–324. doi: 10.1016/j.tins.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Liu Q, Hou C, Zhang H, Fu C, Wang W, Wang B, Li J, Zhao Y, Yang X. Impaired meningeal lymphatic vessels exacerbate early brain injury after experimental subarachnoid hemorrhage. Brain Res. 2021;1769:147584. doi: 10.1016/j.brainres.2021.147584. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Hao J, Yao E, Cao J, Zheng X, Yao D, Zhang C, Li J, Pan D, Luo X, Wang M, Wang W. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun. 2020a;89:357–370. doi: 10.1016/j.bbi.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Gao C, Yuan J, Xiang T, Gong Z, Luo H, Jiang W, Song Y, Huang J, Quan W, Wang D, Tian Y, Ge X, Lei P, Zhang J, Jiang R. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. 2020b;8:16. doi: 10.1186/s40478-020-0888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu X, De la Cruz E, Gu X, Balint L, Oxendine-Burns M, Terrones T, Ma W, Kuo HH, Lantz C, Bansal T, Thorp E, Burridge P, Jakus Z, Herz J, Cleaver O, Torres M, Oliver G. Lymphoangiocrine signals promote cardiac growth and repair. Nature. 2020c;588:705–711. doi: 10.1038/s41586-020-2998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015a;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015b;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R, Nedergaard M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep. 2018;8:2246. doi: 10.1038/s41598-018-20424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Q, Decker Y, Müller A, Ineichen BV, Proulx ST. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med. 2019;216:2492–2502. doi: 10.1084/jem.20190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma W, Oliver G. Lymphatic endothelial cell plasticity in development and disease. Physiology (Bethesda) 2017;32:444–452. doi: 10.1152/physiol.00015.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mader S, Brimberg L. Aquaporin-4 water channel in the brain and its implication for health and disease. Cells. 2019;8:90. doi: 10.3390/cells8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez-Ramirez S, van Rooden S, Charidimou A, van Opstal AM, Wermer M, Gurol ME, Terwindt G, van der Grond J, Greenberg SM, van Buchem M, Viswanathan A. Perivascular spaces volume in sporadic and hereditary (Dutch-Type) cerebral amyloid angiopathy. Stroke. 2018;49:1913–1919. doi: 10.1161/STROKEAHA.118.021137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels:an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 73.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 74.Meng FW, Yu JT, Chen JY, Yang PF. New lymphatic cell formation is associated with damaged brain tissue clearance after penetrating traumatic brain injury. Sci Rep. 2021;11:10193. doi: 10.1038/s41598-021-89616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017;131:2257–2274. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mestre H, Mori Y, Nedergaard M. The brain's glymphatic system:current controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mezey É, Szalayova I, Hogden CT, Brady A, Dósa Á, Sótonyi P, Palkovits M. An immunohistochemical study of lymphatic elements in the human brain. Proc Natl Acad Sci U S A. 2021;118:e2002574118. doi: 10.1073/pnas.2002574118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohammad MG, Tsai VW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, Breit SN, Sawchenko PE, Brown DA. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124:1228–1241. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munk AS, Wang W, Bèchet NB, Eltanahy AM, Cheng AX, Sigurdsson B, Benraiss A, Mäe MA, Kress BT, Kelley DH, Betsholtz C, Møllgård K, Meissner A, Nedergaard M, Lundgaard I. PDGF-B is required for development of the glymphatic system. Cell Rep. 2019;26:2955–2969.e2953. doi: 10.1016/j.celrep.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21(st) century:novel functional roles in homeostasis and disease. Cell. 2020;182:270–296. doi: 10.1016/j.cell.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Owasil R, O'Neill R, Keable A, Nimmo J, MacGregor Sharp M, Kelly L, Saito S, Simpson JE, Weller RO, Smith C, Attems J, Wharton SB, Yuen HM, Carare RO. The pattern of AQP4 expression in the ageing human brain and in cerebral amyloid angiopathy. Int J Mol Sci. 2020;21:1225. doi: 10.3390/ijms21041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papadopoulos Z, Herz J, Kipnis J. Meningeal lymphatics:from anatomy to central nervous system immune surveillance. J Immunol. 2020;204:286–293. doi: 10.4049/jimmunol.1900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K, McKee CA, Sheehan PW, Musiek ES, Xiong C, Coble D, Holtzman DM. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener. 2019;14:11. doi: 10.1186/s13024-019-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrova TV, Koh GY. Organ-specific lymphatic vasculature:from development to pathophysiology. J Exp Med. 2018;215:35–49. doi: 10.1084/jem.20171868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. 2020;369:eaax4063. doi: 10.1126/science.aax4063. [DOI] [PubMed] [Google Scholar]
- 86.Phillips MJ, Needham M, Weller RO. Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J Pathol. 1997;182:457–464. doi: 10.1002/(SICI)1096-9896(199708)182:4<457::AID-PATH870>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 87.Piantino J, Lim MM, Newgard CD, Iliff J. Linking traumatic brain injury, sleep disruption and post-traumatic headache:a potential role for glymphatic pathway dysfunction. Curr Pain Headache Rep. 2019;23:62. doi: 10.1007/s11916-019-0799-4. [DOI] [PubMed] [Google Scholar]
- 88.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pu T, Zou W, Feng W, Zhang Y, Wang L, Wang H, Xiao M. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp Neurobiol. 2019;28:104–118. doi: 10.5607/en.2019.28.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016;39:581–586. doi: 10.1016/j.tins.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rasschaert M, Weller RO, Schroeder JA, Brochhausen C, Idée JM. Retention of gadolinium in brain parenchyma:pathways for speciation, access, and distribution. a critical review. J Magn Reson Imaging. 2020;52:1293–1305. doi: 10.1002/jmri.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, Kahle KT. Glymphatic system impairment in Alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. 2020;26:285–295. doi: 10.1016/j.molmed.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a 'paravascular'fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 95.Riba-Llena I, Jiménez-Balado J, Castañé X, Girona A, López-Rueda A, Mundet X, Jarca CI, Álvarez-Sabin J, Montaner J, Delgado P. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke. 2018;49:1279–1281. doi: 10.1161/STROKEAHA.118.020163. [DOI] [PubMed] [Google Scholar]
- 96.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, Lemieux M, Da Mesquita S, Cugurra A, Fitzpatrick J, Sviben S, Kossina R, Bayguinov P, Townsend RR, Zhang Q, Erdmann-Gilmore P, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184:1000–1016.e1027. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simka M, Latacz P, Czaja J. Possible role of glymphatic system of the brain in the pathogenesis of high-altitude cerebral edema. High Alt Med Biol. 2018;19:394–397. doi: 10.1089/ham.2018.0066. [DOI] [PubMed] [Google Scholar]
- 99.Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689–694. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, Oliver G. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 2014;28:2175–2187. doi: 10.1101/gad.216226.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2018;84:316–324. doi: 10.1016/j.neubiorev.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 102.Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY. Lymphatic drainage system of the brain:A novel target for intervention of neurological diseases. Prog Neurobiol. 2018;163-164:118–143. doi: 10.1016/j.pneurobio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valenza M, Facchinetti R, Steardo L, Scuderi C. Altered waste disposal system in aging and Alzheimer's disease:focus on astrocytic aquaporin-4. Front Pharmacol. 2019;10:1656. doi: 10.3389/fphar.2019.01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Zwam M, Huizinga R, Heijmans N, van Meurs M, Wierenga-Wolf AF, Melief MJ, Hintzen RQ, t Hart BA, Amor S, Boven LA, Laman JD. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol. 2009;217:543–551. doi: 10.1002/path.2476. [DOI] [PubMed] [Google Scholar]
- 106.Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019a;29:176–192. doi: 10.1111/bpa.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Exp Clin Cancer Res. 2019b;38:87. doi: 10.1186/s13046-019-1085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, Förstera B, Peng S, Shi M, Ladrón-de-Guevara A, Delle C, Sigurdsson B, Xavier ALR, Ertürk A, Libby RT, Chen L, Thrane AS, Nedergaard M. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12:eaaw3210. doi: 10.1126/scitranslmed.aaw3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, Tian H, Liu H, Liang D, Qin C, Zhu Q, Meng L, Fu Y, Xu S, Zhai Y, Ding X, Wang X. Impaired meningeal lymphatic flow in NMOSD patients with acute attack. Front Immunol. 2021;12:692051. doi: 10.3389/fimmu.2021.692051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei F, Song J, Zhang C, Lin J, Xue R, Shan LD, Gong S, Zhang GX, Qin ZH, Xu GY, Wang LH. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology. 2019;236:1367–1384. doi: 10.1007/s00213-018-5147-6. [DOI] [PubMed] [Google Scholar]
- 111.Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018;135:363–385. doi: 10.1007/s00401-018-1809-z. [DOI] [PubMed] [Google Scholar]
- 112.Wen YR, Yang JH, Wang X, Yao ZB. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer's disease. Neural Regen Res. 2018;13:709–716. doi: 10.4103/1673-5374.230299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 114.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wojciechowski S, Virenque A, Vihma M, Galbardi B, Rooney EJ, Keuters MH, Antila S, Koistinaho J, Noe FM. Developmental dysfunction of the central nervous system lymphatics modulates the adaptive neuro-immune response in the perilesional cortex in a mouse model of traumatic brain injury. Front Immunol. 2020;11:559810. doi: 10.3389/fimmu.2020.559810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y, Zhong L, Geng J. Neuromyelitis optica spectrum disorder:Pathogenesis, treatment, and experimental models. Mult Scler Relat Disord. 2019;27:412–418. doi: 10.1016/j.msard.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβaccumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue Y, Liu X, Koundal S, Constantinou S, Dai F, Santambrogio L, Lee H, Benveniste H. In vivo T1 mapping for quantifying glymphatic system transport and cervical lymph node drainage. Sci Rep. 2020;10:14592. doi: 10.1038/s41598-020-71582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M, Plautz EJ, Dellinger MT, Stowe AM. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. 2020;40:263–275. doi: 10.1177/0271678X18822921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zeppenfeld DM, Simon M, Haswell JD, D'Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, Iliff JJ. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74:91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 122.Zhang E, Wan X, Yang L, Wang D, Chen Z, Chen Y, Liu M, Zhang G, Wu J, Han H, Fan Z. Omega-3 polyunsaturated fatty acids alleviate traumatic brain injury by regulating the glymphatic pathway in mice. Front Neurol. 2020;11:707. doi: 10.3389/fneur.2020.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y, Cai J, Zhang W, Gong X, Yan S, Zhang K, Luo Z, Sun J, Jiang Q, Lou M. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol. 2020;87:357–369. doi: 10.1002/ana.25670. [DOI] [PubMed] [Google Scholar]
- 124.Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R, Xiao M, Hu G. Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7. doi: 10.1186/s40035-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


