Abstract
Background:
Sepsis is a leading cause of morbidity, mortality, and resource utilization amongst cutaneous T-cell lymphoma (CTCL) patients.
Objective:
To characterize the demographic, clinical, and microbial attributes distinguishing CTCL sepsis patients from other non-Hodgkin lymphoma (NHL) sepsis patients and CTCL patients in general.
Methods:
Two-part retrospective cohort study at an academic medical center, 2001–2019, involving CTCL (n=97) and non-CTCL NHL (n=88) patients admitted with sepsis, and a same-institution CTCL patient database (n=1094). Overall survival was estimated by Kaplan-Meier analyses.
Results:
CTCL sepsis patients were more likely to be older, Black, experience more sepsis episodes, die or be readmitted within 30 days of an inpatient sepsis episode, and develop Gram-positive bacteremia than non-CTCL NHL sepsis patients. Staphylococcus aureus and Escherichia coli were the most frequently speciated organisms in CTCL (26%) and non-CTCL NHL (14%), respectively. No between-group differences were identified regarding sex, presence of central line, chemotherapy use, or disease stage. Compared to general CTCL patients, sepsis patients were Black and exhibited advanced-stage disease, higher body surface area involvement, and higher lactate dehydrogenase levels.
Limitations:
Single institution, retrospective nature may limit generalizability.
Conclusions:
Awareness of CTCL-specific risk factors is crucial for guiding sepsis prevention and improving patient outcomes.
Keywords: cutaneous T-cell lymphoma, sepsis, bacteremia, non-Hodgkin lymphoma, risk factors, outcomes, race, Black patients
Background
Cutaneous T-cell lymphoma (CTCL) progression is associated with profound immune dysregulation and concomitant recurrent skin infections – phenomena that increase patient risk for sepsis and death.1–4 Indeed, infection is cited as the cause of death in up to 60% of CTCL patients, of which the majority are diagnosed with mycosis fungoides (MF) or its leukemic variant Sézary syndrome (SS).5 Further emphasizing the importance of bacteria in CTCL, microbial virulence factors are known to drive disease flares and aggressive antibiotic therapy has been shown to reduce tumor burden and malignant T-cell activity.6, 7 While these data suggest infections in CTCL are tied to disease acceleration and mortality, infection prevention remains a challenge in the management of these patients.8 Moreover, guidelines for identifying CTCL patients at high risk for sepsis are lacking and little is known about the clinical characteristics such as the disease-specific features that predispose patients to severe infections or the most common infectious agents themselves that are associated with poor outcomes in CTCL sepsis.
The spectrum of infections in CTCL is broad, yet it is generally accepted that patients are predisposed to infections secondary to lesional ulceration and tumor development.5, 9, 10 Bacteremia in CTCL has been previously associated with Black race, female sex, advanced disease stage, the presence of invasive lines, and active chemotherapy treatment,4 but these data remain to be validated in larger studies. Moreover, it is unknown whether these risk factors are unique to CTCL or common to other non-Hodgkin lymphomas (NHLs). Infectious causes of death affect one-third of NHL patients and clinical characteristics associated with infection include more advanced stage and chemotherapy-related granulocytopenia.11 As such, efforts are still needed to distill which disease factors are specific to CTCL sepsis versus those that are more generalizable to the oncologic patient population in general. To further characterize CTCL sepsis, we investigated clinical, demographic, and microbe-related features and survival outcomes among CTCL sepsis patients compared to non-CTCL NHL sepsis and general CTCL patient populations.
Methods
Subjects and Matching
This was an Institutional Review Board-approved, single-institution, two-part retrospective cohort study comparing CTCL (n=97; 180 total sepsis episodes) to non-CTCL NHL (n=88; 100 total sepsis episodes) patients with at least one documented sepsis episode, and CTCL sepsis patients to a general cohort of CTCL patients (n=1094). All patients were seen at Northwestern Memorial Hospital (NMH) (Chicago, IL), January 2001 to December 2019. The general CTCL cohort was comprised of patients seen through Northwestern’s multidisciplinary CTCL clinic and diagnosed clinically and pathologically with CTCL by an expert cutaneous lymphoma specialist (JG).
Figure S1 illustrates the patient selection protocol. NMH electronic health record (EHR) data was queried from the Northwestern record repository (Enterprise Data Warehouse) using International Classification of Disease (ICD) 10th edition diagnosis codes for MF (C84.0), SS (C84.1), unspecified CTCL (C84.A), and non-CTCL NHL (C84.4, C84.Z, C84.9, C85). Together, MF/SS comprised 78% of the CTCL patient group. Meanwhile, the non-CTCL cohort included mainly patients with systemic T-cell lymphomas. There was no group overlap, as confirmed by individual chart review. Among these patients, those with at least one post-diagnosis episode of sepsis (A41*), systemic inflammatory response syndrome (SIRS) (R65*), or bacteremia (R78.81) were selected. Exclusion criteria included age <18 or >90 years, no confirmed diagnosis of CTCL or non-CTCL NHL, no documented encounter with dermatology and/or hematology-oncology within the Northwestern Medicine health system, and duplicate or incomplete medical records. All included patients were validated by individual chart review to confirm clinician documentation of sepsis, defined as meeting SIRS criteria in the presence of an infection (documented or probable). While sepsis definitions have changed in recent years, this traditional definition was chosen as it was actively and universally used over the study time period and captures uniform cohorts that allow for clinical comparison.
Data Collection
CTCL and non-CTCL NHL sepsis cohorts were assessed for the presence of at least one documented episode of sepsis, SIRS, or bacteremia per EHR. Date of death was acquired from medical records and/or public obituary notices and death records. Causative microorganisms, including Gram staining, and presence or absence of bacteremia were assessed. Patient age at cancer diagnosis, age at first sepsis episode, sex, self-reported race and ethnicity, cancer subtype, clinical disease stage, lymphoma treatment interventions, treatment with chemotherapy (concurrent with documented sepsis or prior), and history of stem cell transplant (SCT) were captured for identified septic patients. Additionally, number of weeks from cancer diagnosis to initial sepsis episode, initial sepsis episode to death, and cancer diagnosis to death were all assessed. Other measures assessed included the overall number of sepsis episodes, if death occurred within 30 days of sepsis or hospital discharge, the number of sepsis episodes that occurred within 30 days of a prior episode, readmission rates within 30 days of previous hospital discharge, and patient status or outcome (deceased, alive, or unknown). The presence or absence of a central line, line type, number of invasive lines, and the suspected sepsis trigger were also obtained.
A subgroup analysis examining the blood microbes of post-SCT and non-transplanted CTCL and non-CTCL NHL patients was performed. Post-SCT groups included only sepsis episodes that occurred more than 30 days after the date of transplant to minimize capturing peri-transplant, immunosuppression-related sepsis. Lastly, a comparison of clinical and demographic differences of CTCL patients who developed sepsis and the general institutional cohort of CTCL patients was conducted. Patient sex, age at diagnosis, self-reported race, clinical stage, lactate dehydrogenase (LDH), and percent of involved body surface area (BSA) were assessed for each patient within both groups.
Statistical analyses
Differences in demographics, clinical disease characteristics, and sepsis-related data between CTCL and non-CTCL NHL sepsis patients were assessed using Students’ T-tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. These statistical analyses were also used to compare the CTCL sepsis and general institutional CTCL cohorts. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all relevant variables and risk factors. Kaplan-Meier (KM) analyses assessing overall survival (OS) were generated comparing the CTCL and non-CTCL NHL sepsis cohorts using the survival and survminer R packages; significance was based on the logRank (Mantel-Cox) test. The timeline used for all KM survival analyses was date of first positive sepsis to date of death or last known follow-up for each subject. A second-tier survival analysis was completed from initial sepsis to death or last known follow-up based on the overall number of sepsis episodes for Staphylococcus versus non-Staphylococcus bacteremia, Gram-negative versus Gram-positive bacteremia, and blood microbe type (bacterial, viral, or fungal). Statistical analysis and graph generation were completed using R version 4.2.0 and GraphPad Prism version 9.0 (GraphPad, San Diego, CA). Adjusted p-values (q-values) were calculated using the Benjamini-Hochberg method to correct for multiple hypothesis testing.12 Significance was determined based on a false discovery rate threshold of 5% (0.05).
Results
Several demographic, clinical, and microbe-related characteristics distinguished CTCL from non-CTCL NHL sepsis (Tables 1–2; Table S1). Mean age of CTCL patients at first sepsis was 62 versus 56 years in the non-CTCL NHL cohort (p=0.005). Black individuals comprised 57% of the CTCL sepsis cohort versus 15% of the non-CTCL NHL sepsis (p<0.0001) and 9% of the general CTCL cohorts (p<0.0001). While time between cancer diagnosis and first sepsis was longer in CTCL patients compared to non-CTCL NHL (199 versus 76 weeks, p=0.0006), mean time from initial sepsis to death did not differ between groups (113 versus 124 weeks, p=0.70). Amongst patients with a reported blood Gram stain, Gram-positive organisms were most frequently identified, regardless of cohort (Table 2). Whereas Staphylococcus aureus was the most frequently speciated organism in CTCL (26%), Escherichia coli predominated in non-CTCL NHL (14%). Within 30 days of a sepsis episode, CTCL patients had greater overall readmission (12% versus 0%, p=0.0006) and death rates (44% versus 16%, p<0.0001) compared to non-CTCL NHL patients. Furthermore, CTCL patients experienced more sepsis episodes (mean 1.9 versus 1.1, p<0.0001).
Table 1.
Demographic and clinical characteristics differentiating CTCL and non-CTCL NHL sepsis patients. Significant p-values are bolded.
| CTCL | NHL | p-value | OR (95% CI) | |
|---|---|---|---|---|
| N | 97 | 88 | ||
| Mean age (range), y | ||||
| At cancer diagnosis | 59 (19–87) | 55 (22–90) | 0.08a | |
| At first sepsis | 62 (20–88) | 56 (24–90) | 0.005 a | |
| Sex, N (%) | 0.31b | 0.74 (0.41–1.32) | ||
| Male | 49 (51) | 51 (58) | ||
| Female | 48 (49) | 37 (42) | ||
| Race, N (%) | <0.0001 b | |||
| White | 34 (35) | 59 (67) | <0.0001 c | 0.27 (0.14–0.49) |
| Black | 55 (57) | 13 (15) | <0.0001 c | 7.55 (3.70–15.41) |
| Asian | 0 (0) | 4 (5) | 0.05 c | 0.10 (0.005–1.81)d |
| Other | 7 (7) | 8 (9) | 0.79c | 0.78 (0.27–2.24) |
| Unknown | 1 (1) | 4 (5) | 0.19c | 0.22 (0.02–1.99) |
| Ethnicity, N (%) | 0.41b | 1.55 (0.54–4.46) | ||
| Hispanic | 10 (10) | 7 (8) | ||
| Non-Hispanic | 87 (90) | 81 (92) | ||
| CTCL subtype, N (%) | ||||
| Mycosis fungoides | 35 (36) | |||
| Sézary syndrome | 41 (42) | |||
| Gamma delta CTCL | 9 (9) | |||
| CTCL NOS | 12 (12) | |||
| Non-CTCL NHL subtype, N (%) | ||||
| T-cell | 72 (82) | |||
| B-cell | 16 (18) | |||
| Stage: Early versus Late, N (%) | 0.60b | 1.48 (0.55–4.00) | ||
| Early stage e | 11 (11) | 7 (8) | ||
| Late stage f | 86 (89) | 81 (92) | ||
| Treatment, N (%) | 0.01 b | |||
| None | 16 (16) | 7 (8) | 0.12c | 2.29 (0.89–5.85) |
| Skin-directed g | 3 (3) | 0 (0) | 0.25c | 6.56 (0.33–128.65)d |
| Non-chemotherapy systemic h | 36 (37) | 22 (25) | 0.08c | 1.77 (0.94–3.34) |
| Chemotherapy i | 36 (37) | 47 (53) | 0.03 c | 0.51 (0.29–0.93) |
| Stem cell transplant conditioning | 6 (6) | 12 (14) | 0.13c | 0.42 (0.15–1.16) |
| Stem cell transplant, N (%) | 0.26b | 0.71 (0.39–1.29) | ||
| Yes | 33 (34) | 37 (42) | ||
| No | 64 (66) | 51 (58) | ||
| Patient status, N (%) | 0.29b | |||
| Alive | 13 (13) | 18 (20) | 0.24c | 0.60 (0.28–1.31) |
| Dead | 77 (79) | 61 (69) | 0.13c | 1.70 (0.87–3.33) |
| Lost to follow-up | 7 (7) | 9 (10) | 0.60c | 0.68 (0.24–1.92) |
CTCL: cutaneous T-cell lymphoma; NHL: non-Hodgkin lymphoma; NOS: not otherwise specified
T-test
Chi-square
Fisher’s exact
Odds Ratio (OR) Haldane Correction
Early Stage: CTCL stages IA-IIA; NHL stages I-II
Late Stage: CTCL stages IIB-IVB; NHL stages III-IV
Narrowband UVB phototherapy, local radiation therapy, topical corticosteroids, topical retinoids, imiquimod, resiquimod, bexarotene gel, mechlorethamine gel
Immunomodulatory: IFN-alpha, IFN-gamma, oral retinoids, extracorporeal photopheresis (ECP), brentuximab, rituximab, vorinostat, romidepsin, JAK-inhibitors, denileukin difitox; Targeted immunotherapy: mogamulizumab alemtuzumab, pembrolizumab, CAR T-cell therapy; Immunosuppressive: methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, sirolimus, systemic steroids
Doxorubicin, gemcitabine, pentostatin, bendamustine, chlorambucil, cyclophosphamide, vincristine, etoposide, cytarabine, cisplatin
Table 2.
Clinical features of sepsis encounters amongst CTCL and non-CTCL NHL cohorts. Significant p-values are bolded
| CTCL | NHL | p-value | OR (95% CI) | |
|---|---|---|---|---|
| Total sepsis episodes, N | 180 | 100 | ||
| Mean number of sepsis episodes (range) | 1.87 (1–7) | 1.14 (1–3) | <0.0001 a | |
| Mean weeks (range) | ||||
| Cancer diagnosis to initial sepsis | 199 (0–2172) | 76 (0–661) | 0.0006 a | |
| Initial sepsis to death | 113 (0–822) | 124 (0–888) | 0.70a | |
| Cancer diagnosis to death | 308 (1–2174) | 182 (0–1197) | 0.005 a | |
| Death within 30 days of final sepsis episode, N (% patients) | 43 (44) | 14 (16) | <0.0001 a | 2.01 (1.09–3.70) |
| Death within 30 days of final hospital discharge, N (% patients) | 48 (49) | 25 (28) | 0.003 a | 2.47 (1.34–4.55) |
| Sepsis within 30 days of preceding sepsis episode, N (%, patients) | 14 (14) | 1 (1) | 0.0008 a | 14.67 (1.89–114.10) |
| Readmission within 30 days preceding discharge, N (% patients) | 12 (12) | 0 (0) | 0.0006 a | 25.89 (1.51–444.20)d |
| Blood microbe, N (% patients) | 0.97b | |||
| Bacterial | 56 (58) | 53 (60) | 0.77c | 0.90 (0.50–1.62) |
| Viral | 6 (6) | 5 (6) | 1.00c | 1.09 (0.32–3.72) |
| Fungal | 3 (3) | 4 (5) | 0.71c | 0.67 (0.22–3.08) |
| Multiple microbe types | 8 (8) | 6 (7) | 0.79c | 1.23 (0.41–3.69) |
| All cultures negative | 24 (25) | 20 (23) | 0.86c | 2.94 (1.55–5.6) |
| Blood microbe, N (% sepsis episodes) | 0.04 b | |||
| Bacterial | 97 (54) | 63 (63) | 0.17c | 0.69 (0.42–1.13) |
| Viral | 10 (6) | 6 (6) | 1.00c | 0.92 (0.32–2.62) |
| Fungal | 5 (3) | 6 (6) | 0.21c | 0.92 (0.59–3.95) |
| Multiple microbe types | 2 (1) | 4 (4) | 0.19c | 0.36 (0.06–2.21) |
| All cultures negative | 66 (37) | 21 (21) | 0.007 c | 2.18 (1.23–3.85) |
| Blood culture Gram stain, N (% patients) | 0.23b | |||
| Positive | 42 (43) | 33 (38) | 0.46c | 1.27 (0.71–2.29) |
| Negative | 11 (11) | 20 (23) | 0.05 c | 0.43 (0.19–0.97) |
| Mixed (positive + negative) | 9 (9) | 7 (8) | 0.80c | 1.18 (0.42–3.32) |
| Neither | 35 (36) | 28 (32) | 0.64c | 1.21 (0.66–2.23) |
| Blood culture Gram stain, N (% sepsis episodes) | 0.002 b | |||
| Positive | 75 (42) | 40 (40) | 0.80c | 1.07 (0.65–1.76) |
| Negative | 15 (8) | 24 (24) | 0.0005 c | 0.29 (0.14–0.58) |
| Mixed (positive + negative) | 7 (4) | 4 (4) | 1.00c | 0.97 (0.28–3.40) |
| Neither | 83 (46) | 32 (32) | 0.02 c | 1.81 (1.09–3.03) |
CTCL: cutaneous T-cell lymphoma; NHL: non-Hodgkin lymphoma
T-test
Chi-square
Fisher’s exact
Odds Ratio (OR) Haldane Correction
Survival analysis from first sepsis to death revealed blood cultures with non-Staphylococcus microbes, Gram-negative microbes, and viremia or fungemia were associated with worse OS in both groups (p<0.05) (Figure 1). There were no differences in OS between CTCL and non-CTCL NHL sepsis patients based on sex, race, disease stage, presence of a central line, or treatment with chemotherapy (Figure S2). Prior SCT was associated with longer survival in both groups (p=0.0006), despite excluding sepsis episodes that occurred within 30 days of SCT (Figure 1). Analysis of post-SCT and non-SCT sepsis patients revealed post-SCT CTCL patients have greater risk for viremia compared to non-SCT CTCL patients; post-SCT non-CTCL NHL patients have greater propensity for bacteremia compared to those without SCT (p<0.05) (Table S2; Figure S3).
Figure 1.
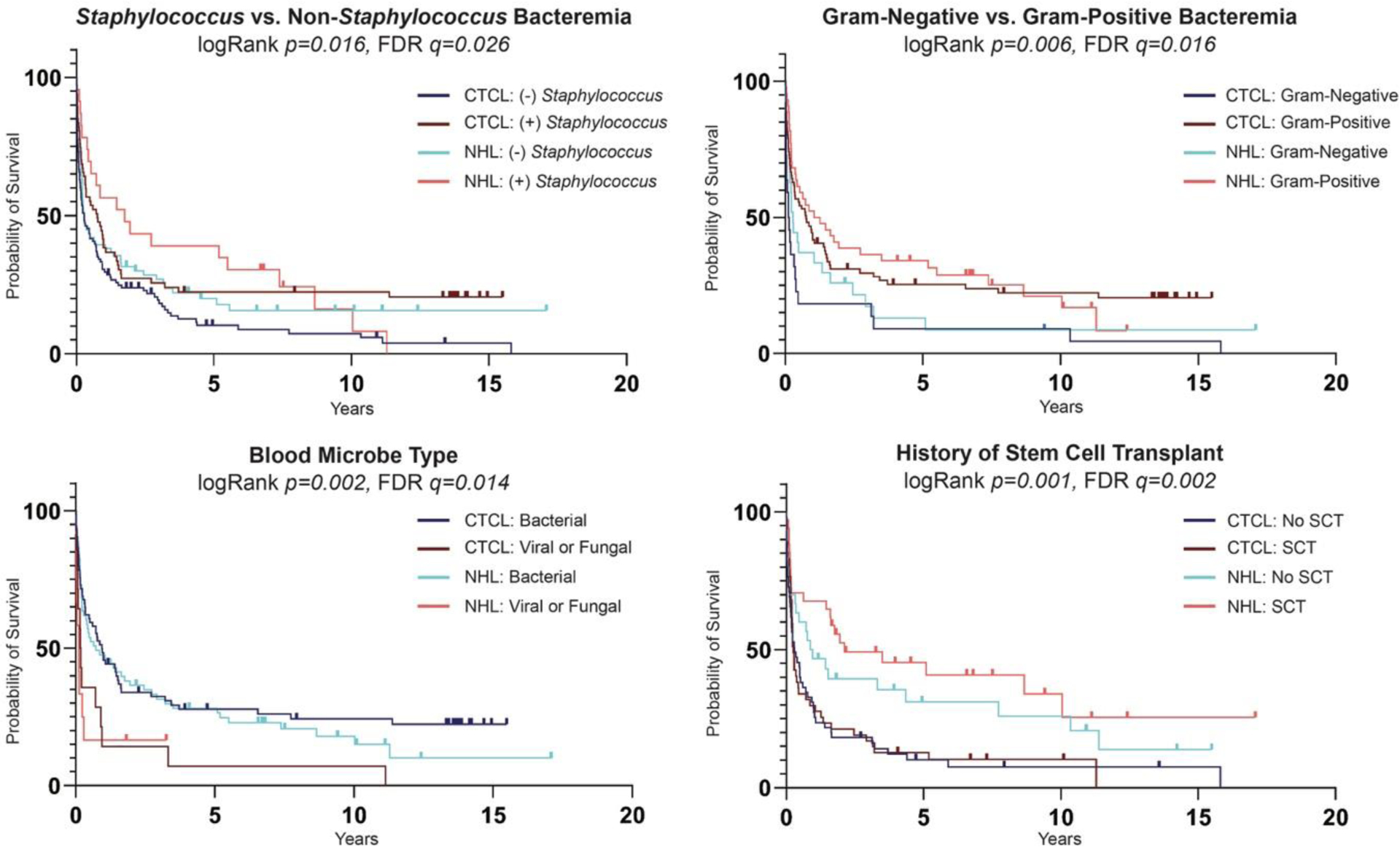
Kaplan-Meier estimates of overall survival amongst CTCL (n=97) and non-CTCL NHL (n=88) patients who developed sepsis for Staphylococcus versus non-Staphylococcus bacteremia; Gram-negative versus Gram-positive bacteremia; blood microbe type (bacterial, viral, fungal); and history of stem cell transplant. Adjusted p-values (q-values) were calculated using false discovery rate (FDR) correction of 0.05.
Compared to our general CTCL cohort, CTCL sepsis patients predominantly identified as Black and exhibited more advanced disease, greater BSA, and higher LDH levels (p<0.0001) (Figure 2a; Table S3). Amongst CTCL sepsis patients, 36% showed histologic features of large cell transformation, which was associated with more frequent sepsis episodes (p=0.040). Although a higher proportion of Black CTCL patients were diagnosed with stage IV disease compared to White CTCL patients in our general cohort (31.4% versus 11.2%; p=0.003), there were no racial differences based on clinical stage among the CTCL patients who developed sepsis (p=0.470) (Figure 2b). Compared to the same respective racial group in the general CTCL cohort, Black CTCL sepsis patients were older (62 versus 52 years, p<0.0001), while White CTCL sepsis patients were the same age or slightly younger (52 versus 59 years, p=0.053).
Figure 2.
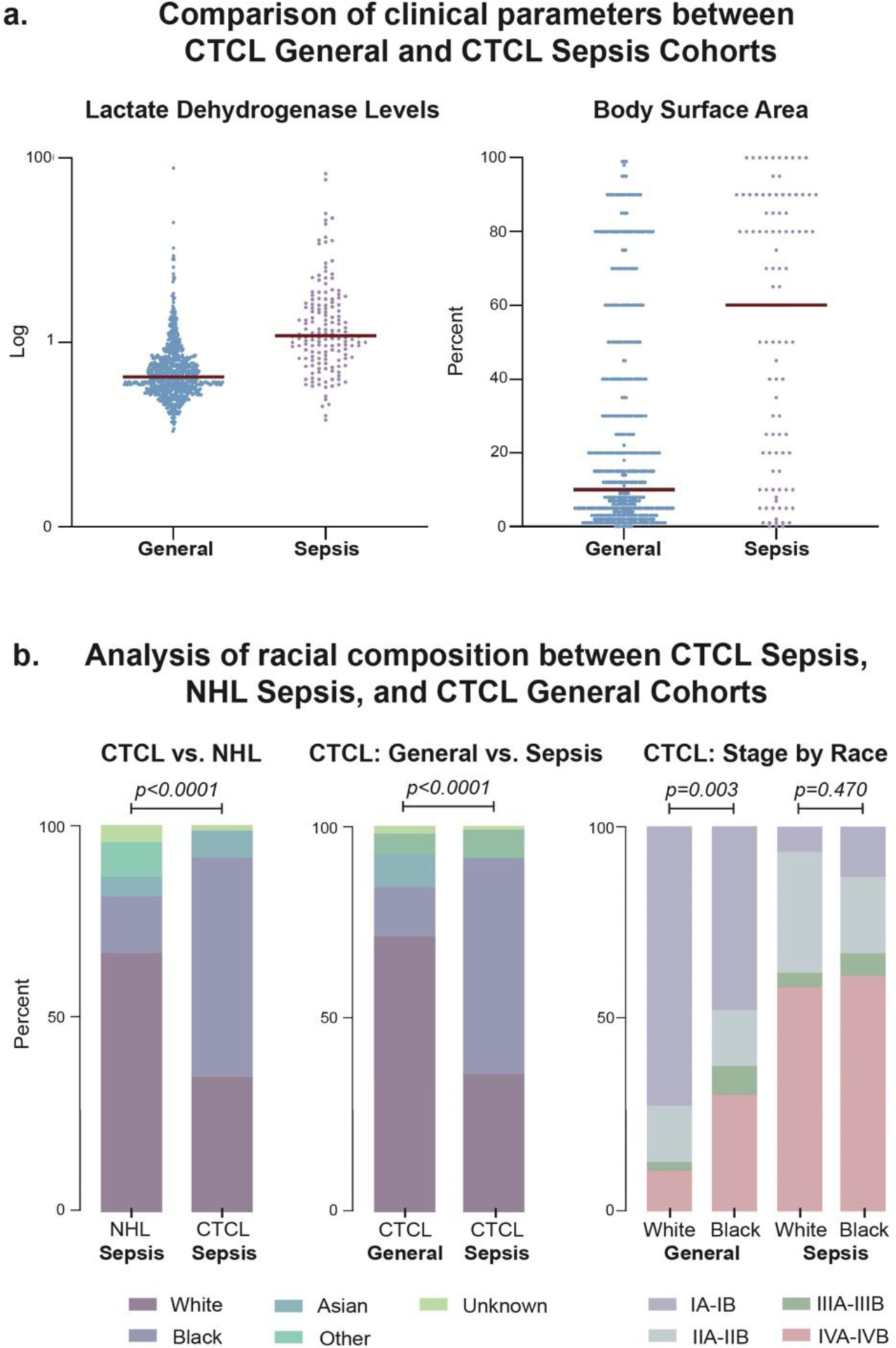
Demographic and clinical characteristics differentiate CTCL sepsis (n=97), non-CTCL NHL sepsis (n=88), and general CTCL (n=1094) cohorts. (a) Comparison of general CTCL and CTCL sepsis cohorts at first presentation and first sepsis episode, respectively, revealed lactate dehydrogenase (LDH) levels and percentage of body surface area of disease involvement trended higher within the sepsis cohort. Dot plot demonstrates LDH values normalized to the upper limit of normal (i.e., 271 units/L at Northwestern). Red horizontal bar signifies group median. (b) Analysis of cohort racial composition demonstrated a larger percentage of the CTCL sepsis group was Black compared to both the non-CTCL NHL and general CTCL groups. Combined analysis of both clinical stage and race revealed a significant proportion of Black patients have advanced-stage disease within the general CTCL cohort; however, no significant difference existed between Black and White sepsis patients based on disease stage distribution.
Discussion
Although it is established that advanced-stage CTCL coincides with greater risk of developing localized and systemic infections,3, 9, 13 limited data is available regarding demographics, common culprit organisms, and overall patient survival in CTCL sepsis. The current literature discusses several modifiable and non-modifiable risk factors which may contribute to increased bacteremia incidence in certain CTCL patients.4, 9 Our data adds further dimension to this epidemiological framework by appropriately controlling for disease features that may influence infection risk in all hematologic malignancies. The direct comparison of majority MF/SS and non-CTCL NHL patients in this study allows us to isolate risk factors that may be specific to CTCL sepsis and sepsis-related mortality, namely Black race and Gram-negative bacterial infection patterns.
Our findings reveal Black patients with CTCL face disproportionally higher rates of sepsis even when compared to other NHLs. Recent evaluation of racial disparities using the Surveillance, Epidemiology, and End Results Registry indicated Black patients comprised roughly 10.8% of NHL14 and 11.68% of CTCL patients,15 compared to 9% in our general CTCL cohort. Meanwhile, Black patients comprised 15% of our non-CTCL NHL sepsis cohort, but 57% of our CTCL sepsis cohort. Previous reports have indicated Black patients have higher rates of sepsis nationally;16 smaller studies suggest CTCL bacteremia incidence is skewed by racial disparities.4, 8, 9 Although the reasons for these discrepancies are unclear, non-White race and darker skin type are also associated with heightened CTCL severity.17 Moreover, Lili and colleagues recently demonstrated the keratinocytes of Black patients exhibit intrinsic pro-inflammatory pathways distinct from the keratinocytes of White patients which may contribute to higher rates of certain inflammatory skin diseases amongst Black individuals.18 Adding to this basal pro-inflammatory risk, CTCL-mediated chronic skin barrier disruption and inflammation may increase sepsis susceptibility in Black patients.
Still, any discussion of race in medicine must consider the social determinants of health. Studies in NHL have identified racial disparities in the presentation and outcomes of various NHL subtypes, including CTCL, B-cell NHLs, and peripheral T-cell lymphoma, thereby suggesting our CTCL and non-CTCL NHL cohorts were likely affected by similar race- and ethnicity-related risk factors.19 While data aimed at distilling the role of socioeconomics in CTCL have suggested no differences in survival exist between racial groups when evaluating healthcare coverage, median household income, poverty rate, and insurance status,20, 21 the findings that Black patients present with more advanced disease at younger ages and Black females have a greater propensity for poorer outcomes certainly carry significance for clinical practice.22, 23 As our controlled dataset indicates sepsis incidence is higher in Black CTCL patients than in Black non-CTCL NHL patients, our findings emphasize that infection risk may be uniquely influenced by CTCL-specific biological phenomena. Further research on this possibility is imperative. Additionally, translation of these findings into heightened awareness regarding Black patient infection risk is warranted at all levels of multidisciplinary CTCL care.
CTCL patients’ propensity to suffer from and succumb to infections comprises another important area of study, as cutaneous lymphomas have the highest 30-day readmission rate of any skin disease24 and – as reflected herein – are more likely to die shortly after sepsis compared to non-CTCL NHL patients. Consistent with the skin barrier disruption in CTCL that predisposes patients to chronic skin impetiginization and bacterial entry into the bloodstream, sepsis in this cohort tended to involve repeated infections with skin-predominant, Gram-positive organisms like S. aureus. However, worse overall survival appeared to be associated with Gram-negative bacteremia, viremia, and fungemia. Prior SCT appeared to improve sepsis outcomes in our CTCL cohort. Bacterial infection types did not differ in those with prior SCT but among microbe types, viremia was significantly more common. Given these findings, blood microbial studies (i.e., bacterial culture results, identification of non-bacterial microbes) could comprise a useful indicator for stratifying CTCL patient mortality risk upon their development of sepsis.
Our data validates previous studies that suggested more advanced and active disease (as indicated by clinical stage, disease BSA involvement, and LDH levels) is a risk factor for bacteremia,4 but it challenges the assertion that chemotherapy treatment, presence of invasive lines, and older age are disease features that uniquely increase sepsis risk in CTCL. We agree that these clinical parameters are associated with vulnerability to infections, yet our current work aims to accurately represent those factors that differentiate sepsis in CTCL because it remains such a devastating complication in this NHL subtype.
Study generalizability may be limited by our single-institution design and data reliability is dependent on EHR accuracy. The applicability of our findings may be limited to MF/SS as 78% of our sepsis cohort had these most common CTCL subtypes. Non-MF/SS CTCL subtypes may also vary in their sepsis characteristics. Furthermore, using ICD-10 codes to guide inclusion criteria for CTCL studies is challenging due to the heterogeneity of CTCL overall. Disease classification in CTCL is complex and continues to evolve; that both general (C84.A) and more specific (C84.0, C84.1) CTCL codes exist leads to the possibility of differential coding practices between clinicians. Some overlap is expected to be present between our CTCL sepsis and general CTCL cohorts, as the latter is a curated dataset of all CTCL patients seen through the Northwestern multidisciplinary CTCL clinic: if a patient was admitted for sepsis and also followed in CTCL clinic, or vice versa, this patient would be included in both cohorts. Additionally, the difference in viremia rates post-SCT in CTCL versus non-CTCL NHL patients may be an artifact of low statistical power, but it is plausible that biologic differences between CTCL and non-CTCL NHL patients who undergo SCT underlie this disparity. Knowing the racial breakdown of non-CTCL NHL patients at our institution would strengthen our conclusions regarding race in this analysis and encompasses an invaluable focus for future study. Finally, as sepsis definitions have changed over time, varying criteria may capture slightly different patient cohorts. Larger, multi-institutional, and prospective datasets will provide greater ability to accurately capture relevant clinical variables.
Including the largest cohort of hospitalized CTCL sepsis patients assessed to date, this study is the first to compare sepsis in CTCL to non-CTCL NHL. CTCL sepsis patients represent a distinctly high-risk group of inpatients compared to non-CTCL NHL given their greater likelihood of short-term sepsis recurrence, hospital readmission, and death. The strategic use of targeted antimicrobial therapies (topical or systemic), plus efforts to repair the skin barrier and reconstitute a healthy cutaneous microbiome could reduce disease burden and modulate the likelihood of superinfection and subsequent sepsis in these patients. Evaluation of racial differences in CTCL remains essential to this research focus.
Supplementary Material
Capsule Summary
How does this article integrate into what was already known?
Cutaneous T-cell lymphoma (CTCL) patients face significant sepsis-related morbidity and mortality, but the clinical characteristics differentiating sepsis in CTCL versus other non-Hodgkin lymphomas remain unknown.
How does it change practice?
CTCL sepsis harbors unique demographic, clinical, and microbial features resulting in a distinctly high-risk group of inpatients that may require earlier or more aggressive intervention.
Acknowledgements
The authors would like to thank the patients whose data comprised this study. Dr. Zhou was supported by a career development award from the Dermatology Foundation, a Cutaneous Lymphoma Foundation Catalyst Research Grant, and an institutional grant from Northwestern University Clinical and Translational Sciences Institute (NUCATS) and National Institute of Health (NIH) (Grant # 5KL2TR001424).
Funding:
Dermatology Foundation, Cutaneous Lymphoma Foundation, National Institute of Health (5KL2TR001424)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to disclose.
IRB approval status: Reviewed and approved by Northwestern University IRB STU00209226
Supplemental Material: https://data.mendeley.com/datasets/7nnvbr35np/1
References
- 1.Fanok MH, Sun A, Fogli LK, Narendran V, Eckstein M, Kannan K et al. Role of Dysregulated Cytokine Signaling and Bacterial Triggers in the Pathogenesis of Cutaneous T-Cell Lymphoma. Journal of investigative dermatology 2018;138:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suga H, Sugaya M, Sato S, Miyagaki T, Ohmatsu H, Kawaguchi M et al. Skin Barrier Dysfunction and Low Antimicrobial Peptide Expression in Cutaneous T-cell Lymphoma. Clinical cancer research 2014;20:4339–48. [DOI] [PubMed] [Google Scholar]
- 3.Scarisbrick JJ. Infections in mycosis fungoides and Sézary syndrome are a frequent cause of morbidity and contribute to mortality. What can be done? British journal of dermatology (1951) 2018;179:1243–4. [DOI] [PubMed] [Google Scholar]
- 4.Allen PB, Switchenko J, Ayers A, Kim E , Lechowicz MJ. Risk of bacteremia in patients with cutaneous T-cell lymphoma (CTCL). Leuk Lymphoma 2020;61:2652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posner LE, Fossieck BE, Eddy JL , Bunn PA. Septicemic complications of the cutaneous T-cell lymphomas. Am J Med 1981;71:210–6. [DOI] [PubMed] [Google Scholar]
- 6.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, Bonefeld CM, Wasik MA, Koralov SB et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins 2013;5:1402–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindahl LM, Willerslev-Olsen A, Gjerdrum LMR, Nielsen PR, Blümel E, Rittig AH et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood 2019;134:1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen PB, Goyal S, Switchenko J, Tarabadkar E, Pouch S, Parikh P et al. Mitigation strategies among cutaneous T-cell lymphoma patients with positive Staphylococcus aureus skin and soft tissue cultures have unclear impacts on the risk of subsequent bacteremia. Leukemia & Lymphoma 2022:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelrod PI, Lorber B , Vonderheid EC. Infections Complicating Mycosis Fungoides and Sézary Syndrome. JAMA : the journal of the American Medical Association 1992;267:1354–8. [PubMed] [Google Scholar]
- 10.Blaizot R, Ouattara E, Fauconneau A, Beylot-Barry M , Pham-Ledard A. Infectious events and associated risk factors in mycosis fungoides/Sézary syndrome: a retrospective cohort study. British Journal of Dermatology 2018;179:1322–8. [DOI] [PubMed] [Google Scholar]
- 11.Ostrow S, Diggs CH, Sutherland J , Wiernik PH. Causes of death in patients with non-Hodgkin’s lymphoma. Cancer 1981;48:779–82. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y , Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 13.Blaizot R, Ouattara E, Fauconneau A, Beylot-Barry M , Pham-Ledard A. Infectious events and associated risk factors in mycosis fungoides/Sézary syndrome: a retrospective cohort study. British journal of dermatology (1951) 2018;179:1322–8. [DOI] [PubMed] [Google Scholar]
- 14.Abodunrin FO, Akinyemi OA, Ojo AS, Elleissy Nasef K, Haupt T, Oduwole A et al. Racial Disparities in Survival Among Non-Hodgkin Lymphoma Patients: An Analysis of the SEER Database (2007–2015). Curēus (Palo Alto, CA) 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA , Linos E. Incidence Trends of Primary Cutaneous T-Cell Lymphoma in the US From 2000 to 2018: A SEER Population Data Analysis. JAMA Oncol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnato AE, Alexander SL, Linde-Zwirble WT , Angus DC. Racial Variation in the Incidence, Care, and Outcomes of Severe Sepsis: Analysis of Population, Patient, and Hospital Characteristics. American journal of respiratory and critical care medicine 2008;177:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen M, LeWitt T, Pang Y, Bagnowski K, Espinosa ML, Choi J et al. Lifestyle, demographic and Skindex measures associated with cutaneous T-cell lymphoma: a single institution cohort study. Archives of Dermatological Research 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lili LN, Klopot A, Readhead B, Baida G, Dudley JT , Budunova I. Transcriptomic Network Interactions in Human Skin Treated with Topical Glucocorticoid Clobetasol Propionate. Journal of investigative dermatology 2019;139:2281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayers AA, Lyu L, Dance K, Ward KC, Flowers CR, Koff JL et al. Characterizing Lymphoma Incidence and Disparities for a Cancer Center Catchment Region. Clin Lymphoma Myeloma Leuk 2019;19:699–708.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller S, Lebowitz E, Pulitzer MP, Horwitz SM, Moskowitz AJ, Dusza S et al. Outcomes and prognostic factors in African American and black patients with mycosis fungoides/Sézary syndrome: Retrospective analysis of 157 patients from a referral cancer center. Journal of the American Academy of Dermatology 2020;83:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buechler CR, Sagher E, Tisack A, Jacobsen G, Lim HW, McHargue C et al. Contribution of Socioeconomic Risk Factors within a Diverse Mycosis Fungoides Cohort from Detroit, MI. Journal of the American Academy of Dermatology 2021. [DOI] [PubMed] [Google Scholar]
- 22.Balagula Y, Dusza SW, Zampella J, Sweren R , Hinds GA. Early-onset mycosis fungoides among African American women: a single-institution study. J Am Acad Dermatol 2014;71:597–8. [DOI] [PubMed] [Google Scholar]
- 23.Sun G, Berthelot C, Li Y, Glass DA 2nd, George D, Pandya A et al. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. J Am Acad Dermatol 2009;60:231–5. [DOI] [PubMed] [Google Scholar]
- 24.Arnold JD, Crockett RM , Kirkorian AY. Hospital readmissions among patients with skin disease: A retrospective cohort study. Journal of the American Academy of Dermatology 2018;79:696–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


