Abstract
Purpose:
Physical inactivity and obesity increase risk for breast cancer recurrence and cardiovascular death; inflammation is hypothesized to mediate these associations.
Methods:
In a 4-arm randomized controlled trial, 318 breast cancer survivors with overweight or obesity were randomized to Exercise Alone, Weight Loss Alone, Exercise plus Weight Loss, or Control for 12-months. Inflammation outcomes included C-reactive protein (CRP), serum amyloid A (SAA), intracellular adhesion molecule-1 (ICAM-1), and vascular adhesion molecule-1 (VCAM-1).
Results:
Compared with Control, Exercise Alone increased ICAM-1 [9.3%; 95% CI: 1.6, 16.9] and VCAM-1 [8.6%; 95% CI: 2.6, 14.5], but did not change CRP or SAA. Compared with Control, Weight Loss Alone reduced CRP [−35.2%; 95% CI: −49.9, −20.7], and SAA [−25.6%; 95% CI: −39.8, −11.9], but did not change ICAM-1 or VCAM-1. Compared with Control, Exercise plus Weight Loss reduced CRP [−44.1%; 95% CI: −57.1, −31.1] and SAA [−26.6%; 95% CI: −40.5, −12.6], but did not change ICAM-1 or VCAM-1. Among 194 participants with elevated CRP at baseline (e.g., >3 mg/L), compared to Control, Weight Loss Alone [0.17; 95% CI: 0.04, 0.30] and Exercise plus Weight Loss [0.31; 95% CI: 0.16, 0.46] increased the probability of achieving normal CRP at month 12. In analyses that consolidated randomized groups, body weight and adiposity reductions, but not change in fitness level, correlated with decreased CRP, SAA, and ICAM-1 levels.
Conclusions:
In breast cancer survivors with overweight or obesity, weight loss or exercise plus weight loss reduced measures of inflammation that are associated with breast cancer recurrence and cardiovascular death.
Keywords: BREAST NEOPLASMS, NEOPLASM RECURRENCE, OBESITY, ADIPOSITY, INFLAMMATION, BIOMARKERS
INTRODUCTION
Breast cancer survivors comprise more than 50% of the 8.8 million female cancer survivors in the United States (1). As this population grows, there will be a significant number of women that have a breast cancer recurrence, with the risk of recurrence at 10–52% depending on tumor subtype and cancer stage (2). Additionally, breast cancer patients have an elevated risk of mortality from cardiovascular diseases (CVDs) compared to the general population; the most common non-cancer cause of death after a breast cancer diagnosis is heart disease (3, 4).
Obesity and physical inactivity are risk factors for breast cancer recurrence and CVD (5, 6). One common pathologic feature of obesity and physical inactivity is inflammation (7, 8). Systemic inflammation is also associated with cancer recurrence and CVD (8, 9). Given that breast cancer survivors with excess body weight are at elevated risk of breast cancer recurrence and CVD, we investigated the role of exercise training, weight loss, and a combination of the two energetic interventions on biomarkers of inflammation in a secondary analysis of the one-year long Women in Steady Exercise Research (WISER) Survivor trial (10–12).
Elevated levels of C-reactive protein (CRP) (7, 13, 14), serum amyloid A (SAA) (15–17), intracellular adhesion molecular 1 (ICAM1) (18–20), and vascular adhesion molecular 1 (VCAM1) (21–23) have been associated with obesity, risk of breast cancer, and CVD. Therefore, we tested the effects of exercise training, weight loss, and a combination of the two energetic interventions on CRP, SAA, ICAM-1, and VCAM-1 in sedentary breast cancer survivors with overweight or obesity diagnosed with lymphedema. We hypothesized that exercise and weight loss would improve biomarkers of inflammation, and that the combined effect of exercise plus weight loss would be larger than exercise alone or weight loss alone.
METHODS
Design
WISER Survivor was a 4-arm randomized controlled trial completed in May 2016 (Groups: Exercise Alone, Weight Loss Alone, Exercise plus Weight Loss, and Control). The study is registered at ClinicalTrials.gov as NCT01515124. The study compared the individual and combined effects of exercise and weight loss in breast cancer survivors with excess body weight and lymphedema. Study design, recruitment, and main results have been described in extensive detail elsewhere (10–12, 24). All measurements were acquired at baseline and 12 month follow up. The protocol was approved by the University of Pennsylvania Institutional Review Board, all participants provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Patients
Eligible participants were breast cancer survivors with a BMI 25–50 kg/m2, age 18–80 years old, cancer free, and having completed curative treatment (surgery, chemotherapy, radiation therapy) more than 6 months before randomization. They also had breast cancer-related lymphedema. Exclusions included use of weight loss medication at the time of enrollment, weight loss ≥ 4.5 kg in the previous 12 weeks, engaging in any resistance exercise or ≥3 bouts of aerobic exercise weekly over the prior 52 weeks, and history of bariatric surgery. The primary aim of the study was to evaluate the effects of the interventions on lymphedema interlimb volume difference (12). This is a report of a pre-planned secondary analysis.
Exercise Intervention
Participants were asked to engage in two weight training sessions and 180 minutes of aerobic exercise per week. Adjustable dumbbells (Powerblock, Inc) were provided for the weight training component of the exercise intervention. In weeks 1–6, supervised weekly instruction from certified fitness professionals focused on safety and gradual increases of the prescribed resistance exercises, and how to safely increase aerobic exercise to 180 min/wk. From weeks 7–52, participants received monthly supervised sessions, in addition to performing two weight training sessions per week at home. Women performed warm-ups, stretching, and 10 repetitions each of 9 resistance exercises. Detailed exercises, sets, repetitions, and progression protocols are available (10). The aerobic exercise prescription remained constant with an exercise prescription of 180 min/wk of unsupervised walking at moderate intensity. All participants were asked to keep a log of their exercises performed. Fitness professionals called participants weekly to provide behavioral counseling and check on adherence.
Weight Loss Intervention
During Weeks 1–24, participants consumed foods from NutriSystem® to promote adherence to a reduced calorie diet of 1200–1500 kcal/d. Participants also attended weekly group meetings where they received behavioral modification instruction from a registered dietitian (25). The weight loss goal was 10% of baseline body weight. During Weeks 20–24 participants transitioned back to consuming regular foods but maintaining the same daily caloric intake target. During Weeks 25–52, participants increased their caloric intake to 1500–2000 kcal/d with the goal of weight loss maintenance. No instructions to maintain a specific percentage of calories from carbohydrates, fats, or protein were given. During this period, group meetings were held once per month, with additional weekly individual telephone calls with the registered dietitian. Adherence to the dietary intervention was recorded by attendance at the in-person sessions. Process measures such as paper or electronic food diaries to self-monitor dietary intake were reviewed by dietitians either in-person or over the phone (10).
Exercise plus Weight Loss Intervention
Participants in this group started with six weeks of exercise instruction, then started the weight loss intervention, both as described above.
Control Group
Participants were asked not to start an exercise or nutrition program while enrolled in the study. If questions arose regarding lifestyle modifications, the participant was directed to the American Cancer Society website or instructed to speak with their physician.
Biomarker Outcome Variables
Biomarker measures for this study were conducted using EDTA plasma samples prepared from fasting blood collections. Plasma was immediately isolated by centrifugation, aliquoted and stored at −80°C. Assay technicians were blinded to participants’ study groups. Fasting plasma CRP, SAA, ICAM-1, and VCAM-1 levels were determined using a multiplex high-sensitivity immunoassay (Meso Scale Discovery, catalog #K15198G). Paired participant samples were run in adjacent wells of the same plate and loaded onto a total of 19 assay plates in numerical order of ID, resulting in each plate containing a randomized number of participant samples by group. Intra-plate coefficients of variance (CV) were: CRP (7.6%), SAA (9.6%), ICAM-1 (7.2%), and VCAM-1 (7.4%), respectively.
Measurements
Body weight and height were assessed on a calibrated scale and stadiometer, respectively. Body composition was measured with total body dual-energy x-ray absorptiometry scans (DXA; Hologic Inc) (24). A modified Bruce protocol with 10 lead EKG was conducted (26). VO2max (ml/kg/min) was estimated based on the last stage of the modified Bruce protocol. Age (years), final stage speed (mph), and maximum recorded heart rate (bpm) during the test were utilized in a predictive algorithm: 15.1 + 21.8 (mph) − 0.327 (bpm) − 0.263 (mph × years) + 0.00504 (bpm × years) (27). Upper body and lower body strength were assessed through 1 RM free weight bench press (with bar) and machine leg press testing, respectively (10). Demographics and clinical characteristics were documented by a combination of self-report, pathology reports, and state cancer registry.
Statistical Analysis
Post hoc power estimates based on the LOOK AHEAD study and HOPE study indicated we would have over 90% power to detect a 1.0 ± 2.0 mg/L difference from the control group in CRP at an alpha level of 0.05 (28, 29). Of the 351 women randomized to participate, 318 had baseline inflammation measures and were included in the analysis (see Supplemental Figure 1, SDC 1). Descriptive statistics are presented as mean ± standard deviation or median [interquartile range]. We assessed the main effect of the intervention on inflammation markers using a repeated measures analysis of covariance model. Models are adjusted for the baseline value of the dependent variable, and randomization stratification factors including age, receipt of radiotherapy, number of lymph nodes resected, lymphedema severity, and body mass index. Unobserved data were multiply imputed using predictive mean matching and analyzed using a repeated measures analysis of covariance model (30). A mixed model that assumes data are missing at random did not reach any different conclusions (results not shown). P values were not adjusted for multiplicity. In a subgroup of participants with elevated CRP level at baseline (> 3mg/L), we quantified the proportion who achieved a CRP level ≤3 mg/L at month 12.(31, 32) Unobserved data at month 12 were multiply imputed using a parametric logistic regression that was adjusted for randomization stratification factors. The relationship between change in hypothesized intervention effect mediators and change in log-transformed biomarker levels was assessed with Pearson correlation.
RESULTS
Participant characteristics are summarized in Table 1. The study population was 36% Black or other minority. There were no significant differences between groups at baseline. Baseline distribution of women less than, or, greater than or equal to, 3 mg/L CRP was not different between groups. Adherence to aerobic exercise was reported at 142 ± 78 and 154 ± 87 minutes per week in the Exercise Alone group and Exercise plus Weight Loss group, respectively. Attendance at supervised exercise sessions was 84 ± 20% and 85 ± 21% in the Exercise Alone group and Exercise plus Weight Loss group, respectively.
Table 1.
Baseline characteristics by randomized group
| Characteristic | Total Sample (n=318) | Control (n=81) | Exercise (n=80) | Diet (n=81) | Exercise & Diet (n=76) |
|---|---|---|---|---|---|
| Age, y | 59.2 (8.6) | 58.7 (8.4) | 59.2 (8.2) | 59.0 (9.0) | 59.9 (8.8) |
| Race, n (%) | |||||
| White | 202 (64%) | 59 (73%) | 47 (59%) | 50 (62%) | 46 (61%) |
| Black | 106 (33%) | 20 (25%) | 32 (40%) | 28 (35%) | 26 (34%) |
| Other | 10 (3%) | 2 (2%) | 1 (1%) | 3 (3%) | 4 (5%) |
| BMI, kg/m2 | 34.1 (5.9) | 34.1 (5.8) | 34.1 (6.3) | 33.8 (5.6) | 34.3 (6.3) |
| Cancer stage*, n (%) | |||||
| DCIS | 22 (7%) | 10 (12%) | 5 (6%) | 4 (5%) | 3 (4%) |
| I | 69 (22%) | 16 (20%) | 23 (29%) | 16 (20%) | 14 (18%) |
| II | 94 (30%) | 21 (26%) | 21 (26%) | 28 (35%) | 24 (32%) |
| III | 65 (20%) | 15 (19%) | 12 (15%) | 19 (23%) | 19 (25%) |
| Unknown | 68 (21%) | 19 (23%) | 19 (24%) | 14 (17%) | 16 (21%) |
| CRP, mg/L | 4.84 [1.86–9.78] | 5.95 [2.26–10.17] | 3.89 [1.76–7.92] | 4.80 [2.10–9.20] | 4.84 [1.86–9.80] |
| CRP, mg/L | |||||
| <3.0 | 124 (39%) | 27 (33%) | 36 (45%) | 31 (38%) | 30 (39%) |
| ≥3.0 | 194 (61%) | 54 (67%) | 44 (55%) | 50 (62%) | 46 (61%) |
| SAA, μg/mL | 6.55 [3.98–11.38] | 6.53 [4.41–11.58] | 6.39 [3.54–10.69] | 6.89 [3.58–10.57] | 6.41 [3.98–11.86] |
| ICAM-1, ng/mL | 491.65 [419.31–580.21] | 500.45 [439.02–583.61] | 482.33 [419.61–573.54] | 488.00 [404.97–554.42] | 505.26 [408.31–603.66] |
| VCAM-1, ng/mL | 523.32 [438.00–635.69] | 546.17 [457.64–657.58] | 513.38 [437.99–593.39] | 513.07 [437.99–593.39] | 530.32 [437.99–635.69] |
Exercise Alone (Exercise), Weight Loss Alone (Diet), and Exercise plus Weight Loss (Exercise & Diet), Ductal carcinoma in situ (DCIS), C-Reactive Protein (CRP), Serum Amyloid A (SAA), Intercellular Adhesion Molecule-1 (ICAM-1), Vascular Cell Adhesion Molecule-1 (VCAM-1).
Cancer stage was captured via self-report and confirmed where available through pathology reports or state cancer registry. Values are mean ± standard deviation, median [interquartile range], or n (%). Percentages may not sum to 100.0% due to rounding error.
Intervention Effects
Table 2 displays the baseline and change values of inflammatory biomarkers. The intervention effect relative to the Control group is also presented. There were no differences between the groups at baseline. At follow up, CRP and SAA levels in the Weight Loss Alone group as well as the Exercise plus Weight Loss group, were significantly lower compared to both the Control group and the Exercise Alone group. Relative to the Control group, CRP levels in the Weight Loss Alone group (−35.2% [CI −49.9, −20.7]) and Exercise plus Weight Loss group (−44.1% [CI −57.1, −31.1]) decreased. SAA levels decreased by a similar amount (−25.6% [CI −39.8, −11.9]) in the Weight Loss Alone and Exercise plus Weight Loss groups (−26.6% [CI −40.5, −12.6]), compared to the Control group. ICAM-1 (9.3% [CI 1.6, 16.9]) and VCAM-1 (8.6% [CI 2.6, 14.5]) levels in the Exercise Alone group were significantly higher compared to the Control group following the intervention. No significant intervention effects on ICAM-1 or VCAM-1 were observed for the Weight Loss Alone or Exercise plus Weight Loss groups.
Table 2.
Change in biomarker endpoints of inflammation by randomized group
| Inflammation Endpoint | Randomized Group | Baseline Geo. Mean (SD) | Geo. Mean Change (SE) | Intervention Main Effect, Treatment Ratio (95% CI) |
|---|---|---|---|---|
| CRP | Control | 1.60 (1.10) | 0.01 (0.08) | 1.00 (Reference) |
| Exercise Alone | 1.30 (1.21) | 0.14 (0.08) | 1.13 (0.88, 1.39) | |
| Weight Loss Alone | 1.48 (1.17) | −0.42 (0.08)a | 0.65 (0.50, 0.79)b,c | |
| Exercise plus Weight Loss | 1.39 (1.14) | −0.57 (0.09)a | 0.56 (0.43, 0.69)b,c | |
| SAA | Control | 2.03 (1.00) | 0.00 (0.07) | 1.00 (Reference) |
| Exercise Alone | 1.79 (0.96) | 0.12 (0.07) | 1.12 (0.91, 1.33) | |
| Weight Loss Alone | 1.92 (1.03) | −0.29 (0.07)a | 0.74 (0.60, 0.88)b,c | |
| Exercise plus Weight Loss | 1.92 (0.85) | −0.30 (0.07)a | 0.73 (0.59, 0.87)b,c | |
| ICAM-1 | Control | 6.27 (0.34) | −0.03 (0.02) | 1.00 (Reference) |
| Exercise Alone | 6.15 (0.40) | 0.06 (0.03)a | 1.09 (1.02, 1.17)b | |
| Weight Loss Alone | 6.20 (0.34) | −0.01 (0.02) | 1.02 (0.95, 1.09) | |
| Exercise plus Weight Loss | 6.23 (0.31) | −0.07 (0.02)a | 0.96 (0.89, 1.03) | |
| VCAM-1 | Control | 6.31 (0.28) | −0.03 (0.02) | 1.00 (Reference) |
| Exercise Alone | 6.21 (0.41) | 0.05 (0.02)a | 1.08 (1.03, 1.14)b | |
| Weight Loss Alone | 6.23 (0.31) | 0.02 (0.02) | 1.05 (0.99, 1.11) | |
| Exercise plus Weight Loss | 6.30 (0.29) | 0.01 (0.02) | 1.03 (0.97, 1.09) |
P<0.05 (two-sided) compared with baseline (within group).
P<0.05 (two-sided) compared with Control.
P<0.05 (two-sided) compared with Exercise Alone.
P<0.05 (two-sided) compared with Weight Loss Alone.
Intervention effect mediators
Given the nature of the study design, we also explored hypothesized intervention effect mediators. We hypothesized that changes in biomarker levels may be related to changes in specific components of body composition. We also hypothesized that changes in biomarker levels may be related to changes in components of fitness. We observed significant positive correlations between changes in bodyweight and composition with changes in CRP, SAA, and ICAM-1 levels (Table 3). While not surprising that a decrease in body weight was associated with decreased inflammation, we unexpectedly observed these associations independent of any concomitant significant association between change in aerobic fitness or strength with change in biomarker levels.
Table 3.
Pearson correlation coefficients for the relationship between change in hypothesized intervention effect mediators and change in log-transformed biomarker level
| Hypothesized Intervention Effect Mediators | Change in Log-Transformed Biomarker Level | |||
|---|---|---|---|---|
| CRP | SAA | ICAM-1 | VCAM-1 | |
| Δ Bodyweight | 0.40 | 0.32 | 0.18 | — |
| Δ Fat Mass | 0.37 | 0.34 | 0.14 | — |
| Δ Visceral Adipose Tissue | 0.31 | 0.19 | — | — |
| Δ Subcutaneous Adipose Tissue | 0.31 | 0.28 | — | — |
| Δ Lean Mass | 0.30 | 0.24 | 0.21 | — |
| Δ Appendicular Lean Mass | 0.23 | — | — | — |
| Δ Aerobic Fitness Capacity | — | — | — | — |
| Δ Bench Strength | — | — | — | — |
| Δ Leg Strength | — | — | — | — |
CRP, C-Reactive Protein; SAA, Serum Amyloid A; ICAM-1, Intercellular Adhesion Molecule-1; VCAM-1, Vascular Cell Adhesion Molecule-1. All correlations shown were statistically significant (p<0.05). The symbol “—” denotes absence of significant correlation.
Clinically relevant improvements in CRP
At baseline, 194 women had CRP levels >3 mg/L. Compared to Control, Weight Loss Alone [0.17; 95% CI: 0.04, 0.30] and Exercise plus Weight Loss [0.31; 95% CI: 0.16, 0.46] increased the probability of achieving normal CRP at month 12 (Figure 1). We observed the largest estimated treatment difference (ETD) in the Exercise plus Weight Loss group (0.31 [95% CI, 0.16, 0.46]).
Figure 1. Change in clinically relevant levels of CRP.
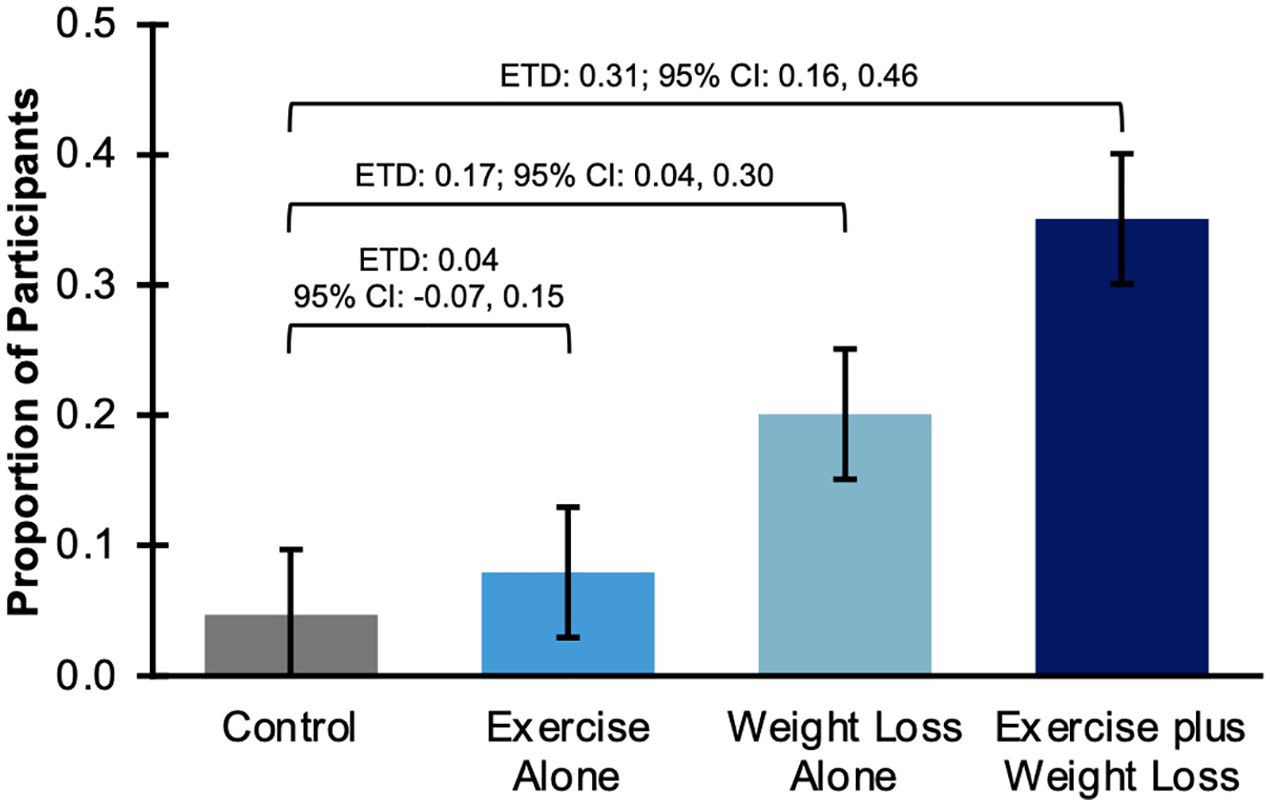
Proportion of study participants with elevated CRP level (>3 mg/L) at baseline who achieved a clinically meaningful reduction (≤3 mg/L) at month 12. ETD, Estimated Treatment Difference; CI, Confidence Interval.
DISCUSSION
Breast cancer survivors with obesity face a 17–46% greater risk of breast cancer recurrence (33), and breast cancer survivors with a BMI ≥ 35 kg/m2 are at 70% greater risk of an incident cardiovascular event (34). We observed that, compared to the control condition, weight loss with or without exercise led to statistically significant and clinically meaningful reductions in CRP and SAA in breast cancer survivors with excess body weight. Changes in inflammation (CRP, SAA, and ICAM-1) may be driven mainly through weight loss regardless of where the weight loss was observed and without changes in aerobic fitness or muscle strength. CRP levels >3 mg/L are associated with a high risk for CVD, and a 1 mg/L increase in CRP level is associated with a 6% increase in risk for cancer mortality (31, 32). We observed that in women with CRP levels > 3mg/L, a combination of caloric restriction and physical activity appears to be most effective. Collectively, our results indicate that breast cancer survivors with excess bodyweight should focus on weight loss as the primary driver of improvements in inflammation, and for women with high CVD risk the addition of exercise to caloric restriction may synergize for improvements in CRP levels.
In our prior literature review of the effects of diet and exercise induced weight loss on biomarkers of inflammation in breast cancer survivors, we found no reports of significant effects on CRP levels after intervention with exercise alone or in combination with dietary restriction (35). However, we induced greater mean levels of weight loss in the WISER Survivor trial caloric restriction groups than did the studies included in our meta-analysis. The average weight loss in the WISER Survivor exercise plus weight loss group was 8.06% of their baseline weight, while in our systematic review only 3 studies (10% of reviewed studies) reported weight loss ≥ 5% (12, 35). Given the known relationship between CRP and adiposity in postmenopausal women (36), it is not surprising that we observed a positive correlation between decreased CRP levels and decreased adiposity. Thus, it is likely that if exercise is intended to have an anti-inflammatory effect, it will need to be dosed in such a way that exercise (or exercise in combination with caloric restriction) decreases adiposity. As previously reported in WISER Survivor, we did not observe changes in body composition in the Exercise Alone group (24). Also consistent with previous reports from the WISER Survivor trial, we observed that a ≥ 10% decrease in body weight was beneficial for improvements in insulin and insulin resistance (37). Further, the addition of exercise to weight loss via caloric restriction was particularly important for breast cancer survivors with clinically abnormal levels of C-peptide (37).
Observed associations, as well as mechanistic pathways, have connected the inflammatory biomarkers SAA, ICAM-1, and VCAM-1 to both cancer and CVD (15, 16, 19, 20, 22, 23). Considerably less is known about their response to changes in energy balance in breast cancer survivors. The Nutrition and Exercise of Women (NEW) study is similar to the WISER Survivor trial with respect to their intervention length (12 months), study design for energy balance (control, exercise, caloric restriction, and the combination of the two), and population sample (postmenopausal women with excess body weight) (38). The NEW study also observed that exercise alone did not improve SAA or CRP levels, and caloric restriction, as well as the combination group, did improve SAA and CRP levels compared to the control group (38).
Although lower levels of ICAM-1 and VCAM-1 are typically considered beneficial (39), findings from some studies conflict with this notion. For example, higher levels of ICAM-1 have been associated with lower breast cancer risk (40); and higher levels of VCAM-1 have been associated with lower risk of CVD (23). Prior exercise and weight loss intervention studies report no change in ICAM-1 or VCAM-1 after intervention (41, 42). Thus, additional studies to elucidate the mechanistic role of ICAM-1 and VCAM-1 in complex pathologies such as cancer recurrence or CVD, on the backdrop of lifestyle modification interventions, are necessary.
The WISER Survivor trial was designed as a 4-armed randomized controlled trial to assess the individual and combined interventions on lymphedema outcomes. As such, the interventions, and particularly the exercise intervention, was prescribed specifically for lymphedema outcomes. Changes in biomarkers of inflammation that predict cancer recurrence and CVD may require a different exercise prescription. Further, our 4-arm randomized controlled trial was not designed to test the interaction effect of diet and exercise such as would be tested in a 2×2 factorial design (i.e. that the effect of one independent variable (e.g. exercise) depends on the level of the other independent variable (e.g. diet)). Future trials might consider a 2×2 factorial design to gain a more thorough understanding of how both components operate together. This is a limitation of our study. Other limitations include generalizability to female breast cancer survivors, the highly selected sample (overweight or obese, lymphedema, Philadelphia region), and only testing one weight loss and one exercise approach.
Approximately 62% of breast cancer survivors have excess body weight, and 68% do not meet recommendations for physical activity (43). While treatment guidelines exist and clinicians often recommend weight loss and increased physical activity to breast cancer survivors, the reality is that energy balance is not supported by care providers in the majority of cancer care settings. As a growing number of cancer treatment centers focus on issues of survivorship, inclusion of evidence-based weight loss and physical activity interventions is strongly encouraged.
In conclusion, with an increasing number of breast cancer survivors, an increased emphasis on lifestyle modification to reduce recurrence and the sequela of breast cancer and breast cancer treatment is warranted. The results of this study demonstrate that reduction of biomarkers of inflammation is more effectively accomplished with a weight loss intervention than an exercise intervention when compared to the control group. However, for participants with clinically impaired CRP levels, a combination of exercise and weight loss may be necessary to normalize CRP levels.
Supplementary Material
SDC 1: Supplemental Digital Content – CONSORT.tif
Acknowledgements
This work was supported, in part, by the National Cancer Institute of the National Institutes of Health under Award Numbers U54-CA155850, U54-CA155435, UL1-TR001878, P30-CA016520. Dr. Sturgeon is supported by grant UL1TR002014 and KL2TR002015 from the National Center for Advancing Translational Sciences. Dr. Brown is supported by grants from National Institute of General Medicine Sciences of the National Institutes of Health under Award Number U54-GM104940, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30-DK072476, and the National Cancer Institute of the National Institutes of Health under Award Numbers R00-CA218603; and R25-CA203650. Compression garments were donated by BSN Medical, and discounted meal replacements were provided by Nutrisystem, Inc. The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of Pennsylvania State University or the National Institutes of Health. The funders of the study had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit manuscript for publication. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Conflict of Interest
Dr. Schmitz reports receiving nonfinancial support from BSN Medical, personal fees from Klose Training, and a licensed patent for a Strength After Breast Cancer course. No other disclosures were reported. Dr. Sarwer has consulting relationships with Ethicon and NovoNordisk which are unrelated to the study.
REFERENCES
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 2.Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016;34(9):927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: a US population-based analysis. Cancer. 2020;126(7):1559–67. [DOI] [PubMed] [Google Scholar]
- 5.Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. [DOI] [PubMed] [Google Scholar]
- 8.Pang Y, Kartsonaki C, Du H, et al. Physical activity, sedentary leisure time, circulating metabolic markers, and risk of major vascular diseases. Circ Genom Precis Med. 2019;12(9):386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur RP, Rubal, Banipal RPS, Vashistha R, Dhiman M, Munshi A. Association of elevated levels of C-reactive protein with breast cancer, breast cancer subtypes, and poor outcome. Curr Probl Cancer. 2019;43(2):123–9. [DOI] [PubMed] [Google Scholar]
- 10.Winkels RM, Sturgeon KM, Kallan MJ, et al. The women in steady exercise research (WISER) survivor trial: the innovative transdisciplinary design of a randomized controlled trial of exercise and weight-loss interventions among breast cancer survivors with lymphedema. Contemp Clin Trials. 2017;61:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturgeon KM, Hackley R, Fornash A, et al. Strategic recruitment of an ethnically diverse cohort of overweight survivors of breast cancer with lymphedema. Cancer. 2018;124(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz KH, Troxel AB, Dean LT, et al. Effect of home-based exercise and weight loss programs on breast cancer-related lymphedema outcomes among overweight breast cancer survivors: the WISER survivor randomized clinical trial. JAMA Oncol. 2019;5(11):1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan DS, Bandera EV, Greenwood DC, Norat T. Circulating C-reactive protein and breast cancer risk-systematic literature review and meta-analysis of prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1439–49. [DOI] [PubMed] [Google Scholar]
- 14.Lagrand WK, Visser CA, Hermens WT, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100(1):96–102. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Kip KE, Marroquin OC, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):726–32. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Sheng J, Fan Y, et al. Association between serum amyloid A levels and cancers: a systematic review and meta-analysis. Postgrad Med J. 2018;94(1115):499–507. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, He X, Shi X, et al. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res. 2010;59(5):323–34. [DOI] [PubMed] [Google Scholar]
- 18.Mulhem A, Moulla Y, Kloting N, et al. Circulating cell adhesion molecules in metabolically healthy obesity. Int J Obes (Lond). 2021;45(2):331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. [DOI] [PubMed] [Google Scholar]
- 21.Isoppo de Souza C, Rosa DD, Ettrich B, et al. Association of adipokines and adhesion molecules with indicators of obesity in women undergoing mammography screening. Nutr Metab (Lond). 2012;9(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38(17):2252–7. [DOI] [PubMed] [Google Scholar]
- 23.Kunutsor SK, Bakker SJL, Dullaart RPF. Soluble vascular cell adhesion molecules may be protective of future cardiovascular disease risk: findings from the PREVEND prospective cohort study. J Atheroscler Thromb. 2017;24(8):804–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JC, Sarwer DB, Troxel AB, et al. A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Res Treat. 2021;189(1):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vetter ML, Wadden TA, Chittams J, et al. Effect of lifestyle intervention on cardiometabolic risk factors: results of the POWER-UP trial. Int J Obes (Lond). 2013;37 Suppl 1(0 1):S19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546–62. [DOI] [PubMed] [Google Scholar]
- 27.Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Phys Ther. 2000;80(8):782–807. [PubMed] [Google Scholar]
- 28.Irwin M, Cartmel B, Gross C, et al. Effect of exercise on weight, body fat, and serum inflammatory biomarkers in breast cancer survivors with aromatase inhibitor arthralgias: the hormones and physical exercise (HOPE) study. J Clin Oncol. 2014;32(15 Suppl 1). [Google Scholar]
- 29.Belalcazar LM, Reboussin DM, Haffner SM, et al. A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33(11):2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little R Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6:287–96. [Google Scholar]
- 31.Johns I, Moschonas KE, Medina J, Ossei-Gerning N, Kassianos G, Halcox JP. Risk classification in primary prevention of CVD according to QRISK2 and JBS3 ‘heart age’, and prevalence of elevated high-sensitivity C reactive protein in the UK cohort of the EURIKA study. Open Heart. 2018;5(2):e000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni P, Yu M, Zhang R, et al. Dose-response association between C-reactive protein and risk of all-cause and cause-specific mortality: a systematic review and meta-analysis of cohort studies. Ann Epidemiol. 2020;51:20–7.e11. [DOI] [PubMed] [Google Scholar]
- 33.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111(2):329–42. [DOI] [PubMed] [Google Scholar]
- 34.Cespedes Feliciano EM, Chen WY, Bradshaw PT, et al. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol. 2019;37(28):2528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruinsma TJ, Dyer AM, Rogers CJ, Schmitz KH, Sturgeon KM. Effects of diet and exercise-induced weight loss on biomarkers of inflammation in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1048–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105(5):564–9. [DOI] [PubMed] [Google Scholar]
- 37.D’Alonzo NJ, Qiu L, Sears DD, et al. WISER survivor trial: combined effect of exercise and weight loss interventions on insulin and insulin resistance in breast cancer survivors. Nutrients. 2021;13(9):3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72(9):2314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmefors H, DuttaRoy S, Rundqvist B, Borjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis--a systematic review. Atherosclerosis. 2014;235(1):150–61. [DOI] [PubMed] [Google Scholar]
- 40.Tobias DK, Akinkuolie AO, Chandler PD, et al. Markers of inflammation and incident breast cancer risk in the Women’s Health Study. Am J Epidemiol. 2018;187(4):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond). 2007;31(6):996–1003. [DOI] [PubMed] [Google Scholar]
- 42.Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc. 2014;62(4):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: Supplemental Digital Content – CONSORT.tif


