Abstract
The metabolites of gut microbiota show favorable therapeutic effects on nonalcoholic fatty liver disease (NAFLD), but the active metabolites and mechanisms against NAFLD have not been documented. The aim of the study was to investigate the active metabolites and mechanisms of gut microbiota against NAFLD by network pharmacology. We obtained a total of 208 metabolites from the gutMgene database and retrieved 1256 targets from similarity ensemble approach (SEA) and 947 targets from the SwissTargetPrediction (STP) database. In the SEA and STP databases, we identified 668 overlapping targets and obtained 237 targets for NAFLD. Thirty-eight targets were identified out of those 237 and 223 targets retrieved from the gutMgene database, and were considered the final NAFLD targets of metabolites from the microbiome. The results of molecular docking tests suggest that, of the 38 targets, mitogen-activated protein kinase 8-compound K and glycogen synthase kinase-3 beta-myricetin complexes might inhibit the Wnt signaling pathway. The microbiota-signaling pathways-targets-metabolites network analysis reveals that Firmicutes, Fusobacteria, the Toll-like receptor signaling pathway, mitogen-activated protein kinase 1, and phenylacetylglutamine are notable components of NAFLD and therefore to understanding its processes and possible therapeutic approaches. The key components and potential mechanisms of metabolites from gut microbiota against NAFLD were explored utilizing network pharmacology analyses. This study provides scientific evidence to support the therapeutic efficacy of metabolites for NAFLD and suggests holistic insights on which to base further research.
Subject terms: Microbiology, Gastroenterology
The community of microorganisms inhabiting the human gut (gastrointestinal tract) is defined as the microbiota, which is estimated to be 100 trillion, including bacteria, viruses, fungi, and protozoa1. The gut microbiota is a significant element in human health and disease and variations in its diversity are associated with an unhealthy diet, medicines, and pathogenic infections as well as chronic kidney disease2,3. Notably, genetically engineered gut bacteria are significant therapeutic resources capable of producing beneficial metabolites for the treatment of chronic diseases such as cancer, autoimmune disorders, metabolic diseases, and beyond NAFLD4. An imbalance in gut microbiota can lead to the progression of some diseases, such as cancer, atherosclerosis, type 1 diabetes, and even nonalcoholic fatty liver disease (NAFLD)5,6. It has been suggested to have relatively stable and diverse distributions with a communal crucial microbiota, including the Firmicutes and Bacteroidetes phyla, as the key dominants7. The microbiota products are related to the occurrence and development of liver complications via diverse mechanisms, such as differential intestinal permeability, persistent inflammatory responses, and secretion of some short-chain fatty acids8. The microbiota products are related to the occurrence and development of liver complications via diverse mechanisms, such as differential intestinal permeability, persistent inflammatory responses, and secretion of some short-chain fatty acids9.
In particular, the gut-related microbiota converts exogenous and endogenous compounds into metabolites via the microbiota and nervous system10. These benefits of the cross-talk between microbiota and the gut can be exerted locally as well as in distant organs due to the systemic circulation of metabolites produced in the intestine11. Furthermore, the gut-liver axis is critical for understanding the mechanism of diverse liver diseases, such as NAFLD, nonalcoholic steatohepatitis (NASH), and the development and occurrence of cirrhosis12. For instance, the progression of NAFLD is related to lipopolysaccharide (LPS) produced by gram-negative bacteria inhabiting the gut13. Likewise, the gut microbiota converts choline into trimethylamine oxide, which exacerbates liver inflammation and damage14,15. This implies that the gut microbiota is critically related to liver diseases caused by inflammation. Over the past few years, the gut microbiota has been an increasingly significant therapeutic strategy for relieving NAFLD due to its great efficacy and low adverse effects16. The metabolites produced by gut microbiota are effective agents for the treatment of NAFLD17. Some microbiota-associated metabolites have been examined to determine either positive or negative effects on the development of NAFLD, even though the number of metabolites of gut microbiota is not completely clear18. Furthermore, the active metabolites of gut microbiota and their pharmacological mechanisms against NAFLD have not yet been reported. Hence, studies on active metabolites transformed by substrates and their mechanism of action should be better defined prior to clinical trials of proposed NAFLD treatments.
We suggest that the systematic methodology of network pharmacology can be used to unravel interactions of multiple components, for gut microbiota analysis, such as microbiota, signaling pathways, targets, and metabolites. Most recently, a report demonstrated that the gut microbiota have anti-fatigue effects by analyzing multiple targets via network pharmacology19. The development and occurrence of NAFLD are dependent on multiple factors that involve inherited characteristics as well as inconsistent microbiota distribution20. Therefore, network pharmacology would seem to be a very effective technology to explore the function of microbiota-related metabolites against diseases.
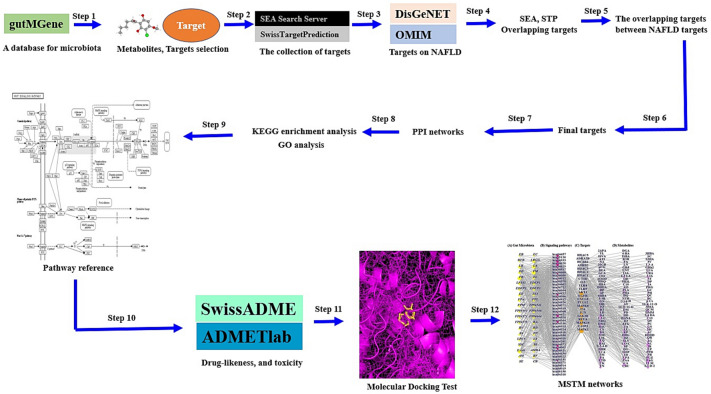
In this study, network pharmacology was utilized to investigate the analysis of a multi-factorial and very complex process, including key microbiota, signaling pathways, targets, and metabolites, in NAFLD. In parallel, we determined the key signaling pathways, targets, and metabolites to alleviate NAFLD. First, metabolites produced by the gut microbiome were identified utilizing a microbiome database, and metabolite-related targets were identified using cheminformatics. Then, NAFLD-related targets were retrieved via a bioinformatics database, and we identified the final targets among the metabolite-related targets and NAFLD targets. Second, we conducted a protein–protein interaction (PPI) network analysis, Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis, and gene ontology (GO) analysis. In key signaling pathways, we performed molecular docking test (MDT) to verify the most stable metabolites, which were identified by drug-likeness and toxicity in the in silico platform. Finally, we analyzed the microbiota-signaling pathways-targets-metabolites (MSTM) networks to identify the most significant components, microbiota, signaling pathways, targets, and metabolites from a holistic perspective. The workflow is represented in Fig. 1.
Figure 1.
The workflow of this study.
Methods
Selection of gut microbiota metabolites and targets
The metabolites and targets of gut microbiota were retrieved by gutMGene v1.0 (http://bio-annotation.cn/gutmgene/) (Accessed on 2 April 2022). The Simplified Molecular Input Line Entry System (SMILES) formats of each metabolite were identified by PubChem (https://pubchem.ncbi.nlm.nih.gov/) (accessed on 3 April 2022).
Identification of core targets against non-alcoholic fatty liver disease
The targets related to metabolites were identified through both similarity ensemble approach (SEA) (http://sea.bkslab.org/) (accessed on 4 April 2022)21 and SwissTargetPrediction (STP) (http://www.swisstargetprediction.ch/) (accessed on 4 April 2022)22 with the “Homo sapiens” setting. The overlapping targets between the SEA and STP databases were considered to be important targets for further analysis. In addition, NAFLD targets were obtained by DisGeNET (https://www.disgenet.org/) (accessed on 4 April 2022)23 and OMIM (accessed on 5 April 2022)24. Significant targets were identified among the metabolite-related targets and NAFLD targets. Then, the core targets were recognized between the significant targets and the gutMGene database.
Construction of the protein–protein interaction network
The PPI network was constructed using R package and was based on final targets in STRING analysis (https://string-db.org/) (accessed on 6 April 2022). A target with the highest degree value in the PPI networks was considered a hub target to control the PPI network against NAFLD.
Analysis of gene ontology and Kyoto encyclopedia of genes and genomes pathways of gut microbiota metabolites
GO analysis was performed to describe the functions of the targets, and consisted of molecular function (MF), biological function (BF), and cellular component (CC) analyses. The KEGG pathway enrichment analysis was used to understand the potential signaling pathways related to the final targets against NAFLD. The bubble plots are based on a rich factor defined as the gene ratio expressed differentially to the total target number in a signaling pathway25.
The preparation of metabolites and targets for molecular docking testing
The metabolites associated with the key target were converted from the .sdf format from PubChem to.pdb format using PyMOL, and we obtained the .pdbqt format via AutoDock. The key target was identified in STRING through RCSB (https://www.rcsb.org/) (accessed on 6 April 2022). The.pdb format obtained by RCSB was converted into .pdbqt format by using AutoDock (http://autodock.scripps.edu/) (accessed on 6 April 2022).
Molecular docking test of metabolites for the key target
The metabolites were docked with the key target utilizing AutoDock 4 by setting up 4 energy ranges and 8 exhaustiveness values as the defaults to acquire 10 different poses of the metabolites26. The center of the key target was x = − 0.861, y = 2.109, z = 1.303. The active site grid box size was set to x = 40 Å, y = 40 Å, and z = 40 Å. Detailed information on 2D binding was generated by LigPlot + 2.2 (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/download.html) (accessed on 7th April 2022)27. The threshold value of MDT was – 6.0 kcal/mol28 and a core metabolite with the lowest Gibbs free energy was selected on the metabolite-target complex in PyMOL.
Evaluation of drug-likeness properties
The drug-likeness properties of the three metabolites were evaluated using SwissAMDE29 and the literature. Commonly, metabolites have hydrophilic properties and have low bioavailability; therefore, we identified their physicochemical properties through an in silico strategy.
Toxicological evaluation by ADMETlab
One of key reason for failure of drug development is the lack of safety caused by some adverse effects: hERG blockers obstruct potassium channels30 and cause human hepatotoxicity31, Ames mutagenicity32, Skin sensitization33, Lethal Dose 50 (LD50) of acute toxicity34, and Drug Induced Liver Injury (DILI)35. Thus, we confirmed the six parameters by using ADMETlab platform36.
Microbiota-signaling pathways-targets-metabolites network analysis
The MSTM networks were constructed as a size plot based on the degree value of each node. In the network plot, yellow circles (nodes) describe the gut microbiota; pink circles (nodes) display the signaling pathways; orange circles (nodes) represent the targets; and violet circles (nodes) represent the metabolites. The size of the yellow circles represents the total number of relationships with signaling pathways, metabolites, and targets; the size of pink circles represents the number of correlations with gut microbiota; the size of orange circles depicts the number of interactions with signaling pathways; and the size of violet circles describes the number of relationships with targets. The merged network was built using R Package.
Results
Acquisition of potential targets and metabolites of gut microbiota
We obtained 208 metabolites from the gutMgene microbiome database. The obtained targets and metabolites were considered significant components to analyze the therapeutic effects of the gut microbiota.
Identification of 38 core targets from gut microbiota metabolites
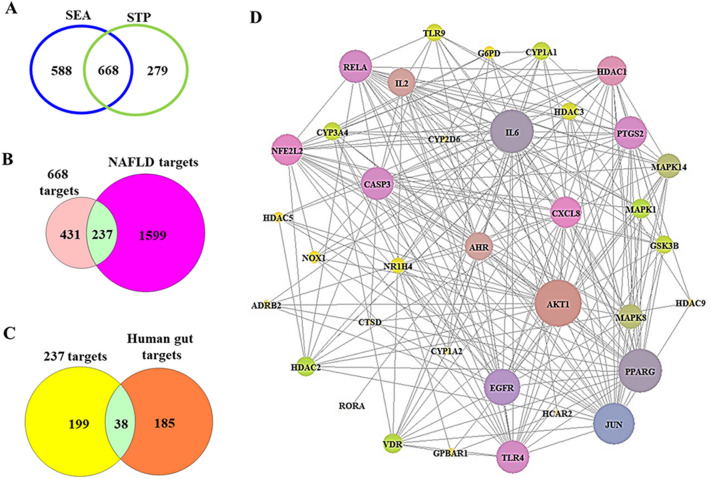
A total of 208 metabolites were analyzed to search for their targets in silico in the SEA and STP databases. We identified 1256 targets from SEA and 947 targets from STP (Fig. 2A), and 668 targets were identified as overlapping targets between the two databases (Fig. 2B). A total of 237 targets among the 668 targets and 1836 NAFLD targets were identified; therefore, 38 core targets were obtained by analysis of the 223 targets (Fig. 2C).
Figure 2.
(A) The number of overlapping 668 targets between SEA and STP database. (B) The number of overlapping 237 targets between the 668 targets and NAFLD-related targets. (C) The number of the final overlapping 38 targets between the 237 targets and gut human targets. (D) The PPI networks (36 nodes and 237 targets).
Protein–protein interaction network analysis
The PPI network consists of 36 nodes and 237 edges (Fig. 2D) in the 38 core targets, the size of which is based on the degree of value (Table 1). Two targets (ADRA2B and ST6GAL1) were not linked to one another in the 38 core targets. Based on the network map, a key target, AKT1, was defined as the uppermost target, followed by IL6, PPARG, JUN, and EGFR, further verifying the significant role of the target against NAFLD.
Table 1.
The degree of value of PPI networks.
| No. | Target | Degree of value | No. | Target | Degree of value |
|---|---|---|---|---|---|
| 1 | AKT1 | 28 | 19 | VDR | 12 |
| 2 | IL6 | 26 | 20 | CYP1A1 | 11 |
| 3 | PPARG | 26 | 21 | CYP3A4 | 11 |
| 4 | JUN | 25 | 22 | GSK3B | 11 |
| 5 | EGFR | 22 | 23 | HDAC3 | 10 |
| 6 | CASP3 | 20 | 24 | TLR9 | 10 |
| 7 | PTGS2 | 20 | 25 | NR1H4 | 9 |
| 8 | RELA | 20 | 26 | G6PD | 7 |
| 9 | TLR4 | 20 | 27 | NOX1 | 7 |
| 10 | CXCL8 | 19 | 28 | HDAC5 | 6 |
| 11 | NFE2L2 | 19 | 29 | ADRB2 | 5 |
| 12 | HDAC1 | 18 | 30 | GPBAR1 | 5 |
| 13 | AHR | 17 | 31 | CYP1A2 | 4 |
| 14 | IL2 | 17 | 32 | CYP2D6 | 4 |
| 15 | MAPK14 | 15 | 33 | HDAC9 | 4 |
| 16 | MAPK8 | 15 | 34 | CTSD | 3 |
| 17 | HDAC2 | 12 | 35 | HCAR2 | 3 |
| 18 | MAPK1 | 12 | 36 | RORA | 1 |
HDAC5, histone deacetylase 5; ADRA2B, alpha-2B adrenergic receptor; HCAR2, hydroxycarboxylic acid receptor 2; ADRB2, adrenoceptor beta 2; HDAC3, histone deacetylase 3; HDAC2, histone deacetylase 2; HDAC1, histone deacetylase 1; CTSD, cathepsin D; IL2, interleukin 2; TLR4, toll-like receptor 4; TLR9, toll-like receptor 9; AKT1, AKT serine/threonine kinase 1; EGFR, epidermal growth factor receptor; CXCL8, C-X-C motif chemokine ligand 8; PTGS2, prostaglandin-endoperoxide synthase 2; MAPK8, mitogen-activated protein kinase 8; IL6, interleukin-6; JUN, jun proto-oncogene, AP-1 transcription factor subunit; GSK3B, glycogen synthase kinase-3 beta; RELA, RELA proto-oncogene, NF-KB subunit; MAPK14, mitogen-activated protein kinase 14; CASP3, caspase 3; MAPK1, mitogen-activated protein kinase 1.
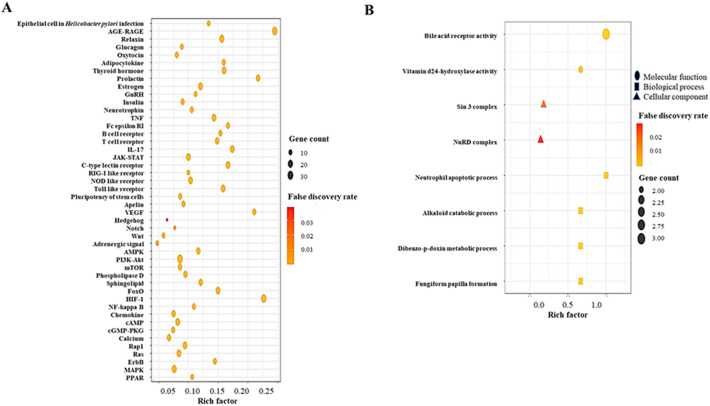
Identification of the 41 Kyoto encyclopedia of genes and genomes pathway enrichments and gene ontology enrichment analysis of the 3 components
To further evaluate the pharmacological mechanism of gut metabolites in the therapeutic strategy of NAFLD, the 38 core targets were investigated by KEGG pathway and GO enrichment analyses. The KEGG pathway enrichment analysis was based on signaling pathways (Table 2), the bubble size of which indicates the number of targets related to the pathway. The 41 signaling pathways of the KEGG pathway enrichment are represented in Fig. 3A, suggesting that the Wnt signaling pathway (Fig. 3B) might function as a potent inhibitive pathway of NAFLD. The GO enrichment analysis consisted of three components: molecular function (MF), biological process (BP), and cellular component (CC).
Table 2.
The targets of 41 signaling pathways related to NAFLD.
| KEGG ID and description | Target genes | False discovery rate |
|---|---|---|
| hsa04620: Toll-like receptor signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, IL6, CXCL8, TLR4, TLR9, MAPK14 | 5.19E−10 |
| hsa04657: IL-17 signaling pathway | MAPK1, RELA, GSK3B, JUN, MAPK8, IL6, PTGS2, CXCL8, MAPK14, CASP3 | 3.26E−13 |
| hsa04933: AGE-RAGE signaling pathway in diabetic complications | MAPK1, AKT1, CASP3, RELA, JUN, MAPK8, IL6, CXCL8, MAPK14, NOX1 | 4.70E−13 |
| hsa04668: TNF signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, IL6, PTGS2, MAPK14, CASP3 | 3.02E−11 |
| hsa04917: Prolactin signaling pathway | AKT1, RELA, GSK3B, MAPK8, MAPK14, MAPK1 | 6.52E−08 |
| hsa04660: T cell receptor signaling pathway | MAPK1, AKT1, RELA, JUN, GSK3B, MAPK1, MAPK14, MAPK8 | 5.3E−10 |
| hsa04625: C-type lectin receptor signaling pathway | MAPK1, AKT1, RELA, JUN, IL6, PTGS2, MAPK8, IL2, MAPK14 | 1.58E−11 |
| hsa04012: ErbB signaling pathway | MAPK1, AKT1, JUN, GSK3B, EGFR, MAPK8 | 0.000000156 |
| hsa04664: Fc epsilon RI signaling pathway | MAPK1, AKT1, MAPK14, MAPK8 | 0.0000462 |
| hsa04066: HIF-1 signaling pathway | MAPK1, AKT1, RELA, EGFR, IL6, TLR4 | 0.000000552 |
| hsa05120: Epithelial cell signaling in Helicobacter pylori infection | RELA, JUN, MAPK8, CXCL8, EFGR, MAPK14, CASP3 | 1.5E−09 |
| hsa04722: Neurotrophin signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, MAPK14 | 3.34E−08 |
| hsa04662: B cell receptor signaling pathway | MAPK1, AKT1, RELA, JUN, GSK3B | 0.00000319 |
| hsa04370: VEGF signaling pathway | MAPK1, AKT1, PTGS2, MAPK14 | 0.0000278 |
| hsa04071: Sphingolipid signaling pathway | MAPK1, AKT1, MAPK1, MAPK8, MAPK14, CTSD | 0.000000861 |
| hsa04068: FoxO signaling pathway | MAPK1, AKT1, IL6, MAPK8, EGFR, MAPK14 | 0.00000132 |
| hsa04919: Thyroid hormone signaling pathway | MAPK1, AKT1, HDAC1, HDAC2, HDAC3, GSK3B | 0.000000974 |
| hsa04064: NF-kappa B signaling pathway | RELA, PTGS2, CXCL8, TLR4 | 0.0002 |
| hsa04920: Adipocytokine signaling pathway | AKT1, RELA, MAPK8 | 0.0011 |
| hsa04062: Chemokine signaling pathway | MAPK1, AKT1, RELA, GSK3B, CXCL8 | 0.00013 |
| hsa04630: JAK-STAT signaling pathway | AKT1, IL2, IL6, EGFR | 0.00092 |
| hsa04926: Relaxin signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, MAPK14, EGFR | 6.55E−08 |
| hsa04622: RIG-I-like receptor signaling pathway | RELA, MAPK8, MAPK14, CXCL8 | 0.0000547 |
| hsa04621: NOD-like receptor signaling pathway | RELA, JUN, MAPK8, IL6, CXCL8, TLR4, MAPK14, MAPK1 | 2.15E−08 |
| hsa04915: Estrogen signaling pathway | MAPK1, AKT1, JUN, EGFR, CTSD | 0.0000312 |
| hsa04024: cAMP signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, HCAR2, ADRB2 | 0.00000112 |
| hsa04910: Insulin signaling pathway | MAPK1, AKT1, GSK3B, MAPK8 | 0.000052 |
| hsa04072: Phospholipase D signaling pathway | MAPK1, AKT1, EGFR, CXCL8 | 0.00071 |
| hsa04150: mTOR signaling pathway | MAPK1, AKT1, GSK3B | 0.0083 |
| hsa04912: GnRH signaling pathway | MAPK1, JUN, MAPK8, EGFR, MAPK14 | 0.00000565 |
| hsa04151: PI3K-Akt signaling pathway | MAPK1, AKT1, RELA, GSK3B, IL2, EGFR, IL6, TLR4 | 0.00000224 |
| hsa04010: MAPK signaling pathway | MAPK1, AKT1, RELA, JUN, MAPK8, EGFR, MAPK14, CASP3 | 0.000000606 |
| hsa04921: Oxytocin signaling pathway | MAPK1, JUN, EGFR, PTGS2 | 0.00073 |
| hsa04371: Apelin signaling pathway | MAPK1, AKT1, HDAC5 | 0.0059 |
| hsa04014: Ras signaling pathway | MAPK1, AKT1, RELA, MAPK8, EGFR | 0.00031 |
| hsa04022: cGMP-PKG signaling pathway | MAPK1, AKT1, ADRB2, ADRA2B | 0.00094 |
| hsa04261: Adrenergic signaling in cardiomyocytes | MAPK1, AKT1, MAPK14, ADRB2 | 0.00071 |
| hsa04015: Rap1 signaling pathway | AKT1, MAPK14, EGFR | 0.0019 |
| hsa04550: Signaling pathways regulating pluripotency of stem cells | MAPK1, AKT1, MAPK14, GSK3B | 0.00062 |
| hsa04330: Notch signaling pathway | HDAC1, HDAC2 | 0.012 |
| hsa04310: Wnt signaling pathway | JUN, MAPK8, GSK3B | 0.0087 |
HDAC5, histone deacetylase 5; ADRA2B, alpha-2B adrenergic receptor; HCAR2, hydroxycarboxylic acid receptor 2; ADRB2, adrenoceptor beta 2; HDAC3, histone deacetylase 3; HDAC2, histone deacetylase 2; HDAC1, histone deacetylase 1; CTSD, cathepsin D; IL2, interleukin 2; TLR4, toll-like receptor 4; TLR9, toll-like receptor 9; AKT1, AKT serine/threonine kinase 1; EGFR, epidermal growth factor receptor; CXCL8, C-X-C motif chemokine ligand 8; PTGS2, prostaglandin-endoperoxide synthase 2; MAPK8, mitogen-activated protein kinase 8; IL6, interleukin-6; JUN, jun proto-oncogene, AP-1 transcription factor subunit; GSK3B, glycogen synthase kinase-3 beta; RELA, RELA proto-oncogene, NF-KB subunit; MAPK14, mitogen-activated protein kinase 14; CASP3, caspase 3; MAPK1, mitogen-activated protein kinase 1.
Figure 3.
(A) KEGG enrichment analysis. (B) GO analysis.
Kyoto encyclopedia of genes and genomes pathway analysis
The Wnt signaling pathway out of the 41 KEGG pathways was the most significant mechanism and indicates the critical targets on the KEGG pathway enrichment diagram37.
Molecular docking test
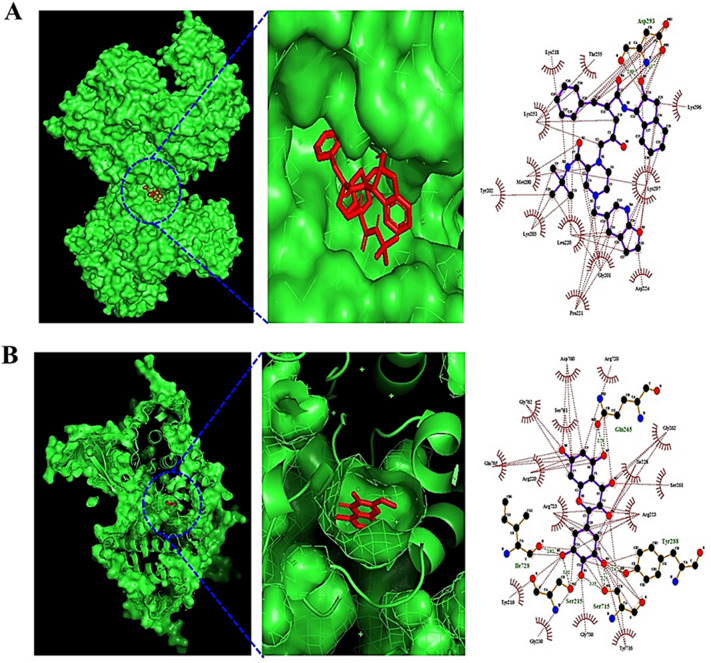
A total of 53 metabolites and three targets (JUN, MAPK8, and GSK3B) linked to the Wnt signaling pathway were identified via KEGG pathway enrichment analysis. MDT was performed to verify the binding affinity of each complex at the molecular level. AutoDockTools-1.5.6 software was used for MDT analysis; the docking scores are displayed in Supplementary Tables 1 and 2. The higher the negative docking score is, the more stable the complex is between the ligand and protein.
The cutoff of AutoDockTools-1.5.6 software is (< -6.0 kcal/mol), which can exert its efficacy on the target28. Between the 53 metabolites and 3 targets, the most stable complexes were JUN-platycodin D (− 9.0 kcal/mol), MAPK8-Compound K (− 8.5 kcal/mol), and GSK3B-myricetin (− 10.6 kcal/mol) (Fig. 4).
Figure 4.
The molecular docking test on key targets of Wnt signaling pathway. (A) compound K-MAPK8 (PDB ID: 4YRB). (B) myricetin-GSK3B (PDB ID: 1J1B).
Identification of drug-likeness properties in silico
The three metabolites (platycodin D, Compound K, and myricetin) were identified by the ADME parameters in silico. Platycodin D violated the drug-likeness properties characterized by Lipinski’s rule, including the topological polar surface area (TPSA) (cutoff value: < 140 Å2). The other two metabolites (Compound K and myricetin) had acceptable drug-likeness properties (Supplementary Table 3). Thus, we suggest that the two compounds can be metabolized by the gut microbiota and could be administered directly as new agents against NAFLD.
Toxicological properties of the two metabolites
The possible toxicological properties of Compound K and myricetin were evaluated by the ADMElab online tool. Both were free of such attributes, which can be a hurdle for drug development (Supplementary Table 4).
Identification of key components in the microbiota-signaling pathways-targets-metabolites network analysis
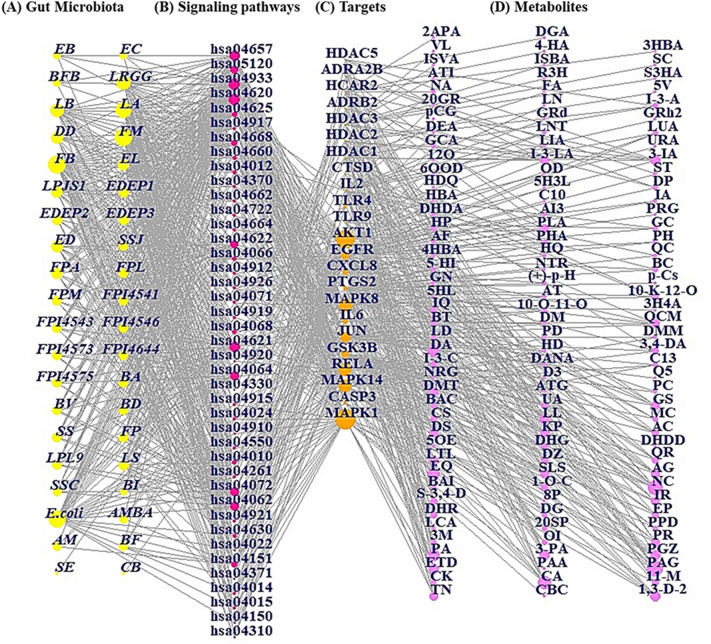
The MSTM network analysis was performed using the R package with the STRING database, comprising 232 nodes (41 microbiota, 41 signaling pathways, 23 targets, and 127 metabolites) and 1047 edges of the network. The green circles represent the gut microbiota, the pink circles represent the signaling pathways, the orange circles depict the targets, and the violet circles describe the metabolites (Fig. 5). The connectivity between nodes indicates the direct relationships of the nodes. The greater the number of linked nodes is, the more significant the function of the microbiota, signaling pathways, targets, or metabolites. Then, we analyzed the degree of value using R package.
Figure 5.
The MSTM networks (229 nodes and 1,044 edges). Yellow circle: gut microbiota; pink circle: signaling pathway; orange circle: target; violet circle: metabolite. (A) Microbiota. Firmicutes: FM; Fusobacteria: FB; Escherichia coli; E.coli; Lactobacillus acidophilus ATCC 4357: LA; Lactobacillus rhamnosus GG: LRGG; Lactobacillus: LB; Dictyostelium discoideum: DD; Enterococcus durans M4-5: ED; Lactobacillus paracasei JS1: LPJS1; Faecalibacterium prausnitzii A2-165: FPA; Eubacterium limosum: EL; Enterococcus durans EP1: EDEP1; Enterococcus durans EP2: EDEP2; Enterococcus durans EP3: EDEP3; Lachnospiraceae: LS; Streptococcus salivarius JIM8772: SSJ; Faecalibacterium prausnitzii L2-6: FPL; Faecalibacterium prausnitzii M 21/2: FPM; Faecalibacterium prausnitzii CNCM I-4541: FPI4541; Faecalibacterium prausnitzii CNCM I-4543: FPI4543, Faecalibacterium prausnitzii CNCM I-4546: FPI4546; Faecalibacterium prausnitzii CNCM I-4573: FPI4573; Faecalibacterium prausnitzii CNCM I-4644: FPI4644; Faecalibacterium prausnitzii CNCM I-4575: FPI4575; Bifidobacterium adolescentis: BA; Bacteroides vulgatus: BV; Bacteroides distasonis: BD; Streptococcus salivarius: SS; Faecalibacterium prausnitzii: FP; Lactobacillus plantarum L9: LPL9; Bacteroides fragilis ATCC 23,745: BF; Streptococcus salivarius CIP102503: SSC; Akkermansia muciniphila ATCC BAA-835: AMBA; Faecalibacterium prausnitzii A2 < U + 2013 > 165: FPA2; Akkermansia muciniphila: AM; Eubacterium: EB; Enterococcus: EC; Bifidobacterium: BFB; Bacteroides: BI; Salmonella enterica: SE; Clostridium butyricum ATCC 19,398: CB. (B) Signaling pathways. hsa04620: Toll-like receptor signaling pathway; hsa04657: IL-17 signaling pathway; hsa04933: AGE-RAGE signaling pathway in diabetic complications; hsa04668: TNF signaling pathway; hsa04917: Prolactin signaling pathway; hsa04660: T cell receptor signa;ing pathway; hsa05120: Epithelial cell signaling in Helicobacter pylori infection; hsa04722: Neurotrophin signaling pathway; hsa04662: B cell receptor signaling pathway; hsa04370: VEGF signaling pathway; hsa04071: Sphingolipid signaling pathway; hsa04068: FoxO signaling pathway; hsa04919: Thyroid hormone signaling pathway; hsa04064: NF-kappa B signaling pathway; hsa04920: Adipocytokine signaling pathway; hsa04062: Chemokine signaling pathway; hsa04630: JAK-STAT signaling pathway; hsa04926: Relaxin signaling pathway; hsa04622: RIG-I-like receptor signaling pathway; hsa04621: NOD-like receptor signaling pathway; hsa04915: Estrogen signaling pathway; hsa04024: cAMP signaling pathway; hsa04910: Insulin signaling pathway; hsa04072: Phospholipase D signaling pathway; hsa04150: mTOR signaling pathway; hsa04912: GnRH signaling pathway; hsa04151: PI3K-Akt signaling pathway; hsa04010: MAPK signaling pathway; hsa04921: Oxytocin signaling pathway; hsa04371: Apelin signaling pathway; hsa04014: Ras signaling pathway; hsa04022: cGMP-PKG signaling pathway; hsa04261: Adrenergic signaling in cardiomyocytes; hsa04015: Rap1 signaling pathway; hsa04310: Wnt signaling pathway; hsa04550: Signaling pathways regulating pluripotency of stem cells; hsa04330: Notch signaling pathway. (C) Targets. HDAC5: Histone deacetylase 5; ADRA2B: Alpha-2B adrenergic receptor; HCAR2: Hydroxycarboxylic acid receptor 2; ADRB2: Adrenoceptor Beta 2; HDAC3: Histone Deacetylase 3; HDAC2: Histone Deacetylase 2; HDAC1: Histone Deacetylase 1; CTSD: Cathepsin D; IL2: Interleukin 2; TLR4: Toll-like receptor 4; TLR9: Toll-like receptor 9; AKT1: AKT Serine/Threonine Kinase 1; EGFR: Epidermal Growth Factor Receptor; CXCL8: C-X-C Motif Chemokine Ligand 8; PTGS2: Prostaglandin-Endoperoxide Synthase 2; MAPK8: Mitogen-Activated Protein Kinase 8; IL6: Interleukin-6; JUN: Jun Proto-Oncogene, AP-1 Transcription Factor Subunit; GSK3B: Glycogen synthase kinase-3 beta; RELA: RELA Proto-Oncogene, NF-KB Subunit; MAPK14: Mitogen-Activated Protein Kinase 14; CASP3: Caspase 3; MAPK1: Mitogen-Activated Protein Kinase 1. (D) Metabolites. Phenylacetylglutamine: PAG; Naringenin chalcone: NC; Caffeic acid: CA; Phenylacetic acid: PA; Equol: EQ; Dihydroisoferulic acid: DA; 1,3-Diphenylpropan-2-ol: 1,3-D-2; Enterodiol: ETD; 3-Phenylpropionic acid: 3-PA; Pioglitazone: PGZ; Lunularin: LL; 3-Indolepropionic acid: 3-IA; Tretinoin: TN; Phloretin: PR; Icaritin: IR; Secoisolariciresinol: SLS; Apigenin: AG; Luteolin: LTL; Diosmetin: DS; Kaempferol: KP; Genistein: GS; Demethyltexasin: DMT; Quercimeritrin: QCM; Phenylalanine: PLA; Indole-3-lactic acid: I-3-LA; 11-Methoxycurvularin: 11-M; Dihydroresveratrol: DHR; Ethyl phenyllactate, (-)-: EP; Stilbene-3,4-diol: S-3,4-D; (S,R)-1-O-caffeoylglycerol: 1-O-C; Daidzein: DZ; Quercetin: QR; Acacetin: AC; Chrysin: CS; Urolithin A: UA; Indole-3-carboxylic acid: I-3-C; 3,4-Dihydroxyphenylacetic acid: 3,4-DA; Isoquercitrin: IQ; 10-Keto-12Z-octadecenoic acid: 10-K-12-O; Compound K: CK; 3-Methyloxindole: 3M; Oxindole: OI; (20S)-Protopanaxadiol: 20SP; Protopanaxadiol: PPD; Diosgenin: DG; Baohuoside I: BAI; Myricetin: MC; Baicalein: BAC; CHEBI:137478: C13; Levodopa: LD; Butyrate: BT; 10-Oxo-11-octadecenoic acid: 10-O-11-O; Baicalin: BC; Phloretic acid: PHA; HPLA: HP; Glycitein: GC; Dopamine: DP; Iuro-a: IA; Indole-3-acrylic acid: I-3-A; Dihydroglycitein: DHG; Leucocianidol: LCA; Ponciretin: PC; Danshensuan A: DANA; Hesperetin dihydrochalcone: HD; Platycodin D: PD; Didemethylmatairesinol: DMM; D-Mannose: DM; Acetic: AT; Genipin: GN; (+)-p-Hydroxyhydratropic acid: (+)-p–H; 5-HIAA: 5-HI; 4-Hydroxyphenylacetic acid: 4-HA; Hydroxyquercitrin: HQ; Quercitrin: QC; Acifran: AF; PhlP: PH; Dihydrocaffeic acid: DHDA; AI3-32,395: AI3; luro-a: IA; Serotonin: ST; Glycocholic acid: GCA; Lacto-N-tetraose: LNT; Nicotinic acid: NA; Colibactin: CBC; Palmitic acid: PAA; 8-Prenylnaringenin: 8P; 5-OH-Equol: 5OE; Dihydrogenistein: DHG; Dihydrodaidzein: DHDD; Arctigenin: ATG; Naringenin: NRG; DIF-3: D3; Q51617483: Q5; 3-Hydroxy-4-methoxybenzenepropanoic acid: 3H4A; 5-(Hydroxy-3-indolyl)lactic acid: 5HL; p-Cresol sulfate: p-Cs; Norathyriol: NTR; Phloroglucinol: PRG; Hydroumbellic acid: HDQ; CHEBI:10980: C10; Hydroquinone: HDQ; 5-hydroxyindole-3-lactic acid: 5H3L; 6′-OH-O-Dma: 6OOD; O-Desmethylangolensin: OD; 10-oxo-12Z-octadecenoic acid: 12O; Lithocholic acid: LIA; Ursodeoxycholic acid: URA; Deoxycholic acid: DEA; p-Cresol glucuronide: PCG; Ginsenoside-Rd: GRD; Ginsenoside Rh2: GRH; 20(R)-Ginsenoside Rh2: 20GR; LNnT: LN; Folic acid: FA; 5-(3,4-Dihydroxyphenyl)-valerolactone: 5V; Acetoin: ATI; (R)-3-Hydroxybutyrate: R3H; (S)-3-Hydroxybutyric acid: S3HA; Isovaleric acid: ISVA; Isobutyric acid: ISBA; Succinate: SC; Valerate: VL; 4-Hydroxybenzoic acid: 4HBA; 3-Hydroxybenzoic acid: 3HBA; 2-Acetoxypropanoic acid: 2APA; D-Glucuronic Acid: DGA.
We discovered that Firmicutes and Fusobacteria are the most significant microbiota, with 586 degrees of value each, the Toll-like receptor signaling pathway is the most significant effector mechanism, with 33 degrees of value, MAPK1 is the uppermost target, with 34 degrees of value, and phenylacetylglutamine is the highest metabolite, with 10 degrees of value. The 4 components exhibited more relationships, suggesting that these components might be the most significant hallmarks in NAFLD.
Discussion
We investigated the interaction between metabolites and gut microbiota via data-driven analysis. Previous research has suggested the use of gut microbiota in NAFLD treatment, but the details of the relevant metabolites and their targets remain unclear. Recently, network-based systems pharmacology has been used for diagnosis of various diseases and identification of target substances38. This study demonstrated that the relevant microbiome-derived metabolites might be detected by using network-based systems pharmacology, and the results of our study support the power of this approach.
In the PPI networks, AKT1, IL6, PPARG, JUN, and EGFR were defined as important targets. AKT inactivation attenuated NAFLD progression and liver tumorigenesis in mouse experiments39. The IL6 level was markedly increased in NAFLD patients, which can exacerbate its severity40. This implies that inactivation of IL6 might be a therapeutic strategy to alleviate NAFLD. Additionally, a study demonstrated that upregulation of PPARG can accelerate the progression of adipogenic hepatic steatosis41. In the NAFLD cellular sample, the expression of JUN was considerably elevated, suggesting that miR-139-5p overexpression is an indirect approach to dampen the JUN expression level42. An animal test suggested that epidermal growth factor receptor (EGFR) activation exacerbates the severity of NAFLD due to dysfunction of lipid metabolism43. Therefore, the five targets may be promising key targets for the treatment of NAFLD via gut microbiota metabolites.
The GO enrichment analysis results suggest that NAFLD targets of metabolites from gut microbiota are mainly related to bile acid receptor activity, vitamin D 24-hydroxylase activity, the Sin3 complex, nucleosome remodeling and the deacetylase (NuRD) complex, the neutrophil apoptotic process, alkaloid catabolic process, dibenzo-p-dioxin metabolic process, and fungiform papilla formation to relieve NAFLD. This analysis sheds light on the functions of metabolites in the treatment of NAFLD.
The results of the KEGG enrichment analysis indicate enrichment in inflammatory-related pathways, such as the IL-17 signaling pathway, AGE-RAGE signaling pathway, C-type lectin receptor signaling pathway, TNF signaling pathway, Toll-like receptor signaling pathway, T-cell receptor signaling pathway, epithelial cell signaling in Helicobacter pylori infection, the NOD-like receptor signaling pathway, neurotrophin signaling pathway, and prolactin signaling pathway. The targets of the key metabolites of gut microbiota associated with NAFLD are also related to inflammation. The relationships of the 10 significant pathways according to the FDR (false discovery rate < 0.05) are briefly discussed. IL-17 signaling pathway: IL-17 signaling aggravated the severity of NAFLD in mouse experiments due to the causal contribution of gut microbiota driving IL-17 production in damaged hepatocytes44. Advanced glycation end-products–receptor advanced glycation end-products (AGE-RAGE) signaling pathway: The upregulation of advanced glycation end-products (AGEs) accelerates the detrimental effects (liver injury, inflammation, and hepatic fibrosis) of NAFLD; therefore, a restrictive regime of AGEs might be a therapeutic strategy to relieve NAFLD45. C-type lectin receptor signaling pathway: C-type lectin is a hallmark to identify the stage of chronic liver disease, which is commonly upregulated in nonalcoholic steatohepatitis (NASH)46. It has been postulated that the overexpression of C-type lectin might induce excessive inflammation in hepatocytes. Tumor necrosis factor (TNF) signaling pathway: the expression level of TNF-α was increased in serum samples of NAFLD patients; in contrast, mice with deleted TNF receptors showed attenuated inflammation, steatosis, and fibrosis47. Toll-like receptor 7 (TLR7) dampened the development of NAFLD, and might be a potential treatment48. T-cell receptor signaling pathway: The dysregulation of T cells leads to the development of NAFLD, which results in cirrhosis and hepatocellular carcinoma49. Epithelial cell signaling in Helicobacter pylori infection: Helicobacter pylori infection might lead to NAFLD due to excessive inflammatory responses and insulin resistance50. NOD-like receptor signaling pathway: NLR induces the innate immune response to defend against foreign bodies, such as microbes or toxic chemicals, and the silencing of NLR can protect against cytokines51. Neurotrophin signaling pathway: The synthesis of brain-derived neurotrophic factor in the central nervous system indirectly enhances NAFLD via adiponectin52. Prolactin signaling pathway: Prolactin decreases lipid accumulation in hepatocytes, which ameliorates inflammation in the liver53. The rich factor (gene-ratio) results in our analysis showed the Wnt signaling pathway to have the lowest rich factor, indicating that the pathway might function as an inhibitive mechanism against NAFLD. Consistent with this result, Wnt antagonists have been shown to be a significant target for inhibiting the progression of NAFLD54.
Our study shows that Compound K and myricetin are promising antagonists that bind stably to MAPK8 and GSK3B in the Wnt signaling pathway, respectively. Compound K is a major metabolite of ginsenoside Rb1, which is converted by the gut microbiota55. Myricetin is a metabolite of myricitrin, which is transformed by Escherichia sp. 12, Escherichia sp. 33, and Enterococcus sp.4556. Furthermore, these metabolites have stable physicochemical properties in common in the systemic circulation and have low toxicity."57.
According to the MSTM networks, the results suggest that 41 microbiota constituents, 41 signaling pathways, 23 targets, and 125 metabolites might exert therapeutic efficacy against NAFLD. The Firmicutes phyla play significant roles in repressing the growth of pathogenic microbes, maintaining a constant immune system58. A group who consumed red wine combined with polyphenols had increased levels of Fusobacteria and Firmicutes, suggesting that the gut microbes might be significant players against cirrhosis59. Moreover, polyphenols play important roles in inhibiting hepatic fat accumulation, which has been confirmed by several in vitro experiments, in vivo tests, and clinical trials60. A finding which has been confirmed that both Firmicutes and Fusobacteria might exert desirable effects on NAFLD. MAPK inhibition attenuates obesity, insulin resistance, and steatosis in NAFLD61. With the highest degree of value of the metabolites of the gut microbiota, phenylacetylglutamine might be a biomarker to sign hepatic dysfunction62.
Our results in this study show that a holistic-based analysis, as integrated science, is a powerful tool for unraveling complex diseases and targets, as concluded by others63. Moreover, the associations and interactions between microbiota and complex chronic diseases can be better understood/elucidated utilizing network pharmacology concepts64.
Conclusion
In summary, this study investigated the key metabolites of gut microbiota in treating NAFLD via a network pharmacology-based study. We revealed that Compound K and myricetin can function as antagonists of the Wnt signaling pathway by docking stably to MAPK8 (also known as JNK) and GSK3B. Our study provides crucial evidence that Compound K converted from ginsenoside Rb1 and myricetin converted from myricitrin can be administered orally as a therapeutic strategy against NAFLD. From a holistic viewpoint, Firmicutes and Fusobacteria, the Toll-like receptor signaling pathway, MAPK1, and phenylacetylglutamine might be important key components and distinctive features of NAFLD in MSTM networks. Thus, we suggest that a systemic approach to the analysis of metabolites of gut microbiota can be an effective methodology to screen therapeutic agents.
Supplementary Information
Acknowledgements
This research was supported by Hallym University Research Fund, the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2018M3A9F3020956, NRF-2019R1I1A3A01060447, NRF-2020R1I1A3073530 and NRF-2020R1A6A1A03043026).
Abbreviations
- BF
Biological function
- CC
Cellular component
- Gut
Gastrointestinal tract
- KEGG
Kyoto encyclopedia of genes and genomes
- LD50
Lethal dose 50
- LPS
Lipopolysaccharide
- LVEF
Left ventricular ejection fraction
- MAFLD
Metabolic dysfunction-associated fatty liver disease
- MDT
Molecular docking test
- MF
Molecular function
- MSTM
Microbiota-signaling pathways-targets-metabolites
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NuRD
Nucleosome remodeling and deacetylase
- PPI
Protein–protein interaction
- T2DM
Type 2 diabetes mellitus
- TMAO
Trimethylamine oxide
- TPSA
Topological polar surface area
Author contributions
K.T.S., and D.J.K.: supervision, project administration, and investigation. K.T.S., and K.K.O.: conceptualization, methodology. K.T.S., K.K.O., and H.G.: formal analysis. B.H.M., S.J.Y., R.G., Y.A.G., S.M.W., J.J.J., S.P.S., E.J.P., M.R.C., S.B.L., M.G.C., G.H.K., M.K.J., J.Y.H., J.A.E., and H.J.P.: visualization, data curation. K.T.S., and K.K.O.: writing (original draft). K.K.O., H.G., and B.H.M.: software, investigation, and data curation. K.T.S., D.J.K., H.G., B.H.M., S.J.Y., H.G., B.H.M., S.J.Y.: validation and writing. K.T.S., and D.J.K.: review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hallym University Research Fund, the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (NRF-2018M3A9F3020956, NRF-2019R1I1A3A01060447, NRF-2020R1I1A3073530 and NRF-2020R1A6A1A03043026).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-27885-w.
References
- 1.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:36–44. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogra SK, Doré J, Damak S. Gut microbiota resilience: Definition, link to health and strategies for intervention. Front. Microbiol. 2020;11:2245. doi: 10.3389/fmicb.2020.572921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Renal Physiol. 2019;316:F1211–F1217. doi: 10.1152/ajprenal.00298.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosoky NS, May-Zhang LS, Davies SS. Engineering the gut microbiota to treat chronic diseases. Appl. Microbiol. Biotechnol. 2020;104:7657–7671. doi: 10.1007/s00253-020-10771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics—A step beyond pre- and probiotics. Nutrients. 2020;12:1–17. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albhaisi SAM, Bajaj JS. The influence of the microbiome on NAFLD and NASH. Clin. Liver Dis. 2021;17:15–18. doi: 10.1002/cld.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlawat S, Sharma KK. Gut–organ axis: a microbial outreach and networking. Lett. Appl. Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333. [DOI] [PubMed] [Google Scholar]
- 8.Schwenger KJ, Clermont-Dejean N, Allard JP. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP Rep. 2019;1:214–226. doi: 10.1016/j.jhepr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 2020;21:1–19. doi: 10.3390/ijms21155214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Zhou J, Wang L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021;11:86. doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017;67:1084–1103. doi: 10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Ohtani N, Kawada N. Role of the gut–liver axis in liver inflammation, fibrosis, and cancer: A special focus on the gut microbiota relationship. Hepatol. Commun. 2019;3:456. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 15.He X, Ji G, Jia W, Li H. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanism and application of metabolomics. Int. J. Mol. Sci. 2016;17:300. doi: 10.3390/ijms17030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayakumar S, Loomba R. Review article: emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Aliment. Pharmacol. Ther. 2019;50:144. doi: 10.1111/apt.15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Liu S, Chen L, Zhao Z, Du S, Dong Q, et al. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Exp. Ther. Med. 2019;18:1935. doi: 10.3892/etm.2019.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Tripathi M, Sinha RA, Singh BK, Yen PM. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021;7:11. doi: 10.20517/2394-5079.2020.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muc-Wierzgon M, Martin-Pelaez S, Zhu H, Wang R, Hua H, Cheng Y, et al. Network pharmacology exploration reveals gut microbiota modulation as a common therapeutic mechanism for anti-fatigue effect treated with maca compounds prescription. Nutrients. 2022;14:1533. doi: 10.3390/nu14081533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennison E, Byrne CD. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2020;27:22–43. doi: 10.3350/cmh.2020.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 22.Daina A, Michielin O, Zoete V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Yu L, Zou F, Hu H, Liu K, Lin Z. Gene expression profiling and functional analysis reveals that p53 pathway-related gene expression is highly activated in cancer cells treated by cold atmospheric plasma-activated medium. PeerJ. 2017;2017:e3751. doi: 10.7717/peerj.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanal P, Patil BM, Chand J, Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat. Prod. Bioprospect. 2020;10:325. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laskowski RA, Swindells MB. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 28.Shityakov S, Förster C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv. Appl. Bioinform. Chem. 2014;7:23–36. doi: 10.2147/AABC.S63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daina A, Zoete V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamothe SM, Guo J, Li W, Yang T, Zhang S. The human ether-a-go-go-related gene (hERG) potassium channel represents an unusual target for protease-mediated damage. J. Biol. Chem. 2016;291:20387. doi: 10.1074/jbc.M116.743138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulliner D, Schmidt F, Stolte M, Spirkl HP, Czich A, Amberg A. Computational models for human and animal hepatotoxicity with a global application scope. Chem. Res. Toxicol. 2016;29:757–767. doi: 10.1021/acs.chemrestox.5b00465. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Cheng F, Chen L, Du Z, Li W, Liu G, et al. In silico prediction of chemical ames mutagenicity. J. Chem. Inf. Model. 2012;52:2840–2847. doi: 10.1021/ci300400a. [DOI] [PubMed] [Google Scholar]
- 33.Alves VM, Muratov E, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, et al. Predicting chemically-induced skin reactions. Part I: QSAR models of skin sensitization and their application to identify potentially hazardous compounds. Toxicol. Appl. Pharmacol. 2015;284:262. doi: 10.1016/j.taap.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei T, Li Y, Song Y, Li D, Sun H, Hou T. ADMET evaluation in drug discovery: 15 Accurate prediction of rat oral acute toxicity using relevance vector machine and consensus modeling. J. Cheminform. 2016;8:1–19. doi: 10.1186/s13321-016-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Dai Z, Chen F, Gao S, Pei J, Lai L. Deep learning for drug-induced liver injury. J. Chem. Inf. Model. 2015;55:2085–2093. doi: 10.1021/acs.jcim.5b00238. [DOI] [PubMed] [Google Scholar]
- 36.Dong J, Wang NN, Yao ZJ, Zhang L, Cheng Y, Ouyang D, et al. Admetlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018;10:1–11. doi: 10.1186/s13321-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2022;51:D587–D589. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gocho Y, Liu J, Hu J, Yang W, Dharia NV, Zhang J, et al. Network-based systems pharmacology reveals heterogeneity in LCK and BCL2 signaling and therapeutic sensitivity of T-cell acute lymphoblastic leukemia. Nat. Cancer. 2021;2:284–299. doi: 10.1038/s43018-020-00167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong SH, Kim HB, Kim MC, Lee JM, Lee JH, Kim JH, et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J. Clin. Investig. 2018;128:1010–1025. doi: 10.1172/JCI95802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieckowska A, Papouchado BG, Li ZZ, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 42.Jin SS, Lin CJ, Lin XF, Zheng JZ, Guan HQ. Silencing lncRNA NEAT1 reduces nonalcoholic fatty liver fat deposition by regulating the miR-139–5p/c-Jun/SREBP-1c pathway. Ann. Hepatol. 2022;27:100584. doi: 10.1016/j.aohep.2021.100584. [DOI] [PubMed] [Google Scholar]
- 43.Choung S, Kim JM, Joung KH, Lee ES, Kim HJ, Ku BJ. Epidermal growth factor receptor inhibition attenuates non-alcoholic fatty liver disease in diet-induced obese mice. PLoS ONE. 2019;14:e0210828. doi: 10.1371/journal.pone.0210828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harley ITW, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Liu H, Xie G, Cai W, Xu J. Identification of hub genes and key pathways of dietary advanced glycation end products-induced non-alcoholic fatty liver disease by bioinformatics analysis and animal experiments. Mol. Med. Rep. 2020;21:685–694. doi: 10.3892/mmr.2019.10872/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schierwagen R, Uschner FE, Ortiz C, Torres S, Brol MJ, Tyc O, et al. The role of macrophage-inducible C-type lectin in different stages of chronic liver disease. Front. Immunol. 2020;11:1352. doi: 10.3389/fimmu.2020.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osawa Y, Kojika E, Hayashi Y, Kimura M, Nishikawa K, Yoshio S, et al. Tumor necrosis factor-α-mediated hepatocyte apoptosis stimulates fibrosis in the steatotic liver in mice. Hepatology Communications. 2018;2:407–420. doi: 10.1002/hep4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Park S, Kim B, Kwon J. Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci. Rep. 2016;6:1–15. doi: 10.1038/srep27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsova P, Bamidele AO, Wang H, Povero D, Revelo XS. Emerging roles of T cells in the pathogenesis of nonalcoholic steatohepatitis and hepatocellular carcinoma. Front. Endocrinol. 2021;12:1432. doi: 10.3389/fendo.2021.760860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Manatsathit W, Jaruvongvanich V, Ungprasert P. Helicobacter pylori and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2018;52:386–391. doi: 10.1097/MCG.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 51.Xu T, Du Y, Fang X-B, Chen H, Zhou D-D, Wang Y, et al. New insights into Nod-like receptors (NLRs) in liver diseases. Int. J. Physiol. Pathophysiol. Pharmacol. 2018;10:1. [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YD, et al. New insight of obesity-associated NAFLD: Dysregulated “crosstalk” between multi-organ and the liver? Genes Dis. 2021;12:1–14. doi: 10.1016/j.gendis.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Ge Z, Wang H, Feng W, Sun X, Chu X, et al. Prolactin improves hepatic steatosis via CD36 pathway. J. Hepatol. 2018;68:1247–1255. doi: 10.1016/j.jhep.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Song K, Srivastava R, Dong C, Go GW, Li N, et al. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015;29:3436–3445. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE. 2013;8:e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du LY, Zhao M, Xu J, Qian DW, Jiang S, Shang EX, et al. Identification of the metabolites of myricitrin produced by human intestinal bacteria in vitro using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Expert Opin. Drug Metab. Toxicol, 2014;10:921–931. doi: 10.1517/17425255.2014.918954. [DOI] [PubMed] [Google Scholar]
- 57.Wong AC, Levy M. New approaches to microbiome-based therapies. mSystems. 2019 doi: 10.1128/mSystems.00122-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jasirwan COM, Lesmana CRA, Hasan I, Sulaiman AS, Gani RA. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health. 2019;38:81. doi: 10.12938/bmfh.18-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guohong-Liu QZ. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann. Hepatol. 2019;18:796–803. doi: 10.1016/j.aohep.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 60.Abenavoli L, Larussa T, Corea A, Procopio AC, Boccuto L, Dallio M, et al. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients. 2021;13:494. doi: 10.3390/nu13020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cicuéndez B, Ruiz-Garrido I, Mora A, Sabio G. Stress kinases in the development of liver steatosis and hepatocellular carcinoma. Mol. Metab. 2021;50:101190. doi: 10.1016/j.molmet.2021.101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delzenne NM, Knudsen C, Beaumont M, Rodriguez J, Neyrinck AM, Bindels LB. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: a focus on the gut-liver axis. Proc. Nutr. Soc. 2019 doi: 10.1017/S0029665118002756. [DOI] [PubMed] [Google Scholar]
- 63.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y, Chen M, Wang Q, Gao L, Feng Y, Wang S, et al. Integrating pharmacology and microbial network analysis with experimental validation to reveal the mechanism of composite sophora colon-soluble capsule against ulcerative colitis. Evid.-Based Complement. Altern. Med. 2020 doi: 10.1155/2020/9521073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).