Abstract
The Staphylococcus aureus genome encodes three ferric uptake regulator (Fur) homologues: Fur, PerR, and Zur. To determine the exact role of PerR, we inactivated the gene by allelic replacement using a kanamycin cassette, creating strain MJH001 (perR). PerR was found to control transcription of the genes encoding the oxidative stress resistance proteins catalase (KatA), alkyl hydroperoxide reductase (AhpCF), bacterioferritin comigratory protein (Bcp), and thioredoxin reductase (TrxB). Furthermore, PerR regulates transcription of the genes encoding the iron storage proteins ferritin (Ftn) and the ferritin-like Dps homologue, MrgA. Transcription of perR was autoregulated, and PerR repressed transcription of the iron homeostasis regulator Fur, which is a positive regulator of catalase expression. PerR functions as a manganese-dependent, transcriptional repressor of the identified regulon. Elevated iron concentrations produced induction of the PerR regulon. PerR may act as a peroxide sensor, since addition of external hydrogen peroxide to 8325-4 (wild type) resulted in increased transcription of most of the PerR regulon, except for fur and perR itself. The PerR-regulated katA gene encodes the sole catalase of S. aureus, which is an important starvation survival determinant but is surprisingly not required for pathogenicity in a murine skin abscess model of infection. In contrast, PerR is not necessary for starvation survival but is required for full virulence (P < 0.005) in this model of infection. PerR of S. aureus may act as a redox sentinel protein during infection, analogous to the in vitro activities of OxyR and PerR of Escherichia coli and Bacillus subtilis, respectively. However, it differs in its response to the metal balance within the cell and has the added capability of regulating iron uptake and storage.
The relationship between invading pathogenic bacteria and their host is dynamic, with bacteria having to rapidly adapt to the hostile and changing environment which they have entered. Metal ion acquisition is essential for pathogen proliferation, and limitation is a nonspecific host response to infection (10), which reduces the ability of bacteria to replicate and increases their susceptibility to clearance by the immune system. Iron is an essential nutrient in vivo and together with manganese is an important cofactor for bacterial antioxidant defense enzymes, e.g., catalase, peroxidase, and superoxide dismutase (SOD) (1, 48). Consequently, bacteria have evolved specialized proteins that monitor metal ion levels and respond accordingly by regulating gene expression (31, 51). These metalloregulatory proteins cluster in four distinct families represented by Fur (ferric uptake regulator), DtxR (diphtheria toxin repressor), MerR, and ArsR (53). The well-characterized DtxR from Corynebacterium diphtheriae (61) and Fur (26) have similar roles with respect to iron homeostasis and toxin synthesis; however, these two proteins share little amino acid homology, and their consensus DNA binding sequences are different.
Four metal ion-dependent repressors have been identified in Bacillus subtilis: three Fur-like proteins, Fur, PerR, and Zur (13, 28), and the recently identified DtxR-like protein, MntR (53). Fur controls iron homeostasis via a regulon of iron transporters, iron siderophore transporters, and siderophore biosynthesis proteins. Zinc homeostasis is maintained by Zur-mediated repression of two operons encoding zinc uptake transporters. MntR is a bifunctional, manganese-responsive regulator of two manganese transporters, MntABCD and MntH, which are both selectively repressed by high levels of Mn(II) while MntR functions as a positive regulator of the mntABCD operon under low-Mn(II) growth conditions (53). MntH belongs to the Nramp family of proteins and has been proposed previously to have a role in the pathogenesis of Salmonella enterica serovar Typhimurium (42).
Genetic evidence revealed that B. subtilis PerR is a manganese- and iron-responsive transcriptional repressor of the genes encoding a catalase, alkyl hydroperoxide reductase (AhpCF); a Dps homologue (MrgA); and heme biosynthesis enzymes (13, 17, 18). Furthermore, PerR was hydrogen peroxide responsive, a property that makes it functionally analogous to OxyR, the well-characterized peroxide stress regulator in Escherichia coli. It was proposed elsewhere that peroxide stress activation of B. subtilis PerR was linked to the bound metal ion, a feature that would separate it mechanistically from OxyR, which is activated by H2O2-catalyzed disulfide bond formation and is not metalliferous (13, 69). A homologue of PerR in the gram-negative pathogen Campylobacter jejuni was similarly shown to repress catalase and AhpC in response to iron; however, regulation by manganese was not investigated (62). The Streptococcus pyogenes PerR was shown to be required for an inducible peroxide stress response (43). Intriguingly, this inducible response did not involve AhpC or MrgA, and it was suggested that a novel mechanism of peroxide stress management may exist in S. pyogenes (43)
Staphylococcus aureus successfully colonizes humans and commonly forms part of the flora of the anterior nares and the skin. Its versatility makes it an important pathogen that can infect a diversity of tissues causing a wide spectrum of diseases (24). Many staphylococcal virulence determinants are known and include adhesins, hemolysins, proteases, and superantigenic toxins that are mainly controlled by the interdependent, global regulators Agr and SarA (16, 20, 44). Intracellular survival in neutrophils (30), endothelial cells (63), epithelial cells (8), and osteoblasts (38) has been described previously, suggesting that both intracellular survival and extracellular multiplication play important roles in the pathogenesis of S. aureus infections. The determinants that promote in vivo survival intracellularly are poorly defined.
S. aureus has homologues of the four main metal ion-dependent repressors, Fur, PerR, Zur, and MntR. S. aureus Fur has been shown to be an iron-dependent repressor in vitro (68). Recently, we have shown that Fur represses the transcription of a number of iron uptake transporters and positively regulates, either directly or indirectly, catalase expression (37). Inactivation of fur was shown to impact on virulence, although this may simply be due to the fact that under some conditions growth rate was affected by the loss of Fur (37). S. aureus Zur mediates zinc homeostasis but is not important for virulence, and the functions of PerR and MntR have yet to be determined. The Staphylococcus epidermidis MntR homologue SirR was identified in vitro as an iron-regulated DtxR-like protein (34).
The importance of in vivo expression of the oxidative stress enzymes catalase and SOD has been suggested through the analysis of clinical isolates with reduced levels of expression of these enzymes (40, 46). In contrast, in vivo expression of one of the two SODs, SodA, was shown not to be important for pathogenicity despite contributing to survival in vitro (21).
In this report, we demonstrate that PerR controls a regulon of oxidative stress resistance and iron storage proteins in S. aureus. Furthermore, we demonstrate that PerR-dependent control of this regulon is important for pathogenicity.
MATERIALS AND METHODS
Media and growth conditions.
S. aureus, and E. coli strains and plasmids are listed in Table 1. E. coli was grown in Luria-Bertani (LB) medium at 37°C. S. aureus was grown at 37°C with shaking at 250 rpm in brain heart infusion (BHI) broth (Oxoid), in chemically defined medium (CDM) (64), or in chemically defined metal-limitation medium (CL) (37). CLR medium consists of CL (which contains 400 μM magnesium sulfate) without glucose and replete with the following metals added at an 0.2 μM final concentration: calcium chloride, copper sulfate, ferrous sulfate, manganese chloride, nickel sulfate, molybdenum sulfate, and zinc sulfate. Colonies from non-Chelex-treated CL agar plates were used to inoculate a CLR preculture. Experimental 25-ml cultures in acid-washed, 250-ml flasks were inoculated at a starting optical density at 600 nm (OD600) of 0.002 prior to growth at 37°C. When included, antibiotics were added at the indicated concentrations: ampicillin, 100 mg liter−1; kanamycin, 50 mg liter−1; neomycin, 50 mg liter−1; and erythromycin and lincomycin, 5 and 25 mg liter−1, respectively.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype, description, or sequencea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | φ80 Δ(lacZ)M15 Δ(argF-lac)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | 56 |
| S. aureus | ||
| 8325-4 | Wild-type strain cured of prophages | Lab stock |
| RN4220 | Restriction-deficient transformation recipient | Lab stock |
| MJH001 | perR::kan | This study |
| MJH002 | ahpC::pAZ106 ahpC+ | This study |
| MJH003 | bcp::pAZ106 bcp+ | This study |
| MJH004 | ftn::pAZ106 ftn+ | This study |
| MJH005 | fur::pAZ106 fur+ | This study |
| MJH006 | katA::pAZ106 katA+ | This study |
| MJH007 | mrgA::pAZ106 mrgA+ | This study |
| MJH008 | perR::pAZ106 perR+ | This study |
| MJH009 | trxB::pAZ106 trxB+ | This study |
| ST16 | katA::Tn917-LTV1 | This study |
| SPW1 | sodA::Tn917-LTV1 | 65 |
| MJH102 | perR::kan ahpC::pAZ106 ahpC+ | This study |
| MJH103 | perR::kan bcp::pAZ106 bcp+ | This study |
| MJH104 | perR::kan ftn::pAZ106 ftn+ | This study |
| MJH105 | perR::kan fur::pAZ106 fur+ | This study |
| MJH106 | perR::kan katA::pAZ106 katA+ | This study |
| MJH107 | perR::kan mrgA::pAZ106 mrgA+ | This study |
| MJH108 | perR::kan perR::pAZ106 | This study |
| MJH109 | perR::kan trxB::pAZ106 trxB+ | This study |
| MJH408 | perR::kan pMAL34 (perR+) | This study |
| MJH418 | 8325-4 pMAL34 (perR+) | This study |
| Plasmids | ||
| pAZ106 | Promoterless lacZ erm insertion vector | 16 |
| pGem3Zf(+) | General cloning vector | Promega |
| pAUL-A | Temperature-sensitive erm integrational shuttle vector | 15 |
| pCU1 | E. coli-S. aureus cat shuttle vector | 7 |
| pMAL5 | pGem3Zf(+) containing a 2-kb perR fragment with engineered ClaI site | This study |
| pMAL7 | pMAL5 containing a kan cassette in ClaI site. | This study |
| pMAL8 | 3.5-kb BamHI-HindIII perR::kan fragment from pMAL7 in pAUL-A | This study |
| pMAL9 | 1.08-kb OL7–OL11 perR promoter-containing fragment in pAZ106 | This study |
| pMAL10 | 0.6-kb OL13–OL14 fur promoter-contaiing fragment in pAZ106 | This study |
| pMAL11 | 1.2-kb OL15–OL16 katA promoter-containing fragment in pAZ106 | This study |
| pMAL12 | 1.2-kb OL17–OL18 ahpCF promoter-containing fragment in pAZ106 | This study |
| pMAL13 | 1.1-kb OL19–OL20 mrgA promoter-containing fragment in pAZ106 | This study |
| pMAL28 | 0.6-kb OL53–OL54 bcp promoter-containing fragment in pAZ106 | This study |
| pMAL29 | 1.4-kb OL60–OL61 ftn promoter-containing fragment in pAZ106 | This study |
| pMAL30 | 1.4-kb OL62–OL63 trxB promoter-containing fragment in pAZ106 | This study |
| pMAL34 | 0.9-kb OL82–OL83 perR gene-containing fragment in pCU1 | This study |
| Primers | ||
| OL7 | AATTGGATCCCATGGTTTGCAACGGGTG | This study |
| OL8 | CCGGAAGCTTATCCTGAGCCAGGATCAAACTCTCCAT | This study |
| OL9 | GAATCAATTGCATCGATTGCGACAAGCAGGCG | This study |
| OL10 | CGCCTCCTTGTCGCAATCGATGCAATTGATTC | This study |
| OL11 | CCAGAATTCGAATCGACTTGATGAGTCTCCATATG | This study |
| OL12 | AATTGGATCCTGTAATGGTTTGCCACTTTGCAG | This study |
| OL13 | CCAGAATTCAGTAGCTTCGCGTTGTGGCGTTAGC | This study |
| OL14 | AATTGGATCCATTACCAAACGGTGAAACGTC | This study |
| OL15 | CCAGAATTCAACGCAGCTTGTTCAACATCC | This study |
| OL16 | AATTGGATCCGACCACAATGCCCAATACAACC | This study |
| OL17 | CCAGAATTCTGAATGTACCACGTTGAGCTAAC | This study |
| OL18 | AATTGGATCCATGGTAAGCGTGGCTTGGCTGC | This study |
| OL19 | CCAGAATTCTACATCATCGCCAGCATTACC | This study |
| OL20 | AATTGGATCCATGGCAATTTCGTGTCGCGAGG | This study |
| OL53 | GGTTGGATCCAATTTCTAATTCAGTCGGTGTACC | This study |
| OL54 | CCAGAATTCTAAATTGTCTCTAAAGTCACAAGC | This study |
| OL60 | AGAAGGATCCGCGTTATAAGCGTTAAAGTCAC | This study |
| OL61 | AGCAGAATTCTGCATGTGCACCTCTGTCG | This study |
| OL62 | AGAAGGATCCAGAACTGATTACGATTGGTAG | This study |
| OL63 | AGCAGAATTCCTGCCAAATACTTTCCGTCATC | This study |
| OL82 | AGAGGATCCACAGCGCATATAACTGGTAATG | This study |
| OL83 | CCAGAATTCCTTATACTCACTTTATGGATAG | This study |
| PEX2 | AGCTCCATGGTCTGACGCTCAGTGGAACGAAAACTC | This study |
| PEX3 | CAGTAGCTTCGCGTTGTGGCGTTAGC | This study |
| PEX4 | GGTGTAATTCTTACGCCTGCTTGTCGC | This study |
| PEX5 | TCTCGGTCTGATACTGGATGCC | This study |
| PEX6 | TTACAACATCTTGTTGATTACTC | This study |
| Tn1 | GGAACGCCGTCTACTTACAAGCAGC | This study |
| Tn2 | CTCACAATAGAGAGATGTCACCGTC | This study |
In primer sequences, the restriction sites are underlined.
Construction of strains.
Derivatives of plasmid pAZ106, an integrating plasmid conferring resistance to erythromycin and containing a promoterless lacZ gene (16), or plasmid pAUL-A, a temperature-sensitive integrating plasmid conferring resistance to erythromycin (15), were constructed using PCR with Pwo polymerase (Roche) and standard cloning techniques (56). A plasmid for disrupting perR was constructed by PCR amplification of a 2-kb perR fragment using primers OL7, OL8, OL9, and OL10 (Table 1) to insert an internal ClaI site by the site-directed mutagenesis method of Higuchi (33). This fragment was cloned into pAUL-A, creating pMAL5, and then a 1.5-kb ClaI fragment containing a kanamycin cassette from pDG782 (32) was inserted to produce pMAL7.
Transcriptional reporter fusions to the perR, fur, katA, ahpC, mrgA, bcp, ftn, and trxB genes were made by PCR amplification of suitable DNA fragments using primers detailed in Table 1 followed by cloning into pAZ106, creating the plasmids pMAL9, pMAL10, pMAL11, pMAL12, pMAL13, pMAL28, pMAL29, and pMAL30, respectively. Typically, between 0.6 and 1.4 kb of DNA encompassing the start and promoter regions was amplified using Pwo polymerase. The purified DNA fragments were digested with BamHI and EcoRI and cloned into plasmid pAZ106 digested with the same enzymes. The perR gene, amplified using primers OL82 and OL83, was cloned into the E. coli-S. aureus shuttle vector pCU1 (7), producing plasmid pMAL34.
All of the above suicide plasmids were integrated into the chromosome of electrocompetent S. aureus RN4220 (58) through homology with the parental copies by a Campbell-type event. These were then transduced into 8325-4 using phage φ11 (50), and individual clones were verified by Southern blotting or genomic DNA sequencing to confirm the structural integrity of the DNA at the integration site.
Random Tn917 insertions into the chromosome of S. aureus 8325-4 were created as described in the work of Watson et al. (65). Screening of transposants for a starvation survival defect was performed on CDM agar as described in the work of Watson et al. (65) with limiting, 0.1% (wt/vol) concentrations of glucose. Comparative starvation survival experiments were performed in glucose-limiting CDM with shaking (250 rpm) at 37°C.
Genomic DNA sequencing.
DNA was isolated from lysed cells of overnight cultures of S. aureus using lysostaphin (100 μg ml−1) and Qiagen genomic DNA columns. Three micrograms of genomic DNA was sequenced from both ends of the transposon insertion using 16 μl of Big-Dye (ABI) premix in a 40-μl volume with 13 pmol of primer Tn1 or Tn2 (Table 1) in a 90-cycle PCR sequencing program (95°C, 30 s; 50°C, 20 s; 60°C, 4 min). PCR sequencing products were purified using DyeEx columns (Qiagen).
β-Galactosidase assays.
Levels of β-galactosidase activity were measured as described previously (37). Briefly, 0.1-ml samples were harvested, and cell pellets were stored at −20°C. Thawed pellets were resuspended in 0.5 ml of ABT buffer (60 mM K2HPO4, 40 mM KH2PO4, 100 mM NaCl). The assay was started with the addition of 50 μl of freshly prepared MUG (4-methylumbelliferyl-β-d-galactoside) (10 mg ml−1), and the assay mixture was incubated at 25°C for 60 min. The assay was stopped with the addition of 0.5 ml of 0.4 M Na2CO3. The stopped assay mixture was then serially diluted in a 50:50 (vol/vol) mixture of ABT and Na2CO3 in 96-well microtiter plates (Nunc). Fluorescence was measured using a Victor plate reader (Wallac) with a 0.1-s count time and calibrated with standard concentrations of MU (4-methylumbelliferone). One unit of β-galactosidase activity was defined as the amount of enzyme that catalyzed the production of 1 pmol of MU min−1 OD600 U−1. Assays were performed on duplicate samples, and the values were averaged. The results presented here were representative of three independent experiments that showed less than 20% variability.
Catalase assay activity gels, H2O2 challenge, and induction.
Catalase activity was detected after electrophoresis on a 10% (wt/vol) native polyacrylamide gel, pH 7.5 (45), of washed cells lysed with lysostaphin (100 μg ml−1). The double-staining method of Wayne and Diaz (66) was used to visualize bands of activity. Catalase activity was assayed spectrophotometrically at 240 nm as described by Beers and Sizer (9) using 50 mM potassium phosphate buffer (pH 7.0) with 19.6 mM hydrogen peroxide. Protein concentration was measured by the method of Bradford (12) using bovine serum albumin (fraction V; Sigma) as the standard. Hydrogen peroxide resistance was assayed as described in the work of Watson et al. (64) with the following modifications. Cells grown to exponential phase in CLR were washed and diluted in phosphate-buffered saline (PBS) to an OD600 of 0.2 and, following challenge with 7.5 mM H2O2, were diluted in PBS containing 10 mg of catalase ml−1 and then serially diluted in PBS. Viability was assessed by overnight growth on BHI agar. Hydrogen peroxide induction of gene expression using lacZ fusions was performed on cultures grown in CLR or Chelex-treated BHI medium to OD600s of 0.2, 2, and 8 by adding H2O2 to a final concentration of 100 or 500 μM with shaking (250 rpm) at 37°C. Samples were removed for assay before and 60 min after the addition of H2O2 and assayed for β-galactosidase.
Cellular protein preparation.
The soluble cellular protein fraction was prepared by pelleting cells and resuspending them in 5 μl per 0.1 OD600 U of 1 M Tris-HCl, pH 6.8, containing 20 μg of lysostaphin (Sigma). After 10 min of incubation at 37°C, an equal volume of Laemmli sample buffer was added before boiling for 10 min. The samples were centrifuged for 5 min before loading on a 10% (wt/vol) polyacrylamide gel. Samples were blotted and N-terminally sequenced as described previously (16).
RNA isolation and transcriptional start site mapping.
Total RNA was isolated from exponential cultures of S. aureus (OD600 = 1.0) using the rapid liquid nitrogen chill method of Arnau et al. (6). Frozen cell pellets, briefly stored at −70°C, were thawed, and RNA was rapidly extracted by cell disruption using the Fast-Prep blue kit (Bio 101) and a Fast-Prep system reciprocal shaker (Bio 101). Primer extension reactions were performed for perR, katA, mrgA, ahpC, and fur as described in the work of Horsburgh and Moir (36) using 100 μg of RNA and 10 pmol of the corresponding primer (PEX2, PEX3, PEX4, PEX5, and PEX6, respectively). The PCR products used for cloning into pAZ106 were purified using a Qiagen PCR kit and then sequenced with a Sequenase 2.0 PCR kit (Amersham) using the same oligonucleotide used for the primer extension reaction.
Virulence testing of strains in a murine skin abscess model.
Virulence of S. aureus strains was tested in an established murine abscess model of infection (16). Briefly, cells were grown to stationary phase in BHI (time, 15 h), then harvested by centrifugation, and washed twice in PBS. The cell numbers were adjusted to 5 × 108 CFU ml−1, and then 200 μl of cell suspension was injected subcutaneously in female 6- to 8-week-old BALB/c mice. The precise inoculum was confirmed by serial dilution and counting on BHI agar. After 7 days, the mice were euthanatized with CO2, and skin lesions were aseptically removed and stored frozen in liquid nitrogen. The lesions were weighed, chopped, and homogenized in a miniblender in 2.5 ml of ice-cold PBS. After 1 h of incubation on ice, the lesions were homogenized again before serial dilution of the suspension, and the total number of bacteria was determined by growth on BHI agar. Statistical significance was evaluated on the percent recovery of strains using Student's t test with a 5% confidence limit.
RESULTS
Identification of perR and katA in S. aureus
The strain ST16 was isolated during a screen for S. aureus Tn917 transposants unable to survive glucose starvation after prolonged incubation on CDM agar plates. Genomic DNA sequencing in both directions from the transposon insertion of ST16 revealed inactivation of the katA gene encoding catalase. The 1,518-bp gene is monocistronic and encodes a protein of 505 amino acids with a molecular mass of 58.3 kDa (57). Upstream of the catalase gene is an imperfect palindrome of 18 bp, suggestive of transcriptional regulation by a member of the Fur family. A homologue of the B. subtilis perR gene, which controls peroxide stress resistance, was identified in the incomplete S. aureus 8325 genomic database (http://www.genome.ou.edu). The 441-bp perR gene is monocistronic and encodes a protein with a predicted molecular mass of 17.2 kDa. To characterize the role of PerR in S. aureus, we introduced a kanamycin resistance cassette into the perR gene using allelic replacement, thereby disrupting the chromosomal copy and creating strain MJH001.
Peroxide resistance of MJH001 (perR) and ST16 (katA).
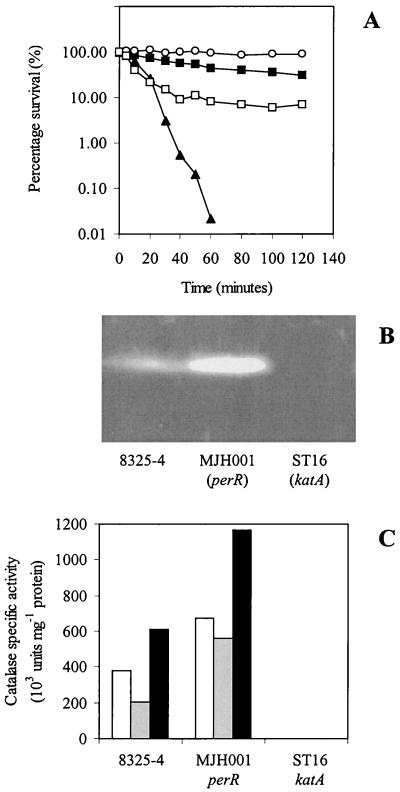
To investigate the importance of PerR and catalase in stress resistance of S. aureus, we examined the effect of adding hydrogen peroxide. MJH001 (perR) and ST16 (katA) mutants contrasted strikingly with 8325-4 (wild type). ST16 (katA) was hypersensitive to hydrogen peroxide, while MJH001 (perR) was more resistant than 8325-4 (wild type) (Fig. 1A). The katA gene encodes the sole catalase (Fig. 1B) of S. aureus, and the observed sensitivity was a consequence of a complete loss of all catalase activity as shown by enzyme assay (Fig. 1C). Database searching also failed to reveal any further catalase homologues in S. aureus. MJH001 (perR) was found to have increased levels of catalase activity (Fig. 1), as was reported elsewhere for B. subtilis (13) and C. jejuni (62) perR mutants. The addition of 20 μM manganese to the growth medium reduced the amount of catalase present in 8325-4 (wild type) and decreased resistance to hydrogen peroxide (Fig. 1A) but did not reduce the amount of catalase or resistance in MJH001 (perR). Addition of 20 μM iron produced the reverse effect, increasing catalase expression in both 8325-4 (wild type) and MJH001 (perR). This contrasts with both B. subtilis and C. jejuni, where PerR functions as both a manganese- and an iron-responsive repressor (13, 62). The increased level of katA expression when MJH001 (perR) was grown with 20 μM iron sulfate added is due to a second regulator of catalase that was identified as Fur (37).
FIG. 1.
(A) Effect of H2O2 (7.5 mM) on washed, exponential-phase CLR-grown cells of 8325-4 (wild type) (▪), MJH001 (perR) (○), and ST16 (katA) (▴) and 8325-4 (wild type) grown in CLR with 20 μM manganese chloride (□). (B) Catalase activity gel. (C) Total catalase activity of washed, lysed, stationary-phase cells after 24 h of growth in CLR medium (open bars), CLR with 20 μM manganese chloride (gray bars), or CLR with 20 μM iron sulfate (black bars).
The role of perR and katA in the growth of S. aureus.
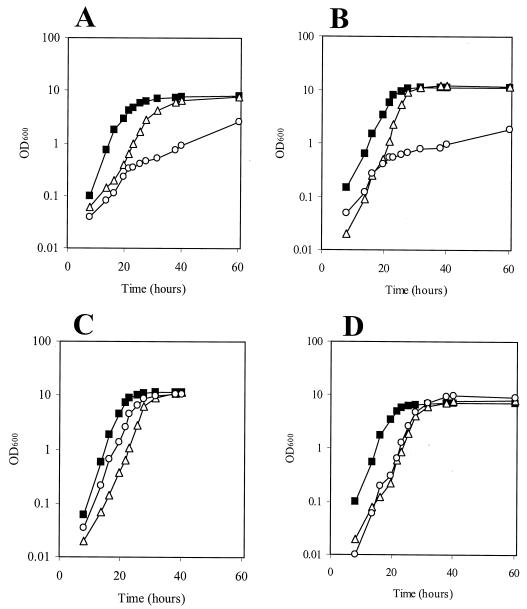
The importance of PerR and catalase for growth in vitro and their effect on the sensitivity of the strains to different metal ions were evaluated by growth in the metal-depleted medium CL. MJH001 (perR) had an increased doubling time compared to that of 8325-4 (wild type) during exponential growth in CL medium (262 and 144 min, respectively) (Fig. 2A). This difference in growth rate was eliminated (doubling time, 133 and 150 min, respectively) by adding increased (20 μM) iron sulfate (Fig. 2B) to the cultures. ST16 (katA) showed a wild-type growth rate in CLR and yet had marked growth defects in CL, where all metal ions (except magnesium) were absent from the culture (Fig. 2A), and in CL with 20 μM iron sulfate (Fig. 2B). Growth in the metal-replete medium CLR produced a wild-type growth rate for ST16 (katA) (Fig. 2C). Further experiments showed that manganese alone was capable of restoring a wild-type growth rate to ST16 (katA) (Fig. 2D). Complementation of the mutation in MJH001 (perR) with the perR gene alone restored catalase activity and manganese-dependent repression to levels similar to those of the wild type (8325-4). This was demonstrated by assaying strains MJH408 (perR::kan [pMAL34 (perR+)]) and MJH418 (8325-4 [pMAL34 (perR+)]) during growth in CLR or CLR containing 20 μM manganese chloride (data not shown).
FIG. 2.
Growth of 8325-4 (wild-type) (▪), MJH001 (perR) (▵,) and ST16 (katA) (○) strains in CL with no added metal ions except magnesium (A), CL with 20 μM iron sulfate (B), CLR (C), and CL with 20 μM manganese chloride (D). All cultures were inoculated at an OD600 of 0.002.
Identification of PerR-regulated genes in S. aureus.
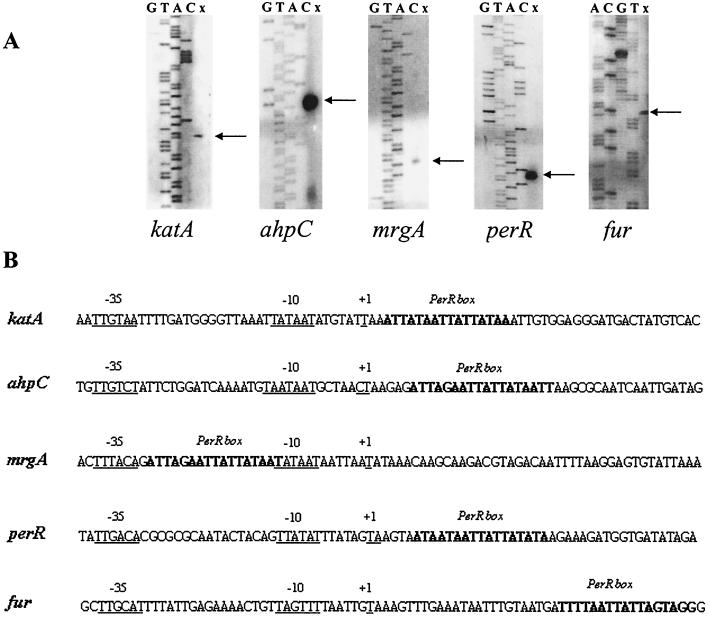
Putative PerR binding sites were identified in the S. aureus incomplete genomic databases based on homology with the putative site upstream of katA (Fig. 3). A number of candidate sites were found upstream of the coding sequences of genes whose protein products were likely to have a role in oxidative stress or metal ion storage (Fig. 3). The fur, ftn, perR, trxB, and bcp genes have not been identified as members of a PerR regulon previously. To determine whether these candidate PerR binding sites were located in the promoter region of the genes, we determined the transcriptional start point for the perR, fur, ahpC, katA, and mrgA genes (Fig. 4). In each case, the PerR binding site was located in the promoter region close to the −35 and −10 elements. The PerR box in the mrgA gene was situated between the −35 and −10 elements in a position consistent with the high level of manganese-dependent transcriptional repression observed in 8325-4 (wild type) compared to that in MJH001 (perR) (Fig. 5).
FIG. 3.
Alignment of the putative PerR boxes identified in the incomplete S. aureus genome databases. Boxes were identified as described previously (37). An S. aureus consensus sequence was compiled from all of the sequences identified and compared to the B. subtilis consensus (19).
FIG. 4.
(A) Mapping of the 5′ end of the perR, katA, mrgA, ahpC, and fur mRNA by primer extension. RNA was isolated from exponential cultures of 8325-4 (wild type) grown in CLR (OD600 of 1.0), and 100 μg of RNA was used for each reaction. The potential transcription start sites are marked with arrows. Lanes G, T, A, and C show the dideoxy sequencing ladder obtained with the same primer used for primer extension (x). (B) Sequence of the identified promoter regions. Potential −35 and −10 regions and transcriptional start sites are underlined. PerR boxes are in boldface.
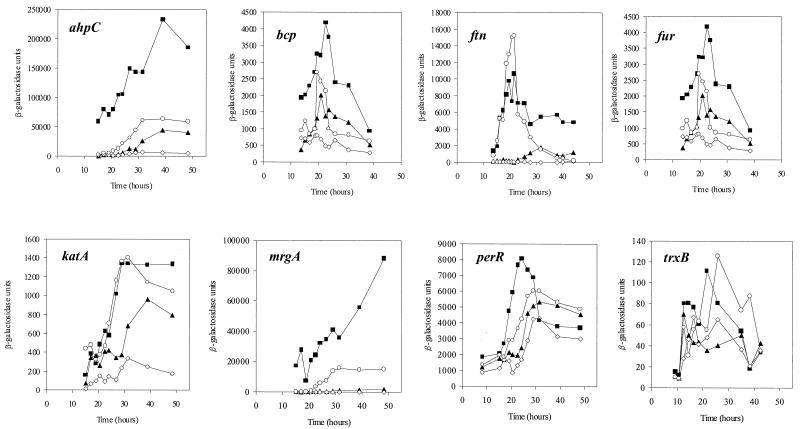
FIG. 5.
Analysis of transcription from promoter-lacZ fusions during growth in CLR medium. 8325-4 (wild type) containing the fusion indicated was grown in CLR medium (▴), CLR with 20 μM manganese (⋄), and CLR with 20 μM iron sulfate (○). Transcription of the lacZ fusions in MJH001 (perR) (▪) is shown on each graph. Samples were removed at the times indicated and sampled for β-galactosidase activity. The strains grew with slightly different growth rates as indicated in Fig. 2, but for reasons of clarity only the changes in β-galactosidase activity are shown. The lacZ fusions shown are MJH002 (ahpC-lacZ), MJH003 (bcp-lacZ), MJH004 (ftn-lacZ), MJH005 (fur-lacZ), MJH006 (katA-lacZ), MJH007 (mrgA-lacZ), MJH008 (perR-lacZ), and MJH009 (trxB-lacZ), and these same fusions in the perR mutant background are MJH102, MJH103, MJH104, MJH105, MJH106, MJH107, MJH108, and MJH109, respectively.
The effect of PerR on transcription of candidate genes.
To determine whether PerR regulated the expression of the identified genes, lacZ fusions were made to each of the eight genes listed in Fig. 3 to monitor transcription from their promoters. All of the identified genes displayed PerR-dependent regulation (Fig. 5) as evinced by significantly increased transcriptional activity from the promoters in MJH001 (perR) compared to that for 8325-4 (wild type). Furthermore, elevated levels of manganese repressed transcription of the genes, with the exception of trxB, in 8325-4 (wild type) (Fig. 5), but not in MJH001 (perR) (data not shown). This is consistent with the known manganese-responsive transcriptional repression activity previously reported for PerR from B. subtilis (18). The addition of calcium, copper, cobalt, nickel, molybdenum, or zinc to CLR had no significant effect on transcription of katA or mrgA (data not shown). Transcription of all of the PerR-regulated genes was found to be consistently greater in CLR with 20 μM iron sulfate added than in CLR medium alone (Fig. 5). Titration of the iron-dependent induction showed concentrations above 2 μM to be effective (data not shown). In the case of katA and ftn, iron-dependent induction was found to be as great as the level of transcription resulting from perR inactivation (Fig. 5). Transcription of katA is positively regulated, either directly or indirectly, by Fur in an iron-dependent manner (37).
SOD activity in S. aureus was not affected by perR inactivation (data not shown). Interestingly, when catalase levels were assayed in a sodA mutant (SPW1) of S. aureus, we found a higher specific activity (698 U mg−1) than that for 8325-4 (wild type) (379 U mg−1). Since this suggested that increased superoxide stress in the cell ultimately leads to activation of the PerR regulon, we tested the effect of paraquat on induction of mrgA. The addition of 10 μM paraquat to an early- or mid-log culture of MJH007 (mrgA-lacZ mrgA+) produced a threefold increase in β-galactosidase activity when assayed 60 min after paraquat addition (data not shown).
Induction of PerR-regulated genes with hydrogen peroxide.
PerR was originally described as a peroxide-responsive repressor and was shown to derepress transcription of the katA, ahpC, hemA, and mrgA genes of B. subtilis after the addition of 100 μM hydrogen peroxide (19). Peroxide induction of the S. aureus PerR regulon lacZ reporter fusions was tested in 8325-4 (wild type) by adding 100 or 500 μM hydrogen peroxide to early- and mid-exponential and early-stationary-phase cultures grown in CLR medium or Chelex-treated BHI. A 100 μM concentration of hydrogen peroxide was found to produce little induction compared to a 500 μM concentration, possibly due to the very high levels of catalase activity present in S. aureus. Using a concentration of 500 μM hydrogen, increased transcription was observed in early- and mid-log-phase cultures grown in CLR for ahpC (threefold), bcp (twofold), ftn (threefold), katA (twofold), mrgA (sixfold), and trxB (twofold) but not for fur or perR (data not shown). Induction was not observed in early-stationary-phase growth.
The effect of PerR and KatA on cellular protein profiles.
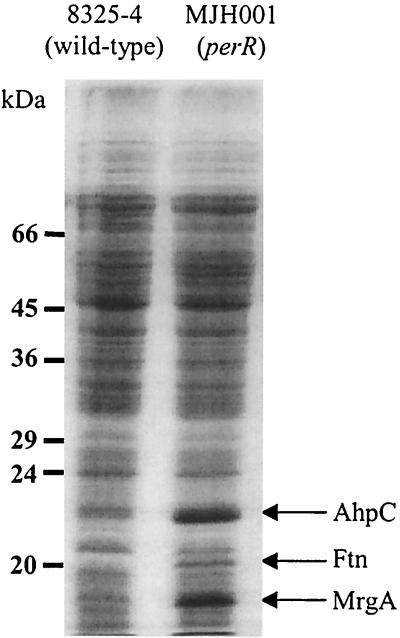
The soluble cellular protein fraction of the strains revealed that MJH001 (perR) overexpressed a number of proteins compared to 8325-4 (wild type) (Fig. 6). The addition of manganese repressed these proteins in 8325-4 (wild type), while iron induced their expression; neither metal affected expression of these proteins in MJH001 (perR) (data not shown). To confirm that the major proteins were members of the PerR regulon, they were N-terminally sequenced. The N-terminal sequence of the 17-kDa (SNQQDVVKEXNQQXANXTVA), 20-kDa (LSKNXLEAL), and 23-kDa (SLINKEILPF) proteins matched the predicted translation of the mrgA, ftn, and ahpC genes of S. aureus, respectively. AhpC was one of four major proteins from S. aureus induced by osmotic upshock after growth in 2.5 M NaCl (5). Apart from AhpC, the other proteins induced during osmotic upshock do not match the sizes of those overexpressed in MJH001 (perR), suggesting that this osmotic induction was not PerR mediated. ST16 (katA) was found to overexpress the same proteins as MJH001 (perR), albeit at a lower level (data not shown). Bsat et al. (14) and Antelmann et al. (3) have shown that inactivation of katA and ahpC in B. subtilis results in derepression of the PerR regulon. From this, it was inferred that PerR senses the accumulation of both hydrogen peroxide and organic hydroperoxide.
FIG. 6.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total proteins from wild-type and MJH001 (perR) strains after growth to stationary phase in CLR at 37°C. The AhpC, Ftn, and MrgA proteins identified by N-terminal sequencing are shown.
Analysis of stationary-phase exoprotein profiles showed no apparent difference between MJH001 (perR) and 8325-4 (wild type). This was confirmed for specific proteins using antisera raised against alpha-hemolysin (Hla) and staphylococcal serine protease A (SspA). In addition, the zones of hemolysis on rabbit blood agar were identical for MJH001 (perR) and 8325-4 (wild type) (data not shown).
The role of PerR and catalase in starvation survival.
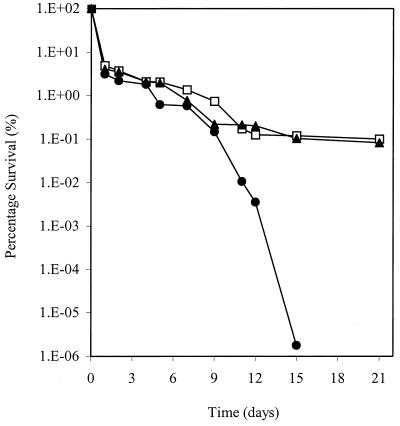
ST16 (katA) was identified in a carbon starvation screen due to its inability to survive extended incubation on glucose-limiting CDM. Catalase is induced postexponentially, and starved S. aureus cells were shown previously to be highly resistant to elevated hydrogen peroxide (64). The reduced viability of ST16 (katA) was demonstrated by prolonged aerobic incubation at 37°C (Fig. 7). In contrast, MJH001 (perR) retained a wild-type level of viability in this assay.
FIG. 7.
Starvation survival of 8325-4 (wild type) (□), MJH001 (perR) (▴), and ST16 (katA) (●) after growth and incubation in CDM containing 0.1% (wt/vol) glucose (glucose limiting). Samples were aseptically removed at the times indicated, and viability was assessed by dilution and counting on BHI agar.
The importance of PerR and catalase in vivo.
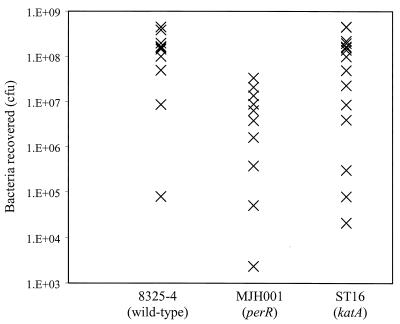
Catalase was previously proposed to be an important virulence determinant in S. aureus (40, 46) since catalase and SOD levels correlated with virulence in clinical isolates. The availability of single defined mutations in sodA (21) and now katA has enabled us to test this fully. While sodA encodes one of two SODs, making the importance of SOD activity in virulence harder to test, katA encodes the sole catalase of S. aureus. Together with MJH001 (perR) and 8325-4 (wild type), we tested the pathogenicity of ST16 (katA) in a murine subcutaneous skin abscess model of infection (Fig. 8). ST16 (katA) was not attenuated in this model (P = 0.124) in two independent experiments (n = 7 and n = 9). Conversely, MJH001 (perR) was significantly attenuated in this model of infection (P < 0.005) and produced smaller lesions (0.237 ± 0.143 g) compared to both 8325-4 (0.528 ± 0.173 g) and ST16 (0.582 ± 0.217 g).
FIG. 8.
Pathogenicity of S. aureus strains in a murine skin abscess model of infection. Approximately 108 CFU of each strain was inoculated subcutaneously into 6- to 8-week-old BALB/c mice; the strains used were 8325-4 (wild type) (n = 10), MJH001 (perR) (n = 10), and ST16 (katA) (n = 16). Seven days after infection, mice were euthanized, lesions were removed and homogenized, and viable bacteria were counted after dilution and growth on BHI agar plates. The mean percent recovery of each strain and the Student t test P values are as follows, respectively: 8325-4 (wild type), 143%; MJH001 (perR), 10.5% and P < 0.005; and ST16 (katA), 86.3% and P = 0.124.
DISCUSSION
S. aureus PerR controls a large regulon of oxidative stress components including catalase, alkyl hydroperoxide reductase, the thioredoxin-dependent thiol peroxidase (Bcp), and thioredoxin reductase. It also regulates the iron storage protein ferritin and the ferritin-like Dps homologue, MrgA. Furthermore, S. aureus PerR has an autoregulatory capacity and modulates expression of the iron homeostasis regulator Fur. In B. subtilis, PerR has so far been shown to have a more specific role as a controller of catalase, alkyl hydroperoxide reductase, MrgA, and heme biosynthesis enzymes.
MJH001 (perR) has a reduced growth rate in CLR medium that contains low levels of iron and manganese. Supplementing the medium with iron negated this defect, and we hypothesize that the observed high level of overexpression of ferritin and MrgA may sequester intracellular iron, effectively limiting free iron in the cell. MrgA in B. subtilis functions as a Dps-like protein. These proteins have the ability to bind DNA (17, 47) and sequester iron to protect the DNA from oxidative damage (29, 67), and B. subtilis MrgA is important for cellular defense against hydrogen peroxide (17).
The indirect control of iron homeostasis by PerR regulation of Fur and the direct control of iron storage proteins allow S. aureus to coordinate the intracellular availability of free iron with the level of antioxidant proteins present in the cell. The deleterious effect of hydroxyl radicals on growth resulting from hydrogen peroxide and Fe(II) participating in the Fenton reaction has been demonstrated clearly in S. aureus (54), and elevated iron levels have been shown to predispose S. aureus to killing by monocytes and macrophages but not polymorphonuclear granulocytes (35, 55). Regulation of fur in E. coli is mediated by a number of regulators including OxyR, the well-characterized, gram-negative bacterial functional analogue of PerR (60).
Expression of the PerR regulon is repressed by elevated manganese concentrations, and this is likely to be due to the antioxidant properties of manganese in vivo. Numerous studies have shown that several manganese complexes can catalyze the disproportionation of hydrogen peroxide (11, 59) while others can act as superoxide scavengers (27). Lactobacillus plantarum maintains high intracellular levels of manganese as a substitute for having enzymes that protect against reactive oxygen species (4). Que and Helmann (53) recently described the function of MntR of B. subtilis as a manganese homeostasis regulator controlling expression of two manganese uptake transporter systems, MntABC and MntH (Nramp); it has a similar role in S. aureus (S. J. Wharton, M. J. Horsburgh, and S. J. Foster, unpublished data). SirR from S. epidermidis is a homologue of MntR, although its role in metal ion homeostasis is not known (34). TroR was recently described as a DtxR-like manganese-dependent regulatory protein that regulates a manganese transport system in Treponema pallidum (52). A direct regulatory link between the PerR and MntR regulons has not been reported, although Kehres et al. (42) have reported that MntH in S. enterica serovar Typhimurium is induced by peroxide and has a putative OxyR binding site. We have shown that there is PerR-dependent control of Fur, the regulator of iron homeostasis in S. aureus (37). Should such a link between PerR and the MntR regulon exist, it would demonstrate a complex interdependence between antioxidant defense and the levels of iron and manganese in the cell. In S. aureus, the importance of PerR as a central regulator of antioxidant defenses and of iron storage proteins was shown by the reduced virulence of a perR mutant in a skin abscess model of infection. Measured levels of manganese in the human body vary from extremely low levels in the skin (0.05 μM) to 30-fold-higher levels in the blood (1.5 to 2.4 μM) and yet-higher levels in the central nervous system (160 to 180 μM) (41, 52). S. aureus will encounter varying concentrations of manganese depending on its location during infection, and these encompass the concentrations over which PerR is active, since titration experiments show that in vitro manganese-dependent PerR repression occurs at and above 1 to 2 μM concentrations (data not shown).
In B. subtilis, PerR was identified as a metal limitation and peroxide-sensing repressor of the genes encoding catalase, MrgA, AhpC, and heme biosynthesis enzymes (13, 17, 18, 19). The S. aureus PerR regulon was shown here to be metal regulated; however, not all of the genes were induced by hydrogen peroxide. Induction was observed only at early and mid-log phase and not the stationary phase of growth. The lack of induction of transcription for perR and fur suggests that some promoters may be noninducible with hydrogen peroxide, a feature that would indicate a complex response to peroxide stress for members of the PerR regulon. Transcription of the perR gene, for example, is autoregulated. The complex regulation of some of the PerR-dependent loci is highlighted by the effect of iron. In some cases, such as katA, the level of transcription after addition of iron was as high as that in a perR mutant background. We have previously demonstrated that katA is positively regulated by Fur in an iron-dependent manner (37).
The intracellular redox-regulating molecules thioredoxin, glutaredoxin, and protein disulfide isomerase maintain cellular redox status and catalyze the formation and reduction of disulfide bonds. PerR-dependent regulation of thioredoxin reductase (TrxB) and a thiol peroxidase (Bcp) further demonstrates the central role of S. aureus PerR in protection from reactive oxygen species. The thiol peroxidase (Bcp) recently characterized by Jeong et al. (39) was shown to reduce linoleic acid hydroperoxide and hydrogen peroxide with the use of thioredoxin as an in vivo immediate electron donor. We note that a second thiol peroxidase encoded in the S. aureus 8325 genome has a putative PerR box (AAGTATTATTTATTATTATTA) with a high level of identity to those in this study and is located in the likely promoter region of this gene. It would thus appear that S. aureus PerR, like OxyR of E. coli (2), has a regulatory role in transcription of genes for the thioredoxin pathway and thiol peroxidases. The gene encoding 3-phosphoglycerate dehydrogenase (pdh) is located in the same operon as bcp, such that it will be under PerR control. The reason for this is unclear but may be a metabolic mechanism for regenerating the reduced NAD that is consumed when a cell is subjected to oxidative stress.
The ability of S. aureus to survive intracellularly in granulocytes has been noted in several studies (25, 49, 70), and recently this was shown to contribute to infection (30). The intracellular location of the staphylococci was shown to be in macropinosome-like vacuoles similar to those seen with salmonellae in epithelial cells and macrophages. S. aureus has also been shown to escape the endosome of epithelial cells (8). It is not yet clear what role oxidative stress resistance has in these intracellular life cycles. However, the ability of PerR to sense hydrogen peroxide and to regulate antioxidant defense and iron storage may be important for coordinating a survival response. The mechanism by which PerR senses hydrogen peroxide has not been determined, although it has been proposed that metal ion oxidation may be involved (13). We are currently characterizing PerR from S. aureus to determine how oxidative stress is perceived by this protein and how this signal is transduced to DNA binding.
Catalase has long been implicated as a virulence determinant in S. aureus (40, 46). We have shown definitively that it has no role in the murine skin abscess model of infection. This, together with our previous work with SodA (21), suggests that these determinants are not important for infection, at least not in the well-characterized skin abscess model that we have studied. We note that the previous studies used undefined clinical isolates and that the levels of both catalase and SOD were reduced in their avirulent isolates. We have not observed any correlation between disruption of katA and sodA levels; in fact, disruption of sodA increased catalase levels. Staphylococcus aureus subsp. anaerobius is an organism closely related to S. aureus and is the etiological agent of lymphadenitis and abscess formation in young sheep and goats (57). The main phenotypic differences between the strains are the lack of catalase and weak aerobic growth in the former (22). Weak aerobic growth was not observed in S. aureus ST16 (katA), and we suggest that the altered characteristics of S. aureus subsp. anaerobius are not due solely to its dysfunctional catalase gene (57).
The ability to survive long-term starvation and desiccation has been implicated as an important factor in the nosocomial transmission of S. aureus and is likely to exacerbate the problem of methicillin-resistant S. aureus (23). We have previously identified a number of starvation survival components required for nutrient recycling, cellular repair, and oxidative stress resistance (65). The identification of catalase as a critical component for maintaining viability in long-term starvation reinforces the importance of protection from reactive oxygen species in this process.
This study has identified oxidative stress resistance and/or metal ion homeostasis to be important in the ability of S. aureus to cause disease. The role of the many PerR-regulated genes and the complex coregulatory processes linking stress resistance, metal ion homeostasis, and pathogenicity await investigation.
ACKNOWLEDGMENTS
We thank the BBSRC (M.J.H.) and the Royal Society (S.J.F.) for funding this research.
We thank the S. aureus Genome Sequencing Project (8325) and B. A. Roe, Y. Qian, A. Dorman, F. Z. Najar, S. Clifton, and J. Iandolo with funding from NIH and the Merck Genome Research Institute. Preliminary sequence data for S. aureus (COL) were obtained from The Institute for Genomic Research website at http://www.tigr.org, accomplished with support from NIH and the Merck Genome Research Institute. We thank Arthur Moir for N-terminal sequencing of proteins.
REFERENCES
- 1.Agranoff D D, Krishna S. Metal ion homeostasis and intracellular parasitism. Mol Microbiol. 1998;8:403–412. doi: 10.1046/j.1365-2958.1998.00790.x. [DOI] [PubMed] [Google Scholar]
- 2.Alamo M P, Jurado J, Gallardo-Madueno R, Monje-Casas F, Holmgren A, Pueyo C. Transcriptional regulation of glutaredoxin and thioredoxin pathways and related enzymes in response to oxidative stress. J Biol Chem. 2000;275:13398–13405. doi: 10.1074/jbc.275.18.13398. [DOI] [PubMed] [Google Scholar]
- 3.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald F S, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong-Buisseret L, Cole M B, Stewart G S A B. A homologue of the Escherichia coli alkyl hydroperoxide reductase AhpC is induced by osmotic upshock in Staphylococcus aureus. Microbiology. 1995;141:1655–1661. doi: 10.1099/13500872-141-7-1655. [DOI] [PubMed] [Google Scholar]
- 6.Arnau J, Sorensen K I, Appel K F, Vogensen F K, Hammer K. Analysis of heat shock gene expression in Lactococcus lactis MG1363. Microbiology. 1996;142:1685–1691. doi: 10.1099/13500872-142-7-1685. [DOI] [PubMed] [Google Scholar]
- 7.Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, Engelke G, Entian K D, Götz F F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus aureus. Eur J Biochem. 1992;204:1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- 8.Bayles K, Wesson C, Liou L, Fox G, Bohach G G, Trumble W. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beers R F, Sizer I W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 10.Beisel W R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977;30:1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- 11.Berlett B S, Chock P B, Yim M B, Stadtman E R. Manganese(II) catalyses the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc Natl Acad Sci USA. 1990;87:389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 15.Chackraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Noterman S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Helmann J D. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol Microbiol. 1995;18:295–300. doi: 10.1111/j.1365-2958.1995.mmi_18020295.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, James L P, Helmann J D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Keramati L, Helmann J D. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc Natl Acad Sci USA. 1995;92:8190–8194. doi: 10.1073/pnas.92.18.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung A, Koomey J M, Butler C, Projan S, Fischetti V. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements M O, Watson S P, Foster S J. Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J Bacteriol. 1999;181:3898–3903. doi: 10.1128/jb.181.13.3898-3903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De la Fuente R, Ruiz-Santa-Quiteria J A, Cid D, Domingo M, Suarez G. Experimental intramammary infection of ewes with Staphylococcus aureus subsp. anaerobius. Res Vet Sci. 1993;54:221–226. doi: 10.1016/0034-5288(93)90061-j. [DOI] [PubMed] [Google Scholar]
- 23.Duckworth G J, Jordens J Z. Adherence and survival properties of an epidemic methicillin-resistant strain of Staphylococcus aureus compared with those of methicillin sensitive strains. J Med Microbiol. 1990;32:195–200. doi: 10.1099/00222615-32-3-195. [DOI] [PubMed] [Google Scholar]
- 24.Easmon C S F, Adlam C. Staphylococci and staphylococcal infections. New York, N.Y: Academic Press; 1983. [Google Scholar]
- 25.Elliot G R, Peterson P K, Verbrugh H A, Freiberg M R, Hoidal J R, Quie P G. Influence of subinhibitory concentrations of penicillin, cephalothin, and clindamycin on Staphylococcus aureus growth in human phagocytic cells. Antimicrob Agents Chemother. 1982;22:781–784. doi: 10.1128/aac.22.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escolar L, Perez-Martin J, De Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulkner K M, Liochev S I, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 28.Gaballa A, Helmann J D. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant R A, Filman D J, Finkel S E, Kolter R, Hogle J M. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- 30.Gresham H D, Lowrance J H, Caver T E, Wilson B S, Cheung A L, Lindberg F P. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 31.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 32.Guerot-Fleury A-M, Shazand N, Frandsen N, Stragier P. Antibiotic resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi R. Using PCR to engineer DNA. In: Erlich H A, editor. PCR technology. Principles and applications for DNA amplification. New York, N.Y: Macmillan; 1989. pp. 61–70. [Google Scholar]
- 34.Hill P J, Cockayne A, Landers P, Morrissey J A, Sims C A, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect Immun. 1998;66:4123–4129. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoepelman I M, Bezemer W A, Vandenbroucke-Grauls C M, Marx J J, Verhoef J. Bacterial iron enhances oxygen radical-mediated killing of Staphylococcus aureus by phagocytes. Infect Immun. 1990;58:26–31. doi: 10.1128/iai.58.1.26-31.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horsburgh M J, Moir A. ςM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol Microbiol. 1999;32:41–50. doi: 10.1046/j.1365-2958.1999.01323.x. [DOI] [PubMed] [Google Scholar]
- 37.Horsburgh M J, Ingham E, Foster S J. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183:468–475. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson M, Ramp W, Nicholson N, Williams A, Nousiainen M. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 39.Jeong W, Cha M, Kim I. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem. 2000;275:2924–2930. doi: 10.1074/jbc.275.4.2924. [DOI] [PubMed] [Google Scholar]
- 40.Kanafi H, Martin S E. Catalase and superoxide dismutase activities in virulent and nonvirulent Staphylococcus aureus isolates. J Clin Microbiol. 1985;21:607–610. doi: 10.1128/jcm.21.4.607-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keen C L, Lonnerdal B, Hurley L S. Manganese. In: Frieden I, editor. Biochemistry of the essential ultratrace elements. New York, N.Y: Plenum Press; 1984. pp. 89–132. [Google Scholar]
- 42.Kehres D G, Zaharik M L, Finlay B B, Maguire M E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 43.King K Y, Horenstein J A, Caparon M G. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J Bacteriol. 2000;182:5290–5299. doi: 10.1128/jb.182.19.5290-5299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornblum J, Kreisworth B N, Projan S J, Ross H, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 45.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 46.Mandell G L. Catalase, superoxide dismutase and virulence of Staphylococcus aureus. J Clin Investig. 1975;55:561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999;18:4361–4371. doi: 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen S L, Black F T, Storgaard M, Obel N. Evaluation of a method for measurement of intracellular killing of Staphylococcus aureus in human neutrophil granulocytes. APMIS. 1995;103:460–468. [PubMed] [Google Scholar]
- 50.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 51.O'Halloran T V. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- 52.Posey J E, Hardham J M, Norris S J, Gherardini F C. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Que Q, Helmann J D. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 54.Repine J E, Fox R B, Berger E M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981;256:7094–7096. [PubMed] [Google Scholar]
- 55.Repine J E, Fox R B, Berger E M, Harada R N. Effect of staphylococcal iron content on the killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981;32:407–410. doi: 10.1128/iai.32.1.407-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Sanz R, Marin I, Ruiz-Santa-Quiteria J A, Orden J A, Cid D, Diez R M, Silhadi K S, Amils R, De La Fuente R. Catalase deficiency in Staphylococcus aureus subsp. anaerobius is associated with natural loss-of-function mutations within the structural gene. Microbiology. 2000;146:465–475. doi: 10.1099/00221287-146-2-465. [DOI] [PubMed] [Google Scholar]
- 58.Schenk S, Ladagga R A. Improved methods for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992;94:133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- 59.Stadtman E R, Berlett B S, Chock P B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci USA. 1990;87:384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storz G, Imlay J A. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 61.Tao X, Schiering N, Zeng H Y, Ringe D, Murphy J R. Iron, DtxR, and regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 62.Van Vliet A H M, Baillon M-L A, Penn C W, Ketley J. Campylobacter jejuni contains two Fur homologs: characterization of iron-responsive regulation of peroxide stress genes by the PerR repressor. J Bacteriol. 1999;181:6371–6376. doi: 10.1128/jb.181.20.6371-6376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vesga O, Groeschel M, Otten M, Brar D, Vann J, Proctor R. Staphylococcus aureus small colony variants are induced by the endothelial intracellular milieu. J Infect Dis. 1996;173:739–745. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]
- 64.Watson S P, Clements M O, Foster S J. Characterization of the starvation survival response of Staphylococcus aureus. J Bacteriol. 1998;180:1750–1758. doi: 10.1128/jb.180.7.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson S P, Antonio M A, Foster S J. Isolation and characterisation of Staphylococcus aureus starvation-induced, stationary phase mutants defective in survival or recovery. Microbiology. 1998;144:3159–3169. doi: 10.1099/00221287-144-11-3159. [DOI] [PubMed] [Google Scholar]
- 66.Wayne L G, Diaz G A. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal Biochem. 1986;157:89–92. doi: 10.1016/0003-2697(86)90200-9. [DOI] [PubMed] [Google Scholar]
- 67.Wolf S G, Frenkiel D, Arad T, Finkel S E, Kolter R, Minsky A. DNA protection by stress-induced biocrystallization. Nature. 1999;400:83–85. doi: 10.1038/21918. [DOI] [PubMed] [Google Scholar]
- 68.Xiong A, Singh V K, Cabrera G, Jayaswal R K. Molecular characterisation of the ferric uptake regulator, Fur, from Staphylococcus aureus. Microbiology. 2000;146:659–668. doi: 10.1099/00221287-146-3-659. [DOI] [PubMed] [Google Scholar]
- 69.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 70.Zimmerli W, Lew P D, Suter S, Wyss M, Waldvogel F A. In vitro efficacy of several antibiotics against intracellular S. aureus in chronic granulomatous disease. Helv Paediatr Acta. 1983;38:51–61. [PubMed] [Google Scholar]