Abstract
The aryl hydrocarbon receptor (AhR) is a key regulator in the microbiome-gut-brain axis, and AhR-active microbial metabolites modulate multiple neuronal responses. We recently demonstrated that 3,3’-diindolylmethane (DIM) and 1,4-dihydroxy-2-naphthoic acid (DHNA), two selective AhR modulators (SAhRMs), act as antidepressants in female mice. Thus, to examine the role of intestinal AhR in depression, anxiety, and spatial learning, this study employed transgenic mice in which the AhR was knockout only in the intestinal epithelium (AhRΔIEC). Additionally, this study examined whether the antidepressant effects of dietary DIM and DHNA is mediated by intestinal AhR. AhRΔIEC and WT female mice were fed daily with vehicle, 20 mg/kg DIM or DHNA for three weeks prior to four weeks of unpredictable chronic mild stress (UCMS). Mice were examined for weight gain, anhedonia-like behavior (sucrose preference test), anxiety levels (open field, light/dark, elevated plus maze, novelty-induced hypophagia, and marble burying tests), and spatial learning (Morris water maze). UCMS reduced weight gain in AhRΔIECs, but not WTs. Moreover, UCMS initially reduced sucrose preference in both AhRΔIECs and WTs, but over 4 weeks of UCMS, AhRΔIECs develop resilience to UCMS-induced anhedonia. Additionally, AhRΔIECs exhibit slightly reduced anxiety in certain tests and faster spatial learning. DIM and DHNA acted as antidepressants in both AhRΔIECs and WTs. Thus, this study suggests that intestinal AhR plays differential roles, mitigating stress effects on weight gain, and increasing stress effects on mood. However, the site of antidepressant action of SAhRMs, such as DIM and DHNA, is not dependent on the expression of intestinal AhR.
Keywords: Aryl hydrocarbon receptor (AhR); Microbiome-gut-brain axis; Depression; Anxiety; Spatial learning; 3,3'-Diindolylmethane (DIM); 1,4-dihydroxy-2-naphthoic acid (DHNA)
Graphical Abstract
1. Introduction
The gut is estimated to host 3.8·1013 microbes, known as the gut microbiota [1, 2]. These gastrointestinal (GI) microflora play an important role in the reciprocal communication between the central nervous system (CNS) and the peripheral intestinal functions and affect activation of neural pathways and mental status: a system known as the microbiome-gut-brain axis [3]. Specifically, gut microbiota disturbance is associated with major depressive disorder (MDD) [4-7]. Transplantation of fecal microbiota from MDD patients into germ-free mice resulted in depression-like behaviors, while fecal microbiota transplantation from healthy individuals decreased depressive and anxiety symptoms in both preclinical and clinical studies [8-10]. Furthermore, administration of the probiotic bacteria Lactobacillus (Firmicutes) and Bifidobacterium (Actinobacteria) to germ-free animals reduced depression- and anxiety-like behaviors [11, 12].
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that binds diverse ligands, including phytochemicals, AhR-active tryptophan metabolites, dietary components, microbiota-derived metabolites, and pharmaceuticals [13-16]. AhR is expressed across many organs and tissues, is recognized for its broader physiological role, and was suggested to play a key role in the microbiome-gut-brain axis [17, 18]. There are several studies demonstrating interactions between the gut microbiome and AhR-active microbial metabolites on physiological and pathological processes in the brain [17, 19-22]. Additionally, intestinal AhR expression can modify the composition of intestinal microbial metabolites [23-25]. Tryptophan diets that are metabolized to AhR ligands or dietary supplements of indole, indoxyl-3-sulfate, indole-3-propionic acid, indole-3-aldehyde and tryptamine regulate neurogenesis and the activity of brain-specific cell types, and attenuate experimentally-induced multiple sclerosis [26-29].
Our recent study demonstrated that orally administered 3,3'-diindolylmethane (DIM) and 1,4-dihydroxy-2-naphthoic acid (DHNA), two selective AhR modulators (SAhRMs), act as antidepressants in female mice [30]. Specifically, female mice were fed with DIM or DHNA before being subjected to unpredictable chronic mild stress (UCMS), an established rodent model of depression with high translational potential and relevance to human depression [31]. Both DIM and DHNA prevented UCMS-induced anhedonia-like behavior, as measured by a decrease in sucrose preference [30], a rodent model of depression-like behavior [32]. Anhedonia, a core feature of MDD, is the decreased ability to experience pleasure [33]. Moreover, 3,7-dihydroxy-2-naphthoic acid (3,7-DHNA), a structurally similar analog of DHNA that is predominantly inactive as an AhR ligand [34], did not prevent UCMS-induced anhedonia-like behavior [30], which suggests AhR plays a role in protecting against the effects of UCMS.
DIM is derived from the ingestion and subsequent dimerization of indole-3-carbinol (I3C, also an AhR agonist) a chemoprotective phytochemical from cruciferous vegetables, which is also available as a dietary supplement [35]. DHNA is a bacteria-derived metabolite produced by Propionibacterium freudenreichii and Lactobacillus casei [36-38]. Given the suggested roles of gut microbiota and AhR in mental states, and particularly in depression, in this study we employed transgenic mice in which the AhR was specifically knocked out only in the intestinal epithelium (AhRΔIEC) to examine the role of intestinal AhR in depression, anxiety, and spatial learning. Additionally, we examine whether the antidepressant effects of dietary DIM and DHNA are dependent on intestinal AhR expression.
2. Methods
2.1. Animals
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Constitutive villin Cre promoter was used to target Cre recombination to epithelial cells in the GI tract in AhR floxed (AhRf/f) mice (villinCre x AhRf/f = AhRΔIEC) [24, 39]. Female C57BL/6J intestinal epithelium-specific AhR knockout mice (AhRΔIEC) and their wildtype (AhRf/f) littermates were used in the study. Mouse breeders and offspring were co-housed to increase the likelihood that the microbiomes of the mice were similar. They were housed in a temperature-controlled (21 +/− 2°C, humidity 45%) vivarium with a 12-hour light/12-hour dark cycle (light on at 7:30 AM) with food and water ad libitum. Unless specifically mentioned, all tests were conducted in rooms containing low illumination (60 W white light) and 40 dB white noise generator.
2.2. SAhRM Treatment
3,3'-Diindolylmethane (DIM) and 1,4-dihydroxy-2-naphthoic acid (DHNA) were purchased from Sigma-Aldrich (St. Louis, MO). Mice were fed with vehicle, 20 mg/kg DIM or 20 mg/kg DHNA dissolved in corn oil and mixed into melted peanut butter [30]. Mice were placed in individual cages for approximately 10-30 minutes until they finished eating and were then returned to their home cage. This method was chosen to avoid the stress of daily gavage injection [40].
2.3. Unpredictable chronic mild stress (UCMS)
The UCMS protocol is described in detail in our recent publication [30]. Briefly, this protocol does not include food or water deprivation. Seven stressors were administered weekly over the course of 4-5 weeks - restraint, wet cage, cage replacement, tilted cage, dampened bedding, empty cage, and light/dark disruption. The order of appearance and daily schedule of each stressor was altered weekly.
2.4. Sucrose preference
Mice were monitored once weekly for their preference to consume sucrose. Once weekly, for 2 consecutive days, animals were individually housed for 4 hrs with access to one bottle of water and one bottle of 2% sucrose. The bottles were placed side by side, were freely available, and their positions were switched daily in order to account for a side preference. Sucrose preference was calculated as [milliliters sucrose solution consumed]/[milliliters sucrose solution + milliliters water consumed]. For each mouse, data was averaged across the 2 consecutive days.
2.5. Open Field Test (OFT)
This test was conducted in an automated optical beam activity monitor [40 x 40 x 30.5 (height) cm] (San Diego Instruments, San Diego, CA). A multiplexor-analyzer, which is interfaced to a PC, simultaneously tracks the interruption of beams from the optical beam activity monitors. The animal’s position in the apparatus is updated every 10 ms. The integration of the data (using PAS Reporter software) about the location of the animals was used to determine the duration of time spent in each zone (in seconds). Subjects were placed in the center of the box to begin a 10-minute test session. The computerized integration of the data was used to score time spent in the center and in the periphery.
2.6. Light/Dark (L/D) test
Based on [41], this test is assessed in the same activity boxes as the OFT, split into an 18x18 cm dark chamber and a light zone. A mouse was placed in the middle of the light chamber and recorded for 10 min. The computerized integration of the data was used to score time spent in the light and dark zones.
2.7. Elevated plus Maze (EPM)
Like in our previous studies [42], the EPM apparatus consists of four arms (87 mm wide, 155 mm long) elevated 63.8 cm above the ground, with two arms enclosed on two sides by 16.3 cm high opaque walls. Mice were placed in the center of the maze facing toward an enclosed arm and recorded for 10 min by an overhead camera. Behaviors were scored from the video for the duration of time spent in the open arms (defined as all four legs having crossed the entrance line to one of the open arms), and the total number of crosses into the center compartment.
2.8. Novelty-Induced Hypophagia (NIH)
Based on [43], mice were introduced in their home cage to diluted condensed milk for 30 minutes daily for 3 consecutive days. Carnation sweetened condensed milk, diluted 1:3 in water, was provided in plastic serological pipettes (10 mL) with attached sippers and rubber stoppers that are mounted to the wire cage lid. On day 4, mice were tested individually in their home cages with low illumination level (50 lux). Each mouse was removed from the cage while the pipette is installed on the cage lid. Testing began immediately upon returning to the home cage. On day 5, mice were tested in a novel cage free of bedding and in bright illumination level (1200 lux). On both testing days, mice were recorded for the latency to the first sip of milk.
2.9. Marble Burying (MB) test
Based on [44], each mouse was placed in a large cage (40 × 24 × 20 cm) filled with bedding 5 cm deep from the cage floor and 20 blue marbles (positioned in 4 × 5 grid) for 30 min. Marbles were counted as buried if 2/3 or more was covered by bedding. Number of buried marbles was recorded.
2.10. Morris water maze (MWM)
Based on [45]. A 36” diameter pool was used as the maze with a 5in2 clear Plexiglas platform placed at the eastern equator 7in from the edge of the pool. The room featured distinct visual cues on the northern, eastern, and western walls, and the experimenter stood in a marked position in the southern side of the room. The time to reach the platform was recorded on six consecutive days. On days 1-5, mice were subjected to 4 daily trial sessions; the start position was rotated across days and sessions, and the time to reach the platform over the 4 daily sessions was averaged. On day 6, the challenge day, a considerably different, previously unused, start position was chosen and the time to reach the platform was recorded.
2.11. Experimental Design
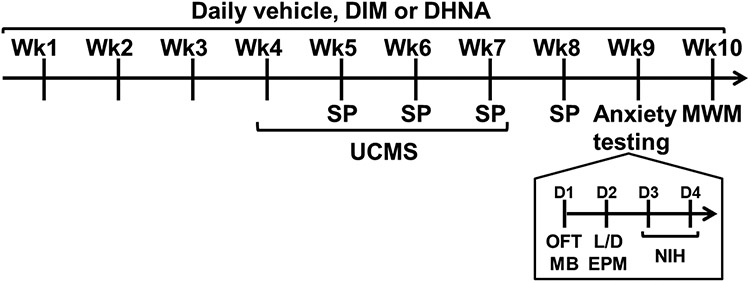
As demonstrated in Fig 1, following a habituation period, mice (8-10 weeks old; n=6-10 per experimental group) were administered daily with vehicle, DIM, or DHNA for 10 weeks. During weeks 4-7 of ligand administration, mice either remained unstressed or were subjected to the UCMS protocol. Sucrose preference tests were conducted once weekly at the end of weeks 5-8 of ligand administration. During week 9 of ligand administration, all mice were tested for their behaviors in the following paradigms in the same order: OFT (Day 1 in the morning), MB (day 1 in the afternoon), L/D test (Day 2 in the morning), EPM (Day 2 in the afternoon), and NIH (days 4 and 5). During week 10, mice were tested in the MWM task.
Fig 1. Experimental design.
Wk = Week; D = Day; Anxiety testing = testing in the Open Field Test (OFT), Marble Burying (MB), Light/Dark (L/D) test, Elevated Plus Maze (EPM), and Novelty-Induced Hypophagia (NIH) paradigms; UCMS = Unpredictable chronic mild stress; SP = sucrose preference test; MWM = Morris water maze.
Results presented in section 3.1 focus on role of intestinal AhR in depression-like behavior, anxiety and cognition. For this comparison we analyzed the data from mice that received vehicle, and this section compares the behaviors of WT/NS (n=10), WT/UCMS (n=10), AhRΔIEC/NS (n=7), and AhRΔIEC/UCMS (n=10). NS = No stress. Results presented in section 3.2 focus on a secondary question of whether intestinal AhR mediated the antidepressant effects of DIM and DHNA. For this comparison we analyzed the data from mice that were subjected to UCMS, and this section compares the behaviors of WT/NL (n=10), WT/DIM (n=6), WT/DHNA (n=6), AhRΔIEC/NL (n=10), AhRΔIEC/DIM (n=9), AhRΔIEC/DHNA (n=9). NL = No Ligand, referring to mice receiving vehicle.
2.12. Data Analysis
For each behavioral test, data was analyzed using MANOVA (IBM SPSE Statistics 27) for the between-group factors of genotype (WT, AhRΔIEC), stress (NS, UCMS), ligand (vehicle, DIM, DHNA) and within-group factors of week or day as applicable. Post hoc contrasts between each treatment group were conducted using the Bonferroni procedure. Differences with p-values of less than 0.05 were deemed statistically significant. Results are presented as mean ± SEM.
3. Results
3.1. Epithelium-specific AhR knockout mice (AhRΔIEC)
3.1.1. UCMS reduces weight gain in AhRΔIEC mice
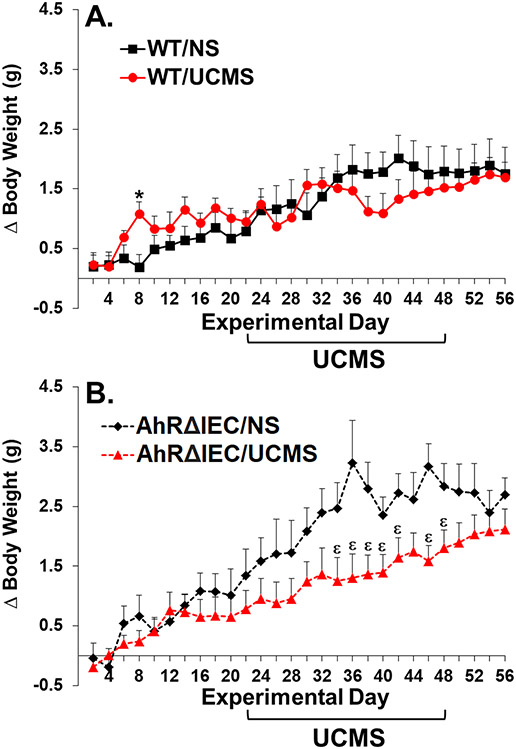
Analysis of weight gain revealed a significant main effect of day (F(27, 891)=48.177, p<0.0001), and significant interactions between genotype and day (F(27, 891)=3.475, p<0.0001) and stress and day (F(27, 891)=4.697, p<0.0001). Although visually it appears that the unstressed AhRΔIEC mice gain more weight than the unstressed WTs, there were no significant differences in weight gain between unstressed WT and AhRΔIEC mice (p>0.5). UCMS did not have a significant effect on weight gain in WT animals (Fig. 2A). Although it appeared to have a minor transient effect at the onset of UCMS, it did not reach statistical significance. In contrast, UCMS reduced weight gain in the AhRΔIEC mice, which became statistically significant toward the end of the second week and continued for the duration of UCMS procedure (Fig. 2B).
Fig 2. UCMS reduces weight gain in AhRΔIEC, but not WT mice.
Mice were unstressed or subjected to UCMS for 4 weeks. (A) WT/NS (n=10) – unstressed WT mice; WT/UCMS (n=10) – WT mice subjected to UCMS; (B) AhRΔIEC/NS (n=7) – unstressed intestinal epithelium AhR KO mice; AhRΔIEC/UCMS (n=10) – intestinal epithelium AhR KO mice subjected to UCMS. (*) Bonferroni post hoc contrast indicates a significant difference from WT/NS (p < 0.05); (ε) Bonferroni post hoc contrast indicates a significant difference from AhRΔIEC/NS (p < 0.05). NS = No Stress. Results are presented as mean + SEM.
3.1.2. Resilience of AhRΔIEC to UCMS-induced anhedonia
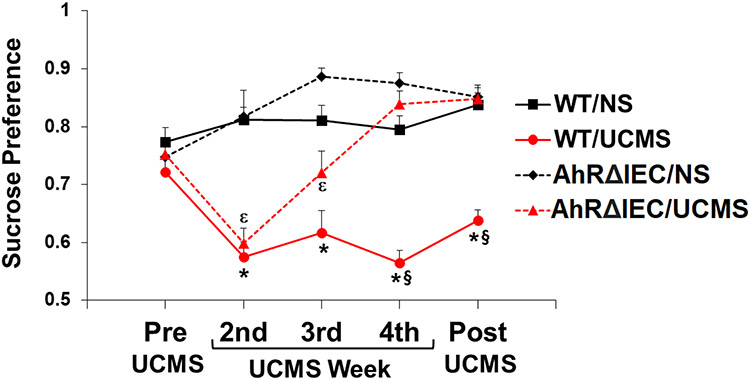
Analysis of sucrose preference revealed significant main effects of genotype (F(1, 33)=26.445, p<0.0001), stress (F(1, 33)=74.576, p<0.0001), and week (F(4, 132)=8.173, p<0.0001), and significant interactions between genotype and stress (F(1, 33)=10.191, p<0.01), genotype and week (F(4, 132)=9.090, p<0.0001), stress and week (F(4, 132)=10.553, p<0.0001), and genotype, stress and week (F(4, 132)=3.523, p<0.05). As illustrated in Fig. 3, no significant differences in sucrose preference between the experimental groups were observed in the pre-test. Two weeks of UCMS induced significant decrease in sucrose preference in both the WT and AhRΔIEC mice. As expected, sucrose preference of the WT mice remained low for the entire duration of the UCMS and one week post-UCMS. However, sucrose preference of the AhRΔIEC mice started to recover by week 3 of UCMS, and by week 4 were indistinguishable from control unstressed mice.
Fig 3. Differences in UCMS-induced anhedonia between AhRΔIEC and WT mice.
Mice were unstressed or subjected to UCMS for 4 weeks. Sucrose preference was monitored before starting UCMS, and at the end of the second, third and fourth week of UCMS, and 1 week post UCMS. WT/NS (n=10) – unstressed WT mice; WT/UCMS (n=10) – WT mice subjected to UCMS; AhRΔIEC/NS (n=7) – unstressed intestinal epithelium AhR KO mice; AhRΔIEC/UCMS (n=10) – intestinal epithelium AhR KO mice subjected to UCMS. (*) Bonferroni post hoc contrast indicates a significant difference from WT/NS (p < 0.05); (ε) Bonferroni post hoc contrast indicates a significant difference from AhRΔIEC7NS (p < 0.05) (§) Bonferroni post hoc contrast indicates a significant difference from AhRΔIEC/UCMS (p < 0.05). Results are presented as mean + SEM.
3.1.3. Effects on anxiety in AhRΔIEC mice
Marble burying test:
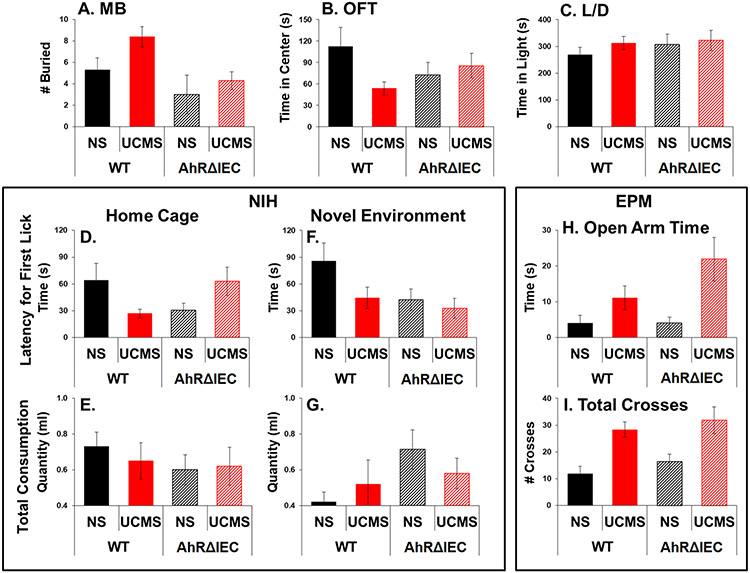
Analysis revealed significant main effects of genotype (F(1, 33)=9.461, p<0.01) and stress (F(1, 33)=4.472, p<0.05). UCMS increased the number of buried marbles, but overall AhRΔIEC mice buried fewer marbles than WT mice (Fig. 4A).
Fig 4. AhRΔIEC mice exhibit slightly reduced anxiety in certain tests.
Mice were unstressed or subjected to UCMS for 4 weeks. Then, mice were examined for number of buried marbles (A); time spent in the center in the OFT (B); time spent in the light compartment in the L/D test (C); latency for the first sip in the NIH test in their home cages (D) and novel environment (F); total consumption of milk in the NIH test in their home cages (E) and novel environment (G); and time spent in the open arm (H) and total crosses (I) in the EPM. Results are presented as mean + SEM.
Novelty-induced hypophagia:
Analysis of the latency for first lick in home cages revealed a significant interaction between genotype and stress (F(1, 33)=4.472, p<0.05). Latency for first lick was shorter for unstressed AhRΔIEC mice compared to unstressed WT mice (Fig. 4D). UCMS had opposing effects in WT and AhRΔIEC mice, reducing the latency in WTs and increasing the latency AhRΔIEC mice. However, no significant effects were observed in the total amount consumed in home cages (Fig 4E) as well as in their behaviors in the novel environment (Fig. 4F and Fig. 4G).
Elevated plus maze:
Analysis revealed a significant main effect of stress (open arm time: F(1, 33)=10.561, p<0.01; open arms entries: F(1, 33)=14.122, p<0.001; total crosses: F(1, 33)=20.904, p<0.0001). However, no significant differences were observed between the WT and AhRΔIEC mice (Fig. 4H and Fig. 4I).
OFT and L/D Test:
No significant effects were observed (Fig. 4B and Fig. 4C). A trend was observed for reduced time spent in the center of the OFT and reduced UCMS-effect on center time in the AhRΔIEC mice, but it did not reach statistical significance.
3.1.4. A slightly faster spatial learning in AhRΔIECs
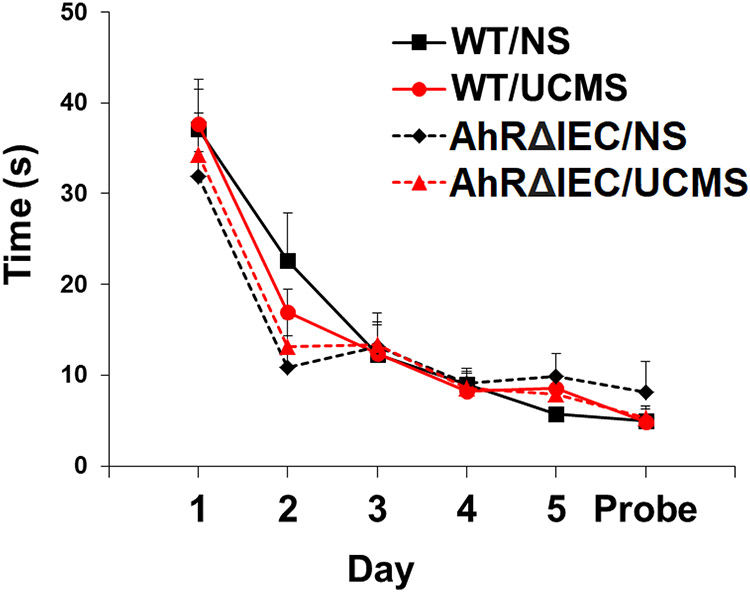
Analysis of MWM performance revealed a significant main effect of day (F(5, 165)=76.998, p<0.0001) and a significant interaction between genotype and day (F(5, 165)=2.515, p<0.05). AhRΔIEC learned the position of the hidden platform slightly faster (Fig. 5). There was no significant effect of UCMS in either WT or AhRΔIEC mice.
Fig 5. AhRΔIEC mice exhibit slightly faster spatial learning.
Mice were unstressed or subjected to UCMS for 4 weeks. Then, mice were examined for six consecutive days for the time to find a hidden platform in the MWM. WT/NS (n=10) – unstressed WT mice; WT/UCMS (n=10) – WT mice subjected to UCMS; AhRΔIEC/NS (n=7) – unstressed intestinal epithelium AhR KO mice; AhRΔIEC/UCMS (n=10) – intestinal epithelium AhR KO mice subjected to UCMS. Results are presented as mean + SEM.
3.2. DIM and DHNA antidepressant effects are not mediated via AhR expressed in the intestinal epithelium
3.2.1. DIM and DHNA prevents UCMS-induced anhedonia in both AhRΔIECs and WTs
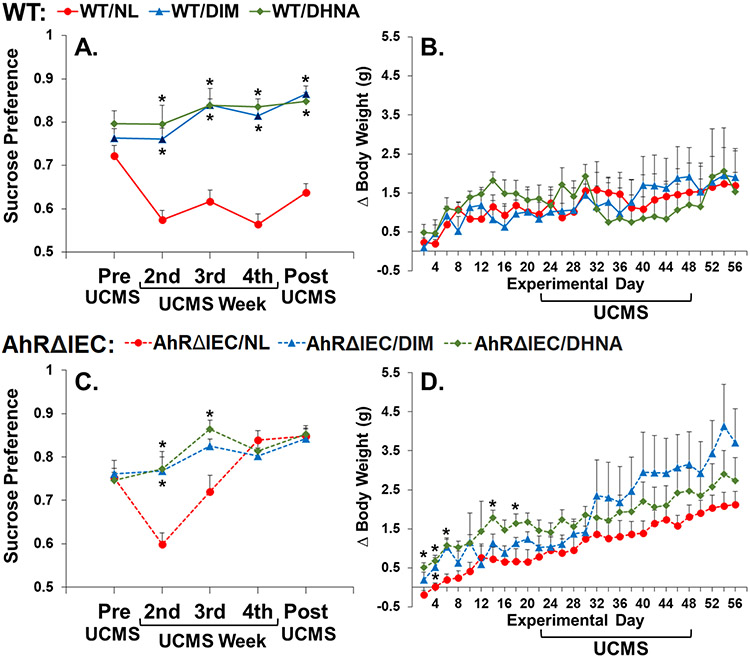
Analysis of sucrose preference revealed significant main effects of genotype (F(1, 44)=9.980, p<0.01), ligand (F(2, 44)=59.303, p<0.0001), and week (F(4, 176)=12.542, p<0.0001), and significant interactions between genotype and ligand (F(2, 44)=18.949, p<0.0001), genotype and week (F(4, 176)=2.988, p<0.05), ligand and week (F(8, 176)=4.149, p<0.0001), and genotype, ligand and week (F(8, 176)=3.471, p<0.001). No significant differences in sucrose preference between the experimental groups were observed in the pre-test. DIM and DHNA prevented UCMS-induced decrease in sucrose preference in both WT (Fig. 6A) and AhRΔIEC (Fig. 6C) mice.
Fig 6. DIM and DHNA act as antidepressants in both AhRΔIEC and WT mice.
Mice received vehicle (referred to as NL = No Ligand), DIM or DHNA, and then were subjected to UCMS for 4 weeks. Sucrose preference was monitored before starting UCMS, and at the end of the second, third and fourth week of UCMS, and 1 week post UCMS. (A) Sucrose preference and (B) weight gain of WT/NL (n=10) – WT mice receiving vehicle; WT/DIM (n=6) – WT mice receiving DIM; WT/DHNA (n=6) – WT mice receiving DHNA. (C) Sucrose preference and (D) weight gain of AhRΔIEC/NL (n=10) – intestinal epithelium AhR KO mice receiving vehicle; AhRΔIEC/DIM (n=9) – intestinal epithelium AhR KO mice receiving DIM; AhRΔIEC/DHNA (n=9) – intestinal epithelium AhR KO mice receiving DHNA. (*) Bonferroni post hoc contrast indicates a significant difference from the genetically corresponding experimental group receiving NL (p < 0.05). Results are presented as mean + SEM.
3.2.2. DIM and DHNA effects on weight gain
Analysis of weight revealed a significant main effect of day (F(27, 1188)=17.591, p<0.0001), and significant interactions between day and genotype (F(27, 1188)=3.809, p<0.0001), and day and ligand (F(54, 1188)=1.633, p<0.01). DIM and DHNA did not have a significant effect on weight gain in WT mice (Fig. 6B). For the AhRΔIECs, mice receiving DHNA gained more weight prior to the beginning of UCMS (Fig. 6D). Although DIM, and to a lesser degree DHNA, appears to mitigate the effects of UCMS on weight gain, given the high within-group varibility, it did not reach statistical significance (Fig. 6D). However, in AhRΔIEC, UCMS significantly reduced weight gain in mice treated with vehcile as compared to unstressed control mice (NL/NS vs. NL/UCMS, Fig. 2B). However, when comparing to NL/NS mice, UCMS did not significanly reduce weight gain in AhRΔIEC mice treated with DIM and DHNA.
4. Discussion
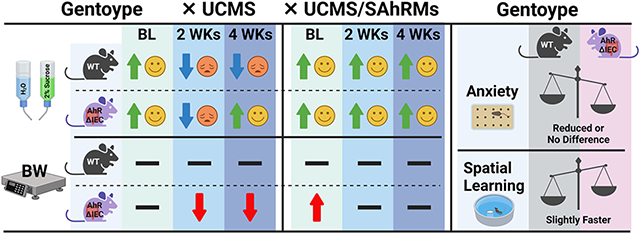
The results of this study suggest that AhR in the intestinal epithelium plays a multifaceted role in stress sensitivity and the development of various symptoms of depression, i.e. anhedonia and somatic effects. We demonstrated that both AhRΔIEC and WT female mice develop anhedonia-like symptoms following UCMS. However, over 4 weeks of UCMS, in contrast to their WT counterparts, female AhRΔIEC mice develop resilience to the anhedonia-like state induced by UCMS. Moreover, UCMS significantly suppressed body weight gain in AhRΔIEC females, but not WTs. Close examination of the timeline revealed that the effects of UCMS on body weight gain in AhRΔIEC mice is persistent, suggesting a significant reduction of food consumption for the entire duration of UCMS. On the other hand, while the desire for pleasurable palatable sucrose was initially reduced it began to recover on week 3 of UCMS. These results suggest that in WT mice intestinal epithelium AhR decreases the sensitivity to somatic effects of stress manifested in body weight gain. In contrast, intestinal epithelium AhR is essential for increasing and maintaining the persistence of stress-induced anhedonic depressive symptoms in WT females.
In this study we examined an anhedonic-like effect (i.e. sucrose preference) as a measure of depression-like effect. Our results demonstrate that the unstressed AhRΔIEC animals have normal sucrose preference. Thus, considering the normal sucrose preference observed in the unstressed AhRΔIEC mice, the short timeline of the effect of UCMS on sucrose preference, and the persistent effect of UCMS on weight gain, it is highly unlikely that the UCMS effects on sucrose preference observed in this study is due to effects on ingestive behaviors. However, future studies should further strengthen the findings of this study by including other measures of depression-like behaviors (such as forced swim test).
Gut microbiota can affect emotional and cognitive functions and has been implicated in mental well-being [3]. Importantly, AhRΔIEC mice have an altered fecal microbiome, which may explain their resilience to UCMS-induced anhedonic behavior [24]. When fed standard low-fat diet, AhRΔIEC females had lower abundance of Bacteroidetes, Bifidobacterium, Clostridiaceae, Coprococcus and Paraprevotella as compared to WTs. Thus, resilience to depression in AhRΔIEC mice is in line with high abundance of Paraprevotella and Clostridiaceae reported in MDD patients [7]. In contrast, low abundance of Bifidobacterium and Coprococcus was reported in MDD patients [7]. Additionally, Bifidobacterium and Lactobacillus were demonstrated to synergistically reduce stress-induced anxiety- and depression-like behaviors [46]. Importantly, these effects were mediated, at least partially, by reducing the abundance of Proteobacteria and Bacteroidetes populations in the gut. Notably, although likely not mediated via Bifidobacterium, lower abundance of Bacteroidetes was observed in the AhRΔIECs [24].
Moreover, AhRΔIEC females had higher levels of fecal tryptamine as compared to WTs [24]. Tryptamine, a tryptophan metabolite, is a well-established ligand for the AhR [47-49], and attenuates the development of multiple sclerosis in animal model [50]. Given that certain AhR ligands act as antidepressants [30], high levels of tryptamine might provide AhRΔIEC females with resilience to the AhRΔIEC anhedonia-like states. Thus, alterations of microbioa and metabolomic profiles in the AhRΔIECs might explain their resistance to stress-induced anhedonia-like symptoms.
AhRΔIEC females buried fewer marbles and took the first sip of milk sooner in the NIH test, suggesting AhRΔIEC mice are less anxious than the WT mice. However, no significant differences were found in other anxiety tests. Thus, these data suggest that intestinal epithelium AhR might contribute to higher levels of anxiety in certain tests. Moreover, AhRΔIEC females exhibit slightly faster spatial learning. It is possible that the reduced anxiety observed in the AhRΔIEC mice could contribute toward faster learning of the spatial task by a decrease in anxiety-related distraction. Alternatively, it has been suggested that gut microbiota affects spatial learning [51-53], and Lactobacillus reduced anxiety and enhanced spatial learning in the MWM [54]. Thus, it is possible that alterations in gut microbiota composition contributed to both reduced anxiety and enhanced spatial learning observed in the AhRΔIECs.
In this study we also examined whether the antidepressant effect of two SAhRMs, DIM and DHNA, is mediated via the intestinal AhR. The AhR receptor is very complex, and ligand-directed signaling via the receptor, especially in the brain, is not fully understood. Various ligands can act as agonists or antagonists in different cells and tissue, and have differential effects in the modulation of different signaling pathways within the same cells [14, 15]. Therefore, in this study we describe this compound as modulators, and focus on their effect as antidepressants. The underlying molecular and cellular mechanisms mediating their antidepressant effects are largely unknown, and should be further investigated in future studies. Notably, the antidepressant effects of DIM and DHNA are not dependent on intestinal AhR expression. DIM and DHNA acted as antidepressants in the AhRΔIEC mice during the first 3 weeks of UCMS when the AhRΔIECs still displayed UCMS-induced anhedonia-like symptoms. In other words, DIM and DHNA have similar effectiveness as antidepressants in both AhRΔIEC and WT mice. Thus, although this study suggests that intestinal AhR contributes to the effects of stress on mood, the site of action of DIM and DHNA is likely not in the gastrointestinal tract. AhR is expressed broadly throughout the brain, and particularly in the frontal cortex, hippocampus, and hypothalamus [55], areas implicated in depression. Notably, the AhR receptor was linked to expression levels of brain-derived neurotrophic factor (BDNF) [56]. In particular, DIM was demonstrated to have neuroprotective and anti-apoptotic properties [57], and increase the expression of BDNF [58]. BDNF levels were demonstrated to be reduced in the hippocampus of rodents following UCMS [59] as well as in individuals suffering from depression [60], and antidepressant treatments increase BDNF levels [61]. Together this suggests DIM and DHNA’s antidepressant properties may involve BDNF, and future studies will examine their effect on BDNF levels following UCMS.
5. Conclusions
Our findings suggest intestinal AhR plays differential roles, mitigating the effects of stress on weight gain, but in contrast has an essential role in increasing and maintaining the effects of stress on mood. Future studies should examine the underlying mechanisms, and whether these effects are mediated via AhR’s role in regulating gut microbiota and metabolomics. Additionally, future studies should further examine the potential role of intestinal AhR in anxiety and cognition. Although intestinal AhR appears to play a role in mood, the site of antidepressant action of SAhRMs, such as DIM and DHNA, is not the intestinal AhR. Future studies should examine the role of BDNF expression levels in the antidepressant effects of AhR ligands. Additionally, stereotaxic injection of a pharmacological antagonist and tissue specific knockouts will be require to determine the site of antidepressant action of AhR ligands.
Highlights.
UCMS reduces weight gain in AhRKOs, but not WTs
AhRKOs, but not WTs, develop resilience to the anhedonic effects of UCMS
AhRKOs exhibit slightly reduced anxiety and faster spatial learning
Intestinal AhR plays differential role in the effects of stress on weight and mood
The antidepressant effect of AhR ligands is not mediated via intestinal AhR
Role of Funding Source
This work was supported by a Seed Grant from Texas A&M University (SE) and supported in part by NIH grant R35 CA197707 (RSC) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention (RSC). The funding source had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have no conflicts of interest
References
- 1.Luckey TD, Introduction to intestinal microecology, Am J Clin Nutr. 25 (1972) 1292–1294. [DOI] [PubMed] [Google Scholar]
- 2.Sender R, Fuchs S, Milo R, Revised Estimates for the Number of Human and Bacteria Cells in the Body, PLoS Biol. 14 (2016) e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG, The Microbiota-Gut-Brain Axis, Physiol Rev. 99 (2019) 1877–2013. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Li J, Gui X, Shi X, Bao Z, Han H, Li MD, Updated review of research on the gut microbiota and their relation to depression in animals and human beings, Mol Psychiatry. (2020) [DOI] [PubMed] [Google Scholar]
- 5.Liu RT, Rowan-Nash AD, Sheehan AE, Walsh RFL, Sanzari CM, Korry BJ, Belenky P, Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults, Brain Behav Immun. S0889-1591 (2020) 31531–31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, Mimura M, Iwanami A, Kishimoto T, Gut microbiota and major depressive disorder: A systematic review and meta-analysis, J Affect Disord. 266 (2020) 1–13. [DOI] [PubMed] [Google Scholar]
- 7.Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS, Altered Composition of Gut Microbiota in Depression: A Systematic Review, Front Psychiatry. 11 (2020) 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P, Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism, Mol Psychiatry. 21 (2016) 786–796. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F, Gut Microbiota Regulates Depression-Like Behavior in Rats Through the Neuroendocrine-Immune-Mitochondrial Pathway, Neuropsychiatr Dis Treat. 16 (2020) 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinna Meyyappan A, Forth E, Wallace CJK, Milev R, Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review, BMC Psychiatry. 20 (2020) 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WH, Chuang HL, Huang YT, Wu CC, Chou GT, Wang S, Tsai YC, Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice, Behav Brain Res. 298 (2016) 202–209. [DOI] [PubMed] [Google Scholar]
- 12.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF, The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication, Neurogastroenterol Motil. 23 (2011) 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockinger B, Shah K, Wincent E, AHR in the intestinal microenvironment: safeguarding barrier function, Nat Rev Gastroenterol Hepatol. (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safe S, Jin UH, Park H, Chapkin RS, Jayaraman A, Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs), Int J Mol Sci. 21 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safe S, Han H, Goldsby J, Mohankumar K, Chapkin RS, Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies, Curr Opin Toxicol. 11-12 (2018) 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifka-Walk HM, Jenkins BR, Kominsky DJ, Amino Acid Trp: The Far Out Impacts of Host and Commensal Tryptophan Metabolism, Front Immunol. 12 (2021) 653208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HU, McPherson ZE, Tan B, Korecka A, Pettersson S, Host-microbiome interactions: the aryl hydrocarbon receptor and the central nervous system, J Mol Med (Berl). 95 (2017) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ, The aryl hydrocarbon receptor and the gut-brain axis, Cellular & molecular immunology. 18 (2021) 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agus A, Planchais J, Sokol H, Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease, Cell Host Microbe. 23 (2018) 716–724. [DOI] [PubMed] [Google Scholar]
- 20.Ma N, He T, Johnston LJ, Ma X, Host-microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling, Gut Microbes. 11 (2020) 1203–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ, The aryl hydrocarbon receptor and the gut-brain axis, Cell Mol Immunol. 18 (2021) 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiboub M, Verburgt CM, Sovran B, Benninga MA, de Jonge WJ, Van Limbergen JE, Nutritional Therapy to Modulate Tryptophan Metabolism and Aryl Hydrocarbon-Receptor Signaling Activation in Human Diseases, Nutrients. 12 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korecka A, Dona A, Lahiri S, Tett AJ, Al-Asmakh M, Braniste V, D'Arienzo R, Abbaspour A, Reichardt N, Fujii-Kuriyama Y, Rafter J, Narbad A, Holmes E, Nicholson J, Arulampalam V, Pettersson S, Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism, NPJ Biofilms Microbiomes. 2 (2016) 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, DeLuca JAA, Menon R, Garcia-Vilarato E, Callaway E, Landrock KK, Lee K, Safe SH, Chapkin RS, Allred CD, Jayaraman A, Effect of diet and intestinal AhR expression on fecal microbiome and metabolomic profiles, Microb Cell Fact. 19 (2020) 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, Li V, Maradana MR, Schiering C, Stockinger B, The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity, Immunity. 49 (2018) 353–362 e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, Quintana FJ, Microglial control of astrocytes in response to microbial metabolites, Nature. 557 (2018) 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kebir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ, Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor, Nat Med. 22 (2016) 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei GZ, Martin KA, Xing PY, Agrawal R, Whiley L, Wood TK, Hejndorf S, Ng YZ, Low JZY, Rossant J, Nechanitzky R, Holmes E, Nicholson JK, Tan EK, Matthews PM, Pettersson S, Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor, Proc Natl Acad Sci U S A. 118 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dopkins N, Becker W, Miranda K, Walla M, Nagarkatti P, Nagarkatti M, Tryptamine Attenuates Experimental Multiple Sclerosis Through Activation of Aryl Hydrocarbon Receptor, Front Pharmacol. 11 (2020) 619265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madison CA, Kuempel J, Albrecht GL, Hillbrick L, Jayaraman A, Safe S, Chapkin RS, Eitan S, 3,3'-Diindolylmethane and 1,4-dihydroxy-2-naphthoic acid prevent chronic mild stress induced depressive-like behaviors in female mice, J Affect Disord. 309 (2022) 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willner P, The chronic mild stress (CMS) model of depression: History, evaluation and usage, Neurobiol Stress. 6 (2017) 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D, Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress, Behav Pharmacol. 17 (2006) 271–287. [DOI] [PubMed] [Google Scholar]
- 33.Ribot T-A, Lapsychologie des sentiments. 1896, Paris: Felix Alcan. [Google Scholar]
- 34.Cheng Y, Jin U-H, Davidson LA, Chapkin RS, Jayaraman A, Tamamis P, Orr A, Allred C, Denison MS, Soshilov A, Weaver E, Safe S, Editor's Highlight: Microbial-Derived 1,4-Dihydroxy-2-naphthoic Acid and Related Compounds as Aryl Hydrocarbon Receptor Agonists/Antagonists: Structure-Activity Relationships and Receptor Modeling, Toxicological sciences : an official journal of the Society of Toxicology. 155 (2017) 458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermillion Maier ML, Siddens LK, Uesugi SL, Choi J, Leonard SW, Pennington JM, Tilton SC, Smith JN, Ho E, Chow HHS, Nguyen BD, Kolluri SK, Williams DE, 3,3'-Diindolylmethane (BioResponse DIM(®)) Exhibits Significant Metabolism Following Oral Dosing in Humans, Drug Metab Dispos. (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentley R, Meganathan R, Biosynthesis of vitamin K (menaquinone) in bacteria, Microbiol Rev. 46 (1982) 241–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isawa K, Hojo K, Yoda N, Kamiyama T, Makino S, Saito M, Sugano H, Mizoguchi C, Kurama S, Shibasaki M, Endo N, Sato Y, Isolation and identification of a new bifidogenic growth stimulator produced by Propionibacterium freudenreichii ET-3, Biosci Biotechnol Biochem. 66 (2002) 679–681. [DOI] [PubMed] [Google Scholar]
- 38.Mori H, Sato Y, Taketomo N, Kamiyama T, Yoshiyama Y, Meguro S, Sato H, Kaneko T, Isolation and structural identification of bifidogenic growth stimulator produced by Propionibacterium freudenreichii, J Dairy Sci. 80 (1997) 1959–1964. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Villatoro EL, DeLuca JAA, Callaway ES, Allred KF, Davidson LA, Hensel ME, Menon R, Ivanov I, Safe SH, Jayaraman A, Chapkin RS, Allred CD, Effects of high-fat diet and intestinal aryl hydrocarbon receptor deletion on colon carcinogenesis, Am J Physiol Gastrointest Liver Physiol. 318 (2020) G451–g463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzales C, Zaleska MM, Riddell DR, Atchison KP, Robshaw A, Zhou H, Sukoff Rizzo SJ, Alternative method of oral administration by peanut butter pellet formulation results in target engagement of BACE1 and attenuation of gavage-induced stress responses in mice, Pharmacology Biochemistry and Behavior. 126 (2014) 28–35. [DOI] [PubMed] [Google Scholar]
- 41.Bourin M, Hascoet M, The mouse light/dark box test, Eur J Pharmacol. 463 (2003) 55–65. [DOI] [PubMed] [Google Scholar]
- 42.Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ, Eitan S, Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal, Behav Pharmacol. 20 (2009) 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS, Antidepressant actions of the exercise-regulated gene VGF, Nature Medicine. 13 (2007) 1476. [DOI] [PubMed] [Google Scholar]
- 44.Deacon RM, Digging and marble burying in mice: simple methods for in vivo identification of biological impacts, Nat Protoc. 1 (2006) 122–124. [DOI] [PubMed] [Google Scholar]
- 45.Vorhees CV, Williams MT, Morris water maze: procedures for assessing spatial and related forms of learning and memory, Nat Protoc. 1 (2006) 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han SK, Kim DH, Lactobacillus mucosae and Bifidobacterium longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis, J Microbiol Biotechnol. 29 (2019) 1369–1374. [DOI] [PubMed] [Google Scholar]
- 47.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S, Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities, Mol Pharmacol. 85 (2014) 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS, Activation of the Ah receptor by tryptophan and tryptophan metabolites, Biochemistry. 37 (1998) 11508–11515. [DOI] [PubMed] [Google Scholar]
- 49.Vikström Bergander L, Cai W, Klocke B, Seifert M, Pongratz I, Tryptamine serves as a proligand of the AhR transcriptional pathway whose activation is dependent of monoamine oxidases, Mol Endocrinol. 26 (2012) 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg J, Mahmoudjanlou Y, Duscha A, Massa MG, Thöne J, Esser C, Gold R, Haghikia A, The immunomodulatory effect of laquinimod in CNS autoimmunity is mediated by the aryl hydrocarbon receptor, J Neuroimmunol. 298 (2016) 9–15. [DOI] [PubMed] [Google Scholar]
- 51.Mosaferi B, Jand Y, Salari AA, Antibiotic-induced gut microbiota depletion from early adolescence exacerbates spatial but not recognition memory impairment in adult male C57BL/6 mice with Alzheimer-like disease, Brain Res Bull. 176 (2021) 8–17. [DOI] [PubMed] [Google Scholar]
- 52.Yu F, Han W, Zhan G, Li S, Xiang S, Zhu B, Jiang X, Yang L, Luo A, Hua F, Yang C, Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice, Aging (Albany NY). 11 (2019) 3262–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O'Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, Cryan JF, Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat, Neuroscience. 339 (2016) 463–477. [DOI] [PubMed] [Google Scholar]
- 54.Luo J, Wang T, Liang S, Hu X, Li W, Jin F, Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat, Sci China Life Sci. 57 (2014) 327–335. [DOI] [PubMed] [Google Scholar]
- 55.Juricek L Coumoul X, The Aryl Hydrocarbon Receptor and the Nervous System, Int J Mol Sci. 19 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CH, Chen CC, Chou CM, Wang CY, Hung CC, Chen JY, Chang HW, Chen YC, Yeh GC, Lee YH, Knockdown of the aryl hydrocarbon receptor attenuates excitotoxicity and enhances NMDA-induced BDNF expression in cortical neurons, J Neurochem. 111 (2009) 777–789. [DOI] [PubMed] [Google Scholar]
- 57.Rzemieniec J, Litwa E, Wnuk A, Lason W, Krzeptowski W, Kajta M, Selective Aryl Hydrocarbon Receptor Modulator 3,3'-Diindolylmethane Impairs AhR and ARNT Signaling and Protects Mouse Neuronal Cells Against Hypoxia, Mol Neurobiol. 53 (2016) 5591–5606. [DOI] [PubMed] [Google Scholar]
- 58.Lee BD, Yoo JM, Baek SY, Li FY, Sok DE, Kim MR, 3,3'-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells, Antioxidants (Basel). 9 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, Fei P, Mu J, Li W, Song J, Hippocampal expression of aryl hydrocarbon receptor nuclear translocator 2 and neuronal PAS domain protein 4 in a rat model of depression, Neurol Sci. 35 (2014) 277–282. [DOI] [PubMed] [Google Scholar]
- 60.Duman RS Monteggia LM, A neurotrophic model for stress-related mood disorders, Biol Psychiatry. 59 (2006) 1116–1127. [DOI] [PubMed] [Google Scholar]
- 61.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT, Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication, Biol Psychiatry. 50 (2001) 260–265. [DOI] [PubMed] [Google Scholar]