Abstract
A synthetic method for the reductive transformation of nitroarenes into ortho-aminated and -annulated products is reported. The method operates via the exhaustive deoxygenation of nitroarenes by an organophosphorus catalyst and a mild terminal reductant to access aryl nitrenes, which after ring expansion are trapped by amine nucleophiles to give dearomatized 2-amino-3H-azepines. Treatment of these ring-expanded intermediates with acyl electrophiles triggers 6π electrocyclization to extrude the nitrogen atom and restore aromaticity of the phenyl ring, delivering via C–H activation 2-aminoanilide and benzimidazole products—important scaffolds in industrially relevant and bioactive molecules.
Graphical Abstract
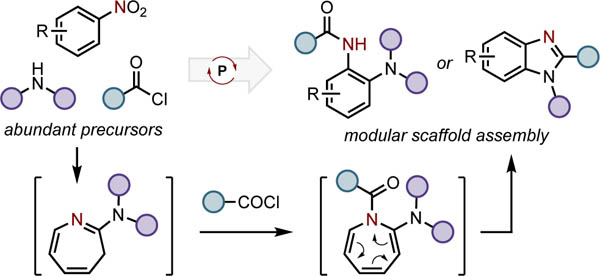
Nitroarenes are readily-accessed and versatile starting materials for synthesis.1 The nitro moiety itself can be transformed through direct reductive transformation2–3,4 or ipso substitution5 to provide routes to a range of synthetically useful benzenoid intermediates (Figure 1A, a). Alternatively, the nitro moiety can be used to facilitate C–H functionalization reactions6 that decorate the aryl periphery with retention of the nitro group.7 Such methods include vicarious nucleophilic substitution (SNArH, Mąkosza reaction), oxidative nucleophilic substitution of hydrogen (ONSH) reactions,8,9 and directed transition metal-catalyzed C–H functionalization (Figure 1A, b).10,11 A valuable subclass of nitroarene functionalization reactions accomplishes both nitro group reduction and proximal C–H functionalization simultaneously, permitting annulation as exemplified in the Bartoli indole synthesis12 and catalytic indole-forming methods (Figure 1A, c).13 To move beyond indole synthesis, a complementary annulative approach to nitroarene C/N-difunctionalization would ideally provide a synthetically modular method for the synthesis of heterocyclic ring systems, providing the synthetic chemist with control over the functionality incorporated into annulated products (Figure 1A, d).
Figure 1.
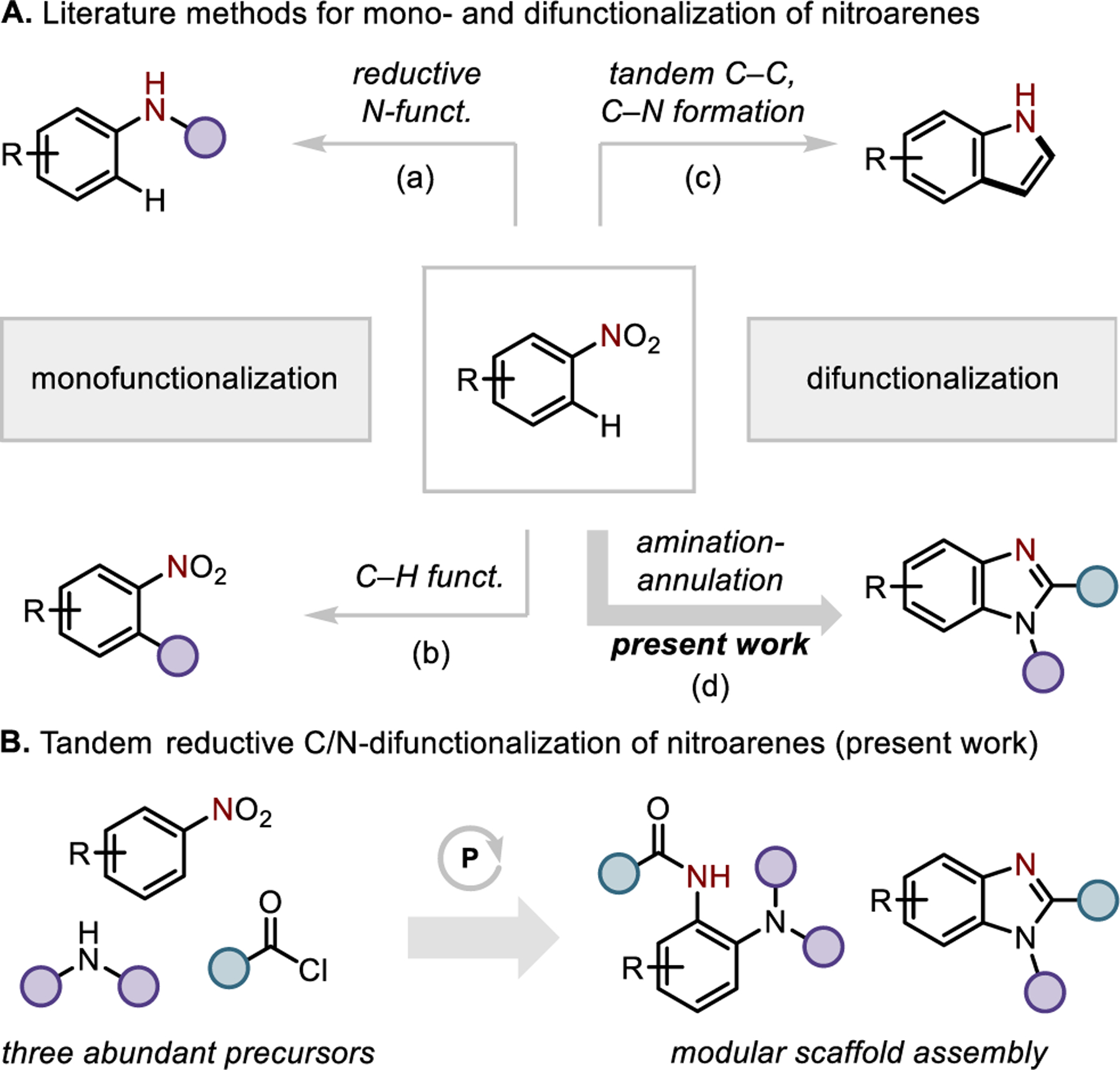
(A) Mono- and difunctionalization of nitroarenes. (B) Reductive C/N-difunctionalization of nitroarenes as modular entry to 2-aminoanillides and benzimidazoles.
Towards this goal, a one-pot, three-component coupling protocol utilizing commercially available components was conceived, which relies on the potential of transient nitrogen intermediates—formed under mild conditions from abundant nitroarenes—to participate both as direct sites of bond formation and as indirect activators of proximal sites (Figure 1B). On the basis of prior work,14 we expected that a redox-active organophosphorus catalyst could drive exhaustive deoxygenation of a nitroarene substrate by P(III)/P(V) cycling15 to yield a high-energy arylnitrene intermediate (Figure 2). Commonly generated by direct photolytic decomposition of phenylazide,16
Figure 2.
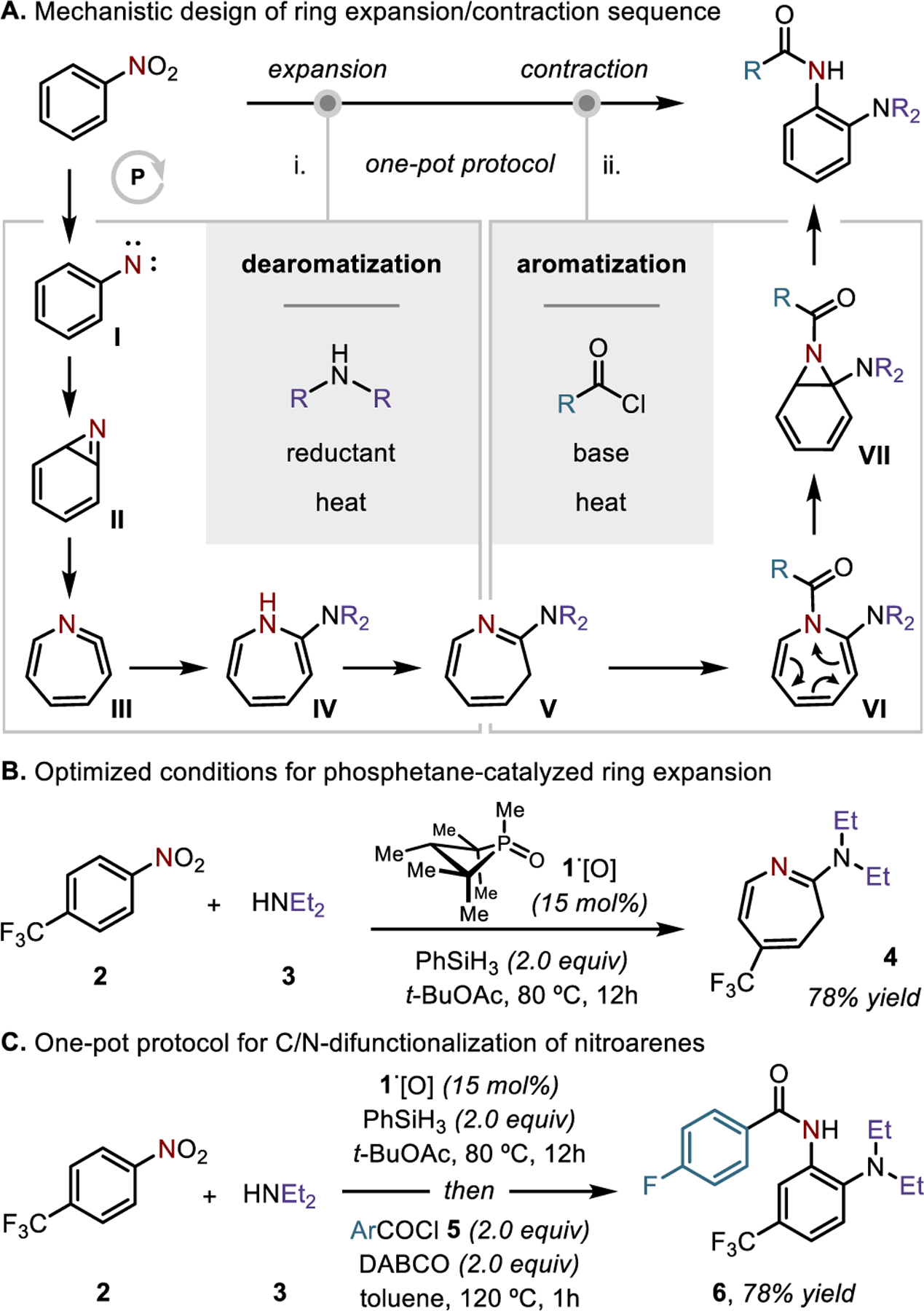
(A) Mechanistic outline for the reported C/N-difunctionalization of nitroarenes. (B) Conditions for the reductive ring expansion of nitroarenes via arylnitrenes. (C) Synthetic conditions for the one-pot C/N-difunctionalization of nitroarenes.
Phenylnitrene17 (I) is well-known to isomerize to benzazirine18 (II) and dehydroazepine19 (III) valence tautomers, which are susceptible to interception by nucleophilic trapping agents such as amines to give 2-amino-3H-azepines (V) by ring expansion.20–21,22 Subsequent isomerization of V was envisioned on the conjecture23 that an azepine-to-azanorcaradiene 6π electrocyclization24 (VI→VII) would be favored by N-acylation, and that decomposition of the fused bicyclic aminal VII could evolve with rearomatization of the arene core.25 The resulting 2-aminoanilide product VIII is then poised to undergo thermal cyclization under established conditions to arrive at the desired benzimidazole product.
Overall, this synthetic approach would result in a reductive amination of the substrate nitroarene, in which both nitro group reduction and C(sp2)–H amination are accomplished through a transient ring expansion of the original aromatic system. The method presents a complement to recent work by Burns,26 which leverages the intermediacy of the azepine ring to perform a nitrogen-for-carbon switch to yield 2-aminopyridines upon ring contraction. Here, we report the development of a one-pot synthetic method for the reductive C/N-difunctionalization of nitroarenes via the intermediacy of high-energy arylnitrenes.27 This ability to generate arylnitrenes from nitroarenes under thermal catalytic conditions enables an expedient entry to 2-aminoanilide and benzimidazole products scaffold useful in pharmaceutical discovery.28,29
In prior work from our group, we established that reductive functionalization of nitroarenes with primary and secondary amine nucleophiles under conditions of P(III)/P(V)=O redox cycling proceeds by direct N-functionalization, culminating in N–N bond formation to yield unsymmetrical hydrazine products.4f However, as exemplified by the reaction of 4-trifluoromethylnitrobenzene (2) and diethylamine (3), we find that related conditions (15 mol % of 1,2,2,3,4,4-hexamethylphosphetane P-oxide 1·[O]30 as catalyst, 2.0 equivalents of phenylsilane as terminal reductant) but with omission of an explicit Brønsted acid31 lead instead to 2-diethylamino-5-trifluoromethyl-3H-azepine (4) in 78% yield. Presumably, under these conditions, the nitroarene 2 is iteratively deoxygenated by the P(III)/P(V)=O redox catalyst to generate the corresponding arylnitrene,4b which then evolves along the sequence indicated in Figure 2A to the 2-amino-3H-azepine. These thermal P(III)/P(V)=O catalytic conditions can be applied broadly for formation of 2-substituted-3H-azepines; a synthetic scope for this conversion is included in the SI (Figures S1 and S2).
Importantly, as shown in Figure 2C, the conditions optimized for the formation of azepine 4 could be extended to a two-part synthetic expansion/contraction synthetic sequence, achieved in a single reaction vessel. Specifically, reaction of nitroarene 2 and diethylamine (3) with 15 mol% of 1,2,2,3,4,4-hexamethyl phosphetane P-oxide (1·[O]) and 2.0 equiv of phenylsilane in t-BuOAc for 12 h, followed by a solvent swap to PhMe and addition of 2.0 equiy each of acyl chloride 5 and DABCO lead directly to isolation of ortho-aminoanilide 6 in 78% yield (0.5 mmol scale). As a synthetic transformation, this manipulation brings together readily available nitroarene substrates like 2 with exogenous amines and acyl electrophiles to assemble highly decorated products in a modular fashion by tandem C/N-difunctionalization of the nitroarene substrate.
The scope and potential utility of this method are exemplified in Figures 3–5. 2-Aminoanilide products are reliably accessed by assembly from a range of nitroarenes, secondary amines, and acyl electrophiles (Figure 3). With respect to the amine component, beyond the use of diethylamine (7, 8) as a trapping amine nucleophile, several of the most prevalent secondary amine heterocycles found in pharmaceuticals including piperidine (9, 10, 14), piperazine (11), and morpholine (15–17) derivatives are also readily accessed. Arylalkylamine (13) derivatives are similarly incorporated, but less nucleophilic diarylamines are insufficiently reactive to trap the benazirine/dihydroazepine intermediate. In terms of electrophilic reaction partners, substituted benzoyl (7–9, 11–12, 14–15) and related heteroaryl derivatives (16) are viable partners, as are alkanoyl (10) and fluoroalkanoyl (13) compounds, providing (fluoro)acylated products in good yields.
Figure 3.
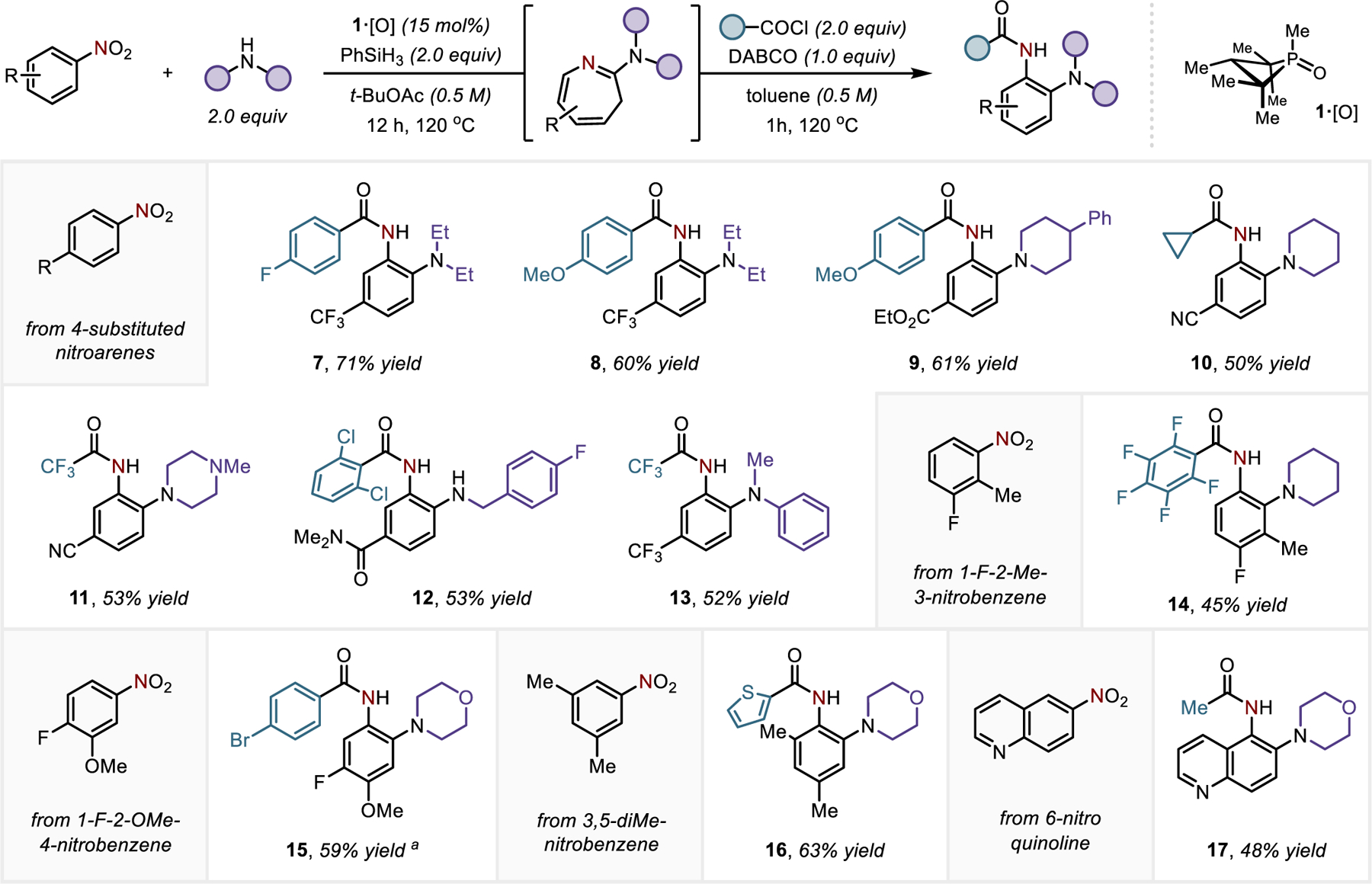
Synthesis of 2-aminoanilides by reductive C/N-difunctionalization of nitroarenes. a 8:1 regioisomeric ratio. See SI for full experimental details.
Figure 5.
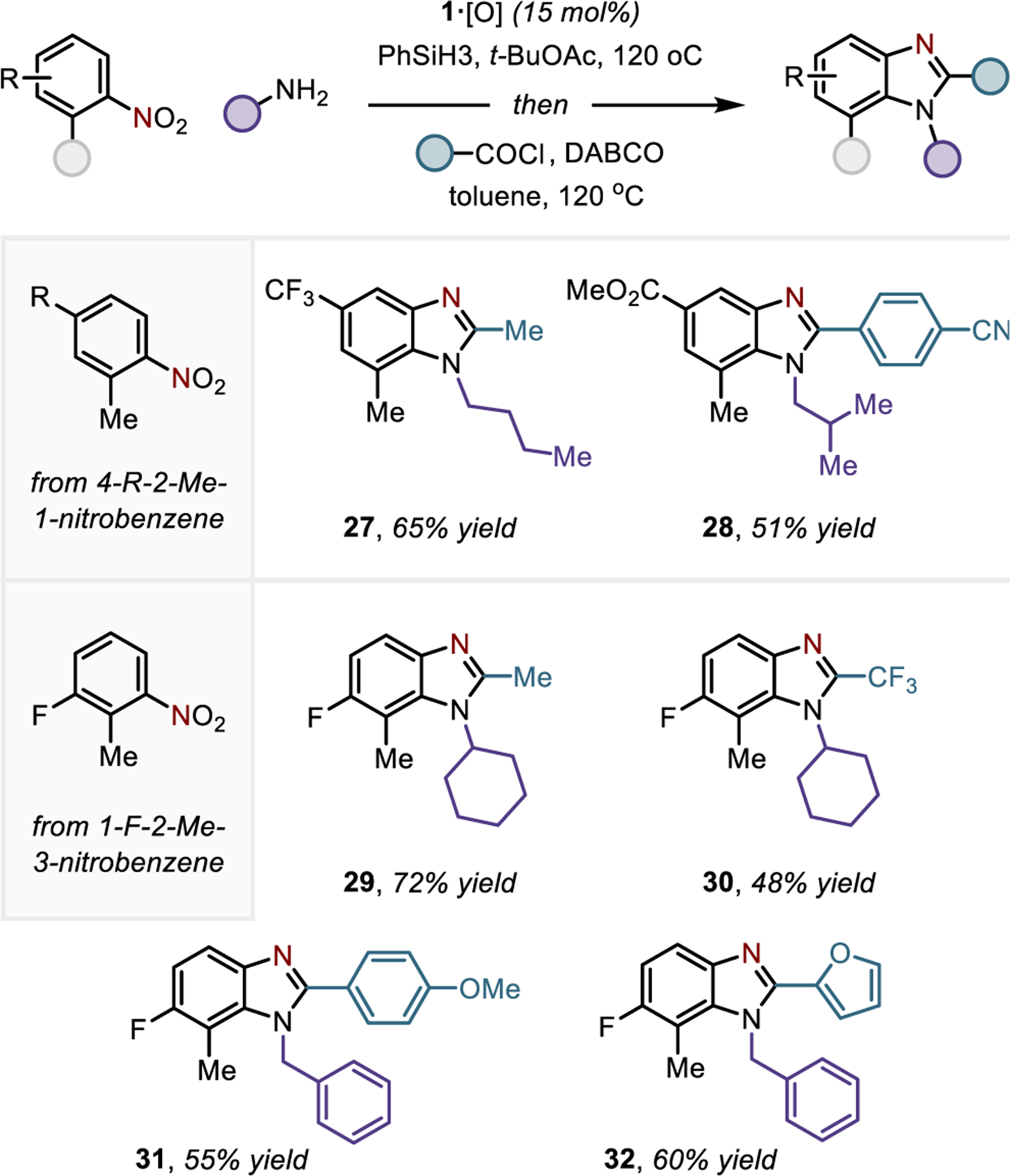
Regiospecific preparation of 1,2,7-benzimidazoles. See SI for full experimental details.
In terms of regiochemistry, nitroarenes bearing a symmetrical substitution patterns only generate one isomer following the ring expansion/contraction reaction sequence (for instance, 7-13, 16). However, for nitroarene substrates with substitution lacking mirror symmetry, two possible regioisomeric products are in principle possible. To a first approximation, the position of apparent C-H amination is dictated by steric considerations. For instance, product 14 is formed in as a single regioisomer, as a result of the insertion of arylnitrene to the sterically less encumbered position distal from the methyl substituent. Nevertheless, in the absence of a large steric bias, electronic effects dominate the regioselectivity, as exemplified by the product 15, in which arylnitrene prefers the insertion at the more electronically rich position. Consistent with electronic arguments, the quinolyl substrate (17) exhibits regioselectivity with apparent C-H amination proximal to the ring fusion. The unobserved regioisomer of the C/N-difunctionalization would need to arise from a benzazirine intermediate that enforces quinoidal structure on the heterocyclic system, whereas as the benzazirine leading to the observed C/N-difunctionalization product does not require disruption of heteroaromatic stabilization of the pyridyl subunit. Taken together, the C/N-difunctionalization method proceeds with predictable regiochemical preference if both steric and electronic factors are adequately considered.
Reactions with primary amines similarly enable the formation of corresponding 2-aminoanilide products, provided that the acyl electrophile employed is sterically encumbered (for instance, 2,6-chlorobenzoyl as in 12). However, when a primary amine nucleophile (e.g. benzylamine) is employed in conjunction with a sterically unencumbered acyl moiety (e.g. trifluoroacyl) then a subsequent cyclodehydration reaction spontaneously ensues under the reaction conditions to furnish a benzimidazole (18, Figure 4). A brief optimization revealed that the azepine contraction and subsequent aromatization/annulation could be achieved in a simple one-pot procedure (Table S2). This strategy could be applied to form benzimidazoles directly from nitroarenes, secondary amines, and acyl electrophiles (Figure 4); benzimidazole derivatives with N-benzyl (18-20, 23-24), N-aryl (21), and N-alkyl (22) substitution could be prepared with the same reductive selectivity for the nitro moiety compared to other reducible (ester 19, amide 24) functionality. With 3-nitroanisole as substrate, the reductive C/N-difunctionalization proceeds with excellent regioselectivity to give 1,2,6-trisubstituted benzimidazoles (25, 26) as the major product. Variation of the acyl electrophile allows control over substitution at the 2-position of the benzimidazole.
Figure 4.
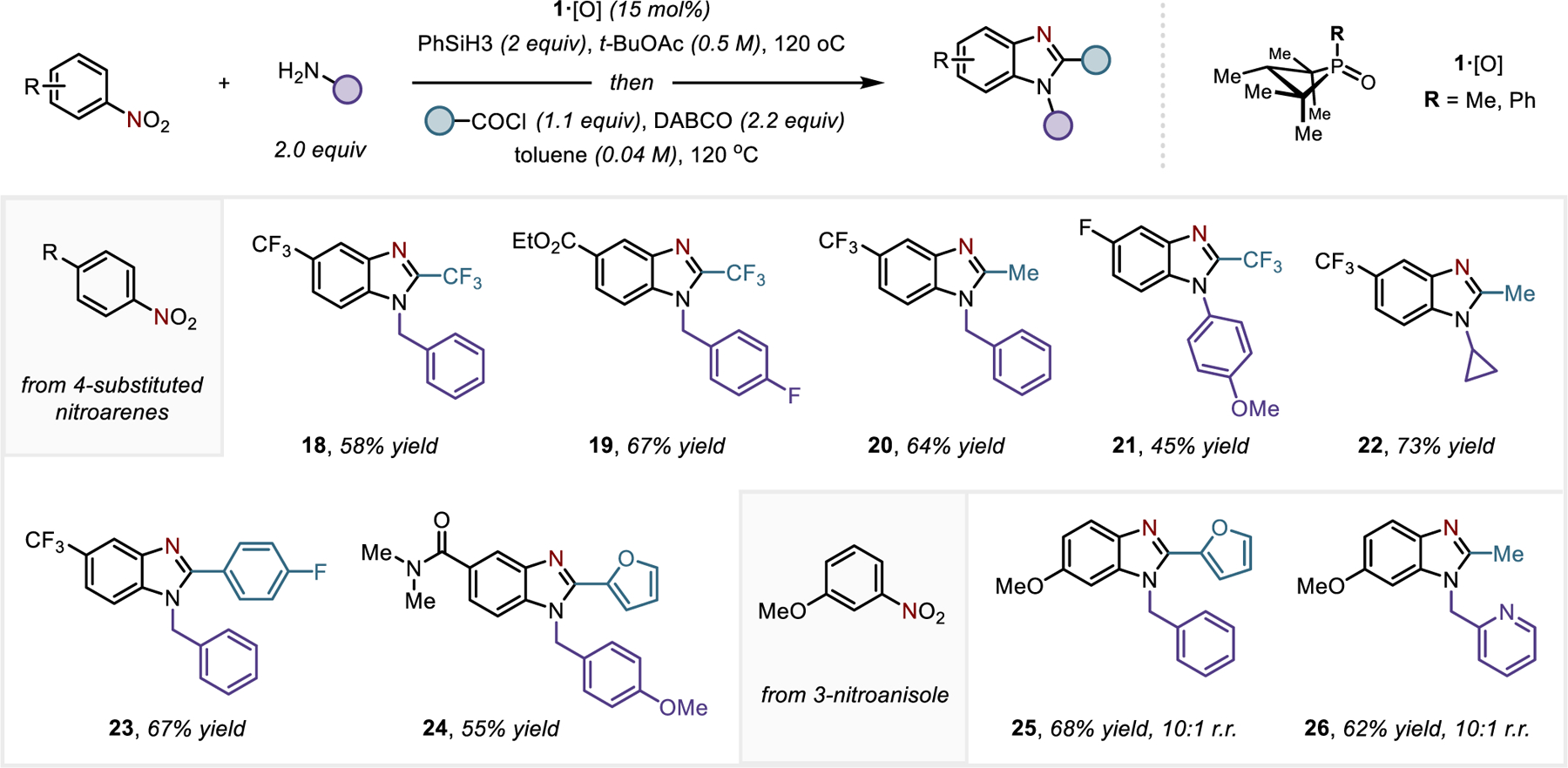
Synthesis of benzimidazoles by reductive C/N-difunctionalization of nitroarenes. See SI for full experimental details.
The modularity and regiochemical predictability of the reductive C/N-difunctionalization allows the benzimidazole to be viewed as a retron accessible by an annulative C/N-difunctionalization transform from simple nitroarenes possessing accessible ortho C-H positions. Practically, this feature may have use for the preparation of benzimidazoles with challenging substitution patterns, for instance where direct N-functionalization of the parent N–H benzimidazole would be unselective or unfavorable due to steric considerations affecting the pseudosymmetry of the N1 and N3 positions.32 In this vein, Figure 5 collects examples of various benzimidazole derivatives synthesized by this protocol with sterically encumbered N-alkyl substituents in the presence of 2-and 7-substitution. Annulated systems with N-substitution next to primary (27, 28, 31, 32) and secondary (29, 30) carbon sites could be synthesized in synthetically useful yields. Collectively, this modular one-pot procedure could be used to access a variety of 2-aminoanilides and benzimidazoles from a range of nitroarenes, primary and secondary amines, and acyl electrophiles.
In summary, the results described above constitute a novel strategy for reductive C–H amination of nitroarenes that leverages tandem C- and N-functionalization to construct valuable 2-aminoanilide and benzimidazole products from readily accessible reaction partners. Integral to the success of this method is the efficient thermal generation of reactive arylnitrenes from nitroarenes, and the ability to achieve ring contraction and aromatization of 3H-azepine intermediates. Taken together, these developments enable new expedient routes to 2-aminoanilides and benzimidazoles via a novel synthetic sequence for C–H functionalization and annulation. In connection with our efforts to explore the biphilic catalytic reactivity of phosphetanes for reductive O-atom transfer processes via the P(III)/P(V)=O redox cycling, this study portends future developments in tandem C/N-difunctionalization of nitroarenes and homologues to access a wide range of elaborated products.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by the National Institutes of Health under Award Number GM114547. G.L. thanks Bristol Myers Squibb for a graduate fellowship. M.N.L. thanks the National Institute of General Medical Sciences of the National Institutes of Health for support under Award Number F32 GM147996. We are grateful to Drs. P. Müller and M. Drance (MIT) for assistance with crystallographic data collection and refinement.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website at http://pubs.acs.org.
General methods and synthetic procedures (.pdf).
1H, 13C, 19F and 31P NMR spectra (.pdf).
The authors declare no competing financial interest
REFERENCES
- 1.Ono N The Nitro Group in Organic Synthesis; Wiley, New York, 2001. [Google Scholar]
- 2.(a) Stoichiometric main group metal approaches, see: Sapountzis I; Knochel P A New General Preparation of Polyfunctional Diarylamines by the Addition of Functionalized Arylmagnesium Compounds to Nitroarenes. J. Am. Chem. Soc 2002, 124, 9390. [DOI] [PubMed] [Google Scholar]; b Doyle W; Staubitz A; Knochel P Mild Synthesis of Polyfunctional Benzimidazoles and Indoles by the Reduction of Functionalized Nitroarenes with Phenylmagnesium Chloride. Chem. Eur. J 2003, 9, 5323. [DOI] [PubMed] [Google Scholar]; c Kopp F; Sapountzis I; Knochel P Preparation of Polyfunctionalized Amines by the Addition of Functionalized Organomagnesium Reagents to Nitrosoarenes. Synlett, 2003, 885. [Google Scholar]; d Sapountzis I; Knochel P A New Method for the Selective Amination of 1,3- and 1,4-Dinitrobenzenes and Protected Nitroanilines Leading to Polyfunctional 1,3- and 1,4- Disubstituted Anilines. Synlett 2004, 955. [Google Scholar]; e Dhayalan V; Saemann C; Knochel P Synthesis of polyfunctional secondary amines by the addition of functionalized zinc reagents to nitrosoarenes. Chem. Commun 2015, 51, 3239. [DOI] [PubMed] [Google Scholar]; f Gao H; Xu Q-L; Ess DH; Kürti L Transition-Metal-Free, Low-Temperature Intramolecular Amination of Aromatic C-H Bonds: Rapid Synthesis of Fused Heterocycles.” Angew. Chem. Int. Ed 2014, 53, 2701. [DOI] [PubMed] [Google Scholar]; g Rauser M; Ascheberg C; Niggemann M Electrophilic Amination with Nitroarenes. Angew. Chem., Int. Ed 2017, 56, 11570. [DOI] [PubMed] [Google Scholar]; h Rauser M; Ascheberg C; Niggemann M Direct Reductive N-Functionalization of Aliphatic Nitro Compounds. Chem. Eur. J 2018, 24, 3970. [DOI] [PubMed] [Google Scholar]; i Rauser M; Warzecha DP; Niggemann M O2-Mediated Oxidation of Aminoboranes through 1,2-N Migration. Angew. Chem., Int. Ed 2018, 57, 5903. [DOI] [PubMed] [Google Scholar]; j Rauser M; Eckert R; Gerbershagen M; Niggemann M Catalyst-Free Reductive Coupling of Aromatic and Aliphatic Nitro Compounds with Organohalides. Angew. Chem., Int. Ed 2019, 58, 6713. [DOI] [PubMed] [Google Scholar]
- 3.(a) Catalytic transition metal approaches, see: Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. [DOI] [PubMed] [Google Scholar]; b Cheung CW; Hu X Amine synthesis via iron-catalysed reductive coupling of nitroarenes with alkyl halides. Nat. Commun 2016, 7, 12494. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cheung CW; Ploeger ML; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal 2017, 7, 7092–7096. [Google Scholar]; d Xiao J; He Y; Ye F; Zhu S Remote Sp3 C–H Amination of Alkenes with Nitroarenes. Chem 2018, 4, 1645–1657. [Google Scholar]; e Suárez-Pantiga S; Hernández-Ruiz R; Virumbrales C; Pedrosa MR; Sanz R Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds. Angew. Chem. Int. Ed 2019, 58, 2129–2133. [DOI] [PubMed] [Google Scholar]
- 4.(a) Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; adosevich AT Intermolecular Reductive C−N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc 2018, 140, 15200–15205. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV=O-Catalyzed Reductive C−N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc 2020, 142, 6786–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nykaza TV; Li G; Yang J; Luzung MR; Radosevich AT PIII/PV=O-Catalyzed Cascade Synthesis of N-Functionalized Azaheterocycles. Angew. Chem. Int. Ed 2020, 59, 4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li G; Qin Z; Radosevich AT P(III)/P(V)-Catalyzed Methylamination of Arylboronic Acids and Esters: Reductive C−N Coupling with Nitromethane as a Methylamine Surrogate. J. Am. Chem. Soc 2020, 142, 16205–16210. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li G; te Grotenhuis C; Radosevich AT Reductive Csp2−N Coupling by PIII/PV=O−Catalysis. Trends Chem 2021, 3, 72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Li G; Miller SP; Radosevich AT PIII/PV=O-Catalyzed Intermolecular N–N Bond Formation: Cross-Selective Reductive Coupling of Nitroarenes and Anilines J. Am. Chem. Soc 2021, 143, 14464–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Li G; Kanda Y; Hong SY; Radosevich AT Enabling Reductive C-N Cross-Coupling of Nitroalkanes and Boronic Acids by Steric Design of P(III)/P(V)=O Catalysts. J. Am. Chem. Soc 2022, 144, 8242–8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Kashihara M; Nakao Y Cross-Coupling Reactions of Nitroarenes. Acc. Chem. Res 2021, 54, 2928–2935; [DOI] [PubMed] [Google Scholar]; b Muto K; Oshita T; Yamaguchi J Transition-Metal-Catalyzed Denitrative Coupling of Nitroarenes. ACS Catal 2020, 10, 9856–9871 [Google Scholar]
- 6.Sinha SK; Guin S; Maiti S; Biswas JP; Porey S; Maiti D Toolbox for Distal C-H Bond Functionalizations in Organic Molecules. Chem Rev 2022, 122, 5682. [DOI] [PubMed] [Google Scholar]
- 7.Terrier F Modern Nucleophilic Aromatic Substitution, Wiley-VCH, Weinheim, 2013. [Google Scholar]
- 8.(a) Mąkosza M; Krzysztof W Nucleophilic Substitution of Hydrogen in Heterocyclic Chemistry. Chem. Rev 2004, 104, 2631; [DOI] [PubMed] [Google Scholar]; b Mąkosza M; Winiarski J Acc. Chem. Res 1987, 20, 282. [Google Scholar]
- 9.(a) For amination by SNArH, see:Katritzky AR; Laurenzo KS Alkylaminonitrobenzenes by Vicarious Nucleophilic Amination with 4-(Alkylamino)-1,2,4,-triazoles. J. Org. Chem 1988, 53, 3978. [Google Scholar]; b Pagoria PF; Mitchell AR; Schmidth RD 1,1,1-Trimethylhydrazinium Iodide: A Novel, Highly Reactive Reagent for Aromatic Amination via Vicarious Nucleophilic Substitution of Hydrogen. J. Org. Chem 1996, 61, 2934–2935. [DOI] [PubMed] [Google Scholar]; c Seko S; Kawamura N Copper-Catalyzed Direct Amination of Nitrobenzenes with O-Alkylhydroxylamines. J. Org. Chem 1996, 61, 442–443. [DOI] [PubMed] [Google Scholar]; d Seko S; Miyake K; Kawamura N A Convenient Copper-Catalyzed Direct Amination of Nitroarenes with O-alkylhydroxylamines. J. Chem. Soc., Perkin Trans 1 1999, 1437. [Google Scholar]
- 10.Senguptam S; Das P C-H Activation Reactions of Nitroarenes: Current Status and Outlook. Org. Biomol. Chem 2021, 19, 8409. [DOI] [PubMed] [Google Scholar]
- 11.(a) Caron L; Campeau L-C; Fagnou K Palladium-Catalyzed Direct Arylation of Nitro-Substituted Aromatics with Aryl Halides. Org. Lett 2008, 10, 4533; [DOI] [PubMed] [Google Scholar]; b Tan E; Montesinos-Margraner M,; Garcia-Morales G; Mayans JG; Echavarren AM Rhodium-catalysed Ortho-alkynylation of Nitroarenes. Chem. Sci 2021, 12, 14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Bartoli G; Dalpozzo R; Nardi M Applications of Bartoli Indole Synthesis. Chem. Soc. Rev 2014, 43, 4728. [DOI] [PubMed] [Google Scholar]; b Bartoli G; Palmieri G; Bosco M; Dalpozzo R The Reaction of Vinyl Grignard Reagents with 2-Substituted Nitroarenes: A New Approach to the Synthesis of 7-Substituted Indoles 1989, 30, 2129. [Google Scholar]
- 13.(a) Penoni A; Nicholas KM A Novel and Direct Synthesis of Indoles via Catalytic Reductive Annulation of Nitroaromatics with Alkynes. Chem. Commun 2002, 484; [DOI] [PubMed] [Google Scholar]; b Özkaya B; Bub CL; Patureau FW Step and redox efficient nitroarene to indole synthesis. Chem. Commun 2020, 56, 13185–13188. [DOI] [PubMed] [Google Scholar]
- 14.(a) Cadogan JIG; Cameron-Wood M; Mackie RK; Searle RJG The reactivity of organophosphorus compounds. Part XIX. Reduction of nitro-compounds by triethyl phosphite: a convenient new route to carbazoles, indoles, indazoles, triazoles, and related compounds. J. Chem. Soc 1965, 4831–4837. [Google Scholar]; b Cadogan JIG Phosphite-Reduction of Aromatic Nitro-Compounds as a Route to Heterocycles. Synthesis 1969, 1969, 11–17. [Google Scholar]; c Cadogan JIG; Todd MJ Reduction of nitro- and nitroso-compounds by tervalent phosphorus reagents. Part IV. Mechanistic aspects of the reduction of 2,4,6-trimethyl-2′-nitrobiphenyl, 2-nitrobiphenyl, and nitrobenzene J. Chem. Soc. C 1969, 2808–2813. [Google Scholar]; d Sundberg RJ Deoxygenation of Nitro Groups by Trivalent Phosphorus. Indoles from o-Nitrostyrenes. J. Org. Chem 1965, 30, 3604–3610. [Google Scholar]; e Nykaza TV; Ramirez A,; Harrison TS; Luzung MR; Radosevich AT Biphilic Organophosphorus-Catalyzed Intramolecular Csp2-H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 2018, 140, 3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Lao Z; Toy PH Catalytic Wittig and aza-Wittig Reactions. Beilstein J. Org. Chem 2016, 12, 2577–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Guo H; Fan YC; Sun Z; Wu Y; Kwon O Phosphine Organocatalysis. Chem. Rev 2018, 118, 10049–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lipshultz JM; Li G; Radosevich AT Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15. J. Am. Chem. Soc 2021, 143, 1699–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xie C; Smaligo AJ; Song XR; Kwon O Phosphorus Based Catalysis. ACS Cent. Sci 2021, 7, 536–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levya E; Platz MS; Moctezuma E Investigation of phenyl azide photochemistry by conventional and time-resolved spectroscopy. Elucidation of intermediates and reaction mechanisms. J. Photochem. Photobiol 2022, 11, 100126. [Google Scholar]
- 17.(a) Borden WT; Gritsan NP; Hadad CM; Karney WL; Kemnitz CR; Platz MS The Interplay of Theory and Experiment in the Study of Phenylnitrene. Acc. Chem. Res 2000, 33, 765–771. [DOI] [PubMed] [Google Scholar]; b Karney WL; Borden WT Ab Initio Study of the Ring Expansion of Phenylnitrene and Comparison with the Ring Expansion of Phenylcarbene. J. Am. Chem. Soc 1997, 119, 1378. [Google Scholar]; c Gritsan NP; Likhotvorik I; Tsao ML; Çelebi N; Platz MS; Karney WL; Kemnitz CR; Borden WT Ring-Expansion Reaction of Cyano-Substituted Singlet Phenyl Nitrenes: Theoretical Predictions and Kinetic Results from Laser Flash Photolysis and Chemical Trapping Experiments. J. Am. Chem. Soc 2001, 123, 1425. [Google Scholar]
- 18.Inui H; Sawada K: Oishi S; Ushida K; McMahon RJ J. Am. Chem. Soc 2013, 135, 10246. [DOI] [PubMed] [Google Scholar]
- 19.Chapman OL; Le Roux JP 1-Aza-1,2,4,6-cycloheptatetraene. J. Am. Chem. Soc 1978, 100, 282–285 [Google Scholar]
- 20.(a) Huisgen R; Vossius D; Appl M Die Thermolyse des Phenylazids in primären Aminen; die Konstitution des Dibenzamils. Chem. Ber 1958, 91, 1–12. [Google Scholar]; b Doering W; Odum RA Ring Enlargement in the Photolysis of Phenyl Azide. Tetrahedron 1966, 22, 81–93 [Google Scholar]
- 21.Iddon B; Meth-Cohn O; Scriven EFV; Suschitzky H; Gallagher PT Developments in Arylnitrene Chemistry: Syntheses and Mechanisms. Angew. Chem. Int. Ed 1979, 18, 900–917. [Google Scholar]
- 22.(a) Previous reports of trivalent phosphorous reagents-mediated 3H-azepine formation from nitroarene, see Streef W, J.; van der Plas C, H. Chemical Evidence for a Didehydroazepine in Reactions of Halogenoazepines with a Strong Base. Heterocycles 1985, 23, 2715. [Google Scholar]; b Ulfa SM; Okamoto H; Satake K Unprecedented Temperature-Dependent Formation of 3- and 7-Methyl-3H-Azepine Derivatives by the Reaction of o-Nitrotoluene with Tributylphosphine in Nucleophilic Media. Chem. Lett 2012, 41, 400–402. [Google Scholar]
- 23.(a) Paquette LA; Kuhla DE; Barrett JH Unsaturated heterocyclic systems. LIII. Thermochemical reactions of 1H-azepine derivatives. 2. Aromatization and sigmatropic migrations involving nitrogen. J. Org. Chem 1969, 34, 2879–2884. [Google Scholar]; b Storer W-D; Hoffmann R Effect of Protonation on Aziridine and Oxirane Bond Strengths. Angew. Chem. Int. Ed 1972, 11¸825–826. [Google Scholar]; c Atherton FR; Lambert RW Nitrenes Generated from Nitro-compounds by Various Phosphorus Reagents in Heterocyclic Synthesis. A Convenient Route to Substituted 3H-Azepines. J Chem Soc Perkin Trans, 1973, 1079–1084. [Google Scholar]; d Anderson DJ; Hassner A; Tang DY Reactions of 3H-Azepines Derived from Cyclopentadienones and 1-Azirines. J. Org. Chem 1974, 39, 3076–3080. [Google Scholar]; e Smalley RK; Strachan WA; Suschitzky H Thermolysis of Aryl Azides in Benzoyl Chloride. Tetrahedron Lett 1974, 10, 825–828. [Google Scholar]
- 24.(a) Jansen H;Slootweg JC;Lammertsma K Valence isomerization of cyclohepta-1,3,5-triene and its heteroelement analogues. Beilstein J. Org. Chem 2011, 7, 1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mandal N; Das A; Hajra C; Datta A Stereoelectronic and Dynamical Effects Dictate Nitrogen Inversion During Valence Isomerization in Benzene Imine. Chem. Sci 2022, 13, 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Paquette LA In Nonbenzenoid Aromatics, Snyder JP, Ed.; Academic Press, New York; Vol. 1, p. 293. [Google Scholar]; b Paquette LA Valence Isomerism in Selected Heterocyclic Systems. Angew. Chem. Int. Ed 1970, 10, 11–20. [Google Scholar]
- 26.Patel SC; Burns NZ Conversion of Aryl Azides to Aminopyridines. J. Am. Chem. Soc 2022, 144, 17797–17802. [DOI] [PubMed] [Google Scholar]
- 27.Gritsan NP; Platz MS Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes. Chem. Rev 2006, 106, 3844–3867 [DOI] [PubMed] [Google Scholar]
- 28.(a) Selected examples of the pharmaceutical importance of 2-amino anilidines: Bailey BL; Nguyen W; Ngo A; Goodman CD; Gancheva MR; Favuzza P; Sanz LM; Gamo FJ; Lowes KN; McFadden GI; et al. Optimisation of 2-(N-Phenyl Carboxamide) Triazolopyrimidine Antimalarials with Moderate to Slow Acting Erythrocytic Stage Activity. Bioorg. Chem 2021, 115, 105244; [DOI] [PubMed] [Google Scholar]; b Getlik M; Smil D; Zepeda-Velázquez C; Bolshan Y; Poda G; Wu H; Dong A; Kuznetsova E; Marcellus R; Senisterra G; Dombrovski L Hajian T; Kiyota T; Schapira M; Arrowsmith CH; Brown PJ; Vedadi M; Al-awar R Structure-Based Optimization of a Small Molecule Antagonist of the Interaction between WD Repeat-Containing Protein 5 (WDR5) and Mixed-Lineage Leukemia 1 (MLL1). J. Med. Chem 2016, 59, 2478–2496; [DOI] [PubMed] [Google Scholar]; c Siqueira RP; Barros M. V. de A.; Barbosa É. de A. A.; Onofre TS; Gonçalves VHS; Pereira HS; Silva Júnior A; de Oliveira LL; Almeida MR; Fietto JLR; et al. Trifluoromethyl Arylamides with Antileukemia Effect and Intracellular Inhibitory Activity over Serine/Arginine-Rich Protein Kinases (SRPKs). Eur. J. Med. Chem 2017, 134, 97. [DOI] [PubMed] [Google Scholar]
- 29.Vitaku E; Smith DT; Njadarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- 30.Nykaza TV; Cooper JC; Radosevich AT “Preparation of anti-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide and Its Application in PIII/PV=O Redox Catalysis.” Org. Synth 2019, 96, 418–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The effect of Brønsted acid in the organophosphorus-catalyzed reductive N–N coupling (ref 4f) is to promote the condensation between nitrosoarene intermediates and amine nucleophiles. When omitting the Brønsted acid additive, the phosphetane-catalyzed deoxygenation of nitrosoarene to nitrene intermediate dominates, leading to 3H-azepine products.
- 32.Ziegler DT; Choi J; Muñoz-Molina JM; Bissember AC; Peters JC; Fu GC A Versatile Approach to Ullmann C-N Couplings at Room Temperature: New Families of Nucleophiles and Electrophiles for Photoinduced, Copper-Catalyzed Processes. J. Am. Chem. Soc 2013, 135, 13107–13112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


