ABSTRACT
Accumulating evidence suggests that the therapeutic role of mesenchymal stem cells (MSCs) in bone diseases is closely related to paracrine-generated extracellular vesicles (EVs). MSC-derived EVs (MSC-EVs) carry proteins, nucleic acids, and lipids to the extracellular space and affect the bone microenvironment. They have similar biological functions to MSCs, such as the ability to repair organ and tissue damage. In addition, MSC-EVs also have the advantages of long half-life, low immunogenicity, attractive stability, ability to pass through the blood-brain barrier, and demonstrate excellent performance with potential practical applications in bone diseases. In this review, we summarise the current applications and mechanisms of MSC-EVs in osteoporosis, osteoarthritis, bone tumours, osteonecrosis of the femoral head, and fractures, as well as the development of MSC-EVs combined with materials science in the field of orthopaedics. Additionally, we explore the critical challenges involved in the clinical application of MSC-EVs in orthopaedic diseases.
Keywords: exosomes, extracellular vesicles, mesenchymal stem cells, orthopaedic diseases, regenerative medicine
Introduction
Bone is one of the largest organ systems in mammals, and accounts for about 15% of body weight in humans, playing a vital role in supporting the body, allowing movement and protecting the internal organs.1 In addition, bone is also responsible for producing blood cells and regulating hormones.2 Bone tissue continuously undergoes cell metabolism with active intercellular interactions.3 Bone diseases include a diverse spectrum of skeletal-related disorders that impair mobility and increase mortality.4 Many bone diseases, especially degenerative bone diseases, lack effective treatment methods.5
Mesenchymal stem cells (MSCs) are the primary source of adult stem cells, which are mesoderm-derived cell subsets with self-renewal and multilineage differentiation potential.6 MSCs can differentiate into various cell types of the mesoderm lineage, such as osteocytes/chondrocytes, adipocytes, fibroblasts, and other embryonic lineages.7 MSCs have significant clinical application advantages compared with several other types of stem cells, including ease of availability (they can be isolated from the stroma of nearly all organs), relatively simple isolation process, and ability to produce soluble growth factors and cytokines by endocrine or paracrine secretion.8 Although MSC therapy is a promising strategy for bone diseases, however, the direct use of MSCs still faces many challenges, including limited cell survival rate and immune rejection. Moreover, MSCs also have unique environmental sensitivity, and their behaviour can change significantly in different microenvironments, which increases the phenotypic uncertainty that exists after MSC transplantation.9-11
Extracellular vesicles (EVs) are nano-sized lipid-bound vesicles released from cells into the extracellular space.12 These vesicles are considered to play essential roles in intercellular communication and immune response.13 Further, accumulating evidence suggests that the therapeutic role of MSCs in bone diseases is closely related to the paracrine-produced EVs.14 MSC-EVs perform similar functions to MSCs, but contain fewer membrane proteins related to immune recognition (such as the major histocompatibility complex), are less immunogenic, and the contents are protected by lipid biomolecules making it possible to maintain their biological activity for a long period. Therefore MSC-EVs combine the advantages of MSCs with those of EVs, making them a promising contender for the treatment of bone disorders.
With the rapid development of deep sequencing technology and diverse proteomic analysis, our understanding of MSCs and MSC-EVs is gradually deepening; through multidisciplinary collaboration, MSC-EVs are expected to play a more important role in treatment of various diseases including bone diseases in the future. In this review, we summarise research progress in understanding the roles of MSC-derived small EVs (mainly exosomes) in bone diseases and their potential as therapeutic targets.
Search Strategy
For this review, electronic searches of the Web of Science and PubMed databases for literature describing the role of MSC-EVs in bone diseases, published before May 2022, were performed using the following terms: 1) (mesenchymal stem cells [Title/Abstract]) AND ((Extracellular vesicles [Title/Abstract]) OR (exosomes [Title/Abstract])) AND (bone [Title/Abstract]); 2) ((mesenchymal stem cells [Title/Abstract]) AND ((extracellular vesicles [Title/Abstract]) OR (exosomes [Title/Abstract])) AND ((osteoporosis [Title/Abstract]) OR (osteoarthritis [Title/Abstract]) OR (necrosis of the femoral head [Title/Abstract]) OR (bone fractures [Title/Abstract])). The results were further screened by title and abstract. We searched for clinical trials at clinicaltrials.gov which were also analysed. Finally, 106 articles were included in this review.
Overview of Mesenchymal Stem Cells and Extracellular Vesicles
As the “origin” of life, stem cells have the potential for self-renewal and multilineage differentiation, with the ability to develop into various terminally-differentiated cells.15 Based on the source of isolation or origin, stem cells can be divided into three categories: 1) embryonic stem cells (ESCs), which are present in the earliest stages of pregnancy or can be generated externally through in vitro fertilisation. ESCs have an unlimited capacity for proliferation and remain undifferentiated, with a capacity to differentiate into all cell types, but the use of ESCs is hampered by their limited sources, tumorigenic risks, and ethical controversies.16 2) Somatic or adult stem cells, present throughout life, are less effective but critical for overall health maintenance. These stem cells were initially discovered in bone marrow but are now believed to exist in nearly every body organ.17 3) Induced pluripotent stem cells are adult cells that have been reprogrammed in the laboratory to resemble ESCs. The self-proliferation and differentiation capabilities of induced pluripotent stem cells are similar to those of ESCs and avoid ethical issues. However, the reprogramming efficiency of induced pluripotent stem cells is limited and they are also tumorigenic.18, 19 The characteristics of different stem cells are summarised in Table 1.
Table 1. Comparison of the characteristics of different stem cells.
| Embryonic stem cells | Induced pluripotent stem cells | Somatic or adult stem cells | |
|---|---|---|---|
| Source | Inner cell mass of the blastocyst | Reprogrammed somatic cells | Tissue-specific protocols from different tissues |
| Differentiation potential | Can differentiate into cell types of all three germ lineages | Can differentiate into cell types of all three germ lineages | Can only differentiate into limited cell types |
| Self-renewal | Complete | Complete | Limited |
| Proliferative capacity | Strongest | Powerful | Weak and cannot be maintained for a long periods |
| Tumorigenicity | Yes | Yes | No |
| Ethical controversy | Yes | No | No |
MSCs are adult stem cells. Identification of MSCs can be traced back to 1966; Professor Friedenstein et al.20 found that transplanted bone marrow cells were able to differentiate into osteoblasts in vivo. In 1991, Professor Caplan21 coined the name mesenchymal stem cells, and this term has been in use ever since. MSCs lack specific markers, so they are usually identified by a combination of expression of CD29, CD73, CD90, CD105, and CD106, but lack of expression of hematopoietic stem cell markers CD34, CD45, etc.22 MSC therapy has many drawbacks including immune rejection, low cell survival rate, embolism, mutagenesis, and inability to cross the blood-brain barrier,23 limiting its application in clinical practice which urges us to find new alternative treatments.
Recent research indicates that the therapeutic actions of MSCs are mediated mostly by their MSC-EVs.24 EVs is a collective term for a class of nanoscale membrane vesicles with a phospholipid bilayer structure, which can be further divided into different subclasses based on their physicochemical properties and biochemical characteristics, classifying them into exosomes, microvesicles, apoptotic vesicles, etc.25 (Table 2).
Table 2. Characteristics of the major types of extracellular vesicles.
| Vesicles | Size (nm) | Density (g/mL) | Origin | Markers | Membrane permeability |
|---|---|---|---|---|---|
| Exosomes | 30–150 | 1.13–1.18 | Endosomes | Tetraspanins (CD9, CD63, CD81), TSG101, ALIX | Impermeable |
| Microvesicles | 200–1000 | 1.16–1.19 | Budding of plasma membrane | Integrins, selectins, CD40 | Impermeable |
| Apoptotic bodies | >1000 | 1.16–1.28 | Release after apoptosis | Propidium iodide positive Phosphatidylserine DNA fragmentation | Permeable |
Note: ALIX: apoptosis-linked gene 2-interacting protein X; TSG101: tumour susceptibility gene 101.
Almost all live cells secrete EVs, which were initially thought to be “rubbish bags” for the excretion of metabolic waste, but as research progressed it has become clear that EVs play an important role in nucleic acid and protein transport, antigen presentation and modification of the cellular microenvironment.26, 27
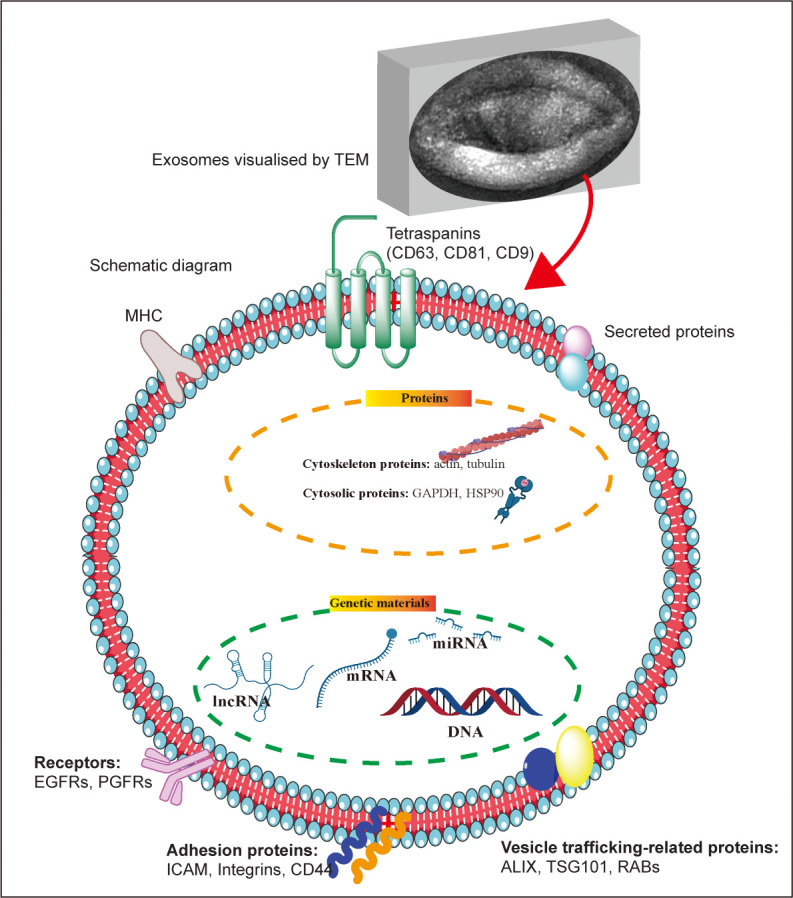
Exosomes are vesicle-like bodies with a diameter of approximately 30–150 nm secreted by living cells, with a density of 1.13–1.18 g/mL in a sucrose gradient, and a saucer-like structure visible under an electron microscope28 (Figure 1). The biogenesis of exosomes involves three main steps: 1) the formation of endocytic vesicles via invagination of the plasma membrane, which then fuse to form early endosomes; 2) early endosomal formation via regulation of the endosomal sorting complex to generate multiple intraluminal vesicles, which then form multivesicular bodies; and 3) fusion of multivesicular bodies with the plasma membrane and release of exosomes into the extracellular space.29
Figure 1. Exosome morphology and structure. The upper image represents the morphology of exosomes (produced by mouse hepatocytes) under a transmission electron microscope; below is a schematic diagram of the structure and composition of exosomes. ALIX: apoptosis-linked gene 2-interacting protein X; EGFR: epidermal growth factor receptor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; HSP90: heat shock protein 90; ICAM: intercellular adhesion molecule; lncRNA: long non-coding RNA; MHC: major histocompatibility complex; miRNA: microRNA; PGFR: platelet-derived growth factor receptor; RAB: Rab GTPases; TEM: transmission electron microscope; TSG101: tumour susceptibility gene 101. This figure was created using Servier Medical Art templates (https://smart.servier.com).
Microvesicles are produced by direct outward budding of the plasma membrane and occur in a diverse range of sizes (100–1000 nm in diameter).30 The cellular origin determines the composition of the microvesicular membrane to a large extent, so that each microvesicle has a membrane composition similar to that of the parent cell. Microvesicle formation is associated with the asymmetric distribution of phospholipids in the cell membrane bilayer, and may be influenced by calcium ion concentration and the junction protein arrestin domain-containing protein 1, but the exact mechanism is unclear.31
Apoptotic bodies form during apoptosis and originate from blebbing of the plasma membrane. Apoptotic vesicles are 500– 2000 nm in diameter, and enclose cytoplasm, organelles and nuclear fragments.32
The Role of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone Homeostasis
Bone homeostasis is the cornerstone of bone health, created by a dynamic balance between osteoclasts, osteoblasts and osteocytes.33 Osteoblasts and chondrocytes originate from the bone marrow MSC (BMSC) lineage. Multiple transcriptional regulators that act as “master switches” orchestrate the commitment of MSCs to tissue-specific cell types. In skeletogenesis, the transcription factors Runx2, osterix, activating transcription factor 4, and distal-less homeobox 5 are known to play critical roles in the cell fate decision process by which MSCs differentiate into osteoblasts via activation of cell type-specific genes.34 Osteoclasts are multinucleated giant cells that differentiate from osteoclast precursor cells derived from haematopoietic stem cell niche-monocyte/macrophage lineage cells in the presence of two critical factors: receptor activator of nuclear factor-κB ligand and macrophage/ monocyte colony-stimulating factor.35
Osteoclasts degrade the bone matrix and collaborate with other bone cells, osteoblasts and osteocytes to remodel bone.36 The predominance of osteoclasts over osteoblasts will result in degradation of the bone matrix, including reduced bone mineral density and loss of bone marrow cells, which is associated with a variety of disorders, including osteoporosis (OP) and rheumatoid arthritis. However, increased osteoblastic activity will result in osteosclerosis.37
In addition, maintaining the equilibrium between osteogenic and adipogenic differentiation of BMSCs is crucial for maintaining bone homeostasis.38 Reduced osteogenic differentiation and enhanced adipogenic differentiation of BMSCs are key factors in the aetiology of OP. One of the directions for the treatment of OP is to inhibit the adipogenic differentiation of BMSCs and promote their osteogenic differentiation to rectify the imbalance of bone metabolism.39, 40 A good overview of the molecules that regulate the osteogenic, adipogenic, and chondrogenic differentiation of MSCs has been published.41-43
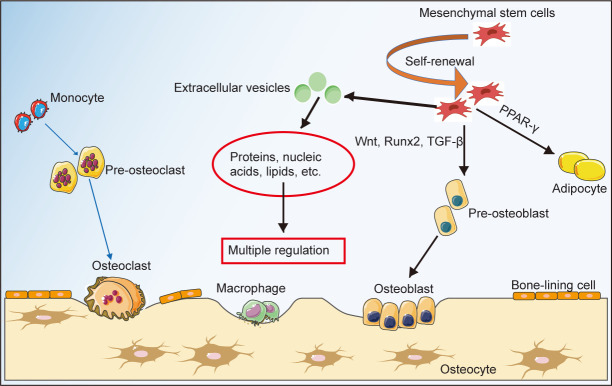
It is suggested that coordination among various bone-related cells contributes to the maintenance of bone homeostasis and bone health. In addition to the direct differentiation of MSCs into pre-osteoblasts, MSC-EVs also play a critical role in the control of bone homeostasis by carrying a range of proteins and RNAs that can regulate bone formation/degradation (Figure 2). We will discuss this in detail in the following sections.
Figure 2. Multi-factors involved in maintaining bone homeostasis. Bone homeostasis is the cornerstone of bone health, created by a dynamic balance between osteoclasts, osteoblasts, osteocytes and the equilibrium between osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells, in which mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles play essential roles. PPAR-γ: peroxisome proliferator-activated receptor γ; TGF-β: transforming growth factor β. This figure was created using Servier Medical Art templates (https://smart.servier.com).
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone Diseases
MSC-EVs have similar biological functions to MSCs and have various properties beneficial for the treatment of bone diseases, such as pro-angiogenic, anti-inflammatory, and promotion of osteogenic differentiation (Figure 3). Below we summarise the research progress and molecular mechanism of MSC-EVs in different bone diseases (Table 3).44-63
Figure 3. Summary of MSC-derived extracellular vesicles in the treatment of bone diseases. Natural/genetic or chemically-modified MSC-derived extracellular vesicles can regulate a series of biological processes by carrying RNA and protein to target cells, and exert therapeutic effects on bone diseases. GPNMB: glycoprotein nonmelanoma clone B; lncRNA: long non-coding RNA; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; MSC: mesenchymal stem cell. This figure was created using Servier Medical Art templates (https://smart.servier.com).
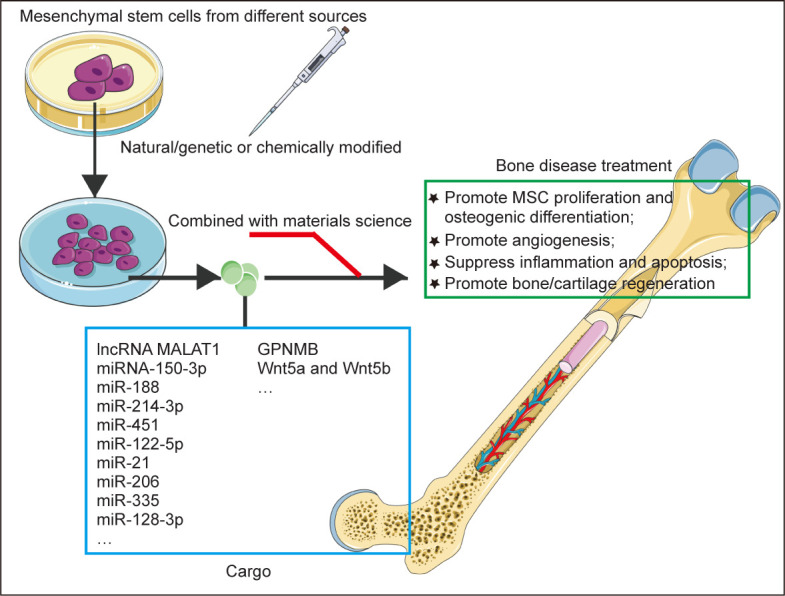
Table 3. Summary of the role of MSC-EVs in orthopaedic diseases.
| Diseases | Source of EVs | Main contents of EVs | Main mechanism | Effect | Reference |
|---|---|---|---|---|---|
| OP | BMSCs | lncRNA MALAT1 | Sponge miR-34c, upregulate SATB2 | Alleviates OP | 44 |
| OP | BMSCs | miRNA-150-3p | Promote osteoblast proliferation and differentiation | Mitigate OP | 45 |
| OP | BMSCs | GPNMB | Activate Wnt/β-catenin signalling | Alleviate OP | 46 |
| OP | BMSCs | miR-188 | Promote osteogenesis and inhibit adipogenic differentiation | Alleviate OP | 47 |
| OP | BMSCs from OVX mice | miR-214-3p | Inhibit type H vessel formation, and bone mineral density and contents | Aggravate OP | 48 |
| ONFH | GC-treated BMSCs | miR-451 | Increase the level of PAI-1 | Aggravate ONFH | 49, 50 |
| ONFH | BMSCs | miR-122-5p | RTK/Ras/MAPK signalling pathway | Promote ONFH healing | 51 |
| ONFH | hUCMSCs | miR-21 | PTEN-AKT pathway | Promote ONFH healing | 52 |
| OA | SMSCs | Wnt5a and Wnt5b | Activate the YAP signalling pathway | Alleviate OA | 53 |
| OA | Ad-MSCs | Unknown | Inhibit inflammation and MMP activity | Alleviate OA | 54 |
| OA | MSCIPFP | miR-100-5p | Inhibit mTOR signalling | Alleviate OA | 55 |
| OS | Ad-MSCs | Unknown | Promote osteosarcoma cell invasion, migration, and proliferation | Promote OS progression | 56 |
| OS | BMSCs | hsa-miR-148a | Promote OS cell proliferation, metastasis and prevent apoptosis | Promote OS progression | 57 |
| OS | BMSCs | lncRNA MALAT1 | lncRNA MALAT1/miR-143/NRSN2/Wnt/β-catenin pathway | Promote OS progression | 58 |
| OS | BMSCs | miR-206 | miR-206/TRA2B | Suppress OS progression | 59 |
| BF | BMSCs | miR-335 | miR-335/VapB/Wnt/β-catenin pathway | Promoted BF recovery | 60 |
| BF | Aged-BMSCs | miR-128-3p | miR-128-3p/Smad8 pathway | Inhibition of BF healing | 61, 62 |
| BF | BMSCs | miR-126 | SPRED1/Ras/Erk pathway | Promoted BF recovery | 63 |
Note: Ad-MSCs: adipose-derived mesenchymal stem cells; AKT: protein kinase B; BD: bone disease; BF: bone fracture; BMSCs: bone marrow mesenchymal stem cells; Erk: extracellular signal-regulated kinase; GC: glucocorticoids; GPNMB: glycoprotein nonmelanoma clone B; hUCMSC: human umbilical cord mesenchymal stem cells; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; lncRNA: long non-coding RNA; MAPK: mitogen-activated protein kinase; MMP: matrix metalloprotease; MSC-EVs: mesenchymal stem cell-derived extracellular vesicles; MSCIPFP: infrapatellar fat pad-derived mesenchymal stem cells; mTOR: mammalian target of rapamycin; OA: osteoarthritis; ONFH: osteonecrosis of the femoral head; OP: osteoporosis; OS: osteosarcoma; OVX: ovariectomised; PAI-1: plasminogen activator inhibitor 1; PTEN: phosphatase and tensin homolog; RTK: receptor tyrosine kinase; Smad8: SMAD family member 8; SMSCs: synovial mesenchymal stem cells; SPRED1: sprouty related EVH1 domain containing 1; TRA2B: transformer 2β; VapB: VAMP associated protein B.
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Osteoporosis
OP has become a serious public health problem, with a high prevalence among the elderly and postmenopausal women, characterised by bone loss and changes in bone structure.64 Fractures caused by OP are a significant source of morbidity and death, causing a serious socioeconomic burden.65
The pathophysiology of OP is complex, including an imbalance between osteoclasts and osteoblasts, decreased osteogenic differentiation and increased adipogenic differentiation of BMSCs.66 Currently, available therapies can only halt the progression of OP. As a result, innovative treatments for OP have garnered more study focus.67 MSC-EVs participate in the treatment of OP through multiple pathways. On the one hand, MSC-EVs inhibit OP by directly regulating the proliferation and activity of osteoblasts. On the other hand, MSC-EVs correct bone metabolism imbalance by promoting osteogenic differentiation and reducing adipogenic differentiation of BMSCs.68
The contents and functions of MSC-EVs in different states are diverse. MSC-EVs sourced from young individuals promote osteogenic differentiation and inhibit osteoclast formation, while from aging individuals, they promote adipogenic differentiation and activate osteoclasts. For example, bone matrix-derived EVs derived from aging organisms enriched with miR-483-5p boost BMSC adipogenic differentiation and bone-lipid imbalance to promote OP. However, EVs derived from bone matrix-derived cells of young organisms have no such function.69 Moreover, the therapeutic effect of MSC-EVs can be further optimised and the side effects can be reduced by genetic/chemical modification combined with material science technology.70, 71
Type H vessels are found near the growth plate in the metaphysis, as well as in the periosteum and endosteum of the diaphysis,72 are strongly positive for CD31 and endomucin (CD31hiEmcnhi), and couple angiogenesis and osteogenesis.73 miR-214-3p expression is significantly elevated in BMSC-EVs from postmenopausal mice, inhibiting type H vessel formation, and decreasing bone mineral density and content.48
Modification of MSC-EVs further enhances their anti-OP effect. For example, after overexpressing miRNA-150-3p in MSCs, co-culture of MSC-EVs with osteoblasts significantly promotes osteoblast proliferation and differentiation.45 EVs released by MSCs that overexpress glycoprotein nonmelanoma clone B (a multifunctional transmembrane glycoprotein) have a similar effect, significantly promoting the proliferation and osteogenic differentiation of BMSCs by activating Wnt/β-catenin signalling and alleviating ovariectomy-induced OP.46 EVs derived from miR-935-modified BMSCs alleviate OP by enhancing osteoblast proliferation and differentiation via targeting of transducer and activator of transcription 1.74
It is suggested that favourable modification of MSC-EVs may cause them to have a more substantial bone protective effect. The inhibitory effect of some nanomaterials on OP is also related to MSC-EVs. For example, bioactive glass nanoparticles promote the release of exosomes rich in long non-coding RNA noncoding repressor of NFAT from BMSCs, inhibit osteoclast differentiation by inhibiting nuclear translocation of activated T cell nuclear factor 1, and alleviate bone loss in OP mice.75
Competing endogenous RNA mechanisms have been confirmed to participate in OP, the long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 in MSC-EVs alleviates OP by up-regulating special AT-rich sequence-binding protein 2 through competitively binding to miR-34c.44 MSC-EVs also inhibit OP by reducing the generation of reactive oxygen species, inhibiting inflammation, promoting DNA repair, delaying aging, and promoting bone regeneration.14
Although MSC-EVs show obvious bone protective effects in vitro and in vivo, the therapeutic ability of native MSC-EVs administered through tail vein injection on bone diseases is minimal because the EVs administered through the circulatory system are mainly concentrated in the liver, spleen and lung.76, 77 Modification of EVs (e.g., binding of BMSC-specific aptamers) to specifically target BMSCs significantly enhances the ability of MSC-EVs to ameliorate OP and promote bone healing.78 In addition to binding specific aptamers to BMSCs, genetically-engineered C-X-C motif chemokine receptor 4-positive exosomes selectively accumulate in the bone marrow. In one study, hybrid nanoparticles were generated by fusing C-X-C motif chemokine receptor 4-positive exosomes with antagomir-188-carrying liposomes. Nanoparticles specifically accumulated in the bone marrow and released antagomir-188, promoting osteogenesis and inhibiting adipogenic differentiation of BMSCs, thereby reversing age-related bone loss and reducing cortical bone porosity in mice.47 Recently scientists developed a new EV delivery system. They coupled Ser-Asp-Ser-Ser-Asp (SDSSD) peptides with human-induced pluripotent stem cell-derived MSC-derived EVs to construct bone-targeted EVs, then transferred the small RNA-siShn3 into bone-targeted EVs. They found that these EVs enhanced slit guidance ligand 3 expression, ultimately promoting osteogenesis, inhibiting osteoclasts and treating OP.79 These studies show that bone-targeted EVs have better effects in treating bone diseases and reduce the impact on other tissues, which is a promising treatment method.
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Femoral Head Necrosis
Osteonecrosis of the femoral head (ONFH) is the result of disrupted blood supply to the femoral head due to various causes, resulting in the alternation of bone cell death and repair, secondary microfractures in the head, subchondral fractures, and stress concentration.80 ONFH is a common cause of hip joint dysfunction or loss in young adults, often requiring hip replacement surgery.81
The main types of ONFH are traumatic ONFH and nontraumatic ONFH. Glucocorticoid (GC)-associated ONFH is the most common form of non-traumatic ONFH. The level of miR-451 is significantly increased in GC-treated MSC-derived exosomes, while the level of miR-133b-3p is significantly decreased. miR-451 increases the level of plasminogen activator inhibitor 1, which inhibits the conversion of plasminogen to plasmin, reduces the level of fibrinolysis in blood vessels, promotes thrombosis, affects the microcirculation of the femoral head, and eventually leads to ischemic ONFH, while in contrast overexpression of miR-133b-3p significantly alleviates GC-induced ONFH by directly targeting plasminogen activator inhibitor 1.49, 50 In addition, miR-122-5p in BMSC-derived exosomes promotes osteoblast proliferation and differentiation by downregulating sprouty2 through the receptor tyrosine kinase/Ras GTPase/mitogen-activated protein kinase signalling pathway, resulting in increases in bone mineral density, trabecular bone volume and mean trabecular bone density of the femoral head, plate thickness increases and promotion of healing of ONFH.51
Through encapsulating small interfering RNAs (siRNAs) which interact with aggravating ONFH-related genes, such as tumour necrosis factor-α, Caspase3, etc., into MSC-derived exosomes, it is possible to promote angiogenesis and mitigate ONFH.82 However, the current research on bone protection by directly encapsulating ONFH-related gene siRNAs into EVs is still mainly in vitro and at the preclinical stages. Its effectiveness and safety need to be further clarified. In addition to BMSC-EVs inhibiting ONFH, human umbilical cord MSC-derived EVs have also been shown to have significant therapeutic effects. For example, research has shown that human umbilical cord MSC-derived exosomes significantly inhibit the apoptosis of MLO-Y4 osteocytes through the miR-21-phosphatase and tensin homolog/protein kinase B (Akt) signalling pathway and attenuate GC-induced ONFH.52
In summary, the current understanding of the role of MSC-EVs in ONFH mainly focuses on the following aspects: 1) During the occurrence of ONFH, some osteoprotective components (mainly miRNAs) in MSC-derived exosomes decrease, while harmful factors increase, this imbalance contributes to the onset and development of ONFH. 2) Natural MSC-EVs may have direct bone protective effects by promoting angiogenesis and inhibiting inflammation. 3) MSC-EVs may have a stronger inhibitory effect on ONFH through genetic (such as encapsulating multiple siRNAs)/chemical modification (osteogenesis induction). 4) Natural/modified MSC-EVs combined with materials science alleviate ONFH.
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Osteoarthritis
steoarthritis (OA) is a joint disease characterised by cartilage destruction and extracellular matrix loss.83 Persistent pain and dysfunction lead to decreased quality of life. The current treatment for OA is mainly the use of non-steroidal anti-inflammatory drugs to relieve symptoms, but the efficacy is limited, and there are many side effects (such as gastric mucosal damage).84
Studies have shown that BMSC-EVs block macrophage activation and reduce inflammation by inhibiting the inflammation-inducing molecule iNOS; moreover MSC-EVs also inhibit chondrocyte apoptosis, and promote proliferation and migration through multiple pathways.85 Infrapatellar fat pad MSC-derived exosomes with high expression of miR-100-5p ameliorate gait abnormalities by targeting mammalian target of rapamycin signalling.55
Wnt5a and Wnt5b carried by synovial MSC-derived EVs alleviate OA by promoting chondrocyte proliferation and migration via activation of the yes-associated protein (YAP) signalling pathway, but at the same time significantly reduce extracellular matrix. Targeted inhibition of the Ras-related protein RalA by high expression of miR-140-5p significantly attenuates this harmful effect, thereby promoting chondrocyte proliferation and migration without affecting extracellular matrix secretion.53 Adipose-derived MSC-derived EVs also exert chondroprotective effects and anti-OA protection through multiple mechanisms, such as reducing the production of inflammatory mediators (including tumour necrosis factor-α, interleukin-6, prostaglandin E2 and nitric oxide), inhibiting matrix metalloprotease activity, and enhancing production of the anti-inflammatory cytokine interleukin-10.54
The therapeutic effects of different stem cell-derived EVs on OA are diverse. Some studies have compared the treatment effect of EVs derived from synovial MSCs and EVs derived from induced pluripotent stem cells. The results showed that both types of EVs promoted chondrocyte proliferation and migration and relieved OA symptoms, but EVs derived from induced pluripotent stem cells had a better effect.86
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone Tumours
The bone microenvironment includes extracellular matrix and various types of cells. Bone microenvironment and cellular crosstalk play essential roles in bone physiology and disease (tumours). Tumour cells and MSCs have complex interactions through intercellular communication mediated by paracrine-generated EVs.87
At present, there are few studies on MSC-EVs in bone tumours. Osteosarcoma (OS) is the most common primary malignant bone tumour among teenagers.88 The high complexity and heterogeneity of OS render it challenging for scientists to find new therapeutic targets.89
Some recent studies highlight the role of MSC-EVs in OS. Adipose-derived MSC-EVs may promote OS cell invasion, migration, and proliferation by augmenting collagen beta (1-O) galactosyl transferase 2 expression, eventually promoting OS progression.56 EVs secreted by MSCs under stress (nutrient deprived) promote OS cell proliferation and metastasis and protect against apoptosis, which may be mediated by a change in the miRNA expression profile (downregulation of hsamiR-195 and hsa-miR-124 in parallel with upregulation of hsa-miR-148a).57
BMSC-EVs carrying metastasis-associated lung adenocarcinoma transcript 1 into OS cells, through a competing endogenous RNA mechanism, reduce the expression of miR-143, increase NRSN2 expression and activate the Wnt/β-catenin pathway, eventually contributing to the progression of OS.58 Another research group found that BMSC-EVs encapsulate long non-coding RNA plasmacytoma variant translocation 1 and transport it into OS cells, and that by sponging miR-183-5p and increasing E-26 transformation-specific (ETS)-related gene expression, BMSC-EVs promote OS growth and metastasis.90 In addition, BMSC-EVs accelerate the progression and metastasis of OS by delivering non-coding RNA activated by DNA damage to OS cells and regulating the miR-30c-5p/Kruppel-like factor 10 axis.91
Although most of the literature suggests that MSC-EVs promote OS, there are a few studies showing the opposite effect. For example, studies have found that MSC-EVs containing miR-206 inhibit OS progression by targeting transformer 2β.59 These contradictory findings may be dependent on the EVs’ cargo, and the culture/in vivo conditions. The existence of these contradictory phenomena is the focus of research.
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone Fracture
Fractures are one of the most prevalent musculoskeletal system injuries. However, roughly 5–10% of patients experience delayed or non-healing of bones as a result of poor fracture repair.92, 93 Differentiation of BMSCs into definite lineages is critical for tissue healing.94
Callus development requires a mild inflammatory response during the initial phases of fracture healing. However, persistent and hyperactive inflammation impedes fracture repair and results in severe tissue damage.95 Programmed death-1-rich EVs secreted by genetically-modified human umbilical vein endothelial cells induce osteogenic differentiation and promote fracture healing by suppressing immunity and reducing inflammation.96
Hypoxic preconditioning of MSCs enhances their paracrine effects. MSCs upregulate miR-126 through high expression of hypoxia-inducible factor 1-α under hypoxic conditions, and miR-126 promotes proliferation, angiogenesis and migration in human umbilical vein endothelial cells via the sprout-related EVH1 domain containing 1/Ras-extracellular signal-regulated kinase pathway and finally promotes bone fracture healing.63
It is widely established that MSCs’ capacity for repair declines with age. Research has shown that miR-128-3p expression is significantly increased in aged-MSC-EVs, through direct targeting of SMAD family member 5 (Smad5; a gene related to endogenous bone morphogenetic protein activity) inhibition of MSC osteogenic differentiation and fracture healing.61, 62 Another study reported that BMSC-EVs promote bone fracture recovery and osteoblast differentiation by releasing miR-335, which targets vesicle-associated membrane protein (VAMP)-associated protein B and activates the Wnt/β-catenin pathway.60 This reminds us that the cargo and functions carried by MSC-EVs in different states are diverse, and we need to choose according to specific needs.
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone Regenerative Medicine
Scientists have long struggled with the research and application of regenerative medicine in bone diseases. A systematic review of 23 studies which evaluated the existing preclinical animal studies97 showed that MSC-EVs improve bone morphology, biomechanics and histological results. With positive effects on cell survival, proliferation and migration, osteogenesis and angiogenesis, MSC-EVs were demonstrated to have a positive impact on bone regeneration.98
The affable modification of MSC-EVs according to different purposes, or combination with material science further increase their application value in bone regeneration. For example, three-dimensional printed titanium alloy scaffolds have the advantages of uniform structure, high strength, low stiffness, high porosity, corrosion resistance and high friction coefficient. Studies have found that EVs secreted by MSCs after osteogenic induction are rich in a series of miRNAs with pro-osteogenic functions, such as hsa-miR-146a-5p, hsamiR-503-5p, hsa-miR-129-5p, hsa-miR-32-5p, through the activation of phosphoinositide 3-kinase/Akt and mitogen-activated protein kinase signalling pathways relies on titanium alloy scaffolds to achieve cell-free bone regeneration, which is expected to be applied in the field of bone repair in the future.99 Zhou et al.100 recently obtained tumour-derived EV membranes by removing the contents of EVs derived from hepatocellular carcinoma cells. A novel lipid nanovesicle was prepared by hybridising tumour-derived EV membrane with phospholipid, which was able to achieve precise delivery to tumour sites and efficient siRNA transfection.
Maintaining the stability and function of EVs in vivo are significant challenges. The cell-free approach that combines exosomes with different types of tissue-engineered scaffolds has significantly circumvented this restriction and has promising application potential.101 EV scaffolds currently used in orthopaedics include titanium scaffolds, hydrogels, nanoparticles, etc.99, 102-108 (Table 4).
Table 4. Application of mesenchymal stem cell-derived extracellular vesicle-integrated biomaterial scaffolds in orthopaedic diseases.
| Source of extracellular vesicles | Extracellular vesicle modification | Scaffolds | Main function | Reference |
|---|---|---|---|---|
| hMSCs | Osteoinduction | 3D-printed titanium alloy scaffolds | Induce osteogenic differentiation of hMSCs | 99 |
| BMSCs | Osteoinduction | Functionalised decalcified bone matrix scaffolds | Pro-angiogenic and pro-osteogenic regeneration | 102 |
| hASCs | Osteoinduction | Polydopamine-coating PLGA scaffolds | Enhance the migration, proliferation and osteogenic differentiation of hBMSCs | 103 |
| hiPS-MSCs | – | Exosome/β-TCP combination scaffold | Activating the PI3K/Akt signalling pathway and promoting osteogenic differentiation | 104 |
| hiPS-MSCs | – | β-TCP scaffolds | Pro-angiogenic and pro-osteogenic differentiation | 105 |
| hucMSCs | – | CHA/SF/GCS/DFPEG hydrogel | Promote osteogenic differentiation | 106 |
| hGMSCs | – | 3D-PLA scaffolds | Promote osteogenic differentiation | 107 |
| hucMSCs | – | HA-ALG hydrogel scaffolds | Promote the proliferation, migration, and osteogenic differentiation | 108 |
Note: 3D: three-dimensional; Akt: protein kinase B; BMSCs: bone marrow mesenchymal stem cells; CHA: coralline hydroxyapatite; DFPEG: difunctionalised polyethylene glycol; GCS: glycol chitosan; HA-ALG: hydroxyapatite-embedded in situ cross-linked hyaluronic acid-alginate; hASCs: human adipose-derived stem cells; hBMSCs: human bone marrow mesenchymal stem cells; hGMSCs: human gingival mesenchymal stem cells; hiPS-MSCs: human-induced pluripotent stem cell-derived mesenchymal stem cells; hMSCs: human mesenchymal stem cell; hucMSCs: human umbilical cord mesenchymal stem cells; PI3K: phosphoinositide 3-kinase; PLA: polylactic acid; PLGA: poly(lactic-co-glycolic acid); SF: silk fibroin; β-TCP: β-tricalcium phosphate.
Conclusion and Perspective
Accumulating evidence suggests that the therapeutic role of MSCs in bone diseases is closely related to the paracrine-generated EVs. MSC-EVs have anti-inflammatory, immune regulation, and angiogenic effects, induce osteogenic differentiation of MSCs, and inhibit adipogenic differentiation. Based on natural MSC-EVs, favourable genetic or chemical modification can enhance their tissue targeting, reduce adverse effects, and further enhance their bone protection. In addition, as an alternative to cell-free therapy, EVs avoid the problems of immune rejection, stemness maintenance and cell senescence in stem cell transplantation therapy, making them promising for the treatment of bone diseases.
While preclinical studies of MSC-EVs have shown that they can achieve multiple bone-protective effects through diverse pathways,97 clinical trials are still in their infancy (Table 5). To date, there are still no MSC-EVs officially approved for the treatment of bone diseases. This is because there remain some critical challenges. For example: 1) The choice of the source of EVs for the treatment of bone diseases; 2) the choice of autologous stem cell or allogeneic stem cell-derived EVs; 3) questions over how to obtain higher purity and concentration of EVs and how to maintain their storage conditions and biological activities; 4) safety of exosomes for the treatment of bone diseases (MSC-derived exosomes are complex in composition and may contain potentially pathogenic components such as toxic proteins and harmful miRNAs, etc.); 5) how to achieve targeted enrichment of EVs in vivo; and 6) lack of large-sample, multi-centre clinical trials confirming essential points such as the effectiveness and safety of EVs.
Table 5. Summary of the clinical trials involving mesenchymal stem cell-derived extracellular vesicles in orthopaedic diseases.
| NCT No. | Title | Status | Phase | Disease | Aim | Intervention/treatment | Source of extracellular vesicles | Sponsor/collaborator |
|---|---|---|---|---|---|---|---|---|
| NCT04223622 | Effects of ASC secretome on human osteochondral explants | Recruiting | Phase I | Osteoarthritis | Development of treatment strategies | Not clear | Adipose-derived stromal cells | Istituto Ortopedico Galeazzi, Italy |
| NCT05060107 | Intra-articular injection of MSC-derived exosomes in knee osteoarthritis | Not yet recruiting | Phase I | Osteoarthritis | Development of treatment strategies | Intra-articular knee injection | Allogeneic mesenchymal stromal cells | Francisco Espinoza, Universidad de los Andes, Chile |
| NCT05101655 | Construction of microfluidic exosome chip for diagnosis of lung metastasis of osteosarcoma | Enrolling by invitation | – | Osteosarcoma | Development of diagnostic markers | – | Plasma | Ruijin Hospital, China |
| NCT03108677 | Circulating exosome RNA in lung metastases of primary high-grade osteosarcoma | Recruiting | – | Osteosarcoma | Development of diagnostic markers | – | Plasma | Ruijin Hospital, China |
Note: ASC: adipose-derived stem cell; MSC: mesenchymal stem cell.
Scientists have extensively explored these issues, such as increasing the targeting of EVs by linking EVs with short peptides or aptamers with target organ affinity. Storage conditions have been continuously optimised and standardised to increase the stability and experimental reproducibility of EVs (Table 5). MSC-EVs’ efficacy, safety, and cost-effectiveness in treating bone diseases will continue to improve.
Footnotes
Author contributions: Literature search and analysis, manuscript draft and figure preparation: ZL; manuscript revision: XH. Both authors read and approved the final manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China (Nos. 81871822, 82072504), and the Postgraduate Scientific Research Innovation Project of Hunan Province (No. CX20210917).
Acknowledgement: The figure in the Graphical Abstract was created with BioRender.com.
Conflicts of interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Han Y., You X., Xing W., Zhang Z., Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasza K., Gurnani P., Hardie K. R., Cámara M., Alexander C. Challenges and solutions in polymer drug delivery for bacterial biofilm treatment: a tissue-by-tissue account. Adv Drug Deliv Rev. 2021;178:113973. doi: 10.1016/j.addr.2021.113973. [DOI] [PubMed] [Google Scholar]
- 3.Yao D., Huang L., Ke J., Zhang M., Xiao Q., Zhu X. Bone metabolism regulation: implications for the treatment of bone diseases. Biomed Pharmacother. 2020;129:110494. doi: 10.1016/j.biopha.2020.110494. [DOI] [PubMed] [Google Scholar]
- 4.Rachner T. D., Khosla S., Hofbauer L. C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W., Shi Y., Wang H., Yu C., Zhu H., Wu A. Sinensetin reduces osteoarthritis pathology in the tert-butyl hydroperoxide-treated chondrocytes and the destabilization of the medial meniscus model mice via the AMPK/mTOR signaling pathway. Front Pharmacol. 2021;12:713491. doi: 10.3389/fphar.2021.713491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullah I., Subbarao R. B., Rho G. J. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy M. B., Moncivais K., Caplan A. I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankrum J. A., Ong J. F., Karp J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triffitt J. T., Wang Q. Stem cell fate and microenvironment. Biomater Transl. 2022;3:1–2. doi: 10.12336/biomatertransl.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Z. L., Yuan Q., Zu X., Liu J. Insights into the role of mitochondria in vascular calcification. Front Cardiovasc Med. 2022;9:879752. doi: 10.3389/fcvm.2022.879752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yáñez-Mó M., Siljander P. R., Andreu Z., Zavec A. B., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J. M., Ghobrial I. M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N. H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., KraljIglic V., Krämer-Albers E. M., Laitinen S., Lässer C., Lener T., Ligeti E., Linē A., Lipps G., Llorente A., Lötvall J., Manček-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-’t Hoen E. N., Nyman T. A., O’Driscoll L., Olivan M., Oliveira C., Pállinger É., Del Portillo H. A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez-Madrid F., Santarém N., Schallmoser K., Ostenfeld M. S., Stoorvogel W., Stukelj R., Van der Grein S. G., Vasconcelos M. H., Wauben M. H., De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S., Xu X., Liang S., Chen Z., Zhang Y., Qian A., Hu L. The application of MSCs-derived extracellular vesicles in bone disorders: novel cell-free therapeutic strategy. Front Cell Dev Biol. 2020;8:619. doi: 10.3389/fcell.2020.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mousaei Ghasroldasht M., Seok J., Park H. S., Liakath Ali F. B., AlHendy A. Stem cell therapy: from idea to clinical practice. Int J Mol Sci. 2022;23:2850. doi: 10.3390/ijms23052850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazari H., Zhang L., Zhu D., Chader G. J., Falabella P., Stefanini F., Rowland T., Clegg D. O., Kashani A. H., Hinton D. R., Humayun M. S. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 2015;48:1–39. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosi T. H., Longaker M. T., Chan C. K. F. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019;7:189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z. L., Lin X. L., Tan L. L., Liu Y. M., Qu K., Wang Z. MicroRNAs: important regulators of induced pluripotent stem cell generation and differentiation. Stem Cell Rev Rep. 2018;14:71–81. doi: 10.1007/s12015-017-9785-6. [DOI] [PubMed] [Google Scholar]
- 19.Jin J. Stem cell treatments. JAMA. 2017;317:330. doi: 10.1001/jama.2016.17822. [DOI] [PubMed] [Google Scholar]
- 20.Friedenstein A. J., Piatetzky S., II, Petrakova K. V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 21.Caplan A. I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 22.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzynska S., Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y., Yang L. Mesenchymal stem cells and extracellular vesicles in therapy against kidney diseases. Stem Cell Res Ther. 2021;12:219. doi: 10.1186/s13287-021-02289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulanger C. M., Loyer X., Rautou P. E., Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 26.Harding C. V., Heuser J. E., Stahl P. D. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 28.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 29.Quiñones-Vico M. I., Sanabria-de la Torre R., Sánchez-Díaz M., Sierra-Sánchez Á., Montero-Vílchez T., Fernández-González A., Arias-Santiago S. The role of exosomes derived from mesenchymal stromal cells in dermatology. Front Cell Dev Biol. 2021;9:647012. doi: 10.3389/fcell.2021.647012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan Z., Pathak D., Venkatesan Kalavai S., Yoshii-Kitahara A., Muraoka S., Bhatt N., Takamatsu-Yukawa K., Hu J., Wang Y., Hersh S., Ericsson M., Gorantla S., Gendelman H. E., Kayed R., Ikezu S., Luebke J. I., Ikezu T. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. 2021;144:288–309. doi: 10.1093/brain/awaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas S. L. N., Breakefield X. O., Weaver A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akers J. C., Gonda D., Kim R., Carter B. S., Chen C. C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chotiyarnwong P., McCloskey E. V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16:437–447. doi: 10.1038/s41574-020-0341-0. [DOI] [PubMed] [Google Scholar]
- 34.Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 36.Feng X., Teitelbaum S. L. Osteoclasts: new insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Bari A. A., Al Mamun A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020;2:668–679. doi: 10.1096/fba.2020-00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J., Zhang W., Ran Q., Xiang Y., Zhong J. F., Li S. C., Li Z. The differentiation balance of bone marrow mesenchymal stem cells is crucial to hematopoiesis. Stem Cells Int. 2018;2018:1540148. doi: 10.1155/2018/1540148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G., Zhuo Y., Tao B., Liu Q., Shang W., Li Y., Wang Y., Li Y., Zhang L., Fang Y., Zhang X., Fang Z., Yu Y. Moderate SMFs attenuate bone loss in mice by promoting directional osteogenic differentiation of BMSCs. Stem Cell Res Ther. 2020;11:487. doi: 10.1186/s13287-020-02004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du G., Cheng X., Zhang Z., Han L., Wu K., Li Y., Lin X. TGF-beta induced key genes of osteogenic and adipogenic differentiation in human mesenchymal stem cells and miRNA-mRNA regulatory networks. Front Genet. 2021;12:759596. doi: 10.3389/fgene.2021.759596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer M. B., Benkusky N. A., Sen B., Rubin J., Pike J. W. Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J Biol Chem. 2016;291:17829–17847. doi: 10.1074/jbc.M116.736538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robert A. W., Marcon B. H., Dallagiovanna B., Shigunov P. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: a comparative transcriptome approach. Front Cell Dev Biol. 2020;8:561. doi: 10.3389/fcell.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolf C. M., Cho E., Tuan R. S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X., Yang J., Lei P., Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany N Y) 2019;11:8777–8791. doi: 10.18632/aging.102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu M., Zhai S., Fu Q., Liu D. Bone marrow mesenchymal stem cells-derived exosomal microRNA-150-3p promotes osteoblast proliferation and differentiation in osteoporosis. Hum Gene Ther. 2021;32:717–729. doi: 10.1089/hum.2020.005. [DOI] [PubMed] [Google Scholar]
- 46.Huang B., Su Y., Shen E., Song M., Liu D., Qi H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. 2021;272:119208. doi: 10.1016/j.lfs.2021.119208. [DOI] [PubMed] [Google Scholar]
- 47.Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Li X., Li J., Zhai L., Liu D., Abdurahman A., Zhang Y., Yokota H., Zhang P. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J. 2021;35:e21150. doi: 10.1096/fj.202001080RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bond J., Adams S., Richards S., Vora A., Mitchell C., Goulden N. Polymorphism in the PAI-1 (SERPINE1) gene and the risk of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;118:2632–2633. doi: 10.1182/blood-2011-05-355206. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Wang Y., Yu X., Bao Y., An L., Wei X., Yu W., Liu B., Li J., Yang J., Xia Y., Liu G., Cao F., Zhang X., Zhao D. Bone marrow mesenchymal stem cell-derived exosomes promote plasminogen activator inhibitor 1 expression in vascular cells in the local microenvironment during rabbit osteonecrosis of the femoral head. Stem Cell Res Ther. 2020;11:480. doi: 10.1186/s13287-020-01991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao W., Ning Y., Xu H. J., Zou W. Z., Hu J., Liu X. Z., Yang Y., Li Z. H. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond) 2019;133:1955–1975. doi: 10.1042/CS20181064. [DOI] [PubMed] [Google Scholar]
- 52.Kuang M. J., Huang Y., Zhao X. G., Zhang R., Ma J. X., Wang D. C., Ma X. L. Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int J Biol Sci. 2019;15:1861–1871. doi: 10.7150/ijbs.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao S. C., Yuan T., Zhang Y. L., Yin W. J., Guo S. C., Zhang C. Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tofiño-Vian M., Guillén M. I., Pérez Del Caz M. D., Silvestre A., Alcaraz M. J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47:11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- 55.Wu J., Kuang L., Chen C., Yang J., Zeng W. N., Li T., Chen H., Huang S., Fu Z., Li J., Liu R., Ni Z., Chen L., Yang L. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Chu Y., Li K., Zhang G., Guo Z., Wu X., Qiu C., Li Y., Wan X., Sui J., Zhang D., Xiang H., Chen B. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353. doi: 10.3389/fcell.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallabhaneni K. C., Hassler M. Y., Abraham A., Whitt J., Mo Y. Y., Atfi A., Pochampally R. Mesenchymal stem/stromal cells under stress increase osteosarcoma migration and apoptosis resistance via extracellular vesicle mediated communication. PLoS One. 2016;11:e0166027. doi: 10.1371/journal.pone.0166027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li F., Chen X., Shang C., Ying Q., Zhou X., Zhu R., Lu H., Hao X., Dong Q., Jiang Z. Bone marrow mesenchymal stem cells-derived extracellular vesicles promote proliferation, invasion and migration of osteosarcoma cells via the lncRNA MALAT1/miR-143/NRSN2/ Wnt/β-catenin axis. Onco Targets Ther. 2021;14:737–749. doi: 10.2147/OTT.S283459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H., Wang J., Ren T., Huang Y., Liang X., Yu Y., Wang W., Niu J., Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi: 10.1016/j.canlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Hu H., Wang D., Li L., Yin H., He G., Zhang Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell Death Dis. 2021;12:156. doi: 10.1038/s41419-021-03430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katakawa Y., Funaba M., Murakami M. Smad8/9 is regulated through the BMP pathway. J Cell Biochem. 2016;117:1788–1796. doi: 10.1002/jcb.25478. [DOI] [PubMed] [Google Scholar]
- 62.Xu T., Luo Y., Wang J., Zhang N., Gu C., Li L., Qian D., Cai W., Fan J., Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnology. 2020;18:47. doi: 10.1186/s12951-020-00601-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W., Li L., Rong Y., Qian D., Chen J., Zhou Z., Luo Y., Jiang D., Cheng L., Zhao S., Kong F., Wang J., Zhou Z., Xu T., Gong F., Huang Y., Gu C., Zhao X., Bai J., Wang F., Zhao W., Zhang L., Li X., Yin G., Fan J., Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Anam A. K., Insogna K. Update on osteoporosis screening and management. Med Clin North Am. 2021;105:1117–1134. doi: 10.1016/j.mcna.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 65.He X. Y., Yu H. M., Lin S., Li Y. Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell Mol Biol Lett. 2021;26:47. doi: 10.1186/s11658-021-00291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu P., Wang W., Li Z., Li Y., Yu X., Tu J., Zhang Z. Ferroptosis: a new regulatory mechanism in osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431. doi: 10.1155/2022/2634431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y., Jin D., Xie W., Wen L., Chen W., Xu J., Ding J., Ren D., Xiao Z. Mesenchymal stem cells-derived exosomes: a possible therapeutic strategy for osteoporosis. Curr Stem Cell Res Ther. 2018;13:362–368. doi: 10.2174/1574888X13666180403163456. [DOI] [PubMed] [Google Scholar]
- 68.An Q., Wu D., Ma Y., Zhou B., Liu Q. Suppression of Evi1 promotes the osteogenic differentiation and inhibits the adipogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. Int J Mol Med. 2015;36:1615–1622. doi: 10.3892/ijmm.2015.2385. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z. X., Luo Z. W., Li F. X., Cao J., Rao S. S., Liu Y. W., Wang Y. Y., Zhu G. Q., Gong J. S., Zou J. T., Wang Q., Tan Y. J., Zhang Y., Hu Y., Li Y. Y., Yin H., Wang X. K., He Z. H., Ren L., Liu Z. Z., Hu X. K., Yuan L. Q., Xu R., Chen C. Y., Xie H. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022;13:1453. doi: 10.1038/s41467-022-29191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murali V. P., Holmes C. A. Mesenchymal stromal cell-derived extracellular vesicles for bone regeneration therapy. Bone reports. 2021;14:101093. doi: 10.1016/j.bonr.2021.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng F., Xue X., Yin Z., Gao F., Wang X., Geng Z. Research progress of exosomes in bone diseases: mechanism, diagnosis and therapy. Front Bioeng Biotechnol. 2022;10:866627. doi: 10.3389/fbioe.2022.866627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng Y., Wu S., Li Y., Crane J. L. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., Li C., Xie L., Crane J., Wan M., Zhen G., Bian Q., Yu B., Chang W., Qiu T., Pickarski M., Duong L. T., Windle J. J., Luo X., Liao E., Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y., Cao X., Li P., Fan Y., Zhang L., Ma X., Sun R., Liu Y., Li W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021;272:119204. doi: 10.1016/j.lfs.2021.119204. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z., Liu X., Zhao F., Yao M., Lin Z., Yang Z., Liu C., Liu Y., Chen X., Du C. Bioactive glass nanoparticles inhibit osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression in the extracellular vesicles derived from bone marrow mesenchymal stem cells. Biomaterials. 2022;283:121438. doi: 10.1016/j.biomaterials.2022.121438. [DOI] [PubMed] [Google Scholar]
- 76.Lai C. P., Mardini O., Ericsson M., Prabhakar S., Maguire C., Chen J. W., Tannous B. A., Breakefield X. O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsharkasy O. M., Nordin J. Z., Hagey D. W., de Jong O. G., Schiffelers R. M., Andaloussi S. E., Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Luo Z. W., Li F. X., Liu Y. W., Rao S. S., Yin H., Huang J., Chen C. Y., Hu Y., Zhang Y., Tan Y. J., Yuan L. Q., Chen T. H., Liu H. M., Cao J., Liu Z. Z., Wang Z. X., Xie H. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884–20892. doi: 10.1039/c9nr02791b. [DOI] [PubMed] [Google Scholar]
- 79.Cui Y., Guo Y., Kong L., Shi J., Liu P., Li R., Geng Y., Gao W., Zhang Z., Fu D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022;10:207–221. doi: 10.1016/j.bioactmat.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petek D., Hannouche D., Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 2019;4:85–97. doi: 10.1302/2058-5241.4.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baig S. A., Baig M. N. Osteonecrosis of the femoral head: etiology, investigations, and management. Cureus. 2018;10:e3171. doi: 10.7759/cureus.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C., Su Y., Ding H., Yin J., Zhu Z., Song W. Mesenchymal stem cells-derived and siRNAs-encapsulated exosomes inhibit osteonecrosis of the femoral head. J Cell Mol Med. 2020;24:9605–9612. doi: 10.1111/jcmm.15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guilak F., Nims R. J., Dicks A., Wu C. L., Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40–50. doi: 10.1016/j.matbio.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roos E. M., Arden N. K. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12:92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 85.Mianehsaz E., Mirzaei H. R., Mahjoubin-Tehran M., Rezaee A., Sahebnasagh R., Pourhanifeh M. H., Mirzaei H., Hamblin M. R. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340. doi: 10.1186/s13287-019-1445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarhadi V. K., Daddali R., Seppänen-Kaijansinkko R. Mesenchymal stem cells and extracellular vesicles in osteosarcoma pathogenesis and therapy. Int J Mol Sci. 2021;22:11035. doi: 10.3390/ijms222011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu X., Qiu B., Yi P., Li H. Overexpression of miR-206 in osteosarcoma and its associated molecular mechanisms as assessed through TCGA and GEO databases. Oncol Lett. 2020;19:1751–1758. doi: 10.3892/ol.2020.11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kempf-Bielack B., Bielack S. S., Jürgens H., Branscheid D., Berdel W. E., Exner G. U., Göbel U., Helmke K., Jundt G., Kabisch H., Kevric M., Klingebiel T., Kotz R., Maas R., Schwarz R., Semik M., Treuner J., Zoubek A., Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 90.Zhao W., Qin P., Zhang D., Cui X., Gao J., Yu Z., Chai Y., Wang J., Li J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany N Y) 2019;11:9581–9596. doi: 10.18632/aging.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He H., Ding M., Li T., Zhao W., Zhang L., Yin P., Zhang W. Bone mesenchymal stem cell-derived extracellular vesicles containing NORAD promote osteosarcoma by miR-30c-5p. Lab Invest. 2022 doi: 10.1038/s41374-021-00691-6. [DOI] [PubMed] [Google Scholar]
- 92.Einhorn T. A., Gerstenfeld L. C. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komatsu D. E., Warden S. J. The control of fracture healing and its therapeutic targeting: improving upon nature. J Cell Biochem. 2010;109:302–311. doi: 10.1002/jcb.22418. [DOI] [PubMed] [Google Scholar]
- 94.Buettmann E. G., McKenzie J. A., Migotsky N., Sykes D. A., Hu P., Yoneda S., Silva M. J. VEGFA from early osteoblast lineage cells (Osterix+) is required in mice for fracture healing. J Bone Miner Res. 2019;34:1690–1706. doi: 10.1002/jbmr.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y., Xiong Y., Yan C., Chen L., Chen D., Mi B., Liu G. Downregulation of microRNA-16-5p accelerates fracture healing by promoting proliferation and inhibiting apoptosis of osteoblasts in patients with traumatic brain injury. Am J Transl Res. 2019;11:4746–4760. [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Z., Xiong Y., Meng W., Hu Y., Chen L., Chen L., Xue H., Panayi A. C., Zhou W., Sun Y., Cao F., Liu G., Hu L., Yan C., Xie X., Lin C., Cai K., Feng Q., Mi B., Liu G. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact Mater. 2022;13:300–311. doi: 10.1016/j.bioactmat.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan S. H. S., Wong J. R. Y., Sim S. J. Y., Tjio C. K. E., Wong K. L., Chew J. R. J., Hui J. H. P., Toh W. S. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 2020;7:100067. doi: 10.1016/j.mtbio.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X., Thomsen P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin Pharmacol Toxicol. 2021;128:18–36. doi: 10.1111/bcpt.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhai M., Zhu Y., Yang M., Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv Sci (Weinh) 2020;7:2001334. doi: 10.1002/advs.202001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou X., Miao Y., Wang Y., He S., Guo L., Mao J., Chen M., Yang Y., Zhang X., Gan Y. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J Extracell Vesicles. 2022;11:e12198. doi: 10.1002/jev2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alqurashi H., Ortega Asencio I., Lambert D. W. The emerging potential of extracellular vesicles in cell-free tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2021;27:530–538. doi: 10.1089/ten.TEB.2020.0222. [DOI] [PubMed] [Google Scholar]
- 102.Xie H., Wang Z., Zhang L., Lei Q., Zhao A., Wang H., Li Q., Cao Y., Jie Zhang W., Chen Z. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep. 2017;7:45622. doi: 10.1038/srep45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li W., Liu Y., Zhang P., Tang Y., Zhou M., Jiang W., Zhang X., Wu G., Zhou Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10:5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7:136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., Hu B., Wang Y., Li X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836–849. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L., Wang J., Zhou X., Sun J., Zhu B., Duan C., Chen P., Guo X., Zhang T., Guo H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 2020;8:564731. doi: 10.3389/fbioe.2020.564731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diomede F., Gugliandolo A., Cardelli P., Merciaro I., Ettorre V., Traini T., Bedini R., Scionti D., Bramanti A., Nanci A., Caputi S., Fontana A., Mazzon E., Trubiani O. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 2018;9:104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang S., Zhu B., Yin P., Zhao L., Wang Y., Fu Z., Dang R., Xu J., Zhang J., Wen N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6:1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]