Abstract
Purpose:
Several studies have shown a lower risk of developing frailty with long-term higher levels of physical activity. However, most these studies lacked repeated measurement over the follow-up period. Therefore, we examined the association between different types of physical activity and in frailty development using repeated measurements.
Methods:
69,642 non-frail women aged 60+ from the Nurses’ Health Study were followed from 1992 to 2016. Leisure time physical activity was assessed biennially. Frailty was defined as having 3+ of the following five criteria from the FRAIL scale: fatigue, low strength, reduced aerobic capacity, having ≥5 illnesses, and ≥5% weight loss. Cox models adjusted for potential confounders were used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for the association between total, moderate intensity, vigorous intensity physical activity, walking and incident frailty.
Results:
During 24 years of follow-up, we documented 16,479 incident frailty cases. Comparing top to bottom quintiles of MET hrs/wk of physical activity, the HR was 0.48 (95% CI=0.45, 0.50) for total physical activity, 0.51 (0.48, 0.54) for moderate, and 0.75 (0.71, 0.79) for vigorous activity (p trend <0.001 for all activities). For each hour/wk increase, HR was 0.56 (0.53, 0.58), 0.51 (0.48, 0.54), and 0.63 (0.58, 0.68) for total, moderate, and vigorous activity, respectively. Walking was the most common activity, and each hour/d increase in walking was associated with an HR of 0.41 (0.38, 0.44) for frailty incidence; this was evident even among those older than 70y and those with pre-existing frailty characteristics.
Conclusions:
Both moderate and vigorous physical activity was associated with a lower risk of frailty. In particular, walking, a broadly accessible activity, was also associated with lower risk.
Keywords: ELDERLY, FRAIL, WOMEN, ACTIVITY
INTRODUCTION
Frailty in older adults is the loss of physical and functional reserve that manifests as decreasing capacity in performing more demanding activities as well as greater vulnerability to even minor health stressors (1). It is often characterized by fatigue, weakness, low physical stamina, and sometimes unintentional weight loss. The prevalence of frailty globally among individuals aged 50 and older, as measured by various frailty indices, was estimated to be approximately 24% (2). In addition, the prevalence in women (29%) was substantially higher than that of men (20%). Frail individuals are at higher risk for falls, poor quality of life, hospitalization, disability and death (3). Physical activity and nutrition have been the main treatment approaches to improve strength, balance, and mobility (4). While intervention trials have shown success in frailty management, trial durations were short, often included participants who were already frail, or only addressed specific components of frailty. Given the substantial prevalence of frailty in the older population, prevention strategies that can be widely and economically implemented are valuable.
A few longitudinal studies have examined the association between physical activity and the development of frailty (5–10). While a lower risk of frailty was observed with higher levels of physical activity, most studies lacked detailed examination of different levels of intensity or specific types of physical activity, and none assessed physical activity beyond a single measure at baseline. In addition, some studies had a relatively short follow-up time (7, 10). An in-depth analysis that encompasses different types of physical activity, including exploration of nuances such as latency and effect modification by age and other risk factors of frailty, would provide more detailed information on the role of low physical activity for frailty development.
In this prospective analysis, we used data from the Nurses’ Health Study (NHS), with repeated measurement of physical activity to examine total, moderate, and vigorous physical activity in relation to risk of frailty in women aged 60 or older. In addition, we examined frailty risk with walking, which is a low-cost activity suitable for older individuals. We also explored several durations of latency, and potential difference in association by risk factors of frailty.
METHODS
Study participants
Women in this analysis were participants of the U.S. Nurses’ Health Study (NHS). This ongoing cohort began in 1976 with 121,700 nurses aged 30–55 y in 11 states (11). Participants self-reported lifestyle and morbidity through a questionnaire every 2 years. In this analysis we included women aged ≥60 years in 1992, with valid dietary information (intake between 500 and 3500kcal/d) from the 1994 food frequency questionnaire and without missing BMI. We used 1992 as baseline for this analysis as it was the first time that frailty characteristics were assessed. Women younger than age 60 in 1992 entered follow-up when they reached age 60 in subsequent questionnaire cycles. After excluding those who were already frail in 1992 or at age 60 (i.e. entry to follow-up), 69,642 women were included for analysis and followed up to 2016. The Harvard School of Public Health and Brigham and Women’s Hospital Institutional Review Board approved the protocol of the study.
Assessment of physical activity
Data on physical activity was self-reported via questions on common leisure time physical activities in the questionnaires in years 1992, 1996, 2000, 2004, 2008, 2012. The validity of the physical activity questions was assessed in a sample of participants using four separate one-week diaries administered over the course of a year (12). The correlation coefficient between activity diaries and the questionnaire was 0.62, indicating that the questions were reasonably valid. Participants reported the average hours spent per week during the past year for each activity. We then assigned a metabolic equivalent task (MET), which indicates the energy cost of that particular activity relative to resting metabolic rate, based on a compendium of physical activity (13), to quantify the intensity of physical activities. Total physical activity was calculated by summing all physical activities in MET-hour per week. Among the specific physical activities, those of 6 METs or higher (jogging, running, swimming, bicycling, calisthenics, other aerobic exercises, squash/racquetball/ tennis) were classified as vigorous physical activity, and those of 3 to <6 METS (walking, other low intensity activity such as yoga, and beginning in year 2000 upper and lower extremities weight training) were considered moderate physical activity (14).
Assessment of frailty
Frailty was defined using the FRAIL scale that includes five self-reported criteria: fatigue, low strength (reduced resistance), reduced aerobic capacity, history of several major chronic illnesses, and a significant weight loss during the previous year (15). The FRAIL scale correlates well with the Fried frailty phenotype, and it has been adapted and validated to be used in several populations (16, 17). The simple structure based on self-reported data, which makes the definition suitable for research purposes and repeated measurements in large cohorts. The FRAIL scale has been shown to be moderately correlated with the Fried index (r= 0.617, p<0.001) (18).
In 1992, 1996, 2000, 2004, 2008 and 2012, participants completed the Medical Outcomes Study Short-Form (SF-36), a 36-item-questionnaire with 8 health dimensions, including physical and mental components (19). From the SF-36, we assessed three frailty criteria with the following questions: a) for fatigue: “Did you have a lot of energy?”, with replies “some of the time” or “none of the time” or with the question “I could not get going” , with responses “moderate amount” or “all of the time”; b) for poor strength (reduced resistance): “In a normal day, is your health a limitation to walk up 1 flight of stairs?”, with responses “yes” or “a lot”; and c) for low aerobic capacity: “In a normal day, is your health a limitation to walk several blocks or several miles?”, with responses “yes” or “a lot”. In addition, the illness criterion was ascertained from the question “In the last 2 years, have you had any of these physician-diagnosed illnesses?” Participants reporting ≥5 of the following diseases were considered to meet this criterion: cancer, hypertension, type 2 diabetes, angina, myocardial infarction, stroke, congestive heart failure, asthma, arthritis, chronic obstructive lung disease, Parkinson’s disease, kidney disease, and depression. Finally, the weight loss criterion was defined as a ≥5% decrease in the weight reported in two consecutive follow-up cycles. At the end of each 4-year follow-up cycle, incident cases of frailty were defined as participants having ≥3 criteria in the FRAIL scale. Missing response in 3 or more components were assumed as missing on frailty status and were excluded. During follow-up, frailty was defined when participants reported having developed 3 criteria, therefore if >=3 missing, it was assumed as missing on frailty and excluded, but if 1 or 2 missing, we were able to assess frailty status using remaining frailty components, assuming missing in each component as not having it.
Assessment of health and lifestyle characteristics
Height was self-reported in 1976 and weight was reported at each biennial questionnaire. The biennial questionnaire also assessed smoking status and the quantity of cigarette use, use of medication for hypertension, diabetes, and hyperlipidemia, and postmenopausal hormones. Diet was assessed at baseline and in 1994, 1998, 2002, 2006, and 2010 using a validated self-administered semi-quantitative FFQ (20) that contained approximately 135 items. Standard portion sizes were provided for each item, and nine frequency choices, ranging from <1 time/month to ≥6 times/day, were available. Highest level of education was assessed in 1992.
Statistical analysis
The primary exposures in this analysis were total, moderate, and vigorous physical activity expressed in MET hours/wk and as duration in hours/wk. Correlations between the different types of physical activity were obtained using Spearman correlation coefficients. We computed cumulative averages from each physical activity assessment year to reduce within-person variation and represent long-term activity habits (21). MET hours/wk were then classified into quintiles and activity duration was classified into specific categories. Hazard ratios (HR) for incident frailty by different levels of physical activity were computed using Cox proportional hazard models. Tests of trends were conducted by modeling physical activity as a continuous variable.
In multivariable analysis, we adjusted for age (in months), energy intake (quintiles), alcohol intake (0, 1 to <5g/d, 5 to <10g/d, 10 to <15g/d, 15+g/d), smoking (never, past, current 1-14 cigarettes/d, 15-24 cigarettes/d, 25+ cigarettes/d), protein intake (quintiles), highest academic degree earned (RN only, bachelor’s degree, graduate degree), predicted lean body mass (22), use of post-menopausal hormone treatment, aspirin, diuretics, beta blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, and other antihypertensive medication, lipid lowering medications, insulin, and oral hypoglycemic medication. The sex-specific lean body mass equation using height, weight, and age showed an R2 of 0.84 when validated against a nationally representative sample of women with DXA-measured body composition (22). To account for dietary quality, we used FFQ data to compute the Alternate Healthy Eating Index-2010 (23) and modeled it in quintiles. Missing data was categorized into a missing indicator. In analysis of moderate and vigorous physical activities, these two activity intensities were mutually adjusted for in the model. Covariates were selected based on their potential as confounders and as predictors of components of the FRAIL score.
We explored potential differences in associations by lean body mass and age with an analysis stratified by lean body mass at the median and age at 70. Test for interaction was performed using the likelihood ratio test comparing regression models with and without interaction terms. We also examined changes in physical activity over 4 years as MET hours/week and duration in hours/week, using little change (i.e. ± 2 MET hrs/week or ± 1 hr/wk) as reference. To explore the lag time between physical activity level and frailty development, and to examine potential reverse causation, we modeled the association with 4, 8, and 12 years of lag time.
Although we did not include any women who were already frail at baseline, some already had one or two preexisting frailty components. Therefore, we repeated the analysis among those with 0, 1, or 2 of any frailty components at baseline to explore if the association of physical activity and frailty may differ depending on the baseline status. We also used the subset of participants without any frailty criterion at baseline to explore the association between physical activity and risk of developing each component of the FRAIL scale.
Walking was the most common physical activity among the participants, and all other physical activities were less often reported (see Supplemental Figure 1, Supplemental Digital Content, Mean MET hrs/week for total and specific physical activities from 1992 to 2012). We examined the overall association between increments of 1 hour/day walking with risk of frailty and each frailty criterion, as well as stratified analysis by lean body mass (median), age (at 70y), by lag time of 4, 8, and 12 years, and by the number of baseline preexisting frailty criteria. We also conducted a stratified analysis by weight training exercises as our biennial questionnaires included 2 questions on arm and leg weight training in 2000. However, less than 50% of our participants reported any weight training since year 2000, therefore the stratified analysis was conducted with classifying weight training as a binary category (none/any).
RESULTS
In up to 24 years of follow-up, we documented 16,479 incident cases of frailty. Total MET hours/wk declined slightly over the follow-up period, but there was an increase in moderate activity and a decline in vigorous activity (see Supplemental Figure 1, Supplemental Digital Content, Mean MET hrs/week for total and specific physical activities from 1992 to 2012). Total physical activity in MET hours/wk was highly correlated with moderate (Spearman r=0.76, p<0.001), and vigorous activity (r=0.79, p<0.001) (see Supplemental Table 1, Supplemental Digital Content, Spearman correlation coefficients of cumulative averages of physical activity), while moderate and vigorous physical activity were moderately correlated (r=0.31, p<0.001). Walking was reported at the highest level among different leisure activities (see Supplemental Figure 1, Supplemental Digital Content) and highly correlated with total physical activity (r=0.93, p<0.001) (Supplemental Table 1, Supplemental Digital Content). Women with higher levels of activity tended to have a lower BMI, were less likely to be current smokers, but they were more likely to use post menopausal hormones, consumed more alcohol, and had better diet quality (Table 1).
Table 1:
Characteristics (mean ± SD) of women by quintiles of total physical activity (MET hour/wk) at age 60
| Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.6 ± 5.8 | 26.0 ± 5.1 | 25.6 ± 4.8 | 25.2 ± 4.5 | 24.4 ± 4.2 |
| Body weight (kg) | 71.4 ± 16.1 | 69.9 ± 14.5 | 69.0 ± 13.6 | 67.8 ± 12.9 | 52.2 ± 12.1 |
| Estimated lean body mass (kg)* | 10.3 ± 5.7 | 9.8 ± 5.2 | 9.5 ± 4.8 | 9.1 ± 4.6 | 8.5 ± 4.3 |
| Estimated fat mass (kg)* | 30.3 ± 10.1 | 29.3 ± 9.0 | 28.6 ± 8.5 | 27.9 ± 8.0 | 26.5 ± 7.4 |
| Current smoker (%) | 4.9 | 3.7 | 3.0 | 2.6 | 2.6 |
| Physical activity (MET hr/wk) | |||||
| Total physical activity | 1.3 ± 1.1 | 5.4 ± 2.1 | 12.2 ± 3.5 | 23.4 ± 5.5 | 54.6 ± 28.0 |
| Moderate physical activity | 1.0 ± 1.0 | 4.0 ± 2.2 | 8.5 ± 4.5 | 15.2 ± 7.8 | 29.1 ± 18.2 |
| Vigorous physical activity | 0.06 ± 0.3 | 0.9 ± 1.8 | 3.3 ± 4.1 | 7.6 ± 7.4 | 24.9 ± 24.0 |
| Walking | 0.9 ± 1.0 | 3.3 ± 2.2 | 6.3 ± 4.5 | 11.0 ± 7.9 | 19.5 ± 13.4 |
| Physical activity (hr/wk) | |||||
| Total physical activity | 0.4 ± 0.4 | 1.5 ± 0.7 | 3.3 ± 1.2 | 6.0 ± 1.9 | 12.4 ± 5.7 |
| Moderate physical activity | 0.4 ± 0.4 | 1.4 ± 0.8 | 2.7 ± 1.5 | 4.7 ± 2.6 | 8.5 ± 5.0 |
| Vigorous physical activity | 0.01 ± 0.04 | 0.1 ± 0.3 | 0.5 ± 0.6 | 1.2 ± 1.2 | 3.9 ± 3.7 |
| Walking | 0.4 ± 0.4 | 1.2 ± 0.8 | 2.2 ± 1.6 | 3.7 ± 2.7 | 6.1 ± 4.0 |
| Medication use (% yes) | |||||
| Post menopausal hormones | 5.5 | 7.2 | 8.3 | 9.8 | 11.7 |
| Diuretics | 18.0 | 19.6 | 20.1 | 19.9 | 17.4 |
| Beta blockers | 24.3 | 25.8 | 24.0 | 23.5 | 20.6 |
| Calcium channel blockers | 18.4 | 18.2 | 17.4 | 16.3 | 14.3 |
| Other antihypertensive medication | 10.1 | 9.9 | 9.4 | 8.8 | 8.3 |
| ACE inhibitors | 9.4 | 10.8 | 11.5 | 11.6 | 11.6 |
| Lipd lowering medications | 51.7 | 53.0 | 51.6 | 49.1 | 45.4 |
| Insulin | 4.4 | 2.7 | 2.1 | 1.5 | 1.1 |
| Oral hypoglycemics | 5.3 | 4.1 | 3.8 | 2.9 | 2.1 |
| Dietary intake | |||||
| Energy adjusted protein (g/d) | 65.3 ± 13.1 | 66.4 ± 12.8 | 67.4 ± 12.6 | 67.9 ± 12.5 | 68.1 ± 12.3 |
| Alcohol (g/d) | 4.7 ± 9.8 | 5.3 ± 10.1 | 6.0 ± 10.4 | 6.6 ± 10.5 | 7.4 ± 11.3 |
| Alternate Healthy Eating Index-2010 score | 57.0 ± 11.1 | 59.1 ± 11.4 | 61.3 ± 11.5 | 63.3 ± 11.8 | 65.7 ± 12.1 |
After multivariable adjustment, total physical activity, moderate, and vigorous physical activity were all inversely associated with risk of frailty (Tables 2 and 3). Comparing top to bottom quintiles of MET hours/week, the HR was 0.48 (95% CI=0.45, 0.50) for total physical activity, 0.51 (0.48, 0.54) for moderate activity, and 0.75 (0.71, 0.79) for vigorous activity (Table 2). Similarly, the HR comparing ≥10.0 hours/wk vs <1.0 hour/wk of total physical activity was 0.40 (0.37, 0.44) (Table 3). The HR comparing ≥ 4.0 vs <1.0 hour/wk of moderate physical activity was 0.58 (0.55, 0.62) and for vigorous physical activity was 0.71 (0.66, 0.76). For each hour/d increase in physical activity, the HR was 0.56 (0.53, 0.58) for total physical activity, 0.51 (0.48, 0.54) for moderate activity and 0.63 (0.58, 0.68) for vigorous activity.
Table 2:
Hazard ratios (95% CI) for risk of frailty according to categories of cumulative average of leisure time physical activity MET hour/wk in women ≥60y
| Quintiles | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p trend | Per 3 MET hr/wk | |
| Total physical activities | |||||||
| Median MET (hour/wk) | 3.2 | 8.6 | 14.7 | 23.3 | 42.2 | ||
| No. of cases | 5598 | 3757 | 3052 | 2386 | 1686 | ||
| Person-time | 221,776 | 223,719 | 223,740 | 224,409 | 224,685 | ||
| Age adjusted | 1 | 0.68 (0.65, 0.71) | 0.56 (0.53, 0.58) | 0.44 (0.42, 0.47) | 0.32 (0.31, 0.34) | <0.001 | 0.92 (0.92, 0.93) |
| Multivariablea | 1 | 0.78 (0.75, 0.81) | 0.69 (0.66, 0.72) | 0.59 (0.56, 0.62) | 0.48 (0.45, 0.50) | <0.001 | 0.95 (0.95, 0.95) |
| Moderate activities | |||||||
| Median MET (hour/wk) | 1.1 | 3.6 | 6.9 | 11.3 | 20.3 | ||
| No. of cases | 5474 | 3793 | 3174 | 2412 | 1626 | ||
| Person-time | 221,978 | 223,048 | 229,698 | 221,618 | 221,987 | ||
| Age adjusted | 1 | 0.69 (0.66, 0.72) | 0.57 (0.55, 0.60) | 0.45 (0.43, 0.47) | 0.31 (0.29, 0.33) | <0.001 | 0.85 (0.84, 0.85) |
| Multivariablea | 1 | 0.80 (0.76, 0.83) | 0.72 (0.69, 0.76) | 0.63 (0.60, 0.66) | 0.51 (0.48, 0.54) | <0.001 | 0.91 (0.90, 0.92) |
| Vigorous activities | |||||||
| Median MET (hour/wk) | 0.0 | 2.3 | 5.5 | 11.1 | 25.5 | ||
| No. of cases | 5052 | 3468 | 3183 | 2708 | 2068 | ||
| Person-time | 249,643 | 193,390 | 225,813 | 226,164 | 223,319 | ||
| Age adjusted | 1 | 0.84 (0.80, 0.87) | 0.72 (0.69, 0.76) | 0.62 (0.59, 0.64) | 0.48 (0.46, 0.51) | <0.001 | 0.93 (0.93, 0.94) |
| Multivariablea | 1 | 0.91 (0.87, 0.95) | 0.85 (0.81, 0.89) | 0.80 (0.76, 0.84) | 0.75 (0.71, 0.79) | <0.001 | 0.97 (0.97, 0.98) |
Multivariable adjusted for age, estimated lean body mass, estimated fat mass, body weight, energy intake, alcohol intake, AHEI-2010 score, post-menopausal hormone use, smoking, highest degree earned, use of the following medications: aspirin, beta blockers, hydrochlorthiazide, ACE inihibitors, calcium channel blockers, other antihypertensive medications, insulin, oral hypoglycemics, lipid lowering medication. Moderate and vigorous activities were mutually adjusted for each other.
Table 3:
Hazard ratios (95% CI) for risk of frailty according to categories of cumulative average of leisure time physical activity time (hour/wk) in women ≥60y
| <1.0 | 1 to <3.0 | 3.0 to <7.0 | 7.0 to <10.0 | ≥10.0 | Per 1 hr/d | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total activity | |||||||
| Median (hr/wk) | 0.5 | 2.0 | 4.5 | 8.2 | 12.5 | ||
| No. of cases | 3832 | 6222 | 5019 | 931 | 475 | ||
| Person-time | 155,474 | 358,170 | 415,386 | 111,743 | 77,557 | ||
| Age adjusted | 1 | 0.65 (0.63, 0.68) | 0.45 (0.43, 0.46) | 0.31 (0.29, 0.34) | 0.25 (0.22, 0.27) | <0.001 | 0.40 (0.38, 0.42) |
| Multivariablea | 1 | 0.76 (0.73, 0.80) | 0.60 (0.57, 0.63) | 0.47 (0.44, 0.50) | 0.40 (0.37, 0.44) | <0.001 | 0.56 (0.53, 0.58) |
| <1.0 | 1 to < 2.0 | 2 to < 4.0 | ≥4.0 | ||||
|
|
|||||||
| Moderate activity | |||||||
| Median (hr/wk) | 0.5 | 1.4 | 2.8 | 5.4 | |||
| No. of cases | 6286 | 4101 | 4058 | 2034 | |||
| Person-time | 292,486 | 261,034 | 322,461 | 242,349 | |||
| Age adjusted | 1 | 0.69 (0.66, 0.71) | 0.54 (0.52, 0.56) | 0.37 (0.35, 0.39) | <0.001 | 0.28 (0.26, 0.30) | |
| Multivariablea | 1 | 0.81 (0.77, 0.84) | 0.71 (0.68, 0.74) | 0.58 (0.55, 0.62) | <0.001 | 0.51 (0.48, 0.55) | |
| Vigorous activity | |||||||
| Median (hr/wk) | 0.2 | 1.4 | 2.7 | 5.6 | |||
| No. of cases | 10,563 | 2964 | 2081 | 871 | |||
| Person-time | 589,465 | 228,530 | 187,951 | 112,383 | |||
| Age adjusted | 1 | 0.73 (0.70, 0.76) | 0.63 (0.60, 0.66) | 0.46 (0.43, 0.49) | <0.001 | 0.34 (0.32, 0.37) | |
| Multivariablea | 1 | 0.85 (0.81, 0.88) | 0.82 (0.78, 0.86) | 0.71 (0.66, 0.76) | <0.001 | 0.63 (0.58, 0.68) | |
Multivariable adjusted for age, BMI, energy intake, alcohol intake, AHEI-2010 score, post-menopausal hormone use, smoking, highest degree earned, use of the following medications: aspirin, beta blockers, hydrochlorthiazide, ACE inihibitors, calcium channel blockers, other antihypertensive medications, insulin, oral hypoglycemics, lipid lowering medication. Moderate and vigorous activities were mutually adjusted for each other.
When we stratified the analyses by median estimated lean body mass, we found that higher total or vigorous physical activity was more strongly associated with a lower risk of frailty among those with lower lean body mass (Table 4). However, the association between moderate physical activity and risk for frailty did not appear to differ by lean body mass. In addition, physical activity was more strongly associated with lower risk of frailty among women <70 years than in those who were older. For example, HR comparing top to bottom quintile for total physical activity was 0.40 (0.36, 0.45) among women <70 years and 0.51 (0.47, 0.54) for those older.
Table 4:
Multivariablea hazard ratio (95% CI) for risk of frailty according to categories of cumulative average leisure time physical activity in women ≥60y stratified by lean body mass and ageb
| Quintiles of physical activity (MET hours/wk) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P trend | P interaction | |
|
| |||||||
| Total MET hours/wk | |||||||
| Lean body mass < median | 1 | 0.74 (0.69, 0.80) | 0.65 (0.60, 0.70) | 0.54 (0.50, 0.58) | 0.44 (0.40, 0.48) | <0.001 | |
| Lean body mass >= median | 1 | 0.80 (0.76, 0.84) | 0.71 (0.67, 0.75) | 0.63 (0.59, 0.67) | 0.51 (0.47, 0.55) | <0.001 | 0.002 |
| Age <70 y | 1 | 0.70 (0.64, 0.75) | 0.59 (0.53, 0.64) | 0.48 (0.43, 0.53) | 0.40 (0.36, 0.45) | <0.001 | |
| Age >= 70y | 1 | 0.81 (0.77, 0.85) | 0.73 (0.69, 0.77) | 0.64 (0.60, 0.67) | 0.51 (0.47, 0.54) | <0.001 | <0.001 |
| Moderate activity MET hours/wk | |||||||
| Lean body mass < median | 1 | 0.76 (0.71, 0.82) | 0.71 (0.66, 0.77) | 0.61 (0.56, 0.66) | 0.49 (0.45, 0.54) | <0.001 | |
| Lean body mass >= median | 1 | 0.81 (0.77, 0.85) | 0.73 (0.69, 0.77) | 0.64 (0.60, 0.68) | 0.53 (0.49, 0.58) | <0.001 | 0.33 |
| Age <70 y | 1 | 0.74 (0.69, 0.81) | 0.62 (0.56, 0.67) | 0.53 (0.48, 0.58) | 0.47 (0.41, 0.53) | <0.001 | |
| Age >= 70y | 1 | 0.82 (0.78, 0.86) | 0.77 (0.73, 0.81) | 0.67 (0.63, 0.70) | 0.53 (0.49, 0.56) | <0.001 | <0.001 |
| Vigorous activity MET hours/wk | |||||||
| Lean body mass < median | 1 | 0.92 (0.86, 0.99) | 0.81 (0.75, 0.87) | 0.75 (0.70, 0.82) | 0.70 (0.65, 0.77) | <0.001 | |
| Lean body mass >= median | 1 | 0.90 (0.85, 0.95) | 0.87 (0.83, 0.92) | 0.83 (0.78, 0.88) | 0.79 (0.73, 0.85) | <0.001 | 0.004 |
| Age <70 y | 1 | 0.84 (0.77, 0.91) | 0.76 (0.70, 0.83) | 0.72 (0.66, 0.79) | 0.64 (0.57, 0.71) | <0.001 | |
| Age >= 70y | 1 | 0.94 (0.89, 0.99) | 0.89 (0.84, 0.93) | 0.83 (0.79, 0.88) | 0.79 (0.75, 0.84) | <0.001 | <0.001 |
| Activity duration | (hour/wk) | ||||||
| <1.0 | 1 to <3.0 | 3.0 to <7.0 | 7.0 to <10.0 | ≥10.0 | |||
|
|
|||||||
| Physical Activity time | |||||||
| Lean body mass < median | 1 | 0.72 (0.67, 0.77) | 0.55 (0.51, 0.59) | 0.43 (0.38, 0.48) | 0.35 (0.30, 0.40) | <0.001 | |
| Lean body mass >= median | 1 | 0.79 (0.75, 0.83) | 0.63 (0.60, 0.67) | 0.50 (0.45, 0.55) | 0.47 (0.41, 0.53) | <0.001 | 0.02 |
| Age <70 y | 1 | 0.70 (0.65, 0.76) | 0.50 (0.46, 0.54) | 0.43 (0.37, 0.50) | 0.40 (0.34, 0.48) | <0.001 | |
| Age >= 70y | 1 | 0.80 (0.76, 0.84) | 0.64 (0.61, 0.68) | 0.49 (0.45, 0.53) | 0.41 (0.36, 0.46) | <0.001 | <0.001 |
| <1.0 | 1 to < 2.0 | 2 to < 4.0 | ≥4.0 | ||||
|
|
|||||||
| Moderate activity time | |||||||
| Lean body mass < median | 1 | 0.81 (0.75, 0.86) | 0.69 (0.64, 0.74) | 0.58 (0.53, 0.63) | <0.001 | ||
| Lean body mass >= median | 1 | 0.80 (0.76, 0.84) | 0.72 (0.69, 0.76) | 0.59 (0.55, 0.64) | <0.001 | 0.25 | |
| Age <70 y | 1 | 0.75 (0.70, 0.81) | 0.62 (0.57, 0.67) | 0.56 (0.50, 0.63) | <0.001 | ||
| Age >= 70y | 1 | 0.83 (0.79, 0.87) | 0.74 (0.71, 0.78) | 0.59 (0.55, 0.63) | <0.001 | <0.001 | |
| Vigorous activity time | |||||||
| Lean body mass < median | 1 | 0.80 (0.74, 0.85) | 0.78 (0.72, 0.84) | 0.67 (0.60, 0.74) | <0.001 | ||
| Lean body mass >= median | 1 | 0.88 (0.84, 0.93) | 0.86 (0.80, 0.91) | 0.76 (0.69, 0.83) | <0.001 | 0.005 | |
| Age <70 y | 1 | 0.81 (0.74, 0.88) | 0.70 (0.63, 0.77) | 0.73 (0.63, 0.84) | <0.001 | ||
| Age >= 70y | 1 | 0.86 (0.82, 0.90) | 0.87 (0.82, 0.92) | 0.71 (0.65, 0.77) | <0.001 | <0.001 | |
Multivariable adjusted for age, BMI, energy intake, alcohol intake, AHEI-2010 score, post-menopausal hormone use, smoking, highest degree earned, use of the following medications: aspirin, beta blockers, hydrochlorthiazide, ACE inihibitors, calcium channel blockers, other antihypertensive medications, insulin, oral hypoglycemics, lipid lowering medication. Moderate and vigorous activities were mutually adjusted for each other.
Lean body mass < median: case = 6129, Lean body mass >= median: case= 10,350; age < 70y: case= 4406, age >=70: case= 12,073
When examining 4- or 8-year change in physical activity, a higher risk of frailty was observed when activity reduction was ≥10 MET hour/wk or ≥ 3 hour/wk compared to women with little (± 2 MET hours/wk or ± 1 hour/wk) change (see Supplemental table 2, Supplemental Digital Content, Multivariable hazard ratio for risk of frailty according to categories of change in leisure time physical activity in women ≥60y). However, a progressively stronger inverse association was observed with increasing levels of physical activity level.
For sensitivity analysis, HR for latency of 4 years, 8 years, and 12 years were computed (see Supplemental table 3, Supplemental Digital Content, Multivariable hazard ratio for risk of frailty according to categories of leisure time physical activity in women ≥60y of different latency periods). While an attenuation of associations was observed with longer latency, the inverse association remained strong (HR comparing 10 hour/wk vs <1.0 hour/wk total physical activity was 0.69 (95% CI=0.65, 0.74) for a latency of 12 years. In addition, we examined the influence of the number of pre-existing frailty criteria at baseline as these women were closer to accumulating a total 3 criteria to meet the frailty definition. The inverse association between physical activity and frailty risk was generally stronger among women with fewer number of pre-existing criteria (Table 5). For example, HR for each 1 hour/d increase of total physical activity was 0.61 (95% CI=0.57, 0.65) among women with no pre-existing frailty criterion versus 0.71 (95%CI= 0.60, 0.84) for those with 2 pre-existing criteria. In examining the association between physical activity and individual frailty criterion among women with no pre-existing frailty criterion, inverse associations across different activity intensities were observed for fatigue, low aerobic capacity, and reduced resistance (see Supplemental table 4, Supplemental Digital Content, Multivariable hazard ratio for risk of specific frailty criteria among women with no pre-existing frailty criterion at baseline).
Table 5:
Multivariablea hazard ratio (95% CI) for risk of frailty according to the number of pre-existing frailty criteria at entry to follow-up
| 0 criterion (case= 7241) | 1 criterion (case=3832) | 2 criteria (case= 1380) | |
|---|---|---|---|
|
|
|||
| METs (per 3 MET hour/wk) | |||
| Total activity | 0.96 (0.95, 0.96) | 0.96 (0.95, 0.96) | 0.97 (0.96, 0.99) |
| Moderate activity | 0.92 (0.91, 0.93) | 0.92 (0.90, 0.94) | 0.94 (0.91. 0.97) |
| Vigorous activity | 0.98 (0.97, 0.99) | 0.98 (0.96, 0.99) | 0.99 (0.97, 1.01) |
| Duration (per 1 hour/d) | |||
| Total activity | 0.61 (0.57, 0.65) | 0.61 (0.55, 0.67) | 0.71 (0.60, 0.84) |
| Moderate activity | 0.55 (0.50, 0.60) | 0.58 (0.50, 0.67) | 0.66 (0.52, 0.84) |
| Vigorous activity | 0.70 (0.63, 0.78) | 0.65 (0.55, 0.77) | 0.79 (0.59, 1.05) |
Multivariable adjusted for age, BMI, energy intake, alcohol intake, AHEI-2010 score, post-menopausal hormone use, smoking, highest degree earned, use of the following medications: aspirin, beta blockers, hydrochlorthiazide, ACE inihibitors, calcium channel blockers, other antihypertensive medications, insulin, oral hypoglycemics, lipid lowering medication. Moderate and vigorous activities were mutually adjusted for each other.
For walking, we found that each hour per day of walking was strongly associated with a lower risk of frailty (HR=0.41, 95% CI=0.38, 0.44) (Figure 1) but was somewhat stronger for women <70 years than for older women (HR 0.36 vs 0.42, p interaction <0.001). The association did not differ by participation of upper or lower extremities weight training (p for interaction = 0.73) (see Supplemental table 5, Supplemental Digital Content, Multivariable hazard ratio risk of quintiles of walking by weight training status). And the inverse association held across different latency periods but attenuated with longer latency (Figure 1). It also remained regardless of the number of pre-existing frailty criteria, but it was slightly weaker with higher number of pre-existing criteria.
Figure 1:
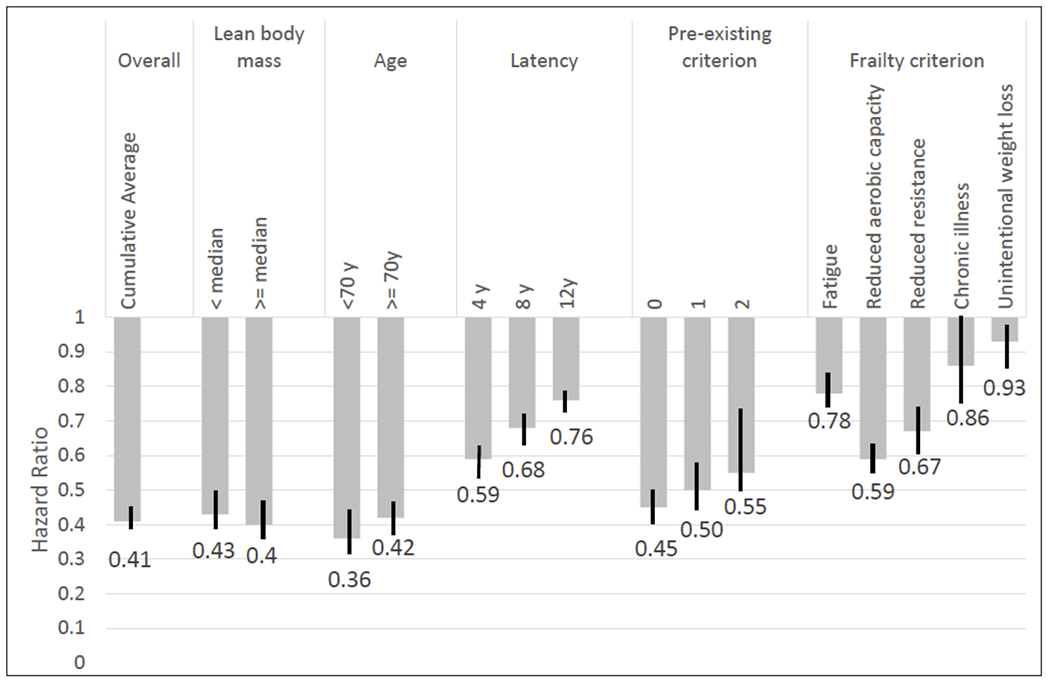
Multivariable hazard ration (95% CI) for risk of frailty according to walking (each 1h/day increment), overall and stratified by participants’ characteristics. Results are also presented for the risk of each individual frailty criterion among women with no pre-existing criterion.
Multivariable adjusted for age, BMI, energy intake, alcohol intake, AHEI-2010 score, post-menopausal hormone use, smoking, highest degree earned, use of the following medications: aspirin, beta blockers, hydrochlorthiazide, ACE inihibitors, calcium channel blockers, other antihypertensive medications, insulin, oral hypoglycemics, lipid lowering medication, non-walking physical activity time.
DISCUSSION
We observed a clear inverse association between physical activity and risk of frailty in women aged 60 and older. Both moderate and vigorous intensity physical activity were associated with a lower frailty risk, even at durations of 30 minutes to <1 hr per day. Notably, walking, the most common physical activity in this cohort of women, was associated with a lower risk of frailty, even among those older than 70y or with one or two pre-existing frailty criteria. Various sensitivity analyses showed that our results were robust in subgroups within the cohort and unlikely to have substantial bias. Development of frailty has a varied progression rate (24). To our knowledge, these results on physical activity and walking are the first to demonstrate an association with lower risk of frailty in different latency periods.
Exercise trials have shown mixed results in reducing frailty risk. A 6-month strength and endurance exercise intervention showed a reduction of frailty score (25) but a longer multicomponent moderate intensity intervention involving strength training and walking carried out up to 3.5 years showed no effect on frailty risk (26). On the other hand, a 12-month intervention among pre-frail individuals showed a significantly lower progression to frailty (27). These trials focused on prescribed exercise programs and the durations were short relative to the development of frailty.
In prospective observational studies, results consistently showed that longer physical activity duration was associated with lower risk of frailty, regardless of how frailty was defined (5–10, 28). However, data were mixed for physical activity intensity. Some studies reported a lower risk for frailty only with vigorous intensity activity (5, 9, 28) but others reported significant inverse associations with both moderate and high intensity activities (6, 8). Our study with repeated measurements of physical activity showed that even moderate duration or moderate intensity physical activity were associated with a meaningful magnitude of lower risk.
Data on specific activity type and risk of frailty are scarce. While a number of studies showed walking was associated with a lower risk of death (29, 30), physical activity trials that targeted frailty used a combination of physical activity type and thus were difficult to compare. Among longitudinal studies, a 7-year Finnish study on city workers showed that conditioning exercise was associated with maintenance of good physical function (28).
Our results add to the body of literature supporting physical activity as a strategy to prevent frailty development. We also observed that a significant inverse association was achieved at moderate durations of activity The lack of difference between moderate and vigorous activity in our findings may suggest that any potential benefit may come from different types of activities. However, as the women in the cohort aged, the intensity of vigorous activity might decline while still performing activities are usually considered as vigorous. Hence the difference in intensity between moderate and vigorous categories of activities might not be greatly different.
Physical activity may reduce the development of frailty by maintaining or improving physical functioning, preventing falls and developing components of the frailty syndrome. Intervention trials generally showed improvements in balance (31), mobility (32, 33), or gait ability (34). However, results were mixed for fall risk, balance, functional ability, and muscle strength (32). Exercise trials appeared to have limited effectiveness in improving sarcopenia (35). On the other hand, resistance training multiple times a week showed improvement in gait and muscle strength. (34).
A major strength of this analysis is the repeated measurement of physical activity to capture changes as these women aged. Similarly, we had detailed and updated lifestyle information for better control of confounders. The long follow-up period allowed for exploration of different latency periods and examination of potential for reverse causation. We were able to examine various subgroups, individual components of frailty, and potential differential association due to pre-existing frailty characteristics.
Although we assessed specific types of physical activity, the list was not comprehensive. However, the list of leisure time physical activity was selected to represent the most common types and a validation study showed them to be suitable for use in this group of women (12). Although work related activity was not captured, women in this analysis were at least 60 years old, and progressively fewer were employed over the course of the follow-up period. We were unable to examine in detail other types of leisure time physical activities, apart from walking, since few women engaged in them.
The study of frailty development is complicated by the lack of a standard definition of frailty and a universally accepted measurement tool for large epidemiological studies. Nevertheless, our frailty definition was based on the FRAIL scale and captured the most currently agreed upon characteristics of frailty (15, 36). Reverse causation is also a possible bias in any observational study of physical activity and frailty development. Individuals becoming frail may have increasing mobility difficulties, and thus reduce their physical activity which could accelerate the progression to frailty. We addressed this possibility by examining different latency periods to capture the time in which physical activity level may not be affected by developing frailty. Although we did observe some attenuation of the inverse association between physical activity and frailty, the association remained significant with a 4-, 8-, or 12-year latency periods. Therefore, our results are unlikely to be due to reverse causation.
Both the World Health Organization (37) and the current Physical Activity Guidelines (14) for Americans recommend older adults to engage in multicomponent activities that include balance and strength training, as well as aerobic activities. Older adults should also aim for at least 150 min/week of moderate intensity or 75 minutes of vigorous intensity activities. Those who are unable due to chronic diseases or disability should aim to be as physical active as their condition allows (14). Our results support the current recommendation on overall activity time and intensity. In addition, walking as an endurance exercise promotes cardiopulmonary fitness and strengthens the lower extremities for better balance ability. It is also low cost and can be undertaken with limited location, time, and equipment.
CONCLUSIONS
In conclusion, physical activity, regardless of moderate or vigorous intensity, was associated with a lower risk of frailty. In particular, we observed an inverse association with walking at a level that is feasible for most people.
Supplementary Material
Figure S1 - Mean MET hrs/week for total and specific physical activities from 1992 to 2012
Table S1 - Spearman correlation coefficients of cumulative averages of physical activity
Table S2 - Multivariable hazard ratio (95% CI) for risk of frailty according to categories of change in leisure time physical activity in women ≥60y
Table S3 - Multivariable hazard ratio (95% CI) for risk of frailty according to categories of leisure time physical activity in women ≥60y of different latency periods
Table S4 - Multivariable hazard ratio (95% CI) for risk of specific frailty criteria among women with no pre-existing frailty criterion at baseline.
Table S5 - Multivariable hazard ratio (95% CI) for risk of quintiles of walking by weight training status
Acknowledgements
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine. This work is funded by the Instituto de Salud Carlos III, State Secretary of R+D+I of Spain, and FEDER/FSE grants FIS 20/1040, and NIH grant UM1 CA186107. None of the authors have any conflicts of interest to disclose.
Conflict of Interest and Funding Source:
This work is funded by the Instituto de Salud Carlos III, State Secretary of R+D+I of Spain, and FEDER/FSE grants FIS 20/1040, and NIH grant UM1 CA186107. None of the authors have any conflicts of interest to disclose. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2020;50(1):96–104. [DOI] [PubMed] [Google Scholar]
- 3.Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd T, Mold F, Jones C, et al. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019;19(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil-Salcedo A, Dugravot A, Fayosse A, et al. Healthy behaviors at age 50 years and frailty at older ages in a 20-year follow-up of the UK Whitehall II cohort: A longitudinal study. PLoS Med. 2020;17(7):e1003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederstrasser NG, Rogers NT, Bandelow S. Determinants of frailty development and progression using a multidimensional frailty index: evidence from the English Longitudinal Study of Ageing. PLoS One. 2019;14(10):e0223799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing. PLoS One. 2017;12(2):e0170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savela SL, Koistinen P, Stenholm S, et al. Leisure-time physical activity in midlife is related to old age frailty. J Gerontol A Biol Sci Med Sci. 2013;68(11):1433–8. [DOI] [PubMed] [Google Scholar]
- 10.Ye B, Chen H, Huang L, et al. Changes in frailty among community-dwelling Chinese older adults and its predictors: evidence from a two-year longitudinal study. BMC Geriatr. 2020;20(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. [DOI] [PubMed] [Google Scholar]
- 14.Services USDoHaH. 2008 Physical Activity Guidelines for Americans. Washington DC. 2018. [Google Scholar]
- 15.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardiner PA, Mishra GD, Dobson AJ. Validity and responsiveness of the FRAIL scale in a longitudinal cohort study of older australian women. J Am Med Fir Assoc. 2015;16(9):781–3. [DOI] [PubMed] [Google Scholar]
- 17.Rosas-Carrasco O, Cruz-Arenas E, Parra-Rodríguez L, García-González AI, Contreras-González LH, Szlejf C. Cross-cultural adaptation and validation of the FRAIL scale to assess frailty in Mexican adults. J Am Med Fir Assoc. 2016;17(12):1094–8. [DOI] [PubMed] [Google Scholar]
- 18.Mijnarends DM, Schols JMGA, Meijers JMM, et al. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: similarities and discrepancies. J Am Med Fir Assoc. 2015;16(4):301–8. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 20.Willett WC. Nutritional Epidemiology. United Kingdom: Oxford University Press; 2013. [Google Scholar]
- 21.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Keum N, Hu FB, et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br J Nutr. 2017;118(10):858–66. [DOI] [PubMed] [Google Scholar]
- 23.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verghese J, Ayers E, Sathyan S, et al. Trajectories of frailty in aging: prospective cohort study. PLoS One. 2021;16(7):e0253976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh T-J, Su S-C, Chen C-W, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trombetti A, Mélany H, Hsu F-C, et al. Effect of Physical Activity on Frailty: Secondary Analysis of a Randomized Controlled Trial. Ann Intern Med. 2018;168(5):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46(3):401–7. [DOI] [PubMed] [Google Scholar]
- 28.Lahti J, Laaksonen M, Lahelma E, Rahkonen O. The impact of physical activity on physical health functioning – a prospective study among middle-aged employees. Prev Med. 2010;50(5):246–50. [DOI] [PubMed] [Google Scholar]
- 29.Hall KS, Hyde ET, Bassett DR, et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act. 2020;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paluch AE, Gabriel KP, Fulton JE, et al. Steps per day and all-cause mortality in middle-aged adults in the Coronary Artery Risk Development in Young Adults study. JAMA Netw Open. 2021;4(9):e2124516–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanmore EK, Mavroeidi A, de Jong LD, et al. The effectiveness and cost-effectiveness of strength and balance Exergames to reduce falls risk for people aged 55 years and older in UK assisted living facilities: a multi-centre, cluster randomised controlled trial. BMC Med. 2019;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn NW, Gmelin T, Santanasto AJ, et al. Impact of baseline fatigue on a physical activity intervention to prevent mobility disability. J Am Geriatr Soc. 2020;68(3):619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadore EL, Rodriguez-Manas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira JS, Pinheiro MB, Fairhall N, et al. Evidence on physical activity and the prevention of frailty and sarcopenia among older people: a systematic review to inform the World Health Organization physical activity guidelines. J PhysAct Health. 2020;17(12):1247–58. [DOI] [PubMed] [Google Scholar]
- 36.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WH [Internet]. Physical Activity Fact Sheet. Geneva: 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/physical-activity. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 - Mean MET hrs/week for total and specific physical activities from 1992 to 2012
Table S1 - Spearman correlation coefficients of cumulative averages of physical activity
Table S2 - Multivariable hazard ratio (95% CI) for risk of frailty according to categories of change in leisure time physical activity in women ≥60y
Table S3 - Multivariable hazard ratio (95% CI) for risk of frailty according to categories of leisure time physical activity in women ≥60y of different latency periods
Table S4 - Multivariable hazard ratio (95% CI) for risk of specific frailty criteria among women with no pre-existing frailty criterion at baseline.
Table S5 - Multivariable hazard ratio (95% CI) for risk of quintiles of walking by weight training status


